Abstract
Bronchobiliary fistula (BBF) is defined as the abnormal connection between the biliary system and the bronchial tree, which presents clinically as an irritant cough with bilioptysis. Many conditions can lead to its development. We present a case of an acquired BBF in a 61-year-old man with a significant history of spilled gallstones from a prior laparoscopic cholecystectomy and subsequent presentation of intermittent right upper quadrant pain and recurrent pneumonia. Imaging studies revealed a liver and subdiaphragmatic abscess with right middle lobe pneumonia and a BBF traversing the right hemidiaphragm. The patient was surgically managed by takedown of fistula with drainage of the abscess and removal of spilled gallstone, followed by a resection of the right middle lobe. While previous studies indicate spilled gallstones are benign, this case demonstrates its potential for serious complications. Therefore, early diagnosis and proper management is essential as BBF has a high morbidity and mortality rate.
Keywords: gastrointestinal surgery, liver disease, pneumonia (respiratory medicine), radiology
Background
A bronchobiliary fistula (BBF) is a pathological communication between the biliary system and the bronchial tree. The patients usually present with chronic cough, bile in the sputum (bilioptysis), right upper quadrant (RUQ) pain, fever, pneumonia and in rare cases with lithoptysis; which is the expectoration of gallstones.1 It has various aetiologies ranging from congenital to acquired causes. The acquired causes include infections, thoracoabdominal trauma, tumours, diseases of the biliary tract and iatrogenic complications.1 2 The mechanisms behind the formation of a BBF is an inflammatory reaction in the right subdiaphragmatic space that leads to abscess formation. This consequently causes a transdiaphragmatic rupture and fistula formation into the bronchial tree.3 Although BBF is a rare occurrence, it is a severe complication with a high mortality and morbidity rate (12.2%).4 Thus, early diagnosis and appropriate management is essential. In addition, BBF has a high chance of recurrence if the subphrenic space is not adequately irrigated and the stone is not removed.5 Most literature on BBF focus on the congenital form or acquired forms secondary to complications from hydatid disease or trauma. Our case is unique, as we demonstrate a BBF secondary to spilled gallstones from a prior laparoscopic cholecystectomy.
Laparoscopic cholecystectomy has become the gold standard for the treatment of cholecystitis and cholelithiasis and has replaced the traditional open cholecystectomy.6 Some of the complications of laparoscopic cholecystectomy include bile duct injury, bleeding, bile leakage, infection and bowel injury. Although, gallbladder perforation occurs in 6%–40% of patients, stone spillage occurs less often.7–9 As opposed to open cholecystectomy; it is strenuous to remove intraperitoneal spilled stones laparoscopically, and thus precaution should be taken to avoid this complication. Many reports indicate that spilled stones in the abdominal cavity are benign; however, 2.3%–8.5% of patients do develop severe complications.7–9 Here, we report a case of a BBF with resultant right middle lobe pneumonia secondary to abscess formation in the liver and subdiaphragmatic space from spilled gallstones.
Case presentation
A 61-year-old man presented to our hospital in June 2019 with a 1-year history of intermittent RUQ abdominal pain, recurrent bronchitis and pneumonia with mucopurulent cough and brown coloured sputum. There was no associated haemoptysis, chest pain, weight loss or fever. He has a significant surgical history of laparoscopic cholecystectomy in June 2018 at an outside institute. At initial presentation in June 2018, an ultrasound of the RUQ was performed for RUQ pain and fever, and demonstrated features of acute calculus cholecystitis. After treating his acute condition, he was scheduled for an elective laparoscopic cholecystectomy. The surgery was complicated by intraoperative spillage of gallstones, which was removed with foreceps as per operative notes.
On postoperative day 2, the patient reported repeated RUQ pain for which a CT of the abdomen was performed, which demonstrated a gallstone adjacent to the right dome of the liver, with minimal free fluid around the stone without evidence of biliary leak (figure 1). He was discharged on postoperative day 4 with stable condition.
