Abstract
Glioblastoma multiforme (GBM), the most frequently occurring malignant brain tumor in adults, remains mostly untreatable. Because of the heterogeneity of invasive gliomas and drug resistance associated with the tumor microenvironment, the prognosis is poor, and the survival rate of patients is low. Communication between GBMs and non-glioma cells in the tumor microenvironment plays a vital role in tumor growth and recurrence. Emerging data have suggested that neural stem cells (NSCs) in the subventricular zone (SVZ) are the cells-of-origin of gliomas, and SVZ NSC involvement is associated with the progression and recurrence of GBM. This review highlights the interaction between SVZ NSCs and gliomas, summarizes current findings on the crosstalk between gliomas and other non-glioma cells, and describes the links between SVZ NSCs and gliomas. We also discuss the role and mechanism of SVZ NSCs in glioblastoma, as well as the interventions targeting the SVZ and their therapeutic implications in glioblastoma. Taken together, understanding the biological mechanism of glioma-NSC interactions can lead to new therapeutic strategies for GBM.
Keywords: Neural stem cells, Glioma, Tumor microenvironment, Communication, Exosomes
Core Tip: This review summarizes current findings on the links between neural stem cells (NSCs) in the subventricular zone (SVZ) and glioblastoma as well as the therapeutic implications of using SVZ NSCs as drug delivery vehicles for targeted glioblastoma multiforme (GBM) therapy and their potential mechanisms. Understanding glioma-NSC interactions will lead to the development of strategies for treating GBM, such as the use of extracellular vesicles/exosomes.
INTRODUCTION
Glioblastoma multiforme (GBM) is the most frequently occurring malignant brain tumor in adults, for which no effective therapy is currently available. Current conventional therapies, such as a combination of surgery and radio- or chemo-therapy, yield poor prognosis and low median survival times of patients[1,2]. In addition, the recurrence of GBM is common or inevitable, and there are no standardized therapeutic approaches for such cases[3]. To improve clinical outcomes, immunotherapy has been successfully performed by activating the immune systems of patients[4], employing chimeric antigen receptor T cells, oncolytic viruses (OV), anti-cytotoxic-T-lymphocyte-associated protein 4, and anti-programmed cell death protein 1, among others[5-8]. However, the therapeutic efficacy remains limited in GBM due to the effects of the tumor microenvironment (TME), which leads to immunosuppression or immune tolerance[1,5,9]. Recent advances integrating metabolomics with genomics or proteomics have provided new insight into the mechanisms that drive the origin and development of tumors, including GBM[10-12], especially the interactions between the tumor and TME, and provide important clues for new therapeutic strategies. Neural stem cells (NSCs), as unique stem cell type in the brain, have the abilities of self-renewal and multi-directional differentiation, and can differentiate into neurons, astrocytes, and oligodendrocytes[13]. NSCs mainly exist in the subventricular zone (SVZ) of the lateral ventricle and dentate gyrus [subgranular zone (SGZ)] of the hippocampus. Furthermore, NSCs create a unique stem cell microenvironment in the SVZ or SGZ region that maintains stem cell homeostasis and stemness and inhibits differentiation[14,15]. Recent studies[16-20] have found that NSCs located in the SVZ might be the cells-of-origin of gliomas, and that SVZ involvement is associated with GBM recurrence in patients. Further, GBMs contacting the SVZ significantly decrease the overall survival (OS) and progression-free survival (PFS) of patients[16-19]. Thus, crosstalk between the oncogenic signaling of tumors and SVZ NSCs might be important in GBM. In this review, we focus on recent advances of the origin and development of GBM and explore novel strategies for GBM treatment. First, we summarize current findings on the crosstalk between gliomas and other non-glioma cells in the tumor niche. Then, we address the recently identified links between NSCs and gliomas and discuss the role and mechanism of SVZ NSCs in glioblastoma. Finally, we provide insight into the interventions targeting the SVZ and their therapeutic implications in glioblastoma. This review provides an overview of current opinions on gliomas.
TUMOR NICHE IN GLIOBLASTOMA
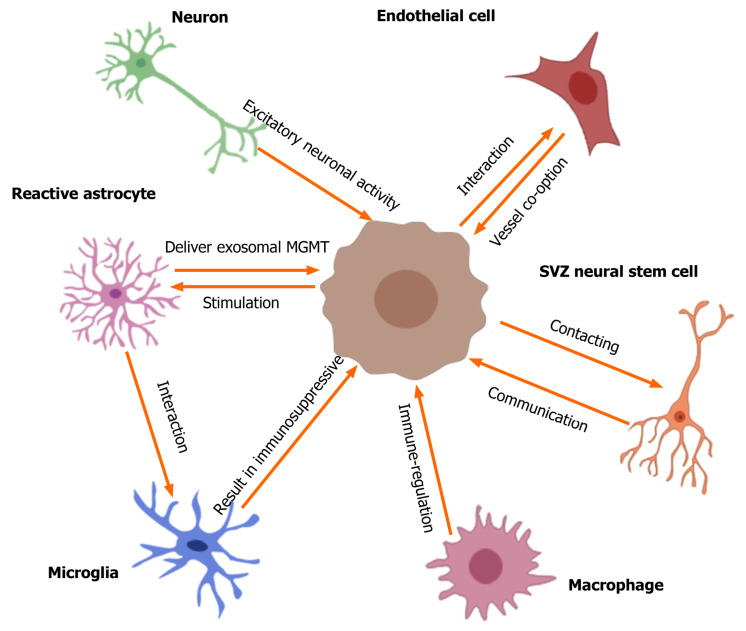
Emerging evidence[5,21-25] suggests that the unique TME involved by different non-glioma cells is critical for glioma growth, invasion, recurrence, and tumor angiogenesis. In particular, the communication or crosstalk between glioblastoma and other non-glioma cells in the TME mediates tumor progression and therapeutic drug resistance[5,25]. The non-glioma cells in the TME or glioma niche contain neurons, normal and reactive astrocytes (RAs), glioma-associated microglia/macrophages, endothelial cells (ECs), neural stem cells, etc.[23-25]. These non-cancer cells secrete proteins or non-protein biomolecules (including nucleic acids, lipids, and nitric oxide) within the TME to regulate glioma growth. Furthermore, glioblastoma cells can recruit non-tumor cells to alter their phenotype to regulate the TME[23-25]. Neurons are the main cell type in the glioma niche. Venkatesh et al[26] found that excitatory neuronal activity affects the growth of glioblastomas, and neurons can mediate the interaction with gliomas mainly via the cytokine NLGN3 secreted by activated neurons. Furthermore, the specific interaction between neurons and gliomas occurs mainly via the bona fide α-amino-3-hydroxy-5-methyl-4 isoxazole propionic acid receptor-dependent neuron–glioma synapses[27]. These findings[26-28] describe the vital role of neurons in the glioma niche and crosstalk between these neurons. Astrocytes, especially reactive astrocytes, are involved in brain injury, tumors, and inflammatory and degenerative diseases[29]. The glioma-astrocyte interaction also plays a vital role in the TME[30,31]. Yu et al[32] showed that glioma cells can stimulate the transformation of normal human astrocytes into RAs in the absence of direct contact, and RAs deliver exosomal O6-alkylguanine DNA alkyltransferase to glioma cells, resulting in temozolomide (TMZ) resistance of gliomas. Furthermore, tumor-associated astrocytes exhibit immunomodulatory properties within the TME[33]. This indicates that reactive astrocytes or glioma-associated astrocytes in the TME mediate the potential interaction with gliomas. The TME of gliomas normally contains infiltrated microglia/macrophages, and the ability of immunoregulation in glioma is positively correlated with the number of tumor-associated microglia/macrophages[34,35]. Chen et al[34] found that a complex composed of circadian regulator, circadian locomotor output cycles kaput, and its heterodimeric partner, brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1, contributes to the interaction between the glioma and microglia. In addition, the authors[36] found that phosphatase and tensin homolog (PTEN) deficiency in glioblastomas significantly increased macrophage infiltration to sustain GBM survival and stimulate tumor angiogenesis. A review by Poon et al[37] summarized the biology of glioblastoma-associated microglia/macrophages. These findings indicate the vital crosstalk of microglia/ macrophages with GBM in the TME. Gliomas have a high metabolic level, and the vasculature or angiogenesis is always abundant in the TME. Griveau et al[38] investigated glioma-vascular interactions by focusing on tumor-stromal and vascular regulation to explore the role of the glial phenotype associated with anti-angiogenic therapy escape[38,39]. Thus, the biological interaction between glioblastomas and non-glioma cells in the tumor niche is important in the development and progression of gliomas and exploration of new therapeutic opportunities. First, we describe the potential crosstalk between gliomas and glioma-associated non-glioma cells, such as neurons, astrocytes, microglia/ macrophages, and ECs (Figure 1); next, we carefully review current findings on the interactions between gliomas and NSCs.
Figure 1.
Crosstalk between gliomas and non-glioma cells in tumor microenvironment. The tumor microenvironment of glioma contains many non-glioma cells around the tumor, such as neurons, astrocytes, oligodendrocytes, immune cells (microglia and macrophages), endothelial cells, stem cells, etc. Active neurons promote the growth of glioblastomas through secreted cytokine NLGN3. Excitatory neuronal activity mediates the neuron-glioma interactions mainly via the bona fide AMPA receptor-dependent neuron–glioma synapse. Glioma cells stimulate normal astrocytes into reactive astrocytes and then reactive astrocytes induce temozolomide-resistance of glioma cells via delivering exosomal MGMT. The interactions of astrocyte-microglia can regulate transcriptional re-programming of microglia, and microglia contribute to sustaining the immunosuppressive microenvironment in glioblastoma multiforme (GBM) for promoting tumor progression. Glioblastomas increase macrophage infiltration, and then infiltrated macrophages promote GBM survival and tumor angiogenesis via secreted cytokines. Olig2-positive glioma cells can be invaded by vessel co-option; the glioma-EC interactions restrict the anti-angiogenic therapy of gliomas. In addition, GBM contacting neural stem cells in the subventricular zone of the lateral ventricles result in a shorter overall survival of patients and increase early recurrence of gliomas. SVZ: Subventricular zone.
