Abstract
Natural killer (NK) cells are cytotoxic lymphocytes of the innate immune system that are capable of killing virally infected and/or cancerous cells. Nearly 20 years ago, NK cell-mediated immunotherapy emerged as a safe and effective treatment approach for patients with advanced-stage leukaemia. Subsequently, the field of NK cell-based cancer therapy has grown exponentially and currently constitutes a major area of immunotherapy innovation. In general, the development of NK cell-directed therapies has two main focal points: optimizing the source of therapeutic NK cells for adoptive transfer and enhancing NK cell cytotoxicity and persistence in vivo. A wide variety of sources of therapeutic NK cells are currently being tested clinically, including haploidentical NK cells, umbilical cord blood NK cells, stem cell-derived NK cells, NK cell lines, adaptive NK cells, cytokine-induced memory-like NK cells and chimeric antigen receptor NK cells. A plethora of methods to augment the cytotoxicity and longevity of NK cells are also under clinical investigation, including cytokine-based agents, NK cell-engager molecules and immune-checkpoint inhibitors. In this Review, we highlight the variety of ways in which diverse NK cell products and their auxiliary therapeutics are being leveraged to target human cancers. We also identify future avenues for NK cell therapy research.
The field of natural killer (NK) cell-based immunotherapy of cancer has reached an exciting juncture. Although these therapies have not yet achieved the same degree of clinical success as adoptive T cell therapies, early preclinical and clinical successes with NK cell therapies have led to increasing enthusiasm in developing their potential. The underlying hypothesis driving this new field has its roots in observations from the transplantation clinic roughly 20 years ago. In this setting, NK cells were found to quickly reconstitute following haematopoietic stem cell transplantation (HSCT) and to possess direct cytotoxic activity against malignant cells. In this Review, we discuss the major NK cell-based modalities for cancer treatment to date. We begin by reviewing several aspects of NK cell biology that pertain to their antitumour function, including their activating and inhibitory receptors, effector attributes, subpopulations, anatomical locations and developmental processes. The potency of any cell therapy is profoundly influenced by the tumour microenvironment (TME) in which it operates, and therefore we also provide an overview of the tumour immune-evasion strategies that most strongly suppress NK cell function; when relevant, we highlight therapeutic interventions aimed at overcoming these tumour-associated barriers. However, the bulk of this Review is focused on perhaps the two most important major factors governing the efficacy of NK cell-based treatments: the choice of cell source and in vivo enhancement of NK cell function. We briefly cover current expansion methods for increasing the scale and standardization of these products. The discussion of in vivo functional enhancement methods includes cytokine-based treatments, NK cell engagers (NKCEs) and immune-checkpoint inhibitors. We conclude by offering our thoughts on the most crucial points for future research and clinical testing of NK cell cancer immunotherapy.
NK cell biology
Who are the natural killers?
NK cells were the first subtype of innate lymphoid cells (ILCs) to be identified and can respond to virally infected and/or transformed cells with a variety of effector functions, chiefly cell killing and production of pro-inflammatory cytokines1,2. NK cells and the other ILC family members — type 1 ILCs (ILC1s), ILC2s and ILC3s — originate from the same common lymphoid progenitor cells as B cells and T cells3. The cytotoxic activity of NK cells makes them functionally most similar to CD8+ T cells, whereas the cytokine production patterns of ILC1, ILC2 and ILC3 populations categorize these cells as functional counterparts of the T helper 1 (TH1), TH2 and TH17 subsets of CD4+ T cells, respectively3. The prevailing model for the development of NK cells posits that bone marrow-derived CD34+CD45RA+ haematopoietic progenitor cells (HPCs) migrate to various anatomical sites where IL-15-dependent signalling drives their maturation into the NK cell (CD3−CD56+) lineage4,5. The sites of NK cell development are numerous and include the spleen, liver, secondary lymphoid organs, thymus, gut, tonsils and uterus4,5. Whether NK cell differentiation at these locations proceeds in a linear or non-linear manner continues to be debated6.
The two most well-characterized subsets of NK cells are the CD56brightCD16− and CD56dimCD16+ populations. CD56bright cells are found at lower numbers in peripheral blood (90% of NK cells in the circulation are CD56dim), although tissue-resident NK cells are predominantly CD56bright (ref.7). CD56bright NK cells are robust cytokine producers and, unless primed by pro-inflammatory cytokines such as IL-15, are weakly cytotoxic8. By contrast, the CD56dim NK cell population can mediate serial killing of infected and/or malignant cells9,10, predominantly via exocytosis of pre-assembled cytolytic granules containing granzyme B and perforin across the immunological synapse, which ultimately induces apoptosis of the target cell. Evidence indicates that granzyme B-dependent killing typically occurs early in a series of kills mediated by an NK cell, whereas later cytolytic events induced by that cell are death receptor-mediated (for example, via Fas ligand and TNF-related apoptosis-inducing ligand (TRAIL) expressed by NK cells)10.
Activating and inhibitory receptors
Unlike B cells and T cells, NK cells do not express somatically rearranged antigen receptors, but rather stochastic combinations of activating receptors and inhibitory receptors; the net balance of stimulatory versus suppressive signals through these various receptors results in either a response to or tolerance of the target cells11 (fig. 1). Crosslinking of activating receptors induces the phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) contained within adapter proteins, which in turn recruit multiple proteins capable of initiating signalling cascades11. Low-affinity IgG Fc region receptor III (FcγRIII), also known as CD16, is the most potent activating receptor expressed by NK cells11. Accordingly, the Fc regions of IgG antibodies on opsonized cells serve to crosslink CD16 molecules and thus activate NK cells through a process termed antibody-dependent cell-mediated cytotoxicity (ADCC)11. Of note, CD16 is the only receptor that can activate NK cells on its own, without any additional activation through other receptors11. CD16 expression is negatively regulated by the metalloproteinase ADAM17, which cleaves this receptor from the surface of NK cells after they are activated in vivo12. Preventing the downregulation of CD16 expression on tumour-infiltrating NK cells is a potential target of therapeutic intervention (fig. 2). Indeed, the safety and efficacy of an ADAM17 inhibitor (INCB7839) in combination with rituximab, a monoclonal antibody (mAb) used to target CD20+ tumour cells for NK cell-mediated ADCC, are currently being investigated in a phase I/II trial involving patients with diffuse large B cell lymphoma (NCT02141451).
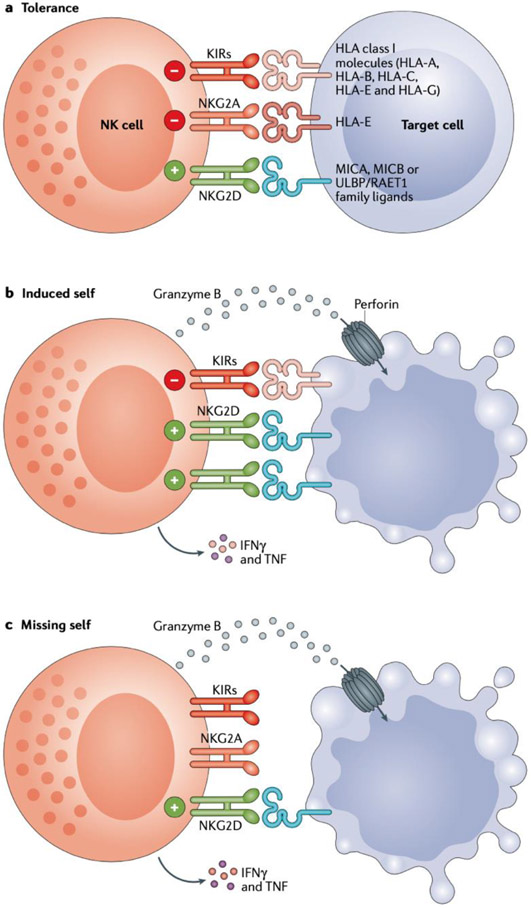
Fig. 1 ∣. NK cells respond to virally infected and transformed cells via balancing signals.
Natural killer (NK) cells express a variety of receptors with activatory or inhibitory functions (or both), and the balance of signalling inputs through these receptors dictates tolerance of or activation of cytotoxicity against the target cells. a ∣ When the overall level of inhibitory receptor signalling outweighs activating receptor signalling, NK cell activation is thwarted, resulting in tolerance of the signal-inducing cell. b ∣ Upon viral infection or transformation, cells typically upregulate stimulatory ligands for NK cell activating receptors such as NKG2D, with the resultant interactions inducing a level of activatory signalling that surpasses that of constitutive signalling through inhibitory receptors (such as killer immunoglobulin-like receptors (KIRs) and NKG2A), which activates NK cell cytokine release and cytotoxicity against the target cell. This scenario is referred to as the ‘induced-self’ response. c ∣ When class I MHC ligands of NK cell inhibitory receptors are downregulated, which commonly occurs in tumour cells, the loss of inhibitory signals and the resulting unabated positive signalling also leads to NK cell activation. This phenomenon is referred to as the ‘missing-self’ response.
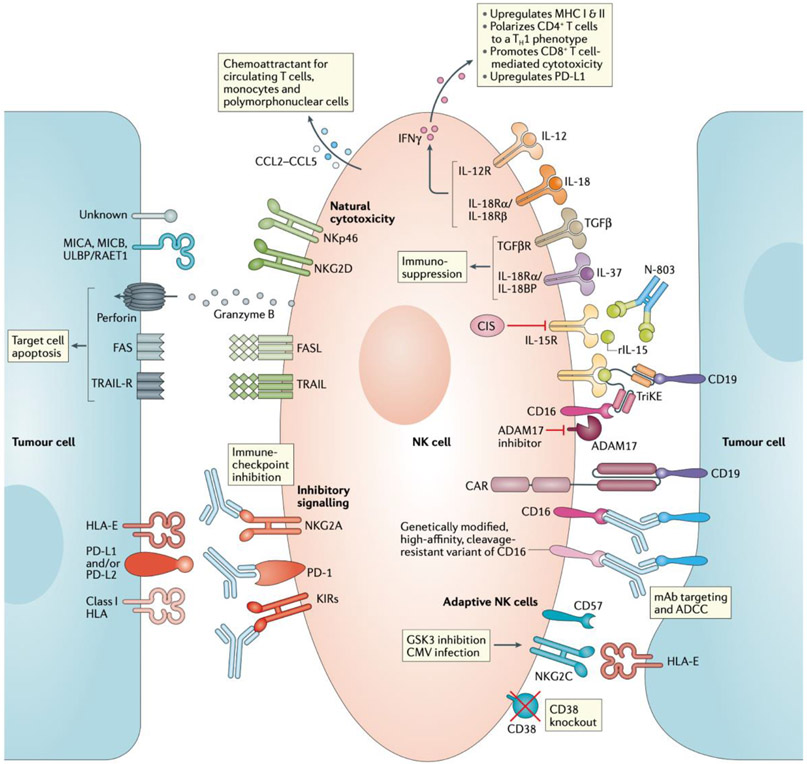
Fig. 2 ∣. Summary of the various approaches to enhancing NK cell effector function.
