Key Points
Question
Among men with symptoms of urinary tract infection (UTI) who are afebrile, is treatment with ciprofloxacin or trimethoprim/sulfamethoxazole for 7 days noninferior to 14 days of treatment with regard to resolution of UTI symptoms?
Findings
In this randomized clinical trial that included 272 men with presumed symptomatic UTI, resolution of initial UTI symptoms by 14 days after completion of active antibiotic therapy occurred in 122 of 131 (93.1%) participants in the 7-day group and 111 of 123 (90.2%) in the 14-day group, a difference that met the prespecified noninferiority margin of 10%.
Meaning
The findings support the use of a 7-day course of ciprofloxacin or trimethoprim/sulfamethoxazole as an alternative to a 14-day course for treatment of afebrile men with suspected UTI.
Abstract
Importance
Determination of optimal treatment durations for common infectious diseases is an important strategy to preserve antibiotic effectiveness.
Objective
To determine whether 7 days of treatment is noninferior to 14 days when using ciprofloxacin or trimethoprim/sulfamethoxazole to treat urinary tract infection (UTI) in afebrile men.
Design, Setting, and Participants
Randomized, double-blind, placebo-controlled noninferiority trial of afebrile men with presumed symptomatic UTI treated with ciprofloxacin or trimethoprim/sulfamethoxazole at 2 US Veterans Affairs medical centers (enrollment, April 2014 through December 2019; final follow-up, January 28, 2020). Of 1058 eligible men, 272 were randomized.
Interventions
Participants continued the antibiotic prescribed by their treating clinician for 7 days of treatment and were randomized to receive continued antibiotic therapy (n = 136) or placebo (n = 136) for days 8 to 14 of treatment.
Main Outcomes and Measures
The prespecified primary outcome was resolution of UTI symptoms by 14 days after completion of active antibiotic treatment. A noninferiority margin of 10% was selected. The as-treated population (participants who took ≥26 of 28 doses and missed no more than 2 consecutive doses) was used for the primary analysis, and a secondary analysis included all patients as randomized, regardless of treatment adherence. Secondary outcomes included recurrence of UTI symptoms and/or adverse events within 28 days of stopping study medication.
Results
Among 272 patients (median [interquartile range] age, 69 [62-73] years) who were randomized, 100% completed the trial and 254 (93.4%) were included in the primary as-treated analysis. Symptom resolution occurred in 122/131 (93.1%) participants in the 7-day group vs 111/123 (90.2%) in the 14-day group (difference, 2.9% [1-sided 97.5% CI, –5.2% to ∞]), meeting the noninferiority criterion. In the secondary as-randomized analysis, symptom resolution occurred in 125/136 (91.9%) participants in the 7-day group vs 123/136 (90.4%) in the 14-day group (difference, 1.5% [1-sided 97.5% CI, –5.8% to ∞]) Recurrence of UTI symptoms occurred in 13/131 (9.9%) participants in the 7-day group vs 15/123 (12.9%) in the 14-day group (difference, –3.0% [95% CI, –10.8% to 6.2%]; P = .70). Adverse events occurred in 28/136 (20.6%) participants in the 7-day group vs 33/136 (24.3%) in the 14-day group.
Conclusions and Relevance
Among afebrile men with suspected UTI, treatment with ciprofloxacin or trimethoprim/sulfamethoxazole for 7 days was noninferior to 14 days of treatment with regard to resolution of UTI symptoms by 14 days after antibiotic therapy. The findings support the use of a 7-day course of ciprofloxacin or trimethoprim/sulfamethoxazole as an alternative to a 14-day course for treatment of afebrile men with UTI.
Trial Registration
ClinicalTrials.gov identifier: NCT01994538
This randomized clinical trial assesses the effect of 14-day antibiotic treatment vs 7 days of antibiotic treatment plus 7 days of placebo among afebrile men with urinary tract infection.
Introduction
Determining the optimal treatment duration for common infectious diseases is an important strategy to preserve the effectiveness of antimicrobials.1 A growing body of evidence has shown that shorter-duration treatment performed similarly to longer-duration treatment for many infections, including pneumonia,2,3 intra-abdominal infections,4 osteomyelitis,5 cellulitis,6 urinary tract infection (UTI) in women,7,8 and others. These findings support prescribing antimicrobials for no longer than needed to resolve manifestations of infection, given that extended treatment often does not provide additional benefit, is costly and inconvenient, and increases the risk of adverse events.9
However, UTI in men lacks a defined optimal treatment duration. Clinical trials demonstrated that for febrile men with UTI, 2 weeks of treatment performed similarly to 4 weeks10; whereas 7 days was inferior to 14 days for short-term but not long-term outcomes.11 A third trial showed that for afebrile men with spinal cord injury and UTI, 3 days of treatment was inferior to 14 days.12 Other studies of afebrile men with UTI have been observational, and longer-duration therapy was not associated with benefit.13,14
Because UTI is among the most common reasons for antimicrobial use,15 definitive evidence that shorter duration treatment is no worse than longer duration treatment has the potential to substantially decrease antimicrobial use, particularly for agents active against gram-negative bacteria, in which emerging resistance is of great concern. To assess whether afebrile men with UTI can be treated with shorter-duration treatment, a randomized, placebo-controlled noninferiority trial of 7 vs 14 days of ciprofloxacin or trimethoprim/sulfamethoxazole for men with clinically diagnosed UTI was conducted.
