Abstract
This protocol describes the allotransplantation of tumors in Drosophila melanogaster using an auto-nanoliter injection apparatus. With the use of an autoinjector apparatus, trained operators can achieve more efficient and consistent transplantation results compared to those obtained using a manual injector. Here, we cover topics in a chronological fashion: from the crossing of Drosophila lines, to the induction and dissection of the primary tumor, transplantation of the primary tumor into a new adult host and continued generational transplantation of the tumor for extended studies. As a demonstration, here we use Notch intracellular domain (NICD) overexpression induced salivary gland imaginal ring tumors for generational transplantation. These tumors can first be reliably induced in a transition-zone microenvironment within larval salivary gland imaginal rings, then allografted and cultured in vivo to study continued tumor growth, evolution, and metastasis. This allotransplantation method can be useful in potential drug screening programs, as well as for studying tumor-host interactions.
Introduction
This protocol provides a step-by-step guidance for allotransplantation of Drosophila larval salivary gland (SG) imaginal ring tumors into abdomens of adult hosts using an auto-nanoliter injection apparatus (e.g., Nanoject). This protocol also provides directions for the subsequent re-allografting of tumors into new generations of adult hosts, which provides opportunities for continued longitudinal study of tumor characteristics, such as tumor evolution and tumorhost interactions. The protocol can also be applied toward drug screening experiments.
This method was developed to improve upon the efficacy of performing tumor allotransplantation in Drosophila using manual injectors1, which are often inconsistent in their suction and injection forces, leading to suboptimal results for tumor allotransplantation. An autoinjector apparatus provides better control and can result in lower rates of fly mortality post-allograft. A trained operator could achieve a host-survival rate of over 90% with the autoinjector, compared to around 80% when the manual injector was used1. The overall tumor acquisition rate is 60%−80% at day 8–12 post-allograft. The average injection time has also been improved from 30–40 s per fly using a manual injector to 20–25 s per fly using the autoinjector.
This protocol is among the first few protocols to use the autoinjector apparatus in Drosophila tumor allotransplantation. A recent study also used the autoinjector for allotransplantation of tumorous neural stem cells2. Previously, the autoinjector apparatus was used in Drosophila to study bacterial virulence3, parasitic infections and host defense4, as well as screening for bioactivity of different compounds5. Our protocol adapts the autoinjector apparatus for tumor injection use and seeks to provide Drosophila researchers with higher quality and more consistent results while saving them considerable time. This protocol can not only be used for the allotransplantation of tumors, but can also be tailored to the allotransplantation of wildtype and mutant tissues of similar caliber6.
The Drosophila NICD tumor used in this protocol was first introduced by Yang et al.7 in the SG imaginal ring transitional zone, a “tumor hotspot” that exhibits high levels of endogenous Janus Kinase/Signal Transducer and Activators of Transcription (JAK-STAT), and c-Jun N-terminal Kinase (JNK) activity. Additionally, the transition zone has high levels of matrix metalloproteinase-1 (MMP1)7, which makes this region particularly conducive to tumorigenesis. Notch pathway activation through NICD overexpression alone is sufficient to consistently initiate tumor formation. These tumors can be subsequently allotransplanted to allow investigation of a broad range of topics, including tumor cell division, invasion, and tumor-host interactions.
Protocol
1. Preparation of SG imaginal ring tumor
Cross adult flies with genotypes of UAS-NICD (Male: 10–15 flies) and Act-Gal4, UAS-GFP/CyO; tub-Gal80ts (Virgin female: 10–15 flies) and allow them to breed for 1 day at 18 °C. The selected adult flies should be 5–9 days old to ensure high fertility.
-
Allow the adult flies to lay eggs in the fly food contained in vials for 24 h at 18 °C, then remove the adult flies.
NOTE: Fly food is prepared using the standard cornmeal food recipe from Drosophila Stock Center8. Each vial should contain around 10mL of fly food.
