Abstract
Despite rapid advances in modern medical technology and significant improvements in survival rates of many cancers, pancreatic cancer is still a highly lethal gastrointestinal cancer with a low 5-year survival rate and difficulty in early detection. At present, the incidence and mortality of pancreatic cancer are increasing year by year worldwide, no matter in the United States, Europe, Japan, or China. Globally, the incidence of pancreatic cancer is projected to increase to 18.6 per 100000 in 2050, with the average annual growth of 1.1%, meaning that pancreatic cancer will pose a significant public health burden. Due to the special anatomical location of the pancreas, the development of pancreatic cancer is usually diagnosed at a late stage with obvious clinical symptoms. Therefore, a comprehensive understanding of the risk factors for pancreatic cancer is of great clinical significance for effective prevention of pancreatic cancer. In this paper, the epidemiological characteristics, developmental trends, and risk factors of pancreatic cancer are reviewed and analyzed in detail.
Keywords: Pancreatic cancer, Epidemiology, Trend, Risk factors, Pancreatic ductal adenocarcinoma
Core Tip: Pancreatic cancer is still a highly lethal gastrointestinal cancer with a low 5-year survival rate and difficulty in early detection. A comprehensive understanding of the risk factors for pancreatic cancer is of great clinical significance for effective prevention of pancreatic cancer. In this review, the latest epidemiology, future trends, and various risk factors of pancreatic cancer are analyzed and summarized, which will provide more guidance and suggestions for the prevention and control of this malignancy.
INTRODUCTION
The pancreas is an about 15-cm-long, spongy, tube-shaped organ located in the upper abdomen between the stomach and spine[1]. A normal healthy pancreas consists of acinar cells secreting digestive enzyme, ductal cells secreting bicarbonate, centro-acinar cells that are the transitional region between acinar and ductal cells, endocrine islets secreting hormone, and relatively inactive stellate cells[2]. Pancreatic cancer occurs when abnormal DNA mutations in the pancreas cause pancreatic cells to uncontrollably grow and divide, forming tumors[3]. Pancreatic cancer is characterized as a fatal disease and one of the most aggressive and lethal malignancies[4,5]. By the time of diagnosis, pancreatic cancer often presents at an advanced stage, and has often spread to other parts of the body. Clinically, pancreatic cancer is the general term for malignant tumor formed in the epithelial cells of glandular structures in the pancreatic ductal cells, referred to as adenocarcinoma[6], and pancreatic ductal adenocarcinoma (PDAC) accounts for more than 90% of pancreatic cancers[7]. Due to the poor survival outcomes, PDAC is the seventh leading cause of global cancer death despite being the 10th most common cancer[8]. Other less common exocrine pancreatic cancers include adenosquamous carcinoma, squamous cell carcinoma, giant cell carcinoma, acinar cell carcinoma, and small cell carcinoma. At present, pancreatic cancer remains a devastating disease whose prognosis has remained largely unchanged over the last two decades[9]. Improvement in patient outcomes will depend on clear knowledge of epidemiology, reasonable prevention, and scientific regulation of early detection[4]. Therefore, it is necessary to understand the epidemiological characteristics, development trends, and risk factors of pancreatic cancer in detail, which will eventually establish rational prevention approaches for clinical benefit.
EPIDEMIOLOGY OF PANCREATIC CANCER
Assessing the latest epidemiologic trends in pancreatic cancer is necessary because it is of great importance for preventive measures and clinical care[10]. Therefore, we present a review of the latest epidemiology of pancreatic cancer.
Pancreatic cancer ranks consistently last among all cancers in terms of prognostic outcomes for patients and is predicted to become the second leading cause of cancer death in some regions[11]. A study including 84275 patients with at least 5 years of follow-up showed that actual 5-year survival rate in patients rose from 0.9% in 1975 to 4.2% in 2011 for all stages of pancreatic cancer, while in surgically resected patients, it increased from 1.5% to 17.4%[12]. In non-resected patients, the actual 5-year survival rate was 0.8% in 1975 and 0.9% in 2011, meaning that it remained roughly the same between 1975 and 2011[12]. The 5-year relative survival rate of pancreatic cancer was 7.2% in China and the lowest level in all cancers[13]. Cancer Stat Facts showed that the 5-year survival rate at the time of diagnosis is approximately 10% in the United States based on data from Surveillance, Epidemiology, and End Results Program 18 between 2010 and 2016[14]. Pancreatic cancer has a poor 5-year survival rate, ranging from 2% to 9%, with little difference between high-income countries and low-income and middle-income countries[11,15]. Therefore, the 5-year survival rate of pancreatic cancer varies globally in different regions and countries, but does not exceed 10%. And it is predicted that patients with nonoperative pancreatic cancer have a lower 5-year survival rate.
According to Cancer Statistics 2021, the American Cancer Society reported approximately 60430 new cases and 48220 deaths for pancreatic cancer in the United States, ranking third after lung and bronchus cancer and colorectal cancer[16]. In the 28 countries of the European Union (EU), it was estimated that approximately 111500 people (55000 in males and 56500 in females) will die from pancreatic cancer by 2025, and the number of recorded deaths from the cancer in 2010 will increase by almost 50% (45% in men and 49% in women), and it has been projected that pancreatic cancer may become the third leading cause of cancer death in the EU after lung and colorectal cancers[17]. Global Cancer Statistics 2018 showed that the incidence and mortality of pancreatic cancer were 458918 and 432242 in 2018 in the world, respectively, and deaths account for about 94.2% of new cases[18]. Pancreatic cancer remains the seventh leading cause of cancer death globally, and Global Cancer Statistics 2020 showed that, globally, a total of 495773 new cases and 466003 related deaths were reported for pancreatic cancer in 2020, with almost as many mortality as incidence[19]. The systematic analysis for the 2017 Global Burden of Disease Study showed that the number of incident cases and deaths from pancreatic cancer in both genders increased 2.3-fold from 195000 incident cases and 196000 deaths in 1990 to 448000 incident cases and 441000 deaths in 2017 globally[15]. These reports indicate a gradual increase in the number of incident cases and deaths from pancreatic cancer.
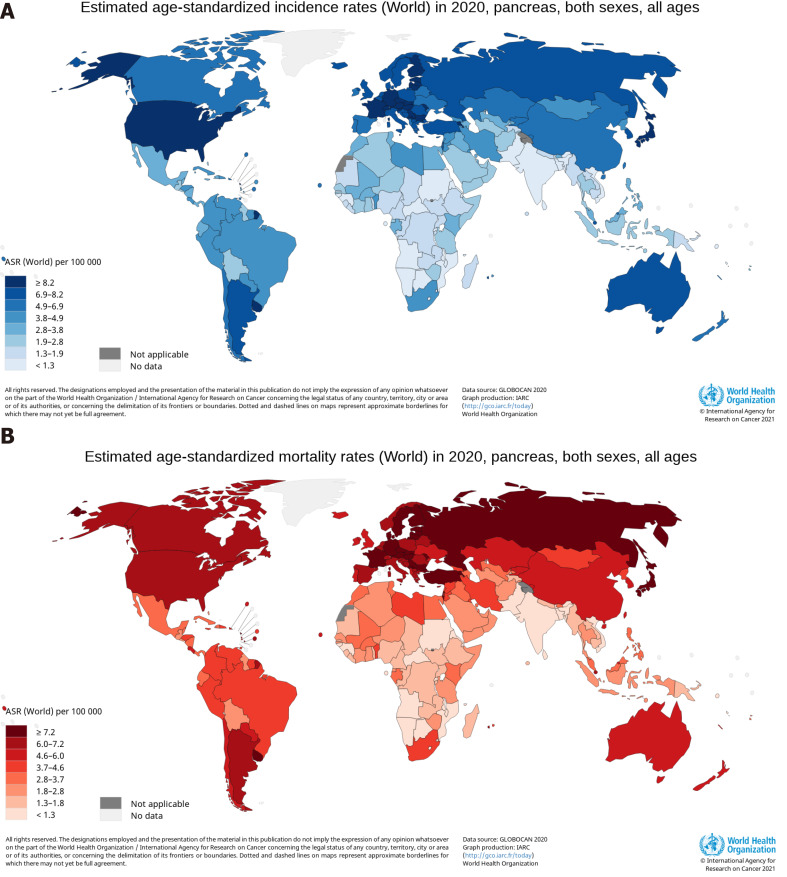
Average age-standardized rates (ASRs) of pancreatic cancer incidence and mortality vary widely across regions of the world[19]. The ASR of the incidence was highest in Eastern Europe, with 9.9 per 100000, followed by Western Europe (9.8), Northern America (9.3), Southern Europe (8.4), Northern Europe (8.3), Australia/New Zealand (7.9), Micronesia/Polynesia (7.7), and Western and Eastern Asia (7.0)[19]. The ASR of the mortality was highest in Western Europe, with 7.4 per 100000, followed by Northern America (6.9), Northern Europe (6.7), Australia/New Zealand (6.7), Southern Europe (8.4), Eastern Europe (5.6), Eastern Asia (4.8), and Western Asia (4.4)[19]. The human development index (HDI) is a composite index that measures three dimensions: Life expectancy, education period, and access to essential sources for a suitable and reasonable life[20]. The ASRs of pancreatic cancer incidence and mortality in regions with a very high HDI were significantly higher than medium or low HDI regions[19]. The low ASRs of the incidence and mortality were found mainly in South-Central Asia (1.5 per 100000, 0.9 per 100000), Eastern Africa (2.0, 1.7), Middle Africa (2.0, 1.2), Western Africa (2.2, 1.8), Melanesia (2.9, 1.7), and South-Eastern Asia (2.9, 1.8), all of which are medium or low HDI regions[19]. The top six countries for pancreatic cancer incidence were Hungary (ASR, 11.2), Uruguay (ASR, 10.7), Japan (ASR, 9.9), Slovakia (ASR, 9.6), Czechia (ASR, 9.5), and Austria (ASR, 9.0), with 9.0 and greater per 100000, and a total of 21 countries, including the United States (ASR, 8.2), had an ASR of the incidence between 8.1 and 8.9 per 100000, as shown in Figure 1A[21]. The ASR of pancreatic cancer mortality was highest in Hungary and Uruguay, both at 10.2 per 100000, and a total of 26 countries, not including the United States (ASR, 6.6), had an ASR of the incidence between 7.2 and 8.6 per 100000, as shown in Figure 1B[21]. The proportion of estimated new cases for pancreatic cancer in China was relatively high in East China (9.4 per 100000), Northeast (9.4), Northwest (6.8), and North China (5.3), and was comparatively low in Central China (5.2), Southwest (4.3), and South China (3.6), having obvious regional characteristics[13]. Age-standardized rates of pancreatic cancer were 3-fold to 4-fold higher in higher HDI countries, compared with lower HDI countries[18]. The higher incidence and mortality rates of pancreatic cancer were reported in countries and regions with higher levels of HDI and Gross Domestic Product (GDP) per capita, and the coefficients of determination (R2) of HDI and GPD per capita were high for the incidence and mortality[22]. The higher incidence and mortality rates of pancreatic cancer in countries with higher HDI indicates the importance that paying more attention and implementing appropriate programme to reduce risk factors acts as an effective measure to control the incidence and mortality of the cancer[23].
Figure 1.
Maps showing estimated age-standardized rates of incidence and mortality for pancreatic cancer worldwide in 2020, including both sexes and all ages. A: Incidence; B: Mortality. Citation: Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global Cancer Observatory: Cancer Today. [cited 20 Jan 2021]. In: International Agency for Research on Cancer [Internet]. Available from: https://gco.iarc.fr/today. Copyright ©International Agency for Research on Cancer 2021. Published by World Health Organization[21].
TRENDS OF PANCREATIC CANCER
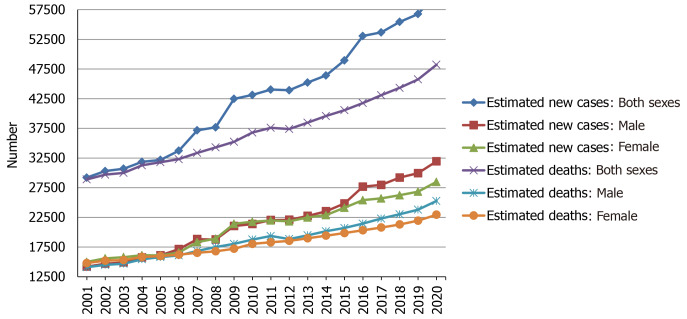
Over the two decades from 2001 to 2020, the estimated new cases and deaths of pancreatic cancer have been increasing year by year in the United States, and the same trend has been observed among men and women, as shown in Figure 2. Using statistical models for analysis, in the United States, age-adjusted rates of new cases for pancreatic cancer remained stable from 2008 to 2017, and age-adjusted rates of death increased by an average of 0.3% each year from 2009 to 2018[14]. Prediction of pancreatic cancer incidence burden from the 28 member states of the EU and other selected countries around the world showed that in 2025, 2030, 2035, and 2040, the incidence will be 557688, 639030, 726740, and 815276, respectively, with growth rates of 21.5%, 39.2%, 58.4%, and 77.7%[24]. The incidence and mortality of pancreatic cancer in Africa will increase by 18327 and 17744 in 2040, respectively, with growth rates of 114.1% and 114.8%, the rates of which will be highest in the world, followed by Latin America and the Caribbean (incidence: + 99.3%; mortality: 101.0%)[25]. However, in 2040, the growth rates of the incidence and mortality in Europe will be lowest at 29.3% and 31.6%, respectively[25]. Based on China and India, both countries in Asia with more than one billion population, the incidence and mortality in Asia will increase by 190532 and 182127 in 2040, respectively, which will be the largest increase in terms of number[25]. In addition, standardized mortality rate of pancreatic cancer increased from 1.30 per 100000 to 3.32 per 100000 over 1991-2014 and might reach the peak in the ensuing 5 years in China, and the mortality rate was higher among elderly people and in urban and northeast/eastern regions than among young people and in rural and middle/western regions[26]. The incidence of pancreatic cancer was 12.1 per 100000 in 2010 and is predicted to increase to 15.1 and 18.6 per 100000 in 2030 and 2050, respectively, with an average annual growth of 1.1%[27]. In the age-stratified analysis, the over 65 years group will have the highest projected incidence (31.9 per 100000) in 2050, and the incidence is projected to increase gradually in the sex-stratified analysis, with an average annual growth of 1.3% in males and 0.9% in females[27].
