This systematic review and meta-analysis synthesizes whether patients with mental health disorders are at increased risk of COVID-19 mortality compared with patients without mental health disorders.
Key Points
Question
Is there a significant association between mental health disorders and COVID-19–related mortality?
Findings
In this systematic review and meta-analysis of 16 observational studies in 7 countries with 19 086 patients, mental health disorders were associated with increased COVID-19 mortality according to both pooled crude and adjusted odds ratios. Patients with severe mental health disorders had the highest odds ratios.
Meaning
These findings suggest that patients with COVID-19 and mental health disorders should be targeted as a high-risk population for severe forms of COVID-19, requiring enhanced preventive and disease management strategies.
Abstract
Importance
Heterogeneous evidence exists for the association between COVID-19 and the clinical outcomes of patients with mental health disorders. It remains unknown whether patients with COVID-19 and mental health disorders are at increased risk of mortality and should thus be targeted as a high-risk population for severe forms of COVID-19.
Objective
To determine whether patients with mental health disorders were at increased risk of COVID-19 mortality compared with patients without mental health disorders.
Data Sources
For this systematic review and meta-analysis, MEDLINE, Web of Science, and Google Scholar were searched from inception to February 12, 2021. Bibliographies were also searched, and the corresponding authors were directly contacted. The search paradigm was based on the following combination: (mental, major[MeSH terms]) AND (COVID-19 mortality[MeSH terms]). To ensure exhaustivity, the term mental was replaced by psychiatric, schizophrenia, psychotic, bipolar disorder, mood disorders, major depressive disorder, anxiety disorder, personality disorder, eating disorder, alcohol abuse, alcohol misuse, substance abuse, and substance misuse.
Study Selection
Eligible studies were population-based cohort studies of all patients with identified COVID-19 exploring the association between mental health disorders and mortality.
Data Extraction and Synthesis
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline was used for abstracting data and assessing data quality and validity. This systematic review is registered with PROSPERO.
Main Outcomes and Measures
Pooled crude and adjusted odds ratios (ORs) for the association of mental health disorders with mortality were calculated using a 3-level random-effects (study/country) approach with a hierarchical structure to assess effect size dependency.
Results
In total, 16 population-based cohort studies (data from medico-administrative health or electronic/medical records databases) across 7 countries (1 from Denmark, 2 from France, 1 from Israel, 3 from South Korea, 1 from Spain, 1 from the UK, and 7 from the US) and 19 086 patients with mental health disorders were included. The studies covered December 2019 to July 2020, were of good quality, and no publication bias was identified. COVID-19 mortality was associated with an increased risk among patients with mental health disorders compared with patients without mental health disorders according to both pooled crude OR (1.75 [95% CI, 1.40-2.20]; P < .05) and adjusted OR (1.38 [95% CI, 1.15-1.65]; P < .05). The patients with severe mental health disorders had the highest ORs for risk of mortality (crude OR: 2.26 [95% CI, 1.18-4.31]; adjusted OR: 1.67 [95% CI, 1.02-2.73]).
Conclusions and Relevance
In this systematic review and meta-analysis of 16 observational studies in 7 countries, mental health disorders were associated with increased COVID-19–related mortality. Thus, patients with mental health disorders should have been targeted as a high-risk population for severe forms of COVID-19, requiring enhanced preventive and disease management strategies. Future studies should more accurately evaluate the risk for patients with each mental health disorder. However, the highest risk seemed to be found in studies including individuals with schizophrenia and/or bipolar disorders.
Introduction
More than 100 million people have been infected with SARS-CoV-2, and almost 2.5 million have died of COVID-19 worldwide.1 These numbers probably underestimate COVID-19 deaths by 50% owing to misclassified dementia and cardiovascular or metabolic deaths.2 Patients with mental health disorders may be at particular risk of poor COVID-19 outcomes. Patients with mental health disorders can have multiple comorbidities that have been identified as risk factors for severe COVID-19: diabetes, hypertension, chronic obstructive respiratory disease, and end-stage kidney disease.3 Mental health disorders are also associated with socioeconomic deprivation4 and reduced access to care,5 2 important factors of poor COVID-19 outcomes.6
Data on the risks of poor COVID-19 outcomes among patients with mental health disorders continue to evolve from the first reports. Several population-based studies from South Korea, the US, and France revealed that severe mental health disorders (defined across studies by schizophrenia spectrum disorders and/or bipolar disorders) are risk factors for increased COVID-19 mortality.7,8,9 The results were less clear for other mental health disorders (ie, depressive disorders, anxiety disorders, eating disorders, and personality disorders).8,9,10,11 Determining whether patients with mental health disorders are at high risk of severe COVID-19 is an urgent research priority and can alert health policy makers and lead to adaptions in preventive care and disease management strategies to meet their health needs.12 A preliminary meta-analysis has concluded that patients with mental health disorders were at increased risk of COVID-19 mortality.13 However, this meta-analysis had methodological issues because the interdependency of effect sizes was not handled, in particular within each country. Moreover, mental health disorders were not analyzed separately, and there may be important discrepancies between diagnoses, which should guide health strategy priorities.
We carried out a systematic review and meta-analysis to synthesize the accumulating research on mental health disorders and COVID-19. The primary objective was to determine whether patients with mental health disorders are at increased risk of COVID-19 mortality compared with patients without mental health disorders. The secondary objectives were to determine whether patients with mental health disorders are at increased risk of intensive care unit (ICU) admission and which specific mental health disorders were associated with the risk of COVID-19 mortality.
Methods
Literature Search Strategy
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.14 Systematic bibliographic searches were carried out according to the Cochrane methodology. This project was registered in PROSPERO (CRD42021238038).
The search paradigm was based on the PubMed interface (MEDLINE database) and adapted for 2 databases: ScienceDirect and Google Scholar. The search paradigm was based on the following combination: (mental, major[MeSH terms]) AND (COVID-19 mortality[MeSH terms]). To ensure exhaustivity, the term mental was replaced by psychiatric, schizophrenia, psychotic, bipolar disorder, mood disorders, major depressive disorder, anxiety disorder, personality disorder, eating disorder, alcohol abuse, alcohol misuse, substance abuse, and substance misuse.
The last search was carried out on February 22, 2021. The reference lists and bibliographies of relevant reviews and articles retrieved from the database searches were manually searched for additional eligible articles. The authors of the relevant studies were contacted when additional data or clarifications were required.
Eligibility
The inclusion criteria were as follows: (1) articles in any language and with any date of publication; (2) original research articles; (3) population-based studies based on medico-administrative health databases or a health care data warehouse; (4) studies that included participants with a diagnosis of mental health disorders according to the DSM or any version of the International Classification of Diseases (ICD); (5) studies on patients with clinical or biological diagnosis of COVID-19; and (6) articles with mortality and ICU admission data.
The titles and abstracts were screened by 2 researchers (G.F. and D.E.-E.). The full texts of the articles were then reviewed to determine whether they would be included by authors (G.F., D.E.-E., and L.B.).