Allow the eggs to incubate for 6 days at 18 °C. During this period, the larvae will hatch.
-
Transfer the vials containing larvae to a 29 °C incubator and incubate for another 7 days.
NOTE: This incubation step is optional depending on the specific experimental design.
2. Preparation of adult wild type Drosophila for allotransplantation
Anesthetize wild type or appropriate mutant adult flies with 100% CO2 and sort flies based on sexes. Both male and female flies can be used as tumor hosts.
Secure a 5 cm long piece of fly tape to a microscope slide with the sticky side up by fixing it with two smaller pieces of tape, one at each end.
-
Immobilize the flies by adhering their wings to the tape. Use forceps while maneuvering the flies.
-
Repeat the above step for 60–80 adult flies used as allograft acceptors.
NOTE: It is best to organize flies into neat rows, with their body axis aligned parallel to each other for a more time-efficient injection process later. Figure 1 shows rows of host flies taped down in this manner.
-
Figure 1: Host flies taped and secured for allotransplantation.
The host flies are taped down by their wings and oriented neatly to prepare for the subsequent transplantation procedure.
3. Assembly of the autoinjector apparatus
-
Connect both the autoinjector apparatus and power cord to the controller box.
Set the injection volume to 59.8 nL. This will help maintain the appropriate amount of suction and injection forces during allotransplantation.
-
Place the controller box and the autoinjector on opposite sides of the light microscope.
NOTE: For right-handed operators, the control box should be placed on the left side of the microscope with the injector on the right side. Vice versa for left-handed operators.
-
Prepare the 3.5” glass capillary for use by clipping off the closed end with forceps.
Process one end of the glass capillary using a four-step micropipette puller and heat to a narrow, closed end using the following specifications in the instrument: Heat = 650, Force = 200, and Distance = 8. Neatly place the capillaries into the puller apparatus and run the program after inputting the above settings.
