Abstract
Arynes, strained cyclic alkynes, and strained cyclic allenes were validated as plausible intermediates in the 1950s and 1960s. Despite initially being considered mere scientific curiosities, these transient and highly reactive species have now become valuable synthetic building blocks. This Perspective highlights recent advances in the field that have allowed access to structural and stereochemical complexity, including recent breakthroughs in asymmetric catalysis.
Keywords: Arynes, Cyclic allenes, Benzyne, Silyl triflates, Medicinal chemistry, Catalysis
Introduction
In 1902, a provocative proposal was put forth by Stoermer and Kahlert, who contemplated the intermediacy of benzofuranyne 1 (Figure 1).1 Although the existence of 1 would ultimately be called into question,2−4 the proposal led chemists to consider if triple bonds could exist in small rings. Roughly 50 years later, benzyne (2) and cyclohexyne (3) were validated experimentally, thanks to pioneering efforts by Roberts,5,6 Wittig,7 and Huisgen,8 in particular. Soon thereafter, in 1966, Wittig showed that 1,2-cyclohexadiene (4), an unusual-looking counterpart to benzyne (2) and cyclohexyne (3), could be generated and intercepted in cycloaddition processes.9 These results were striking at the time, as 2–4 possess functional groups (i.e., alkynes and allenes) that would ordinarily be linear. The bent nature of these species renders them transient and highly reactive intermediates that initially received little use in chemical synthesis. However, over the past few decades, the field of arynes and related strained intermediates has undergone a substantial period of growth, as shown in Figure 1.10
Figure 1.
Historical perspective, growth of “aryne” or “benzyne” chemistry, and select synthetic applications.
The synthetic utility of arynes and related strained intermediates is often underappreciated, perhaps owing to common misconceptions regarding safety or high reactivity (and consequently, misconceptions regarding low selectivity in aryne reactions). Conversely, we highlight the impact of arynes and related intermediates in the syntheses of 5–9. Popular ligands, such as XPhos (5), are made using aryne chemistry.11 The medicinal chemistry route toward Chantix (6) was enabled by an aryne cyclization.12 Syngenta has prepared isopyrazam (7), an important fungicide, using an aryne Diels–Alder reaction on multi-kilogram scale.13 Lastly, there are numerous examples of arynes and related intermediates in total synthesis,14−16 such as the use of an aryne insertion in Sarpong’s synthesis of cossonidine (8) and a cyclohexyne insertion in Carreira’s synthesis of guanacastepene N (9).17,18 As these examples highlight, arynes and related intermediates can be used to build two new bonds in a single transformation. Moreover, it should be noted that arynes can be generated under exceedingly mild, safe, and operationally simple fluoride-based reaction conditions, thanks to the advent of Kobayashi silyl triflates.19−22 In turn, chemists have sought to leverage the synthetic utility of these once controversial species, in addition to deepening our fundamental understanding through electrophilicity studies23 and regioselectivity studies.24−26
Rather than providing a comprehensive overview of the field, several of which are available elsewhere,14−16,20,27−37 this Perspective examines some of the quickly emerging areas of aryne chemistry and related strained intermediates. We assess the following recent advances: (a) the use of heterocyclic strained alkynes to construct scaffolds of value to medicinal and materials chemistry, (b) access to quaternary stereocenters using noncatalytic reactions of arynes and cyclic alkynes, (c) catalytic asymmetric reactions of arynes, (d) the assembly of intricate heterocyclic scaffolds via strained cyclic allene intermediates, and (e) stereospecific and catalytic asymmetric transformations of strained cyclic allenes. We hope this discussion underscores the current excitement in the field and, moreover, helps to draw researchers into an area that continues to rapidly evolve with opportunities for discovery.
Use of Heterocyclic Alkynes in the Synthesis of Medicinal and Materials Scaffolds
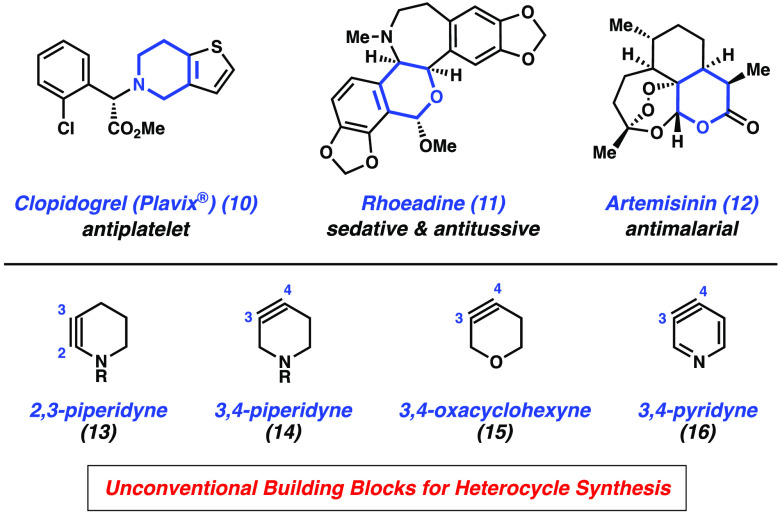
New strategies to access heterocyclic compounds, especially those with a high degree of sp3-rich character, remain highly sought after due to the numerous applications of heterocycles in drugs, agrochemicals, natural products, and materials.38−43 Just within the pharmaceutical industry, the vast majority of small-molecule drugs approved by the U.S. Food and Drug Administration contain a nitrogen or oxygen-containing heterocycle.44,45 A few examples are Plavix (10), an antiplatelet, rhoeadine (11), a sedative and antitussive, and the antimalarial drug artemisinin (12) (Figure 2).
Figure 2.
Representative natural products and pharmaceuticals containing heterocycles and representative nitrogen- and oxygen-containing strained cyclic alkynes.
Strained cyclic alkynes have emerged as valuable building blocks to access sp3-rich39 medicinally relevant heterocycles (Figure 2).46−49 Heteroatom-containing cyclohexyne derivatives, such as 2,3-piperidynes 13, 3,4-piperidynes 14, and oxacyclohexyne 15 have been far less studied than related aryne counterparts, (e.g., 3,4-pyridyne (16)).50−53 For comparison, the 3,4-pyridyne (16) was first disclosed in 1955,54 but studies involving 3,4-piperidynes were only reported recently. Specifically, the Danheiser group reported access to 13 in 201447 and our group described generation and interception of 14 and 15 in 2015 and 2016, respectively.48,49 These studies demonstrate the synthetic value of transient intermediates 13–15.
The initial breakthrough in this area by Danheiser and co-workers involved piperidyne precursor 17 (Figure 3). Upon treatment of 17 with CsF or KF, in the presence of a cycloaddition partner, a variety of (3 + 2) and (2 + 2) cycloadducts 20 were obtained. Presumably, these reactions proceed via the regioselective trapping of cyclic alkyne 19. Three examples are shown to highlight the diverse products (i.e., 22, 24, and 26) that can be obtained using this methodology. Moreover, each of the cycloadducts shown were isolated as one major constitutional isomer, with the significant regioselectivity of the reaction being attributed to the electronic effect of the nitrogen atom.
Figure 3.
Selected trapping experiments involving piperidyne 19.
Our laboratory had simultaneously engaged in complementary studies, which were geared toward accessing cyclic alkynes 28 (Figure 4). After developing synthetic routes to the requisite silyl triflates 27, we performed experiments to generate and trap the corresponding heterocyclic alkynes. As summarized, strained intermediate trapping via (4 + 2) and (3 + 2) cycloadditions led to an array of interesting heterocyclic products, such as aza- and oxa-bicycles 30 and 32, respectively, isooxazolines 34 and 35, pyrazole 36, and triazole 37. Where applicable, reactions occurred regioselectively, indicative of initial bond formation occurring at C4 of the reactive strained intermediate. It should be emphasized that by strategically varying the trapping partner, one can utilize common silyl triflate precursors (i.e., 27) to rapidly arrive at a variety of diverse heterocyclic scaffolds, including previously unknown scaffolds and analogs of medicinally relevant compounds.55−58
Figure 4.
Selected trapping experiments of heterocyclic alkynes using silyl triflate precursors.
An attractive feature related to strained cyclic alkyne methodologies is the ability to make reliable predictions regarding regioselectivities using the aryne distortion model. This model, proposed by Houk and co-workers via our earlier collaborative studies,25,33 shows that nonsymmetrical cyclic alkynes are geometrically distorted in their ground state. Nucleophilic addition occurs more favorably at the alkyne terminus with a larger internal angle (i.e., more distorted toward linearity), which correlates to a lower calculated distortion energy seen in the corresponding transition state. Moreover, larger differences between the internal angles typically correlate with regioselectivities in trapping experiments. Thus, by analyzing the ground state structure of a strained cyclic alkyne, one can typically make meaningful regioselectivity predictions. In the cases of piperidyne 14 and oxacyclohexyne 15, both react with a preference for C4 addition (Figure 5). Consistent with the distortion model, the ground state geometries of 14 and 15 shown by DFT calculations demonstrate that the C4 alkyne terminus is more distorted toward linearity. Furthermore, the difference between the internal angles of 14 and 15 are 12° and 15°, respectively. The greater angle difference of 15° seen in 15 is consistent with observed regioselectivities. For example, as shown in Figure 4, the nitrone trapping proceeds with higher selectivity when using 15 compared to 14.
Figure 5.
