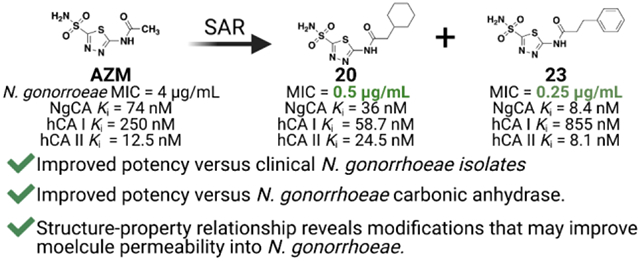
Abstract
Neisseria gonorrhoeae is an urgent threat to public health in the United States and around the world. Many of the current classes of antibiotics to treat N. gonorrhoeae infection are quickly becoming obsolete due to increased rates of resistance. Thus, there is a critical need for alternative antimicrobial targets and new chemical entities. Our team has repurposed the FDA-approved carbonic anhydrase inhibitor scaffold of acetazolamide to target N. gonorrhoeae and the bacteria’s essential carbonic anhydrase, NgCA. This study established both structure-activity and structure-property relationships that contribute to both antimicrobial activity and NgCA activity. This ultimately led to molecules 20 and 23, which displayed minimum inhibitory concentration values as low as 0.25 μg/mL equating to an 8- to 16-fold improvement in anti-gonococcal activity compared to acetazolamide. These analogs were determined to be bacteriostatic against the pathogen and likely on-target against NgCA. Additionally, they did not exhibit any detrimental effects in cellular toxicity assays against both a human endocervical (End1/E6E7) cell line or colorectal adenocarcinoma cell line (Caco-2) at concentrations up to 128 μg/mL. Taken together, this study presents a class of anti-gonococcal agents with the potential to be advanced for further evaluation in N. gonorrhoeae infection models.
Keywords: Carbonic anhydrase inhibitors, Neisseria gonorrhoeae, antibiotics, drug discovery
Graphic:
Structure-activity relationship studies for acetazolamide-based carbonic anhydrase inhibitors with activity against Neisseria gonorrhoeae.
Gonorrhea is a sexually transmitted disease caused by the bacterial pathogen Neisseria gonorrhoeae that colonizes urogenital, anal, and nasopharyngeal tissues. The World Health Organization (WHO) estimated there were 87 million new cases of gonorrhea in adults worldwide in 2016.1 In the United States specifically, the Centers for Disease Control and Prevention (CDC) reported a 67% increase in gonorrhea cases between 2013 – 2018 with a record 583,404 cases reported in 2018 alone.2,3 However, this is believed to be an underestimation as gonorrhea can present as both symptomatic and asymptomatic. It is projected that asymptomatic colonization makes up more than half of the infected individuals at any one time, and it is this version that greatly promotes transmission of the pathogen.4 Both versions wreak havoc on world health care systems causing pelvic inflammatory disease, infertility and ectopic pregnancies.5 The bacteria can also be transmitted from mother to child during birth and lead to blindness.6 If left untreated, N. gonorrhoeae can cause blood infection known as gonococcemia resulting in disseminated gonococcal infection which can lead to a variety of clinical symptoms including skin infection, arthritis or endocarditis.7,8
Pathogenic N. gonorrhoeae strains are increasingly resistant to common front-line antibiotics. The WHO surveillance program reports resistance to available antibiotics including β-lactams, tetracyclines and quinolines.9 Since 2010 the CDC has recommended treatment with a combination of oral azithromycin and intramuscular injection of ceftriaxone, to which resistance has been documented as well.10 For this reason, along with potential negative affects the dual therapy has on commensal microbiota, the CDC has recently recommended using only a single 500 mg injection of ceftriaxone, removing azithromycin from the treatment regimen, for uncomplicated N. gonorrhoeae infection.11 This recommendation leaves no effective oral therapeutic option. Rampant resistance has caused the CDC and the WHO each to classify N. gonorrhoeae as a superbug12 and a future with an untreatable gonococcal infection is a real possibility.13 Therefore, the CDC has listed drug-resistant N. gonorrhoeae at the highest possible threat level to public health.14 Additionally, the WHO identifies N. gonorrhoeae as a high priority pathogen and has called for an international collaborative effort to combat the drug-resistant gonorrhea.10 Work to develop a vaccine toward N. gonorrhoeae is still in the discovery phase and questions remain regarding the effectiveness of immune response in mucosal membranes15. Furthermore, existing antibiotics such as delafloxacin and the clinical molecule solithromycin both were investigated against gonorrhea in clinical trials, but neither met the criteria for non-inferiority relative to current treatment options.16,17 There is some progress being made as the oral agents zolifladacin18 and gepotidacin19 have each passed Phase 2 clinical trials for treatment of uncomplicated gonorrhea and are entering Phase 3. Nonetheless, there remains an inadequate number of both antibacterial agents and molecular targets for treating gonorrhea and underscores the critical unmet need for safe and effective oral alternatives.
Carbonic anhydrases (CAs) are a group of zinc-metalloenzymes that consist of α, β, and γ-sub-families, among others, found in all kingdoms of life. CAs catalyze the essential reaction of converting carbon dioxide (CO2) and water to bicarbonate, HCO3−, and a proton.20 In humans there are 16 α-CA isoforms with broad tissue distribution that carry out this reaction. The reaction is relevant in many physiological processes such as transport of CO2 from metabolizing tissues to excretion in the lungs,21 maintaining pH and CO2 homeostasis in various tissues,22 and regulating electrolyte secretion in various tissues and organs.23–25 These roles have made CAs prime drug targets as FDA-approved carbonic anhydrase inhibitors are used to treat various disorders including glaucoma,26,27 as a diuretic for kidney health,28 treatment for congestive heart failure,29 and recently certain isoforms have gained momentum as promising cancer targets.30–32
Aside from human targets, CAs have been identified in a handful of parasites and pathogenic bacteria,33,34 including Vibrio cholera,35 Burkholderia spp36,37 and N. gonorrhoeae. In bacteria, altering bicarbonate homeostasis was revealed to perturb the proton motive force and reduce bacterial fitness.38 It was first observed in 1967 that Neisseria spp, including N. gonorrhoeae, were susceptible to the FDA-approved CAIs acetazolamide and ethoxzolamide (AZM and EZM, Figure 1).39 Thirty years later the N. gonorrhoeae CA (NgCA) was first cloned40 and a crystal structure was published a year later.41 NgCA was further characterized enzymatically42 and genomic data identified NgCA as an essential enzyme in N. gonorrhoeae in 2014.43 NgCA is classified as an α-CA that resides may reside in the periplasmic space, is required for maintaining CO2 and pH homeostasis for the organism and has been suggested to be a valuable new drug target to treat gonorrhea.33 However, until now, there have been no reported efforts to target NgCA as a means to combat this pathogen. Thus, building on our previous work to target carbonic anhydrases in vancomycin-resistant enterococci (VRE),44 we report carbonic anhydrase inhibitors that display sub-nanomolar activity against NgCA and potency of < 1 μg/mL toward various N. gonorrhoeae clinical isolates. Herein, our efforts for evaluation of this carbonic anhydrase inhibitor scaffold for activity against N. gonorrhoeae and NgCA, off-target activity at human CAs, mechanism of action, and human cell toxicity are reported.
Figure 1.
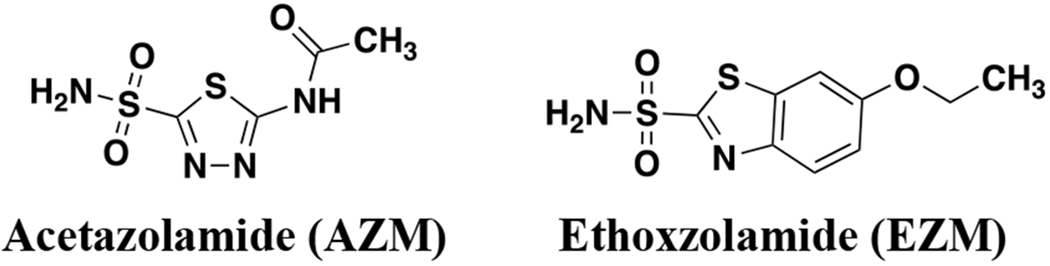
FDA-approved carbonic anhydrase inhibitors with antimicrobial activity against N. gonorrhoeae.
RESULTS
Chemistry
Analogs in this study (1 – 31) were synthesized previously as part of our efforts to develop inhibitors for VRE.44 In brief, AZM was used as the starting reagent for this series of molecules. The acetamide group was cleaved under acidic conditions to provide the free amine. This amine then served as a diversification point for amide coupling or reductive amination reactions to yield the final analogs for testing in the various assays (Scheme S1 and S2). Analogs 32 and 33 were purchased from commercial vendors and characterized to confirm identity and purity. Details are provided in supporting information.
For the purposes of a fluorescence polarization (FP) assay to assess inhibitor binding to NgCA and human carbonic anhydrases (hCAs), a fluorescein-linked AZM probe (FITC-AZM, Figure 2A) was designed. This molecule was synthesized starting with intermediate 1 and coupling with 6-azidohexanoic acid to form the azide intermediate 35 (Scheme S3). This intermediate was then reduced using H2 and Pd/C and the corresponding free amine was coupled with fluorescein isothiocyanate (5-isomer) to provide the desired FITC-AZM probe utilized in the FP assays.
Figure 2.
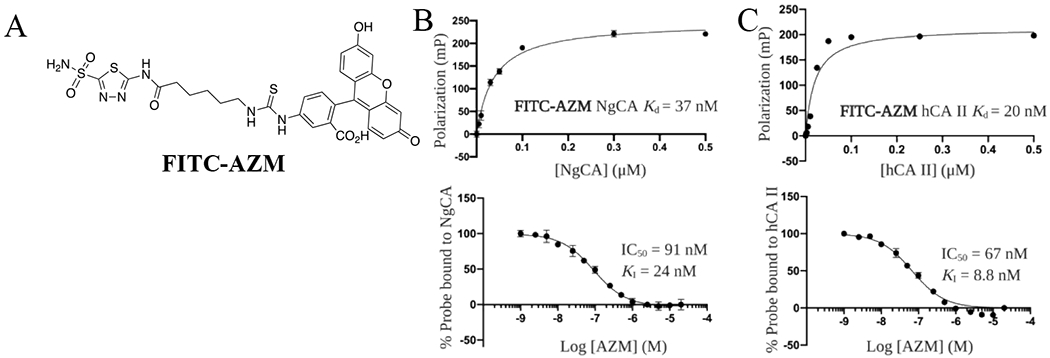
Fluorescent probe and FP assay development. (A) Structure of FITC-AZM fluorescent probe. (B) Titration curve of NgCA with 10 nM FITC-AZM (top), Competition assay for AZM to displace FITC-AZM probe from NgCA (bottom). (C) Titration curve of hCA II with 10 nM FITC-AZM (top), Competition assay for AZM to displace FITC-AZM probe from hCA II (bottom).
Carbonic anhydrase fluorescence polarization assay
Analogs were assessed in two orthogonal assays to determine inhibitory constants (Ki) against NgCA: 1) a fluorescence-polarization (FP) competition assay and 2) a catalytic CO2 hydration activity assay.45,46 The FP assay provides a more accessible means to assess Ki compared to the catalytic CO2 hydration assay, which requires stopped-flow instrumentation. However, it should be noted that the Ki determined from FP is from competition of a fluorophore-labeled tracer while the Ki from the hydration assay is generated from inhibition data. Nonetheless, both assays were performed in parallel to understand how analogs bind to or inhibit NgCA in vitro.
The dissociation constants (Kds) for FITC-AZM to NgCA, as well as human carbonic anhydrase I (hCA I) and II (hCA II), were determined by titration of protein while maintaining the concentration of probe and polarization values were recorded according to published protocols.47,48 The titrations for NgCA (Figure 2B, top panel) and hCA II (Figure 2C, top panel) were fit to a one site – specific binding non-linear regression model and dissociation constants were calculated (hCA I shown in Figure S1). These Kd values were used to select optimal conditions to maximize dynamic range for a competitive FP assay (FITC-AZM and protein concentrations shown in Table S1). Next, analogs were tested in dose-response to determine an IC50 value for displacement of FITC-AZM from each carbonic anhydrase with data for AZM shown in Figure 2B and C (bottom panels; hCA I shown in Figure S1). The Ki values for all analogs were determined by entering the resulting IC50 values, probe Kds, and protein concentrations into the FP Ki equation developed by Nikolovska-Coleska et al49 and are presented in Table 1. The IC50 values, 95% confidence intervals and Kis for all CAs tested for each analog are provided in Table S2.
Table 1.
