Abstract
Objectives
To determine the variation in SARS-CoV-2 seroprevalence in school children and the relationship with self-reported symptoms.
Design
Baseline measurements of a longitudinal cohort study (Ciao Corona) from June to July 2020.
Setting
55 schools stratified by district in the canton of Zurich, Switzerland.
Participants
2585 children (1339 girls; median age: 11 years, age range: 6–16 years), attending grades 1–2, 4–5 and 7–8.
Main outcome measures
Variation in seroprevalence of SARS-CoV-2 in children across 12 cantonal districts, schools and grades, assessed using Luminex-based test of four epitopes for IgG, IgA and IgM (Antibody Coronavirus Assay, ABCORA 2.0). Clustering of cases within classes. Association of seropositivity and symptoms. Comparison with seroprevalence in adult population, assessed using Luminex-based test of IgG and IgA (Sensitive Anti-SARS-CoV-2 Spike Trimer Immunoglobulin Serological test).
Results
Overall seroprevalence was 2.8% (95% CI 1.5% to 4.1%), ranging from 1.0% to 4.5% across districts. Seroprevalence in grades 1–2 was 3.8% (95% CI 2.0% to 6.1%), in grades 4–5 was 2.4% (95% CI 1.1% to 4.2%) and in grades 7–8 was 1.5% (95% CI 0.5% to 3.0%). At least one seropositive child was present in 36 of 55 (65%) schools and in 44 (34%) of 131 classes where ≥5 children and ≥50% of children within the class were tested. 73% of children reported COVID-19-compatible symptoms since January 2020, with the same frequency in seropositive and seronegative children for all symptoms. Seroprevalence of children and adults was similar (3.2%, 95% credible interval (CrI) 1.7% to 5.0% vs 3.6%, 95% CrI 1.7% to 5.4%). The ratio of confirmed SARS-CoV-2 cumulative incidence-to-seropositive cases was 1:89 in children and 1:12 in adults.
Conclusions
SARS-CoV-2 seroprevalence was low in children and similar to that in adults by the end of June 2020. Very low ratio of diagnosed-to-seropositive children was observed. We did not detect clustering of SARS-CoV-2-seropositive children within classes, but the follow-up of this study will shed more light on transmission within schools.
Trial registration number
Keywords: paediatric infectious disease & immunisation, infection control, epidemiology, virology, community child health
Strengths and limitations of this study.
This study presents the results of a regionally representative cohort of children, randomly selected on school and class levels, and thus, allowing the analysis of clustering of cases within classes and schools.
This cross-sectional analysis estimates the seroprevalence in children in June–July in Switzerland, from a period when there is very little evidence about SARS-CoV-2 seroprevalence in children globally.
Serological test with high sensitivity and specificity was used, and Bayesian hierarchical models were applied to estimate seroprevalence, adjusting for test accuracy parameters.
Self-reported symptoms might be subject to recall-bias, particularly when reporting retrospectively for a period of >6 months.
Introduction
The transmission of SARS-CoV-2 in the school setting is not well understood,1 partly as schools were closed in many countries during the peaks of the pandemic, partly due to lack of representative studies with random sampling. Anecdotal evidence and case studies suggest that outbreaks can happen in schools,2–4 but it is not clear whether they represent outlier events or widely underdiagnosed spread of the infection. The effect of school closures on the community transmission of SARS-CoV-2 ranges from minimal to substantial,5 with some modelling studies even predicting an increase of the total number of deaths.6 Currently existing or planned population-based studies focusing on SARS-CoV-2 spread in schools are few and small-sized.7 8
In this study, we present the results of the first cross-sectional analysis of a large cohort of children from randomly selected schools and classes in the canton of Zurich, Switzerland. The cohort study follows the seroprevalence, symptoms, sociodemographic and lifestyle factors of enrolled children from June 2020 to April 2021. The participating children were enrolled from 16 June 2020 to 9 July 2020. Schools in Switzerland were closed for a relatively short period (16 March to 10 May) compared with other countries, and lock-down measures were mild. Restaurants, bars and non-essential shops and services were closed on 17 March and events or meetings with >5 people prohibited on 20 March, but no strict confinement at home implemented. These measures were gradually lifted in April–May 2020.
The aim of this analysis is to present the overall estimate of seroprevalence in children and its variation across districts, schools, grades and classes, the association of seroprevalence with self-reported symptoms, and the clustering of seropositive children within classes.