From top right, clockwise: pro-inflammatory cytokines such as IL-12 and IL-18 enhance NK cell effector function and cytokine secretion, whereas anti-inflammatory cytokines such as IL-37 and TGFβ suppress NK cell function. IL-15 is a crucial homeostatic cytokine for NK cells; cytokine-inducible SH2-containing protein (CIS) is a negative regulator of IL-15 signalling and thus a potential therapeutic target. Recombinant IL-15 (rIL-15) and the IL-15 superagonist N-803 promote NK cell proliferation and persistence within the tumour microenvironment. NK cell engagers (for example, tri-specific killer cell engagers (TriKEs) that also include an IL-15 domain to enhance NK cell activation) and monoclonal antibodies (mAbs) capable of binding to and activating CD16 (low-affinity IgG Fc region receptor III) on NK cells enable the targeting of these cells towards tumour cells expressing specific antigens, such as CD19. Disintegrin and metalloproteinase domain-containing protein 17 (ADAM17) inhibitors reduce CD16 cleavage from the cell surface and might, therefore, enhance NK cell activity. Genetically modified cell products, such as chimeric antigen receptor (CAR) NK cells or NK cells with modified forms of CD16 (for example, FT-516), are also being investigated as a means of improving the antitumour activity of adoptive NK cell therapies. Moreover, GSK3 inhibition and cytomegalovirus (CMV) infection give rise to hyperfunctional NKG2C+CD57+ adaptive NK cells, which have been associated with favourable clinical outcomes. Knockout of CD38 in adoptively transferred NK cells can prevent antibody-dependent cell-mediated cytotoxicity (ADCC)-related fratricide during treatment with mAbs targeting this protein, such as daratumumab. Immune- checkpoint inhibitors could potentially relieve suppression of NK cell- mediated cytotoxicity by preventing inhibitory signalling through PD-1, NKG2A and killer immunoglobulin-like receptors (KIRs). Ultimately, signalling induced by Fas ligand (FASL) and TNF-related apoptosis-inducing ligand (TRAIL) on NK cells can trigger apoptosis of target cells. In addition, activation of members of the natural cytotoxicity receptor family, such as NKp46 and NKG2D, results in the release of preformed cytolytic granules containing granzyme B and perforin. NK cells also secrete chemokines that attract multiple subsets of immune cells with potential antitumour functions.
In addition to CD16, members of the natural cytotoxicity receptor family, including NKp30, NKp40, NKp44 and NKp46, can induce activation signals in NK cells; these receptors directly bind to viral, bacterial and/or tumour-associated ligands to potentiate cytokine production and cell killing13. NKG2D and NKG2C are both additional activating receptors with important translational implications as therapeutic targets. NKG2D is a widely studied homodimeric C-type lectin family receptor with known ligands on human tumours and virally infected cells, including MHC class I polypeptide-related sequence A (MICA), MICB and the RAET1/ULBP family of proteins. NKG2C forms a dimer with CD94 and is unique among NK cell activating receptors in that its activation is dependent on binding to HLA-E, a ligand that is typically associated with suppressive signalling via the NK cell inhibitory receptor NKG2A. NKG2C is also renowned as the receptor that recognizes human cytomegalovirus (HCMV) peptides and mediates the expansion of a hyperfunctional subset of NK cells termed adaptive NK cells14,15.
NK cells also express a diverse repertoire of inhibitory receptors, which provide immunological self-tolerance and negative-feedback mechanisms that counteract the stimulatory signals from activating receptors16. Multiple families of NK cell inhibitory receptors exist and their cognate ligands are generally class I HLA molecules (HLA-A, HLA-B, HLA-C, HLA-E and HLA-G). Many of these receptors have intracellular domains containing immunoreceptor tyrosine-based inhibition motifs (ITIMs), which serve as docking sites for membrane proximal phosphatases that dampen activating receptor signalling and thus NK cell activation16. The killer immunoglobulin-like receptor (KIR) family is composed of 14 polymorphic receptors: six activating (2DS1–2DS5 and 3DS1), seven inhibitory (2DL1–2DL3, 2DL5 and 3DL1–3DL3), and one with both activating and inhibitory functions (2DL4)17. As alluded to above, the C-type lectin family member NKG2A that can bind to HLA-E is another major inhibitory receptor expressed by NK cells. Unlike KIRs, neither NKG2A nor HLA-E are polymorphic, which might facilitate the generation of therapeutic agents that block their interaction16. Indeed, blocking the interactions of NKG2A, KIRs and other inhibitory receptors with their cognate ligands expressed in tumours is an interesting strategy for NK cell-based cancer therapy, as discussed in more detail later in this Review.
Antitumour NK cells
Mechanisms of effector function
NK cells have multiple functions that can restrict the growth and spread of cancerous cells. Circulating NK cells can be recruited to sites of tumorigenesis under the direction of pro-inflammatory chemokines produced by innate and adaptive immune cells in the TME. CXCR3–CXCR4, CX3CR1 and CCR3–CCR5 are the main chemotactic receptors differentially expressed by NK cell subsets that respond to chemokine gradients generated as a result of the immune response to cancer18.
Upon entry into the TME, NK cells can kill cancer cells via the ‘missing-self ’ mechanism. As mentioned previously, NK cell activation is suppressed by the binding of inhibitory receptors to class I HLA (MHC I) molecules. However, many cancer cells downregulate expression of MHC I molecules in order to evade detection by cytotoxic CD8+ T cells; therefore, NK cells can identify and respond to cells of this missing-self phenotype, owing to a lack of MHC I-induced signalling via inhibitory receptors and a consequent increase in activatory signalling, ultimately resulting in lysis of the target cells19 (fig. 1). Thus, NK cells have therapeutic potential in the setting in which T cells cannot recognize cancer cells as a result of MHC I downregulation.
ADCC is another key mechanism of NK cell-mediated killing of cancer cells, and this activity can be exploited through the administration of therapeutic mAbs directed at tumour-associated antigens. Indeed, evidence suggests that NK cell-mediated ADCC is an important mechanism contributing to the successes achieved with therapeutic mAbs, such as rituximab and trastuzumab, in patients with haematological or solid cancers20,21. Several of these mAbs are in broad use and have changed the practice of cancer care, thus demonstrating the considerable therapeutic potential of NK cells.
As well as directly inducing cytotoxicity, NK cells also respond to transformed cells by producing pro-inflammatory cytokines, including IFNγ and TNF. These pleiotropic proteins have potent anti-proliferative, anti-angiogenic and pro-apoptotic effects on cancer cells, in addition to their ability to enhance cytotoxic CD8+ T cell responses22.
Immunosuppression in the TME
Despite their activity in controlling tumour growth, NK cells are susceptible to multiple immunosuppressive mechanisms that are active in the TME. Many cancer-associated soluble immunosuppressive molecules negatively affect NK cell function, including IL-10, indoleamine 2,3-dioxygenase, prostaglandin E2 and macrophage migration inhibitory factor23. Transforming growth factor-β (TGFβ) is one of the most studied molecules with immunosuppressive effects on NK cells. TGFβ can be produced by multiple immune cell subsets in the TME, including regulatory T (Treg) cells, myeloid-derived suppressor cells (MDSCs) and tumour-associated macrophages (TAMs), as well as cancer cells themselves24. This pleiotropic cytokine is known to downregulate multiple aspects of NK cell function, including cytokine secretion, degranulation, metabolism and mTOR signalling25-27. Furthermore, inhibition of TGFβ preserves the antitumour activity of NK cells in preclinical models of acute myeloid leukaemia (AML) and colon cancer28. TGFβ-mediated suppression is not restricted to NK cells, given that this cytokine also prevents naive T cell differentiation, suppresses effector T cell function and hinders antigen presentation by dendritic cells25. Antagonism of TGFβ is, therefore, a potential strategy to improve the efficacy of immunotherapy29,30. Indeed, bi-functional ‘traps’ simultaneously targeting TGFβ and immune-checkpoint proteins are being tested in several ongoing clinical trials (for example, NCT03631706).
Hypoxia is another considerable barrier to NK cell cytotoxic activity in the TME. Large solid tumours are not well vascularized and, therefore, often contain extensive regions with low oxygen concentrations, wherein NK cell activity is substantially — but not completely — abrogated. Such conditions result in downregulation of activating receptors and death receptors, degradation of granzyme B via autophagy and decreases in cytokine secretion by NK cells31,32. Various treatments designed to overcome hypoxia in the TME, including hypoxia-induced prodrugs and HIF-1 targeting, are in clinical development (for example, NCT01746979).
When shed from tumours through immune-escape processes, soluble ligands for NK cell activating receptors can act as suppressors, rather than activators, of NK cell activity. Specifically, high serum levels of MICA and MICB correlate strongly with unfavourable clinical outcomes across multiple cancers33,34. Binding of these soluble ligands to NKG2D not only prevents NK cells from recognizing MICA and MICB presented on the surface of tumour cells but also results in downregulation of the expression of this activating receptor by tumour-infiltrating NK cells35. Notably, mAb-based targeting of MICA and MICB on the surface of tumours can prevent shedding of these ligands, promote ADCC of tumour cells and inhibit tumour growth in humanized mouse models of melanoma36.
IL-37 (also known as IL-1 family member 7 (IL-1F7)) secreted by immunosuppressive Treg cells in the TME is another soluble factor known to negatively regulate the antitumour activity of NK cells37. Importantly, hyper- functional adaptive NK cells possess intrinsic resistance to the inhibitory effects of IL-37, and this population of cells is currently being evaluated in clinical trials as a source of therapeutic NK cells38 (Table 1). The IL-37 co-receptor, single Ig IL-1-related receptor (SIGIRR, also known as IL-1 receptor 8) is a novel immune-checkpoint protein expressed by NK cells, and negatively regulates their antitumour and antiviral activities39. Genetic disruption of SIGIRR in NK cells increases resistance to hepatic carcinogenesis and haematogenous metastasis of sarcomas and colorectal cancers to the lungs and liver, respectively, in mouse models39.
Table 1 ∣.