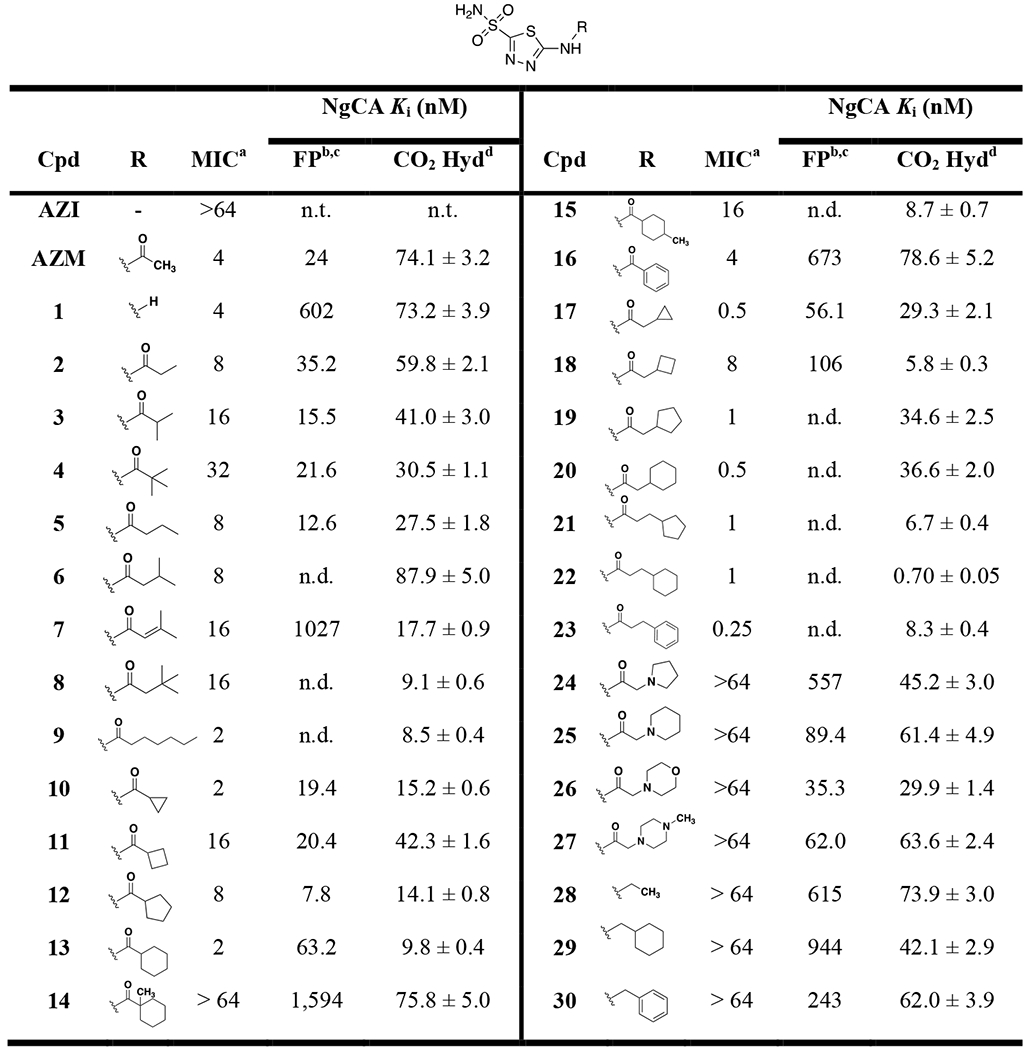
Minimum inhibitory concentrations (MICs) and NgCA Ki values for AZM-based analogs against N. gonorrhoeae 181
 |
AZI = azithromycin, AZM = acetazolamide.
MIC values against N. gonorrhoeae strain CDC 181 in μg/mL.
Fluorescence-polarization competition Ki values determined from the mean of one experiment performed in triplicate and IC50 values input into equation from Nikolovska-Coleska et al49. Based on the calculation theory and assay parameters the values obtained have a reliable lower limit of 33 nM for IC50. Any IC50 value < 33 nM unable to generate accurate Ki and thus is listed as not determined, n.d.
IC50 values obtained from FP competition assay presented in Table S2 and expressed as both mean and as 95% confidence interval to demonstrate precision.
Catalytic CO2 hydration assay Ki determined from the mean of one experiment in triplicate and IC50 values entered into Cheng-Prusoff equation82. Values reported are ± standard error of the mean.
Structure-activity relationship studies
Analogs were tested for antimicrobial activity versus the azithromycin-resistant clinical N. gonorrhoeae strain CDC 181 to determine minimum inhibitory concentrations (MICs) and the aforementioned NgCA FP and CO2 hydration assay in support of structure-activity relationship studies. AZM displayed an initial MIC value of 4 μg/mL and Ki values of 24 nM and 74.1 nM for the FP and CO2 assays, respectively (Table 1). Removal of the acetamide group in 1 provided no change in antimicrobial activity or CO2 hydration Ki, but did reduce the FP Ki significantly. The Kis for this analog reinforce the caveats related to the FP assay when compared to the catalytic assay as one assay is measuring the competition with FITC-AZM while the other is measuring the competition with CO2. Increase of lipophilic bulk in place of the methyl group on AZM reduced activity versus N. gonorrhoeae as the substituents were ranked in terms of potency in the order of methyl (AZM) > ethyl (2) > iso-propyl (3) > tert-butyl (4). Interestingly, the opposite trend was observed for the Ki values as the methyl and ethyl derivatives were the least potent, particularly in the CO2 hydration assay. Extending the alkyl chain away from the carbonyl by a single carbon for analogs 5 - 8 did not result in a discernible change in antimicrobial potency; however, these analogs were observed to be more active against NgCA. The n-hexyl derivative 9 displayed an improvement over AZM with an MIC value of 2 μg/mL and this was the first molecule that paired improved antimicrobial activity with improved activity against NgCA (CO2 hydration Ki = 8.5 nM). Among this first set of analogs it was observed that linear alkyl chains were preferred over branched alkane counterparts for anti-gonococcal activity; however, the branched alkane generally outperformed the nearest neighbor linear alkane analogs in the NgCA Ki assays.
The next set of analogs featured cyclic alkanes and provided an interesting trend as both the smallest ring-size, cyclopropyl (10), and largest ring size, cyclohexyl (13), displayed MIC values of 2 μg/mL. The intermediate ring sizes lagged in activity with the cyclopentyl (12) MIC at 8 μg/mL and the cyclobutyl (11) at 16 μg/mL. The Ki values for the CO2 hydration assay also followed this general trend as the cyclobutyl analog 11 was 3- to 4-fold less potent against NgCA compared to the other ring sizes while the cyclohexyl 13 was the most potent (CO2 Ki values of 42.3 and 9.8 nM, respectively). The same antimicrobial trend (cyclopropyl = cyclohexyl > cyclopentyl > cyclobutyl) was observed again for the nearest neighbor analogs in which a methylene was inserted between the cyclic alkane and the carbonyl (17 – 20). This latter set of cycloalkane substituted analogs yielded the most potent of the study to this point as both the cyclopropyl (17) and cyclohexyl (20) containing derivatives displayed MIC values of 0.5 μg/mL each, an 8-fold improvement over AZM. The SAR regarding the in vitro NgCA activity was relatively flat for this set as most remained in the 30 – 35 nM range, with the exception of the cyclobutyl derivative 18, which was the most potent in the CO2 hydration assay but the least potent in the FP assay.
The trend of alkyl branching that led to reduced antimicrobial activity was again observed for analogs 13 – 15. The cyclohexamide derivative 13 exhibited an MIC value of 2 μg/mL and was among the most potent against NgCA (CO2 Ki = 9.8 nM) while installation of a methyl at the 1-position carbon to provide a quaternary carbon directly adjacent to the carbonyl (14) resulted in reduction of both antimicrobial and NgCA activities (MIC > 64 μg/mL; Ki = 75.8 nM), although NgCA potency was still comparable to AZM. Moving the methyl to the 4-position on the cyclohexane ring (15) ring also resulted in an 8-fold reduction of antimicrobial activity compared to 13 but improved NgCA potency to KI = 8.7 nM. These modifications generally maintained in vitro activity against NgCA and suggest the presence of a quaternary carbon may have an effect on N. gonorrhoeae permeability. Meanwhile, substitution of an aromatic phenyl group (16) in place of the cyclohexane provided a two-fold reduction of antimicrobial activity and 7-fold reduction of NgCA activity. However, the opposite trend was observed when the pendant group was extended away from the carbonyl by two methylenes as the phenyl derivative 23 outperformed the cyclohexyl containing 22 by 4-fold; thus, yielding the most potent anti-gonococcal agent in this study with an MIC value of 0.25 μg/mL. Three of the most potent analogs in terms of antimicrobial activity (21 – 23) were also among the most potent with regards to Ki against NgCA.
Interestingly, insertion of heteroatoms, such as nitrogen and oxygen, into the cyclic alkane ring systems retained activity versus NgCA with the Ki range of 30 – 60 nM. However, these changes abolished antimicrobial activity as the polar pendant groups such as pyrrolidine (24), piperidine (25), morpholine (26), and piperazine (27) all had MICs values > 64 μg/mL. The importance of the carbonyl on the amide for antimicrobial activity was also noted as three analogs (28 – 30) containing an amine linkage, rather than amide, all exhibited significant reduction of antimicrobial activity with MIC values of > 64 μg/mL. Yet again, these analogs all displayed NgCA inhibitory activity comparable to AZM and other active analogs. This set of derivatives emphasizes the disconnect that often may arise between in vitro inhibitory data and whole-cell bacterial efficacy in Gram-negative pathogens, suggesting the molecules may have reduced permeability properties in N. gonorrhoeae.
To round out the SAR observations with respect to antimicrobial activity three analogs were tested with modifications to other regions of the scaffold (Figure 3). The first substituted a sulfone (31) in place of the sulfonamide and this molecule was inactive against N. gonorrhoeae. The next two analogs contained alterations to the central thiadiazole heterocyclic core of the scaffold. Analog 32 replaced the 4-nitrogen directly flanking the sulfonamide substituent with a carbon resulting in a thiazole scaffold and led to an 8.5-fold reduction of NgCA potency in the CO2 hydration assay and a complete loss of anti-gonococcal activity. The same was true for analog 33 in which the thiadiazole was replaced with a phenyl aromatic core.
Figure 3.
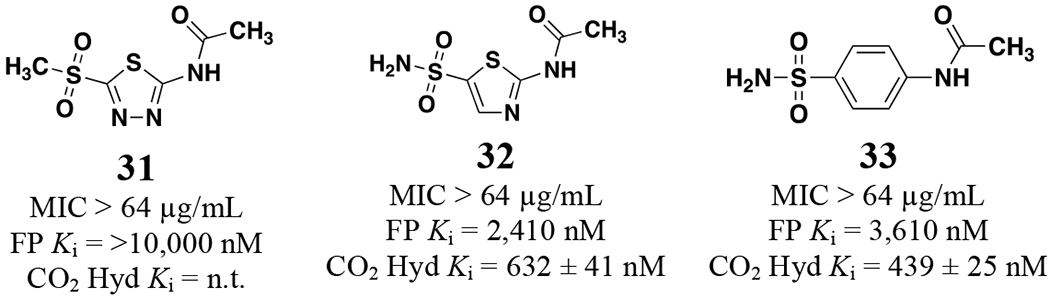
Analogs 31 – 33 against N. gonorrhoeae CDC 181 and NgCA Ki values.
To summarize the observed SAR, there was a clear trend that increased alkyl branching, particularly directly adjacent to the amide carbonyl, was detrimental to the anti-gonococcal activity. This effect was diminished as the branched carbon was extended away from the carbonyl. Cyclic alkanes provided mixed results with the cyclopropyl and cyclohexyl derivatives being preferred. Extending these cyclic alkanes away from the carbonyl improved activity by approximately 4-fold. The best performing analogs in combined antimicrobial and NgCA activity were 20, 22, and 23, of which each contained at least one-methylene linker with either a cyclohexyl or phenyl pendant group. Insertion of nitrogen or oxygen into the cyclic alkane rings was not tolerated for antimicrobial activity and the carbonyl was also shown to be essential for potency, even though these modifications had no impact on NgCA activity.
Anti-gonococcal activity against additional drug-resistant and -sensitive N. gonorrhoeae strains
The activity of two of the best performing analogs from the SAR study, 20 and 23, as well as AZM and azithromycin were assessed against a broad panel of 30 N. gonorrhoeae clinical isolates (strain information in Table S3). As shown in Table 2, analog 20 was the most potent overall displaying MIC values ranging from 0.06 – 2 μg/mL, inhibiting 50% of the isolates tested (MIC50) at the concentration of 0.5 μg/mL and 90% of the tested isolates (MIC90) at 2 μg/mL. Analog 23 and AZM provided a range of MICs from 0.5 μg/mL to 4 μg/mL, with a lone strain for 23 dropping to 0.25 μg/mL. Both molecules displayed the same MIC50 and MIC90 values of 2 and 4 μg/mL, respectively. Azithromycin inhibited the tested strains at concentrations ranging from 0.125 μg/mL to 16 μg/mL, with a lone strain not exhibiting an MIC > 64 μg/mL. Azithromycin displayed MIC50 and MIC90 (1 μg/mL and 4 μg/mL, respectively) values comparable to AZM, 20 and 23. Although the MIC50 and MIC90 values for 20 are modest improvements over azithromycin, the analog showed the potential to outperform azithromycin against many isolates by 2 – 16-fold. These results also indicate the molecules exhibited similar potencies against azithromycin-sensitive and -resistant strains.
Table 2.
