Abstract
Objective
To investigate the prognostic value of preoperative cardiac magnetic resonance imaging (MRI) for long-term major adverse cardiac and cerebrovascular events (MACCEs) in patients undergoing tricuspid valve (TV) surgery for functional tricuspid regurgitation (TR).
Materials and Methods
The preoperative cardiac MR images, New York Heart Association functional class, comorbidities, and clinical events of 78 patients (median [interquartile range], 59 [51–66.3] years, 28.2% male) who underwent TV surgery for functional TR were comprehensively reviewed. Cox proportional hazards analyses were performed to assess the associations of clinical and imaging parameters with MACCEs and all-cause mortality.
Results
For the median follow-up duration of 5.4 years (interquartile range, 1.2–6.6), MACCEs and all-cause mortality were 51.3% and 23.1%, respectively. The right ventricular (RV) end-systolic volume index (ESVI) and the systolic RV mass index (RVMI) were higher in patients with MACCEs than those without them (77 vs. 68 mL/m2, p = 0.048; 23.5 vs. 18.0%, p = 0.011, respectively). A high RV ESVI was associated with all-cause mortality (hazard ratio [HR] per value of 10 higher ESVI = 1.10, p = 0.03). A high RVMI was also associated with all-cause mortality (HR per increase of 5 mL/m2 RVMI = 1.75, p < 0.001). After adjusting for age and sex, only RVMI remained a significant predictor of MACCEs and all-cause mortality (p < 0.05 for both). After adjusting for multiple clinical variables, RVMI remained significantly associated with all-cause mortality (p = 0.005).
Conclusion
RVMI measured on preoperative cardiac MRI was an independent predictor of long-term outcomes in patients who underwent TV surgery for functional TR.
Keywords: Tricuspid regurgitation, Functional tricuspid regurgitation, Right ventricle, Right ventricular mass index, Cardiac MRI
INTRODUCTION
Functional tricuspid regurgitation (TR) is a sequel of left-sided heart disease or pulmonary hypertension, and it can cause right ventricular (RV) dysfunction and remodeling with tethering of the tricuspid valve (TV) leaflets, tricuspid annular dilation, and/or papillary muscle displacement [1]. Therefore, functional TR is frequently found in mitral valve disease, where it accounts for 30–50% of patients with severe mitral valve regurgitation [2,3]. In the past, surgical treatment of the mitral valve in these patients was considered to be a correction of tricuspid pathology; conservative treatment was therefore recommended for functional TR [4,5], and its significance was largely ignored. Severe TR is associated with a worse long-term prognosis, regardless of the presence or absence of left ventricular systolic dysfunction or pulmonary hypertension [2,6]. Therefore, surgical and interventional treatment for TR has been receiving attention [7].
It has been recommended that the timing of surgical treatment for functional TR should be determined using the duration of left-sided surgery and RV enlargement or dysfunction [8]. However, severe functional TR also leads to RV dilatation and dysfunction [1,9]. Therefore, accurate and timely evaluation of RV function and dimensions is important. Clinical decision-making in patients with TR is based on the categorization of TR by echocardiography, but this remains a challenging task with limitations [10]. The current American Society of Echocardiography guidelines suggest the potential role of cardiac magnetic resonance imaging (MRI), which has been used as a gold standard for the determination of RV morphology and function because it provides excellent myocardial definition and high accuracy and reproducibility for the evaluation of the RV [11].
Previous studies on preoperative imaging modalities for functional TR have indicated the role of cardiac MRI [9,12]. The preoperative cardiac MRI-based RV ejection fraction (RVEF) was predictive of adverse outcomes in patients with severe functional TR in a previous study [12], but the study population was limited to those with isolated TR, despite the frequent association of TR with mitral valve disease. Therefore, it was not clear if other cardiac MR-derived parameters, including the RVEF, are associated with poor outcomes in patients undergoing TV surgery for functional TR with or without significant left-sided heart disease (i.e., moderate to severe mitral or aortic regurgitation or stenosis). Therefore, the purpose of this study was to investigate the prognostic value of factors derived from preoperative cardiac MRI for predicting the long-term outcomes of patients who underwent TV surgery for functional TR.
MATERIALS AND METHODS
Study Patients
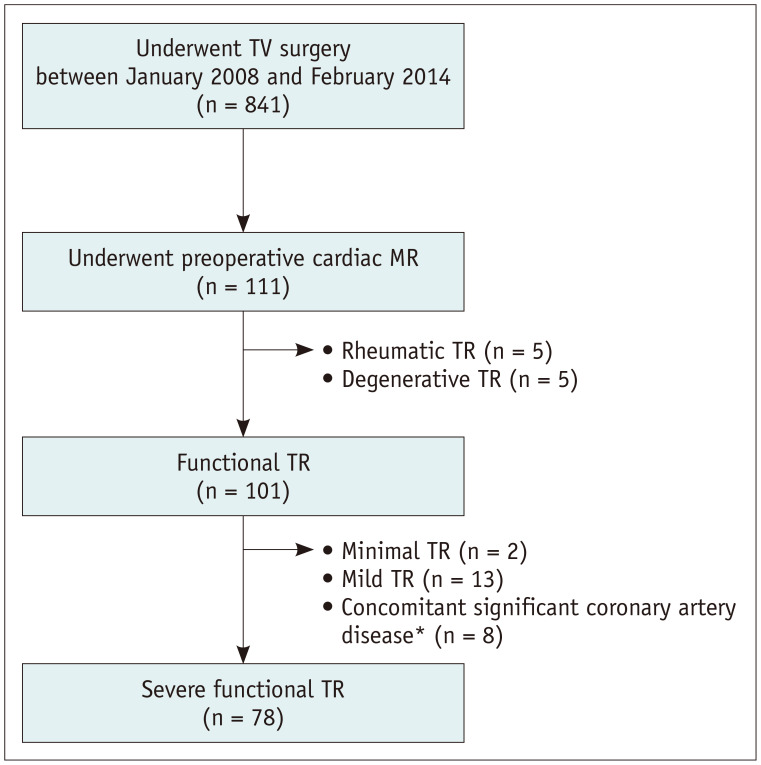
This retrospective study was approved by the Institutional Review Board of Asan Medical Center Hospital, and the requirement for informed consent was waived (approval number: 2015-0470). Between January 2008 and February 2014, 841 patients underwent TV surgery at our hospital (Fig. 1). Among them, 111 had undergone preoperative cardiac MRI, and 78 with severe functional TR were finally identified after excluding those with mild to moderate TR and/or concomitant significant coronary artery disease. The surgeries were performed by four experienced cardiac surgeons, each with more than 8 years of experience. Patients with primary TR were excluded based on imaging or surgical findings. Primary TR was considered as TR due to structural deterioration of TV leaflets and/or chordae by rheumatic, degenerative, congenital, infective, traumatic, or iatrogenic causes. Functional TR was defined as incomplete coaptation of TV leaflets secondary to the deformation of the TV apparatus, especially the dilatation of the TV annulus, and consequent TR jet area in the right atrium and combined systolic flow reversal in the hepatic veins [13]. The patients' clinical findings, including the New York Heart Association (NYHA) class before TR surgery, preoperative echocardiography results, and the presence of comorbidities, were thoroughly reviewed. Medical records of the perioperative period, including laboratory findings, medication use, vital signs, and operation records, were also reviewed. Follow-up data, including mortality data, were obtained from the electronic medical records through May 22, 2019, and the nationwide data on death certification provided by the National Statistical Office were reviewed. The study endpoints were major adverse cardiovascular or cerebrovascular events (MACCEs) and all-cause mortality. The MACCEs were defined as all-cause death, myocardial infarction, stroke, rehospitalization due to cardiac causes, and reintervention or surgery.