Figure 2.
Estimated new cases and deaths from 2001 to 2020 in the United States. The data is from Cancer Statistics that the American Cancer Society estimates the numbers of new cancer cases and deaths in the United States from 2001 to 2020[16,28-47].
The number of years of life lost (YLL) is a measure of premature mortality, taking into account simultaneously the number of deaths and life expectancy at age of death, and projection of YLL due to premature mortality (i.e., time-based approach) provides a comprehensive outlook of the fatal burden at a population level[27]. Due to premature death in individuals with pancreatic cancer, the total YLL was 5604 years in 2010 and is projected to increase to 9784 in 2030 and 14247 in 2050, with an average annual growth of 2.1%[27]. In the age-stratified analysis, the 40-64 years group will have the highest projected YLL (7588 years) in 2050, and the YLL is projected to increase gradually in the sex-stratified analysis, with an average annual growth of 2.1% in males and 2.2% in females[27].
In conclusion, pancreatic cancer, like other cancers such as lung, liver, and stomach cancer, will cause a huge economic burden to all countries and related populations in the next 20 years, especially China having a huge population, which is still a developing country. In order to reverse these trends and improve the prognosis of patients with pancreatic cancer, the most simple, direct, and effective way is to understand the risk factors affecting the occurrence and development of pancreatic cancer in detail, which provides comprehensive and reasonable guidance and suggestions for the prevention of pancreatic cancer, and offers reliable and feasible ideas for the early screening of pancreatic cancer.
CAUSES AND RISK FACTORS OF PANCREATIC CANCER
Pancreatic intraepithelial neoplasias (PanINs) are noninvasive epithelial proliferations in smaller pancreatic ducts, which progress from PanIN-1 (low-grade) to PanIN-2 (intermediate-grade) to PanIN-3 (high-grade)[48]. The differentiation of normal epithelium into PanIN-1/PanIN-2 and then into PanIN-3/invasive pancreatic cancer requires a considerable period of development, while the development process before high-grade PanIN-3 and invasive pancreatic cancer is the golden stage of preventing pancreatic cancer through effective interventions. Therefore, a thorough and comprehensive understanding of pancreatic cancer risk factors is of great practical significance for the prevention of pancreatic cancer. The exact cause of pancreatic cancer is unknown, but many non-modifiable and modifiable risk factors are associated with development of pancreatic cancer. Non-modifiable risk factors include age, gender, ethnicity, ABO blood group, microbiota, diabetes mellitus (DM), and family history and genetic susceptibility, while modifiable risk factors include smoking, alcohol drinking, dietary factors, pancreatitis, obesity, infection, and socioeconomic status and insurance. The influence of these factors on the occurrence, progression, and invasion of pancreatic cancer is analyzed and summarized as follows.
NON-MODIFIABLE RISK FACTORS
Age
Both 89.4% of new cases of pancreatic cancer and 92.6% of deaths occur in patients over 55 years of age in the United States, the new cases are most frequently diagnosed among people 65-74 years of age with a median age at diagnosis of 70 years, and the percent of deaths is also highest among people of the same age group with a median age at death of 72 years[14]. The proportions at 40-64 years and over 65 years of age were 47.9% and 48.6% in diagnosed patients with pancreatic cancer in China[49]. The mortality rates of patients aged under 30, 30-44, 45-59, 60-74, and 75 and above among males are 0.1, 1.4, 10.1, 19.3, and 14.6 per 100000 in China, respectively[50], meaning that male pancreatic cancer population over 60 years of age has a higher mortality rate. The reference does not provide the mortality rates of pancreatic cancer in the five broad age groups in women and both sexes[50]. Worldwide, it is extremely rare for pancreatic cancer to be diagnosed before the age of 30, so it is typically a disease of the elderly. The risk factor also determines the need for screening and early detection of pancreatic cancer among the population over a certain age.
Gender
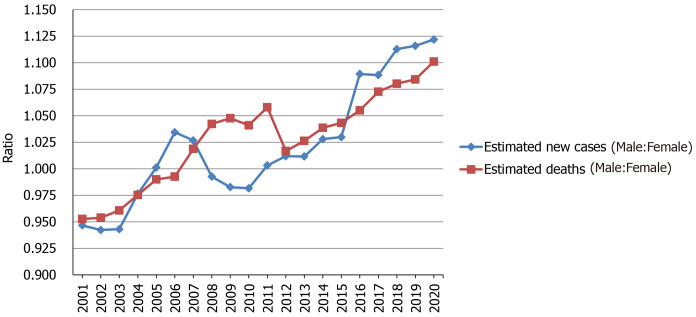
In the United States, the new cases of pancreatic cancer is 31950 among males and 28480 among females in 2020, and the deaths is 25270 among males and 22950 among females[16]. In China, the age-standardized respective incidence and mortality rate are 52.2 and 45.6 per 100000 among men in 2015, and 37.9 and 33.8 per 100000 among women[50]. On a global scale, the new cases are 243033 among men and 215885 among women in 2018, and the deaths are 226910 among men and 205332 among women[18]. The global respective incidence and mortality rates are 5.5 and 5.1 per 100000 among men in 2018, and 4.0 and 3.8 per 100000 among women[18]. Globally, the respective cumulative risk of developing pancreatic cancer and dying from it from birth to 74 years is 0.65% and 0.59% among males in 2018, and 0.45% and 0.41% among females[18]. The ratio of male to female for estimated new cases and deaths increased in the United States from 2001 to 2020, as shown in Figure 3. Thus, the worldwide incidence and mortality of pancreatic cancer are higher among males than females.
Figure 3.
Ratio of male to female for estimated new cases and deaths from 2001 to 2020 in the United States. The data is from Cancer Statistics that the American Cancer Society estimates the numbers of new cancer cases and deaths in the United States from 2001 to 2020[16,28-47].
ABO blood group
The ABO antigens were first described by Landsteiner as erythrocyte antigens in 1900[51]. The antigens of ABO blood group are glycoproteins that are expressed on red blood cells and various epithelial cells, including the urothelium and gastrointestinal mucosa[52]. The phenotypic A and B antigens are terminal carbohydrates synthesized by the addition of monosaccharides catalyzed by a series of specific glycosyltransferases, and the phenotype O is characterized by deficiency of A and B glycosyltransferases[52]. There is growing evidence that ABO blood group may also be associated with carcinogenesis or progression of pancreatic cancer. A consortia-based evaluation and replication study showed that non-O blood group was associated with an increased risk of pancreatic cancer compared with blood group O[53]. A register-based cohort study showed that blood group A was associated with an increased risk of pancreatic cancer[54]. There was a significantly higher risk for developing pancreatic cancer in Chinese patients with the A or AB blood types than for those with type O[55]. El Jellas et al[56] reported that the prevalence of blood type A and subtype A1 was highest among the unresected cases, the unresected cases had the lowest frequency of blood group O, and patients with blood group O survived longer than non-O patients in the group of unresected cases. A study at Shanghai Pancreatic Cancer Institute showed that Chinese Han population with blood type A were more likely to develop pancreatic cancer, but people with blood type B were less likely to develop pancreatic neuroendocrine tumors and other types of pancreatic masses, compared with those with blood type O[57]. Hofmann et al[51] reported that patients with blood type O had more often well-differentiated PDAC compared with blood type non-O, and they elucidated the novel interaction between blood type immunoglobulin M isoagglutinins and PDAC O-GalNAc glycoproteins, which may contribute to the pathogenesis and progression of pancreatic cancer. Accordingly, the risk for people with blood type O to develop pancreatic cancer is lower than those with other blood types. In addition, the ABO allele that determines blood type A has two major subtypes, namely, A1 and A2, and the association of A1 but not A2 with pancreatic cancer could therefore suggest that the activity of blood type A glycosyltransferase plays a role in carcinogenesis[56]. The study showed that the A2 subtype has a single base deletion near the carboxyl terminal, and introducing the single base deletion into the expression construct of A1 transferase cDNA significantly reduced the activity of A transferase in DNA-transfected HeLa cells[58]. Therefore, clarifying the etiological mechanism between the risk of pancreatic cancer and ABO blood type may provide a new perspective for the treatment of this disease.
Ethnicity
The burden of exocrine pancreatic disease, including pancreatic cancer, pancreatitis, and pancreatic cyst, differs among various ethnicities, and African-Americans and certain indigenous populations are at the greatest risk of developing these diseases[59]. Huang et al[60] observed that African-Americans, Native Americans, and Japanese-Americans had higher rates of developing pancreatic cancer, but no difference between Latino- and European-Americans, and found that African-Americans had a 20% greater risk of pancreatic cancer than European-Americans even after adjusting for known risk factors. In the studies including few minority patients, the neutrophil-to-lymphocyte ratio (NLR) is associated with a reduced overall survival in pancreatic cancer patients, and NLR > 5 was significantly associated with a worse overall survival compared with NLR ≤ 5[61]. Patients with an NLR ≤ 5 were also more likely to develop locally advanced disease than metastatic cancers and primary tumor located in the head or neck of the pancreas, while patients with an NLR > 5 were more likely to have liver metastases and albumin < 3.4 g/dL, suggesting that elevated NLR is an independent marker for poor prognosis and a potentially valuable factor[61]. Patients with an NLR ≤ 5 were more likely to be non-Hispanic Black, while patients with an NLR > 5 were more likely to be non-Hispanic White or Hispanic[61], suggesting that there are different predispositions and outcomes for pancreatic cancer between non-Hispanic Black and non-Hispanic White or Hispanic. Gad et al[62] found that the incidence of pancreatic cancer among Asian-Americans, especially malignancies of the body and tail of the pancreas, as well as the mortality based on the incidence, was overall on the rise in an epidemiological study, without respect to age, sex, or stage subgroup. Amaral et al[63] also emphasized the importance of the influence of ethnicity on somatic mutations in Brazilian patients with PDAC. To elucidate the reasons for the racial differences in the incidence of pancreatic cancer may help us improve the understanding and prevention of this disease.
Microbiota
Oral microbiota: Several epidemiological studies have found the direct relationship between oral bacteria and pancreatic cancer[64]. Farrell et al[65] reported that the levels of two bacteria biomarkers (Neisseria elongate and Streptococcus mitis) were lower in patients with pancreatic cancer than in healthy controls, and found that the combination of the two bacteria biomarkers distinguished pancreatic cancer patients from healthy subjects with an area under the curve value of 0.90, sensitivity of 96.4%, and specificity of 82.1%. Torres et al[66] found that the ratio of Leptotrichia to Porphyromonas in the saliva of pancreatic cancer patients was significantly higher than that in healthy individuals or those with other disease. Fan et al[67] found that Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans were associated with a higher risk of pancreatic cancer, and phylum Fusobacteria and its genus Leptotrichia were associated with a decreased risk of this cancer. Olson et al[68] reported that the mean relative proportions of Firmicutes and related taxa were higher in patients with pancreatic cancer, while the mean relative proportions of Proteobacteria and related taxa were higher in controls.
Gut microbiota: Studies have confirmed that gut microbiota is associated with recognized risk factors for pancreatic cancer, such as obesity and type II diabetes, suggesting the relationship between gut bacteria and pancreatic cancer[64]. In recent decades, multiple and highly complex effects of gut microbiota on pancreatic cancer have been identified as potential risk factors for the development and progression of this tumor[69]. A prospective study, for the first time, analyzed gut microbial profile in Chinese pancreatic cancer cohorts by MiSeq sequencing, revealing a significant decline in gut microbial diversity and a unique microbial profile in pancreatic cancer, due in part to the decline in alpha diversity[70]. Additionally, the microbial profile changed in pancreatic cancer, with an increase in certain pathogens and lipopolysaccharides (LPS)-producing bacteria and a decrease in probiotics and butyrate-producing bacteria[70]. LPS might play a pro-inflammatory pro-tumor role by activating the nuclear factor-κappa B (NF-κB) pathway, producing proinflammatory cytokines [tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, and IL-1] and leading to liver inflammatory and oxidative damage[71]. After LPS treatment, Ras activity in cells prepared from acinar-Ras mice was greatly elevated and maintained at a high level for a long time, and severe chronic pancreatitis and PanIN lesions were induced in acinar-Ras mice, accompanied by sustained elevated Ras activity, whereas there was no observed effects in control mice, suggesting that LPS treatments led to fibrosis and PanIN formation in the presence of oncogenic Ras[72]. Therefore, LPS may have a greater pathological impact on patients carrying cells expressing oncogenic RAS, which may explain individual differences in response to infection, suggesting the association between chronic bacterial infectious diseases and colon and pancreatic cancers[72]. As a member of the RAS family of GTP-binding proteins, KRAS mediates a wide variety of cellular functions including proliferation, differentiation, and survival[73], and the prevalence of oncogenic KRAS mutation in PDAC ranges from 88% to 100%[74]. Consistent with a central pathogenic role of the KRASG12D mutation, mice engineered with pancreas-specific expression of this activated KRAS allele sustain classical PanIN lesions that can progress to PDAC in the appropriate tumor suppressor background[73]. Thomas et al[75] found that the proportion of poorly differentiated PDAC in the microbiota-intact mice was higher than that in microbiota-depleted mice (89.75 vs 34.8%, respectively), demonstrated that the intestinal microbiota accelerated pancreatic carcinogenesis in the KRASG12D/PTENlox/+ mice model of pancreatic cancer, and considered that the intestinal microbiota had a long-distance role on PDAC progression. In addition, based on crucial genera associated with pancreatic cancer, gut microbial markers might achieve an excellent classification capacity between pancreatic cancer and healthy controls, suggesting that the specific alterations of gut microbiota might become non-invasive biomarkers for pancreatic cancer diagnosis[70].
