Abstract
BACKGROUND
Direct metagenomic next-generation sequencing (mNGS) of clinical samples is an effective method for the molecular diagnosis of infection. However, its role in the diagnosis of patients with acute respiratory distress syndrome (ARDS) of an unknown infectious etiology remains unclear.
CASE SUMMARY
A 33-year-old man was admitted to our center for a cough and febrile sensation. Shortly after admission, the patient was diagnosed with ARDS and treated in the intensive care unit. Subsequently, chest computed tomography features suggested an infection. mNGS was performed and the results were indicative of an infection caused by adenovirus type 7. The patient recovered after receiving appropriate treatment.
CONCLUSION
mNGS is a promising tool for the diagnosis of ARDS caused by infectious agents. However, further studies are required to develop strategies for incorporating mNGS into the current diagnostic process for the disease.
Keywords: Acute respiratory distress syndrome, Metagenomic next-generation sequencing, Adenovirus, Case report
Core Tip: Direct metagenomic next-generation sequencing (mNGS), is useful for infection diagnosis, and has potential for the diagnosis of acute respiratory distress syndrome (ARDS) of unknown infectious etiology. This case report describes the successful use of mNGS for the diagnosis of a patient with ARDS. At his first presentation, the patient was suspected of having an infection based on the results of chest computed tomography. mNGS indicated an infection by adenovirus type 7. The patient recovered with the appropriate treatment. This case highlights the usefulness of mNGS for the diagnosis of ARDS caused by infectious agents.
INTRODUCTION
Acute respiratory distress syndrome (ARDS) is a life-threatening condition with a mortality rate of 20%-40%[1-4]. ARDS may result from various factors, and the treatment recommendations are largely based on the cause[5,6]. Therefore, ARDS patients would benefit from targeted treatment following the identification of all possible causes of the disease. However, the etiology of ARDS is unclear in 5% to 10% of patients[7-9].
Direct metagenomic next-generation sequencing (mNGS) of clinical samples is an effective method for determining the molecular diagnosis of infection[10]. As infection remains the most common cause of ARDS[11], mNGS has potential for the diagnosis of ARDS of unknown etiology. We report an ARDS case of unknown infectious etiology that was diagnosed with mNGS. We believe ARDS patients may benefit from the application of mNGS in clinical practice.
CASE PRESENTATION
Chief complaints
In January 2020, a 33-year-old man was admitted to the hospital because of a cough and fever lasting for 3 d.
History of present illness
After admission, community-acquired pneumonia was diagnosed in the patient and empiric antibiotic treatment with cephalosporin, oseltamivir, and moxifloxacin, was initiated. Remission was not achieved after 6 d of treatment. In addition, the patient showed a poor response to imipenem after 3 d of treatment. The patient was subsequently transferred to our center. His temperature dropped gradually following tigecycline and levofloxacin administration, the dyspnea and lesions continued to progress. Because of the rapid deterioration of his condition, the patient was admitted to the intensive care unit (ICU) on March 5, 2020 where he was intubated for mechanical ventilation (inspiratory pressure, 25 cm H2O; positive end-expiratory pressure, 18 cm H2O).
History of past illness
He had no previous medical history.
Physical examination
Upon admission to ICU, his vital signs were, respiratory rate, 40 beats/min; heart rate, 150 beats/min; and pulse oxygen saturation (SpO2), 78%. Auscultation of the lungs revealed extensive moist rales.
Laboratory examination
Laboratory test results (with normal values in parentheses) were hemoglobin 153 g/L (110-150 g/L), white blood cells (WBC) 9.3 × 109/L (3.5-9.5 × 109/L), lymphocytes 0.57 × 109/L (1.1-3.2 × 109/L), platelets 74 × 109/L (100-350 × 109/L), C-reactive protein 17.1 mg/L (< 8.0 mg/L), procalcitonin 0.27 ng/mL, CD3+ T cells 606/mm3 (500-1500/mm3), CD4+ T cells 333/mm3 (300-1200/mm3), CD8+ T cells 286/mm3 (238-874/mm3), antinuclear antibodies and antineutrophil cytoplasmic antibodies were negative. Arterial blood gas analysis for hypoxemia and hypocapnia found pH 7.34, pCO2 24.6 mmHg, pO2 80 mmHg, bicarbonate 12.8 mmol/L, base excess −11.1 mmol/L, lactate, 3.5 mmol/L, oxygenation index, 80 mmHg (400-500 mmHg). Tests for human immunodeficiency virus, mycoplasma, chlamydia, streptococcus, and legionella were all negative.