Data Extraction
The following data were extracted: first author, timing (period of inclusion of cases and follow-up), databases, population, study setting, COVID-19 criteria inclusion, mental health disorders group definition, mean age, percentage of men, mortality rate, mortality crude odds ratio (OR), mortality adjusted OR, ICU admission crude OR, and ICU admission adjusted OR.
Two researchers (G.F. and D.E.-E.) extracted data from the included studies systematically using a predesigned extraction form. Each discrepancy in data extraction was examined by the first and last authors (G.F. and L.B.) to reach a consensus.
Assessment of Risk of Bias
To assess the risk of bias, we used the Newcastle-Ottawa Quality Assessment Scale. A good quality score required 3 or 4 stars for the selected item, 1 or 2 stars for the comparability item, and 2 or 3 stars for the outcomes item. A fair quality score required 2 stars for selection, 1 or 2 stars for comparability, and 2 or 3 stars for outcomes. A poor quality score required 0 or 1 star(s) for the selection, 0 stars for comparability, or 0 or 1 star(s) for outcome.
Statistical Analyses
A random-effects model was used to calculate the pooled crude and adjusted log ORs with 95% CIs for mortality and ICU admission. When available, we used the numbers of events and the sample sizes instead of the value of the crude ORs.15 Because studies may show a certain degree of overlap in observations (several ORs per study and a potential higher-level unit by country), we used a 3-level random-effects (study/country) approach with a hierarchical structure.
We also investigated sources of heterogeneity using Cochran Q and I2 statistics. I2 is reported as a percentage out of 100%, whereby 0% to 40% denotes that the heterogeneity might not be important, 30% to 60% indicates moderate heterogeneity, 50% to 90% represents to substantial heterogeneity, and 75% to 100% indicates considerable heterogeneity.16 Publication bias was assessed graphically with a funnel plot and statistically with modified Egger regression test computed by including the standard error as a predictor of effect sizes in the multilevel model.17
For subgroup analyses, we explored the association of the following study characteristics with outcomes: mean age older than 65 years vs 65 years and younger; inpatients and outpatients vs only inpatients; laboratory-confirmed COVID-19 vs ICD criteria inclusion; and severe mental health disorders (defined as schizophrenia spectrum disorder ± bipolar disorders if bipolar disorders were distinguished from other mood disorders) vs nonsevere mental health disorders (defined as mood disorders, anxiety disorders, personality disorders, eating disorders, and alcohol and substance misuses). All analyses were performed in R using the metafor package.18 Two-sided P values were significant at .05.
Results
Search Strategy
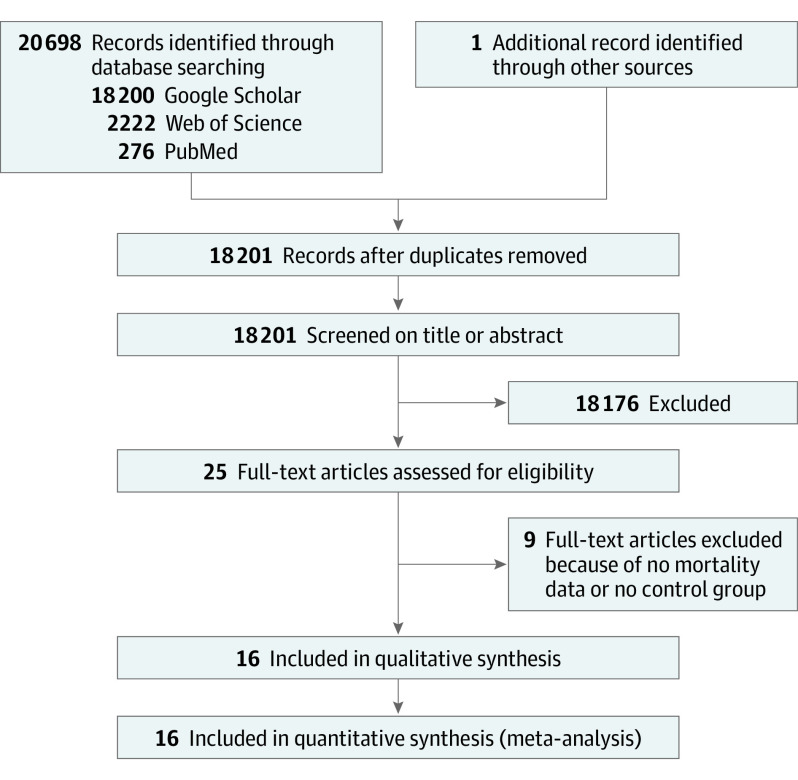
We identified 18200 studies from the database search and no additional records through other sources. Sixteen studies met the eligibility criteria7,8,9,10,11,19,20,21,22,23,24,25,26,27 (1 unpublished study by G.F. et al based on the French national hospital database) and were included in the quantitative analysis (Figure 1). A Swedish study28 was not included because no data on patients with COVID-19 were available at the time of the analyses of the present work. Overall, the data of 19 086 patients with mental health disorders and COVID-19 were analyzed. It was not possible to determine the total number of individual patients, as some studies had overlaps in their samples.
Figure 1. Flowchart.
Study Characteristics
The study and patient characteristics are presented in Table 1 and Table 2. One study was carried out in Denmark,24 2 in France (including the unpublished study by G.F. et al),7 1 in Israel,26 3 in South Korea,9,10,11 1 in Spain,19 1 in the UK,24 and 7 in the US.8,20,21,22,23,27,29 The definition of the mental health disorders group varied across countries. The 3 South Korean studies,9,10,11 2 US studies,21,27 and the UK study25 included a large definition of mental health disorders (almost all F00-F90 codes) with addictions mixed with mental health disorders. Two US studies22,23 included only patients with addictions (with no information on their mental status), and the Danish study24 analyzed addictions separately from mental health disorders. No study reported on patients with the combination of a mental health disorder and addiction. It was possible to distinguish severe mental health disorders (as defined in the Methods section) in 6 studies (including the unpublished study by G.F. et al).7,8,9,24,26 Only patients with schizophrenia were analyzed in the French and Israeli studies,7,26 and patients with schizophrenia were included in a schizophrenia spectrum diagnosis group in 3 studies8,9,26 and were combined with patients with bipolar disorders in 1 study.24 Patients with bipolar disorders were analyzed separately in only 1 unpublished study by G.F. et al, and were included in the mood disorder group in 2 studies.8,19 The last study, published as a letter, did not describe the mental health disorders of the patients in the mental health disorders group.20
Table 1. Study Characteristics.