ROLE OF SVZ NSCS IN GLIOMAGENESIS
Emerging data[16-19,40] have revealed that SVZ NSCs are closely related to glioblastoma development and progression, and GBM may arise from the accrual of gene mutations in NSCs. Jiang et al[41] found that GBMs associated with glial fibrillary acidic protein-expressing SVZ NSCs in mice showed accelerated tumor development, higher malignancy, and lesser drug resistance in comparison to those in the control group. In addition, TERT promoter mutation can permit the protracted self-renewal of cells and may induce gliomagenesis of NSCs[20,42,43]. Currently, NSCs in the SVZ are considered as potential cells-of-origin in gliomas[44-47]. In the next sections, we address the possible gene mutations in SVZ NSCs inducing gliomagenesis.
P53 or IDH1 mutation in SVZ NSCs
Recent findings[20,46,48-50] showed that SVZ NSCs that have acquired mutations in the tumor protein p53 or IDH1 gene can result in uncontrolled proliferation and tumorigenesis. Furthermore, p53 deficiency can induce the accumulation of oncogenic alterations[51,52]. Wang et al[53] showed the presence of mutant p53 proteins in SVZ NSCs, and that subsequent expression of mutant p53-expressing Olig2+ transit-amplifying progenitor-like cells was associated with the initiation of glioma formation. Modrek et al[54] introduced IDH1R132H, P53 short hairpin (shRNA), and α-thalassemia/mental retardation syndrome X-linked shRNA into human NSCs and found that these oncogenic hits blocked NSC differentiation and increased invasiveness, thus representing early drivers of gliomagenesis. Pirozzi et al[55] reported that mouse NSCs expressing IDH1R132H displayed reduced proliferation within the SVZ due to p53-mediated cell-cycle arrest and underwent neuronal differentiation. Bardella et al[56] conditionally expressed IDH1R132H in the SVZ of the adult mouse brain, and the mice developed hydrocephalus and dilated lateral ventricles (LVs), with the accumulation of 2-hydroxyglutarate and reduced α-ketoglutarate. Besides, stem cell populations were expanded, and mutant SVZ cells displayed features similar to those of gliomas[56]. Lee et al[20] sought direct molecular genetic evidence associated with GBMs that had originated from SVZ NSCs in clinical patients. They utilized brain tissue from 28 patients with IDH wild-type GBM or other types of brain tumors to perform deep sequencing and found low-level GBM driver mutations in healthy SVZ tissue away from the tumor in 56.3% of patients with IDH wild-type GBM. Moreover, astrocyte-like NSCs carrying the driver mutations led to high-grade malignant gliomas in a genome-edited mouse model[20]. These results show that P53 or IDH1 mutation in SVZ NSCs drives gliomagenesis by disrupting the characteristics and phenotypes of SVZ NSCs.
Other gene mutations in SVZ NSCs
Many genes or molecules such as tumor oncogenes and transcription factors involved in biological functions may affect the development of glioblastoma[46,47]. Abel et al[57] found that infiltrating glioma cells may be derived from SVZ NSCs that are transformed by activation of the oncogenic K-Ras. Daniel et al[58] showed that PI3K activation in NSCs can drive the initiation of tumorigenesis. Liu et al[50] found that overexpression of the nuclear receptor, tailless, inhibited age-dependent exhaustion of NSCs in mice, induced migration of stem cells from the SVZ niche, and led to the development of gliomas. Yang et al[59] found that loss of the transcriptional repression factor, Capicua, promoted gliomagenesis via aberrant NSC proliferation and differentiation. The transcription factors Forkhead Box G1 (FOXG1) and sex-determining region Y-box 2 (SOX2) are frequently overexpressed in GBMs. Bulstrode et al[60] demonstrated that FOXG1-null cells showed increased astrocyte differentiation and SOX2 ablation attenuated NSC proliferation, which suggests that FOXG1 and SOX2 play complementary, but distinct, roles in GBM self-renewal. The Y-box binding protein 1 (YB-1) is vital gene in brain development and is upregulated in glioblastomas[61,62]. Fotovati et al[63] showed that YB-1 was also overexpressed in the SVZ region of the mouse fetal brain; indeed, YB-1 knockout mice displayed reduced expression of NSC markers in the SVZ, as well as reduced neurosphere growth, but showed enhanced NSC differentiation[63]. These data indicate the importance of oncogenes or cancer-associated transcription factors in SVZ NSCs involved in the genesis of GBM.
Undifferentiated NSCs
Recently, undifferentiated NSCs, especially intermediate progenitor cells, rather than NSCs, have been considered as the cells-of-origin of glioma tumors[64]. Llaguno et al[47] edited glioblastoma-relevant tumor suppressors, neufibromatosis type 1 (Nf1), transformation-related protein p53, and PTEN by a tamoxifen-inducible Cre-recombinase in late-stage neuronal progenitors, neuroblasts, and differentiated neurons, respectively, but found no evidence of glioma formation. They showed that mainly early neural progenitor cells were responsible for gliomagenesis[47]. Liu et al[65] mutated concurrent p53/Nf1 in NSCs to establish gliomagenesis in mice by using mosaic analysis with double markers (MADM). The results showed that only oligodendrocyte precursor cells expressed aberrant/malignant growth and led to gliomagenesis, determined by tracing in MADM-based lineage analysis[65]. This suggests that undifferentiated stem cells, or oligodendrocyte precursor cells, are susceptible to tumorigenesis.
Thus, taken together, although many glioma-associated oncogene or transcription factor mutations in SVZ NSCs are responsible for the development of glioblastoma, understanding the role and potential mechanisms of SVZ NSCs driving GBM genesis or progression will be very meaningful for developing novel therapeutic interventions.
MECHANISM OF SVZ NSCS IN GLIOBLASTOMA
Patients with GBM with high isotropic p values in the SVZ region with high fluid-attenuated inversion recovery indicated tumor infiltration involving the SVZ region[66]. Emerging data[67,68] confirmed that GBMs in close contact with the SVZ possessed aggressive characteristics, furthermore, the SVZ region may be an independent predictor of lower OS and PFS and early recurrence in patients with GBM. Therefore, the mechanism of the interaction between GBMs and SVZ NSCs should be carefully evaluated.
Evidence for SVZ involved in GBM progression
Recent studies suggested that patients with GBMs in contact with the lateral ventricle-SVZ region have lower survival rates than those with GBMs contacting the subgranular zone, corpus callosum, or cortex[16,17]. Furthermore, Şuşman et al[69] found a significant difference in the PFS of patients with GBM who were administered with high radiotherapy doses within the LV-SVZ region. Chen et al[70] investigated 102 patients with GBM who had undergone surgical resection followed by adjuvant intensity-modulated radiation therapy and concomitant TMZ, and found that the recurrence of GBM was significantly related to the proximity to neurogenic regions (SVZ)[70]. To identify the potential molecules in the SVZ associated with GBM progression, Gollapalli et al[71] used proteomics techniques (two-dimensional difference gel electrophoresis and liquid chromatography-tandem mass spectrometry) to investigate the differences between SVZ+ (contacting) and SVZ− (non-contacting) GBM subtypes. Both serum and tissue proteomic analyses revealed significant alterations in various proteins associated with disease pathobiology, including lipid proteins, cytoskeletal, lipid binding, and cell-cycle-regulating proteins[71]. In addition, because of the similarities between tumor-initiating, GBM-derived neural stem (GNS) cells and genetically normal NSCs in vitro[72], Okawa et al[73] performed quantitative proteomics to compare total proteome and secreted proteome between GNS cells and NSCs. They identified 447 proteins in the total proteome and 138 proteins in the secreted proteome that were differentially expressed in GNSs and NSCs. Gene enrichment analysis mainly included extracellular matrix interactions, focal adhesion, cell motility, and cell signaling. They suggested that cell-matrix and cell-cell adhesion molecules play crucial roles in tumor infiltration[73]. Thus, these findings provide clinical and molecular evidence for SVZ NSCs in the regulation of GBM progression.
Gliomas invade SVZ region via chemoattractants secreted by NSCs
However, the mechanism of SVZ NSCs in glioma progression remains unclear, specifically the interactive biological functions between SVZ NSCs and GBMs. Qin et al[74] focused on the role or action of NSCs/neural progenitor cells (NPCs) on glioma cells, and found that the CM from SVZ NPCs had a chemoattractant effect on glioma cells. Through proteomic and functional analyses, they identified a chemoattractant complex secreted by SVZ NPCs, which included the neurite outgrowth-promoting factor, pleiotrophin (PTN), and its binding partners, secreted protein acidic and rich in cysteine (SPARC)/SPARC-like protein 1 and heat shock protein 90-beta. The chemoattractant complex promoted tumor invasion by activating Rho/Rho-associated protein kinase signaling in gliomas. Furthermore, PTN was expressed at high levels in the SVZ, and its knockdown by shRNA in vivo remarkably reduced the ability of glioma to invade the SVZ[74]. This study mainly proposed that NSCs in the SVZ induced high-grade gliomas to invade the SVZ region by secreting specific chemoattractant factors, and considered that the cytokine PTN is a potential target for glioma therapy. These results provide an experimental basis for glioma invasion of the SVZ region. Thus, targeting the interaction process between GBM and SVZ NSCs can represent a novel strategy to curtail the malignant potential of SVZ NSCs and restrict the progression of gliomas.
Gliomas promote tumor transformation of NSCs by extracellular vesicles delivery
Many studies[75-78] have shown that extracellular vesicles (EVs)/exosomes play an important role in intercellular communication. In addition, EVs/exosomes derived from gliomas or non-glioma cells in the TME are involved in tumor cell proliferation, invasion, malignancy, and drug resistance owing to their functions delivering mRNA, microRNAs, or proteins[79,80]. Wang et al[81] added glioblastoma-derived EVs to culturing with NSCs and found that NSCs de-differentiated into tumor-promoting cells. They found that these transformed cells had higher proliferative, migratory, and clonogenic activities than naïve cells, and accelerated tumor formation in vivo. Using single-cell transcriptome sequencing analysis, they identified several key genes in the transformed NSCs, including S100B, CXCL14, EFEMP1, SCRG1, GLIPR1, HMGA1, and CD44[81]. This study preliminarily shows that EVs secreted by gliomas can regulate and promote tumor transformation of SVZ NSCs by gene delivery, suggesting an origin for glioma recurrence. However, the targets or potential links between SVZ NSCs and gliomas are unclear and require further investigation and experimental validation.