MICs for Selected Analogs against a panel of N. gonorrhoeae clinical isolates
| MIC (μg/mL) |
||||
|---|---|---|---|---|
| N. gonorrhoeae strains | AZM | 20 | 23 | AZI |
| CDC 165 | 4 | 1 | 4 | 2 |
| CDC 166 | 4 | 1 | 2 | 2 |
| CDC 167 | 2 | 0.5 | 4 | 4 |
| CDC 168 | 4 | 0.5 | 4 | 1 |
| CDC 169 | 4 | 0.25 | 2 | 1 |
| CDC 170 | 1 | 0.125 | 0.5 | 1 |
| CDC 171 | 2 | 0.5 | 2 | 1 |
| CDC 173 | 1 | 0.125 | 0.5 | 0.5 |
| CDC 175 | 2 | 0.25 | 2 | 4 |
| CDC 176 | 1 | 0.25 | 1 | 0.5 |
| CDC 177 | 1 | 0.5 | 2 | 0.5 |
| CDC 178 | 2 | 1 | 0.5 | 2 |
| CDC 179 | 4 | 2 | 4 | 4 |
| CDC 180 | 2 | 0.5 | 2 | 0.5 |
| CDC 181 | 2 | 0.5 | 0.25 | >64 |
| CDC 182 | 4 | 0.5 | 2 | 1 |
| CDC 183 | 2 | 0.25 | 2 | 1 |
| CDC 184 | 2 | 0.25 | 2 | 1 |
| CDC 185 | 4 | 0.5 | 2 | 1 |
| CDC 187 | 2 | 0.25 | 2 | 4 |
| CDC 189 | 2 | 0.06 | 1 | 0.5 |
| CDC 190 | 2 | 0.125 | 1 | 1 |
| CDC 197 | 4 | 2 | 4 | 2 |
| CDC 202 | 4 | 2 | 4 | 16 |
| CDC 211 | 1 | 2 | 4 | 1 |
| ATCC 700825 | 0.5 | 0.5 | 0.5 | 0.25 |
| MS11 | 0.5 | 0.125 | 1 | 0.25 |
| WHO-K | 1 | 0.25 | 1 | 0.25 |
| WHO-X | 1 | 0.25 | 1 | 0.25 |
| WHO-W | 2 | 0.25 | 1 | 0.125 |
| aMIC50 | 2 | 0.5 | 2 | 2 |
| bMIC90 | 4 | 2 | 4 | 4 |
AZI = azithromycin, AZM = acetazolamide.
MIC50: minimum inhibitory concentration at which the compound/drug inhibited 50% of the tested strains.
MIC90: minimum inhibitory concentration at which the compound/drug inhibited 90% of the tested strains.
Mechanism of action studies
Previous studies have suggested AZM’s antimicrobial properties against N. gonorrhoeae may be dependent on inhibition of the essential α-carbonic anhydrase, NgCA.39 CO2 is the substrate for carbonic anhydrases; therefore, high levels of the gas will out compete an inhibitor of NgCA and the bacteria should exhibit reduced susceptibility.39,50 To confirm that the new analogs maintained on-target activity at NgCA we performed MIC assays for N. gonorrhoeae in both standard lab culture conditions in ambient atmosphere and in conditions that contain 5% CO2. This assay was performed against two strains of N. gonorrhoeae and the results for representative analogs are presented in Table 3. MIC values for multiple strains of N. gonorrhoeae in presence and absence of CO2 are presented in Table S4.
Table 3.
MICs of molecules under normal and CO2 conditions
| N. gonorrhoeae CDC 181a | N. gonorrhoeae CDC 178a | |||
|---|---|---|---|---|
| Cpd | normalb | CO2c | normalb | CO2c |
| AZM | 4 | >64 | 2 | >64 |
| AZI | >64 | >64 | 2 | 2 |
| 20 | 0.5 | >64 | 1 | >64 |
| 23 | 0.25 | >64 | 0.25 | >64 |
AZM = acetazolamide; AZI = azithromycin.
MIC values in μg/mL.
indicates standard conditions in ambient air.
indicates incubation in presence of 5% CO2.
It was observed that in two strains of N. gonorrhoeae, one susceptible to azithromycin and one resistant, that the efficacy of AZM and analogs 20 and 23 was significantly reduced in the presence of CO2. As a control to ensure that the CO2 conditions did not cause unintended drug-resistance to drugs with a different mechanism of action, azithromycin was also tested and displayed no change in efficacy against N. gonorrhoeae strain CDC 178 when cultured in CO2 conditions compared to normal ambient air. The analogs did show weak antimicrobial activity (range of 16 – 32 μg/mL) in the CO2 conditions against a few strains (Table S4), however, the difference between ambient and CO2 conditions was still an 8- to 16-fold reduction in potency. It is too early to determine if this result indicates a secondary target and further testing is taking place to understand the difference in carbonic anhydrase inhibitor susceptibility in these strains. Nonetheless, these results suggest the primary intracellular target for the sulfonamide scaffold is likely NgCA.
The analogs next were assessed in a killing kinetics assay to determine whether the scaffold exhibits bacteriostatic or bactericidal activity against N. gonorrhoeae ATCC 70085 strain. The positive control azithromycin displayed bactericidal activity consistent with previous reports (Figure 4).51–53 The carbonic anhydrase inhibitors all displayed bacteriostatic properties against N. gonorrhoeae over the time course of the experiment and were found to have significantly reduced the bacterial burden compared to DMSO (negative control). After 24 hours, analogs 20 and 23 reduced N. gonorrhoeae load by 1.9- and 2.6-log10 units, respectively. At the 24-hour time point, 20 and 23 were found to reduce the bacterial CFU count by 3- and 3.8-log10, respectively as compared to DMSO. The analogs 20 and 23 also outperformed AZM reducing the bacterial burden by 2.4- and 3.1- log10-reduction, respectively as compared to AZM. It is worth noting that the CFU count for analog 20 slightly increased at 24 hours. We isolated the colonies and performed and MIC assay on these isolates and did not find any shift in the MIC values. Consequently, this rebounding at 24 hours could be attributed to the concentration of 20 potentially becoming diminished below the MIC at that timepoint and suggests frequent dosing for analog 20.
Figure 4.
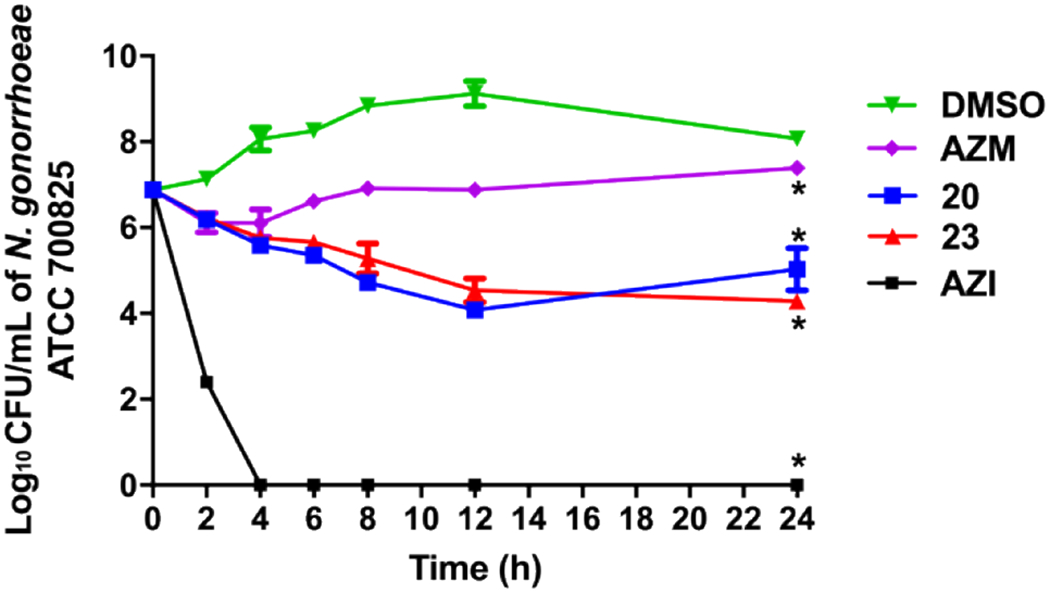
Time-kill assay of carbonic anhydrase inhibitors and azithromycin (tested in triplicates, at 10 × MIC) against N. gonorrhoeae ATCC 700825. DMSO (vehicle) served as a negative control. The error bars represent standard deviation values for each test agent studied. The data were analyzed via a two-way ANOVA with post-hoc Dunnett’s test for multiple comparisons. An asterisk (*) indicates a statistically significant difference (P<0.05) between treatment with drugs/compounds compared to DMSO treatment (negative control).
Inhibition of human carbonic anhydrases
Humans express at least 16 α-carbonic anhydrase (hCA) isoforms in various tissues.54 Given that these enzymes are ubiquitous throughout the human body, it is critical to gain an understanding for how the new analogs inhibit these enzymes to assess potential drug distribution and toxicity liabilities. To do this, we measured the Ki values for all analogs versus two representative hCAs; hCA I and hCA II. These hCAs were chosen as they are widely distributed in various tissues including in erythrocytes. This factor may affect systemic distribution of the molecules in vivo as hCA I and hCA II have been shown to act as a sink and partition carbonic anhydrase inhibitors into erythrocytes as opposed to plasma.55 NgCA shares 26.7% sequence identity with hCA I and 25.6% identity with hCA II; however, there is high identity of residues within the active sites (alignment shown in Figure S2).
In general, most analogs maintained nanomolar range activity against both hCAs. AZM was found to be almost 6-fold more selective for hCA II over NgCA while it was less selective for hCA I (Table 4). Although the selectivity window was able to be narrowed to approximately equipotent values between NgCA and hCA II, rarely was it observed that the new analogs were more potent against NgCA. In fact, only four analogs (9, 12, 13, and 15) exhibited greater than 1.5-fold selectivity for NgCA over hCA II. Analogs 22 and 23, among the most potent in terms of antimicrobial activity, were also among the most potent against both NgCA and hCA II, with 22 reaching sub-nanomolar potency against each.
Table 4.
Inhibitory constants for analogs against NgCA and hCAs
| CO2 Hydration KI (nM)a |
CO2 Hydration KI (nM)a |
CO2 Hydration KI (nM)a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cpd | NgCA | hCA I | hCA II | Cpd | NgCA | hCA I | hCA II | Cpd | NgCA | hCA I | hCA II |
| AZM | 74.1 ± 3.2 | 250 ± 11 | 12.5 ± 0.8 | 12 | 14.1 ± 0.8 | 76.5 ± 3.6 | 26.2 ± 1.4 | 24 | 45.2 ± 3.0 | 64.7 ± 4.5 | 58.1 ± 2.2 |
| 1 | 73.2 ± 3.9 | 8,600b ± 300 | 60b ± 3 | 13 | 9.8 ± 0.4 | 63.3 ± 3.7 | 20.2 ± 1.5 | 25 | 61.4 ± 4.9 | 53.4 ± 2.8 | 20.9 ± 1.6 |
| 2 | 59.8 ± 2.1 | 235 ± 14 | 37.2 ± 1.9 | 14 | 75.8 ± 5.0 | 117 ± 9.4 | 47.7 ± 3.2 | 26 | 29.9 ± 1.4 | 14c ± 0.9 | 0.9c ± 0.04 |
| 3 | 41.0 ± 3.0 | 180 ± 6 | 30.9 ± 1.3 | 15 | 8.7 ± 0.7 | 152 ± 13 | 26 ± 1.8 | 27 | 63.6 ± 2.4 | 9.6c ± 0.8 | 1.6c ± 0.1 |
| 4 | 30.5 ± 1.1 | 167 ± 6.9 | 9.5 ± 0.8 | 16 | 78.6 ± 5.2 | 109 ± 7 | 29.0 ± 1.5 | 28 | 73.9 ± 3.0 | 701 ± 34 | 47.2 ± 4.1 |
| 5 | 27.5 ± 1.8 | 213 ± 14 | 22.3 ± 1.9 | 17 | 29.3 ± 2.1 | 372 ± 19 | 7.6 ± 0.6 | 29 | 42.1 ± 2.9 | 1,135 ± 98 | 78.4 ± 4.7 |
| 6 | 87.9 ± 5.0 | 215 ± 17 | 55.6 ± 3.7 | 18 | 5.8 ± 0.3 | 77.8 ± 6.9 | 4.9 ±0.3 | 30 | 62.0 ± 3.9 | 1,623 ± 126 | 104 ± 9.1 |
| 7 | 17.7 ± 0.9 | 367 ± 14 | 23.6 ± 1.4 | 19 | 34.6 ± 2.5 | 86.3 ± 5.9 | 19.4 ± 1.1 | 31 | >10,000 | n.d. | n.d. |
| 8 | 9.1 ± 0.6 | 231 ± 15 | 7.3 ± 0.60 | 20 | 36.6 ± 2.0 | 58.7 ± 4.1 | 24.5 ± 1.7 | 32 | 632 ± 41 | 465 ± 11 | 97.2 ± 11 |
| 9 | 8.5 ± 0.4 | 328 ± 14 | 22.7 ± 1.8 | 21 | 6.7 ± 0.4 | 190 ± 18 | 10.2 ± 0.7 | 33 | 439 ± 41 | 1,331 ± 11 | 67.7 ± 11 |
| 10 | 15.2 ± 0.6 | 157 ± 13 | 24.6 ± 1.3 | 22 | 0.70 ± 0.05 | 945 ± 37 | 0.32 ± 0.02 | ||||
| 11 | 42.3 ± 1.6 | 125 ± 11 | 41.5 ± 2.0 | 23 | 8.3 ± 0.4 | 855 ± 76 | 8.1 ± 0.70 | ||||
AZM = acetazolamide;
Catalytic CO2 hydration assay Ki determined from the mean of one experiment performed in triplicate and IC50 values entered into Cheng-Prusoff equation. Values reported are ± standard error of the mean.