Fig. 1. Flowchart for patient selection.
*Two or three vessel disease on preoperative coronary angiography. TR = tricuspid regurgitation, TV = tricuspid valve
Echocardiography
Preoperative echocardiographic examinations were performed according to standard guidelines using commercially available equipment (General Electric, Philips Healthcare, Siemens) [14]. Routine four-chamber, left and right two-chamber, and short-axis views were acquired, and the TR jet area, peak TR velocity, and pulmonary artery systolic pressure, estimated by the peak trans-tricuspid pressure gradient, were carefully obtained. The qualitative grading of TR was performed by echocardiography experts according to the guidelines of the Journal of the American Society of Echocardiography (three grades: mild, moderate, and severe) [15].
Cardiac MRI
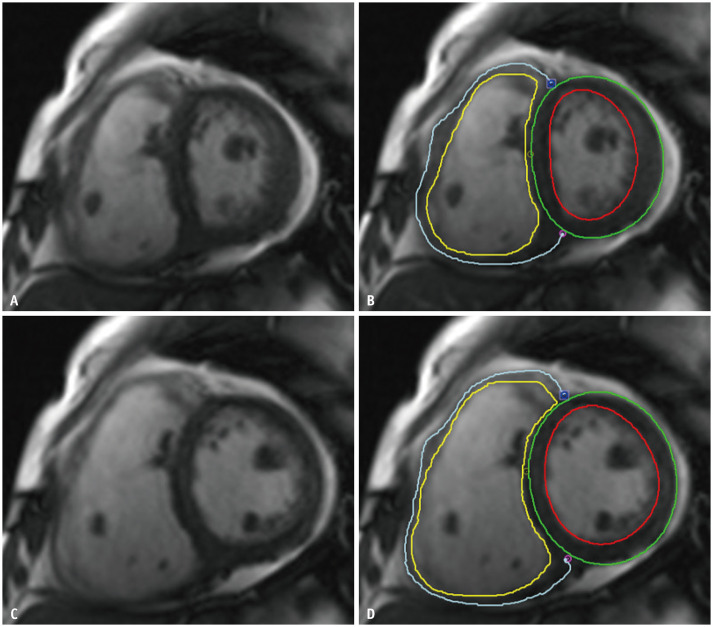
The median interval between cardiac MRI and TV surgery was 8 days (interquartile range, 3–24). All cardiac MRI scans were performed using a 1.5T scanner (Magnetom Avanto; Siemens Healthcare) with standard protocols [16]. Steady-state free-precession cine images were obtained during a patient's breath-hold, and both ventricular wall motions were imaged. The MRI parameters included the following: repetition time/echo time, 37.1/1.9 ms; flip angle, 68°; matrix, 256 × 256; field of view, 253 × 300 mm. The late gadolinium enhancement (LGE) images were obtained 10 minutes after the administration of 0.2 mmoL/kg intravenous gadolinium chelate contrast agent with magnitude-only inversion recovery and phase-sensitive inversion recovery sequence reconstruction. The MRI parameters included the following: repetition time/echo time, 700.0/1.2 ms; flip angle, 80°; matrix, 256 × 256; field of view, 238 × 349; inversion time, 300 ms for nulling of the normal myocardial signal. The contrast agent was administered to 96.2% (75 of 78) of the patients. The entire short-axis images of the heart were obtained using an 8-mm thickness without an intersection gap from the heart apex to above the valve plane, with the entire ventricular volumes being included. LV and RV volumetric analyses were performed by one radiologist (a board-certified radiologist with 6 years of experience in cardiovascular imaging) using dedicated software (CVI42; Circle Cardiovascular Imaging; Fig. 2). The papillary muscles and trabeculations were excluded, as described in a previous study [17]. Another radiologist (a resident in training with 3 years of experience in cardiovascular imaging) independently conducted RV volumetric analysis using the same dedicated software to evaluate the reproducibility of the RV functional parameters derived from cardiac MRI. The LV and RV end-diastolic and end-systolic volume, stroke volume, ejection fraction, and myocardial masses were measured, and the myocardial masses were assessed in both end-systolic and end-diastolic phase images. Ventricular volume indices and myocardial mass indices were calculated by normalizing the patients' body surface areas. For quantification of LV LGE, endomyocardial and epimyocardial borders were manually drawn. LGE was calculated semiautomatically as a percentage of LV mass and was defined as presenting an intensity five standard deviation above the normal myocardium (dedicated using a region of interest).
Fig. 2. Short-axis MR images with RV manual contouring during end-systole (A, B) and end-diastole phases (C, D).
Yellow and blue lines indicate endomyocardial and epimyocardial RV borders, respectively. Left ventricular endomyocardial and epimyocardial borders are drawn in red and green lines, respectively. Myocardial trabeculation and papillary muscles are excluded from the contouring. RV = right ventricle
Statistical Analysis
Continuous variables are presented as median and interquartile range, and categorical data are presented as numbers with percentages in parentheses. For comparisons between patients with and without MACCEs, the student's t test and the Mann-Whitney U-test were used for continuous data, and the chi-square test or Fisher's exact test was used for categorical variables. Cox proportional hazards analysis was used to identify the predictors of MACCEs or all-cause mortality. Variables with a p < 0.10, in the univariable analyses, were included in the features for the multivariable models. To avoid multicollinearity, RV function parameters were not simultaneously entered into the multivariable models. Receiver operating characteristic (ROC) curves for predicting MACCEs or all-cause mortality were drawn for the statistically significant RV parameters measured on cardiac MRI. The areas under the ROC curves (AUCs) were assessed to evaluate the predictive power of the RV parameters. Kaplan-Meier curves for MACCE-free survival and overall survival (i.e., without all causes of mortality) were created using cut-off values for the RV parameters obtained from the ROC curves. The log-rank test was used to compare the curves stratified by the optimal cut-off values. The optimal cut-off values were determined using the minimum p value method [18]. Interclass correlation coefficients (ICCs) were used to evaluate the agreement of the two observers during the RV volumetric analysis. ICC values between 0.50 and 0.75 were considered indicative of moderate agreement, those between 0.75 and 0.90 were considered indicative of good agreement, and those above 0.90 were indicative of excellent agreement [19]. Statistical analyses were performed using SPSS (version 21.0; IBM Corp.) and the R package (version 3.1.1; R Foundation of Statistical Imaging). p value < 0.05 was considered indicative of statistical significance.