INTERVENTIONS TARGETING THE SVZ (NSCS) FOR GLIOBLASTOMA TREATMENT
Recent data revealed that GBM contacting the SVZ region presented highly aggressive characteristics, and radiotherapy received within the SVZ region increased the PFS of patients with GBM. Furthermore, SVZ NSCs not only contribute to neurogenesis and play an important role in nerve regeneration[13,82], but also have a tumor-homing property and can be used to deliver drugs for tumor treatment[83-86]. The pre-clinical and clinical studies of interventions using SVZ/NSCs for glioblastoma treatment are shown in Table 1.
Table 1.
Pre-clinical and clinical studies of interventions using subventricular zone/neural stem cells for glioblastoma treatment
|
Ref.
|
Experiment
|
Clinical
|
Interventions
|
Doses
|
Subjects
|
Outcomes
|
Mechanism
|
| [87] | - | Yes | Adjuvant radiation therapy for the SVZ (NSCs) | Ipsilateral 48.7 Gy | Patients = 116 | Improved PFS and OS in patients with GBM after GTR | - |
| [88] | Yes | - | Adjuvant TMZ/XRT of the SVZ (NSCs) | 50-100 mg/kg TMZ, and 1-2 Gy | Mice, n = 6 per cohort | SVZ NSCs tolerated chemoradiation | - |
| [89] | - | Yes | Adjuvant chemoradiation therapy for the SVZ (NSCs) | Ipsilateral (High dose > 59.4 Gy) | Patients = 173 | Improved PFS and OS in patients with high ipsilateral doses | - |
| [90] | - | Yes | Adjuvant radiation therapy for the SVZ (NSCs) | Ipsilateral High dose > 57.4 Gy | Patients = 50 | Negatively impacted on OS in IDH wild type GBM | - |
| [91] | - | Yes | DWI evaluated before and after adjuvant chemoradiation | No data | Patients = 40 | Increasing in ipsilesional ADCL associated with shorter PFS and OS | - |
| [96] | Yes | - | NSCs modified by IL-4 | 89 ng/5 × 105 cells per 48 h | Mice, n = 5-7 rats, n = 12-33 per group | Strong anti-tumor effects and long-term survival of animals | Produced IL-4 |
| [97] | Yes | - | hNSCs overexpressed BMP4 (hNSCs-BMP4) | No data | Mice, n = 10 per group | Inhibited tumor growth and prolonged survival | BMP/Smad1 pathway |
| [99] | Yes | - | Modified iNSC with anticancer molecule TRAIL | 7.5 × 105 cells per mouse | Mice, n = 12 per group | Decreased tumor growth and extended the survival | Secreted anticancer molecule TRAIL |
| [100] | Yes | - | h-iNSCTE transduced with TRAIL and TK | 7.5 × 105 cells per mouse | Mice, n = 12 per group | Inhibited GBM growth and prolonged the median survival | Secreted cytotoxic molecules TRAIL and TK |
| [85] | Yes | - | HB1.F3.CD NSCs combined with intraperitoneal injection of 5-FC | 1 × 104, 5 × 104, 1 × 105 cells per mouse, 500 mg/kg 5-FC | Mice, n = 12 per group | Inhibited GBM growth and prolonged the survival | Converted prodrug 5-FC to active 5-FU |
| [101] | - | Yes | HB1.F3.CD NSCs combined with oral administration of 5-FC | 1 × 107, 5 × 107 cells, 75-150 mg/kg/day 5-FC | Patients = 15 | Confirmed the safety and ability of NSCs to target brain tumors and locally produce chemotherapy | Convert prodrug 5-FC to active 5-FU |
| [102] | Yes | - | HB1.F3.CD NSCs-TRAIL combined with intraperitoneal injection of Lan C | 2 × 105 cells, 1 mg/kg Lan C | Mice, n = 10 per group | Induced tumorregression | Lan C sensitized GBM to TRAIL |
| [105] | Yes | - | HB1.F3.CD NSCs loaded with CRAd-Survivin-pk7 | 5 × 105 cells, with 50 IU per cell of CRAd-S-pk7 | Mice, n = 7 per group | Increased the median survival of mice | Overcame major limitations of OVs in vivo |
| [106] | Yes | - | HB1.F3.CD NSCs-CRAd-S-pk7 combined with intraperitoneal injection of NACA | 4 × 105 cells, 250 mg/kg/day NACA | Mice, n = 6-7 per group | Extended the median survival of mice | Enhanced OVs production and distribution in vivo |
| [109] | Yes | - | Overexpressed CXCR4 in NSCs and loaded with CRAd-S-pk7 | 5 × 105 cells | Mice, n = 8 per group | Extended the survival | SDF-1/CXCR4 pathway |
| [110] | Yes | - | NSCs loaded CRAd-S-pK7 combined with intraperitoneal injection of MT | 5 × 105 cells, 50 μg/g MT | Mice, n = 8 per group | Improved the survival of GBM-bearing mice | Prolonged thepersistence of NSCs in the nasal cavities |
| [114] | Yes | - | HB1.F3.CD NSCs loaded with MSN-Dox | 2.5 × 105 cells | Mice, n = 4-8 per group | Prolonged the median survival of mice | Self-destructing mechanism |
| [116] | Yes | - | Scaffold GEMs/tNSCstk | 1 × 106 cells per scaffold | Mice, n = 5 per group | Increased cell viability and improved the survival | Reduced residual tumor volumes |
| [117] | Yes | - | tNSC-TRAIL and/or tNSC–TK | 7 × 105–1.4 × 106 cells | Mice, n = 4-13 per group | Inhibited tumor growth and survival | Secreted cytotoxic molecules TRAIL and/or TK |
SVZ: Subventricular zone; NSCs: Neural stem cells; PFS: Progression free survival; OS: Overall survival; GTR: Gross total resection; TMZ: Temozolomide; XRT: X-irradiation; IDH: Isocitrate dehydrogenase; DWI: Diffusion-weighted imaging; ADC: Apparent diffusion coefficient; IL-4: Interleukin-4; BMP4: Bone morphogenetic protein 4; iNSC: Induced neural stem cell; TRAIL: Tumor necrosis factor-α-related apoptosis-inducing ligand; h-iNSCTE: Human fibroblasts into tumor-homing early-stage induced neural stem cells; TK: Thymidine kinase; 5-FC: 5-fluorocytosine; 5-FU: 5-fluorouracil; Lan C: Lanatoside C; OV: Oncolytic adenovirus; CRAd-S-pk7: CRAd-Survivin-pk7; NACA: Novel antioxidant thiol, N-acetylcysteine amide; MT: Methimazole; MSN-Dox: Doxorubicin-loaded mesoporous silica nanoparticles; GEMs: Gelatin matrices; tNSC: Tumoricidal neural stem cells; tNSCstk: Engineered tNSCs to express the prodrug/enzyme thymidine kinase.
Radiotherapy for the SVZ region in glioma intervention
Currently, in addition to surgery and chemotherapy, radiation therapy has been used as a standard treatment strategy for patients with GBM in the clinic, and can be used to target the SVZ region. Chen et al[87] retrospectively analyzed 116 patients with surgically resected glioblastoma and found that the PFS and OS of patients significantly improved with a mean radiation dose of 40 Gy to the ipsilateral SVZ. This result suggests that targeting the SVZ region was necessary for treating GBM. To determine whether SVZ NSCs can tolerate radiation therapy, Cameron et al[88] combined the chemotherapy drug TMZ with X-irradiation in mice, and found that chemoradiation resulted in type A neuroblast apoptosis, but not NSC death. Furthermore, type A cells can be repopulated within the V-SVZ in vivo by sufficient recovery time[88]. Animal experiments suggested that SVZ NSCs could tolerant standard chemoradiation therapy. However, high radiation therapy doses to the ipsilateral SVZ may not be effective in patients with GBM[89]. Muracciole et al[90] found that high radiation doses > 57.4 Gy to ipsilateral NSCs and > 35 Gy to contralateral SVZ negatively impacted the OS of IDH-wild-type glioblastoma patients[90]. Moreover, Cho et al[91] found that the apparent diffusion coefficient with lower Gaussian distribution values of ipsilesional SVZ increased after chemoradiation, leading to a poor PFS and OS of patients.
Therefore, although radiotherapy can be used to target the SVZ area, some problems warrant further consideration. First, the SVZ area is very small, making it difficult to accurately control the dose to the targeted SVZ. In particular, a high dose of radiation therapy may result in adverse effects. Second, due to the fact that SVZ NSCs are physiologically involved in the replenishment and repair of injured nerve tissue, radiation therapy-induced damage to NSCs in the SVZ may affect the repair capability of neurological functions. Therefore, it is necessary to develop novel gene-targeted therapeutic methods to precisely target glioma and avoid potential side effects.
NSCs loading anticancer molecules for targeted therapy of gliomas
The tumor-homing ability of NSCs has been confirmed to enable NSCs to migrate toward and co-localize within the tumor islets in vivo[85,92-94]. Glass et al[95] reported that endogenous NSCs in mice migrated from the SVZ toward gliomas and surrounded them. They injected red fluorescent protein-labeled GL261 cells into transgenic mice with a promoter for nestin (nestin-GFP) to explore the association between endogenous NSCs and gliomas. They found that nestin-GFP cells surrounded the tumors and expressed early precursor markers; furthermore, the tumor-associated precursor cells originated from the SVZ[95].
