Dear Editor,
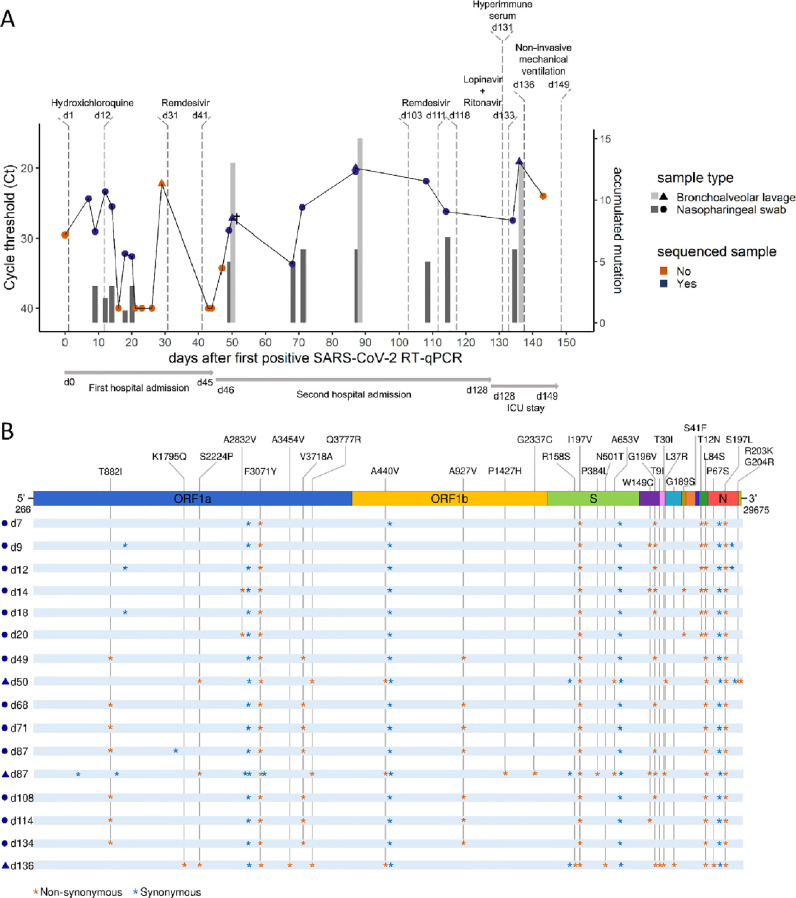
In this journal, Walsh and colleagues1 recently reviewed the evidence supporting that immunocompromised patients may remain positive for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection for long periods of time, up to 20 days. Here, we report the case of an immunocompromised patient with clinically diagnosed X-linked agammaglobulinemia (XLA) (Supplementary Material) who was persistently infected with SARS-CoV-2 for almost five months. He was admitted to the hospital on the 14th April 2020 with left bilobar pneumonia, reporting cough, chronic diarrhoea, and fever over the previous four days, and a nasopharyngeal (NP) sample tested positive for SARS-CoV-2 by RT-qPCR (Fig. 1 A). In the hospital, he was treated with hydroxychloroquine, two courses of remdesivir, lopinavir/ritonavir, antibiotics, antifungal treatments, and glucocorticoids. Infectious SARS-CoV-2 was successfully cultured from a bronchoalveolar lavage (BAL) sample on day 50, showing that the virus was actively replicating in the lower respiratory airways (Supplementary Material). On day 133, he was treated with hyperimmune serum from a convalescent patient. Despite treatments and two coronavirus disease 2019 test negativizations, the patient stayed in the hospital most of the time and died in the intensive care unit from multiorgan failure and shock on day 149 (10th September 2020) (Fig. 1A). See the Supplementary Material for further details.
Fig. 1.
