Abstract
Objectives
To assess the antibody response to BNT162b2 mRNA COVID-19 vaccine in a cohort of health-care workers (HCW), comparing individuals with previous severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and SARS-CoV-2-naive individuals.
Methods
HCW were tested at T0 (day of first dose), T1 (day of second dose) and T2 (2–3 weeks after second dose) for IgG anti-nucleocapsid protein, IgM anti-spike protein and IgG anti-receptor binding domain (IgG-RBD-S). The antibody response was compared between four main groups: group A, individuals with previous infection and positive antibodies at baseline; group B, individuals with the same history but negative antibodies; group C, individuals with no infection history but positive antibodies; group D, naive individuals. Repeated measures analysis was used to compare results over time-points.
Results
A total of 1935 HCW were included. Median IgG-RBD-S titre was significantly higher for group A (232 individuals) than for group B (56 individuals) both at T1 (A: 22 763 AU/mL, interquartile range (IQR) 14 222–37 204 AU/mL; B: 1373 AU/mL, IQR 783–3078 AU/mL, p 0.0003) and T2 (A: 30 765 AU/mL, IQR 19 841–42 813 AU/mL; B: 13 171 AU/mL, IQR 2324–22 688 AU/mL, p 0.0038) and for group D (1563 individuals; 796 AU/mL, IQR 379–1510 AU/mL at T1; 15 494 AU/mL, IQR 9122–23 916 AU/mL at T2, p < 0.0001 for both time-points). T1 values of group A were also significantly higher than T2 values of group D (p < 0.0001). Presence of symptoms, younger age and being female were associated with stronger antibody response. HCW infected in March showed a significantly stronger response (T1: 35 324 AU/mL, IQR 22 003–44 531 AU/mL; T2: 37 648 AU/mL, IQR 27 088–50 451 AU/mL) than those infected in November (T1: 18 499 AU/mL, IQR 11 492–27 283 AU/mL; T2: 23 210 AU/mL, IQR 18 074–36 086 AU/mL, p < 0.0001 for both time-points.
Conclusions
Individuals with past SARS-CoV-2 infection had a strong antibody response after one single vaccine shot. A single dose might be sufficient for this group, regardless of the time elapsed since infection; however, the clinical correlation with antibody response needs to be studied.
Keywords: Antibody response, BNIT162b2 mRNA COVID-19 vaccine, COVID-19, Health-care workers, SARS-CoV-2, Vaccine
Introduction
Real-world data demonstrating the effectiveness of vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [[1], [2], [3]] are badly needed, not only to understand the impact of mass vaccination on virus transmission and death tolls, but also to collect evidence useful for specific recommendations. For example, the antibody response after a single dose of the BNT162b2 mRNA coronavirus disease 2019 (COVID-19) (Pfizer/BioNTech) and mRNA-1273 (Moderna) vaccines in individuals with previous SARS-CoV-2 infection was comparable to or even stronger than that observed after the second dose in virus-naive people [4,5]. Based on this, some European countries recommend the administration of a single dose of BNT162b2 mRNA COVID-19, mRNA-1273 and ChAdOx1-S vaccines in individuals with previous SARS-CoV-2 infection [6]. This recommendation is beneficial in settings where scarce availability of vaccine supplies is hampering a rapid progression of the immunization campaigns [7].
Some cohort studies demonstrated that re-infections are rare in the 7 months following natural SARS-CoV-2 infection [8], providing the basis to delay vaccination after recovery from the infection, as recommended in some countries [6,9]. Other real-world data could, however, be important to fully address this and other aspects of the vaccination policies.
Primary objectives of this study are to describe the prevalence of the infection before vaccination, and the antibody response to the BNT162b2 mRNA COVID-19 vaccine in a cohort of health-care workers (HCW) in a single hospital, comparing different subgroups at different time-points. An exploratory analysis evaluated the adverse effects (AE).
