Abstract
Osteoporosis is a serious public health concern worldwide. Herba epimedii has been used for centuries and even thousands of years to treat osteoporotic conditions. Icariin, a flavonol glycoside, is one of the major active ingredients. In this study, we have shown that icariin protected against glucocorticoid-induced osteoporotic changes in SaoS-2 cells and mice. We have also shown that dexamethasone (a glucocorticoid) suppressed and icariin induced DEC1, a structurally distinct helix-loop-helix protein. DEC1 overexpression promoted whereas DEC1 knockdown decreased osteogenic activity. Likewise, DEC1 overexpression and knockdown inversely regulated the expression of β-catenin and PIK3CA, an essential player in the Wnt/β-catenin and PI3K/Akt signaling pathways, respectively. Interestingly, DKK1, an inhibitor of Wnt/β-catenin signaling inhibitor, and LY294002, an inhibitor of PI3K/Akt signaling, abolished the induction of DEC1 by icariin. It is established that these two pathways are interconnected by the phosphorylation status of GSK3β. Dexamethasone decreased but icariin increased GSK3β phosphorylation. Finally, DEC1 deficient mice developed osteoporotic phenotypes. Taken together, it is concluded that DEC1 likely supports the action of icariin against glucocorticoid induced osteoporosis with an involvement of the PI3K/Akt/GSK3β/β-catenin integrated signaling pathway.
Keywords: differentiated embryonic chondrocyte expressed gene 1 (DEC1), glucocorticoid, icariin, osteogenesis, Icariin (PubChem CID: 5318997), Dexamethasone (PubChem CID: 5743), Prednisolone acetate (PubChem CID: 5834)
Graphical Abstract
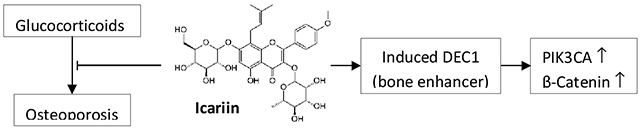
1. Introduction
Osteoporosis (OP) is the most common cause for a broken bone among the elderly and characterized by decreased bone mass and deteriorated bone microarchitecture. Glucocorticoids (GCs) are commonly used anti-inflammatory drugs, but their use has been associated with bone fractures. As a matter of fact, glucocorticoid induced osteoporosis is the most common iatrogenic osteoporosis and affects as many as 50% patients who receive chronic GC therapy. The precise molecular mechanisms remain to be determined. Nevertheless, GCs have been shown to impair the proliferation of osteoblasts, enhancing their apoptosis and inhibiting the mesenchymal transdifferentiation into osteoblasts [1–3].
Herba epimedii has been used for centuries to treat osteoporosis in China, Japan and Korea [4]. Icariin (ICA, C33H40O15; molecular weight: 676.67) is one of the primary active ingredients. This flavonol glycoside has various pharmacological activities including antioxygenation [5], immunoregulation [6], antitumor [7], and neuroprotection [8,9]. Icariin has also been shown to enhance osteogenic differentiation, primarily by upregulated expression of bone morphogenetic protein-2 (BMP-2) and runt-related transcription factor 2 (Runx2) [10–12]. In mice with collagen-induced arthritis [13], icariin was found to suppress cartilage and bone degradation , although the precise mechanism remains to be determined.
Human differentiated embryonic chondrocyte expressed gene 1 (DEC1) belongs to a structurally distinct class of basic helix-loop-helix (bHLH) proteins [14]. DEC1 has been linked to a number of physiological processes including circadian rhythmicity, metabolic homeostasis and many cellular events such as proliferation, differentiation, and apoptosis [15–22]. It has been reported that overexpression of DEC1 in growth plate chondrocytes at the prehypertrophic stage increased the mRNA levels of Indian hedgehog, Runx2, and type X collagen. Alkaline phosphatase activity was increased and mineralization was elevated [22]. Moreover, overexpression of DEC1 accelerated chondrogenic differentiation of mesenchymal stem cells (MSCs). Conversely, knockdown of DEC1 suppressed the expression of osteoblastic phenotype in the induced MSCs [23]. Recently, we have demonstrated that dexamethasone, a widely used glucocorticoid, markedly downregulated DEC1 [24]. In this study, we have established that icariin protected against glucocorticoid induced osteoporosis with a potential involvement of DEC1. Dexamethasone downregulated but icariin upregulated DEC1. DEC1 overexpression promoted whereas DEC1 knockdown decreased osteogenic activity. Likewise, DEC1 overexpression and knockdown inversely regulated the expression of β-catenin and PIK3CA. Interestingly, DKK1, an inhibitor of Wnt/β-catenin signaling inhibitor, and LY294002, an inhibitor of PI3K/Akt signaling, abolished the induction of DEC1 by icariin. Dexamethasone decreased but icariin increased the phosphorylation GSK3β, an interconnector between Wnt/β-catenin and PI3K/Akt signaling. Finally, DEC1 deficient mice developed osteoporotic phenotypes.
2. Materials and methods
2.1. Materials
McCoy’s 5A, dexamethasone, RU-486 and 5,6-Dichlorobenzimidazole 1-β-D-ribofuranoside were purchased from Sigma (St. Louis, MO, USA). The fetal bovine serum was purchased from HyClone (Logan, Utah, USA). 60-day prednisolone slow-release pellet was purchased from Innovative Research of America (Sarasota, FL, USA). Icariin was from J&K scientific LTD (Beijing, China). Cetylpyridinium chloride was from Aladdin (Shanghai, China). L-ascorbic acid was from Gibco BRL (Gaithersburg, MD, USA). β-glycerophosphate was from Santa Cruz (CA, USA). Alkaline Phosphatase Detection Kit , ALP staining kit were purchased from Jiancheng bioengineering institute (Nanjing, China). Bicinchoninic (BCA) protein assay reagent was purchased from Thermo-Fisher Scientific (Waltham, MA, USA). Nitrocellulose membranes was from Bio-Rad (Hercules, CA, USA). Antibody against β-catenin was purchased from BD (San Diego, CA, USA); antibodies against p-ser473-Akt, Akt, PI3Kp110α were purchased from Santa Cruz (CA, USA); antibodies against Runx2, p-ser9-GSK3β, GSK3β, H3, the goat anti-mice Alexa Fluor 488 or goat anti-rabbit TRITC and DAPI were purchased from Bioworld (St. Louis, MN, USA); and antibody against GAPDH was from Abcam (Cambridge, UK). Horseradish peroxidase conjugated secondary antibody was from Pierce (Rockford, IL, USA). The antibody against DEC1, plasmid FlagDEC1 or FlagCMV2 were described elsewhere [26, 45]. ECL Western blotting detection system, HiScript II Q RT SuperMix were purchased from Vazyme biotech co., ltd (Nanjing, China). FastStar Universal SYBR Green Master was from Roche (Indianapolis, IN, USA). Primers were validated by Invitrogen Biotechnology Co. Ltd. GenJet™ In Vitro DNA Transfection Reagent II was from SignaGen (Gaithersburg, USA). The packaged lentiviral vectors containing shRNA against DEC1 (3 virus strains) or lentiviral vector (as control, shRNA) were purchased from Shanghai Genechem Co.,Ltd. (Shanghai, China). DAB Horseradis Peroxidase Color Development was from Kit Boster (Wuhan, China).
2.2. Cell culture
SaoS-2 cells obtained from American Type Culture Collection were routinely cultured in McCoy’s 5A, supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 0.1 mg/ml streptomycin and incubated in a humidified atmosphere of 95% air and 5% CO2 at 37°C. The culture medium was changed every other day.
2.3. ALP activity assay
The SaoS-2 cells were seeded into 6-well plates at the density of 5×105 cells per well. The cells were treated with dexamethasone or icariin for 24 h. ALP activity was detected by Alkaline Phosphatase Detection Kit [25] according to the instructions. The absorbance of each sample was normalized based on the protein content. The protein concentrations were determined by Bicinchoninic (BCA) protein assay reagent.
2.4. ALP staining
SaoS-2 cells were seeded into 12-well plates at the density of 1×105 cells per well overnight. After 24 h treatment of dexamethasone or icariin, the cells were fixed in 4% paraformaldehyde for 10 min and washed thrice with PBS. The histochemical detection of ALP was performed with an ALP staining kit. The stained cultures were photographed and then incubated with 10% of cetylpyridinium chloride for 30 minutes’ moderate shaking. The absorbance of 100μl dyed solution was measured at 490 nm.
2.5. Alizarin Red S staining
The differentiation were induced by the osteoinductive conditioned medium, which was composed of 5% of FBS-MyCcoy’s 5A supplemented with 50 μg/ml L-ascorbic acid, 10 mM β-glycerophosphate and 10−8M dexamethasone. The induction lasted for 14 days with changes of culture medium (drug treatment kept the same) every 2-3 days. After being induced for corresponding time, the cells were fixed in ice-cold 95% ethanol for 30 min at −20 °C and were stained with 1% Alizarin Red S (ARS), pH 4.2, for 30 min at room temperature. The stained cultures were photographed and quantified via extraction with cetylpyridinium chloride.
2.6. Western blot analysis
The cell lysates were resolved by 10% of SDS-polyacrylamide gel electrophoresis and transferred electrophoretically to nitrocellulose membranes. Nonspecific binding was blocked with 5% BSA in TBST for 2 h at room temperature. The membranes were then incubated with the following primary antibodies overnight at 4°C: Antibody against β-catenin; antibodies against p-ser473-Akt, Akt, PI3Kp110α; antibodies against Runx2, p-ser9-GSK3β, GSK3β, H3; and antibody against GAPDH. The preparation of the antibody against DEC1 was described previously [26]. The primary antibodies were subsequently localized with horseradish peroxidase conjugated secondary antibody for 1h at room temperature. The protein bands were visualized with the ECL Western blotting detection system. The chemiluminescent signal was captured by Kodak Image Station 2000.