Selected ongoing clinical trials of therapeutic NK cell products
| Agent | Cell source |
Treatment approach | Malignancy | Study phase (status) |
ClinicalTrials.gov identifier (trial name) |
|---|---|---|---|---|---|
| CAR NK cells | |||||
| NK-92/5.28.z cells | NK-92 cells | Intracranial injection upon repeat surgery or biopsy | Recurrent HER2+ glioblastoma or gliosarcoma | I (recruiting) | NCT03383978 (CAR2BRAIN) |
| iC9/CD19-CAR-CD28-zeta- 2A-IL-15 NK cells | UCB NK cells | In combination with lymphodepleting chemotherapy | CD19+ R/R B cell malignancies | I/II (recruiting) | NCT03056339 |
| PD-L1-targeted high-affinity NK (PD-L1.t-haNK) | NK-92 cells | NA | R/R advanced-stage solid tumours | I (recruiting) | NCT04050709 (QUILT 3.064) |
| In combination with N-803 | Locally advanced or metastatic pancreatic cancer | II (recruiting) | NCT04390399 (QUILT 88) | ||
| CD19-targeted high-affinity NK (CD19.t-haNK) | NK-92 cells | NA | Diffuse large B cell lymphoma | I (not yet recruiting) | NCT04052061 (QUILT 3.061) |
| Genetically modified NK-92 cells | |||||
| NK cells expressing high-affinity variant of CD16 (haNK) | NK-92 cells | Combined with NANT cancer vaccine, avelumab (anti-PD-L1 antibody) and various other agents | R/R advanced stage triple-negative breast cancer | Ib/II (active, not recruiting) | NCT03387085 (QUILT-3.067) |
| Combined with an IL-15 superagonist (N-803) and avelumab | Merkel cell carcinoma that has progressed after ICI | I (recruiting) | NCT03853317 (QUILT-3.063) | ||
| Combined with NANT cancer vaccine, avelumab and various other agents | Squamous cell carcinoma (of the lung or head and neck) that has progressed after platinum-based chemotherapy and ICI | Ib/II (active, not recruiting) | NCT03387111 (QUILT-3.090) | ||
| Combined with NANT cancer vaccine, avelumab and various other agents | R/R pancreatic cancer | I (active, not recruiting) | NCT03586869 (QUILT-3.080) | ||
| iPSC-derived NK cells | |||||
| FT500 (off-the-shelf iPSC NK cells) | iPSCs | Monotherapy or combination with PD-1–PD-L1 ICI (using nivolumab, pembrolizumab or atezolizumab) | R/R advanced-stage solid or haematological cancer | I (recruiting) | NCT03841110 |
| FT516 (iPSC NK cells expressing high-affinity, non-cleavable CD16 (hnCD16)) | iPSCs | Monotherapy for AML or in combination with anti-CD20 antibodies (rituximab or obinutuzumab) for B cell lymphoma | R/R AML or B cell lymphoma | I (recruiting) | NCT04023071 |
| FT596 (iPSC NK cells expressing an anti-CD19 CAR, hnCD16 and IL-15) | iPSCs | Monotherapy or combination with anti-CD20 antibodies (rituximab or obinutuzumab) | R/R chronic lymphocytic leukaemia or B cell lymphoma | I (recruiting) | NCT04245722 |
| CIML NK cells | |||||
| CIML NK cell infusions | PB NK cells | In combination with IL-2 | Myeloid disease that has relapsed after haploidentical HSCT | I (recruiting) | NCT04024761 |
| After T cell donor lymphocyte infusion |
AML that has relapsed after allogeneic HSCT | I (recruiting) | NCT03068819 | ||
| With IL-2 or N-803 | R/R AML or myelodysplastic syndrome | I/II (recruiting) | NCT01898793 | ||
| With N-803 | R/R AML | II (recruiting) | NCT02782546 | ||
| Adaptive NK cells | |||||
| CMV-MVA Triplex vaccine | NA | Administered on days 28 and 56 after autologous HSCT, in order to enhance adaptive NK cell reconstitution | Lymphoma or multiple myeloma | I (completed) | NCT03383055 |
| Adaptive NK cells (cont.) | |||||
| FATE-NK100 | PB NK cells | Combined with subcutaneous IL-2 | R/R AML | I (active, not recruiting) | NCT03081780 |
| Intraperitoneal delivery with IL-2 | Recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer | I (recruiting) | NCT03213964 | ||
| Monotherapy, or combination with cetuximab (anti-EGFR antibody) or trastuzumab (anti-HER2 antibody) | Advanced-stage solid tumours | I (active, not recruiting) | NCT03319459 | ||
| UCB HPC-derived NK cells | |||||
| UCB NK cells | UCB CD34+ HPC-derived NK cells | With or without low-dose or high-dose subcutaneous IL-2 | R/R AML | I/II (not yet recruiting) | NCT04347616 (NK4AML) |
| Monotherapy by intraperitoneal infusion | Recurrent ovarian carcinomas | I (recruiting) | NCT03539406 (INTRO) | ||
Owing to the large number of trials in each category, example trials have been selected to illustrate the research and trials mentioned in this Review. Example trials have been selected for larger cohort size, trial status (preference for active trials) and a focus on therapies that most directly test modalities affecting NK cell function in vivo (excluding trials testing multiple agents simultaneously). Studies with an unknown status, or that have been suspended, terminated or withdrawn without results have been omitted from this list. AML, acute myeloid leukaemia; CAR, chimeric antigen receptor; CIML, cytokine-induced memory-like; CMV-MVA, cytomegalovirus-modified vaccinia Ankara; HPC, haematopoietic progenitor cell; HSCT, haematopoietic stem cell transplantation; iC9, inducible caspase-9; ICI, immune-checkpoint inhibition; iPSC, induced pluripotent stem cell; NA, not applicable/available; NK, natural killer; PB, peripheral blood; R/R, relapsed and/or refractory; t-haNK, NK-92 cells expressing high-affinity CD16 and a CAR; UCB, umbilical cord blood; zeta, CD3ζ domain.
Cytokine-inducible SH2-containing protein (CIS), encoded by CISH, is an intracellular negative regulator of IL-15 signalling that is upregulated upon NK cell or T cell activation and is being investigated as a therapeutic target in tumour-infiltrating NK cells40 (fig. 2). In mice, genetic deletion of Cish renders NK cells hypersensitive to IL-15, resulting in enhanced NK cell survival, proliferation, IFNγ secretion and cytotoxicity towards tumours40.
Thus, a growing body of work demonstrates effective methods of overcoming the myriad defences adopted by tumours against NK cells and the inhibitory effects of the TME on these cells. These strategies have the potential to enhance the effectiveness of the NK cell-based therapeutic approaches discussed in the following sections of this Review.
Adoptive NK cell therapy
Autologous versus haploidentical
Autologous NK cell infusions were the first major focus of adoptive NK cell therapy owing to the convenience of using the patient’s own blood as the cell source, lack of a requirement for immunosuppressive therapy and the low risk of graft-versus-host disease (GvHD), a potentially lethal post-transplantation condition caused by donor T cell destruction of host tissues. Studies of this approach have revealed that the infused cells are able to expand in vivo but fail to mount potent responses against haematological or solid cancers, perhaps owing in part to the inhibitory effects of interactions between the autologous NK cells and self MHC I molecules41,42. Furthermore, patients who received the infusions were heavily pretreated prior to cell collection and therapy, which might have negatively affected NK cell expansion and function41. These findings prompted a shift in focus of many groups from autologous to allogeneic NK cell therapies.
In the mid-20th century, advances in HLA-matched allogeneic HSCT revolutionized the treatment of patients with haematological malignancies, and this approach remains the only curative therapy for most patients with high-risk disease43. The use of fully HLA-matched donors poses logistical barriers to HSCT; however, the widespread use of HLA haploidentical (half-matched) or partially matched umbilical cord blood (UCB) grafts enables a suitable donor to be found for most patients. Indeed, T cell depletion from grafts, intensified immunosuppressive therapy and grafts containing large numbers of CD34+ HPCs obviate the need for a fully HLA-matched transplant44. Nevertheless, morbidities from transplantation and disease relapse remain major causes of treatment failure. Thus, safe, specific and effective cell therapies are needed to mitigate these limitations or perhaps to replace HSCT entirely for patients with certain cancers.
A major role for NK cells in the HSCT setting was elucidated in 2002 by Ruggeri et al.45, who demonstrated that mismatches between inhibitory KIRs expressed by NK cells in haploidentical grafts and host HLA ligands unleashed alloreactive NK cell cytotoxicity against AML cells in mouse models and patients in a setting of T cell depletion and high CD34+ cell doses. Several years later, infusions of haploidentical NK cells following non-myeloablative chemotherapy were found to be capable of inducing complete remissions (CRs) in patients with AML, outside the setting of HSCT46. The correlation between KIR–HLA ligand mismatch and tumour control was also replicated in this study46, although the number of patients was low, and the results of a study by our group in a larger cohort of patients did not confirm this correlation47,48. Additionally, NK cell expansion and invivo persistence for up to 1 month was only observed in patients who received lymphodepleting conditioning treatment with both high-dose cyclophosphamide and fludarabine, but not with fludarabine alone, prior to NK cell infusion46. Cell expansion was associated with a marked increase in serum levels of endogenous IL-15, which highlighted a role for this homeostatic cytokine in NK cell immunotherapy46. These findings underscore the importance of preparatory conditioning regimens for NK cell expansion, persistence and efficacy. In both patients with solid malignancies and patients with haematological malignancies, such nonmyeloablative conditioning chemotherapy is administered to deplete endogenous host lymphocyte populations that compete with the infused NK cells by acting as ‘cytokine sinks’. In patients with haematological malignancies, conditioning chemotherapy also serves to directly deplete tumour cells, thereby reducing the tumour burden, which might increase the likelihood that the NK cells can completely eliminate the disease. Thus, the importance of lymphodepleting agents is well established, although they are associated with characteristic toxicities, including neutropenia49; therefore, safer and more selective approaches are needed to optimize and expand the clinical use of adoptive NK cell therapy.
Alternative allogeneic NK cell sources
Umbilical cord blood NK cells.
Allogeneic peripheral blood NK cells are only one of numerous potential sources of therapeutic NK cells. NK cells account for ~10% of all lymphocytes in peripheral blood, whereas they constitute up to 30% of the lymphocytes in UCB50,51; thus, UCB is a robust source of therapeutic effector NK cells. UCB NK cells produce similar amounts of IFNγ and TNF as peripheral blood NK cells in response to various stimuli, although they have weaker cytotoxic activity against K562 leukaemia cells50,51. This difference is hypothesized to reflect decreased expression of adhesion molecules and activating receptors, such as CD2 and CD16, respectively, and increased expression of the inhibitory receptor NKG2A on UCB NK cells relative to their peripheral blood counterparts50-52. Nevertheless, the therapeutic efficacy of UCB NK cells is currently being evaluated in several clinical trials (such as NCT01619761 and NCT02280525). UCB is also a rich source of HPCs and can therefore serve as a substrate for the in vitro differentiation of therapeutic NK cells with desirable phenotypes, including adaptive NK cells, which is emerging as an exciting new avenue of immunotherapy research and development53.
NK cell lines.
Clonal NK cell lines, such as NK-92, KHYG-1 and YT cells, are an alternative source of allogeneic NK cells, and the NK-92 cell line has been extensively tested in clinical trials54,55. NK-92 cells are easily expanded using multiple good manufacturing practice-compliant methodologies, with doubling times between 24 and 36 hours55. However, these cells are aneuploid and therefore genetically unstable, which requires them to be irradiated prior to infusion. Irradiated NK-92 cells have been observed to kill tumour cells in patients with cancer, although irradiation limits the in vivo persistence of these cells to a maximum of 48 hours — presenting a potential obstacle to durable clinical efficacy56. NK-92 cells lack expression of CD16, although an NK-92-derived product (haNK) has been genetically engineered to express a high-affinity variant of CD16 as well as endogenous IL-2 in order to enhance effector function57. This product is currently being tested in four clinical trials involving patients with a variety of malignancies, in combination with the anti-PD-L1 antibody avelumab plus a cancer vaccine (NANT) or an IL-15 superagonist (Table 1).
Stem cell-derived NK cells.
To date, most adoptive NK cell therapies have involved the use of peripheral blood NK cells, UCB NK cells or NK-92 cells, but each of these cell sources has important drawbacks, as outlined above. Indeed, owing to issues related to costs, delays in blood collection, variability among donors and heterogeneity of leukocytes in donor blood, strong interest is being focused on shifting from traditional allogeneic cell sources to stem cell-derived NK cells that can be used as standardized ‘off-the-shelf ’ therapies for any patient, regardless of HLA haplotype. One particularly attractive approach to circumventing these issues involves the generation of NK cells from CD34+ HPCs58. Not only does this approach enable the production of virtually unlimited amounts of homogeneous NK cells, but the cells are also much more amenable to genetic manipulation than primary NK cells.
Stem cell-derived NK cell products from multiple sources are currently being tested clinically, including those originating from UCB stem cells or induced pluripotent stem cells (iPSCs) (Table 1). Spanholtz et al.53 have developed a method to expand UCB CD34+ HPCs 2,000-fold and generate a product composed of >90% functional NK cells. This production process occurs in closed, large-scale bioreactors, making it a valuable tool for the generation of clinical grade NK cells for adoptive transfer53.