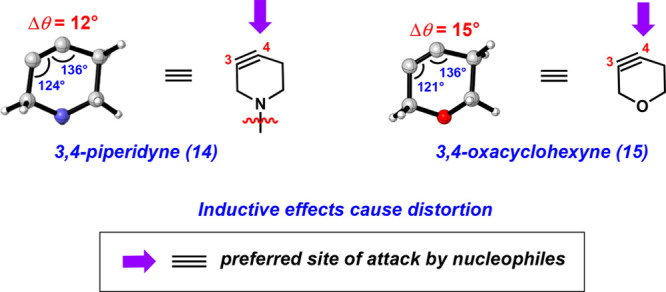
Regioselectivity of piperidyne 14 and oxacyclohexyne 15 trappings.
These methods demonstrate the value of strained heterocyclic alkynes for the synthesis of medicinally relevant scaffolds. Using common building blocks, rapid structural diversification can be achieved, with regioselectivity being predicted using simple calculations. Moreover, this chemistry provides a new and unusual strategy for accessing decorated heterocycles (i.e., using new strained intermediates). We expect these reports to influence future retrosynthetic analyses for the synthesis of heterocycles seen commonly in pharmaceuticals.
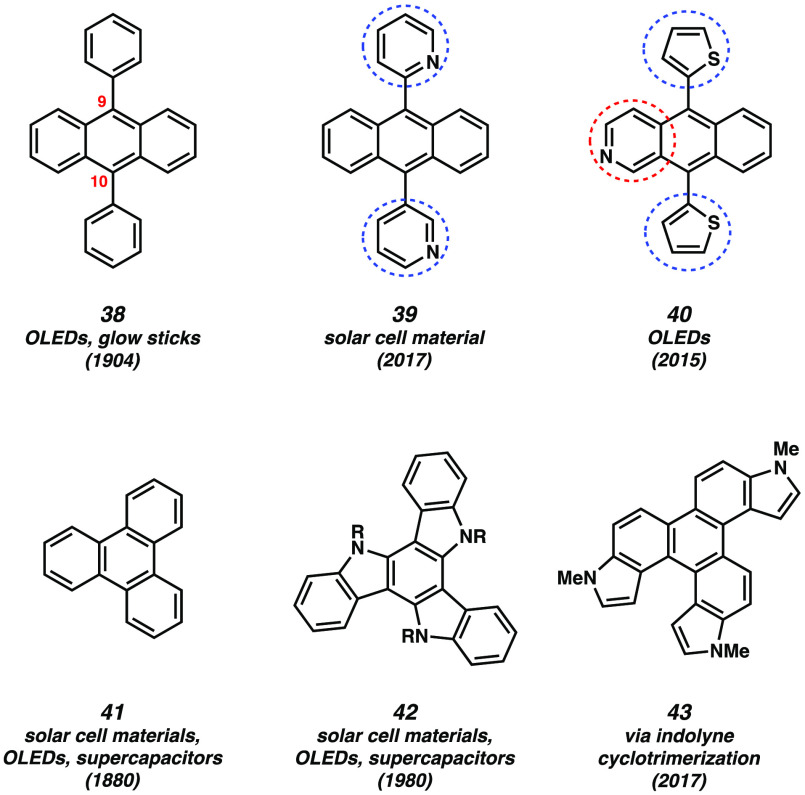
In addition to their value for the synthesis of medicinally relevant scaffolds, strained heterocyclic alkynes have seen recent utility in the synthesis of aromatic structures relevant to materials chemistry. Highly conjugated small molecules and polycyclic hydrocarbon frameworks have many potential applications in the field of organic electronics and material science.59,60 For example, these conjugated small molecules have been widely used in light-emitting diodes (OLEDs),61−64 field-effect transistors (OFETs),65−68 and photovoltaics (OPVs).69−71 Two common conjugated small molecules are 9,10-diphenylanthracene (38) and triphenylene (41) (Figure 6).72−75 Heteroatom-containing derivatives of these compounds, such as 39, 40, 42, and 43, have been gaining interest as the presence of heteroatoms can modulate electronic properties.76−78 New synthetic methods to access novel heterocyclic conjugated materials are highly desirable. As such, in 2017, our laboratory synthesized heterohelicene7943 (and derivatives) using an indolyne cyclotrimerization reaction.80,81 Indole trimers have been used in various materials applications.78,82−87 Building on these studies, we sought to access heteroatom-containing 9,10-diarylanthracene scaffolds using arynes and strained alkyne building blocks.
Figure 6.
Notable polycyclic aromatic hydrocarbons.
In 2019, we disclosed a modular strategy toward N-containing 9,10-diphenylanthracene derivatives, wherein all four aryl rings could be easily modified.88 The sequence was inspired by the collective seminal studies by Steglich, Nuckolls, and Wudl pertaining to double-aryne annulations of oxadiazinones.89−91 As shown in Figure 7, our approach involved two steps. First, 3,4-piperidyne (14), generated in situ from the corresponding silyl triflate, undergoes reaction with oxadiazinones 44 to give pyrones 45 through a Diels–Alder/retro-Diels–Alder sequence.92 The oxadiazinones are easily prepared with two different aryl substituents from readily available starting materials.93 In a second Diels–Alder/retro-Diels–Alder sequence, pyrones 45, which are bench stable and isolable, react with an aryne or nonaromatic strained cyclic alkyne (i.e., 46) to deliver polycyclic hydrocarbon frameworks 47. 48–50 are representative products. As needed, subsequent oxidation can be performed to access more highly conjugated derivatives.
Figure 7.
Modular strategy to access 9,10-diphenylanthracene derivatives and representative products 48–50.
Figure 8 highlights two additional aspects of this chemistry. In the first, 51–53 were shown to react at room temperature in the presence of CsF. This three-component coupling obviates the need to isolate a pyrone intermediate and delivers 54 in 56% yield. Additionally, the methodology could be used to access 55, a novel heterocyclic PAH scaffold that fluoresces, displaying a blue emission. In the presence of acid, pyridinium salt 56 forms, which displays an orange emission. Stimuli responsive materials are useful in a host of applications such as pH fluorescence sensors94,95 and solid-state fluorescent switches.96
Figure 8.
Utilizing arynes to access 9,10-diphenylanthracene derivatives.
The chemistry described above pertaining to PAHs showcases an exciting new direction of modern strained intermediate chemistry. Notably, Hosoya and co-workers published a complementary method in 2019 that utilizes a variety of strained intermediates, including thienobenzyne, to access PAH skeletons.97 Our group has published a follow-up study as well, which provides additional experimental results, as well as a computational mechanistic investigation.98 These efforts demonstrate that heterocyclic strained intermediates can be strategically leveraged through a cycloaddition cascade sequence to rapidly generate PAH scaffolds. The transformation enables access to structurally diverse products through the formation of four carbon–carbon bonds. It is expected that this chemistry and variants thereof will prompt the development of related methods that rely on strained intermediates to access compounds of value to materials chemistry.
Use of Arynes and Cyclic Alkynes to Access Quaternary Stereocenters (Noncatalytic)
The majority of reported methodologies and synthetic applications that utilize strained cyclic alkynes are intermolecular reactions that generate achiral or racemic products. However, efforts have been put forth to generate enantioenriched products, including those that rely on the use of chiral auxiliaries or reagents. For example, reports by Lautens and co-workers99,100 and Barrett et al.101,102 demonstrate stereocontrolled intermolecular reactions of arynes for the introduction of tertiary stereocenters using Oppolzer or Schöllkopf reagents, respectively. As accessing quaternary stereocenters is especially valuable,103−106 we were interested in utilizing arynes and strained cyclic alkynes to access stereodefined quaternary centers in an intermolecular fashion.
Our efforts, which were reported in 2018,107 concerned the reaction between α-ketoester 57 and strained cyclic alkyne 46 to yield α-substituted product 58, as shown in Figure 9. Prior efforts to achieve this transformation in a racemic sense were accompanied by concomitant C–C bond fragmentation,108 so we considered an alternative, two-step approach. First, α-ketoester 57 would be treated with amine 59 to afford the corresponding enamine 60. Enamine 60 could then be used to trap aryne 46, affording α-substituted product 58 after hydrolysis. We envisioned that employing a chiral amine (i.e., 59) would render the reaction diastereoselective and yield 58 in enantioenriched form. It should be noted that enantioselective α-arylation of β-ketoesters, one of the net transformations we hoped to develop, had remained a challenging synthetic problem.109−116
Figure 9.
Intercepting arynes and cyclic alkynes for the installation of stereodefined quaternary stereocenters.
At the time of our study, the use of enamines and strained cyclic alkynes to construct quaternary stereocenters was unknown; thus, the racemic arylation117−119 was first developed (Figure 10). Benzylamine was condensed onto ketoester 61 to afford enamine 62 (R = Bn). Next, enamine 62 was used to trap benzyne, which was generated in situ from silyl triflate 53 using CsF in DME. Following hydrolysis with 1 M HCl, α-arylated product 63 was obtained in 92% yield. To effect the desired stereoselective variant, we ultimately arrived at the use of an anthracenyl derivative (i.e., R = 67). Several products that were obtained are shown in Figure 10 (i.e., 64–66), which highlight the use of heterocyclic arynes and cyclic alkynes as trapping agents, as well as a 7-membered β-ketoester.
Figure 10.
Selected substrate scope of α-arylation methodology.