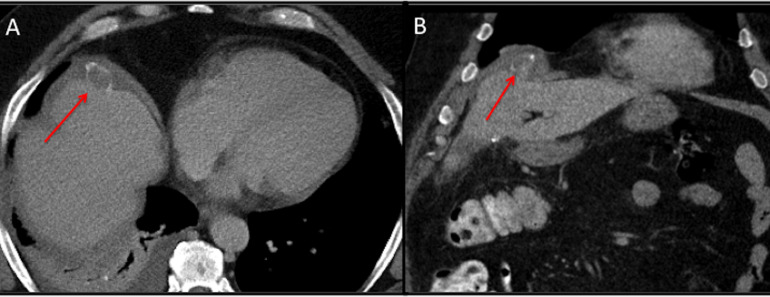
Figure 1.
CT (2018) of the abdomen axial (A) and coronal (B) reformats demonstrated a dropped gallstone adjacent to the right dome of the liver (red arrow), with minimal postoperative free fluid around the stone.
Since his laparoscopic cholecystectomy, the patient presented with recurrent pneumonia. He was investigated with multiple sputum cultures which grew Escherichia coli ESBL (Extended spectrum beta-lactamase) and was repeatedly treated with multiple antibiotics until he presented to our hospital.
Investigations
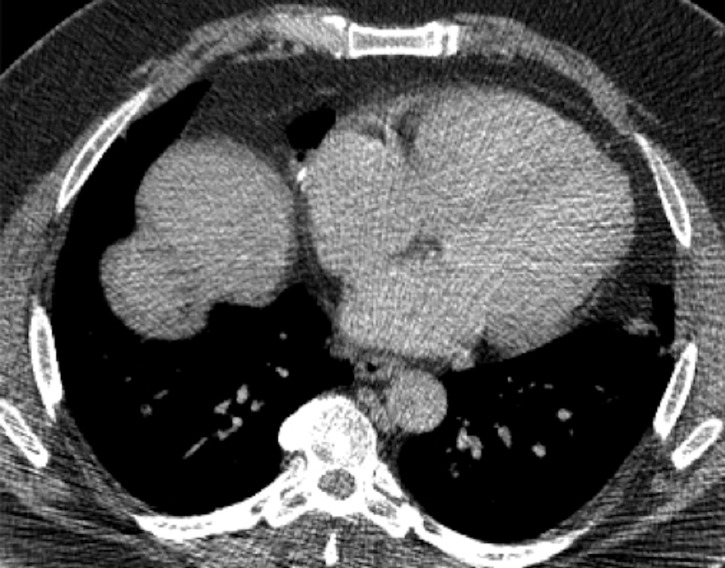
In June 2019, a CT scan of the chest, abdomen and pelvis was performed at our hospital due to persistence of symptoms, which showed a spilled gallstone in the right hepatic dome with an abscess in the right lobe (segment 8) of the liver extending into the right subphrenic space complicated by a fistula traversing the diaphragm resulting in a right middle lobe pneumonia and abscess (figure 2A). A BBF was suspected, and further evaluation with gadoxetic acid contrast MR cholangiography (MRC) was recommended. Meanwhile, the liver function tests showed elevated total bilirubin levels, aspartate transaminase and alanine transaminase enzymes (table 1). This supports that there is ongoing inflammation in the liver secondary to the liver abscess. However, the alkaline phosphatase and gamma-glutamyl transferase levels were within normal limits, and thus indicating that there was no biliary obstruction in this patient. Albumin levels were within normal limits. In addition, in further sputum analysis, bilirubin and ESBL were found, and thus reinforcing the presence of a BBF in our patient as bilioptysis is pathognomonic for this condition and ESBL tends to favour growth where bilirubinate stones are present.