| Source | Timing | Database | Population | Study setting | COVID-19 criteria inclusion | Mental health disorders group definition |
|---|---|---|---|---|---|---|
| Denmark | ||||||
| Reilev et al,24 2020 | February 27 to May 19, 2020 | Danish Microbiology Database linked to the Danish administrative and health care registries | Whole Danish population tested for SARS-CoV-2 infection | Inpatients and outpatients | Positive reverse transcription–polymerase chain reaction test | Mental health disorders (including schizophrenia and bipolar disorders); alcohol misuse; substance use |
| France | ||||||
| Fond et al,7 2021 | February 1 to June 9, 2020 | French national hospital database for acute care | Whole French population hospitalized for COVID-19 aged ≥15 y | Inpatients | COVID-19 ICD-10 codes (U07.1 or U07.2), respiratory symptoms (U07.10 or U07.11), and a length of hospital stay >24 h | Schizophrenia |
| Unpublished study by Fond et al | February 1 to June 9, 2020 | French national hospital database for acute care. In this unpublished study, data were anonymized and can be reused for research purposes. No informed consent was necessary because all data were anonymous. This study was declared to the French National Data Protection Commission in accordance with the methodological reference MR005 (declaration number: 2203797). |
Whole French population hospitalized for COVID-19 aged ≥15 y | Inpatients | COVID-19 ICD-10 codes (U07.1 or U07.2), respiratory symptoms (U07.10 or U07.11), and a length of hospital stay >24 h | Bipolar disorders |
| Israel | ||||||
| Tzur Bitan et al,26 2021 | NA | Israeli health care database | 5 Million citizens (half of the population) | Inpatients and outpatients | Positive reverse transcription–polymerase chain reaction test | Schizophrenia |
| South Korea | ||||||
| Lee et al,9 2020 | January 1 to May 15, 2020 | South Korean national health insurance claims database | 98% Of the whole population aged >20 y | Inpatients and outpatients | Positive reverse transcription–polymerase chain reaction test | One of the following mental health disorders: nonaffective psychotic disorders; affective psychotic disorders; anxiety- and stress-related disorders; alcohol or drug misuse; mood disorders without psychotic symptoms; eating disorders and personality disorders |
| Lee et al,11 2020 | January 1 to April 10, 2020 | South Korean National Health Insurance Review and Assessment Service database | Individuals aged ≥65 y | Inpatients and outpatients | Laboratory-confirmed COVID-19 | Schizophrenia, psychotic disorders; bipolar disorders; depressive disorder; anxiety disorder; panic disorder, acute stress disorder; insomnia; dementia; organic mental disorder; psychoactive substance dependence; psychoactive substance use disorder; psychoactive substance-induced organic mental disorder; psychosomatic factor in physical condition |
| Jeon et al,10 2021 | December 1, 2019, to May 15, 2020 | South Korean national health insurance claims database linked to the Korea Disease Control and Prevention Agency database | Whole population without age restriction | Inpatients and outpatients | Diagnostic code (specific to the database) | Mental health disorders (included schizophrenia spectrum disorder and mood disorders) |
| Spain | ||||||
| Poblador-Plou et al,19 2020 | March 4 to May 17, 2020 | PRECOVID database (created to follow patients with positive COVID-19 test results) linked to Aragon Health System | Spanish region of Aragon | Inpatients and outpatients | Laboratory-confirmed COVID-19 | Bipolar disorders; depressive disorders; anxiety disorders |
| United Kingdom | ||||||
| Yang et al,25 2020 | January 31 to May 31, 2020 | The UK Biobank | 502 507 Participants, aged 40 to 69 y, from England, Scotland, and Wales | Inpatients | Positive reverse transcription–polymerase chain reaction test | Psychotic disorders; depressive disorders; anxiety; stress-related disorder; alcohol and substance misuse |
| United States | ||||||
| Allen et al,22 2020 | January 1 to October 26, 2020 | NYULH linked to NYULH’s COVID-19 deidentified clinical database, which includes data from 4 acute care hospitals | Individuals in greater New York City | Inpatients and outpatients | Positive reverse transcription–polymerase chain reaction test | Any alcohol or substance use disorder excluding nicotine dependence |
| Baillargeon et al,23 2021 | February 20 to July 31, 2020 | TriNetX Research Network platform database, which includes data from 35 health care organizations including hospitals, primary care clinics, and specialty treatment institutions | Approximately 54 million patients aged ≥18 y | Inpatients and outpatients | COVID-19 ICD-10 codes (B34.2, B97.29, J12.81, U07.1, U07.2) or laboratory-confirmed COVID-19 | Substance use disorder |
| Egede et al,21 2021 | Unknown beginning to July 2020 | Froedtert/Medical College of Wisconsin Epic medical record database, which includes data from 5 hospitals and 40 health centers and clinics | One-quarter of the population of southeastern Wisconsin | Inpatients and outpatients | Positive reverse transcription–polymerase chain reaction test | Any mental health disorders with or without physical illness including schizophrenia spectrum disorder, mood disorders (including bipolar disorders and depressive disorders), alcohol misuse and drug misuse |
| Nemani et al,8 2021 | March 3 to May 31, 2020 | NYULH electronic health record system database, which includes data from more than 260 outpatient office sites and 4 acute care hospitals | Individuals from Manhattan, Brooklyn, and Long Island, New York | Inpatients and outpatients | Positive reverse transcription–polymerase chain reaction test | Schizophrenia spectrum disorder mood disorders (including bipolar disorders and depressive disorders); anxiety disorders |
| Li et al,20 2020 | February 15 to May 27, 2020 | Yale New Haven Health System database, which includes data from a 5-hospital system | Individuals in the northeast of the United States | Inpatients and outpatients | NA | Psychiatric diagnoses (nonspecified) |
| Wang et al,27 2021 | Unknown beginning to July 29, 2020 | IBM-Watson Health Explorys database, which includes data from a 360-hospital system across 50 states | 20% Of the United States population | Inpatients and outpatients | Diagnostic code (specific to the database) | Psychiatric diagnoses; schizophrenia; bipolar disorders; depressive disorders; attention-deficit/hyperactivity disorder; substance use disorder |
Abbreviations: ICD-10, International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; NA, not available; NYULH, NYU Langone Health.
Table 2. Crude and Adjusted Mortality Odds Ratio .