Another attractive aspect of this reaction methodology is the one-pot variant shown in Figure 11. Treatment of ketoester 68 with chiral amine 67 yielded the corresponding enamine, which was not isolated. This intermediate was then subjected to silyl triflate 53 and CsF, followed by the addition of aqueous 1 M HCl. The product, ketoester 68, was isolated in 68% yield and 92% ee. Of note, amine 67 was recovered in 67% yield. Overall, this methodology provided a means to access stereodefined quaternary stereocenters by the interception of strained intermediates in intermolecular processes. As will be discussed later in this review, an elegant catalytic asymmetric variant of this methodology has subsequently been developed.120
Figure 11.
α-Arylation reaction demonstrated in one-pot.
The synthesis of natural products and their derivatives also provides an opportunity to use arynes in complex settings, as has been well demonstrated in the literature.14,16,33,34 With regard to several recent efforts, the Hoye group has used arynes generated from hexadehydro-Diels–Alder reactions with readily abundant natural products to access complex derivatives.121 Additionally, our laboratory has performed late-stage intermolecular aryne cycloadditions to access derivatives of strictosidine, the last common biosynthetic precursor to all monoterpene indole alkaloids.122 More commonly, natural products have been accessed through diastereoselective intramolecular aryne trappings of enantioenriched substrates. Early on, our laboratory used an “indolyne” cyclization to build the complex bridged bicyclic core of coveted welwitindolinone natural products.123−127 Subsequently, we targeted the tubingensin alkaloids, wherein we envisioned using an aryne cyclization to access a quaternary center.
Tubingensins A and B (70 and 71, respectively) are complex indole diterpenoids first isolated from Aspergillus tubingensis in 1989 (Figure 12).128,129 Both natural products feature disubstituted carbazole moieties. In tubingensin A (70), the carbazole is fused to a cis-decalin containing four contiguous stereocenters, two of which are vicinal quaternary stereocenters. Tubingensin B (71), however, contains a carbazole fused to a [3.2.2]-bridged bicycle containing five stereocenters, four of which are contiguous. As an additional layer of complexity, three of the stereocenters are quaternary, two of which are vicinal. Moreover, tubingensins A and B (70 and 71, respectively) possess antiviral activity against herpes simplex virus type 1 (HSV-1) along with pesticidal activity.128,129 At the time we began our efforts, there were no total syntheses of tubingensins A or B, although Li and Nicolaou reported an elegant route to (±)-70 in 2012.130
Figure 12.

Tubingensin alkaloids and natural products with quaternary stereocenters introduced using aryne chemistry.
Given the intriguing structural features of tubingensins A and B, our group proposed synthetic routes to these compounds during a “Molecule of the Month” (MOM) exercise in a 2009 group meeting. Several routes that would employ aryne intermediates were proposed, some of which were tested in the laboratory. Although our initial strategies proved unsuccessful in the laboratory, they helped us appreciate the challenge of establishing quaternary stereocenters in complex frameworks using arynes. Prior studies had largely focused on the use of arynes to access tertiary stereocenters.122,131−134 With regard to setting quaternary centers of natural products and drug candidates using arynes, notable precedent was available in the synthesis of crinine (72)135 and ibutamoren mesylate (73)136 (Figure 12). There were no such examples where scaffolds bearing vicinal quaternary stereocenters, like those seen in the tubingensin alkaloids, were accessed using aryne cyclizations.
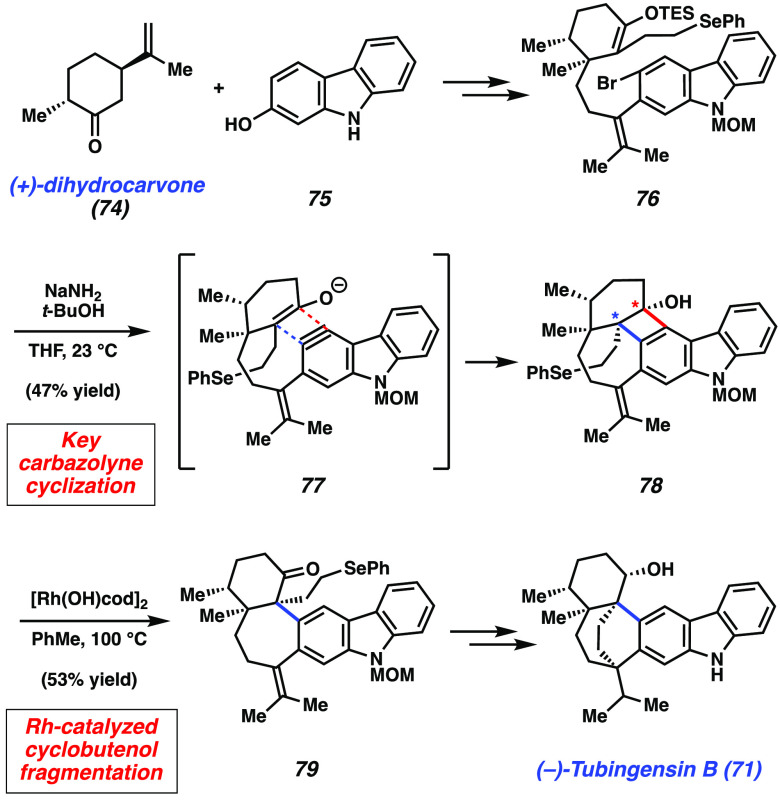
Following our initial total synthesis of (+)-tubingensin A (70),137 we were well-poised to address the more complex family member, (−)-tubingensin B (71). As summarized in Figure 13, (+)-dihydrocarvone (74), an abundant chiral terpene building block, and 2-hydroxycarbazole (75) were used as starting materials and elaborated to give carbazolyne precursor 76. Of note, 76 possesses all of the carbons that would be needed to complete the total synthesis. This key intermediate was subjected to sodium amide and tert-butanol in THF138,139 at 23 °C to effect carbazolyne cyclization. Although we were expecting to form a single C–C bond, we instead observed the formation of two new C–C bonds as evident by product cyclobutenol 78. Although this outcome was undesired, the formation of 78 highlights the structural complexity accessible using aryne chemistry. The reaction likely proceeds via a formal [2 + 2] cycloaddition (e.g., 77) and occurs with excellent diastereoselectivity. Nonetheless, this transformation served to establish the necessary vicinal quaternary stereocenter framework, construct the natural product’s seven-membered ring, and preserve the phenylselenide moiety necessary for a late-stage radical cyclization. To complete the synthesis, cyclobutenol 78 was subjected to Murakami’s Rh-catalyzed fragmentation conditions,140 which delivered the ketone 79 in 53% yield via fragmentation of the cyclobutenol ring. In turn, ketone 79 could be elaborated to (−)-tubingensin B (71).141 This effort demonstrates the utility of highly reactive aryne intermediates for the assembly of sterically congested carbon–carbon bonds in natural products.
Figure 13.
Overview of (−)-tubingensin B total synthesis.
Arynes in Asymmetric Catalysis
The aforementioned section highlights two key tactics for accessing enantioenriched products using aryne intermediates. More specifically, chiral auxiliaries, chiral reagents, or enantioenriched substrates have been the most common and successfully used to build intricate scaffolds and quaternary stereocenters. In turn, chemists have questioned: is it possible to leverage asymmetric catalysis in aryne trapping experiments? Such processes may be very challenging, as they could require two transiently generated intermediates,29,32 including a highly reactive aryne, to come together and undergo a productive reaction with stereocontrol. In addition to the fundamental scientific interest of using a fleeting aryne intermediate in a catalytic reaction, there are also practical advantages of asymmetric catalysis.142−144 We highlight three key studies in this emerging field involving the synthesis of helicenes (axial chirality)145,146 and the introduction of quaternary stereocenters (point chirality).120
A breakthrough involving the use of arynes in asymmetric catalysis was reported by Guitián and co-workers in 2006.145 More specifically, the authors studied [2 + 2 + 2] cycloadditions of arynes and alkynes to access enantioenriched helicenes. Accessing helicenes in an enantioselective manner has remained an important area of research79,147,148 and prior aryne-based approaches had given rise to racemic products.149,150 As shown in Figure 14, silyl triflate 80 was treated with CsF, alkyne 81, catalytic Pd2(dba)3, and BINAP to afford helicene 82. The reaction likely proceeds through a palladium-catalyzed cyclotrimerization of two in situ generated aryne intermediates (from silyl triflate 80) and alkyne 81. Although the yield was modest, helicene 82 was obtained in 67% ee. This study demonstrated an important proof-of-concept with regard to arynes and their use in asymmetric catalysis.
Figure 14.
Enantioselective synthesis of helicenes using arynes and asymmetric catalysis.
Building upon this pioneering study and their own prior work in this area,151 the Kamikawa group published an enantioselective synthesis of helicenes in 2020.146 For example, as shown in Figure 15, a cyclotrimerization was effected between aryne 83 and alkyne 84 using Pd2dba3·CHCl3, CsF, and (S)-QUINAP (86).152 This transformation features a design similar to the aforementioned example by Guitián and co-workers, and affords triple helicene (M,P,M)-85 in 49% yield and 96% ee. Notably, despite using racemic aryne precursor 83, both terminal helicenes exclusively formed the (M)-[5]-helicenyl moiety, which suggests a kinetic resolution or dynamic kinetic resolution occurs in the reaction. Overall, this study achieved the first enantioselective synthesis of a triple helicene, while also providing the first practical catalytic asymmetric aryne trapping reaction.
Figure 15.
Enantioselective synthesis of triple helicenes via the catalytic asymmetric trapping of arynes.
