Abstract
Introduction:
HIV testing at birth of HIV-exposed infants (HEIs) may improve the identification of infants infected with HIV in utero and accelerate antiretroviral treatment (ART) initiation.
Methods:
ICAP at Columbia University supported implementation of a national pilot of HIV testing at birth (0–7 days) in Eswatini at 2 maternity facilities. Dried blood spot (DBS) samples from neonates of women living with HIV (WLHIV) were collected and processed at the National Molecular Reference Laboratory using polymerase chain reaction (PCR). Mothers received birth test results at community health clinics. We report data on HIV birth testing uptake and outcomes for HIV-positive infants from the initial intensive phase (October 2017–March 2018) and routine support phase (April–December 2018).
Results:
During the initial intensive pilot phase, 1669 WLHIV delivered 1697 live-born HEI at 2 health facilities and 1480 (90.3%) HEI received birth testing. During the routine support phase, 2546 WLHIV delivered and 2277 (93.5%) HEI received birth testing. Overall October 2017–December 2018, 22 (0.6%) infants of 3757 receiving birth testing had a positive PCR test, 15 (68.2%) of whom were successfully traced and linked for confirmatory testing (2 infants were reported by caregivers to have negative follow-up HIV tests). Median time from birth test to receipt of results by the caregiver was 13 days (range: 8–23). Twelve (60.0%) of 20 infants confirmed to be HIV-positive started ART at median age of 17.5 days (12–43). One mother of an HIV-positive infant who was successfully traced refused ART following linkage to care and another child died after ART initiation. Three infants (15.0%) had died by the time their mothers were reached and 4 (15.0%) infants were never located.
Conclusion:
This pilot of universal birth testing in Eswatini demonstrates the feasibility of using a standard of care approach in a low resource and high burden setting. We document high uptake of testing for newborns among HIV-positive mothers and very few infants were found to be infected through birth testing.
Keywords: children, health systems, HIV testing, early infant diagnosis, birth testing, early antiretroviral treatment initiation
Early HIV diagnosis and immediate initiation of antiretroviral therapy (ART) in infants are both critical for their survival and long-term health. Studies have demonstrated a peak in mortality in the first months of life for infants with HIV infection who are not on treatment, with up to 20% dying before 3 months of age.1 The benefits of earlier treatment initiation were demonstrated in the Children with HIV Early Antiretroviral Therapy (CHER) study which found 76% reduced mortality in infants started on ART at a median of 7.4 weeks compared to those initiating later.2 Delays in time to ART initiation are of a particular concern for infants with in utero HIV infection as they have significantly higher risk of death compared with infants with intrapartum and postpartum infection.3,4
The World Health Organization (WHO) recommends HIV testing of all HIV-exposed infants (HEIs) at 4–8 weeks followed by ongoing testing until cessation of breast-feeding5; however, in 2017 across 23 prevention of mother-to-child transmission (PMTCT) focus countries, only 52% of HEI received testing within the first 2 months of life.6 Among infants tested, there are also considerable delays in receipt of results by caregivers, confirmatory testing and initiation of ART for those with HIV infection. Most infant HIV diagnosis before 18 months of age relies on dried blood spot (DBS) specimens processed at central laboratories and requires caregivers to return to clinics weeks or months later to get results. Time from specimen collection to receipt of results by caregivers has been shown to range from 38 to 92 days7–11 and in many countries, only half of caregivers of children tested ever receive results.7,12
One solution to reduce loss to follow-up and delays experienced within conventional EID programs is point of care (POC) polymerase chain reaction (PCR) testing for infant diagnosis. In a multicounty POC study on EID turnaround time and ART initiation among HEI, median time from specimen collection to return of results to caregivers was reduced from 55 to 0 days and the proportion of infants identified as having HIV infection who started ART within 60 days increased from 43% to 92% (P < 0.0001).13 Another approach to improve the infant HIV diagnostic cascade is testing infants at birth to identify those at the highest risk for disease progression earlier in life. A study of birth testing in Mozambique using the POC approach found that the combination of testing at birth and 4–6 weeks identified 16% more infections compared to 4–6 week testing alone.14 Birth testing has been included as a consideration in WHO guidelines, but has not been widely implemented in resource limited settings making routine program experience very limited.
We report findings from a national pilot of birth testing of HEI in the Kingdom of Eswatini (formerly, Swaziland) led by the Ministry of Health (MOH) and supported by the US Centers for Disease Control and Prevention (CDC) and ICAP at Columbia University. While Eswatini is one of the countries most affected by the HIV epidemic, it has made a remarkable progress expanding access to HIV care and treatment services.15 In 2018, 79% of pregnant women received antiretroviral medication for PMTCT and 78% of HEI received HIV testing in the first 2 months of life.16 Eswatini also has a high proportion of facility-based deliveries (91%)17 making it a feasible setting in which to implement birth testing. The addition of birth testing could further improve the survival of HEI through early identification and rapid ART initiation for infants with in utero HIV infection. We describe the national birth test pilot and evaluation results, including uptake of birth testing, time to ART initiation for children identified as having in utero HIV infection and estimate the impact of birth testing on uptake of 6–8 week HIV testing.