Because current gene therapies are unable to infiltrate the brain parenchyma and hard-to-reach glioblastoma core site, NSCs have been used to load therapeutic molecules for targeted treatment of gliomas. Benedetti et al[96] transferred IL-4 to C57BL6J mouse NSCs and injected them into the brains of mice to establish a glioblastoma model. They found that the survival of tumor-bearing mice was significantly extended, which was also observed in Sprague-Dawley rats with C6 glioblastomas[96]. Liu et al[97] overexpressed bone morphogenetic protein 4 (BMP4) in hNSCs (hNSCs-BMP4) and found that the cells inhibited gliomas in vitro and in vivo by secreting BMP4. These findings suggest effective approaches based on loading of NSCs with therapeutically effective molecules for glioma treatment. In recent years, transdifferentiation (TD) has been successfully used in somatic cell reprogramming[98]. Bagó et al[99] generated TD-derived induced NSCs (iNSCs) by transdifferentiating fibroblasts in mice, and found that the iNSCs not only rapidly homed and migrated to glioblastomas in vitro and in vivo but also successfully delivered the anticancer molecule, tumor necrosis factor α–related apoptosis-inducing ligand (TRAIL), leading to a significant decrease in the growth of xenograft glioblastoma and prolongation of the median survival times of mice[99]. Next, they[100] also engineered human iNSCs by TD of human fibroblasts to deliver the cytotoxic agents TRAIL and TK (thymidine kinase). The cytotoxic h-iNSCs rapidly migrated to human GBM cells and penetrated GBM spheroids, significantly reducing the size of solid human GBM xenografts and prolonging the median survival of mice[100]. These results suggest that NSCs can be used as a cell platform for glioma-homing cytotoxic therapy.
Pre-clinical and clinical applications of human NSCs for GBM treatment
HB1.F3.CD, a cytosine deaminase (CD)–expressing clonal human NSC line that can convert the prodrug 5-fluorocytosine (5-FC) to active chemotherapeutic 5-fluorouracil (5-FU), has been approved by the United States Food and Drug Administration for use in human clinical trials. Aboody et al[85] used HB1.F3.CD and 5-FC to treat tumor-bearing mice and showed that the average tumor volume of mice was significantly decreased, with no difference in toxicity. This result confirmed the efficacy of an allogeneic NSC-mediated enzyme/prodrug-targeted therapy in high-grade glioma. Portnow et al[101] reported the first-in-human study (NCT01172964) in patients with recurrent, high-grade glioma by retrovirally transducing HB1.F3.CD.C21 (CD-NSCs) to express cytosine deaminase stably. Fifteen patients with recurrent, high-grade glioma underwent intracranial administration of CD-NSCs during tumor resection or biopsy. After oral administration of 5-FC, CD-NSCs produced 5-FU locally in the brain in a 5-FC-dose-dependent manner by intracerebral microdialysis with no dose-limiting toxicity. Furthermore, autopsy results revealed that CD-NSCs that had migrated to distant tumor sites were non-tumorigenic[101]. These findings demonstrate the initial safety and proof-of-concept of NSCs in targeting brain tumors. In addition, the cardiac glycoside lanatoside C (Lan C) sensitizes glioma cells to the anticancer agent, TRAIL. Teng et al[102] showed that HB1.F3.CD engineered to express TRAIL migrated towards tumors in mice and induced tumor regression in combination with Lan C. Oncolytic adenoviral virotherapy exhibits limitations, such as a poor viral distribution and infiltration throughout tumors[103,104]. Ahmed et al[105] used HB1.F3.CD loaded with the oncolytic adenovirus, CRAd-Survivin-pk7 (CRAd-S-pk7), and found that OV-loaded HB1.F3.CD cells effectively migrated to the contralateral hemisphere of mice, inhibited the progression of clinically relevant human-derived glioma models, and prolonged the median survival times of mice compared to OV alone[105]. Furthermore, Kim et al[106] used the novel anti-oxidant thiol, N-acetylcysteine amide (NACA), to prevent OV-mediated potential toxicity, and found that NACA combined with CRAd-S-pk7 significantly increased NSC activity, enhanced CRAd-S-pk7 production, and improved the therapeutic efficacy in vivo[106]. Currently, NSCs loaded with CRAd-S-pk7 have been used in a clinical trial in patients with GBM (NCT03072134).
Other pre-clinical strategies of NSCs
Intranasal delivery of therapeutics to the brain is a novel strategy[107,108]. Dey et al[109] utilized hypoxic preconditioning or overexpression of CXCR4 to enhance the tumor-targeting ability of NSCs. They found that NSCs intranasally delivered oncolytic virus into glioma efficiently and extended the survival of mice. Spencer et al[110] found that methimazole (MT), a US-FDA-approved compound, effectively disrupted the olfactory epithelium, delayed clearance, and kept cells in the nasal cavity. After MT injection, oncolytic virus-loaded NSCs delivered intranasally significantly improved the survival of GBM-bearing mice[110-112]. Thus, intranasal delivery as a novel pharmacologic strategy can employ the non-invasive NSCs-based therapeutic platform to optimize the treatment.
Mesoporous silica nanoparticles (MSNs) have controlled-release capabilities and non-toxic features. Cheng et al[113] conjugated MSNs with 111In and administered 111In-MSN labeled NSCs into glioma-bearing mice via either intracranial or systemic injection. Their results revealed that 111In-MSN-NSCs actively migrated toward glioma xenografts[113]. Cheng et al[114] employed a pH-sensitive, MSN-doxorubicin (Dox)-loaded NSC delivery system for delaying drug release and non-invasively trigger programmed cell death. They found that MSN-Dox-loaded HB1.F3.CD cells efficiently preserved their migratory function and released MSN-Dox conjugates, causing significant toxicity to glioma cells, glioma apoptosis, and animal survival[114]. These results suggest a multimodal, controlled-release, therapeutic strategy.
Engineered tumoricidal neural stem cells (tNSCs) show potential for treating aggressive brain glioblastoma[94,99-101,115]. Sheets et al[116] optimized and used HB1.F3.CD cells to prepare a polymeric scaffold [nanofibrous electrospun poly (L-lactic acid) scaffolds]. They found that the polymeric scaffold significantly extended tNSC persistence in the cavity of a mouse model of human GBM resection/recurrence as the tNSCs migrated from the scaffolds into the tumors, both in vitro and in vivo. After engineering tNSCs with the prodrug/enzyme TK and transplanting them into the post-operative cavity of mice, the researchers found that the residual tumor volume of mice was markedly reduced, and the median survival times were extended[116]. Satterlee et al[117] used organotypic brain slice explants and distinct human glioma types to create a novel hybrid tumor model and then evaluated the efficacy of iNSCs loaded with TRAIL or enzyme-prodrug therapy. They found that tNSC-TRAIL significantly decreased tumor growth and promoted the survival of the animals[117]. These findings suggest a new strategy and model for testing targeted GBM therapy.Overall, as an effective drug delivery platform, NSCs can be modified for delivering various anti-tumor agents, including apoptotic agents, oncolytic viruses, or prodrug-activating enzymes, and optimized to improve their therapeutic benefits in glioblastomas.
CONCLUSION
With the development of gene-targeted therapy and further studies demonstrating the role of the TME in tumor progression, crosstalk between glioma and its microenvironment has been recognized, especially the communication of glioma and non-glioma cells. Recently, pre-clinical and clinical experiments confirmed that SVZ NSCs are closely related to glioma origin and progression through gene mutation and factor delivery. In general, gliomas are separated by a long distance from the SVZ region and may interact paracrine pathways, such as secreted cytokines and EVs. However, studies demonstrating an interaction between gliomas and SVZ are only preliminary, and the crosstalk mechanism remains unclear. In particular, SVZ NSCs are generally in a resting state but can be activated by brain disease or nerve damage. When gliomas occur, which can induce a state of intracranial stress, SVZ NSCs may be activated through the TME. However, how the microenvironment of glioma stimulates SVZ NSCs, and how SVZ NSCs react to the glioma, as well as the potential mechanisms, need further exploration (Figure 2). Thus, the genetic mutations or secreted factors associated with both GBMs and SVZ NSCs should be further examined.
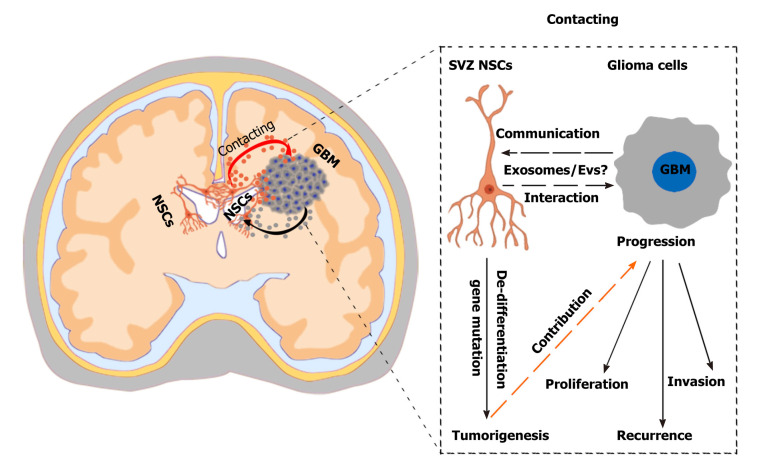
Figure 2.
Challenges and effects of neural stem cells in the subventricular zone in glioma progression. A hypothetical scenario shows the crosstalk between neural stem cells (NSCs) in the subventricular zone (SVZ) and glioblastoma multiformes and the effects of SVZ NSCs in glioma progression. SVZ NSCs can exert chemoattractant effects on glioma cells through secretion of chemoattractant complex factors [such as pleiotrophin, HSP90B, and secreted protein acidic and rich in cysteine (SPARC)/SPARC-like protein 1], and glioblastomas can induce SVZ NSCs to de-differentiate into tumor-promoting cells via glioblastoma-derived extracellular vesicles (EVs). The SVZ NSC-glioma interactions are mainly mediated by the secreted factors, especially EVs/exosomes. Understanding the biological mechanisms mediated by cytokines and EVs/exosomes will help to discover new therapeutic strategy. NSCs: Neural stem cells; SVZ: Subventricular zone; GBM: Glioblastoma multiforme.
In summary, as specialized stem cells in the nervous system, NSCs play vital roles in regulating physiopathological functions of the brain, including glioma development and progression. Studies of the interaction between SVZ NSCs and GBMs may reveal new molecular, epigenetic, and genetic characteristics that can be employed for combination therapy. Further research is needed to verify the mechanisms and advantages of SVZ NSCs in glioma progression and discover specific gene target treatment to increase the survival of patients with GBM. By exploring how gliomas stimulate the activation of SVZ NSCs, and how SVZ NSCs regulate the development and progress of gliomas, particularly the interaction mechanism of glioma-NSC mediated by secreted EVs/exosomes or factors, potential therapeutic strategies can be developed to treat gliomas.