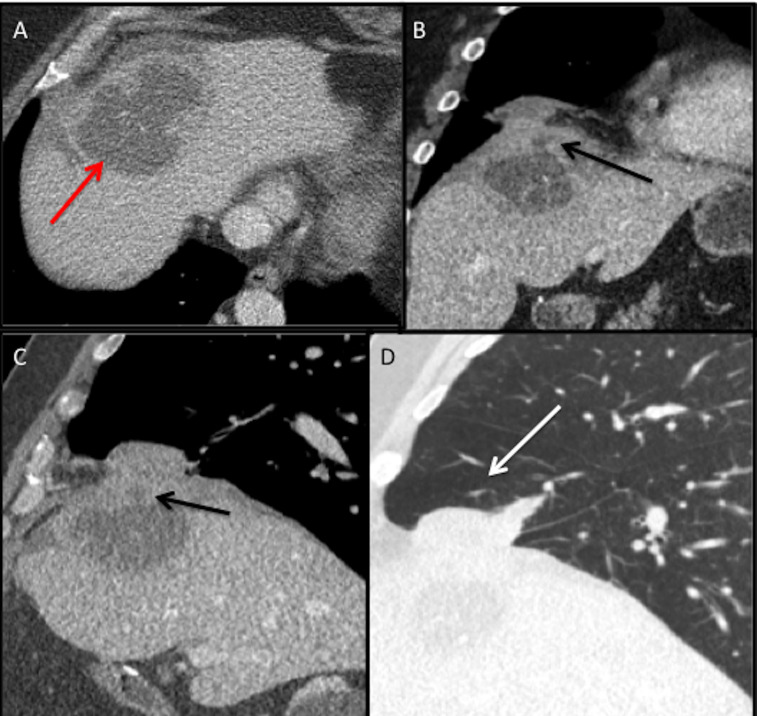
Figure 2.
CT (2019) of the chest and abdomen axial (A) section demonstrates a large liver abscess (red arrow) with a spilled gallstone in the hepatic dome. Coronal (B) and sagittal soft tissue window (C) and sagittal lung window (D) reformats demonstrate the liver abscess extending into the subphrenic space with fistulous tract through the diaphragm (black arrow) with associated right middle lobe pneumonia and abscess (white arrow).
Table 1.
Liver function tests (LFTs) of this patient
| LFT | Patient value | Normal value |
| Total bilirubin | 1.6 mg/dL (H) | 0.1–1.2 mg/dL |
| Direct bilirubin | 0.3 mg/dL | 0.0–0.3 mg/dL |
| AST | 73 IU/L (H) | 5–40 IU/L |
| ALT | 73 IU/L (H) | 5–50 IU/L |
| ALP | 30 IU/L | 20–140 IU/L |
| GGT | 18 IU/L | 0–30 IU/L |
| Albumin | 3.9 g/dL | 3.5–5.0 g/dL |
ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; GGT, gamma-glutamyl transferase.
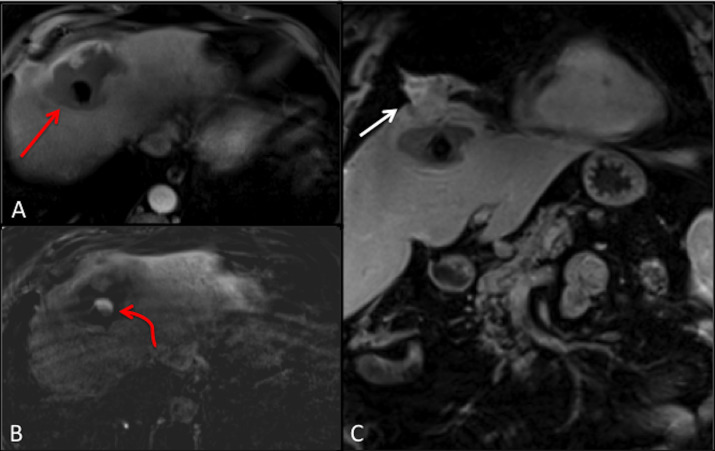
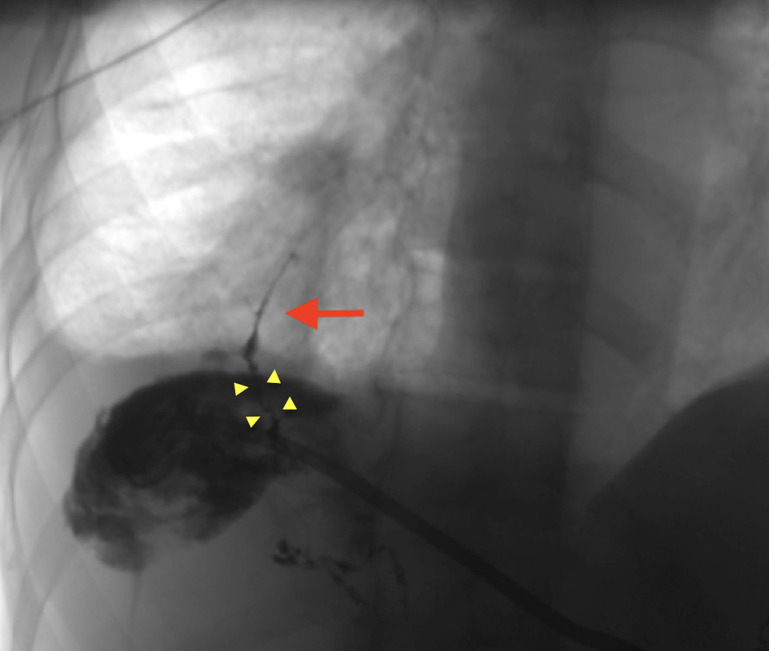
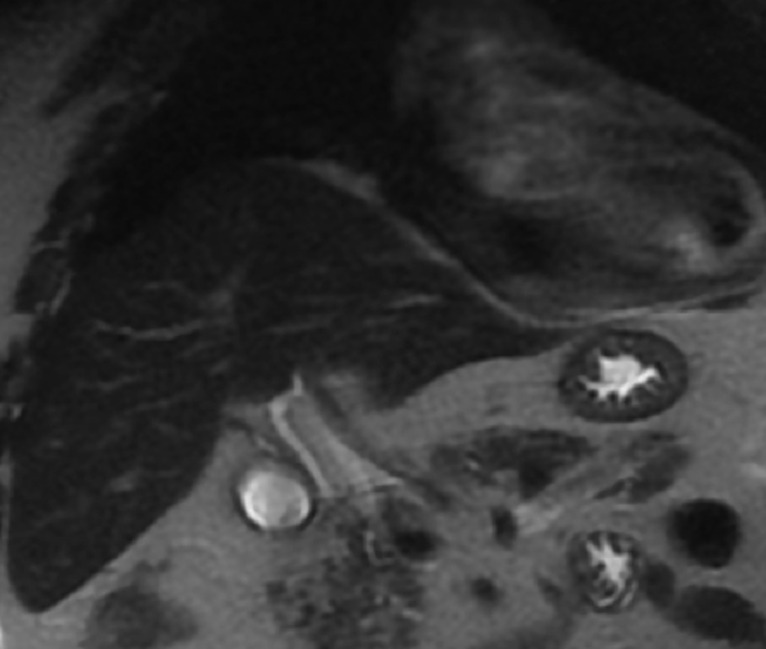
MRC with gadoxetic acid contrast agent (figure 3), which is an imaging modality used to confirm the diagnosis of BBF, was performed. The venous phase T1-weighted axial section of the MRC (figure 3A) demonstrated an abscess in the right lobe of the liver extending into the right subphrenic space and a fistulous tract through the diaphragm with associated right middle lobe pneumonia and abscess. A spilled gallstone was noted in the subphrenic abscess. In addition, the hepatobiliary phase (figure 3B) showed extravasation of contrast from the biliary tree into the liver and the subphrenic space. This prompted further investigation via a three-dimensional (3D) abscessogram. The 3D percutaneous transhepatic abscessogram (figure 4) was performed by interventional radiology where contrast was injected into the liver and evaluated under fluoroscopy. The results show a streak of contrast connecting the biliary system to the bronchial tree was well demonstrated. This confirmed the presence of a transdiaphragmatic communication between the biliary system and the bronchial tree. Moreover, the extension of the contrast from the liver abscess into the subdiaphragmatic space was also noted.
Figure 3.