2.7. Quantitative real-time polymerase chain reaction (qRT-PCR)
SaoS-2 cells were treated with dexamethasone (10−5M) or icariin (10−7M) and 5,6-Dichlorobenzimidazole 1-β-D-ribofuranoside (DRB) 5 μM for 9 h separately and together. Reverse transcription was carried out by using HiScript II Q RT SuperMix. The qRT-PCR was performed by using FastStar Universal SYBR Green Master with the 7300 Real-time PCR System (Applied Biosystems). The primers used in each reaction were as follows: GAPDH forward, 5’-ACCACAGTCCATGCCATCAC-3’; GAPDH reverse, 5’-TCCACCACCCTGTTGCTGTA-3’; PIK3CA forward, 5′-CCTGATCTTCCTCGTGCTGCTC-3′ and PIK3CA reverse, 5′-ATGCCA ATGGACAGTGTTCCTCTT-3′. All data were normalized to the GAPDH.
2.8. Immunofluorescence
Cells were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100 in PBS containing 5% BSA. After being blocked with 5%BSA for 1 h, the cells were incubated with anti-β-catenin and anti-DEC1 antibody overnight at 4°C. Subsequently, the cells were washed three times with PBS and incubated with goat anti-mice Alexa Fluor 488 or goat anti-rabbit TRITC for 1 h, followed by the incubation with DAPI for 15min. The fluorescence signal was captured by using fluorescence microscope (BX53, Olympus, Tokyo, Japan).
2.9. Transient transfection experiment
SaoS-2 cells were plated in 35mm dishes at the density of 1×106 cells per dish. The transfection was conducted by GenJet™ In Vitro DNA Transfection Reagent II. The transfection mixtures contained 1500 ng FlagDEC1 or FlagCMV2 plasmid). After 6 h, the transfection medium was replaced with fresh medium. And after another 18 h, the cells were harvested and the osteogenic phenotypes were determined by ALP activity, ALP staining, Western blot and immunofluorescence.
2.10. Construction of DEC1 knockdown stable cell line with virus infection
The day before infection, 1×106 cells were seeded into 35mm dishes. The lentivirus infection with the best one of the three virus strains was carried out at MOIs of 20 for 24 h. After 48 h, the transduction medium was discarded. After being selected with 5μg/ml puromycin for at least 2 weeks, the DEC1 knockdown stable cells were ready for experiments. When the shDEC1 stable cells were seeded into plates and cultured for 24 h, they were harvested. And the osteogenic phenotypes were determined by ALP activity, ALP staining, Western blot and immunofluorescence.
2.11. Animals and experimental procedures
6-week-old male C57BL/6 mice were purchased from Jiangsu Province’s Medical Experimental Animal Center. Mice were kept at 24°C with a 12 hour/12 hour light/dark cycle, allowed free access to tap water and maintained with a diet of Formular-M07. And efforts were made to minimize animals suffering and to reduce the number of animals used for the experiments. 60-day prednisolone slow-release pellet for 5 mg/kg were administered by subcutaneous implantation (PDL, n=16)[27], and the control mice had sham implantation with placebo (SHAM, n=8). At the 4th week after implantation, half of PDL mice were orally administered icariin (250mg/kg/day, 60d) (PDL+ICA, n=8). DEC1 KO mice (RBRC04841) were obtained from RIKEN BioResource Center. And homozygous DEC1−/− mice were obtained through crossing male and female heterozygous DEC1+/− mice. DEC1+/+ mice came from littermate. The present studies on our samples were conducted in accordance with the guidelines of the Institute for Laboratory Animal Research of Nanjing Medical University. The protocol was approved by the Committee on the Ethics of Animal Experiments of Nanjing Medical University (Permit Number: 14030106).
2.12. Micro-CT and Histomorphometric Analyses
Tibias were fixed in 4% paraformaldehyde overnight, and analyzed by micro-CT with a SkyScan scanner (Skyscan 1172, Bruker, Inc., Kontich, Belgium). Micro-CT scanning was performed at the resolution 9μm, X-ray 100 kVp volts, source current 60 μA, and exposure 250 ms. Trabecular parameters including bone volume faction (BV/TV), trabecular thickness (Tb.Th,mm) and number (Tb.N, 1/mm), and structure model index (SMI) were evaluated. The 3D BMD (mg/cm3) was also measured. The 2D grayscale CT images were reconstructed in 1120×1120 pixel matrices by using NRecon ver.1.6.1.5 (SkyScan) and 3D microstructural parameters were calculated [27].
2.13. Immunohistochemistry
The tibiae tissues were fixed in 4% paraformaldehyde in PBS and were decalcified in 10% ethylene diamine tetraacetic acid (EDTA) for 2 weeks. After that, they were dehydrated, embedded in paraffin, and sectioned to 5μm thickness. And then the tissue sections were dewaxed in xylene and then rehydrated through graded increasing ethanol solutions. The sections were incubated in a target antigen retrieval solution (0.01M citric acid buffer solution) at 95°C for 20 min. The slides blocked in 5% BSA were incubated with anti-DEC1 antibody overnight at 4°C. And then, goat anti-rabbit IgG conjugated with horseradish peroxidase was added for 30min at room temperature. The bands appeared with DAB Horseradis Peroxidase Color Development Kit. Immunoreactivity was detected by using a positive microscope light microscope (BX53, Olympus, Tokyo, Japan). Three visions were randomly selected to analyze immunopositive cells density by using microbrightfield Stereo Investigator software.
2.14. Statistical analysis
The results are represented as mean ± standard error of mean after at least three separate experiments. Data were analyzed by oneway ANOVA through the SPSS software. P<0.05 was considered as statistically significant.
3. Results
3.1. Dexamethasone (DEX) inhibits whereas icariin (ICA) accelerates osteogenic differentiation in SaoS-2 cells.
It was reported that the glucocorticoid DEX exerted both stimulating and inhibiting activities toward osteogenesis depending on the concentrations [28]. In this study, we initially tested whether DEX at various concentrations differentially altered the activity of alkaline phosphatase (ALP), a critical enzyme for bone formation. SaoS-2 cells were treated for 24 h and the activity and expression of ALP were determined. As shown in Figs. 1A and 1B, DEX markedly decreased ALP activity based on biochemical detection (Fig. 1A) and cell staining (Fig. 1B). The decrease occurred in a concentration-dependent manner. To link decreased ALP activity to altered bone minimization, SaoS-2 cells underwent osteoinduction for 14 days, and calcium deposits were determined. As shown in Fig. 1C, calcium deposits were significantly decreased in osteoinduced cells.
Fig. 1. Dexamethasone (DEX) inhibits whereas icariin (ICA) accelerates osteogenic differentiation in SaoS-2 cells.
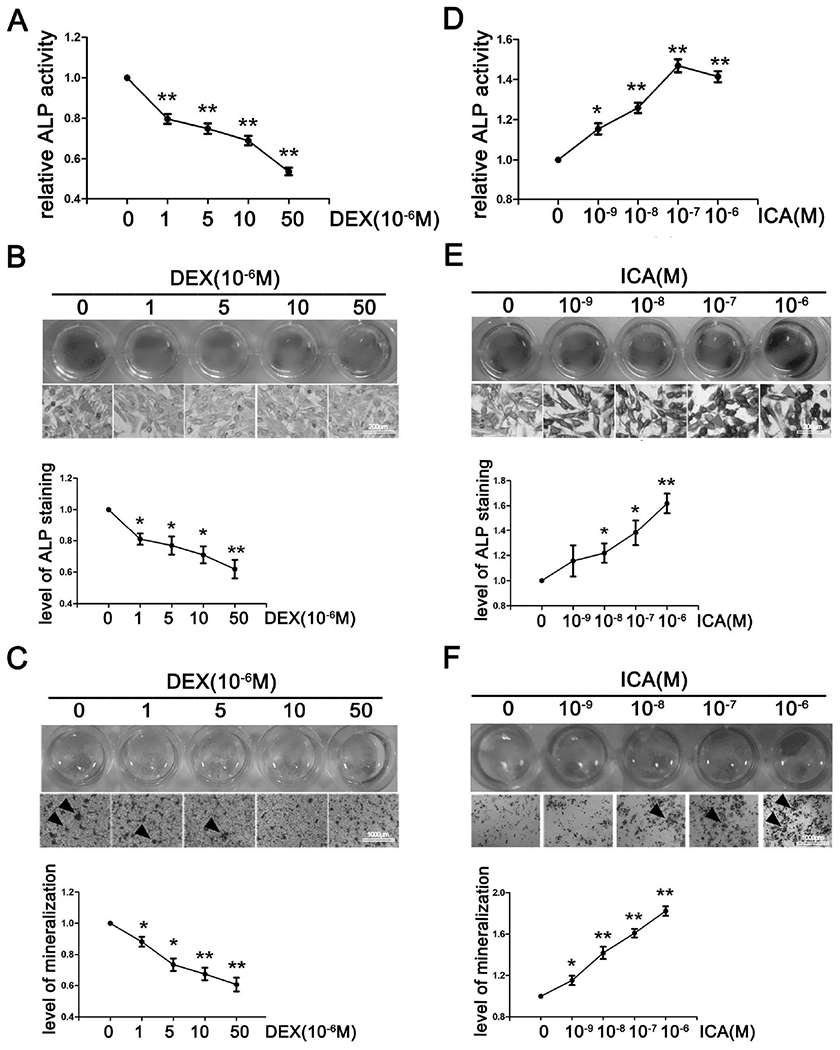
(A)(D) ALP activity of SaoS-2 cells incubated with solvent, DEX or ICA for 24h. (B)(E) ALP staining of SaoS-2 cells incubated with solvent, DEX or ICA for 24h. (C)(F) ARS staining in SaoS-2 cells cultured by the osteoinductive conditioned medium containing solvent, DEX or ICA for 14 days with changes of treatment medium every 2-3 days. All experiments were repeated at least three times, and the data were expressed as mean ± SD.*p < 0.05, **p <0.01 vs 0 concentration.
