Abstract
Antipsychotic monotherapy (APM) is considered best-acceptable treatment option regardless of antipsychotic class and formulation types for treating schizophrenia. However, antipsychotic polypharmacy (APP) has been also widely utilized in routine clinical practice. Despite APP has some clinical benefits it has also numerous pitfalls in relation with increased total number and doses of APs leading to adverse events as well as decrease of treatment adherence and persistence resulting in poor clinical outcomes. Recent introduction of long-acting injectable antipsychotics (LAIs) to the market has offered a chance for better medication adherence/persistence and also provided a simplification of treatment regime leading to more stabilized treatment for schizophrenia patients. When we cannot stay away from APP in the treatment of schizophrenia, clinicians need to find more proper APP regimens and thereby utilization of APP in efficient way should be a practical strategy to benefit schizophrenia patient in a real world treatment setting. With this regard, LAIs can be one of available APP regimen for treatment of schizophrenia in routine practice since their clinical utility and pharmacokinetic stability over oral APs have been well-elaborated today. However, when we have to commence LAIs as a part of APP with oral APs or other LAIs, every effort should be made before doing so whether or not validated and available treatment options or other clinical factors were not done or evaluated yet. Any treatment guidelines do not support APP regardless of the formulation of APP regimen or address two or more LAIs for treatment of schizophrenia till today.
Keywords: Long-acting injectable antipsychotic, Schizophrenia, Monotherapy, Polypharmacy, Guideline, Routine practice.
INTRODUCTION
Schizophrenia is a chronic, recurrent and debilitating mental illness needing a longstanding maintenance therapy which requires a strict drug compliance since poor drug compliance is usually associated with worsening of stabilized psychotic symptoms, recurrence and relapse, adverse events from abrupt withdrawal of medications, overall leading to poor clinical course and outcomes [1,2].
The gold standard treatment for schizophrenia is antipsychotic treatment and most treatment guidelines have proposed antipsychotic monotherapy (APM), while antipsychotic polypharmacy (APP) is substantially prescribed for treatment of schizophrenia patients in clinical practice due to a number of clinical reasons. Indeed, APP has been known to be utilized for 10 to 20% of schizophrenia in outpatient basis, while 40% of schizophrenia in inpatient basis. However, the prevalence of APP is quite differentially estimated in routine practice in accordance with different treatment settings, study methodologies, geographic distribution across the world [3-6].
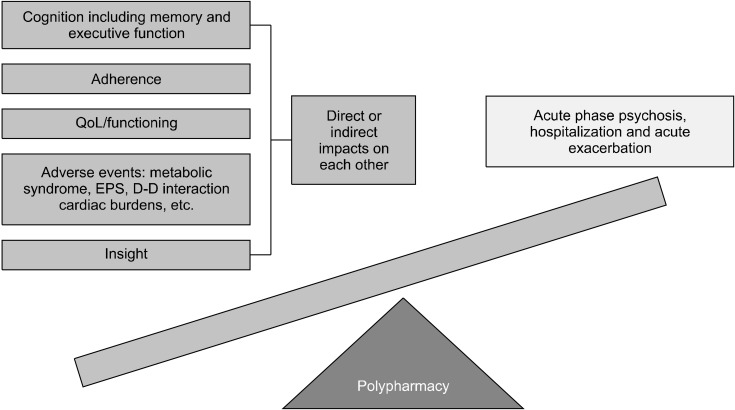
Why do clinicians use the APP for treatment of schizophrenia against the advice of most treatment guideline from numerous academic societies and authorities? Pop-ular treatment guidelines [7-14] recommend the use of APP in specific and limited clinical cases as seen in Table 1. According to numerous studies, clinicians prescribe APP for treatment of schizophrenia, mainly for control of positive psychotic symptoms [15]. Other reasons include amelioration of negative symptoms, improvement of cognitive function, reduction of rehospitalization, cure for specific comorbid symptoms such as depression and anxiety, reduction of total antipsychotic doses of APM, counteracting side effects (SEs), and so on [16-19]. However, APP has also clear pitfalls in treatment of schizophrenia in routine practice. It may increase risk of developing high-dose treatment of AP, SEs and increase of medical costs as well [17,20-22]. Figure 1 illustrates the advantages and disadvantages of APP in routine practice.
Table 1.
APM is recommended across the guidelines in the initial treatment of acute schizophrenic episode
| Society | Guideline title | Statement |
|---|---|---|
| WFSBP 2012 | WFSBP Guidelines for Biological Treatment of Schizophrenia, Part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance | -APM should be the first-line treatment -APP should be a strategy of last resort for TRS |
| NICE 2014 | NICE: psychosis and schizophrenia in adults | -No routine APP except for short periods -Need to check serum level of AP |
| RANZCP 2016 | RANZCP clinical practice guidelines for the treatment of schizophrenia and related disorders | -APP should be in variant form of transition process for AP switching |
| American Psychiatric Association (2020) | The American Psychiatric Association Practice Guideline for the Treatment of Patients with schizophrenia | -Clozapine trial should be considered in the first place. -APP maybe helpful for emergency visits and rehospitaliza-tion rates as compared to monotherapy. -No evidence that APP is any more harmful than using APM, beyond the common side effects from each drug |
| CPA 2017 | Guidelines for the Pharmacotherapy of Schizophrenia in Adults | -Clozapine should be first considered if two different APMs fail -There is insufficient evidence to make any specific recom-mendation for APP |
| BAP 2020 | Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the BAP | -APP should be used with a closely monitored and short-term trial after a lack of response to several adequate trials of APM -No more availability of other evidence-based treatments in-cluding clozapine failure |
| TMAP 2007 | The TMAP antipsychotic algorithm for schizophrenia: 2006 update | -Clozapine APP trial should be placed before initiation of APP with non-clozapine AP |
WFSBP, World Federation of Societies of Biologic Psychiatry; NICE, the National Institute for Health and Care Excellence; RANZCP, Royal Australian and New Zealand College of Psychiatrists; CPA, Canadian Psychiatric Association; BAP, British Association for Psychopharmacology; TMAP, Texas Medication Algorithm Project; APM, antipsychotic monotherapy; APP, antipsychotic polypharmacy; TRS, treatment-resistant schizophrenia; AP, antipsychotic.
Fig. 1.
Pros and cons of antipsy-chotic polypharmacy. QoL, quality of life; EPS, extrapyra-midal side effects.
According to many previous studies, long-acting injectable antipsychotics (LAIs) have been found to reduce the poor drug compliance compared to oral antipsychotics and thereby help to keep maintenance antipsychotic treatment which is an essential part for treatment of schizophrenia patients to achieve best clinical outcomes including symptomatic remission/response and functional recovery [23,24]. In fact LAIs showed comparable or better effectiveness and tolerability compared to oral antipsychotics in treatment of schizophrenia (i.e., significant reduction of relapse/recurrence, rehospitalization and improvement of some functional capacities) [25-27].
LAIs are one of the ideal treatment options for treatment of schizophrenia reflecting the guidance from many crucial treatment guidelines and consensus among renowned experts [25,28]. Despite the advice for advocating APM from most treatment guideline, clinicians cannot stay away from APP but in reality, they need APP in routine practice or specific clinical situations in the treatment of schizophrenia [16].
Can we expect any potential roles of LAIs as one of replacement agent against oral APs in APP for the treatment of schizophrenia? This review focuses on the potentiality and prospect of LAIs in the treatment of schizophrenia patients who need APP in routine clinical practice.
THE TREND OF APP IN THE TREATMENT OF SCHIZOPHRENIA PATIENTS OVER THE WORLD
According to the recent systematic review based on operational criteria using 147 studies [29], there were substantial differences in the prevalence and yearly trend of APP among continents; North America (16%) showed the lowest pooled APP rates compared those from Oceania (16.4%), Asia (32%), and Europe (23%). Despite the wide variations among geographic regions, global median rate of APP was found to be approximately 20% in the study [29].
The reasons for substantial differences in the prevalence of APP among continents are unclear. However, one of the plausible explanations should be availability of second generation APs (SGAs) which have significantly different pharmacodynamics (PD) and pharmacokinetic (PK) profile expanding their clinical usage for more tuned therapeutic targets and objectives such as augmentation of therapeutic benefit and reduction (counteract) of specific SEs from the first AP treatment. Differences in health insurance among countries, cultural backgrounds favoring polypharmacy between countries, and different use of new formulation of APs might also contribute to such differential usage trend of APP among continents [29,30].