METHODS
Birth Test Pilot Description
In 2017, the Eswatini MOH commenced a national pilot of HIV birth testing which was conducted at 5 maternity facilities (those offering labor and delivery services) across the country’s 4 regions. The national pilot utilized 2 approaches to birth testing,1 POC testing with on-site PCR and standard of care (SOC) testing using DBS processed at the National Molecular Reference Laboratory (NMRL). During the national pilot, ICAP provided technical support for the SOC birth testing approach at 2 of the 5 pilot sites, Mankayane Government Hospital (MGH) and the Raleigh Fitkin Memorial Hospital (RFM). MGH and RFM are the only government run maternity facilities in the Manzini region and, in 2015, 46% of all deliveries in Eswatini were in Manzini (some deliveries were in private facilities).17 RFM is located near the capital city serving urban/periurban areas and is a national referral hospital. MGH is located in a rural area approximately 1 hour from Mbabane.
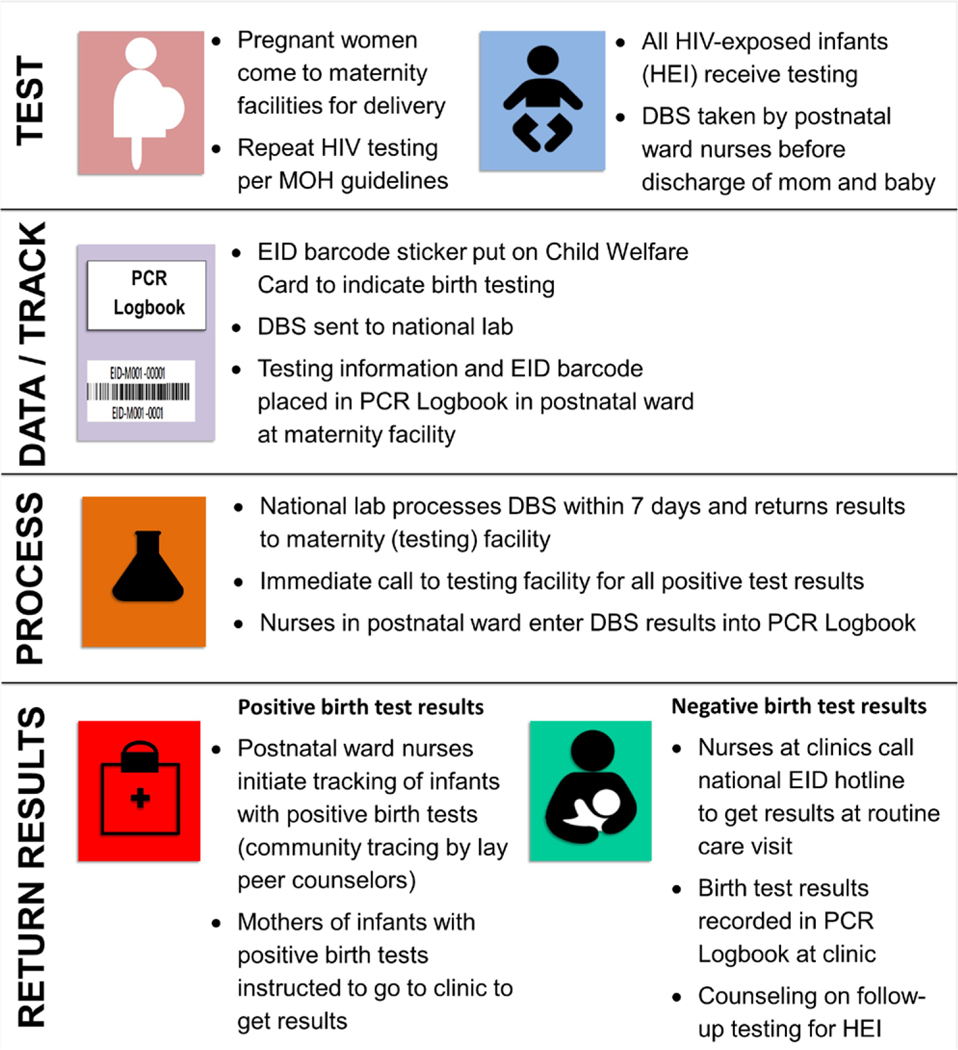
The first 6 months of the pilot (October 2017–March 2018) was the initial intensive phase during which birth testing was introduced, followed by routine support for continued birth testing as part of overall PMTCT technical support funded through the US President’s Emergency Plan For AIDS Relief (PEPFAR) and CDC (starting April and ongoing as of March 2020). Figure 1 illustrates the SOC model of birth testing implemented for the pilot. Before the start of the pilot, ICAP conducted a 2-day training for healthcare workers (HCWs) from the participating hospitals. ICAP also provided on-site training for nurses at the 43 Maternal, Neonatal and Child Health (MNCH) community clinics in the Manzini region to familiarize them with the birth test pilot. During the intensive pilot phase, 2 Nurse Mentors conducted site visits to provide supportive supervision up to 4 days a week at each facility.