Herba epimedii has been used for centuries to treat osteoporosis [4], and ICA is one of the primary active ingredients. Significantly, ICA reportedly promoted the proliferation of MSCs and their osteogenic differentiation [29], critical indicators for bone formation. Consistent with the previous finding, ICA was found to increase ALP activity and its cellular staining (Figs. 1D and 1E). The increase was detected in cells treated with as low as 1 nM ICA. As expected, ICA significantly increased calcium deposits (Fig. 1F). These results concluded that DEX inhibited but ICA accelerated osteogenic differentiation.
3.2. ICA antagonizes glucocorticoids-induced osteoporotic phenotypes.
We next tested whether ICA antagonizes DEX-induced osteoporotic changes. SaoS-2 cells were seeded and treated with DEX or plus ICA for 24 h or 14 days (osteoinduction), the ALP activity and the level of mineralization were determined respectively. As shown in Figs. 2A and 2B, ICA cotreatment significantly abolished DEX-induced decrease of ALP activity. DEX decreased the level of mineralization by as much as 50% and ICA cotreatment completely reversed the decrease (Fig. 2C). More importantly, RU-486, a glucocorticoid receptor antagonist, could not abolish the action of ICA on DEX-induced decrease of ALP activity (Fig. S2), indicating that ICA exerted osteogenic activity independently of GR antagonism.
Fig. 2. Icariin mitigates glucocorticoid induced osteoporosis in vitro and in vivo.
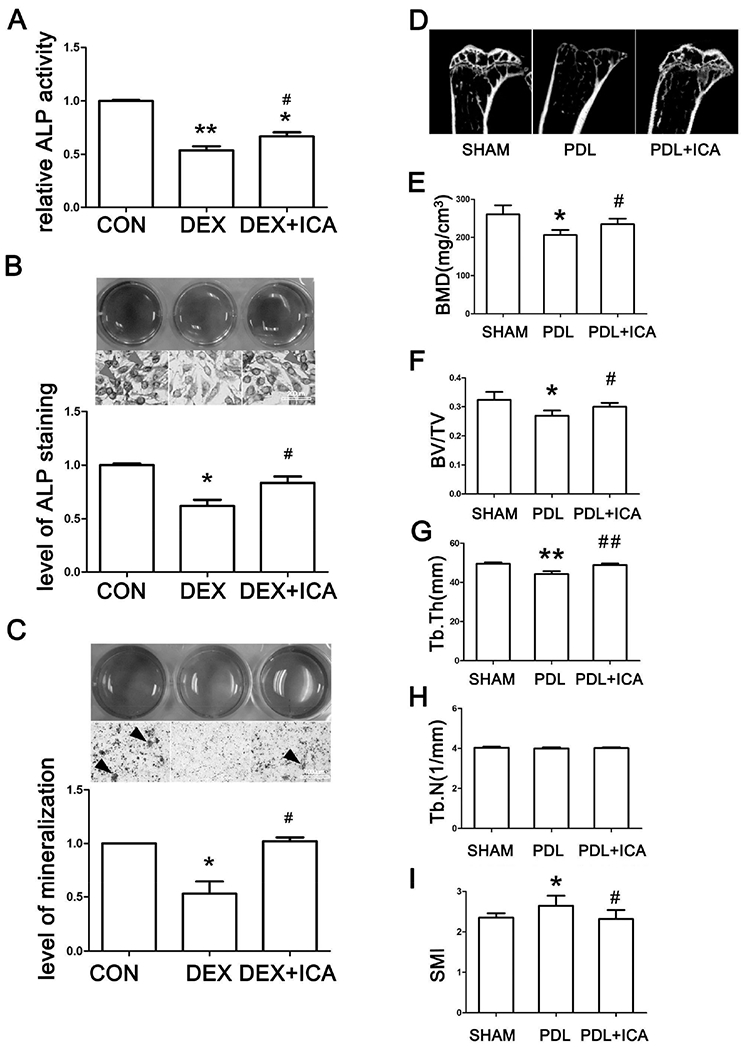
(A) (B) ALP activity and ALP staining after SaoS-2 cells being treated with same volume of DMSO (CON), 10μM DEX, and 10μM DEX plus 10−7M icariin (DEX+ICA) for 24 h. (C) ARS staining of SaoS-2 cells cultured by the osteoinductive conditioned medium containing solvent, DEX or DEX+ICA for 14 days with changes of fresh treatment medium every 2-3 days. All experiments in (A, B, C) were repeated at least three times, and the data were expressed as mean ± SD. *p < 0.05, **p <0.01 vs CON, #p < 0.05 vs DEX. (D) Representative micro-CT images of the tibia. (E) BMD assessed by micro-CT. (F)(G)(H)(I) Histogram of trabecular structure parameters of proximal tibia. *p < 0.05, **p <0.01 vs SHAM, #p < 0.05, ##p <0.01 vs PDL; n=8 for each group.
In order to gain in vivo significance of ICA-osteoblastic activity, we tested ICA for its ability to antagonize the osteolytic changes induced by prednisolone. Namely mice were subcutaneously implanted with slow-release prednisolone pellets [30] alone or with oral administration of ICA (initiated at the 4th week after the implantation), and bone formation was determined. Representative micro-CT images showed that the trabecular bone volume and cortical bone thickness decreased in prednisolone treated mice (Fig. 2D). More detailed structural changes also revealed a bone loss in prednisolone group mice. The trabecular bone mineral density (BMD) in prednisolone group decreased by 20.8% (Fig. 2E). The bone volume fraction (BV/TV) decreased by 16.8% and the trabecular thickness (Tb.Th) decreased by 10.6% in prednisolone group mice (Figs. 2F, 2G). However, no difference was detected in the trabecular number (Tb.N) (Fig. 2H). In agreement with these changes, a 12.5% increase in the structure model index (SMI) was detected (Fig. 2I), indicating a tendency (plate- to rod-like) to osteoporosis in prednisolone group mice. Importantly, all of the osteolytic changes induced by prednisolone implantation were significantly or fully reversed by ICA co-administration. For example, BV/TV and Tb.Th increased by 11.3% and 10.3% respectively in PDL+ICA group compared to those in PDL group (Figs. 2F, 2G). These results conclude that ICA is an effective agent against GIOP, a major adverse effect associated with the use of glucocorticoids.
3.3. ICA reverses DEX-downregulation of Runx2 and DEC1.
Runx2 is a major bone-specific transcription factor and established to drives the maturation of osteoblasts [31,32]. We next tested whether DEX downregulates Runx2 and whether the downregulation can be abolished by ICA. In addition, the expression of DEC1 was determined as well. As shown in Figs. 3A and 3B, both Runx2 and DEC1 were downregulated by DEX but upregulated by ICA. The altered expression occurred in a concentration-dependent manner. Importantly, the downregulation by DEX was partially but significantly reversed by ICA for both Runx2 and DEC1 (Fig. 3C). In order to specify the altered expression of DEC1 in vivo, we determined DEC1 expression in the tibia bone from three groups of mice (SHAM, PDL and PDL+ICA) by immunohistochemistry. DEC1 expression in tibia bone marrow side decreased markedly in the PDL group compared to that in the SHAM group, and cotreatment with ICA rescued the decrease (Fig. 3D). The in vivo data linked DEC1 downregulation to GIOP.
Fig. 3. ICA reverses DEX-downregulation of Runx2 and DEC1.
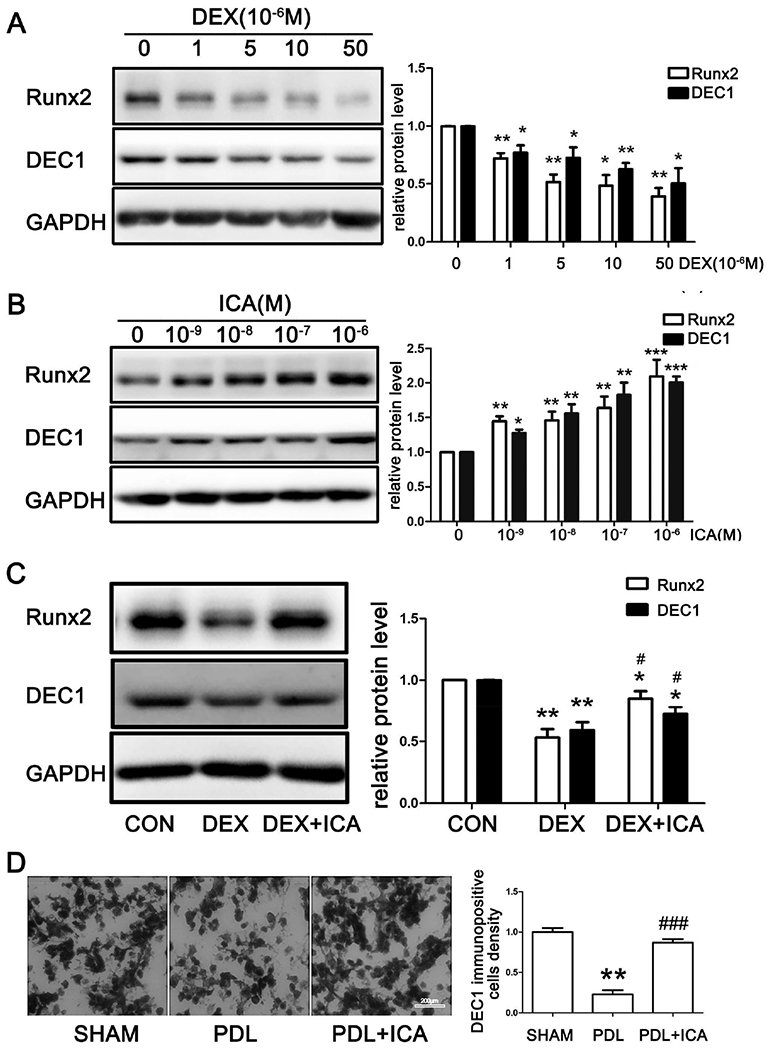
(A)(B)(C) Analysis of Runx2 and DEC1 expression by western blot in SaoS-2 cells treated with dexamethasone or icariin for 24 h separately and together. All experiments were repeated at least three times, and the data were expressed as mean ± SD. *p < 0.05, **p <0.01,***p<0.001 vs CON, #p < 0.05 vs DEX. (D) Percentage of DEC1 positive cells in tibia bone marrow side by immunohistochemistry. Mouse samples were from three groups (SHAM, PDL and PDL+ICA). **p < 0.01 vs SHAM, ###p < 0.001 vs PDL; n=3 for each group.
