Abstract
Sulfuretin (SFR), which is isolated from Rhus verniciflua, Toxicodendron vernicifluum, Dahlia, Bidens tripartite and Dipterx lacunifera, is one of the most important natural flavonoids. This compound is known to have numerous biological activities; among these, the antioxidant activity has not been thoroughly studied yet. In this study, the hydroperoxyl scavenging activity of SFR was examined by using density functional theory calculations. SFR is predicted to be an excellent HOO• scavenger in water at pH = 7.40 with koverall = 4.75 × 107 M−1 s−1, principally due to an increase in the activity of the anionic form following the single-electron transfer mechanism. Consistently, the activity of the neutral form is more prominent in the non-polar environment with koverall = 1.79 × 104 M−1 s−1 following the formal hydrogen transfer mechanism. Thus, it is predicted that SFR exhibits better HOO• antiradical activity than typical antioxidants such as resveratrol, ascorbic acid or Trolox in the lipid medium. The hydroperoxyl radical scavenging of SFR in the aqueous solution is approximately 530 times faster than that of Trolox and similar to ascorbic acid or resveratrol. This suggests that SFR is a promising radical scavenger in physiological environments.
Keywords: sulfuretin, DFT study, antioxidants, antiradical activity, flavonoids
1. Introduction
Sulfuretin (SFR, figure 1) is a natural flavonoid present in numerous plant species, including Rhus verniciflua [1,2], Toxicodendron vernicifluum [3], Dahlia, Bidens tripartite and Dipterx lacunifera [4]. This compound is known to have numerous biological activities such as amelioration of rheumatoid arthritis symptoms [5], antimutagenic [6], antiplatelet [7], anti-cancer [8,9], anti-inflammatory effects [5,10], liver protection [11], anti-ageing effect for skin [12], anti-obesity effect [12] and antioxidant activity [2,13–15].
Figure 1.
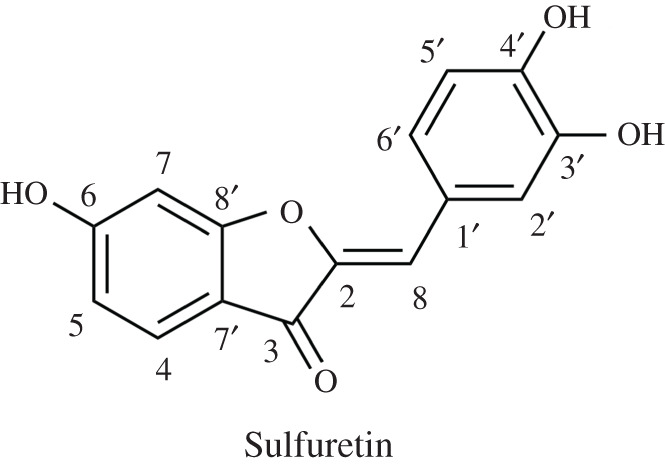
Molecular structure and atomic numbering of SFR.
Jung and co-workers [2] reported that SFR presented strong antioxidant activity in the DPPH (2,2-diphenyl-1-picrylhydrazyl) assay and total anti-ROS (reactive oxygen species) activity with IC50 = 8.52 and 0.73 µM, respectively. The DPPH inhibition of SFR was about two times higher than that of L-ascorbic acid, whereas the total ROS inhibition is about five times stronger than Trolox. SFR also presented significant activity against ONOO− and HO• radicals [2]. Chen et al. [14] also reported that SFR has good DPPH, ABTS•+ (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) and HO• radical scavenging activity that is higher than butylated hydroxytoluene (BHT).
Although the antioxidant activity of SFR is broadly examined experimentally [2,14], there are no studies on the mechanism and kinetics of its antiradical activity, particularly in physiological environments. Computer calculations offer a convenient way to predict the antioxidant activity of organic compounds in physiological media [16–23]. In this context and as a continuation of our previous studies [18,24,25], we set out in this work to evaluate the HOO• antiradical activity of SFR by a combination of thermodynamic and kinetic calculations. This study also considered the effects of solvents on the antioxidant properties of SFR in comparison with some typical antioxidants.