Imaging examination
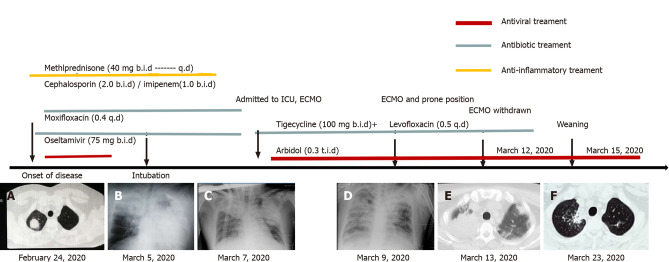
Upon admission, chest computed tomography (CT) was suggestive of an infection (Figure 1A). On day 6, the chest CT findings indicated the disease had progressed (Figure 1B), and imaging indicated that the lesions (Figure 1C) had progressed. In addition, a chest radiograph showed bilateral diffuse infiltration (Figure 1D).
Figure 1.
Clinical course and outcome of a patient with acute respiratory distress syndrome of unknown etiology. A: February 24, 2020; B: March 5, 2020; C: March 7, 2020; D: March 9, 2020; E: March 13, 2020; F: March 23, 2020. ECMO: Extracorporeal membrane oxygenation; ICU: Intensive care unit.
FINAL DIAGNOSIS
The patient was diagnosed with severe pneumonia with ARDS and extracorporeal membrane oxygenation (ECMO) support was initiated. The patient was kept in the prone position. Additionally, abnormal bleeding was found in his hands. On the first ICU day, anticoagulant with heparin was begun and the bleeding disappeared after 5 d. Sputum and bronchoalveolar fluid (BALF) were collected and sent for mNGS, both of which supported a diagnosis of adenovirus type 7 infection.
TREATMENT
The patient was treated with oral arbidol (20 mg q8h) and comprehensive rehabilitation, including psychological counseling. He improved gradually, as evidenced by the oxygenation and chest CT scan results (Figure 1E). On ICU day 5 in the ICU, ECMO was withdrawn successfully, and ventilator support was continued until ICU day 8.
OUTCOME AND FOLLOW-UP
Biomarkers, including WBC and lymphocytes and the Sequential Organ Failure Assessment score, Acute Physiology and Chronic Health Evaluation II score, and Disseminated Intravascular Coagulation score were all found to be normal. Sixteen days after admission, the patient was discharged without any complications (Figure 1F).
DISCUSSION
Although mNGS is widely used in clinical practice[12], its role in ARDS of unknown etiology remains unclear. Our data confirm that mNGS may be a useful tool for the management of ARDS cases with unknown etiology. Identifying the etiology would certainly be helpful for choosing the appropriate treatment and improving the outcomes of patients with ARDS[13]. However, it is usually difficult to determine the etiology of viral infection from respiratory specimens. One of the most common infectious agents is adenovirus, which is a double-stranded DNA virus widely distributed in nature and mainly transmitted through the respiratory tract. It not only causes upper respiratory tract infection, but also pneumonia. More than 80% of adenovirus infections occur in children under 4 years of age because of defects in humoral immunity[14]. Although adenovirus infections are generally self-limiting, severe and disseminated infections can occur in individuals with immunocompromised conditions, such as transplantation, human immunodeficiency virus infection, and congenital immunodeficiency. Outbreaks of adenovirus pneumonia in immunocompetent patients have been occasionally reported in military recruits and adults in long-term care facilities[15-18].
In the last few decades, ARDS cases caused by adenovirus types 1, 3, 4, 6, and 55 have been reported[19,20]. However, limited data are available on adenovirus type 7 infections. Hence, this case was reviewed and presented for its possible significance.
The usefulness of many diagnostic tools for identifying the cause of ARDS has been evaluated, such as imaging (CT, magnetic resonance imaging, and positron emission tomography-CT) and microbiological examinations (culture, serological assays, and molecular assays such as polymerase chain reaction)[21]. However, those techniques are inadequate for the diagnosis of ARDS. mNGS of clinical samples may be superior to current diagnostic technologies because it has the potential to identify both known and unknown infectious agents in a single application. In general, mNGS appears to be a promising method for investigating the cause of an infection. For example, Takeuchi et al[22] found that in patients with severe respiratory diseases, mNGS had a diagnostic sensitivity of 88.89% and a specificity of 74.07%[22]. It was demonstrated that mNGS is a sensitive method for the detection of causative pathogens in children with severe nonresponding pneumonia[22]. Another study showed that BALF mNGS had a sensitivity of 81.3% for the detection of infectious agents[23]. Interestingly, in our study, sputum and BALF mNGS were both positive for adenovirus type 7. Hence, these two results corroborated each other and were used to confirm the diagnosis of the disease.