Materials and methods
Study setting and participants
The study was carried out at the IRCCS Sacro Cuore Don Calabria hospital, Negrar, Verona, Italy between 1 January and 30 March 2021. All consenting HCW supplied blood samples upon administration of the first (T0) and the second (T1) dose of vaccine, and 2–3 weeks after the second dose (T2). At T2 the study investigators collected information with a previously piloted questionnaire on possible AE. The questionnaire included questions about symptoms related to SARS-CoV-2 for HCW reporting previous infection.
The study population comprises all HCW for whom serological results were available at least at one time point. Subgroups were:
Group A: Seropositive exCOVID-19: HCW with a confirmed (i.e. RT-PCR-based) previous infection with SARS-CoV-2 and any positive serology at T0.
Group B: Seronegative exCOVID-19: HCW with a confirmed (i.e. RT-PCR-based) previous infection with SARS-CoV-2 and all serology tests negative at T0.
Group C: Suspected previous infections: HCW without a documented previous infection with SARS-CoV-2 (that is, periodically tested with PCR as per the hospital protocol, and resulting negative) but any positive serology at T0.
Group D: Naive individuals: no previous infection and negative serology tests.
Ethical issues
The study protocol received ethical clearance from the local ethics committee (Comitato Etico per la Sperimentazione Clinica delle Province di Verona e Rovigo) on 13 January 2021 (study protocol n. 17985). Participants received written and oral information and signed an informed consent form.
Laboratory tests
Serum samples were tested for: IgG anti-nucleocapsid protein (IgG-N), IgM anti-spike protein (IgM-S) and IgG anti-receptor-binding domain, including neutralizing antibodies (IgG-RBD-S). Laboratory procedures are detailed in the Supplementary material (Supplementary File 1).
Primary outcomes were: (a) estimation of baseline prevalence before vaccination, calculated as the proportion of HCW found to be serologically positive at T0 over the total number of HCW tested; and (b) antibody response to vaccination at T1 and T2 of the different study groups, calculated as the median values (interquartile range (IQR)) of antibodies.
For the exploratory analysis on AE, the outcome was the prevalence of AE following the first (D1) and the second (D2) doses of vaccine, calculated as the number of AE over the number of participants who responded to the questionnaire. The proportion of each specific AE was reported as the number of each AE over the number of participants reporting an AE. The frequency of the total number of AE and of each specific AE was compared between study groups.
Statistical analysis
Repeated measures analysis was used to compare test results over study time-points, broken down by study groups. The analysis was performed in SAS software version 9.4 using the procedure glm. It included different models, each one evaluating one of the covariates considered (study group, presence of symptoms, age, sex), on the whole cohort or in a specific subgroup. Each model evaluated the possible influence of the considered covariate on the test results and a time effect, i.e. the magnitude of change in test results over time. The resulting ‘interaction time variable’ indicated whether the covariate included in each model had a significant (p < 0.05) effect on test results in at least one of the study time-points. The time-point(s) where the effect was found was/were reported in the pertinent column(s) (T0, T1, T2), with some details about the favouring condition (for instance, indicating whether being female or male was associated with a significantly higher value of antibodies).
We also used Friedman test (G) followed by Wilcoxon rank-sum test (U) in the post-hoc analysis or McNemar's test for paired proportions to compare results between time-points and strata. Values of p were adjusted (adj. p) appropriately in the case of multiple comparisons.
Results
Participants
A total of 2209 out of 2311 (95.6%) HCW gave written consent to the the study upon administration of the first dose of vaccine (between 1 January and 25 February 2021). However, clinical data were available for only 1935 individuals, who represent the final cohort. There were 232, 56, 84 and 1563 individuals in groups A (Seropositive exCOVID-19), B (Seronegative exCOVID-19), C (Suspected previous infections) and D (Naive individuals), respectively.
Of the 1935 HCW, 1225 (63.3%) were women. Women were more represented in all study groups, ranging from 45 out of 84 (53.6%) of all HCW with suspected previous infections to 38 out of 56 (67.9%) of Seronegative exCOVID-19. There were no significant differences in sex distribution between study groups (p 0.256, χ2 test). Median age at T0 was 45 years (IQR 33–53 years), and did not differ significantly between study groups (p 0.3691, Kruskal–Wallis test).