| Source | Topic | No. | Age, mean (SD) | % | OR (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mortality | ICU admission | ||||||||
| Men | Mortality | Crude | Adjusted | Crude | Adjusted | ||||
| Denmark | |||||||||
| Reilev et al,24 2020 | SZ and BD | 76 | NA | NA | 2.3 | 3.8 (2.1-7.0) | 2.5 (1.2-5.1) | NA | NA |
| Alcohol misuse | 298 | NA | NA | 6.4 | 2.7 (1.9-3.9) | 1.8 (1.2-2.7) | NA | NA | |
| Substance use | 185 | NA | NA | 3.6 | 2.4 (1.5-3.8) | 1.8 (1.1-3.2) | NA | NA | |
| France | |||||||||
| Fond et al,7 2021 | SZ | 823 | 69.2 (14.6) | 48.8 | 25.6 | 1.25 (1.05-1.49) | 1.30 (1.08-1.56) | 0.78 (0.65-0.94) | 0.75 (0.62-0.91) |
| Unpublished study by Fond et al | BD | 480 | 70.9 (13.5) | 37.9 | 26.0 | 1.30 (1.16-1.45) | 1.29 (1.15-1.44) | 1.08 (0.97-1.20) | 1.02 (0.91-1.14) |
| Israel | |||||||||
| Tzur-Bitan et al,26 2021 | SZ | 649 | NA | NA | 3.4 | NA | NA | NA | NA |
| South Korea | |||||||||
| Lee et al,9 2020 | MD | 1443 | 59.5 (17.2) | 39.6 | 6.7 | 1.39 (1.01-1.95) | 1.38 (1.00-1.95) | 1.17 (0.82-1.68) | 1.18 (0.82-1.70) |
| Severe MDa | 404a | 58.7 (17.3)a | 55.9a | NAa | NAa | NAa | NAa | NAa | |
| Lee et al,11 2020 | MD | 255 | 77.2 (7.5) | 42.7 | 13.7 | 1.38 (0.86-2.21) | NA | NA | NA |
| Jeon et al,10 2021 | MD | 928 | 61.2 (18.2) | 40.2 | 6.03 | 7.12 (4.87-10.39) | 3.93 (2.57-6.03) | NA | NA |
| SZa | 159a | NAa | NAa | 3.8a | NAa | 2.25 (0.36-14.03)a | NAa | NAa | |
| BD and MDDa | 273a | NAa | NAa | 4.4a | NAa | 2.33 (0.96-5.66)a | NAa | NAa | |
| Spain | |||||||||
| Poblador-Plou et al,19 2020 | Men with BD and MDD | 70 | NA | 100 | NA | NA | 1.38 (0.98-1.95) | NA | NA |
| Men with AD | 38 | NA | 100 | NA | NA | 0.80 (0.53-1.21) | NA | NA | |
| Women with BD and MDD | 132 | NA | 0 | NA | NA | 1.46 (1.12-1.91) | NA | NA | |
| Women with AD | 83 | NA | 0 | NA | NA | 1.21 (0.90-1.64) | NA | NA | |
| United Kingdom | |||||||||
| Yang et al,25 2020 | MD | 442 | NA | NA | 27.1 | NA | NA | NA | NA |
| United States | |||||||||
| Allen et al,22 2020 | Alcohol and substance use disorder | 395 | NA | NA | NA | 1.20 (0.76-1.90) | 0.91 (0.53-1.57) | 3.20 (2.29-4.48) | 2.61 (1.80-3.79) |
| Baillargeon et al,23 2020 | Substance use disorder | 5450 | 53.8 (17.3) | 52.4 | 4.7 | NA | 1.00 (0.84-1.20) | NA | NA |
| Egede et al,21 2021 | MD with physical illness | 505 | 54.0 (17.8) | 33.5 | 7.7 | 1.52 (0.99-2.32) | 1.35 (0.85-2.08) | NA | NA |
| MD without physical illness | 52 | 37.7 (15.8) | 34.6 | 1.9 | 0.39 (0.05-2.86) | 1.08 (0.15-8.05) | NA | NA | |
| Nemani et al,8 2021 | SZ | 75 | 59.7 (15.0) | 56.0 | 26.7 | 2.93 (1.75-4.92) | 2.67 (1.48-4.80) | NA | NA |
| BD and MDD | 564 | 62.3 (18.7) | 39.9 | 18.4 | 1.82 (1.45-2.29) | 1.14 (0.87-1.49) | NA | NA | |
| AD | 360 | 54.9 (19.3) | 39.7 | 10.8 | 0.98 (0.70-1.38) | 0.96 (0.65-1.40) | NA | NA | |
| Li et al,20 2020 | MD | 473 | NA | NA | 44.8 | 2.3 (1.8-2.9) | 1.5 (1.1-1.9) | NA | NA |
| Wang et al,27 2021 | MD | 3430 | NA | NA | 8.5 | NA | NA | NA | NA |
| Alcohol and substance use disorder | 1880 | NA | NA | 9.6 | NA | NA | NA | NA | |
Abbreviations: AD, anxiety disorder; BD, bipolar disorder; ICU, intensive care unit; MD, mental health disorder; MDD, major depressive disorder; NA, not available; OR, odds ratio; SZ, schizophrenia.
Subgroup analyses.
Quality assessment
The study quality is presented in eTable 1 in the Supplement. All studies had a good quality score.
Meta-analysis
The forest plots of the adjusted ORs are presented in Figure 2 (mental health disorders) and Figure 3 (severe mental health disorders). The funnel plot is presented in eFigure 1 in the Supplement and shows no publication bias (Egger test > 0.05). COVID-19 mortality was associated with an increase in patients with mental health disorders compared with patients without mental health disorders (pooled adjusted OR, 1.38 [95% CI, 1.15-1.65]; I2 = 0%). In the studies including only patients with severe mental health disorders (schizophrenia spectrum disorders and/or bipolar disorders), the pooled adjusted OR was 1.67 (95% CI, 1.02-2.73; I2 = 27.3%). In the other studies that included patients with all mental health disorders and addictions, the pooled adjusted OR was 1.34 (95% CI, 1.08-1.65; I2 = 0%). All P values were significant (P < .05). The ICD codes are presented in eTable 2 in the Supplement and adjustment factors are in eTable 3 in the Supplement.
Figure 2. Forest Plot of the Association Between Mental Health Disorders and Mortality (Adjusted Odds Ratio).
