Abstract
Exposed endoscopic full-thickness resection (EFTR) without laparoscopic assistance is a minimally invasive natural orifice transluminal endoscopic surgery technique that is emerging as a promising effective and safe alternative to surgery for the treatment of muscularis propria-originating gastric submucosal tumors. To date, various techniques have been used for the closure of the transmural post-EFTR defect, mainly consisting in clip- and endoloop-assisted closure methods. However, the recent advent of dedicated tools capable of providing full-thickness defect suture could further improve the efficacy and safety of the exposed EFTR procedure. The aim of our review was to evaluate the efficacy and safety of the different closure methods adopted in gastric-exposed EFTR without laparoscopic assistance, also considering the recent advent of flexible endoscopic suturing.
Keywords: Endoscopic full-thickness resection, Exposed endoscopic full-thickness resection, Full-thickness resection, Natural orifice transluminal endoscopic surgery, Endoscopic surgery, Endoscopic suturing
Core Tip: Exposed endoscopic full-thickness resection (EFTR) without laparoscopic assistance is a natural orifice transluminal endoscopic surgery technique that is emerging as a promising alternative to surgery for the treatment of muscularis propria-originating gastric submucosal tumors. To date, transmural post-EFTR defect closure has been achieved mainly by means of hemostatic devices, such as clips only or clips combined with endoloops. However, the recent advent of dedicated tools capable of providing full-thickness defect suture could further improve the efficacy and safety of the exposed EFTR procedure. Our review aimed to evaluate the efficacy and safety of the different closure techniques adopted in gastric-exposed EFTR without laparoscopic assistance, also considering the recent advent of flexible endoscopic suturing.
INTRODUCTION
Exposed endoscopic full-thickness resection (EFTR), previously reported as pure free-hands or standard EFTR, is a scarless natural orifice transluminal endoscopic surgery (NOTES) technique that is emerging as a promising minimally invasive alternative to surgery for the treatment of muscularis propria-originating gastric submucosal tumors (G-SMTs)[1,2].
In 2006, Ikeda and colleagues first illustrated EFTR by the use of the endoscopic submucosal dissection (ESD) technique on a porcine stomach[3]. Subsequently, the technique was translated into clinical practice by Zhou et al[4], who reported successful resection of 26 G-SMTs.
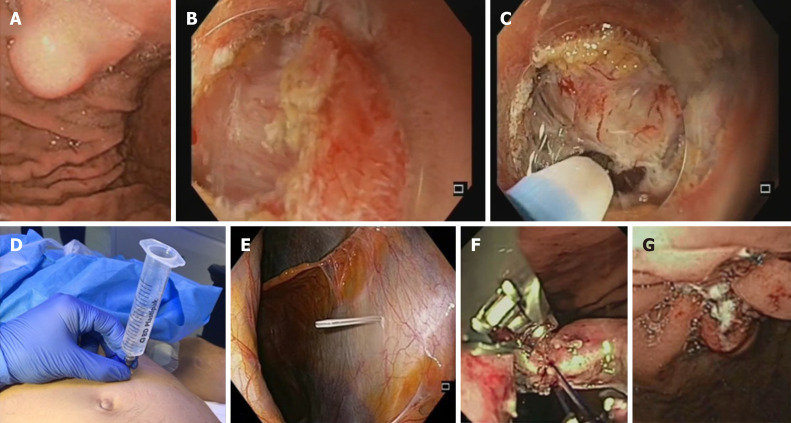
Main steps of the exposed EFTR procedure are described as follows[4]: A: submucosal injection followed by precutting the mucosal and submucosal layer around the tumor with standard ESD technique; B: full-thickness resection of the tumor, including the serosal layer, with creation of an intentional perforation; and C: transmural wall defect closure by the use of clips or other suturing techniques. The exposed EFTR technique is illustrated in Figure 1.
Figure 1.
Technical illustration of the exposed endoscopic full-thickness resection technique with defect closure by means of endoscopic suturing system. A: Endoscopic view of gastric submucosal lesion; B: Precutting and removal of the mucosal and submucosal layer after submucosal injection, in order to expose the tumor; C: Exposed endoscopic full-thickness resection of the tumor and creation of “active perforation”; D and E: Capnoperitoneum management using percutaneously inserted angiocatheter; F: Transmural defect closure with the OverStitch endoscopic suturing system; G: Final apposition of the tissue margins.
The term “exposed” is thus derived from the temporary exposure of the peritoneal cavity to the gastrointestinal (GI) lumen[5]. Indeed, this “cut then close” technique provides the intentional creation of an active perforation to achieve a complete endoscopic resection, followed by wall patency restoration. Effective full-thickness defect closure is a key step of the exposed EFTR procedure in order to prevent delayed perforation, peritonitis, abdominal infection, and the need for surgical intervention. However, though a full-thickness defect closure is currently strongly advised when performing exposed EFTR[5], to date post-EFTR defect closure has been achieved mainly by the use of hemostatic tools, such as trough-the-scope (TTS) clips or clips combined with endoloops.
The aim of our study was to review the current evidence concerning the various closure techniques adopted in gastric exposed EFTR without laparoscopic assistance, also taking into account the recent advent of flexible endoscopic suturing.