When they had SARS-CoV-2 infection, 205 out of 232 (88.4%) HCW who answered in the group of Seropositive exCOVID-19 and 27 out of 54 (50%) HCW in the Seronegative exCOVID-19 group presented symptoms (p < 0.0001). None of the Seronegative exCOVID-19 HCW were admitted to hospital for COVID-19 symptoms, whereas six out of the 205 (2.9%) symptomatic individuals in the group of Seropositive exCOVID-19 HCW were hospitalized.
Antibody response
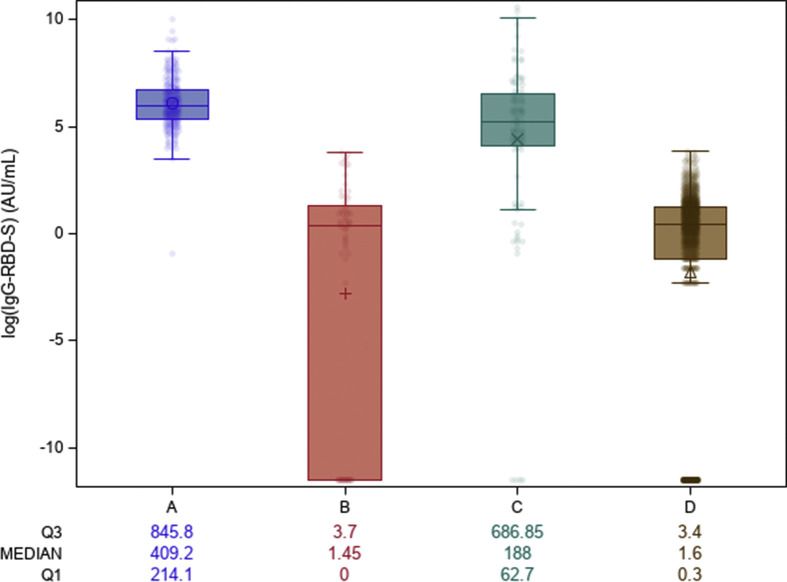
At T0, 293 out of 1935 HCW (15.1%) had positive IgG-RBD-S. Median values were significantly different between all study groups (p < 0.0001), with the exception of Seronegative exCOVID-19 and Naive individuals (p 0.998) (Fig. 1 ).
Fig. 1.
IgG-RBD-S antibodies at baseline (T0) in the different groups. Group A: Seropositive exCOVID-19 health-care workers (HCW); Group B: Seronegative exCOVID-19 HCW; Group C: HCW with Suspected previous infections; Group D: Naive individuals. The values below the figure are reported as medians of IgG-RBD-S (AU/mL) and interquartile range. The figures have been created in logarithmic (log 10) scale.
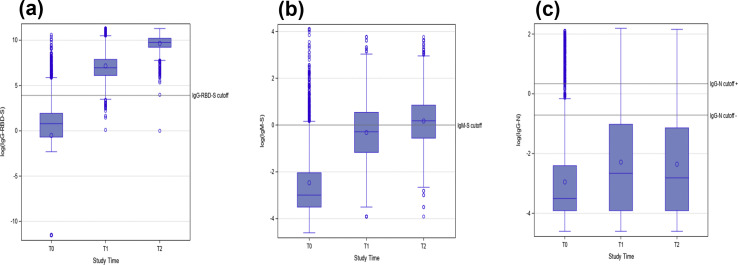
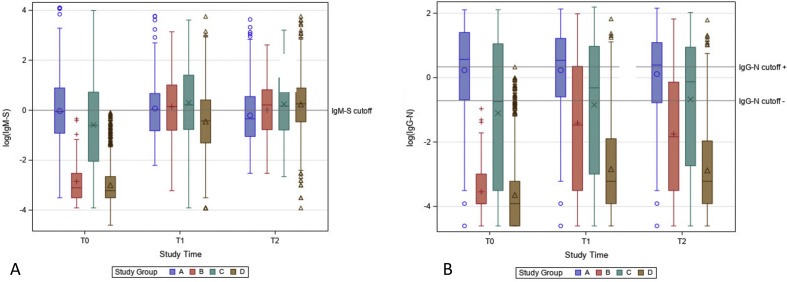
IgG-N was positive in 174 out of 1935 (9%) HCW. IgM-S was positive in 146 out of 1935 (7.5%) HCW. Considering the whole cohort, IgM-S and IgG-RBD-S values significantly increased from T0 to T1 and from T1 to T2 (G p < 0.0001, U adj. p 0.0020), whereas IgG-N significantly increased from T0 to T1 (G p < 0.0001, U adj. p 0.0020) but not from T1 to T2 (U adj p 0.3075) (Fig. 2 ). The dynamics of IgM-S and IgG-N in each subgroup are shown in the Supplementary material (Fig.S1).