FIGURE 1.
Eswatini national pilot of birth testing, standard of care approach supported by ICAP.
Birth testing was conducted at the 2 maternity facilities by nurses already employed by the MOH (no additional nurses were hired). DBS specimens were collected from neonates of HIV-positive mothers within postnatal wards before discharge and testing of infants was also conducted in the neonatal intensive care unit (NICU) at RFM (MGH does not have a NICU). The pilot aimed to conduct birth testing for all HIV-exposed neonates up to 7 days of age, including infants born outside of health facilities brought for care within the first week. DBS specimens were dried, packaged and sent several times per week to the NMRL for PCR testing (COBAS AmpliPrep/COBAS TaqMan HIV-1 Qualitative Test, version 2.0). The existing MOH PCR Logbook was used for documenting birth testing. National guidelines called for prioritized processing of all HIV tests for infants up to 18 months of age within 7 days. During the intensive phase, an additional NMRL Data Manager was hired to assist with data entry into the NMRL electronic database and, all birth test results in the NMRL database were printed at the facilities and returned to the postnatal wards for documentation in the PCR Logbook.
The date of birth test sample collection was recorded on the Child Health Card (CHC), the national patient-held record and a barcode sticker matching the sample sent to the laboratory was also affixed to the CHC. All mothers of infants receiving birth testing were instructed at the time of testing that they would receive results when they attended the routine 7-day postnatal visit at MNCH clinics where they receive postnatal care and infants receive routine healthcare. A small information sheet was affixed to the CHC with instructions for HCWs at MNCH clinics on how to call the existing NMRL EID hotline to retrieve results for infants who had birth testing. Using the information on the CHC, mothers of infants tested at birth should have been able to receive test results at any health facility in Eswatini.
Per standard operating procedures for all EID testing, positive birth test results were immediately reported to the testing facility by phone. All mothers of infants with HIV-positive birth test results were then called by nurses from the 2 maternity facilities. Mothers of infants with positive birth test results were told to immediately go to an MNCH clinic to get their child’s birth test results (results were not given by phone). If mothers could not be reached by phone, lay peer educators conducted home visits to locate women at the address on record at the health facility.
Mothers of infants with HIV-negative birth test results were not tracked, but received results when they brought infants for follow-up care at MNCH clinics. When HCWs called and received birth test results, these were recorded on the CHC held by the mother and in the PCR Logbook at the MNCH facility. At the time of results receipt, mother of infants with negative birth tests were counseled by HCWs about the importance of follow-up EID testing per national guidelines. During the intensive phase, the date and name of the MNCH clinics calling the NMRL EID hotline to retrieve birth test results were tracked in a paper-based logbook (calls continued during the routine phase and results were given to HCWs but call information was not recorded).
Procedures for conducting birth testing, recording test and result information and returning results (both positive and negative) were the same in the intervention and routine phases. During the routine support phase, Nurse Mentors continued to visit MGH and RFM at least once per week to support birth testing as part of support for PMTCT services. At RFM, testing was not offered routinely in the NICU as a result of limited available staff. The return of negative birth test results to postnatal wards also differed; instead of all results being printed and returned to postnatal wards automatically, during the routine phase, according to standard practice in Eswatini, results were only received from the laboratory when patients return to retrieve them. Positive results were still relayed by phone to postnatal wards immediately by the NMRL staff.
Evaluation and Analysis
We report data from the national birth test pilot for the Manzini region from 2 periods: the intensive phase and the routine support phase described above. The intensive phase analysis includes 6 months of data from MGH and RFM from October 2017 through March 2018. The routine support phase includes 9 months of data from MGH and RFM, April through December 2018. For the intensive phase, patient-level data were abstracted for all HIV-positive women from the Maternity Register which was then linked to infant testing data in the PCR Logbook. We describe the characteristics of women living with HIV (WLHIV) who delivered at MGH and RFM (including women who experienced stillbirth), infant test results (for all live births), turnaround time from specimen collection to recorded results at the testing facility and time to location of caregivers and ART initiation among HIV-positive infants during the intensive phase. We also report the number of calls to the NMRL EID hotline to retrieve birth test results. For the routine support phase, routinely collected PEPFAR monitoring, evaluation and reporting indicators, and Eswatini national MOH reporting data were assessed to measure birth testing uptake and results, completion of recorded results at the maternity facilities and turnaround time from birth test to location/tracking of caregivers and ART initiation of HIV-positive infants.