ACKNOWLEDGEMENTS
We really appreciate Dr. Ouyang SS for assistance with the valuable suggestions and image editing.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interests for this article.
Manuscript source: Invited manuscript
Peer-review started: February 26, 2021
First decision: April 20, 2021
Article in press: June 18, 2021
Specialty type: Cell biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lei T S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Xing YX
Contributor Information
Gui-Long Zhang, Department of Neurosurgery, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou 510260, Guangdong Province, China.
Chuan-Fang Wang, Department of Neurosurgery, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou 510260, Guangdong Province, China.
Cheng Qian, Department of Neurosurgery, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou 510260, Guangdong Province, China.
Yun-Xiang Ji, Department of Neurosurgery, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou 510260, Guangdong Province, China.
Ye-Zhong Wang, Department of Neurosurgery, The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou 510260, Guangdong Province, China. wangyezhong@gzhmu.edu.cn.
References
- 1.Shergalis A, Bankhead A 3rd, Luesakul U, Muangsin N, Neamati N. Current Challenges and Opportunities in Treating Glioblastoma. Pharmacol Rev . 2018;70:412–445. doi: 10.1124/pr.117.014944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet . 2018;392:432–446. doi: 10.1016/S0140-6736(18)30990-5. [DOI] [PubMed] [Google Scholar]
- 3.Desjardins A, Gromeier M, Herndon JE 2nd, Beaubier N, Bolognesi DP, Friedman AH, Friedman HS, McSherry F, Muscat AM, Nair S, Peters KB, Randazzo D, Sampson JH, Vlahovic G, Harrison WT, McLendon RE, Ashley D, Bigner DD. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N Engl J Med . 2018;379:150–161. doi: 10.1056/NEJMoa1716435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Xu T, Huang Q, Jin W, Chen J. Immunotherapy for Malignant Glioma: Current Status and Future Directions. Trends Pharmacol Sci . 2020;41:123–138. doi: 10.1016/j.tips.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Pine AR, Cirigliano SM, Nicholson JG, Hu Y, Linkous A, Miyaguchi K, Edwards L, Singhania R, Schwartz TH, Ramakrishna R, Pisapia DJ, Snuderl M, Elemento O, Fine HA. Tumor Microenvironment Is Critical for the Maintenance of Cellular States Found in Primary Glioblastomas. Cancer Discov . 2020;10:964–979. doi: 10.1158/2159-8290.CD-20-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye L, Park JJ, Dong MB, Yang Q, Chow RD, Peng L, Du Y, Guo J, Dai X, Wang G, Errami Y, Chen S. In vivo CRISPR screening in CD8 T cells with AAV-Sleeping Beauty hybrid vectors identifies membrane targets for improving immunotherapy for glioblastoma. Nat Biotechnol . 2019;37:1302–1313. doi: 10.1038/s41587-019-0246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, Wang AC, Ellingson BM, Rytlewski JA, Sanders CM, Kawaguchi ES, Du L, Li G, Yong WH, Gaffey SC, Cohen AL, Mellinghoff IK, Lee EQ, Reardon DA, O'Brien BJ, Butowski NA, Nghiemphu PL, Clarke JL, Arrillaga-Romany IC, Colman H, Kaley TJ, de Groot JF, Liau LM, Wen PY, Prins RM. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med . 2019;25:477–486. doi: 10.1038/s41591-018-0337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Starr R, Chang WC, Aguilar B, Alizadeh D, Wright SL, Yang X, Brito A, Sarkissian A, Ostberg JR, Li L, Shi Y, Gutova M, Aboody K, Badie B, Forman SJ, Barish ME, Brown CE. Chlorotoxin-directed CAR T cells for specific and effective targeting of glioblastoma. Sci Transl Med . 2020;12 doi: 10.1126/scitranslmed.aaw2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiffer D, Annovazzi L, Casalone C, Corona C, Mellai M. Glioblastoma: Microenvironment and Niche Concept. Cancers (Basel) . 2018;11 doi: 10.3390/cancers11010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mai WX, Gosa L, Daniels VW, Ta L, Tsang JE, Higgins B, Gilmore WB, Bayley NA, Harati MD, Lee JT, Yong WH, Kornblum HI, Bensinger SJ, Mischel PS, Rao PN, Clark PM, Cloughesy TF, Letai A, Nathanson DA. Cytoplasmic p53 couples oncogene-driven glucose metabolism to apoptosis and is a therapeutic target in glioblastoma. Nat Med . 2017;23:1342–1351. doi: 10.1038/nm.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klughammer J, Kiesel B, Roetzer T, Fortelny N, Nemc A, Nenning KH, Furtner J, Sheffield NC, Datlinger P, Peter N, Nowosielski M, Augustin M, Mischkulnig M, Ströbel T, Alpar D, Ergüner B, Senekowitsch M, Moser P, Freyschlag CF, Kerschbaumer J, Thomé C, Grams AE, Stockhammer G, Kitzwoegerer M, Oberndorfer S, Marhold F, Weis S, Trenkler J, Buchroithner J, Pichler J, Haybaeck J, Krassnig S, Mahdy Ali K, von Campe G, Payer F, Sherif C, Preiser J, Hauser T, Winkler PA, Kleindienst W, Würtz F, Brandner-Kokalj T, Stultschnig M, Schweiger S, Dieckmann K, Preusser M, Langs G, Baumann B, Knosp E, Widhalm G, Marosi C, Hainfellner JA, Woehrer A, Bock C. The DNA methylation landscape of glioblastoma disease progression shows extensive heterogeneity in time and space. Nat Med . 2018;24:1611–1624. doi: 10.1038/s41591-018-0156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bi J, Chowdhry S, Wu S, Zhang W, Masui K, Mischel PS. Altered cellular metabolism in gliomas - an emerging landscape of actionable co-dependency targets. Nat Rev Cancer . 2020;20:57–70. doi: 10.1038/s41568-019-0226-5. [DOI] [PubMed] [Google Scholar]
- 13.Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtås S, van Roon-Mom WM, Björk-Eriksson T, Nordborg C, Frisén J, Dragunow M, Faull RL, Eriksson PS. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science . 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 14.Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell . 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Ortega JA, Sirois CL, Memi F, Glidden N, Zecevic N. Oxygen Levels Regulate the Development of Human Cortical Radial Glia Cells. Cereb Cortex . 2017;27:3736–3751. doi: 10.1093/cercor/bhw194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mistry AM, Dewan MC, White-Dzuro GA, Brinson PR, Weaver KD, Thompson RC, Ihrie RA, Chambless LB. Decreased survival in glioblastomas is specific to contact with the ventricular-subventricular zone, not subgranular zone or corpus callosum. J Neurooncol . 2017;132:341–349. doi: 10.1007/s11060-017-2374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mistry AM, Hale AT, Chambless LB, Weaver KD, Thompson RC, Ihrie RA. Influence of glioblastoma contact with the lateral ventricle on survival: a meta-analysis. J Neurooncol . 2017;131:125–133. doi: 10.1007/s11060-016-2278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwan K, Schneider JR, Patel NV, Boockvar JA. Tracing the Origin of Glioblastoma: Subventricular Zone Neural Stem Cells. Neurosurgery . 2019;84:E15–E16. doi: 10.1093/neuros/nyy512. [DOI] [PubMed] [Google Scholar]
- 19.Yamaki T, Shibahra I, Matsuda KI, Kanemura Y, Konta T, Kanamori M, Yamakawa M, Tominaga T, Sonoda Y. Relationships between recurrence patterns and subventricular zone involvement or CD133 expression in glioblastoma. J Neurooncol . 2020;146:489–499. doi: 10.1007/s11060-019-03381-y. [DOI] [PubMed] [Google Scholar]
- 20.Lee JH, Lee JE, Kahng JY, Kim SH, Park JS, Yoon SJ, Um JY, Kim WK, Lee JK, Park J, Kim EH, Lee JH, Chung WS, Ju YS, Park SH, Chang JH, Kang SG. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature . 2018;560:243–247. doi: 10.1038/s41586-018-0389-3. [DOI] [PubMed] [Google Scholar]
- 21.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells. Cancer Cell . 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Nakano I, Garnier D, Minata M, Rak J. Extracellular vesicles in the biology of brain tumour stem cells--Implications for inter-cellular communication, therapy and biomarker development. Semin Cell Dev Biol . 2015;40:17–26. doi: 10.1016/j.semcdb.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Jung E, Alfonso J, Osswald M, Monyer H, Wick W, Winkler F. Emerging intersections between neuroscience and glioma biology. Nat Neurosci . 2019;22:1951–1960. doi: 10.1038/s41593-019-0540-y. [DOI] [PubMed] [Google Scholar]
- 24.Gao X, Zhang Z, Mashimo T, Shen B, Nyagilo J, Wang H, Wang Y, Liu Z, Mulgaonkar A, Hu XL, Piccirillo SGM, Eskiocak U, Davé DP, Qin S, Yang Y, Sun X, Fu YX, Zong H, Sun W, Bachoo RM, Ge WP. Gliomas Interact with Non-glioma Brain Cells via Extracellular Vesicles. Cell Rep. 2020;30:2489–2500.e5. doi: 10.1016/j.celrep.2020.01.089. [DOI] [PubMed] [Google Scholar]