However, the gradual increasing trend of APP usage has been currently and consistently reported over few decades regardless of geographic regions. According to the study investigating the 10-year trends in the treatment and outcomes of patients with first-episode schizophrenia in Denmark [30], the percentage of patients treated with APP increased significantly using any of the two criteria (“total polypharmacy” defined as receiving at least two APs within 2 months, increased by 23% from 33.3% to 56.2%; “long-term polypharmacy” defined as exclusion of cross-tapering situation and renewed new APs within four months from the last prescription, increased by 20% from 16.7% to 37.1%). Another interesting finding was that the use and average dosage of APs (defined daily dose, DDD) also increased significantly as SGAs became the most widely prescribed AP among classes (SGA + SGA > SGA + FGA (first generation antipsychotic) > FGA + FGA). As for APM, an increase in yearly DDD was also found, however, it was significantly higher in APP than in APM by more than twice.
WHY DO WE NEED TO CONSIDER LAIs AS A REGIMEN FOR APP IN ROUTINE PRACTICE
Real Data Regarding LAIs as Being a Part of APP
One of the major advantages of LAIs is to offer the best-available APM strategy enhancing treatment adherence as well as simplifying the complexity of treatment regimens in the treatment of schizophrenia. Despite inadequate current evidence strongly supporting the use of LAIs as an APP regimen, emerging claims data studies and some small studies have been suggesting the relative popularity of APP with LAI and oral APs in routine practice.
Interestingly, a recent prospective, observational study investigating the effectiveness of aripiprazole once-monthly (AOM) for those initiated or switched to AOM was conducted in 53 schizophrenia patients in Canada [21]. According to the results, the use of AOM significantly improved all psychopathologies and functioning level measured by the Positive and Negative Syndrome Scale and Global Assessment of Functioning scores as well as adverse events (AEs) from baseline to month 6. Intriguingly the total number of APs was also significantly reduced after a 6-month treatment with AOM (3.0 to 2.1), clearly indicating that AOM usage could be positively associated with potential treatment adherence, health care costs or tolerability issues. Such AOM’s effect regarding the reduction of AP number was also replicated in another 12-month retrospective study in different geographic region [25]. The study was conducted for those treated with two or more APs or different LAIs (≥ 3 months duration) before the first injection of AOM. After switch to AOM for 12-month, the number of APs before AOM was 2.4, while it significantly decreased to 0.7, indicating 58.3% reduction. Indeed, such reducing effect was already notable in early time at the study and maintained throughout the whole observation period; the number of concurrent APs were 1.3 at baseline, 0.4 at month 3, and 0.5 at month 6. Furthermore, numerical decreases of other concomitant psychotropics including mood stabilizers, antianxiety drugs, and antiparkinsonian drugs were also found, indicating that the clinical utility of AOM in patients with APP in daily practice could be extended to substantial amelioration and simplification of the whole treatment regimen beyond APs. Such two studies provided preliminary findings regarding the beneficial influence of AOM on reduction of total psychotropics including APs as well as offering the co-administration trend of AOM and existing ongoing APs in routine practice since the number of concurrent APs were still approximately two and one, respectively, after switch to AOM for 6-month and 12- month in such two studies. Both 6 months and 12 months are longer to be in a transition period to AOM monotherapy, but rather showing a form of APP comprising AOM and oral APs.
Likewise, previous claim data-based studies [31,32] have also shown the co-prescription pattern of LAIs and other APs proposing that APP consisting of LAIs and other APs may potentially prevalent than we think in real treatment setting. Doshi et al. [32] has conducted an observa-tional, retrospective cohort study using administrative claim data for investigating the frequency and duration of concurrent oral APs in 340 Medicaid patients receiving LAIs. Among all patients initiated LAIs including fluphenazine decanoate (FD), haloperidol decanoate (HD), risperidone LAI (RLAI), and paliperidone palmitate (PP), approximately 76% had a concurrent oral APs in the 6 months post-hospital discharge. There were substantial differences in the frequency of combining any oral APs, where 80.0%, 80.4%, 88.9%, and 58.8% for FD, HD, RLAI, and PP, respectively. Almost 3 out of 4 patients initiated LAIs still took concurrent oral APs according to the results. Further to say, oral APs overlapped with LAIs for an average of 56.5 days, corresponding to an average of 65.7% of LAIs covered days, in which PP users with concurrent usage of any oral APs had the lowest overlapping days (53.4%), while it was highest in the users with HD (72.1%). It should be also notable that patients taking concurrent oral APs were mostly on their LAIs, while the first generation LAIs were frequently on oral SGAs which is commonly observed APP pattern for the use of different pharmacological profile between APs. In another similar study using retrospective chart review in a single center [31], LAIs/oral APs APP was defined as the use of both agents outside the manufacturer’s recommendations for titration and overlap for a certain period. Among 79 patients met study selection criteria, 27.8% of patients (n = 22) were found as LAIs/oral AP APP. Among such patients with LAIs/oral APs APP, approximately 50% of patients were on the same AP in both formula type. Interestingly patients with LAIs/oral AP APP were half as likely to be on a maximum dose LAI compared with those on LAI monotherapy (13% vs. 26%) indicating that LAI/oral AP APP is being used without maximization of a single agent in 87% of patients which represents the need of therapeutic dose optimization. Notably 68% of patients took LAI/oral AP APP for more than 1 year based on medical records, indicating that clinicians may be reluctant to change long-term medication regimen not to hinder patients’ stability or measurement-based reassessments were not done for a while, despite of the fact that 59% of patients had a history of attempt to reduction of oral APs’ doses. According to detailed documentation, the reasons for LAI/oral AP APP were diverse as follows: no rationale for 32%, failure of previous tapering of oral APs for 23%, refractory cases for 17%, preference of patients for 14%, and slow tapering process for 14%. Furthermore, as for the LAI/oral AP APP types by class, 56%, 26%, 11%, and 7% were on SGA + SGA, FGA + SGA, FGA + FGA, and SGA + FGA, respectively.
Netley et al. [21] analyzed 427 patient cases who received a combined 1,480 injections including aripiprazole, fluphenazine, haloperidol, risperidone and paliperidone LAIs by retrospective chart review to find whether or not clinicians utilize LAIs in accordance with the published labeling information in their practice in one health system at Indiana and Ohio involving 9 hospitals. Based on the results, consistency rates following the labeling information provided and approved by the manufacturer and authority were 71.2% for indication, 67.4% for trial of oral APs, 94.4% for dose of LAI, 84.5% for frequency of LAI shot, and 93.9% for titration method. Interestingly among patients those were able to track within the health system, a majority had a LAI treatment duration less than 90 days, possibly indicating that LAIs were acutely and shortly combined to oral APs for control of severe psychotic symptoms or improvement of adherence when patients are released from involuntary admission or holds. Such data also suggest that off-label usage and flexible utilization of LAIs may be frequently done potentially by the clinical preference and experience of clinicians, patients’ factors, and other diverse clinical factors.
One previous study (n = 90) has investigated the risks and benefits of switching from two to one AP among patients on two non-clozapine oral APs, and among those on combinations involving either clozapine or an injectable APs [33]. In the study, approximately one out of five patients were already being treated with APP involving LAIs with non-clozapine oral APs, also proposing the prevalent use of APP including LAIs and oral APs in routine practice.
LAIs are Better than Oral APs in Prevention of Relapse and Treatment Adherence
It is well-known that LAIs have superiority in relapse and rehospitalization over oral APs in a number of clinical trials and meta-analysis [34-38] despite some debates on this issue [39] due to diverse possible biases such as study methodology, expectation bias, natural illness course, and time effect [40]; indeed, the superiority of LAIs over oral APs were not found in some randomized and controlled clinical trials (RCTs), while LAIs were superior to oral APs in most mirror-image and some large cohort studies, indicating a mandatory change on study design in consideration of formula difference. A possible solution would be the implementation of a practical effectiveness trial in which post-randomization involvement should be kept to a minimum for better reflection of routine practice [40].