LITERATURE SEARCH
A comprehensive literature search of the PubMed (MEDLINE) and EMBASE electronic databases for the period January 1998 (the year EFTR was first described)-November 2020 was carried out in order to identify relevant studies reporting on gastric exposed EFTR without laparoscopic assistance. The medical literature was searched using the terms "endoscopic full-thickness resection”, “EFTR”, and “exposed endoscopic full-thickness resection”. The search strategy was limited to human studies and articles written in English. Meeting abstracts and studies in which the results of each adopted closure technique could not be extrapolated were excluded. In the event of studies from same institute and suspicion of cohort overlapping, only the study which included the highest number of patients over the longest enrollment period was considered for inclusion.
CLOSURE TECHNIQUES FOR GASTRIC POST-EFTR DEFECT
Clip closure method
Zhou et al[4] first reported successful post-EFTR defect closure with the use of several standard TTS metallic clips in 26 cases. Clip closure of the gastric wall defect with a ‘‘side to center’’ method was used when the size of the defect was smaller than the width of the open clip, while the so called “suction-clip-suture’’ technique was adopted in cases of defect diameter larger than the width of the open clip. Finally, larger defects were managed by means of the omental-patch method, providing suction of the greater omentum into the stomach and its clipping with the gastric mucosa, as previously reported[6]. Gastric defects resulting from the resection of lesions up to 4.5 cm in diameter (mean 2.8 cm) were sealed by the above mentioned techniques. The Zhongshan group reported no cases of delayed perforation, peritonitis or abdominal abscess occurring after EFTR[4]. In line with these results, in 2014, the efficacy and safety of the clip closure method was reported by Huang et al[7] in a cohort of 35 patients. Subsequently, successful clip-only closure technique was reported in 48 cases of gastric EFTR. Of note, the authors reported the application of a mean of 8.14 ± 4.08 (range 3-20) TTS titanium clips. Furthermore, after full-thickness resection of lesions larger than 3 cm a mean number of 12.0 ± 5.5 (range 6-18) clips was needed for the wound closure[8]. Since then, feasibility and efficacy of the clip-closure method adoption for the post-EFTR defect have been widely reported[9-11].
Conversely, Dong et al[12] described the occurrence of peritonitis and abdominal abscess in one out of 10 patients undergoing gastric exposed EFTR, probably due to premature clip falling-off. Furthermore, with regard to major adverse events, one case of delayed bleeding and one case of abdominal infection were reported across the included study. The first required laparoscopic suturing due to evidence of hemorrhage on the serosal surface of the surgical site[13], while the latter resolved after antibiotic treatment[14].
Intriguingly, the use of a foreign body forceps delivered through a dual-channel endoscope was proposed in a small case series, in order to facilitate and reduce the time spent in post-EFTR defect closure[15].
Results of the included studies in which clip-closure method was performed are summarized in Table 1.
Table 1.
Summary of studies reporting post-endoscopic full-thickness resection defect closure by the use of endoclips
| Ref. | Study design | Lesions, n | Mean tumor size (range), cm | Site (cardia/antrum/ body/fundus) | R0 | Surgical conversion | Suture technique | Suture technical success | Mean operation time (range), min | Mean suture time (range), min | Major adverse events |
| Zhou et al[4], 2011 | R | 26 | 2.8 ± 1.3 (1.2-4.5) | 0/0/14/12 | 26 | 0 | Clips +/- omental- patch | 26 | 105 (60-145) | - | 0 |
| Huang et al[7], 2014 | R | 35 | 2.8 (2.0-4.5) | 0/0/21/14 | 35 | 0 | Clips +/- omental- patch | 35 | 90 (60-155) | - | 0 |
| Dong et al[12], 2014 | R | 10 | 1.65 ± 0.59 (0.80-2.50) | 1/1/1/7 | 10 | 0 | Clips | 10 | 120 (60-180) | - | Peritonitis and abdominal abscess (n = 1) |
| Feng et al[8], 2014 | R | 48 | 1.59 ± 1.01 (0.50-4.80) | 0/1/7/40 | 48 | 0 | Clips | 48 | 59.7 (30-270) | - | 0 |
| Wu et al[9], 2015 | R | 50 | 3.40 ± 0.83 (2.50-5.00) | 0/13/23/14 | 50 | 0 | Clips +/- omental- patch | 50 | 85 (55-155) | - | 0 |
| Tang et al[15], 2016 | R | 6 | - | 0/1/2/3 | - | 0 | CFCM | 6 | - | 14.83 ± 1.94 (-) | 0 |
| Lu et al[10], 2016 | R | 62 | 2.23 ± 1.80 (0.60-6.00) | 0/0/29/33 | 61 | 0 | Clips | 62 | NA (n = 30): 85 (40-180); TWC (n = 21): 45 (25-90); LA (n = 11): 40 (30-75) | - | 0 |
| Tan et al[13], 2017 | R | 32 | 1.54 ± 0.66 (-) | 0/0/7/25 | - | 1 | Clips | 62 | 69.1 ± 27.0 (-) | 6.3 ± 1.6 (-) | Delayed bleeding (n = 1) |
| Abe et al[11], 2018 | R | 14 | - | - | 14 | 3 | Clips | 11 | - | - | 0 |
| Zhao et al[14], 2019 | R | 85 | 1.60 ± 0.88 (-) | 6/4/20/55 | 81 | 0 | Clips | 85 | - | - | Abdominal infection (n = 1) |
R: Retrospective; CFCM: Clips assisted with foreign body forceps clip method.