Five-month longitudinal study of SARS-CoV-2 positive samples collected from a XLA-immunocompromised patient.(A) Chronological visualization of samples collected throughout the course of infection until patient death (day 149). RT-qPCR cycle threshold (Ct) are shown for collected nasopharyngeal swab (NP, circle) and bronchoalveolar (BAL, triangle) samples, with sequenced samples highlighted in blue. Vertical bars represent the accumulated number of mutations in the sequenced genome compared to the consensus viral sequence obtained from the first NP sample (day 9). † At day 50, a BAL sample was shown to have actively replicating SARS-CoV-2 viruses. (B) Graphical representation of SARS-CoV-2 whole-genome consensus sequences with synonymous (blue asterisks) and nonsynonymous mutations (orange asterisks) identified as compared to the Wuhan-Hu-1 reference sequence (NC_045512.2). Only non-synonymous mutations are identified with the amino acid changes in the figure. On day 50, in the N gene, the amino acid substitution S197L is replaced by S197T. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article)
Throughout the period described, 26 respiratory samples were collected from the patient (22 NP swabs and 4 BAL samples) (Fig. 1A). A urine, faeces, and peripheral blood sample (on day 44), and another peripheral blood sample (on day 87) were collected, but viral genome was not detected in any of their RNA extractions. SARS-CoV-2 viral genomes of a subset of 13 NP and 3 BAL samples were sequenced using alternative methodologies (Supplementary Material). All genomes were assigned to the PANGO lineage A.2 (Clade 19B), which was predominant in Spain during the early months of the pandemic.2 Assignation of the sequences to the same lineage suggests that the patient had a single viral infection event. Synonymous and non-synonymous mutations accumulated throughout the course of the infection in NP and BAL samples (Spearman correlation, r = 0.77, p = 0.00072) (Fig. 1B). Different constellations of mutations were observed in the sequences isolated from NP and BAL samples, suggesting compartmentalization of viral subpopulations evolving independently. The median mutation rate -accumulated mutations per day since diagnosis- was 0.09 mutations/day, higher than the originally estimated for SARS-CoV-2 (0.06 mutations/day3) (One-sample Wilcoxon test, p = 0.005), indicating accelerated mutation rate during infection. There was no significant difference in the mutation rate calculated for NP and BAL samples (Mann-Whitney U test, p = 0.18).
On day 0, viral genome sequences harboured the characteristic mutational pattern of lineage A.2 (ORF1a:F3701Y, ORF3a:G196V, ORF8:L84S, N:S197L) in addition to two other substitutions and four synonymous mutations (Fig. 1B). In particular, the spike (S) gene sequence was characterized by the I197V substitution and one synonymous mutation. These were the only two mutations observed in the S gene throughout the course of this five-month infection period in NP samples. However, we observed a different evolutionary pattern in BALs. On day 50, the A653V substitution was observed. This was found as part of the mutational pattern of two variants spreading in France4 and Germany5 at the beginning of 2021. On day 87, the P384L in the receptor-binding domain emerged but disappeared together with the A653V at day 136, five days after treatment with hyperimmune serum, when R158S and N501T emerged. Strikingly, the N501T is associated with an increased binding affinity of the S protein to the human angiotensin-converting enzyme 2 (ACE2) receptor and has been identified as an escape mutation against anti-SARS-CoV-2 neutralizing antibodies (NAbs).6 Interestingly, the position R158 of the S protein is part of the N-terminal domain (NTD) antigenic supersite, a region being recognized by all known NAbs directed to the NTD,7 and the R158S has been included among the escape mutations of anti-SARS-CoV-2 monoclonal NAbs targeting the NTD of the S protein.8 Besides, we highlight the emergence, on day 50, of the G204R in the nucleocapsid (N) gene, a mutation characteristic of the P.2 lineage, and, on day 136, of the K1795Q in the ORF1a and the P67S in the N gene, which are distinctive signatures of P.1 and B.1.617.3 lineages, respectively.