2. Computational details
All calculations were carried out with Gaussian 09 suite of programs [26]. M06–2X/6–311 + +G(d,p) model chemistry was used for all calculations [27–29]. It was demonstrated before that the M06–2X functional is one of the most reliable methods to study thermodynamics and kinetics of radical reactions, particularly in physiological environments [19,28,30,31]. The solvation model density (SMD) method was used for including the effects of water and pentyl ethanoate in the computations [17,18,24,32–34]. The kinetic calculations were performed following the quantum mechanics-based test for the overall free radical scavenging activity (QM-ORSA) protocol [17,34], using the conventional transition state theory (TST) and 1 M standard state at 298.15 K [18,34–40]. The details of the method are shown in the electronic supplementary material, table S1.
3. Results and discussion
3.1. The HOO• antiradical activity of SFR in the gas phase
3.1.1. Thermodynamic evaluation
For SFR that contains OH and moieties, the antioxidant activity may follow either of the four main mechanisms: the formal hydrogen transfer (FHT), the sequential proton loss electron transfer (SPLET), the single-electron transfer proton transfer (SETPT) and radical adduct formation (RAF) [41,42]. The first three pathways are defined by the following thermodynamic parameters: bond dissociation enthalpy (BDE), proton affinity (PA) and ionization energy (IE), respectively. The Gibbs free energy change of the addition reaction is calculated directly for the RAF mechanism. Thus, the BDE, IE and PA values of SFR were first calculated in the gas phase, and the results are shown in table 1.
Table 1.
The calculated thermodynamic parameters (BDEs, PAs and IEs) of SFR in the gas phase.
| positions | BDE | PA | IE |
|---|---|---|---|
| O6−H | 90.7 | 323.4 | 174.6 |
| O3′−H | 80.5 | 327.9 | |
| O4′−H | 77.5 | 320.9 |
As per table 1, the lowest BDE value was predicted for O4′−H at 77.5 kcal mol−1. This value is lower than that of natural antioxidants such as viniferifuran (82.7 kcal mol−1) [43], resveratrol (83.9 kcal mol−1) [43], puerarin (87.3 kcal mol−1) [44] and vanillic acid (85.2 kcal mol−1) [45]. The lowest PA and IE values are about 4.14 and 2.25 times higher than the BDE value. Thus, based on the computed data, the antioxidant activity of SFR is predicted to favour the FHT pathway, at least in apolar and low-dielectric environments.
To confirm that FHT is indeed the preferred pathway of the HOO• antiradical activity of SFR, the Gibbs free energy of the SFR + HOO• reaction was calculated according to each of the four mechanisms: FHT, single-electron transfer (SET, the first step of the SETPT mechanism), sequential proton (SP, the first step of the SPLET) and RAF (table 2). It was found that the HOO• antiradical activity of SFR is only clearly spontaneous for FHT at O3′(O4′)−H bonds and RAF at the C8 position (ΔGo < 0), whereas the RAF reaction at C2 with ΔGo = 1.1 kcal mol−1 cannot be clearly excluded based on thermodynamics alone and therefore it was also included in the kinetic study. The other reactions are clearly not spontaneous with high positive ΔGo values. The ΔGo values for the reactions following the SP and SET pathways are much higher than those of the FHT mechanism. Thus, the calculated data suggest that the HOO• antiradical activity of SFR may follow either FHT or RAF mechanism (at O3′(4′)−H and C2/C8 positions, respectively), and these pathways should be investigated in the kinetic study.
Table 2.
Calculated ΔGo (kcal/mol) of the SFR + HOO• reactions according to the FHT, SP, RAF and SET mechanisms in the gas phase.
| positions | FHT | SP | SET | RAF |
|---|---|---|---|---|
| O6−H | 4.8 | 170.8 | 152.1 | — |
| O3′−H | −4.9 | 176.1 | — | |
| O4′−H | −7.7 | 169.2 | — | |
| C2 | — | — | 1.1 | |
| C8 | — | — | −4.6 |
3.1.2. Kinetic study
Based on the above results, the kinetics of the SFR + HOO• reaction in the gas phase was investigated for the thermodynamically favourable positions and mechanisms according to the QM-ORSA protocol [17], and the data are presented in table 3 and figure 2.
Table 3.
