Key Points
Question
What were the frequencies of thrombocytopenia, heparin-induced thrombocytopenia, and platelet factor 4/heparin antibodies in patients with cerebral venous sinus thrombosis prior to the COVID-19 pandemic?
Findings
In a descriptive analysis of a retrospective consecutive sample of 865 patients with cerebral venous sinus thrombosis from 1987 to 2018, baseline thrombocytopenia was observed in 8.4% of patients, and heparin-induced thrombocytopenia was diagnosed in 0.1%. In a convenience sample subset of 93 patients with plasma available for additional laboratory analysis (including 8 who had thrombocytopenia), none had platelet factor 4/heparin antibodies.
Meaning
These findings may inform investigations of the possible association between the ChAdOx1 nCoV-19 (AstraZeneca/Oxford) and Ad26.COV2.S (Janssen/Johnson & Johnson) COVID-19 vaccines and cerebral venous sinus thrombosis with thrombocytopenia.
Abstract
Importance
Cases of cerebral venous sinus thrombosis in combination with thrombocytopenia have recently been reported within 4 to 28 days of vaccination with the ChAdOx1 nCov-19 (AstraZeneca/Oxford) and Ad.26.COV2.S (Janssen/Johnson & Johnson) COVID-19 vaccines. An immune-mediated response associated with platelet factor 4/heparin antibodies has been proposed as the underlying pathomechanism.
Objective
To determine the frequencies of admission thrombocytopenia, heparin-induced thrombocytopenia, and presence of platelet factor 4/heparin antibodies in patients diagnosed with cerebral venous sinus thrombosis prior to the COVID-19 pandemic.
Design, Setting, and Participants
This was a descriptive analysis of a retrospective sample of consecutive patients diagnosed with cerebral venous sinus thrombosis between January 1987 and March 2018 from 7 hospitals participating in the International Cerebral Venous Sinus Thrombosis Consortium from Finland, the Netherlands, Switzerland, Sweden, Mexico, Iran, and Costa Rica. Of 952 patients, 865 with available baseline platelet count were included. In a subset of 93 patients, frozen plasma samples collected during a previous study between September 2009 and February 2016 were analyzed for the presence of platelet factor 4/heparin antibodies.
Exposures
Diagnosis of cerebral venous sinus thrombosis.
Main Outcomes and Measures
Frequencies of admission thrombocytopenia (platelet count <150 ×103/μL), heparin-induced thrombocytopenia (as diagnosed by the treating physician), and platelet factor 4/heparin IgG antibodies (optical density >0.4, in a subset of patients with previously collected plasma samples).
Results
Of 865 patients (median age, 40 years [interquartile range, 29-53 years], 70% women), 73 (8.4%; 95% CI, 6.8%-10.5%) had thrombocytopenia, which was mild (100-149 ×103/μL) in 52 (6.0%), moderate (50-99 ×103/μL) in 17 (2.0%), and severe (<50 ×103/μL) in 4 (0.5%). Heparin-induced thrombocytopenia with platelet factor 4/heparin antibodies was diagnosed in a single patient (0.1%; 95% CI, <0.1%-0.7%). Of the convenience sample of 93 patients with cerebral venous sinus thrombosis included in the laboratory analysis, 8 (9%) had thrombocytopenia, and none (95% CI, 0%-4%) had platelet factor 4/heparin antibodies.
Conclusions and Relevance
In patients with cerebral venous sinus thrombosis prior to the COVID-19 pandemic, baseline thrombocytopenia was uncommon, and heparin-induced thrombocytopenia and platelet factor 4/heparin antibodies were rare. These findings may inform investigations of the possible association between the ChAdOx1 nCoV-19 and Ad26.COV2.S COVID-19 vaccines and cerebral venous sinus thrombosis with thrombocytopenia.
This retrospective analysis examined the frequency of thrombocytopenia and heparin-induced thrombocytopenia among patients with cerebral venous sinus thrombosis (CVST) before COVID-19 to help elucidate the association of ChAdOx1 nCoV-19 and Ad26.COV2.S vaccines and CVST thrombocytopenia.