According to a meta-analysis investigating 25 mirror-image studies, LAIs showed clear superiority over oral APs in prevention of admission by risk ration (RR) of 0.43 and reduction of the number of hospitalizations by RR of 0.38. Such beneficial effects of LAIs over oral APs were also replicated in claims data studies as well [34]. Nonadherence (proportion of days covered less than 0.80), discontinuation (continuous medication gap ≥ 60 days), and schizophrenia-related rehospitalization were compared between LAIs users and oral APs users, all in the 6 months after discharge [34]. The non-adherent proportion of patients were significantly lower in LAIs user than in oral APs users (51.8% vs. 67.7%). The proportions of patients with a 60-day continuous gap in medication (23.8% vs. 39.4%) and rehospitalization (19.1% vs. 25.3%, p = 0.01) were also significantly lower in LAIs users than in oral APs users [34]. Interestingly the magnitude of such differences was increased when comparing SGA LAIs users with those of oral APs. For instance, when examined separately, only patients receiving SGA LAIs (absolute odds ratio, AOR = 0.59,) and not FGA LAIs (AOR = 0.90) had a statistically significant reduction in odds of rehospitalization, possibly indicating differential effect between SGA and FGA LAIs due to differences in pharmacological profile.
Numerous studies have also replicated the superiority of LAIs over oral APs in terms of adherence, all-cause discontinuation, rehospitalization, cost effectiveness, and functioning in the treatment of schizophrenia as well as bipolar disorder regardless of geographic regions conducted clinical trials and studies [34-38].
LAIs can Achieve and Maintain Stable Therapeutic Dose
Based on previously mentioned meta-analysis [41], the proposed minimal dose for prevention of relapse was found to be 200 CPZeq mg/d, and also even when the dose was reduced up to 40% from the initial maintaining dose, psychopathological worsening was not observed if the AP dose reduction was adequate to occupy at least 50% (especially > 50 years) to 65% of D2 receptor prospectively measured by Carbon 11-labeled raclopride positron emission tomography [42]. Such effects of therapeutic dose maintenance regarding relapse prevention was also replicated in numerous studies and other meta-analyses [43]. Uchida et al. [43] conducted a meta-analysis including 13 studies with 1,395 subjects to investigate the differential efficacy between standard dose AP (DDD) vs. low dose AP (≥ 50% to < 1 DDD) or very low dose AP (< 50% DDD) for relapse prevention in schizophrenia where very low-dose AP was inferior to the standard-dose AP (number-need-to-harm = 9) and a trend-level of significance was found between standard-dose AP and the low-dose AP therapy favoring standard-dose AP over low-dose AP (p = 0.05). This data clearly indicate the AP dose reduction should be possible but clinicians need to keep the dose in therapeutic range of individual AP, at least corresponding to CPZeq dose of 200 mg/d or more.
Meanwhile 44% of patients identified as treatment-re-sistance schizophrenia by treating physicians were found to have either subtherapeutic (25%) or undetec-table (19%) AP levels, which was significantly associated with black ethnicity, shorter duration of current treatment and APs other than olanzapine and amisulpride [44], indicating serious issue of under-treatment and/or poor compliance of patients with schizophrenia. Such inappropriate treatment trends have been consistently found in many other studies. Lopez et al. [45] has investigated the level of disagreement between clinicians’ routine assessments of medication status and plasma levels of commonly prescribed APs in acutely ill patients (n = 105) who visited an emergency room. According to the results, urgent evaluation of AP status predicted therapeutic and nontherapeutic antipsychotic levels at rates of 41.5% and 75%, respectively, indicating frequent misleading by usual assessments of AP intake status.
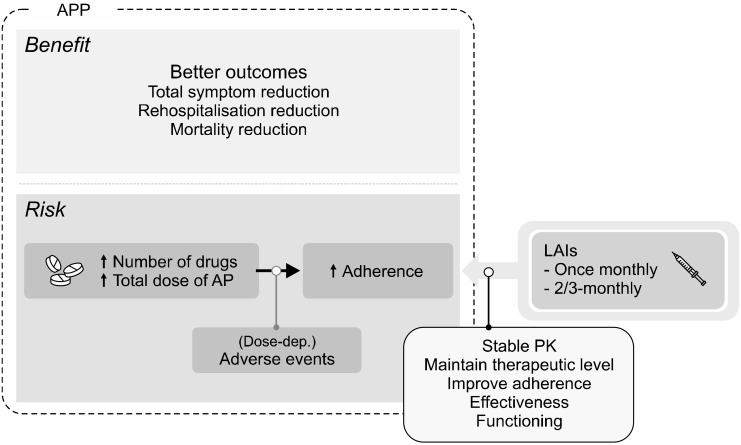
Likewise, substantial proportion of previous researches reported similar findings supporting a significant correlation between plasma concentration and/or large fluctuations in peak-to-trough plasma concentration with increased tolerability issues [46]. Indeed, it was proposed that improved adherence with LAIs compared with oral APs may have been associated with stable serum concentrations and a subsequent significant reduction in AEs [47]. As previously reported, if plasma concentration correlates with downstream pharmacologic effect on D2-re-ceptor occupancy, AP formulations with smaller plasma- concentration fluctuations should lead to less fluctuations in D2-receptor occupancy resulting in an improved PD profile [46]. Further, peak-to-trough fluctuations are higher and more unstable in formulations with a shorter half-life than in those with longer half-life since half-life represents the time for plasma drug level to decrease by half [46]. Additionally the advantages of small plasma- concentration fluctuations often seen in controlled-release formulations include reduced peak concentrations which is related to reduced risk for developing AEs, increased trough concentrations which is associated with lowering a risk for subtherapeutic drug levels, and eventually more chance for achieving an optimal adherence by balancing risk-benefit profile, when stable and proper selection of AP dose is achieved, compared to immediate-release formulations [46,48,49]. In line with previous reports [46,49], the PK profile of RLAI administered every 2 weeks captured a stable steady-state AP levels falling in the interval observed with an equivalent oral AP dose but with lower and less fluctuations (i.e., 1/2 weeks vs 1/day) [50], which was also similarly observed in different LAIs including AOM [51]. Figure 2 illustrates a potential role of LAIs as a part of APP in the treatment of schizophrenia.
Fig. 2.
Potential role of long-acting injectable antipsychotics (LAIs) as being a part of antipsychotic poly-pharmacy (APP) regimen. PK, pharmacokinetic.
Which LAIs should be Better for APP Regimen?
Currently available and popular LAIs include AOM, RLAI, PP, Olanzapine Pamoate (OP) and HD in current practice, which have different pharmacological profile along with slightly different injection interval, requirement of oral AP at the early phase, AEs, injection sites, post-injection monitoring, cost, and dosing schedule. Table 2 summarizes pharmacological characteristics of LAIs [47-56].
Table 2.
Pharmacological characteristics of popular LAIA
| Agents | Half-life (day) | Tmax (day) | Formulation technology | Frequency | Oral AP stabilization | Note |
|---|---|---|---|---|---|---|
| RLAIA | 3−6 | 28−35 | Microsphere preparation | BM | 3 weeks | Needs refrigeration |
| OP | 30 | 7 | Crystalline salt with OZP and pamoic acid | OM | No need | Post-injection delirium |
| PP1 | 24−49 | 13 | Nanocrystal | OM | No need | Needs two consecutive loading doses of PP1 with first 234 mg and then 156-mg dose after 7 to 10 days, necessarily on del-toid muscle |
| PP3 | 84−95 | 30−33 | Larger nanocrystals | 3-monthly | No need | Stabilized on the PP1 prepara-tion before the first of PP3 |
| AOM | 29.9−46.5 | 6.5−7.1 | Polymorphic mono-hydrate−water preparation | OM | 2 weeks | - |
| AL | 29.2−34.9 | 3−5 | Prodrug approach | OM, 6-week, 2-monthly | 3 weeks | - |
| HD | 21 | 1−6 | A sesame oil formulation | OM | Oral AP stabilization at least 3 weeks and up to 3 months; no needs if loading doses are applied | Needs initiation of a 25 mg IM test dose, followed one week later by 50 mg IM, after which once-monthly |
LAIAs, long acting injectable antipsychotics; AP, antipsychotic; RLAIA, risperidone LAIA; OP, olanzapine pamoate; PP1, paliperidone palmitate once-monthly; PP3, PP 3-monthly; AOM, aripiprazole once-monthly; AL, aripiprazole lauroxil; HD, haloperidol decanoate; BW, biweekly; OZP, olanzapine; OM, onece-monthly; IM, intramuscular; SCZ, schizophrenia.