Calculated ΔH (kcal/mol), activation Gibbs free energies (ΔG≠, kcal/mol), tunnelling corrections (κ), kEck (M−1 s−1) and branching ratios (Γ, %) for the HOO• + SFR reaction in the gas phase.
| mechanism | positions | ΔH | ΔG≠ | κ | kEck | Γ |
|---|---|---|---|---|---|---|
| FHT | O3′−H | 2.3 | 11.6 | 39.6 | 8.43 × 105 | 23.0 |
| O4′−H | 2.0 | 11.2 | 72.1 | 2.83 × 106 | 77.0 | |
| RAF | C2 | 7.1 | 17.1 | 1.5 | 2.83 | 0.0 |
| C8 | 8.6 | 17.7 | 1.5 | 9.03 × 10−1 | 0.0 | |
| koverall | 3.67 × 106 |
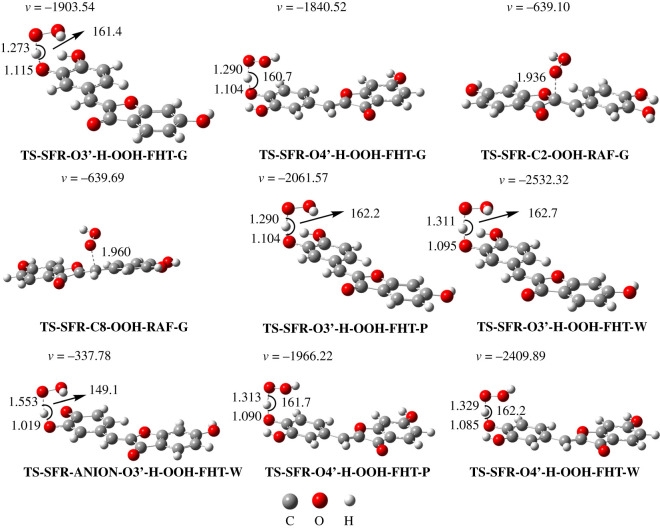
Figure 2.
The optimized transition state (TS) structures following the FHT and RAF mechanisms of the SFR + HOO• reaction (G: gas phase; W: water; P: pentyl ethanoate).
It is apparent that the HOO• antiradical activity of SFR occurs mostly by the H-abstraction of the O4′−H bond (ΔG≠ = 11.2 kcal/mol; kEck = 2.83 × 106 M−1 s−1; Γ = 77.0%). That is more than three times higher contribution than the hydrogen abstraction of the O3′−H bond (ΔG≠ = 11.6 kcal mol−1; kEck = 8.43 × 105 M−1 s−1; Γ = 23.0%). By contrast, the addition of the radical does not make any contribution (Γ = 0%) at either the C2 or C8 positions. This result is in good agreement with previous studies in phenolic compounds [46–48]. We can conclude that the HOO• antiradical activity of SFR is dominated by the FHT mechanism at the O3′(4′)–H bond; therefore, this is further analysed in physiological environments.
3.2. The HOO• antiradical activity of SFR in physiological environments
3.2.1. Acid–base equilibrium
Previous studies showed that the deprotonation of the OH bonds plays a key role in the HOO• antiradical activity of phenolic compounds in the aqueous solution [30,34,49]. The spontaneous dissociation of acidic moieties practically eliminates the activation energy barrier of the first step of the SPLET mechanism, simplifying it to direct electron transfer, and for this reason, this pathway can become energetically favoured in aqueous solution for the dissociated species. Thus, in this study, the deprotonation of SFR must also be considered. The PA values (table 1) showed that the site most likely to dissociate is the O4′−H bond. Thus, this bond was used to calculate the pKa of SFR. The pKa was computed following the literature [49,50], and the results are shown in figure 3. The calculated pKa value was 7.47. Thus, under physiologically relevant conditions (pH = 7.40), SFR has both neutral (HA, 54.0%) and anionic (A−, 46.0%) forms. Therefore, in the physiological environments, these states were used for the kinetic investigation.
Figure 3.