Gadoxetic acid MR cholangiography venous phase T1-weighted axial section (A) demonstrates a liver abscess extending into the right subphrenic space with spilled gallstone (red arrow), axial section hepatobiliary phase (B) demonstrates spillage of contrast into the liver and the subphrenic abscess (curved red arrow). Postcontrast T1-weighted coronal section (C) demonstrates transdiaphragmatic extension of the liver abscess into the right middle of the lung (white arrow).
Figure 4.
Three-dimensional percutaneous transhepatic abscessogram evaluation demonstrated contrast tracking from the biliary system (four yellow arrowheads) into the bronchial tree (red arrow) confirming bronchobiliary fistula. Also, contrast is seen spilling into the liver abscess and the subphrenic space.
The patient was then scheduled for surgery for the correction of BBF and removal of spilled gallstones. During the surgery, the subdiaphragmatic abscess was visualised, drained and irrigated. A single large stone was found in the hepatic dome and was removed in an endocatch bag. A transdiaphragmatic takedown of the BBF was performed with a right middle lobe wedge resection. There were no immediate postoperative complications, and the patient was discharged 5 days later.
Treatment
The patient was then scheduled for a laparoscopic surgery for the correction of BBF and removal of spilled gallstones. A left subcostal incision was made, and a 5 mm optical trocar and 0° scope was entered into the abdominal cavity. After the layers of the abdominal wall were visualised, a 30°, 5 mm scope was introduced, and a diagnostic laparoscopy was performed. Prior to approaching the subphrenic space, the liver and anterior abdominal adhesions were removed, which allowed for better visualisation. After the liver was mobilised off of the anterior abdominal wall, the subdiaphragmatic abscess was visualised, drained and irrigated. A single large stone was found in the hepatic dome and was removed via an endocatch bag from the right midclavicular port. The abscess cavity and the RUQ were extensively irrigated. After transdiaphragmatic takedown of the BFF, the patient underwent a video-assisted thoracoscopic exploration with wedge resection of the right middle lobe to obliterate the fistulous tract and the affected portion of the lung. There were no immediate postoperative complications, and the patient was discharged 5 days later.
Even though ERCP (Endoscopic retrograde cholangiopancreatography) is the mainstay of treatment for the retrieval of biliary stones and drainage of abscesses, this patient had several more challenges that could not be fulfilled by an ERCP. This includes the fact that a single large stone was found outside the biliary tract in the right subphrenic space, which cannot be accessed by the ERCP procedure. The removal of this stone was crucial as it acted as the main nidus for the abscess formation. Moreover, this patient had a fistulous tract with an already established right middle lobe lung abscess. So, it was essential to takedown this fistulous tract to prevent further damage to the lung. Therefore, a transabdominal laparoscopic approach can meet the requirement of the removal of the stone, drainage of the abscess as well as the takedown of the fistulous tract. ERCP was also not considered to visualise this pathology as the diagnosis was already established by imaging methods such as CT, MRC and 3D percutaneous transhepatic abscessogram as well as additional sputum analysis.
Outcome and follow-up
A follow-up CT, which was performed in November 2019, showed postoperative changes such as minimal loss of lung volume and minimal scar tissue secondary to the takedown of the transdiaphragmatic fistulous tract and right middle lobe wedge resection (figure 5). No residual subdiaphragmatic abscess was seen. In December 2020, a follow-up MRI of the abdomen showed complete resolution of the subphrenic collection (figure 6). The patient remains asymptomatic.
Figure 5.
A follow-up CT of the chest and abdomen axial section shows postoperative changes such as minimal loss of lung volume and scar tissue related to the takedown of the transdiaphragmatic fistulous tract and right middle lobe wedge resection.
Figure 6.
A follow-up MRI of the abdomen coronal section T2-weighted image shows complete resolution of right subphrenic abscess.
Discussion
A BBF is an abnormal communication between the biliary tract and the bronchial tree. Patients usually present with generalised symptoms such as a chronic cough, bilioptysis, RUQ pain, fever and in rare cases with mild jaundice and lithoptysis. Moreover, patients can also present with bronchitis or pneumonia due to the intense inflammatory reaction caused by the irritant nature of bile on the bronchial mucosa as well as the nature of bilirubinate stones, which act as a nidus for E. coli.10 A high degree of clinical suspicion, radiological imaging and sputum analysis for the presence of bilirubin or gallstones is needed to confirm the diagnosis of BBF. BBF is also associated with a high morbidity and mortality rate, prompting immediate management.