There have been no clear evidence supporting a superiority of one specific LAI over other LAI since no sufficient comparative trials have been conducted [57], which has been also reported in the recent real-world study (similar efficacy among PP, AOM, and RLAI for rehospitalization) [58]. Therefore it should be most reasonable for clinicians to utilize any pertinent LAI in accordance with preference and clinical experience of physician, patient’s urge, clinical situation, comorbid condition, economic status, past patient’s personal and family history to APs if available, and the goal of APP such as increasing adherence/persistence under full pharmacological understandings including PK and PD profile of currently available LAIs in the market because they have substantial differences in pharmacological property since they originated from similar chemical characteristics of their own oral APs [59].
However, we can have some clues in the selection of LAIs as one of APP regimen for the treatment of schizophrenia since there have been intriguing data from diverse clinical studies as following. For instance, AOM has been recently approved for treatment of schizophrenia as such one of LAIs [28,60]. In a number of RCTs including well-designed registration trials, it has been found to be effective and tolerable for treatment of schizophrenia patients leading to possible replacement of oral AP treatment which is usually associated with early dropout and poor persistence/compliance leading to frequent easy recurrence and relapse [61-65]. According to recent studies [59,66-69], AOM showed a superiority over competitive LAI, PP, especially regarding improvement of quality of life (QoL) and functional capacity as well as reduction of psychopathology symptoms and AEs such as sexual dysfunction. Functional recovery is the primary objective in contemporary treatment of schizophrenia since it is highly and significantly associated with the well-being of patients with schizophrenia. The beneficial effects of AOM on functional recovery and improvement of QoL have maintained without worsening for 1 years which was found in a long-term open trials [70]. Indeed, AOM has differential pharmacological profile compared to PP showing some clinical differences in low propensity of extrapyramidal side effects (EPS), negative symptoms, anxiety, depression and cognitive function due to its unique pharmacological profile including D2 partial agonist, partial 5-HT1A agonist, 5-HT2A antagonist, 5-HT2C partial agonist, 5-HT6 antagonist, weak partial agonist at 5-HT7, and 5-HT2B inverse agonist [71]. However, it should be completely premature to say that AOM may have a superiority over PP in terms of QoL and functional capacity due to a dearth of direct comparative trials since all such direct-comparison trials were sponsored by the manufacturer, Otsuka. Hence, we definitely need more adequately-powered and well-designed independent clinical trials whether such differences between AOM and PP would be replicated.
Likewise treatment retention rates were found to be significantly different by severity of schizophrenia among AOM, RLAI, and PP in a recent retrospective cohort study [72], in which AOM was significantly beneficial in all cause discontinuation in mild cases of schizophrenia than PP (hazard ration, HR = 0.36) and RLAI (HR = 0.4), while PP (HR = 2.6) and RLAI (HR = 1.67) were superior to AOM in moderate to severe cases of schizophrenia. Such reversed trend of findings by disease severity was also similar in terms of hospitalization despite of no statistical differences between AOM, PP, and RLAI in the study.
A recent independent RCT [73] has compared AOM and PP in the treatment of schizophrenia and bipolar disorder comorbid with substance use disorder (AOM, n = 51; PP, n = 50) where AOM significantly more improved craving and QoL than PP at the 1-year follow-up. Indeed there have been no study result proposing that oral paliperidone had effects on craving, whereas numerous studies have reported the beneficial effects of oral aripiprazole on amelioration of craving of alcohol in human [74] and animal [75], possibly due to profound differences in perspectives of involvement in dopaminergic neurotransmission between AOM and PP.
As described before, the inconsistency between LAIs usage following labeling information was observed in the recent study in which there were some clinically intriguing points for inquiry on oral AP titration, injection frequency, and LAI dose adjustment needing future researches [21]. For instance, the inconsistency rate on oral AP trial of AOM was almost 30%, while it was approximately 50% in RLAI and the inconsistency rate on injection interval of AOM was almost 40% (more frequent visit), while it was only approximately 5% in PP. In addition, approximately 50% of the patients who had any LAIs administered did not return for next injection or failed to show follow-up visit and most patients who returned to follow-up visit had a short LAI treatment duration less than 3 months, indicating that clinicians may also use LAI as a short-term control for acute psychotic symptoms and/or prescribed a single-injection before discharge to prevent acute worsening.
Interestingly a recent large cohort study has clearly found that the most effective APP regimen regarding reduction of hospitalization should be clozapine/aripiprazole compared to any numerous APP regimen [19]. Indeed, a recent case study [76] reported a successful APP with clozapine and AOM (400 mg/d) for a patient whose psychotic symptoms were not sufficiently controlled by adequate dose of clozapine monotherapy (545 mg/dL) for 4 months. This data suggests that clozapine/AOM polypharmacy should be effective alternative treatment approach at least for a subgroup of patients with schizo-phrenia.
OP has been also widely used as one of LAIs for treatment of schizophrenia [77], however, it has a substantial differences compared to AOM, PP, and RLAI since it has a strong potential of sedation, liability for developing weight gain, and requirement of close post-injection monitoring after injection, at least for 3 hours due to its tendency for developing post-injection delirium/sedation syndrome (PDSS) which may lead to serious accident and injury in patients [77]. Indeed, the prevalence of PDSS ranges from 0.07 to 1.4% after its approval in EU 2008 and USA 2009. The PDSS includes various notorious neuropsychiatric symptoms such as deep sedation, confusion, ataxia, delirium and cloud of consciousness, and the mechanism is related to exposure to toxic concentration of OP due to unintended partial intravascular injection or blood vessel injury during the injection [78]. Based on data with approximately 45,000 OP given to 2,054 patients, onset of PDSS ranged from immediate to 3 to 5 hours after injection (median onset time = 25 minutes after injection) and the recovery time ranged from 1.5 to 72 hours [78]. However, it should be notable that 3-hour close monitoring time did not significantly impacted on patients’ satisfaction with continued long-term treatment with OP based on the results from post-hoc analysis for a 6-month fixed dose RCT and a 6-year open-label safety study [79]. Intramuscular/intravenous olanzapine has been successfully and commonly utilized to treat schizophrenia patients with acute agitation in emergency department or acute admission situation [80,81].
RLAI has been mostly widely used for treatment of schizophrenia since 2003 [82]. It needs shortest injection interval among LAIs. Thus it requires repeated injections every 2 weeks after which steady-state levels are usually reached by weeks 6 to 8. RLAI may be suitable for patients who need frequent visit to clinic less than 4 weeks interval for frequent assessment and observation by clinicians. In addition, despite few studies have compared LAI with oral APs in recent onset schizophrenia, RLAI has been tested in comparison with oral APs including risperidone and haloperidol for patients who experienced their first episode of schizophrenia where the remission rate of RLAI (64.0%) was higher than oral APs (40.4%) [83]. Further, it was also easily accepted by such patients and showed increased adherence in first-episode patients being treated by oral APs (less than 4 months) [84].
DISCUSSION
When clinicians decided to prescribe LAIs as a regimen of APP, there should be some possible clinical issues in relation with existing oral APs or LAIs. In a transition period from oral AP to LAI, overlapping period of LAI and previous oral AP/own oral AP of LAI should be considered due to PK of LAIs since some LAIs need stabilization phase with oral AP. For instance, AOM needs at least 2 weeks of oral AP stabilization is needed to secure proper therapeutic dose level. RLAI and HD also need at least 21 days of oral AP initiation/stabilization period, while PP and OP do not need such overlap with oral APs due to their rapid dissolution effects [85]. Hence, clinician may use PP or OP for acute addition to oral APs or rapid replacement of oral AP to complement APP. There are also LAIs with 2-month or 3-month injection interval today. PP also has a formulation 3-monthly injection, while aripiprazole has 2-monthly injection as well. Those would be beneficial for APP patients who do not like frequent injections, are insightful, and do not need frequent clinic visit due to stabilized clinical condition.