Introduction
Cases of thromboses at unusual sites with associated thrombocytopenia have recently been reported that developed within 4 to 28 days of vaccination with the COVID-19 vaccines ChAdOx1 nCov-19 (AstraZeneca/Oxford) and Ad26.COV2.S (Janssen/Johnson & Johnson).1,2,3 Many of the reported patients had cerebral venous sinus thrombosis (CVST). After temporary suspension of vaccination with ChAdOx1 nCov-19 in several European countries, vaccination was resumed but restricted to older age groups in most of these countries following a benefit and risk assessment by the European Medicines Agency.4 Vaccination with Ad26.COV2.S was resumed after a temporary halt in the United States following recommendations by the Food and Drug Administration and the Centers for Disease Control and Prevention.5
In a study involving 10 patients who developed thrombosis and thrombocytopenia (median platelet count nadir, 20 ×103/μL , interquartile range [IQR], 12-64 ×103/μL) 5 to 16 days after vaccination with ChAdOx1 nCov-19, all tested patients had developed antibodies against platelet factor 4 (PF4), which were strongly platelet activating in a functional test, despite the absence of prior heparin treatment.1 To describe this specific response, the authors coined the term vaccine-induced immune thrombotic thrombocytopenia. The response suggests a disease mechanism similar to spontaneous heparin-induced thrombocytopenia (HIT), a form of autoimmune HIT, which has been previously reported to cause CVST, as well as other forms of thrombosis.6,7,8,9
Among patients diagnosed with deep vein thrombosis and pulmonary embolism prior to the COVID-19 pandemic, the estimated proportion with thrombocytopenia was 14%.10 The frequency of thrombocytopenia, HIT, and PF4/heparin antibodies in CVST prior to the COVID-19 pandemic are unknown. Since this information may guide investigations of the possible association between the ChAdOx1 nCoV-19 and Ad26.COV2.S vaccines and CVST with thrombocytopenia, this study sought to elucidate these questions using data of the pre–COVID-19 pandemic era from the International Cerebral Venous Sinus Thrombosis Consortium.11,12
Methods
This retrospective study examined the frequency of thrombocytopenia, HIT in particular, in a large multicenter cohort of consecutive patients with CVST recruited prior to the COVID-19 pandemic. Each center had permission from their ethical review board for the collection of observational data. Written informed consent was obtained if required under applicable national laws (eTable 1 in the Supplement). The presence of PF4/heparin antibodies was separately tested in previously collected plasma samples in a subset of patients with CVST.11 Ethical approval for the laboratory analyses was previously granted.
Study Design and Participants
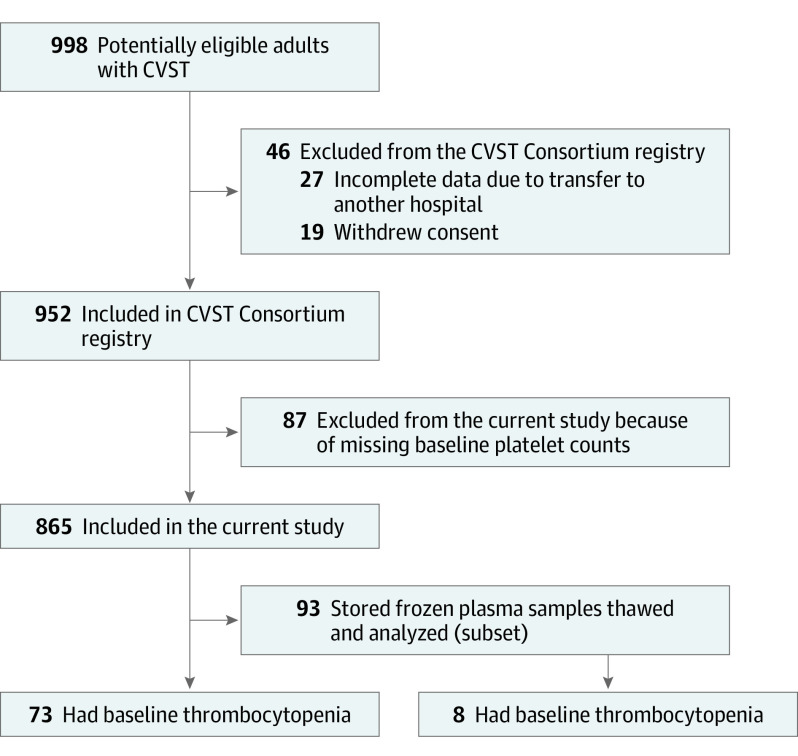
Data were included from consecutive adult patients with CVST from 7 hospitals participating in the International Cerebral Venous Sinus Thrombosis Consortium registry: Helsinki University Hospital, Finland; Amsterdam UMC, the Netherlands; Inselspital Bern University Hospital, Switzerland; Sahlgrenska University Hospital, Sweden; National Institute Manuel Velasco Suarez, Mexico; Hamadan University of Medical Science, Iran; and Hospital Dr Calderón Guardia, Costa Rica. Details of this cohort have been published.13 Start of recruitment varied per hospital, the earliest being August 1987 (eTable 2 in the Supplement). Patients diagnosed up to March 2018 were included. Patients who declined or withdrew consent, had incomplete data due to transfer to another hospital, or had a missing baseline platelet count were excluded.