The initial reprogramming of adult cells to pluripotency in order to enable their differentiation into NK cells and expansion to generate the final product is a unique feature of the iPSC-based methodology58. This approach standardizes the starting material and provides greater homogeneity and reproducibility in the development and administration of NK cell therapies. In 2009, Woll et al.59 reported that iPSC-derived NK cells are more potently cytotoxic and have greater anticancer activity than UCB NK cells in several mouse models of leukaemia. More recently, Hermanson et al.60 reported that iPSC-derived NK cells are as effective as peripheral blood NK cells in increasing the median survival duration of mice harbouring ovarian cancer xenografts, thus demonstrating their efficacy against solid tumours. The investigators of one clinical trial are currently recruiting patients with haematological or solid cancers to test the safety of these off-the-shelf iPSC-derived NK cells, either as monotherapy or in combination with immune-checkpoint inhibition (ICI) (Table 1). In addition, several genetically modified iPSC-derived NK cell products are under clinical investigation in patients with a variety of cancers (Table 1). These products include iPSC-derived NK cells expressing a high-affinity CD16 variant that is also resistant to proteolytic cleavage by ADAM17 (fig. 2) or that have knockout of CD38 in order to prevent ADCC-related ‘fratricide’ of the infused cells when used in parallel with therapeutic mAbs targeting this tumour-associated antigen (for example, daratumumab in the context of multiple myeloma)61. Additionally, iPSC-derived NK cells triple-gene-modified to express high-affinity, cleavage-resistant CD16, a chimeric antigen receptor (CAR) targeting CD19 and a membrane-bound IL-15 receptor signalling complex (in order to promote their persi tence and survival) are being evaluated in a clinical trial involving patients with B cell lymphoma or chronic lymphocytic leukaemia (CLL) (Table 1).
CAR NK cells.
Following the clinical successes achieved with CAR T cell therapies, substantial effort has been applied to exploring the efficacy of CAR NK cell products (fig. 2) and the potential advantages they offer over their T cell counterparts. CAR T cells are derived from autologous T cells collected from the patient’s blood and genetically modified ex vivo to express a synthetic receptor construct with an extracellular antigen-binding domain, typically an antibody single-chain variable fragment (scFv), targeting a specific tumour-associated antigen. This extracellular domain is linked to intracellular ITAM-containing signalling domains derived from components of the T cell receptor complex and/or co- stimulatory molecules, which potently activate cytotoxicity towards the target cells upon antigen binding. The two FDA-approved CAR T cell therapies, tisagenlecleucel and axicabtagene ciloleucel, have achieved objective response rates (ORRs) of >80% in patients with relapsed and/or refractory acute lymphocytic leukaemia (ALL) and B cell non-Hodgkin lymphoma, respectively62-64. Despite this impressive clinical efficacy, several shortcomings of CAR T cell therapies support the development of alternative approaches. These obstacles include severe adverse events (cytokine-release syndrome (CRS) and neurological toxicities), exorbitant costs, and inefficiencies of T cell isolation, modification and expansion65. CAR NK cells are generating considerable enthusiasm owing in large part to their potential to circumvent many of these issues, perhaps including the problematic toxicities, while recapitulating the robust anticancer effects of CAR T cells66. However, further testing of this therapeutic approach is required.
Liu et al.67 have generated a CAR NK cell product from UCB NK cells retrovirally transduced to express genes encoding an anti-CD19 CAR, IL-15 and an inducible caspase-9 suicide switch that enables eradication of the genetically engineered cells in vivo (if and when required) through administration of a small-molecule dimerizing agent. Data from preclinical studies of this product demonstrated effective killing of CD19-expressing primary leukaemia cells in vitro, substantial prolongation of NK cell survival owing to IL-15 expression and rapid elimination upon triggering of the suicide switch67. In patients with CD19+ B cell lymphoma or CLL, this CAR NK cell product was associated with a 64% CR rate (7 of 11 patients), without any major adverse effects (no CRS, neurological toxicities or GvHD)68. Despite these encouraging results, firm conclusions regarding the efficacy of the cell product cannot be drawn because many of the patients received other therapeutic interventions before and after CAR NK cell infusion.
Primary NK cells are not ideal substrates for the generation of CAR cell products, owing to challenges faced during cell isolation, transduction and expansion. Thus, current clinical trials of CAR NK cells are focused mainly on products derived from stem cell or progenitor sources69. Numerous clinical trials of CAR NK-92 cells are underway (Table 1), but the requirement for irradiation and consequent persistence issues are potential limitations to the clinical efficacy of these products. CAR NK cells derived from iPSCs, such as the triple-gene-modified products described above, are a promising alternative. Li et al.70 generated a novel iPSC-derived CAR NK cell product targeting mesothelin, which is a cell-surface antigen overexpressed in many solid tumours, and containing an NKG2D transmembrane domain, an NK cell receptor 2B4 (CD244) co-stimulatory domain and a CD3ζ activatory domain that potentiates tumour cell killing. In a mouse xenograft model of human ovarian cancer, this CAR NK cell product slowed tumour growth and prolonged survival relative to the effects of peripheral blood NK cells, iPSC-derived NK cells or CAR T cells expressing the same construct70. A number of phase I trials of CAR NK cells from various sources, including autologous peripheral blood NK cells, UCB NK cells, NK-92 cells and iPSCs, and that were designed to target a variety of cancers, such as AML, ALL or other B cell malignancies, non-small-cell lung cancer (NSCLC), ovarian cancer or glioblastoma, are currently active (for examples, see Table 1). The safety of these agents has not yet been studied in large-cohort trials, although the infrequent severe toxicities observed with NK cell therapies to date supports the notion that CAR NK cells will circumvent the life-threatening adverse effects of CAR T cells, which probably reflects differences in biological responses between NK cells and T cells following activation through CARs.
Enhancing cell priming and expansion
Regardless of the choice of cell source, large numbers of NK cells are required for a single therapeutic infusion, which are costly and time-consuming to generate using traditional cytokine-based methods of cell expansion. Thus, more efficient and inexpensive means to expand NK cells in vitro are of considerable clinical interest, and several groups have reported promising results in this area. One of the first notable methods of ex vivo NK cell expansion utilized a K562 leukaemia feeder cell line modified to express membrane-bound IL-15 (ref.71). Denman et al.72 subsequently improved upon this method using a K562 cell line expressing membrane-bound IL-21 and 4-1BBL, reporting a mean 47,967-fold expansion over the course of 6 weeks, without any signs of cell senescence or loss of cytotoxic activity. Infusions of NK cells expanded with this IL-21– 4-1BBL K562 feeder cell line have been tested clinically and deemed safe for use in patients73.
Adaptive NK cells are another emerging source of therapeutic NK cells. Adaptive NK cells are a naturally occurring cell population that expands in humans upon HCMV infection or reactivation74. This expansion process is driven by interactions between HCMV UL40-derived peptide antigen–HLA-E complexes and NKG2C on NK cells, which result in the proliferation of NKG2ChiCD57+ NK cells that downregulate certain proteins involved in intracellular signalling, including PLZF, SYK and FcεRIγ75. Adaptive NK cells have several unique effector characteristics, including enhanced ADCC, augmented cytokine responses and intrinsic resistance to the immunosuppressive effects of MDSCs and Treg cells38. Furthermore, HCMV reactivation in patients with leukaemia following HSCT has been associated with expansion of adaptive NK cells and correlated with survival outcomes (1-year relapse rate and disease-free survival of 26% and 55%, respectively, versus 35% and 46% in those without HCMV reactivation; P = 0.05 and P = 0.04)76. Moreover, adaptive NK cells have been detected in patients’ blood >1 year after HSCT, and the presence of >2.5 NKG2C+CD57+ NK cells per microlitre of blood at 6 months after HSCT was associated with a strong trend towards lower 2-year relapse rates (16% versus 46%; P = 0.06), independently of HCMV serostatus or reactivation, suggesting clinically relevant intrinsic advantages of adaptive NK cell persistence76. Efforts to expand or enrich this hyperfunctional population of NK cells ex vivo have yielded promising results. Culturing of peripheral blood NK cells isolated from healthy HCMV-seropositive individuals together with IL-15 and a pharmacological GSK3 inhibitor expanded a population of NK cells with the adaptive phenotype77 (fig. 2). The resulting NK cells were CD57+SYKloFcεRIγlo, produced elevated amounts of IFNγ and TNF, and had improved natural cytotoxicity as well as enhanced ADCC in a mouse xenograft model of ovarian cancer77. The efficacy of these adaptive NK cells in patients with various cancers is currently being evaluated in three clinical trials (Table 1). Nevertheless, the optimal method of generating off-the-shelf adaptive NK cell products remains unclear. The antitumour efficacy of iPSC-derived adaptive NK cells, generated using a 44-day manufacturing process that includes 2 weeks of expansion using IL-21–4-1BBL K562 feeder cells, is currently being tested in patients with various solid or haematological malignancies (NCT03841110 and NCT04106167). Efforts to incorporate the attributes of adaptive NK cells into iPSCs through genetic engineering are also ongoing (NCT04023071 and NCT04245722) (Table 1).
Cytokine-induced memory-like (CIML) NK cells are another option for allogeneic cell therapy and have unique advantages over other types of NK cell products. CIML NK cells are generated ex vivo through brief priming with IL-12, IL-15 and IL-18, which yields NK cells with enhanced responsiveness to cytokine and activating receptor stimulation that persists for weeks to months. Infusion of these CIML NK cells into mice bearing AML xenografts results in robust antitumour effects and markedly prolonged survival78. In a phase I trial79, CIML NK cells had a good safety profile, expanded in vivo and induced remission in 44% of evaluable patients (four of nine) with AML.
Enhancing NK cell activity in vivo
Cytokine-dependent NK cell activation
NK cells are unique among lymphocytes in their ability to lyse tumour cells independent of prior sensitization; however, priming with homeostatic and/or pro-inflammatory cytokines endows NK cells with a number of enhanced effector functions that promote antitumour immunity and increase their persistence in vivo80 — two crucial characteristics of effective cell therapies. IL-2 and IL-15 have been identified as key cytokines that upregulate the activity of NK cells.
IL-2-based priming.
Early clinical studies testing the effects of cytokines as immunotherapies for cancer were mainly focused on IL-2, owing to the promising ability of this cytokine to activate antitumour immunity observed in preclinical studies. In the early 1980s, several groups found that treating human peripheral blood mononuclear cells with IL-2 results in the expansion of a population of lymphokine-activated killer (LAK) cells, a group composed mainly of T cells and NK cells, that have potent cytotoxicity against syngeneic and allogeneic tumour cells81,82. Additionally, when injected repeatedly at low doses following adoptive LAK cell transfer, IL-2 substantially reduced the number and size of metastases in mouse models of pulmonary osteosarcoma83,84. Furthermore, although early studies of IL-2-primed LAK cell infusion in patients with a variety of malignancies revealed no discernible effects on tumour growth85, the results of a study reported in 1985 by Rosenberg et al.86 demonstrated that infusion with autologous LAK cells plus recombinant IL-2 (rIL-2) induced objective tumour regression in 44% of patients. This finding spurred a wave of studies testing IL-2-based immunotherapy.