Pancreatic microbiota: A number of different bacterial taxa are found in pancreatic tissue contents, including those known to inhabit the oral cavity, which suggest that the pancreas is not a sterile organ[76]. The relative abundance of bacterial taxa at the genus level in the pancreas has a substantial between-person variability, and bacterial composition of the pancreatic duct, head, and tail as well as the duodenum was highly similar in the same individuals[76]. The study showed that the community composition of the microbiota in the human pancreas failed to discriminate between normal and disease states, and that the acquisition of pancreatic bacteria is not a physiologic process, even under conditions of intestinal inflammation[75]. Del Castillo et al[76] found that the presence and relative abundance of Lactobacillus were lower in pancreatic tissue of cancer subjects and the relative abundance of periodontal-related pathogens was higher in cancer subjects, when compared with noncancer subjects. Pushalkar et al[77] found that the bacteria (Enterococcus faecalis and Escherichia coli) could migrate into the pancreas, confirmed that the abundance of intrapancreatic bacteria in both mice and patients with PDAC was markedly greater compared with normal pancreas by 16S rRNA fluorescence in situ hybridization and qPCR analysis, and consider that the bacteria promote the progression of pancreatic oncogenesis in both preinvasive and invasive models[77]. Maekawa et al[78] mainly detected Enterococcus and Enterobacter species in bile, demonstrating that Enterococcus and Enterobacter can survive in pancreatic juice and/or bile, and found that 29 of 36 pancreatic juice samples were positive for bacterial DNA[78]. Enterococcus faecalis was also found in pancreatic tissue from patients with chronic pancreatitis and pancreatic cancer, and serum antibodies to capsular polysaccharide of Enterococcus faecalis were elevated in patients with chronic pancreatitis[78]. They demonstrated that Enterococcus faecalis is involved in the progression of chronic pancreatitis using the model mice with caerulein-induced chronic pancreatitis, which may ultimately result in the development of pancreatic cancer[78].
In addition, Pushalkar et al[77] also found that the microbiome regulates immunogenicity in PDAC and programs tumor-associated macrophages via Toll-like receptor signaling to induce immune tolerance, and bacterial communities are distinct between early and advanced PDAC. Geller et al[79] demonstrated that bacteria are a component of the PDAC tumor microenvironment, and estimated that bacteria colonized PDAC samples had an average of one bacterium per 146 human cells. Their results indicated that PDACs contain bacteria that can potentially modulate tumor sensitivity to gemcitabine, and they considered that the bacteria play a critical role in mediating resistance to chemotherapy[79]. By studying colon cancer models, they found that bacteria can metabolize the chemotherapeutic drug gemcitabine (2′,2′-difluorodeoxycytidine) into its inactive form (2′,2′-difluorodeoxyuridine), which depends on the expression of a long isoform of bacterial cytidine deaminase, seen primarily in γ-proteobacteria[79].
The above various microorganisms have different influences on the occurrence, development, and invasion of pancreatic cancer in different ways. Therefore, the study on the influence of microorganisms on pancreatic cancer will provide more new insights to reveal its etiology. And studying the effects of microorganisms on pancreatic cancer has the potential to be used as a target for the regulation of disease progression and treatment.
Family history and genetic susceptibility
Hereditary pancreatic cancer includes inherited cancer syndromes with a recognized known germline mutation associated with an increased risk of pancreatic cancer and familial pancreatic cancer with two or more cases of pancreatic cancer in their families[80]. Pancreatic cancer associated with hereditary syndromes or familial pancreatic cancer accounts for about 10% of cases[81]. Family history of pancreatic cancer was associated with an increased pancreatic cancer risk when compared with cancer-free family history, with the risk being greater when ≥ 2 first-degree relatives suffered pancreatic cancer and among current smokers[82]. Members of familial pancreatic cancer kindreds having at least one first-degree relative affected by pancreatic cancer had a 9-fold increased risk of developing pancreatic cancer, whereas members of sporadic pancreatic cancer kindreds having a first-degree relative with pancreatic cancer did not have an increased risk[83]. Risk was higher among members of familial pancreatic cancer kindreds with a young-onset patient (< 50 years) in the kindred than those without a young-onset case in the kindred[84].
Genetic mutations associated with an increased risk of pancreatic cancer include STK11/LKB1, CDKN2A (p16), BRCA1/2, PRSS1/SPINK1/CFTR, mismatch repair genes (MLH1/MSH6/MSH2/PMS2), ATM, and PALB2 (a new pancreatic cancer susceptibility gene)[85]. And pancreatic cancer is also found to be associated with these familial cancer syndromes for which genetic mutations correspond, such as Peutz-Jeghers syndrome (STK11/LKB1), familial atypical multiple mole melanoma (CDKN2A), hereditary breast cancer ovarian cancer syndrome (BRCA1/2), and hereditary non-polyposis colorectal carcinoma syndrome (MLH1/MSH6/MSH2/PMS2)[25,85].
A study from Mayo Clinic showed that the aggregate prevalence was 36/302 (11.9%) for all cases with any positive PDAC family history[86]. Seven PDAC-associated genes (ATM, BRCA1, BRCA2, CDKN2A, MSH2, PALB2, and PMS2) and four genes with no known PDAC association (BARD1, CHEK2, MUTYH/MUY, and NBN) were identified as pathogenic variants in the study[86]. Kindreds with at least one pair of first-degree relatives who were affected by PDAC were considered FPC, and kindreds with at least two affected blood relatives that did not meet the FPC definition were considered “familial non-FPC”[86]. Thirty-six (12%) patients carried at least one pathogenic variant in one of 11 genes, and the probabilities of carriers with pathogenic variant among FPC patients and familial non-FPC patients were 14% and 9%, respectively[86]. Pathogenic variants (n) identified in PDAC patients were BRCA2 (11), ATM (8), CDKN2A (4), CHEK2 (4), MUTYH/MYH (3 heterozygotes, not biallelic), BRCA1 (2), and 1 each in BARD1, MSH2, NBN, PALB2, and PMS2[86]. Regardless of FPC status, multiple susceptibility gene testing may be necessary in PDAC patients with a family history of pancreatic cancer, which will provide genetic risk counseling for families[86]. Therefore, the study of the family characteristics and genetic features of pancreatic cancer is of great clinical significance in identifying the susceptible population of pancreatic cancer, screening the high-risk individuals of pancreatic cancer, and early diagnosis of pancreatic cancer.
Diabetes mellitus
In comparison with patients without diabetes, those who were recently diagnosed with diabetes had an nearly 7-fold increase in risk of developing pancreatic cancer[87]. Either hyperglycaemia or diabetes is found among as many as 80% of patients, both of which can be detected in the presymptomatic phase, and on the contrary, older patients with new-onset diabetes have about an 8-fold higher risk of developing pancreatic cancer than the general population, suggesting a “dual causality” between diabetes and PDAC[88], in that both long-standing type 2 DM (T2DM) is a risk factor of developing PDAC and PDAC is assumed to be a cause of diabetes in many cases[89]. A multiethnic cohort study also showed that recent-onset diabetes is a manifestation of pancreatic cancer and long-standing diabetes is a risk factor of developing this cancer[90]. At present, the prevalence of diabetes in China is on the rise and has the largest diabetes epidemic worldwide, and in 2013, the estimated total prevalence was 10.9% for diabetes and 35.7% for prediabetes among adults in China, indicating the importance of diabetes as a public health problem in China[91]. In 2011-2012, the prevalence of diabetes was estimated from 12% to 14% among US adults, and participants who were non-Hispanic black, non-Hispanic Asian, and Hispanic had a higher prevalence[92]. The global spread of this enormous medical burden further highlights the necessity to better understand the pathophysiological relationship between T2DM and pancreatic cancer.
A growing body of epidemiological and experimental evidence shows that chronic hyperinsulinaemia increases the risk of cancers of the colon and endometrium, and probably other tumours (such as pancreas and kidney)[93]. Hyperinsulinemia, especially intrapancreatic, due to obesity and insulin resistance in patients with prediabetes or early T2DM may believably conduce to the observed increased risk of developing PDAC[89]. The high level of islet hormones in blood directly reaches groups of acinar and ductal cells and acts on insulin-like growth factor-1 (IGF-1) receptors to promote survival and proliferation of acinar and ductal cells[89]. The characteristics of T2DM patients and the overwhelming majority of obese individuals are insulin resistance with ensuing hyperinsulinemia and high levels of IGF-1, which can act as potent growth-promoting factors[94]. In addition, Butler et al[95] reported that replication of pancreatic duct cells in lean subjects with T2DM had a 4-fold increase compared with lean non-diabetic controls, suggesting that the increased risk of pancreatitis and pancreatic cancer in T2DM is driven by replication of chronically increased pancreatic duct cell replication[95].
The desmoplastic response attribute to production and proliferation of extracellular matrix proteins in tumor-associated fibroblasts, activating pancreatic stellate cells (PaSC)[94]. Yang et al[96] reported that progression of high-fat diet-induced PDAC in mice is associated with hyperglycemia, hyperinsulinemia, and PaSC activation, and found that the pancreas from patients with T2DM showed substantial collagen deposition and activated PaSC in islet and peri-islet exocrine pancreas compared with normal control[96]. Both quiescent and activated PaSC coexpress insulin and IGF-1 receptors, the expression of which was modulated by both insulin and glucose[96]. Insulin induces rapid tyrosine autophosphorylation of insulin/IGF-1 receptors at specific kinase domain activation loop sites, activates Akt/mTOR/p70S6K signaling, and inactivates FoxO1, a transcription factor suppressing cell growth[96]. In activated PaSC, insulin promotes cell proliferation and production of extracellular matrix proteins, and specific inhibition of mTORC1 and mTORC2 can abolish the above effects, suggesting that increased local glucose and insulin concentrations are associated with obesity and T2DM promotes PaSC growth and fibrosing responses[96]. In premalignant H6c7-kras cells, hyperglycemia increases secretion and signaling of transforming growth factor beta1 (TGF-β1) and induces properties of cancer stem cells depending on TGF-β1-signaling, suggesting that hyperglycemia promotes pancreatic ductal epithelial cells to acquire the properties of mesenchymal and cancer-stem cells by activating TGF-β signaling[97]. Li et al found that patients with A blood type who also had DM had a greater odds of having pancreatic cancer, and further research is needed to confirm the results and to identify the mechanisms by which A blood type and DM jointly contribute to the risk of pancreatic cancer progression and development[55].
There are many mechanisms that explain the effect of DM on pancreatic cancer and the relationship between the two. However, in order to reveal the real relationship between diabetes and pancreatic cancer, it still needs to be further studied to provide new strategies for the prevention of pancreatic cancer.
MODIFIABLE RISK FACTORS
Smoking
Epidemiological studies have shown that many causative factors are associated with pancreatic cancer, and cigarette smoking has the strongest positive association with the risk of developing the cancer[98]. Due to smoking, the estimated prevalence was about 30% in many parts of the world and the risk of pancreatic cancer was doubled in smokers, whereas the population-attributable risk caused by smoking is about 25% for pancreatic cancer, meaning that the overall burden of this cancer would be reduced if smoking was completely eliminated[99]. Patients with pancreatic cancer who smoked prior to diagnosis had an about 40% increased hazard for death compared with those who never smoked[100]. And long-term smoking portended worse outcomes for current smokers, but former smokers experienced outcomes similar to those who had never smoked, suggesting that quitting smoking can have potential beneficial effects[100]. A large European case-control study confirmed that current smokers had a 72% increased risk of developing pancreatic cancer compared with never-smokers, and the study also endorsed that around 16% of all pancreatic cancer diagnoses could be avoided through tobacco preventive measures in terms of attributable risk[101]. And analysis of dose-response relationships confirmed that higher smoking intensity, longer smoking duration, and increased cumulative dose levels were associated with a further increased risk of pancreatic cancer, whereas smoking cessation led to a gradual decline in the risk of pancreatic cancer[101]. Smoking also notably increases the risk of developing pancreatic cancer in individuals with a family history of this cancer[82]. These studies suggest that smoking cessation has a potential benefit to improve survival for patients with pancreatic cancer and helpfully prevent pancreatic cancer in those at risk.