PF4/heparin antibodies were tested in samples of frozen citrated plasma of a subset of patients participating in a previous study on CVST performed at the Amsterdam UMC and Inselspital, Bern University Hospital, between September 2009 and February 2016.11 This study had aimed to investigate prediction of CVST by admission D-dimer levels. The study cohort consisted of consecutive adult patients who were suspected of having CVST; were not receiving anticoagulant treatment prior to admission; and had not had a diagnosis of deep vein thrombosis, pulmonary embolism, ischemic stroke, or myocardial infarction in the 3 months prior to admission.
Data Collection and Definitions
Data on clinical manifestations and ancillary investigations were obtained with a standardized case report form. Diagnosis of CVST was confirmed with computed tomographic venography, magnetic resonance imaging with magnetic resonance venography, catheter angiography, or autopsy, in accordance with international guidelines.14,15 Intracerebral hemorrhage was defined as hemorrhagic infarction or intracerebral hematoma.
Platelet count was measured in venous blood samples as part of routine clinical care. The first platelet count performed upon hospital arrival, within a maximum of 48 hours after admission, was used for analysis. Thrombocytopenia was defined as a platelet count of less than 150 ×103/μL; normal platelet count, 150 to 450 ×103/μL, and thrombocytosis as a platelet count of more than 450 ×103/μL. Thrombocytopenia was further categorized into mild (platelet count, 100-149 ×103/μL), moderate (50-99 ×103/μL), or severe (<50 ×103/μL).16 For all patients with thrombocytopenia, additional details on presumed underlying causes were obtained through retrospective analysis of the medical records by a local investigator. For all patients in whom immune thrombocytopenic purpura (ITP) was identified by the local investigator, a hematologist (J.A.K.H.) and vascular internist (S.M.) independently reviewed details of the diagnosis and assessed the possibility of a missed diagnosis of autoimmune HIT.
For the laboratory analysis, an IgG-specific enzyme-linked immunosorbent assay (ELISA) was performed to assess the presence of PF4/heparin IgG antibodies (Lifecodes PF4 IgG, Immucor GTI Diagnostics) according to manufacturer’s instructions, on the frozen citrated plasma collected on hospital admission for CVST from a subset of patients. All samples were drawn prior to start of heparin treatment and were processed and stored frozen at −80 °C immediately after withdrawal, a process by which the stability of PF4/heparin antibodies is maintained.17 An optical density of more than 0.4 was classified as a positive result.
Outcomes
The main outcomes of the study were the frequencies of thrombocytopenia, HIT, and PF4/heparin antibodies among patients with CVST prior to the COVID-19 pandemic.
Statistical Analysis
A power calculation was not performed for this retrospective analysis but instead data from all patients with an available baseline platelet count were included. Because of the descriptive nature of the study, patients with a missing baseline platelet count were excluded rather than having their platelet counts imputed. Demographics and clinical characteristics of patients with available and missing baseline platelet counts, respectively, can be found in eTable 3 in the Supplement. In the primary analysis, frequencies of thrombocytopenia, HIT, and PF4/heparin antibodies were assessed. Because all patients with an unknown cause of thrombocytopenia were classified as “HIT unlikely,” they were included in the calculation of the frequency of HIT as non-HIT patients, and no variables were missing for the frequency calculations. The Wilson method was used to calculate 95% CIs for these proportions using the following formula: (p + 1.962/2n ± 1.96√{[p(1 −p)]/n + 1.962/4n2})/(1 + 1.962/n) where p is the proportion and n is the sample size.18 In a secondary analysis, clinical and imaging characteristics of patients with thrombocytopenia and patients with a normal platelet count were described. For these descriptive and exploratory analyses, missing data for each variable were again described and no statistical tests were applied. Analyses were performed in RStudio (version 1.2.1335) with the Hmisc package.