Previously reported by Di Cesare Mannelli et al.81
Previously reported by Turkmen et al.80
An increase in the selectivity window was able to be achieved over hCA I. The initial Ki value for AZM versus hCA I was 250 nM, equating to 3.3-fold selectivity for NgCA. Analogs 22 and 23 each exhibited reduced hCA I activity (hCA I Ki = 945 and 855 nM, respectively). This, coupled with improved activity toward NgCA, provided selectivity windows in favor of NgCA that were > 1,300-fold for 22 and 100-fold for 23.
Several analogs provided carbonic anhydrase targeting data points even though they were inactive against the bacteria. It is interesting to note that the polar analogs 24 – 27 were essentially equipotent against NgCA but became increasingly more potent against hCA I and II. To this point, the N-methylpiperazine analog 27 was the least active against NgCA (Ki = 63.6 nM) but had single-digit nanomolar potency toward the human isoforms (Ki values for hCA I and II of 9.6 and 1.6 nM, respectively). The effect of the amide carbonyl on Ki also differed among the three carbonic anhydrases. Removal of the amide carbonyl provided little change in Ki (3-fold or less) versus NgCA across three analogs 28 – 30 when compared to the carbonyl containing counterparts. Removal of the carbonyl had a greater effect versus the human carbonic anhydrases with 30 representing the least potent analog against both hCA I and II (KI values of 1,623 and 104 nM, respectively). This amounts to a reduction of activity against hCA I by almost 15-fold compared to the matched molecular pair analog 16.
Two analogs explored modification to the central thiadiazole core. Molecule 32, in which a nitrogen is replaced with a carbon to provide a thiazole core, displayed an 8.5-fold reduction in NgCA activity compared to AZM with a Ki value of 632 nM. This also led to a 7.7-fold decrease in potency against hCA II (Ki = 97.2 nM). The modification had less effect on hCA I with only about 1.8-fold decrease in activity. Finally, replacing the heterocyclic core with a phenyl core (33) was still not preferred for any of the carbonic anhydrases with approximately 5-fold loss in KI across all three carbonic anhydrases.
In summation, the SAR trends generally aligned for NgCA and hCA II while there was greater flexibility for improvement of selectivity against hCA I. The propensity for analog potency to align more between NgCA and hCA II is likely attributed to the conservation of active site threonine residue, Thr204 in NgCA and Thr200 hCA II. This residue was been shown to participate in polar interactions with the ligand, particularly the 4-nitrogen of the thiadiazole as shown in the AZM complex with hCA II56 (PDB: 3HS4). Alternatively, hCA I has a histidine at that corresponding residue location (PDB: 1AZM) that is shown to participate in π – π stacking interactions with AZM that are weaker than hydrogen-bonds.57 Thus, the likely conservation of the hydrogen bond between ligands and the threonine accounts for the observation NgCA and hCA II potency tracks together. Future work will incorporate structure-based design to build in selectivity against the human carbonic anhydrases.
Assessment of human cell toxicity for analogs 20 and 23
To be considered as viable leads the molecules should display little-to-no toxicity against relevant human cell lines at concentrations greater than the MIC values. Thus, to prepare for future in vivo experiments an assessment of cell toxicity using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (MTS) was performed in two human cell lines. N. gonorrhoeae is known to invade epithelial cells of the genital tract and cross the epithelial barrier into the subepithelial space58; therefore, one cell line used in the assay was endocervical End/E6E7 cells. The second cell line used were human colorectal adenocarcinoma epithelial cells (Caco-2) to determine potential for detrimental effects on the gastrointestinal tract upon oral dosing for future in vivo efficacy assays. Cells were dosed at concentrations of 8 – 128 μg/mL and assessed for viability. Compounds 20 and 23 displayed no toxic effects against either End1/E6E7 endocervical or Caco-2 cells compared to DMSO control at doses up to 128 μg/mL after 24 hours of incubation at 37 °C (Figure 5). These concentrations represent more than 256-fold and 64-fold higher values than the respective MIC50s for 20 and 23. It should be noted that Caco-2 cells have high endogenous expression of human CA XIII59, to which AZM has a Ki value of 16 nM.60 Literature search could not identify the human CA isoforms present in the End1/E6E7 cell line; however, a report suggests a related cell line studied for endocervical cancer expresses human CA IX.61 Regardless, the molecules derived in this study showed no adverse cell toxicity at the highest doses tested.
Figure 5.
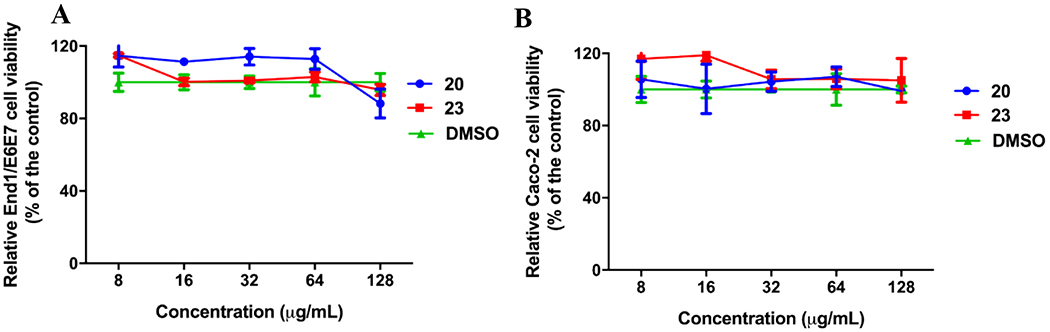
Human cell toxicity of carbonic anhydrase inhibitor analogs 20 (blue) and 23 (red) compared to DMSO control (green). (A) Cell viability in human endocervical (End1/E6E7) cells using the MTS; 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay. (B) Cell viability in human colorectal ladenocarcinoma (Caco-2) cells. Results are presented as percent viable cells relative to DMSO (negative control to determine a baseline measure for the cytotoxic impact of each compound). The absorbance values represent an average of three samples analyzed for each compound. Error bars represent standard deviation values. Data were analyzed via a two-way ANOVA with post hoc Dunnett’s test for multiple comparisons.
DISCUSSION
An investigation of a set of carbonic anhydrase inhibitors for efficacy against N. gonorrhoeae was carried out. Previous studies had reported that AZM possessed antimicrobial activity against N. gonorrhoeae and it was hypothesized that the intracellular target was the α-carbonic anhydrase, NgCA.39 With this in mind, our team assessed a set of carbonic anhydrase inhibitors developed by our lab for activity against N. gonorrhoeae. It was shown that these molecules have anti-gonococcal properties with MICs ranging from 0.06 μg/mL – 32 μg/mL. Analog 23 displayed the greatest potency against the azithromycin-resistant N. gonorrhoeae strain CDC 181 followed by analogs 17 and 20. The antimicrobial effects generated by these molecules are significantly reduced when higher levels of CO2 are present within the culture suggesting the activity is likely mediated primarily via NgCA inhibition. Even with this hypothesis we must note that sulfonamide containing drugs used to be a common therapeutic option to treat gonorrhea in the 1930’s through mid-1940’s and they were determined to inhibit bacterial dihydropteroate synthase (DHPS);62 however, by the late-1940’s >90% of gonococcal strains were resistant to sulfonamides.63–65 If a second target was potentially contributing to the antimicrobial activity of the molecules presented herein, like DHPS, we would expect analogs to maintain an appreciable level antimicrobial activity in higher CO2 levels, as in the case of azithromycin. Further, in order to investigate the possibility of DHPS inhibition as a target for our analogs we tested the antibacterial activity of sulfamethoxazole, sulfdoxine, and sulfathiazole against three N. gonorrhoeae strains. As depicted in Table S5, sulfa drugs did not inhibit the bacterial strains tested, under both CO2 and non-CO2 conditions at concentrations up to >64 μg/mL while CAIs – AZM, 20, and 23 – inhibited the test strains under non-CO2 conditions with MIC values ranging from 0.125 μg/mL to 1 μg/mL.
Preliminary evidence based on previous literature and the reduced susceptibility to the molecules in CO2 conditions suggests that the intracellular target for these molecules is likely NgCA. Specific analog SAR observations may also support this hypothesis. First, the observation that the modification of the sulfonamide to a sulfone abrogates both antimicrobial activity as well as in vitro activity against all three CAs tested supports this claim. Structural and mechanism of action studies for CAIs have shown that inhibition is mediated primarily through coordination of the active site Zn2+ by a chelating moiety,66–68 in this case a sulfonamide. Our data shows that modification of this functional group (as in analog 31) eliminates both the ability of the molecules to bind the target NgCA (Figure 6), and in turn reduces antimicrobial activity, supporting the hypothesis that the intracellular target is NgCA.
Figure 6.
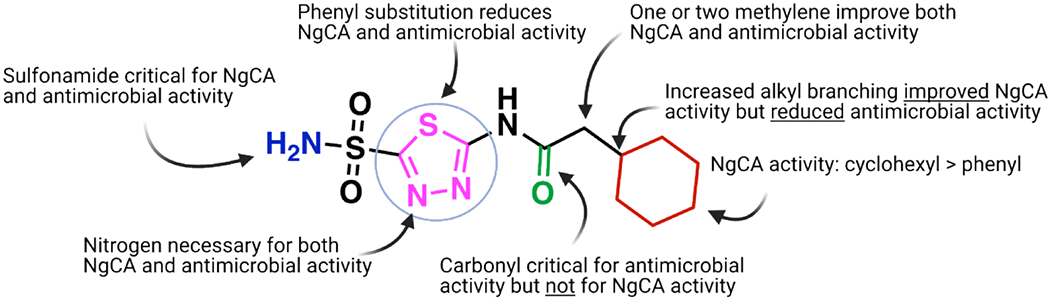
Summary of SAR observations as they relate to either NgCA activity and antimicrobial activity.
Another SAR data point that supports this hypothesis is that modification of central thiadiazole ring reduced activity in both assays. Analog 32 investigated the role of the 4-nitrogen in the thiadiazole ring on both in vitro CA and antimicrobial activity. The nitrogen is documented to participate in a hydrogen bond with the side chain of Thr200 in hCA II56 and this threonine is conserved in NgCA (Figure S2). Modification to the thiazole ring in 32 reduced activity in both NgCA and hCA II by 5–6-fold compared to AZM, suggesting the change had the same detrimental effect on each CA and that these analogs may interact with NgCA similar to how CAIs bind to hCA II. This resulted in 32 displaying the second highest Ki in the CO2 hydration assay behind the sulfone derivative described above. If the concurrent loss in NgCA potency and antimicrobial activity are related it further bolsters the claim that NgCA is the intracellular target. However, this analysis does not account for any potential changes to N. gonorrhoeae compound permeability that could also affect the antimicrobial potency. Studies are ongoing to quantify bacterial permeability of analogs in N. gonorrhoeae and to confirm on-target inhibition in a cellular context.
Observed SAR on the amide functionality for antimicrobial activity (Figure 6) generally preferred linear alkanes with reduced steric bulk. The impact of alkyl branching was most noticeable on analogs with the branched carbon directly next to the carbonyl. This is illustrated by the congeneric series of analogs ranking in order of activity of H (1) = methyl (AZM) > ethyl (2) > iso-propyl (3) > tert-butyl (4) with increased alkyl branching trending to poorer MIC values. A modification that appears to have a large impact on antimicrobial efficacy is the presence of a quaternary carbon. Three analogs that possess quaternary carbons (4, 8, and 14) were all less potent in antimicrobial assays than their counterparts that contained reduced alkyl branching. For example, when comparing the cyclohexyl containing analog 13 (MIC = 2 μg/mL) there was a steep decrease in activity for the quaternary carbon containing nearest analog 14 (MIC > 64 μg/mL). With respect to in vitro NgCA activity the increase in alkyl bulk generally improved inhibition of the enzyme. Among the linear alkane analogs the tert-butyl representatives, 4 and 8, displayed the most potent Ki values (30.5 and 9.1 nM, respectively) within their respective congeneric series yet were the least potent in antimicrobial assays. This trend for in vitro activity was not observed with the cyclohexyl containing matched molecular pair 13 and 14. The quaternary carbon containing analog 14 was less potent against NgCA compared to 13. However, 14 still maintained NgCA activity (Ki = 75.8 nM) comparable to AZM, and many other analogs, yet the molecule was inactive in antimicrobial assays at concentrations up to 64 μg/mL. Conversely, the tertiary carbon derivative 13 displayed an MIC value of 2 μg/mL. It would seem the divergence in NgCA and antimicrobial activity among these analogs indicates that increased alkyl branching at these positions may be detrimental to the permeability of the molecules to access the intracellular target.
