Abstract
A 22-year-old woman presented to the emergency room with right lower abdominal pain. A CT scan suggested potential appendicitis and perforation. She had no relevant medical or surgical history, and she last had vaginal sex 4 years prior to admission. During surgery, turbid fluid, secondary inflammatory changes, and dilated, fluid-filled fallopian tubes pointed to a diagnosis of pelvic inflammatory disease (PID), so she was started on azithromycin, metronidazole and piperacillin/tazobactam. The following day, she continued to have abdominal pain and developed tachycardia, hypotension, a marked leukemoid response, haemoconcentration, third space fluid accumulation and acidosis. Culture results led to her being further diagnosed with Clostridium perfringens PID with peritonitis and toxic shock syndrome. A gynaecological infection of C. perfringens leading to toxic shock syndrome is both extremely rare and highly fatal. Her antibiotics were changed to meropenem and clindamycin, and she slowly made a full recovery.
Keywords: obstetrics and gynaecology, general surgery, pelvic inflammatory disease, infectious diseases
Background
Clostridium is an important Gram-positive anaerobic pathogen in humans, and Clostridium perfringens is the most common isolated species.1 Clostridial toxic shock caused by C. perfringens or C. sordellii is a rare and generally fatal condition among pregnant and non-pregnant reproductive-age women with genital tract infections. Recent gynaecological cases have carried a grave prognosis, with an overall mortality rate of over 95% in known patients.2 In comparison with other anaerobic bacteria, C. perfringens is rarely associated with pelvic inflammatory disease (PID) and other infections of the genital tract of women.3
PID is an infection of the upper genital tract that occurs primarily in young, sexually active women. PID encompasses a wide array of infectious processes of the endometrium, fallopian tubes, ovaries and pelvic peritoneum.4 Once diagnosed, prompt treatment of PID is critical as a delay in treatment increases the risk of infertility, ectopic pregnancy and sepsis.5 6
Case presentation
The patient was a 22-year-old woman with no significant medical history who presented to the emergency room with abdominal pain in her right lower quadrant that began at 16:00 1 day prior to admission. The abdominal pain was constant throughout the night, which prompted her to visit a freestanding emergency room. During the visit, a CT scan was performed that reported possible appendicitis with perforation. She was then transported to the emergency department of a local hospital. Her abdominal pain was associated with a mild fever, but she denied chills, nausea, vomiting, diarrhoea, chronic abdominal pain, dysuria, bleeding or any other genitourinary symptoms. She had no significant medical or surgical history and had an allergy to fentanyl. She was a gravida 0 and last had vaginal sexual intercourse 4 years prior to admission; she denied vaginal douching. Her last menstrual period was 2 weeks prior to admission. On physical examination, her abdomen was soft and diffusely tender with guarding; no rebound tenderness was appreciated. The rest of her physical examination was unremarkable.
Investigations
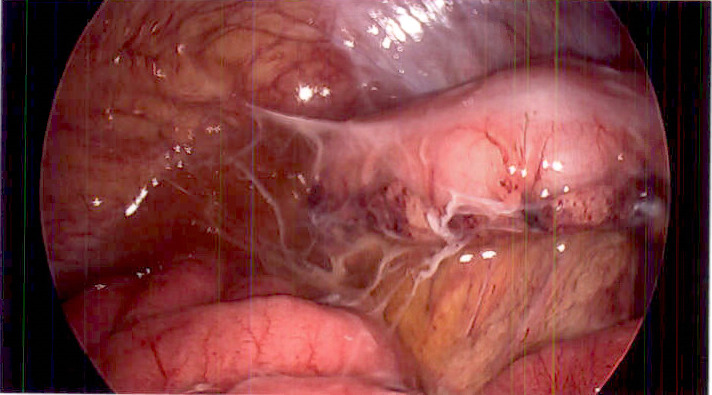
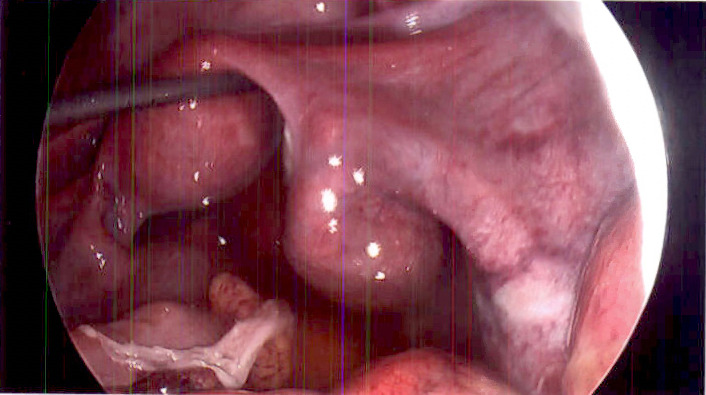
