Abstract
In this study, a cell wall-associated extracellular electron transfer (EET) was determined in the thermophilic Geobacillus sp. to utilize iron as a terminal electron acceptor. The direct extracellular transfer of its electrons was primarily linked to the cell wall cytochrome-c and diffusible redox mediators like flavins during the anoxic condition. Based on the azo dye decolouration and protein film voltammetry, it was revealed that, in the absence of surface polysaccharide and diffusible mediators, the cell wall-associated EET pathway was likely to be a favorable mechanism in Geobacillus sp. Since the permeability of such redox molecule is primarily limited to the cell wall, the electron transfer occurs by direct contact with cell wall-associated cytochrome and final electron acceptor. Furthermore, transfer of electrons with the help of redox shuttling molecules like riboflavin from cytochrome to cells, vice versa indicates that Geoabcillus sp. has adopted this unique pathway during an anoxic environment for its respiration.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-02917-2.
Keywords: Extracellular electron transfer, Thermophiles, Redox proteins, Mediators, Flavins
Introduction
In bioelectrochemistry, the dissimilarity metal reduction by microbes is inter-related, as ubiquitous membrane proteins, conductive materials, and shuttling compounds are usually involved (Coursolle et al. 2010; Fuller et al. 2014; García-Angulo 2017; Kotloski and Gralnick 2013; von Canstein et al. 2008; Wu et al. 2014, 2016). Several species of Gram-negative bacterial genera, including Shewanella spp. and Geobacter spp., are proposed as they are associated with dissimilatory redox activity (Lovley 1991; Lovley and Phillips 1988). Under anaerobic conditions, bioenergetics is usually accomplished by the transfer of its electrons(e−) from quinol moiety to a series of inner/outer membrane-bound multiheme c-type cytochromes before it reaches to final electron acceptors (Fernandes et al. 2017; Fuller et al. 2014; Hernandez and Newman 2001; Zhao and Gurumurthy 2016; Zhao et al. 2020). Generally, for such concept, bacteria usually develop two distinct mechanisms to deliver their electrons, i.e., (1) direct contact with metal oxides using membrane-bound c-type cytochromes (Mtr, OMCs) (Coursolle et al. 2010; Hernandez and Newman 2001; Zhao and Gurumurthy 2016) and (2) by indirectly through soluble extracellular electron shuttles such as flavins and humic substances (Brutinel and Gralnick 2012; Fuller et al. 2014; Kotloski and Gralnick 2013; Lovley et al. 1996; Wu et al. 2014, 2016). The literature evidence suggests that the electron transfer from flavins is a reversible mechanism and will be carried out multiple redox cycles widely synthesized in various bacterial groups (Nevin and Lovley 2002), including thermophiles and archaea. To date, species like Shewanella spp., (von Canstein et al. 2008) and several methanotrophs (Balasubramanian et al. 2010) are able utilize flavins (i.e., flavin mononucleotide [FMN] and riboflavin) as their electron shuttles to reduce metal oxides. On the other hand, electrons released into the outer environment are always dependent on the bacteria type; for example, Gram-positive bacteria consist of thick rigid cell walls, which act as a barrier to their movement (Wu et al. 2014). Hence, the difference in the membrane topology of Gram-positive and Gram-negative bacteria, it is difficult to predict that the electron flow in the respiratory chain outside of the cell membranes. However, in Gram-negative bacteria, several compounds, including dual reduction (precipitate) pattern, were observed at the cell surface and also in the inner compartments for uranium (VI) and Cr (VI) (Shi et al. 2011; Wall and Krumholz 2006). Hence, microbially reduced uranium, Cr (III), and dimethyl sulfoxide (DMSO) are found at the cell surface and in the periplasm (Cologgi et al. 2011).
However, there is no detailed information available on extracellular electron transfer mechanisms (EET) in thermophiles. Thermophiles catalyze the dissimilatory redox reactions for ferrous iron are distinct, both phylogenetically and in their physiology. Our understanding of thermophiles utilizes Fe (III) as a terminal electron acceptor in anaerobic conditions is still unclear. It has been known that iron is a paradigm for versatile dissimilatory metabolism by microbes (Fuller et al. 2014; Lovley 1991; Nevin and Lovley 2002; Richter et al. 2012). In soil, iron occurs either in the form of Fe (III) under relatively oxic conditions and Fe (II) under reducing conditions (Lloyd 2003; Lovley 1991).
On the other hand, at pH 8 and above, most Fe (III) has become insoluble, and hence, certain microbes have been known to promote extracellular respiration in anoxic conditions (Fuller et al. 2014). Furthermore, the temperature is also a challenging factor along with pH as they grow between 45 and 65 °C compared to the mesophilic counterpart (Popova et al. 2002). Henceforth, we studied here growth characteristics of a thermophilic Geobacillus sp. under Fe (III)-containing anoxic medium. Furthermore, cell wall-associated EET in Fe (III) reduction was also analyzed using various analytical methods. The obtained results revealed that a thermophilic bacterium, Geobacillus sp., could reduce Fe (III) by transferring its electrons extracellularly via cell wall-associated cytochrome and riboflavins to the terminal electron acceptor.
Materials and methods
Bacterial growth medium
All the experiments were maintained in strict anaerobic conditions with nitrogen (N2). Geobacillus sp. used in this study was previously isolated (Gurumurthy and Neelagund 2010). Fuller and co-group described the medium used in this study with slight modifications (Fuller et al. 2014). The headspaces, reagents, solutions, and medium were flushed with nitrogen (N2) for 30 min in glass serum bottles before use and sealed with butyl rubber stoppers and aluminum crimps. Aerobically grown cells (Geobacillus sp.) from a mid-log phase that were collected from the PBTA (1.5% peptone, 0.2% beef extract, 0.5% tryptone, 0.1% NaCl, 2% agar, and pH 7.0) medium (Gurumurthy and Neelagund 2010) were then washed thrice with 10 mM phosphate buffer saline (PBS). Yeast extract free medium containing NaH2PO4·H2O (0.356 g/L) and KCl (0.1 g/L) was prepared. The pH of the medium was adjusted to 8.0 with 1 N NaOH and sterilized.