Interestingly, the hypothesis that increased alkyl branching could reduce permeability may be further supported by SAR observations against Gram-positive VRE for this same set of analogs. As previously reported for this series the trend of increasing alkyl branching steadily improved antimicrobial activity against the Enterococcus faecium.44 Direct comparison of MIC values between the two pathogens are shown in Table 5. For example, against E. faecium strain HM-965 the two congeneric series that increased alkyl branching yielded stepwise improvements to anti-enterococcal activity, while the opposite was observed in N. gonorrhoeae as described above. These analogs are hypothesized to inhibit the α-CA in E. faecium (α-EfCA) and in vitro data collection against this enzyme is ongoing. Therefore, there are currently no in vitro potency comparisons that can be made for the analogs between NgCA and α-EfCA, nonetheless, based on sequence data alignment (Figure S2) the sequence identity within the two active sites is 67% with key residues known for CAI interaction, the threonine in particular, remaining intact. Thus, we would suspect similar in vitro potency trends to be observed for these analogs in both NgCA and α-EfCA. If this assumption is indeed shown to be true then the variance in antimicrobial potency between the Gram-positive and Gram-negative pathogens may be less due to difference in target inhibition and more so due to differences in accessibility to the intracellular targets. The physicochemical properties that drive permeability of molecules into N. gonorrhoeae is an active arm of study within this project and may illuminate the properties necessary for improved permeability.
Table 5.
MIC comparison between N. gonorrhoeae and E. faecium
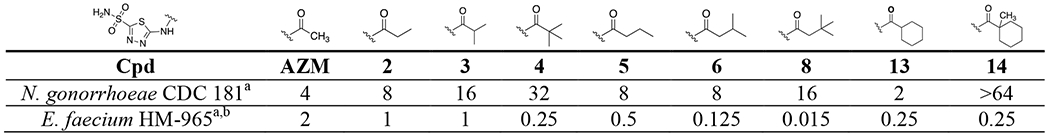 |
MIC values in μg/mL.
Values reported by Kaur et al.44
Another observation may link cyclization of alkyl functional groups to improved N. gonorrhoeae permeability. In general, if a tertiary carbon was branched as part of a linear alkane the antimicrobial activity was less potent than if that tertiary center was incorporated within a cycloalkyl ring. For example, comparing the iso-propyl analog 3 (MIC = 16 μg/mL) with cyclized matched molecular pairs listed in order of increasing ring-size: 10, 11, 12, and 13 were comparable or much improved in antimicrobial activity compared to the iso-propyl counterpart all while displaying similar NgCA activity. This observation suggests alkane cyclization may improve permeability compared to the linear alkanes and will be investigated further.
The discrepancy between whole-cell antibacterial activity and in vitro data against NgCA, particularly for the molecules that are not active against the pathogen, suggests a physicochemical property of these inactive molecules may cause reduced permeability into the Gram-negative bacteria. We attempted to rationalize this difference in activity by applying the eNTRy rules for Gram-negative Escherichia coli bacteria developed by Richter et al69. All molecules were submitted for analysis using the online portal associated with the Richter manuscript, www.entry-way.org. Almost all molecules analyzed fit at least two of the three criteria that are correlated with improved Gram-negative permeability, those being globularity < 0.25 and < 5 rotatable bonds. However, none of the analogs contain a primary amine and there were no clear trends for any metrics that would correlate with antimicrobial activity (spreadsheet with values provided in supporting information). The antimicrobial active sulfonamide analogs in the present work lack a primary amine, or any positive charge for that matter. In fact, the sulfonamide of AZM has a reported pKa of 7.270 while the proton on the amide has a predicted pKa of 6.6 (MarvinSketch version 18.28, ChemAxon, https://chemaxon.com). These values indicate the majority of molecules in solution would carry a net negative charge at physiological pH and this, in theory, may limit their ability to enter through the such porins. However, it is important to note that these rules were developed in E. coli as the model organism and there likely may be differences in the porins between E. coli and N. gonorrhoeae that could limit the applicability of the eNTRy rules to N. gonorrhoeae. For example, the current drug of choice to treat gonococcal infection is ceftriaxone, which is marketed as a di-sodium salt, and would be predominantly anionic at physiological pH. Additionally, previous reports have demonstrated negatively-charged molecules are capable of flux through the two porins associated with N. gonorrhoeae,71 although these studies were performed in liposomal systems with recombinantly express porins and not in a whole-cell context. Nonetheless, these studies and ours indicate the presence of a primary amine and avoidance of a negatively charged analog may not be as important for permeability in N. gonorrhoeae.
Even though the scaffold may not fully adhere to the eNTRy rules it does appear to have an intrinsic ability to permeate the Gram-negative outer membrane even while lacking a positive charge. Previous work by O’Shea and Moser looked into the effect both cLogD7.4 and total polar surface area (tPSA) had on Gram-negative active FDA-approved antibiotics.72 To investigate whether these metrics have an effect on the activity of our analogs we used the program QikProp (Meastro release 2020-4;Schrödinger, LLC; New York, NY) to generate the predicted octanol/water partition coefficient (QPLogPo/w) and the tPSA for each analog. We then plotted these metrics in a 3-dimensional scatter plot with tPSA on the X-axis, QPLogPo/w on the Y-axis and MIC value on the Z-axis (Figure 7). We introduced a 4th dimension in bubble size that corresponds to the Ki values for each analog against NgCA in the CO2 hydration assay. We then added a 5th dimension by color coding the bubbles into cohorts according to the following: green = analogs with MIC < 64 μg/mL and NgCA Ki < 100 nM; red = polar pendant groups (24 – 27); yellow = contain quaternary carbon (4, 8, and 14); gray = no carbonyl on the amide (28 – 30); and violet = NgCA Ki > 400 nM (32 and 33). Analog 31 was left out of the analysis as the sulfone is known to abrogate carbonic anhydrase activity and it was inactive against the bacteria. We also colored AZM black as a point of reference from the hit molecule.
Figure 7.
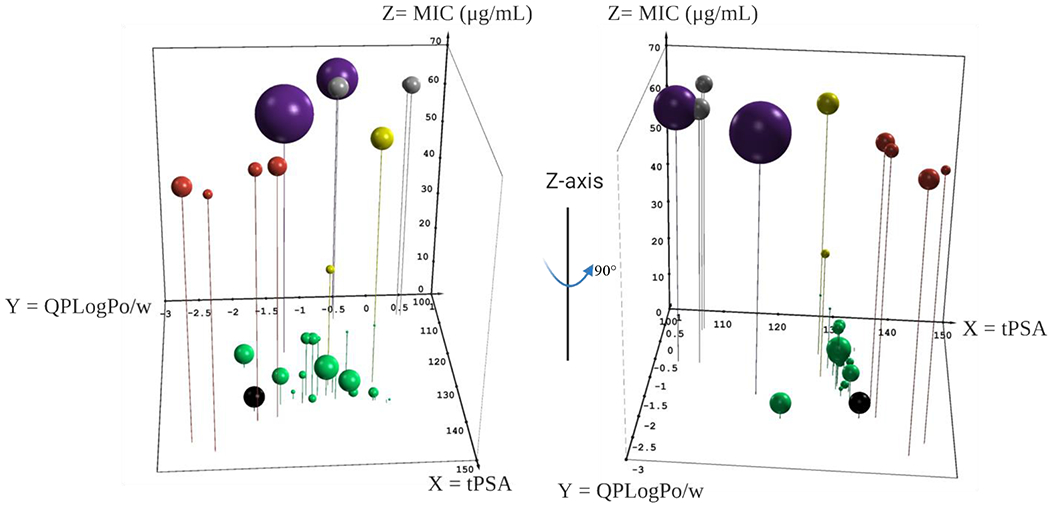
Three-dimensional scatterplot to assess the relationship between physicochemical properties and antimicrobial activity. QikProp metrics tPSA (Å, X-axis), QPLogPo/w (Y-axis) and MIC (μg/mL, Z-axis). Size of bubble corresponds to Ki (nM) versus NgCA as determined by CO2 hydration assay. Analogs with: < 64 μg/mL and < 100 nM Ki (green); contain quaternary carbon (yellow); polar pendant groups (red); no carbonyl (gray); and reduced NgCA activity (violet). AZM is shown in black. Plot was made in Excel using the 5dchart add-in (www.5dchart.com).
What was observed is that molecules maintaining physicochemical properties of QPLogPo/w between 0 and −2 and tPSA between 120 and 135 Å2 (Figure 7, green spheres) as well as NgCA Ki < 100 nM generally maintained antimicrobial activity against N. gonorrhoeae at MIC values ≤ 16 μg/mL. Exceptions to this trend were analogs 4, 8 and 14 (yellow spheres), which contained quaternary carbons and maintained sub-100 nM Ki versus NgCA but displayed MICs of 32, 16, and >64 μg/mL, respectively. In fact, analog 8 (Ki = 9.1 nM) was among the most potent of the entire study against NgCA but had an MIC of only 16 μg/mL; however, within this congeneric series the MIC values did correlate to NgCA potency. These observations, along with those discussed prior, suggest that perhaps the quaternary carbon limits molecule permeability into N. gonorrhoeae. Exceptions were also noted for a few analogs containing a tertiary carbon, but these analogs never had MIC values worse than 16 μg/mL and reduced antimicrobial potency was not applicable to all tertiary carbon containing analogs.
In the O’Shea and Moser analysis, the authors noted that for sulfa-class antibacterials the average tPSA was 112 Å2, which would suggest that the tPSA lower limit for Gram-negative permeability for this scaffold is likely < 120 Å2. Five analogs in our set had tPSA values below this threshold but were still inactive against N. gonorrhoeae. Digging deeper, two of these analogs, 32 and 33 (purple spheres), had steep reductions in NgCA activity (Ki > 400 nM) that may explain the reduced antimicrobial activity. The other three analogsbelow 120 Å2 with no antimicrobial activity were observed to lack a carbonyl on the amide (28 – 30, gray spheres). These molecules maintained potent NgCA activity but did not have antibacterial effect. It is unclear if the removal of the carbonyl reduces permeability; however, this modification would increase both the rotatable bond count and flexibility of the analogs, two trends that would be detrimental to Gram-negative permeability according to the eNTRy rules.
Finally, four analogs containing polar pendant groups (24 – 27) are presented in red spheres. These analogs also were active against NgCA in the CO2 hydration assays at comparable levels to the rest of the set; however, they were inactive against the bacteria. In observing where these molecules reside in terms of physicochemical property space, they are the most polar with QPLogPo/w < −1.4 and tPSA > 135 Å2. Thus, it is reasonable to presume that the increased polarity of these molecules has crept outside of the range necessary for N. gonorrhoeae permeability. While this initial analysis offers clues to the properties that may provide entry into N. gonorrhoeae, a more exhaustive study to correlate structure-property relationship with permeability into the bacteria is necessary.
The analog set maintained potent activity against the two representative human carbonic anhydrases, hCA I and hCA II. At this point of the project, it is unclear whether this would be a liability for the class. Generally, FDA-approved carbonic anhydrase inhibitors are relatively non-toxic and require high doses before they exhibit side-effects.73 For example, a phase 1 trial showed that 90% of patients tolerated > 1 g/day of AZM for 6 months with 45% tolerating up to 4 g/day over the same time frame.74 Moreover, when side effects are observed in the clinic they tend to be attributed to age75,76 or drug-drug interaction with salicylate.77 Furthermore, a common length of an antimicrobial drug dosing regimen would likely be in the range of days to a few weeks at most. In theory, this acute dosing timeline could reduce the impact of any negative side-effects associated with inhibition human carbonic anhydrases. Even though inhibition of human carbonic anhydrases may not prove to be a liability in terms of toxicity, it could have a profound effect on molecule distribution in vivo. Carbonic anhydrase inhibitors are known to partition quite readily into red blood cells that contain hCA I and hCA II, which effectively forms a sink to sequester the drug.55,78 Thus, our ongoing project will be focused on reduction of binding to human isoforms as this would likely lead to improved pharmacokinetics and decrease the size of the dose needed for any in vivo effect.
In additional antimicrobial testing, the carbonic anhydrase inhibitors showed potential that was on par or better than azithromycin. Analog 20 consistently outperformed azithromycin against a panel of 30 N. gonorrhoeae isolates and posted improved MIC50 and MIC90 values compared to the positive control. While azithromycin was bactericidal, AZM, 20 and 23 were found to possess bacteriostatic properties over the course of a 24-hour incubation and reduced CFU load of N. gonorrhoeae by least 3-log units compared to the DMSO treated controls. When tested for cytotoxicity in human cells the scaffold was shown to be non-toxic against the human endocervical cell line End1/E6E7 and Caco-2 cell lines at concentrations up to 128 μg/mL. Moreover, both 20 and 23 also retain favorable drug-like physicochemical properties with molecular weights around 300 Da and low predicted QPLogPo/w’s. While these molecules are yet to be assessed in in vitro ADME experiments, the structurally similar analog 22 has been tested in various assays. This molecule exhibited solubility > 30 μM in PBS, was highly permeable across a Caco-2 monolayer, and was detected in plasma of mice after 10 mg/kg oral dosing.44 It did show a propensity to be modified in human liver microsome assay with 62% of the molecule remaining after 1 hr. Analogs 20 and 23 occupy similar chemical property space compared to 22 so we would expect these molecules to possess a similar in vitro ADME and in vivo pharmacokinetic profile to 22, although these experiments are planned to confirm this. Nonetheless, the favorable physicochemical properties of the analogs in this study may translate to reduced risk for triage due to toxicity and/or solubility as they are advanced beyond pre-clinical studies.