BBF has many aetiologies from the congenital form to acquired causes. The acquired causes can be either obstructive or non-obstructive. Obstructive causes include biliary tract diseases such as cholelithiasis, tumours, postsurgical stenosis or postoperative complications.1 On the other hand, the non-obstructive mechanism is most likely due to trauma.3 The most common mechanisms for the formation of BBF are infections such as hydatid cysts, echinococcosis and amebiasis, which can erode into the thoracic cavity via the diaphragm and cause a fistula.2 Another mechanism behind the formation of the fistula is a cholangitic abscess formed as a result of the inflammatory reaction in the subdiaphragmatic space.3 This will in turn rupture through the diaphragm and into the pulmonary cavity. Boyd5 stated that a fistula between the subphrenic space and the lung cavity occurs in a predictable location; in the right posterior subphrenic space and into the basal segments of the right lower lobe. It is also postulated that there are three routes for gallstone migration; via congenital diaphragmatic defects, through the lymphatic channels of Ranvier and via the transdiaphragmatic route that forms due to infection and inflammation.11
We present a rare case of an acquired BBF with resultant right middle lobe abscess and pneumonia secondary to intraperitoneal gallstone spillage from a laparoscopic cholecystectomy. Laparoscopic cholecystectomy has become the gold standard for the treatment of cholelithiasis and has replaced the traditional open cholecystectomy as it is less invasive and has faster recovery times.6 Although the procedure is relatively safe, gallbladder perforation still occurs. Of the procedures that resulted in stone spillage, 13%–32% of them lead to lost stones.7 In most cases, spilled gallstones are considered as innocuous. However, it is now recognised that the stones trapped in the right subdiaphragmatic space can lead to increased risk as these stones are prone to be a niche for infection as this area cannot be cleared by the gastrointestinal immune mechanism.7 Thoracic complications of spilled gallstones are less frequent than abdominal complications.12 In addition, they are more common in elderly patients due to their weaker immune system. The documented complications of spilled gallstones include abscess formation, bacteremia, small bowel obstruction, granulomas, small bowel fistula, chest wall fistula and incarceration within a hernial sac.6 These complications can present within a few months or can take up to 20 years to manifest. In our patient, the time period between the laparoscopic cholecystectomy and the complication of BBF was 1 year.
The literature on the sequelae of spilled gallstones includes case reports of chest wall fistulas, peritoneo-cutaneous fistulas, hepato-colonic fistulas and limited cases of BBFs.7 13 14 On review of the literature, there were 24 cases from 1993 to 2020, where patients faced thoracic complications from retained abdominal gallstones.11 12 Among them, only 10 case reports presented with cholelithoptysis as a postoperative complication from spilled stones during a laparoscopic cholecystectomy.15–2 Not only does our case demonstrate that BBF can manifest as one of the serious complications of spilled gallstones, but it also harbours unique features. Our patient presented with a BBF and pneumonia in the right middle lobe as opposed to the right lower lobe, which is the postulated location. Also, our patient had several sputum cultures positive for E. coli. This is the characteristic of bilirubinate stones as they are most likely to be a nidus for bacteria, especially E. coli.7 8 Patients can present with an array of symptoms from generalised to specific. Thus, it is important to have good documentation of prior surgeries and adequate clinical suspicion to make the initial diagnosis of BBF. However, proper imaging is the best method to confirm the diagnosis so that a tailored management plan can be implemented.
