Abstract
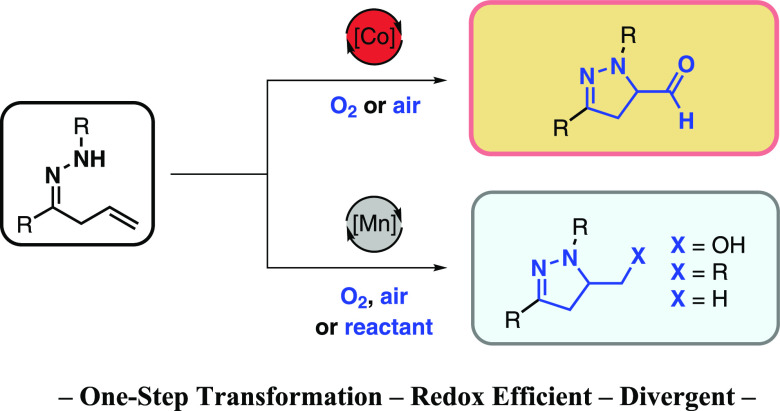
Manganese- and cobalt-catalyzed aminocyclization reactions of unsaturated hydrazones are reported. Whereas manganese catalysis provides access to pyrazoline and tetrahydropyridazine alcohols, cobalt catalysis for the first time paves the way for the selective formation of pyrazoline aldehydes. Furthermore, various functional groups including hydroperoxide, thiol derivatives, iodide, and bicyclopentane may be introduced via manganese-catalyzed ring-forming aminofunctionalization. A progesterone receptor antagonist was prepared using the aminocyclization protocol.
Keywords: Aminocyclization, Cobalt, Manganese, Pyrazolines, Hydrazones, Oxygen, Aldehyde
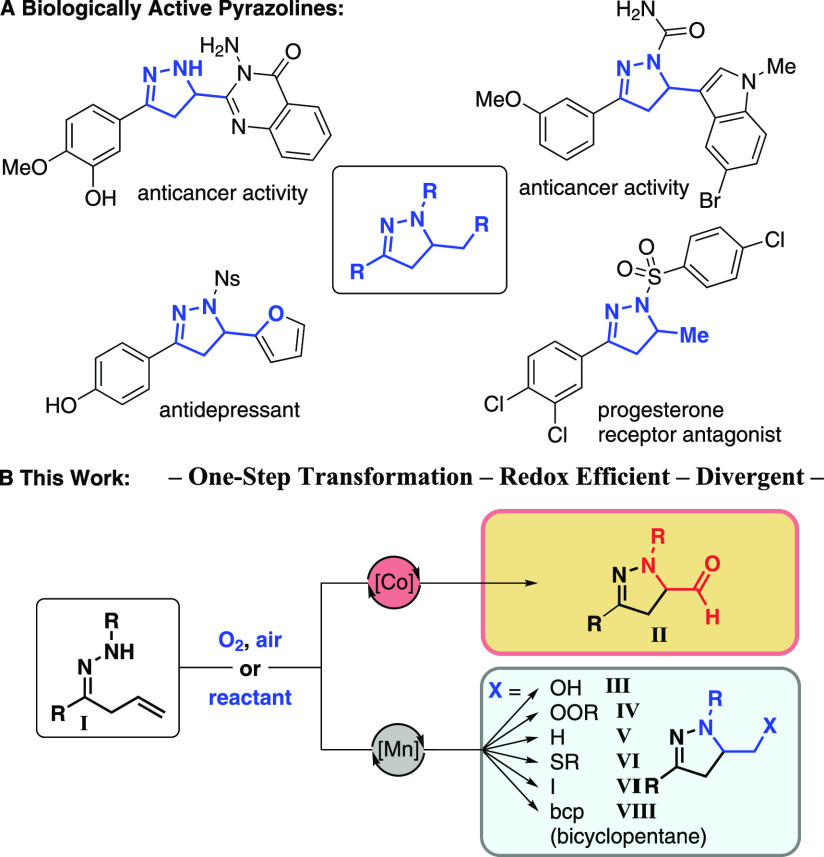
Azoles such as pyrazoles are important building blocks in modern pharmaceutical and agrochemical industry.1−6 Partially saturated counterparts, in particular 2-pyrazolines, are gaining recognition as promising scaffolds,7,9 as they offer great opportunity for structural diversification, which has proven to be key in modern drug development.17,18 In parallel, there has been great interest in building blocks that provide possibilities for design beyond the two-dimensional space of traditional (hetero)aromatic rings.17,18 Accordingly, leads incorporating 2-pyrazolines have appeared in drug discovery programs for treatment of a wide range of diseases, including cancer,10,12 diabetes,15 and malaria.13 They have also shown anti-inflammatory,11 -microbial,13 and -fungal activity (Scheme 1A).14 Hence, approaches that lead to this scaffold with diverse functional groups are especially valuable. Herein we report selective manganese- and cobalt-catalyzed aminocyclization reactions of unsaturated hydrazones I that provide a wide variety of functionalized pyrazolines, including aldehydes, alcohols, peroxide, thiol derivatives, iodide, and bicyclopentane (II–VIII, Scheme 1B).
Scheme 1. Biologically Active Pyrazolines and Cyclization Reactions of Unsaturated Hydrazones.