Fig. 2.
Values of IgG-RBD-S (a), IgM-S (b), IgG-N (c) antibodies over time, in the whole cohort. IgG-RBD-S results (a) were reported as AU/mL. IgG-N and IgM-S results were reported in an S/C index (S: sample, C: calibrator). A logarithmic scale (log 10) was used to report the results in the figure.
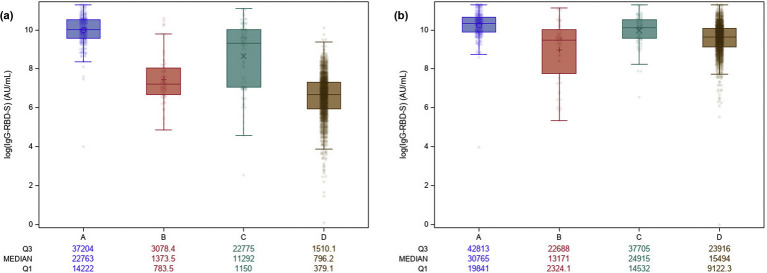
At T1, a marked rise in the IgG-RBD-S values was observed for Seropositive exCOVID-19 HCW, which differed significantly from Seronegative exCOVID-19 HCW (p 0.0003) and Naive individuals (p < 0.0001) (Fig. 3 a). Conversely, similar T1 values were observed between Seropositive exCOVID-19 HCW and those with Suspected previous infections (p 0.1422). Individuals in the Suspected previous infections group presented values also significantly higher than those found in Naive individuals (p < 0.0001).
Fig. 3.
Dynamics of IgG-RBD-S antibodies over time in the different groups. (a) Values at T1; (b) values at T2. Group A: Seropositive exCOVID-19 health-care workers (HCW); Group B: Seronegative exCOVID-19 HCW; Group C: HCW qith Suspected previous infections; Group D: Naive individuals. The values below the figure are reported as medians of IgG-RBD-S (AU/mL) and interquartile range. The figures have been created in logarithmic (log 10) scale.
At T2, a significant difference was still observed in the IgG-RBD-S values of Seropositive exCOVID-19 HCW versus Seronegative exCOVID-19 HCW (p 0.0038) and versus Naive individuals (p < 0.0001), and between HCW with Suspected previous infections and Naive individuals (p < 0.0001) (Fig. 3b). Similar values were instead found between Seronegative exCOVID-19 HCW and Naive individuals (p 0.8133).
Moreover, T1 values of Seropositive exCOVID-19 HCW were significantly higher than T2 values of Naive individuals (p < 0.0001).
Additional factors that could possibly impact on the antibody response were explored with the repeated measures analysis (Table 1 ).
Table 1.