The patient was promptly taken to surgery where she underwent a laparoscopic appendectomy for suspected ruptured appendicitis. During surgery, her appendix was removed and was found to have secondary inflammatory changes with no evidence of rupture. Erythema was found on the small bowel peritoneal lining and continued down to her pelvis and over her bladder and uterus. There was also evidence of peritonitis with no significant purulence; however, a turbid-appearing fluid was found throughout the abdomen (figure 1). The fallopian tubes were dilated and fluid-filled, which suggested the possibility of PID (figure 2). No tubo-ovarian abscesses were visualised. The appendix was sent to pathology, and a culture of the abdominal fluid was obtained before abdominal irrigation and closure. The pathology report confirmed acute appendicitis and did not include any mention of appendix rupture. Based on the findings from the laparoscopic appendectomy, primary PID was suspected.
Figure 1.
Turbid peritoneal fluid and global visceral oedema with filmy adhesions.
Figure 2.
Laparoscopic uterus with dilated distal fallopian tubes.
Treatment
Due to the suspected diagnosis of PID, the on-call obstetrics and gynaecology physician was consulted; the patient was placed on intravenous azithromycin (1000 mg), metronidazole (500 mg) and piperacillin/tazobactam (3.375 g). That evening, her white blood cell count was 46.3×109/L, red blood cell count was 7.10×1012/L, haemoglobin was 221 g/L, haematocrit was 71.0% and lactic acid was 9.2 mmol/L (table 1). The following morning (postoperative day 1), her white blood cell count was 49.7×109/L, red blood cell count was 7.25×1012/L, manual haematocrit was 68%, neutrophils were 82% and bands were 10%. Her temperature was 37.8°C, pulse was 147 beats/min and blood pressure was 104/64 mm Hg. She reported lower abdominal pain (6/10), nausea, vomiting and an episode of syncope when using the bathroom. Due to her worsening labs and symptoms, systemic inflammatory response syndrome was suspected, and the infectious disease physician was consulted. Early culture growth showed a Gram-positive rod which led to the suspected diagnosis of PID with peritonitis and toxic shock syndrome due to either C. sordellii or C. perfringens. Her antibiotics were then switched to meropenem (1 g) and clindamycin (900 mg). Later that evening, the patient went into shock and was found to be acidotic with a blood pH of 7.06; her serum lactate level was 5.7 mmol/L. She was intubated, started on vasopressors and was aggressively volume resuscitated. Two days later, conclusive culture results confirmed C. perfringens; the specific strain of C. perfringens was not able to be determined.
Table 1.
Laboratory results of the patient from presentation to predischarge with reference ranges
| Variable | Day of surgery | Postoperative day 1 | Postoperative day 27 | Reference range |
| White blood cells ×109/L | 46.3 | 49.7 | 8.1 | 4.5–11.0 |
| Red blood cells ×1012/L | 7.1 | 7.25 | 2.44 | 3.5–5.5 |
| Haemoglobin (g/L) | 221 | – | 76 | 120–160 |
| Haematocrit (%) | 71 | 68 | 22.5 | 36–46 |
| Neutrophil (%) | – | 82 | 72 | 54–62 |
| Bands (%) | – | 10 | 5 | 3–5 |
| Lactic acid (mmol/L) | 9.2 | 5.7 | – | 0.5–2.2 |
Outcome and follow-up
During the rest of her hospital visit, the patient developed volume overload and significant third spacing from fluid resuscitation. She also developed acute renal failure as reflected by the rise of her serum creatinine to a maximum value of 6.95 mg/dL; this prompted initiation of dialysis. Fortunately, she was later taken off of pressors and dialysis and extubated. The medical disciplines, which participated in this patient’s care included: general surgery, gynaecology, critical care medicine/pulmonology, infectious diseases and nephrology. She slowly gained back her strength and was discharged 29 days after admission. After discharge, the patient continued to regain her strength and was functioning near her initial baseline 3 months after initial presentation.