Methods
This double-blind, placebo-controlled, randomized trial was conducted at 2 US Veterans Affairs (VA) medical centers (in Minnesota and Texas), with the second center added midstudy to increase enrollment. Study sites were the Minneapolis VA Medical Center (hereafter, Minneapolis) and the Michael E DeBakey VA Medical Center in Houston (hereafter, Houston). The institutional review board at each site approved the study protocol, and all participants provided written informed consent. The study protocol is available online (Supplement 1 and Supplement 2).
Patients
Patients were eligible for enrollment if they were aged 18 years or older, male, treated in the outpatient setting, afebrile, and initially prescribed 7 to 14 days of ciprofloxacin or trimethoprim/sulfamethoxazole for UTI treatment by their clinician. Eligibility also required new onset of at least 1 of the following: dysuria, frequency of urination, urgency of urination, hematuria, costovertebral angle (CVA) tenderness; or perineal, flank, or suprapubic pain. Treatment needed to be completely or almost completely in the outpatient setting; up to 24 hours of inpatient observation was allowed. Ciprofloxacin or trimethoprim/sulfamethoxazole were chosen because at the time of study initiation they accounted for 90% of male UTI treatment in the VA system13 and to facilitate blinding. Exclusion criteria were prior UTI treatment within 14 days, symptoms that the treating clinician attributed to a non-UTI cause, documented temperature of 38 °C (≥100.4 °F), and growth of an organism in urine that was not susceptible to the agent initially prescribed (trimethoprim/sulfamethoxazole or ciprofloxacin). A urine culture was not required for enrollment, although it was encouraged in institutional clinical guidance. If clinicians opted to change the initially prescribed ciprofloxacin or trimethoprim/sulfamethoxazole prior to enrollment due to culture results or other parameters, the patient was considered ineligible. Race and Latino ethnicity information were collected as required by the VA; race and ethnicity status were determined by asking participants to self-identify by choosing from prespecified categories.
Participants who were being treated for UTI as part of usual clinical care were identified by performing daily searches of each center's electronic databases for a series of diagnostic codes and prescriptions for ciprofloxacin or trimethoprim/sulfamethoxazole. During the study period, all patients who presented to the outpatient setting with urinary concerns were provided with an information sheet notifying them of possible contact for a research study and that they could opt out of such contact. Study personnel reviewed the records of all patients identified by the daily database search as potentially being treated for UTI, then contacted those who appeared to meet inclusion criteria to confirm eligibility. Eligible patients were offered enrollment via 3 methods: in-person clinic enrollment, home enrollment via mail, or in-person enrollment at home.
In-person clinic enrollment entailed coming to the medical center prior to day 8 of UTI treatment for a visit with a study coordinator. At this visit written informed consent was obtained, and the patient was dispensed a study medication to take for 1 week, starting on treatment day 8 (ie, for days 8-14 of treatment). The study medication was either the same antimicrobial as initially prescribed (but from a different manufacturer), or a placebo tablet of similar size. The active antimicrobials were purchased from commercial vendors by the research pharmacy at the Minneapolis VA Medical Center, whereas the placebo tablets were purchased from the VA Cooperative Studies Program Clinical Research Pharmacy Coordinating Center in Albuquerque, New Mexico. Participants were told that the study medication would look different from their initially prescribed medication, and that they had an equal chance of receiving active antimicrobial or placebo for days 8 through 14 of treatment. Participants receiving placebo for days 8 through 14 were the 7-day treatment group, whereas those receiving active antimicrobials for days 8 through 14 were the 14-day treatment group.
Mail enrollment was used for patients who lived remotely or who preferred an at-home visit for other reasons. A study coordinator contacted each potential participant by phone to confirm eligibility and interest, then sent study paperwork via an overnight delivery service. The next day, a coordinator reviewed the forms with the participant via another phone conversation and specified instructions to return the signed forms using the provided prepaid overnight mailer. Upon receipt of signed forms, the patient was randomized to the 7- or 14-day study group and study medication (active antimicrobial vs placebo) was shipped overnight or hand delivered.
In-person enrollment at the participant’s home was facilitated for those who were reached too late to receive the study medication by day 8 of UTI treatment. For these participants, a study coordinator traveled to the home to conduct the study visit and brought the study medication assigned for days 8 through 14, with randomization occurring prior to the study visit.