To examine the potential impact of birth testing on uptake of EID testing at 6–8 weeks, we utilized data from the NMRL to identify all HIV tests conducted in the Manzini region among infants between 4 and 10 weeks of age (excluding confirmatory tests) along with data on the number of women living with HIV who delivered at the 2 maternities in the Manzini region (as a proxy for the number of HEI born during the same period). We calculated the proportion of HEI tested at 6–8 weeks among all HEI born in Manzini for 2 periods: November 2016 through May 2017 representing the period before birth testing and November 2017 through May 2018, which was during the birth test pilot. Chi-square statistics were used to compare the estimated proportion of HEI tested in the pre-implementation and implementation periods. The evaluation received ethical approval from the Columbia University Medical Center Institutional Review Board and the Eswatini National Health Research Review Board, and administrative review by the CDC Associate Director of Science Office. The protocol was reviewed in accordance with the Centers for Disease Control and Prevention (CDC) human research protection procedures and was determined to be research, but CDC investigators did not interact with human subjects or have access to identifiable data or specimens for research purposes. Statistical analyses were performed using SAS 9.4.
RESULTS
Intensive Phase
During the intensive phase of the pilot (October 2017–March 2018), a total of 1669 WLHIV delivered at MGH and RFM (Table 1). The median age of WLHIV delivering was 29 years [interquartile range (IQR) 24–33]. Almost all WLHIV had prior knowledge of their HIV-positive status (98.3%) and 96.1% of these women were on ART. An additional 14 (0.8%) women who had previously tested negative and 15 (0.9%) women who had unknown status before delivery were found to be HIV-positive at delivery. Among 1697 infants delivered, including 15 sets of twins, 58 (3.4%) were stillbirths (Table 1). Among 1639 live born infants, 48.0% were female and median birth weight was 3.1 kg (IQR 2.8–3.4).
TABLE 1.
Characteristics of HIV-positive Women Who Delivered at 2 Health Facilities in Manzini (N = 1669), Infants Delivered (N = 1697) and Infants Who Received Birth Testing (N =1480), Eswatini, October 2017–March 2018 (Intensive Phase)
| All | Mankayane Government Hospital | Raleigh Fitkin Memorial Hospital | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| 1669 | 100.0 | 406 | 24.3 | 1263 | 75.7 | |
| Age, median years (IQR) | 29 (24–33) | 28 (24–32) | 29 (24–33) | |||
| 14–19 | 78 | 4.7 | 18 | 4.4 | 60 | 4.8 |
| 20–25 | 445 | 26.7 | 115 | 28.3 | 330 | 26.1 |
| 26–40 | 1111 | 66.6 | 268 | 66.0 | 843 | 66.7 |
| >40 | 29 | 1.7 | 5 | 1.2 | 24 | 1.9 |
| Missing | 6 | 0.4 | 0 | 0.0 | 6 | 0.5 |
| Parity, median (IQR) | 2 (1–3) | 2 (1–2) | 1 (1–2) | |||
| First live delivery (parity = 1) | 246 | 14.8 | 72 | 17.7 | 174 | 13.8 |
| Missing | 4 | 0.2 | 0 | 0.0 | 4 | 0.3 |
| HIV status at L&D | ||||||
| Known HIV+ | 1640 | 98.3 | 403 | 99.3 | 1237 | 97.9 |
| Previously negative | 14 | 0.8 | 3 | 0.7 | 11 | 0.9 |
| Unknown | 15 | 0.9 | 0 | 0.0 | 15 | 1.2 |
| ART status | ||||||
| ART during ANC* | 1603 | 960 | 386 | 95.1 | 1217 | 96.4 |
| Initiated in L&D | 35 | 2.1 | 6 | 1.5 | 29 | 2.3 |
| Not on ART | 7 | 0.4 | 0 | 0.0 | 7 | 0.6 |
| Incomplete/missing | 24 | 1.4 | 14 | 3.4 | 10 | 0.8 |
| HEI delivered (including twins) | 1697 | 100.0 | 412 | 24.3 | 1285 | 75.7 |
| Infant NVP administered in L&D | ||||||
| Started in L&D | 1580 | 93.1 | 378 | 91.7 | 1202 | 93.5 |
| Not given | 69 | 4.1 | 6 | 1.5 | 63 | 4.9 |
| Incomplete/missing | 48 | 2.8 | 28 | 6.8 | 20 | 1.6 |
| Mode of delivery | ||||||
| Vaginal | 1404 | 82.7 | 326 | 79.1 | 1078 | 83.9 |
| Cesarean section | 190 | 11.2 | 60 | 14.6 | 130 | 10.1 |
| Born before admission | 103 | 6.1 | 26 | 6.3 | 77 | 6.0 |
| Live births | 1639 | 96.6 | 399 | 96.8 | 1240 | 96.5 |
| Infant sex (live infants) | ||||||
| Male | 838 | 51.1 | 200 | 50.1 | 638 | 51.5 |
| Female | 787 | 48.0 | 196 | 49.1 | 591 | 47.7 |
| Missing | 14 | 0.9 | 3 | 0.8 | 11 | 0.9 |
| Median birth weight, kg (IQR) (live infants) | 3.1 (2.8–3.4) | 3.1 (2.7–3.4) | 3.1 (2.8–3.4) | |||
| Infant feeding initiated | ||||||
| Exclusive breastfeeding | 1613 | 98.4 | 396 | 99.2 | 1217 | 98.1 |
| Exclusive replacement | 16 | 1.0 | 1 | 0.3 | 15 | 1.2 |
| Missing | 10 | 0.6 | 2 | 0.5 | 8 | 0.6 |
| Infants tested at birth (% out of all live born HEI) | 1480 | 90.3 | 317 | 79.4 | 1163 | 93.7 |
| Infants test results in maternity register | 1449 | 97.9 | 310 | 97.8 | 1139 | 97.9 |
| Birth test results October 2017–March 2018 | ||||||
| Negative | 1450 | 98.0 | 315 | 99.4 | 1135 | 97.6 |
| Positive | 5 | 0.3 | 0 | 0.0 | 5 | 0.4 |
| Indeterminate | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Results not documented | 25 | 1.7 | 2 | 0.6 | 23 | 2.0 |