Methods
Study setting
The protocol for this longitudinal cohort study was reported elsewhere.9 The study is part of a large nationally coordinated research network Corona Immunitas in Switzerland.10 11
The study took place from 16 June 2020 to 9 July 2020, in the canton of Zurich, Switzerland. The canton of Zurich comprises 1.5 million residents, roughly 18% of the Swiss population, and includes both urban and rural settings, as well as an ethnically and linguistically diverse population. The first preventive measures in schools were introduced on 16 March 2020, when physical attendance of schools was stopped. Schools were partly reopened on 10 May, with a combination of online and on-site teaching with preventive measures (eg, teaching in smaller groups, school attendance every second day, sports and large group activities limited). Schools were fully reopened on 7 June, with minimal preventive measures (eg, recommended social distancing for teachers, reduction of group events) and otherwise regular operation (eg, full classrooms with desks mostly facing forward) until the end of the school year on 17 July. The prevention measures implemented in schools after 10 May were school-specific and based on the federal and cantonal guidelines.12
Study procedures
Schools were selected based on a full list of schools provided by the educational department of the canton. Primary schools were randomly selected by a computer programme from the list of all primary schools in the respective district. The closest secondary school (often in the same building or area) was then selected. The random sample of primary and secondary schools was stratified by geographic district. Within schools, randomly selected classes in lower school level (grades 1–2, attended by children aged 6–9 years), middle school level (grades 4–5, attended by children aged 9–13 years) and upper school level (grades 7–8, attended by children aged 12–16 years) were invited. Invited grades and classes were selected to ensure that the same cohort of children within the class can be followed until April 2021. Therefore, grades 1–2, 4–5 and 7–8 (but not grades 3, 6 and 9) were included, as they normally stay in the same school and class for the next school-year. We aimed to enrol at least three classes and 40 children per school level. As we were only able to test the children at schools, a major exclusion criterion was a suspected or confirmed infection with SARS-CoV-2 in the given child on the testing date, precluding attendance of school.
Study information, link to study website (www.ciao-corona.ch), and informational videos in multiple languages for schools, parents and children were sent to school principals and further to families of children from the selected classes. Children were enrolled and venous blood samples were taken in schools between 16 June 2020 and 9 July 2020. Questionnaires with information on sociodemographics and symptoms compatible with SARS-CoV-2 infection from January to June 2020 were completed online for the majority of children by their parents in June–July 2020 (for 3% of children in August–September).
In total, 55 of 156 invited primary and secondary schools agreed to participate, and 2585 children in 273 of 274 invited classes (no children participated in one class). Venous blood samples were collected from 2484 children (a sufficient amount of blood could not be obtained from 101 children). An online questionnaire, containing sociodemographic, health, symptoms and quality of life information for the children, and sociodemographic and symptoms information for the household members were completed for 2288 of all enrolled children. Questionnaires were not filled for 297 children, after several email and phone call reminders.
Serological tests
The primary outcome of the study was the serological results of blood serum samples analysed using ABCORA 2.0 binding assay of the Institute of Medical Virology of the University of Zurich based on the Luminex technology.13 The test analyses IgG, IgM and IgA against four SARS-CoV-2 targets (receptor-binding domain (RBD), spike proteins S1 and S2 and the nucleocapsid protein (N), yielding 12 different measurements. Cut-off values were established against prepandemic plasma allowing a high sensitivity (93.3%) and specificity (99.6%). Samples were defined as seropositive for SARS-CoV-2 if at least 2 of the 12 parameters were above the cut-off.
SARS-CoV-2 seroprevalence in children was compared with that estimated in a random sample from the general population, adjusting for age group and sex, in the same region in June–July 2020. The adult study, like all studies of the Swiss-wide research programme Corona Immunitas10 11, used the Sensitive Anti-SARS-CoV-2 Spike Trimer Immunoglobulin Serological (SenASTrIS) test developed by the Centre Hospitalier Universitaire Vaudois, the Swiss Federal Institute of Technology in Lausanne and the Swiss Vaccine Center.14 The test also uses Luminex technology to detect IgG and IgA antibodies binding to the entire trimeric S protein of SARS-CoV-2 and with demonstrated 98.3% sensitivity and 98.4% specificity for the combined testing of IgG and IgA (result declared as positive when either or both were positive).14 To compare the seroprevalence estimates in children and adult cohorts, blood serum samples of 2476 children were also analysed using the SenASTrIS test (samples were insufficient for analysis with the second test for 8 children) and compared with a random population sample of 857 adults who took part in the second phase of the Switzerland-wide Corona Immunitas research programme.11
Seroprevalence was also compared with the cumulative incidence of reverse transcription (RT)-PCR-confirmed SARS-CoV-2 infections in adults and children, based on official statistics up to the beginning of June.15
Statistical analysis
Statistical analysis included descriptive statistics and Bayesian hierarchical modelling to estimate seroprevalence.16 The Bayesian approach allowed to account for the sensitivity and specificity of the SARS-CoV-2 antibody test and the hierarchical structure of cohort (individual and school levels). The model (Bayesian logistic regression) was adjusted for participants’ grade and geographic district of the school, and included random effects for school levels (lower, middle and upper). To compute an estimate representative for the population of the canton of Zurich, we applied poststratification weights, which adjusted for the total population size of the specific school level and the geographic district. The model and weighing procedure are described in detail in online supplemental appendix 1.