Randomization
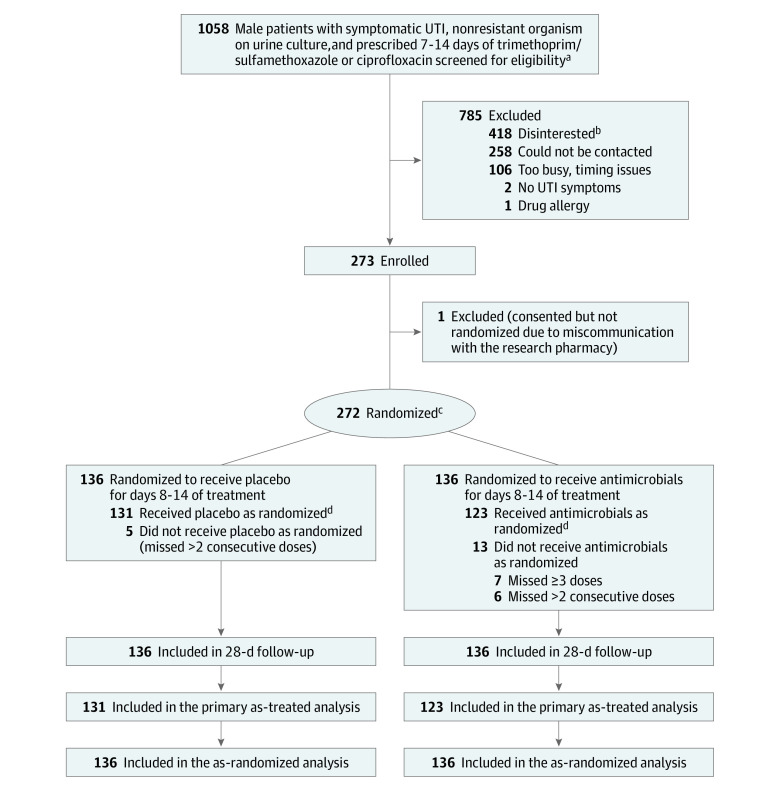
Randomization was completed by the research pharmacists using computer-generated random numbers, stratified by presence of a urinary catheter, study site, and the initially prescribed antibiotic (trimethoprim/sulfamethoxazole vs ciprofloxacin). Staff who enrolled and obtained consent from patients were not aware of treatment allocation. Randomization was performed 1:1 using blocks of 4 and according to predetermined randomization schedules that were controlled by the study pharmacist at each site (Figure). Patients who were initially prescribed more than 7 days of treatment were asked to return the excess antimicrobial doses to the study coordinator or to dispose of them.
Figure. Enrollment, Randomization, and Flow of Patients in the Trial.
aAll participants received 7 days of trimethoprim/sulfamethoxazole or ciprofloxacin before being randomized to receive an additional 7 days of the same antimicrobial or placebo.
bVarious reasons were given for disinterest; however, 29 expressed disinterest specifically because they did not want to change treatment duration.
cRandomization was stratified by urinary catheter use, study drug, and study site.
dIndicates that participants adhered to taking at least 26 of 28 assigned medication doses and missed no more than 2 consecutive doses.
UTI indicates urinary tract infection.
Interventions
All participants initially continued the antibiotic prescribed by their treating clinician for 7 days then continued antibiotic therapy or placebo for days 8 through 14 of treatment, depending on their randomization group. Medications were dosed on a twice-daily schedule. Participants randomized to the placebo group for days 8 through 14 were considered the 7-day treatment group, whereas those randomized to receive active antimicrobials for days 8 through 14 were the 14-day treatment group.
Participants received scheduled telephone visits with study personnel (blinded to their randomization group) on treatment day 14 (the day their study medication stopped) and again on days 7, 14, and 28 after medication was stopped. During each call, participants were asked about their presenting UTI symptoms, if they had sought UTI treatment since completing study medication, and whether they had experienced new UTI symptoms or adverse effects of antibiotics. On the last day of taking study medication, participants self-reported protocol adherence, which was defined as having taken at least 26 of 28 total doses (14 days of twice-daily dosing) and missing no more than 2 consecutive doses.
Outcomes
The primary end point was resolution of the initial UTI symptoms by day 14 after completion of active antibiotic treatment, a clinical end point used in prior UTI trials.7,10,12,16 To assess this end point uniformly, irrespective of assigned treatment duration, patients were asked about UTI symptoms on days 7 and 14 after completion of study medication. After study closure and unblinding of treatment assignment, each participant’s outcome assessment (which corresponded to 14 days after completion of active antibiotic treatment) was used for the primary outcome. For the shorter-duration (placebo) group, the assessment was 7 days after completion of study medication, and assessment was 14 days after completion of study medication for those who received the longer-duration treatment.
As a post hoc sensitivity analysis, resolution of initial UTI symptoms was evaluated at the same time in both groups relative to the time of initiation of antibiotics, in this case, 14 days after stopping study medication. Participants who were uncertain or noncommittal regarding symptom resolution were classified as having unresolved symptoms if they sought reassessment or retreatment for UTI after study medication completion; they were classified as having resolved UTI symptoms if further medical care was not sought.
Prespecified secondary outcomes included participant-reported recurrence of UTI symptoms (after initial symptom resolution) and adverse events through 28 days after stopping study medication. Intestinal carriage of antimicrobial-resistant gram-negative bacilli was also a secondary outcome that is not reported in this article. Adverse events were elicited by telephone interview and medical record review. An order for Clostridiodes difficile testing was used to indicate suspected C difficile infection.