Despite the reported good efficacy in post-EFTR defect closure, TTS clips were originally designed for achieving hemostasis, thus being technically unable to create tissue approximation with full-thickness closure. Indeed, endoclips realize a mucosal and submucosal apposition only, whereas muscularis propriae and serosa apposition is not achievable due to the superficial bite of the clips[16]. In addition, as reported in one of the included studies[12], clips may prematurely drop off the gastric mucosa due to both peristalsis and the radial force of the large post-EFTR defect, resulting in delayed perforation and severe complications. Finally, clip closure method appears to be strongly operator-dependent[4].
Endoloop-assisted closure method
In 2013, Shi et al[17] developed a new endoloop and metallic clip interrupted-suture method for repair of large gastric post-EFTR defects. Through the use of a two-channel endoscope, an endoloop was anchored with two clips to the opposite sides of the defect margins and tightened in order to approximate the defect borders. Thus, defect closure was achieved by the application of more endoloops with the same technique. If necessary, additional clips were placed to obtain complete wound closure. Successful gastric defect closure by this method was retrospectively reported in all 20 patients who underwent EFTR for G-SMT with a mean size of 1.47 ± 0.72 cm (range 0.4–3 cm). No severe complications, such as delayed perforation or bleeding, were reported. A median suture time of 10 min (range 8-20 min) was reported[17].
Ye et al[18] reported the efficacy and safety of a different endoloop-assisted closure technique in 51 cases of gastric post-EFTR defects. By means of this method, a standard clip closure of the defect was realized and then reinforced by endoloop ligature of all clips together.
In 2014, Zhang et al[19] retrospectively evaluated a new closure method for large post-EFTR defects, called endoscopic purse-string suture. Through a double-channel endoscope, an endoloop was anchored onto the circumferential margin of the gastric wall defect using several clips. Thus, final defect repair was achieved by tightening the endoloop. The use of additional clip in case of not accurately placed clip or not tight purse-string suture was reported by the authors. Among 29 gastric cases, this closure method was technically feasible in all cases and no severe complications were reported. The feasibility and safety of the above-mentioned closure method was subsequently also reported across two retrospective studies[15,20]. In addition, the application of the EPSS method using a novel endoloop (LeClamp Loop-20 and Loop-30; Leo, Changzhou, China) by means of a single-channel endoscope was subsequently illustrated[21].
In a pilot prospective study enrolling 13 cases of gastric EFTR, a novel and simplified endoscopic grasp-and-loop closure method using an endoloop assisted with grasping forceps was evaluated. By the use of a dual-channel upper endoscope, defect margins were grasped by the use of an alligator grasping forceps passed through an open endoloop. The grasper was thus retracted and the base of the created pseudo pedunculated lesion was secured by means of the endoloop. This “lift-and-closure” technique was effective in all cases. However, median tumor size was 1.5 cm only (range 0.5-3.5 cm)[22].
In 2018, Wu and colleagues developed a new closure method called prepurse-string suture (p-EPSS), using a single-channel gastroscope. An endoloop was anchored onto different sides of the normal mucosa proximal to the resection edge with several clips. Another endoloop was anchored onto the lesion, and the gastric extra-luminal lesion was turned endoluminal by pulling the endoloop. The defect was finally sutured by immediately tightening the endoloop following resection, in order to reduce the time of peritoneal exposure to gastric content. Feasibility and safety of the p-EPSS method was reported in all 25 cases[23].
Table 2 summarizes the results of the included studies reporting on endoloop-assisted closure method in gastric EFTR.
Table 2.
Summary of studies reporting post-endoscopic full-thickness resection defect closure by the use of endoclips combined with endoloops
| Ref. | Study design | Lesions, n | Mean tumor size (range), cm | Site (cardia/antrum/ body/fundus) | R0 | Surgical conversion | Suture technique | Suture technical success | Mean operation time (range), min | Mean suture time (range), min | Major adverse events |
| Shi et al[17], 2013 | R | 20 | 1.47 ± 0.87 (0.40-3.00) | 0/1/7/12 | 20 | 0 | EMCIS | 20 | - | 10 (8-20) | 0 |
| Ye et al[18], 2014 | R | 51 | 2.40 ± 0.73 (1.30-3.50) | 0/1/22/28 | 50 | 1 (resection failure) | Clips + endoloop ligature | 50 | 52 (30-125) | - | 0 |
| Zhang et al[19], 2014 | R | 29 | 1.9 ± 1.1 (0.3–4.2) | 0/0/2/27 | 29 | 0 | EPSS | 29 | 55.7 ± 15.4 (35–95) | - | 0 |
| Tang et al[15], 2016 | R | 12 | - | 0/1/4/7 | - | 0 | EPSS | 12 | - | 22.42 ± 5.73 | 0 |
| Shi et al[21], 2017 | R | 68 | 2.60 ± 0.50 (2.00-3.50) | 0/0/0/68 | 68 | 0 | EPSS | 68 | 41 (23-118) | 13 (9-21) | Delayed bleeding (n = 1) |
| Hu et al[22], 2017 | P | 13 | 1.50 ± 1.00 (0.50-3.50) | 0/0/2/11 | 13 | 0 | GAL | 13 | 43.5 (20-80) | 9.4 (3–18) | 0 |
| Wu et al[23], 2018 | R | 25 | 1.70 ± 1.00 (0.50-4.50) | 0/0/7/18 | 25 | 0 | p-EPSS | 25 | 31 (-) | - | 0 |
| Li et al[20], 2019 | R | 28 | 1.55 ± 0.4 (-) | 0/0/9/19 | - | 0 | EPSS | 28 | - | - | 0 |
R: Retrospective; EMCIS: Endoloop and metallic clip interrupted-suture; EPSS: Endoscopic purse-string suture; GAL: Grasp-and-loop; p-EPSS: Prepurse-string suture.