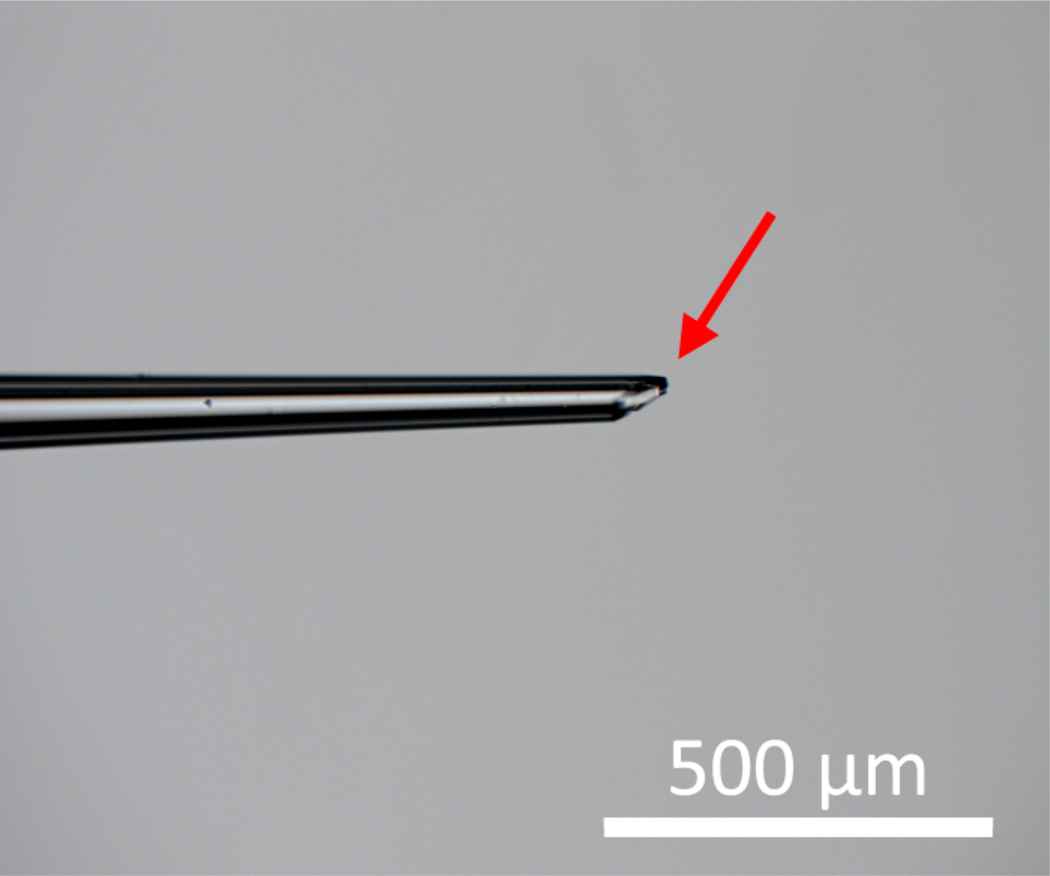
Use forceps to clip the capillary at an approximately 60° angle to make a sharper end for easy entry into the adult fly abdomen1. See Figure 2 for an example of a well-clipped capillary.
Lightly unscrew the cap of the injector. Hold down the Empty button to advance the injector needle until 70%−80% of its total length is showing. To accelerate capillary advancement, press the Fill button once while simultaneously holding down the Empty button.
-
Use a syringe to fill the glass capillary with mineral oil. Then, carefully insert the glass capillary onto the injector needle until the former is firmly attached to the rubber stopper of the injector. Now screw the injector cap tight.
Wipe off the mineral oil residue on the external surface of the glass capillary cover to avoid contaminating the medium during allotransplantation.
Figure 2: A well-clipped injection capillary.
The red arrow points to a sharp edge needed to effectively pierce the abdominal cuticle of adult Drosophila hosts.
4. Dissection of the SG imaginal ring tumor
-
Select one of the larvae and transfer it to a dissection plate filled with 100 μL of Schneider’s Medium to prepare for SG imaginal ring tumor dissection.
NOTE: Only the specimens harboring the tumor will remain as larvae. This is because tumor growth delays larval development and progression9. Two thirds of the specimens will not harbor the tumor and will thus have progressed to pupae/adults.
For dissection and allotransplantation purposes, use a stereomicroscope with a 10x-20x magnification range.
Using one pair of forceps to hold the mid-section of the larval body, pinch the larval head using another pair of forceps and apply a stretching force lengthwise.
Locate the Y-shaped SG of the larvae and isolate it from the rest of the larval tissue10.
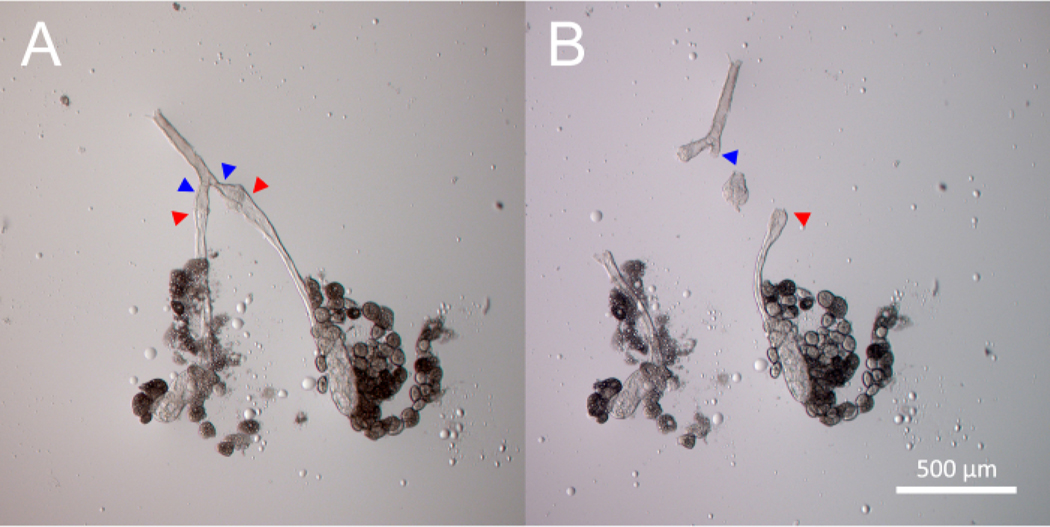
Dissect and isolate the SG imaginal ring tumor by removing the adjacent tissue. See Figure 3 for a depiction of this dissection process.