Results
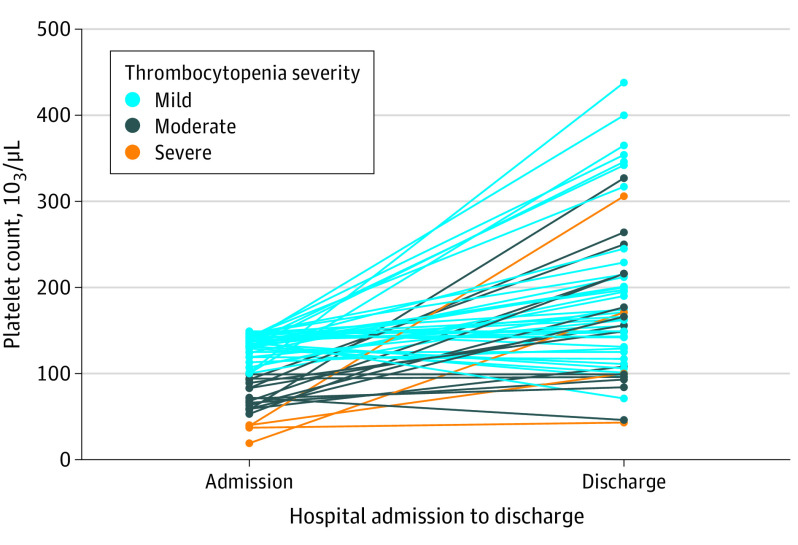
Of 998 eligible patients with CVST, 46 were excluded from the CVST Consortium registry (19 declined or withdrew consent, and 27 had incomplete data due to transfer to another hospital, Figure 1). Of the 952 patients included in the CVST Consortium registry, 87 (9.1%) patients with a missing baseline platelet count were excluded. Of the remaining 865 patients, 73 (8.4%) had thrombocytopenia, 751 (86.8%) had a normal platelet count, and 41 (4.7%) had thrombocytosis. The 95% CI for frequency of thrombocytopenia ranged from 6.8% to 10.5%. Of the 73 patients with thrombocytopenia, 52 (71%) had mild, 17 (23%) had moderate, and 4 (5%) had severe thrombocytopenia. Admission and discharge platelet counts for the 56 patients with thrombocytopenia at admission and available discharge platelet counts are presented in Figure 2. Thirty-six patients (49%) with thrombocytopenia and 540 (72%) with a normal platelet count were women. The number of patients with thrombocytopenia who had a diagnosis of cancer was 19 (26%); anemia, 29 (40%); and history of thrombosis, 13 (18%), whereas, the number of patients with a normal platelet count who had a diagnosis of cancer was 57 (8%); anemia, 147 (20%); and history of thrombosis, 61 (8%; Table 1). Focal neurological deficits at presentation were seen in 55 patients (75%) with thrombocytopenia and in 439 patients (59%) with a normal platelet count, and intracerebral hemorrhage was present in 32 (44%) with thrombocytopenia and 235 (32%) with a normal platelet count.
Figure 1. Patient Selection.
CVST indicates cerebral venous sinus thrombosis.
Figure 2. Admission and Discharge Platelet Counts for 56 Patients With Cerebral Venous Sinus Thrombosis With Thrombocytopenia at Admission and Available Discharge Platelet Count.
Change in platelet count between admission and discharge is provided for 56 of 73 patients with thrombocytopenia at admission (platelet count at discharge was missing for the remaining 17 patients). Mild thrombocytopenia: platelet count 100 to 149 ×103/μL; moderate thrombocytopenia: platelet count 50 to 99 ×103/μL; severe thrombocytopenia: platelet count <50 ×103/μL.
Table 1. Baseline Demographics and Clinical Characteristics of 865 Patients With Cerebral Venous Sinus Thrombosis Prior to the COVID-19 Pandemic.