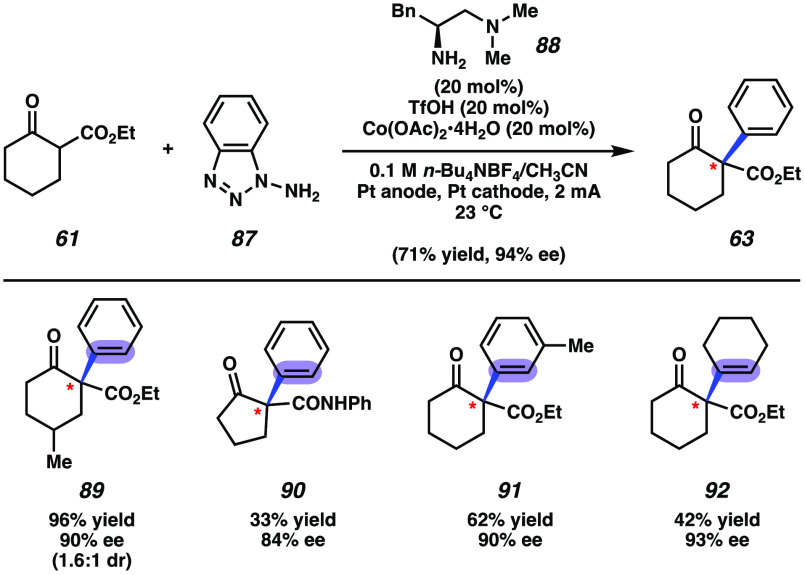
Also in 2020, the Luo group reported a pioneering study in the catalytic asymmetric trapping of arynes and related strained intermediates.120 Their methodology strategically blends organocatalysis and electrochemistry to achieve the α-functionalization of 1,3-dicarbonyl substrates and establish point chiral stereochemistry. For example, treatment of ketoester 61 with 1-aminobenzotriazole (87) and amine catalyst 88, under their optimal electrochemical oxidation conditions, afforded α-arylated ketoester 63 in 71% yield and 94% ee (Figure 16). Mechanistically, the reaction is thought to proceed by way of an intermediate enamine species, which reacts with the aryne generated by electrochemical oxidation of 87. The cobalt catalyst is proposed to bind the aryne triple bond and stabilize excess benzyne intermediate that is generated.153−155 Products 89–92 provide further examples of the products that were generated using this methodology. These studies demonstrate an electrochemical oxidation approach to access benzyne and related strained intermediates, which nicely complements prior studies involving the use of nonelectrochemical oxidation conditions for aryne generation.156−160 Moreover, this study demonstrates that arynes can be engaged in asymmetric catalysis to provide access to point chiral products in synthetical useful enantioselectivities. The ability to access stereodefined quaternary stereocenters is especially notable.
Figure 16.
Catalytic enantioselective α-arylation of 1,3-dicarbonyls.
Use of Strained Cyclic Allenes to Access Polycyclic Scaffolds
Whereas arynes and strained cyclic alkynes have been studied extensively, a related counterpart, strained cyclic allenes (i.e., 4, 93–94, Figure 17), have received significantly less attention. Of note, the discovery of 1,2-cyclohexadiene (4) by Wittig was reported in 1966,9 only 9 years after the validation of cyclohexyne (and 13 years after the validation of benzyne in 1953).14,27,31 Theoretical investigations of these species have been reported161−169 as well as synthetic advances, most notably by Christl.170−176 In a key finding, Guitián and co-workers developed Kobayashi precursors to strained cyclic allenes and demonstrated their utility in several transformations, such as [4 + 2] cycloadditions.177,178 Subsequently, our laboratory and West’s laboratory expanded the synthetic utility of Kobayashi cyclic allene precursors to encompass other intermolecular cycloadditions, especially (3 + 2) cycloadditions.179−182
Figure 17.
Strained cyclic allenes and representative trapping reactions.
Here, we feature selected recent efforts aimed at harnessing strained cyclic allenes to build complex polycyclic scaffolds bearing heteroatoms and sp3 centers. Generally speaking, these reactions proceed by treating silyl triflate precursors 95 with a fluoride source to generate cyclic allenes 96. In situ trapping with cycloaddition partners 18 provides adducts 97 (Figure 17). As will be discussed in the remaining sections of this Perspective, strained cyclic allene chemistry provides exciting opportunities for study related to regiochemistry, pertaining to which olefin of the allene undergoes reaction, and stereochemistry, due to the inherent chirality of cyclic allenes and generation of a new sp3 center.
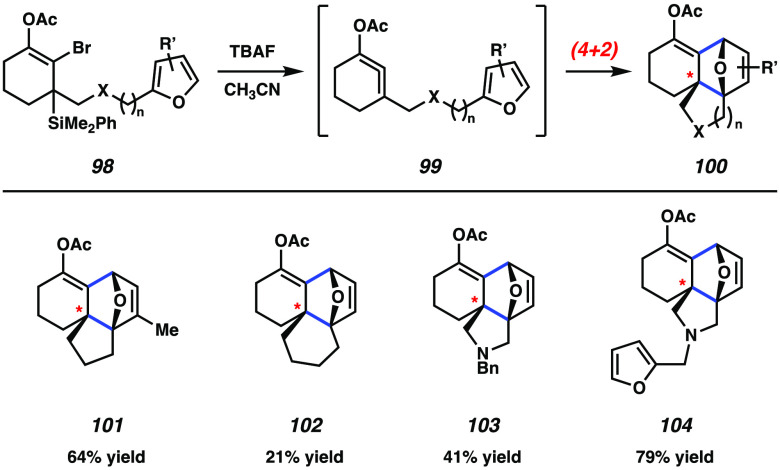
A striking example of cyclic allene chemistry was reported by West and co-workers in 2019, where in situ generated 1,2-cyclohexadienes underwent (4 + 2) cycloaddition with tethered furans to access complex tetracyclic products (Figure 18).183 Substrates 98 were subjected to TBAF to afford cycloadducts 100, presumably by way of cyclic allene intermediate 99. The products contain three stereocenters, set in a relative sense, including a quaternary center. Representative products 101 and 102 highlight that the linker length could be varied (n = 1 or 2). In addition, heteroatoms were tolerated in the linker, as shown by cycloadducts 103 and 104. Of note, the transformations proceeded diastereoselectively in favor of the endo product and regioselectively, with the latter being governed by the tether. This methodology provides the first examples of an intramolecular cycloaddition with a six-membered cyclic allene and showcases the structural complexity that is accessible using cyclic allene chemistry.
Figure 18.
Intramolecular Diels–Alder reactions afford tetracyclic products.
Our laboratory was particularly interested in heterocyclic allenes and the possibility of accessing them using Kobayashi-type precursors. Many heterocyclic allenes had been accessed previously, but never using a silyl triflate as the precursor.184−190 After developing syntheses of the appropriate precursors to azacyclic and oxacyclic allenes (i.e., 105 and 106, respectively), we demonstrated their use in cycloaddition reactions, with select results shown in Figure 19.191,192 Operationally, reactions are performed by treating the appropriate silyl triflate precursor (105, X = NCbz; or 106, X = O) with a trapping agent 18 in the presence of CsF at 23 °C, ultimately leading to cycloadducts 108. Three cycloadducts arising from azacyclic allene precursor 105 are shown reflective of (4 + 2), (3 + 2), and (2 + 2) cycloadditions (entries 1–3). The cycloadducts, 109, 111, and 112, are formed in good yield and useful diastereoselectivities (when applicable). Similarly, oxacyclic allene precursor 106 could be employed in (4 + 2), (3 + 2), and (2 + 2) cycloadditions, thus giving rise to cycloadducts 114, 116, and 118, respectively (entries 4–6). Overall, by varying the heterocyclic allene precursor and the trapping agent, one can now use strained cyclic allene chemistry as a strategy to synthesize structurally complex heterocyclic compounds.
Figure 19.
Representative azacyclic and oxacyclic allene trapping experiments.
Whereas the results shown in Figure 19 showcase reactions of unsubstituted strained cyclic allenes, another important facet of these compounds is observing and understanding what happens when substituents are present on the allene carbons in intermolecular trapping experiments. Our laboratory has probed this issue in the context of azacyclic allenes,191 with key results provided in Figure 20. Silyl triflate 119, 122, and 125 were accessed as precursors to the corresponding cyclic allenes 120, 123, and 126, in order to probe the influence of electron-donating and electron-withdrawing substituents (i.e., alkyl and ester groups, respectively). As shown by the formation of 121, cycloaddition occurs on the olefin of 120 distal to the methyl group, whereas the cycloaddition takes place on the olefin proximal to the ester of 123. When both methyl and ester substituents were present, cycloaddition occurs proximal to the ester and distal to the methyl of cyclic allene 126, as one might expect in this matched scenario (125 + 113 → 127). All three reactions proceeded in good yields and excellent diastereoselectivities to give structurally complex products, including two with quaternary centers (i.e., 124 and 127), further highlighting the synthetic utility of this methodology. In a collaboration with the Houk group at UCLA, a distortion-interaction analysis demonstrated that interaction energies, related to electron-factors, likely govern regioselectivity.191 With regard to diastereoselectivity, reactions proceed with endo selectivity with respect to the unreactive cyclic allene double bond.191,193 Given the synthetic utility of this methodology and the ability to understand the regiochemical and stereochemical outcomes, we expect this methodology will see increased usage in chemical synthesis. For example, Schreiber and co-workers have recently harnessed heterocyclic allene chemistry for the synthesis of DNA-encoded libraries.194
Figure 20.