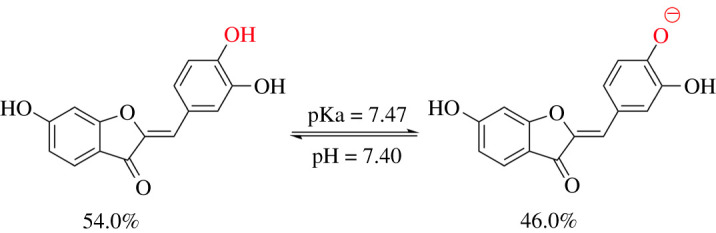
The acid dissociation equilibrium of SFR.
3.2.2. Kinetic study
Based on the results of the kinetic calculations in the gas phase, the HOO• antiradical activity in non-polar environments was modelled by the hydrogen transfer mechanism at the O3′(O4′)−H bonds. In the aqueous environment, the SET mechanism was also investigated for the deprotonated state of SFR. The overall rate constants (koverall) were computed following the QM-ORSA protocol [17,33], (table 4) according to equations (3.1) and (3.2).
Table 4.
Calculated ΔG≠ (kcal mol−1), tunnelling corrections (κ), the nuclear reorganization energy (λ, kcal mol−1) rate constants (kapp, kf, and koverall M−1 s−1), molar fractions (f) and branching ratios (Γ, %) at 298.15 K, in the SFR + HOO• reaction in pentyl ethanoate and water solvents.
| mechanism | pentyl ethanoate |
water |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔG≠ | κ | kapp | Γ | ΔG≠ | κ | kapp | f | kf** | Γ | ||
| SET | 6.6 | 15.6* | 8.90 × 107 | 0.460 | 4.09 × 107 | 86.2 | |||||
| HAT | O3′−H | 15.0 | 106.9 | 6.90 × 103 | 38.5 | 16.0 | 744.5 | 9.20 × 103 | 0.540 | 4.97 × 103 | 0.0 |
| O4′−H | 14.9 | 163.1 | 1.10 × 104 | 61.5 | 15.5 | 202.8 | 5.30 × 103 | 0.540 | 2.86 × 103 | 0.0 | |
| O3′−H (anion) | 7.8 | 1.2 | 1.42 × 107 | 0.460 | 6.53 × 106 | 13.7 | |||||
| koverall | 1.79 × 104 | 4.75 × 107 | |||||||||
*λ; **kf = f.kapp; Γ = k.100/koverall.
In the lipid medium
| 3.1 |
In water at pH = 7.40
| 3.2 |
As shown in table 4, the HOO• antiradical activity of SFR in the polar solvent is excellent with the koverall = 4.75 × 107 M−1 s−1. Similarly, in the lipid medium, SFR exhibits good activity with koverall = 1.79 × 104 M−1 s−1. It was found that the SET of anion A− plays a principal role (kf = 4.09 × 107 M−1 s−1, Γ = 86.2%) in the radical scavenging activity of SFR. The H-abstraction of the anion state contributes about 13.7% to the overall rate constants. The rate constants for the H-abstraction of O3′(O4′)−H bonds against HOO• radical are kf = 4.97 × 103 and 2.86 × 103 M−1 s−1, respectively; however, these reactions do not make any contributions (approx. 0%) to the activity of SFR. Based on the results, SFR is better HOO• radical scavenger than typical antioxidants Trolox, ascorbic acid and resveratrol in lipid phase (reference lipid phase activities: koverall = 3.40 × 103 M−1 s−1 [33], koverall = 5.71 × 103 M−1 s−1 [17] and koverall = 1.31 × 104 M−1 s−1 [46], respectively). In aqueous solution, the HOO• antiradical activity of SFR is approximately 530 times faster than that of Trolox (k = 8.96 × 104 M−1 s−1) [33] and fairly similar to other well-known natural antioxidants, i.e. ascorbic acid (k = 9.97 × 107 M−1 s−1) [17] and resveratrol (k = 5.62 × 107 M−1 s−1) [46]. Thus, the results suggest that SFR is a promising antioxidant in physiological media.