| No./total (%) of patients | ||||
|---|---|---|---|---|
| Thrombocytopenia: platelet count <150 ×103/μL (n = 73) | Normal: platelet count 150-450 ×103/μL (n = 751) | Thrombocytosis: platelet count >450 ×103/μL (n = 41) | Subset with plasma samples analyzed (n = 93)a | |
| Demographics | ||||
| Age, median (IQR), y | 40 (27-59) | 40 (29-52) | 43 (34-54) | 40 (30-51) |
| Sex | ||||
| Men | 37/73 (51) | 211/751 (28) | 13/41 (32) | 21/93 (22) |
| Women | 36/73 (49) | 540/751 (72) | 28/41 (68) | 72/93 (77) |
| Risk factors | ||||
| Oral contraceptive useb | 18/36 (50) | 269/536 (50) | 7/26 (27) | 47/72 (65) |
| Anemia | 29/73 (40) | 147/742 (20) | 17/40 (43) | 19/91 (21) |
| Cancer | 19/73 (26) | 57/750 (8) | 7/41 (17) | 10/93 (11) |
| Previous thrombosis | 13/72 (18) | 61/745 (8) | 3/41 (7) | 11/93 (12) |
| Any infection | 11/73 (15) | 85/749 (11) | 6/40 (15) | 14/93 (15) |
| Genetic thrombophilia | 9/60 (15) | 102/670 (15) | 3/36 (8) | 12/82 (15) |
| Pregnancy or recent deliveryb,c | 2/36 (6) | 64/540 (12) | 3/28 (11) | 3/72 (4) |
| Clinical characteristics | ||||
| Focal deficits | 55/73 (75) | 439/747 (59) | 12/40 (30) | 74/93 (80) |
| Headache | 52/71 (73) | 650/743 (87) | 36/41 (88) | 89/93 (96) |
| Seizure(s) | 29/72 (40) | 235/747 (31) | 8/40 (20) | 34/93 (37) |
| Coma | 6/73 (8) | 44/750 (6) | 3/41 (7) | 8/93 (9) |
| Imaging characteristics | ||||
| Intracerebral hemorrhage | 32/73 (44) | 235/747 (31) | 16/41 (39) | 41/93 (44) |
| Focal cerebral edema only | 13/73 (18) | 151/747 (20) | 6/41 (15) | 20/93 (22) |
| Thrombosis locationd | ||||
| Lateral sinuse | 48/72 (67) | 557/751 (74) | 31/41 (76) | 79/93 (85) |
| Superior sagittal sinus | 43/72 (60) | 388/751 (52) | 18/41 (44) | 56/93 (60) |
| Cortical veins | 17/72 (24) | 160/751 (21) | 4/41 (10) | 18/93 (19) |
| Straight sinus | 9/72 (13) | 127/751 (17) | 5/41 (12) | 24/93 (26) |
| Deep venous systemf | 5/72 (7) | 82/751 (11) | 4/41 (10) | 19/93 (20) |
Abbreviation: IQR, interquartile range.
Patients are a subset of the other 3 categories in this Table: 8 (9%) had thrombocytopenia, 81 (87%) had a normal platelet count, and 4 (4%) had thrombocytosis.
Percentage of women.
Delivery less than 12 weeks prior to onset of cerebral venous sinus thrombosis.
Multiple locations possible.
That is, transverse or sigmoid sinus.
That is, vein of Galen, basal vein of Rosenthal, internal cerebral vein, inferior longitudinal sinus, thalamostriate, or caudate veins.
Among patients with thrombocytopenia at admission, the thrombocytopenia was newly diagnosed in 47 patients (64%). Among those with an identified cause of thrombocytopenia (n = 47, 64%), the most common causes were infection (n = 12, 16%), hematologic cancer (n = 12, 16%), and drug-induced thrombocytopenia (n = 9, 12%, Table 2). Details on the specific infections, hematologic cancer types, and drugs presumed to cause thrombocytopenia in these patients are available in eTable 4 in the Supplement. Among 26 patients, the cause of thrombocytopenia could not be identified, of whom 23 had mild and 3 had moderate thrombocytopenia, and none had a decrease in platelet count of more than 40% during admission.
Table 2. Clinical Manifestations, Etiology, and Outcomes of Thrombocytopenia in 73 Patients With Cerebral Venous Sinus Thrombosis and Thrombocytopenia.