RESULTS
Patient Characteristics
The baseline clinical characteristics of the patients are summarized in Table 1. The median follow-up duration for all the patients was 5.4 years (interquartile range, 1.2–6.6 years). MACCEs occurred in 40 patients during the median follow-up of 1.4 years (interquartile range, 0.1–4.6 years). A breakdown of the MACCEs is provided in Supplementary Table 1. The all-cause mortality rate was 23.1% (n = 18). Half of the patients (51.3%) had concurrent significant mitral valve disease, and 9.0% had aortic valve disease. However, the percentages of patients with and without MACCEs who had left heart disease were not significantly different. Among the 78 patients, 39 (50.0%) underwent tricuspid annuloplasty, 14 (17.9%) underwent tricuspid valvuloplasty, 24 (30.8%) had TV replacement, and one (1.2%) had both tricuspid annuloplasty and valvuloplasty. Forty (47.4%) patients had undergone prior valve surgery (TV surgery, 7; mitral valve surgery, 22; aortic valve surgery, 8). The operation-related results and outcomes are described in the Supplementary Table 2.
Table 1. Baseline Clinical Characteristics according to MACCE.
| All Patients (n = 78) |
Patients without MACCE (n = 38) |
Patients with MACCE (n = 40) |
P | ||
|---|---|---|---|---|---|
| Age, years | 59.0 (51.0–66.3) | 56.0 (46.5–64.5) | 62.0 (55.0–66.8) | 0.08 | |
| Sex, male | 22 (28.2) | 10 (26.3) | 12 (30.0) | 0.72 | |
| Comorbidity | |||||
| DM | 14 (17.9) | 7 (18.4) | 7 (17.5) | 0.92 | |
| Hypertension | 18 (23.1) | 9 (23.7) | 9 (22.5) | 0.90 | |
| Hyperlipidemia | 13 (16.7) | 9 (23.7) | 4 (10.0) | 0.11 | |
| Dialysis | 2 (2.6) | 0 (0.0) | 2 (5.0) | 0.49 | |
| Congestive hepatopathy | 2 (2.6) | 0 (0.0) | 2 (5.0) | 0.49 | |
| Atrial fibrillation | 45 (57.7) | 19 (50.0) | 26 (65.0) | 0.18 | |
| COPD | 2 (2.6) | 1 (2.6) | 1 (2.5) | 0.97 | |
| Medication use | |||||
| ACE inhibitor or ARB | 39 (50.0) | 16 (42.1) | 23 (57.5) | 0.26 | |
| Beta blocker | 20 (25.6) | 12 (31.6) | 8 (20.0) | 0.30 | |
| Diuretics | 63 (80.8) | 28 (73.7) | 35 (87.5) | 0.16 | |
| Digoxin | 41 (52.6) | 19 (50.0) | 22 (55.0) | 0.82 | |
| Calcium channel blocker | 13 (16.7) | 6 (15.8) | 7 (17.5) | 1.00 | |
| Antiplatelet | 10 (12.8) | 6 (15.8) | 4 (10.0) | 0.51 | |
| Anticoagulant | 47 (60.3) | 21 (55.3) | 26 (65.0) | 0.49 | |
| Antidiabetic agent | 14 (17.9) | 7 (18.4) | 7 (17.5) | 0.92 | |
| Antidyslipidemic agent | 13 (16.7) | 9 (23.7) | 4 (10.0) | 0.11 | |
| Laboratory findings | |||||
| Hemoglobin, g/dL | 12.3 (10.9–13.2) | 12.4 (10.7–13.4) | 12.1 (11.0–13.1) | 0.62 | |
| eGFR, mL/min/1.73 m2 | 71.0 (58.8–90.0) | 78.5 (65.8–90.0) | 68.0 (55.3–86.0) | 0.11 | |
| Aspartate aminotransferase, IU/mL | 26.0 (21.0–32.0) | 24.0 (21.0–30.0) | 29.0 (20.0–33.0) | 0.14 | |
| Alanine aminotransferase, IU/mL | 19.0 (14.0–22.3) | 18.5 (14.0–22.3) | 19.0 (12.0–22.0) | 0.76 | |
| Prothrombin time, INR | 1.2 (1.1–1.5) | 1.1 (1.0–1.5) | 1.2 (1.1–1.5) | 0.28 | |
| Initial vital signs | |||||
| Systolic BP, mm Hg | 109.5 (100.0–117.0) | 108.0 (100.0–117.3) | 110.0 (100.0–113.0) | 0.74 | |
| Diastolic BP, mm Hg | 67.5 (60.0–74.3) | 67.0 (60.0–73.3) | 69.0 (60.0–77.0) | 0.92 | |
| Heart rate | 69.0 (60.0–80.0) | 66.0 (60.0–80.0) | 70.0 (61.0–80.0) | 0.43 | |
| Respiratory rate | 18.0 (18.0–20.0) | 18.0 (18.0–20.0) | 18.0 (18.0–20.0) | 0.48 | |
| Body temperature, ℃ | 36.4 (36.2–36.6) | 36.4 (36.2–36.6) | 36.4 (36.2–36.7) | 0.79 | |
| Preoperative NYHA classification | 0.13 | ||||
| I | 16 (20.5) | 7 (18.4) | 9 (22.5) | ||
| II | 38 (48.7) | 23 (60.5) | 15 (37.5) | ||
| III | 22 (28.2) | 8 (21.1) | 14 (35.0) | ||
| IV | 2 (2.6) | 0 (0.0) | 2 (5.0) | ||
| Other valve pathology | |||||
| Moderate to severe MS or MR | 40 (51.3) | 17 (44.7) | 23 (57.5) | 0.26 | |
| Moderate to severe AS or AR | 7 (9.0) | 4 (10.5) | 3 (7.5) | 0.71 | |
| Moderate pulmonary regurgitation | 4 (5.1) | 1 (2.6) | 3 (7.5) | 0.62 | |
| Operation type | 0.98 | ||||
| Tricuspid annuloplasty | 39 (50.0) | 20 (52.6) | 19 (47.5) | ||
| Tricuspid valvuloplasty | 14 (17.9) | 7 (18.4) | 7 (17.5) | ||
| TV replacement | 24 (30.8) | 11 (28.9) | 13 (32.5) | ||
| Tricuspid annuloplasty & valvuloplasty | 1 (1.2) | 0 (0.0) | 1 (2.5) | ||
| Combined operation | |||||
| Mitral valve surgery | 47 (60.3) | 22 (57.9) | 25 (62.5) | 0.68 | |
| Aortic valve surgery | 9 (11.5) | 4 (10.5) | 5 (12.5) | 1.00 | |
| Pulmonary valve surgery | 2 (2.6) | 1 (2.6) | 1 (2.5) | 1.00 | |
| Maze operation | 40 (51.3) | 19 (50.0) | 21 (52.5) | 0.83 | |
| Left atrial reduction | 21 (26.9) | 10 (26.3) | 11 (27.5) | 0.91 | |
| Right atrial reduction | 25 (32.1) | 9 (23.7) | 16 (40.0) | 0.12 | |
| Glenn shunt | 3 (3.8) | 1 (2.6) | 2 (5.0) | 1.00 | |
| Number of TV operations* | 0.76 | ||||
| Redo | 18 (23.1) | 7 (18.4) | 11 (27.5) | ||
| Trido | 5 (6.4) | 3 (7.9) | 2 (5.0) | ||
| Previous valve operation | |||||
| TV operation history | 7 (9.0) | 3 (7.9) | 4 (10.0) | 1.00 | |
| Mitral valve operation history | 22 (28.2) | 8 (21.1) | 14 (35.0) | 0.17 | |
| Aortic valve operation history | 8 (9.3) | 3 (7.9) | 5 (12.5) | 0.71 | |
Data are median and interquartile range, or number with percentage in parentheses. *Number of surgeries includes the dedicated operation after obtaining cardiac MRI. ACE = angiotensin-converting enzyme, AR = aortic regurgitation, ARB = angiotensin II receptor blocker, AS = aortic stenosis, BP = blood pressure, COPD = chronic obstructive pulmonary disease, DM = diabetes mellitus, eGFR = estimated glomerular filtration rate, INR = international normalized ratio, MACCE = major adverse cardiovascular event, MR = mitral regurgitation, MS = mitral stenosis, NYHA = New York Heart Association, TV = tricuspid valve
Echocardiography and Cardiac MR Parameters
Among the echocardiographic parameters, only the TR jet area showed a significant difference in the patient groups; it was larger in patients with MACCEs than in those without MACCEs (21.0% vs. 16.0%, p = 0.007; Table 2). The RV ESVI measured on MRI was higher in patients with than those without MACCEs (77 vs. 68, p = 0.048), but the RV end-diastolic volume index (EDVI) showed no significant difference in the two groups. Systolic RV mass index (RVMI) was higher in patients with than in those without MACCEs (23.5 vs. 18.0%, p = 0.011). No MRI-derived LV functional parameters were significantly different in the two groups. The reproducibility of the RV functional parameters derived from cardiac MRI ranged from good to excellent (Table 3).