4. Conclusion
The hydroperoxyl radical scavenging activity of SFR was investigated using DFT calculations. The results showed that SFR has excellent HOO• antiradical activity with koverall = 4.75 × 107 M−1 s−1 in water at pH = 7.40 by the SET pathway of the anion state, and good/moderate HOO• scavenging activity in lipid environment (koverall = 1.79 × 104 M−1 s−1) by the FHT mechanism via the O3′(O4′)–H bonds. The hydroperoxyl antiradical activity of SFR is better than Trolox, ascorbic acid and resveratrol in the lipid medium. This activity of SFR is approximately 530 times faster than that of Trolox and relatively similar to ascorbic acid and resveratrol in the polar environment. Thus, SFR can be a useful natural antioxidant in physiological environments.
Supplementary Material
Data accessibility
All relevant necessary data to reproduce all results in the paper are within the main text, electronic supplementary material and the Dryad Digital Repository: https://doi.org/10.5061/dryad.t4b8gtj1z [51].
The data are provided in the electronic supplementary material [52].
Authors' contributions
N.T.H., D.T.N.H., D.P.H. and H.V.T. carried out the molecular laboratory work, participated in data analysis, carried out sequence alignments, participated in the design of the study and drafted the manuscript; L.P.H. carried out the statistical analyses and collected field data; A.M. and Q.V.V. conceived of the study, designed the study, coordinated the study and helped draft the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests
Funding
This research is funded by the Vietnamese Ministry of Education and Training under project number B2021-DNA-16 (Q.V.V.).
References
- 1.Park HJ, Kwon SH, Kim GT, Lee KT, Choi JH, Choi JW, Park KY. 2000. Physicochemical and biological characteristics of flavonoids isolated from the heartwoods of Rhus verniciflua. Kor. J. Pharrnacogn. 31, 345-350. [Google Scholar]
- 2.Jung MJ, Chung HY, Kang SS, Choi JH, Bae KS, Choi JS. 2003. Antioxidant activity from the stem bark of Albizzia julibrissin. Arch. Pharm. Res. 26, 458-462. ( 10.1007/BF02976862) [DOI] [PubMed] [Google Scholar]
- 3.Kim JH, Shin YC, Ko SG. 2014. Integrating traditional medicine into modern inflammatory diseases care: multitargeting by Rhus verniciflua Stokes. Mediators Inflamm. 2014, 1-17. ( 10.1155/2014/154561) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orhan N, İçöz ÜG, Altun L, Aslan M. 2016. Anti-hyperglycaemic and antioxidant effects of Bidens tripartita and quantitative analysis on its active principles. Iran. J. Basic Med. Sci. 19, 1114-1124. [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YR, Hwang JK, Koh HW, Jang KY, Lee JH, Park JW, Park BH. 2012. Sulfuretin, a major flavonoid isolated from Rhus verniciflua, ameliorates experimental arthritis in mice. Life Sci. 90, 799-807. ( 10.1016/j.lfs.2012.04.015) [DOI] [PubMed] [Google Scholar]
- 6.Park KY, Jung GO, Lee KT, Choi J, Choi MY, Kim GT, Jung HJ, Park HJ. 2004. Antimutagenic activity of flavonoids from the heartwood of Rhus verniciflua. J. Ethnopharmacol. 90, 73-79. ( 10.1016/j.jep.2003.09.043) [DOI] [PubMed] [Google Scholar]
- 7.Jeon WK, et al. 2006. Anti-platelet effects of bioactive compounds isolated from the bark of Rhus verniciflua Stokes. J. Ethnopharmacol. 106, 62-69. ( 10.1016/j.jep.2005.12.015) [DOI] [PubMed] [Google Scholar]
- 8.Kim JM, et al. 2013. Suppression of TPA-induced tumor cell invasion by sulfuretin via inhibition of NF-κB-dependent MMP-9 expression. Oncol. Rep. 29, 1231-1237. ( 10.3892/or.2012.2218) [DOI] [PubMed] [Google Scholar]