Furthermore, 5 mL/L of each standard vitamin and mineral mixture were added to the above medium through a 0.45 µM membrane filter. In addition to this, Fe (III) citrate (2 g/L) and glucose (20 mM) were added (through 0.45 µM membrane filter) as a sole source of electron acceptors and donors, respectively. Cells were then transferred aseptically to a sterilized medium. Finally, alternative electron donors such as acetate, L-lactate, ethanol, methanol were added (through 0.45 µM membrane filter) at the same concentration as glucose as separate tests. All the bottles were incubated between 7 and 10 days at 55 °C; the changes in the color of the medium from red to black indicated iron reduction by the bacteria.
Fe (III) reduction and sensitive redox assay
Ferrozine assay was performed for Fe (III) reduction activity in membrane fractions. The chromophore formed by ferrous iron and ferrozine was measured at 562 nm (Moody and Dailey 1983; Myers and Myers 1992). In addition, the presence of redox mediators was determined by testing rates of decolouration of the azo dye Direct Blue 53 (von Canstein et al. 2008).
Electron microscopy
For scanning electron microscope, 2 mL of anaerobic culture samples was collected from a serum bottle and pelleted by centrifugation at 13,300×g for 5 min. The pellets were then resuspended in deionized water to remove the remaining Na2CO3 and re-centrifuged for 5 min. For cell fixation, 2% glutaraldehyde was used in the copper crucible. The SEM analysis was performed on FEI Quanta 650 FEG-ESEM scanning electron microscope. Energy-dispersive X-ray spectra were collected with an Oxford X-max 80 SDD (liquid nitrogen-free) energy-dispersive X-ray spectroscopy (EDS) detector, and images were obtained in secondary electron imaging mode.
Extraction of cell wall-associated proteins
Cell wall-associated proteins are isolated from both aerobically and anaerobically grown Geobacillus sp. by the previously described methods for Gram-positive bacteria (Gurumurthy and Neelagund 2010). In brief, the cell pellets were centrifuged at 6000×g for 20 min at 4 °C and kept in an ice bath for 5 min before resuspension. The pellets were then resuspended in the 5 mL of ice-chilled 0.1 M Tris–EDTA containing 1.0 mM PMSF and followed by treatment with 1.15 mL of ice-cold mutanolysin. Further centrifugation at 6000×g for 20 min at 4 °C each time of resuspension, the pellets were then transferred to a sterile microcentrifuge tube and incubated for two hours at 37 °C in a shaker (200 rpm). After digestion, the samples were re-centrifuged at 14,000×g for 5 min at room temperature. Finally, the solubilized cell wall-associated proteins from the supernatants were collected and stored in aliquots at − 20 °C for experimental uses (Cole et al. 2008).
Protein film voltammetry (PFV)
A standard PFV procedure was performed as described previously by Anderson and co-group with minor modifications (Anderson et al. 2001). The standard Cytochrome c, riboflavin, FMN, and FAD were purchased from Sigma (Sigma Inc., USA). The cyclic voltammetry studies were performed on CHI 660D (CH Instruments, Texas, USA) using three-electrode systems. The electrodes used were a silica polished glassy carbon electrode, a platinum wire, and a saturated Ag/AgCl (3 mol L−1 KCl) as the working (WE), counter, and reference electrodes (RE), respectively. All the potentials recorded in the study are vs the saturated Ag/AgCl in PBS 10 mmol L−1, pH = 8 at 55 °C under N2–CO2 (vol/vol, 80:20) atmosphere. The aliquots were allowed to cool to room temperature and drop-casted with 1 μL of 1% Nafion on the Glassy carbon (GC) electrode. The same procedure was employed for standards that were dissolved previously in 10 mM PBS with subsequent filtering through 0.22 µM membrane filters. Cyclic Voltammetry (CV) parameters were used are; Ei − 0.6 V; Ef: − 0.6 V; scan rate was 10 mV s−1. For Differential pulse voltammetry (DPV) to study the oxidation reactions, the staircase was 0.004 V, the initial potential was Ei − 0.60 V, and the final potential was Ef − 0.20 V (Wu et al. 2014). The parameters were unchanged during experiments with 10 mM Fe (III) citrate into the electrochemical cell. All the tests were performed under dark conditions.
Homogenization and cell wall-associated redox proteins/flavin analysis
All the optical measurements were recorded in UV 1200 spectrophotometer (MAPADA, China). The crude cell wall-associated proteins from the aliquots were precipitated with trichloroacetic acid (TCA). The precipitated proteins were dialyzed in cellulose-membrane (10 kDa) and then were centrifuged at 5000 × g for 5 min at 4 °C to collect the pellets. The pellets were then suspended in a small amount of phosphate buffer pH 8.0 (10 mM) and lyophilized. The microplate direct dilution method and the following spectroscopic method were employed to determine protein concentration (Mulla et al. 2016).
Furthermore, the absorption spectrum and HPLC were performed for the redox mediators, such as flavins identification and discrimination from the crude cell wall extracts (Wu et al. 2014). The method employed for flavins as follows for UPLC–Qtof/MS analysis. In brief, an ACQUITY BEH C18 (1.7 µm, 50 mm) reverse phase column (Waters, MA) and fluorescence detector (Waters) was used with an excitation wavelength of 440 nm and an emission wavelength of 525 nm. The column wash was performed with 200 μL of 1:1 water/acetonitrile followed by 600 μL 95:5 water/acetonitrile wash. The linear gradient separation was performed with ethanol:water (%, 50:50) mobile phase at the flow rate of 1 mL/min at 40 °C. 2 μL of samples was injected, followed by column wash as described previously. Eluted compounds were analyzed by MS and MS–MS using a Thermo Electron LCQ Ion Trap Spectrometer (Thermo Scientific) operated in the positive ion mode.