3.4. DEC1 regulates osteogenic differentiation and bone mass formation.
We next performed a number of experiments to functionally link DEC1 expression to osteogenic activities. First, cells were transfected with DEC1 or the corresponding vector, and the activity of ALP was determined. As shown in Figs. 4A and 4B, the activity of ALP and its cellular staining intensity were markedly higher in DEC1-transfected cells compared with those in vector-transfected cells. The level of Runx2 protein was much higher in DEC1-transfected cells as well (Fig. 4C). It should be noted that the DEC1 transfection efficiency was relatively high (~50%).
Fig. 4. DEC1 regulates osteogenic differentiation and bone mass formation.
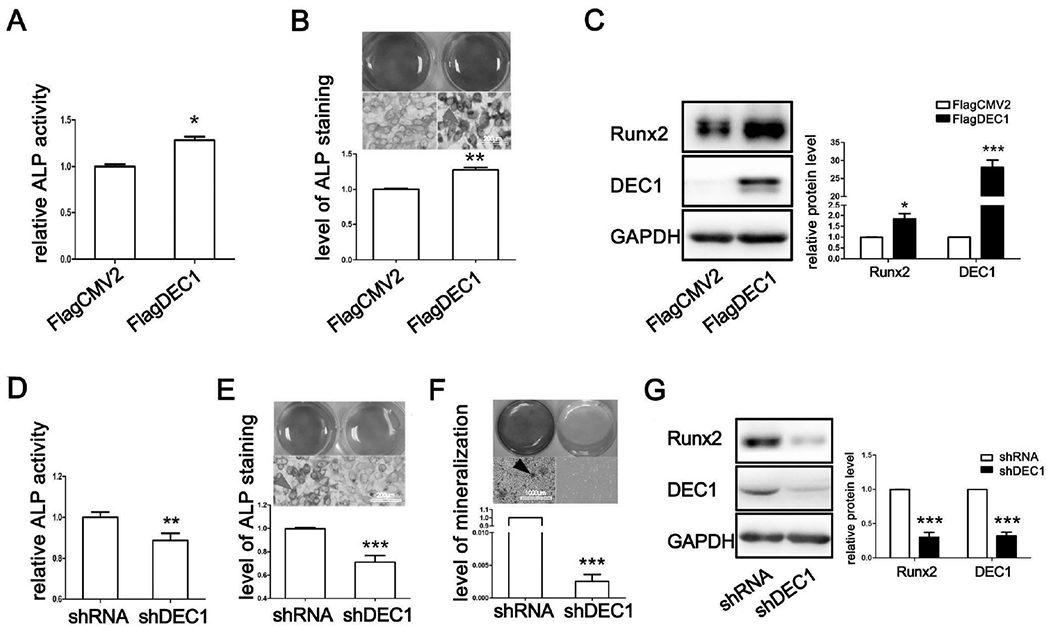
(A)(D) ALP activity in SaoS-2 cells transfected with DEC1 or shDEC1. (B)(E) ALP staining in SaoS-2 cells transfected with DEC1 or shDEC1. (C)(G) Analysis of Runx2 and DEC1 expression by western blot. (F) ARS staining in shDEC1 stable transfected SaoS-2 cells incubated with the osteoinductive conditioned medium for 14 days with changes of medium every 2-3 days. All experiments were repeated at least three times, and the data were expressed as mean ± SD. *p < 0.05, **p <0.01, ***p <0.001 vs vector (FlagCMV2 or shRNA).
To complement the overexpression study, we performed DEC1 knockdown experiment. SaoS-2 cells were lentivirally transduced to shDEC1, and then the activity of ALP and the level of mineralization were determined. As shown in Figs. 4D and 4E, the activity of ALP and its cellular staining intensity were significantly decreased. Consistent with the reduced ALP activity, DEC1 knockdown completely abolished extracellular matrix mineralization (Fig. 4F). In addition, the expression of Runx2 protein was reduced by 75.58% (Fig. 4G). It should be noted that DEC1 knockdown efficiency was more than 70% (Fig. 4G).
3.5. β-catenin is involved in the osteogenesis induced by DEC1.
To gain signaling insight regarding DEC1 induced osteogenesis, we examined a potential role of DEC1 in β-catenin signaling, a major pathway in bone homeostasis [33]. As expected, DEX significantly decreased the level of β-catenin protein (Fig. 5A) and the decrease occurred in the nucleus and cytoplasm (Fig. 5B). The significant decrease of β-catenin was further confirmed by immunofluorescence analysis (Fig. 5C). Likewise, DEX decreased DEC1 expression with the nuclear DEC1 being decreased to a greater extent (Figs. 5B and 5C). ICA, on the other hand, increased the expression of β-catenin in the nucleus and cytoplasm (Figs. 5D and 5E). Interestingly, ICA significantly increased nuclear DEC1, but caused little changes in cytoplasmic DEC1 (Fig. 5E). Nevertheless, ICA at 10−7M significantly increased the nuclear presence of both β-catenin and DEC1 based on immunofluorescence analysis (Fig. 5F).
Fig. 5. β-catenin is involved in the osteogenesis induced by DEC1.
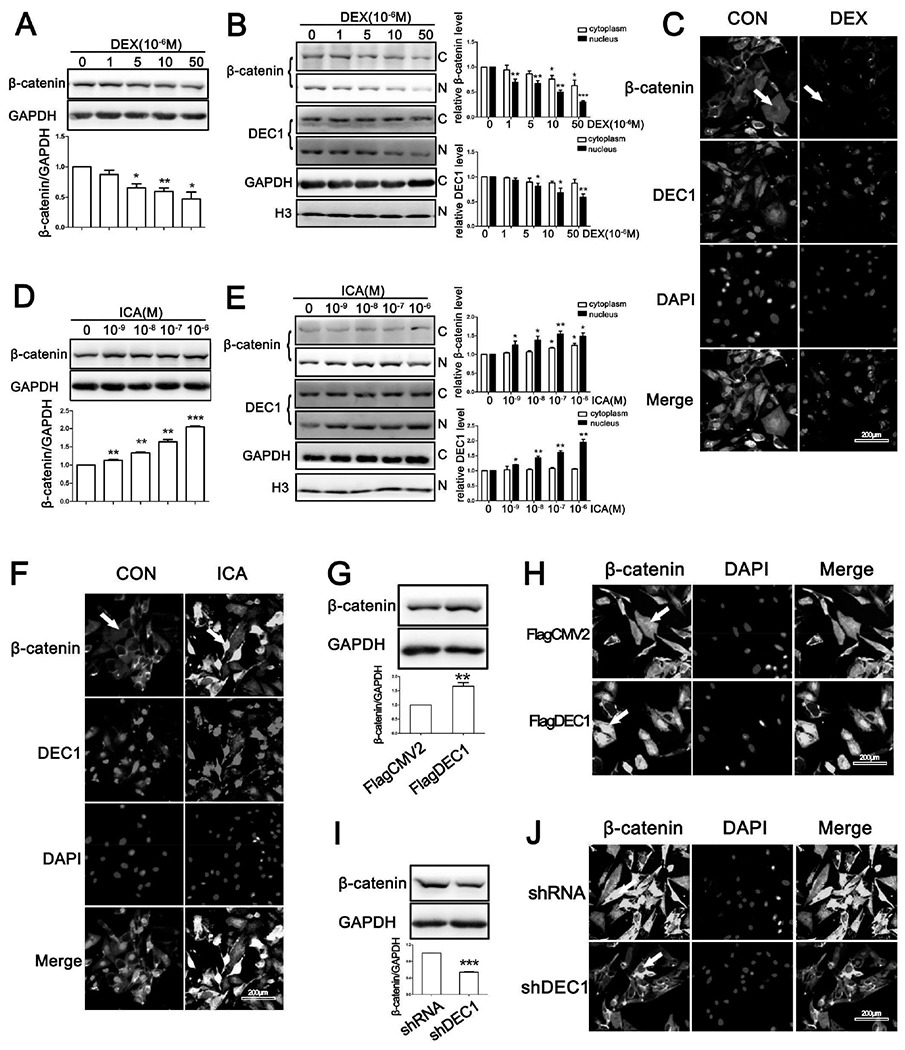
(A)(D) Analysis of total β-catenin protein expression in SaoS-2 cells treated with dexamethasone or icariin for 24 h. (B)(E) Analysis of β-catenin expression in cytoplasm and nucleus in SaoS-2 cells treated with dexamethasone or icariin for 24 h. (C)(F) Representative images of β-catenin and DEC1 immunofluorescence in SaoS-2 cells treated with dexamethasone (10μM) or icariin (10−7M) for 24 h. (G)(I) Analysis of β-catenin protein expression in SaoS-2 cells. (H)(J) Representative images of β-catenin immunofluorescence in SaoS-2 cells. All experiments were repeated at least three times, and the data were expressed as mean ± SD. *p < 0.05, **p <0.01, ***p <0.001 vs 0 concentration or vector (FlagCMV2 or shRNA).