- 25.Broekman ML, Maas SLN, Abels ER, Mempel TR, Krichevsky AM, Breakefield XO. Multidimensional communication in the microenvirons of glioblastoma. Nat Rev Neurol . 2018;14:482–495. doi: 10.1038/s41582-018-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, Gibson EM, Mount CW, Polepalli J, Mitra SS, Woo PJ, Malenka RC, Vogel H, Bredel M, Mallick P, Monje M. Neuronal Activity Promotes Glioma Growth through Neuroligin-3 Secretion. Cell . 2015;161:803–816. doi: 10.1016/j.cell.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venkatesh HS, Morishita W, Geraghty AC, Silverbush D, Gillespie SM, Arzt M, Tam LT, Espenel C, Ponnuswami A, Ni L, Woo PJ, Taylor KR, Agarwal A, Regev A, Brang D, Vogel H, Hervey-Jumper S, Bergles DE, Suvà ML, Malenka RC, Monje M. Electrical and synaptic integration of glioma into neural circuits. Nature . 2019;573:539–545. doi: 10.1038/s41586-019-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim-Fat MJ, Wen PY. Glioma progression through synaptic activity. Nat Rev Neurol . 2020;16:6–7. doi: 10.1038/s41582-019-0290-1. [DOI] [PubMed] [Google Scholar]
- 29.Liddelow SA, Barres BA. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity . 2017;46:957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Priego N, Zhu L, Monteiro C, Mulders M, Wasilewski D, Bindeman W, Doglio L, Martínez L, Martínez-Saez E, Ramón Y Cajal S, Megías D, Hernández-Encinas E, Blanco-Aparicio C, Zarzuela E, Muñoz J, Fustero-Torre C, Piñeiro-Yáñez E, Hernández-Laín A, Bertero L, Poli V, Sanchez-Martinez M, Menendez JA, Soffietti R, Bosch-Barrera J, Valiente M. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat Med . 2018;24:1024–1035. doi: 10.1038/s41591-018-0044-4. [DOI] [PubMed] [Google Scholar]
- 31.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci . 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu T, Wang X, Zhi T, Zhang J, Wang Y, Nie E, Zhou F, You Y, Liu N. Delivery of MGMT mRNA to glioma cells by reactive astrocyte-derived exosomes confers a temozolomide resistance phenotype. Cancer Lett . 2018;433:210–220. doi: 10.1016/j.canlet.2018.06.041. [DOI] [PubMed] [Google Scholar]
- 33.Henrik Heiland D, Ravi VM, Behringer SP, Frenking JH, Wurm J, Joseph K, Garrelfs NWC, Strähle J, Heynckes S, Grauvogel J, Franco P, Mader I, Schneider M, Potthoff AL, Delev D, Hofmann UG, Fung C, Beck J, Sankowski R, Prinz M, Schnell O. Tumor-associated reactive astrocytes aid the evolution of immunosuppressive environment in glioblastoma. Nat Commun . 2019;10:2541. doi: 10.1038/s41467-019-10493-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen P, Hsu WH, Chang A, Tan Z, Lan Z, Zhou A, Spring DJ, Lang FF, Wang YA, DePinho RA. Circadian Regulator CLOCK Recruits Immune-Suppressive Microglia into the GBM Tumor Microenvironment. Cancer Discov . 2020;10:371–381. doi: 10.1158/2159-8290.CD-19-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miranda A, Hamilton PT, Zhang AW, Pattnaik S, Becht E, Mezheyeuski A, Bruun J, Micke P, de Reynies A, Nelson BH. Cancer stemness, intratumoral heterogeneity, and immune response across cancers. Proc Natl Acad Sci USA . 2019;116:9020–9029. doi: 10.1073/pnas.1818210116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen P, Zhao D, Li J, Liang X, Chang A, Henry VK, Lan Z, Spring DJ, Rao G, Wang YA, DePinho RA. Symbiotic Macrophage-Glioma Cell Interactions Reveal Synthetic Lethality in PTEN-Null Glioma. Cancer Cell. 2019;35:868–884.e6. doi: 10.1016/j.ccell.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poon CC, Sarkar S, Yong VW, Kelly JJP. Glioblastoma-associated microglia and macrophages: targets for therapies to improve prognosis. Brain . 2017;140:1548–1560. doi: 10.1093/brain/aww355. [DOI] [PubMed] [Google Scholar]
- 38.Griveau A, Seano G, Shelton SJ, Kupp R, Jahangiri A, Obernier K, Krishnan S, Lindberg OR, Yuen TJ, Tien AC, Sabo JK, Wang N, Chen I, Kloepper J, Larrouquere L, Ghosh M, Tirosh I, Huillard E, Alvarez-Buylla A, Oldham MC, Persson AI, Weiss WA, Batchelor TT, Stemmer-Rachamimov A, Suvà ML, Phillips JJ, Aghi MK, Mehta S, Jain RK, Rowitch DH. A Glial Signature and Wnt7 Signaling Regulate Glioma-Vascular Interactions and Tumor Microenvironment. Cancer Cell. 2018;33:874–889.e7. doi: 10.1016/j.ccell.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai HH, Niu J, Munji R, Davalos D, Chang J, Zhang H, Tien AC, Kuo CJ, Chan JR, Daneman R, Fancy SP. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science . 2016;351:379–384. doi: 10.1126/science.aad3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roesler R. Interplay between neural stem cells and glioblastoma: possible role of neurotrophin signaling. Clin Transl Oncol . 2019;21:1578–1579. doi: 10.1007/s12094-019-02206-8. [DOI] [PubMed] [Google Scholar]
- 41.Jiang Y, Marinescu VD, Xie Y, Jarvius M, Maturi NP, Haglund C, Olofsson S, Lindberg N, Olofsson T, Leijonmarck C, Hesselager G, Alafuzoff I, Fryknäs M, Larsson R, Nelander S, Uhrbom L. Glioblastoma Cell Malignancy and Drug Sensitivity Are Affected by the Cell of Origin. Cell Rep . 2017;18:977–990. doi: 10.1016/j.celrep.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Ham SW, Jeon HY, Jin X, Kim EJ, Kim JK, Shin YJ, Lee Y, Kim SH, Lee SY, Seo S, Park MG, Kim HM, Nam DH, Kim H. TP53 gain-of-function mutation promotes inflammation in glioblastoma. Cell Death Differ . 2019;26:409–425. doi: 10.1038/s41418-018-0126-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alcantara Llaguno SR, Parada LF. Cell of origin of glioma: biological and clinical implications. Br J Cancer . 2016;115:1445–1450. doi: 10.1038/bjc.2016.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kusne Y, Sanai N. The SVZ and Its Relationship to Stem Cell Based Neuro-oncogenesis. Adv Exp Med Biol . 2015;853:23–32. doi: 10.1007/978-3-319-16537-0_2. [DOI] [PubMed] [Google Scholar]
- 45.Alderton GK. Tumorigenesis: the origins of glioma. Nat Rev Cancer . 2011;11:627. doi: 10.1038/nrc3129. [DOI] [PubMed] [Google Scholar]
- 46.Matarredona ER, Pastor AM. Neural Stem Cells of the Subventricular Zone as the Origin of Human Glioblastoma Stem Cells. Therapeutic Implications. Front Oncol . 2019;9:779. doi: 10.3389/fonc.2019.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alcantara Llaguno S, Sun D, Pedraza AM, Vera E, Wang Z, Burns DK, Parada LF. Cell-of-origin susceptibility to glioblastoma formation declines with neural lineage restriction. Nat Neurosci . 2019;22:545–555. doi: 10.1038/s41593-018-0333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Modrek AS, Bayin NS, Placantonakis DG. Brain stem cells as the cell of origin in glioma. World J Stem Cells . 2014;6:43–52. doi: 10.4252/wjsc.v6.i1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JH, Lee JH. The origin-of-cell harboring cancer-driving mutations in human glioblastoma. BMB Rep . 2018;51:481–483. doi: 10.5483/BMBRep.2018.51.10.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu HK, Wang Y, Belz T, Bock D, Takacs A, Radlwimmer B, Barbus S, Reifenberger G, Lichter P, Schütz G. The nuclear receptor tailless induces long-term neural stem cell expansion and brain tumor initiation. Genes Dev . 2010;24:683–695. doi: 10.1101/gad.560310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gil-Perotin S, Marin-Husstege M, Li J, Soriano-Navarro M, Zindy F, Roussel MF, Garcia-Verdugo JM, Casaccia-Bonnefil P. Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors. J Neurosci . 2006;26:1107–1116. doi: 10.1523/JNEUROSCI.3970-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z, Stommel JM, Dunn KL, Wiedemeyer R, You MJ, Brennan C, Wang YA, Ligon KL, Wong WH, Chin L, DePinho RA. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature . 2008;455:1129–1133. doi: 10.1038/nature07443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y, Yang J, Zheng H, Tomasek GJ, Zhang P, McKeever PE, Lee EY, Zhu Y. Expression of mutant p53 proteins implicates a lineage relationship between neural stem cells and malignant astrocytic glioma in a murine model. Cancer Cell . 2009;15:514–526. doi: 10.1016/j.ccr.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Modrek AS, Golub D, Khan T, Bready D, Prado J, Bowman C, Deng J, Zhang G, Rocha PP, Raviram R, Lazaris C, Stafford JM, LeRoy G, Kader M, Dhaliwal J, Bayin NS, Frenster JD, Serrano J, Chiriboga L, Baitalmal R, Nanjangud G, Chi AS, Golfinos JG, Wang J, Karajannis MA, Bonneau RA, Reinberg D, Tsirigos A, Zagzag D, Snuderl M, Skok JA, Neubert TA, Placantonakis DG. Low-Grade Astrocytoma Mutations in IDH1, P53, and ATRX Cooperate to Block Differentiation of Human Neural Stem Cells via Repression of SOX2. Cell Rep . 2017;21:1267–1280. doi: 10.1016/j.celrep.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pirozzi CJ, Carpenter AB, Waitkus MS, Wang CY, Zhu H, Hansen LJ, Chen LH, Greer PK, Feng J, Wang Y, Bock CB, Fan P, Spasojevic I, McLendon RE, Bigner DD, He Y, Yan H. Mutant IDH1 Disrupts the Mouse Subventricular Zone and Alters Brain Tumor Progression. Mol Cancer Res . 2017;15:507–520. doi: 10.1158/1541-7786.MCR-16-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bardella C, Al-Dalahmah O, Krell D, Brazauskas P, Al-Qahtani K, Tomkova M, Adam J, Serres S, Lockstone H, Freeman-Mills L, Pfeffer I, Sibson N, Goldin R, Schuster-Böeckler B, Pollard PJ, Soga T, McCullagh JS, Schofield CJ, Mulholland P, Ansorge O, Kriaucionis S, Ratcliffe PJ, Szele FG, Tomlinson I. Expression of Idh1R132H in the Murine Subventricular Zone Stem Cell Niche Recapitulates Features of Early Gliomagenesis. Cancer Cell . 2016;30:578–594. doi: 10.1016/j.ccell.