bmjopen-2020-047483supp001.pdf (55.2KB, pdf)
The factor of diagnosed-to-seropositive children was calculated as the ratio of RT-PCR-confirmed cumulative incidence by the end of June 2020 and the estimated seroprevalence. We assessed the clustering of seropositive children within classes, school levels and schools by studying the distribution of classes with zero, one or more seropositive children. As the probability of detecting a seropositive child increases with more children tested, we separately assessed the proportion of classes with at least one seropositive child among all class and among classes with ≥5 participating children and ≥50% of children participating from the class.
Patient and public involvement
Several school principals were consulted during the development of the protocol to ensure feasibility of the planned study procedures. Early feedback was collected from invited children and parents to adapt the communication strategies and channels. Numerous online informational sessions, encouraging open exchange and feedback, were organised for invited and enrolled school principals, personnel and parents of the children. Results of individual tests were communicated to the participants, and overall study results disseminated to participating schools. Findings will be disseminated in lay language in the national and local press, to the national and regional educational and public health departments and on the website of the study (www.ciao-corona.ch).
Results
In total, 55 schools and 2585 children were recruited (1339 (52%) girls; median age: 11 years, age range: 6–16 years), 754 (29%) in the lower school level, 899 (35%) in the middle school level and 932 (36%) in the upper school level. Mean participation rate was 50% of the invited children within invited classes (range from 0% to 94% (0–21 children), IQR 32%–63%). Venous blood was collected and analysed for 2484 children (1278 (51%) girls, median age: 11 years, age range: 6–16 years).
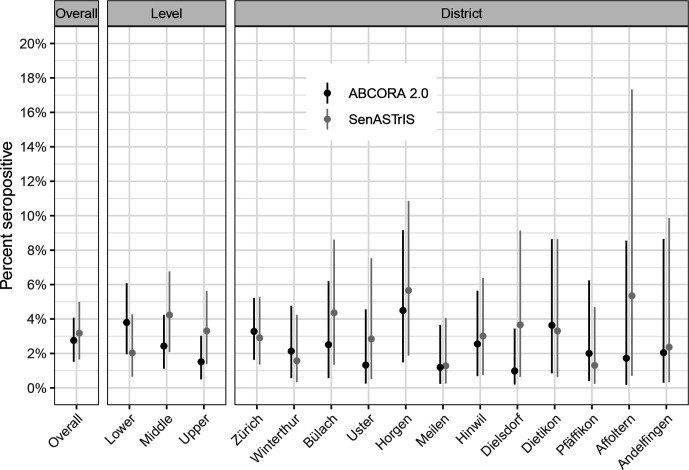
Seventy-four children had SARS-CoV-2 antibodies, resulting in overall weighted seroprevalence of 2.8% (95% credible interval (CrI) 1.5% to 4.1%), ranging from 1.0% to 4.5% in districts, as measured using ABCORA test (figure 1). Seroprevalence was 3.8% (95% CrI 2.0% to 6.1%) in grades 1–2, 2.4% (95% CrI 1.1% to 4.2%) in grades 4–5 and 1.5% (95% CrI 0.5% to 3.0%) in grades 7–8 (figure 1).
Figure 1.
Overall, school level and district estimates of SARS-CoV-2 seroprevalence in children. Weighted point estimates and 95% credible intervals are shown. Districts are ordered by population size. ABCORA 2.0—primary estimate based on the ABCORA 2.0 of the Institute of Medical Virology, University of Zurich test. SenASTrIS—estimate based on the SenASTrIS test. SenASTrIS, Sensitive Anti-SARS-CoV-2 Spike Trimer Immunoglobulin Serological test.
Seroprevalence of children, as measured with the SenASTrIS test of IgG and IgA combined, was very similar to the seroprevalence of randomly selected adults in the same region in June–July 2020 (children 3.2%, 95% CrI 1.7% to 5.0% vs adults 3.6%, 95% CrI 1.7% to 5.4%). The estimates of seroprevalence in different school levels and districts were somewhat different from those estimated with the primary ABCORA 2.0 test (figure 1); however, the CrIs of the estimates overlapped. Seroprevalence measured with SenASTriS test was 2.0% (95% CrI 0.6% to 4.3%) in grades 1–2, 4.2% (95% CrI 2.1% to 6.8%) in grades 4–5 and 3.3% (95% CrI 1.4% to 5.6%) in grades 7–8.