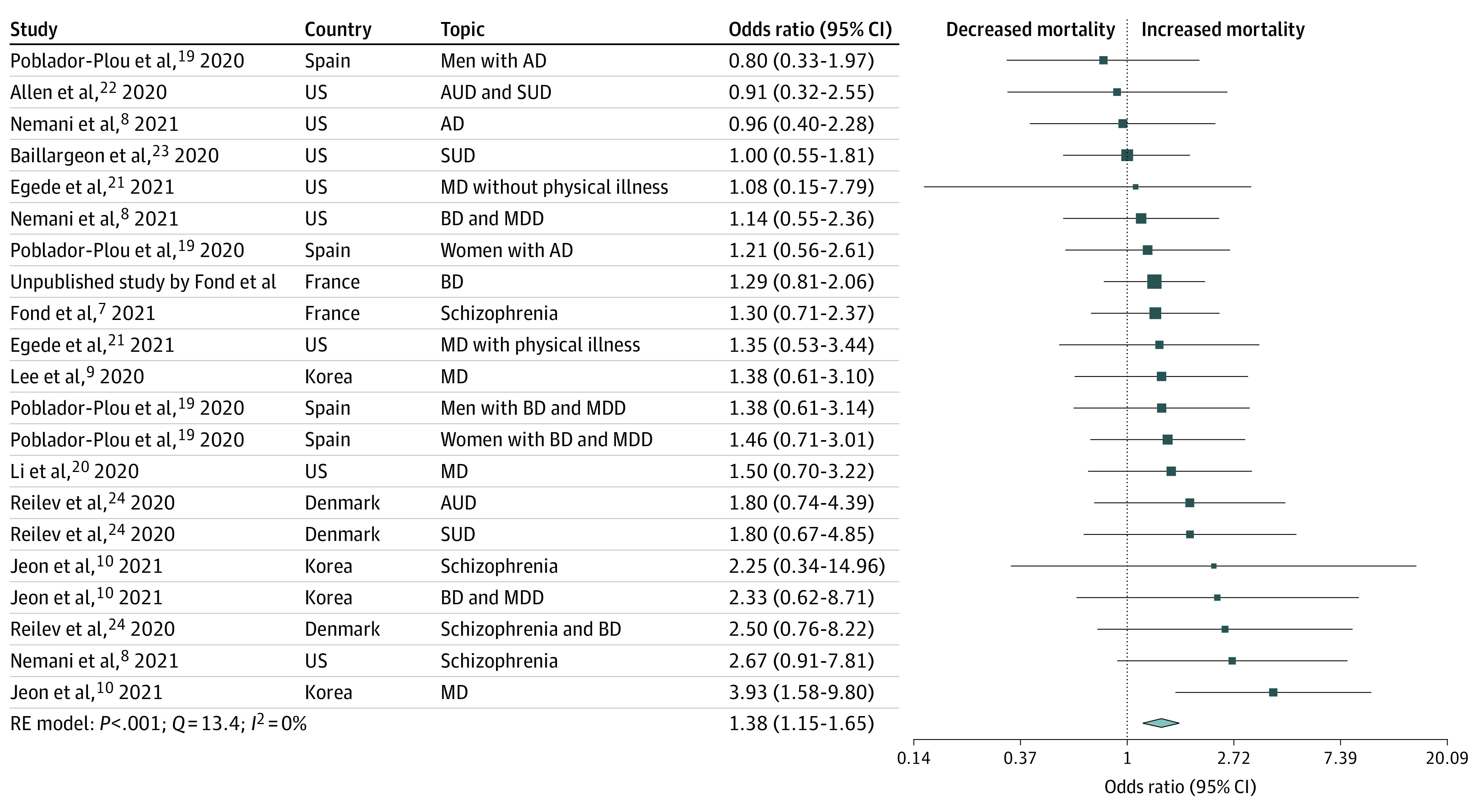
AD indicates anxiety disorder; AUD, alcohol use disorder; BD, bipolar disorder; MD, mental health disorder; MDD, major depressive disorder; SUD, substance use disorder.
Figure 3. Forest Plot of the Association Between Severe Mental Health Disorders and Mortality (Adjusted Odds Ratio).
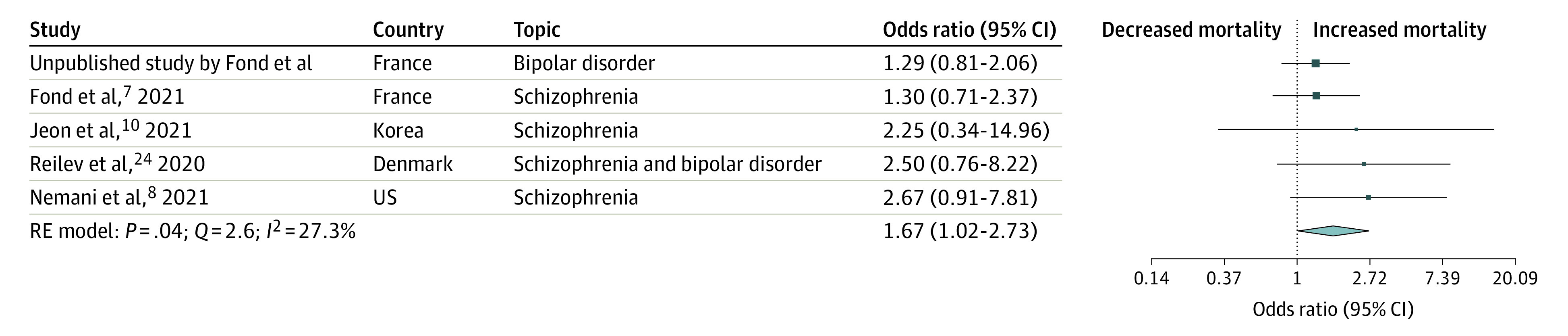
Severe mental health disorders were defined as schizophrenia spectrum disorder and/or bipolar disorder.
The forest plots of the crude ORs are presented in eFigure 2 in the Supplement and confirm the abovementioned results (pooled crude OR, 1.75 [95% CI, 1.40-2.19]; I2 = 26.1%). In studies including only patients with severe mental health disorders, the pooled crude OR was 2.26 (95% CI, 1.18-4.31; I2 = 55.5%; eFigure 3 in the Supplement). In the other studies that included patients with all mental health disorders and addictions, the pooled crude OR was 1.72 (95% CI, 1.45-2.04; I2 = 0%). All P values were significant (P < .05).
The subgroup analyses did not report any significant difference in either the crude or adjusted OR. Comparing patients with each mental health disorder in head-to-head comparisons or patients with severe mental health disorders with those with nonsevere mental health disorders was not possible because in all studies including patients with nonsevere mental health disorders except 1,8 patients with nonsevere mental health disorders were combined with patients severe mental health disorders.9,19,20,21,27,30
The number of studies reporting ICU admission (4 studies) was not sufficient to perform a meta-analysis. Among this small number of studies, the results were very heterogeneous and contradictory between countries, with an OR less than 1 in France7 for schizophrenia, 1 in South Korea9 for all mental health disorders (1 unpublished study by G.F. et al), and more than 1 in the US for patients with alcohol and substance misuse.22
Discussion
We confirmed that mental health disorders were associated with increased COVID-19–related mortality from population-based data from 7 countries on 3 continents (North America, Europe, and Asia). Thus, patients with mental health disorders should have been targeted as a high-risk population for severe forms of COVID-19, requiring enhanced preventive and disease management strategies. However, future studies should evaluate the risk for each mental health disorder, which could not be determined with the current published data.
The different random-effects models confirmed the association between mental health disorders and increased COVID-19–related mortality for both pooled crude and adjusted ORs. The confirmation of increased mortality by adjusted ORs suggests that patients with mental health disorders are at higher risk of poor COVID-19 outcomes than patients without mental health disorders independent of the main clinical risk factors for severe COVID-19 (eg, age, obesity, smoking addiction, kidney disease, cardiovascular and cerebrovascular disease, and chronic obstructive pulmonary disease). This suggests that other factors lead to this health inequity in patients with mental health disorders, including several factors such as barriers to access to care, social determinants of health, immunological disturbances, and the effects of psychotropic drugs.
Several studies have reported important barriers to COVID-19–related somatic care in patients with mental health disorders in various countries.31,32,33,34,35,36 Therefore, it is likely that these barriers may have influenced access to care during the pandemic and thus had an effect on COVID-19 prognosis in patients with mental health disorders. A wide range of social factors (eg, socioeconomic status, family or household composition, and environmental factors) were also reported to be associated with increased COVID-19 mortality37 and are known to be highly influential in patients with mental health disorders.38 These factors need to be explored in depth in future works on mental health disorders and COVID-19, and they need to be considered for health policies.