Table 2. Baseline Echocardiography and Cardiac MRI Parameters according to MACCE.
| Parameters | All Patients (n = 78) |
Patients without MACCE (n = 38) |
Patients with MACCE (n = 40) |
P | ||
|---|---|---|---|---|---|---|
| Echocardiography | ||||||
| Right-side parameters | ||||||
| TV or TR jet area, cm2 | 19.0 (14.0–26.0) | 16.0 (13.3–21.8) | 21.0 (16.5–34.0) | 0.01 | ||
| Peak TR velocity, m/s | 2.0 (2.7–3.6) | 3.1 (2.7–3.6) | 3.0 (2.6–3.6) | 0.79 | ||
| PA systolic pressure, mm Hg | 36.0 (29.0–52.0) | 37.0 (29.5–51.3) | 36.0 (26.5–57.8) | 0.79 | ||
| RV tissue Doppler (S′), cm/s | 9.9 (8.2–11.2) | 9.9 (8.4–11.4) | 9.9 (8.2–11.3) | 0.78 | ||
| Left-side parameters | ||||||
| LVEF, % | 58.5 (54.3–63.0) | 58.3 (54.4–63.8) | 58.5 (53.8–62.6) | 0.67 | ||
| LV ESVI, mL/m2 | 41.5 (28.0–58.3) | 41.0 (29.3–54.5) | 39.5 (27.0–63.3) | 0.88 | ||
| LV EDVI, mL/m2 | 99.5 (72.5–135.0) | 98.0 (75.0–124.8) | 98.5 (63.8–138.3) | 0.80 | ||
| LVMI, g/m2 | 104.8 (82.1–140.0) | 101.0 (74.5–137.0) | 110.2 (84.5–138.9) | 0.29 | ||
| Cardiac MRI | ||||||
| TV annulus maximum diameter, mm | 45.1 (40.7–49.9) | 44.8 (40.4–48.9) | 45.5 (40.9–51.5) | 0.42 | ||
| TV annulus minimum diameter, mm | 38.5 (34.7–42.1) | 36.8 (33.5–41.1) | 40.1 (36.6–42.2) | 0.09 | ||
| Right-side parameters | ||||||
| RVEF, % | 46.5 (37.0–52.3) | 46.0 (41.0–53.3) | 45.5 (35.0–52.0) | 0.38 | ||
| RV ESVI, mL/m2 | 71.5 (51.5–94.3) | 68.0 (47.3–87.3) | 77.0 (58.5–95.0) | 0.048 | ||
| RV EDVI, mL/m2 | 126.0 (102.0–174.5) | 123.0 (87.0–150.0) | 133.5 (110.8–186.3) | 0.10 | ||
| Systolic RVMI, g/m2 | 20.0 (15.8–25.0) | 18.0 (14.0–21.8) | 23.5 (17.0–26.3) | 0.01 | ||
| Diastolic RVMI, g/m2 | 25.0 (21.0–32.0) | 23.5 (19.5–28.3) | 26.5 (22.0–33.0) | 0.05 | ||
| Left-side parameters | ||||||
| LVEF, % | 48.0 (41.0–55.3) | 50.0 (42.0–56.8) | 46.5 (37.0–55.0) | 0.17 | ||
| LV ESVI, mL/m2 | 56.0 (37.0–76.3) | 55.5 (37.0–70.5) | 57.0 (39.3–77.8) | 0.43 | ||
| LV EDVI, mL/m2 | 104.0 (80.0–145.5) | 99.5 (74.3–141.0) | 108.0 (80.0–149.8) | 0.69 | ||
| Systolic LVMI, g/m2 | 50.0 (41.0–67.3) | 48.5 (41.0–64.0) | 54.5 (43.0–68.8) | 0.12 | ||
| Diastolic LVMI, g/m2 | 58.0 (48.0–72.3) | 57.0 (47.0–70.3) | 58.5 (51.0–78.3) | 0.20 | ||
| LV LGE, % | 1.0 (0.0–1.0) | 0.0 (0.0–1.4) | 0.5 (0.0–3.2) | 0.06 | ||
Data are median and interquartile range, or number with percentage in parentheses. EDVI = end-diastolic volume index, EF = ejection fraction, ESVI = end-systolic volume index, LGE = late gadolinium enhancement, LV = left ventricle, MACCE = major adverse cardiac and cerebrovascular event, MI = mass index, PA = pulmonary artery, RV = right ventricle, TR = tricuspid regurgitation, TV = tricuspid valve
Table 3. ICCs for Inter-Observer Agreement on RV Functional Parameters Derived from Cardiac MRI.
| Variables | ICC | P |
|---|---|---|
| RVEF | 0.986 (0.974–0.949) | < 0.001 |
| RV EDVI | 0.835 (0.682–0.918) | < 0.001 |
| RV ESVI | 0.973 (0.945–0.987) | < 0.001 |
| Diastolic RVMI | 0.919 (0.838–0.961) | < 0.001 |
| Systolic RVMI | 0.915 (0.829–0.959) | < 0.001 |
Data in parentheses are 95% confidence intervals. EDVI = end-diastolic volume index, EF = ejection fraction, ESVI = end-systolic volume index, ICC = interclass correlation coefficient, MI = mass index, RV = right ventricle
Outcome Analysis
Table 4 lists the univariable predictors of MACCEs and all-cause mortality. Among the clinical parameters, old age and dialysis status were associated with MACCEs and all-cause mortality. Among the echocardiography parameters, the TR jet area revealed an association with MACCEs and all-cause mortality, whereas LV EDV and LV ESV were associated with all-cause mortality. Among the cardiac MRI parameters, diastolic and systolic RVMI were associated with MACCEs and all-cause mortality. RVEF, RV EDVI, RV ESVI, LV?EDVI, LV ESVI, and diastolic and systolic LVMI showed associations with all-cause mortality, but LV LGE as a % of LV mass was the only left-sided parameter associated with MACCEs.