Repeated measures analysis of variables possibly associated with the antibody response
| Model | Marginal means (MM) and p values |
|||
|---|---|---|---|---|
| Main effect |
Interaction Time∗Variable | |||
| T0 Group (MM) |
T1 Group (MM) |
T2 Group (MM) |
||
| Groups A B C D | Favours A (946.09) vs C (531.96) vs B (6.00) vs D (2.86) p 0.001 | Favours A (27 010.79) vs C (14 524.94) vs B (46 15.33) vs D (1186.27) p < 0.001 | Favours A (33 819.57) vs C (27 784.78) vs D (18 165.73) vs B (16 190.64) p < 0.001 | Interaction Time (T0 T1 T2) and Group signficant at p < 0.001 |
| Symptoms (Group A) | Symptoms (1015.78) vs No Symptoms (424.74) p 0.1650 | Favours Symptoms (27 923.57) vs No Symptoms (20 181.90) p 0.0271 | Symptoms (30 049.14) vs No Symptoms (34 323.54) p 0.2615 |
Interaction Time (T0 T1 T2) and Symptoms signficant at p < 0.001 |
| Age (all groups) | 18–30 y (65.70) vs 31–40 y (111.17) vs 41–50 y (170.26) vs 51–60 y (173.02) vs 60 y (165.35) or older p 0.2769 | 18–30 y (5051.60) vs 31–40 y (4766.96) vs 41–50 y (4580.01) vs 51–60 y (5473.81) vs 60 y (4551.94) or older p 0.7398 | 18–30 y (24 573.30) vs 31–40 y (21 270.20) vs 51–60 y (19 293.61) vs 41–50 y (18 851.72) vs 60 y or older (16 779.32) p < 0.0001 | Interaction Time (T0 T1 T2) and Age groups signficant at p < 0.001 |
| Sex (all groups) | Female (132.32) vs Male (147.75) p 0.6866 | Female (5009.80) vs Male (4793.47) p 0.6794 | Female (21 617) vs Male (18 254.82) p | Interaction Time (T0 T1 T2) and Sex signficant at p < 0.001 |
Group A, Seropostive exCOVID-19 health-care workers (HCW); group B, Seronegative exCOVID-19 HCW; group C, suspected previous infections; group D, naive individuals. Marginal means are means of IgG-RBD-S extracted from the model, representing average response for each considered variable (reported in the column “Model”).
The time variable column in Table 1 shows that for all considered models, a significant difference was found in at least one time-point. The variable Groups A B C and D confirmed that a significant difference in the antibody response was observed between study groups, and the extent of the response was highest in group A (Seropositive exCOVID-19) at all time-points. The presence of symptoms during past SARS-CoV-2 infection was associated with a boosted antibody response both at T1 and T2. Age was associated with antibody response at T2, with the youngest individuals (age range 18–30 years) showing the highest values of antibodies. The downtrend with increasing age was not respected for the age range 51–60 years, which seemed to perform better than the range 41–50 years. Women had significantly higher antibody levels compared with men at T2. Symptoms in association with belonging to the Seropositive exCOVID-19 group caused a significant difference in the antibody response at T1 only.
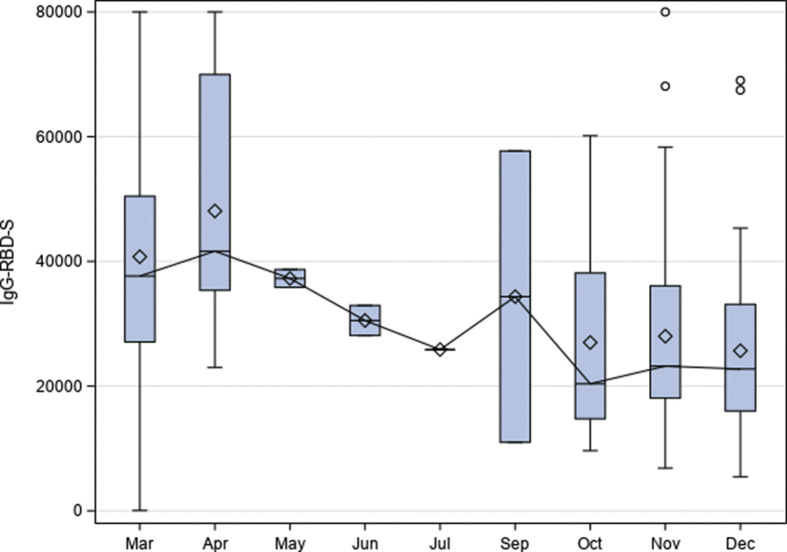
Time elapsed since SARS-CoV-2 infection (considering Seropositive and Seronegative exCOVID-19 HCW) influenced the antibody response (Fig. 4 ). At T1, a similar response was observed between HCW infected in March and those infected in April (p 0.592, Kruskal–Wallis test) and between September/October/November and December (p 0.91). Considering the largest groups, i.e. March and November, at T0 we found that the former group had a significantly lower median antibody level than the latter (p 0.0081), but antibody response was significantly higher in HCW infected in March both at T1 and T2 (p < 0.0001 both comparisons).