Endoloops were originally created as hemostatic tools for the prevention of bleeding following resection of pedunculated polyps. Compared with endoclips alone, their adoption in combination with standard clips may allow the management of larger post-EFTR defects and may reinforce the wound closure. However, though being a relatively simple technique, it is unable to provide a full-thickness suture of the gastric wall, creating mucosal and submucosal approximation only[16,24].
Over-the-scope clip closure method
In 2015, Guo et al[25] first retrospectively reported feasibility and safety of post-EFTR wound closure by means of the over-the-scope clip (OTSC) system (Ovesco Endoscopy GmbH, Tuebingen, Germany). In 23/23 patients, complete defect closure was achieved with only one OTSC. The success rate of defect closure was 100%, with an average closure time of 4.9 min only (range 2–12 min). No patients experienced major adverse events. By the use of this closure technique, gastric perforation edges were clamped with twin graspers and then drawn into the transparent cap of the OTSC device for full aspiration. Finally, the OTSC closure system was released in order to achieve full-thickness closure of the defect. Of note, only tumors smaller the 2 cm in diameter (range 0.6-2.0 cm) were included in the study.
In line with these data, technical success and safety of post-EFTR OTSC-closure were reported in a small case series by Wang et al[26] in 2019.
Recently, closure of perforations left after EFTR for fundic G-SMTs with an average size of 2.4 cm was successfully reported. Of interest, closure was performed with the OTSC system plus additional TTS clips in 8 out of 20 of the retrospectively reviewed cases, while OTSC only was adopted in 12 cases[27].
Results of the included studies with regard to OTSC-closure method are summarized in Table 3.
Table 3.
Summary of studies reporting post-endoscopic full-thickness resection defect closure by the use of over-the-scope clips
| Ref. | Study design | Lesions, n | Mean tumor size (range), cm | Site (cardia/antrum/ body/fundus) | R0 | Surgical conversion | Suture technique | Suture technical success | Mean operation time (range), min | Mean suture time (range), min | Major adverse events | |
| Guo et al[25], 2015 | R | 23 | 1.21 ± 0.47 (0.6-2.0) | 0/3/9/11 | 23 | 0 | OTSC | 23 | 40.5 ± 25.8 (16–104) | 4.9 ± 2.2 (2–12) | 0 | |
| Wang et al[26], 2019 | CS | 2 | 1.1 (1-1.2) | 0/0/1/1 | 2 | 0 | OTSC | 2 | 108.5 (48-121) | 43 (16-70) | 0 | |
| Hu et al[27], 2020 | R | 20 | 2.4 ± 0.26 (-) | 0/0/0/20 | 20 | 0 | OTSC +/- clips | 20 | 130.6 ± 51.9 (-) | - | 0 | |
R: Retrospective; OTSC: Over-the-scope clip; CS: Case series.
As opposed to both TTS clips and endoloops, the Ovesco OTSC system was specifically designed to manage gastrointestinal perforations and leaks, and has the significant advantage of realizing a full-thickness closure, incorporating the muscularis propria layer[28,29]. Despite its higher cost (P = 0.001), OTSC closure has been associated with a significantly shorter hospital stay (P = 0.047), compared with standard clips-only closure method[30]. Furthermore, OTSC is relatively quick and simple to use compared with the above-mentioned closure methods. Of note, in cases of OTSC-related complications occurrence or need for re-therapy after incomplete EFTR, safe and effective OTSC removal mainly by means of a dedicated bipolar direct current grasping device (remOVE system, Ovesco, Tuebingen, Germany) has been reported[31]. However, the use of the OTSC closure method is limited mainly to defects smaller then 20-25 mm, due to the relatively small internal diameter of the device[32-34]. Thus, the placement of additional TTS clips for the closure completion is often needed because of the frequently large size of the post-EFTR defect, resulting in a “partial” full-thickness repair only. In addition, OTSC cannot be repositioned once deployed[24].
Endosuturing closure method
Evidence concerning post-EFTR defect closure by means of the OverStitch endoscopic suturing system (ESS) (Apollo Endosurgery, Austin, TX, United States) is still limited, consisting in a few case series and a handful of case reports only. Nevertheless, successful full-thickness closure with the ESS was achieved in all reported cases across the included studies, both as primary[35-39] or rescue closure method[40-42]. No major adverse events were observed.
Results of the included studies reporting the use of endosuturing for the post-EFTR defect are presented in Table 4.
Table 4.