Several observations suggest that IL-2-based immunotherapy directly engages the anticancer activities of NK cells. First, early studies in mouse models demonstrated that tumour regression upon LAK cell infusion is predominantly mediated by NK cells87. In addition, NK cells are the predominant lymphocytes observed in the blood during the first month of immune reconstitution in patients undergoing HSCT after lymphodepleting chemotherapy with high-dose cyclophosphamide (60 mg/kg per day) for 2 days followed by fludarabine (25 mg/m2 per day) for 5 days (Hi-Cy/Flu)88. Thus, researchers hypothesized that the use of IL-2 to enhance NK cell function in these contexts would mitigate toxicities caused by excessive T cell activation while maintaining antitumour activity. However, clinical trials designed to test this hypothesis led to concerns about the efficacy and safety of this approach. Indeed, administration of low-dose IL-2 after autologous or allogeneic HSCT resulted in robust expansion of NK cells but without any enhancement in antitumour efficacy, although the failure in the context of autologous HSCT was probably attributable to interactions between NK cell inhibitory receptors and self MHC I molecules within the host that dampened antitumour activity89,90. Notably, high doses of IL-2 can induce life-threatening adverse effects, including vascular leak syndrome, heart failure and liver toxicity91-93. Additionally, low-dose IL-2 can activate and expand an immunosuppressive population of Treg cells94-96.
IL-2 belongs to a family of cytokines that also includes IL-4, IL-7, IL-9 and IL-15, all of which can bind to and activate receptor complexes that include the common γ chain (γc, also known as CD132). Both NK cells and T cells express CD132 and the IL-2 receptor β chain (IL-2Rβ, also known as CD122), which can form a dimeric receptor complex; however, Treg cells typically express a high-affinity trimeric IL-2 receptor complex, which also contains IL-2Rα (also known as CD25) that amplifies IL-2-derived signals97. Thus, the limited in vivo efficacy of low-dose IL-2 infusions is speculated to be at least partially attributable to the inhibitory effects of immunosuppressive cytokines produced by Treg cells on tumour-infiltrating lymphocytes. Cumulatively, these observations highlighted a need for alternatives to IL-2 for NK cell therapy combinations and provided the rationale for the evaluation of a recombinant IL-2– diphtheria toxin fusion protein as a means of ablating host Treg cells following lymphodepleting Hi-Cy/Flu chemotherapy and haploidentical NK cell infusion in patients with AML. In a small cohort of patients, this treatment increased NK cell expansion in vivo relative to that observed in patients receiving NK cell infusion and IL-2 after lymphodepleting chemotherapy47. Despite the small sample size, this finding highlights the need to optimize the cytokine milieu of the TME in order to potently activate antitumour immunity while also enhancing the function of NK cells specifically.
IL-15-based priming.
Over the past decade, IL-15 has emerged as a promising substitute for IL-2. IL-2 and IL-15 are closely related homeostatic cytokines, and both require CD132 and CD122 heterodimers to signal. Unlike IL-2, however, IL-15 signalling via CD132 and CD122 heterodimers expressed by the target cell is dependent on transpresentation of this cytokine in complex with IL-15 receptor-α (IL-15Rα) expressed on the surface of another cell98-100. IL-15 rarely circulates unbound to IL-15Rα in vivo for several reasons, including the high affinity of IL-15 for IL-15Rα and the inefficient post-translational processing of this cytokine101. In initial studies in syngeneic mouse models of various cancers, including melanoma, colorectal cancer, lymphoma and lung adenocarcinoma, rIL-15 was well tolerated, expanded NK cell and CD8+ T cell populations, promoted tumour regression and decreased metastasis102-104. Despite being associated with mild-to-moderate toxicities, including diarrhoea, loss of appetite and neutropenia observed in rhesus macaques, rIL-15 entered clinical trials, with the US National Cancer Institute ranking this agent number 1 in their 2008 list of immunotherapies with the highest potential for anticancer efficacy105.
Since then, rIL-15 has been tested in multiple clinical trials both as monotherapy and as an adjuvant to adoptive cell therapies. Furthermore, both intravenous and subcutaneous injections have been tested for toxicities and levels of circulating IL-15. Without prior lymphodepletion, rIL-15 monotherapy administered by either route was well tolerated, induced robust expansion of NK cells and CD8+ T cells, but was not associated with any objective responses106,107. By contrast, Hi-Cy/Flu lymphodepletion and haploidentical NK cell infusion followed by intravenous (0.75 μg/kg) or subcutaneous (2.0 μg/kg) injections of rIL-15, induced CRs in 32% and 40% of patients with relapsed and/or refractory AML, respectively. However, 56% of the cohort receiving subcutaneous rIL-15 developed CRS (compared with 0% following intravenous administration), although this toxicity had no effect on disease response and was responsive to treatment with corticosteroids and/or the anti-IL-6 receptor antibody tocilizumab108. These results suggest that the loss of a cytokine sink after lymphodepletion results in decreased elimination of IL-15, which favours NK cell activation but — along with high subcutaneous depot dosing — might activate residual myeloid cells that mediate CRS by releasing pro-inflammatory cytokines such as IL-6 and IL-1. Additionally, NK cell persistence was superior to that observed in studies with IL-2, although rejection of the infused cells beyond day 14 was common, perhaps as a result of rIL-15-induced stimulation of host CD8+ T cells108. This finding illustrates the complexities of the competitive immunological environment associated with the adoptive transfer of allogeneic cells. Future dosing schedules of rIL-15 should take into account the pharmacodynamic properties of this agent in vivo and incorporate methods intended to selectively minimize the activation of host CD8+ T cells that can mediate rejection of allogeneic NK cells.
In addition to rIL-15, several other approaches to harnessing the immunostimulatory effect of IL-15 are currently being tested clinically (fig. 2). Because the therapeutic effects of IL-15 are dependent on IL-15Rα transpresentation and are thus limited by IL-15Rα bioavailability, multiple strategies have been developed to deliver these molecules in complex with each other. For example, a heterodimeric IL-15–IL-15Rα fusion protein construct (hetIL-15) is currently in phase I testing in patients with metastatic cancer (NCT02452268 and NCT04261439) (Table 2). N-803 (formerly ALT-803), an IL-15 ‘superagonist’ consisting of rIL-15 bound to a bivalent IL-15Rα–IgG1-Fc fusion protein, is being tested in multiple clinical trials, including in combination with adoptive NK cell therapies (NCT03899480, NCT04290546, NCT03853317, NCT04390399, NCT03050216, NCT02890758, NCT02465957, NCT01898793 and NCT02782546) (Table 2). The rIL-15 component of N-803 is mutated to increase its affinity for CD122–CD132 receptors, and the Fc portion is intended to prolong the in vivo half-life as well as increase lymphoid homing, recycling and retention of this agent. In a large number of mouse models of cancer, N-803 induced marked tumour regression and prolonged survival compared with rIL-15 (refs109,110). N-803 is also being explored as a combination therapy with immune-checkpoint inhibitors and tumour-targeting mAbs (NCT02384954, NCT03228667 and NCT02523469), with the rationale of enhancing NK cell as well as T cell activity and enhancing ADCC, respectively. In a phase I trial involving a small cohort of 21 patients with NSCLC, N-803 plus the anti-PD-1 mAb nivolumab induced or reinduced objective responses (ORR 29% overall, and 27% in those with relapsed and/or refractory disease after prior anti-PD-1 therapy), with no increase in the risk of immune-related adverse events compared with that associated with nivolumab alone111. In patients with relapsed and/or refractory indolent non-Hodgkin lymphoma, N-803 in combination with the anti-CD20 mAb rituximab resulted in an ORR of 48%, with responses observed in patients with rituximab-refractory disease112.
Table 2 ∣.
Selected clinical trials of nK cell engagers and nK cell-directed cytokine therapies
| agent | Treatment approach |
Malignancy | Study phase (status) |
ClinicalTrials.gov identifier (trial name) |
|---|---|---|---|---|
| NK cell-engager molecules | ||||
| GTB-3550 (CD16/ IL-15/CD33 TriKE) | Monotherapy | CD33+ high-risk myelodysplastic syndrome, R/R AML or advanced-stage systemic mastocytosis | I/II (recruiting) | NCT03214666 |
| AFM13 (tetravalent bi-specific chimeric anti-CD30/CD16a antibody) | Monotherapy | R/R CD30+ peripheral T cell lymphoma or transformed mycosis fungoides | II (recruiting) | NCT04101331 (REDIRECT) |
| Monotherapy | R/R CD30+ Hodgkin lymphoma | I (completed) | NCT01221571 | |
| In combination with pembrolizumab (anti-PD-1 antibody) | R/R CD30+ Hodgkin lymphoma | I (completed) | NCT02665650 (KEYNOTE- 206) | |
| DF1001 (anti-HER2 TriNKET) | Monotherapy or in combination with pembrolizumab | Advanced-stage HER2+ solid tumours | I/II (recruiting) | NCT04143711 |
| Cytokine-based treatments | ||||
| N-803 (IL-15 superagonist) | Monotherapy | Advanced-stage melanoma, non-small-cell lung carcinoma, renal cell carcinoma or head and neck squamous cell carcinoma | I (completed) | NCT01946789 |
| Subcutaneous recombinant IL-15 | Following infusion of IL-15-activated haploidentical NK cells | R/R AML | II (completed; has results) | NCT02395822 |
| NIZ985 (soluble IL-15/IL-15 receptor-α heterodimer (hetIL-15)) | In combination with spartalizumab (anti-PD-1 antibody) | Solid tumours, lymphoma or melanoma | I/Ib (recruiting) | NCT04261439 |
Owing to the large number of trials in each category, example trials have been selected to illustrate the research and trials mentioned in this Review. Example trials have been selected for larger cohort size, trial status (preference for active trials) and a focus on therapies that most directly test modalities affecting NK cell function in vivo (excluding trials testing multiple agents simultaneously). Studies with an unknown status, or that have been suspended, terminated or withdrawn without results have been omitted from this list. AML, acute myeloid leukaemia; NK, natural killer; R/R, relapsed and/or refractory; TriKE, tri-specific killer engager; TriNKET, tri-specific NK cell engagement therapy.
NK cell-directed immune cell engagers
Various immune-evasion mechanisms limit the extent of NK cell engagement of tumour cells in vivo and are a major hurdle to achieving broadly effective NK cell therapies113. In an attempt to enhance the natural cytotoxicity of tumour-infiltrating NK cells, many groups have generated molecules that bring such cells into contact with tumour cells in an antigen-specific manner. These molecules are typically bi-specific or tri-specific engager proteins composed of multiple antibody-derived antigen-targeting domains (typically scFvs), such that one domain targets an NK cell activating receptor and another binds to a specific tumour-associated antigen. For example, our group has generated a tri-specific killer engager (TriKE) consisting of two scFvs, one targeting CD16 on NK cells and the other targeting CD33 on AML cells, linked by an IL-15 domain that is intended to enhance NK cell survival and proliferation114 (fig. 2). CD16 ligation alone does not induce proliferation or prolong the survival of NK cells in vivo, and we hypothesize that NKCEs containing both CD16-targeting and IL-15 domains will improve the in vivo expansion and persistence of the engaged NK cells, which is of crucial clinical importance114. Moreover, this anti-CD16, IL-15 and anti-CD33 TriKE (GTB-3550) enhances several other important aspects of NK cell effector function, with improved migratory abilities, increased serial killing and a decreased time to first kill observed in preclinical studies115. Following the demonstration of robust antitumour and pro-proliferative effects in mouse xenograft models of human myelodysplastic syndrome115, GTB-3550 is currently being evaluated in a phase I trial involving patients with relapsed and/or refractory AML or myelodysplastic syndrome (Table 2).