For identification, any cytochromes type-c in the samples of aliquots were first dialyzed against 20 mMHEPES, pH 7.5, 100 mMNaCl, 0.5% (wt/vol) 3-[(3-cholamidopropyl) dimethylammonio]-1-propane sulfonate (CHAPS) detergent and concentrated by ultrafiltration (10 kDa cutoff membrane) and stored at − 20 °C. The concentration of cytochromes type-c was determined by UV–visible spectroscopy of the air-equilibrated proteins using experimentally determined millimolar absorbance coefficient (ε410 nm = 1260 mM−1 cm−1). For quantification of the number of covalently ligated c-type hemes attached to cytochrome-c, conversion into pyridine derivatives was achieved by incubating protein (3 μM) with pyridine (2.1 M) and NaOH (75 mM) in the water at room temperature for 15 min. Sodium dithionite and potassium ferricyanide were then added to separate aliquots of the resulting solution such that the final concentrations of protein, reductant, and oxidant were 2.5 μM, 1.5 mM, and 750 μM, respectively. Heme content was determined using the difference molar absorption coefficient of 19.1 mM−1 cm−1 at 550 nm for the pyridine ferrihemochrome minus the pyridine ferrihemochrome (Berry and Trumpower 1987). After dialysis, the concentrated proteins were resuspended in 1X SDS loading buffer and boiled for 5 min at 95 °C to detect c-type cytochromes. Furthermore, the cytochrome proteins were separated using a 10% SDS-PAGE (Laemmli 1970). Staining of heme was performed using 3,3′,5,5′-tetramethylbenzidine dihydrochloride (TMB) (Thomas et al. 1976). The exact molecular weight of Cyt-c was determined from the peak position in MALDI-TOF–MS (Ultraflex TOF-TOF Bruker Daltonics, Bremen, Germany) described by the service provider.
Results and discussion
Bacterial growth and Fe (III) reduction
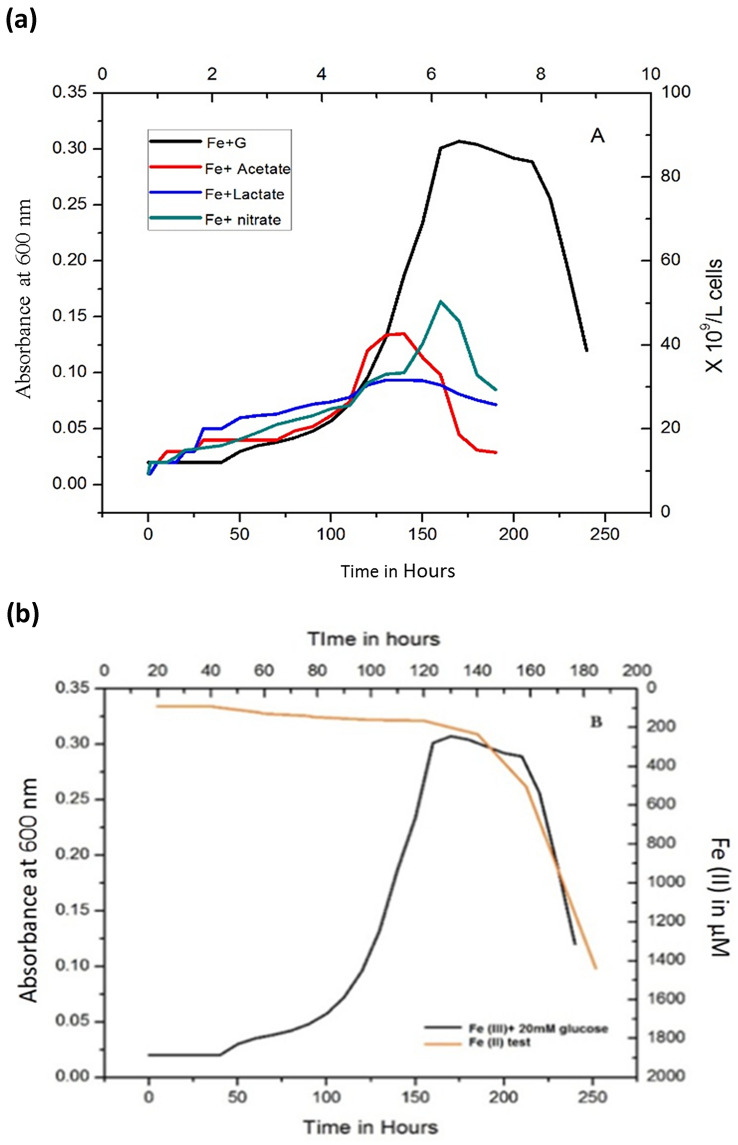
Since the discovery of role of extracellular electron transfer (EET) in bacteria (Hernandez and Newman 2001; Reguera et al. 2005) and some yeasts (Wu et al. 2014, 2016), various mechanisms have been postulated. In contrast to Gram-positive strains (Carlson et al. 2012; Dana 1997), the mechanism of EET coupled with the metal reduction in Gram-negative Shewanella (Brutinel and Gralnick 2012; Fernandes et al. 2017; Gurav et al. 2020; Hernandez and Newman 2001; Kotloski and Gralnick 2013; Lovley 1991; Myers and Myers 1992; Tokunou et al. 2016; von Canstein et al. 2008; White et al. 2013) and Geobacter strains was widely studied (Cologgi et al. 2011; Gurumurthy and Neelagund 2010; Hernandez and Newman 2001; Lovley 1991; Richter et al. 2012; Zhao and Gurumurthy 2016). Although, several reports were demonstrated on EET-dependent metal reduction for alkaliphiles (Fuller et al. 2014) and thermophiles (Carlson et al. 2012; Lusk 2019; Mohan et al. 2014; Torres et al. 2010). However, there is no concluding evidence that supports the respiratory pathway for electron transfer was available. In this concern, we undertook this research to understand the basics of the EET mechanism in thermophiles. It was known that some strains of Gram-positive thermophile Geobacillus spp. exhibit a respiratory metabolism (Gurumurthy et al. 2019). Moreover, they are capable of anaerobic growth on a nitrate-containing medium (Popova et al. 2002). However, none of the reports available previously concerns the dissimilatory metal reduction (DMR) properties and redox behavior of Geobacillus spp. In this study, the thermophilic bacteria Geobacillus sp. grew anaerobically at 55 °C, pH 8.0 for 240 h with glucose, and Fe (III) as an electron donor and acceptor, respectively (Fig. 1a). The lag phase, where the number of cells/L stayed roughly constant for 48 h of initial inoculation, was observed. Then, the cell numbers exponentially increased to a peak of ∼ 90 × 109 cells/L; after that, declination was observed between 210 and 240 h. The same trend for the initial lag phase was observed for acetate and L-lactate, but 40 h earlier. However, their declination was rapid, and measurements were completed between 160 and 170 h of cultivation. With glucose in the medium, only a negligible Fe (II) concentration was recorded until 120 h of incubation, and when it reaches a maximum of ∼ 1450 μM Fe (II) concentration post 120–170 h of incubation (Fig. 1B). The pH value of 8.0 was unchanged throughout the time of incubation and analysis. Unlike glucose, acetate, and lactate, the bacteria could not utilize ethanol and methanol as electron donors for Fe (III) reduction. Previously, Fuller and co-group demonstrated that the alkaliphilic bacterial growth was observed only in sucrose or ethanol along with the low concentration of yeast extract in the culture medium. On the other hand, in the absence of these, bacterial growth deteriorates to some extent (Fuller et al. 2014). In the present study, our observation suggested that the lack of base supplements such as yeast extract in culture medium indicated that a unique metabolic process adaptation in Geobacillus sp. during Fe (III) reduction. Moreover, with the use of various alternative electron donors, the bacteria can only grow and reduce Fe (III) with either glucose: ethanol and acetate: ethanol (as appropriate) with the subsequent transfer. However, any combination of methanol and lactate did not show any vital growth and Fe (III) reduction.