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abel TW, Clark C, Bierie B, Chytil A, Aakre M, Gorska A, Moses HL. GFAP-Cre-mediated activation of oncogenic K-ras results in expansion of the subventricular zone and infiltrating glioma. Mol Cancer Res . 2009;7:645–653. doi: 10.1158/1541-7786.MCR-08-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Daniel PM, Filiz G, Brown DV, Christie M, Waring PM, Zhang Y, Haynes JM, Pouton C, Flanagan D, Vincan E, Johns TG, Montgomery K, Phillips WA, Mantamadiotis T. PI3K activation in neural stem cells drives tumorigenesis which can be ameliorated by targeting the cAMP response element binding protein. Neuro Oncol . 2018;20:1344–1355. doi: 10.1093/neuonc/noy068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang R, Chen LH, Hansen LJ, Carpenter AB, Moure CJ, Liu H, Pirozzi CJ, Diplas BH, Waitkus MS, Greer PK, Zhu H, McLendon RE, Bigner DD, He Y, Yan H. Cic Loss Promotes Gliomagenesis via Aberrant Neural Stem Cell Proliferation and Differentiation. Cancer Res . 2017;77:6097–6108. doi: 10.1158/0008-5472.CAN-17-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bulstrode H, Johnstone E, Marques-Torrejon MA, Ferguson KM, Bressan RB, Blin C, Grant V, Gogolok S, Gangoso E, Gagrica S, Ender C, Fotaki V, Sproul D, Bertone P, Pollard SM. Elevated FOXG1 and SOX2 in glioblastoma enforces neural stem cell identity through transcriptional control of cell cycle and epigenetic regulators. Genes Dev . 2017;31:757–773. doi: 10.1101/gad.293027.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao Y, Fotovati A, Lee C, Wang M, Cote G, Guns E, Toyota B, Faury D, Jabado N, Dunn SE. Inhibition of Y-box binding protein-1 slows the growth of glioblastoma multiforme and sensitizes to temozolomide independent O6-methylguanine-DNA methyltransferase. Mol Cancer Ther . 2009;8:3276–3284. doi: 10.1158/1535-7163.MCT-09-0478. [DOI] [PubMed] [Google Scholar]
- 62.Faury D, Nantel A, Dunn SE, Guiot MC, Haque T, Hauser P, Garami M, Bognár L, Hanzély Z, Liberski PP, Lopez-Aguilar E, Valera ET, Tone LG, Carret AS, Del Maestro RF, Gleave M, Montes JL, Pietsch T, Albrecht S, Jabado N. Molecular profiling identifies prognostic subgroups of pediatric glioblastoma and shows increased YB-1 expression in tumors. J Clin Oncol . 2007;25:1196–1208. doi: 10.1200/JCO.2006.07.8626. [DOI] [PubMed] [Google Scholar]
- 63.Fotovati A, Abu-Ali S, Wang PS, Deleyrolle LP, Lee C, Triscott J, Chen JY, Franciosi S, Nakamura Y, Sugita Y, Uchiumi T, Kuwano M, Leavitt BR, Singh SK, Jury A, Jones C, Wakimoto H, Reynolds BA, Pallen CJ, Dunn SE. YB-1 bridges neural stem cells and brain tumor-initiating cells via its roles in differentiation and cell growth. Cancer Res . 2011;71:5569–5578. doi: 10.1158/0008-5472.CAN-10-2805. [DOI] [PubMed] [Google Scholar]
- 64.Walton NM, Snyder GE, Park D, Kobeissy F, Scheffler B, Steindler DA. Gliotypic neural stem cells transiently adopt tumorigenic properties during normal differentiation. Stem Cells . 2009;27:280–289. doi: 10.1634/stemcells.2008-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu C, Sage JC, Miller MR, Verhaak RG, Hippenmeyer S, Vogel H, Foreman O, Bronson RT, Nishiyama A, Luo L, Zong H. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell . 2011;146:209–221. doi: 10.1016/j.cell.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Dijken BRJ, Yan JL, Boonzaier NR, Li C, van Laar PJ, van der Hoorn A, Price SJ. Subventricular Zone Involvement Characterized by Diffusion Tensor Imaging in Glioblastoma. World Neurosurg . 2017;105:697–701. doi: 10.1016/j.wneu.2017.06.075. [DOI] [PubMed] [Google Scholar]
- 67.Tomita T, Akimoto J, Haraoka J, Kudo M. Clinicopathological significance of expression of nestin, a neural stem/progenitor cell marker, in human glioma tissue. Brain Tumor Pathol . 2014;31:162–171. doi: 10.1007/s10014-013-0169-6. [DOI] [PubMed] [Google Scholar]
- 68.Altmann C, Keller S, Schmidt MHH. The Role of SVZ Stem Cells in Glioblastoma. Cancers (Basel) . 2019;11 doi: 10.3390/cancers11040448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Şuşman S, Leucuţa DC, Kacso G, Florian ŞI. High dose vs low dose irradiation of the subventricular zone in patients with glioblastoma-a systematic review and meta-analysis. Cancer Manag Res . 2019;11:6741–6753. doi: 10.2147/CMAR.S206033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen L, Chaichana KL, Kleinberg L, Ye X, Quinones-Hinojosa A, Redmond K. Glioblastoma recurrence patterns near neural stem cell regions. Radiother Oncol . 2015;116:294–300. doi: 10.1016/j.radonc.2015.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gollapalli K, Ghantasala S, Kumar S, Srivastava R, Rapole S, Moiyadi A, Epari S, Srivastava S. Subventricular zone involvement in Glioblastoma - A proteomic evaluation and clinicoradiological correlation. Sci Rep . 2017;7:1449. doi: 10.1038/s41598-017-01202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res . 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 73.Okawa S, Gagrica S, Blin C, Ender C, Pollard SM, Krijgsveld J. Proteome and Secretome Characterization of Glioblastoma-Derived Neural Stem Cells. Stem Cells . 2017;35:967–980. doi: 10.1002/stem.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qin EY, Cooper DD, Abbott KL, Lennon J, Nagaraja S, Mackay A, Jones C, Vogel H, Jackson PK, Monje M. Neural Precursor-Derived Pleiotrophin Mediates Subventricular Zone Invasion by Glioma. Cell . 2017;170:845–859.e19. doi: 10.1016/j.cell.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Y, Kim MS, Jia B, Yan J, Zuniga-Hertz JP, Han C, Cai D. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature . 2017;548:52–57. doi: 10.1038/nature23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yan W, Wu X, Zhou W, Fong MY, Cao M, Liu J, Liu X, Chen CH, Fadare O, Pizzo DP, Wu J, Liu L, Chin AR, Ren X, Chen Y, Locasale JW, Wang SE. Cancer-cell-secreted exosomal miR-105 promotes tumour growth through the MYC-dependent metabolic reprogramming of stromal cells. Nat Cell Biol . 2018;20:597–609. doi: 10.1038/s41556-018-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, Xia H, Man Q, Zhong W, Antelo LF, Wu B, Xiong X, Liu X, Guan L, Li T, Liu S, Yang R, Lu Y, Dong L, McGettigan S, Somasundaram R, Radhakrishnan R, Mills G, Kim J, Chen YH, Dong H, Zhao Y, Karakousis GC, Mitchell TC, Schuchter LM, Herlyn M, Wherry EJ, Xu X, Guo W. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature . 2018;560:382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Janas AM, Sapoń K, Janas T, Stowell MH. Exosomes and other extracellular vesicles in neural cells and neurodegenerative diseases. Biochim Biophys Acta . 2016;1858:1139–1151. doi: 10.1016/j.bbamem.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 79.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol . 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 80.Xia X, Wang Y, Huang Y, Zhang H, Lu H, Zheng JC. Exosomal miRNAs in central nervous system diseases: biomarkers, pathological mediators, protective factors and therapeutic agents. Prog Neurobiol . 2019;183:101694. doi: 10.1016/j.pneurobio.2019.101694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang J, Liu J, Sun G, Meng H, Wang J, Guan Y, Yin Y, Zhao Z, Dong X, Yin S, Li H, Cheng Y, Wu H, Wu A, Yu X, Chen L. Glioblastoma extracellular vesicles induce the tumour-promoting transformation of neural stem cells. Cancer Lett . 2019;466:1–12. doi: 10.1016/j.canlet.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 82.Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci . 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- 83.Ahmed AU, Alexiades NG, Lesniak MS. The use of neural stem cells in cancer gene therapy: predicting the path to the clinic. Curr Opin Mol Ther . 2010;12:546–552. [PMC free article] [PubMed] [Google Scholar]