Even though these molecules represent promising starting points for validating carbonic anhydrase inhibition as a viable strategy to target N. gonorrhoeae there are improvements still to be made. Future work will include investigation of properties that drive permeability into the bacteria as well as structure-guided design of new inhibitors to improve NgCA activity, reduce hCA activity and limit sites of metabolic liability. Ultimately, these future directions will advance the scaffold to experiments to assess the molecule for in vivo efficacy animal models for N. gonorrhoeae infection.
CONCLUSION
We report a new class of sulfonamide-based carbonic anhydrase inhibitors with antimicrobial efficacy against the drug-resistant pathogen N. gonorrhoeae. The original hit AZM displayed an MIC of 4 μg/mL and subsequent analogs produced a range of activity against the bacteria with 20 and 23 rising above the rest to exhibit MIC values as low as 0.25 μg/mL. This translated to an MIC50 value of 0.5 μg/mL for 20 against a panel of 30 clinical isolates and it outperformed azithromycin with respect to antimicrobial activity. It differed from azithromycin in terms of killing kinetics as the carbonic anhydrase inhibitors were found to be bacteriostatic while azithromycin is bactericidal. Moreover, we hypothesize the intracellular target for the molecules is likely the α-carbonic anhydrase, NgCA. Several SAR trends were observed in both MIC and in vitro inhibition assays and at times these SAR trends diverged in opposite directions when comparing antibacterial activity to NgCA potency. Additional analysis of structure-property relationship relative to antimicrobial activity also highlighted the potential roles polarity and structural modifications, such as quaternary carbons and lack of an amide carbonyl, may have on permeability across N. gonorrhoeae outer membrane. Ultimately, analogs that lacked a quaternary carbon and maintained tPSA < 135 Å2 trended toward increased potency in both antimicrobial and NgCA assays. Analogs were also assessed against two representative human carbonic anhydrases and were shown to maintain appreciable activity against those enzymes. Finally, molecules 20 and 23 were shown to be non-toxic against two relevant human cell lines. The data provided highlights the potential of the class of molecules to be added to the arsenal of effective therapeutics for the treatment of N. gonorrhoeae.
METHODS
Chemistry
All analogs tested for activity in antimicrobial assays and in vitro binding assays were previously synthesized as part of a related project reported by Kaur et al.44 All synthetic procedures can be found in that publication. Characterization and purification data for all analogs, as well as the synthetic procedure for FITC-AZM, are provided in the supporting information of this manuscript. Spectral data for FITC-AZM is also provided.
Biological Evaluation
Bacterial strains, media and chemicals
N. gonorrhoeae strains (Table S3) used in the study were clinical isolates obtained from the CDC and the American Type Culture Collection (ATCC). Drugs used in this study were purchased from chemical vendors: acetazolamide (Sigma-Aldrich, MO) and azithromycin (TCI America, OR). Compounds were synthesized in our lab and prepared in stock concentrations in DMSO. Media and reagents were purchased commercially: brucella broth, IsoVitaleX and chocolate II agar (Becton, Dickinson and Company, MD), yeast extract and dextrose (Fisher Bioreagents, NJ), protease peptone and nicotinamide adenine dinucleotide (NAD) (Sigma-Aldrich, MO), hematin, tween 80 and pyridoxal (Chem-Impex International, IL) and phosphate buffered saline (PBS) (Fisher Scientific, MA).
Antibacterial activity of synthesized compounds against N. gonorrhoeae strains
The minimum inhibitory concentrations (MICs) of acetazolamide and analogs were determined using the broth microdilution assay, as previously described.51–53 Briefly, N. gonorrhoeae strains were grown overnight on chocolate II agar plates. Bacterial cells were then suspended in phosphate-buffered saline (PBS) to achieve a turbidity equivalent to a 1.0 McFarland standard which was diluted in brucella broth supplemented with yeast extract, dextrose, proteose-peptone, NAD, pyrodixal, hematin and IsoVitaleX to reach a bacterial count of about 1 × 106 CFU/mL. Drugs and compounds were added and serially diluted along the plates. Media containing bacteria (without test agents) were included in the assays as a control. Plates were then incubated aerobically, and in presence of 5% CO2 for 24 hours at 37 °C prior to recording the MIC as observed visually. MICs reported are the minimum concentrations of the compounds and drugs that completely inhibited the visual growth of bacteria.
Transformation of plasmid into E. coli
The His-NgCA plasmid in a pET-15b vector was ordered from GenScript and transformed into BL21(DE3) competent E. coli cells (New England Biolabs, catalog no. C2527I) This was plated onto an ampicillin agar plate and grown overnight at 37 °C. Colonies were picked from the agar platesand grown overnight in LB media at 37 °C with shaking at 250 rpm. These cultures were used to make glycerol stocks by mixing 20% glycerol and 80% culture and placed in −80 for future protein expressions.
Recombinant expression and purification of NgCA
His-NgCA starter cultures were made in autoclaved LB media containing 100 μg/mL ampicillin from the glycerol stock (made as described above) and grown at 37 °C with shaking overnight at 250 rpm. An aliquot of 10 mL of the starter culture was inoculated into each liter of autoclaved LB media containing 100 μg/mL ampicillin and 1 mM ZiCl2. These cultures were grown at 37 °C and shaken at 250 rpm. Upon reaching an OD600 of 0.4 – 0.8, the cultures were induced with 300 μL of 1.0 M isopropyl β-D-thiogalactopyranoside (IPTG) and then grown overnight at 16 °C with shaking at 150 rpm. Cultures were spun down at 4000 x g for 20 minutes. Bacterial pellets were resuspended in lysis buffer (1 x PBS containing 1 mM DTT). The bacterial cells were then lysed by sonication. Lysed cells were pelleted by centrifuging them at 14000 x g for 1 hour. The supernatant of the pelleted His-NgCA sample was loaded onto a nickel-NTA column pre-equilibrated with 50 mM Tris, 150 mM NaCl, and 1 mM DTT pH = 7.4. His-NgCA was eluted from the column using a gradient of 0-500mM imidazole in the same equilibration buffer. Fractions were collected and analyzed by SDS-PAGE to determine which fractions containing the His-NgCA. These fractions were combined and dialyzed at 4 °C against 50 mM Tris, 150 mM NaCl containing 1mM DTT pH = 7.4. NgCA was concentrated using Amicon Ultra Centrifugal Filters and further purified through size-exclusion chromatography with an S100 column using running buffer [50mM Tris, 150mM NaCl, and 1mM DTT (pH 7.4)].
Determination of the Kd of FITC-AZM to NgCA, hCA I, and hCA II
All fluorescence polarization binding assays were performed in 384-well black bottom plates using an assay buffer consisting of 50 mM Tris, 150 mM NaCl, and 1mM DTT (pH 7.4). His-NgCA was purified as described above and hCA I and hCA II were ordered from Millipore Sigma (hCA I Catalog# C4396-5MG; hCA II Catalog # C6624-500UG) and diluted in assay buffer to concentrations used in the assay in assay buffer. The initial Kd of the probe to His-NgCA, hCA I, and hCA II were determined by titrating the respective proteins (at 2 x concentration) into 25 μL solution containing 20 nM probe for final concentration in the well of 10 nM FITC-AZM and protein concentrations ranging in 14 dilutions ranging from 0 – 2.0 μM. Each concentration of each protein was performed in technical triplicate. Each Kd determination assay plate was covered in foil and incubated together with shaking for 15 minutes at room temperature. After 15 minutes, an additional incubation of 15 minutes was completed without shaking. Assay plates were then read on a Synergy Neo 2 (BioTek) using FP settings (excitation wavelength 485 nm and emission wavelength of 530 nm) with autogain setting on probe alone control wells (autogain polarization values set to 20 and intensity set to 10,000). After a baseline correction analysis, the polarization values were plotted against the protein concentrations and a one-site specific binding model (Prism 9.0.0) was fit to the data to determine the Kd. The protein concentration utilized in the competitive-FP assay was determined at ~70% asymptotic polarization values in this initial binding experiment.
Competitive Fluorescence Polarization Assay with Carbonic Anhydrase Inhibitors
The competitive FP assay was performed with final protein assay concentrations of 50 nM His-NgCA or 50 nM hCA II or 125 nM hCA II (20 μL of 2.5x concentrations were first added to the wells, along with necessary controls as detailed below. 20 μL of 25 μM probe was then added to the protein in a dark room. Subsequently, 10 μL of a titration of carbonic anhydrase inhibitor was added in a dark room. Controls for this assay consisted of 1) probe alone, 2) probe + protein, 3) highest concentration of inhibitor alone, and 4) highest concentration of inhibitor + probe. Each concentration of inhibitor was performed in technical triplicate. Controls 3 and 4 were used to determine if there were any internal fluorescence/polarization effects of the inhibitor or if any quenching of the probe was occurring and none was observed. Experimental data was input into Prism 9.0.0 software. Concentrations were transformed to log10 based values and the control 1 and 2 (listed above) were normalized to 0 and 100% probe bound to protein, respectively. The data was then fit to non-linear response (log [inhibitor] vs. normalized response) from which the IC50s were derived. Estimations of Kis were completed by inputting the IC50 values for each inhibitor, protein concentration, FITC-AZM concentration and Kd into the FP Ki calculation derived by Nikolovska-Coleska et al.49
Carbonic anhydrase CO2 hydration catalytic assay and Ki determination
The assay was performed according to previously published protocols.46,79–81 Recombinant hCA I and hCA II were purchased from Millipore Sigma (hCA I Catalog# C4396-5MG; hCA II Catalog # C6624-500UG). Ki values were determined from inputting the IC50 values into the Cheng-Prusoff82 equation for Ki from catalytic inhibition constants.
Killing kinetics assay
In order to determine the mode of killing of carbonic anhydrase inhibitors and analogs a standard time kill kinetics assay was performed against N. gonorrhoeae ATCC 700825 as described previously.83–85 N. gonorrhoeae was grown in brucella supplemented broth to logarithmic phase and further diluted to reach an initial inoculum of 6 × 106 CFU/mL. Acetazolamide, analogs and azithromycin (positive control) were then added (at 10 × MIC in triplicates), and further incubated in the ambient air at 37°C for 24 hours. Bacteria exposed to DMSO (solvent of drugs) alone served as a negative control. An aliquot from each sample was collected from each treatment after the corresponding times of incubation and subsequently serially diluted and plated onto chocolate II agar plates. Plates were incubated for 24 h at 37°C before viable CFU/mL was determined.
Cytotoxicity assessment against human endocervical End1/E6E7 and Caco-2 cell lines.
Compounds were assayed (at concentrations of 8, 16, 32, 64 and 128 μg/mL) against human endocervical (End/E6E7) cells to determine their potential toxic effect to the human endocervix cells in vitro. Briefly, cells were cultured in serum-free keratinocyte medium (KSFM) supplemented with 0.05 mg/ml bovine pituitary extract, 0.1 ng/ml epidermal growth factor, and 44.1 mg/liter calcium chloride, at 37 °C with CO2 (5%). The cells were incubated aerobically with the compounds (in triplicates) in a 96-well plate at 37 °C for 24 hours. Control cells received DMSO (the solvent of the compounds) alone at a concentration equal to that in drug-treated wells to determine the baseline measure of the cytotoxic impact of the compounds. The assay reagent MTS 3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) (Promega, Madison, WI, USA) was subsequently added and the plate was incubated for three hours. Absorbance readings (at OD490) were recorded using a kinetic microplate reader (Molecular Devices, Sunnyvale, CA, USA).
For human colorectal adenocarcinoma (Caco-2) cell line toxicity the assay went as described previousy.44 Briefly, cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), non-essential amino acids (1X), penicillin-streptomycin at 37 °C with CO2 (5%). Control cells received DMSO (the solvent of the compounds) alone at a concentration equal to that in drug-treated wells to determine the baseline measurement of the cytotoxic impact of the compounds. The cells were incubated with the compounds (in triplicates) in a 96-well plate at 37 °C with 5% CO2 for 24 hours. The assay reagent MTS (Promega, Madison, WI, USA) was subsequently added and the plate was incubated for four hours. Absorbance readings (at OD490) were recorded using a kinetic microplate reader (Molecular Devices, Sunnyvale, CA, USA).
For each cell line, the quantity of viable cells after treatment with each compound was expressed as a percentage of the viability relative to DMSO-treated control cells (average of triplicate wells ± standard deviation). A two-way ANOVA with post-hoc Dunnett’s test for multiple comparisons determined no statistically significant difference between treatments with 20 or 23 as compared to DMSO-treated cells.