Fig. 1.
Growth characteristics of Geobacillus sp. in Fe (III) citrate containing an anoxic medium. a The pattern of growth with different 20 mM electron donors at 55 °C, pH 8. G glucose (black), A acetate (red), L-lactate (blue) simulated at same condition. b Fe (II) increased concentration in the medium was seen after 120 h of incubation and reached 170 h
Furthermore, the aerobically grown Gram-positive Geobacillus sp. produces surface polysaccharides. However, to our surprise, the production was utterly absent in the anoxic condition (Fig. 2A, B). The reason behind this is unclear. However, it may be proposed that bacteria evolved a mechanism to preserve the energy required for polysaccharide production during anaerobic respiration. This evidence led us to explore the functionary mechanism adopted by this thermophile for Fe (III) and other metals reduction due to the direct exposure of its cell surface. The black precipitates were separated and further analyzed for size using SEM revealed that approximately 5.0 μm in size and flattered structure, which was similar to previously reported morphology of Vivianite [Fe3(PO4)2·8H2O] (Dana 1997; Fuller et al. 2014). Vivianite is a typical mineral formed during Fe (III) reduction in a medium containing high concentrations of soluble phosphate (Bae and Lee 2013). Furthermore, the EDS analysis of crystals gave distinct spectral peaks for the O, P, and Fe peaks (Fig. 2C).
Fig. 2.
Geobacillus sp. SEM images recovered during the post lag phase with Fe (III) containing anoxic medium (a). Clear images showed the lack of surface polysaccharide around the Geobacillus sp. b Surface polysaccharide (EPS) produced cell grown aerobically. c EDS spectra of crystals/precipitates were showing Fe peaks
Protein film electrochemistry
Cyclic voltammetry studies revealed that cell wall extracts of Geobacillus sp. can transfer electron species with the Glassy carbon electrode (GC). Similar redox potential was observed for both standard and extract riboflavin at − 0.44 V, which showed a slight shift compared to other Gram-positive bacteria and yeast (Wu et al. 2014). For cytochrome, the redox potential was recorded at − 0.23 V when grown in the presence of Fe (III) citrate (Fig. 3). However, this redox potential for cytochrome-c was not reported previously, and it might be due to the temperature sensitivity experiments that may affect the blanked energy equilibrium at a considerable rate. In addition, the limitation in the concentration availability of the redox rate enhanced within the cell wall content may harm the structural features or due to the drawbacks of isolation of procedure. Nevertheless, this evidence was confirmed that the extract contains riboflavin, and cytochromes are the possible mode of electron transfer mechanism that existed in the Geobacillus sp.
Fig. 3.
Protein film voltammetry (PFV) of cell wall extract of Geobacillus sp. grown in Fe(III) citrate anoxic medium showing potential for riboflavin (− 0.44 V) and cytochrome (− 0.23 V). The red line indicates the blank extract
Analysis of extracellular respiration pathway
The presence of cytochrome and other redox-active molecules after Fe (III) reduction, the cell wall extracts, and the medium supernatant were investigated using an azo dye decolouration method described for Shewanella (von Canstein et al. 2008). A positive test for dye discolouration was observed in cell wall extracts isolated from the culture with Fe (III) reduction, but not for culture supernatant and control Geobacillus sp. cell wall extract. The protein yield in reduced cell wall extract was low; however, the presence of redox-sensitive molecules was observed by the faint decolouration of azo dye. This direct evidence suggested that various shuttling compounds or outer membrane proteins are metabolically produced by Geobacillus sp. for the Fe (III) redox activity.
On the other hand, the absence of redox peaks and negative for azo dye decolouration for supernatants indicate that the cell wall of Geobacillus sp. may be acting as a barrier for non-movement of compounds communicating outside the cell. Hence, further analysis of supernatants was omitted due to the absence of any redox mediators. To investigate whether a membrane-bound electron-shuttling pathway was involved in Fe (III) reduction by Geobacillus, the proteins spectral properties of cell wall extract after dialysis and flavin moiety before TCA precipitation was studied. Scanning of the extract over a wavelength range from 200 to 700 nm revealed spectral features similar to riboflavin and cytochromes-c (Fuller et al. 2014). The presence of three absorption peaks for reduced cytochrome at 550, 521 nm as α and β peaks, respectively, and a Soret (γ) peak at 414 nm indicated that cytochrome-c was in the reduced form [before and after Fe (II) formation]. However, upon the addition of dithionite, the Soret (γ) peak shifts to 419 nm, and defined α and β peaks are observed at 547 and 520 nm. The pyridine hemochrome displays an α absorption peak at 550 nm, typical of low-spin c-type cytochromes (Fig. 4) (Berry and Trumpower 1987). The quantitative analysis revealed that 10.8 heme C per mg total cytochrome-c (average value from five repeat experiments) was covalently attached, as it correlates with the ten predicted protein sequence responsible for CXXCH heme attachment.
Fig. 4.