Based on the cumulative incidence of SARS-CoV-2 RT-PCR-confirmed cases by the end of June (0.03% for children and 0.24% for adult populations), the ratio of diagnosed-to-seropositive in children was 1:89, compared with a ratio of 1:12 in the adult population.
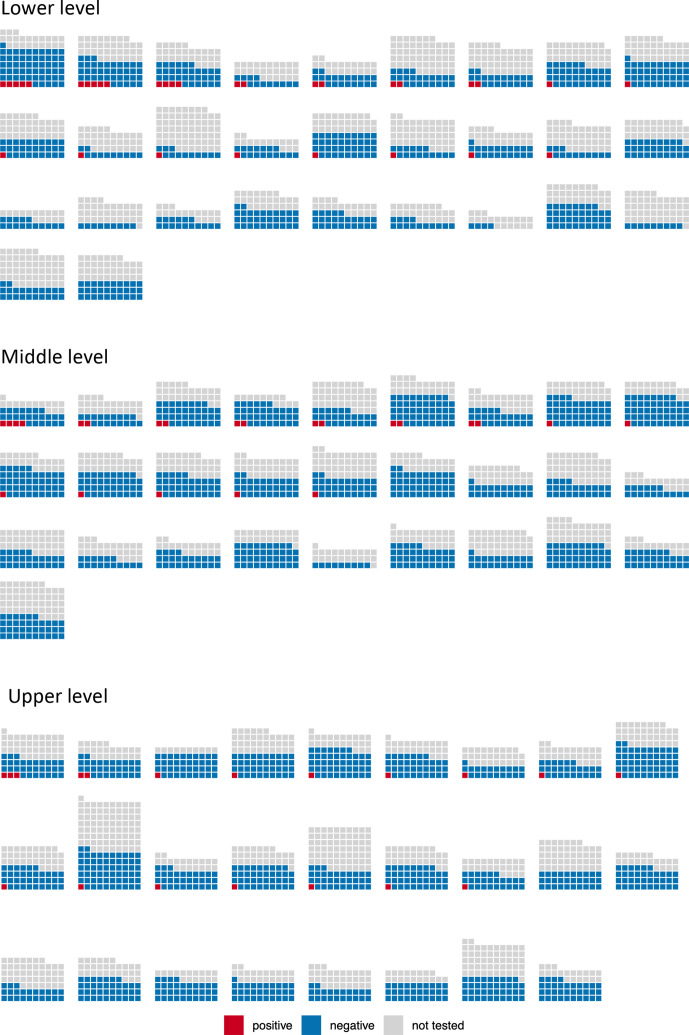
At least one seropositive child was present in 36 of 55 (65%) of the tested schools. Within the levels of the schools, at least one seropositive child was present in the lower level of 17 of 29 (59%) schools, in the middle level of 14 of 28 (50%) schools and in the upper level of 16 of 25 (64%) schools (figure 2).
Figure 2.
Seropositive children in the tested schools and school levels. Each little square illustrates an invited child. Each block of squares illustrates a school level in a school. Lower and middle levels are both taught in the primary schools; however, lower and middle levels of the same school are not matched in this graph due to protection of participant privacy. The distribution of the invited, tested and seropositive children is depicted only on the school level in the figures of this article to preserve deidentification and privacy of the participants.
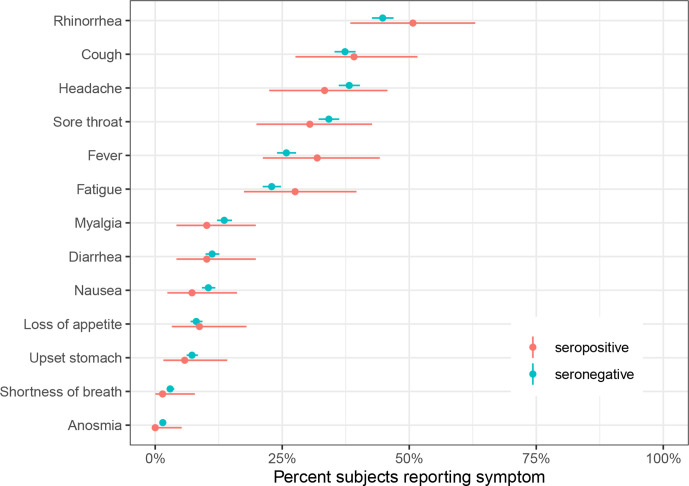
No sex differences in seroprevalence were noted (2.8% (95% CrI 1.6% to 4.1%) in girls and 2.7% (95% CrI 1.5% to 4.0%) in boys). 73% of children reported any SARS-CoV-2-compatible symptoms, such as cough, fever, fatigue or diarrhoea (see figure 3 for the full list), between January and June 2020. None of the symptoms were more frequent in seropositive than in seronegative children (figure 3).