Fig. 4.
Antibody response at T1 in relation to the time elapsed since severe acute respiratory syndrome coronavirus 2 infection. Year was 2020. IgG-RBD-S results (a) were reported as AU/mL. The number of infected health-care workers (HCW) in each month was as follows: March, 81; April, 16; May, 2; June, 2; July, 1; September, 2; October, 15; November, 78; December, 35.
With a multivariate analysis, we explored a possible impact of other variables such as sex, age, symptoms, that could explain the higher response in people infected earlier, but none of those considered were found significant.
Adverse events
Patients reporting at least one AE were 1565 out of 1935 (80.9%) at D1 and 1686 out of 1935 (87.1%) at D2 (McNemar test, p < 0.0001). One Naive HCW died 5 days after the second dose of vaccine; causality assessment is ongoing. No other serious AE were registered.
Symptoms frequently reported are shown in Table 2
Table 2.
Frequency of adverse events reported following the first (D1) and the second (D2) dose of vaccine
| Adverse event | D1 n/N (%) | D2 n/N (%) | p (McNemar test) |
|---|---|---|---|
| Fatigue | 289/1935 (14.9) | 331/1935 (17.1) | <0.0001 |
| Fever | 51/1935 (2.6) | 433/1953 (22.4) | <0.0001 |
| Headache | 203/1935 (10.5) | 515/1935 (26.6) | <0.0001 |
| Malaise | 111/1935 (5.7) | 588/1935 (30.4) | <0.0001 |
| Myalgia | 140/1935 (7.2) | 640/1935 (33.1) | <0.0001 |
| Pain at injection site | 1475/1935 (75.3) | 1331/1935 (68.8) | <0.0001 |
At D1, the frequency of AE was significantly higher in Seropositive exCOVID-19 HCW compared with Seronegative exCOVID-19 HCW (p < 0.0001, pairwise comparison, Bonferroni p adj), in Seropositive exCOVID-19 HCW compared with Naive individuals (p 0.0001), in suspected previous infections compared with Seronegative exCOVID-19 HCW (p 0.0177), and in Naive individuals compared with Seronegative exCOVID-19 HCW (p 0.0052). At D2, there was still a significantly different frequency of AE between Seropositive and Seronegative exCOVID-19 HCW (p < 0.0001), and between Naive individuals and Seronegative exCOVID-19 HCW (p < 0.0001). All systemic symptoms were significantly more frequent at D2 compared with D1.
Discussion
We report the antibody response after the first and the second doses of BNT162b2 mRNA COVID-19 vaccine in a cohort of HCW and compare the results between different subgroups in relation to their previous infection history (exCovid and naive individuals) and baseline antibody status. Other variables influencing the antibody response were explored, with age, sex, presence of symptoms during SARS-CoV-2 infection and time elapsed since natural infection being significantly associated. Finally, frequency of AE was reported, in relation to the dose received and the subgroup.
A first overview of antibody status at baseline and at T1 and T2 in the whole cohort was performed with three different antibody tests. A prevalence of 15% was found with IgG-RBD-S, decreasing to 9% and 7.5% for IgG-N and IgM-S, respectively. The different proportions could be explained by different durations over time of each antibody class (e.g. IgM-S lasting about 2.5 months from natural infection) [[10], [11], [12]]. As expected, the different antibodies also showed different dynamics at T1 and T2, in relation to their target protein/domain. The IgG-N targets the nucleocapsid protein, hence it can be useful to evaluate the response to natural infection; indeed, a median value above the cut-off was observed at baseline only for group A. On the other hand, IgM-S and IgG-RBD-S showed a significantly increasing trend across time-points that was particularly marked for IgG-RBD-S that and not included neutralizing antibodies.