Table 4. Univariable Predictors of MACCE and All-Cause Mortality.
| Variables | MACCE | All-Cause Mortality | |||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||||
| Clinical parameters | |||||||
| Age, year | 1.03 (1.00–1.05) | 0.07 | 1.04 (1.00–1.08) | 0.09 | |||
| Male sex | 1.00 (0.51–1.98) | 0.99 | 1.94 (0.76–4.92) | 0.17 | |||
| Comorbidity (absence vs. presence except for NYHA classification) | |||||||
| DM | 1.20 (0.53–2.74) | 0.67 | 1.28 (0.36–4.55) | 0.70 | |||
| Hypertension | 1.10 (0.52–2.33) | 0.80 | 1.84 (0.64–5.30) | 0.26 | |||
| Hyperlipidemia | 0.50 (0.18–1.40) | 0.19 | 0.32 (0.04–2.44) | 0.27 | |||
| Dialysis | 7.48 (1.67–33.39) | 0.01 | 32.33 (5.28–197.92) | < 0.001 | |||
| Congestive hepatopathy | 3.05 (0.72–12.87) | 0.13 | 3.81 (0.50–29.19) | 0.20 | |||
| Atrial fibrillation | 1.41 (0.73–2.70) | 0.30 | 1.26 (0.48–3.26) | 0.64 | |||
| NYHA classification | |||||||
| I | Baseline | - | Baseline | ||||
| II | 0.60 (0.26–1.37) | 0.22 | 0.87 (0.26–2.90) | 0.82 | |||
| III | 1.15 (0.49–2.70) | 0.74 | 0.88 (0.24–3.28) | 0.85 | |||
| IV | 2.12 (0.46–9.89) | 0.34 | 2.61 (0.29–23.95) | 0.40 | |||
| Echocardiography | |||||||
| Echo TR jet area, cm2 | 1.05 (1.02–1.08) | 0.003 | 1.06 (1.01–1.10) | 0.01 | |||
| Peak TR velocity, m/s | 1.00 (0.63–1.58) | 1.00 | 1.15 (0.62–2.11) | 0.66 | |||
| LVEF, % | 0.99 (0.96–1.03) | 0.61 | 0.97 (0.92–1.01) | 0.16 | |||
| LV EDVI, mL/m2 | 1.00 (0.99–1.01) | 0.62 | 1.01 (1.00–1.02) | 0.04 | |||
| LV ESVI, mL/m2 | 1.00 (0.99–1.02) | 0.90 | 1.02 (1.01–1.04) | 0.01 | |||
| Cardiac MRI | |||||||
| TV annulus maximum diameter, mm | 1.00 (0.97–1.04) | 0.83 | 1.02 (0.98–1.06) | 0.38 | |||
| TV annulus minimum diameter, mm | 1.01 (0.98–1.05) | 0.57 | 1.00 (0.94–1.06) | 0.95 | |||
| Right-side parameters | |||||||
| RVEF, % | 0.99 (0.96–1.02) | 0.33 | 0.96 (0.92–1.01) | 0.09 | |||
| RV EDVI, mL/m2 | 1.002 (1.00–1.01) | 0.37 | 1.01 (1.00–1.01) | 0.05 | |||
| RV ESVI, mL/m2 | 1.004 (1.00–1.01) | 0.27 | 1.01 (1.00–1.02) | 0.03 | |||
| Diastolic RVMI, g/m2 | 1.04 (1.00–1.08) | 0.04 | 1.12 (1.06–1.18) | < 0.001 | |||
| Systolic RVMI, g/m2 | 1.06 (1.02–1.11) | 0.009 | 1.12 (1.06–1.18) | < 0.001 | |||
| Left-side parameters | |||||||
| LVEF, % | 0.99 (0.96–1.02) | 0.35 | 0.97 (0.93–1.02) | 0.25 | |||
| LV EDVI, mL/m2 | 1.00 (0.99–1.01) | 0.91 | 1.01 (1.00–1.02) | 0.07 | |||
| LV ESVI, mL/m2 | 1.005 (0.99–1.02) | 0.39 | 1.02 (1.00–1.04) | 0.04 | |||
| Diastolic LVMI, g/m2 | 1.009 (0.99–1.02) | 0.24 | 1.03 (1.01–1.05) | 0.001 | |||
| Systolic LVMI, g/m2 | 1.01 (1.00–1.03) | 0.17 | 1.04 (1.02–1.06) | 0.001 | |||
| LV LGE, % | 1.03 (1.00–1.07) | 0.03 | 0.99 (0.94–1.05) | 0.76 | |||
Data in parentheses are 95% CIs. HR values are for 1 unit value for each variable. CI = confidence interval, DM = diabetes mellitus, EDVI = end-diastolic volume index, EF = ejection fraction, ESVI = end-systolic volume index, HR = hazard ratio, LGE = late gadolinium enhancement, LV = left ventricle, MACCE = major adverse cardiac and cerebrovascular event, MI = mass index, NYHA = New York Heart Association, RV = right ventricle, TR = tricuspid regurgitation, TV = tricuspid valve
Cox proportional hazard analyses adjusted for age, sex, TR jet area, and dialysis were performed for three cardiac MRI-measured parameters: systolic RVMI, RV ESVI, and RVEF (Table 5). A systolic RVMI of 5 g/m2 was associated with a 34% higher risk of MACCEs and a 75% higher risk of all-cause mortality (p = 0.009 and p < 0.001, respectively). A 10 mL/m2 higher RV ESVI was associated with 10% higher all-cause mortality (p = 0.03), whereas a 5% higher RVEF was associated with an 18% lower all-cause mortality (p = 0.09). The association between systolic RVMI and MACCEs did not change when adjusted for age, sex, and TR jet area, likewise the association with all-cause mortality until adjustment for dialysis. However, RV ESVI and RVEF revealed no association when adjusted for age and sex.