The typical absorption spectrum of cell wall extract showing absorbance for riboflavin and cytochrome at respective locations corresponds to the standards. For cytochrome /protein analysis, the CW extract was precipitated with TCA and dialyzed. The dialysate was then subjected to spectral observation at a range of 200–600 nm. For flavin analysis, before TCA precipitation, crude CW extract was used for analysis
Furthermore, riboflavin also yields three absorption peaks between 250 and 500 nm at 270, 350, and 446 nm. Similar absorption peaks were also observed in riboflavin using sodium borate buffer at pH 7.52 (Bartzatt and Wol 2014). However, none of the other spectral peaks was found. Thus, it indicates that these cytochrome and riboflavin molecules are the primary pathway for redox electron transfer across the bacterial cell wall.
To conform this results, we developed a standard redox-active elution pattern in HPLC using commercially available riboflavin alone. A single faint yellow color separation peak at 12 min indicates the presence of the riboflavin in the cell wall extract of the bacterium (Fig. 5). On the other hand, results from LCMS also revealed the presence of the single m/z 380 peaks for the 18-min fraction corresponding to riboflavin. Furthermore, the migration pattern of cytochrome-c in SDS-PAGE revealed approximately 55 kDa band in both CMB and heme staining, which was smaller in molecular weight compared to MtrC from Shewanella oneidensis MR-1 (Fig. 6A and B) (Hartshorne et al. 2007). A single MALDI spectral peak also confirms the exact weight of cytochromes-c (m/z 55,197), confirming the PAGE results (Fig. 6C). Based on these results, the possible direct mechanistic electron transfer pathway adopted by Geobacillus sp. during anoxic Fe (III) reduction was depicted precisely in Fig. S1 (Supplementary information).
Fig. 5.
High-performance liquid chromatography (HPLC) chromatogram of cell wall extract showing riboflavin moiety (CW-RF). The elution was determined at 12 min after the injection of samples—a similar chromatogram for also shown for standard riboflavin (RF Std)
Fig. 6.
a SDS-PAGE of cell wall extract cytochrome shows the typical band at 55 kDa compared to standards. b Heme staining/peroxidase activity of native/non-reduced cytochrome
The bacteria involved in metal reduction using electron transfer pathways that could transfer extracellularly is debated among the scientific communities. Some of the bacteria capable of iron redox transformation are currently characterized in detail (Fuller et al. 2014; Lloyd 2003; Reguera et al. 2005). In contrast, Gram-negative dissimilatory metal reduction bacteria (DMRB) strains were extensively studied for indirect pathways using electron shuttles (Marsili et al. 2008; von Canstein et al. 2008) or by direct reduction through surface-mediated electron transfer involving cytochromes (Lloyd 2003; Lovley 1991) or conductive pili (Reguera et al. 2005). However, only a few studies were available in thermophilic bacterial communities (Carlson et al. 2012). The multiheme cytochrome pathway is the only known mechanism for EET involving Cysts in Gram-positive thermophiles from Thermincola potens (Lusk 2019). However, this pathway does not explain the observation of direct long-range EET present in Thermincola ferriacetica and some other Gram-positive thermophiles because it does not consider the transfer of electrons through a conductive or semi-conductive extracellular matrix (Lusk et al. 2015, 2016; Parameswaran et al. 2013). Other Gram-positive thermophiles include Thermoanaerobacter ethanolicus (Holmes et al. 2016) or Thermoanaerobacter pseudethanolicus (Lusk et al. 2015), C. ferrireducens (Gavrilov et al. 2021), also known for an exoelectrogenic character in nature. Besides, various research has shown that OMCs, Mtr, and other Cysts are the typical EET cascade like that of Gram-negative mesophiles has been documented (Bird et al. 2011; Dalla Vecchia et al. 2014; Lusk et al. 2015). Furthermore, the prevalence of OmhA and SmhA expression in C. ferrireducens as extracellular electron-transferring proteins are likely involved in exoelectrogenesis and a distinct SmhB is likely involved in the reduction of soluble Fe (III). Moreover, it was revealed that none of these cytochromes is not associated with porin–cytochrome complexes or pilin–cytochrome assemblies, and are phylogenetically distinct (Gavrilov et al. 2021).
Herein, an unusual Fe (III) reduction pathway was determined through direct contact of respiratory proteins associated with the thermophilic Geobacillus sp. cell wall. Though the cells were grown anaerobically at 55 °C under a mild alkaline environment, Fe (III) reduction was observed much faster than previously reported alkalophilic bacteria (Fuller et al. 2014). Moreover, the use of complex organic compounds such as yeast extract was omitted due to the possible interference as electron mediators (Fuller et al. 2014). Various alternative electron donors such as acetate, L-lactate, ethanol, and methanol were also studied in the case of Fe (III) reduction. However, lower productive Fe (II) precipitation was observed in acetate and L-lactate, indicating the specific metabolic, respiratory system adapted to the glucose. Other donors such as ethanol and methanol are unresponsive for Fe (III) reduction even in a combined attempt with lactate and acetate (data not shown). Furthermore, the rapid detection of Fe (II) in ferrozine assay (less than 2 min) indicating the less possible interference of any other electron donors or acceptors in the system. The azo dye decolourization test yielded negative for culture supernatant because a thick and rigid cell wall of Gram-positive restricts the movement of compounds across the cell. In addition, the thermal degradation of cellular components at a higher temperature was an affecting factor after 240 h during the death phase or sporulation.
Furthermore, the absence of EPS around the cell in the anoxic environment lacks our understanding of the metabolic properties in these thermophiles. This mechanism may be believed the same as energy-conserving hydrogenase (Ech) activity in Thermoanaerobacter kivui, where cytochromes and proton gradient mechanism were absent due to the H+ dependency (Hess et al. 2014). Since, the energy limitation and availability of substrates under an anaerobic environment may suppress the expression of specific gene sequences for EPS production. Expect the potential generated by flavoprotein and cytochrome; no other potential was observed for cell wall extracted protein films. The potential for riboflavin was similar to the previously reported Gram-positive bacteria (Wu et al. 2014). Riboflavin occurs as a constituent of the two flavin prosthetic groups of flavoproteins, i.e., FAD and FMN. These flavins can undergo oxidation–reduction reactions through the stepwise reversible addition of two electrons via the semiquinone form to the colorless reduced form (von Canstein et al. 2008). In cytochrome, the potential at − 0.23 V lies in the typical range for type-c cytochromes of other bacteria (Kracke et al. 2015).