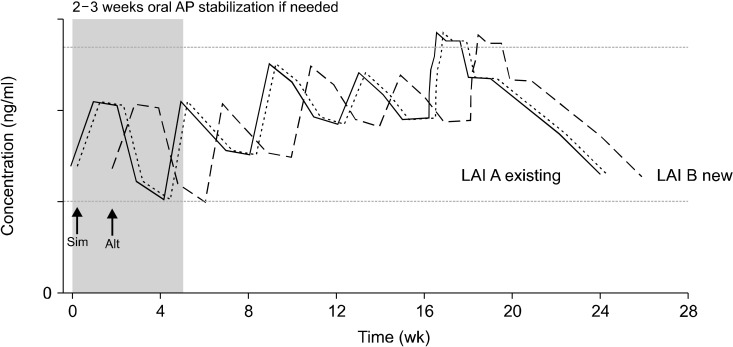
When combining certain LAI with existing LAI with or without existing oral AP, clinicians should be more careful since there are a dearth of data regarding the mixed use of two or more LAIs. However some case series reports proposed the clinical utility of diverse combination of two different LAIs without further AEs including PP [86-88], RLAI [88,89], OP [86,88,90], and HD [90] in clinical practice. Even in one case report, it was used for adolescent patients with severe psychosis and aggression where clozapine was not a viable therapeutic option and the results endorsed the effectiveness of two LAIs combination without showing any tolerability issues [88]. Two different LAI OM formulation can be administered simultaneously every 4 weeks or alternatively every 2 weeks depending on clinical situation and patients’ preference where simultaneous injection can be useful for those want less frequent shots and are relatively stabilized, while alternating injection can be beneficial for patients who need more close observation and are sensitive to simultaneous injection [91]. The proper usage of two LAIs should be utilization of lowest efficacious doses closely monitoring risks associated with AEs under clinicians’ best reflection of cost-effectiveness since LAIs combination is not covered by insurance yet. However we should keep in mind that a single LAI, preferably with a longer dosing interval and less AE should be left and maintained to minimize risks of tolerability issues if patients’ symptoms are meaningfully ameliorated. Figure 3 illustrates the putative administration method of two different LAI.
Fig. 3.
Hypothetical injection interval when combining two or more long-acting injectable antipsychotics (LAIA) once-monthly. Solid line means LAI A existing injection and two dashed lines indicate LAI B newly injected. New LAI B can be administered simultaneously (tight dashed line, Sim) every 4 weeks along with an existing LAI A or injected alternatively (wide dashed line, Alt) every 2 weeks with existing LAI A depending on clinical situation and patients’ preference. Simultaneous injection can be useful for those want less frequent shots and are relatively in stable clinical status, while alternating injection can be beneficial for patients who need more close observation, are more compliant and are sensitive to injection pain. In case of switching from existing LAI A/B to LAI A/C, a new LAI C can be administered in place of the next injection of existing LAI B (not shown in figure). AP, antipsychotic polypharmacy.
When switching one LAI to another LAI for any reasons in a patient at steady-state on two LAIs, later LAI should be injected in place of the next injection of existing LAI.
There has been no evidence to support the use of specific LAI over other LAI in the treatment of schizophrenia in terms of efficacy, however, clinicians may differentially utilize individual LAI for target symptoms or objectives based on unique pharmacological differences among LAIs. For instance, AOM has some beneficial PD in terms of efficacy and tolerability in association with negative symptoms, cognition, and functional recovery as well as reduction of EPS, weight gain and sexual dysfunction, compared with other LAIs such as RLAI, PPAs, HD, and OP [13,35,92]. RLAI was found to be effective in the treatment of first-episode schizophrenia as well as shown a superior remission rate in comparison with oral AP [83].
As for cost-effectiveness, AOM and PP were compared in some studies resulting in mixed findings [35,93-96]. Indeed, comparative studies using claim-data or inde-pendent researches comparing the cost-effectiveness between AOM and PP failed to show a superiority of one over the other till today. Such cost-effectiveness studies on LAIs should be further investigated in more adequately-powered and well-designed RCTs.
Indeed, LAI monotherapy is still the optimal treatment option given the recommendation from all major treatment guidelines. Currently available treatment guidelines do not give any formal suggestion or recommendation to address on LAI polypharmacy in combination with existing oral APs as well as on two or more LAIs combination therapy. Literatures also exist that LAI monotherapy may have superiority over LAI polypharmacy on some points such compliance and development of AEs. In a recent retrospective cohort study (n = 397) [97], 97 took LAI monotherapy and 300 patients had LAI/oral AP polypharmacy in which the mean CPZeq of LAI monotherapy and LAI/oral AP polypharmacy were 416.8 mg and 1147.9 mg, respectively. The time to discontinuation for any reason was significantly longer in the LAI monotherapy group (HR = 0.46) than in the LAI/oral AP polypharmacy and no significant difference was found in the mean clinical global impression-Improvement scale (CGI-I) between the LAI monotherapy group and the LAI/oral AP polypharmacy (3.3 vs. 3.4), indicating that LAI/oral AP polypharmacy may have more risks of developing poor compliance and high-dose treatment, although such data should be more replicated in subsequent studies.
To summarize, when clinicians have to commence LAIs as a part of polypharmacy, every effort should be made before to do so with meticulous re-evaluation of available treatment options or other clinical factors not being considered or investigated yet. It is not easy decision for clinicians to take LAIs polypharmacy in routine practice since no formal guidelines or even expert consensus has not been established till today. Based on currently available data and clinical practice, APP with LAIs can be potentially usable but not for all patients in routine practice. However, it could be one of another viable treatment options for treating patients with difficult-to-treat, partial response to clozapine/unusable case of clozapine treatment and compliance issues at best today. Needless to say, more data coming from adequately-powered and well- designed RCTs, naturalistic researches, direct compara-tive studies and observational cohort studies may clearly address these interesting points near future.
Footnotes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: Chi-Un Pae, Changsu Han. Supervision: Soo-Jung Lee, Won-Myong Bahk, Ashwin A. Patkar, Prakash S. Masand. Writing−original draft: Chi-Un Pae. Writing−review & editing: Changsu Han, Soo-Jung Lee, Won-Myong Bahk, Ashwin A. Patkar, Prakash S. Masand.
References
- 1.Fountoulakis KN, Panagiotidis P, Theofilidis AT, Nimatoudis I. One-year outcome of first vs. later episode schizophrenia: a real-world naturalistic study. Clin Psychopharmacol Neurosci. 2020;18:434–444. doi: 10.9758/cpn.2020.18.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katona L, Czobor P, Bitter I. Real-world effectiveness of antipsychotic monotherapy vs. polypharmacy in schizophrenia: to switch or to combine? A nationwide study in Hungary. Schizophr Res. 2014;152:246–254. doi: 10.1016/j.schres.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 3.Park JH, Hong JS, Kim SM, Min KJ, Chung US, Han DH. Effects of amisulpride adjunctive therapy on working memory and brain metabolism in the frontal cortex of patients with schizophrenia: a preliminary positron emission tomography/com-puterized tomography investigation. Clin Psychopharmacol Neurosci. 2019;17:250–260. doi: 10.9758/cpn.2019.17.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JS, Yun JY, Kang SH, Lee SJ, Choi JH, Nam B, et al. Korean Medication Algorithm for Schizophrenia 2019, second revision: treatment of psychotic symptoms. Clin Psychopharmacol Neurosci. 2020;18:386–394. doi: 10.9758/cpn.2020.18.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oltra JAE. Improving therapeutic interventions of schizophrenia with advances in stem cell technology. Clin Psychopharmacol Neurosci. 2020;18:352–361. doi: 10.9758/cpn.2020.18.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong M, Zeng LN, Zhang Q, Yang SY, Chen LY, Najoan E, et al. Prescription of antipsychotic and concomitant medications for adult Asian schizophrenia patients: findings of the 2016 Research on Asian Psychotropic Prescription Patterns (REAP) survey. Asian J Psychiatr. 2019;45:74–80. doi: 10.1016/j.ajp.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, et al. World Federation of Societies of Biological Psychiatry (WFSBP) Task Force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13:318–378. doi: 10.3109/15622975.2012.696143. [DOI] [PubMed] [Google Scholar]