| Median days from date of test to results returned to postnatal ward (IQR) | 12 (8–17) | 12 (7–17) | 12 (7–17) | |||
| Median days from date of test to results returned to postnatal ward for HIV+ infants (IQR) | 9 (9–13) | N/A | 9 (9–13) | |||
| Calls to NMRL hotline | 268 | 18.1% | 55 | 17.4% | 213 | 18.3% |
| Calls from within Manzini | 236 | 88.1% | 47 | 85.5% | 189 | 88.7% |
| Calls from outside Manzini | 32 | 11.9% | 8 | 14.5% | 24 | 11.3% |
| Median time from DOB to results call (days) | 20 (8–44) | 13 (8–42) | 32 (8–45) | |||
“ART during ANC” includes women on ART who became pregnant and continued ART during pregnancy and women newly identified as HIV-positive during pregnancy who initiated ART.
ANC indicates antenatal care; L&D, labor and delivery; NVP, nevirapine.
In total, 1480 infants (90.3%) received birth testing across the 2 facilities, including 44 infants who had infant testing information but could not be linked to a woman in the maternity register (among infants with linked maternal data, 90.0% received a birth test). The proportion of infants receiving a birth test in the intensive phase was higher at RFM, 93.7% compared to 79.4% at MGH (Table 1). Among all infants tested in the intensive phase, 5 had positive results (see further information below on positive infants), 1450 (98.0%) had negative tests and 25 (1.7%) children were missing recorded test results. Test results were recorded in the PCR Logbooks in the postnatal wards at MGH and RFM for 98.3% of all tested infants and the median time from the date of specimen collection to recording of results was 12 days (IQR 8–17); for the 5 infants who tested HIV-positive at birth, the median time to recording of results in the PCR Logbook was 9 days (IQR 9–13). During the intensive phase, there were 268 calls to the NMRL EID hotline from community health clinics to receive birth test results representing 18.1% of all infants tested. Almost all calls (88.1%) were from health clinics within the Manzini region. The median time between date of birth and the call to get birth test results was 20 days (IQR 8–44) (Table 1).
Routine Support Phase
From April through December 2018, during the routine support phase, 2546 WLHIV delivered and 2435 HEIs were admitted to the postnatal wards (infants admitted to intensive care units were not included) (Table 2). Among all infants in the postnatal wards, 2277 (93.5%) received birth testing. Results for birth test were returned and recorded for 1972 (86.6%) infants (66.2% at MGH and 94.3% at RFM). In total, 17 (0.8%) infants with results had positive PCR results in the 9 months of routine support, while 1955 (99.2%) had negative and none had indeterminate results (ie, inconclusive laboratory result that could not be used to determine positive or negative status) (Table 2).
TABLE 2.
Routinely Collected Birth Test Data From 2 Maternity Facilities in Manzini, Eswatini, April Through December 2018 (Routine Support Phase)
| Total | Mankayane Government Hospital | Raleigh Fitkin Memorial Hospital | |
|---|---|---|---|
| HIV-positive women delivered | 2546 | 697 | 1849 |
| HIV-exposed infants admitted to the postnatal ward | 2435 | 693 | 1742 |
| HIV-exposed infant received birth testing (%) | 2277 (93.5%) | 627 (90.5%) | 1650 (94.7%) |
| Birth test results recorded in maternity facility register (%) | 1971 (86.6%) | 415 (66.2%) | 1556 (94.3%) |
| Negative birth test results (% out of results returned) | 1955 (99.2%) | 414 (99.8%) | 1541 (99.0%) |
| Positive birth test results (% out of results returned) | 17 (0.9%) | 1 (0.2%) | 16 (1.0%) |
| Positive birth test results (% out of all tested) | 17 (0.7%) | 1 (0.2%) | 16 (1.0%) |
| Indeterminate birth test results (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Infants Testing Positive at Birth
During the full evaluation period (October 2017 through December 2018), 22 (0.6%) infants of the 3757 who received birth testing had a positive PCR test. Among the 22 infants positive at birth, 15 (68.2%) mothers were successfully traced and linked for confirmatory testing per national guidelines. The median time from birth testing to receipt of results by caregivers (among those successfully traced) was 13 days (range 8–23 days). Two infants (9.1%) of those successfully traced were reported by mothers to have had negative test results at 6 weeks (data were not available to verify; additionally, information on uptake of infant prophylaxis at the time of confirmatory testing was not available). Twelve (60.0%) of the 20 infants confirmed to be HIV-positive started ART at a median age of 17.5 days (range of 12–43 days). One mother of an HIV-positive infant who was successfully traced refused ART following confirmatory testing and another child died after ART initiation. Three infants (15.0%) had died by the time their mothers were reached and four (15.0%) infants were never located.