Pyrazolines have commonly been prepared via 1,3-dipolar cycloadditions19,21 or condensation reactions of enones and hydrazines.20,25,26 Recently, Cu-catalyzed oxidative cyclization of unsaturated hydrazones has been reported to give pyrazolines.27 However, this process affords a mixture of aldehydes II (20%), alcohols III (18%), and hydroperoxides IV (40%), thus requiring a subsequent reductive step to convert the mixture into alcohol products. There have also been reports on the use of acridinium28 and ruthenium29 photocatalysis to furnish pyrazolines, such as III and V.30−37 Collectively, these approaches demonstrate the general interest in methods for the preparation of functionalized pyrazolines. However, convenient access is desirable not only to alcohols but also to an expanded set of products that include other groups. These would be especially useful because they may serve as linchpins for further synthetic elaboration. In this respect, selective and efficient access to aldehydes, such as II in Scheme 1B, has not been reported, despite the fact that they act as a gateway to other functionalities, such as carboxylic acids, amides, nitriles, amines, and heterocycles. Chemler reported the aerobic copper-catalyzed cyclization of 4-pentenylsulfonamides to yield 2-formylpyrrolidines, which were then subjected to oxidative C–C bond cleavage and further transformed into 2-pyrrolidinones.38,39
Catalysis by first-row transition metals has gained significant attention because of their low cost and natural abundance.43 The use of manganese and cobalt catalysis remains relatively underexplored for olefin functionalizations in comparison with other transition metals such as copper, palladium, and nickel,45−47 yet it offers great opportunities. We have been inspired by one of the earliest examples of preparatively useful cobalt-catalyzed olefin functionalization, namely, the Mukaiyama hydration,48,49 and related processes.53,56
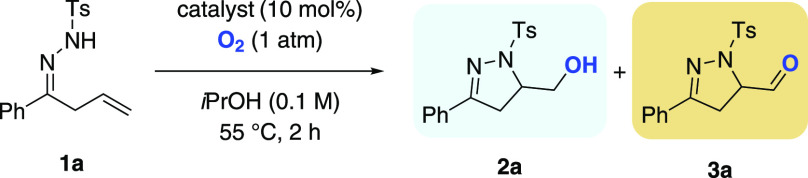
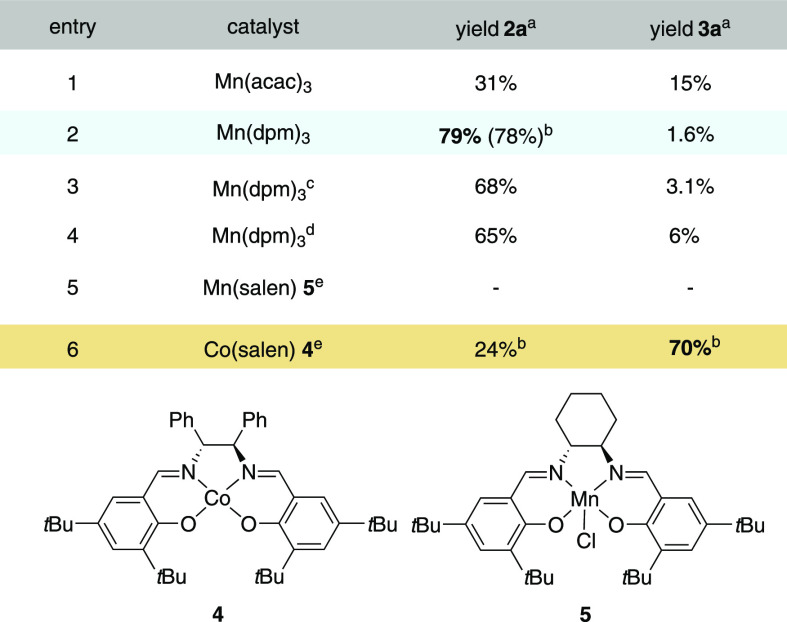
Our prospecting studies commenced by examination of hydrazone 1a as a prototype in a variety of cyclization reactions (Table 1). Extensive optimization studies58 revealed that treatment of 1a with Mn(acac)3 (10 mol %) under an oxygen atmosphere (1 atm) in isopropanol (0.1 M) at 55 °C for 2 h afforded pyrazoline alcohol 2a in 31% yield alongside the corresponding aldehyde 3a in 15% yield (Table 1, entry 1).59 Switching to Mn(dpm)3 (dpm = dipivaloylmethanato) significantly improved the reaction outcome and selectivity, providing 2a in 79% yield and 3a in merely 1.6% yield (2a:3a ratio = 98:2) (entry 2). The use of air via a gas inlet instead of a pure oxygen atmosphere furnished alcohol 2a in 68% yield (entry 3). This result was satisfying, as especially on larger scales the handling of molecular oxygen can be hazardous.60 Lowering the reaction temperature to 25 °C was also feasible with an elongated reaction time (12 h, 65% yield; entry 4). In examining other catalysts (see the Supporting Information), we observed that cobalt salen 4 resulted in a change in the reaction outcome, affording aldehyde 3a in 70% yield, whereas manganese salen 5 did not lead to product formation (entries 5 and 6) .58,61