For suspicion of spilled gallstones, the initial imaging method of choice is ultrasound as it has a high sensitivity for stone visualisation, particularly low calcium density stones.24 However, due to its limited beam penetration, deeper stones/abscesses cannot be visualised easily.24 CT and MRI can also be to used to obtain additional images of the stones. In CT, pigmented stones that are radio opaque can be seen clearly. On the other hand, cholesterol stones are difficult to identify due to their low attenuation.24 On T1-MRI, cholesterol stones present as hyperintense or isointense. On T2-MRI-weighted images, they present as hypointense.6
For a suspected BBF, CTs of the thoracic and upper abdominal seem to be the best choice for the initial diagnosis.4 In addition, contrast-enhanced MRC and hepatobiliary iminodiacetic acid scan have also been proven to show a definitive diagnosis.4 Percutaneous transhepatic cholangio has also been used to provide direct photographic evidence.2 Imaging plays an important role as identifying a dropped stone in the centre of the abscess is crucial in the decision-making for further management.
After the initial diagnosis of BBF, it is important to encourage the patients to seek prompt management. Any abscesses should be drained and irrigated, and the stones should be removed via percutaneous or surgical methods.12 Less invasive techniques are preferred but sometimes open surgery is conducted. Some of the most common treatment options for BBF are thoracic surgery, abdominal surgery, interventional radiology and antibiotic therapy.12
In conclusion, this case report highlights the fact that BBF can present as a severe postoperative complication from spilled gallstones during laparoscopic cholecystectomy. Therefore, caution should be taken to avoid this complication and if spillage does occur, the stones should be meticulously removed. Moreover, proper documentation of spilled gallstones should be kept. Finally, any patient presenting with right-sided thoracic or abdominal symptoms after laparoscopic cholecystectomy should undergo imaging to rule out complications. This will ultimately aid in prompt management and reduce the morbidity and mortality rates.
Learning points.
Bronchobiliary fistulas secondary to spilled gallstones in the right subdiaphragmatic space is a serious postoperative complication as these stones cannot be cleared by the gastrointestinal immune mechanism.
Special precaution should be taken to avoid spillage of gallstones during laparoscopic cholecystectomy.
The presence of unretrieved spilled gallstones should be documented in the patient’s medical records and should be relayed to their primary care physician.
Any patient presenting with symptoms after laparoscopic cholecystectomy should undergo imaging to determine the definitive diagnosis and management.
Footnotes
Contributors: Both authors have contributed equally to the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Baig S, Mandal A, Sen S. Bronchobiliary fistula. J Minim Access Surg 2008;4:111. 10.4103/0972-9941.45208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liao G-Q, Wang H, Zhu G-Y, et al. Management of acquired bronchobiliary fistula: a systematic literature review of 68 cases published in 30 years. World J Gastroenterol 2011;17:3842. 10.3748/wjg.v17.i33.3842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harnoss JM, Yung R, Brodsky RA, et al. Bronchobiliary fistula and lithoptysis after endoscopic retrograde cholangiopancreatography and liver biopsy in a patient with paroxysmal nocturnal hemoglobinuria. Am J Respir Crit Care Med 2013;187:451–4. 10.1164/ajrccm.187.4.451a [DOI] [PubMed] [Google Scholar]
- 4.Eryigit H, Oztas S, Urek S, et al. Management of acquired bronchobiliary fistula: 3 case reports and a literature review. J Cardiothorac Surg 2007;2:52. 10.1186/1749-8090-2-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyd DP. Bronchobiliary and bronchopleural fistulas. Ann Thorac Surg 1977;24:481–7. 10.1016/S0003-4975(10)63443-1 [DOI] [PubMed] [Google Scholar]