Sample Size
Four infectious disease physicians, all with multiple publications regarding UTI management, were asked to provide a value for a noninferiority margin they would consider acceptable for a proposed trial of afebrile men with UTI. Based on their responses (range, 10%-20%, with no process to achieve consensus), a noninferiority margin of 10% was selected, which required 290 participants to be able to detect this difference with 85% power and a 1-sided α of .025. A 90% success rate in the 14-day group was assumed. This margin is equal to or more stringent than those used in UTI studies with similar populations and severity of illness.11,17,18
Statistical Analysis
The primary noninferiority analysis compared the proportion of participants with resolution of UTI symptoms by day 14 after completion of active antimicrobial treatment, limited to the as-treated population (participants who took ≥26 of 28 doses and missed no more than 2 consecutive doses). Exact 1-sided confidence interval of the difference in the proportions of symptom resolution between treatment duration groups was calculated using the method of Shan and Wang19 and implemented in the R package ExactCIdiff. The primary analysis used a 1-sided 97.5% CI for noninferiority, which was established if the lower bound of the 1-sided 97.5% CI did not cross the noninferiority margin of −10% difference in symptom resolution. A secondary, as-randomized analysis was performed that included all randomized participants analyzed in the groups to which they were randomized. Analyses of secondary outcomes were performed using a common 2-tailed superiority hypothesis test of differences in proportions (2-sample test for equality of proportions with continuity correction) with α = .05 and with 2-sided 95% CIs. Because the analyses of secondary outcomes were considered exploratory, there was no correction for type I error.
Given the low rates of missing data, complete case analyses were performed. Post hoc subgroup analyses were performed to assess for any differential effect of type of antibiotic drug treatment, pretreatment bacteriuria count, and study site on the primary outcome. A generalized linear model was used to assess the interaction effect between treatment duration and drug treatment (ciprofloxacin vs trimethoprim/sulfamethoxazole) on the primary outcome. A separate generalized linear model was used to assess the interaction effect between treatment duration and pretreatment bacteriuria, with bacteriuria categorized as no growth, any growth, and high-count (>100 000 colony-forming units [CFU]/mL). A generalized linear mixed model with site included as a random effect was used to examine the interaction between treatment duration and study site (Minneapolis vs Houston). R version 4.0.2 (R Core Team) was used for all statistical analyses.
Results
From April 2014 through December 2019 (Minneapolis), and from January 2018 through December 2019 (Houston), 273 total patients consented to randomization, and 272 were randomized to receive 7 vs 14 days of treatment. Enrollment stopped after funding expired. One participant consented but was not randomized due to a miscommunication with the research pharmacy. Enrollment was conducted in person at the Minneapolis or Houston VA Medical Center for 130 (47.8%) participants, by mail for 136 (50.0%), and by home visit for 6 (2.2%). Overall, 76% of participants (207/272) were enrolled at the Minneapolis site, and 24% (66/272) at the Houston site.
All randomized participants underwent follow-up at day 28 after completion of study medication (Figure). There were no missing data for all primary and most secondary outcomes; more than 98% of follow-up calls to assess adverse events were completed as planned per protocol.
Median age for the 272 participants was 69 years (interquartile range, 62-73 years); 18.0% were Black, 2.2% Native American, 77.9% White, 1.1% multiple races, and 0.7% declined to answer. Most (93.0%) were not of Hispanic/Latino ethnicity, 4.8% were of Hispanic/Latino ethnicity, and 2.2% declined to answer. These demographic factors, baseline comorbid conditions, and Charlson Comorbidity Index scores were evenly distributed by group (Table 1). For 253 (93.0%) of the 272 participants, the treating clinician obtained a pretreatment urinalysis; whereas for 239 (87.9%) participants, the clinician obtained a pretreatment urine culture. Of the 239 urine cultures, 145 (60.7%) yielded more than 100 000 CFU/mL, 39 (16.3%) yielded lower colony counts, and 55 (23.0%) had no growth. Among the 145 cultures with more than 100 000 CFU/mL, Escherichia coli was the most commonly isolated organism (59/145 [40.7%]; Table 2). The clinician-prescribed antimicrobial choice was 57% for ciprofloxacin (156/272) and 43% for trimethoprim/sulfamethoxazole (116/272).
Table 1. Baseline Demographics and Comorbid Conditionsa.