Patients with schizophrenia and/or bipolar disorders had the highest risk of COVID-19 mortality. This may be explained by the particular immunological profile of these patients. Variation in the human leukocyte antigen complex is one of the most consistently replicated findings in genome-wide association studies in patients with schizophrenia and bipolar disorders.39 Human leukocyte antigen predominantly regulates viral infection, especially COVID-19.40 Genetic variability across major human leukocyte antigen class I genes may contribute to differences in the immune response to COVID-19, and an inappropriate T-cell response has been implicated in severe COVID-19 outcomes.39,41 Abnormal cytokine levels have also been found in the cerebrospinal fluid of patients with schizophrenia and bipolar disorders,42 who are also at higher risk of hypovitaminosis D,43,44 contributing to poor COVID-19 prognosis.45,46 Antipsychotic treatments have shown inconsistent pro- or anti-inflammatory properties, modulating anti-inflammatory (interleukin 4 and interleukin 10) or pro-inflammatory cytokines (interleukin 17, tumor necrosis factor, interferon γ), which results in immune function alterations.47,48
In all studies that analyzed patients with depressive disorders, patients with acute major depressive disorder (F32*) and those with recurrent major depressive disorder (F33*) were combined,8,9,10 but it is probable that patients with these different forms of the disorder have different risks of COVID-19 mortality. An acute major depressive episode is common, and patients can achieve full remission with or without treatment and without later consequences. In contrast, recurrent major depressive disorder is a severe mental health disorder that may strongly affect functioning and vulnerability to severe COVID-19 events, even between acute episodes. Patients with recurrent major depressive episodes have impaired immune defenses from the onset of illness.49 However, the administrative databases included in the present work did not include information on the mood state of the patients. It is possible that a current major depressive disorder at COVID-19 onset was the real culprit of increased COVID-19 mortality, and this hypothesis should be further explored. Anxiety disorders have also been associated with immune-inflammatory disturbances50; however, the only study that separately explored patients with anxiety disorders did not find an increased risk of COVID-19 mortality in this population.8
While addictions are classically distinguished from mental health disorders, patients with addiction were combined with those with mental health disorders in some studies.10,11,51 Studies analyzing patients misusing alcohol and substances found an increased risk in those patients,22,23 suggesting that attention should be paid to these patients. Long-term use of tobacco, alcohol, and other drugs is associated with cardiovascular (arrhythmias, cardiac insufficiency, and myocardial infarction), pulmonary (chronic obstructive pulmonary disease, pulmonary hypertension), and metabolic (diabetes, hypertension) diseases,52,53 all of which are risk factors for COVID-19 infection and worse outcomes. Of note, opioid use disorder is a particular concern in the US. It is estimated that approximately 70 000 people died of an opioid overdose in 2019, and opioids have respiratory depressant effects that could be particularly lethal in the case of COVID-19.29 As addictions and mental health disorders are frequently comorbid, the contribution of each to mortality risk should be clarified in future studies.
Our study has highlighted that there is a lack of data and discrepant data on ICU admission. Increased ICU admission was found in US patients with COVID-19 with alcohol and/or substance use disorders22, while decreased ICU admission was found in French patients with COVID-19 with schizophrenia.7 ICU admission is an important indicator because it can provide information on the allocation of scarce medical resources. Triage may become necessary when the demand for ICU resources exceeds supply.54 Severity of illness, initial ward or team the patient was referred from, and do-not-resuscitate order status/patient preference are modifiable factors that could be improved in patient with mental health disorders.55 Addressing a do-not-resuscitate order is complicated in mental health disorders owing to increased social isolation and the absence of relatives. Advance directives should be implemented in routine mental health to guarantee the respect of patients’ wishes, but this remains challenging.56 Reducing the time of access to the hospital for mental health disorders may prevent them from being admitted with very serious conditions. Specific training on mental health disorders for ICU staff could also deeply reduce mental illness stigma and improve ICU admission of patients with mental health disorders.57 Future studies should determine the ability of patients with mental health disorders in obtaining health resources in the COVID-19 pandemic.
Strengths and Limitations
The publication bias was reduced at its minimum by the inclusion of population-based approaches of unselected cohorts. Population-based studies allowed complete individual-level ascertainment without restricting analysis to those treated at hospitals and irrespective of socioeconomic differences. Studies yielding no data on patients with positive COVID-19 test results28 were not included to reduce the heterogeneity of the findings.
Overall, these results should be interpreted with several caveats. Most of the included studies were carried out during the first peak of the COVID-19 pandemic. At this time, testing was largely restricted in some countries (such as the US and France) to symptomatic and high-risk people, while it was provided to all citizens in South Korea, which may explain discrepancies in mortality rates. However, it did not seem to influence the risk of increased mortality in patients with mental health disorders. The important variations in mental health disorder definitions across studies limited our analyses for each mental health disorder and contributed to the heterogeneity of our findings. One study included patients with insomnia and dementia in the mental health disorder group.11 Insomnia is very common, while dementia is associated with COVID-19 prognosis and should be analyzed separately.58 One study (published as a letter) did not clearly define its mental health disorder groups.20
In light of these issues, we recommend distinguishing data for patients with different mental health disorders in future studies to better understand which patients are at increased risk of COVID-19 mortality. The definition of control groups also varied across studies: some control groups excluded patients with mental health disorders, while others only included patients with or without 1 disorder in a direct head-to-head comparison. We did not obtain information on the stage of illness (acute vs stabilized), which could have affected the risk for severe COVID-19 outcomes. Comorbidities such as obesity or tobacco smoking may often be underreported in medico-administrative databases, thus causing an underestimation of the prevalence of these specific issues. Most of the studies lacked data on social deprivation, which is likely to influence the risk of developing severe COVID-19 or dying.59 It was not possible to determine if the deaths were directly caused by COVID-19. No treatment was proven to be effective during the first wave of the COVID-19 pandemic; thus, no treatment data were included in the present analysis because they were available in only 1 study.
Conclusions
In this systematic review and meta-analysis of 16 observational studies involving 19 086 patients with mental health disorders in 7 countries, mental health disorders were associated with increased COVID-19–related mortality after adjustment for the main clinical risk factors for severe COVID-19. Thus, patients with mental health disorders should have been targeted as a high-risk population for severe forms of COVID-19, requiring enhanced preventive and disease management strategies. Future studies should evaluate the risk for each mental health disorder and confirm that patients with schizophrenia and bipolar disorders are at the highest risk of mortality.