Regioselectivity investigation of substituted azacyclic allenes.
Strained Cyclic Allenes in Enantioselective and Stereospecific Reactions
The aforementioned studies demonstrate that cyclic allenes can undergo regio- and diastereoselective trapping to give polycyclic heterocyclic products. In an emerging area, efforts have also been put forth to control absolute stereochemistry in reactions of strained cyclic allenes. Seminal studies performed by Christl and co-workers demonstrated that enantioenriched cyclic allenes can be generated and intercepted using the Skattebøl rearrangement.171,172 Our laboratory devised the alternative approach shown in Figure 21.191,192 Silyl triflates 128 would be prepared with control of absolute stereochemistry and subjected to mild fluoride-based conditions. This could lead to the transmission of stereochemical information to cyclic allenes 129, which in turn could undergo stereospecific trapping with 18 to yield cycloadducts 130. Of note, this overall process would proceed with the transfer of point chirality from 128 to 130, via the intermediacy of axially chiral intermediates (i.e., 129), with potential ablation of the sole stereocenter present in the substrate. This approach involving enantioenriched silyl triflate precursors had not been examined previously.
Figure 21.
Transfer of stereochemical information in allene cycloadditions.
As shown in Figure 22, the success of the aforementioned approach was validated in the context of both aza- and oxacyclic allenes. In the first example, silyl triflate (+)-119 was accessed in >99% ee by performing preparative chiral supercritical fluid chromatography (SFC) on a synthetic precursor. Under standard cyclic allene generation and trapping conditions, with diene 31 as the trapping agent, a Diels–Alder adduct was obtained in 98% ee (98% stereoretention).191 In the second example, silyl triflate (−)-132 was obtained in 81% ee. The absolute stereochemistry was introduced by performing asymmetric allylic alkylation195−199 of a ketone precursor in collaboration with Stoltz and co-workers, thus obviating the need for preparative chiral chromatography. Nonetheless, cyclic allene generation and trapping provided (+)-134 in 81% ee, reflective of >99% stereoretention.192 Collectively, these results demonstrate the feasibility of transferring stereochemical information from the silyl triflate to the cycloadduct through an axially chiral cyclic allene, while providing access to enantioenriched polycyclic products.
Figure 22.
Stereospecific cycloadditions of heterocyclic allenes using enantioenriched silyl triflates.
An exciting new approach to control the absolute stereochemistry in reactions of cyclic allenes was recently demonstrated. This strategy was motivated by an interest in cyclic allene racemization, in contrast to the aforementioned approach involving transfer of stereochemical information. A key result that prompted this study is shown in Figure 23. Silyl triflate 122, accessible in 87% ee (via chiral separation of a precursor), was subjected to cyclic allene generation and trapping.191 However, cycloadduct 135 was obtained racemically. This result suggested the importance of substituent effects and that racemization could plausibly outcompete cyclic allene trapping. Interestingly, computational studies suggest that ester-substituted cyclic allene 122 undergoes racemization readily, with an estimated barrier of only 14.1 kcal/mol.191 Thus, efforts were put forth toward developing an asymmetric trapping of cyclic allene intermediates using transition metal catalysis.
Figure 23.
Cyclic allene racemization.
Only one prior example of a transition metal-mediated strained cyclic allene reaction was known in the literature, which involved a cyclotrimerization process.177 Our laboratory has since developed two variants of asymmetric transition metal-catalyzed annulations of cyclic allenes, as shown in Figure 24. It was found that, under nickel-catalyzed annulation conditions,200 cyclic allene precursor 136 and benzotriazinone 137 afforded tricycle 139 in 85% yield and 94% ee.201 More recently, we demonstrated that reaction of silyl triflate 140 and iodopyridine 141 under palladium-catalyzed annulation conditions afforded tricycle 143 in 64% yield and 90% ee.202 These transformations are thought to proceed via the reaction of an in situ generated, racemic cyclic allene and a transient metallocycle formed by oxidative addition. Mechanistic details, including how absolute stereochemistry is introduced, have been proposed for the nickel-catalyzed annulation based on computations.201 As such, these methodologies not only provide an advance in strained cyclic allene chemistry but also are some of the few catalytic asymmetric reactions across the larger family of transiently generated cyclic intermediates (e.g., arynes, cyclic alkynes, cyclic allenes).
Figure 24.
Catalytic asymmetric reactions of cyclic allenes.
Outlook and Future Directions
The study of arynes, strained cyclic alkynes, and strained cyclic allenes has seen tremendous growth in recent years. As highlighted in this Perspective, such strained intermediates were contemplated as early as 1902 and validated 50+ years later. In the modern era, these species can be strategically employed in a host of impressive synthetic applications. The increase in practical use can be attributed to a combination of factors, including the mild conditions used for their generation, the commercial availability of silyl triflate precursors,203 and advances in our ability to understand and predict how strained cyclic intermediates will react in complexity-generating transformations.24−26
What does the future hold for arynes, cyclic alkynes, and cyclic allenes? We have shown in this Perspective how these transient species can be strategically leveraged to build complexity in the form of polycyclic scaffolds. Notably, many of newly developed methods discussed herein have potential applications in drug discovery and materials as well as in the synthesis of heterocycles, natural products, and sp3-rich compounds. Moreover, key advances to control absolute stereochemistry using asymmetric catalysis recently emerged and represent a particularly exciting area for future reaction development. As such, we curiously and enthusiastically await further advances from the synthetic community in the chemistry of arynes, strained cyclic alkynes, and strained cyclic allenes.
Acknowledgments
The authors thank the NIH-NIGMS (R01-GM132432 and R35-GM139593 to N.K.G. and T32-GM136614 for M.S.M.), the Trueblood Family (N.K.G.), the Foote family (S.M.A., L.G.W., M.S.M.), the California Tobacco-Related Disease Research Program (T29DT0359 to S.M.A.), the NSF (DGE-1650604 to L.G.W.), and the University of California, Los Angeles, for financial support. The Houk laboratory is gratefully acknowledged for their insight and collaborations throughout the studies described.
Author Contributions
† L.G.W. and M.S.M. contributed equally.
The authors declare no competing financial interest.
References
- Stoermer R.; Kahlert B. Ueber das 1- und 2-Brom-cumaron. Ber. Dtsch. Chem. Ges. 1902, 35, 1633–1640. 10.1002/cber.19020350286. [DOI] [Google Scholar]
- Reinecke M. G. Hetarynes. Tetrahedron 1982, 38, 427–498. 10.1016/0040-4020(82)80092-6. [DOI] [Google Scholar]
- Kauffmann T. The Hetarynes. Angew. Chem., Int. Ed. Engl. 1965, 4, 543–557. 10.1002/anie.196505431. [DOI] [Google Scholar]
- Kauffmann T.; Wirthwein R. Progress in the Hetaryne Field. Angew. Chem., Int. Ed. Engl. 1971, 10, 20–33. 10.1002/anie.197100201. [DOI] [Google Scholar]
- Roberts J. D.; Simmons H. E.; Carlsmith L. A.; Vaughan C. W. Rearrangment in the Reaction of Chlorobenzene-1-C14 with Potassium Amide. J. Am. Chem. Soc. 1953, 75, 3290–3291. 10.1021/ja01109a523. [DOI] [Google Scholar]
- Scardiglia F.; Roberts J. D. Evidence for Cyclohexyne as an Intermediate in the Coupling of Phenyllithium with 1-Chlorocyclohexene. Tetrahedron 1957, 1, 343–344. 10.1016/0040-4020(57)88011-9. [DOI] [Google Scholar]
- Wittig G. Fortschritte auf dem Gebiet der Organischen Aniono-Chemie. Angew. Chem. 1954, 66, 10–17. 10.1002/ange.19540660103. [DOI] [Google Scholar]
- Huisgen R.; Knorr R. Sind Die Benz-ine Verschiedener Provenienz Identischi?. Tetrahedron Lett. 1963, 4, 1017–1021. 10.1016/S0040-4039(01)90765-8. [DOI] [Google Scholar]
- Wittig G.; Fritze P. On the Intermediate Occurrence of 1,2-Cyclohexadiene. Angew. Chem., Int. Ed. Engl. 1966, 5, 846. 10.1002/anie.196608461. [DOI] [Google Scholar]
- The graph in Figure 1 is based on a SciFinder search for the research topics “benzyne” or “aryne” (accessed 2020-10-20).
- Mauger C. C.; Mignani G. A. An Efficient and Safe Procedure for the Large-Scale Pd-Catalyzed Hydrazonation of Aromatic Chlorides Using Buchwald Technology. Org. Process Res. Dev. 2004, 8, 1065–1071. 10.1021/op049832y. [DOI] [Google Scholar]
- Coe J. W.; Brooks P. R.; Wirtz M. C.; Bashore C. G.; Bianco K. E.; Vetelino M. G.; Arnold E. P.; Lebel L. A.; Fox C. B.; Tingley F. D.; Schulz D. W.; Davis T. I.; Sands S. B.; Mansbach R. S.; Rollema H.; O’Neill B. T. 3,5-Bicyclic Aryl Piperidines: A Novel Class of α4β2 Neuronal Receptor Partial Agonist for Smoking Cessation. Bioorg. Med. Chem. Lett. 2005, 15, 4889–4897. 10.1016/j.bmcl.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Schleth F.; Vettiger T.; Rommel M.; Tobler H.. Process for the Preparation of Pyrazole Carboxylic Acid Amides. International patent WO2011131544A1, 2011.