Several other NKCE molecules have been shown to have potent antitumour effects in preclinical studies. Gauthier et al.116 described a novel trifunctional NKCE platform consisting of mAb fragments targeting CD16, NKp46 and the tumour-associated antigens CD19, CD20 or EGFR. The anti-CD20 NKCE was effective at clearing invasive, solid, B cell lymphoma (Raji) xenografts in mice and had greater efficacy than ADCC-inducing mAbs targeting the same tumour antigens116. The redirected optimized cell killing (ROCK) platform comprises tetravalent bi-specific engager molecules consisting of two diabodies (scFv dimers). The ROCK engager AFM13 targets the lymphoma-associated antigen CD30 and CD16a, which is a transmembrane form of CD16 expressed by NK cells, mast cells, monocytes and macrophages (as opposed to CD16b, a glycosylphosphatidylinositol anchored form expressed exclusively by neutrophils). Importantly, the CD16a-binding portion of AFM13 does not interfere with the Fc-binding capacity of this antigen, which provides opportunities for combinations with tumour-targeting IgG mAbs to enhance tumour cell killing via ADCC. AFM13 is currently being tested in multiple phase I and/or phase II trials encompassing a variety of solid and haematological malignancies (NCT02321592, NCT03192202, NCT01221571, NCT04101331, NCT02665650 and NCT04074746) (Table 2). DF1001 is one of several agents belonging to the tri-specific NKCE therapies (TriNKETs) platform. The safety and efficacy of this HER2-targeting TriNKET are being investigated in patients with various advanced-stage HER2+ solid tumours, including in combination with the anti-PD-1 antibody pembrolizumab (Table 2). At least four other TriNKETs, targeting antigens associated with haematological malignancies, are currently in earlier stages of development117. Although the number of NKCE therapies that have reached clinical testing is small, several other companies are developing similar products that are expected to enter clinical trials in the near future116.
Immune-checkpoint inhibition
Initial studies involving ICI have mainly been focused on the disinhibition of antitumour T cells, although clinical application of this therapeutic paradigm to specifically enhance the activity of NK cells is garnering considerable interest (fig. 2). For example, the humanized anti-NKG2A mAb monalizumab is currently being tested alone and in combination with other immune-checkpoint inhibitors in patients with a variety of cancers, after preclinical data indicated that this agent strongly enhances NK cell and T cell cytotoxicity118 (Supplementary Table 1).
Clinical trials of KIR antagonists have generated mixed results, which were disappointing considering the promising activity of such agents against AML and multiple myeloma in mouse models119,120. For example, the KIR2D-specific mAb IPH2101 (targeting KIR2DL1, KIR2DL2 and KIR2DL3) was found to induce NK cell dysfunction (less degranulation and cytokine production ex vivo) in patients with smouldering multiple myeloma involved in a phase II trial that was suspended due to lack of efficacy (NCT01248455)121,122 (Supplementary Table 1). One reason for this surprising result is related to the finding that phagocytes were able to remove KIR2D from the surface of NK cells via FcγRI-mediated trogocytosis122. To achieve full effector function, NK cells must undergo a developmental process termed ‘education’, which requires signalling through inhibitory receptors; therefore, loss of KIR2D disrupted these education signals, thereby abrogating the antitumour activity of NK cells122. In addition to the clinical data from patients with smouldering multiple myeloma, a phase I trial of IPH2101 in patients with AML revealed no efficacy despite the agents being well tolerated123. Similarly, the results of a phase Ib trial of combined KIR (KIR2DL1, KIR2DL2 and KIR2DL3) and PD-1 inhibition with lirilumab and nivolumab, respectively, indicate no increase in efficacy against classic Hodgkin lymphoma, non-Hodgkin lymphoma or multiple myeloma relative to that observed with combined CTLA-4 and PD-1 inhibition (NCT01592370)124. Despite these discouraging results, clinical trials of KIR inhibitors have not been categorically negative. Indeed, the combination of lirilumab and nivolumab yielded promising clinical activity (ORRs 24%) in 29 patients with advanced-stage head and neck squamous cell carcinoma (NCT01714739)125. Such observations have increased confidence in several other ongoing clinical trials of anti-KIR mAbs (Supplementary Table 1).
T cell immunoreceptor with Ig and ITIM domains (TIGIT) is another inhibitory receptor expressed by NK cells as well as T cells — with ligands overexpressed on human tumours126,127 — that has been found to be overexpressed on human tumour-infiltrating NK cells128. Inhibition of TIGIT reversed NK cell exhaustion and promotes NK cell-dependent antitumour immunity in several mouse models128. Several active clinical trials have been designed to test the efficacy and/or safety of anti-TIGIT agents alone and in combination with other immune-checkpoint inhibitors (such as NCT03119428, NCT04150965, NCT04047862, NCT04256421, NCT03563716 and NCT04294810).
Similar to TIGIT, the NK cell and T cell inhibitory receptor CD96 has been found at higher levels on NK cells in tumour tissue than on NK cells in peritumoural tissues129. Additionally, higher levels of CD96 expression on NK cells in hepatocellular carcinoma specimens has been associated with an unfavourable prognosis129. CD96 inhibitors have yet to enter clinical trials, but preclinical data have indicated an ability of such agents to suppress experimental or spontaneous metastases in several mouse models130.
The major successes achieved with anti-PD-1 and anti-PD-L1 mAbs in overcoming T cell tolerance of human cancers have raised the question of whether these treatments similarly reinvigorate exhausted antitumour NK cells. Indeed, NK cells have been identified as key mediators of anti-PD-L1 mAb-induced cytotoxicity against several cancers in preclinical models, including PD-L1+ triple-negative breast cancer and PD-L1− leukaemia, via mechanisms including ADCC of anti-PD-L1 mAb-opsonized tumour cells or activation of PD-L1+ NK cells by these mAbs, respectively131,132. PD-1 is upregulated on NK cells in the TME of multiple human cancers, although tumour-infiltrating NK cells do not express PD-1 to the same degree as the previously mentioned receptors133-137. Nevertheless, NK cells are indispensable for the full anticancer activity of both anti-PD-1 and anti-PD-L1 mAbs in several mouse models of cancer138. Furthermore, PD-1+ tumour-infiltrating NK cells have dysfunctional phenotypes ex vivo, and higher levels of PD-1 expression on NK cells in several gastrointestinal cancers are correlated with an unfavourable patient prognosis, suggesting a reasonable rationale for therapeutic intervention to target PD-1 on endogenous or adoptive NK cells135,139.
T cell immunoglobulin mucin receptor 3 (TIM-3, also known as hepatitis A virus cellular receptor 2) is another immune-checkpoint protein with inhibitory properties that is expressed by various leukocytes, including NK cells, and binds to a ligand, galectin-9, that is known to be expressed on human tumours140,141. Increased TIM-3 expression on NK cells from patients with lung adenocarcinoma predicted an unfavourable prognosis, and ex vivo TIM-3 inhibition of such NK cells enhanced their cytotoxicity and IFNγ production142. Similar results of TIM-3 inhibition in NK cells from patients with advanced-stage melanoma independently underscore the therapeutic potential of this approach143. Currently, several clinical trials are testing TIM-3 inhibitors in patients with various cancers, although none involves combinations with other, more specific approaches to NK cell therapy (NCT03311412, NCT03066648, NCT03489343, NCT03680508, NCT03961971, NCT04370704, NCT02817633, NCT03099109, NCT03744468 and NCT04139902).
Lymphocyte activation gene 3 protein (LAG-3) is an immune-checkpoint receptor that is expressed broadly across leukocyte populations, including NK cells, activated T cells, B cells and plasmacytoid dendritic cells, and is known to induce inhibitory signalling upon binding to MHC class II molecules144-147. Preclinical evidence suggests that LAG-3 synergistically regu lates tumour immune escape with PD-1, and that LAG-3 inhibition enhances T cell-mediated anticancer immunity148-151. However, the role of LAG-3 in regulating NK cell-mediated antitumour immunity is less clear. Studies investigating the effects of LAG-3 inhibition on human NK cells are lacking, and published material in this area does not report any enhancement of activity, although evidence indicates that low LAG-3 expression on NK cells is associated with immune control of HIV152,153. By contrast, data from studies of murine NK cells suggest a positive role for LAG-3 in mediating cytotoxicity against tumour cell lines (indicating that this protein might act as an activating receptor in mice)154, highlighting the need to further investigate this area. Driven by the encouraging preclinical effects of LAG-3 inhibition on antitumour T cells, several clinical trials are testing the efficacy of this approach in patients with various malignancies (including NCT01968109, NCT04150965, NCT04140500 and NCT03625323).
Conclusions
NK cell therapies are emerging as a promising area of clinical research, with manageable safety profiles and preliminary signs of efficacy in patients with certain cancers. Specifically, several groups have independently confirmed the potent antitumour activity of adoptive allogeneic NK cells in patients with AML, although randomized controlled trials with larger cohorts of patients are needed to validate these early results. Additionally, such studies might shed light on the roles of the NK cells themselves and identify the optimal form of lymphodepleting chemotherapy, which also contributes to the antitumour activity. Several crucial issues must be addressed to maximize the effectiveness of future therapeutic NK cell products. First, we need to understand how immunosuppressive factors such as low glucose availability, low oxygen concentrations and certain cell populations in the TME (predominantly MDSCs, Treg cells and TAMs) suppress the antitumour functions of lymphocytes. Circumventing and ablating these barriers is a current focus of research efforts and must continue to be studied as NK cell therapies evolve. The absence of growth factors necessary for NK cell proliferation and survival is another factor that limits the activity of these cells in the TME. Future treatments must not only target NK cells towards tumour cells, but also provide these lymphocytes with essential survival factors, such as IL-15, that increase their persistence in vivo. These factors can be co-administered with adoptive cell infusions or the NK cells themselves can be modified to secrete or present membrane-bound factors. Additionally, it is becoming increasingly clear that non-specific activation of NK cells, even in the presence of homeostatic cytokines such as IL-15 or IL-2, does not generate the robust antitumour effects observed with NK cells redirected towards tumour-associated antigens using immune cell-engager molecules or CARs.
Finally, traditional cell therapies sourced from individual donors pose several logistical impediments, and thus off-the-shelf products will be important to achieving widespread access to adoptive NK cell therapies in the cancer community. NK cells are particularly amenable to off-the-shelf strategies because: (1) unlike T cells, they do not necessarily require presentation of antigens by MHC molecules for target cell recognition and killing; (2) they can be selected to avoid inhibitory receptor-mediated suppression (through KIR–HLA mismatching to mimic the missing-self phenotype); and (3) they are derived from cells not previously exposed to cancer-induced immunosuppression that is encountered in the autologous setting. We expect that new modalities for the large-scale generation of NK cell products derived from physiological NK cell populations, HPCs or iPSC will circumvent these issues and provide a path for broader use in the treatment of patients with cancer. Ultimately, combinatorial approaches using NK cells with antibodies, immune cell engagers, CARs and proteins designed to modulate inhibitory checkpoints and enhance tumour homing need to be tested. The goal is to find an NK cell-based therapeutic strategy that is safe and has the definitive efficacy that will be required for widespread clinical adoption. The promise in this area has been a motivating factor in the development of diverse pharmaceutical and cell therapy approaches to exploit the inherent anticancer activities of NK cells. The field eagerly awaits clinical results in the coming years; these key data will provide researchers and clinicians with much needed insights into how the potential of NK cell-based cancer therapies could be maximized.
Supplementary Material
Key points.
Natural killer (NK) cell-based therapies are emerging as safe and efficacious treatments for some cancers.
Generally, the two main considerations relating to NK cell therapies are the choice of NK cell source and the method of in vivo enhancement of NK cell function; determining approaches to optimize both of these aspects is of high clinical interest.
Therapeutic NK cells include haploidentical NK cells, chimeric antigen receptor NK cells, stem cell-derived NK cells, umbilical cord blood NK cells, NK cell lines, adaptive NK cells and cytokine-induced memory-like NK cells.