Summary of studies reporting post-endoscopic full-thickness resection defect closure by the use of endoscopic suturing systems
| Ref. | Study design | Lesions, n | Mean tumor size (range), cm | Site (cardia/antrum/ body/fundus) | R0 | Surgical conversion | Suture technique | Suture technical success | Mean operation time (range), min | Mean suture time (range), min | Major adverse events |
| Andalib et al[35], 2018 | CS | 7 | - | - | - | 0 | ESS | 7 | - | - | 0 |
| Xu et al[36], 2018 | CR | 1 | 2.4 | 0/0/0/1 | 1 | 0 | ESS | 1 | - | - | 0 |
| Granata et al[37], 2018 | CR | 1 | - | 0/0/1/0 | - | 0 | ESS (3 running sutures) | 1 | - | - | 0 |
| Dedania et al[38], 2018 | CR | 1 | 1.5 | 0/0/1/0 | . | 0 | ESS (2 running sutures) | 1 | - | - | 0 |
| Inayat et al[40], 2019 | CS | 3 | 2.35 (1.85-3.20) | 2/0/0/1 | - | 0 | Clips omental-patch + ESS | 3 | - | - | 0 |
| Kerdsirichairat et al[41], 2019 | CR | 1 | - | 1/0/0/0 | 1 | 0 | Clips omental-patch + ESS | 1 | - | - | 0 |
| Sachdev et al[42], 2020 | CS | 2 | 3.15 (2.8-3.5) | 2/0/0/0 | - | 0 | Clips omental-patch + ESS | 2 | - | - | 0 |
| Modayil et al[39], 2020 | CR | 1 | 2.5 | 0/0/0/1 | 1 | 0 | ESS (1 running suture) | 1 | - | - | 0 |
CS: Case series; ESS: Endoscopic suturing system; CR: Case report.
Compared with TTS clips or endoloops that were designed for hemostasis, endosuturing device was specifically created for full-thickness tissue approximation. Furthermore, its superiority compared with other counterparts in creating full-thickness closure of transmural defects has been shown[43-46]. Though technically demanding and requiring dedicated training, suturing closure with ESS creates a full-thickness “surgical-quality” suture through all layers of the GI wall by the placement of durable full-thickness sutures that incorporates a muscle layer with a stable reliable construct. Either continuous or interrupted nonabsorbable sutures can be created according to defect size, shape, and location. In addition, defects larger than 20-30 mm in diameter, not amenable by the use of the OTSC closure method, can be successfully repaired with the ESS[45]. Currently, the main limitation of flexible endoscopic suturing is likely represented by its high cost. However, all in all, exposed EFTR with endosuturing closure seems to be less expensive than traditional surgery. The cost effectiveness of post-EFTR defect closure by means of endosuturing needs to be further investigated in light of its potential capability to reduce adverse events, hospitalization, and need for surgery.
CONCLUSION
Exposed EFTR is a “cut then close” NOTES technique providing the intentional creation of an active perforation. Thus, defect closure is a crucial step, with a key role in the final outcome. Conversely, in the non-exposed EFTR procedure the resection of the lesion is performed after the plication of the GI tract wall with the use of dedicated full-thickness suturing devices, principally represented by the full-thickness resection device (FTRD; Ovesco Endoscopy, Tuebingen, Germany), consisting of an OTSC preloaded into a cap with an integrated snare. The advantages of this “close then cut” technique consist mainly in the potential avoidance of both intra-peritoneal dissemination of tumor cells and spillage of gastrointestinal luminal content into the peritoneum. In addition, this approach has greater technical simplicity, with faster operating time. However, compared with exposed EFTR, the FTRD is limited by a lower R0 resection rate, likely due to the impossibility of a “real-time” and direct visualization of the perimetral cutting margins. Also, the clip cannot be reverted once released, and is limited for small-size lesions (< 25 mm)[5,47].
To date, post-EFTR transmural defects closure has been achieved in large part by means of either TTS clips or clips combined with endoloops. However, a reliable full-thickness defect closure is not achievable with the above-mentioned techniques, due to the superficial bite of the clips. Accordingly, concerns regarding effective and reliable defect closure achievement remain unresolved, likely limit the worldwide application of the exposed EFTR procedure, especially within Western countries.
In our opinion, the recent advent of dedicated devices for tissue-approximation, such as the OTSC system and the OverStitch ESS, could help in overcoming these concerns. However, due to the frequently large size defect resulting from exposed EFTR, OTSC use in this scenario is partially limited, whereas flexible endosuturing could represent the natural evolution of the exposed EFTR endosurgical technique, providing secure “surgical-quality” full-thickness closure of defects even larger than 20-30 mm.
Large prospective studies are needed to clarify the role of both OTSC and flexible endoscopic suturing in gastric exposed EFTR.
Footnotes
Conflict-of-interest statement: No conflict of interest to declare.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: European Society of Gastrointestinal Endoscopy; Italian Association of Hospital Gastroenterologists and Digestive Endoscopists; and Italian Society for Digestive Endoscopy.
Peer-review started: February 6, 2021
First decision: March 16, 2021
Article in press: June 16, 2021
Specialty type: Surgery
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dumoulin FL S-Editor: Zhang H L-Editor: A P-Editor: Li JH
Contributor Information
Antonino Granata, Digestive Endoscopy Service, Department of Diagnostic and Therapeutic Services, IRCCS–ISMETT, Palermo 90127, Italy.
Alberto Martino, Department of Gastroenterology and Digestive Endoscopy, AORN “Antonio Cardarelli”, Napoli 80131, Italy. alberto-martino@libero.it.
Dario Ligresti, Digestive Endoscopy Service, Department of Diagnostic and Therapeutic Services, IRCCS–ISMETT, Palermo 90127, Italy.
Francesco Paolo Zito, Department of Gastroenterology and Digestive Endoscopy, AORN “Antonio Cardarelli”, Napoli 80131, Italy.