- Tadross P. M.; Stoltz B. M. A Comprehensive History of Arynes in Natural Product Total Synthesis. Chem. Rev. 2012, 112, 3550–3577. 10.1021/cr200478h. [DOI] [PubMed] [Google Scholar]
- Gampe C. M.; Carreira E. M. Arynes and Cyclohexyne in Natural Product Synthesis. Angew. Chem., Int. Ed. 2012, 51, 3766–3778. 10.1002/anie.201107485. [DOI] [PubMed] [Google Scholar]
- Takikawa H.; Nishii A.; Sakai T.; Suzuki K. Aryne-Based Strategy in the Total Synthesis of Naturally Occurring Polycyclic Compounds. Chem. Soc. Rev. 2018, 47, 8030–8056. 10.1039/C8CS00350E. [DOI] [PubMed] [Google Scholar]
- Kou K. G. M.; Pflueger J. J.; Kiho T.; Morrill L. C.; Fisher E. L.; Clagg K.; Lebold T. P.; Kisunzu J. K.; Sarpong R. A Benzyne Insertion Approach to Hetisine-Type Diterpenoid Alkaloids: Synthesis of Cossonidine (Davisine). J. Am. Chem. Soc. 2018, 140, 8105–8109. 10.1021/jacs.8b05043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gampe C. M.; Carreira E. M. Total Syntheses of Guanacastepenes N and O. Angew. Chem., Int. Ed. 2011, 50, 2962–2965. 10.1002/anie.201007644. [DOI] [PubMed] [Google Scholar]
- Himeshima Y.; Sonoda T.; Kobayashi H. Fluoride-Induced 1,2-Elimination of O-Trimethylsilylphenyl Triflate to Benzyne Under Mild Conditions. Chem. Lett. 1983, 12, 1211–1214. 10.1246/cl.1983.1211. [DOI] [Google Scholar]
- Shi J.; Li L.; Li Y. o-Silylaryl Triflates: A Journey of Kobayashi Aryne Precursors. Chem. Rev. 2021, 121, 3892–4044. 10.1021/acs.chemrev.0c01011. [DOI] [PubMed] [Google Scholar]
- Kelleghan A. V.; Busacca C. A.; Sarvestani M.; Volchkov I.; Medina J. M.; Garg N. K. Safety Assessment of Benzyne Generation from a Silyl Triflate Precursor. Org. Lett. 2020, 22, 1665–1669. 10.1021/acs.orglett.0c00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For the use of silyl tosylates as strained intermediate precursors, see the following and references therein:; McVeigh M. S.; Kelleghan A. V.; Yamano M. M.; Knapp R. R.; Garg N. K. Silyl Tosylate Precursors to Cyclohexyne, 1,2-Cyclohexadiene, and 1,2-Cycloheptadiene. Org. Lett. 2020, 22, 4500–4504. 10.1021/acs.orglett.0c01510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine Nathel N. F.; Morrill L. A.; Mayr H.; Garg N. K. Quantification of the Electrophilicity of Benzyne and Related Intermediates. J. Am. Chem. Soc. 2016, 138, 10402–10405. 10.1021/jacs.6b06216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina J. M.; Mackey J. L.; Garg N. K.; Houk K. N. The Role of Aryne Distortions, Steric Effects, and Charges in Regioselectivities of Aryne Reactions. J. Am. Chem. Soc. 2014, 136, 15798–15805. 10.1021/ja5099935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong P. H.-Y.; Paton R. S.; Bronner S. M.; Im G.-Y. J.; Garg N. K.; Houk K. N. Indolyne and Aryne Distortions and Nucleophilic Regioselectivities. J. Am. Chem. Soc. 2010, 132, 1267–1269. 10.1021/ja9098643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im G.-Y. J.; Bronner S. M.; Goetz A. E.; Paton R. S.; Cheong P. H.-Y.; Houk K. N.; Garg N. K. Indolyne Experimental and Computational Studies: Synthetic Applications and Origins of Selectivities of Nucleophilic Additions. J. Am. Chem. Soc. 2010, 132, 17933–17944. 10.1021/ja1086485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig G. 1,2-Dehydrobenzene. Angew. Chem., Int. Ed. Engl. 1965, 4, 731–737. 10.1002/anie.196507311. [DOI] [Google Scholar]
- Wenk H. H.; Winkler M.; Sander W. One Century of Aryne Chemistry. Angew. Chem., Int. Ed. 2003, 42, 502–528. 10.1002/anie.200390151. [DOI] [PubMed] [Google Scholar]
- Dhokale R. A.; Mhaske S. B. Transition-Metal-Catalyzed Reactions Involving Arynes. Synthesis 2018, 50, 1–16. 10.1055/s-0036-1589517. [DOI] [Google Scholar]
- Bhunia A.; Yetra S. R.; Biju A. T. Recent Advances in Transition-Metal-Free Carbon–Carbon and Carbon–Heteroatom Bond-Forming Reactions Using Arynes. Chem. Soc. Rev. 2012, 41, 3140–3152. 10.1039/c2cs15310f. [DOI] [PubMed] [Google Scholar]
- Dubrovskiy A. V.; Markina N. A.; Larock R. C. Use of Benzyne for the Synthesis of Heterocycles. Org. Biomol. Chem. 2013, 11, 191–218. 10.1039/C2OB26673C. [DOI] [PubMed] [Google Scholar]
- Guitián E.; Pérez D.; Peña D. Palladium-Catalyzed Cycloaddition Reactions of Arynes. Top. Organomet. Chem. 2005, 14, 109–146. 10.1007/b104128. [DOI] [Google Scholar]
- Bronner S. M.; Goetz A. E.; Garg N. K. Understanding and Modulating Indolyne Regioselectivities. Synlett 2011, 2011, 2599–2604. 10.1055/s-0031-1289561. [DOI] [Google Scholar]
- Goetz A. E.; Garg N. K. Enabling the Use of Heterocyclic Arynes in Chemical Synthesis. J. Org. Chem. 2014, 79, 846–851. 10.1021/jo402723e. [DOI] [PubMed] [Google Scholar]
- Goetz A. E.; Shah T. K.; Garg N. K. Pyridynes and Indolynes as Building Blocks for Functionalized Heterocycles and Natural Products. Chem. Commun. 2015, 51, 34–45. 10.1039/C4CC06445C. [DOI] [PubMed] [Google Scholar]
- Although outside of the scope of this Perspective, an important area of modern aryne chemistry is the hexadehydro-Diels–Alder (HDDA) reaction. For a review, see:; Diamond O. J.; Marder T. B. Methodology and Applications of the Hexadehydro-Diels–Alder (HDDA) reaction. Org. Chem. Front. 2017, 4, 891–910. 10.1039/C7QO00071E. [DOI] [Google Scholar]
- Another important field outside of the scope of this Perspective involves biorthogonal reactions of cyclic alkynes in medium sized rings. For a pertinent review, see:; Chupakhin E. G.; Krasavin M. Y. Achievements in the Synthesis of Cyclooctynes for Ring Strain-Promoted [3 + 2] Azide-Alkyne Cycloaddition. Chem. Heterocycl. Compd. 2018, 54, 483–501. 10.1007/s10593-018-2295-x. [DOI] [Google Scholar]
- Gilchrist T. L. Synthesis of Aromatic Heterocycles. J. Chem. Soc., Perkin Trans. 1 1999, 2849–2866. 10.1039/a808162j. [DOI] [Google Scholar]
- Lovering F.; Bikker J.; Humblet C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- McGrath N. A.; Brichacek M.; Njardarson J. T. A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives. J. Chem. Educ. 2010, 87, 1348–1349. 10.1021/ed1003806. [DOI] [Google Scholar]
- Ritchie T. J.; Macdonald S. J. F. The Impact of Aromatic Ring Count on Compound Developability – Are Too Many Aromatic Rings a Liability in Drug Design?. Drug Discovery Today 2009, 14, 1011–1020. 10.1016/j.drudis.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Dinges J.; Lamberth C.. Bioactive Heterocyclic Compounds Classes: Agrochemicals; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Lovering F. Escape from Flatland 2: Complexity and Promiscuity. MedChemComm 2013, 4, 515–519. 10.1039/c2md20347b. [DOI] [Google Scholar]
- Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- Delost D. M.; Smith D. T.; Anderson B. J.; Njardarson J. T. From Oxiranes to Oligomers: Architectures of U.S. FDA Approved Pharmaceuticals Containing Oxygen Heterocycles. J. Med. Chem. 2018, 61, 10996–11020. 10.1021/acs.jmedchem.8b00876. [DOI] [PubMed] [Google Scholar]
- Medina J. M.; McMahon T. C.; Jimeńez-Oseś G.; Houk K. N.; Garg N. K. Cycloadditions of Cyclohexynes and Cyclopentyne. J. Am. Chem. Soc. 2014, 136, 14706–14709. 10.1021/ja508635v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlais S. F.; Danheiser R. L. N-Tosyl-3-azacyclohexyne. Synthesis and Chemistry of a Strained Cyclic Ynamide. J. Am. Chem. Soc. 2014, 136, 15489–15492. 10.1021/ja509055r. [DOI] [PubMed] [Google Scholar]
- McMahon T. C.; Medina J. M.; Yang Y.-F.; Simmons B. J.; Houk K. N.; Garg N. K. Generation and Regioselective Trapping of a 3,4-Piperidyne for the Synthesis of Functionalized Heterocycles. J. Am. Chem. Soc. 2015, 137, 4082–4085. 10.1021/jacs.5b01589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah T. K.; Medina J. M.; Garg N. K. Expanding the Strained Alkyne Toolbox: Generation and Utility of Oxygen-Containing Strained Alkynes. J. Am. Chem. Soc. 2016, 138, 4948–4954. 10.1021/jacs.6b01986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz A. E.; Garg N. K. Regioselective Reactions of 3,4-Pyridynes Enabled by the Aryne Distortion Model. Nat. Chem. 2013, 5, 54–60. 10.1038/nchem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enamorado M. F.; Ondachi P. W.; Comins D. L. A Five-Step Synthesis of (S)-Macrostomine from (S)-Nicotine. Org. Lett. 2010, 12, 4513–4515. 10.1021/ol101887b. [DOI] [PubMed] [Google Scholar]
- Saito N.; Nakamura K.-i.; Shibano S.; Ide S.; Minami M.; Sato Y. Addition of Cyclic Ureas and 1-Methyl-2-oxazolidone to Pyridynes: A New Approach to Pyridodiazepines, Pyridodiazocines, and Pyridooxazepines. Org. Lett. 2013, 15, 386–389. 10.1021/ol303352q. [DOI] [PubMed] [Google Scholar]
- Saito N.; Nakamura K.-i.; Sato Y. 1,3-Dipolar Cycloaddition of Pyridynes and Azides: Concise Synthesis of Triazolopyridines. Heterocycles 2014, 88, 929–937. 10.3987/COM-13-S(S)86. [DOI] [Google Scholar]
- Levine R.; Leake W. W. Rearrangement in the Reaction of 3-Bromopyridine with Sodium Amide and Sodioacetophenone. Science 1955, 121, 780. 10.1126/science.121.3152.780. [DOI] [PubMed] [Google Scholar]
- Vaca M. J. A.; Gil J. I. A.; Chrovian C. C.; Coate H. R.; Angelis M. D.; Dvorak C. A.; Gelin C. F.; Letavic M. A.; Savall B. M.; Soyode-Johnson A.; Stenne B. M.; Swanson D. M.. P2X7 Modulators. US20140275015, September 18, 2014.
