Abstract
Background
With the onset of the COVID-19 pandemic in 2020, hospital clinical teams have realised that there is a need for a rapid, accurate testing facility that will allow them to move patients quickly into isolation rooms or specific COVID-19 cohort wards as soon as possible after admission.
Methods
Starting from July 2020, PCR-based test platforms, which could test 4–8 samples in parallel with turnaround (sample-to-result) times of 50–80 min, were placed in a satellite laboratory. This laboratory was on the same floor and within walking distance to the acute respiratory admissions ward. It was staffed by a team of three mid-Band 4 staff that split a 0700–2200 h-work day, 7 days a week, with 2 senior supervisors. Urgent sample testing was decided upon by the clinical teams and requested by phone. The test results were entered manually in real-time as they became available, and sent electronically to the requesting ward teams.
Results
The daily/monthly PCR positive test numbers approximately followed the local and national UK trend in COVID-19 case numbers, with the daily case numbers being reflective of the November and December 2020 surges. Test results were used to rapidly segregate positive patients into dedicated COVID-19 ward areas to minimise risk of potential nosocomial transmission in crowded waiting areas. Testing capacity was sufficient to include cases with uncertain diagnosis likely to require hospital admission. Following completion of other admission processes, based on these rapid test results, patients were allocated to dedicated COVID-19 positive or negative cohort wards.
Conclusions
This rapid testing facility reduced unnecessary ‘length-of-stay’ in a busy acute respiratory ward. In the current absence of a treatment for mild-to-moderate COVID-19, on which patients could be discharged home to complete, the rapid test facility has become a successful aid to patient flow and reduced exposure and nosocomial transmission.
Keywords: SARS-CoV-2, COVID-19, Rapid testing, Triage, Patient flow, Nosocomial transmission, Infection control, Diagnostics
Introduction
With the onset of the COVID-19 pandemic in 2020, hospital clinical teams have realised that there is a need for a rapid, accurate testing facility that will allow them to move patients quickly into isolation rooms or specific COVID-19 cohort wards as soon as possible after admission, to reduce the risk of transmission to non-COVID-19 patients, as well as to staff and visitors (Brendish et al., 2020), similar to what is now done in some hospitals for seasonal influenza (Public Health England, 2019a).
This led to the development of an on-site rapid diagnostic service, run by laboratory-trained staff, who could receive a limited daily number of urgent samples for immediate testing.
This satellite rapid-testing laboratory model may be of particular use in hospitals that are spread over several geographical sites where all diagnostic laboratory services are based only at one site.
Materials and methods
Testing platforms and assays
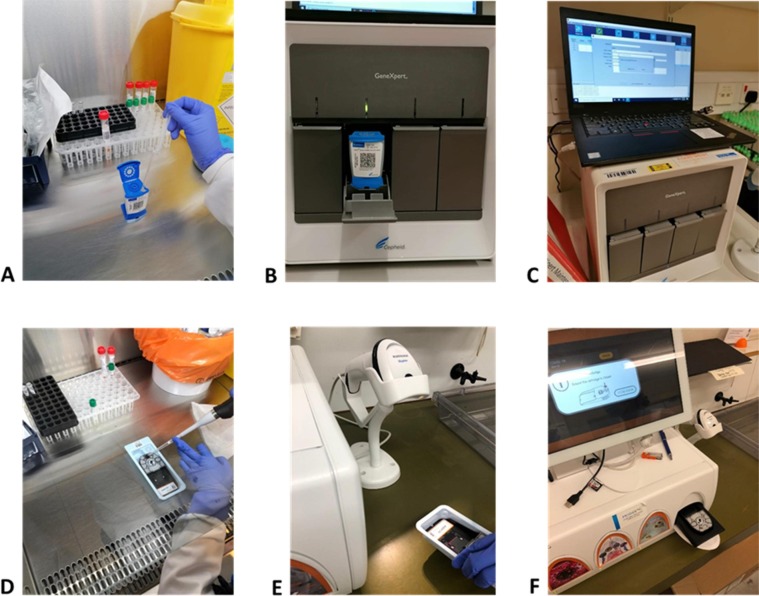
Starting from July 2020, initially, a single PCR-based test platform (Cepheid GeneXpert, Woodburn Green, Bucks., UK) was used, targeting the SARS-CoV-2 N2 and E genes (Fig. 1 A-C). This allowed up to 8 tests to be performed at the same time, with a turnaround time of about 52 min, by a single operator. Initial daily test quotas were limited to 30–33, and it was left to admitting ward how to prioritise the testing for their patients.