| Variable | 7-Day antimicrobial + 7-day placebo group (n = 136)b,c | 14-Day antimicrobial group (n = 136)c |
|---|---|---|
| Age, median (IQR), y | 70 (62-73) | 70 (62-75) |
| Raced,e | (n = 135) | (n = 135) |
| White | 107 (79) | 105 (78) |
| Black | 26 (19) | 23 (17) |
| Native American | 1 (1) | 5 (4) |
| Multiple races | 1 (1) | 2 (1) |
| Hispanic/Latino ethnicityd,f | 5/132 (4) | 8/134 (6) |
| Charlson comorbidity index, median (IQR)g | 1 (0-2) | 1 (0-2) |
| Urinary tract–related comorbidities | (n = 136) | (n = 136) |
| Any prior UTI | 84 (62) | 78 (57) |
| Prostatic hypertrophy | 56 (41) | 47 (35) |
| Urinary incontinence | 44 (32) | 52 (38) |
| Intermittent catheter use | 24 (18) | 23 (17) |
| Prostate cancer | 21 (15) | 23 (17) |
| Urethral stricture | 17 (13) | 16 (12) |
| Prior prostatitis | 16 (12) | 18 (13) |
| Indwelling catheter use | 8 (6) | 8 (6) |
| Nonurinary comorbidities | (n = 136) | (n = 136) |
| Diabetes | 46 (34) | 60 (44) |
| Cerebrovascular accident | 13 (10) | 5 (4) |
| Chronic kidney disease | 8 (6) | 14 (10) |
| Spinal cord injury | 5 (4) | 6 (4) |
| HIV | 2 (1) | 2 (1) |
| Most common symptoms associated with UTI diagnosis | (n = 136) | (n = 136) |
| Dysuria | 93 (68) | 88 (65) |
| Frequency | 80 (59) | 70 (51) |
| Urgency | 52 (39) | 39 (29) |
Abbreviations: IQR, interquartile range; UTI, urinary tract infection.
Values are reported as No. (%) unless otherwise indicated.
This group received a placebo on days 8 through 14.
Percent calculations were based on 136 participants in each treatment group, except for the subcategories of race and the Hispanic/Latino ethnicity category.
Race and ethnicity were self-reported by participants, who chose from fixed categories.
Percent values for the subcategories of race are based on 135 participants in each treatment group.
Participants had the option to report as Hispanic Black or Hispanic White. In this case, all were Hispanic White.
Charlson Comorbidity Index was calculated using 22 comorbid conditions as documented in the medical record (score range, 0 [no comorbidities] to >20 [multiple comorbidities with increased mortality risk]). Expected 10-year survival for patients with a score of 1 is 73%; with a score of 2, expected 10-year survival is 52%.20
Table 2. Distribution of Organisms Isolated From 145 Urine Cultures With Growth at Greater Than 100 000 Colony-Forming Units/mLa.
| Organism isolated | No. (%) | |
|---|---|---|
| 7-Day antimicrobial + 7-day placebo group (n=70) | 14-Day antimicrobial group (n-75) | |
| Escherichia coli | 30 (43) | 29 (39) |
| Klebsiella species | 11 (16) | 12 (16) |
| Enterococcus species | 7 (10) | 6 (8) |
| Coagulase-negative staphylococci | 6 (9) | 8 (11) |
| Citrobacter species | 3 (4) | 3 (4) |
| Morganella morganii | 3 (4) | 1 (1) |
| Streptococcus species | 3 (4) | 2 (3) |
| Enterobacter species | 2 (3) | 2 (3) |
| Proteus mirabilis | 2 (3) | 2 (3) |
| Serratia marcescens | 2 (3) | 1 (1) |
| Staphylococcus aureus | 1 (1) | 2 (3) |
| Aerococcus urinae | 1 (1) | 1 (1) |
| Gram-positive bacilli, not further identified | 1 (1) | 1 (1) |
| Pseudomonas aeruginosa | 0 | 2 (3) |
| Salmonella species | 0 | 1 (1) |
No culture yielded more than 1 species. All isolated organisms were known (n = 139) or inferred (n = 16) to be susceptible to the prescribed antimicrobial (ciprofloxacin or trimethoprim/sulfamethoxazole). Susceptibility was inferred using facility-specific antimicrobial susceptibility data. Patients with urinary tract infections caused by nonsusceptible organisms were excluded from enrollment.
Primary Outcome
The primary outcome, resolution of UTI symptoms by day 14 after completion of active antibiotic treatment, occurred overall in 233/254 (91.7%) participants in the as-treated population and in 248/272 (91.2%) participants in the as-randomized population. By treatment group, in the as-treated population, symptom resolution occurred in 122/131 (93.1%) participants in the 7-day group vs 111/123 (90.2%) in the 14-day group (difference, 2.9% [1-sided 97.5% CI, −5.2% to ∞]), meeting the noninferiority criterion. In the as-randomized population, symptom resolution occurred in 125/136 (91.9%) participants in the 7-day group vs 123/136 (90.4%) in the 14-day group (difference, 1.5% [1-sided 97.5% CI, –5.8% to ∞]), also meeting the noninferiority criterion (Table 3). In the post hoc sensitivity analysis evaluating symptom resolution 14 days after stopping study medication (active antibiotic or placebo), rather than by day 14 after completion of active antibiotic treatment as originally performed, the overall proportion of participants with symptom resolution was unchanged from the original analysis (125/136 for the 7-day treatment group; 123/136 for the 14-day treatment group).