- Ashton W. T.; Caldwell C. G.; Mathvink R. J.; Ok H. O.; Reigle L. B.; Weber A. E.. 3-Amino-4-phenylbutanoic Acid Derivatives as Dipeptidyl Peptidase Inhibitors for the Treatment or Prevention of Diabetes. WO2004064778, August 5, 2004.
- Thompson F.; Maillet P.; Damiano T.; Cherrier M. P.; Clerc F.. New Tetrahydropyrazolo(3,4-c)pyridine Derivatives are Kinase Modulators Used Especially for Treating Cancer. FR2857362, Jan 14, 2005. [Google Scholar]
- Blatt L. M.; Seiwert S.; Beigelman L.; Kercher T.; Kennedy A. L.; Andrews S. W.. Novel Inhibitors of Hepatitis C Virus Replication. WO2008005511, January 10, 2008.
- Facchetti A. π-Conjugated Polymers for Organic Electronics and Photovoltaic Cell Applications. Chem. Mater. 2011, 23, 733–758. 10.1021/cm102419z. [DOI] [Google Scholar]
- Forrest S. R. The Path to Ubiquitous and Low-Cost Organic Electronic Appliances on Plastic. Nature 2004, 428, 911–918. 10.1038/nature02498. [DOI] [PubMed] [Google Scholar]
- Kulkarni A. P.; Tonzola C. J.; Babel A.; Jenekhe S. A. Electron Transport Materials for Organic Light-Emitting Diodes. Chem. Mater. 2004, 16, 4556–4573. 10.1021/cm049473l. [DOI] [Google Scholar]
- Duan L.; Hou L.; Lee T.-W.; Qiao J.; Zhang D.; Dong G.; Wang L.; Qiu Y. Solution Processable Small Molecules for Organic Light-Emitting Diodes. J. Mater. Chem. 2010, 20, 6392–6407. 10.1039/b926348a. [DOI] [Google Scholar]
- Tao Y.; Yang C.; Qin J. Organic Host Materials for Phosphorescent Organic Light-Emitting Diodes. Chem. Soc. Rev. 2011, 40, 2943–2970. 10.1039/c0cs00160k. [DOI] [PubMed] [Google Scholar]
- Yu T.; Liu L.; Xie Z.; Ma Y. Progress in Small-Molecule Luminescent Materials for Organic Light-Emitting Diodes. Sci. China: Chem. 2015, 58, 907–915. 10.1007/s11426-015-5409-7. [DOI] [Google Scholar]
- Mas-Torrent M.; Rovira C. Novel Small Molecules for Organic Field-Effect Transistors: Towards Processability and High Performance. Chem. Soc. Rev. 2008, 37, 827–838. 10.1039/b614393h. [DOI] [PubMed] [Google Scholar]
- Allard S.; Forster M.; Souharce B.; Thiem H.; Scherf U. Organic Semiconductors for Solution-Processable Field-Effect Transistors (OFETs). Angew. Chem., Int. Ed. 2008, 47, 4070–4098. 10.1002/anie.200701920. [DOI] [PubMed] [Google Scholar]
- Sirringhaus H. 25th Anniversary Article: Organic Field-Effect Transistors: The Path Beyond Amorphous Silicon. Adv. Mater. 2014, 26, 1319–1335. 10.1002/adma.201304346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back J. Y.; Kim Y.; An T. K.; Kang M. S.; Kwon S.-K.; Park C. E.; Kim Y.-H. Synthesis and Electrical Properties of Novel Oligomer Semiconductors for Organic Field-Effect Transistors (OFETs): Asymmetrically End-Capped Acene-heteroacene Conjugated Oligomers. Dyes Pigm. 2015, 112, 220–226. 10.1016/j.dyepig.2014.07.008. [DOI] [Google Scholar]
- Mishra A.; Bäuerle P. Small Molecule Organic Semiconductors on the Move: Promises for Future Solar Energy Technology. Angew. Chem., Int. Ed. 2012, 51, 2020–2067. 10.1002/anie.201102326. [DOI] [PubMed] [Google Scholar]
- Lin Y.; Li Y.; Zhan X. Small Molecule Semiconductors for High-Efficiency Organic Photovoltaics. Chem. Soc. Rev. 2012, 41, 4245–4272. 10.1039/c2cs15313k. [DOI] [PubMed] [Google Scholar]
- Roncali J.; Leriche P.; Blanchard P. Molecular Materials for Organic Photovoltaics: Small is Beautiful. Adv. Mater. 2014, 26, 3821–3838. 10.1002/adma.201305999. [DOI] [PubMed] [Google Scholar]
- Haller A.; Guyot A. Sur le γ-diphenylanthracene et le Dihydrure de γ-Diphenylanthracene Symetriques. C. R. Acad. Sci. 1904, 138, 1251–1254. [Google Scholar]
- Carmel J. H.; Ward J. S.; Cooper M. M. A Glowing Recommendation: A Project-Based Cooperative Laboratory Activity to Promote Use of the Scientific and Engineering Practices. J. Chem. Educ. 2017, 94, 626–631. 10.1021/acs.jchemed.6b00628. [DOI] [Google Scholar]
- Jo W. J.; Kim K.-H.; No H. C.; Shin D.-Y.; Oh S.-J.; Son J.-H.; Kim Y.-H.; Cho Y.-K.; Zhao Q.-H.; Lee K.-H.; Oh H.-Y.; Kwon S.-K. High Efficient Organic Light Emitting Diodes Using New 9,10-Diphenylanthracene Derivatives Containing Bulky Substituents on 2,6-Position. Synth. Met. 2009, 159, 1359–1364. 10.1016/j.synthmet.2009.03.007. [DOI] [Google Scholar]
- Schmidt H.; Schultz G. II. Ueber Diphenylbenzole. Justus Liebigs Ann. Chem. 1880, 203, 118–137. 10.1002/jlac.18802030107. [DOI] [Google Scholar]
- Markiewicz J. T.; Wudl F. Perylene, Oligorylenes, and Aza-Analogs. ACS Appl. Mater. Interfaces 2015, 7, 28063–28085. 10.1021/acsami.5b02243. [DOI] [PubMed] [Google Scholar]
- Anthony J. E. Functionalized Acenes and Heteroacenes for Organic Electronics. Chem. Rev. 2006, 106, 5028–5048. 10.1021/cr050966z. [DOI] [PubMed] [Google Scholar]
- Stępień M.; Gońka E.; Żyła M.; Sprutta N. Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds: Synthetic Routes, Properties, and Applications. Chem. Rev. 2017, 117, 3479–3716. 10.1021/acs.chemrev.6b00076. [DOI] [PubMed] [Google Scholar]
- Shen Y.; Chen C. F. Helicenes: Synthesis and Applications. Chem. Rev. 2012, 112, 1463–1535. 10.1021/cr200087r. [DOI] [PubMed] [Google Scholar]
- Lin J. B.; Shah T. K.; Goetz A. E.; Garg N. K.; Houk K. N. Conjugated Trimeric Scaffolds Accessible from Indolyne Cyclotrimerizations: Synthesis, Structures, and Electronic Properties. J. Am. Chem. Soc. 2017, 139, 10447–10455. 10.1021/jacs.7b05317. [DOI] [PubMed] [Google Scholar]
- Peña D.; Escudero S.; Pérez D.; Guitián E.; Castedo L. Efficient Palladium-Catalyzed Cyclotrimerization of Arynes: Synthesis of Triphenylenes. Angew. Chem., Int. Ed. 1998, 37, 2659–2661. . [DOI] [PubMed] [Google Scholar]
- Ji L.; Fang Q.; Yuan M.-S.; Liu Z.-Q.; Shen Y.-X.; Chen H.-F. Switching High Two-Photon Efficiency: From 3,8,13-Substituted Triindole Derivatives to Their 2,7,12-Isomers. Org. Lett. 2010, 12, 5192–5195. 10.1021/ol102057t. [DOI] [PubMed] [Google Scholar]
- Goḿez-Lor B.; Alonso B.; Omenat A.; Serrano J. L. Electroactive C3 Symmetric Discotic Liquid-Crystalline Triindoles. Chem. Commun. 2006, 5012–5014. 10.1039/B611965D. [DOI] [PubMed] [Google Scholar]
- Talarico M.; Termine R.; García-Frutos E. M.; Omenat A.; Serrano J. L.; Goḿez-Lor B.; Golemme A. New Electrode-Friendly Triindole Columnar Phases with High Hole Mobility. Chem. Mater. 2008, 20, 6589–6591. 10.1021/cm8020512. [DOI] [Google Scholar]