The presence of antibodies at baseline influenced the response to the vaccine. Indeed, those with a history of natural infection and positive serology at baseline (Seropositive exCOVID-19 HCW) showed significantly higher IgG-RBD-S levels compared with those with the same history but negative serology at baseline (Seronegative exCOVID-19 HCW), at both T1 and T2. Moreover, the response of Seropositive exCOVID-19 HCW was similar to that of HCW without a history of previous infection but with positive serology at baseline (suspected previous infections). It is possible that some Seronegative exCOVID-19 HCW might have been misdiagnosed with the infection (false-positive PCR), and that suspected previous infections may include individuals who had infection without molecular evidence.
The antibody level in Seropositive exCOVID-19 HCW at T1 was significantly higher than in Naive individuals at T2, as was already reported by smaller studies [1,[13], [14], [15]]. Moreover, here we found that having symptoms during SARS-CoV-2 infection was also significantly associated with a boosted response. It might hence be questioned whether a single vaccine shot could be proposed for those with a history of symptomatic infection, rather than those with barely any signs of infection. A serological baseline assessment to recommend a single rather than a double dose would also be supported by our results, although this would probably be impracticable.
Other factors shown to influence the antibody response were age, sex and time elapsed from natural infection to vaccination. The latter variable is of particular interest in relation to vaccination policy. Indeed, at the moment of writing this work, the Italian guidelines recommended a single dose of BNT162b2 mRNA COVID-19 vaccine in people who had the infection from 3 to 6 months before vaccination. This approach is not entirely supported by our findings. Our results clearly show that the single shot might be still recommended for people infected even 9 months before, and probably more.
Adverse events, mostly local reactions, were reported by the large majority of HCW, and were significantly more frequent after the second vaccine dose. Overall, AE were more frequent than was reported in the phase 2/3 randomized clinical trial assessing efficacy and safety of the BNT162b2 vaccine [16] and other cohort studies [17,18]. Although we did not collect data about the grading of AE, we observed no serious AE with the exception of the individual who died and for whom causality assessment is ongoing.
Main limitation of this study is the use of IgG-RBD-S antibodies as a surrogate of neutralizing antibodies, although a correlation between IgG-RBD-S and neutralizing antibodies has been found previously [[19], [20], [21]]. Also, the number of infected individuals was small in several months (i.e. between first and second epidemic waves, mirroring the epidemiological situation in Italy), limiting the understanding of the antibody response of HCW infected between 9 and 4 months before vaccination. However, the comparison between March and November (the months with the largest number of patients of the first and second wave, respectively) was useful to evaluate the antibody response in relation to time from natural infection. The sample size of the different groups was different, because of the composition of the whole cohort. However, we believe that the trend of antibody response was well-depicted also for smaller groups, though limiting further stratification.
A strength of this study was the size of our cohort, which was large enough to permit stratification into subgroups, thus providing results that may be relevant to inform vaccination policy.
Author contributions
DB conceived the work, analysed and interpreted the data, and wrote the first draft; CP conceived the work, analysed and interpreted the data, and contributed to the first draft; FG conceived the work, interpreted the data and critically revised the draft; AA contributed to data collection and interpretation and critically revised the manuscript; DM, GB, TU, LM, NR, PR, CC, FT, ST, ER, MDegani, MDeiana and MP contributed to data collection and interpretation; RS performed the statistical analysis and contributed to the first draft; ZB conceived the work, analysed and interpreted the data, contributed to the first draft. All authors approved the final version of the manuscript.
Transparency declaration
All authors declare no conflict of interest. This work was supported by the Italian Ministry of Health: “Ricerca Finalizzata” COVID-2020-12371675 and “Fondi Ricerca Corrente” L1P5.
Acknowledgements
We are grateful to all staff of the Department who gave support for the implementation of this study, including other personnel from the ward and from the laboratory, and nurses and administrative staff.
Editor: A. Kalil
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.07.024.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Multimedia component 1
Multimedia component 2
Fig. S1.
Values of IgG-RBD-S (A), IgM-S (B), IgG-N (C) antibodies over time, for each subgroup.