To shed light on whether DEC1 is involved in the nuclear accumulation of β-catenin, cells with DEC1 overexpression or knocked down were analyzed for the nuclear presence of β-catenin. Cells transfected with FlagDEC1 markedly increased the expression of β-catenin (Fig. 5G) and the increased occurred primarily in the nucleus (Fig. 5H). In contrast, DEC1 knockdown considerably decreased the expression of β-catenin (Figs. 5I and 5J).
3.6. DEX and ICA inversely regulate the PIK3CA/Akt/GSK3β/β-catenin signaling cascade in osteogenic differentiation.
In the absence of an upstream Wnt signal, Glycogen synthase kinase-3β (GSK-3β) phosphorylates residues near the amino terminus of β-catenin, targeting β-catenin for ubiquitin dependent proteolysis [34]. Various kinases phosphorylate GSK3-β at ser9 including Akt [35]. We next examined whether DEX and ICA inversely impact the phosphorylation status of GSK-3β and Akt. As shown in Fig. 6A, DEX decreased p-ser9-GSK3β and p-ser473-Akt in a dose dependent manner. Interestingly, DEX also decreased the protein level of PIK3CA (i.e., PI3Kp110α), an upstream regulator of the Akt/GSK3β/β-catenin signaling cascade. PIK3CA is the catalytic subunit of phosphatidylinositol-3 kinase (PI3K). In contrast to DEX, ICA significantly increased the expression of PIK3CA and the phosphorylation of its downstream targets: Akt and GSK3β (Fig. 6B). These data suggested that DEX and ICA antagonistically regulate osteogenic differentiation through the PIK3CA/Akt/GSK3β/β-catenin signaling cascade.
Fig. 6. DEX and ICA inversely regulate the PIK3CA/Akt/GSK3β/β-catenin signaling cascade in osteogenic differentiation.
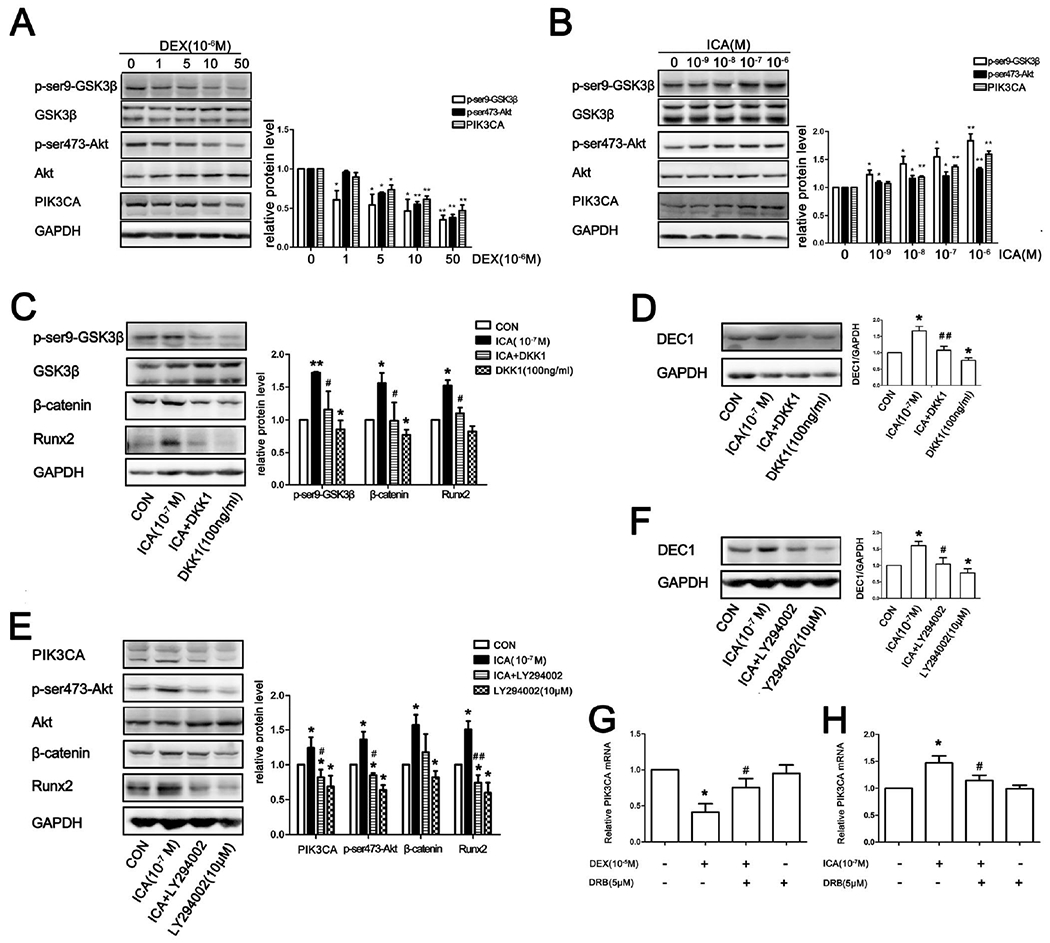
(A)(B)Western blot analysis in SaoS-2 cells treated with DEX or ICA for 3h. (C)(D) Western blot analysis of SaoS-2 cells in the presence or absence of DKK1 and ICA alone or in combination for 24 h. (E)(F) Western blot analysis of SaoS-2 cells in the presence or absence of LY294002 and ICA alone or in combination for 24h. (G)(H) Effect of DRB on the transactivation of PIK3CA. All experiments were repeated at least three times, and the data were expressed as mean ± SD. *p < 0.05, **p < 0.01 vs CON, #p < 0.05, ##p <0.01 vs DEX or ICA.
To further confirm an involvement of this cascade in ICA-mediated osteogenic activity, we tested dickkopf related protein 1 (DKK1), an inhibitor of Wnt/β-catenin pathway, for the ability to reverse the osteogenic effect of ICA. SaoS-2 cells were treated with ICA, DKK1 or both for 3 h (protein phosphorylation) or 24 h (expression). Then, the protein levels in the cascade were determined. As shown in Fig. 6C, DKK1 reduced the ICA-elevated phosphorylation of GSK-3β and β-catenin. DKK1 also attenuated ICA-mediated upregulation of Runx2 (Fig. 6C). Interestingly, LY294002, a potent inhibitor of the PI3K/Akt pathway, comparably altered as DKK1 the expression of such genes as DEC1, Runx2 and β-catenin (Figs, 6C–F), particularly related to the antagonistic activity against ICA (osteogenic effect). These results conclude that DEC1 is a critical player in osteogenic process and does so in collaboration with an integral cascade of the PIK3CA/Akt/GSK3β/β-catenin signaling pathway. It should be noted that 5,6-dichlorobenzimidazole 1-β-D-ribofuranoside (DRB), an RNA synthesis inhibitor, abolished the altered expression of PIK3CA mRNA by DEX and ICA (Figs. 6G and 6H), suggesting that both DEX and ICA regulated PIK3CA expression by transcription.
3.7. DEC1 upregulates PIK3CA and enhances phosphorylation of its downstream targets: Akt and GSK3β.
We next tested whether DEC1 is involved in the transcriptional regulation of PIK3CA. SaoS-2 cells were transfected or virally transduced to overexpress or knockdown DEC1, and the expression of PIK3CA was determined. As shown in Fig. 7A, DEC1 overexpression significantly increased the protein expression of PIK3CA. In contrast, knockdown of DEC1 decreased its expression (Fig. 7B). Importantly, the opposing regulated expression of DEC1 inversely altered the phosphorylation status of Akt and GSK3β, two downstream targets of PIK3CA. DEC1 overexpression increased but DEC1 knockdown decreased the phosphorylation of both Akt and GSK3β (Figs. 7A and 7B).
Fig. 7. DEC1 is involved in the regulation of PIK3CA and downstream targets: Akt and GSK3β.