| No./total (%) of patients with thrombocytopenia (n = 73) | |
|---|---|
| New thrombocytopenia at CVST diagnosisa | 47/73 (64) |
| Clinical manifestations | |
| Any symptom or sign | 6/70 (9) |
| Petechiae | 3/68 (4) |
| Mucosal bleeding | 3/68 (4) |
| Other bleedingb | 2/68 (3) |
| Baseline laboratory findings | |
| Admission platelet count, median (IQR), ×103/μL | 129 (94-140) |
| Thrombocytopeniac | |
| Mild (100-149 ×103/μL) | 52/73 (71) |
| Moderate (50-99 ×103/μL) | 17/73 (23) |
| Severe (<50 ×103/μL) | 4/73 (5) |
| Hemoglobin, median (IQR), g/dL | 12.9 (11.3-14.2) |
| White blood cell count, median (IQR) [No.], ×103/μL | 9.0 (5.9-13.1) [63] |
| Presumed cause of thrombocytopeniad | |
| Infection | 12/73 (16) |
| Hematologic cancer | 12/73 (16) |
| Drug-induced (other than heparin) | 9/73 (12) |
| Alcohol-induced | 6/73 (8) |
| ITP | 6/73 (8) |
| Antiphospholipid syndromee | 3/73 (4) |
| Hereditary thrombocytopenia | 2/73 (3) |
| Heparin-induced thrombocytopenia | 1/73 (1) |
| Otherf | 5/73 (7) |
| Unknowng | 26/73 (36) |
| Outcome thrombocytopenia | |
| Platelets normalized at dischargeh | 35/56 (63) |
| Platelet count at discharge, median (IQR) [No.], ×103/μL | 166 (115-216) [56] |
Abbreviations: CVST, cerebral venous sinus thrombosis; IQR, interquartile range; ITP, immune thrombocytopenic purpura.
SI conversion factor: To convert hemoglobin from g/dL to mmol/L, multiply by 0.6206.
That is, thrombocytopenia was not known from prior blood tests or medical history.
Excluding intracerebral hemorrhage secondary to CVST. One patient had oropharyngeal bleeding; 1 patient had thoracic bleeding and tamponade as a complication of local urokinase treatment of CVST.
Platelet factor 4/heparin antibodies were analyzed in 7 (1 as part of routine care) patients with mild, 2 with moderate, and 1 (as part of routine care) with severe thrombocytopenia.
Multiple causes possible.
Anticardiolipin antibodies repeatedly positive upon testing more than 12 weeks apart (n = 2), positive antiphospholipid antibodies tested at a different hospital recorded without further available information (n = 1).
That is, suspected myelodysplastic syndrome, gestational thrombocytopenia, systemic lupus erythematosus, nutrient deficiency, and thoracic bleeding.
Twenty-three (88%) had mild and 3 (12%) had moderate baseline thrombocytopenia.
Of the 17 patients with a missing platelet count at discharge, 15 (88%) had mild and 2 (12%) had moderate baseline thrombocytopenia.
There were 6 patients in whom ITP was diagnosed by the local physicians. After reevaluation of available data, ITP was confirmed by both adjudicators for 5 of 6 patients. In the other patient, the adjudicators determined that autoimmune HIT could not be ruled out. The patient was an 18-year-old man with CVST, pulmonary embolism, extracranial bleeding, and thrombocytopenia (baseline platelet count, 71 ×103/μL). Despite immunosuppressive therapy including intravenous immunoglobulins, the patient deteriorated and died 4 days after admission. PF4/heparin antibodies were not determined.
Two other patients were tested for PF4/heparin antibodies as part of routine care, 1 of whom tested positive. This patient was a 35-year-old woman who had an extra-uterine pregnancy that was treated with curettage and uterine embolization. During this hospitalization, she received a prophylactic dose of low-molecular weight heparin. Thirteen days later she was diagnosed with CVST. Her platelet count at that time was 37 ×103/μL. The patient was found to have platelet activating PF4/heparin antibodies and was diagnosed with HIT (based on a positive particle centrifugation immunoassay with a titer of 1:32; an Immunocor GTI anti-PF4/heparin antibody ELISA optical density of >3.000; and a positive heparin-induced platelet aggregation test result) and was treated with lepirudin and endovascular thrombectomy. However, her clinical condition worsened, and she died 3 weeks later due to uncontrollable intracranial hypertension. Thus, HIT was conclusively diagnosed in 1 of 73 patients (1%) with CVST and thrombocytopenia and in 1 of 865 patients (0.1%; 95% CI, <0.1%-0.7%) in the entire CVST cohort.
Of the 93 patients whose plasma samples were analyzed, 8 of 93 (9%) had baseline thrombocytopenia (6 with mild, 2 with moderate, and none with severe thrombocytopenia). Baseline demographics and clinical characteristics of this subset of patients are detailed in Table 1. None of the 93 patients tested positive for PF4/heparin antibodies (95% CI, 0%-4%). The median optical density of anti-PF4 IgG was 0.106 (interquartile range, 0.088-0.142; range, 0.064-0.357).
Discussion
In this study involving 865 patients with CVST prior to the COVID-19 pandemic, thrombocytopenia was uncommon at the time of presentation. In many patients, thrombocytopenia could be explained by comorbidities, like cancer or infection, or by use of alcohol or certain medications. HIT was conclusively identified in only 1 patient. Because patients with CVST were not routinely screened for HIT, additional plasma samples from a subset of 93 patients were tested for the presence of PF4/heparin antibodies, without a single positive test result.