Discussion
The pathogen identified by pelvic cavity fluid culture was C. perfringens. In this case, the source of the infection and origin of the bacteria is not clear. As C. perfringens can be a naturally occurring anaerobic member of the gastrointestinal microbiota, it is biologically plausible that this bacterium originated from the gastrointestinal tract (possibly the appendix), and that a microperforation led to seeding of the adjacent internal reproductive structures.7 Many gynaecological conditions can mimic acute appendicitis, and this makes the definitive diagnosis unclear. Pelvic pathology may also be confused with other intra-abdominal disease processes.8 As a result, clinically differentiating acute appendicitis from PID remains difficult. Because of this, the theory of a gastrointestinal origin of the bacteria cannot be confirmed or refuted. A perforated appendix was not suspected intraoperatively. The patient arrived from an outside facility where a CT of the abdomen and pelvis suggested a perforated appendix due to ‘fat stranding changes’ in the appendiceal area. However, at the time of surgery, there was no gross evidence of appendiceal rupture or infection. As stated by the primary general surgeon in the operative report, ‘…there was no obvious perforation to the appendix and peeling it up and off the pelvic side wall to see it. There was significant inflammatory change that was secondary to it.’ At laparoscopic survey, the peritoneal fluid appeared turbid with global peritoneal oedema and erythema that was grossly visible on the visceral surfaces (figure 1). Bilateral swollen distal fallopian tubes with no overt hydrosalpinges were also visualised (figure 2). While the pathological examination of the appendix resulted in a diagnosis of ‘acute appendicitis’, there was no gross or microscopic evidence of perforation, nor was a hard faecalith found at cross-section. It is hypothesised that appendicitis caused by C. perfringens would present as a grossly affected appendix with likely gangrenous change, but this was not observed at the time of surgery. Alternatively, it is also possible that the appendiceal inflammation was reactionary (secondary) as a response to the infected tube.
This patient did not have any other known risk factors for a C. perfringens infection. Most cases documented in medical literature follow various pregnancy outcomes, including full-term birth, stillbirth, spontaneous abortion and induced abortion; it is rarely found in non-pregnant women as seen in this presented case.2 3 The patient last had vaginal penetrative sex 4 years prior to presentation, and she did not possess an intrauterine device. She did not report recent tampon use. Nonetheless, as C. perfringens may be carried vaginally in 3%–18% of healthy women, it is biologically plausible that an ascending infection occurred at the precise opportune time to result in PID.2 Although we cannot definitively pinpoint the origin of infection (gastrointestinal or genitourinary tract), this case highlights the importance of recognising the possibility of this type of infection in a deteriorating patient with presumed PID. With most case reports and published literature stating a possible mortality rate between 70% and 100% with C. perfringens toxic shock, this case adds to the evidence that survival and full recovery are still possible in the otherwise healthy adult patient.9
C. perfringens is classified by strain types, and it is separated into five groups denoted by A–E. These groups are established based on the toxins each one can produce. The major toxins used for strain classification are alpha toxin, beta toxin, epsilon toxin and iota toxin.10 Unfortunately, our hospital microbiology laboratory does not routinely analyse for, nor identify, the specific toxin type. Despite direct communication with the laboratory director, this test was unable to be performed within our system. Nonetheless, treatment and supportive care provided followed published guidelines and recommendations.
C. sordellii and C. perfringens are both associated with catastrophic, undiagnosed infectious gynaecological illnesses among women of childbearing age, and C. perfringens can often be falsely diagnosed as C. sordellii.11 This is significant as one study found that 1 in 200 deaths of women of childbearing age was due to C. sordellii toxic shock syndrome (CSTS). However, in the same study, 3 of the 5 cases of CSTS originally attributed to C. sordellii were later found to be attributed to C. perfringens.11 Thus, C. perfringens remains a relevant and important pathogen to consider in young women of childbearing age. Nonetheless, the presence of C. sordellii and C. perfringens in the vagina and rectum is still relatively rare, so any form of screening or prophylactic approach to prevent infection is not recommended.2 12
The patient discussed in this case report mirrored the typical clinical signs associated with clostridial toxic shock syndrome. Typical clinical findings and symptoms include abdominal pain, tachycardia, hypotension, third space fluid accumulation, haemoconcentration and a marked leukemoid response without fever.3 Given that clostridial infections are highly fatal, clinicians should be aware of these symptoms and keep C. perfringens and C. sordellii in the differential diagnosis for cases of peritonitis, PID and toxic shock syndrome, even when the patient does not have the typical risk factors or epidemiological background.