- 9.Antal DS, et al. 2016. Effects of cyclodextrin complexation on the anti-cancer effects of Cotinus coggygria extract and its constituents, butein and sulfuretin. Rev. de Chim. 67, 1618-1622. [Google Scholar]
- 10.Lee DS, Jeong GS, Li B, Park H, Kim Y-C. 2010. Anti-inflammatory effects of sulfuretin from Rhus verniciflua Stokes via the induction of heme oxygenase-1 expression in murine macrophages. Int. Immunopharmacol. 10, 850-858. ( 10.1016/j.intimp.2010.04.019) [DOI] [PubMed] [Google Scholar]
- 11.Lu YT, Xiao YF, Li YF, Li J, Nan FJ, Li JY. 2019. Sulfuretin protects hepatic cells through regulation of ROS levels and autophagic flux. Acta Pharmacol. Sin. 40, 908-918. ( 10.1038/s41401-018-0193-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S, et al. 2019. Sulfuretin prevents obesity and metabolic diseases in diet induced obese mice. Biomol. Ther. 27, 107-116. ( 10.4062/biomolther.2018.090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JC, Lim KT, Jang YS. 2002. Identification of Rhus verniciflua Stokes compounds that exhibit free radical scavenging and anti-apoptotic properties. Biochim. Biophys. Acta Gen. Subj. 1570, 181-191. ( 10.1016/S0304-4165(02)00196-4) [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Wang C, Zhou H, Tao R, Ye J, Li W. 2017. Antioxidant capacity and identification of the constituents of ethyl acetate fraction from Rhus verniciflua Stokes by HPLC-MS. Nat. Prod. Res. 31, 1573-1577. ( 10.1080/14786419.2016.1277353) [DOI] [PubMed] [Google Scholar]
- 15.Kim DH, Kim MJ, Kim DW, Kim GY, Kim JK, Gebru YA, Choi HS, Kim YH, Kim MK. 2019. Changes of phytochemical components (urushiols, polyphenols, gallotannins) and antioxidant capacity during Fomitella fraxinea–mediated fermentation of Toxicodendron vernicifluum bark. Molecules 24, 683. ( 10.3390/molecules24040683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galano A, Raúl Alvarez-Idaboy J. 2019. Computational strategies for predicting free radical scavengers' protection against oxidative stress: where are we and what might follow? Int. J. Quantum Chem. 119, e25665. ( 10.1002/qua.25665) [DOI] [Google Scholar]
- 17.Galano A, Alvarez-Idaboy JR. 2013. A computational methodology for accurate predictions of rate constants in solution: application to the assessment of primary antioxidant activity. J. Comput. Chem. 34, 2430-2445. ( 10.1002/jcc.23409) [DOI] [PubMed] [Google Scholar]
- 18.Vo QV, Bay MV, Nam PC, Quang DT, Flavel M, Hoa NT, Mechler A. 2020. Theoretical and experimental studies of the antioxidant and antinitrosant activity of syringic acid. J. Org. Chem. 85, 15 514-15 520. ( 10.1021/acs.joc.0c02258) [DOI] [PubMed] [Google Scholar]
- 19.Carreon-Gonzalez M, Vivier-Bunge A, Alvarez-Idaboy JR. 2019. Thiophenols, promising scavengers of peroxyl radicals: mechanisms and kinetics. J. Comput. Chem. 40, 2103-2110. ( 10.1002/jcc.25862) [DOI] [PubMed] [Google Scholar]
- 20.Leopoldini M, Russo N, Toscano M. 2011. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 125, 288-306. ( 10.1016/j.foodchem.2010.08.012) [DOI] [Google Scholar]
- 21.Galano A, Mazzone G, Alvarez-Diduk R, Marino T, Alvarez-Idaboy JR, Russo N. 2016. Food antioxidants: chemical insights at the molecular level. Annu. Rev. Food Sci. Technol. 7, 335-352. ( 10.1146/annurev-food-041715-033206) [DOI] [PubMed] [Google Scholar]
- 22.Ghosh D, Acharya A, Tiwari SC, Krylov AI. 2012. Toward understanding the redox properties of model chromophores from the green fluorescent protein family: an interplay between conjugation, resonance stabilization, and solvent effects. J. Phys. Chem. B 116, 12 398-12 405. ( 10.1021/jp305022t) [DOI] [PubMed] [Google Scholar]