Figure 3.
Self-reported symptoms in seropositive and seronegative children in January–June 2020. Point estimates and 95% CIs are shown.
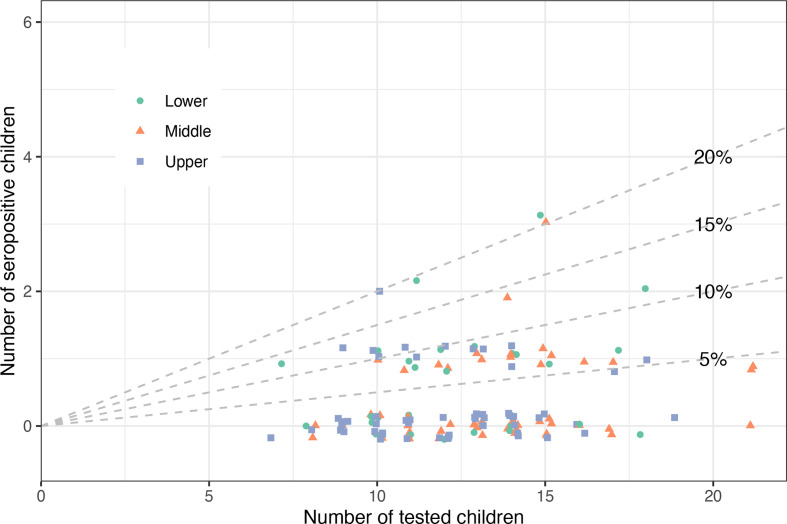
At least one seropositive child was present in 34% (44/131) of classes where ≥5 children and ≥50% of children within the class were tested (figure 4). Among the classes with at least one seropositive child, 38 (86%) had only one, 4 (9%) had two and 2 (5%) had three seropositive children. When considering all classes regardless of participation rate, 24% (65/273) of classes had at least one seropositive child.
Figure 4.
Clustering of seropositive children in classes: number and proportion of seropositive children in the tested classes. Each dot represents a class. Diagonal lines partition the figure into classes with 0%–5% of tested seropositive children (below the 5% line), 5%–10% of tested seropositive children (between 5% and 10% lines), etc. Only classes where at least five children and at least 50% of the class were tested are shown. The distribution of seropositive children in the enrolled classes is only presented in an aggregated form rather than clustered within schools to preserve the deidentification and privacy of the participants.
Discussion
In this study of randomly sampled schools and classes, we found variation in seroprevalence in children aged 6–16 years across districts, schools and classes by July 2020, but no indication of major transmission and outbreaks within classes and schools. The overall seroprevalence was not different from a randomly selected adult population living in the same region—pointing to striking underdiagnosis of SARS-CoV-2 infection in children, with only 1 in 89 cases diagnosed. Contrary to the studies of symptomatic infections17 and some other population-based studies of seroprevalence,16 18 there was a trend of higher seroprevalence in younger children as measured using the main ABCORA test (the trend was not present in seroprevalence estimated based on the SenASTriS test). The presence of symptoms was very common (three of four children reported one or several symptoms compatible with a COVID-19 infection) and importantly not specific to the seropositive children. Although no outbreaks were reported in schools at the time of testing in the canton of Zurich (comprising 18% of the Swiss population), seropositive children were detected in more than half of the tested schools and a third of all tested classes. However, the vast majority of classes with seropositive children had only a single seropositive child among the tested children, reflecting low prevalence and no significant clustering within classes after the re-opening of the schools.
By the time of conducting this study, there were few studies focusing on SARS-CoV-2 spread in schools.18–20 Most of the reported evidence consisted of cases studies of outbreaks in specific schools or reports of contact tracing of index cases in educational settings. Most of the studies reported low secondary attack rates in schools4 21 but also some conflicting observations of outbreaks.2 22 Although some studies of seroprevalence had included children,16 23 24 they mostly focused on households and the general population. The management of SARS-CoV-2 transmission in schools was therefore highly debated.25 26 This study is unique as one of the first major studies reporting variation in seroprevalence in children from randomly selected schools in a country where the general lock-down on a population level was mild and short (1 month), and school closure lasted only for 2 months.