As an avoidable risk factor, smoking is of particular concern, and elucidating the mechanisms involved would significantly reduce the number of PDAC cases diagnosed each year[102]. Smoking-induced inflammation was accompanied by enhanced activation of PaSC and elevated levels of serum retinoic acid-binding protein 4, suggesting increased bioavailability of retinoic acid that is conducive to differentiation of myeloid-derived suppressor cells to tumor-associated macrophages and dendritic cells[103]. And smoking exposure also leads to partial suppression of the immune system in the early progression of pancreatic cancer[103]. In xenografts of patient-derived pancreatic cancer, nicotine intervention promoted growth and metastasis of tumor, and it was confirmed that nicotine reduced survival by enhancing paracrine HGF-MET signaling in the pancreatic cancer microenvironment[104]. In addition, nicotine induced dedifferentiation of acinar cells by activating AKT-ERK-MYC signaling, thereby inhibiting the activity of Gata6 promoter and losing GATA6 protein, and subsequently causing loss of acinar differentiation and over-activation of oncogenic K-Ras[105]. And metformin could inhibit nicotine-induced carcinogenesis of the pancreas and tumor growth by up-regulating GATA6 expression and promoting programmed differentiation of acinar cell[105]. Benzo(a)pyrenes, polycyclic aromatic hydrocarbons, and tobacco-specific nitrosamines are several carcinogens identified in tobacco smoke, most of which play a genotoxic role by formation of DNA adducts and generation of reactive oxygen species, leading to mutations in vital genes such as K-Ras and p53[106]. Nicotine and other carcinogenic components in tobacco smoke can directly promote growth of tumor cells, change cross-talk between tumor and stromal cells within the tumor microenvironment, and enhance infiltration of myeloid-derived suppressor cells[100]. Therefore, the study and elucidation of carcinogenic mechanism of carcinogens in tobacco smoke will contribute to the treatment and prevention of pancreatic cancer caused by related factors.
Alcohol drinking
East Asians have a high proportion in inefficient metabolism of acetaldehyde, so alcohol drinking may play a more important role in the developing pancreatic cancer among East Asians[107]. According to many studies, there is no doubt that the risk of pancreatic cancer is associated with high alcohol consumption (more than three drinks per day), but no association was found with low-to-moderate alcohol consumption[25]. A population-based study demonstrated that heavy alcohol consumption and binge drinking increased estimated risk of developing pancreatic cancer among males but not among females[108]. It may also be the reason why pancreatic cancer has a higher incidence and mortality in men than in women. And the study also suggested that either binge or consistent heavy alcohol consumption persistently increased the risk of developing pancreatic cancer regardless of the temporal proximity between alcohol consumption and diagnosis of pancreatic cancer[108]. A large prospective study suggested that baseline and lifetime alcohol consumption was positively associated with the risk of developing pancreatic cancer, and the estimated risk for beer and spirits/liquors was more apparent than wine[109]. Alcohol plays an independent role in promoting PDAC associated with fibrosis formed by a stellate cell-independent mechanism and further boosts formation of PanIN lesion and induction of M2 macrophages in the context of chronic pancreatitis[110]. This is an important finding, namely, M2 macrophages suppress the directed immune mechanisms of cancer and block the recruitment of T cells into the tumor, further promoting cancer progression[111]. Mice that expressed mutant K-ras gene developed early and advanced forms of the most common pancreatic cancer in humans[112]. Specific mutations in the K-ras oncogene may be more commonly found in alcohol consumers with pancreatic cancer, and may be initiators or terminators of pancreas cancer associated with heavy alcohol consumption[108]. Additionally, alcohol might promote the development of cancer by inducing oxidative stress and lipid peroxidation, and alcohol abuse may also accelerate the progression of tumor by boosting pancreatic inflammation[112].
Two NAD-dependent enzymes, namely, alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH), are mainly involved in alcohol metabolism in human[113]. In the body, alcohol is first converted into acetaldehyde by ADH oxidation, and acetaldehyde is then converted into non-toxic acetate by ALDH oxidation for excretion[113]. In the human liver, there are two kind of ALDHs, including ALDH1 (cytosolic) and ALDH2 (mitochondrial), and only mitochondrial ALDH2 oxidizes acetaldehyde to acetate at physiological concentrations[114]. The variant ALDH2*2 allele can significantly decrease ALDH2 enzyme activity and affect alcohol response, which is a striking genetic polymorphism[113].
In many East Asian countries, the ALDH2*2 allele associated with reduced enzyme activity is found in 30%-50% of the population, resulting in the inefficient metabolism of the carcinogenic acetaldehyde generated from alcohol metabolism[107]. Thus, acetaldehyde accumulates in ALDH2*2 carriers, even after a moderate intake of ethanol (0.5 g/kg)[115], also leading to a higher risk of developing alcohol-related cancers in individuals carrying the ALDH2*2 allele. Kanda et al[116] found that the impact of alcohol on pancreatic cancer risk was associated with rapid production or high accumulation of acetaldehyde, indicating that acetaldehyde may play a substantial role in the potential mechanism of pancreatic cancer[116]. This can also provide guidance and help to prevent pancreatic cancer for people of different groups and races.
Dietary factors
In general, diets high in fruits, vegetables, and other plant-based foods can reduce the risk of developing pancreatic cancer, while dietary patterns rich in meat and animal products can increase the risk of the cancer[107]. Intake of meat, especially red meat, cooked meat at high temperature, and meat-associated heterocyclic amines, and overall mutagenic activity may induce the development of exocrine pancreatic cancer[117]. Foods are often heat processed and may contain advanced glycation end products (AGEs) that can be formed by a nonenzymatic reaction between reducing sugars and free amino groups on proteins, peptides, and amino acids[118]. When the carbonyl group of reducing sugar like glucose or its oxidation or lipid peroxidation products react with the ε-amino group of lysine or the guanidino group of arginine, AGEs can be formed, including imidazolones, pentosidine, pyrraline, and Nε-(carboxymethyl)lysine (CML)[118]. AGEs accumulate in sera and tissues during the ageing process because of glycolytic and oxidative reactions, reduced activity of the detoxification systems, cigarette smoking, and consumption of high-temperature-processed foods[8]. Excessively high concentrations of AGEs in human tissue and circulation accelerate oxidative stress and inflammation, which may play a pathogenic role[118]. Exogenous AGEs, in particular those derived from the diet, have been claimed to contribute to several disease processes, including cancer in general and, specifically PDAC[8]. CML is frequently used as a marker for AGEs in general[119]. Jiao et al[120] proposed a novel mechanism that consumption of heat-treated red meat can cause chronic inflammation and subsequently lead to pancreatic cancer, and considered that consumption of dietary CML-AGE was associated with a modest increase in risk of pancreatic cancer for men, which might partly explain the positive correlation between red meat and pancreatic cancer. A model experiment showed that AGEs markedly accelerated the development of pancreatic cancer and inhibition of AGE prevented the tumor-promoting effect of diabetes[8]. Therefore, exogenous AGEs from processed/ grilled/baked foods may be involved in the genesis and development of pancreatic cancer.
Many nutrients and phytochemicals in fruits and vegetables have antioxidant, anti-mutagenic, and anti-carcinogenic properties, especially for water-soluble vitamins[121]. Additionally, isothiocyanates found in cruciferous vegetables powerfully induce the detoxification enzymes, and assist in the removal of potential carcinogens[121]. Higher intake of fruit and vegetables is associated with a reduced risk of pancreatic cancer[122], and cruciferous vegetable intake might be inversely associated with pancreatic cancer risk[123]. A meta-analysis of epidemiological studies showed that high intake of dietary fiber was associated with a risk reduction of pancreatic cancer[124]. A European Prospective Investigation Into Cancer-Norfolk study showed that high-fiber diet altered a positive correlation between red/processed meats and the PDAC development but not those with lower fiber intake, and fiber intake made few alteration for the PDAC risk in past and current smokers[125]. This study showed that fiber intake may be beneficial for those with high meat intake, but the findings do not suggest that high fiber intake can protect against the PDAC development[125]. Therefore, further prospective cohort studies are needed to investigate the effect of fiber intake on the development and progression of pancreatic cancer. A low-fat dietary intervention was associated with a reduced incidence of pancreatic cancer in overweight or obese women in the Women’s Health Initiative Dietary Modification trial[126]. Therefore, it is of great significance for the prevention of pancreatic cancer by adjusting the diet of pancreatic cancer susceptible population. And it is worth further investigating how both diet and lifestyle may work together to promote or inhibit pancreatic cancer.
Pancreatitis
The majority of burden in exocrine pancreatic disease arises from acute pancreatitis, chronic pancreatitis, and pancreatic cancer[127]. Acute pancreatitis, an inflammatory disease of exocrine pancreas, is associated with injury and necrosis of tissue[128]. It has been reported that acute pancreatitis may be an early symptom of pancreatic cancer[129]. A nationwide matched-cohort study in Denmark showed that patients hospitalized with acute pancreatitis had an increased risk of developing pancreatic cancer compared with age- and gender-matched controls in the general population[130]. Rijkers et al[131] found that patients who suffered a first incident of acute pancreatitis and had no further progression to chronic pancreatitis had a 0.4% risk of developing pancreatic cancer, but the risk of pancreatic cancer increased 9-fold for those who progressed to chronic pancreatitis, suggesting that screening for pancreatic cancer after a first incident of acute pancreatitis, especially in patients who had further progression to chronic pancreatitis, could potentially result in more curable resections and improved survival[131]. The risk of pancreatic cancer increased markedly after an initial diagnosis of acute pancreatitis, regardless of its type, and gradually decreases with passage of time[132]. An increase in the number of recurrent episodes of acute pancreatitis was associated with an increased risk of developing pancreatic cancer[132].
Patients who have an episode of acute pancreatitis are 20%-30% more likely to have one or more relapse, and approximately 10% of the relapsing cases progress to chronic pancreatitis[133]. Chronic pancreatitis is a progressive inflammatory disease, and causes pancreatic parenchyma to be replaced by fibrous tissue, resulting in a loss of acinar and islet cells[134]. Chronic pancreatitis may cause mild or asymptomatic debilitating pain, attack(s) of acute pancreatitis, endocrine and/or exocrine deficiency, local and/or systemic complications, and pancreatic cancer[135]. In recent decades, there is accumulating evidence that longstanding pre-existing chronic pancreatitis is a strong risk factor of developing pancreatic cancer[25]. Low body mass index and pancreatic exocrine insufficiency in patients with chronic pancreatitis define a high-risk population with latent PDAC[136]. In comparison with the general population, patients with chronic pancreatitis had a significantly increased risk of pancreatic cancer, especially those with an older age at onset and a > 60 pack-year smoking history[137]. Five years after diagnosis, the risk of pancreatic cancer increased nearly 8-fold in patients with chronic pancreatitis, but the association diminishes with long-term follow-up[138]. The same risk trend was observed in patients with recurrent acute pancreatitis and chronic pancreatitis, suggesting the need for close follow-up in the first few years after diagnosis of these two types of pancreatitis to avoid neglect of pancreatic cancer. Patients who need surgery to treat chronic pancreatitis have a very high risk of developing pancreatic cancer, and early surgical intervention can play a role in preventing the progression of chronic pancreatitis to pancreatic cancer[139]. Chronic pancreatitis patients with de novo postoperative diabetes have a high suspicion index of developing pancreatic cancer after surgery[139].
Patients with early-onset pancreatitis caused by genetic factors appear to have a higher risk of developing pancreatic cancer[140]. Mutations of susceptibility genes in chronic pancreatitis can determine hereditary pancreatitis, idiopathic chronic pancreatitis, and cystic fibrosis, and Cazacu et al[140] found that mutations of cystic fibrosis transmembrane conductance regulator (CFTR) genes modestly increase the risk of pancreatic cancer in a meta-analysis. A total of 50080 patients were diagnosed with pancreatic cancer, of which 14.8% (7420 cases) were diagnosed with idiopathic pancreatitis prior to the diagnosis of cancer[141]. After pancreatitis diagnosis, six risk factors significantly associated with pancreatic cancer diagnosis included age between 40 and 90 years, African-American race, male sex, smoking, obesity, and DM, suggesting that it may be warranted to screen patients older than 40 years with unclear etiology of pancreatitis, especially for African-Americans and male population[141].
The study of the promoting effect of pancreatitis on the development of pancreatic cancer is beneficial to the early detection of pancreatic cancer, and can provide more guidance for the prevention of pancreatic cancer. However, in order to better understand the promoting role of pancreatitis on pancreatic cancer, more studies are needed to clarify the mechanism of pancreatitis in the development of pancreatic cancer.
Obesity
Obesity has been more and more recognized as a strong but modifiable risk factor for pancreatic cancer[142]. Relevant studies have confirmed that obesity is associated with an increased incidence of pancreatic cancer and potentially worse outcomes of this cancer[142]. A cohort study with pooled analysis found that central obesity was associated with increased mortality of pancreatic cancer, independent of body mass index, and also suggested that being overweight or obese during early adulthood may have a significant impact on the mortality risk of pancreatic cancer later in life[143]. A nationwide study including 1.79 million Israeli adolescents showed that obesity (≥ 95th percentile) was associated with an increased risk of pancreatic cancer later in life among both men and women compared with normal weight (5th to- < 85th percentile)[144].