Table 5. Adjusted Cox Proportional Hazards Analysis of MACCE and All-Cause Mortality in Relation to Cardiac MRI-Derived RVMI, RV ESVI, and RVEF.
| Parameter | MACCE | All-Cause Mortality | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Systolic RVMI, per increase of 5 g/m2 | |||||||
| Univariable | 1.34 | 1.08–1.65 | 0.009 | 1.75 | 1.34–2.29 | < 0.001 | |
| Age and sex adjusted | 1.44 | 1.13–1.86 | 0.004 | 1.87 | 1.33–2.62 | < 0.001 | |
| Age, sex, and TR jet area adjusted | 1.30 | 0.97–1.75 | 0.09 | 1.96 | 1.30–2.94 | < 0.001 | |
| Age, sex, TR jet area, and dialysis adjusted | 1.28 | 0.94–1.73 | 0.11 | 2.01 | 1.28–3.16 | 0.002 | |
| RV ESVI, per increase of 10 mL/m2 | |||||||
| Univariable | 1.04 | 0.97–1.12 | 0.27 | 1.10 | 1.01–1.20 | 0.03 | |
| Age and sex adjusted | 1.06 | 0.98–1.15 | 0.15 | 1.09 | 0.98–1.21 | 0.10 | |
| Age, sex, and TR jet area adjusted | 0.95 | 0.84–1.07 | 0.41 | 1.15 | 0.90–1.23 | 0.56 | |
| Age, sex, TR jet area, and dialysis adjusted | 0.95 | 0.84–1.07 | 0.40 | 1.03 | 0.88–1.22 | 0.69 | |
| RVEF, per increase of 5% | |||||||
| Univariable | 0.93 | 0.79–1.08 | 0.33 | 0.82 | 0.66–1.03 | 0.09 | |
| Age and sex adjusted | 0.89 | 0.75–1.07 | 0.22 | 0.86 | 0.67–1.11 | 0.26 | |
| Age, sex, and TR jet area adjusted | 0.94 | 0.79–1.11 | 0.46 | 0.89 | 0.69–1.16 | 0.40 | |
| Age, sex, TR jet area, and dialysis adjusted | 0.94 | 0.79–1.12 | 0.47 | 0.92 | 0.71–1.20 | 0.54 | |
CI = confidence interval, EF = ejection fraction, ESVI = end-systolic volume index, HR = hazard ratio, MACCE = major adverse cardiac and cerebrovascular event, MI = mass index, RV = right ventricle, TR = tricuspid regurgitation
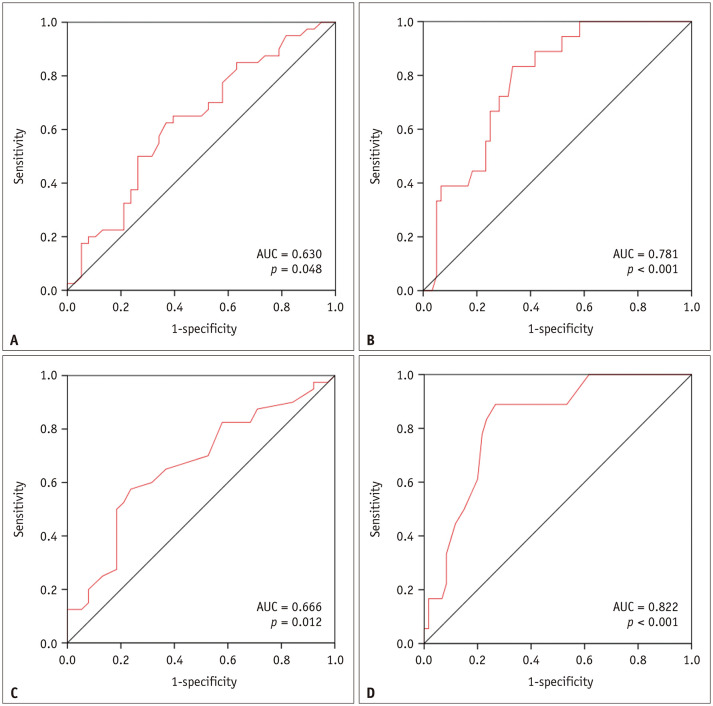
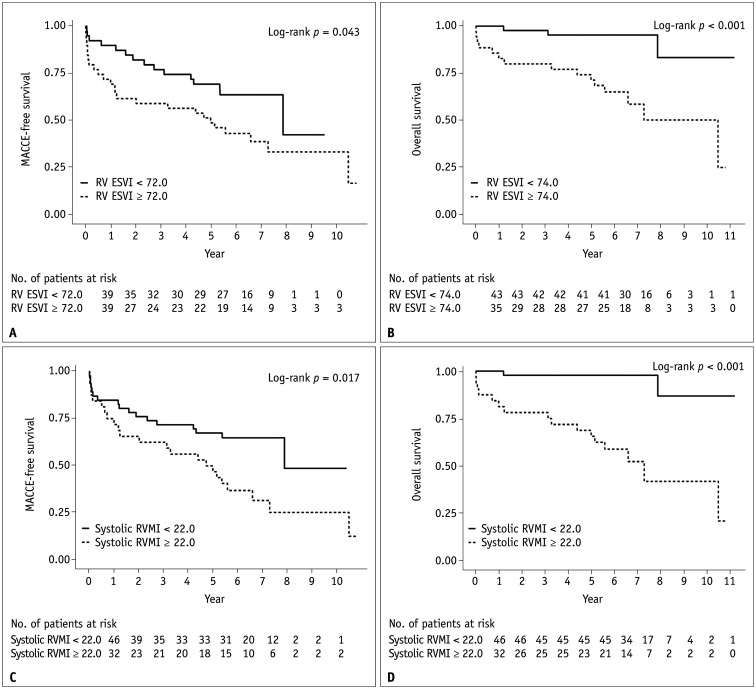
On ROC curve analysis, the AUC, cut-off value, sensitivity, and specificity of RV ESVI were 0.630, 72.0 mL/m2, 62.5%, and 63.2%, respectively, for predicting MACCEs and 0.781, 74.0 mL/m2, 83.3%, and 66.7%, respectively, for predicting all-cause mortality (Fig. 3). The corresponding values for systolic RVMI were 0.666, 22.0 g/m2, 57.5%, and 76.3%, respectively, for predicting MACCEs and 0.822, 22.0 g/m2, 88.9%, and 73.3%, respectively, for predicting all-cause mortality. For RVEF, the AUCs were < 0.5 for predicting MACCEs and all-cause mortality (0.437 and 0.364, respectively). Stratifying the patients by the cut-off values determined by the ROC analysis, we conducted a Kaplan-Meier curve analysis to determine MACCE-free survival and overall survival (Fig. 4). The curves showed statistically significant differences between the two groups when stratified by RV ESVI cut-off values of 72.0 mL/m2 for the prediction of MACCEs and 74.0 mL/m2 for the prediction of all-cause mortality (log-rank, p = 0.043 and p < 0.001, respectively); the same was observed when stratified by systolic RVMI cut-offs of 22.0 g/m2 for MACCE and all-cause mortality predictions (log-rank, p = 0.017 and p < 0.001, respectively).
Fig. 3. ROC curves for the cardiac MRI-derived right ventricular end-systolic volume index for predicting (A) MACCEs and (B) all-cause mortality, and ROC curves for the cardiac MRI-derived systolic right ventricular mass index for predicting (C) MACCE and (D) all-cause mortality.
AUC = area under the ROC curve, MACCE = major adverse cardiac and cerebrovascular event, ROC = receiver operating characteristic
Fig. 4. Kaplan-Meier curves for MACCE-free survival and overall survival (i.e., without all causes of mortality) stratified by the cardiac MRI-derived (A, B) RV ESVI and (C, D) systolic RVMI.
ESVI = end-systolic volume index, MACCE = major adverse cardiac and cerebrovascular event, MI = mass index, RV = right ventricle
DISCUSSION
This retrospective observational study found that in patients who underwent TV surgery for severe functional TR, cardiac MRI-measured RV ESVI and systolic RVMI were significantly higher in those with than in those without MACCEs or all-cause mortality. Systolic RVMI was also an independent predictor of MACCEs and all-cause mortality, whereas RV ESVI was a predictor of all-cause mortality but not MACCEs.