Table 3. Primary and Secondary Outcomes.
| Characteristic | No./total No. (%) | Absolute difference, % (1-sided 97.5% CI)a | |
|---|---|---|---|
| Resolution of UTI symptoms 14 days after stopping active antimicrobials | 7-Day antimicrobial + 7-day placebo group | 14-Day antimicrobial group | |
| As-treated population (primary analysis) | 122/131 (93.1) | 111/123 (90.2) | 2.9 (–5.2 to ∞) |
| As-randomized population | 125/136 (91.9) | 123/136 (90.4) | 1.5 (–5.8 to ∞) |
| Recurrence of UTI symptoms within 28 days of stopping study medication (secondary outcome) | 7-Day antimicrobial + 7-day placebo group | 14-Day antimicrobial group | Absolute difference, % (2-sided 95% CI)b |
| As-treated population | 13/131 (9.9) | 15/123 (12.9) | –3.0 (–10.8 to 6.2) |
| As-randomized population | 14/136 (10.3) | 23/136 (16.9) | –6.6 (–15.5 to 2.2) |
Abbreviation: UTI, urinary tract infection.
The primary analysis used a 1-sided 97.5% CI for noninferiority, which was established if the lower bound of the 1-sided 97.5% CI did not cross the noninferiority margin of −10% difference in symptom resolution.
The secondary outcome was analyzed using a 2-tailed superiority hypothesis test of differences in proportions (2-sample test for equality of proportions with continuity correction) with α = .05 and with 2-sided 95% CIs.
Secondary Outcome
In the as-treated population, the proportion of participants who reported recurrence of UTI symptoms was not significantly different between the 7-day group (13/131 [9.9%]) vs the 14-day group (15/123 [12.9%]) (difference, −3.0% [2-sided 95% CI, −10.8% to 6.2%]; P = .70). Comparable results were observed in the as-randomized population (10.3% recurrence in the 7-day group vs 16.9% in the 14-day group) (difference, −6.6% [2-sided 95% CI, −15.5% to 2.2%]; P = .20).
Adverse Events
In the as-treated-population, the proportion of participants who reported any adverse event was 26/131 (19.8%) in the 7-day group vs 29/123 (23.6%) in the 14-day group. In the as-randomized population, the proportion of participants who reported an adverse event was slightly higher overall than in the as-treated population (22.4% vs 21.7%). The proportion of participants with an adverse event for each group in the as-randomized population was 28/136 (20.6%) in the 7-day group vs 33/136 (24.3%) in the 14-day group. Individual adverse events are detailed in Table 4; only participants with diabetes (N = 64) or those taking warfarin (N = 10) were assessed for abnormal blood glucose values or effects to warfarin dosing. Among all participants, the most common adverse event was diarrhea, reported in 8.8% of participants in both treatment groups. Of the 64 participants with diabetes, 13 (20.3%) reported abnormal blood glucose values, with no notable differences between treatment groups. Of the 13 participants with dysglycemia, 11 (84.6%) had been prescribed ciprofloxacin and 2 (15.4%) were prescribed trimethoprim/sulfamethoxazole.
Table 4. Adverse Events Among the 272 Participants in the As-Randomized Population in Relation to Treatment Group.
| Adverse event | Participants, No./total No. (%)a | |
|---|---|---|
| 7-Day antimicrobial + 7-day placebo group (n = 136) | 14-Day antimicrobial group (n = 136) | |
| Warfarin dosing affectedb | 2/4 (50) | 3/6 (50) |
| Abnormal blood glucose levelsc | 7/28 (25) | 6/36 (17) |
| Diarrhea | 12 (9) | 12 (9) |
| Nausea | 8 (6) | 9 (7) |
| Headache | 4 (3) | 4 (3) |
| Dizziness | 3 (2) | 7 (5) |
| Muscle/joint aches | 2 (1) | 6 (4) |
| Tested for Clostridiodes difficile | 2 (1) | 0 |
| Allergy | 0 | 4 (3) |
| ≥1 Adverse event | 28 (21) | 33 (24) |
The percent values shown in each cell are based on the absolute values in that same cell, not for the number of participants in each study group.
Assessed only in the 10 participants taking warfarin.
Assessed only in the 64 participants with diabetes.
Post Hoc Analyses
Exploratory post hoc subgroup analyses were used to assess whether the likelihood of symptom resolution differed by type of antibiotic drug treatment, pretreatment bacteriuria count, and study site in both the overall and the 7- vs 14-day populations. Overall, symptom resolution occurred in 147/156 (94.2%) participants receiving ciprofloxacin vs 101/116 (87.1%) receiving trimethoprim/sulfamethoxazole. Using a generalized linear model with treatment duration, type of antibiotic drug treatment, and an interaction variable, the effect of drug treatment was not statistically significant: 147/156 (94.2%) with ciprofloxacin vs 101/116 (87.1%) with trimethoprim/sulfamethoxazole (P = .054). The treatment duration by drug treatment interaction was not statistically significant (P value for interaction, .37), indicating that any drug effect on symptom resolution did not significantly differ by treatment duration.