eTable 1. Newcastle–Ottawa quality assessment scale
eTable 2. Codes used for mental health disorders definitions according to the International Statistical Classification of Diseases and Related Health Problems
eTable 3. Matching and adjustment factors
eFigure 1. Funnel plot (adjusted odds ratios). The Egger’s test is non-significant (p>0.05)
eFigure 2. Forest plot of the association between mental health disorders and mortality (crude Odds Ratio)
eFigure 3. Forest plot of the association between severe mental health disorders and mortality (crude Odds Ratio)
References
- 1.Johns Hopkins University of Medicine. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Accessed February 20, 2021. https://coronavirus.jhu.edu/map.html
- 2.Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L. Excess deaths from COVID-19 and other causes, March-April 2020. JAMA. 2020;324(5):510-513. doi: 10.1001/jama.2020.11787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams R, Jenkins DA, Ashcroft DM, et al. Diagnosis of physical and mental health conditions in primary care during the COVID-19 pandemic: a retrospective cohort study. Lancet Public Health. 2020;5(10):e543-e550. doi: 10.1016/S2468-2667(20)30201-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kivimäki M, Batty GD, Pentti J, et al. Association between socioeconomic status and the development of mental and physical health conditions in adulthood: a multi-cohort study. Lancet Public Health. 2020;5(3):e140-e149. doi: 10.1016/S2468-2667(19)30248-8 [DOI] [PubMed] [Google Scholar]
- 5.Knickman J, Krishnan R, Pincus H. Improving access to effective care for people with mental health and substance use disorders. JAMA. 2016;316(16):1647-1648. doi: 10.1001/jama.2016.13639 [DOI] [PubMed] [Google Scholar]
- 6.Lone NI, McPeake J, Stewart NI, et al. ; Scottish Intensive Care Society Audit Group . Influence of socioeconomic deprivation on interventions and outcomes for patients admitted with COVID-19 to critical care units in Scotland: a national cohort study. Lancet Reg Health Eur. 2021;1:100005. doi: 10.1016/j.lanepe.2020.100005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fond G, Pauly V, Leone M, et al. Disparities in intensive care unit admission and mortality among patients with schizophrenia and COVID-19: a national cohort study. Schizophr Bull. 2021;47(3):624-634. doi: 10.1093/schbul/sbaa158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemani K, Li C, Olfson M, et al. Association of psychiatric disorders with mortality among patients With COVID-19. JAMA Psychiatry. 2021;78(4):380-386. doi: 10.1001/jamapsychiatry.2020.4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SW, Yang JM, Moon SY, et al. Association between mental illness and COVID-19 susceptibility and clinical outcomes in South Korea: a nationwide cohort study. Lancet Psychiatry. 2020;7(12):1025-1031. doi: 10.1016/S2215-0366(20)30421-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeon H-L, Kwon JS, Park S-H, Shin J-Y. Association of mental disorders with SARS-CoV-2 infection and severe health outcomes: nationwide cohort study. Br J Psychiatry. Published online January 7, 2021. doi: 10.1192/bjp.2020.251 [DOI] [PubMed] [Google Scholar]
- 11.Lee DY, Cho J, You SC, et al. Risk of mortality in elderly coronavirus disease 2019 patients with mental health disorders: a nationwide retrospective study in South Korea. Am J Geriatr Psychiatry. 2020;28(12):1308-1316. doi: 10.1016/j.jagp.2020.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Hert M, Mazereel V, Detraux J, Van Assche K. Prioritizing COVID-19 vaccination for people with severe mental illness. World Psychiatry. 2021;20(1):54-55. doi: 10.1002/wps.20826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toubasi AA, AbuAnzeh RB, Tawileh HBA, Aldebei RH, Alryalat SAS. A meta-analysis: the mortality and severity of COVID-19 among patients with mental disorders. Psychiatry Res. 2021;299:113856. doi: 10.1016/j.psychres.2021.113856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Shamseer L, Clarke M, et al. ; PRISMA-P Group . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang B-H, Hoaglin DC. Meta-analysis of odds ratios: current good practices. Med Care. 2017;55(4):328-335. doi: 10.1097/MLR.0000000000000696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(1):1-48.20808728 [Google Scholar]
- 19.Poblador-Plou B, Carmona-Pírez J, Ioakeim-Skoufa I, et al. ; EpiChron Group . Baseline chronic comorbidity and mortality in laboratory-confirmed COVID-19 cases: results from the PRECOVID study in Spain. Int J Environ Res Public Health. 2020;17(14):E5171. doi: 10.3390/ijerph17145171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li L, Li F, Fortunati F, Krystal JH. Association of a prior psychiatric diagnosis with mortality among hospitalized patients with coronavirus disease 2019 (COVID-19) infection. JAMA Netw Open. 2020;3(9):e2023282. doi: 10.1001/jamanetworkopen.2020.23282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egede J, Campbell JA, Walker RJ, Garacci E, Dawson AZ, Egede LE. Relationship between physical and mental health comorbidities and COVID-19 positivity, hospitalization, and mortality. J Affect Disord. 2021;283:94-100. doi: 10.1016/j.jad.2021.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen B, El Shahawy O, Rogers ES, Hochman S, Khan MR, Krawczyk N. Association of substance use disorders and drug overdose with adverse COVID-19 outcomes in New York City: January-October 2020. J Public Health (Oxf). Published online December 26, 2020. doi: 10.1093/pubmed/fdaa241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baillargeon J, Polychronopoulou E, Kuo Y-F, Raji MA. The impact of substance use disorder on COVID-19 outcomes. Psychiatr Serv. 2021;72(5):578-581. doi: 10.1176/appi.ps.202000534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reilev M, Kristensen KB, Pottegård A, et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol. 2020;49(5):1468-1481. doi: 10.1093/ije/dyaa140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H, Chen W, Hu Y, et al. Pre-pandemic psychiatric disorders and risk of COVID-19: a UK Biobank cohort analysis. Lancet Healthy Longev. 2020;1(2):e69-e79. doi: 10.1016/S2666-7568(20)30013-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzur Bitan D, Krieger I, Kridin K, et al. COVID-19 prevalence and mortality among schizophrenia patients: a large-scale retrospective cohort study. Schizophr Bull. Published online February 19, 2021. doi: 10.1093/schbul/sbab012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry. 2021;20(1):124-130. doi: 10.1002/wps.20806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maripuu M, Bendix M, Öhlund L, Widerström M, Werneke U. Death associated with coronavirus (COVID-19) infection in individuals with severe mental disorders in Sweden during the early months of the outbreak: an exploratory cross-sectional analysis of a population-based register study. Front Psychiatry. 2021;11:609579. doi: 10.3389/fpsyt.2020.609579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang QQ, Kaelber DC, Xu R, Volkow ND. COVID-19 risk and outcomes in patients with substance use disorders: analyses from electronic health records in the United States. Mol Psychiatry. 2021;26(1):30-39. doi: 10.1038/s41380-020-00880-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SW, Yang JM, Moon SY, et al. Association between mental illness and COVID-19 in South Korea: a post-hoc analysis. Lancet Psychiatry. 2021;8(4):271-272. doi: 10.1016/S2215-0366(21)00043-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gervaix J, Haour G, Michel M, Chevreul K. Impact of mental illness on care for somatic comorbidities in France: a nation-wide hospital-based observational study. Epidemiol Psychiatr Sci. 2019;28(5):495-507. doi: 10.1017/S2045796018000203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Björk Brämberg E, Torgerson J, Norman Kjellström A, Welin P, Rusner M. Access to primary and specialized somatic health care for persons with severe mental illness: a qualitative study of perceived barriers and facilitators in Swedish health care. BMC Fam Pract. 2018;19(1):12. doi: 10.1186/s12875-017-0687-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Hert M, Cohen D, Bobes J, et al. Physical illness in patients with severe mental disorders: II: barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. 2011;10(2):138-151. doi: 10.1002/j.2051-5545.2011.tb00036.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fond G, Salas S, Pauly V, et al. End-of-life care among patients with schizophrenia and cancer: a population-based cohort study from the French national hospital database. Lancet Public Health. 2019;4(11):e583-e591. doi: 10.1016/S2468-2667(19)30187-2 [DOI] [PubMed] [Google Scholar]