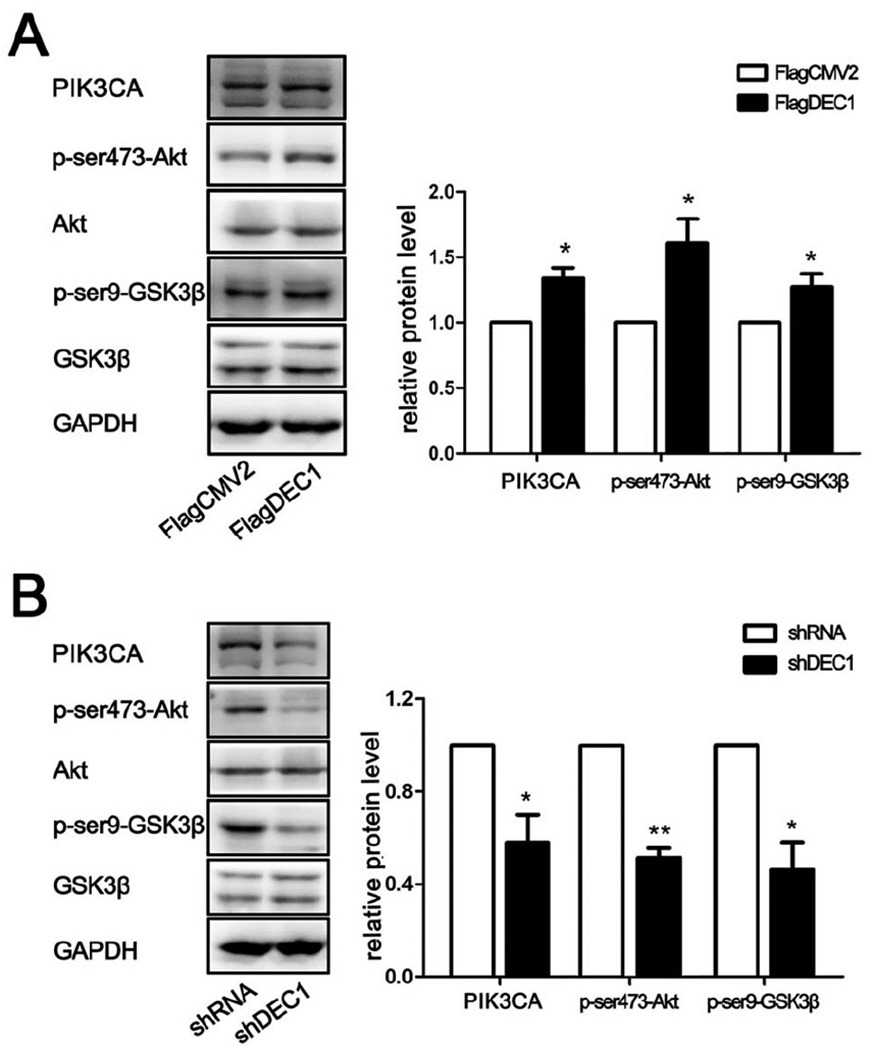
(A)(B) Western blot analysis in SaoS-2 cells transfected with DEC1 or shDEC1. All experiments were repeated at least three times, and the data were expressed as mean ± SD. *p < 0.05, **p <0.01 vs vector (FlagCMV2 or shRNA).
3.8. There is a tendency to the osteoporosis in DEC1−/− mice.
To validate the osteogenic involvement of DEC1 in vivo, we examined the bone microarchitecture of tibia in DEC1−/− and DEC1+/+ mice (18 week old). Compared with DEC1+/+ mice, DEC1−/− mice had reduced trabecular bone volume and cortical bone thickness (Fig. 8A). DEC1−/− mice exhibited a 37.0% decrease in trabecular BMD compared to DEC1+/+ mice (Fig. 8B). BV/TV decreased by 21.9% in DEC1−/− mice compared to that in DEC1+/+ mice (Fig. 8C). Tb.Th decreased by 13.4% in DEC1−/− mice, but there was no significant difference in Tb.N between DEC1−/− and DEC1+/+ mice (Fig. 8C). Consistent with these changes, SMI increased by 8.6%, indicating tendency to the osteoporosis in DEC1−/− mice. The genotypes of the mice were verified by the protocol provided by RIKEN BioResource Center (Fig. 8D).
Fig. 8. There is a tendency to the osteoporosis in DEC1−/− mice.
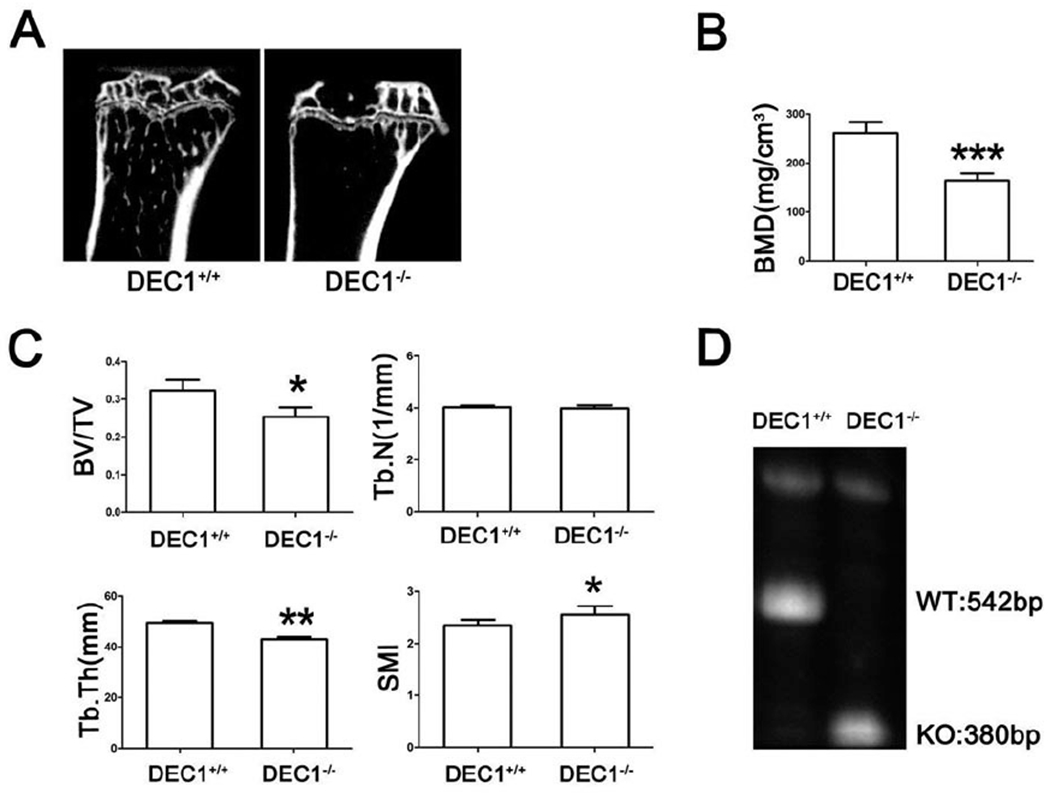
(A) Representative micro-CT images of the tibia. (B) BMD assessed by micro-CT. (C) BV/TV; Tb.Th; Tb.N; SMI of proximal tibia. (D) Detection genotype by PCR. *p < 0.05, **p <0.01, ***p <0.01 vs DEC1+/+; n=6 for each group.
4. Discussion
Chronic use of glucocorticoids is the most common cause of iatrogenic osteoporosis, and it is estimated that as many as 50% patients with glucocorticoid therapy develop at least some osteoporotic conditions [36–38]. The precise mechanisms remain to be determined, but increased osteoclastogenesis and decreased calcium absorption are recognized to be important contributing factors [39]. Helix-loop-helix (HLH) proteins constitute a superfamily of transcription factors and many of them have been implicated in bone homeostasis [40,41]. Some HLH proteins such as such as USFs and c-Myc positively regulate osteogenesis [41], whereas others such as Twist1 negatively regulate osteogenesis [40].
In this study, we have shown that ICA protected against glucocorticoid induced osteoporosis, presumably through DEC1, a structurally distinct HLH transcription factor. Several lines of evidence from this study support this conclusion. Firstly, glucocorticoids DEX and prednisolone were both found to induce osteoporotic changes in SaoS-2 cells and mice (Fig. 1, Fig. 2), but these changes were effectively prevented by ICA (Fig. 2), an established osteogenic agent [42–44]. Importantly, both glucocorticoids decreased the expression but ICA increased the expression of DEC1 (Figs. 1–3). Secondly, glucocorticoids-mediated downregulations of DEC1 in SaoS-2 cells and mice were effectively reversed by ICA (Figs. 3C and 3D). Thirdly, DEC1 overexpression increased the activity of ALP, the expression of Runx2, and mineralization (Figs. 4A–D), and the opposite was true with DEC1 knockdown. And finally, DEC1−/− mice exhibited decreased BMD, BV/TV, Tb.Th accompanied by increased SMI (Figs. 4I and 4J), linking DEC1 directly to a positive role in osteogenesis.
The precise molecular mechanisms whereby DEC1 functions as an osteogenic transcription factor remain to be determined, particularly in relation to the osteogenic effect of ICA. It appears that DEC1 supported the action of ICA by interplaying with several signaling pathways: notably the Wnt/β-catenin and the PI3K/Akt signaling cascades. While ICA significantly induced DEC1, the induction was abolished by DKK1, a negative regulator of the Wnt/β-catenin signaling pathway (Fig. 6D). On the other hand, DEC1 overexpression increased and DEC1 knockdown decreased the levels of β-catenin (Figs. 5G–J). Likewise, the upregulation of DEC1 by ICA was abolished by LY294002, an inhibitor of the PI3K/Akt signaling (Fig. 6F), and DEC1 overexpression and knockdown inversely affected the expression of PIK3CA (Figs. 7A and 7B). Together, these findings suggest that DEC1 reciprocally interacted with the Wnt/β-catenin and PI3K/Akt signaling pathways, leading to enhanced osteogenesis.