There have been two biological mechanisms that were proposed to explain the underlying association between obesity and risk of pancreatic cancer, including inflammation and hormonal misbalance[142]. Many human cancers result directly from chronic inflammation, and inflammation has emerged to be a key mediator of pancreatic cancer development[145]. Changes in the fibro-inflammatory microenvironment are the major feature of obesity-associated pancreatic tumors[146]. Obesity is a pro-inflammatory condition, and both hypertrophied adipocytes and immune cells (primarily lymphocytes and macrophages) residing in adipose tissue contribute to increased circulating levels of pro-inflammatory cytokines like TNF-α, IL-6, leptin, and adiponectin[147]. The imbalance between these finely regulated pro-inflammatory and anti-inflammatory bioactive molecules leads to changes in tissue microenvironment, which further have influence on cell proliferation, apoptosis, cell invasion, and angiogenesis[148]. For example, Hertzer et al[149] reported that conditional KRASG12D mice with a high fat, high calorie diet exhibited significantly increased inflammation in the peri-pancreatic fat accompanied by elevated levels of several inflammatory cytokines, such as IL6, IL13, and IFN-γ, suggesting that obesity-associated inflammation in peri-pancreatic fat may accelerate pancreatic neoplasia in the model mice[149].
Obesity is also often associated with insulin resistance and T2DM, along with raised levels of insulin and IGF-1[142]. Insulin resistance is a hallmark of T2DM, in which insulin fails to trigger adequate glucose uptake, resulting in accumulation of glucose in bloodstream and raised levels of insulin[150]. Hyperglycemia can enhance the availability of nutrients to cancer cells which metabolize glucose through the Warburg effect[151]. Islet adaptation enhances hormone production, processing, and secretion in the setting of obesity[146]. Even moderate overall and abdominal obesity and weight gain during adulthood were independently associated with an increased risk of developing hyperinsulinemia in non-diabetic middle-aged men[152]. Hyperinsulinemia causes a rise of IGF-1 which activates PI3K/MAPK/mTOR pathways after binding with its receptor, or the IGF receptor[148]. Overactivation of these pathways can activate the Ras/ERK pathway, leading to an increase in cell division, and IGF-1 activating PI3K/AKT/mTOR pathways promotes proliferation and inhibits apoptosis[142].
The mutation of oncogenic KRAS is the major event in pancreatic cancer and permanently activates KRAS protein, and then the protein serves on a molecular switch to activate various signaling pathways and transcription factors in cells, inducing cell proliferation, migration, transformation, and survival[153]. In comparison with lean KC mice, the pancreas of obese KC mice showed an increase in activation of KRAS downstream pathways, including MAPK and PI3K/AKT/ mTORC1[154]. Chung et al[146] found that β-cell aberrantly expressed peptide hormone cholecystokinin in response to obesity and showed that islet cholecystokinin promoted oncogenic KRAS-driven tumorigenesis in pancreatic duct.
Infection
Helicobacter pylori (H. pylori) found mainly in the stomach is a Gram-negative microaerophilic pathogen that chronically infects as much as half the world's population[155]. H. pylori infection is associated with a variety of malignancies, such as gastric cancer, premalignant lesions of the stomach (atrophic gastritis and intestinal metaplasia), gastric lymphoma, pancreatic cancer, colorectal cancer, and laryngeal cancer[156]. With an estimated prevalence between 25% and 50% in Westernized countries, H. pylori could result in 4% to 25% of all cases with pancreatic cancer in these countries[157]. H. pylori infection is closely, albeit weakly, associated with the development of pancreatic cancer, and the association is prominent in Europe and East Asia, but less so in North America[158]. Cytotoxin-associated antigen A (CagA), a 120-145-kDa protein, was for the first time described as a virulence factor of H. pylori related to peptic ulcers[159]. A risk of pancreatic cancer increased in individuals with seropositivity for CagA-negative H. pylori, whereas the risk decreased in individuals with seropositivity for CagA-positive H. pylori[160]. CagA-negative strains of H. pylori might be a causative factor of pancreatic cancer[161]. Xiao et al[158] reported that CagA-positive H. pylori strains appear not to be associated with pancreatic cancer.
The net effects of H. pylori colonization in the gastric antrum are paracrine disinhibition of antral G-cell function, hypergastrinemia, and hyperacidity[162]. The risk of pancreatic cancer is increased by long-term conditions of excess gastric/ duodenal acidity[162]. Gastric acid drives pancreatic bicarbonate secretion and, a consequence of hyperchlorhydria and suppressed somatostatin increases bicarbonate output from the pancreas in H. pylori carriers[162]. Low-level, prolonged generating of secretin or pancreatic bicarbonate increases the activity and turnover rate of ductular epithelial cell to sufficiently enhance the carcinogenic effect of environmental or endogenous N-nitroso carcinogens[162]. Asymptomatic H. pylori colonization, non-ulcer dyspepsia, or duodenal ulcers, and exposure to N-nitroso carcinogens via dietary or other routes in individuals would increase the risk of developing pancreatic cancer by increasing basal secretors or pancreatic bicarbonate[162]. CagA injected into gastric parietal cells through the interaction of H. pylori with integrin results in the activation of extracellular regulated protein kinases (ERK1/2) to further mobilize NF-κB p50 homodimers into the nucleus, leading to the inhibition of gastric H,K-adenosine triphosphatase (H,K-ATPase) α subunit transcription and the repression of gastric acid secretion[163]. Seropositivity of CagA-positive H. pylori was shown to protect against pancreatic cancer when compared to CagA-negative H. pylori, suggesting that differential modification of CagA-negative vs CagA-positive strains of H. pylori on chronic gastric acidity may be involved in modulating the risk of pancreatic cancer[160].
Socioeconomic status and insurance
In contrast with the traditional biomedical model, the bio-psycho-social-medical model highlights the significant role of socioeconomic status in health care services, including insurance status, marital status, and poverty level[164]. A study suggested that African-Americans, and in some cases Hispanics, had lower rates of surgery, less accessed to aggressive stage specific treatment, and underwent surgery at low volume hospitals and/or by lower volume surgeons, which might contribute to the differences in outcomes[165]. In addition, underinsured or uninsured patients also tended to receive less aggressive treatment[165]. A study of a total of 83902 patients with pancreatic cancer showed that patients with lower socioeconomic status were less likely to undergo surgical resection among patients with localized/regional pancreatic cancer[166]. Among patients with localized/regional pancreatic cancer who underwent surgical resection, patients with higher socioeconomic status have better overall survival, and patients with lower socioeconomic status have worse pancreatic cancer-specific survival compared with patients with higher socioeconomic status[166]. These findings suggest that racial differences in treatment and outcomes might be attributable to socioeconomic, insurance, and geographic factors[165]. The study of pancreatic cancer cases from the National Cancer Database from 2004 to 2015 showed that private insurance was associated with more treatment and better survival, higher education was associated with earlier treatment, and treatment was less and delayed among African-Americans despite later diagnosis[167]. After adjusting for socioeconomic status, African-Americans had about the same rate of survival overall at integrated facilities and the survival was improved, suggesting that higher socioeconomic status was associated with better treatment and survival[167]. A pan-cancer analysis showed that socioeconomic status was strongly associated with 1-mo postoperative mortality in primary solid tumors, and the risk of dying was high within 1-mo after surgery in socioeconomically disadvantaged people[164]. And underserved populations around the world often face similar barriers to cancer treatment, largely reflecting inequalities in social factors[165]. Therefore, socioeconomic status plays an extremely important role in the prevention and prognosis of pancreatic cancer, and the formulation of policies targeting low socioeconomic status patients may improve the low 5-year survival rate of pancreatic cancer.
CONCLUSION
Over the next 10 years to 20 years, an increase in pancreatic cancer is inevitable. At the same time, in the face of the characteristics of high mortality and difficult early diagnosis of pancreatic cancer, early prevention of pancreatic cancer through understanding the risk factors for pancreatic cancer is an economical and effective means, which is to prevent pancreatic cancer in advance. In view of the non-modifiable factors affecting pancreatic cancer, we may screen the susceptible population to pancreatic cancer, and provide reliable screening strategies and reasonable diagnostic ideas for the early diagnosis of pancreatic cancer. By studying the modifiable risk factors that affect pancreatic cancer, we may provide earlier interventions to prevent pancreatic cancer so that it can be possibly blocked in its early stages of canceration, thus significantly reducing the incidence of pancreatic cancer. Globally, a comprehensive prevention and control strategy for pancreatic cancer should include effective tobacco-control policy, recommendations for healthier lifestyles, and enlarging coverage of screening, education and vaccination programmes to better improve public awareness of the need to take precautions.
ACKNOWLEDGEMENTS
We sincerely thank International Agency for Research on Cancer for granting us permission to use Figure 1 in this paper.
Footnotes
Conflict-of-interest statement: All the authors of this paper declare that there is no conflict of interest to declare.
Manuscript source: Invited manuscript
Peer-review started: February 8, 2021
First decision: March 6, 2021
Article in press: June 15, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sperti C, Takemura N S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Wang LL
Contributor Information
Jian-Xiong Hu, Intensive Care Unit (ICU), Affiliated Hospital of Putian University, Putian 351100, Fujian Province, China.
Cheng-Fei Zhao, School of Pharmacy and Medical Technology, Putian University, Putian 351100, Fujian Province, China; Key Laboratory of Pharmaceutical Analysis and Laboratory Medicine in University of Fujian Province, Putian University, Putian 351100, Fujian Province, China. zhaochengfei209@163.com.
Wen-Biao Chen, Department of Basic Medicine, Quanzhou Medical College, Quanzhou 362011, Fujian Province, China.
Qi-Cai Liu, Department of Reproductive Medicine Centre, First Affiliated Hospital of Fujian Medical University, Fuzhou 350005, Fujian Province, China.
Qu-Wen Li, Department of Priority Laboratory for Zoonoses Research, Fujian Center for Disease Control and Prevention, Fuzhou 350001, Fujian Province, China.
Yan-Ya Lin, Intensive Care Unit (ICU), Affiliated Hospital of Putian University, Putian 351100, Fujian Province, China.
Feng Gao, Department of Pathology, First Affiliated Hospital of Fujian Medical University, Fuzhou 350005, Fujian Province, China.
References
- 1.PDQ Adult Treatment Editorial Board. Pancreatic Cancer Treatment (Adult) (PDQ®): Health Professional Version 2002. [Google Scholar]
- 2.Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH, Neoptolemos JP. Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 3.US Preventive Services Task Force, Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, Curry SJ, Doubeni CA, Epling JW Jr, Kubik M, Landefeld CS, Mangione CM, Pbert L, Silverstein M, Simon MA, Tseng CW, Wong JB. Screening for Pancreatic Cancer: US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA. 2019;322:438–444. doi: 10.1001/jama.2019.10232. [DOI] [PubMed] [Google Scholar]
- 4.Luo W, Tao J, Zheng L, Zhang T. Current epidemiology of pancreatic cancer: Challenges and opportunities. Chin J Cancer Res. 2020;32:705–719. doi: 10.21147/j.issn.1000-9604.2020.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jagadeesan B, Haran PH, Praveen D, Chowdary PR, Aanandhi MV. A comprehensive review on pancreatic cancer. Res J Pharm Technol . 2021;14:552–554. [Google Scholar]
- 6.Aier I, Semwal R, Sharma A, Varadwaj PK. A systematic assessment of statistics, risk factors, and underlying features involved in pancreatic cancer. Cancer Epidemiol. 2019;58:104–110. doi: 10.1016/j.canep.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Jin C, Bai L. Pancreatic cancer–current situation and challenges. Gastroenterol Hepatol Lett . 2020;2:1–3. [Google Scholar]
- 8.Menini S, Iacobini C, Vitale M, Pesce C, Pugliese G. Diabetes and Pancreatic Cancer-A Dangerous Liaison Relying on Carbonyl Stress. Cancers (Basel) 2021;13 doi: 10.3390/cancers13020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hand F, Conlon KC. Pancreatic cancer. Surg (Oxford) 2019;37:319–326. [Google Scholar]
- 10.Huang J, Lok V, Ngai CH, Zhang L, Yuan J, Lao XQ, Ng K, Chong C, Zheng ZJ, Wong MCS. Worldwide Burden of, Risk Factors for, and Trends in Pancreatic Cancer. Gastroenterology. 2021;160:744–754. doi: 10.1053/j.gastro.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 11.McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846–4861. doi: 10.3748/wjg.v24.i43.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bengtsson A, Andersson R, Ansari D. The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data. Sci Rep. 2020;10:16425. doi: 10.1038/s41598-020-73525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao C, Gao F, Li Q, Liu Q, Lin X. The Distributional Characteristic and Growing Trend of Pancreatic Cancer in China. Pancreas. 2019;48:309–314. doi: 10.1097/MPA.0000000000001222. [DOI] [PubMed] [Google Scholar]
- 14.Surveillance , Epidemiology , and End Results Program. Cancer Stat Facts: Pancreatic Cancer. [cited 20 Jan 2021]. In: National Cancer Institute [Internet]. Available from: https://seer.cancer.gov/statfacts/html/pancreas.html .
- 15.GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:934–947. doi: 10.1016/S2468-1253(19)30347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 17.Ferlay J, Partensky C, Bray F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. 2016;55:1158–1160. doi: 10.1080/0284186X.2016.1197419. [DOI] [PubMed] [Google Scholar]
- 18.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 19.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 20.Conceição P. Human Development Report 2020. [cited 10 Mar 2021]. In: United Nations Development Programme (UNDP) [Internet]. Available from: http://hdr.undp.org/en/2020-report .
- 21.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global Cancer Observatory: Cancer Today. [cited 20 Jan 2021]. In: International Agency for Research on Cancer [Internet]. Available from: https://gco.iarc.fr/today .