Together, these data indicate that HIT-associated CVST was very rare prior to the COVID-19 pandemic. These observations suggest that the severe thrombocytopenia reported in association with CVST in many of the cases occurring after ChAdOx1 nCov-19 and Ad.26.COV2.S vaccination is unusual, as is the presence of PF4/heparin antibodies. This may indicate that these CVST cases were associated with the vaccine.1,2,3 Laboratory investigation with a binding assay (ELISA) to determine the presence of anti–PF4/heparin antibodies is essential in these cases because rapid and chemiluminescence immunoassays may produce false-negative results.19
These findings should not be interpreted as justification to halt use of the ChAdOx1 nCov-19 or Ad.26.COV2.S vaccines. Reported rates of thrombotic complications with thrombocytopenia associated with the vaccine remain low, whereas rates of morbidity and mortality of COVID-19 are much higher.4,5,20
Limitations
This study has several limitations. First, clinical data for this multicenter cohort were collected retrospectively, and information on race/ethnicity was not obtained. Baseline platelet count was not always available, nor was the underlying cause of thrombocytopenia identified or investigated in all cases. This was especially true among patients with mild thrombocytopenia, who constituted 23 of 26 patients with thrombocytopenia of unknown cause. Second, patients were not routinely screened for HIT; thus, it is possible that the point estimate of 0.1% is an underestimation of the frequency of HIT among CVST cases prior to the COVID-19 pandemic. One case was identified in whom autoimmune HIT could not be reliably excluded, but PF4/heparin antibodies were not measured for this patient. However, because analysis of the 93 plasma samples from a convenience sample did not yield a single positive result for PF4/heparin antibodies, and thrombocytopenia was present in only 8% of patients overall, it is unlikely that this potential underrepresentation can fully explain the difference between the post–COVID-19 vaccination CVST with HIT-like disease1,2 and the pre–COVID-19 CVST cohort in this study. Third, of the subset of 93 patients whose plasma samples were analyzed for PF4/heparin antibodies, only 8 had thrombocytopenia and none had severe thrombocytopenia. Fourth, each participating hospital initiated the inclusion of patients with CVST at different time points for a variable length of time. However, because more than 60% of included patients were diagnosed after 2010, the data are presumed to be representative of present-day patients with CVST. Fifth, the data did not allow for the calculation of a population-based incidence rate of CVST with thrombocytopenia. Sixth, despite the CVST Consortium including one of the largest data sets of patients with CVST, due to its rarity, the cohort size remained limited.
Conclusions
In patients with cerebral venous sinus thrombosis prior to the COVID-19 pandemic, baseline thrombocytopenia was uncommon, and heparin-induced thrombocytopenia and platelet factor 4/heparin antibodies were rare. These findings may inform investigations of the possible association between the ChAdOx1 nCoV-19 and Ad26.COV2.S COVID-19 vaccines and cerebral venous sinus thrombosis with thrombocytopenia.