- 23.Solntsev KM, Ghosh D, Amador A, Josowicz M, Krylov AI. 2011. What drives the redox properties of model green fluorescence protein chromophores? J. Phys. Chem. Lett. 2, 2593-2597. ( 10.1021/jz2011397) [DOI] [Google Scholar]
- 24.Vo QV, Bay MV, Nam PC, Mechler A. 2019. Is indolinonic hydroxylamine a promising artificial antioxidant? J. Phys. Chem. B 123, 7777-7784. ( 10.1021/acs.jpcb.9b05160) [DOI] [PubMed] [Google Scholar]
- 25.Vo QV, Nam PC, Bay MV, Thong NM, Cuong ND, Mechler A. 2018. Density functional theory study of the role of benzylic hydrogen atoms in the antioxidant properties of lignans. Sci. Rep. 8, 12361. ( 10.1038/s41598-018-30860-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frisch MJ, et al. 2009. Gaussian 09. Wallingford, CT: Gaussian, Inc. [Google Scholar]
- 27.Zhao Y, Schultz NE, Truhlar DG. 2006. Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J. Chem. Theory Comput. 2, 364-382. ( 10.1021/ct0502763) [DOI] [PubMed] [Google Scholar]
- 28.Zhao Y, Truhlar DG. 2008. How well can new-generation density functionals describe the energetics of bond-dissociation reactions producing radicals? J. Phys. Chem. A 112, 1095-1099. ( 10.1021/jp7109127) [DOI] [PubMed] [Google Scholar]
- 29.Zhao Y, Truhlar DG. 2008. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215-241. ( 10.1007/s00214-007-0310-x) [DOI] [Google Scholar]
- 30.Galano A, Alvarez-Idaboy JR. 2014. Kinetics of radical-molecule reactions in aqueous solution: a benchmark study of the performance of density functional methods. J. Comput. Chem. 35, 2019-2026. ( 10.1002/jcc.23715) [DOI] [PubMed] [Google Scholar]
- 31.Boulebd H, Khodja IA, Bay MV, Hoa NT, Mechler A, Vo QV. 2020. Thermodynamic and kinetic studies of the radical scavenging behavior of hydralazine and dihydralazine: theoretical insights. J. Phys. Chem. B 124, 4123-4131. ( 10.1021/acs.jpcb.0c02439) [DOI] [PubMed] [Google Scholar]
- 32.Alvarez-Idaboy JRl, Galano A. 2012. On the Chemical repair of DNA radicals by glutathione: hydrogen vs electron transfer. J. Phys. Chem. B 116, 9316-9325. ( 10.1021/jp303116n) [DOI] [PubMed] [Google Scholar]
- 33.Alberto ME, Russo N, Grand A, Galano A. 2013. A physicochemical examination of the free radical scavenging activity of Trolox: mechanism, kinetics and influence of the environment. Phys. Chem. Chem. Phys. 15, 4642-4650. ( 10.1039/c3cp43319f) [DOI] [PubMed] [Google Scholar]
- 34.Dzib E, Cabellos JL, Ortíz-Chi F, Pan S, Galano A, Merino G. 2019. Eyringpy: a program for computing rate constants in the gas phase and in solution. Int. J. Quantum Chem. 119, e25686. ( 10.1002/qua.25686) [DOI] [Google Scholar]
- 35.Evans MG, Polanyi M. 1935. Some applications of the transition state method to the calculation of reaction velocities, especially in solution. Trans. Faraday Soc. 31, 875-894. ( 10.1039/tf9353100875) [DOI] [Google Scholar]
- 36.Eyring H. 1935. The activated complex in chemical reactions. J. Chem. Phys. 3, 107-115. ( 10.1063/1.1749604) [DOI] [Google Scholar]
- 37.Truhlar DG, Hase WL, Hynes JT. 1983. Current status of transition-state theory. J. Phys. Chem. 87, 2664-2682. ( 10.1021/j100238a003) [DOI] [Google Scholar]
- 38.Furuncuoglu T, Ugur I, Degirmenci I, Aviyente V. 2010. Role of chain transfer agents in free radical polymerization kinetics. Macromolecules 43, 1823-1835. ( 10.1021/ma902803p) [DOI] [Google Scholar]
- 39.Vélez E, Quijano J, Notario R, Pabón E, Murillo J, Leal J, Zapata E, Alarcón G. 2009. A computational study of stereospecifity in the thermal elimination reaction of menthyl benzoate in the gas phase. J. Phys. Org. Chem. 22, 971-977. ( 10.1002/poc.1547) [DOI] [Google Scholar]
- 40.Dzib E, Cabellos JL, Ortiz-Chi F, Pan S, Galano A, Merino G. 2018. Eyringpy 1.0.2. Cinvestav, Mérida, Yucatán.