This study describes an XLA-immunocompromised patient with prolonged SARS-CoV-2 infection, supporting evidence that these patients undergo viral shedding for long periods of time.9 , 10 The patient presented RT-qPCR negative NP samples in different time intervals throughout the course of infection that either matched to a positive BAL sample or were followed by a positive RT-qPCR sample. This indicates that a negative RT-qPCR result in NP samples may not imply remission from infection.9 Viral genome sequencing revealed an accelerated intra-host viral evolution. Different mutations were accumulated in samples collected from NPs and BALs throughout the course of infection, which may point to viral adaptation to the upper and lower respiratory airways. Several host factors may account for this phenomenon, such as temperature and immune response disparities and/or differences in the ACE2 expression. Furthermore, it is worth noting that the mutations emerging in the lower respiratory tract were not detected by sequencing NP samples. Thus, the emergence of potentially worrying viral variants may be underestimated by sequencing standards focusing on NP samples. The emergence of substitutions linked to immune evasion in the BAL sample collected three days after treatment with hyperimmune serum is remarkable. Of note, the presence of the same or other mutations of interest in the NP samples days after hyperimmune serum treatment could not be ruled out. In fact, we were able to sequence only one NP sample 24 h after treatment, which may not be enough time to observe a possible viral population shifting in these samples. One limitation is that we have no data on the Abs composition and SARS-CoV-2 neutralizing activity of the hyperimmune serum used. Lastly, the emergence of mutations distinctive of currently circulating SARS-CoV-2 variants of concern (VOCs) support the hypothesis for long-term viral shedding in immunocompromised patients as one possible mechanism for the emergence of VOCs.
CRediT authorship contribution statement
Laura Ciuffreda: Investigation, Formal analysis, Data curation, Writing – review & editing. José M. Lorenzo-Salazar: Investigation, Formal analysis, Data curation, Writing – review & editing. Julia Alcoba-Florez: Supervision, Data curation, Writing – review & editing. Héctor Rodriguez-Pérez: Investigation, Writing – review & editing. Helena Gil-Campesino: Investigation, Data curation, Writing – review & editing. Antonio Íñigo-Campos: Investigation, Writing – review & editing. Diego García-Martínez de Artola: Investigation, Data curation, Writing – review & editing. Agustín Valenzuela-Fernández: Formal analysis, Funding acquisition, Writing – review & editing. Marcelino Hayek-Peraza: Data curation, Writing – review & editing. Susana Rojo-Alba: Investigation, Writing – review & editing. Marta Elena Alvarez-Argüelles: Investigation, Writing – review & editing. Oscar Díez-Gil: Data curation, Writing – review & editing. Rafaela González-Montelongo: Investigation, Writing – review & editing. Carlos Flores: Supervision, Writing – review & editing, Formal analysis, Data curation, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
This work was supported by Cabildo Insular de Tenerife [Grants CGIEU0000219140 and “Apuestas científicas del ITER para colaborar en la lucha contra la COVID-19″]; the agreement with Instituto Tecnológico y de Energías Renovables (ITER) to strengthen scientific and technological education, training research, development and innovation in Genomics, Personalized Medicine and Biotechnology [Grant No. OA17/008]; Instituto de Salud Carlos III [Grant No. FI18/00230 and PI20/00876] and Ministerio de Ciencia e Innovación [Grant No. RTI2018–093747-B-100 and RTC-2017–6471–1], co-funded by the European Regional Development Fund (ERDF), “A way of making European” from the European Union; Lab P2+ facility [Grant No. UNLL10–3E-783], co-funded by the ERDF and “Fundación CajaCanarias”; and the Spanish HIV/AIDS Research Network [Grant No. RIS-RETIC, RD16/0025/0011], co-funded by Instituto de Salud Carlos III and by the ERDF; and RIS-3 Canarias Strategy – “María del Carmen Betancourt y Molina” Program, “Consejería de Economía, Conocimiento y Empleo, Gobierno de Canarias” [Grant No. ProID2020010093]. The funders had no role in the study design, collection, analysis and interpretation of data, in the writing of the manuscript or in the decision to submit the manuscript for publication.
Acknowledgment
We deeply acknowledge the University Hospital Nuestra Señora de Candelaria (HUNSC) and the Instituto Tecnológico y de Energías Renovables (ITER) board of directors for their strong support and assistance in accessing diverse resources used in the study.