Supplementary Material
Scheme S1. Synthetic route for analogs 1 – 30
Scheme S2. Synthetic route for analog 31
Scheme S3. Synthetic route for FITC-AZM
Figure S1. FITC-AZM binding curve and AZM displacement for hCA I
Table S1. Binding affinity and concentrations for carbonic anhydrase fluorescence polarization competition
Table S2. Carbonic anhydrase fluorescence polarization competition assay data
Table S3. N. gonorrhoeae strains used in the study
Table S4. Anti-gonococcal activity against additional drug-resistant and -sensitive N. gonorrhoeae strains in both ambient and increased CO2 conditions
Table S5. MICs (μg/mL) of sulfa drugs as compared to CAIs against N. gonorrhoeae strains
Figure S2. Sequence alignment for a-carbonic anhydrase
Procedure for sequence alignments
Characterization and Purity for final analogs
Spectra for FITC-AZM
Synopsis:
Hewitt et al. report the characterization of a set of carbonic anhydrase inhibitors based onteh FDA-approved scaffold of acetazolamide for activity against the Gram-negative pathogen Neisseria gonorrhoeae. The in vitro inhibition constants against the N. gonorrhoeae carbonic anhydrase and two human carbonic anhydrase isoforms are reported. The article provides insight into the structure-activity relationship for this class of antimicrobial agents and identifies structural modifications that may reduce permeability into the Gram-negative pathogen.
ACKNOWLEDGEMENTS
The authors thank Jason Scott for assistance in computing QikProp metrics. The research program was partially funded by a Purdue Institute for Drug Discovery Programmatic Grant (M.N.S and D.P.F.) and NIH/NIAID 1R01AI148523 (M.N.S and D.P.F.). This work was also supported by the Italian Ministry for University and Research, grant FISR2019_04819 BacCAD (C.T.S.). Figures created using Biorender.com.
Dr. Flaherty also dedicates this work to his mentor Professor Jonathan L. Vennerstrom, who has demonstrated an unwavering commitment to combating neglected tropical disease with passion and humility, on the occasion of his 65th birthday.
Footnotes
Supporting information
Supporting information is available free of charge at https://pubs.acs.org.
REFERENCES
- (1).World Health Organization (2020) Multi-drug resistant gonorrhoea Fact Sheet, WHO, Geneva, https://www.who.int/news-room/fact-sheets/detail/multi-drug-resistant-gonorrhoea. [Google Scholar]
- (2).Bowen VB, Braxton J, Davis DW, Flagg EW, Grey J, Grier L, Harvey A, Kidd S, Kreisel K, Llata E, (2019) Sexually Transmitted Disease Surveillance 2018. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- (3).Kersh EN, Pham CD, Papp JR, Myers R, Steece R, Kubin G, Gautom R, Nash EE, Sharpe S, Gernert KM, (2020) Expanding US laboratory capacity for Neisseria gonorrhoeae antimicrobial susceptibility testing and whole-genome sequencing through the CDC’s antibiotic resistance laboratory network. J. Clin. Microbiol 58, e01461–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Rice PA, Shafer WM, Ram S, Jerse AE, Neisseria gonorrhoeae: drug resistance, mouse models, and vaccine development . Annu. Rev. Microbiol 2017, 71 (1), 665–686. [DOI] [PubMed] [Google Scholar]
- (5).Little JW, (2006) Gonorrhea: Update. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontology 101, 137–143. [DOI] [PubMed] [Google Scholar]
- (6).Sandström I, (1987) Etiology and diagnosis of neonatal conjunctivitis. Acta Pædiatrica 76, 221–227. [DOI] [PubMed] [Google Scholar]
- (7).Masi AT, Eisenstein BI(1981) Disseminated gonococcal infection (DGI) and gonococcal arthritis (GCA): II. Clinical manifestations, diagnosis, complications, treatment, and prevention. In Seminars in arthritis and rheumatism. pp 173–197, Elsevier, Amsterdam, Netherlands. [DOI] [PubMed] [Google Scholar]
- (8).Kerle KK, Mascola JR, Miller TA, (1992) Disseminated gonococcal infection. Am. Fam. Physician 45, 209–214. [PubMed] [Google Scholar]
- (9).Unemo M, Del Rio C, Shafer WM, (2016) Antimicrobial resistance expressed by Neisseria gonorrhoeae: a major global public health problem in the 21st Century. In Emerging Infections, 10th ed. pp 213–273, American Society for Microbiology, Washington, D.C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Wi T, Lahra MM, Ndowa F, Bala M, Dillon JAR, Ramon-Pardo P, Eremin SR, Bolan G, Unemo M, (2017) Antimicrobial resistance in Neisseria gonorrhoeae: global surveillance and a call for international collaborative action. PLoS Med. 14, 1–16. 10.1371/journal.pmed.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Cyr SS, Barbee L, Workowski KA, Bachmann LH, Pham C, Schlanger K, Torrone E, Weinstock H, Kersh EN, Thorpe P, (2020) Update to CDC’s treatment guidelines for gonococcal infection. Morb. Mortal. Wkly. Rep 69, 1911–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).World Health Organization (2014) Antimicrobial resistance: global report on surveillance; World Health Organization, Geneva. [Google Scholar]
- (13).Bolan G, Sparling PF, Wasserheit JN, (2012) The emerging threat of untreatable gonococcal infection. N. Engl. J. Med 366, 485–487. [DOI] [PubMed] [Google Scholar]
- (14).Centers for Disease Control and Prevention (2019) Antibiotic resistance threats in the United States, CDC, Atlanta, GA. [Google Scholar]
- (15).Wetzler LM, Feavers IM, Gray-Owen SD, Jerse AE, Rice PA, Deal CD, (2016) Summary and recommendations from a National Institute of Allergy and Infectious Diseases (NIAID) workshop on “Gonorrhea vaccines: the way forward.” Clin. Vaccine Immunol 23, 656–663. 10.1128/cvi.00230-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Lewis DA, (2019) New treatment options for Neisseria gonorrhoeae in the era of emerging antimicrobial resistance. Sex. Health 16, 449–456. 10.1071/SH19034. [DOI] [PubMed] [Google Scholar]
- (17).Hook EW, Golden MR, Taylor SN, Henry E, Tseng C, Workowski KA, Swerdlow J, Nenninger A, Cammarata S, (2019) Efficacy and safety of single-dose oral Delafloxacin compared with intramuscular Ceftriaxone for uncomplicated gonorrhea treatment: an open-label, noninferiority, phase 3, multicenter, randomized Study. Sex. Transm. Dis 46, 279–286. 10.1097/OLQ.0000000000000971. [DOI] [PubMed] [Google Scholar]
- (18).Taylor SN, Marrazzo J, Batteiger BE, Hook EW, Seña AC, Long J, Wierzbicki MR, Kwak H, Johnson SM, Lawrence K, Mueller J, (2018) Single-dose Zoliflodacin (ETX0914) for treatment of urogenital gonorrhea. N. Engl. J. Med 379, 1835–1845. 10.1056/nejmoa1706988. [DOI] [PubMed] [Google Scholar]
- (19).Taylor SN, Morris DH, Avery AK, Workowski KA, Batteiger BE, Tiffany CA, Perry CR, Raychaudhuri A, Scangarella-Oman NE, Hossain M, Dumont EF, (2018) Gepotidacin for the treatment of uncomplicated urogenital gonorrhea: a phase 2, randomized, doseranging, single-oral dose evaluation. Clin. Infect. Dis 67, 504–512. 10.1093/cid/ciy145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Supuran CT, (2008) Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov 7, 168–181. 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- (21).Nishimori I, Minakuchi T, Onishi S, Vullo D, Cecchi A, Scozzafava A, Supuran CT, (2007) Carbonic anhydrase inhibitors: cloning, characterization, and inhibition studies of the cytosolic isozyme III with sulfonamides. Bioorg. Med. Chem 15, 7229–7236. [DOI] [PubMed] [Google Scholar]
- (22).Vullo D, Franchi M, Gallori E, Antel J, Scozzafava A, Supuran CT, (2004) Carbonic anhydrase inhibitors. Inhibition of mitochondrial isozyme V with aromatic and heterocyclic sulfonamides. J. Med. Chem 47, 1272–1279. [DOI] [PubMed] [Google Scholar]
- (23).Koch A, Woodbury DM, (1960) Carbonic anhydrase inhibition and brain electrolyte composition. Am. J. Physiol. Content 198, 434–440. [DOI] [PubMed] [Google Scholar]
- (24).Becker B, (1954) Decrease in intraocular pressure in man by a carbonic anhydrase inhibitor, Diamox*: a preliminary report. Am. J. Ophthalmol 37, 13–15. [DOI] [PubMed] [Google Scholar]
- (25).Maren TH, (1967) Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol. Rev 47, 595–781. [DOI] [PubMed] [Google Scholar]
- (26).Supuran CT, Scozzafava A, Conway J, (2004) Carbonic anhydrase: its inhibitors and activators; CRC press, Washington, D.C. [Google Scholar]
- (27).Sugrue MF, (2000) Pharmacological and ocular hypotensive properties of topical carbonic anhydrase inhibitors. Prog. Retin. Eye Res 19, 87–112. [DOI] [PubMed] [Google Scholar]
- (28).Kyllönen MS, Parkkila S, Rajaniemi H, Waheed A, Grubb JH, Shah GN, Sly WS, Kaunisto K, (2003) Localization of carbonic anhydrase XII to the basolateral membrane of H+-secreting cells of mouse and rat kidney. J. Histochem. Cytochem 51, 1217–1224. [DOI] [PubMed] [Google Scholar]
- (29).Ellison DH, (2001) Diuretic therapy and resistance in congestive heart failure. Cardiology 96, 132–143. [DOI] [PubMed] [Google Scholar]
- (30).Švastová E, Hulíková A, Rafajová M, Zat’ovičová M, Gibadulinová A, Casini A, Cecchi A, Scozzafava A, Supuran CT, Pastorek J, (2004) Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 577, 439–445. [DOI] [PubMed] [Google Scholar]
- (31).Cecchi A, Hulikova A, Pastorek J, Pastorekova S, Scozzafava A, Winum JY, Montero JL, Supuran CT, (2005) Carbonic anhydrase inhibitors. Sulfonamides inhibit isozyme IX mediated acidification of hypoxic tumors. Fluorescent sulfonamides design as probes of membrane-bound carbonic anhydrase isozymes involvement in tumorigenesis. J. Med. Chem 48, 4834–4841. [DOI] [PubMed] [Google Scholar]
- (32).Winum J-Y, Vullo D, Casini A, Montero J-L, Scozzafava A, Supuran CT, (2003) Carbonic anhydrase inhibitors: Inhibition of transmembrane, tumor-associated isozyme IX, and cytosolic isozymes I and II with aliphatic sulfamates. J. Med. Chem 46, 5471–5477. [DOI] [PubMed] [Google Scholar]
- (33).Supuran CT, (2011) Bacterial carbonic anhydrases as drug targets: toward novel antibiotics? Front. Pharmacol 2, 1–6. 10.3389/fphar.2011.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Supuran CT, Capasso C, (2020) Antibacterial carbonic anhydrase inhibitors: an update on the recent literature. Expert Opin. Ther. Pat 30, 963–982. [DOI] [PubMed] [Google Scholar]
- (35).Mancuso F, De Luca L, Angeli A, Berrino E, Del Prete S, Capasso C, Supuran CT, Gitto R, (2020) In silico-guided identification of new potent inhibitors of carbonic anhydrases expressed in Vibrio cholerae. ACS Med. Chem. Lett 11, 2294–2299. 10.1021/acsmedchemlett.0c00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Del Prete S, Nocentini A, Supuran CT, Capasso C, (2020) Bacterial ι-carbonic anhydrase: a new active class of carbonic anhydrase identified in the genome of the gram-negative bacterium Burkholderia territorii. J. Enzyme Inhib. Med. Chem 35, 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Angeli A, Ferraroni M, Pinteala M, Maier SS, Simionescu BC, Carta F, Del Prete S, Capasso C, Supuran CT, (2020) Crystal structure of a tetrameric type II β-carbonic anhydrase from the pathogenic bacterium Burkholderia pseudomallei. Molecules 25, 2269–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Farha MA, French S, Stokes JM, Brown ED, (2018) Bicarbonate alters bacterial susceptibility to antibiotics by targeting the proton motive force. ACS Infect. Dis 4, 382–390. 10.1021/acsinfecdis.7b00194. [DOI] [PubMed] [Google Scholar]
- (39).Sanders E, Maren TH, (1967) Inhibition of carbonic anhydrase in neisseria: effects on enzyme activity and growth. Mol. Pharmacol 3, 204–215. [PubMed] [Google Scholar]
- (40).Chirica LC, Elleby B, Jonsson B, Lindskog S, (1997) The complete sequence, expression in Escherichia coli, purification and some properties of carbonic anhydrase from Neisseria gonorrhoeae. Eur. J. Biochem 244, 755–760. [DOI] [PubMed] [Google Scholar]