- 6.Helme S, Samdani T, Sinha P. Complications of spilled gallstones following laparoscopic cholecystectomy: a case report and literature overview. J Med Case Rep 2009;3:8626. 10.4076/1752-1947-3-8626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaster RS, Berger AJ, Ahmadi-Kashani M, et al. Chronic cutaneous chest wall fistula and gallstone empyema due to retained gallstones. BMJ Case Rep 2014;2014:bcr2013010159. 10.1136/bcr-2013-010159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sathesh-Kumar T, Saklani AP, Vinayagam R, et al. Spilled gall stones during laparoscopic cholecystectomy: a review of the literature. Postgrad Med J 2004;80:77–9. 10.1136/pmj.2003.006023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodfield JC, Rodgers M, Windsor JA. Peritoneal gallstones following laparoscopic cholecystectomy: incidence, complications, and management. Surg Endosc 2004;18:1200–7. 10.1007/s00464-003-8260-4 [DOI] [PubMed] [Google Scholar]
- 10.Chua HK, Allen MS, Deschamps C, et al. Bronchobiliary fistula: principles of management. Ann Thorac Surg 2000;70:1392–4. 10.1016/S0003-4975(00)01693-3 [DOI] [PubMed] [Google Scholar]
- 11.Fontaine JP, Issa RA, Yantiss RK, et al. Intrathoracic gallstones: a case report and literature review. JSLS 2006;10:375–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Perrone G, Giuffrida M, Tarasconi A, et al. Thoracic complications from retained abdominal gallstones after laparoscopic cholecystectomy: is it always mandatory a thoracic approach? Ulus Travma Acil Cerrahi Derg 2021;27:95–103. 10.14744/tjtes.2020.07280 [DOI] [PubMed] [Google Scholar]
- 13.Kumar S. Peritoneo-cutaneous fistula from spilled gall bladder calculus following laparoscopic cholecystectomy. Clin Case Rep 2017;5:720–2. 10.1002/ccr3.903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens JL, Laliotis A, Gould SWT. Hepatocolonic fistula: a rare consequence of retained gallstones after laparoscopic cholecystectomy. Ann R Coll Surg Engl 2013;95:139–41. 10.1308/003588413X13629960048550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Downie GH, Robbins MK, Souza JJ, et al. Cholelithoptysis. A complication following laparoscopic cholecystectomy. Chest 1993;103:616–7. 10.1378/chest.103.2.616 [DOI] [PubMed] [Google Scholar]
- 16.Lee VS, Paulson EK, Libby E, et al. Cholelithoptysis and cholelithorrhea: rare complications of laparoscopic cholecystectomy. Gastroenterology 1993;105:1877–81. 10.1016/0016-5085(93)91087-X [DOI] [PubMed] [Google Scholar]
- 17.Thompson J, Pisano E, Warshauer D. Cholelithoptysis: an unusual complication of laparoscopic cholecystectomy. Clin Imaging 1995;19:118–21. 10.1016/0899-7071(94)00038-E [DOI] [PubMed] [Google Scholar]
- 18.Barnard SP, Pallister I, Hendrick DJ, et al. Cholelithoptysis and empyema formation after laparoscopic cholecystectomy. Ann Thorac Surg 1995;60:1100–2. 10.1016/0003-4975(95)00404-9 [DOI] [PubMed] [Google Scholar]
- 19.Breslin AB, Wadhwa V. Cholelithoptysis: a rare complication of laparoscopic cholecystectomy. Med J Aust 1996;165:373–4. 10.5694/j.1326-5377.1996.tb125021.x [DOI] [PubMed] [Google Scholar]
- 20.Chan SY, Osborne AW, Purkiss SF. Cholelithoptysis: an unusual complication following laparoscopic cholecystectomy. Dig Surg 1998;15:707–8. 10.1159/000018663 [DOI] [PubMed] [Google Scholar]
- 21.Baldó X, Serra M, Belda J, et al. Cholelithoptysis as spontaneous resolution of a pulmonary solitary nodule. Eur J Cardiothorac Surg 1998;14:445–6. 10.1016/s1010-7940(98)00194-8 [DOI] [PubMed] [Google Scholar]
- 22.Chopra P, Killorn P, Mehran RJ. Cholelithoptysis and pleural empyema. Ann Thorac Surg 1999;68:254–5. 10.1016/S0003-4975(99)00498-1 [DOI] [PubMed] [Google Scholar]
- 23.Hanna SJ, Barakat O, Watkin S. Cholelithoptysis: an unusual delayed complication of laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg 2004;11:190–2. 10.1007/s00534-002-0822-7 [DOI] [PubMed] [Google Scholar]
- 24.Ramamurthy NK, Rudralingam V, Martin DF, et al. Out of sight but kept in mind: complications and Imitations of dropped gallstones. American Journal of Roentgenology 2013;200:1244–53. 10.2214/AJR.12.9430 [DOI] [PubMed] [Google Scholar]