Michele Amata, Digestive Endoscopy Service, Department of Diagnostic and Therapeutic Services, IRCCS–ISMETT, Palermo 90127, Italy.
Giovanni Lombardi, Department of Gastroenterology and Digestive Endoscopy, AORN “Antonio Cardarelli”, Napoli 80131, Italy.
Mario Traina, Digestive Endoscopy Service, Department of Diagnostic and Therapeutic Services, IRCCS–ISMETT, Palermo 90127, Italy.
References
- 1.Antonino G, Alberto M, Michele A, Dario L, Fabio T, Mario T. Efficacy and safety of gastric exposed endoscopic full-thickness resection without laparoscopic assistance: a systematic review. Endosc Int Open. 2020;8:E1173–E1182. doi: 10.1055/a-1198-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C, Gao Z, Shen K, Cao J, Shen Z, Jiang K, Wang S, Ye Y. Safety and efficiency of endoscopic resection versus laparoscopic resection in gastric gastrointestinal stromal tumours: A systematic review and meta-analysis. Eur J Surg Oncol. 2020;46:667–674. doi: 10.1016/j.ejso.2019.10.030. [DOI] [PubMed] [Google Scholar]
- 3.Ikeda K, Mosse CA, Park PO, Fritscher-Ravens A, Bergström M, Mills T, Tajiri H, Swain CP. Endoscopic full-thickness resection: circumferential cutting method. Gastrointest Endosc. 2006;64:82–89. doi: 10.1016/j.gie.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 4.Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD, Zhong YS, Chen WF, Zhang YQ, Qin WZ, Hu JW, Liu JZ. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc. 2011;25:2926–2931. doi: 10.1007/s00464-011-1644-y. [DOI] [PubMed] [Google Scholar]
- 5.ASGE Technology Committee, Aslanian HR, Sethi A, Bhutani MS, Goodman AJ, Krishnan K, Lichtenstein DR, Melson J, Navaneethan U, Pannala R, Parsi MA, Schulman AR, Sullivan SA, Thosani N, Trikudanathan G, Trindade AJ, Watson RR, Maple JT. ASGE guideline for endoscopic full-thickness resection and submucosal tunnel endoscopic resection. VideoGIE. 2019;4:343–350. doi: 10.1016/j.vgie.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minami S, Gotoda T, Ono H, Oda I, Hamanaka H. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video) Gastrointest Endosc. 2006;63:596–601. doi: 10.1016/j.gie.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Huang LY, Cui J, Lin SJ, Zhang B, Wu CR. Endoscopic full-thickness resection for gastric submucosal tumors arising from the muscularis propria layer. World J Gastroenterol. 2014;20:13981–13986. doi: 10.3748/wjg.v20.i38.13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng Y, Yu L, Yang S, Li X, Ding J, Chen L, Xu Y, Shi R. Endolumenal endoscopic full-thickness resection of muscularis propria-originating gastric submucosal tumors. J Laparoendosc Adv Surg Tech A. 2014;24:171–176. doi: 10.1089/lap.2013.0370. [DOI] [PubMed] [Google Scholar]
- 9.Wu CR, Huang LY, Guo J, Zhang B, Cui J, Sun CM, Jiang LX, Wang ZH, Ju AH. Clinical Control Study of Endoscopic Full-thickness Resection and Laparoscopic Surgery in the Treatment of Gastric Tumors Arising from the Muscularis Propria. Chin Med J (Engl) 2015;128:1455–1459. doi: 10.4103/0366-6999.157651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J, Jiao T, Li Y, Zheng M, Lu X. Facilitating retroflexed endoscopic full-thickness resection through loop-mediated or rope-mediated countertraction (with videos) Gastrointest Endosc. 2016;83:223–228. doi: 10.1016/j.gie.2015.08.063. [DOI] [PubMed] [Google Scholar]
- 11.Abe N, Takeuchi H, Ohki A, Hashimoto Y, Mori T, Sugiyama M. Comparison between endoscopic and laparoscopic removal of gastric submucosal tumor. Dig Endosc. 2018;30 Suppl 1:7–16. doi: 10.1111/den.13010. [DOI] [PubMed] [Google Scholar]
- 12.Dong HY, Wang YL, Jia XY, Li J, Li GD, Li YQ. Modified laparoscopic intragastric surgery and endoscopic full-thickness resection for gastric stromal tumor originating from the muscularis propria. Surg Endosc. 2014;28:1447–1453. doi: 10.1007/s00464-013-3375-8. [DOI] [PubMed] [Google Scholar]
- 13.Tan Y, Tang X, Guo T, Peng D, Tang Y, Duan T, Wang X, Lv L, Huo J, Liu D. Comparison between submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric stromal tumors originating from the muscularis propria layer. Surg Endosc. 2017;31:3376–3382. doi: 10.1007/s00464-016-5350-7. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Pang T, Zhang B, Wang L, Lv Y, Ling T, Zhang X, Huang Q, Xu G, Zou X. Retrospective Comparison of Endoscopic Full-Thickness Versus Laparoscopic or Surgical Resection of Small (≤ 5 cm) Gastric Gastrointestinal Stromal Tumors. J Gastrointest Surg. 2020;24:2714–2721. doi: 10.1007/s11605-019-04493-6. [DOI] [PubMed] [Google Scholar]