DEC1 is a sequence specific transcription factor, and we have shown previously that DEC1 acted on E-box or Sp1 element [45–47]. The interaction with the Sp1 element, in contrast to that with the E-box, was mediated by a complex that contained DEC1 and Sp1 proteins. Importantly, the interaction with E-box led to transcriptional repression, whereas the interaction with Sp1 led to transactivation. It is therefore assumed that the observed upregulation of β-catenin and PIK3CA was likely achieved through a DEC1-Sp1 complex. Interestingly, β-catenin reportedly interacted with Sp1 [48]. It is likely that DEC1, Sp1 and β-catenin form a functional trimer, although it remains to be determine whether binding to Sp1 is mutually exclusive between DEC1 and β-catenin. In addition to β-catenin and PIK3CA, DEC1 was also found to upregulate Runx2, and probably ALP (Figs. 4). It was reported that mouse Runx2 promoter contains functional E-box, although this E-box has a single nucleotide substitution [49,50]. In addition, a functional Sp1 element was characterized in the rat Runx2 promoter [51]. Likewise, several Sp1 sites were characterized in the rat ALP promoter. Together, these findings suggest that Runx2 and ALP are DEC1 regulated genes.
In addition to supporting the osteogenic activity of ICA, DEC1 likely functions as a common target of osteogenic agents, and precise mechanisms of the support may vary. For example, we have shown that DEC1 exerted potent antiapoptotic activities through upregulating the gene of survivin [52]. In this study, we have shown that decreased expression of DEC1 was closely related to cell number in tibia bone marrow (Fig. 3D). Nevertheless, calcium, a known bone-enhancing agent, significantly induced the expression of DEC1 [53]. Likewise, retinoic acid, a DEC1 inducer, was shown to induce osteogenic differentiation of mesenchymal cells [54,55]. It should be noted that retinoic acid was also reported to exert osteolytic activities [56]. The precise mechanisms remain to be determined on the conflicting findings. It is likely that retinoic acid possesses osteogenic and osteolytic activities depending on the concentrations and types of models.
Herba epimedii has been used for centuries and even thousands of years to treat osteoporotic conditions, and ICA is one of the primary active ingredients vitro [4–9]. In this study, we have provide several lines of evidence that support a major role of DEC1 in ICA-osteogenic activity. Osteoporosis is a serious public health concern worldwide. Supplements such as calcium are commonly used to prevent osteoporotic fractures. However, limitations remain, particularly for those who take drugs such as proton pump inhibitors. Our study validates ICA as an effective alternative to control osteoporotic conditions.
Supplementary Material
Fig. S1. The differential effects of dexamethasone on ALP activity in SaoS-2 cells
ALP activity of SaoS-2 cells incubated with solvent, various concentrations of DEX for 24 h. The experiment was repeated at least three times, and the data were expressed as mean ± SD. *p < 0.05, **p <0.01 vs 0 concentration.
Fig. S2. Icariin exerts osteogenic activity independently of GR antagonism.
ALP staining of SaoS-2 cells incubated with solvent, 10μM DEX, (10μM DEX + 0.1μM ICA), (10μM DEX + 0.1μM ICA + 0.1μM RU-486) and (10μM DEX + 0.1μM ICA + 1μM RU-486) for 24 h. The experiment was repeated at least three times, and the data were expressed as mean ± SD. **p <0.01 vs DMSO, ##p <0.01 vs DEX.
Acknowledgments
This study was supported by the Natural Science Foundation of China (Nos. 81573503, 81373443) and the Major Project of Jiangsu Provincial Department of Education (No. 13KJA310003) to J.Y. as well as NIH grants R01GM061988 and R01EB018748 to B.Y.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflicts of interest to this work.
References
- [1].Shi C, Huang P, Kang H, Hu B, Qi J, Jiang M, et al. , Glucocorticoid inhibits cell proliferation in differentiating osteoblasts by microRNA-199a targeting of WNT signaling, J Mol Endocrinol. 54 (2015) 325–337. [DOI] [PubMed] [Google Scholar]
- [2].Ding H, Wang T, Xu D, Cha B, Liu J, Li Y, Dexamethasone-induced apoptosis of osteocytic and osteoblastic cells is mediated by TAK1 activation. Biochem Biophys Res Commun. 460 (2015) 157–163. [DOI] [PubMed] [Google Scholar]
- [3].Contador D, Ezquer F, Espinosa M, Arango-Rodriguez M, Puebla C, Sobrevia L, et al. , Dexamethasone and rosiglitazone are sufficient and necessary for producing functional adipocytes from mesenchymal stem cells, Exp Bio Med. 240 (2015) 1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jiang F, Wang XL , Wang NL, Yao XS, Two new flavonol glycosides from Epimedium koreanum Nakai. J Asian Nat Prod Res. 11 (2009) 401–409. [DOI] [PubMed] [Google Scholar]
- [5].Sze SC, Tong Y, Ng TB, Cheng CL, Cheung HP, Herba Epimedii: anti-oxidative properties and its medical implications, Molecules. 15 (2010) 7861–7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chi L, Gao W, Shu X, Lu X, A natural flavonoid glucoside, icariin, regulates Th17 and alleviates rheumatoid arthritis in a murine model., Mediators Inflamm. 2014 (2014) 392062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang DC, Liu JL , Ding YB, Xia JG, Chen GY, Icariin potentiates the antitumor activity of gemcitabine in gallbladder cancer by suppressing NF-kappaB, Acta Pharmacol Sin, 34 (2013) 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li F, Gao B, Dong H, Shi J, Fang D, Icariin induces synoviolin expression through NFE2L1 to protect neurons from ER stress-induced apoptosis, PloS one. 10 (2015) e0119955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang L, Shen C, Chu J, Zhang R, Li Y, Li L, Icariin decreases the expression of APP and BACE-1 and reduces the beta-amyloid burden in an APP transgenic mouse model of Alzheimer’s disease, Int Bio Sci. 10 (2014) 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li XF, Xu H, Zhao YJ, Tang DZ, Xu GH, Holz J, et al. , Icariin Augments Bone Formation and Reverses the Phenotypes of Osteoprotegerin-Deficient Mice through the Activation of Wnt/ beta -Catenin-BMP Signaling, Evid Based Complement Alternat Med. 2013 (2013) 652317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hsieh TP, Sheu SY, Sun JS, Chen MH, Liu MH, Icariin isolated from Epimedium pubescens regulates osteoblasts anabolism through BMP-2, SMAD4, and Cbfa1 expression. Phymedicine. 17 (2010) 414–423. [DOI] [PubMed] [Google Scholar]
- [12].Zhao J, Ohba S, Shinkai M, Chung UI, Nagamune T, Icariin induces osteogenic differentiation in vitro in a BMP- and Runx2-dependent manner, Biochem Biophy Res Commun, 369 (2008) 444–448. [DOI] [PubMed] [Google Scholar]
- [13].Sun P, Liu Y, Deng X, Yu C, Dai N, Yuan X, et al. , An inhibitor of cathepsin K, icariin suppresses cartilage and bone degradation in mice of collagen-induced arthritis, Phytomedicine 20 (2003) 975–979. [DOI] [PubMed] [Google Scholar]
- [14].Shen M, Kawamoto T, Yan W, Nakamasu K, Tamagami M, Koyano Y, et al. , Molecular characterization of the novel basic helix-loop-helix protein DEC1 expressed in differentiated human embryo chondrocytes, Biochem Biophy Res Commun. 36 (1997)294–198. [DOI] [PubMed] [Google Scholar]
- [15].Liu Y, Wang L, Lin XY, Wang J, Yu JH, Miao Y, et al. , The transcription factor DEC1 (BHLHE40/STRA13/SHARP-2) is negatively associated with TNM stage in non-small-cell lung cancer and inhibits the proliferation through cyclin D1 in A549 and BE1 cells, Tumour biol. 34 (2013) 1641–1650. [DOI] [PubMed] [Google Scholar]
- [16].Shi XH, Zheng Y, Sun Q, Cui J, Liu QH, Qu F, et al. , DEC1 nuclear expression: a marker of differentiation grade in hepatocellular carcinoma, World J Gastroenterol. 17 (2011) 2037–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Seino H, Wu Y, Morohashi S, Kawamoto T, Fujimoto K, Kato Y, et al. , Basic helix-loop-helix transcription factor DEC1 regulates the cisplatin-induced apoptotic pathway of human esophageal cancer cells, Biomed Res. 36 (2015) 89–96. [DOI] [PubMed] [Google Scholar]
- [18].Baier PC, Brzozka MM, Shahmoradi A, Reinecke L, Kroos C, Wichert SP, et al. , Mice lacking the circadian modulators SHARP1 and SHARP2 display altered sleep and mixed state endophenotypes of psychiatric disorders, PloS one. 9 (2014) e110310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kato Y, Kawamoto T, Fujimoto K, Noshiro M, DEC1/STRA13/SHARP2 and DEC2/SHARP1 coordinate physiological processes, including circadian rhythms in response to environmental stimuli, Curr Top Dev Biol. 110 (2014) 339–372. [DOI] [PubMed] [Google Scholar]
- [20].Shen L, Cui A, Xue Y, Cui Y, Dong X, Gao Y, et al. , Hepatic differentiated embryo-chondrocyte-expressed gene 1 (Dec1) inhibits sterol regulatory element-binding protein-1c (Srebp-1c) expression and alleviates fatty liver phenotype, J Biol Chem. 289 (2014) 23332–23342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Martinez-Llordella M, Esensten JH, Bailey-Bucktrout SL, Lipsky RH, Marini A, Chen J, et al. , CD28-inducible transcription factor DEC1 is required for efficient autoreactive CD4+ T cell response, J Exp Med 210 (2013)1603–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shen M, Yoshida E, Yan W, Kawamoto T, Suardita K, Koyano Y, et al. , Basic helix-loop-helix protein DEC1 promotes chondrocyte differentiation at the early and terminal stages, J Biol Chem. 277 (2002) 50112–50120. [DOI] [PubMed] [Google Scholar]