Moreover, a small 55 kDa MW cytochrome-c may not limit the electron-transferring function but also appears to function similarly to other Geobacter and S. oneidensis OMC. So far, it is known that the respiratory electrons enter the periplasm of Geobacter cells through a small soluble triheme cytochrome PpcA, which serves as an intermediary periplasmic electron-transferring protein. Currently, in the genome of G. sulfurreducens has been identified genes for at least 30 outer membrane cytochromes, out of which only five (OmcB, OmcS, OmcT, OmcE, OmcZ), including S. oneidensis MtrC, was involved in ferric iron reduction (Coursolle et al. 2010; Hartshorne et al. 2007; Richter et al. 2012). G. sulfurreducens OmcB is an essential protein involved in the reduction of both insoluble iron species and ferric citrate (Leang et al. 2003). The deletion of the omcB gene leads to sharply decreased growth rates in media with ferric citrate and no growth with ferric oxide as the electron acceptor (Leang et al. 2003). In contrast, EET pathway of Gram-positive T. ferriacetica, it was proposed that the three multiheme c-cytochromes, Tfer_0070 (ImdcA), Tfer_0075 (CwcA), and Tfer_1887 (PdcA), are involved. Based on the electrochemical characterization, only ImdcA and PdcA were studied in support of the pathway. Another protein, CwcA, could not be stabilized in solution, and based on the homology with the OmcS, a structural model for CwcA was developed, providing a molecular perspective into the mechanisms of electron transfer across the peptidoglycan layer in Thermincola (Faustino et al. 2021).
Conclusions
In this study, an associated pathway of extracellular electron transfer (EET) was identified in the thermophilic Geobacillus sp., which reduces Fe (III) terminal electron acceptor. Fe (III) reduction is mainly dependent on bacterial growth in anoxic conditions. In the absence of EPS and other diffusible redox mediators, it was revealed that Geobacillus sp. might have evolved an alternative mechanism for direct extracellular electron transfer (DEET). The un-diffusible cell wall redox cytochrome-c (55 kDa) and the associated riboflavin is responsible for the anoxic Fe (III) reduction. LCMS and electrochemical analysis revealed prominence of these two redox molecules in the cell wall extract based on redox-sensitive decolourization. Further, the pathway was conceptualized as the terminal electron acceptor directly attaches the cell wall-associated donor cytochrome and riboflavins, an electron shuttle acting between the membrane and cell wall of the bacterium. Hence, in a thermophilic bacterium, the cell wall-associated pathway is only favorable in an extracellular metal reduction under anoxic conditions.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We want to thank Dr. Shivayogeeswar Neelagund, Associate Professor, Department of Biochemistry, Kuvempu University, for his constant support throughout the process. DMG would like to thank all colleagues from the Department of Biotechnology, GM Institute of Technology, Davangere. SIM would like to thank all colleagues from the Department of Biochemistry, School of Applied Science, REVA University, Bangalore.
Author contributions
DMG and SIM conceived and designed the study. DMG and SIM performed experimental works. DMG, LFRF, AK, GDS, MB, SKG and SIM analyzed the data. DMG, VDR and SIM wrote the paper. GDS, UG, MB, and SKG helped to revise the final draft. All the authors read and approved the final manuscript.
Funding
Not applicable.
Declarations
Conflict of interest
The authors ensure no conflict of interest exist.
Contributor Information
Dummi Mahadevan Gurumurthy, Email: drgurumurthydm@gmit.ac.in.
Sikandar I. Mulla, Email: sikandar.mulla@gmail.com, Email: sikandar.mulla@reva.edu.in
References
- Anderson LJ, Richardson DJ, Butt JN. Catalytic protein film voltammetry from a respiratory nitrate reductase provides evidence for complex electrochemical modulation of enzyme activity. Biochemistry. 2001;40:11294–11307. doi: 10.1021/bi002706b. [DOI] [PubMed] [Google Scholar]
- Bae S, Lee W. Biotransformation of lepidocrocite in the presence of quinones and flavins. Geochim Cosmochim Acta. 2013;114:144–215. doi: 10.1016/j.gca.2013.03.041. [DOI] [Google Scholar]
- Balasubramanian R, Levinson BT, Rosenzweig AC. Secretion of flavins by three species of methanotrophic bacteria. Appl Environ Microbiol. 2010;76:7356–7358. doi: 10.1128/AEM.00935-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzatt R, Wol T (2014) Detection and assay of Vitamin B-2 (Riboflavin) in alkaline borate buffer with UV/Visible spectrophotometry. Int Sch Res Notices 2014:Article ID 453085 [DOI] [PMC free article] [PubMed]
- Berry EA, Trumpower BL. Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal Biochem. 1987;161:1–15. doi: 10.1016/0003-2697(87)90643-9. [DOI] [PubMed] [Google Scholar]
- Bird LJ, Bonnefoy V, Newman DK. Bioenergetic challenges of microbial iron metabolisms. Trends Microbiol. 2011;19:330–340. doi: 10.1016/j.tim.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Brutinel ED, Gralnick JA. Shuttling happens: soluble flavin mediators of extracellular electron transfer in Shewanella. Appl Microbiol Biotechnol. 2012;93:41–48. doi: 10.1007/s00253-011-3653-0. [DOI] [PubMed] [Google Scholar]
- Carlson HK, Iavarone AT, Gorur A, Yeo BS, Tran R, Melnyk RA, Mathies RA, Auer M, Coates JD. Surface multiheme c-type cytochromes from Thermincola potens and implications for respiratory metal reduction by Gram-positive bacteria. Proc Natl Acad Sci USA. 2012;109:1702–1707. doi: 10.1073/pnas.1112905109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JN, Djordjevic SP, Walker MJ. Isolation and solubilization of gram-positive bacterial cell wall-associated proteins. In: Posch A, editor. 2D PAGE: sample preparation and fractionation. Totowa: Humana Press; 2008. pp. 295–311. [DOI] [PubMed] [Google Scholar]