Although manifest clinical disease of COVID-19 is much less prevalent in children than in adults17 27 28 and preliminary evidence points to lower susceptibility of children compared with adults,18 our results indicate very similar seroprevalence in adults and children. Similar seroprevalence in adults and school-aged children was found in another region in Switzerland in April16 and in November 2020.29 Intriguingly, we observed a not statistically significant trend of younger children having higher seroprevalence than older children, when measured with the comprehensive ABCORA 2.0 test. Infections with circulating human coronaviruses (hCoVs) are common in childhood and antibodies to hCoVs 229E, NL63, OC43 and HKU1A are prevalent in the human population and particularly children.30 31 Cross-reactivity with hCoVs was thus considered in the development of both serology tests used in this study and both tests detect SARS-CoV-2 with high specificity (99.6% for ABCORA 2.0 and 98.4% for SenASTrIS). Importantly, to adjust for the possibility of a few false-positive results, we employed a Bayesian hierarchical model, which adjusts for the accuracy parameters of the tests to estimate population-level seroprevalence. Higher seroprevalence in younger children could be compatible with less feasible social distancing behaviour and possibly more vigorous immune response to the virus in early age. The trend could also reflect a chance finding, as substantial proportion of false positives is expected in the low-seroprevalence setting, and it was not observed in the SenASTriS test results. Further testing of the cohort and forthcoming studies will show if this trend is observed in the future.
The frequency of both more specific (eg, fever and cough) and less specific (rhinorrhea, headache and nausea) symptoms32 was not different among seropositive and seronegative children. In general, symptoms, particularly rhinorrhea, cough, headache and sore throat, were reported frequently, with three of four children reporting any symptoms within the last 6 months in both groups. Anosmia was not reported by any of the seropositive children. The specificity of COVID-19-compatible symptoms, therefore, seems to be lower in children than in adults. Moreover, the range of symptoms reported in children was shown to be different than in adults.33 Somewhat unspecific symptoms in children, contrary to more specific symptoms in adults,34 could partly explain the high proportion of seropositive children who were not previously diagnosed with RT-PCR. Furthermore, testing indications by health authorities were cautious both for children and adults during the first half of 2020. Testing was recommended only for children with acute upper respiratory tract infection symptoms or acute anosmia, and not recommended in children with rhinitis only or without symptoms.35
Particular strengths of the design of this study are school-based random sampling, hierarchical data structure and large sample size, allowing to identify clusters within district, school, grade and class levels. Testing was done in schools and study information presented in multiple formats, including videos in multiple languages, to minimise selection bias within enrolled children. The participation rate of 50% can be considered rather high for a study in children involving venous blood sampling, and additional children from the invited classes will have the opportunity to be enrolled in subsequent testing phases (October/November 2020, March/April 2021), further increasing participation rate and the size of the cohort.
The study has a few limitations. First, the retrospective evaluation of symptoms more than 6 months could have been subject to recall bias. Second, the study enrolled only 35% (55/156) of the invited schools. Commonly stated reasons for non-participation on school level were constraints in time and human resources and competing participation in other studies. Participation rate on school level varied significantly depending on the district, and for a few districts, a maximum of three invitation rounds was needed to recruit a regionally representative sample. Third, although the schools were open for 1–2 months directly before the study, they were closed for the 2 months of the highest community transmission of SARS-CoV-2 in the canton of Zurich.15 Therefore, the measured seroprevalence in children might be dominated by infections in households rather than school. The follow-up of this study will shed more light on transmission in schools. Finally, we do not have information on how many eligible children did not attend the testing due to acute infection with SARS-CoV-2. We did not provide testing for such children at home or an alternative date due to limited resources. However, reported total weekly incidence of SARS-CoV-2 infections in the canton of Zurich ranged from 42 to 185 cases (among 1.5 million residents) and 7 to 15 cases among people aged <20 years old during the testing period. Considering that, in comparison, more than 700 total weekly cases were reported in March–April 2020, we believe that excluding acutely infected children during the testing period could not lead to substantial underestimation of the seroprevalence.
In conclusion, clustering of SARS-CoV-2-seropositive children within classes and schools was not prominent shortly after re-opening of schools in this large population-based study. Seroprevalence was similar to adults, resulting in strikingly fewer diagnosed infections in comparison to the seropositive cases in children than in adults. Considering the time window required for SARS-CoV-2 antibodies to form, this study reflects infection of SARS-CoV-2 until approximately end of May 2020, covering 4 months of SARS-CoV-2 infection in the community, with 2 months of school closure and mild lock-down policy. The subsequent testing of parents and school personnel and the follow-up of the children cohort in fall 2020 will yield further evidence on the observed trends and of the spread of SARS-CoV-2 within and outside schools.
Supplementary Material
Footnotes
AU, TR and IAA contributed equally.