Uptake of 6- to 8-week EID testing
In the pre-implementation period (November 2016 through May 2017), before birth testing, routinely collected data show that 1995 HIV-positive women delivered in the Manzini region and 1551 HIV tests were conducted for infants between 4 and 10 weeks of age; indicating an estimated 77.7% of HEI received 6–8 week EID testing. For the period during implementation (November 2017 through May 2018), 2197 HIV-positive women delivered in Manzini and 1621 HIV tests were performed on infants at 4–10 weeks of life indicating that approximately 73.8% of HEI received 6–8 week EID testing (P < 0.01).
DISCUSSION
This evaluation demonstrates the feasibility of testing HEI at birth in Eswatini using a SOC approach with DBS specimen collection and central laboratory processing. Existing nurses working in the postnatal wards were able to counsel WLHIV and collect specimens from more than 90% of HEI, and the national laboratory successfully processed several thousand additional PCR tests. Few HIV infections were identified, less than 1% of HEI tested at birth had positive results. Among the 20 infants found to have in utero HIV infection, just over half (60%) initiated ART. Our analysis of estimated uptake of 6–8 week HEI testing in the Manzini region before and during the birth test pilot suggests that there may have been a modest decrease in coverage from 78% to 74% following the introduction of birth testing.
While South Africa has introduced birth testing nationally, few other high HIV burden countries have attempted roll-out of these services. Eswatini was well positioned to implement birth testing given its high coverage of ART among pregnant women, maternity delivery and EID testing at 6–8 weeks.18 The ICAP-supported pilot project in Manzini used a similar approach to the South African model including DBS specimen collection and central laboratory processing (rather than POC).19 Although one of the facilities in this pilot experienced challenges in the intensive phase with only 79% coverage of birth testing, both facilities had >90% coverage in the routine support phase. The initial implementation challenges at MCH included reservation among HCW to test newborns, incorrectly sized lancets for neonate testing and acute periods of staffing shortages. The barriers were overcome through supportive mentoring to ensure postnatal ward nurses were comfortable conducting birth testing, in addition to procurement of appropriate supplies. These results indicate that this additional service was possible with the existing nursing staff in the postnatal wards. Data from the roll-out of birth testing in South Africa also showed improved performance over time with only 39% of HEI tested at birth initially, which increased to 93% in later months.19
Relatively, few HIV-positive infants were found at birth in the Manzini region of Eswatini during the 15-month evaluation period. Only 22 infants among 3757 tested at birth having reactive tests (0.6%). Studies from Lesotho and South Africa, including POC approaches, have also shown low positivity among infants tested at birth.10,19–21 A study from Botswana examining targeted birth testing using maternal risk factors (<8 weeks of ART in pregnancy and lack viral suppression) found 3.3% positivity.22 This type of targeted approach, while potentially difficult to implement, could lead to significantly higher yield from birth testing. The very low positivity of infants tested at birth in this evaluation in Eswatini, as well as in South Africa, likely reflect the success of expanding ART coverage for pregnant women which has reduced the rate of new in utero infections. In settings with successful antenatal ART coverage, the bulk of new pediatric infections are occurring postnatally during breast-feeding.16 Our evaluation did not include a cost-effectiveness analysis of birth testing; however, a 2016 study from South Africa determined that HIV testing at birth and 6–8 weeks was more cost-effective than 6–8 week testing alone.23 Further evaluations of the impact of birth testing on long-term survival and cost-effectiveness are needed to define the role of birth testing in the context of infant diagnosis programs.
There are few data from other birth testing initiatives in similar settings with which to compare our findings. The median age at ART initiation after a positive birth test that we observed in Eswatini was somewhat higher (18 days) compared to a study of birth testing in Johannesburg where median age at treatment start was 9 days (range 6–25)20 While it is encouraging that so many children in Eswatini with in utero infection were able to initiate treatment within the first month, 8 children with positive initial PCR tests did not start ART, including 3 who died, 4 who could not be located and 1 who was linked to care but never brought back to start treatment. The low proportion of children linked for confirmatory testing and ART initiation demonstrates that inclusion of birth testing in EID programming will be insufficient to improve outcomes among all children with in utero HIV infection. It is not unlikely that challenges preventing women from successfully engaging in ART services during pregnancy also impede uptake of pediatric care. Our findings suggest the need for more intensive interventions for mothers of infants with in utero infection who may be at high risk of loss to follow-up.