However, several limitations may exist in mNGS performed directly on clinical samples. First, standard operating procedures for mNGS are urgently needed and further improvement of the NGS workflow may be required. Second, because of the cost and complexity, mNGS is rather challenging and hardly feasible for a large population. Third, the abundance threshold that indicates a pathogenic infection is hard to define, even when supported by clinical characteristics.
CONCLUSION
In conclusion, mNGS appears to be an appropriate tool for the diagnosis of patients with ARDS of unknown etiology after extensive diagnostic procedures and despite empirical treatment. However, further studies are required to develop strategies to incorporate mNGS into the current diagnostic algorithm.
Footnotes
Informed consent statement: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editor of this journal.
Conflict-of-interest statement: The authors have no competing interests to declare.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: March 18, 2021
First decision: April 14, 2021
Article in press: May 7, 2021
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lucchesi A S-Editor: Fan JR L-Editor: Filipodia P-Editor: Wang LYT
Contributor Information
Xiao-Juan Zhang, Department of Intensive Care Unit, The First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China.
Jia-Yin Zheng, Department of Intensive Care Unit, The First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China.
Xin Li, Department of Infectious Disease, The First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China.
Ying-Jian Liang, Department of Intensive Care Unit, The First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China.
Zhi-Dan Zhang, Department of Infectious Disease, The First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China. 13998318999@163.com.
References
- 1.Vaquer S, de Haro C, Peruga P, Oliva JC, Artigas A. Systematic review and meta-analysis of complications and mortality of veno-venous extracorporeal membrane oxygenation for refractory acute respiratory distress syndrome. Ann Intensive Care. 2017;7:51. doi: 10.1186/s13613-017-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moreira A, Naqvi R, Hall K, Emukah C, Martinez J, Moreira A, Dittmar E, Zoretic S, Evans M, Moses D, Mustafa S. Effects of mesenchymal stromal cell-conditioned media on measures of lung structure and function: a systematic review and meta-analysis of preclinical studies. Stem Cell Res Ther. 2020;11:399. doi: 10.1186/s13287-020-01900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bersten AD, Edibam C, Hunt T, Moran J Australian and New Zealand Intensive Care Society Clinical Trials Group. Incidence and mortality of acute lung injury and the acute respiratory distress syndrome in three Australian States. Am J Respir Crit Care Med. 2002;165:443–448. doi: 10.1164/ajrccm.165.4.2101124. [DOI] [PubMed] [Google Scholar]
- 4.Luhr OR, Antonsen K, Karlsson M, Aardal S, Thorsteinsson A, Frostell CG, Bonde J. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. The ARF Study Group. Am J Respir Crit Care Med. 1999;159:1849–1861. doi: 10.1164/ajrccm.159.6.9808136. [DOI] [PubMed] [Google Scholar]
- 5.Cho YJ, Moon JY, Shin ES, Kim JH, Jung H, Park SY, Kim HC, Sim YS, Rhee CK, Lim J, Lee SJ, Lee WY, Lee HJ, Kwak SH, Kang EK, Chung KS, Choi WI Korean Society of Critical Care Medicine; Korean Academy of Tuberculosis and Respiratory Diseases Consensus Group. Clinical Practice Guideline of Acute Respiratory Distress Syndrome. Tuberc Respir Dis (Seoul) 2016;79:214–233. doi: 10.4046/trd.2016.79.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Confalonieri M, Salton F, Fabiano F. Acute respiratory distress syndrome. Eur Respir Rev. 2017;26 doi: 10.1183/16000617.0116-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 8.Marquette CH, Copin MC, Wallet F, Neviere R, Saulnier F, Mathieu D, Durocher A, Ramon P, Tonnel AB. Diagnostic tests for pneumonia in ventilated patients: prospective evaluation of diagnostic accuracy using histology as a diagnostic gold standard. Am J Respir Crit Care Med. 1995;151:1878–1888. doi: 10.1164/ajrccm.151.6.7767535. [DOI] [PubMed] [Google Scholar]