Contributors: SK and MAP initiated the project and preliminary design, with support of JF. SK, MAP, CB, TR, RJ, JB, AF and AU developed the design and methodology. SK, RJ, AU, TR, JB, AF and CC recruited study participants, collected and managed the data. SRH performed statistical analysis. AT, MH, MSchw, MScha and IA developed the serology analysis plan, supervised, conducted and evaluated the serology tests. AU wrote the first draft of the article. All authors contributed to the design of the study and interpretation of its results, and revised and approved the article for intellectual content. SK is the guarantor and accepts full responsibility for the work and the conduct of the study, had access to the data and controlled the decision to publish. The corresponding author SK attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. AT, JF, MAP and SK contributed as last authors.
Funding: This study is part of Corona Immunitas research network, coordinated by the Swiss School of Public Health (SSPH+), and funded by fundraising of SSPH+ that includes funds of the Swiss Federal Office of Public Health and private funders (ethical guidelines for funding stated by SSPH+ are respected), by funds of the Cantons of Switzerland (Vaud, Zurich and Basel) and by institutional funds of the Universities. Additional funding, specific to this study is available from the University of Zurich Foundation.
Disclaimer: The funder/sponsor did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. All authors had full access to all data analysis outputs (reports and tables) and take responsibility for their integrity and accuracy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Data are still being collected for the cohort study Ciao Corona. Deidentified participant data might be available on reasonable request by email to the corresponding author at later stages of the study.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was approved by the Ethics Committee of the Canton of Zurich, Switzerland (2020-01336). All participants provided written informed consent before being enrolled in the study.
References
- 1.Viner RM, Russell SJ, Croker H, et al. School closure and management practices during coronavirus outbreaks including COVID-19: a rapid systematic review. Lancet Child Adolesc Health 2020;4:397–404. 10.1016/S2352-4642(20)30095-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stein-Zamir C, Abramson N, Shoob H, et al. A large COVID-19 outbreak in a high school 10 days after schools’ reopening, Israel, May 2020. Eurosurveillance 2020;25:2001352. 10.2807/1560-7917.ES.2020.25.29.2001352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez AS, Hill M, Antezano J, et al. Transmission Dynamics of COVID-19 Outbreaks Associated with Child Care Facilities - Salt Lake City, Utah, April-July 2020. MMWR Morb Mortal Wkly Rep 2020;69:1319–23. 10.15585/mmwr.mm6937e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macartney K, Quinn HE, Pillsbury AJ, et al. Transmission of SARS-CoV-2 in Australian educational settings: a prospective cohort study. Lancet Child Adolesc Health 2020;4:807–16. 10.1016/S2352-4642(20)30251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh S, Chowdhury A, Russell S, et al. Do school closures reduce community transmission of COVID-19? A systematic review of observational studies. medRxiv 2021. 10.1101/2021.01.02.21249146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice K, Wynne B, Martin V, et al. Effect of school closures on mortality from coronavirus disease 2019: old and new predictions. BMJ 2020;371:m3588. 10.1136/bmj.m3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ladhani S, Ramsay M, Amirthalingam G, et al. COVID-19 surveillance in children attending preschool, primary and secondary schools, 2020. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/891762/sKID_protocol_v1.3.pdf
- 8.Armann JP, Unrath M, Kirsten C, et al. Anti-SARS-CoV-2 IgG antibodies in adolescent students and their teachers in Saxony, Germany (SchoolCoviDD19): very low seropraevalence and transmission rates. medRxiv 2020:2020.07.16.20155143. 10.2139/ssrn.3651210 [DOI] [Google Scholar]
- 9.Ulyte A, Radtke T, Abela IA, et al. Seroprevalence and immunity of SARS-CoV-2 infection in children and adolescents in schools in Switzerland: design for a longitudinal, school-based prospective cohort study. Int J Public Health 2020;65:1549–57. 10.1007/s00038-020-01495-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ISRCTN . Corona Immunitas: a nationwide program of antibody studies of SARS-CoV-2 in the Swiss population (ISRCTN18181860). Available: http://www.isrctn.com/ISRCTN18181860 [Accessed 28 Aug 2020].
- 11.West EA, Anker D, Amati R, et al. Corona Immunitas: study protocol of a nationwide program of SARS-CoV-2 seroprevalence and seroepidemiologic studies in Switzerland. Int J Public Health 2020;65:1529–48. 10.1007/s00038-020-01494-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Information for public schools . Preventive regulations | Canton of Zurich. Available: https://www.zh.ch/de/gesundheit/coronavirus/informationen-rund-um-schulen-kitas-heime/coronavirus-volksschule.html#-1212670983 [Accessed 13 Nov 2020].
- 13.Institute of medical virology . IMV SARS-CoV-2 Antikörper Differenzierung (ABCORA). Available: https://www.virology.uzh.ch/dam/jcr:0f9bbdba-e215-4e42-a877-131a04a2e175/IMVABCORASerologie.pdf [Accessed 16 Dec 2020].