Patient’s perspective.
The team of doctors who worked on my case and every staff member that was on my case did everything they could to get me to where I am today. I am healthy, all my lab numbers are back to normal, and had they not fought for me and done everything in their power to keep me alive, I wouldn't be here today.
Learning points.
Clostridium perfringens is an important pathogen in women of childbearing age.
Toxic shock syndrome due to C. perfringens is a rapidly progressive and fatal condition that requires prompt treatment with antibiotics and supportive measures.
C. sordellii and C. perfringens should be an early differential diagnosis in patients with peritonitis, pelvic inflammatory disease or toxic shock syndrome, even in patients who do not fit the typical clinical picture.
Footnotes
Contributors: The patient was under the care of HC with assistance from BDC. The report was written by BDC with assistance from NP under the supervision of HC. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer-reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Leal J, Gregson DB, Ross T, et al. Epidemiology of Clostridium species bacteremia in Calgary, Canada, 2000-2006. J Infect 2008;57:198–203. 10.1016/j.jinf.2008.06.018 [DOI] [PubMed] [Google Scholar]
- 2.Zane S, Guarner J. Gynecologic clostridial toxic shock in women of reproductive age. Curr Infect Dis Rep 2011;13:561–70. 10.1007/s11908-011-0207-7 [DOI] [PubMed] [Google Scholar]
- 3.Bergan T. Anaerobic bacteria as cause of infections in female genital organs. Scand J Gastroenterol Suppl 1983;85:37–47. [PubMed] [Google Scholar]
- 4.Curry A, Williams T, Penny ML. Pelvic inflammatory disease: diagnosis, management, and prevention. Am Fam Physician 2019;100:357–64. [PubMed] [Google Scholar]
- 5.Dulin JD, Akers MC. Pelvic inflammatory disease and sepsis. Crit Care Nurs Clin North Am 2003;15:63–70. 10.1016/S0899-5885(02)00031-X [DOI] [PubMed] [Google Scholar]
- 6.Hillis SD, Joesoef R, Marchbanks PA, et al. Delayed care of pelvic inflammatory disease as a risk factor for impaired fertility. Am J Obstet Gynecol 1993;168:1503–9. 10.1016/S0002-9378(11)90790-X [DOI] [PubMed] [Google Scholar]
- 7.Brook I. Microbiology and management of abdominal infections. Dig Dis Sci 2008;53:2585–91. 10.1007/s10620-007-0194-6 [DOI] [PubMed] [Google Scholar]
- 8.Boyd CA, Riall TS. Unexpected gynecologic findings during abdominal surgery. Curr Probl Surg 2012;49:195–251. 10.1067/j.cpsurg.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulino C, Silvestre J, Pereira J. Clostridium perfringens sepsis with massive intravascular haemolysis: a rare presentation. J Med Case Rep 2012;3:207–10. [Google Scholar]
- 10.Freedman JC, Theoret JR, Wisniewski JA, et al. Clostridium perfringens type A-E toxin plasmids. Res Microbiol 2015;166:264–79. 10.1016/j.resmic.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho CS, Bhatnagar J, Cohen AL, et al. Undiagnosed cases of fatal Clostridium-associated toxic shock in Californian women of childbearing age. Am J Obstet Gynecol 2009;201:451–7. 10.1016/j.ajog.2009.05.023 [DOI] [PubMed] [Google Scholar]
- 12.Chong E, Winikoff B, Charles D, et al. Vaginal and rectal Clostridium sordellii and Clostridium perfringens presence among women in the United States. Obstet Gynecol 2016;127:360–8. 10.1097/AOG.0000000000001239 [DOI] [PMC free article] [PubMed] [Google Scholar]