- 9.Pan C, Liu L, Xie JF, Qiu HB. Acute Respiratory Distress Syndrome: Challenge for Diagnosis and Therapy. Chin Med J (Engl) 2018;131:1220–1224. doi: 10.4103/0366-6999.228765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manso CF, Bibby DF, Mohamed H, Brown DWG, Zuckerman M, Mbisa JL. Enhanced Detection of DNA Viruses in the Cerebrospinal Fluid of Encephalitis Patients Using Metagenomic Next-Generation Sequencing. Front Microbiol. 2020;11:1879. doi: 10.3389/fmicb.2020.01879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estenssoro E, Dubin A, Laffaire E, Canales H, Sáenz G, Moseinco M, Pozo M, Gómez A, Baredes N, Jannello G, Osatnik J. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med. 2002;30:2450–2456. doi: 10.1097/00003246-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Cao K, Wei Y, Qian Y, Liang J, Dong D, Tang J, Zhu Z, Gu Q, Yu W. Metagenomic next-generation sequencing in the diagnosis of severe pneumonias caused by Chlamydia psittaci. Infection. 2020;48:535–542. doi: 10.1007/s15010-020-01429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huppert LA, Matthay MA, Ware LB. Pathogenesis of Acute Respiratory Distress Syndrome. Semin Respir Crit Care Med. 2019;40:31–39. doi: 10.1055/s-0039-1683996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lynch JP 3rd, Fishbein M, Echavarria M. Adenovirus. Semin Respir Crit Care Med. 2011;32:494–511. doi: 10.1055/s-0031-1283287. [DOI] [PubMed] [Google Scholar]
- 15.Dudding BA, Wagner SC, Zeller JA, Gmelich JT, French GR, Top FH Jr. Fatal pneumonia associated with adenovirus type 7 in three military trainees. N Engl J Med. 1972;286:1289–1292. doi: 10.1056/NEJM197206152862403. [DOI] [PubMed] [Google Scholar]
- 16.Klinger JR, Sanchez MP, Curtin LA, Durkin M, Matyas B. Multiple cases of life-threatening adenovirus pneumonia in a mental health care center. Am J Respir Crit Care Med. 1998;157:645–649. doi: 10.1164/ajrccm.157.2.9608057. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez JL, Binn LN, Innis BL, Reynolds RD, Lee T, Mitchell-Raymundo F, Craig SC, Marquez JP, Shepherd GA, Polyak CS, Conolly J, Kohlhase KF. Epidemic of adenovirus-induced respiratory illness among US military recruits: epidemiologic and immunologic risk factors in healthy, young adults. J Med Virol. 2001;65:710–718. doi: 10.1002/jmv.2095. [DOI] [PubMed] [Google Scholar]
- 18.Lion T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev. 2014;27:441–462. doi: 10.1128/CMR.00116-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luyt CÉ, Combes A, Trouillet JL, Nieszkowska A, Chastre J. Virus-induced acute respiratory distress syndrome: epidemiology, management and outcome. Presse Med. 2011;40:e561–e568. doi: 10.1016/j.lpm.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfortmueller CA, Barbani MT, Schefold JC, Hage E, Heim A, Zimmerli S. Severe acute respiratory distress syndrome (ARDS) induced by human adenovirus B21: Report on 2 cases and literature review. J Crit Care. 2019;51:99–104. doi: 10.1016/j.jcrc.2019.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dembinski R, Mielck F. [ARDS - An Update - Part 1: Epidemiology, Pathophysiology and Diagnosis] Anasthesiol Intensivmed Notfallmed Schmerzther. 2018;53:102–111. doi: 10.1055/s-0043-107166. [DOI] [PubMed] [Google Scholar]
- 22.Takeuchi S, Kawada JI, Horiba K, Okuno Y, Okumura T, Suzuki T, Torii Y, Kawabe S, Wada S, Ikeyama T, Ito Y. Metagenomic analysis using next-generation sequencing of pathogens in bronchoalveolar lavage fluid from pediatric patients with respiratory failure. Sci Rep. 2019;9:12909. doi: 10.1038/s41598-019-49372-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Ding S, Lei C, Qin J, Guo T, Yang D, Yang M, Qing J, He W, Song M, Zhang Y, Zeng H, Qin Q, Yang L, Long Y, Chen Y, Ma B, Ouyang R, Chen P, Luo H. Blood and Bronchoalveolar Lavage Fluid Metagenomic Next-Generation Sequencing in Pneumonia. Can J Infect Dis Med Microbiol. 2020;2020:6839103. doi: 10.1155/2020/6839103. [DOI] [PMC free article] [PubMed] [Google Scholar]