- 22.Wong MCS, Jiang JY, Liang M, Fang Y, Yeung MS, Sung JJY. Global temporal patterns of pancreatic cancer and association with socioeconomic development. Sci Rep. 2017;7:3165. doi: 10.1038/s41598-017-02997-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodarzi E, Dehkordi AH, Beiranvand R, Naemi H, Khazaei Z. Epidemiology of the Incidence and Mortality of Pancreas Cancer and its Relationship with the Human Development Index (HDI) in the World: An Ecological Study in 2018. Curr Pharm Des. 2020;26:5163–5173. doi: 10.2174/1381612826666200713170047. [DOI] [PubMed] [Google Scholar]
- 24.Maisonneuve P. Epidemiology and burden of pancreatic cancer. Presse Med. 2019;48:e113–e123. doi: 10.1016/j.lpm.2019.02.030. [DOI] [PubMed] [Google Scholar]
- 25.Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10:10–27. doi: 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia X, Du P, Wu K, Xu Z, Fang J, Xu X, Lin K. Pancreatic Cancer Mortality in China: Characteristics and Prediction. Pancreas. 2018;47:233–237. doi: 10.1097/MPA.0000000000000976. [DOI] [PubMed] [Google Scholar]
- 27.Cho J, Petrov MS. Pancreatitis, Pancreatic Cancer, and Their Metabolic Sequelae: Projected Burden to 2050. Clin Transl Gastroenterol. 2020;11:e00251. doi: 10.14309/ctg.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 29.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 30.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 31.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ American Cancer Society. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 32.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 33.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 34.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 35.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 36.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 37.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 38.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 39.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 40.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 41.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 42.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 43.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 44.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 45.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 46.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 47.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 48.Zhao C, Gao F, Weng S, Liu Q. Pancreatic cancer and associated exosomes. Cancer Biomark. 2017;20:357–367. doi: 10.3233/CBM-170258. [DOI] [PubMed] [Google Scholar]
- 49.Zheng R, Zeng H, Zhang S, Chen T, Chen W. National estimates of cancer prevalence in China, 2011. Cancer Lett. 2016;370:33–38. doi: 10.1016/j.canlet.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 51.Hofmann BT, Stehr A, Dohrmann T, Güngör C, Herich L, Hiller J, Harder S, Ewald F, Gebauer F, Tachezy M, Precht C, Izbicki JR, Bockhorn M, Wagener C, Wolters-Eisfeld G. ABO blood group IgM isoagglutinins interact with tumor-associated O-glycan structures in pancreatic cancer. Clin Cancer Res. 2014;20:6117–6126. doi: 10.1158/1078-0432.CCR-14-0716. [DOI] [PubMed] [Google Scholar]
- 52.Ben Q, Wang K, Yuan Y, Li Z. Pancreatic cancer incidence and outcome in relation to ABO blood groups among Han Chinese patients: a case-control study. Int J Cancer. 2011;128:1179–1186. doi: 10.1002/ijc.25426. [DOI] [PubMed] [Google Scholar]
- 53.Antwi SO, Bamlet WR, Pedersen KS, Chaffee KG, Risch HA, Shivappa N, Steck SE, Anderson KE, Bracci PM, Polesel J, Serraino D, La Vecchia C, Bosetti C, Li D, Oberg AL, Arslan AA, Albanes D, Duell EJ, Huybrechts I, Amundadottir LT, Hoover R, Mannisto S, Chanock SJ, Zheng W, Shu XO, Stepien M, Canzian F, Bueno-de-Mesquita B, Quirós JR, Zeleniuch-Jacquotte A, Bruinsma F, Milne RL, Giles GG, Hébert JR, Stolzenberg-Solomon RZ, Petersen GM. Pancreatic cancer risk is modulated by inflammatory potential of diet and ABO genotype: a consortia-based evaluation and replication study. Carcinogenesis. 2018;39:1056–1067. doi: 10.1093/carcin/bgy072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vasan SK, Hwang J, Rostgaard K, Nyrén O, Ullum H, Pedersen OBV, Erikstrup C, Melbye M, Hjalgrim H, Pawitan Y, Edgren G. ABO blood group and risk of cancer: A register-based cohort study of 1.6 million blood donors. Cancer Epidemiol. 2016;44:40–43. doi: 10.1016/j.canep.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 55.Li X, Xu H, Gao P. ABO Blood Group and Diabetes Mellitus Influence the Risk for Pancreatic Cancer in a Population from China. Med Sci Monit. 2018;24:9392–9398. doi: 10.12659/MSM.913769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El Jellas K, Hoem D, Hagen KG, Kalvenes MB, Aziz S, Steine SJ, Immervoll H, Johansson S, Molven A. Associations between ABO blood groups and pancreatic ductal adenocarcinoma: influence on resection status and survival. Cancer Med. 2017;6:1531–1540. doi: 10.1002/cam4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu M, Ji S, Xu W, Liu W, Qin Y, Xiang J, Hu Q, Sun Q, Zhang Z, Xu X, Yu X. ABO Blood Group and the Risk of Pancreatic Neoplasms in Chinese Han Population: A Study at Shanghai Pancreatic Cancer Institute. Pancreas. 2019;48:e65–e66. doi: 10.1097/MPA.0000000000001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamamoto F, McNeill PD, Hakomori S. Human histo-blood group A2 transferase coded by A2 allele, one of the A subtypes, is characterized by a single base deletion in the coding sequence, which results in an additional domain at the carboxyl terminal. Biochem Biophys Res Commun. 1992;187:366–374. doi: 10.1016/s0006-291x(05)81502-5. [DOI] [PubMed] [Google Scholar]
- 59.Cervantes A, Waymouth EK, Petrov MS. African-Americans and Indigenous Peoples Have Increased Burden of Diseases of the Exocrine Pancreas: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2019;64:249–261. doi: 10.1007/s10620-018-5291-1. [DOI] [PubMed] [Google Scholar]
- 60.Huang BZ, Stram DO, Le Marchand L, Haiman CA, Wilkens LR, Pandol SJ, Zhang ZF, Monroe KR, Setiawan VW. Interethnic differences in pancreatic cancer incidence and risk factors: The Multiethnic Cohort. Cancer Med. 2019;8:3592–3603. doi: 10.1002/cam4.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shusterman M, Jou E, Kaubisch A, Chuy JW, Rajdev L, Aparo S, Tang J, Ohri N, Negassa A, Goel S. The Neutrophil-to-Lymphocyte Ratio is a Prognostic Biomarker in An Ethnically Diverse Patient Population with Advanced Pancreatic Cancer. J Gastrointest Cancer. 2020;51:868–876. doi: 10.1007/s12029-019-00316-8. [DOI] [PubMed] [Google Scholar]
- 62.Gad MM, Găman MA, Saad AM, Al-Husseini MJ, Shehata OA, Saleh MA, Nelson AD, Simons-Linares CR. Temporal trends of incidence and mortality in Asian-Americans with pancreatic adenocarcinoma: an epidemiological study. Ann Gastroenterol. 2020;33:210–218. doi: 10.20524/aog.2020.0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amaral NS, Resende V, Dos Santos JS, Lima LF, Moraes DC, Friedman E, DE Marco L, Bastos-Rodrigues L. Impact of Ethnicity on Somatic Mutation Rates of Pancreatic Adenocarcinoma. In Vivo. 2018;32:1527–1531. doi: 10.21873/invivo.11410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li P, Shu Y, Gu Y. The potential role of bacteria in pancreatic cancer: a systematic review. Carcinogenesis. 2020;41:397–404. doi: 10.1093/carcin/bgaa013. [DOI] [PubMed] [Google Scholar]
- 65.Farrell JJ, Zhang L, Zhou H, Chia D, Elashoff D, Akin D, Paster BJ, Joshipura K, Wong DT. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut. 2012;61:582–588. doi: 10.1136/gutjnl-2011-300784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Torres PJ, Fletcher EM, Gibbons SM, Bouvet M, Doran KS, Kelley ST. Characterization of the salivary microbiome in patients with pancreatic cancer. PeerJ. 2015;3:e1373. doi: 10.7717/peerj.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fan X, Alekseyenko AV, Wu J, Peters BA, Jacobs EJ, Gapstur SM, Purdue MP, Abnet CC, Stolzenberg-Solomon R, Miller G, Ravel J, Hayes RB, Ahn J. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut. 2018;67:120–127. doi: 10.1136/gutjnl-2016-312580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olson SH, Satagopan J, Xu Y, Ling L, Leong S, Orlow I, Saldia A, Li P, Nunes P, Madonia V, Allen PJ, O'Reilly E, Pamer E, Kurtz RC. The oral microbiota in patients with pancreatic cancer, patients with IPMNs, and controls: a pilot study. Cancer Causes Control. 2017;28:959–969. doi: 10.1007/s10552-017-0933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Q, Jin M, Liu Y, Jin L. Gut Microbiota: Its Potential Roles in Pancreatic Cancer. Front Cell Infect Microbiol. 2020;10:572492. doi: 10.3389/fcimb.2020.572492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ren Z, Jiang J, Xie H, Li A, Lu H, Xu S, Zhou L, Zhang H, Cui G, Chen X, Liu Y, Wu L, Qin N, Sun R, Wang W, Li L, Zheng S. Gut microbial profile analysis by MiSeq sequencing of pancreatic carcinoma patients in China. Oncotarget. 2017;8:95176–95191. doi: 10.18632/oncotarget.18820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Darnaud M, Faivre J, Moniaux N. Targeting gut flora to prevent progression of hepatocellular carcinoma. J Hepatol. 2013;58:385–387. doi: 10.1016/j.jhep.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 72.Daniluk J, Liu Y, Deng D, Chu J, Huang H, Gaiser S, Cruz-Monserrate Z, Wang H, Ji B, Logsdon CD. An NF-κB pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J Clin Invest. 2012;122:1519–1528. doi: 10.1172/JCI59743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–1249. doi: 10.1101/gad.1415606. [DOI] [PubMed] [Google Scholar]
- 74.Ying H, Dey P, Yao W, Kimmelman AC, Draetta GF, Maitra A, DePinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2016;30:355–385. doi: 10.1101/gad.275776.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomas RM, Gharaibeh RZ, Gauthier J, Beveridge M, Pope JL, Guijarro MV, Yu Q, He Z, Ohland C, Newsome R, Trevino J, Hughes SJ, Reinhard M, Winglee K, Fodor AA, Zajac-Kaye M, Jobin C. Intestinal microbiota enhances pancreatic carcinogenesis in preclinical models. Carcinogenesis. 2018;39:1068–1078. doi: 10.1093/carcin/bgy073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Del Castillo E, Meier R, Chung M, Koestler DC, Chen T, Paster BJ, Charpentier KP, Kelsey KT, Izard J, Michaud DS. The Microbiomes of Pancreatic and Duodenum Tissue Overlap and Are Highly Subject Specific but Differ between Pancreatic Cancer and Noncancer Subjects. Cancer Epidemiol Biomarkers Prev. 2019;28:370–383. doi: 10.1158/1055-9965.EPI-18-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, Mohan N, Aykut B, Usyk M, Torres LE, Werba G, Zhang K, Guo Y, Li Q, Akkad N, Lall S, Wadowski B, Gutierrez J, Kochen Rossi JA, Herzog JW, Diskin B, Torres-Hernandez A, Leinwand J, Wang W, Taunk PS, Savadkar S, Janal M, Saxena A, Li X, Cohen D, Sartor RB, Saxena D, Miller G. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018;8:403–416. doi: 10.1158/2159-8290.CD-17-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maekawa T, Fukaya R, Takamatsu S, Itoyama S, Fukuoka T, Yamada M, Hata T, Nagaoka S, Kawamoto K, Eguchi H, Murata K, Kumada T, Ito T, Tanemura M, Fujimoto K, Tomita Y, Tobe T, Kamada Y, Miyoshi E. Possible involvement of Enterococcus infection in the pathogenesis of chronic pancreatitis and cancer. Biochem Biophys Res Commun. 2018;506:962–969. doi: 10.1016/j.bbrc.2018.10.169. [DOI] [PubMed] [Google Scholar]