Table 1. Selected Optimization Results for the Mn- and Co-Catalyzed Cyclizations.
Determined by 1H NMR spectroscopy with 1,3,5-trimethoxybenzene as the internal standard.
Isolated yield.
Air was continuously introduced into the reaction mixture.
The reaction was conducted at 25 °C for 12 h.
The reaction was conducted at 25 °C for 1 h.
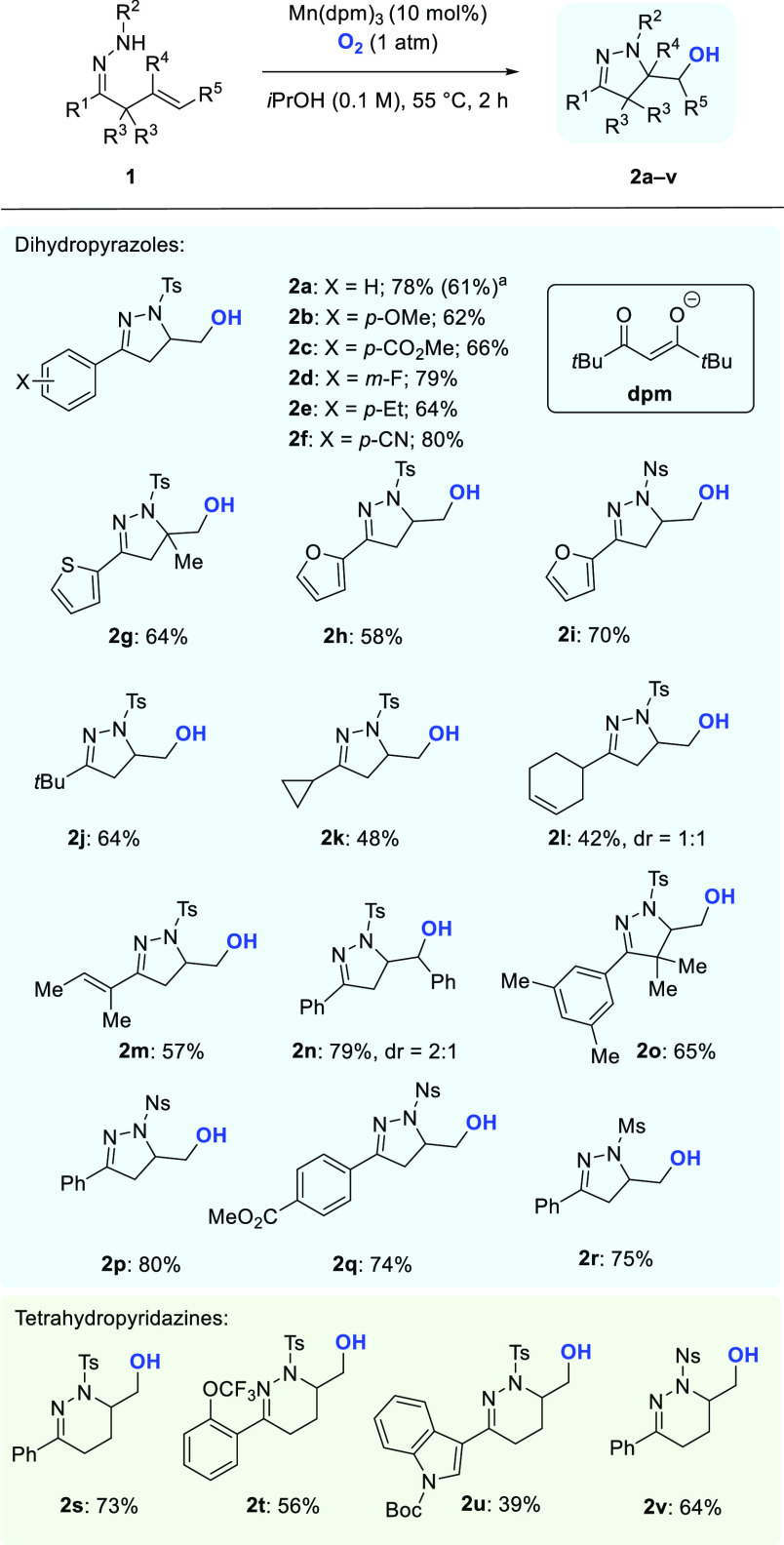
With the optimized reaction parameters in hand for cyclization and selective formation of alcohols, the scope of the reaction was investigated (Scheme 2). Various β,γ-unsaturated aryl- and heteroarylhydrazones were submitted to the established reaction conditions, and N-heterocycles 2a–i were obtained in 58–80% yield. Esters and nitriles were well-tolerated in the cyclization reaction, and no difference in reactivity was observed for substrates incorporating electron-donating and -withdrawing substituents. The use of alkylhydrazones as substrates led to the formation of pyrazolines 2j–l in 42–64% yield. In the presence of additional olefins, which could participate in competitive cyclizations, only 5-exo-trig cyclization was observed, and N-heterocycles 2l and 2m were isolated in 42% and 57% yield, respectively. Substrates with substituents on the alkyl chain (1g, 1n, and 1o) were also employed and provided, after cyclization, pyrazolines 2g, 2n, and 2o in 64–79% yield. Replacing the N-tosyl group with N-nosyl (p-nitrophenylsulfonyl) or N-mesyl (methylsulfonyl) was also possible, giving rise to pyrazolines 2i and 2p–r in 70–80% yield. When γ,δ-unsaturated hydrazones were submitted to the reaction conditions, tetrahydropyridazines 2s–v were obtained in 39–73% yield.
Scheme 2. Mn-Catalyzed Cyclizations.
The reaction was conducted on a 4 mmol scale.
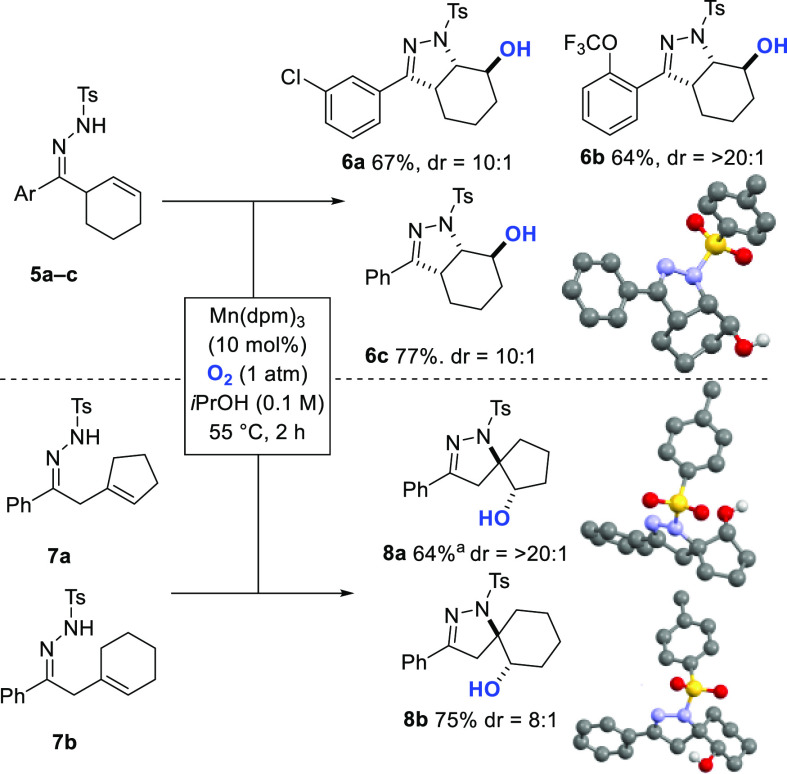
We then investigated substrates in which the alkene partner was embedded within a ring, which would lead to ring-fused or spiro-pyrazolines (Scheme 3). Hydrazones 5a–c as starting materials provided valuable 5,6-fused bicyclic rings 6a–c possessing an anti relative configuration (64–77% yield, dr 10:1 to >20:1), as determined by 1H NMR, X-ray, and 1D NOE data (Scheme 3).62,63 When cyclopentene- and cyclohexene-substituted hydrazones 7a and 7b were used, [4.4] and [4.5] spirocycles 8a and 8b were prepared in high yields with excellent diastereoselectivity (64% and 75% yield, dr >20:1 and 8:1, respectively; Scheme 3).
Scheme 3. Preparation of Fused and Spiro Pyrazolines.
Mn(dpm)3 (20 mol %) was used.