The key finding of our study is that systolic RVMI is an independent prognostic factor, and this differs from the outcome of a previous study in which RVEF was found to be an independent prognostic factor [12]. We believe that this finding was due to the heterogeneity of our study population. In contrast to a previous study conducted on an isolated functional TR group [12], more than half of our patients had moderate to severe mitral stenosis or regurgitation. TR is common in patients with mitral valve disease, with more than one-third of patients with mitral stenosis having moderate to severe TR [1]. TR consequentially causes pulmonary hypertension by increasing left atrial pressure [20]; the mean pulmonary arterial systolic pressure was 36.0 mm Hg in the patients in our study, suggesting a mild degree of pulmonary hypertension. Although pulmonary hypertension was mild in our patients, we consider that increased RVMI, instead of RVEF or ESVI, could be an early reflection of the adverse effect of pulmonary hypertension in the right ventricle. Additionally, only two patients (2.6%) had an initial NYHA classification of IV, and the overall LV function in our patients was normal. Moreover, because more than half of our patients underwent TV surgery because of concomitant left heart disease, the patients had a chance for correction before RV decompensation. This may be the reason why the RVEF or RV ESVI parameters suggesting RV dilatation and dysfunction did not show a difference between the patients with and without MACCEs.
Current guidelines recommend the surgical treatment of severe functional TR for patients who undergo left-sided heart surgery or those with symptomatic severe primary TR [21]. Therefore, RV function is an important factor for determining the timing of intervention and predicting outcomes in TR. In the case of surgery for isolated TR, increased RV EDVI and decreased RV function (measured as the ratio of the sum of the isovolumic relaxation time and isovolumic contraction time divided by ejection time) on echocardiography were independent predictors of clinical outcomes [22,23]. Regarding cardiac MRI, a study of isolated severe functional TR patients showed that RVEF and RV ESVI measured on preoperative cardiac MRI were independent predictive factors for cardiac death and major postoperative cardiac events after TV surgery [12]. However, the role of RVMI was not evaluated in the present study. As TR is persistent, chronic volume overload of the RV causes progressive RV remodeling and dysfunction, and the consequent papillary muscle displacement and tethering worsen TR in a vicious cycle [20,24]. RVMI is associated with a normal course of adaptation to an increased RV afterload in pulmonary hypertension, as revealed in a previous cardiac MRI study that showed a correlation between RV mass and pulmonary artery systolic pressure [25,26]. Proportional increases in RV mass, which are related to RV dilatation, prevent the ventricular wall from the effects of high wall tension, and they represent progressive adaptation [27]. In this context, a high RVMI may be indicative of chronic RV remodeling, and this parameter is an independent predictor of MACCEs and all-cause mortality. Furthermore, pulmonary hypertension is associated with adverse outcomes in patients with TR and after surgical or interventional treatments for TR [28,29]; the prognostic implications of RVMI are similar to those of pulmonary hypertension. Therefore, the additional prognostic information derived from cardiac MRI may facilitate appropriate decisions on the operative timing during the preoperative stage.
Our study has several limitations. First, because this was a retrospective study performed at a single center, selection bias should be considered. We only included patients who underwent preoperative MRI, and patients with poor general conditions who could not undergo MRI may have been excluded. Second, patients with concomitant valve diseases in the aortic or mitral valves were not excluded, and the presence of concurrent valve disease was evaluated as a factor that could affect outcomes after TV surgery. TR patients frequently have left-sided valvular heart disease, and we wished to evaluate such patients after finding that concurrent left-sided valve disease was not associated with TV surgery outcomes. However, in a recent study, the outcomes were related to the type of surgery (repair vs. replacement) rather than concomitancy, and the rates of TV repair in the groups with and without MACCEs were not significantly different [30]. Therefore, we consider that this heterogeneity in our study population did not have sufficient impact to skew the results. Third, echocardiographic parameters for RV function are considered unreliable [31]; however, TR severity is a known prognostic factor for worse long-term outcomes in patients without surgery [6,32]. In our study, the TR jet area was significantly larger in patients with than in those without MACCEs, and this was a significant factor for predicting MACCEs and all-cause mortality in the univariable Cox analysis. Therefore, we adjusted for the TR jet area and other clinical parameters (including age, sex, dialysis, and congestive hepatopathy) for the Cox analysis. Other echocardiographic parameters related to RV function (i.e., tricuspid annular plane systolic excursion and RV tissue Doppler [S′]) were not available for most patients; therefore, we could not evaluate and adjust for them. Finally, although we evaluated preoperative MRI parameters and found that RVMI was associated with an adverse outcome after TV surgery, we could not suggest the optimal surgical timing for severe functional TR. Further studies are warranted to determine the optimal surgical timing and predict outcomes.
In conclusion, systolic RVMI measured by cardiac MRI was independently associated with long-term outcomes in patients who underwent TV surgery for functional TR. We believe that this parameter could help in determining the appropriate timing of surgical correction of severe TR in patients with or without asymptomatic or mild to moderate left heart valve disease.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Joon-Won Kang, Hyun Jung Koo.
- Data curation: Yura Ahn, Hyun Jung Koo, Won Jin Choi.
- Formal analysis: Yura Ahn, Hyun Jung Koo, Won Jin Choi.
- Investigation: Yura Ahn, Hyun Jung Koo, Won Jin Choi.
- Methodology: Yura Ahn, Hyun Jung Koo, Won Jin Choi.
- Supervision: Joon-Won Kang.
- Validation: Joon-Won Kang.
- Visualization: Yura Ahn, Hyun Jung Koo, Won Jin Choi.
- Writing—original draft: Yura Ahn, Hyun Jung Koo.
- Writing—review & editing: Joon-Won Kang, Won Jin Choi, Dae-Hee Kim, Jong-Min Song, Duk-Hyun Kang, Jae-Kwan Song, Joon Bum Kim, Sung-Ho Jung, Suk Jung Choo, Cheol Hyun Chung, Jae Won Lee, Dong Hyun Yang.
Supplement
The Supplement is available with this article at https://doi.org/10.3348/kjr.2020.1084.