- 79.Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, Gavert N, Zwang Y, Cooper ZA, Shee K, Thaiss CA, Reuben A, Livny J, Avraham R, Frederick DT, Ligorio M, Chatman K, Johnston SE, Mosher CM, Brandis A, Fuks G, Gurbatri C, Gopalakrishnan V, Kim M, Hurd MW, Katz M, Fleming J, Maitra A, Smith DA, Skalak M, Bu J, Michaud M, Trauger SA, Barshack I, Golan T, Sandbank J, Flaherty KT, Mandinova A, Garrett WS, Thayer SP, Ferrone CR, Huttenhower C, Bhatia SN, Gevers D, Wargo JA, Golub TR, Straussman R. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357:1156–1160. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chang MC, Wong JM, Chang YT. Screening and early detection of pancreatic cancer in high risk population. World J Gastroenterol. 2014;20:2358–2364. doi: 10.3748/wjg.v20.i9.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ohmoto A, Yachida S, Morizane C. Genomic Features and Clinical Management of Patients with Hereditary Pancreatic Cancer Syndromes and Familial Pancreatic Cancer. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20030561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Molina-Montes E, Gomez-Rubio P, Márquez M, Rava M, Löhr M, Michalski CW, Molero X, Farré A, Perea J, Greenhalf W, Ilzarbe L, O'Rorke M, Tardón A, Gress T, Barberà VM, Crnogorac-Jurcevic T, Domínguez-Muñoz E, Muñoz-Bellvís L, Balsells J, Costello E, Huang J, Iglesias M, Kleeff J, Kong B, Mora J, Murray L, O'Driscoll D, Poves I, Scarpa A, Ye W, Hidalgo M, Sharp L, Carrato A, Real FX, Malats N PanGenEU Study Investigators. Risk of pancreatic cancer associated with family history of cancer and other medical conditions by accounting for smoking among relatives. Int J Epidemiol. 2018;47:473–483. doi: 10.1093/ije/dyx269. [DOI] [PubMed] [Google Scholar]
- 83.Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, Griffin C, Cameron JL, Yeo CJ, Kern S, Hruban RH. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–2638. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 84.Brune KA, Lau B, Palmisano E, Canto M, Goggins MG, Hruban RH, Klein AP. Importance of age of onset in pancreatic cancer kindreds. J Natl Cancer Inst. 2010;102:119–126. doi: 10.1093/jnci/djp466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ghiorzo P. Genetic predisposition to pancreatic cancer. World J Gastroenterol. 2014;20:10778–10789. doi: 10.3748/wjg.v20.i31.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chaffee KG, Oberg AL, McWilliams RR, Majithia N, Allen BA, Kidd J, Singh N, Hartman AR, Wenstrup RJ, Petersen GM. Prevalence of germ-line mutations in cancer genes among pancreatic cancer patients with a positive family history. Genet Med. 2018;20:119–127. doi: 10.1038/gim.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang BZ, Pandol SJ, Jeon CY, Chari ST, Sugar CA, Chao CR, Zhang ZF, Wu BU, Setiawan VW. New-Onset Diabetes, Longitudinal Trends in Metabolic Markers, and Risk of Pancreatic Cancer in a Heterogeneous Population. Clin Gastroenterol Hepatol 2020; 18: 1812-1821. :e7. doi: 10.1016/j.cgh.2019.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10:88–95. doi: 10.1016/S1470-2045(08)70337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andersen DK, Korc M, Petersen GM, Eibl G, Li D, Rickels MR, Chari ST, Abbruzzese JL. Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer. Diabetes. 2017;66:1103–1110. doi: 10.2337/db16-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Setiawan VW, Stram DO, Porcel J, Chari ST, Maskarinec G, Le Marchand L, Wilkens LR, Haiman CA, Pandol SJ, Monroe KR. Pancreatic Cancer Following Incident Diabetes in African Americans and Latinos: The Multiethnic Cohort. J Natl Cancer Inst. 2019;111:27–33. doi: 10.1093/jnci/djy090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D, Zhou M, Tang X, Hu Y, Wang L. Prevalence and Ethnic Pattern of Diabetes and Prediabetes in China in 2013. JAMA. 2017;317:2515–2523. doi: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988-2012. JAMA. 2015;314:1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 93.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 94.Eibl G, Cruz-Monserrate Z, Korc M, Petrov MS, Goodarzi MO, Fisher WE, Habtezion A, Lugea A, Pandol SJ, Hart PA, Andersen DK Consortium for the Study of Chronic Pancreatitis. Diabetes , and Pancreatic Cancer. Diabetes Mellitus and Obesity as Risk Factors for Pancreatic Cancer. J Acad Nutr Diet. 2018;118:555–567. doi: 10.1016/j.jand.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Butler AE, Galasso R, Matveyenko A, Rizza RA, Dry S, Butler PC. Pancreatic duct replication is increased with obesity and type 2 diabetes in humans. Diabetologia. 2010;53:21–26. doi: 10.1007/s00125-009-1556-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang J, Waldron RT, Su HY, Moro A, Chang HH, Eibl G, Ferreri K, Kandeel FR, Lugea A, Li L, Pandol SJ. Insulin promotes proliferation and fibrosing responses in activated pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2016;311:G675–G687. doi: 10.1152/ajpgi.00251.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rahn S, Zimmermann V, Viol F, Knaack H, Stemmer K, Peters L, Lenk L, Ungefroren H, Saur D, Schäfer H, Helm O, Sebens S. Diabetes as risk factor for pancreatic cancer: Hyperglycemia promotes epithelial-mesenchymal-transition and stem cell properties in pancreatic ductal epithelial cells. Cancer Lett. 2018;415:129–150. doi: 10.1016/j.canlet.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 98.Ghadirian P, Lynch HT, Krewski D. Epidemiology of pancreatic cancer: an overview. Cancer Detect Prev. 2003;27:87–93. doi: 10.1016/s0361-090x(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 99.Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol. 2009;6:699–708. doi: 10.1038/nrgastro.2009.177. [DOI] [PubMed] [Google Scholar]
- 100.Yuan C, Morales-Oyarvide V, Babic A, Clish CB, Kraft P, Bao Y, Qian ZR, Rubinson DA, Ng K, Giovannucci EL, Ogino S, Stampfer MJ, Gaziano JM, Sesso HD, Cochrane BB, Manson JE, Fuchs CS, Wolpin BM. Cigarette Smoking and Pancreatic Cancer Survival. J Clin Oncol. 2017;35:1822–1828. doi: 10.1200/JCO.2016.71.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Molina-Montes E, Van Hoogstraten L, Gomez-Rubio P, Löhr M, Sharp L, Molero X, Márquez M, Michalski CW, Farré A, Perea J, O'Rorke M, Greenhalf W, Ilzarbe L, Tardon A, Gress TM, Barberà VM, Crnogorac-Jurcevic T, Muñoz-Bellvis L, Domínguez-Muñoz E, Balsells J, Costello E, Iglesias M, Kleeff J, Kong B, Mora J, O'Driscoll D, Poves I, Scarpa A, Yu J, Ye W, Hidalgo M, Carrato A, Lawlor R, Real FX, Malats N PanGenEU Study Investigators. Pancreatic Cancer Risk in Relation to Lifetime Smoking Patterns, Tobacco Type, and Dose-Response Relationships. Cancer Epidemiol Biomarkers Prev. 2020;29:1009–1018. doi: 10.1158/1055-9965.EPI-19-1027. [DOI] [PubMed] [Google Scholar]
- 102.Weissman S, Takakura K, Eibl G, Pandol SJ, Saruta M. The Diverse Involvement of Cigarette Smoking in Pancreatic Cancer Development and Prognosis. Pancreas. 2020;49:612–620. doi: 10.1097/MPA.0000000000001550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kumar S, Torres MP, Kaur S, Rachagani S, Joshi S, Johansson SL, Momi N, Baine MJ, Gilling CE, Smith LM, Wyatt TA, Jain M, Joshi SS, Batra SK. Smoking accelerates pancreatic cancer progression by promoting differentiation of MDSCs and inducing HB-EGF expression in macrophages. Oncogene. 2015;34:2052–2060. doi: 10.1038/onc.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Delitto D, Zhang D, Han S, Black BS, Knowlton AE, Vlada AC, Sarosi GA, Behrns KE, Thomas RM, Lu X, Liu C, George TJ, Hughes SJ, Wallet SM, Trevino JG. Nicotine Reduces Survival via Augmentation of Paracrine HGF-MET Signaling in the Pancreatic Cancer Microenvironment. Clin Cancer Res. 2016;22:1787–1799. doi: 10.1158/1078-0432.CCR-15-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hermann PC, Sancho P, Cañamero M, Martinelli P, Madriles F, Michl P, Gress T, de Pascual R, Gandia L, Guerra C, Barbacid M, Wagner M, Vieira CR, Aicher A, Real FX, Sainz B Jr, Heeschen C. Nicotine promotes initiation and progression of KRAS-induced pancreatic cancer via Gata6-dependent dedifferentiation of acinar cells in mice. Gastroenterology 2014; 147: 1119-33. :e4. doi: 10.1053/j.gastro.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 106.Schaal C, Chellappan SP. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol Cancer Res. 2014;12:14–23. doi: 10.1158/1541-7786.MCR-13-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tsai HJ, Chang JS. Environmental Risk Factors of Pancreatic Cancer. J Clin Med. 2019;8 doi: 10.3390/jcm8091427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gupta S, Wang F, Holly EA, Bracci PM. Risk of pancreatic cancer by alcohol dose, duration, and pattern of consumption, including binge drinking: a population-based study. Cancer Causes Control. 2010;21:1047–1059. doi: 10.1007/s10552-010-9533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Naudin S, Li K, Jaouen T, Assi N, Kyrø C, Tjønneland A, Overvad K, Boutron-Ruault MC, Rebours V, Védié AL, Boeing H, Kaaks R, Katzke V, Bamia C, Naska A, Trichopoulou A, Berrino F, Tagliabue G, Palli D, Panico S, Tumino R, Sacerdote C, Peeters PH, Bueno-de-Mesquita HBA, Weiderpass E, Gram IT, Skeie G, Chirlaque MD, Rodríguez-Barranco M, Barricarte A, Quirós JR, Dorronsoro M, Johansson I, Sund M, Sternby H, Bradbury KE, Wareham N, Riboli E, Gunter M, Brennan P, Duell EJ, Ferrari P. Lifetime and baseline alcohol intakes and risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition study. Int J Cancer. 2018;143:801–812. doi: 10.1002/ijc.31367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu S, Chheda C, Ouhaddi Y, Benhaddou H, Bourhim M, Grippo PJ, Principe DR, Mascariñas E, DeCant B, Tsukamoto H, Pandol SJ, Edderkaoui M. Characterization of Mouse Models of Early Pancreatic Lesions Induced by Alcohol and Chronic Pancreatitis. Pancreas. 2015;44:882–887. doi: 10.1097/MPA.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Korc M, Jeon CY, Edderkaoui M, Pandol SJ, Petrov MS Consortium for the Study of Chronic Pancreatitis. Diabetes , and Pancreatic Cancer (CPDPC) Tobacco and alcohol as risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2017;31:529–536. doi: 10.1016/j.bpg.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Setiawan VW, Monroe K, Lugea A, Yadav D, Pandol S. Uniting Epidemiology and Experimental Disease Models for Alcohol-Related Pancreatic Disease. Alcohol Res. 2017;38:173–182. [PMC free article] [PubMed] [Google Scholar]
- 113.Chang JS, Hsiao JR, Chen CH. ALDH2 polymorphism and alcohol-related cancers in Asians: a public health perspective. J Biomed Sci. 2017;24:19. doi: 10.1186/s12929-017-0327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Klyosov AA, Rashkovetsky LG, Tahir MK, Keung WM. Possible role of liver cytosolic and mitochondrial aldehyde dehydrogenases in acetaldehyde metabolism. Biochemistry. 1996;35:4445–4456. doi: 10.1021/bi9521093. [DOI] [PubMed] [Google Scholar]
- 115.Chen YC, Peng GS, Tsao TP, Wang MF, Lu RB, Yin SJ. Pharmacokinetic and pharmacodynamic basis for overcoming acetaldehyde-induced adverse reaction in Asian alcoholics, heterozygous for the variant ALDH2*2 gene allele. Pharmacogenet Genomics. 2009;19:588–599. doi: 10.1097/FPC.0b013e32832ecf2e. [DOI] [PubMed] [Google Scholar]
- 116.Kanda J, Matsuo K, Suzuki T, Kawase T, Hiraki A, Watanabe M, Mizuno N, Sawaki A, Yamao K, Tajima K, Tanaka H. Impact of alcohol consumption with polymorphisms in alcohol-metabolizing enzymes on pancreatic cancer risk in Japanese. Cancer Sci. 2009;100:296–302. doi: 10.1111/j.1349-7006.2008.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stolzenberg-Solomon RZ, Cross AJ, Silverman DT, Schairer C, Thompson FE, Kipnis V, Subar AF, Hollenbeck A, Schatzkin A, Sinha R. Meat and meat-mutagen intake and pancreatic cancer risk in the NIH-AARP cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:2664–2675. doi: 10.1158/1055-9965.EPI-07-0378. [DOI] [PubMed] [Google Scholar]
- 118.Nguyen HT, van der Fels-Klerx HJ, van Boekel MAJS. Nϵ-(carboxymethyl) lysine: A review on analytical methods, formation, and occurrence in processed food, and health impact. Food Rev Int . 2014;30:36–52. [Google Scholar]
- 119.Ames JM. Determination of N epsilon-(carboxymethyl)lysine in foods and related systems. Ann N Y Acad Sci. 2008;1126:20–24. doi: 10.1196/annals.1433.030. [DOI] [PubMed] [Google Scholar]
- 120.Jiao L, Stolzenberg-Solomon R, Zimmerman TP, Duan Z, Chen L, Kahle L, Risch A, Subar AF, Cross AJ, Hollenbeck A, Vlassara H, Striker G, Sinha R. Dietary consumption of advanced glycation end products and pancreatic cancer in the prospective NIH-AARP Diet and Health Study. Am J Clin Nutr. 2015;101:126–134. doi: 10.3945/ajcn.114.098061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Salem AA, Mackenzie GG. Pancreatic cancer: A critical review of dietary risk. Nutr Res. 2018;52:1–13. doi: 10.1016/j.nutres.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 122.Wu QJ, Wu L, Zheng LQ, Xu X, Ji C, Gong TT. Consumption of fruit and vegetables reduces risk of pancreatic cancer: evidence from epidemiological studies. Eur J Cancer Prev. 2016;25:196–205. doi: 10.1097/CEJ.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 123.Li LY, Luo Y, Lu MD, Xu XW, Lin HD, Zheng ZQ. Cruciferous vegetable consumption and the risk of pancreatic cancer: a meta-analysis. World J Surg Oncol. 2015;13:44. doi: 10.1186/s12957-015-0454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mao QQ, Lin YW, Chen H, Qin J, Zheng XY, Xu X, Xie LP. Dietary fiber intake is inversely associated with risk of pancreatic cancer: a meta-analysis. Asia Pac J Clin Nutr. 2017;26:89–96. doi: 10.6133/apjcn.102015.03. [DOI] [PubMed] [Google Scholar]