- 35.Moore S, Shiers D, Daly B, Mitchell AJ, Gaughran F. Promoting physical health for people with schizophrenia by reducing disparities in medical and dental care. Acta Psychiatr Scand. 2015;132(2):109-121. doi: 10.1111/acps.12431 [DOI] [PubMed] [Google Scholar]
- 36.Sherrill E, Gonzales G. Recent changes in health insurance coverage and access to care by mental health status, 2012-2015. JAMA Psychiatry. 2017;74(10):1076-1079. doi: 10.1001/jamapsychiatry.2017.2697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karmakar M, Lantz PM, Tipirneni R. Association of social and demographic factors with COVID-19 incidence and death rates in the US. JAMA Netw Open. 2021;4(1):e2036462. doi: 10.1001/jamanetworkopen.2020.36462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye J, Wen Y, Sun X, et al. Socioeconomic deprivation index is associated with psychiatric disorders: an observational and genome-wide gene-by-environment interaction analysis in the UK Biobank cohort. Biol Psychiatry. 2021;89(9):888-895. doi: 10.1016/j.biopsych.2020.11.019 [DOI] [PubMed] [Google Scholar]
- 39.Purcell SM, Wray NR, Stone JL, et al. ; International Schizophrenia Consortium . Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748-752. doi: 10.1038/nature08185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamouza R, Krishnamoorthy R, Leboyer M. Understanding the genetic contribution of the human leukocyte antigen system to common major psychiatric disorders in a world pandemic context. Brain Behav Immun. 2021;91:731-739. doi: 10.1016/j.bbi.2020.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song J-W, Zhang C, Fan X, et al. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat Commun. 2020;11(1):3410. doi: 10.1038/s41467-020-17240-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang AK, Miller BJ. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr Bull. 2018;44(1):75-83. doi: 10.1093/schbul/sbx035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valipour G, Saneei P, Esmaillzadeh A. Serum vitamin D levels in relation to schizophrenia: a systematic review and meta-analysis of observational studies. J Clin Endocrinol Metab. 2014;99(10):3863-3872. doi: 10.1210/jc.2014-1887 [DOI] [PubMed] [Google Scholar]
- 44.Anglin RES, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100-107. doi: 10.1192/bjp.bp.111.106666 [DOI] [PubMed] [Google Scholar]
- 45.Di Nicola M, Dattoli L, Moccia L, et al. Serum 25-hydroxyvitamin D levels and psychological distress symptoms in patients with affective disorders during the COVID-19 pandemic. Psychoneuroendocrinology. 2020;122:104869. doi: 10.1016/j.psyneuen.2020.104869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hastie CE, Mackay DF, Ho F, et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab Syndr. 2020;14(4):561-565. doi: 10.1016/j.dsx.2020.04.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Amin MM, Nasir Uddin MM, Mahmud Reza H. Effects of antipsychotics on the inflammatory response system of patients with schizophrenia in peripheral blood mononuclear cell cultures. Clin Psychopharmacol Neurosci. 2013;11(3):144-151. doi: 10.9758/cpn.2013.11.3.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Himmerich H, Schönherr J, Fulda S, Sheldrick AJ, Bauer K, Sack U. Impact of antipsychotics on cytokine production in-vitro. J Psychiatr Res. 2011;45(10):1358-1365. doi: 10.1016/j.jpsychires.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 49.Çakici N, Sutterland AL, Penninx BWJH, Dalm VA, de Haan L, van Beveren NJM. Altered peripheral blood compounds in drug-naïve first-episode patients with either schizophrenia or major depressive disorder: a meta-analysis. Brain Behav Immun. 2020;88:547-558. doi: 10.1016/j.bbi.2020.04.039 [DOI] [PubMed] [Google Scholar]
- 50.Costello H, Gould RL, Abrol E, Howard R. Systematic review and meta-analysis of the association between peripheral inflammatory cytokines and generalised anxiety disorder. BMJ Open. 2019;9(7):e027925. doi: 10.1136/bmjopen-2018-027925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee EE, Ancoli-Israel S, Eyler LT, et al. Sleep disturbances and inflammatory biomarkers in schizophrenia: focus on sex differences. Am J Geriatr Psychiatry. 2019;27(1):21-31. doi: 10.1016/j.jagp.2018.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friedman H, Newton C, Klein TW. Microbial infections, immunomodulation, and drugs of abuse. Clin Microbiol Rev. 2003;16(2):209-219. doi: 10.1128/CMR.16.2.209-219.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bahorik AL, Satre DD, Kline-Simon AH, Weisner CM, Campbell CI. Alcohol, cannabis, and opioid use disorders, and disease burden in an integrated health care system. J Addict Med. 2017;11(1):3-9. doi: 10.1097/ADM.0000000000000260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anantham D, Chai-Lim C, Zhou JX, Phua GC. Operationalization of critical care triage during a pandemic surge using protocolized communication and integrated supportive care. J Intensive Care. 2020;8(1):59. doi: 10.1186/s40560-020-00475-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.James FR, Power N, Laha S. Decision-making in intensive care medicine: a review. J Intensive Care Soc. 2018;19(3):247-258. doi: 10.1177/1751143717746566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zelle H, Kemp K, Bonnie RJ. Advance directives in mental health care: evidence, challenges and promise. World Psychiatry. 2015;14(3):278-280. doi: 10.1002/wps.20268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murch R. Improving attitudes to mental health patients in ICU. Nurs N Z. 2016;22(8):30-31. [PubMed] [Google Scholar]
- 58.Liu N, Sun J, Wang X, Zhao M, Huang Q, Li H. The Impact of dementia on the clinical outcome of COVID-19: a systematic review and meta-analysis. J Alzheimers Dis. 2020;78(4):1775-1782. doi: 10.3233/JAD-201016 [DOI] [PubMed] [Google Scholar]
- 59.Abrams EM, Szefler SJ. COVID-19 and the impact of social determinants of health. Lancet Respir Med. 2020;8(7):659-661. doi: 10.1016/S2213-2600(20)30234-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Newcastle–Ottawa quality assessment scale
eTable 2. Codes used for mental health disorders definitions according to the International Statistical Classification of Diseases and Related Health Problems
eTable 3. Matching and adjustment factors
eFigure 1. Funnel plot (adjusted odds ratios). The Egger’s test is non-significant (p>0.05)
eFigure 2. Forest plot of the association between mental health disorders and mortality (crude Odds Ratio)
eFigure 3. Forest plot of the association between severe mental health disorders and mortality (crude Odds Ratio)