MACCE
Operation-Related Results and Outcomes
References
- 1.Shiran A, Sagie A. Tricuspid regurgitation in mitral valve disease incidence, prognostic implications, mechanism, and management. J Am Coll Cardiol. 2009;53:401–408. doi: 10.1016/j.jacc.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 2.Koelling TM, Aaronson KD, Cody RJ, Bach DS, Armstrong WF. Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. Am Heart J. 2002;144:524–529. doi: 10.1067/mhj.2002.123575. [DOI] [PubMed] [Google Scholar]
- 3.Cohen SR, Sell JE, McIntosh CL, Clark RE. Tricuspid regurgitation in patients with acquired, chronic, pure mitral regurgitation. I. Prevalence, diagnosis, and comparison of preoperative clinical and hemodynamic features in patients with and without tricuspid regurgitation. J Thorac Cardiovasc Surg. 1987;94:481–487. [PubMed] [Google Scholar]
- 4.Braunwald NS, Ross J, Jr, Morrow AG. Conservative management of tricuspid regurgitation in patients undergoing mitral valve replacement. Circulation. 1967;35:I63–I69. doi: 10.1161/01.cir.35.4s1.i-63. [DOI] [PubMed] [Google Scholar]
- 5.Sade RM, Castaneda AR. The dispensable right ventricle. Surgery. 1975;77:624–631. [PubMed] [Google Scholar]
- 6.Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43:405–409. doi: 10.1016/j.jacc.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 7.Nickenig G, Kowalski M, Hausleiter J, Braun D, Schofer J, Yzeiraj E, et al. Transcatheter treatment of severe tricuspid regurgitation with the edge-to-edge MitraClip technique. Circulation. 2017;135:1802–1814. doi: 10.1161/CIRCULATIONAHA.116.024848. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 9.Kim HK, Kim YJ, Park EA, Bae JS, Lee W, Kim KH, et al. Assessment of haemodynamic effects of surgical correction for severe functional tricuspid regurgitation: cardiac magnetic resonance imaging study. Eur Heart J. 2010;31:1520–1528. doi: 10.1093/eurheartj/ehq063. [DOI] [PubMed] [Google Scholar]
- 10.Hahn RT, Thomas JD, Khalique OK, Cavalcante JL, Praz F, Zoghbi WA. Imaging assessment of tricuspid regurgitation severity. JACC Cardiovasc Imaging. 2019;12:469–490. doi: 10.1016/j.jcmg.2018.07.033. [DOI] [PubMed] [Google Scholar]
- 11.Delhaye D, Remy-Jardin M, Teisseire A, Hossein-Foucher C, Leroy S, Duhamel A, et al. MDCT of right ventricular function: comparison of right ventricular ejection fraction estimation and equilibrium radionuclide ventriculography, part 1. AJR Am J Roentgenol. 2006;187:1597–1604. doi: 10.2214/AJR.05.1193. [DOI] [PubMed] [Google Scholar]
- 12.Park JB, Kim HK, Jung JH, Klem I, Yoon YE, Lee SP, et al. Prognostic value of cardiac MR imaging for preoperative assessment of patients with severe functional tricuspid regurgitation. Radiology. 2016;280:723–734. doi: 10.1148/radiol.2016151556. [DOI] [PubMed] [Google Scholar]
- 13.Dreyfus GD, Martin RP, Chan KM, Dulguerov F, Alexandrescu C. Functional tricuspid regurgitation: a need to revise our understanding. J Am Coll Cardiol. 2015;65:2331–2336. doi: 10.1016/j.jacc.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance imaging (CMR) protocols, society for cardiovascular magnetic resonance: board of trustees task force on standardized protocols. J Cardiovasc Magn Reson. 2008;10:35. doi: 10.1186/1532-429X-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catalano O, Antonaci S, Opasich C, Moro G, Mussida M, Perotti M, et al. Intra-observer and interobserver reproducibility of right ventricle volumes, function and mass by cardiac magnetic resonance. J Cardiovasc Med (Hagerstown) 2007;8:807–814. doi: 10.2459/JCM.0b013e32801105ef. [DOI] [PubMed] [Google Scholar]
- 18.Unal I. Defining an optimal cut-point value in ROC analysis: an alternative approach. Comput Math Methods Med. 2017;2017:3762651. doi: 10.1155/2017/3762651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badano LP, Muraru D, Enriquez-Sarano M. Assessment of functional tricuspid regurgitation. Eur Heart J. 2013;34:1875–1885. doi: 10.1093/eurheartj/ehs474. [DOI] [PubMed] [Google Scholar]
- 21.Alqahtani F, Berzingi CO, Aljohani S, Hijazi M, Al-Hallak A, Alkhouli M. Contemporary trends in the use and outcomes of surgical treatment of tricuspid regurgitation. J Am Heart Assoc. 2017;6:e007597. doi: 10.1161/JAHA.117.007597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JB, Jung SH, Choo SJ, Chung CH, Lee JW. Clinical and echocardiographic outcomes after surgery for severe isolated tricuspid regurgitation. J Thorac Cardiovasc Surg. 2013;146:278–284. doi: 10.1016/j.jtcvs.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Topilsky Y, Khanna AD, Oh JK, Nishimura RA, Enriquez-Sarano M, Jeon YB, et al. Preoperative factors associated with adverse outcome after tricuspid valve replacement. Circulation. 2011;123:1929–1939. doi: 10.1161/CIRCULATIONAHA.110.991018. [DOI] [PubMed] [Google Scholar]
- 24.Fukuda S, Song JM, Gillinov AM, McCarthy PM, Daimon M, Kongsaerepong V, et al. Tricuspid valve tethering predicts residual tricuspid regurgitation after tricuspid annuloplasty. Circulation. 2005;111:975–979. doi: 10.1161/01.CIR.0000156449.49998.51. [DOI] [PubMed] [Google Scholar]
- 25.van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;28:1250–1257. doi: 10.1093/eurheartj/ehl477. [DOI] [PubMed] [Google Scholar]
- 26.Saba TS, Foster J, Cockburn M, Cowan M, Peacock AJ. Ventricular mass index using magnetic resonance imaging accurately estimates pulmonary artery pressure. Eur Respir J. 2002;20:1519–1524. doi: 10.1183/09031936.02.00014602. [DOI] [PubMed] [Google Scholar]
- 27.Badagliacca R, Poscia R, Pezzuto B, Nocioni M, Mezzapesa M, Francone M, et al. Right ventricular remodeling in idiopathic pulmonary arterial hypertension: adaptive versus maladaptive morphology. J Heart Lung Transplant. 2015;34:395–403. doi: 10.1016/j.healun.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Lurz P, Orban M, Besler C, Braun D, Schlotter F, Noack T, et al. Clinical characteristics, diagnosis, and risk stratification of pulmonary hypertension in severe tricuspid regurgitation and implications for transcatheter tricuspid valve repair. Eur Heart J. 2020;41:2785–2795. doi: 10.1093/eurheartj/ehaa138. [DOI] [PubMed] [Google Scholar]
- 29.Di Mauro M, Foschi M, Tancredi F, Guarracini S, Di Marco M, Habib AM, et al. Additive and independent prognostic role of abnormal right ventricle and pulmonary hypertension in mitral-tricuspid surgery. Int J Cardiol. 2018;252:39–43. doi: 10.1016/j.ijcard.2017.11.031. [DOI] [PubMed] [Google Scholar]
- 30.Wong WK, Chen SW, Chou AH, Lee HA, Cheng YT, Tsai FC, et al. Late outcomes of valve repair versus replacement in isolated and concomitant tricuspid valve surgery: a nationwide cohort study. J Am Heart Assoc. 2020;9:e015637. doi: 10.1161/JAHA.119.015637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bleeker GB, Steendijk P, Holman ER, Yu CM, Breithardt OA, Kaandorp TA, et al. Assessing right ventricular function: the role of echocardiography and complementary technologies. Heart. 2006;92 Suppl 1:i19–i26. doi: 10.1136/hrt.2005.082503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JW, Song JM, Park JP, Lee JW, Kang DH, Song JK. Long-term prognosis of isolated significant tricuspid regurgitation. Circ J. 2010;74:375–380. doi: 10.1253/circj.cj-09-0679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MACCE
Operation-Related Results and Outcomes