- Cologgi DL, Lampa-Pastirk S, Speers AM, Kelly SD, Reguera G. Extracellular reduction of uranium via Geobacter conductive pili as a protective cellular mechanism. Proc Natl Acad Sci USA. 2011;108:15248–15252. doi: 10.1073/pnas.1108616108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coursolle D, Baron DB, Bond DR, Gralnick JA. The Mtr respiratory pathway is essential for reducing flavins and electrodes in Shewanella oneidensis. J Bacteriol. 2010;192:467–474. doi: 10.1128/JB.00925-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla Vecchia E, Shao PP, Suvorova E, Chiappe D, Hamelin R, Bernier-Latmani R. Characterization of the surfaceome of the metal-reducing bacterium Desulfotomaculum reducens. Front Microbiol. 2014;5:432. doi: 10.3389/fmicb.2014.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE Dana JD, Gaines RV. Dana’s new mineralogy: the system of mineralogy of James Dwight Dana and Edward Salisbury Dana. 8. Hoboken: Wiley-Blackwell; 1997. [Google Scholar]
- Faustino MM, Fonseca BM, Costa NL, Lousa D, Louro RO, Paquete CM. Crossing the wall: characterization of the multiheme cytochromes involved in the extracellular electron transfer pathway of Thermincola ferriacetica. Microorganisms. 2021;9:293. doi: 10.3390/microorganisms9020293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes AP, Nunes TC, Paquete CM, Salgueiro CA. Interaction studies between periplasmic cytochromes provide insights into extracellular electron transfer pathways of Geobacter sulfurreducens. Biochem J. 2017;474:797–808. doi: 10.1042/BCJ20161022. [DOI] [PubMed] [Google Scholar]
- Fuller SJ, McMillan DGG, Renz MB, Schmidt M, Burke IT, Stewart DI. Extracellular electron transport-mediated Fe(III) reduction by a community of alkaliphilic bacteria that use flavins as electron shuttles. Appl Environ Microbiol. 2014;80:128–137. doi: 10.1128/aem.02282-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Angulo VA. Overlapping riboflavin supply pathways in bacteria. Crit Rev Microbiol. 2017;43:196–209. doi: 10.1080/1040841X.2016.1192578. [DOI] [PubMed] [Google Scholar]
- Gavrilov SN, Zavarzina DG, Elizarov IM, Tikhonova TV, Dergousova NI, Popov VO, Lloyd JR, Knight D, El-Naggar MY, Pirbadian S, Leung KM, Robb FT, Zakhartsev MV, Bretschger O, Bonch-Osmolovskaya EA. Novel extracellular electron transfer channels in a gram-positive thermophilic bacterium. Front Microbiol. 2021;11:597818. doi: 10.3389/fmicb.2020.597818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurav R, Bhatia SK, Choi TR, Kim HJ, Song HS, Park SL, Lee SM, Lee HS, Kim SH, Yoon JJ, Yang YH. Utilization of different lignocellulosic hydrolysates as carbon source for electricity generation using novel Shewanella marisflavi BBL25. J Clean Prod. 2020;277:124084. doi: 10.1016/j.jclepro.2020.124084. [DOI] [Google Scholar]
- Gurumurthy DM, Neelagund SE. Geobacillus sp. Iso 5, a novel amylase-producing thermophile from thermal springs in Konkan region of Southern. Indian J Earth Sci. 2010;21:319–322. doi: 10.1007/s12583-010-0248-0. [DOI] [Google Scholar]
- Gurumurthy DM, Bharagava RN, Kumar A, Singh B, Ashfaq M, Saratale GD, Mulla SI. EPS bound flavins driven mediated electron transfer in thermophilic Geobacillus sp. Microbiol Res. 2019;229:126324. doi: 10.1016/j.micres.2019.126324. [DOI] [PubMed] [Google Scholar]
- Hartshorne RS, et al. Characterization of Shewanella oneidensis MtrC: a cell-surface decaheme cytochrome involved in respiratory electron transport to extracellular electron acceptors. J Biol Inorg Chem. 2007;12:1083–1094. doi: 10.1007/s00775-007-0278-y. [DOI] [PubMed] [Google Scholar]
- Hernandez ME, Newman DK. Extracellular electron transfer. Cell Mol Life Sci. 2001;58:1562–1571. doi: 10.1007/PL00000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess V, Poehlein A, Weghoff MC, Daniel R, Müller V. A genome-guided analysis of energy conservation in the thermophilic, cytochrome-free acetogenic bacterium Thermoanaerobacter kivui. BMC Genomics. 2014;15:1139. doi: 10.1186/1471-2164-15-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes DE, Dang Y, Walker DJF, Lovley DR. The electrically conductive pili of Geobacter species are a recently evolved feature for extracellular electron transfer. Microb Genom. 2016;2:000072. doi: 10.1099/mgen.0.000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloski NJ, Gralnick JA. Flavin electron shuttles dominate extracellular electron transfer by Shewanella oneidensis. Mbio. 2013;4:e00553–e1512. doi: 10.1128/mBio.00553-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kracke F, Vassilev I, Krömer JO. Microbial electron transport and energy conservation—the foundation for optimizing bioelectrochemical systems. Front Microbiol. 2015;6:575. doi: 10.3389/fmicb.2015.00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of Bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leang C, Coppi MV, Lovley DR. OmcB, a c-Type Polyheme Cytochrome, involved in Fe(III) reduction in Geobacter sulfurreducens. J Bacteriol. 2003;185:2096–2103. doi: 10.1128/JB.185.7.2096-2103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd JR. Microbial reduction of metals and radionuclides. FEMS Microbiol Rev. 2003;27:411–425. doi: 10.1016/S0168-6445(03)00044-5. [DOI] [PubMed] [Google Scholar]
- Lovley DR. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991;55:259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley DR, Phillips EJP. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988;54:1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley DR, Coates JD, Blunt-Harris EL, Phillips EJP, Woodward JC. Humic substances as electron acceptors for microbial respiration. Nature. 1996;382:445–448. doi: 10.1038/382445a0. [DOI] [Google Scholar]