- 41.Galano A. 2015. Free radicals induced oxidative stress at a molecular level: the current status, challenges and perspectives of computational chemistry based protocols. J. Mex. Chem. Soc. 59, 231-262. ( 10.29356/jmcs.v59i4.81) [DOI] [Google Scholar]
- 42.Zheng YZ, Deng G, Liang Q, Chen DF, Guo R, Lai RC. 2017. Ror2 signaling regulates Golgi structure and transport through IFT20 for tumor invasiveness. Sci. Rep. 7, 1-11. ( 10.1038/s41598-016-0028-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shang Y, Zhou H, Li X, Zhou J, Chen K. 2019. Theoretical studies on the antioxidant activity of viniferifuran. New J. Chem. 43, 15 736-15 742. ( 10.1039/C9NJ02735A) [DOI] [Google Scholar]
- 44.Zhou H, Li X, Shang Y, Chen K. 2019. Radical scavenging activity of puerarin: a theoretical study. Antioxidants 8, 590. ( 10.3390/antiox8120590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen J, Yang J, Ma L, Li J, Shahzad N, Kim CK. 2020. Re-epithelialization and immune cell behaviour in an ex vivo human skin model. Sci. Rep. 10, 1-9. ( 10.1038/s41598-019-56847-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iuga C, Alvarez-Idaboy JRl, Russo N. 2012. Antioxidant activity of trans-resveratrol toward hydroxyl and hydroperoxyl radicals: a quantum chemical and computational kinetics study. J. Org. Chem. 77, 3868-3877. ( 10.1021/jo3002134) [DOI] [PubMed] [Google Scholar]
- 47.Cordova-Gomez M, Galano A, Alvarez-Idaboy JR. 2013. Piceatannol, a better peroxyl radical scavenger than resveratrol. RSC Adv. 3, 20 209-20 218. ( 10.1039/c3ra42923g) [DOI] [Google Scholar]
- 48.Vo QV, Nam PC, Van Bay M, Thong NM, Mechler A. 2019. A theoretical study of the radical scavenging activity of natural stilbenes. RSC Adv. 9, 42 020-42 028. ( 10.1039/C9RA08381B) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vo QV, Hoa NT, Nam PC, Quang DT, Mechler A. 2020. In silico evaluation of the radical scavenging mechanism of mactanamide. ACS Omega 5, 24 106-24 110. ( 10.1021/acsomega.0c03646) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galano A, et al. 2016. Empirically fitted parameters for calculating pKa values with small deviations from experiments using a simple computational strategy. J. Chem. Inf. Model. 56, 1714-1724. ( 10.1021/acs.jcim.6b00310) [DOI] [PubMed] [Google Scholar]
- 51.Hoa NT, Hang DTN, Hieu DP, Van Truong H, Hoang LP, Mechler A, Vo QV. 2021. Data from: The hydroperoxyl radical scavenging activity of sulfuretin: insights from theory. Dryad Digital Repository. ( 10.5061/dryad.t4b8gtj1z) [DOI] [PMC free article] [PubMed]
- 52.Hoa NT, Hang DTN, Hieu DP, Van Truong H, Hoang LP, Mechler A, Vo QV. 2021. The hydroperoxyl radical scavenging activity of sulfuretin: insights from theory. FigShare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Hoa NT, Hang DTN, Hieu DP, Van Truong H, Hoang LP, Mechler A, Vo QV. 2021. Data from: The hydroperoxyl radical scavenging activity of sulfuretin: insights from theory. Dryad Digital Repository. ( 10.5061/dryad.t4b8gtj1z) [DOI] [PMC free article] [PubMed]
- Hoa NT, Hang DTN, Hieu DP, Van Truong H, Hoang LP, Mechler A, Vo QV. 2021. The hydroperoxyl radical scavenging activity of sulfuretin: insights from theory. FigShare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
All relevant necessary data to reproduce all results in the paper are within the main text, electronic supplementary material and the Dryad Digital Repository: https://doi.org/10.5061/dryad.t4b8gtj1z [51].
The data are provided in the electronic supplementary material [52].