Repeat steps 4.1–4.4 for an additional 10 to 20 SG imaginal ring tumors based on research needs.
Figure 3: Dissection of the primary salivary gland ImR tumor.
The process of dissecting and isolating two primary salivary gland ImR tumors is demonstrated chronologically from Panel (A) to Panel (B), using two separate incisions. Panel (A) shows the salivary gland before tumor dissection. The red arrowheads indicate the first incision points. The blue arrowheads indicate the second incision points. The tumor lies between the red and blue arrowheads. Panel (B) shows the isolated ImR tumor after the two incisions are made to separate it from normal salivary gland tissue.
5. Allograft of primary SG imaginal ring tumor
-
Submerge the capillary into the Schneider’s Medium containing the primary SG imaginal ring tumors. Hold the Fill button to fill the glass capillary with Schneider’s Medium all the way to the top 0.5 cm segment. This top segment should remain filled with mineral oil.
NOTE: Accelerate this process by pressing the Empty button once while simultaneously holding down the Fill button.
-
Locate a primary tumor and press the Fill button until the tumor is suctioned into the capillary.
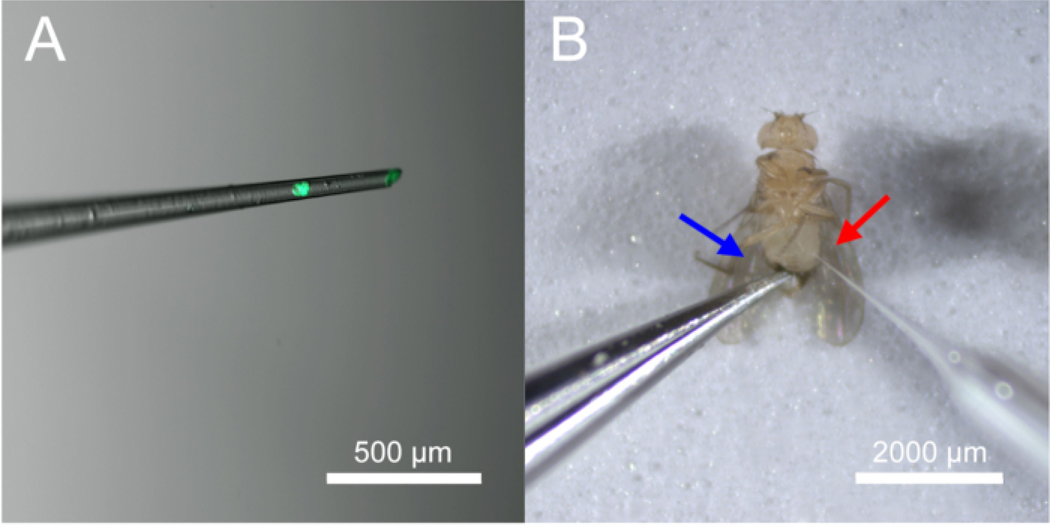
Ensure that the tumor sits at the tip of the capillary or several millimeters away from the tip of the capillary. This helps avoid the tumor drifting and becoming lost in the solution contained within the capillary. See Figure 4A for a demonstration of the appropriate tumor location as it sits in the capillary.