- (41).Huang S, Xue Y, Sauer-Eriksson E, Chirica L, Lindskog S, Jonsson BH, (1998) Crystal structure of carbonic anhydrase from Neisseria gonorrhoeae and its complex with the inhibitor acetazolamide. J. Mol. Biol 283, 301–310. 10.1006/jmbi.1998.2077. [DOI] [PubMed] [Google Scholar]
- (42).Elleby B, Chirica LC, Tu C, Zeppezauer M, Lindskog S, (2001) Characterization of carbonic anhydrase from Neisseria gonorrhoeae. Eur. J. Biochem 268, 1613–1619. 10.1046/j.1432-1327.2001.02031.x. [DOI] [PubMed] [Google Scholar]
- (43).Remmele CW, Xian Y, Albrecht M, Faulstich M, Fraunholz M, Heinrichs E, Dittrich MT, Müller T, Reinhardt R, Rudel T, (2014) Transcriptional landscape and essential genes of Neisseria gonorrhoeae. Nucleic Acids Res 42, 10579–10595. 10.1093/nar/gku762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Kaur J, Cao X, Abutaleb NS, Elkashif A, Graboski AL, Krabill AD, AbdelKhalek AH, An W, Bhardwaj A, Seleem MN, Flaherty DP, (2020) Optimization of acetazolamide-based scaffold as potent inhibitors of vancomycin-resistant enterococcus. J. Med. Chem 63, 9540–9562. 10.1021/acs.jmedchem.0c00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Khalifah RG, (1971) The carbon dioxide hydration activity of carbonic anhydrase I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem 246, 2561–2573. [PubMed] [Google Scholar]
- (46).Petreni A, De Luca V, Scaloni A, Nocentini A, Capasso C, Supuran CT, (2021) Anion inhibition studies of the Zn(II)-bound ι-carbonic anhydrase from the Gram-negative bacterium Burkholderia territorii. J. Enzyme Inhib. Med. Chem 36, 372–376. 10.1080/14756366.2020.1867122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Rossi AM, Taylor CW, (2011) Analysis of protein-ligand interactions by fluorescence polarization. Nat. Protoc 6, 365–387. 10.1038/nprot.2011.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Elbaum D, Nair SK, Patchan MW, Thompson RB, Christianson DW, (1996) Structure-based design of a sulfonamide probe for fluorescence anisotropy detection of zinc with a carbonic anhydrase-based biosensor. J. Am. Chem. Soc 118, 8381–8387. 10.1021/ja954102e. [DOI] [Google Scholar]
- (49).Nikolovska-Coleska Z, Wang R, Fang X, Pan H, Tomita Y, Li P, Roller PP, Krajewski K,, Saito NG, Stuckey JA, Wang S, (2004) Development and optimization of a binding assay for the XIAP BIR3 domain using fluorescence polarization. Anal. Biochem 332, 261–273. 10.1016/j.ab.2004.05.055. [DOI] [PubMed] [Google Scholar]
- (50).Nafi BM, Miles RJ, Butler LO, Carter ND, Kelly C, Jeffery S, (1990) Expression of carbonic anhydrase in neisseriae and other heterotrophic bacteria. J. Med. Microbiol 32, 1–7. 10.1099/00222615-32-1-1. [DOI] [PubMed] [Google Scholar]
- (51).Alhashimi M, Mayhoub A, Seleem MN, (2019) Repurposing salicylamide for combating multidrug-resistant Neisseria gonorrhoeae. Antimicrob. Agents Chemother 63, e01225–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Elkashif A, Seleem MN, (2020) Investigation of auranofin and gold-containing analogues antibacterial activity against multidrug-resistant Neisseria gonorrhoeae. Sci. Rep 10, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Seong YJ, Alhashimi M, Mayhoub A, Mohammad H, Seleem MN, (2020) Repurposing fenamic acids drugs to combat multidrug-resistant Neisseria gonorrhoeae. Antimicrob. Agents Chemother 64, e02206–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Mishra CB, Tiwari M, Supuran CT, (2020) Progress in the development of human carbonic anhydrase inhibitors and their pharmacological applications: where are we today? Med. Res. Rev 40, 2485–2565. 10.1002/med.21713. [DOI] [PubMed] [Google Scholar]
- (55).Wallace SM, Riegelman S, (1977) Uptake of acetazolamide by human erythrocytes in vitro. J. Pharm. Sci 66, 729–731. [DOI] [PubMed] [Google Scholar]
- (56).Sippel KH, Robbins AH, Domsic J, Genis C, Agbandje-Mckenna M, McKenna R, (2009) High-resolution structure of human carbonic anhydrase II complexed with acetazolamide reveals insights into inhibitor drug design. Acta Crystallogr. Sect. F 65, 992–995. 10.1107/S1744309109036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Chakraverty S, Kannan KK, (1994) Refined structures of three sulfonamide drug complexes of human carbonic anhydrase I enzyme. J. Mol. Biol 234, 298–309. [DOI] [PubMed] [Google Scholar]
- (58).Lu P, Wang S, Lu Y, Neculai D, Sun Q, Van Der Veen SA, (2019) Subpopulation of intracellular Neisseria gonorrhoeae escapes autophagy-mediated killing inside epithelial cells. J. Infect. Dis 219, 133–144. 10.1093/infdis/jiy237. [DOI] [PubMed] [Google Scholar]
- (59).Klijn C, Durinck S, Stawiski EW, Haverty PM, Jiang Z, Liu H, Degenhardt J, Mayba O, Gnad F, Liu JA, (2015) Comprehensive transcriptional portrait of human cancer cell lines. Nat. Biotechnol 33, 306. [DOI] [PubMed] [Google Scholar]
- (60).Di Fiore A, Monti SM, Hilvo M, Parkkila S, Romano V, Scaloni A, Pedone C, Scozzafava A, Supuran CT, De Simone G, (2009) Crystal structure of human carbonic anhydrase XIII and its complex with the inhibitor acetazolamide. Proteins Struct. Funct. Bioinforma 74, 164–175. [DOI] [PubMed] [Google Scholar]
- (61).Loncaster JA, Harris AL, Davidson SE, Logue JP, Hunter RD, Wycoff CC, Pastorek J, Ratcliffe PJ, Stratford IJ, West CML, (2001) Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 61, 6394–6399. [PubMed] [Google Scholar]
- (62).Woods DD, (1940) The relation of p-aminobenzoic acid to the mechanism of the action of sulphanilamide. Br. J. Exp. Pathol 21, 74–90 [Google Scholar]
- (63).Unemo M, Shafer WM, (2014) Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin. Microbiol. Rev 27, 587–613. 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Kampmeier RH, (1983) Introduction of sulfonamide therapy for gonorrhea. Sex. Transm. Dis 10, 81–84. [DOI] [PubMed] [Google Scholar]
- (65).Dunlop EMC, (1949) Gonorrhoea and the sulphonamides. Br. J. Vener. Dis 25, 81–83. [PMC free article] [PubMed] [Google Scholar]
- (66).Bertini I, Luchinat C, Scozzafava A, (1982) Carbonic anhydrase: an insight into the zinc binding site and into the active cavity through metal substitution. In: Biochemistry. Structure and Bonding vol 48. pp 45–92, Springer, Berlin, Heidellberg. [Google Scholar]
- (67).Supuran CT, Scozzafava A, Casini A, (2003) Carbonic anhydrase inhibitors. Med. Res. Rev 23, 146–189. 10.1002/med.10025. [DOI] [PubMed] [Google Scholar]
- (68).Glockner S, Ngo K, Wagner B, Heine A, Klebe G, (2020) The influence of varying fluorination patterns on the thermodynamics and kinetics of benzenesulfonamide binding to human carbonic anhydrase II. Biomolecules 2020, 10, 509–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Richter MF, Drown BS, Riley AP, Garcia A, Shirai T, Svec RL, Hergenrother PJ, (2017) Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 545, 299–304. 10.1038/nature22308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Maynard RL, (2001) The Merck Index, 13th ed. pp 11, Merck & Co, Inc. Whitehouse Station, NJ. [Google Scholar]
- (71).Olesky M, Zhao S, Rosenberg RL, Nicholas RA, (2006) Porin-mediated antibiotic resistance in Neisseria gonorrhoeae: ion, solute, and antibiotic permeation through PIB proteins with PenB mutations. J. Bacteriol 188, 2300–2308. 10.1128/JB.188.7.2300-2308.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).O’Shea R, Moser HE, (2008) Perspective physicochemical properties of antibacterial compounds: implications for drug discovery. J. Med. Chem 51, 2871–2878. [DOI] [PubMed] [Google Scholar]
- (73).Dollery CT, (1991) Therapeutic drugs, vol. 2. Churchill Livingstone, Edinburgh, Scotland. [Google Scholar]
- (74).Ten Hove MW, Friedman DI, Patel AD, Irrcher I, Wall M, McDermott MP, (2016) Safety and tolerability of acetazolamide in the idiopathic intracranial hypertension treatment trial. J. Neuro-Ophthalmology 36, 13–19. 10.1097/WNO.0000000000000322. [DOI] [PubMed] [Google Scholar]
- (75).Chapron D, Sweeney K, Feig P, Kramer P, (1985) Influence of advanced age on the disposition of acetazolamide. Br. J. Clin. Pharmacol 19, 363–371. 10.1111/j.1365-2125.1985.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Gomolin H, Chapron J, (1992) Elucidating acetazolamide renal clearance. J. Clin. Pharmacol 32, 1028–1032. [DOI] [PubMed] [Google Scholar]
- (77).Sweeney KR, Chapron DJ, Brandt JL, Gomolin IH, Feig PU, Kramer PA, (1986) Toxic interaction between acetazolamide and salicylate: case reports and a pharmacokinetic explanation. Clin. Pharmacol. Ther 40, 518–524. [DOI] [PubMed] [Google Scholar]
- (78).Wallace SM, Shah VP, Riegelman S, (1977) GLC analysis of acetazolamide in blood, plasma, and saliva following oral administration to normal subjects. J. Pharm. Sci 66, 527–530. 10.1002/jps.2600660416. [DOI] [PubMed] [Google Scholar]
- (79).Vullo D, Del Prete S, Osman SM, De Luca V, Scozzafava A, Alothman Z, Supuran CT, Capasso C, (2014) Sulfonamide inhibition studies of the γ-carbonic anhydrase from the oral pathogen Porphyromonas gingivalis. Bioorganic Med. Chem. Lett 24, 240–244. 10.1016/.bmcl.2013.11.030. [DOI] [PubMed] [Google Scholar]
- (80).Turkmen H, Durgun M, Yilmaztekin S, Emul M, Innocenti A, Vullo D, Scozzafava A, Supuran CT, (2005) Carbonic anhydrase inhibitors. Novel sulfanilamide/acetazolamide derivatives obtained by the tail approach and their interaction with the cytosolic isozymes I and II, and the tumor-associated isozyme IX. Bioorg. Med. Chem. Lett 15, 367–372. [DOI] [PubMed] [Google Scholar]
- (81).Di Cesare Mannelli L, Micheli L, Carta F, Cozzi A, Ghelardini C, Supuran CT, (2016) Carbonic anhydrase inhibition for the management of cerebral ischemia: in vivo evaluation of sulfonamide and coumarin inhibitors. J. Enzyme Inhib. Med. Chem 31, 894–899. 10.3109/14756366.2015.1113407. [DOI] [PubMed] [Google Scholar]
- (82).Yung-Chi C, Prusoff WH, (1973) Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol 22, 3099–3108. 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- (83).Hamann HJ, Abutaleb NS, Pal R, Seleem MN, Ramachandran PV, (2020) β, γ-Diaryl α-methylene-γ-butyrolactones as potent antibacterials against methicillin-resistant Staphylococcus aureus. Bioorg. Chem 2020, 104, 104183–104190. [DOI] [PubMed] [Google Scholar]
- (84).Elsebaei MM, Abutaleb NS, Mahgoub AA, Li D, Hagras M, Mohammad H, Seleem MN, Mayhoub AS, (2019) Phenylthiazoles with nitrogenous side chain: an approach to overcome molecular obesity. Eur. J. Med. Chem 182, 111593–111602. [DOI] [PubMed] [Google Scholar]
- (85).Abutaleb NS, Seleem MN, (2020) Repurposing the antiamoebic drug diiodohydroxyquinoline for treatment of Clostridioides difficile infections. Antimicrob. Agents Chemother 64, e02115–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scheme S1. Synthetic route for analogs 1 – 30
Scheme S2. Synthetic route for analog 31
Scheme S3. Synthetic route for FITC-AZM
Figure S1. FITC-AZM binding curve and AZM displacement for hCA I
Table S1. Binding affinity and concentrations for carbonic anhydrase fluorescence polarization competition
Table S2. Carbonic anhydrase fluorescence polarization competition assay data
Table S3. N. gonorrhoeae strains used in the study
Table S4. Anti-gonococcal activity against additional drug-resistant and -sensitive N. gonorrhoeae strains in both ambient and increased CO2 conditions
Table S5. MICs (μg/mL) of sulfa drugs as compared to CAIs against N. gonorrhoeae strains
Figure S2. Sequence alignment for a-carbonic anhydrase
Procedure for sequence alignments
Characterization and Purity for final analogs
Spectra for FITC-AZM