- 15.Tang AL, Liao XQ, Shen SR, Xiao DH, Yuan YX, Wang XY. Application of clips assisted with foreign body forceps in defect closure after endoscopic full-thickness resection. Surg Endosc. 2016;30:2127–2131. doi: 10.1007/s00464-015-4414-4. [DOI] [PubMed] [Google Scholar]
- 16.Mangiavillano B, Viaggi P, Masci E. Endoscopic closure of acute iatrogenic perforations during diagnostic and therapeutic endoscopy in the gastrointestinal tract using metallic clips: a literature review. J Dig Dis. 2010;11:12–18. doi: 10.1111/j.1751-2980.2009.00414.x. [DOI] [PubMed] [Google Scholar]
- 17.Shi Q, Chen T, Zhong YS, Zhou PH, Ren Z, Xu MD, Yao LQ. Complete closure of large gastric defects after endoscopic full-thickness resection, using endoloop and metallic clip interrupted suture. Endoscopy. 2013;45:329–334. doi: 10.1055/s-0032-1326214. [DOI] [PubMed] [Google Scholar]
- 18.Ye LP, Yu Z, Mao XL, Zhu LH, Zhou XB. Endoscopic full-thickness resection with defect closure using clips and an endoloop for gastric subepithelial tumors arising from the muscularis propria. Surg Endosc. 2014;28:1978–1983. doi: 10.1007/s00464-014-3421-1. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Wang X, Xiong G, Qian Y, Wang H, Liu L, Miao L, Fan Z. Complete defect closure of gastric submucosal tumors with purse-string sutures. Surg Endosc. 2014;28:1844–1851. doi: 10.1007/s00464-013-3404-7. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Meng Y, Ye S, Wang P, Liu F. Usefulness of the thread-traction method in endoscopic full-thickness resection for gastric submucosal tumor: a comparative study. Surg Endosc. 2019;33:2880–2885. doi: 10.1007/s00464-018-6585-2. [DOI] [PubMed] [Google Scholar]
- 21.Shi D, Li R, Chen W, Zhang D, Zhang L, Guo R, Yao P, Wu X. Application of novel endoloops to close the defects resulted from endoscopic full-thickness resection with single-channel gastroscope: a multicenter study. Surg Endosc. 2017;31:837–842. doi: 10.1007/s00464-016-5041-4. [DOI] [PubMed] [Google Scholar]
- 22.Hu JW, Ge L, Zhou PH, Li QL, Zhang YQ, Chen WF, Chen T, Yao LQ, Xu MD, Chu Y. A novel grasp-and-loop closure method for defect closure after endoscopic full-thickness resection (with video) Surg Endosc. 2017;31:4275–4282. doi: 10.1007/s00464-017-5473-5. [DOI] [PubMed] [Google Scholar]
- 23.Wu N, Liu S, Chen M, Zeng X, Wang F, Zhang J, She Q. The prepurse-string suture technique for gastric defect after endoscopic full-thickness resection (with video) Medicine (Baltimore) 2018;97:e12118. doi: 10.1097/MD.0000000000012118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akimoto T, Goto O, Nishizawa T, Yahagi N. Endoscopic closure after intraluminal surgery. Dig Endosc. 2017;29:547–558. doi: 10.1111/den.12839. [DOI] [PubMed] [Google Scholar]
- 25.Guo J, Liu Z, Sun S, Liu X, Wang S, Ge N, Wang G, Qi Y. Endoscopic full-thickness resection with defect closure using an over-the-scope clip for gastric subepithelial tumors originating from the muscularis propria. Surg Endosc. 2015;29:3356–3362. doi: 10.1007/s00464-015-4076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, Li P, Ji M, Wang Y, Zhu S, Liu L, Zhang S. Comparison of two methods for endoscopic full-thickness resection of gastrointestinal lesions using OTSC. Minim Invasive Ther Allied Technol. 2019;28:268–276. doi: 10.1080/13645706.2019.1602544. [DOI] [PubMed] [Google Scholar]
- 27.Hu J, Ge N, Wang S, Guo J, Liu X, Wang G, Sun S. Direct endoscopic full-thickness resection for submucosal tumors with an intraluminal growth pattern originating from the muscularis propria layer in the gastric fundus. BMC Gastroenterol. 2020;20:70. doi: 10.1186/s12876-020-01215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Renteln D, Schmidt A, Vassiliou MC, Gieselmann M, Caca K. Natural orifice transluminal endoscopic surgery gastrotomy closure with an over-the-endoscope clip: a randomized, controlled porcine study (with videos) Gastrointest Endosc. 2009;70:732–739. doi: 10.1016/j.gie.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Voermans RP, van Berge Henegouwen MI, Bemelman WA, Fockens P. Novel over-the-scope-clip system for gastrotomy closure in natural orifice transluminal endoscopic surgery (NOTES): an ex vivo comparison study. Endoscopy. 2009;41:1052–1055. doi: 10.1055/s-0029-1215231. [DOI] [PubMed] [Google Scholar]
- 30.Yang F, Wang S, Sun S, Liu X, Ge N, Wang G, Guo J, Liu W, Feng L, Ma W. Factors associated with endoscopic full-thickness resection of gastric submucosal tumors. Surg Endosc. 2015;29:3588–3593. doi: 10.1007/s00464-015-4113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ou YH, Kong WF, Li LF, Chen PS, Deng SH, He FJ, Peng QQ, Yue H. Methods for Endoscopic Removal of Over-the-Scope Clip: A Systematic Review. Can J Gastroenterol Hepatol. 2020;2020:5716981. doi: 10.1155/2020/5716981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiland T, Fehlker M, Gottwald T, Schurr MO. Performance of the OTSC System in the endoscopic closure of iatrogenic gastrointestinal perforations: a systematic review. Surg Endosc. 2013;27:2258–2274. doi: 10.1007/s00464-012-2754-x. [DOI] [PubMed] [Google Scholar]