For the 239 participants who had a pretreatment urinary culture performed, post hoc subgroup analyses were done by assessing the likelihood of symptom resolution in relation to level of pretreatment bacteriuria, stratified as high-count bacteriuria (>100 000 CFU/mL; 145 participants), any bacteriuria (184 participants), and no bacteriuria (55 participants). Among participants with high-count bacteriuria who received 14 days of treatment, 69/75 (92.0%) had symptom resolution vs 65/70 (92.9%) who received 7 days of treatment. Participants with any bacteriuria had similar results, with 83/91 (91.2%) in the 7-day group vs 84/93 (90.3%) in the 14-day group having symptom resolution. Among participants with no bacteriuria, 28/30 (93.3%) reported symptom resolution in the 7-day group vs 25/25 in the 14-day group. The treatment duration by level of pretreatment bacteriuria interaction effect in a generalized linear model was not significant (P = .53), indicating that level of pretreatment bacteriuria did not differentially affect symptom resolution under the 2 treatment durations.
A post hoc subgroup analysis regarding the differential effect of study site (Minneapolis vs Houston) on treatment response using a generalized linear mixed model, with site modeled as a random effect, yielded a nonsignificant treatment duration by study site interaction effect (P = .88), indicating that the study site did not differentially affect treatment response.
Discussion
In this randomized, double-blinded, placebo-controlled trial of 7 vs 14 days of antibiotic treatment with ciprofloxacin or trimethoprim/sulfamethoxazole for afebrile men with UTI, 7 days of treatment was noninferior to 14 days for the primary outcome of symptom resolution. There was no statistically significant difference in the secondary outcome of participant-reported recurrence of UTI symptoms, and reported adverse events were not notably different between groups.
This study was limited to ciprofloxacin and trimethoprim/sulfamethoxazole because at the time of study initiation, these agents accounted for 90% of treatment courses for UTI among ambulatory male patients in the VA.13 Inclusion of additional agents, such as amoxicillin-clavulanate or nitrofurantoin was considered, use of which is observed to be increasingly common, but this was not done for several reasons. First, the data supporting use of these agents for UTIs in men are relatively sparse. Second, inclusion of these drugs would have introduced substantial heterogeneity into the study population. Third, inclusion of these drugs would have posed additional barriers to achieving blinding, which relied on availability of the same agent but with a different appearance from the original, which would have been very challenging.
This trial was conducted as a pragmatic trial of treatment duration among male veterans who had been diagnosed with and were being treated for UTI by clinical staff. As such, the trial was designed to reflect routine clinical practice, in which clinicians do not consistently order a urine culture when managing a case of suspected UTI. Although, for such patients, institutional guidance at both study sites recommends a pretherapy urine culture, this recommendation is not followed consistently. Accordingly, 12.1% of participants had no pretreatment urine culture evaluated. Inclusion of patients without true UTI might have diluted any effect of treatment duration or choice of agent. However, in the sensitivity analysis, rates of symptom resolution across strata of pretreatment urine culture results (high-count bacteriuria, any bacteriuria, and no growth) were not significantly different; within each stratum, there were no significant differences in outcomes comparing short-duration vs long-duration treatment. This suggests that inclusion of participants with low-count bacteriuria or a negative urine culture did not confound the relationship between treatment duration and success to an important extent.
Flexible recruitment methods were used, including enrollment by mail or at home for 52.2% of participants. This enabled enrollment of patients who viewed the burden of driving to and from the medical center as too onerous. It also allowed enrollment of participants whose initial clinical encounter for UTI symptoms occurred on weekends or during off-hours, enrollment of whom otherwise would have required additional staff and resources.
Limitations
This study has several limitations. First, because of its pragmatic nature, some patients may not have had a UTI, which would bias the results to finding no significant difference. Second only 2 antibiotics were assessed, albeit the 2 most commonly used for men with UTI in the VA. Third, this study was conducted in the VA system and may not be generalizable to nonveterans. Fourth, enrollment fell short of the planned 290 participants, possibly decreasing power to detect a clinically significant difference. Fifth, the noninferiority margin was based on expert opinion rather than evidence. Sixth, there was an imbalance in participants not taking medications as directed, 5 in the 7-day group vs 13 in the 14-day group; these participants were removed from the as-treated population but remained in the as-randomized population for which results were similar. Seventh, this study began in 2014 and ended in 2019; while this spans a considerable time, the question of how long to treat an afebrile man with UTI remains relevant, and ciprofloxacin and trimethoprim/sulfamethoxazole are still commonly used to treat UTI.14
Conclusions
Among afebrile men with suspected UTI, treatment with ciprofloxacin or trimethoprim/sulfamethoxazole for 7 days was noninferior to 14 days of treatment with regard to resolution of UTI symptoms by 14 days after antibiotic therapy. The findings support the use of a 7-day course of ciprofloxacin or trimethoprim/sulfamethoxazole as an alternative to a 14-day course for treatment of afebrile men with UTI.