- 8.Barnes TR, Drake R, Paton C, Cooper SJ, Deakin B, Ferrier IN, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psycho-pharmacol. 2020;34:3–78. doi: 10.1177/0269881119889296. [DOI] [PubMed] [Google Scholar]
- 9.Galletly C, Castle D, Dark F, Humberstone V, Jablensky A, Killackey E, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust N Z J Psychiatry. 2016;50:410–472. doi: 10.1177/0004867416641195. [DOI] [PubMed] [Google Scholar]
- 10.Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 Suppl):1–56. [PubMed] [Google Scholar]
- 11.Remington G, Addington D, Honer W, Ismail Z, Raedler T, Teehan M. Guidelines for the pharmacotherapy of schizophrenia in adults. Can J Psychiatry. 2017;62:604–616. doi: 10.1177/0706743717720448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stahl SM. Emerging guidelines for the use of antipsychotic polypharmacy. Rev Psiquiatr Salud Ment. 2013;6:97–100. doi: 10.1016/j.rpsm.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Keepers GA, Fochtmann LJ, Anzia JM, Benjamin S, Lyness JM, Mojtabai R, et al. The American Psychiatric Association Practice Guideline for the treatment of patients with schizo-phrenia. Am J Psychiatry. 2020;177:868–872. doi: 10.1176/appi.ajp.2020.177901,. [DOI] [PubMed] [Google Scholar]
- 14.National Institute for Health and Care Excellence, author. Psychosis and schizophrenia in adults: prevention and management [Internet] National Institute for Health and Care Excellence; London: 2014. Feb 12, [cited at 2020 Aug 20]. https://www.nice.org.uk/guidance/cg178 . [PubMed] [Google Scholar]
- 15.Hatta K, Hasegawa H, Imai A, Sudo Y, Morikawa F, Katayama S, et al. Real-world effectiveness of antipsychotic monotherapy and polytherapy in 1543 patients with acute-phase schizophrenia. Asian J Psychiatr. 2019;40:82–87. doi: 10.1016/j.ajp.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Guinart D, Correll CU. Antipsychotic polypharmacy in schizophrenia: why not? J Clin Psychiatry. 2020;81:19ac13118. doi: 10.4088/JCP.19ac13118,. [DOI] [PubMed] [Google Scholar]
- 17.Sun F, Stock EM, Copeland LA, Zeber JE, Ahmedani BK, Morissette SB. Polypharmacy with antipsychotic drugs in patients with schizophrenia: trends in multiple health care systems. Am J Health Syst Pharm. 2014;71:728–738. doi: 10.2146/ajhp130471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galling B, Roldán A, Hagi K, Rietschel L, Walyzada F, Zheng W, et al. Antipsychotic augmentation vs. monotherapy in schizophrenia: systematic review, meta-analysis and meta- regression analysis. World Psychiatry. 2017;16:77–89. doi: 10.1002/wps.20387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tiihonen J, Taipale H, Mehtälä J, Vattulainen P, Correll CU, Tanskanen A. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76:499–507. doi: 10.1001/jamapsychiatry.2018.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roh D, Chang JG, Kim CH, Cho HS, An SK, Jung YC. Antipsychotic polypharmacy and high-dose prescription in schizophrenia: a 5-year comparison. Aust N Z J Psychiatry. 2014;48:52–60. doi: 10.1177/0004867413488221. [DOI] [PubMed] [Google Scholar]
- 21.Aly El-Gabry DM, Abdel Aziz K, Okasha T, Azzam H, Okasha A. Antipsychotic polypharmacy and its relation to metabolic syndrome in patients with schizophrenia: an Egyptian study. J Clin Psychopharmacol. 2018;38:27–33. doi: 10.1097/JCP.0000000000000815. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi H, Suzuki T, Remington G, Uchida H. Antipsychotic polypharmacy and corrected QT interval: a systematic review. Can J Psychiatry. 2015;60:215–222. doi: 10.1177/070674371506000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang JG, Roh D, Kim CH. Association between therapeutic alliance and adherence in outpatient schizophrenia patients. Clin Psychopharmacol Neurosci. 2019;17:273–278. doi: 10.9758/cpn.2019.17.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee Y, Lee MS, Jeong HG, Youn HC, Kim SH. Medication adherence using electronic monitoring in severe psychiatric illness: 4 and 24 weeks after discharge. Clin Psychopharmacol Neurosci. 2019;17:288–296. doi: 10.9758/cpn.2019.17.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pae CU, Han C, Bahk WM, Lee SJ, Patkar AA, Masand PS. Effectiveness and tolerability of switching to Aripiprazole Once Monthly from antipsychotic polypharmacy and/or other long acting injectable antipsychotics for patients with schizophrenia in routine practice: a retrospective, observation study. Clin Psychopharmacol Neurosci. 2020;18:153–158. doi: 10.9758/cpn.2020.18.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamei H, Homma Y, Takeuchi I, Hajitsu G, Tozawa K, Hatano M, et al. Acceptance of the deltoid muscle injection of aripiprazole long-acting injectable in the patients with schizo-phrenia. Clin Psychopharmacol Neurosci. 2020;18:49–57. doi: 10.9758/cpn.2020.18.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee D, Lee BC, Choi SH, Kang DH, Jon DI, Jung MH. Effects of paliperidone palmitate on healthcare utilization and costs for patients with schizophrenia: a claim-based mirror-image study in South Korea. Clin Psychopharmacol Neurosci. 2020;18:303–310. doi: 10.9758/cpn.2020.18.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang SM, Han C, Lee SJ, Patkar AA, Masand PS, Pae CU. Schizophrenia relapse and the clinical usefulness of once- monthly aripiprazole depot injection. Neuropsychiatr Dis Treat. 2014;10:1605–1611. doi: 10.2147/NDT.S52486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallego JA, Bonetti J, Zhang J, Kane JM, Correll CU. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res. 2009;138:18–28. doi: 10.1016/j.schres.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Correll CU, Gallego JA. Antipsychotic polypharmacy: a comprehensive evaluation of relevant correlates of a long-standing clinical practice. Psychiatr Clin North Am. 2012;35:661–681. doi: 10.1016/j.psc.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimitropoulos E, Drogemuller L, Wong K. Evaluation of concurrent oral and long-acting injectable antipsychotic prescribing at the Minneapolis veterans affairs health care system. J Clin Psychopharmacol. 2017;37:605–608. doi: 10.1097/JCP.0000000000000755. [DOI] [PubMed] [Google Scholar]
- 32.Doshi JA, Pettit AR, Stoddard JJ, Zummo J, Marcus SC. Concurrent oral antipsychotic drug use among schizophrenia patients initiated on long-acting injectable antipsychotics post-hospital discharge. J Clin Psychopharmacol. 2015;35:442–446. doi: 10.1097/JCP.0000000000000353. [DOI] [PubMed] [Google Scholar]
- 33.Constantine RJ, Andel R, McPherson M, Tandon R. Is the risk of antipsychotic polypharmacy discontinuation dependent on the agents used? Psychiatry Res. 2018;263:238–244. doi: 10.1016/j.psychres.2017.09.050. [DOI] [PubMed] [Google Scholar]
- 34.Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsy-chotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsy-chotics following hospital discharge. J Manag Care Spec Pharm. 2015;21:754–768. doi: 10.18553/jmcp.2015.21.9.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodgson RE. Evaluating the cost and clinical effectiveness of long-acting, injectable aripiprazole and paliperidone palmitate once a month in a real-world setting. Clinicoecon Outcomes Res. 2019;11:517–524. doi: 10.2147/CEOR.S191198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwata N, Inagaki A, Sano H, Niidome K, Kojima Y, Yamada S. Treatment persistence between long-acting injectable versus orally administered aripiprazole among patients with schizophrenia in a real-world clinical setting in Japan. Adv Ther. 2020;37:3324–3336. doi: 10.1007/s12325-020-01396-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahlich J, Olbrich K, Wilk A, Wimmer A, Wolff-Menzler C. Hospitalization rates and therapy costs of German schizophrenia patients who are initiated on long-acting injectable medication: a mirror-image study. Clin Drug Investig. 2020;40:355–375. doi: 10.1007/s40261-020-00900-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin CH, Chen FC, Chan HY, Hsu CC. Time to rehospitalization in patients with schizophrenia receiving long-acting injectable antipsychotics or oral antipsychotics. Int J Neuropsy-chopharmacol. 