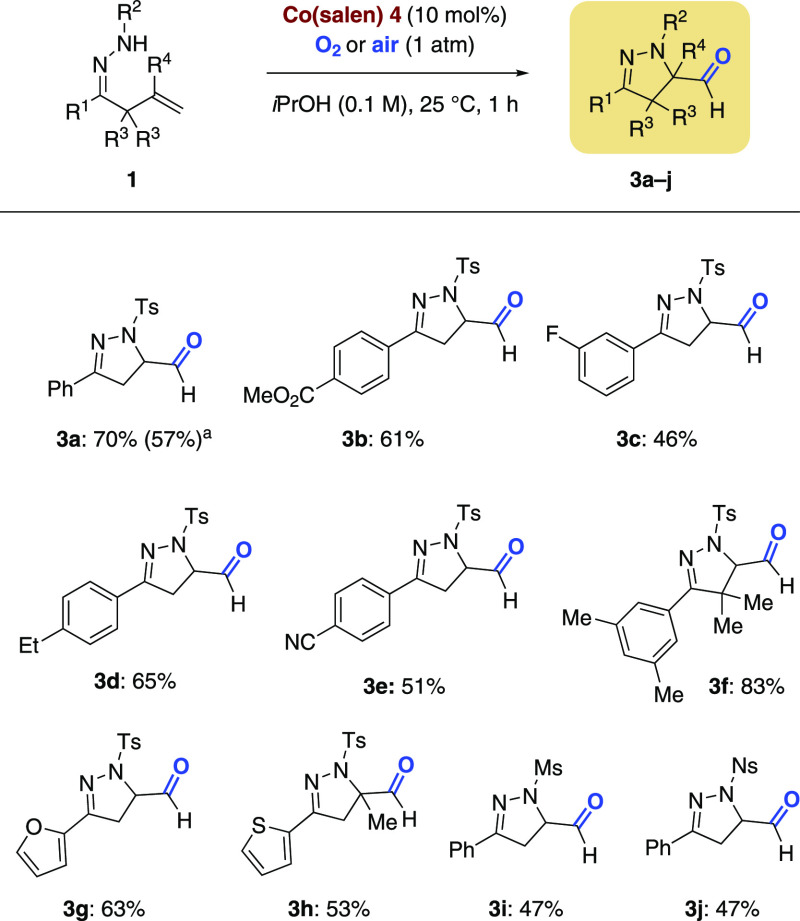
During the optimization studies aimed at preparation of the primary alcohol product shown in Table 1, we observed that the formation of aldehyde 3a was preferred with the use of Co-salen 4 as catalyst (see Table 1, entry 6). Given the rather limited number of examples of cyclization reactions of olefins that produce aldehydes, we set out to investigate the scope of this transformation (Scheme 4). Various functional groups including nitriles and esters were well-tolerated, yielding aldehydes 3a–f in 46–83% yield.64 Replacing the oxygen atmosphere by air via a gas inlet led to 3a in 57% yield. When furan and thiophene hydrazones were employed, pyrazolines 3g and 3h were isolated in 63% and 53% yield, respectively. Other sulfonamides could be used, such as N-mesyl and N-nosyl, yielding aldehydes 3i and 3j, both in 47% yield (Scheme 4). We speculate that the cobalt catalyst mediates cyclization, formation of a terminal hydroperoxide, and its collapse to aldehydes 3a–j.65
Scheme 4. Co-Catalyzed Cyclizations.
Air was continuously introduced into the reaction mixture.
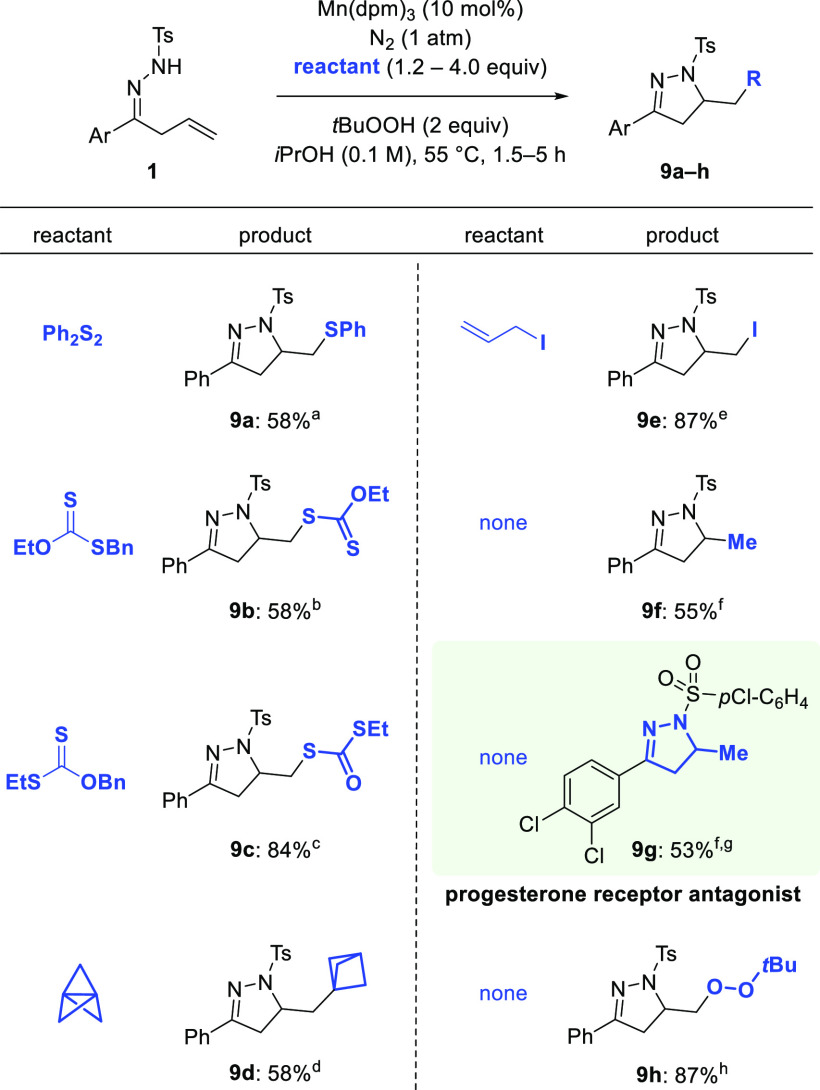
We then proceeded to examine the use of other reactive traps instead of oxygen (Scheme 5). After prospecting experiments, we found a standard set of conditions in which stirring 1a with various reactants in the presence of Mn(dpm)3 (10 mol %) and tBuOOH (2 equiv) in iPrOH under N2 (1 atm) gave a variety of adducts. In the presence of diphenyl disulfide (2 equiv) thioether 9a was formed in 58% yield. The reaction of 1 with S-benzyl O-ethyl carbonodithioate and O-benzyl S-ethyl carbonodithioate afforded xanthate 9b and carbonodithioate 9c in 58% and 84% yield, respectively. With [1.1.1]propellane, bicyclopentane derivative 9d was obtained in 58% yield. The use of allyl iodide afforded primary iodide 9e in 87% yield. Interestingly, in the absence of additional reactants, treatment of 1a with Mn(dpm)3 (10 mol %) and tBuOOH (2 equiv) in iPrOH under N2 (1 atm) provided 9f in 55% yield.66 When the appropriate unsaturated hydrazone was employed as the starting material it was possible to prepare pyrazoline 9g, a progesterone receptor antagonist,9 in 53% yield. In cyclizations leading to 9f and 9g, iPrOH acts as a hydrogen donor, as described in the early work of Mukaiyama.61,67 When the reaction was conducted in DCE instead of iPrOH, tert-butylhydroperoxide quenched the reactive intermediate to give dialkyl peroxide 9h in 87% yield (Scheme 5).
Scheme 5. Mn-Catalyzed Aminofunctionalizations.
Diphenyl disulfide as the reactant.
S-benzyl O-ethyl carbonodithioate as the reactant.
O-benzyl S-ethyl carbonodithioate as the reactant.
[1.1.1]propellane as the reactant.
allyl iodide as the reactant.
No reactant.
Mn(dpm)3 (30 mol %) was used.
No reactant; DCE was used as the solvent.