- Lusk BG. Thermophiles; or, the Modern Prometheus: the importance of extreme microorganisms for understanding and applying extracellular electron transfer. Front Microbiol. 2019;10:818. doi: 10.3389/fmicb.2019.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusk BG, Khan QF, Parameswaran P, Hameed A, Ali N, Rittmann BE, Torres CI. Characterization of Electrical current-generation capabilities from thermophilic bacterium Thermoanaerobacter pseudethanolicus using xylose, glucose, cellobiose, or acetate with fixed anode potentials. Environ Sci Technol. 2015;49:14725–14731. doi: 10.1021/acs.est.5b04036. [DOI] [PubMed] [Google Scholar]
- Lusk BG, Parameswaran P, Popat SC, Rittmann BE, Torres CI. The effect of pH and buffer concentration on anode biofilms of Thermincola ferriacetica. Bioelectrochemistry. 2016;112:47–52. doi: 10.1016/j.bioelechem.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR. Shewanella secretes flavins that mediate extracellular electron transfer. Proc Natl Acad Sci USA. 2008;105:3968–3973. doi: 10.1073/pnas.0710525105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan SV, Velvizhi G, Krishna KV, Babu ML. Microbial catalyzed electrochemical systems: a bio-factory with multi-facet applications. Bioresour Technol. 2014;165:355–364. doi: 10.1016/j.biortech.2014.03.048. [DOI] [PubMed] [Google Scholar]
- Moody MD, Dailey HA. Aerobic ferrisiderophore reductase assay and activity stain for native polyacrylamide gels. Anal Biochem. 1983;134:235–239. doi: 10.1016/0003-2697(83)90290-7. [DOI] [PubMed] [Google Scholar]
- Mulla SI, Wang H, Sun Q, Hu A, Yu CP. Characterization of triclosan metabolism in Sphingomonas sp. strain YL-JM2C. Sci Rep. 2016;6:21965. doi: 10.1038/srep21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CR, Myers JM. Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J Bacteriol. 1992;174:3429–3438. doi: 10.1128/jb.174.11.3429-3438.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin KP, Lovley DR. Mechanisms for Fe(III) oxide reduction in sedimentary environments. Geomicrobiol J. 2002;19:141–159. doi: 10.1080/01490450252864253. [DOI] [Google Scholar]
- Parameswaran P, Bry T, Popat SC, Lusk BG, Rittmann BE, Torres CI. Kinetic, electrochemical, and microscopic characterization of the thermophilic, anode-respiring bacterium Thermincola ferriacetica. Environ Sci Technol. 2013;47:4934–4940. doi: 10.1021/es400321c. [DOI] [PubMed] [Google Scholar]
- Popova NA, Nikolaev YA, Tourova TP, Lysenko AM, Osipov GA, Verkhovtseva NV, Panikov NS. Geobacillus uralicus, a new species of thermophilic bacteria. Microbiology. 2002;71:335–341. doi: 10.1023/A:1015867014278. [DOI] [PubMed] [Google Scholar]
- Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR. Extracellular electron transfer via microbial nanowires. Nature. 2005;435:1098–1101. doi: 10.1038/nature03661. [DOI] [PubMed] [Google Scholar]
- Richter K, Schicklberger M, Gescher J. Dissimilatory reduction of extracellular electron acceptors in anaerobic respiration. Appl Environ Microbiol. 2012;78:913–921. doi: 10.1128/AEM.06803-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Belchik SM, Plymale AE, Heald S, Dohnalkova AC, Sybirna K, Bottin H, Squier TC, Zachara JM, Fredrickson JK. Purification and Characterization of the [NiFe]-Hydrogenase of Shewanella oneidensis MR-1. Appl Environ Microbiol. 2011;77:5584–5590. doi: 10.1128/AEM.00260-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PE, Ryan D, Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976;75:168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Tokunou Y, Hashimoto K, Okamoto A. Acceleration of extracellular electron transfer by alternative redox-active molecules to riboflavin for outer-membrane cytochrome c of Shewanella oneidensis MR-1. J Phys Chem C. 2016;120:16168–16173. doi: 10.1021/acs.jpcc.6b00349. [DOI] [Google Scholar]
- Torres CI, Marcus AK, Lee H-S, Parameswaran P, Krajmalnik-Brown R, Rittmann BE. A kinetic perspective on extracellular electron transfer by anode-respiring bacteria. FEMS Microbiol Rev. 2010;34:3–17. doi: 10.1111/j.1574-6976.2009.00191.x. [DOI] [PubMed] [Google Scholar]
- von Canstein H, Ogawa J, Shimizu S, Lloyd JR. Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl Environ Microbiol. 2008;74:615–623. doi: 10.1128/AEM.01387-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall JD, Krumholz LR. Uranium Reduction. Annu Rev Microbiol. 2006;60:149–166. doi: 10.1146/annurev.micro.59.030804.121357. [DOI] [PubMed] [Google Scholar]
- White GF, Shi Z, Shi L, Wang Z, Dohnalkova AC, Marshall MJ, Fredrickson JK, Zachara JM, Butt JN, Richardson DJ, Clarke TA. Rapid electron exchange between surface-exposed bacterial cytochromes and Fe(III) minerals. Proc Natl Acad Sci U S A. 2013;110:6346–6351. doi: 10.1073/pnas.1220074110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Xiao Y, Wang L, Zheng Y, Chang K, Zheng Z, Yang Z, Varcoe JR, Zhao F. Extracellular electron transfer mediated by flavins in gram-positive Bacillus sp. WS-XY1 and Yeast Pichia stipitis. Electrochim Acta. 2014;146:564–567. doi: 10.1016/j.electacta.2014.09.096. [DOI] [Google Scholar]
- Wu S, Xiao Y, Song P, Wang C, Yang Z, Slade RCT, Zhao F. Riboflavin-mediated extracellular electron transfer process involving Pachysolen tannophilus. Electrochim Acta. 2016;210:117–121. doi: 10.1016/j.electacta.2016.05.139. [DOI] [Google Scholar]
- Zhao F, Gurumurthy DM. 2016. Chapter 16—Resources recovery from wastewater based on extracellular electron transfer. In: Environmental materials and waste. Academic Press, pp 391–412. 10.1016/B978-0-12-803837-6.00016-0
- Zhao J, Li F, Cao Y, Zhang X, Chen T, Song H, Wang Z. Microbial extracellular electron transfer and strategies for engineering electroactive microorganisms. Biotechnol Adv. 2020 doi: 10.1016/j.biotechadv.2020.107682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.