2019;22:541–547. doi: 10.1093/ijnp/pyz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kishimoto T, Robenzadeh A, Leucht C, Leucht S, Watanabe K, Mimura M, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40:192–213. doi: 10.1093/schbul/sbs150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kane JM, Kishimoto T, Correll CU. Assessing the comparative effectiveness of long-acting injectable vs. oral antipsychotic medications in the prevention of relapse provides a case study in comparative effectiveness research in psychiatry. J Clin Epidemiol. 2013;66(8 Suppl):S37–S41. doi: 10.1016/j.jclinepi.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tani H, Takasu S, Uchida H, Suzuki T, Mimura M, Takeuchi H. Factors associated with successful antipsychotic dose reduction in schizophrenia: a systematic review of prospective clinical trials and meta-analysis of randomized controlled trials. Neuropsychopharmacology. 2020;45:887–901. doi: 10.1038/s41386-019-0573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graff-Guerrero A, Rajji TK, Mulsant BH, Nakajima S, Caravaggio F, Suzuki T, et al. Evaluation of antipsychotic dose reduction in late-life schizophrenia: a prospective dopamine D2/3 receptor occupancy study. JAMA Psychiatry. 2015;72:927–934. doi: 10.1001/jamapsychiatry.2015.0891. [DOI] [PubMed] [Google Scholar]
- 43.Uchida H, Suzuki T, Takeuchi H, Arenovich T, Mamo DC. Low dose vs standard dose of antipsychotics for relapse prevention in schizophrenia: meta-analysis. Schizophr Bull. 2011;37:788–799. doi: 10.1093/schbul/sbp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCutcheon R, Beck K, Bloomfield MA, Marques TR, Rogdaki M, Howes OD. Treatment resistant or resistant to treatment? Antipsychotic plasma levels in patients with poorly controlled psychotic symptoms. J Psychopharmacol. 2015;29:892–897. doi: 10.1177/0269881115576688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez LV, Shaikh A, Merson J, Greenberg J, Suckow RF, Kane JM. Accuracy of clinician assessments of medication status in the emergency setting: a comparison of clinician assessment of antipsychotic usage and plasma level determination. J Clin Psychopharmacol. 2017;37:310–314. doi: 10.1097/JCP.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 46.Sheehan JJ, Reilly KR, Fu DJ, Alphs L. Comparison of the peak- to-trough fluctuation in plasma concentration of long-acting injectable antipsychotics and their oral equivalents. Innov Clin Neurosci. 2012;9:17–23. [PMC free article] [PubMed] [Google Scholar]
- 47.Bai YM, Ting Chen T, Chen JY, Chang WH, Wu B, Hung CH, et al. Equivalent switching dose from oral risperidone to risperidone long-acting injection: a 48-week randomized, prospective, single-blind pharmacokinetic study. J Clin Psychiatry. 2007;68:1218–1225. doi: 10.4088/jcp.v68n0808. [DOI] [PubMed] [Google Scholar]
- 48.Ereshefsky L, Mascarenas CA. Comparison of the effects of different routes of antipsychotic administration on pharmacokinetics and pharmacodynamics. J Clin Psychiatry. 2003;64 Suppl 16:18–23. [PubMed] [Google Scholar]
- 49.Eerdekens M, Van Hove I, Remmerie B, Mannaert E. Pharmacokinetics and tolerability of long-acting risperidone in schizophrenia. Schizophr Res. 2004;70:91–100. doi: 10.1016/j.schres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Mannaert E, Vermeulen A, Remmerie B, Bouhours P, Levron JC. Pharmacokinetic profile of long-acting injectable risperidone at steady-state: comparison with oral administration. Encephale. 2005;31(5 Pt 1):609–615. doi: 10.1016/s0013-7006(05)82420-0. [DOI] [PubMed] [Google Scholar]
- 51.Raoufinia A, Baker RA, Eramo A, Nylander AG, Landsberg W, Kostic D, et al. Initiation of aripiprazole once-monthly in patients with schizophrenia. Curr Med Res Opin. 2015;31:583–592. doi: 10.1185/03007995.2015.1006356. [DOI] [PubMed] [Google Scholar]
- 52.Hard ML, Mills RJ, Sadler BM, Wehr AY, Weiden PJ, von Moltke L. Pharmacokinetic profile of a 2-month dose regimen of aripiprazole lauroxil: a phase I study and a population pharmacokinetic model. CNS Drugs. 2017;31:617–624. doi: 10.1007/s40263-017-0447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hard ML, Wehr AY, Sadler BM, Mills RJ, von Moltke L. Population pharmacokinetic analysis and model-based simulations of aripiprazole for a 1-day initiation regimen for the long-acting antipsychotic aripiprazole lauroxil. Eur J Drug Metab Pharmacokinet. 2018;43:461–469. doi: 10.1007/s13318-018-0488-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jann MW, Ereshefsky L, Saklad SR. Clinical pharmacokinetics of the depot antipsychotics. Clin Pharmacokinet. 1985;10:315–333. doi: 10.2165/00003088-198510040-00003. [DOI] [PubMed] [Google Scholar]
- 55.Cruz MP. Aripiprazole lauroxil (Aristada): an extended-release, long-acting injection for the treatment of schizophrenia. P T. 2016;41:556–559. [PMC free article] [PubMed] [Google Scholar]
- 56.Di Lorenzo R, Brogli A. Profile of olanzapine long-acting injection for the maintenance treatment of adult patients with schizophrenia. Neuropsychiatr Dis Treat. 2010;6:573–581. doi: 10.2147/NDT.S5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Lorenzo R, Ferri P, Cameli M, Rovesti S, Piemonte C. Effectiveness of 1-year treatment with long-acting formulation of aripiprazole, haloperidol, or paliperidone in patients with schizophrenia: retrospective study in a real-world clinical setting. Neuropsychiatr Dis Treat. 2019;15:183–198. doi: 10.2147/NDT.S189245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.García-Carmona JA, Simal-Aguado J, Campos-Navarro MP, Valdivia-Muñoz F, Galindo-Tovar A. Long-acting injectable antipsychotics: analysis of prescription patterns and patient characteristics in mental health from a Spanish real-world study. Clin Drug Investig. 2020;40:459–468. doi: 10.1007/s40261-020-00913-7. [DOI] [PubMed] [Google Scholar]
- 59.Pae CU, Wang SM, Han C, Bahk WM, Lee SJ, Patkar AA, et al. Comparison between long-acting injectable aripiprazole versus paliperidone palmitate in the treatment of schizophrenia: systematic review and indirect treatment comparison. Int Clin Psychopharmacol. 2017;32:235–248. doi: 10.1097/YIC.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 60.Park MH, Han C, Pae CU, Lee SJ, Patkar AA, Masand PS, et al. Aripiprazole treatment for patients with schizophrenia: from acute treatment to maintenance treatment. Expert Rev Neurother. 2011;11:1541–1552. doi: 10.1586/ern.11.151. [DOI] [PubMed] [Google Scholar]
- 61.Kane JM, Sanchez R, Perry PP, Jin N, Johnson BR, Forbes RA, et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week, multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2012;73:617–624. doi: 10.4088/JCP.11m07530. [DOI] [PubMed] [Google Scholar]
- 62.Fleischhacker WW, Sanchez R, Perry PP, Jin N, Peters- Strickland T, Johnson BR, et al. Aripiprazole once-monthly for treatment of schizophrenia: double-blind, randomised, non- inferiority study. Br J Psychiatry. 2014;205:135–144. doi: 10.1192/bjp.bp.113.134213. [DOI] [PubMed] [Google Scholar]
- 63.Kane JM, Peters-Strickland T, Baker RA, Hertel P, Eramo A, Jin N, et al. Aripiprazole once-monthly in the acute treatment of schizophrenia: findings from a 12-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75:1254–1260. doi: 10.4088/JCP.14m09168. [DOI] [PubMed] [Google Scholar]