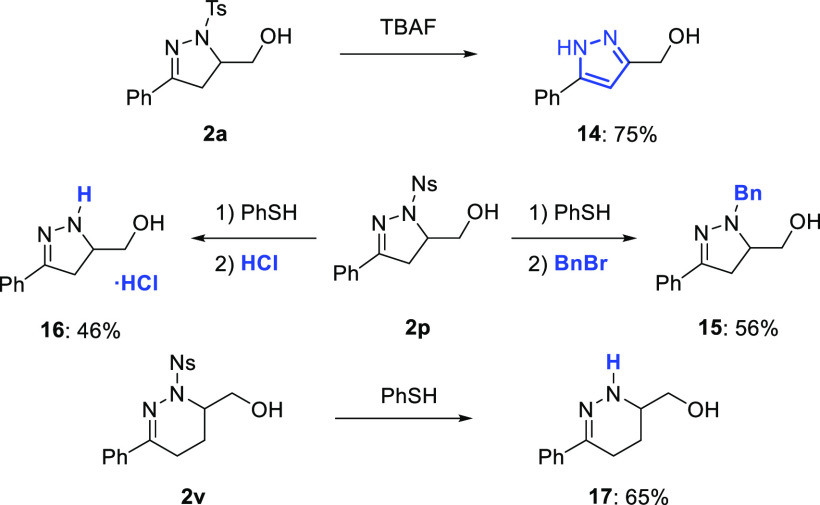
Finally, various synthetic transformations were performed using pyrazoline and tetrahydropyridazine alcohols (Scheme 6).62 Reaction of 2a with Bu4NF led to elimination of the N-tosyl group, which provided pyrazole 14 in 75% yield. When N-nosyl pyrazoline 2p was treated with thiophenol at room temperature in the presence of K2CO3 followed by benzyl bromide, pyrazoline 15 was obtained in 56% yield.68 It was also possible to prepare the corresponding hydrochloride salt 16 in 46% yield by addition of 2 M HCl in dioxane after sulfonamide cleavage.69 Removal of the N-nosyl group from tetrahydropyridazine 2v could also be carried out, affording azine 17 in 65% yield (Scheme 6).
Scheme 6. Derivatization of the Pyrazolines and Tetrahydropyridazine.
In summary, we have disclosed manganese- and cobalt-catalyzed cyclization reactions of unsaturated hydrazones that gave divergent access to a range of complex and highly functionalized N-heterocycles. Whereas aerobic manganese catalysis led to the formation of pyrazoline and tetrahydropyridazine alcohols, a cobalt–salen catalyst for the first time allowed the preparation of pyrazoline aldehydes. Addition of various reactants to the cyclization reaction paved the way for the formation of a variety of functionalized pyrazolines as well as a progesterone receptor antagonist. Finally, synthetic transformations of the prepared products were performed, demonstrating the utility of the cyclization protocol. We are in the process of further developing cyclization reactions that lead to versatile aldehyde products, and the results will be reported as they become available.
Acknowledgments
This work was funded by the European Research Council (OLECAT, Grant ID 833540). M.B. thanks the Deutsche Forschungsgemeinschaft (DFG) for a postdoctoral fellowship. We are grateful to Dr. N. Trapp and M. Solar for X-ray crystallographic analysis.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.1c00176.
The authors declare no competing financial interest.
Supplementary Material
References
- Lednicer D.Drugs Based on Five-Membered Heterocycles. In Strategies for Organic Drug Synthesis and Design, 2nd ed.; Wiley: Hoboken, NJ, 2009; pp 239–318. [Google Scholar]
- Baumann M.; Baxendale I. R.; Ley S. V.; Nikbin N. An overview of the key routes to the best selling 5-membered ring heterocyclic pharmaceuticals. Beilstein J. Org. Chem. 2011, 7, 442–495. 10.3762/bjoc.7.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberth C. Heterocyclic chemistry in crop protection. Pest Manage. Sci. 2013, 69, 1106–1114. 10.1002/ps.3615. [DOI] [PubMed] [Google Scholar]
- Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- Taylor A. P.; Robinson R. P.; Fobian Y. M.; Blakemore D. C.; Jones L. H.; Fadeyi O. Modern advances in heterocyclic chemistry in drug discovery. Org. Biomol. Chem. 2016, 14, 6611–6637. 10.1039/C6OB00936K. [DOI] [PubMed] [Google Scholar]
- Heravi M. M.; Zadsirjan V. Prescribed drugs containing nitrogen heterocycles: an overview. RSC Adv. 2020, 10, 44247–44311. 10.1039/D0RA09198G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For reviews, see:; a Liu X. H.; Ruan B. F.; Li J.; Chen F. H.; Song B. A.; Zhu H. L.; Bhadury P. S.; Zhao J. Synthesis and Biological Activity of Chiral Dihydropyrazole: Potential Lead for Drug Design. Mini-Rev. Med. Chem. 2011, 11, 771–821. 10.2174/138955711796355285. [DOI] [PubMed] [Google Scholar]; b Matiadis D.; Sagnou M. Pyrazoline Hybrids as Promising Anticancer Agents: An Up-to-Date Overview. Int. J. Mol. Sci. 2020, 21, 5507. 10.3390/ijms21155507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For recent examples, see:; a Jones D. G.; Liang X.; Stewart E. L.; Noe R. A.; Kallander L. S.; Madauss K. P.; Williams S. P.; Thompson S. K.; Gray D. W.; Hoekstra W. J. Discovery of non-steroidal mifepristone mimetics: Pyrazoline-based PR antagonists. Bioorg. Med. Chem. Lett. 2005, 15, 3203–3206. 10.1016/j.bmcl.2005.05.001. [DOI] [PubMed] [Google Scholar]; b Wang P.-F.; Qiu H.-Y.; Baloch S. K.; Gong H.-B.; Wang Z.-C.; Zhu H.-L. Synthesis, Biological Evaluation, and Docking of Dihydropyrazole Sulfonamide Containing 2-hydroxyphenyl Moiety: A Series of Novel MMP-2 Inhibitors. Chem. Biol. Drug Des. 2015, 86, 1405–1410. 10.1111/cbdd.12604. [DOI] [PubMed] [Google Scholar]; c Korablina D. D.; Vorozhtsov N. I.; Sviridova L. A.; Kalenikova E. I.; Medvedev O. S. Pharmacological Activity of 4,5-Dihydropyrazole Derivatives. Pharm. Chem. J. 2016, 50, 281–295. 10.1007/s11094-016-1438-6. [DOI] [Google Scholar]; d Sebastian J. Dihydropyrazole and dihydropyrrole structures based design of Kif15 inhibitors as novel therapeutic agents for cancer. Comput. Biol. Chem. 2017, 68, 164–174. 10.1016/j.compbiolchem.2017.03.006. [DOI] [PubMed] [Google Scholar]; e Mishra V. K.; Mishra M.; Kashaw V.; Kashaw S. K. Synthesis of 1,3,5-trisubstituted pyrazolines as potential antimalarial and antimicrobial agents. Bioorg. Med. Chem. 2017, 25, 1949–1962. 