Trial Protocol Version 1.0; August 15, 2013
Trial Protocol 9.0; February 19, 2019
Data Sharing Statement
References
- 1.Barlam TF, Cosgrove SE, Abbo LM, et al. Executive summary: implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):1197-1202. doi: 10.1093/cid/ciw217 [DOI] [PubMed] [Google Scholar]
- 2.Chastre J, Wolff M, Fagon JY, et al. ; PneumA Trial Group . Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290(19):2588-2598. doi: 10.1001/jama.290.19.2588 [DOI] [PubMed] [Google Scholar]
- 3.Uranga A, España PP, Bilbao A, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med. 2016;176(9):1257-1265. doi: 10.1001/jamainternmed.2016.3633 [DOI] [PubMed] [Google Scholar]
- 4.Sawyer RG, Claridge JA, Nathens AB, et al. ; STOP-IT Trial Investigators . Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med. 2015;372(21):1996-2005. doi: 10.1056/NEJMoa1411162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gariani K, Pham TT, Kressmann B, et al. Three versus six weeks of antibiotic therapy for diabetic foot osteomyelitis: a prospective, randomized, non-inferiority pilot trial. Clin Infect Dis. Published online November 26, 2020. doi: 10.1093/cid/ciaa1758 [DOI] [PubMed] [Google Scholar]
- 6.Hepburn MJ, Dooley DP, Skidmore PJ, Ellis MW, Starnes WF, Hasewinkle WC. Comparison of short-course (5 days) and standard (10 days) treatment for uncomplicated cellulitis. Arch Intern Med. 2004;164(15):1669-1674. doi: 10.1001/archinte.164.15.1669 [DOI] [PubMed] [Google Scholar]
- 7.Gupta K, Hooton TM, Roberts PL, Stamm WE. Short-course nitrofurantoin for the treatment of acute uncomplicated cystitis in women. Arch Intern Med. 2007;167(20):2207-2212. doi: 10.1001/archinte.167.20.2207 [DOI] [PubMed] [Google Scholar]
- 8.Gupta K, Hooton TM, Naber KG, et al. ; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases . International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-e120. doi: 10.1093/cid/ciq257 [DOI] [PubMed] [Google Scholar]
- 9.Tamma PD, Turnbull AE, Milstone AM, Lehmann CU, Sydnor ER, Cosgrove SE. Ventilator-associated tracheitis in children: does antibiotic duration matter? Clin Infect Dis. 2011;52(11):1324-1331. doi: 10.1093/cid/cir203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulleryd P, Sandberg T. Ciprofloxacin for 2 or 4 weeks in the treatment of febrile urinary tract infection in men: a randomized trial with a 1 year follow-up. Scand J Infect Dis. 2003;35(1):34-39. doi: 10.1080/0036554021000026988 [DOI] [PubMed] [Google Scholar]
- 11.van Nieuwkoop C, van der Starre WE, Stalenhoef JE, et al. Treatment duration of febrile urinary tract infection: a pragmatic randomized, double-blind, placebo-controlled non-inferiority trial in men and women. BMC Med. 2017;15(1):70. doi: 10.1186/s12916-017-0835-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dow G, Rao P, Harding G, et al. A prospective, randomized trial of 3 or 14 days of ciprofloxacin treatment for acute urinary tract infection in patients with spinal cord injury. Clin Infect Dis. 2004;39(5):658-664. doi: 10.1086/423000 [DOI] [PubMed] [Google Scholar]
- 13.Drekonja DM, Rector TS, Cutting A, Johnson JR. Urinary tract infection in male veterans: treatment patterns and outcomes. Arch Intern Med. 2012;173(1):62-68. doi: 10.1001/2013.jamainternmed.829 [DOI] [PubMed] [Google Scholar]
- 14.Germanos GJ, Trautner BW, Zoorob RJ, et al. No clinical benefit to treating male urinary tract infection longer than seven days: an outpatient database study. Open Forum Infect Dis. 2019;6(6):ofz216. doi: 10.1093/ofid/ofz216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta K, Grigoryan L, Trautner B. Urinary tract infection. Ann Intern Med. 2017;167(7):ITC49-ITC64. doi: 10.7326/AITC201710030 [DOI] [PubMed] [Google Scholar]
- 16.Talan DA, Stamm WE, Hooton TM, et al. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis pyelonephritis in women: a randomized trial. JAMA. 2000;283(12):1583-1590. doi: 10.1001/jama.283.12.1583 [DOI] [PubMed] [Google Scholar]
- 17.Kronenberg A, Bütikofer L, Odutayo A, et al. Symptomatic treatment of uncomplicated lower urinary tract infections in the ambulatory setting: randomised, double blind trial. BMJ. 2017;359:j4784. doi: 10.1136/bmj.j4784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veve MP, Wagner JL, Kenney RM, Grunwald JL, Davis SL. Comparison of fosfomycin to ertapenem for outpatient or step-down therapy of extended-spectrum β-lactamase urinary tract infections. Int J Antimicrob Agents. 2016;48(1):56-60. doi: 10.1016/j.ijantimicag.2016.04.014 [DOI] [PubMed] [Google Scholar]
- 19.Shan G, Wang W.. ExactCIdiff: an R package for computing exact confidence intervals for the difference of two proportions. The R Journal. 2013;5(2): 62-70. doi: 10.32614/RJ-2013-026 [DOI] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol Version 1.0; August 15, 2013
Trial Protocol 9.0; February 19, 2019
Data Sharing Statement