Locate an adult fly immobilized to the tape on the microscope slide. Using forceps, gently hold down the lower abdomen. Then, pierce the lower lateral cuticle of the abdomen with the capillary. Press the Empty button until the tumor enters the new host abdomen. See Figure 4B for a demonstration of this technique.
-
Using forceps, gently pinch the wings of the host up to remove it from the tape. Place the host into a new vial with fresh food. It is best to place the vial sideways for the initial 24 h after injection. Each vial should only contain up to a maximum of 20 flies.
NOTE: Some host flies will have missing wings and other wounds on their bodies after injection and may stick to the fly food if the vial is placed upright.
Repeat steps 5.2 through 5.4 to transplant the remaining primary tumors into their new adulthosts.
Dispose capillaries into sharps’ container and clean the mineral oil residue from the exterior of the autoinjector before replacing the apparatus back into its box.
Store the vial of hosts at room temperature for 1 day, then transfer the vial to an incubation chamber at 29 °C. Transfer fly hosts to new vials every 2–3 days.
Monitor the fly hosts daily and calculate survival rates. After a week, tumors should be visible under a stereo microscope with fluorescence adapter and can be continually monitored for their size and progression.
Figure 4: Appropriate tumor location within the capillary and injection of tumor into Drosophila host abdomen.
Panel (A) shows the most appropriate tumor location within the capillary. The tumor expresses eGFP (488 nm). Panel (B) shows the injection process. The red arrow indicates the tumor injection site. The blue arrow shows the placement of forceps to help hold down the terminalia of the fly for easier injection.
6. Re-allograft of transplanted tumors
Around 10–14 days post-allograft, screen for tumors that have grown in the host abdomens using a fluorescence microscope.
-
Anesthetize a host with CO2 and place it in a dissection plate filled with 100 μL Schneider’s Medium. Dissect the grown allografted tumor out of the host using two pairs of forceps.
Use one pair of forceps to hold down the abdomen and the other pair to incise open the abdominal cuticle, exposing the allografted tumor1.
Carefully isolate the tumor from the attached host tissues as much as possible using fluorescence markers as guidance.
Repeat step 6.2 to prepare for two to three additional allografted tumors.
Transfer the dissection plate containing the harvested tumors onto the stage of a light microscope.
Use sterile needles and dissect the tumors into smaller pieces that are appropriate for the capillary size.
-
Repeat steps 4.1–4.5 to prepare the new generation of adult hosts and repeat steps 5.1–5.7 to complete the allotransplantation of the tumors.
NOTE: Success rates are generally higher for nonprimary tumors compared with those of primary tumors.
Repeat steps 6.1–6.6 for every subsequent generation of flies used in the study.
NOTE: Choose Drosophila host lines appropriate for the experimental needs.
Representative Results
Here, we carried out generational allotransplantation of SG imaginal ring tumors using the nanoliter injection autoinjector apparatus and conducted subsequent tumor live-imaging with a confocal laser scanning microscope, which allowed for a deeper dive into topics of tumor growth, tumor cell migration, and tumor-host interactions. When mounting flies, glue them to a microscope slide and restrain them via a polydimethylsiloxane (PDMS) block11.