10.1016/j.bmc.2017.02.025. [DOI] [PubMed] [Google Scholar]; f Abdelrahmman K.; El-Behairy M. F.; Alsherbiny M. A.; Mazeed T. E. In vitro activity of dihydropyrazole derivatives against Candida species. Bull. Fac. Pharm. 2018, 56, 80–82. 10.1016/j.bfopcu.2017.11.002. [DOI] [Google Scholar]; g Shi J.; Gu Z.; Jurica E. A.; Wu X.; Haque L. E.; Williams K. N.; Hernandez A. S.; Hong Z.; Gao Q.; Dabros M.; Davulcu A. H.; Mathur A.; Rampulla R. A.; Gupta A. K.; Jayaram R.; Apedo A.; Moore D. B.; Liu H.; Kunselman L. K.; Brady E. J.; Wilkes J. J.; Zinker B. A.; Cai H.; Shu Y.-Z.; Sun Q.; Dierks E. A.; Foster K. A.; Xu C.; Wang T.; Panemangalore R.; Cvijic M. E.; Xie C.; Cao G. G.; Zhou M.; Krupinski J.; Whaley J. M.; Robl J. A.; Ewing W. R.; Ellsworth B. A. Discovery of Potent and Orally Bioavailable Dihydropyrazole GPR40 Agonists. J. Med. Chem. 2018, 61, 681–694. 10.1021/acs.jmedchem.7b00982. [DOI] [PubMed] [Google Scholar]; h Tripathi A. C.; Upadhyay S.; Paliwal S.; Saraf S. K. Derivatives of 4,5-dihydro(1H)pyrazoles as possible MAO-A inhibitors in depression and anxiety disorders: synthesis, biological evaluation and molecular modeling studies. Med. Chem. Res. 2018, 27, 1485–1503. 10.1007/s00044-018-2167-z. [DOI] [Google Scholar]
- Lovering F.; Bikker J.; Humblet C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- Carreira E. M.; Fessard T. C. Four-Membered Ring-Containing Spirocycles: Synthetic Strategies and Opportunities. Chem. Rev. 2014, 114, 8257–8322. 10.1021/cr500127b. [DOI] [PubMed] [Google Scholar]
- For reviews, see:; a Stanley L. M.; Sibi M. P. Enantioselective Copper-Catalyzed 1,3-Dipolar Cycloadditions. Chem. Rev. 2008, 108, 2887–2902. 10.1021/cr078371m. [DOI] [PubMed] [Google Scholar]; b Farooq S.; Ngaini Z. One-Pot and Two-Pot Synthesis of Chalcone Based Mono and Bis-Pyrazolines. Tetrahedron Lett. 2020, 61, 151416. 10.1016/j.tetlet.2019.151416. [DOI] [Google Scholar]
- For selected examples, see:; a Chen J.-R.; Dong W.-R.; Candy M.; Pan F.-F.; Jörres M.; Bolm C. Enantioselective Synthesis of Dihydropyrazoles by Formal [4 + 1] Cycloaddition of in Situ-Derived Azoalkenes and Sulfur Ylides. J. Am. Chem. Soc. 2012, 134, 6924–6927. 10.1021/ja301196x. [DOI] [PubMed] [Google Scholar]; b Rueping M.; Maji M. S.; Küçük H. B.; Atodiresei I. Asymmetric Brønsted Acid Catalyzed Cycloadditions—Efficient Enantioselective Synthesis of Pyrazolidines, Pyrazolines, and 1,3-Diamines from N-Acyl Hyrazones and Alkenes. Angew. Chem., Int. Ed. 2012, 51, 12864–12868. 10.1002/anie.201205813. [DOI] [PubMed] [Google Scholar]; c Zhang D.-Y.; Shao L.; Xu J.; Hu X.-P. Copper-Catalyzed Asymmetric Formal [3 + 2] Cycloaddition of Propargylic Acetates with Hydrazines: Enantioselective Synthesis of Optically Active 2-Pyrazolines. ACS Catal. 2015, 5, 5026–5030. 10.1021/acscatal.5b01283. [DOI] [Google Scholar]; d Hu S.; Du S.; Yang Z.; Ni L.; Chen Z. Synthesis of Multi-substituted Dihydropyrazoles by Copper-Mediated [4 + 1] Cycloaddition Reaction of N-Sulfonylhydrazones and Sulfoxonium Ylides. Adv. Synth. Catal. 2019, 361, 3124–3136. 10.1002/adsc.201900212. [DOI] [Google Scholar]
- Léavai A. Synthesis of 2-pyrazolines by the reactions of α,β-unsaturated aldehydes, ketones, and esters with diazoalkanes, nitrile imines, and hydrazines. J. Heterocycl. Chem. 2002, 39, 1–13. 10.1002/jhet.5570390101. [DOI] [Google Scholar]
- Yusuf M.; Jain P. Synthetic and biological studies of pyrazolines and related heterocyclic compounds. Arabian J. Chem. 2014, 7, 553–596. 10.1016/j.arabjc.2011.09.013. [DOI] [Google Scholar]
- Chen S.; Chen W.; Chen X.; Chen G.; Ackermann L.; Tian X. Copper(I)-Catalyzed Oxyamination of β,γ-Unsaturated Hydrazones: Synthesis of Dihydropyrazoles. Org. Lett. 2019, 21, 7787–7790. 10.1021/acs.orglett.9b02733. [DOI] [PubMed] [Google Scholar]
- Hu X.-Q.; Chen J.; Chen J.-R.; Yan D.-M.; Xiao W.-J. Organophotocatalytic Generation of N- and O-Centred Radicals Enables Aerobic Oxyamination and Dioxygenation of Alkenes. Chem. - Eur. J. 2016, 22, 14141–14146. 10.1002/chem.201602597. [DOI] [PubMed] [Google Scholar]
- Hu X.-Q.; Chen J.-R.; Wei Q.; Liu F.-L.; Deng Q.-H.; Beauchemin A. M.; Xiao W.-J. Photocatalytic Generation of N-Centered Hydrazonyl Radicals: A Strategy for Hydroamination of β,γ-Unsaturated Hydrazones. Angew. Chem., Int. Ed. 2014, 53, 12163–12167. 10.1002/anie.201406491. [DOI] [PubMed] [Google Scholar]