Ethical approval
The University Hospital Nuestra Señora de Candelaria (Santa Cruz de Tenerife, Spain) review board approved the study (ethics approval number: CHUNSC_2020_24).
Authors Contribution
JAF and CF conceived the idea, the experimental design and supervised the project. LC, JMLS, HRP, HGC, AIC, RGM, and DGMA conducted the sequencing experiments. SRA and MEAA conducted the viral culture experiments. MHP, JAF, HGC, ODG, and DGMA collected patient data. LC, JMLS, AVF, and CF performed the analysis and interpreted the results. AVF and CFA obtained the funding. LC drafted the first version of the manuscript and prepared the figures. All authors contributed to manuscript revision and read and approved the submitted version.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2021.07.028.
Appendix. Supplementary materials
References
- 1.Walsh K.A., Spillane S., Comber L., Cardwell K., Harrington P., Connell J., et al. The duration of infectiousness of individuals infected with SARS-CoV-2. J Infect [Internet]. 2020;81(6):847–856. doi: 10.1016/j.jinf.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; Available from https://doi.org/10.1016/j.jinf.2020.10.009.
- 2.Latif AA, Mullen JL, Alkuzweny M, Tsueng G, Cano M, Haag E, et al. A.2 Lineage report [Internet]. outbreak.info. 2021 [cited 2021 Jun 21]. Available from: https://outbreak.info/situation-reports?pango=A.2.
- 3.Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol [Internet]. 2021;19(7):409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; Available from https://doi.org/10.1038/s41579-021-00573-0.
- 4.Colson P., Levasseur A., Delerce J., Pinault L., Dudouet P., Devaux C., et al. Spreading of a new SARS-CoV-2 N501Y spike variant in a new lineage. Clin Microbiol Infect [Internet]. 2021 doi: 10.1016/j.cmi.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; Available from https://doi.org/10.1016/j.cmi.2021.05.006.
- 5.Mallm J.P., Bundschuh C., Kim H., Weidner N., Steiger S., Lander I., et al. Local emergence and decline of a SARS-CoV-2 variant with mutations L452R and N501Y in the spike protein. medRxiv [Internet]. 2021 [Google Scholar]; Available from: https://www.medrxiv.org/content/early/2021/04/29/2021.04.27.21254849.
- 6.Wang R., Chen J., Gao K., Wei G.W. Vaccine-escape and fast-growing mutations in the United Kingdom, the United States, Singapore, Spain, India, and other COVID-19-devastated countries. Genomics [Internet]. 2021;113(4):2158–2170. doi: 10.1016/j.ygeno.2021.05.006. https://www.sciencedirect.com/science/article/pii/S0888754321001798 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCallum M., De Marco A., Lempp F.A., Tortorici M.A., Pinto D., Walls A.C., et al. N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. Cell [Internet]. 2021 Apr 29;184(9):2332–2347.e16. Available from: 10.1016/j.cell.2021.03.028 [DOI] [PMC free article] [PubMed]
- 8.Suryadevara N., Shrihari S., Gilchuk P., VanBlargan L.A., Binshtein E., Zost S.J., et al. Neutralizing and protective human monoclonal antibodies recognizing the N-terminal domain of the SARS-CoV-2 spike protein. Cell [Internet]. 2021;184(9) doi: 10.1016/j.cell.2021.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]; 2316–2331.e15. Available from https://doi.org/10.1016/j.cell.2021.03.029.
- 9.Choi B., Choudhary M.C., Regan J., Sparks J.A., Padera R.F., Qiu X., et al. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med [Internet]. 2020;383(23):2291–2293. doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]; Available from: http://www.nejm.org/doi/10.1056/NEJMc2031364.
- 10.Baang J.H., Smith C., Mirabelli C., Valesano A.L., Manthei D.M., Bachman M.A., et al. Prolonged severe acute respiratory syndrome coronavirus 2 replication in an immunocompromised patient. J Infect Dis [Internet]. 2021;223(1):23–27. doi: 10.1093/infdis/jiaa666. [DOI] [PMC free article] [PubMed] [Google Scholar]; Available from https://doi.org/10.1093/infdis/jiaa666.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.