Unfortunately, our evaluation was not able to measure time from testing to return of results to caregivers of infants with negative test results as we lacked data on receipt of results at community health clinics. Very few calls were made to the NMRL EID hotline to receive birth test results (<20% of infants tested), but most calls were within 20 days of testing. It is not clear whether the low number of calls to the NRML hotline from community health clinics suggests few women returned to clinics to get birth test results or whether HCW assumed women not actively traced had negative results and therefore did not feel the need to call. We also do not have information on whether women whose infants were presumed to have negative birth test results were counseled on returning for HIV testing at 6–8 weeks. Effective strategies are needed to ensure that all birth test results are received and that caregivers are counseled on the importance of follow-up testing.
Finally, an important concern related to the introduction of birth testing is the potential impact on follow-up testing. Birth testing can only identify infants with in utero HIV infection and all infants who test negative at birth must receive 6–8 week testing (to identify intrapartum infection) and subsequent testing until the cessation of breast-feeding. An unintended consequence of birth testing could be reduced uptake of 6–8 week testing as a result of misunderstanding about the need for further testing among caregivers. Our analysis found somewhat lower estimated proportions of 6–8 week testing in the Manzini region after the introduction of birth testing; 78% in the period before birth testing was introduced and 74% after. While our findings were statistically significant, it should be noted that we were only able to estimate 6–8 week testing uptake in the Manzini region using routinely collected data. It is possible that secular trends or differences in data quality may have resulted in these findings. Data from South Africa found that compared to infants not tested at birth, those who received targeted birth testing (based on maternal risk factors) were less likely to receive follow-up EID (73% vs. 85%, P < 0.0001).24 While the data remain limited, greater efforts may be needed to ensure that caregivers of HEI understand the meaning of birth test results and need for follow-up testing.
While this pilot demonstrated the feasibility of a universal approach to birth testing for HEI in a high burden setting using the SOC approach, there were some important implementation challenges described above which contributed to initial poor performance in the offering of birth testing. Return of results was challenging in Eswatini because women delivered in maternity facilities but received follow-up care for themselves and infants at community health clinics. There is also high mobility among women in Eswatini who often return to rural areas after delivery. A further challenge was our reliance on routinely collected data which is subject to high rates of missingness and the lack of a unique patient identifier linking mothers and infants and for laboratory data which would have allowed for the examination of follow-up testing of HEI. We also do not have information on why some infants were not tested and cannot identify the extent to which this may have resulted from health systems-related issues (shortage of staff, lack of supplies, etc.) or whether some women refused and for what reasons. Further research is needed to identify both structural challenges and individual-level barriers among HIV-positive women. Given the low proportion of infants who tested HIV-positive at birth, even in a high burden setting, the development of screening tools may be warranted to target testing as a means of increasing the efficiency of birth testing and to reduce costs. In addition, long-term follow-up data on infants tested at birth are also needed to measure the impact of this intervention. Finally, birth testing is not being supported as an intervention for all high HIV burden settings, so our findings may be limited in their generalizability to other countries with high rates of institutional delivery and expanded ART coverage for pregnant women.
CONCLUSIONS
The data from this evaluation provide important information about the feasibility of scaling up universal birth testing using an SOC approach in a low resource and high burden setting, and may also inform efforts to model the impact of birth testing efforts in similar high burden settings.25 Uptake was high, but relatively few infants were diagnosed with in utero HIV infection in Eswatini. Just over half of the children identified as having HIV infection at birth started ART within the first month of life which should contribute to improving their chances of survival. Greater efforts are needed to track all mothers of young children living with HIV and to ensure they are linked to care and start treatment.
Acknowledgments
Supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC) under the terms of Cooperative Agreement Numbers U2GGH000994.