- For cyclizations of β,γ-unsaturated hydrazones, which contain an α-quaternary center, to give pyrazolines, see:; a Duan X.-Y.; Yang X.-L.; Fang R.; Peng X.-X.; Yu W.; Han B. Hydrazone Radical Promoted Vicinal Difunctionalization of Alkenes and Trifunctionalization of Allyls: Synthesis of Pyrazolines and Tetrahydropyridazines. J. Org. Chem. 2013, 78, 10692–10704. 10.1021/jo4016908. [DOI] [PubMed] [Google Scholar]; b Zhao Q.-Q.; Chen J.; Yan D.-M.; Chen J.-R.; Xiao W.-J. Photocatalytic Hydrazonyl Radical-Mediated Radical Cyclization/Allylation Cascade: Synthesis of Dihydropyrazoles and Tetrahydropyridazines. Org. Lett. 2017, 19, 3620–3623. 10.1021/acs.orglett.7b01609. [DOI] [PubMed] [Google Scholar]; c Chen M.; Wang L.-J.; Ren P.-X.; Hou X.-Y.; Fang Z.; Han M.-N.; Li W. Copper-Catalyzed Diamination of Alkenes of Unsaturated Ketohydrazones with Amines. Org. Lett. 2018, 20, 510–513. 10.1021/acs.orglett.7b03401. [DOI] [PubMed] [Google Scholar]; d Wang L.-J.; Ren P.-X.; Qi L.; Chen M.; Lu Y.-L.; Zhao J.-Y.; Liu R.; Chen J.-M.; Li W. Copper-Mediated Aminoazidation, Aminohalogenation, and Aminothiocyanation of β,γ-Unsaturated Hydrazones: Synthesis of Versatile Functionalized Pyrazolines. Org. Lett. 2018, 20, 4411–4415. 10.1021/acs.orglett.8b01620. [DOI] [PubMed] [Google Scholar]; e Liu R.-H.; Wang Z.-Q.; Wei B.-Y.; Zhang J.-W.; Zhou B.; Han B. Cu-Catalyzed Aminoacyloxylation of Unactivated Alkenes of Unsaturated Hydrazones with Manifold Carboxylic Acids toward Ester-Functionalized Pyrazolines. Org. Lett. 2018, 20, 4183–4186. 10.1021/acs.orglett.8b01507. [DOI] [PubMed] [Google Scholar]; f Chen M.; Qi L.; Chen J.-M.; Ren P.-X.; Du J.; Wang L.-J.; Li W. Synthesis of acyloxyl pyrazolines by copper-mediated aminoacyloxylation of unsaturated ketohydrazones. Org. Biomol. Chem. 2018, 16, 5136–5143. 10.1039/C8OB01114A. [DOI] [PubMed] [Google Scholar]
- For an example using phenylhydrazones, see:Zhu M.-K.; Chen Y.-C.; Loh T.-P. Radical-Mediated Diamination of Alkenes with Phenylhydrazine and Azodicarboxylates: Highly Diastereoselective Synthesis of trans-Diamines from Cycloalkenes. Chem. - Eur. J. 2013, 19, 5250–5254. 10.1002/chem.201203832. [DOI] [PubMed] [Google Scholar]
- For an example using hypervalent iodine, see:Hu X.-Q.; Feng G.; Chen J.-R.; Yan D.-M.; Zhao Q.-Q.; Wei Q.; Xiao W.-J. PhI(OAc)2-mediated functionalisation of unactivated alkenes for the synthesis of pyrazoline and isoxazoline derivatives. Org. Biomol. Chem. 2015, 13, 3457–3461. 10.1039/C5OB00029G. [DOI] [PubMed] [Google Scholar]
- Wdowik T.; Chemler S. R. Direct Synthesis of 2-Formylpyrrolidines, 2-Pyrrolidinones and 2-Dihydrofuranones via Aerobic Copper-Catalyzed Aminooxygenation and Dioxygenation of 4-Pentenylsulfonamides and 4-Pentenylalcohols. J. Am. Chem. Soc. 2017, 139, 9515–9518. 10.1021/jacs.7b05680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For related Au/Fe- or Cu-catalyzed cyclizations, see:; a de Haro T.; Nevado C. Flexible Gold-Catalyzed Regioselective Oxidative Difunctionalization of Unactivated Alkenes. Angew. Chem., Int. Ed. 2011, 50, 906–910. 10.1002/anie.201005763. [DOI] [PubMed] [Google Scholar]; b Wang H.; Wang Y.; Liang D.; Liu L.; Zhang J.; Zhu Q. Copper-Catalyzed Intramolecular Dehydrogenative Aminooxygenation: Direct Access to Formyl-Substituted Aromatic N-Heterocycles. Angew. Chem., Int. Ed. 2011, 50, 5678–5681. 10.1002/anie.201100362. [DOI] [PubMed] [Google Scholar]; c Toh K. K.; Wang Y.-F.; Ng E. P. J.; Chiba S. Copper-Mediated Aerobic Synthesis of 3-Azabicyclo[3.1.0]hex-2-enes and 4-Carbonylpyrroles from N-Allyl/Propargyl Enamine Carboxylates. J. Am. Chem. Soc. 2011, 133, 13942–13945. 10.1021/ja206580j. [DOI] [PubMed] [Google Scholar]; d Peng H.; Akhmedov N. G.; Liang Y.-F.; Jiao N.; Shi X. Synergistic Gold and Iron Dual Catalysis: Preferred Radical Addition toward Vinyl–Gold Intermediate over Alkene. J. Am. Chem. Soc. 2015, 137, 8912–8915. 10.1021/jacs.5b05415. [DOI] [PubMed] [Google Scholar]
- For recent reviews, see:; a Gandeepan P.; Müller T.; Zell D.; Cera G.; Warratz S.; Ackermann L. 3d Transition Metals for C–H Activation. Chem. Rev. 2019, 119, 2192–2452. 10.1021/acs.chemrev.8b00507. [DOI] [PubMed] [Google Scholar]; b St John-Campbell S.; Bull J. A. Base Metal Catalysis in Directed C(sp3)–H Functionalisation. Adv. Synth. Catal. 2019, 361, 3662–3682. 10.1002/adsc.201900532. [DOI] [Google Scholar]
- The Chemistry of Organomanganese Compounds; Rappoport Z., Marek I.,Eds.; Wiley, 2011. [Google Scholar]
- Dhungana R. K.; Kc S.; Basnet P.; Giri R. Transition Metal-Catalyzed Dicarbofunctionalization of Unactivated Olefins. Chem. Rec. 2018, 18, 1314–1340. 10.1002/tcr.201700098. [DOI] [PubMed] [Google Scholar]
- Cobalt Catalysis in Organic Synthesis: Methods and Reactions; Hapke M., Hilt G., Eds.; Wiley-VCH: Weinheim, Germany, 2020. [Google Scholar]
- Isayama S.; Mukaiyama T. A New Method for Preparation of Alcohols from Olefins with Molecular Oxygen and Phenylsilane by the Use of Bis(acetylacetonato)cobalt(II). Chem. Lett. 1989, 18, 1071–1074. 10.1246/cl.1989.1071. [DOI] [Google Scholar]