- 64.Kane JM, Zhao C, Johnson BR, Baker RA, Eramo A, McQuade RD, et al. Hospitalization rates in patients switched from oral anti-psychotics to aripiprazole once-monthly: final efficacy analysis. J Med Econ. 2015;18:145–154. doi: 10.3111/13696998.2014.979936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kane JM, Schooler NR, Marcy P, Correll CU, Achtyes ED, Gibbons RD, et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77:1–8. doi: 10.1001/jamapsychiatry.2020.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Naber D, Hansen K, Forray C, Baker RA, Sapin C, Beillat M, et al. Qualify: a randomized head-to-head study of aripiprazole once-monthly and paliperidone palmitate in the treatment of schizophrenia. Schizophr Res. 2015;168:498–504. doi: 10.1016/j.schres.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 67.Potkin SG, Loze JY, Forray C, Baker RA, Sapin C, Peters- Strickland T, et al. Multidimensional assessment of functional outcomes in schizophrenia: results from QUALIFY, a head-to- head trial of aripiprazole once-monthly and paliperidone palmitate. Int J Neuropsychopharmacol. 2017;20:40–49. doi: 10.1093/ijnp/pyw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Potkin SG, Loze JY, Forray C, Baker RA, Sapin C, Peters- Strickland T, et al. Reduced sexual dysfunction with aripiprazole once-monthly versus paliperidone palmitate: results from QUALIFY. Int Clin Psychopharmacol. 2017;32:147–154. doi: 10.1097/YIC.0000000000000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Potkin SG, Loze JY, Forray C, Baker RA, Sapin C, Peters- Strickland T, et al. Relationship between response to aripiprazole once-monthly and paliperidone palmitate on work readiness and functioning in schizophrenia: a post-hoc analysis of the QUALIFY study. PLoS One. 2017;12:e0183475. doi: 10.1371/journal.pone.0183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Naber D, Baker RA, Eramo A, Forray C, Hansen K, Sapin C, et al. Long-term effectiveness of aripiprazole once-monthly for schizophrenia is maintained in the QUALIFY extension study. Schizophr Res. 2018;192:205–210. doi: 10.1016/j.schres.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 71.Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, et al. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharma-cology. 2003;28:1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki H, Hibino H, Inoue Y, Takaya A. Comparisons of the effects of second-generation antipsychotics long-acting injections on treatment retention according to severity of patient condition. Asian J Psychiatr. 2018;37:64–66. doi: 10.1016/j.ajp.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 73.Cuomo I, Kotzalidis GD, de Persis S, Piacentino D, Perrini F, Amici E, et al. Head-to-head comparison of 1-year aripiprazole long-acting injectable (LAI) versus paliperidone LAI in comorbid psychosis and substance use disorder: impact on clinical status, substance craving, and quality of life. Neuropsychiatr Dis Treat. 2018;14:1645–1656. doi: 10.2147/NDT.S171002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown ES, Jeffress J, Liggin JD, Garza M, Beard L. Switching outpatients with bipolar or schizoaffective disorders and substance abuse from their current antipsychotic to aripiprazole. J Clin Psychiatry. 2005;66:756–760. doi: 10.4088/jcp.v66n0613. [DOI] [PubMed] [Google Scholar]
- 75.Feltenstein MW, Altar CA, See RE. Aripiprazole blocks reinstatement of cocaine seeking in an animal model of relapse. Biol Psychiatry. 2007;61:582–590. doi: 10.1016/j.biopsych.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 76.Balcioglu YH, Gokcay H, Yesilkaya UH. One plus one sometimes equals more than two: long-acting injectable aripiprazole adjunction in clozapine-resistant schizophrenia. Clin Neuropharmacol. 2020;43:166–168. doi: 10.1097/WNF.0000000000000404. [DOI] [PubMed] [Google Scholar]
- 77.Lindenmayer JP. Long-acting injectable antipsychotics: focus on olanzapine pamoate. Neuropsychiatr Dis Treat. 2010;6:261–267. doi: 10.2147/ndt.s3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Detke HC, McDonnell DP, Brunner E, Zhao F, Sorsaburu S, Stefaniak VJ, et al. Post-injection delirium/sedation syndrome in patients with schizophrenia treated with olanzapine long-acting injection, I: analysis of cases. BMC Psychiatry. 2010;10:43. doi: 10.1186/1471-244X-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Anand E, Berggren L, Landry J, Tóth Á, Detke HC. Clinical outcomes with olanzapine long-acting injection: impact of the 3-hour observation period on patient satisfaction and well- being. Neuropsychiatr Dis Treat. 2016;12:2737–2743. doi: 10.2147/NDT.S107266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martel ML, Klein LR, Rivard RL, Cole JB. A large retrospective cohort of patients receiving intravenous olanzapine in the emergency department. Acad Emerg Med. 2016;23:29–35. doi: 10.1111/acem.12842. [DOI] [PubMed] [Google Scholar]
- 81.Kishi T, Matsunaga S, Iwata N. Intramuscular olanzapine for agitated patients: a systematic review and meta-analysis of randomized controlled trials. J Psychiatr Res. 2015;68:198–209. doi: 10.1016/j.jpsychires.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 82.Rainer MK. Risperidone long-acting injection: a review of its long term safety and efficacy. Neuropsychiatr Dis Treat. 2008;4:919–927. doi: 10.2147/ndt.s3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Emsley R, Medori R, Koen L, Oosthuizen PP, Niehaus DJ, Rabinowitz J. Long-acting injectable risperidone in the treatment of subjects with recent-onset psychosis: a preliminary study. J Clin Psychopharmacol. 2008;28:210–213. doi: 10.1097/JCP.0b013e318167269d. [DOI] [PubMed] [Google Scholar]
- 84.Weiden PJ, Schooler NR, Weedon JC, Elmouchtari A, Sunakawa A, Goldfinger SM. A randomized controlled trial of long-acting injectable risperidone vs continuation on oral atypical antipsychotics for first-episode schizophrenia patients: initial adherence outcome. J Clin Psychiatry. 2009;70:1397–1406. doi: 10.4088/JCP.09m05284yel. [DOI] [PubMed] [Google Scholar]
- 85.Jann MW, Penzak SR. Long-acting injectable second-generation antipsychotics: an update and comparison between agents. CNS Drugs. 2018;32:241–257. doi: 10.1007/s40263-018-0508-6. [DOI] [PubMed] [Google Scholar]
- 86.Wartelsteiner F, Hofer A. Treating schizophrenia with 2 long- acting injectable antipsychotic drugs: a case report. J Clin Psychopharmacol. 2015;35:474–475. doi: 10.1097/JCP.0000000000000358. [DOI] [PubMed] [Google Scholar]
- 87.Ross C, Fabian T. College of Psychiatric and Neurologic Pharmacists 16th Annual Meeting. Colorado Springs; USA: 2013. Apr 21-24, High dose haloperidol decanoate augmentation with paliperidone palmitate. [Google Scholar]
- 88.McInnis P, Kasinathan J. Combination long-acting injectable (LAI) antipsychotic medication in adolescents with severe psychosis and aggression: a case series. Australas Psychiatry. 2019;27:160–164. doi: 10.1177/1039856218815744. [DOI] [PubMed] [Google Scholar]
- 89.Ladds B, Cosme R, Rivera F. Concurrent use of two depot antipsychotic medications in schizophrenia. Internet J Psychiatry. 2009;1:1. [Google Scholar]
- 90.Scangos KW, Caton M, Newman WJ. Multiple long-acting injectable antipsychotics for treatment-resistant schizophrenia: case report. J Clin Psychopharmacol. 2016;36:283–285. doi: 10.1097/JCP.0000000000000505. [DOI] [PubMed] [Google Scholar]
- 91.Diefenderfer LA. When should you consider combining 2 long-acting injectable antipsychotics? Curr Psychiatry. 2017;16:42–46. [Google Scholar]
- 92.Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–962. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 93.Gozlan G, Lecardeur L, Monfort AS, Doz M, Ortiz I, Larroumets P, et al. [Cost-effectiveness analysis of aripiprazole once-monthly versus paliperidone palmitate once-monthly in the treatment of schizophrenia in France] Encephale. 2018;44:496–503. doi: 10.1016/j.encep.2018.10.001. French. [DOI] [PubMed] [Google Scholar]
- 94.Citrome L, Kamat SA, Sapin C, Baker RA, Eramo A, Ortendahl J, et al. Cost-effectiveness of aripiprazole once-monthly compared with paliperidone palmitate once-monthly injectable for the treatment of schizophrenia in the United States. J Med Econ. 2014;17:567–576. doi: 10.3111/13696998.2014.917089. [DOI] [PubMed] [Google Scholar]
- 95.Sapin C, Hartry A, Kamat SA, Beillat M, Baker RA, Eramo A. Corrigendum: Pharmacoeconomic comparison of aripiprazole once-monthly and paliperidone palmitate from a head-to-head clinical trial in schizophrenia: a US analysis. Drugs Context. 2017;6:212504. doi: 10.7573/dic.212504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Druais S, Doutriaux A, Cognet M, Godet A, Lançon C, Levy P, et al. Cost effectiveness of paliperidone long-acting injectable versus other antipsychotics for the maintenance treatment of schizophrenia in France. Pharmacoeconomics. 2016;34:363–391. doi: 10.1007/s40273-015-0348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Suzuki H, Hibino H, Inoue Y, Takaya A. Comparisons of the effects of long-acting injectable monotherapy and combination therapy of long-acting injectable treatment with oral antipsychotics on treatment retention in patients with chronic schizophrenia. Asian J Psychiatr. 2019;39:112–113. doi: 10.1016/j.ajp.2018.12.016. [DOI] [PubMed] [Google Scholar]