eTable 1. Ethical review and informed consent procedure in each participating center
eTable 2. Cerebral venous sinus thrombosis Consortium cohorts: inclusion periods and frequencies of thrombocytopenia
eTable 3. Baseline demographics and clinical characteristics of patients with cerebral venous sinus thrombosis before the era of COVID-19 with and without available baseline platelet count
eTable 4. Details on specific infections, hematologic cancer types, and drugs presumed to cause thrombocytopenia among 30 patients with cerebral venous sinus thrombosis
References
- 1.Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. Published online April 9, 2021. doi: 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. Published online April 9, 2021. doi: 10.1056/NEJMoa2104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.See I, Su JR, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA. 2021;325(24):2448-2456. doi: 10.1001/jama.2021.7517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Medicines Agency . Assessment report: procedure under Article 5(3) of regulation (EC) No 726/2004—vaxzevira. Accessed May 5, 2021. https://www.ema.europa.eu/en/documents/referral/use-vaxzevria-prevent-covid-19-article-53-procedure-assessment-report_en.pdf
- 5.MacNeil JR, Su JR, Broder KR, et al. Updated recommendations from the Advisory Committee on Immunization Practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):651-656. doi: 10.15585/mmwr.mm7017e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greinacher A, Selleng K, Warkentin TE. Autoimmune heparin-induced thrombocytopenia. J Thromb Haemost. 2017;15(11):2099-2114. doi: 10.1111/jth.13813 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen TH, Medvedev N, Delcea M, Greinacher A. Anti-platelet factor 4/polyanion antibodies mediate a new mechanism of autoimmunity. Nat Commun. 2017;8:14945. doi: 10.1038/ncomms14945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greinacher A. Me or not me? the danger of spontaneity. Blood. 2014;123(23):3536-3538. doi: 10.1182/blood-2014-04-566836 [DOI] [PubMed] [Google Scholar]
- 9.Moores G, Warkentin TE, Farooqi MAM, Jevtic SD, Zeller MP, Perera KS. Spontaneous heparin-induced thrombocytopenia syndrome presenting as cerebral venous sinus thrombosis. Neurol Clin Pract. Published online January 14, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Micco P, Ruiz-Giménez N, Nieto JA, et al. ; RIETE investigators . Platelet count and outcome in patients with acute venous thromboembolism. Thromb Haemost. 2013;110(5):1025-1034. doi: 10.1160/TH13-04-0352 [DOI] [PubMed] [Google Scholar]
- 11.Heldner MR, Zuurbier SM, Li B, et al. Prediction of cerebral venous thrombosis with a new clinical score and D-dimer levels. Neurology. 2020;95(7):e898-e909. doi: 10.1212/WNL.0000000000009998 [DOI] [PubMed] [Google Scholar]
- 12.Lindgren E, Silvis SM, Hiltunen S, et al. Acute symptomatic seizures in cerebral venous thrombosis. Neurology. 2020;95(12):e1706-e1715. doi: 10.1212/WNL.0000000000010577 [DOI] [PubMed] [Google Scholar]
- 13.Silvis SM, Reinstra E, Hiltunen S, et al. ; International CVT Consortium . Anaemia at admission is associated with poor clinical outcome in cerebral venous thrombosis. Eur J Neurol. 2020;27(4):716-722. doi: 10.1111/ene.14148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saposnik G, Barinagarrementeria F, Brown RD Jr, et al. ; American Heart Association Stroke Council and the Council on Epidemiology and Prevention . Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192. doi: 10.1161/STR.0b013e31820a8364 [DOI] [PubMed] [Google Scholar]
- 15.Ferro JM, Bousser MG, Canhão P, et al. ; European Stroke Organization . European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis—endorsed by the European Academy of Neurology. Eur J Neurol. 2017;24(10):1203-1213. doi: 10.1111/ene.13381 [DOI] [PubMed] [Google Scholar]
- 16.Williamson DR, Albert M, Heels-Ansdell D, et al. ; PROTECT collaborators, the Canadian Critical Care Trials Group, and the Australian and New Zealand Intensive Care Society Clinical Trials Group . Thrombocytopenia in critically ill patients receiving thromboprophylaxis: frequency, risk factors, and outcomes. Chest. 2013;144(4):1207-1215. doi: 10.1378/chest.13-0121 [DOI] [PubMed] [Google Scholar]
- 17.Warkentin TE. Laboratory diagnosis of heparin-induced thrombocytopenia. Int J Lab Hematol. 2019;41(suppl 1):15-25. doi: 10.1111/ijlh.12993 [DOI] [PubMed] [Google Scholar]
- 18.Wilson EB. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209-212. doi: 10.1080/01621459.1927.10502953 [DOI] [Google Scholar]
- 19.Nazy I, Sachs UJ, Arnold DM, et al. Recommendations for the clinical and laboratory diagnosis of VITT against COVID-19: Communication from the ISTH SSC Subcommittee on Platelet Immunology. J Thromb Haemost. 2021;19(6):1585-1588. doi: 10.1111/jth.15341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson N, Kvalsvig A, Barnard LT, Baker MG. Case-fatality risk estimates for COVID-19 calculated by using a lag time for fatality. Emerg Infect Dis. 2020;26(6):1339-1441. doi: 10.3201/eid2606.200320 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Ethical review and informed consent procedure in each participating center
eTable 2. Cerebral venous sinus thrombosis Consortium cohorts: inclusion periods and frequencies of thrombocytopenia
eTable 3. Baseline demographics and clinical characteristics of patients with cerebral venous sinus thrombosis before the era of COVID-19 with and without available baseline platelet count
eTable 4. Details on specific infections, hematologic cancer types, and drugs presumed to cause thrombocytopenia among 30 patients with cerebral venous sinus thrombosis