- For reviews, see:; a Waser J.; Gaspar B.; Nambu H.; Carreira E. M. Hydrazines and Azides via the Metal-Catalyzed Hydrohydrazination and Hydroazidation of Olefins. J. Am. Chem. Soc. 2006, 128, 11693–11712. 10.1021/ja062355+. [DOI] [PubMed] [Google Scholar]; b Crossley S. W. M.; Obradors C.; Martinez R. M.; Shenvi R. A. Mn-, Fe-, and Co-Catalyzed Radical Hydrofunctionalizations of Olefins. Chem. Rev. 2016, 116, 8912–9000. 10.1021/acs.chemrev.6b00334. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zweig J. E.; Kim D. E.; Newhouse T. R. Methods Utilizing First-Row Transition Metals in Natural Product Total Synthesis. Chem. Rev. 2017, 117, 11680–11752. 10.1021/acs.chemrev.6b00833. [DOI] [PubMed] [Google Scholar]; d Green S. A.; Crossley S. W. M.; Matos J. L. M.; Vásquez-Céspedes S.; Shevick S. L.; Shenvi R. A. The High Chemofidelity of Metal-Catalyzed Hydrogen Atom Transfer. Acc. Chem. Res. 2018, 51, 2628–2640. 10.1021/acs.accounts.8b00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For cobalt-catalyzed aerobic cyclizations of unsaturated alcohols that provide tetrahydrofurans, see:; a Inoki S.; Mukaiyama T. A Convenient Method for the Stereoselective Preparation of trans-2-Hydroxymethyltetrahydrofurans by the Oxidative Cyclization of 5-Hydroxy-1-alkenes with Molecular Oxygen Catalyzed by Cobalt(II) Complex. Chem. Lett. 1990, 19, 67–70. 10.1246/cl.1990.67. [DOI] [Google Scholar]; b Palmer C.; Morra N. A.; Stevens A. C.; Bajtos B.; Machin B. P.; Pagenkopf B. L. Increased Yields and Simplified Purification with a Second-Generation Cobalt Catalyst for the Oxidative Formation of trans-THF Rings. Org. Lett. 2009, 11, 5614–5617. 10.1021/ol9023375. [DOI] [PubMed] [Google Scholar]; c Schuch D.; Fries P.; Dönges M.; Pérez B. M.; Hartung J. Reductive and Brominative Termination of Alkenol Cyclization in Aerobic Cobalt-Catalyzed Reactions. J. Am. Chem. Soc. 2009, 131, 12918–12920. 10.1021/ja904577c. [DOI] [PubMed] [Google Scholar]
- For cobalt- and manganese-catalyzed aerobic cyclizations of unsaturated oximes that provide dihydroisoxazoles, see:; a Li W.; Jia P.; Han B.; Li D.; Yu W. Cobalt-catalyzed aerobic oxidative cyclization of β,γ-unsaturated oximes. Tetrahedron 2013, 69, 3274–3280. 10.1016/j.tet.2013.02.032. [DOI] [Google Scholar]; b Yamamoto D.; Oguro T.; Tashiro Y.; Soga M.; Miyashita K.; Aso Y.; Makino K. Manganese-Promoted Oxidative Cyclization of Unsaturated Oximes Using Molecular Oxygen in Air under Ambient Conditions. Eur. J. Org. Chem. 2016, 2016, 5216–5219. 10.1002/ejoc.201600998. [DOI] [Google Scholar]
- For full screening details, see the Supporting Information.
- Determined by 1H NMR analysis using trimethoxybenzene as an internal standard.
- Hone C. A.; Roberge D. M.; Kappe C. O. The Use of Molecular Oxygen in Pharmaceutical Manufacturing: Is Flow the Way to Go?. ChemSusChem 2017, 10, 32–41. 10.1002/cssc.201601321. [DOI] [PubMed] [Google Scholar]
- TEMPO trapping experiments led to the formation of the corresponding TEMPO adduct (see the Supporting Information). We tentatively posit a reaction mechanism in which a nitrogen-centered radical is formed and then undergoes cyclization to give a carbon-centered radical intermediate that is trapped by oxygen. Depending on the nature of the catalyst, this species could collapse to either alcohol 2a or aldehyde 3a. Similar reaction pathways of metal peroxo species were described in early work by Mukaiyama. See:Mukaiyama T.; Isayama S.; Inoki S.; Kato K.; Yamada T.; Takai T. Oxidation-Reduction Hydration of Olefins with Molecular Oxygen and 2-Propanol Catalyzed by Bis(acetylacetonato)cobalt(II). Chem. Lett. 1989, 18, 449–452. 10.1246/cl.1989.449. [DOI] [Google Scholar]; However, at this point in time we cannot rule out reactions of Mn– or Co–hydrazone adducts with olefins, with trapping of the corresponding organometal intermediates by oxygen.
- For further details, see the Supporting Information.
- CCDC 2078368 (6c), CCDC 2078369 (8a), CCDC 2078370 (8b), CCDC 2078371 (9d), and CCDC 2078372 (14) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- Reduction of 3a to alcohol 2a using NaBH4 (1.1 equiv, THF (0.1 M), 25 °C, 1 h) followed by chiral SFC analysis of the alcohol did not show enantiomeric enrichment.
- When the cobalt-catalyzed reaction was conducted at 0 °C, exclusive formation of the hydroperoxide was first observed. Upon warming to room temperature, formation of aldehyde 3a and alcohol 2a took place. The hydroperoxide was not observed when Mn(dpm)3 was used. We postulate that while in both reactions the hydroperoxide may be formed, in the presence of Mn it undergoes rapid reduction to the alcohol, thus precluding oxidation to the aldehyde.
- We were also interested to find out whether 9f can be prepared via a condensation reaction. However, treatment of (E)-1-phenylbut-2-en-1-one with N-tosylhydrazide (1.1 equiv) in MeOH at 25 °C for 12 h gave 9f in merely 5% yield.
- Inoki S.; Kato K.; Takai T.; Isayama S.; Yamada T.; Mukaiyama T. Bis(trifluoroacetylacetonato)cobalt(II) Catalyzed Oxidation-Reduction Hydration of Olefins Selective Formation of Alcohols from Olefins. Chem. Lett. 1989, 18, 515–518. 10.1246/cl.1989.515. [DOI] [Google Scholar]
- Mish M. R.; Guerra F. M.; Carreira E. M. Asymmetric Dipolar Cycloadditions of Me3SiCHN2. Synthesis of a Novel Class of Amino Acids: Azaprolines. J. Am. Chem. Soc. 1997, 119, 8379–8380. 10.1021/ja971708p. [DOI] [Google Scholar]
- Also, HCl salt 16 is thermally sensitive and does not store well.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.