Fig. 1.
(A-C): Cepheid GeneXpert testing process, showing (from left to right), the pipetting of the swab VTM sample into the GeneXpert test cartridge, which is then loaded into the GeneXpert platform, and the output. (D-F): MobiDiag testing process showing (from left to right), the pipetting of the mNAT medium mixed with the swab VTM sample into the Novodiag test cartridge, which is then loaded into the Novodiag platform, and the output.
The sensitivity of the Cepheid was 100% compared to the standard laboratory test (AusDiagnostics SARS-CoV-2 PCR assay, AusDiagnostics Ltd., Chesham, UK) (Attwood et al., 2020). This sensitivity score of 100% is the same as that reported by a recent Cochrane study using the same Cepheid GeneXpert Xpress platform and assay (Dinnes et al., 2020). Therefore there was no further confirmatory testing using the laboratory-based (AusDiagnostics) assay, which would have delayed the reporting of any positive results.
Later on (December 2020), to increase the testing capacity, a second platform was available (NovoDiag/MobiDiag, High Wycombe, Bucks., UK) targeting the SARS-CoV-2N and ORF1ab genes (Fig. 1 D-F). This was another PCR-based platform with a longer turnaround time of 1 h 20 min that allowed up to 4 tests to be performed at the same time.
Samples
Both platforms utilised respiratory (oro-naso-pharyngeal) swabs collected into standard virus transport media (VTM) (Virocult, MWE, Corsham, UK and/or Remel, ThermoFisher, UK). Initially, on the Cepheid, only SARS-CoV-2 was the test target, but later an updated cartridge also targeting influenza A and B and RSV was substituted.
Staff
The laboratory team consisted of three mid-Band 4 staff that split a 0700–2200 h-work day, 7 days a week, with 2 senior supervisors. The test results were manually inputted in real-time as and when ready (i.e. not in batches at fixed time intervals) into an electronic laboratory information system (LIS) that sent the results back to the ward teams who requested the testing. The overall staff costs were approximately £7667/month, at this time, which included pension, national insurance and overtime contributions
Results
The daily/monthly PCR positive test numbers approximately followed the local and national UK trend in COVID-19 case numbers, with the daily case numbers being reflective of the Nov and Dec 2020 surges (Fig. 2 ).
Fig. 2.
Daily, monthly positive rate for the rapid test (‘rapid’) compared to the local hospital (‘UHL’) positivity rate, showing a similar trend.
Between March 2020 and February 2021 an average of 1726 patients were seen on the Clinical Decision Unit (CDU) per month, of which 264/month were COVID positive. The average length of stay (LOS) on the unit was 15 h.
Test results were used to rapidly segregate positive patients into dedicated COVID-19 ward areas to minimise risk of potential nosocomial transmission in crowded waiting areas. Testing capacity was sufficient to include cases with uncertain diagnosis likely to require hospital admission. Following completion of admission processes patients were allocated to dedicated COVID-19 positive or negative downstream wards.
Discussion
Rapid diagnostic testing for other viruses, such as seasonal influenza have shown benefits in earlier initiation of therapy, patient triage and cohorting (You et al., 2017, Mac et al., 2020, Melhuish et al., 2020), and even possible earlier discharge in those at low risk of related complications who are able to complete their antiviral treatment (e.g. oseltamivir, zanamivir), at home.
In fact, with seasonal influenza, which has a safe antiviral treatment option for even moderate disease, empirical treatment is recommended (Mac et al., 2020, Melhuish et al., 2020, Public Health England, 2019b), and is often cheaper than the cost of an initial rapid molecular test (e.g. 10 days oseltamivir costs: ~£15, cost of rapid PCR test ~£20–40). The risk with purely empirical treatment is that some of this treatment will be used on non-influenza infections, and this ‘wasted’ treatment cost may increase the overall expense of the empirical treatment approach versus a more targeted ‘rapid test and treat’ approach.