- 33.von Renteln D, Schmidt A, Vassiliou MC, Rudolph HU, Caca K. Endoscopic full-thickness resection and defect closure in the colon. Gastrointest Endosc. 2010;71:1267–1273. doi: 10.1016/j.gie.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 34.Manta R, Manno M, Bertani H, Barbera C, Pigò F, Mirante V, Longinotti E, Bassotti G, Conigliaro R. Endoscopic treatment of gastrointestinal fistulas using an over-the-scope clip (OTSC) device: case series from a tertiary referral center. Endoscopy. 2011;43:545–548. doi: 10.1055/s-0030-1256196. [DOI] [PubMed] [Google Scholar]
- 35.Andalib I, Yeoun D, Reddy R, Xie S, Iqbal S. Endoscopic resection of gastric gastrointestinal stromal tumors originating from the muscularis propria layer in North America: methods and feasibility data. Surg Endosc. 2018;32:1787–1792. doi: 10.1007/s00464-017-5862-9. [DOI] [PubMed] [Google Scholar]
- 36.Xu MM, Angeles A, Kahaleh M. Endoscopic full-thickness resection of gastric stromal tumor: one and done. Endoscopy. 2018;50:E42–E43. doi: 10.1055/s-0043-121566. [DOI] [PubMed] [Google Scholar]
- 37.Granata A, Bisello M, Cipolletta F, Ligresti D, Traina M. Endoscopic Wedge Gastrectomy of a Gastric Subepithelial Tumor and Closure of the Gastric Wall Defect With the Overstitch Suturing System. Surg Innov. 2018;25:542–543. doi: 10.1177/1553350618779670. [DOI] [PubMed] [Google Scholar]
- 38.Dedania B, Mistry T, Buryanek J, Singhal S. Endoscopic full-thickness resection of a gastric subepithelial tumor. VideoGIE. 2018;3:79–80. doi: 10.1016/j.vgie.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Modayil RJ, Zhang X, Khodorskiy D, Stavropoulos SN. Advanced resection and closure techniques for endoscopic full-thickness resection in the gastric fundus. VideoGIE. 2020;5:61–63. doi: 10.1016/j.vgie.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inayat F, Aslam A, Grunwald MD, Hussain Q, Hurairah A, Iqbal S. Omental Patching and Purse-String Endosuture Closure after Endoscopic Full-Thickness Resection in Patients with Gastric Gastrointestinal Stromal Tumors. Clin Endosc. 2019;52:283–287. doi: 10.5946/ce.2018.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kerdsirichairat T, Vosoughi K, Ichkhanian Y, Ngamruengphong S, Kalloo AN, Kumbhari V, Khashab MA. Endoscopic full-thickness resection with omental patch closure for a gastric stromal tumor in the gastric cardia. Endoscopy. 2019;51:E278–E279. doi: 10.1055/a-0885-9031. [DOI] [PubMed] [Google Scholar]
- 42.Sachdev AH, Iqbal S, Ribeiro IB, de Moura DTH. Use of omental patch and endoscopic closure technique as an alternative to surgery after endoscopic full thickness resection of gastric intestinal stromal tumors: A series of cases. World J Clin Cases. 2020;8:120–125. doi: 10.12998/wjcc.v8.i1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goto O, Sasaki M, Ishii H, Horii J, Uraoka T, Takeuchi H, Kitagawa Y, Yahagi N. A new endoscopic closure method for gastric mucosal defects: feasibility of endoscopic hand suturing in an ex vivo porcine model (with video) Endosc Int Open. 2014;2:E111–E116. doi: 10.1055/s-0034-1377180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kantsevoy SV, Bitner M, Hajiyeva G, Mirovski PM, Cox ME, Swope T, Alexander K, Meenaghan N, Fitzpatrick JL, Gushchin V. Endoscopic management of colonic perforations: clips versus suturing closure (with videos) Gastrointest Endosc. 2016;84:487–493. doi: 10.1016/j.gie.2015.08.074. [DOI] [PubMed] [Google Scholar]
- 45.Kantsevoy SV, Bitner M, Davis JM, Hajiyeva G, Thuluvath PJ, Gushchin V. Endoscopic suturing closure of large iatrogenic colonic perforation. Gastrointest Endosc. 2015;82:754–755. doi: 10.1016/j.gie.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 46.Ge PS, Thompson CC. The Use of the Overstitch to Close Perforations and Fistulas. Gastrointest Endosc Clin N Am. 2020;30:147–161. doi: 10.1016/j.giec.2019.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brewer Gutierrez OI, Akshintala VS, Ichkhanian Y, Brewer GG, Hanada Y, Truskey MP, Agarwal A, Hajiyeva G, Kumbhari V, Kalloo AN, Khashab MA, Ngamruengphong S. Endoscopic full-thickness resection using a clip non-exposed method for gastrointestinal tract lesions: a meta-analysis. Endosc Int Open. 2020;8:E313–E325. doi: 10.1055/a-1073-7593. [DOI] [PMC free article] [PubMed] [Google Scholar]