- 125.Koulouris AI, Luben R, Banim P, Hart AR. Dietary Fiber and the Risk of Pancreatic Cancer. Pancreas. 2019;48:121–125. doi: 10.1097/MPA.0000000000001191. [DOI] [PubMed] [Google Scholar]
- 126.Jiao L, Chen L, White DL, Tinker L, Chlebowski RT, Van Horn LV, Richardson P, Lane D, Sangi-Haghpeykar H, El-Serag HB. Low-fat Dietary Pattern and Pancreatic Cancer Risk in the Women's Health Initiative Dietary Modification Randomized Controlled Trial. J Natl Cancer Inst. 2018;110 doi: 10.1093/jnci/djx117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Raimondi S, Lowenfels AB, Morselli-Labate AM, Maisonneuve P, Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;24:349–358. doi: 10.1016/j.bpg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 128.Habtezion A, Gukovskaya AS, Pandol SJ. Acute Pancreatitis: A Multifaceted Set of Organelle and Cellular Interactions. Gastroenterology. 2019;156:1941–1950. doi: 10.1053/j.gastro.2018.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li S, Tian B. Acute pancreatitis in patients with pancreatic cancer: Timing of surgery and survival duration. Medicine (Baltimore) 2017;96:e5908. doi: 10.1097/MD.0000000000005908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kirkegård J, Cronin-Fenton D, Heide-Jørgensen U, Mortensen FV. Acute Pancreatitis and Pancreatic Cancer Risk: A Nationwide Matched-Cohort Study in Denmark. Gastroenterology. 2018;154:1729–1736. doi: 10.1053/j.gastro.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 131.Rijkers AP, Bakker OJ, Ahmed Ali U, Hagenaars JCJP, van Santvoort HC, Besselink MG, Bollen TL, van Eijck CH Dutch Pancreatitis Study Group. Risk of Pancreatic Cancer After a Primary Episode of Acute Pancreatitis. Pancreas. 2017;46:1018–1022. doi: 10.1097/MPA.0000000000000879. [DOI] [PubMed] [Google Scholar]
- 132.Sadr-Azodi O, Oskarsson V, Discacciati A, Videhult P, Askling J, Ekbom A. Pancreatic Cancer Following Acute Pancreatitis: A Population-based Matched Cohort Study. Am J Gastroenterol. 2018;113:1711–1719. doi: 10.1038/s41395-018-0255-9. [DOI] [PubMed] [Google Scholar]
- 133.Serrano J, Andersen DK, Forsmark CE, Pandol SJ, Feng Z, Srivastava S, Rinaudo JAS Consortium for the Study of Chronic Pancreatitis. Diabetes , and Pancreatic Cancer (CPDPC) Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer: From Concept to Reality. Pancreas. 2018;47:1208–1212. doi: 10.1097/MPA.0000000000001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ketwaroo GA, Freedman SD, Sheth SG. Approach to patients with suspected chronic pancreatitis: a comprehensive review. Pancreas. 2015;44:173–180. doi: 10.1097/MPA.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 135.Yadav D, Park WG, Fogel EL, Li L, Chari ST, Feng Z, Fisher WE, Forsmark CE, Jeon CY, Habtezion A, Hart PA, Hughes SJ, Othman MO, Rinaudo JAS, Pandol SJ, Tirkes T, Serrano J, Srivastava S, Van Den Eeden SK, Whitcomb DC, Topazian M, Conwell DL Consortium for the Study of Chronic Pancreatitis. Diabetes , and Pancreatic Cancer (CPDPC) PROspective Evaluation of Chronic Pancreatitis for EpidEmiologic and Translational StuDies: Rationale and Study Design for PROCEED From the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas. 2018;47:1229–1238. doi: 10.1097/MPA.0000000000001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vujasinovic M, Dugic A, Maisonneuve P, Aljic A, Berggren R, Panic N, Valente R, Pozzi Mucelli R, Waldthaler A, Ghorbani P, Kordes M, Hagström H, Löhr JM. Risk of Developing Pancreatic Cancer in Patients with Chronic Pancreatitis. J Clin Med. 2020;9 doi: 10.3390/jcm9113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hao L, Zeng XP, Xin L, Wang D, Pan J, Bi YW, Ji JT, Du TT, Lin JH, Zhang D, Ye B, Zou WB, Chen H, Xie T, Li BR, Zheng ZH, Wang T, Guo HL, Liao Z, Li ZS, Hu LH. Incidence of and risk factors for pancreatic cancer in chronic pancreatitis: A cohort of 1656 patients. Dig Liver Dis. 2017;49:1249–1256. doi: 10.1016/j.dld.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 138.Kirkegård J, Mortensen FV, Cronin-Fenton D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am J Gastroenterol. 2017;112:1366–1372. doi: 10.1038/ajg.2017.218. [DOI] [PubMed] [Google Scholar]
- 139.Zheng Z, Chen Y, Tan C, Ke N, Du B, Liu X. Risk of pancreatic cancer in patients undergoing surgery for chronic pancreatitis. BMC Surg. 2019;19:83. doi: 10.1186/s12893-019-0537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cazacu IM, Farkas N, Garami A, Balaskó M, Mosdósi B, Alizadeh H, Gyöngyi Z, Rakonczay Z Jr, Vigh É, Habon T, Czopf L, Lazarescu MA, Erőss B, Sahin-Tóth M, Hegyi P. Pancreatitis-Associated Genes and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Pancreas. 2018;47:1078–1086. doi: 10.1097/MPA.0000000000001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Syed A, Babich O, Thakkar P, Patel A, Abdul-Baki H, Farah K, Morrissey S, Mitre M, Dhawan M, Kochhar G, Kulkarni A, Thakkar S. Defining Pancreatitis as a Risk Factor for Pancreatic Cancer: The Role, Incidence, and Timeline of Development. Pancreas. 2019;48:1098–1101. doi: 10.1097/MPA.0000000000001367. [DOI] [PubMed] [Google Scholar]
- 142.Xu M, Jung X, Hines OJ, Eibl G, Chen Y. Obesity and Pancreatic Cancer: Overview of Epidemiology and Potential Prevention by Weight Loss. Pancreas. 2018;47:158–162. doi: 10.1097/MPA.0000000000000974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Genkinger JM, Kitahara CM, Bernstein L, Berrington de Gonzalez A, Brotzman M, Elena JW, Giles GG, Hartge P, Singh PN, Stolzenberg-Solomon RZ, Weiderpass E, Adami HO, Anderson KE, Beane-Freeman LE, Buring JE, Fraser GE, Fuchs CS, Gapstur SM, Gaziano JM, Helzlsouer KJ, Lacey JV Jr, Linet MS, Liu JJ, Park Y, Peters U, Purdue MP, Robien K, Schairer C, Sesso HD, Visvanathan K, White E, Wolk A, Wolpin BM, Zeleniuch-Jacquotte A, Jacobs EJ. Central adiposity, obesity during early adulthood, and pancreatic cancer mortality in a pooled analysis of cohort studies. Ann Oncol. 2015;26:2257–2266. doi: 10.1093/annonc/mdv355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zohar L, Rottenberg Y, Twig G, Katz L, Leiba A, Derazne E, Tzur D, Eizenstein S, Keinan-Boker L, Afek A, Kark JD. Adolescent overweight and obesity and the risk for pancreatic cancer among men and women: a nationwide study of 1.79 million Israeli adolescents. Cancer. 2019;125:118–126. doi: 10.1002/cncr.31764. [DOI] [PubMed] [Google Scholar]
- 145.Shadhu K, Xi C. Inflammation and pancreatic cancer: An updated review. Saudi J Gastroenterol. 2019;25:3–13. doi: 10.4103/sjg.SJG_390_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chung KM, Singh J, Lawres L, Dorans KJ, Garcia C, Burkhardt DB, Robbins R, Bhutkar A, Cardone R, Zhao X, Babic A, Vayrynen SA, Dias Costa A, Nowak JA, Chang DT, Dunne RF, Hezel AF, Koong AC, Wilhelm JJ, Bellin MD, Nylander V, Gloyn AL, McCarthy MI, Kibbey RG, Krishnaswamy S, Wolpin BM, Jacks T, Fuchs CS, Muzumdar MD. Endocrine-Exocrine Signaling Drives Obesity-Associated Pancreatic Ductal Adenocarcinoma. Cell 2020; 181: 832-847. :e18. doi: 10.1016/j.cell.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Makki K, Froguel P, Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:139239. doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rawla P, Thandra KC, Sunkara T. Pancreatic cancer and obesity: epidemiology, mechanism, and preventive strategies. Clin J Gastroenterol. 2019;12:285–291. doi: 10.1007/s12328-019-00953-3. [DOI] [PubMed] [Google Scholar]
- 149.Hertzer KM, Xu M, Moro A, Dawson DW, Du L, Li G, Chang HH, Stark AP, Jung X, Hines OJ, Eibl G. Robust Early Inflammation of the Peripancreatic Visceral Adipose Tissue During Diet-Induced Obesity in the KrasG12D Model of Pancreatic Cancer. Pancreas. 2016;45:458–465. doi: 10.1097/MPA.0000000000000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pothuraju R, Rachagani S, Junker WM, Chaudhary S, Saraswathi V, Kaur S, Batra SK. Pancreatic cancer associated with obesity and diabetes: an alternative approach for its targeting. J Exp Clin Cancer Res. 2018;37:319. doi: 10.1186/s13046-018-0963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Alemán JO, Eusebi LH, Ricciardiello L, Patidar K, Sanyal AJ, Holt PR. Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterology. 2014;146:357–373. doi: 10.1053/j.gastro.2013.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lakka HM, Salonen JT, Tuomilehto J, Kaplan GA, Lakka TA. Obesity and weight gain are associated with increased incidence of hyperinsulinemia in non-diabetic men. Horm Metab Res. 2002;34:492–498. doi: 10.1055/s-2002-34788. [DOI] [PubMed] [Google Scholar]
- 153.Buscail L, Bournet B, Cordelier P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2020;17:153–168. doi: 10.1038/s41575-019-0245-4. [DOI] [PubMed] [Google Scholar]
- 154.Eibl G, Rozengurt E. KRAS, YAP, and obesity in pancreatic cancer: A signaling network with multiple loops. Semin Cancer Biol. 2019;54:50–62. doi: 10.1016/j.semcancer.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Debraekeleer A, Remaut H. Future perspective for potential Helicobacter pylori eradication therapies. Future Microbiol. 2018;13:671–687. doi: 10.2217/fmb-2017-0115. [DOI] [PubMed] [Google Scholar]
- 156.Best LM, Takwoingi Y, Siddique S, Selladurai A, Gandhi A, Low B, Yaghoobi M, Gurusamy KS. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev. 2018;3:CD012080. doi: 10.1002/14651858.CD012080.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Maisonneuve P, Lowenfels AB. Risk factors for pancreatic cancer: a summary review of meta-analytical studies. Int J Epidemiol. 2015;44:186–198. doi: 10.1093/ije/dyu240. [DOI] [PubMed] [Google Scholar]
- 158.Xiao M, Wang Y, Gao Y. Association between Helicobacter pylori infection and pancreatic cancer development: a meta-analysis. PLoS One. 2013;8:e75559. doi: 10.1371/journal.pone.0075559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004;4:688–694. doi: 10.1038/nrc1433. [DOI] [PubMed] [Google Scholar]
- 160.Risch HA, Lu L, Kidd MS, Wang J, Zhang W, Ni Q, Gao YT, Yu H. Helicobacter pylori seropositivities and risk of pancreatic carcinoma. Cancer Epidemiol Biomarkers Prev. 2014;23:172–178. doi: 10.1158/1055-9965.EPI-13-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu H, Chen YT, Wang R, Chen XZ. Helicobacter pylori infection, atrophic gastritis, and pancreatic cancer risk: A meta-analysis of prospective epidemiologic studies. Medicine (Baltimore) 2017;96:e7811. doi: 10.1097/MD.0000000000007811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Risch HA. Etiology of pancreatic cancer, with a hypothesis concerning the role of N-nitroso compounds and excess gastric acidity. J Natl Cancer Inst. 2003;95:948–960. doi: 10.1093/jnci/95.13.948. [DOI] [PubMed] [Google Scholar]
- 163.Smolka AJ, Backert S. How Helicobacter pylori infection controls gastric acid secretion. J Gastroenterol. 2012;47:609–618. doi: 10.1007/s00535-012-0592-1. [DOI] [PubMed] [Google Scholar]
- 164.Sun W, Zhou H, Cheng M, Zhuang S, Qiu Z. Association between Socioeconomic Status and One-Month Mortality after Surgery in 20 Primary Solid Tumors: a Pan-Cancer Analysis. J Cancer. 2020;11:5449–5455. doi: 10.7150/jca.46088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Noel M, Fiscella K. Disparities in Pancreatic Cancer Treatment and Outcomes. Health Equity. 2019;3:532–540. doi: 10.1089/heq.2019.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Abdel-Rahman O. Impact of socioeconomic status on presentation, treatment and outcomes of patients with pancreatic cancer. J Comp Eff Res. 2020;9:1233–1241. doi: 10.2217/cer-2020-0079. [DOI] [PubMed] [Google Scholar]
- 167.Zhu F, Wang H, Ashamalla H. Racial and Socioeconomic Disparities in the Treatments and Outcomes of Pancreatic Cancer Among Different Treatment Facility Types. Pancreas. 2020;49:1355–1363. doi: 10.1097/MPA.0000000000001688. [DOI] [PubMed] [Google Scholar]