For COVID-19, the identification of potentially infectious cases currently facilitates management in dedicated ward areas and prevents cross-transmission in potentially crowded waiting areas through reduced LOS. In the present absence of a treatment suitable for mild to moderate COVID-19, on which patients could be discharged home to complete, the rapid test facility essentially becomes an aid to patient flow and reduced nosocomial transmission.
Calculating the precise cost-effectiveness of such rapid testing facilities is challenging and beyond the scope of this paper. However, for acute NHS services, the costed patient event is the episode – which can include an aggregate of the costs incurred by bed day. This cost will have a currency of a healthcare resource group code (HRG) and any episode-related cost will be driven by the exact time spent on individual wards. Wards in turn may differ in their cost due to the average level of acuity, where acuity describes the level of resource a patient uses due to their condition.
Reduced waiting for test results in acute admission areas is therefore highly likely to reduce costs associated with time spent in such environments. It is also likely that further cost benefits will accrue due to reduction of nosocomial COVID-19 infections and any related associated downstream costs of such events. This estimated cost benefit may well change further over time, as new antiviral options are developed, and as the COVID-19 vaccine rollout continues.
Further into the future, if the virus becomes more seasonal and causes milder disease (Phillips, 2021, Murray and Piot, 2021), a more empirical treatment approach may work, as with seasonal influenza.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Attwood L.O., Francis M.J., Hamblin J., Korman T.M., Druce J., Graham M. Clinical evaluation of AusDiagnostics SARS-CoV-2 multiplex tandem PCR assay. J. Clin. Virol. 2020;128:104448. doi: 10.1016/j.jcv.2020.104448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brendish N.J., Poole S., Naidu V.V., Mansbridge C.T., Norton N.J., Wheeler H., Presland L., Kidd S., Cortes N.J., Borca F., Phan H., Babbage G., Visseaux B., Ewings S., Clark T.W. Clinical impact of molecular point-of-care testing for suspected COVID-19 in hospital (COV-19POC): a prospective, interventional, non-randomised, controlled study. Lancet Respir. Med. 2020;8(12):1192–1200. doi: 10.1016/S2213-2600(20)30454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnes J., Deeks J.J., Adriano A., et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2020;8(8):CD013705. doi: 10.1002/14651858.CD013705. Update in: Cochrane Database Syst. Rev. 2021 Mar 24;3:CD013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac S., O’Reilly R., Adhikari N.K.J., Fowler R., Sander B., Kalendar R. Point-of-care diagnostic tests for influenza in the emergency department: A cost-effectiveness analysis in a high-risk population from a Canadian perspective. PLoS ONE. 2020;15(11):e0242255. doi: 10.1371/journal.pone.0242255.r006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhuish A., Vargas-Palacios A., Yaziji N., Selfridge J., Pisavadia M., Sagoo G.S., Minton J. Cost evaluation of point-of-care testing for community-acquired influenza in adults presenting to the emergency department. J. Clin. Virol. 2020;129:104533. doi: 10.1016/j.jcv.2020.104533. [DOI] [PubMed] [Google Scholar]
- Murray C.J.L., Piot P. The Potential Future of the COVID-19 Pandemic: Will SARS-CoV-2 Become a Recurrent Seasonal Infection? JAMA. 2021;325(13):1249–1250. doi: 10.1001/jama.2021.2828. [DOI] [PubMed] [Google Scholar]
- Phillips N. The coronavirus is here to stay – here's what that means. Nature. 2021;590(7846):382–384. doi: 10.1038/d41586-021-00396-2. [DOI] [PubMed] [Google Scholar]
- Public Health England, 2019. Point of care tests for influenza and other respiratory viruses. Updated 28 October 2019. https://www.gov.uk/government/publications/point-of-care-tests-for-influenza-and-other-respiratory-viruses (accessed 9 June, 2021).
- Public Health England, 2019. Guidance on use of antiviral agents for the treatment and prophylaxis of seasonal influenza. September 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/833572/PHE_guidance_antivirals_influenza_201920.pdf (accessed 9 June, 2021).
- You J.H.S., Tam L.-P., Lee N.L.S., Qiu C. Cost-effectiveness of molecular point-of-care testing for influenza viruses in elderly patients at ambulatory care setting. PLoS ONE. 2017;12(7):e0182091. doi: 10.1371/journal.pone.0182091.t003. [DOI] [PMC free article] [PubMed] [Google Scholar]