C.A.T., E.J.A. and H.D. designed the evaluation; F.T., A.M., S.S., M.C., H.N. and S.S. provided implementation support for the pilot activities and data collection; S.M. provided oversight of the pilot and provided input on the evaluation design and analysis for the Eswatini government; H.D., T.A., C.R. and E.R. oversaw the project, reviewed the evaluation protocol and analytic approach for the CDC; C.A.T. developed the analytic approach and conducted the analyses; all authors contributed to manuscript writing and review.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Bourne DE, Thompson M, Brody LL, et al. Emergence of a peak in early infant mortality due to HIV/AIDS in South Africa. AIDS. 2009;23:101–106. [DOI] [PubMed] [Google Scholar]
- 2.Violari A, Cotton MF, Gibb DM, et al. ; CHER Study Team. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zijenah LS, Moulton LH, Iliff P, et al. ; ZVITAMBO Study Group. Timing of mother-to-child transmission of HIV-1 and infant mortality in the first 6 months of life in Harare, Zimbabwe. AIDS. 2004;18:273–280. [DOI] [PubMed] [Google Scholar]
- 4.Becquet R, Marston M, Dabis F, et al. ; UNAIDS Child Survival Group. Children who acquire HIV infection perinatally are at higher risk of early death than those acquiring infection through breastmilk: a meta-analysis. PLoS One. 2012;7:e28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Organization WH. HIV Diagnosis and ARV Use in HIV-Exposed Infants: A Programmatic Update. Geneva, Switzerland: WHO; 2018. [Google Scholar]
- 6.UNAIDS. Start Free Stay Free AIDS Free: 2018 Progress Report. Geneva, Switzerland: UNAIDS; 2018. [Google Scholar]
- 7.Mugambi ML, Deo S, Kekitiinwa A, et al. Do diagnosis delays impact receipt of test results? Evidence from the HIV early infant diagnosis program in Uganda. PLoS One. 2013;8:e78891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manumbu S, Smart LR, Mwale A, et al. Shortening turnaround times for newborn HIV testing in rural tanzania: a report from the field. PLoS Med. 2015;12:e1001897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiam A, Gill MM, Hoffman HJ, et al. Conventional early infant diagnosis in Lesotho from specimen collection to results usage to manage patients: where are the bottlenecks? PLoS One. 2017;12:e0184769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill MM, Hoffman HJ, Mokone M, et al. Assessing very early infant diagnosis turnaround times: findings from a birth testing pilot in Lesotho. AIDS Res Treat. 2017;2017:2572594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutcliffe CG, van Dijk JH, Hamangaba F, et al. Turnaround time for early infant HIV diagnosis in rural Zambia: a chart review. PLoS One. 2014;9:e87028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phiri NA, Lee HY, Chilenga L, et al. Early infant diagnosis and outcomes in HIV-exposed infants at a central and a district hospital, Northern Malawi. Public Health Action. 2017;7:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bianchi F, Cohn J, Sacks E, et al. Evaluation of a routine point-of-care intervention for early infant diagnosis of HIV: an observational study in eight African countries. The lancet HIV. 2019. [DOI] [PubMed] [Google Scholar]
- 14.Meggi B, Vojnov L, Mabunda N, et al. Performance of point-of-care birth HIV testing in primary health care clinics: an observational cohort study. PLoS One. 2018;13:e0198344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nkambule RN, Nuwagaba-Biribonwoha H, Mnisi Z, et al. Substantial progress in confonting the HIV epidemic in Swaziland: first evidence of national impact. In: The International AIDS Society (IAS) Conference on HIV Science; July 23–26, 2017; Paris, France. 2017. [Google Scholar]
- 16.UNAIDS. Start free stay free AIDS free. In: HIV/AIDS. JUNPo, ed. Geneva, Switzerland: UNAIDS; 2019. [Google Scholar]
- 17.Ministry of Health KoS. Annual National Sexual and Reproductive Health Program Report. Mbabane, Eswatini: Ministry of Health, Kingdom of Swaziland; 2015. [Google Scholar]
- 18.UNICEF. Eswatini: Key demographic indicators. 2018. New York: UNICEF. Available at: https://data.unicef.org/country/swz/. [Google Scholar]
- 19.Moyo F, Haeri Mazanderani A, Barron P, et al. Introduction of routine HIV birth testing in the South African national consolidated guidelines. Pediatr Infect Dis J. 2018;37:559–563. [DOI] [PubMed] [Google Scholar]
- 20.Technau KG, Strehlau R, Patel F, et al. 12-month outcomes of HIV-infected infants identified at birth at one maternity site in Johannesburg, South Africa: an observational cohort study. Lancet HIV. 2018;5:e706–e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spooner E, Govender K, Reddy T, et al. Point-of-care HIV testing best practice for early infant diagnosis: an implementation study. BMC Public Health. 2019;19:731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibrahim M, Maswabi K, Ajibola G, et al. Targeted HIV testing at birth supported by low and predictable mother-to-child transmission risk in Botswana. J Int AIDS Soc. 2018;21:e25111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francke JA, Penazzato M, Hou T, et al. Clinical impact and cost-effectiveness of diagnosing HIV infection during early infancy in South Africa: test timing and frequency. J Infect Dis. 2016;214:1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunning L, Kroon M, Fourie L, et al. Impact of birth HIV-PCR testing on the uptake of follow-up early infant diagnosis services in Cape Town, South Africa. Pediatr Infect Dis J. 2017;36:1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiu A, Modi S, Rivadeneira ED, et al. Optimizing infant HIV diagnosis in resource-limited settings: modeling the impact of HIV DNA PCR testing at birth. J Acquir Immune Defic Syndr. 2016;73:454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]