Auxiliary methods for enhancing the therapeutic activity of NK cells in vivo include cytokine-based agents, NK cell-engager molecules (such as TriKEs, ROCK engagers, NKCEs and TriNKETs) and immune-checkpoint inhibitors.
Potential advantages that NK cell therapies have over T cell therapies include more manageable safety profiles and fewer graft restrictions (for example, no requirement for autologous cells, providing opportunities for off-the-shelf products).
NK cell therapies remain subject to important immunosuppressive barriers in the tumour microenvironment; the future success of these therapies will require a better understanding of how these suppressive factors operate and how they can be overcome.
Footnotes
Competing interests
J.S.M. consults for and holds stock in Fate Therapeutics and GT Biopharma. These competing interests have been reviewed and managed by the University of Minnesota in accordance with its conflict of interest policy. J.A.M. declares no competing interests.
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s41571-020-0426-7.
References
- 1.Herberman RB, Nunn ME, Holden HT & Lavrin DH Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int. J. Cancer 16, 230–239 (1975). [DOI] [PubMed] [Google Scholar]
- 2.Kiessling R, Klein E & Wigzell H “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol 5, 112–117 (1975). [DOI] [PubMed] [Google Scholar]
- 3.Colonna M Innate lymphoid cells: diversity, plasticity, and unique functions in immunity. Immunity 48, 1104–1117 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scoville SD, Freud AG & Caligiuri MA Modeling human natural killer cell development in the era of innate lymphoid cells. Front. Immunol 8, 360 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Male V et al. Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. J. Immunol 185, 3913–3918 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cichocki F, Grzywacz B & Miller JS Human NK cell development: one road or many? Front. Immunol 10, 2078 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melsen JE, Lugthart G, Lankester AC & Schilham MW Human circulating and tissue-resident CD56bright natural killer cell populations. Front. Immunol 7, 262 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner JA et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J. Clin. Invest 127, 4042–4058 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanier LL, Le AM, Civin CI, Loken MR & Phillips JH The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J. Immunol 136, 4480–4486 (1986). [PubMed] [Google Scholar]
- 10.Prager I et al. NK cells switch from granzyme B to death receptor-mediated cytotoxicity during serial killing. J. Exp. Med 216, 2113–2127 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryceson YT, March ME, Ljunggren H-G & Long EO Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev 214, 73–91 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romee R et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood 121, 3599–3608 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrow AD, Martin CJ & Colonna M The natural cytotoxicity receptors in health and disease. Front. Immunol 10, 909 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zingoni A et al. NKG2D and its ligands: “one for all, all for one”. Front. Immunol 9, 476 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlums H et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 42, 443–456 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long EO Negative signaling by inhibitory receptors: the NK cell paradigm. Immunol. Rev 224, 70–84 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parham P, Norman PJ, Abi-Rached L & Guethlein LA Human-specific evolution of killer cell immunoglobulin-like receptor recognition of major histocompatibility complex class I molecules. Phil. Trans. R. Soc. B 367, 800–811 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernardini G, Antonangeli F, Bonanni V & Santoni A Dysregulation of chemokine/chemokine receptor axes and NK cell tissue localization during diseases. Front. Immunol 7, 402 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kärre K NK cells, MHC class I molecules and the missing self. Scand. J. Immunol 55, 221–228 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Weng W-K & Levy R Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol 21, 3940–3947 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Varchetta S et al. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res. 67, 11991–11999 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Castro F, Cardoso AP, Gonçalves RM, Serre K & Oliveira MJ Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front. Immunol 9, 847 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vitale M, Cantoni C, Pietra G, Mingari MC & Moretta L Effect of tumor cells and tumor microenvironment on NK-cell function. Eur. J. Immunol 44, 1582–1592 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Molgora M et al. The yin-yang of the interaction between myelomonocytic cells and NK cells. Scand. J. Immunol 88, e12705 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batlle E & Massagué J Transforming growth factor-β signaling in immunity and cancer. Immunity 50, 924–940 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaiatz-Bittencourt V, Finlay DK & Gardiner CM Canonical TGF-β signaling pathway represses human NK cell metabolism. J. Immunol 200, 3934–3941 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Viel S et al. TGF-β inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci. Signal 9, ra19 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Otegbeye F et al. Inhibiting TGF-beta signaling preserves the function of highly activated, in vitro expanded natural killer cells in AML and colon cancer models. PLoS One 13, e0191358 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravi R et al. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFβ enhance the efficacy of cancer immunotherapy. Nat. Commun 9, 741 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knudson KM et al. M7824, a novel bifunctional anti-PD-L1/TGFβ Trap fusion protein, promotes anti- tumor efficacy as monotherapy and in combination with vaccine. Oncoimmunology 7, e1426519 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terrén I, Orrantia A, Vitallé J, Zenarruzabeitia O & Borrego F NK cell metabolism and tumor microenvironment. Front. Immunol 10, 2278 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parodi M et al. Hypoxia modifies the transcriptome of human NK cells, modulates their immunoregulatory profile, and influences NK cell subset migration. Front Immunol. 9, 2358 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doubrovina ES et al. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J. Immunol 171, 6891–6899 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Wu JD et al. Prevalent expression of the immunostimulatory MHC class I chain-related molecule is counteracted by shedding in prostate cancer. J. Clin. Invest 114, 560–568 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raulet DH, Gasser S, Gowen BG, Deng W & Jung H Regulation of ligands for the NKG2D activating receptor. Annu. Rev. Immunol 31, 413–441 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrari de Andrade L et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science 359, 1537–1542 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bufler P et al. A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc. Natl Acad. Sci. USA 99, 13723–13728 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarhan D et al. Adaptive NK cells resist regulatory T-cell suppression driven by IL37. Cancer Immunol. Res 6, 766–775 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molgora M et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and antiviral activity. Nature 551, 110–114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delconte RB et al. CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nat. Immunol 17, 816–824 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Veluchamy JP et al. The rise of allogeneic natural killer cells as a platform for cancer immunotherapy: recent innovations and future developments. Front. Immunol 8, 631 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parkhurst MR, Riley JP, Dudley ME & Rosenberg SA Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin. Cancer Res 17, 6287–6297 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henig I & Zuckerman T Hematopoietic stem cell transplantation—50 years of evolution and future perspectives. Rambam Maimonides Med. J 5, e0028 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luznik L et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol. Blood Marrow Transpl 14, 641–650 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruggeri L et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295, 2097–2100 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Miller JS et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105, 3051–3057 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Bachanova V et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood 123, 3855–3863 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mills CD & North RJ Expression of passively transferred immunity against an established tumor depends on generation of cytolytic T cells in recipient. Inhibition suppressor T cells. J. Exp. Med 157, 1448–1460 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sorror ML et al. Comparing morbidity and mortality of HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative and myeloablative conditioning: influence of pretransplantation comorbidities. Blood 104, 961–968 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Dalle J-H et al. Characterization of cord blood natural killer cells: implications for transplantation and neonatal infections. Pediatric Res. 57, 649–655 (2005). [DOI] [PubMed] [Google Scholar]
- 51.Luevano M et al. The unique profile of cord blood natural killer cells balances incomplete maturation and effective killing function upon activation. Hum. Immunol 73, 248–257 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Tanaka H et al. Analysis of natural killer (NK) cell activity and adhesion molecules on NK cells from umbilical cord blood. Eur. J. Haematol 71, 29–38 (2003). [DOI] [PubMed] [Google Scholar]
- 53.Spanholtz J et al. Clinical-grade generation of active NK cells from cord blood hematopoietic progenitor cells for immunotherapy using a closed-system culture process. PLoS One 6, e20740 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arai S et al. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy 10, 625–632 (2008). [DOI] [PubMed] [Google Scholar]
- 55.Gong JH, Maki G & Klingemann HG Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 8, 652–658 (1994). [PubMed] [Google Scholar]
- 56.Suck G et al. NK-92: an ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol. Immunother 65, 485–492 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jochems C et al. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget 7, 86359–86373 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knorr DA et al. Clinical-scale derivation of natural killer cells from human pluripotent stem cells for cancer therapy. Stem Cells Transl Med. 2, 274–283 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woll PS et al. Human embryonic stem cells differentiate into a homogeneous population of natural killer cells with potent in vivo antitumor activity. Blood 113, 6094–6101 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hermanson DL et al. Induced pluripotent stem cell-derived natural killer cells for treatment of ovarian cancer. Stem Cells 34, 93–101 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagai Y et al. CD38 knockout primary NK cells to prevent fratricide and boost daratumumab activity [abstract]. Blood 134 (Suppl. 1), 870 (2019). [Google Scholar]
- 62.Schuster SJ et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med 380, 45–56 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Maude SL et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med 378, 439–448 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neelapu SS et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med 377, 2531–2544 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shah NN & Fry TJ Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol 16, 372–385 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.[No authors listed] Natural killer cells for cancer immunotherapy: a new CAR is catching up. EBioMedicine 39, 1–2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu E et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 32, 520–531 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu E et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med 382, 545–553 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carlsten M & Childs RW Genetic manipulation of NK cells for cancer immunotherapy: techniques and clinical implications. Front. Immunol 6, 266 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Y, Hermanson DL, Moriarity BS & Kaufman DS Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell 23, 181–192.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fujisaki H et al. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 69, 4010–4017 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Denman CJ et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE 7, e30264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ojo EO et al. Membrane bound IL-21 based NK cell feeder cells drive robust expansion and metabolic activation of NK cells. Sci. Rep 9, 1–12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Paust S, Blish CA & Reeves RK Redefining memory: building the case for adaptive NK cells. J. Virol 91, e00169–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rölle A, Meyer M, Calderazzo S, Jäger D & Momburg F Distinct HLA-E peptide complexes modify antibody-driven effector functions of adaptive NK cells. Cell Rep. 24, 1967–1976.e4 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Cichocki F et al. CD56dimCD57+NKG2C+NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia 30, 456–463 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cichocki F et al. GSK3 inhibition drives maturation of NK cells and enhances their antitumor activity. Cancer Res. 77, 5664–5675 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berrien-Elliott MM, Wagner JA & Fehniger TA Human cytokine-induced memory-like (CIML) NK cells. J. Innate Immun 7, 563–571 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Romee R et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl Med 8, 357ra123 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams R et al. Role of recipient CD8+ T cell exhaustion in the rejection of adoptively transferred haploidentical NK cells. Blood 128, 503 (2016). [Google Scholar]