References
- 1.Abu Jabal K., Ben-Amram H., Beiruti K., Batheesh Y., Sussan C., Zarka S., et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2021;vol. 26 doi: 10.2807/1560-7917.ES.2021.26.6.2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall V.J., Foulkes S., Saei A., Andrews N., Oguti B., Charlett A., et al. COVID-19 vaccine coverage in health-care workers in england and effectiveness of BNT162b2 mRNA vaccine against infection (siren): a prospective, multicentre, cohort study. Lancet. 2021;397(10286):1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F., et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384(14):1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manisty C., Otter A.D., Treibel T.A., McKnight Á., Altmann D.M., Brooks T., et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397(10279):1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ECDC Overview of the implementation of COVID-19 vaccination strategies and vaccine deployment plans in the EU/EEA. https://www.ecdc.europa.eu/en/publications-data/overview-implementation-covid-19-vaccination-strategies-and-vaccine-deployment. 2021 Available at:
- 7.Kluge H., McKee M. COVID-19 vaccines for the European region: an unprecedented challenge. Lancet. 2021;397(10286):1689–1691. doi: 10.1016/S0140-6736(21)00709-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall V.J., Foulkes S., Charlett A., Atti A., Monk E.J.M., Simmons R., et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397:1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salute Md. Vaccini anti COVID-19. http://www.salute.gov.it/portale/nuovocoronavirus/dettaglioFaqNuovoCoronavirus.jsp?lingua=italiano&id=249. 2021 Available at:
- 10.Seow J., Graham C., Merrick B., Acors S., Pickering S., Steel K.J.A, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5:1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iyer A.S., Jones F.K., Nodoushani A., Kelly M., Becker M., Slater D., et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci Immunol. 2020:5. doi: 10.1126/sciimmunol.abe0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qu J., Wu C., Li X., Zhang G., Jiang Z., Li X., et al. Profile of immunoglobulin G and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:2255–2258. doi: 10.1093/cid/ciaa489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anichini G., Terrosi C., Gandolfo C., Gori Savellini G., Fabrizi S., Miceli G.B., et al. SARS-CoV-2 antibody response in persons with past natural infection. N Engl J Med. 2021;385(1):90–92. doi: 10.1056/NEJMc2103825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradley T., Grundberg E., Selvarangan R. medRxiv : the preprint server for health sciences. 2021. Antibody responses boosted in seropositive healthcare workers after single dose of SARS-CoV-2 mRNA vaccine. [Google Scholar]
- 15.Gobbi F., Buonfrate D., Moro L., Rodari P., Piubelli C., Caldrer S., et al. Antibody response to the BNT162b2 mRNA COVID-19 vaccine in subjects with prior SARS-CoV-2 infection. Viruses. 2021;13:422. doi: 10.3390/v13030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadali R.A., Janagama R., Peruru S., Malayala S.V. Side effects of BNT162b2 mRNA COVID-19 vaccine: a randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis. 2021;106:376–381. doi: 10.1016/j.ijid.2021.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S.H., Wi Y.M., Yun S.Y., Ryu J.S., Shin J.M., Lee E.H., et al. Adverse events in healthcare workers after the first dose of ChAdOx1 nCoV-19 or BNT162b2 mRNA COVID-19 vaccination: a single center experience. J Korean Med Sci. 2021;36:e107. doi: 10.3346/jkms.2021.36.e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian L., Elsheikh E.B., Patrone P.N., Kearsley A.J., Gaigalas A.K., Inwood S., et al. Towards quantitative and standardized serological and neutralization assays for COVID-19. Int J Mol Sci. 2021:22. doi: 10.3390/ijms22052723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turbett S.E., Anahtar M., Dighe A.S., Beltran W.G., Miller T., Scott H., et al. Evaluation of three commercial SARS-CoV-2 serologic assays and their performance in two-test algorithms. J Clin Microbiol. 2020;59 doi: 10.1128/JCM.01892-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterhoff D., Glück V., Vogel M., Schuster P., Schütz A., Neubert P., et al. A highly specific and sensitive serological assay detects SARS-CoV-2 antibody levels in COVID-19 patients that correlate with neutralization. Infection. 2021;49:75–82. doi: 10.1007/s15010-020-01503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1
Multimedia component 2