- [23].Iwata T, Kawamoto T, Sasabe E, Miyazaki K, Fujimoto K, Noshiro M, et al. , Effects of overexpression of basic helix-loop-helix transcription factor Dec1 on osteogenic and adipogenic differentiation of mesenchymal stem cells, Eur J Cell Biol. 5 (2006) 423–431. [DOI] [PubMed] [Google Scholar]
- [24].Hu JH, Mao Z, Shang W, Yue HT, Wang YW, Wu LL, et al. , Down regulation of differentiated embryo-chondrocyte expressed gene 1 is related to the decrease of osteogenic capacity, Curr Drug Targets. 15 (2014) 432–441. [DOI] [PubMed] [Google Scholar]
- [25].Ren Y, Han SY, Li PP, Effects of anastrozole combined with Shuganjiangu decoction on osteoblast-like cell proliferation, differentiation and OPG/RANKL mRNA expression, Chin Cancer Res. 24 (2012) 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li Y, Song X, Ma Y, Liu J, Yang D, Yan B, DNA binding, but not interaction with Bmal1, is responsible for DEC1-mediated transcription regulation of the circadian gene mPer1. Biochem J. 382 (2004) 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, et al. , Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee, J Bone Miner Res. 28 (2013) 2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ishida Y, Heersche JN, Glucocorticoid-induced osteoporosis: both in vivo and in vitro concentrations of glucocorticoids higher than physiological levels attenuate osteoblast differentiation, J Bone Miner Res. 13 (1998) 1822–1826. [DOI] [PubMed] [Google Scholar]
- [29].Fan JJ, Cao LG, Wu T, Wang DX, Jin D, Jiang S, et al. , The dose-effect of icariin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cells, Molecules. 16 (2011) 10123–10133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yoon HY, Cho YS, Jin Q, Kim HG, Woo ER, Chung YS, Effects of Ethyl Acetate Extract of Poncirus trifoliata Fruit for Glucocorticoid-Induced Osteoporosis, Biomol Ther. 20 (2012) 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Saravanan S, Vimalraj S, Vairamani M, Selvamurugan N, Role of Mesoporous Wollastonite (Calcium Silicate) in Mesenchymal Stem Cell Proliferation and Osteoblast Differentiation: A Cellular and Molecular Study, J Biomed Nanotechnol. 11 (2015) 1124–1138. [DOI] [PubMed] [Google Scholar]
- [32].Lee ZH, Kim HJ, Ryoo HM, A Novel Osteogenic Activity of Suberoylanilide Hydroxamic Acid is Synergized by BMP-2, J Bone Metab. 22 (2015) 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments, Nat Med. 19 (2013)179–192. [DOI] [PubMed] [Google Scholar]
- [34].Burgers TA, Williams BO. Regulation of Wnt/beta-catenin signaling within and from osteocytes, Bone. 54 (2013) 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McCubrey JA, Steelman LS, Bertrand FE, Davis NM, Sokolosky M, Abrams SL, et al. , GSK-3 as potential target for therapeutic intervention in cancer, Oncotarget. 5 (2014) 2881–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Frenkel B, White W, Tuckermann J. Glucocorticoid-Induced Osteoporosis, Adv Exp Med Bio. 872 (2015) 179–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McGee-Lawrence ME, Carpio LR, Schulze RJ, Pierce JL, McNiven MA, Farr JN, et al. , Hdac3 Deficiency Increases Marrow Adiposity and Induces Lipid Storage and Glucocorticoid Metabolism in Osteochondroprogenitor Cells, J Bone Miner Res. 31 (2016) 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ko JY, Chuang PC, Ke HJ, Chen YS, Sun YC, Wang FS, MicroRNA-29a mitigates glucocorticoid induction of bone loss and fatty marrow by rescuing Runx2 acetylation, Bone. 81 (2015) 80–88. [DOI] [PubMed] [Google Scholar]
- [39].Fraser LA, Adachi JD. Glucocorticoid-induced osteoporosis: treatment update and review, Ther Adv Musculoskelet Dis. 1 (2009) 71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Quarto N, Senarath-Yapa K, Renda A, Longaker MT, TWIST1 silencing enhances in vitro and in vivo osteogenic differentiation of human adipose-derived stem cells by triggering activation of BMP-ERK/FGF signaling and TAZ upregulation, Stem cells. 33 (2015) 833–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang Y, Hassan MQ, Li ZY, Stein JL, Lian JB, Wijnen AJ, et al. , Intricate gene regulatory networks of helix-loop-helix (HLH) proteins support regulation of bone-tissue related genes during osteoblast differentiation, J Cell biochem. 105 (2008) 487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Luo Z, Liu M, Sun L, Rui F, Icariin recovers the osteogenic differentiation and bone formation of bone marrow stromal cells from a rat model of estrogen deficiency-induced osteoporosis, Mol Med Rep. 12 (2015) 382–388. [DOI] [PubMed] [Google Scholar]
- [43].Feng R, Feng L, Yuan Z, Wang D, Wang F, Tan B, et al. Icariin protects against glucocorticoid-induced osteoporosis in vitro and prevents glucocorticoid-induced osteocyte apoptosis in vivo, Cell Biochem Biophys. 67 (2013) 189–197. [DOI] [PubMed] [Google Scholar]
- [44].Liang W, Lin M, Li X, Li C, Gao B, Gan H, et al. , Icariin promotes bone formation via the BMP-2/Smad4 signal transduction pathway in the hFOB 1.19 human osteoblastic cell line, Int J Mol Med 30 (2012) 889–895. [DOI] [PubMed] [Google Scholar]
- [45].Li Y, Xie M, Song X, Gragen S, Sachdeva K, Wan Y et al. , DEC1 negatively regulates the expression of DEC2 through binding to the E-box in the proximal promoter. J Biol Chem. 278 (2003) 16899–16907. [DOI] [PubMed] [Google Scholar]
- [46].Li Y, Song X, Ma Y, Liu J, Yang D, Yan B. DNA binding, but not interaction with Bmal1, is responsible for DEC1-mediated transcription regulation of the circadian gene mPer1. Biochem J 382 (2004) 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Li Y, Xie M, Yang J, Yang D, Deng R, Wan Y, et al. , The expression of antiapoptotic protein survivin is transcriptionally upregulated by DEC1 primarily through multiple sp1 binding sites in the proximal promoter, Oncogene. 25 (2006) 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kumawat K, Menzen MH, Slegtenhorst RM, Halayko AJ, Schmidt M, Gosens R, TGF-beta-activated kinase 1 (TAK1) signaling regulates TGF-beta-induced WNT-5A expression in airway smooth muscle cells via Sp1 and beta-catenin, PloS one. 9 (2014) e94801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hovhannisyan H, Zhang Y, Hassan MQ, Wu H, Glackin C, Lian JB, et al. , Genomic occupancy of HLH, AP1 and Runx2 motifs within a nuclease sensitive site of the Runx2 gene, J Cell Physiol. 28 (2013) 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Li Y, Xie M, Song X, Gragen S, Sachdeva K, Wan Y, et al. DEC1 negatively regulates the expression of DEC2 through binding to the E-box in the proximal promoter. J Bio Chem. 278 (2003) 16899–16907. [DOI] [PubMed] [Google Scholar]
- [51].Zhang Y, Hassan MQ, Xie RL, Hawse JR, Spelsberg TC, Montecino M, et al. , Co-stimulation of the bone-related Runx2 P1 promoter in mesenchymal cells by SP1 and ETS transcription factors at polymorphic purine-rich DNA sequences (Y-repeats), J Bio Chem. 284 (2009) 3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Li Y, Xie M, Yang J, Yang D, Deng R, Wan Y, et al. , The expression of antiapoptotic protein survivin is transcriptionally upregulated by DEC1 primarily through multiple sp1 binding sites in the proximal promoter, Oncogene. 25 (2006) 3296–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ortiz J, Chou LL, Calcium upregulated survivin expression and associated osteogenesis of normal human osteoblasts, J Biomed Mater Res A. 100 (2012) 1770–1776. [DOI] [PubMed] [Google Scholar]
- [54].Malladi P, Xu Y, Yang GP, Longaker MT, Functions of vitamin D, retinoic acid, and dexamethasone in mouse adipose-derived mesenchymal cells, Tissue Eng. 12 (2006) 2031–2040. [DOI] [PubMed] [Google Scholar]
- [55].Zhang S, Chen X, Hu Y, Wu J, Cao Q, Chen S, et al. , All-trans retinoic acid modulates Wnt3A-induced osteogenic differentiation of mesenchymal stem cells via activating the PI3K/AKT/GSK3beta signalling pathway, Mol Cell Endocrinol. 422 (2016) 243–253. [DOI] [PubMed] [Google Scholar]
- [56].Conaway HH, Pirhayati A, Persson E, Pettersson U, Svensson O, Lindholm C, et al. , Retinoids stimulate periosteal bone resorption by enhancing the protein RANKL, a response inhibited by monomeric glucocorticoid receptor, J Biol Chem. 286 (2011) 31425–31436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. The differential effects of dexamethasone on ALP activity in SaoS-2 cells
ALP activity of SaoS-2 cells incubated with solvent, various concentrations of DEX for 24 h. The experiment was repeated at least three times, and the data were expressed as mean ± SD. *p < 0.05, **p <0.01 vs 0 concentration.
Fig. S2. Icariin exerts osteogenic activity independently of GR antagonism.
ALP staining of SaoS-2 cells incubated with solvent, 10μM DEX, (10μM DEX + 0.1μM ICA), (10μM DEX + 0.1μM ICA + 0.1μM RU-486) and (10μM DEX + 0.1μM ICA + 1μM RU-486) for 24 h. The experiment was repeated at least three times, and the data were expressed as mean ± SD. **p <0.01 vs DMSO, ##p <0.01 vs DEX.