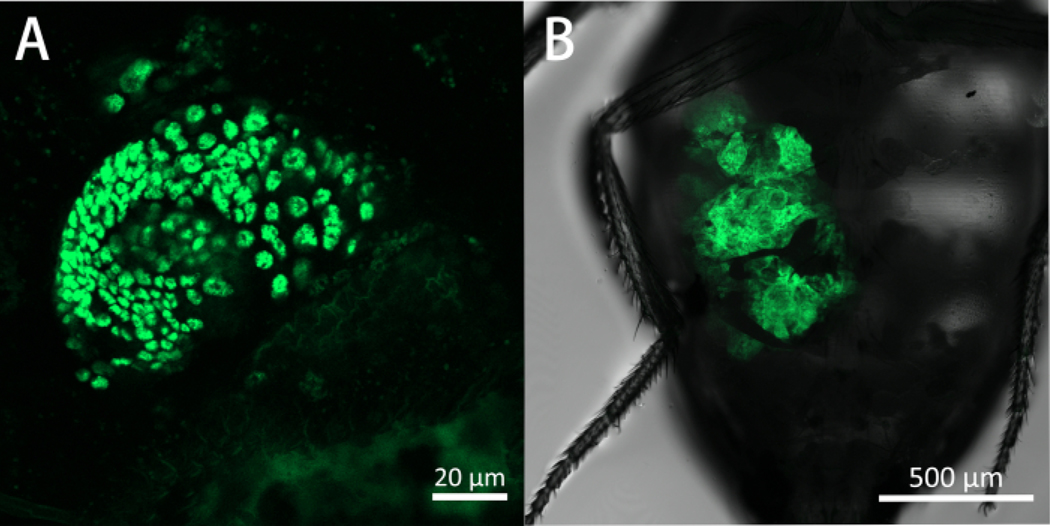
Figure 5A features a live imaging capture of a 1st generation (G1) SG imaginal ring tumor growing in an adult host abdomen on day 10 post-allotransplantation. This level of imaging can be used to track the process of tumor division. Figure 5B depicts a 6th generation (G6) SG imaginal ring tumor occupying a large portion of the host abdomen on day 10 post-allotransplantation. Imaging at this stage may help reveal tumor growth patterns, as well as its migration and invasion behaviors. It is important to note that even though this image was captured using a confocal laser scanning microscope, a stereomicroscope with a GFP fluorescence adapter could also be used at 2x to 5x magnification, depending on the tumor size.
Figure 5: A G1 and G6 ImR tumor seen in WT Drosophila host abdomen on day 10 post-allotransplantation.
These are ventral views of the fly abdomen with the transplanted tumors in green. Panel (A) shows a G1 tumor on day 10 post-allotransplantation expressing eGFP (488 nm). Panel (A) is captured using a confocal microscope using a 20x lens with 0.8 NA, and 3x zoom. Panel (B) shows a G6 tumor on day 10 post-allotransplantation expressing eGFP (488 nm). Panel (B) is captured using a confocal microscope using a 5x lens with 0.25 NA, and 1x scan zoom.
Discussion
Tumor allotransplantation can help researchers address certain problems that arise during Drosophila tumor growth and progression. One such challenge is the circumvention of premature deaths of tumor-bearing larvae or adults during primary tumor culture12. In this context, continued tumor allotransplantation allows tumors to grow indefinitely, which facilitates longitudinal studies of tumor growth, metastasis, and evolution. Tumor allotransplantation is also useful for assessing various aspects of host-tumor interactions7,13. Host genotypes can be manipulated prior to tumor allograft to allow for evaluation of the host effect on tumor growth and migration, and on tumor-induced cachexia14,15. Fly hosts with different genotypes may exhibit distinct manifestations of cachexia-like wasting in response to the same tumor. Post-allotransplantation, the tumor hosts can be mounted in preparation for in vivo imaging using a protocol adapted from Koyama et al. and Ji et al.11,16.
The application of the autoinjector apparatus toward Drosophila tumor allotransplantation provides a convenient and straightforward protocol that possesses enhanced efficiency. As compared to the manual injector1, this method allows for reproducible and large-scale allotransplantations, which can expedite and standardize tumor behavior studies and drug screening procedures. This improved method produces impressive host survival and tumor yield rates. A trained researcher can achieve post-allograft host survival rates of >90%. Tumor yield rates can differ depending on whether the tumor is primary or re-allografted. Researchers can expect to achieve tumor yield rates of >50% for primary tumors and >70% for re-allografted tumors. In addition, this method reduces injection time per host fly by nearly 50% compared to the manual injector method.