- 81.Grimm EA, Mazumder A, Zhang HZ & Rosenberg SA Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J. Exp. Med 155, 1823–1841 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lotze MT et al. Lysis of fresh and cultured autologous tumor by human lymphocytes cultured in T-cell growth factor. Cancer Res. 41, 4420–4425 (1981). [PubMed] [Google Scholar]
- 83.Mule JJ, Shu S, Schwarz SL & Rosenberg SA Adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant interleukin-2. Science 225, 1487–1489 (1984). [DOI] [PubMed] [Google Scholar]
- 84.Rosenberg SA, Mulé JJ, Spiess PJ, Reichert CM & Schwarz SL Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J. Exp. Med 161, 1169–1188 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lotze MT, Line BR, Mathisen DJ & Rosenberg SA The in vivo distribution of autologous human and murine lymphoid cells grown in T cell growth factor (TCGF): implications for the adoptive immunotherapy of tumors. J. Immunol 125, 1487–1493 (1980). [PubMed] [Google Scholar]
- 86.Rosenberg SA et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med 313, 1485–1492 (1985). [DOI] [PubMed] [Google Scholar]
- 87.Phillips JH, Gemlo BT, Myers WW, Rayner AA & Lanier LL In vivo and in vitro activation of natural killer cells in advanced cancer patients undergoing combined recombinant interleukin-2 and LAK cell therapy. J. Clin. Oncol 5, 1933–1941 (1987). [DOI] [PubMed] [Google Scholar]
- 88.Hercend T et al. Characterization of natural killer cells with antileukemia activity following allogeneic bone marrow transplantation. Blood 67, 722–728 (1986). [PubMed] [Google Scholar]
- 89.Burns LJ et al. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I/II trial. Bone Marrow Transpl. 32, 177–186 (2003). [DOI] [PubMed] [Google Scholar]
- 90.Miller JS, Prosper F & McCullar V Natural killer (NK) cells are functionally abnormal and NK cell progenitors are diminished in granulocyte colony- stimulating factor-mobilized peripheral blood progenitor cell collection. Blood 90, 3098–3105 (1997). [PubMed] [Google Scholar]
- 91.Soiffer RJ, Murray C, Gonin R & Ritz J Effect of low-dose interleukin-2 on disease relapse after T-cell depleted allogeneic bone marrow transplantation. Blood 84, 964–971 (1994). [PubMed] [Google Scholar]
- 92.Smith KA Interleukin-2: inception, impact, and implications. Science 240, 1169–1176 (1988). [DOI] [PubMed] [Google Scholar]
- 93.Rosenberg SA et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N. Engl. J. Med 316, 889–897 (1987). [DOI] [PubMed] [Google Scholar]
- 94.Shah MH et al. A phase I study of ultra low dose interleukin-2 and stem cell factor in patients with HIV infection or HIV and cancer. Clin. Cancer Res 12, 3993–3996 (2006). [DOI] [PubMed] [Google Scholar]
- 95.Zorn E et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT- dependent mechanism and induces the expansion of these cells in vivo. Blood 108, 1571–1579 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hirakawa M et al. Low-dose IL-2 selectively activates subsets of CD4+ Tregs and NK cells. JCI Insight 1, e89278 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Malek TR The biology of interleukin-2. Annu. Rev. Immunol 26, 453–479 (2008). [DOI] [PubMed] [Google Scholar]
- 98.Grabstein KH et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science 264, 965–968 (1994). [DOI] [PubMed] [Google Scholar]
- 99.Carson WE et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med 180, 1395–1403 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dubois S, Mariner J, Waldmann TA & Tagaya Y IL-15Rα recycles and presents IL-15 in trans to neighboring cells. Immunity 17, 537–547 (2002). [DOI] [PubMed] [Google Scholar]
- 101.Bergamaschi C et al. Circulating IL-15 exists as heterodimeric complex with soluble IL-15Rα in human and mouse serum. Blood 120, e1–e8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kobayashi H et al. Role of trans-cellular IL-15 presentation in the activation of NK cell- mediated killing, which leads to enhanced tumor immunosurveillance. Blood 105, 721–727 (2005). [DOI] [PubMed] [Google Scholar]
- 103.Tang F et al. Activity of recombinant human interleukin-15 against tumor recurrence and metastasis in mice. Cell Mol. Immunol 5, 189–196 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Klebanoff CA et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc. Natl Acad. Sci. USA 101, 1969–1974 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cheever MA Twelve immunotherapy drugs that could cure cancers. Immunol. Rev 222, 357–368 (2008). [DOI] [PubMed] [Google Scholar]
- 106.Conlon KC et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J. Clin. Oncol 33, 74–82 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miller JS et al. A first-in-human phase I study of subcutaneous outpatient recombinant human IL15 (rhIL15) in adults with advanced solid tumors. Clin. Cancer Res 24, 1525–1535 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cooley S et al. First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv. 3, 1970–1980 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu W et al. Efficacy and mechanism-of-action of a novel superagonist interleukin-15: interleukin-15 receptor αSu/Fc fusion complex in syngeneic murine models of multiple myeloma. Cancer Res. 73, 3075–3086 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim PS et al. IL-15 superagonist/IL-15RαSushi-Fc fusion complex (IL-15SA/IL-15RαSu-Fc; ALT-803) markedly enhances specific subpopulations of NK and memory CD8+ T cells, and mediates potent anti-tumor activity against murine breast and colon carcinomas. Oncotarget 7, 16130–16145 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wrangle JM et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open- label, phase 1b trial. Lancet Oncol. 19, 694–704 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fehniger TA et al. First-in-human phase I combination of the IL-15 receptor super agonist complex ALT-803 with a therapeutic (anti-CD20) monoclonal antibody (mAb) for patients with relapsed or refractory indolent non-Hodgkin lymphoma (iNHL) [abstract]. Cancer Res. 78 (Suppl. 13), CT146 (2018). [Google Scholar]
- 113.Melaiu O, Lucarini V, Cifaldi L & Fruci D Influence of the tumor microenvironment on NK cell function in solid tumors. Front. Immunol 10, 3038 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vallera DA et al. IL15 trispecific killer engagers (TriKE) make natural killer cells specific to CD33+ targets while also inducing persistence, in vivo expansion, and enhanced function. Clin. Cancer Res 22, 3440–3450 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sarhan D et al. 161533 TriKE stimulates NK-cell function to overcome myeloid-derived suppressor cells in MDS. Blood Adv. 2, 1459–1469 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gauthier L et al. Multifunctional natural killer cell engagers targeting nkp46 trigger protective tumor immunity. Cell 177, 1701–1713.e16 (2019). [DOI] [PubMed] [Google Scholar]
- 117.Genetic Engineering & Biotechnology News. Merck & Co. partners with Dragonfly on NK-based cancer immunotherapies. GEN https://www.genengnews.com/news/merck-co-partners-with-dragonfly-on-nk-based-cancer-immunotherapies (2018). [Google Scholar]
- 118.André P et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell 175, 1731–1743.e13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Romagné F et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood 114, 2667–2677 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Benson DM et al. IPH2101, a novel anti-inhibitory KIR antibody, and lenalidomide combine to enhance the natural killer cell versus multiple myeloma effect. Blood 118, 6387–6391 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Korde N et al. A phase II trial of pan-KIR2D blockade with IPH2101 in smoldering multiple myeloma. Haematologica 99, e81–e83 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carlsten M et al. Checkpoint inhibition of KIR2D with the monoclonal antibody IPH2101 induces contraction and hyporesponsiveness of NK cells in patients with myeloma. Clin. Cancer Res 22, 5211–5222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vey N et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood 120, 4317–4323 (2012). [DOI] [PubMed] [Google Scholar]
- 124.Armand P et al. A phase 1b study of dual PD-1 and CTLA-4 or KIR blockade in patients with relapsed/ refractory lymphoid malignancies. Leukemia 10.1038/s41375-020-0939-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bristol Myers Squibb. Interim phase 1/2 data show encouraging clinical benefit for lirilumab in combination with Opdivo (nivolumab) in patients with advanced platinum refractory squamous cell carcinoma of the head and neck. BMS https://news.bms.com/press-release/bristolmyers/interim-phase-12-data-show-encouraging-clinical-benefit-lirilumab-combina (2016). [Google Scholar]
- 126.Bevelacqua V et al. Nectin like-5 overexpression correlates with the malignant phenotype in cutaneous melanoma. Oncotarget 3, 882–892 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gao J, Zheng Q, Xin N, Wang W & Zhao C CD155, an onco-immunologic molecule in human tumors. Cancer Sci. 108, 1934–1938 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang Q et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol 19, 723–732 (2018). [DOI] [PubMed] [Google Scholar]
- 129.Sun H et al. Human CD96 correlates to natural killer cell exhaustion and predicts the prognosis of human hepatocellular carcinoma. Hepatology 70, 168–183 (2019). [DOI] [PubMed] [Google Scholar]
- 130.Blake SJ et al. Suppression of metastases using a new lymphocyte checkpoint target for cancer immunotherapy. Cancer Discov. 6, 446–459 (2016). [DOI] [PubMed] [Google Scholar]
- 131.Dong W et al. The mechanism of anti-PD-L1 antibody efficacy against PD-L1 negative tumors identifies NK cells expressing PD-L1 as a cytolytic effector. Cancer Discov. 9, 1422–1437 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Juliá EP, Amante A, Pampena MB, Mordoh J & Levy EM Avelumab, an IgG1 anti-PD-L1 immune checkpoint inhibitor, triggers NK cell-mediated cytotoxicity and cytokine production against triple negative breast cancer cells. Front. Immunol 9, 2140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Benson DM Jr et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 116, 2286–2294 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Beldi-Ferchiou A et al. PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget 7, 72961–72977 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pesce S et al. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: a phenotypic and functional characterization. J. Allergy Clin. Immunol 139, 335–346.e3 (2017). [DOI] [PubMed] [Google Scholar]
- 136.Li Y et al. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol. Cancer 15, 55 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vari F et al. Immune evasion via PD-1/PD-L1 on NK-cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL. Blood 131, 1809–1819 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hsu J et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Invest 128, 4654–4668 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu Y et al. Increased expression of programmed cell death protein 1 on NK cells inhibits NK-cell-mediated anti-tumor function and indicates poor prognosis in digestive cancers. Oncogene 36, 6143–6153 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Türeci Ö, Schmitt H, Fadle N, Pfreundschuh M & Sahin U Molecular definition of a novel human galectin which is immunogenic in patients with Hodgkin’s disease. J. Biol. Chem 272, 6416–6422 (1997). [DOI] [PubMed] [Google Scholar]
- 141.Folgiero V et al. TIM-3/Gal-9 interaction induces IFNγ-dependent IDO1 expression in acute myeloid leukemia blast cells. J. Hematol. Oncol 8, 36 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xu L et al. Increased Tim-3 expression in peripheral NK cells predicts a poorer prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Int. Immunopharmacol 29, 635–641 (2015). [DOI] [PubMed] [Google Scholar]
- 143.da Silva IP et al. Reversal of NK cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol. Res 2, 410–422 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Triebel F et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med 171, 1393–1405 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Baixeras E et al. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J. Exp. Med 176, 327–337 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huard B, Prigent P, Tournier M, Bruniquel D & Triebel F CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur. J. Immunol 25, 2718–2721 (1995). [DOI] [PubMed] [Google Scholar]
- 147.Khan M, Arooj S & Wang H NK cell-based immune checkpoint inhibition. Front. Immunol 11, 167 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Workman CJ & Vignali DAA Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223). J. Immunol 174, 688–695 (2005). [DOI] [PubMed] [Google Scholar]
- 149.Woo S-R et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 72, 917–927 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Blackburn SD et al. Coregulation of CD8+ T cell exhaustion during chronic viral infection by multiple inhibitory receptors. Nat. Immunol 10, 29–37 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Maçon-Lemaître L & Triebel F The negative regulatory function of the lymphocyte-activation gene- 3 co-receptor (CD223) on human T cells. Immunology 115, 170–178 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huard B, Tournier M & Triebel F LAG-3 does not define a specific mode of natural killing in human. Immunol. Lett 61, 109–112 (1998). [DOI] [PubMed] [Google Scholar]
- 153.Taborda NA et al. Short communication: low expression of activation and inhibitory molecules on NK cells and CD4+ T cells is associated with viral control. AIDS Res. Hum. Retroviruses 31, 636–640 (2015). [DOI] [PubMed] [Google Scholar]
- 154.Miyazaki T, Dierich A, Benoist C & Mathis D Independent modes of natural killing distinguished in mice lacking Lag3. Science 272, 405–408 (1996). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.