- 14.Fenwick C, Croxatto A, Coste AT, et al. Changes in SARS-CoV-2 spike versus nucleoprotein antibody responses impact the estimates of infections in population-based seroprevalence studies. J Virol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canton of Zurich . Numbers and Facts on COVID-19 [Kanton Zürich. Zahlen & Fakten zu COVID-19]. Available: https://www.zh.ch/de/gesundheit/coronavirus/zahlen-fakten-covid-19.html?keyword=covid19#/home [Accessed 13 Nov 2020].
- 16.Stringhini S, Wisniak A, Piumatti G, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet 2020;396:313–9. 10.1016/S0140-6736(20)31304-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Lusignan S, Dorward J, Correa A, et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of general practitioners research and surveillance centre primary care network: a cross-sectional study. Lancet Infect Dis 2020;20:1034–42. 10.1016/S1473-3099(20)30371-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viner RM, Mytton OT, Bonell C, et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults. JAMA Pediatr 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu W, Li X, Dozier M, et al. What is the evidence for transmission of COVID-19 by children in schools? A living systematic review. SSRN Journal 2020. 10.2139/ssrn.3710604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munro APS, Faust SN. COVID-19 in children. Curr Opin Infect Dis 2020. [DOI] [PubMed] [Google Scholar]
- 21.Heavey L, Casey G, Kelly C, et al. No evidence of secondary transmission of COVID-19 from children attending school in Ireland, 2020. Eurosurveillance 2020;25:2000903. 10.2807/1560-7917.ES.2020.25.21.2000903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szablewski CM, Chang KT, Brown MM, et al. SARS-CoV-2 Transmission and Infection Among Attendees of an Overnight Camp - Georgia, June 2020. MMWR Morb Mortal Wkly Rep 2020;69:1023–5. 10.15585/mmwr.mm6931e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. The Lancet 2020;396:535–44. 10.1016/S0140-6736(20)31483-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waterfield T, Watson C, Moore R, et al. Seroprevalence of SARS-CoV-2 antibodies in children - A prospective multicentre cohort study. medRxiv 2020. 10.1101/2020.08.31.20183095 [DOI] [PubMed] [Google Scholar]
- 25.Esposito S, Principi N. School closure during the coronavirus disease 2019 (COVID-19) pandemic: an effective intervention at the global level? JAMA Pediatr 2020;174:921–2. 10.1001/jamapediatrics.2020.1892 [DOI] [PubMed] [Google Scholar]
- 26.Christakis DA. School Reopening-The pandemic issue that is not getting its due. JAMA Pediatr 2020;174:928. 10.1001/jamapediatrics.2020.2068 [DOI] [PubMed] [Google Scholar]
- 27.Götzinger F, Santiago-García B, Noguera-Julián A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health 2020;4:653–61. 10.1016/S2352-4642(20)30177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Posfay-Barbe KM, Wagner N, Gauthey M, et al. COVID-19 in children and the dynamics of infection in families. Pediatrics 2020;146. 10.1542/peds.2020-1576. [Epub ahead of print: 26 May 2020]. [DOI] [PubMed] [Google Scholar]
- 29.Stringhini S, Zaballa M-E, Perez-Saez J, et al. Seroprevalence of anti-SARS-CoV-2 antibodies after the second pandemic peak. Lancet Infect Dis 2021;21:600–1. 10.1016/S1473-3099(21)00054-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou W, Wang W, Wang H, et al. First infection by all four non-severe acute respiratory syndrome human coronaviruses takes place during childhood. BMC Infect Dis 2013;13:433. 10.1186/1471-2334-13-433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng KW, Faulkner N, Cornish GH, et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 2020;370:eabe1107. 10.1126/science.abe1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viner RM, Ward JL, Hudson LD, et al. Systematic review of reviews of symptoms and signs of COVID-19 in children and adolescents. Arch Dis Child 2020:2020.10.16.20213298. 10.1136/archdischild-2020-320972 [DOI] [PubMed] [Google Scholar]
- 33.Mayor S. COVID-19: UK studies find gastrointestinal symptoms are common in children. BMJ 2020;370:m3484. 10.1136/bmj.m3484 [DOI] [PubMed] [Google Scholar]
- 34.Struyf T, Deeks JJ, Dinnes J, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database Syst Rev 2020;7:CD013665. 10.1002/14651858.CD013665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.COVID-19 Fragen und Antworten Teil 8 - pädiatrie schweiz. Available: https://www.paediatrieschweiz.ch/news/covid-19-fragen-und-antworten-teil-8/ [Accessed 23 Feb 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-047483supp001.pdf (55.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Data are still being collected for the cohort study Ciao Corona. Deidentified participant data might be available on reasonable request by email to the corresponding author at later stages of the study.