- 84.Mutukula N, Elkabetz Y. "Neural Killer" Cells: Autologous Cytotoxic Neural Stem Cells for Fighting Glioma. Cell Stem Cell . 2017;20:426–428. doi: 10.1016/j.stem.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 85.Aboody KS, Najbauer J, Metz MZ, D'Apuzzo M, Gutova M, Annala AJ, Synold TW, Couture LA, Blanchard S, Moats RA, Garcia E, Aramburo S, Valenzuela VV, Frank RT, Barish ME, Brown CE, Kim SU, Badie B, Portnow J. Neural stem cell-mediated enzyme/prodrug therapy for glioma: preclinical studies. Sci Transl Med . 2013;5:184ra59. doi: 10.1126/scitranslmed.3005365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ahmed AU, Tyler MA, Thaci B, Alexiades NG, Han Y, Ulasov IV, Lesniak MS. A comparative study of neural and mesenchymal stem cell-based carriers for oncolytic adenovirus in a model of malignant glioma. Mol Pharm . 2011;8:1559–1572. doi: 10.1021/mp200161f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen L, Guerrero-Cazares H, Ye X, Ford E, McNutt T, Kleinberg L, Lim M, Chaichana K, Quinones-Hinojosa A, Redmond K. Increased subventricular zone radiation dose correlates with survival in glioblastoma patients after gross total resection. Int J Radiat Oncol Biol Phys . 2013;86:616–622. doi: 10.1016/j.ijrobp.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cameron BD, Traver G, Roland JT, Brockman AA, Dean D, Johnson L, Boyd K, Ihrie RA, Freeman ML. Bcl2-Expressing Quiescent Type B Neural Stem Cells in the Ventricular-Subventricular Zone Are Resistant to Concurrent Temozolomide/X-Irradiation. Stem Cells . 2019;37:1629–1639. doi: 10.1002/stem.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee P, Eppinga W, Lagerwaard F, Cloughesy T, Slotman B, Nghiemphu PL, Wang PC, Kupelian P, Agazaryan N, Demarco J, Selch MT, Steinberg M, Kang JJ. Evaluation of high ipsilateral subventricular zone radiation therapy dose in glioblastoma: a pooled analysis. Int J Radiat Oncol Biol Phys . 2013;86:609–615. doi: 10.1016/j.ijrobp.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 90.Muracciole X, El-Amine W, Tabouret E, Boucekine M, Barlier A, Petrirena G, Harivony T, Solignac L, Chinot OL, Macagno N, Figarella-Branger D, Padovani L. Negative Survival Impact of High Radiation Doses to Neural Stem Cells Niches in an IDH-Wild-Type Glioblastoma Population. Front Oncol . 2018;8:426. doi: 10.3389/fonc.2018.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cho N, Wang C, Raymond C, Kaprealian T, Ji M, Salamon N, Pope WB, Nghiemphu PL, Lai A, Cloughesy TF, Ellingson BM. Diffusion MRI changes in the anterior subventricular zone following chemoradiation in glioblastoma with posterior ventricular involvement. J Neurooncol . 2020;147:643–652. doi: 10.1007/s11060-020-03460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shah K, Bureau E, Kim DE, Yang K, Tang Y, Weissleder R, Breakefield XO. Glioma therapy and real-time imaging of neural precursor cell migration and tumor regression. Ann Neurol . 2005;57:34–41. doi: 10.1002/ana.20306. [DOI] [PubMed] [Google Scholar]
- 93.Staflin K, Honeth G, Kalliomäki S, Kjellman C, Edvardsen K, Lindvall M. Neural progenitor cell lines inhibit rat tumor growth in vivo. Cancer Res . 2004;64:5347–5354. doi: 10.1158/0008-5472.CAN-03-1246. [DOI] [PubMed] [Google Scholar]
- 94.Walzlein JH, Synowitz M, Engels B, Markovic DS, Gabrusiewicz K, Nikolaev E, Yoshikawa K, Kaminska B, Kempermann G, Uckert W, Kaczmarek L, Kettenmann H, Glass R. The antitumorigenic response of neural precursors depends on subventricular proliferation and age. Stem Cells . 2008;26:2945–2954. doi: 10.1634/stemcells.2008-0307. [DOI] [PubMed] [Google Scholar]
- 95.Glass R, Synowitz M, Kronenberg G, Walzlein JH, Markovic DS, Wang LP, Gast D, Kiwit J, Kempermann G, Kettenmann H. Glioblastoma-induced attraction of endogenous neural precursor cells is associated with improved survival. J Neurosci . 2005;25:2637–2646. doi: 10.1523/JNEUROSCI.5118-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, Galli R, Selleri S, Di Meco F, De Fraja C, Vescovi A, Cattaneo E, Finocchiaro G. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med . 2000;6:447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- 97.Liu S, Yin F, Zhao M, Zhou C, Ren J, Huang Q, Zhao Z, Mitra R, Fan W, Fan M. The homing and inhibiting effects of hNSCs-BMP4 on human glioma stem cells. Oncotarget . 2016;7:17920–17931. doi: 10.18632/oncotarget.7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lujan E, Chanda S, Ahlenius H, Südhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci U S A . 2012;109:2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bagó JR, Alfonso-Pecchio A, Okolie O, Dumitru R, Rinkenbaugh A, Baldwin AS, Miller CR, Magness ST, Hingtgen SD. Therapeutically engineered induced neural stem cells are tumour-homing and inhibit progression of glioblastoma. Nat Commun . 2016;7:10593. doi: 10.1038/ncomms10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bagó JR, Okolie O, Dumitru R, Ewend MG, Parker JS, Werff RV, Underhill TM, Schmid RS, Miller CR, Hingtgen SD. Tumor-homing cytotoxic human induced neural stem cells for cancer therapy. Sci Transl Med . 2017;9 doi: 10.1126/scitranslmed.aah6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Portnow J, Synold TW, Badie B, Tirughana R, Lacey SF, D'Apuzzo M, Metz MZ, Najbauer J, Bedell V, Vo T, Gutova M, Frankel P, Chen M, Aboody KS. Neural Stem Cell-Based Anticancer Gene Therapy: A First-in-Human Study in Recurrent High-Grade Glioma Patients. Clin Cancer Res . 2017;23:2951–2960. doi: 10.1158/1078-0432.CCR-16-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Teng J, Hejazi S, Badr CE, Tannous BA. Systemic anticancer neural stem cells in combination with a cardiac glycoside for glioblastoma therapy. Stem Cells . 2014;32:2021–2032. doi: 10.1002/stem.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Thaci B, Ahmed AU, Ulasov IV, Tobias AL, Han Y, Aboody KS, Lesniak MS. Pharmacokinetic study of neural stem cell-based cell carrier for oncolytic virotherapy: targeted delivery of the therapeutic payload in an orthotopic brain tumor model. Cancer Gene Ther . 2012;19:431–442. doi: 10.1038/cgt.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kanai R, Rabkin SD, Yip S, Sgubin D, Zaupa CM, Hirose Y, Louis DN, Wakimoto H, Martuza RL. Oncolytic virus-mediated manipulation of DNA damage responses: synergy with chemotherapy in killing glioblastoma stem cells. J Natl Cancer Inst . 2012;104:42–55. doi: 10.1093/jnci/djr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ahmed AU, Thaci B, Tobias AL, Auffinger B, Zhang L, Cheng Y, Kim CK, Yunis C, Han Y, Alexiades NG, Fan X, Aboody KS, Lesniak MS. A preclinical evaluation of neural stem cell-based cell carrier for targeted antiglioma oncolytic virotherapy. J Natl Cancer Inst . 2013;105:968–977. doi: 10.1093/jnci/djt141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim CK, Ahmed AU, Auffinger B, Ulasov IV, Tobias AL, Moon KS, Lesniak MS. N-acetylcysteine amide augments the therapeutic effect of neural stem cell-based antiglioma oncolytic virotherapy. Mol Ther . 2013;21:2063–2073. doi: 10.1038/mt.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reitz M, Demestre M, Sedlacik J, Meissner H, Fiehler J, Kim SU, Westphal M, Schmidt NO. Intranasal delivery of neural stem/progenitor cells: a noninvasive passage to target intracerebral glioma. Stem Cells Transl Med . 2012;1:866–873. doi: 10.5966/sctm.2012-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim ID, Shin JH, Kim SW, Choi S, Ahn J, Han PL, Park JS, Lee JK. Intranasal delivery of HMGB1 siRNA confers target gene knockdown and robust neuroprotection in the postischemic brain. Mol Ther . 2012;20:829–839. doi: 10.1038/mt.2011.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dey M, Yu D, Kanojia D, Li G, Sukhanova M, Spencer DA, Pituch KC, Zhang L, Han Y, Ahmed AU, Aboody KS, Lesniak MS, Balyasnikova IV. Intranasal Oncolytic Virotherapy with CXCR4-Enhanced Stem Cells Extends Survival in Mouse Model of Glioma. Stem Cell Reports . 2016;7:471–482. doi: 10.1016/j.stemcr.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Spencer D, Yu D, Morshed RA, Li G, Pituch KC, Gao DX, Bertolino N, Procissi D, Lesniak MS, Balyasnikova IV. Pharmacologic modulation of nasal epithelium augments neural stem cell targeting of glioblastoma. Theranostics . 2019;9:2071–2083. doi: 10.7150/thno.29581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Florek J, Caillard R, Kleitz F. Evaluation of mesoporous silica nanoparticles for oral drug delivery - current status and perspective of MSNs drug carriers. Nanoscale . 2017;9:15252–15277. doi: 10.1039/c7nr05762h. [DOI] [PubMed] [Google Scholar]
- 112.Hartono SB, Phuoc NT, Yu M, Jia Z, Monteiro MJ, Qiao S, Yu C. Functionalized large pore mesoporous silica nanoparticles for gene delivery featuring controlled release and co-delivery. J Mater Chem B . 2014;2:718–726. doi: 10.1039/c3tb21015d. [DOI] [PubMed] [Google Scholar]
- 113.Cheng SH, Yu D, Tsai HM, Morshed RA, Kanojia D, Lo LW, Leoni L, Govind Y, Zhang L, Aboody KS, Lesniak MS, Chen CT, Balyasnikova IV. Dynamic In Vivo SPECT Imaging of Neural Stem Cells Functionalized with Radiolabeled Nanoparticles for Tracking of Glioblastoma. J Nucl Med . 2016;57:279–284. doi: 10.2967/jnumed.115.163006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cheng Y, Morshed R, Cheng SH, Tobias A, Auffinger B, Wainwright DA, Zhang L, Yunis C, Han Y, Chen CT, Lo LW, Aboody KS, Ahmed AU, Lesniak MS. Nanoparticle-programmed self-destructive neural stem cells for glioblastoma targeting and therapy. Small . 2013;9:4123–4129. doi: 10.1002/smll.201301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, Breakefield XO, Snyder EY. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA . 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sheets KT, Ewend MG, Mohiti-Asli M, Tuin SA, Loboa EG, Aboody KS, Hingtgen SD. Developing Implantable Scaffolds to Enhance Neural Stem Cell Therapy for Post-Operative Glioblastoma. Mol Ther . 2020;28:1056–1067. doi: 10.1016/j.ymthe.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Satterlee AB, Dunn DE, Lo DC, Khagi S, Hingtgen S. Tumoricidal stem cell therapy enables killing in novel hybrid models of heterogeneous glioblastoma. Neuro Oncol . 2019;21:1552–1564. doi: 10.1093/neuonc/noz138. [DOI] [PMC free article] [PubMed] [Google Scholar]