This procedure has its limitations, however, mainly due to inconsistency of tumor incision, injection location and wound size. If the primary tumors are not cut into uniformly sized fragments prior to allotransplantation, certain fly hosts can receive larger fragments than others. This is a confounding factor affecting studies that aim to track the rate of tumor growth. This can potentially be mitigated by measuring the differential rate of tumor growth at two-day intervals post-allograft. In addition, during the injection, the operator should choose a consistent site in the abdominal cuticle across all host flies. This helps mitigate another confounding variable that may affect fly host survival and the final location of tumor attachment.
Acknowledgments
We thank former lab members Dr. Sheng-An Yang and Mr. Juan-Martin Portilla for their contribution in developing this protocol. We are grateful for Dr. Yan Song’s lab at Peking University School of Life Sciences for sharing their protocol on manual allotransplantation. We also thank Mr. Calder Ellsworth and Mr. Everest Shapiro for critical reading of the manuscript.
WMD received funding (GM072562, CA224381, CA227789) for this work from National Institute of Health (https://www.nih.gov/) and funding (IOS-155790) from the National Science Foundation (htps://nsf.gov/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosures
There are no conflicts of interest to declare among the authors.
References
- 1.Rossi F, Gonzalez C. Studying tumor growth in Drosophila using the tissue allograft method. Nature Protocols. 10 (10), 1525–1534 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Magadi SS et al. Dissecting Hes-centred transcriptional networks in neural stem cell maintenance and tumorigenesis in Drosophila</ em>. Development. 147 (22), dev191544 (2020). [DOI] [PubMed] [Google Scholar]
- 3.Haller S, Limmer S, Ferrandon D in Pseudomonas Methods and Protocols. Springer. 723–740 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Letinić B, Kemp A, Christian R, Koekemoer L. Inoculation protocol for the African malaria vector, Anopheles arabiensis, by means of nano-injection. African Entomology. 26 (2), 422–428 (2018). [Google Scholar]
- 5.Mejia M, Heghinian MD, Busch A, Marí F, Godenschwege TA Paired nanoinjection and electrophysiology assay to screen for bioactivity of compounds using the Drosophila melanogaster giant fiber system. Journal of Visualized Experiments: JoVE. (62), e3597 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miles WO, Dyson NJ, Walker JA Modeling tumor invasion and metastasis in Drosophila. Disease Models & Mechanisms. 4 (6), 753 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang SA, Portilla JM, Mihailovic S, Huang YC, Deng WM Oncogenic notch triggers neoplastic tumorigenesis in a transition-zone-like tissue microenvironment. Developmental Cell. 49 (3), 461–472.e465 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloomington Drosophila Stock Center. BDSC Cornmeal Food. (2020). [Google Scholar]
- 9.Garelli A, Gontijo AM, Miguela V, Caparros E, Dominguez M. Imaginal discs secrete insulin-like peptide 8 to mediate plasticity of growth and maturation. Science. 336 (6081), 579–582 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Kennison JA Dissection of larval salivary glands and polytene chromosome preparation. CSH Protocols. 2008 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Ji H, Han C. LarvaSPA, a method for mounting drosophila larva for long-term time-lapse imaging. Journal of Visualized Experiments: JoVE. (156) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirzoyan Z. et al. Drosophila melanogaster: a model organism to study cancer. Frontiers in Genetics. 10, 51 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bangi E. Drosophila at the intersection of infection, inflammation, and cancer. Frontiers in Cellular and Infection Microbiology. 3, 103 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saavedra P, Perrimon N. Drosophila as a model for tumor-induced organ wasting. Advances in Experimental Medicine and Biology. 1167, 191–205 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Figueroa-Clarevega A, Bilder D. Malignant drosophila tumors interrupt insulin signaling to induce cachexia-like wasting. Developmental Cell. 33 (1), 47–55 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koyama LAJ et al. Bellymount enables longitudinal, intravital imaging of abdominal organs and the gut microbiota in adult Drosophila. PLOS Biology. 18 (1), e3000567 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]