- Su P.-Y.; Huang L.-B.; Liu J.-M.; Chen Y.-F.; Xiao L.-M.; Kuang D.-B.; Mayor M.; Su C.-Y. A Multifunctional Poly-N-vinylcarbazole Interlayer in Perovskite Solar Cells for High Stability and Efficiency: a Test with New Triazatruxene-based Hole Transporting Materials. J. Mater. Chem. A 2017, 5, 1913–1918. 10.1039/C6TA09314K. [DOI] [Google Scholar]
- Bajpai M.; Yadav N.; Kumar S.; Srivastava R.; Dhar R. Incorporation of Liquid Crystalline Triphenylene Derivative in Bulk Heterojunction Solar Cell with Molybdenum Oxide as Buffer Layer for Improved Efficiency. Liq. Cryst. 2016, 43, 928–936. 10.1080/02678292.2016.1149239. [DOI] [Google Scholar]
- Ramos F. J.; Rakstys K.; Kazim S.; Graẗzel M.; Nazeeruddin M. K.; Ahmad S. Rational Design of Triazatruxene-based Hole Conductors for Perovskite Solar Cells. RSC Adv. 2015, 5, 53426–53432. 10.1039/C5RA06876B. [DOI] [Google Scholar]
- Darzi E. R.; Barber J. S.; Garg N. K. Cyclic Alkyne Approach to Heteroatom-Containing Polycyclic Aromatic Hydrocarbon Scaffolds. Angew. Chem., Int. Ed. 2019, 58, 9419–9424. 10.1002/anie.201903060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steglich W.; Buschmann E.; Gansen G.; Wilschowitz L. Herstellung und Reaktionen von 2,5-Diphenyl-6-oxo-1,3,4-oxadiazin. Synthesis 1977, 1977, 252–253. 10.1055/s-1977-24339. [DOI] [Google Scholar]
- Miao Q.; Chi X.; Xiao S.; Zeis R.; Lefenfeld M.; Siegrist T.; Steigerwald M. L.; Nuckolls C. Organization of Acenes with a Cruciform Assembly Motif. J. Am. Chem. Soc. 2006, 128, 1340–1345. 10.1021/ja0570786. [DOI] [PubMed] [Google Scholar]
- Chun D.; Cheng Y.; Wudl F. The Most Stable and Fully Characterized Functionalized Heptacene. Angew. Chem., Int. Ed. 2008, 47, 8380–8385. 10.1002/anie.200803345. [DOI] [PubMed] [Google Scholar]
- Rickborn B. The Retro-Diels–Alder Reaction Part II. Dienophiles with One or More Heteroatom. Org. React. 1998, 53, 223–629. 10.1002/0471264180.or053.02. [DOI] [Google Scholar]
- Tîntas M. L.; Diac A. P.; Soran A.; Terec A.; Grosu I.; Bogdan E. Structural Characterization of New 2-Aryl-5-Phenyl-1,3,4-Oxadiazin-6-ones and their N-Aroylhydrazone Precursors. J. Mol. Struct. 2014, 1058, 106–113. 10.1016/j.molstruc.2013.11.005. [DOI] [Google Scholar]
- Liu X.; Liu J.; Zheng B.; Yan L.; Dai J.; Zhuang Z.; Du J.; Guo Y.; Xiao D. N-Doped Carbon Dots: Green and Efficient Synthesis on a Large-Scale and Their Application in Fluorescent pH Sensing. New J. Chem. 2017, 41, 10607–10612. 10.1039/C7NJ01889D. [DOI] [Google Scholar]
- Ma Q.-J.; Li H.-P.; Yang F.; Zhang J.; Wu X.-F.; Bai Y.; Li X.-F. A Fluorescent Sensor for Low pH Values Based on a Covalently Immobilized Rhodamine–Napthalimide Conjugate. Sens. Actuators, B 2012, 166, 68–74. 10.1016/j.snb.2011.12.025. [DOI] [Google Scholar]
- Tan L.; Mo S.; Fang B.; Cheng W.; Yin M. Dual Fluorescence Switching of a Rhodamine 6G-Naphthalimide Conjugate with High Contrast in the Solid State. J. Mater. Chem. C 2018, 6, 10270–10275. 10.1039/C8TC03654C. [DOI] [Google Scholar]
- Meguro T.; Chen S.; Kanemoto K.; Yoshida S.; Hosoya T. Modular Synthesis of Unsymmetrical Doubly-Ring-Fused Benzene Derivatives Based on a Sequential Ring Construction Strategy Using Oxadiazinones as a Platform Molecule. Chem. Lett. 2019, 48, 582–585. 10.1246/cl.190118. [DOI] [Google Scholar]
- Ramirez M.; Darzi E. R.; Donaldson J. S.; Houk K. N.; Garg N. K. Cycloaddition Cascades of Strained Alkynes and Oxadiazinones. Angew. Chem., Int. Ed. 2021, 10.1002/anie.202105244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockendorff C.; Sahli S.; Olsen M.; Milhau L.; Lautens M. Synthesis of Dihydronaphthalenes via Aryne Diels–Alder Reactions: Scope and Diastereoselectivity. J. Am. Chem. Soc. 2005, 127, 15028–15029. 10.1021/ja055498p. [DOI] [PubMed] [Google Scholar]
- Webster R.; Lautens M. Conformational Effects in Diastereoselective Aryne Diels–Alder Reactions: Synthesis of Benzo-Fused [2.2.1] Heterobicycles. Org. Lett. 2009, 11, 4688–4691. 10.1021/ol9019869. [DOI] [PubMed] [Google Scholar]
- Jones E. P.; Jones P.; Barrett A. G. M. Asymmetric Synthesis of α-Aryl Amino Acids; Aryne-Mediated Diastereoselective Arylation. Org. Lett. 2011, 13, 1012–1015. 10.1021/ol1030469. [DOI] [PubMed] [Google Scholar]
- Jones E. P.; Jones P.; White A. J. P.; Barrett A. G. M. Asymmetric Synthesis of Quaternary Aryl Amino Acid Derivatives via a Three-Component Aryne Coupling Reaction. Beilstein. Beilstein J. Org. Chem. 2011, 7, 1570–1576. 10.3762/bjoc.7.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quasdorf K. W.; Overman L. E. Catalytic Enantioselective Synthesis of Quaternary Carbon Stereocentres. Nature 2014, 516, 181–191. 10.1038/nature14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Han S.-J.; Liu W.-B.; Stoltz B. M. Catalytic Enantioselective Construction of Quaternary Stereocenters: Assembly of Key Building Blocks for the Synthesis of Biologically Active Molecules. Acc. Chem. Res. 2015, 48, 740–751. 10.1021/ar5004658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockley S. E.; Holder J. C.; Stoltz B. M. Palladium-Catalyzed Asymmetric Conjugate Addition of Arylboronic Acids to α,β-Unsaturated Cyclic Electrophiles. Org. Process Res. Dev. 2015, 19, 974–981. 10.1021/acs.oprd.5b00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X.-P.; Cao Z.-Y.; Wang Y.-H.; Zhou F.; Zhou J. Catalytic Enantioselective Desymmetrization Reactions to All-Carbon Quaternary Stereocenters. Chem. Rev. 2016, 116, 7330–7396. 10.1021/acs.chemrev.6b00094. [DOI] [PubMed] [Google Scholar]
- Picazo E.; Anthony S. M.; Giroud M.; Simon A.; Miller M. A.; Houk K. N.; Garg N. K. Arynes and Cyclic Alkynes as Synthetic Building Blocks for Stereodefined Quaternary Centers. J. Am. Chem. Soc. 2018, 140, 7605–7610. 10.1021/jacs.8b02875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambar U. K.; Stoltz B. M. The Direct Acyl-Alkylation of Arynes. J. Am. Chem. Soc. 2005, 127, 5340–5341. 10.1021/ja050859m. [DOI] [PubMed] [Google Scholar]
- Altman R. A.; Hyde A. M.; Huang X.; Buchwald S. L. Orthogonal Pd- and Cu-Based Catalyst Systems for C- and N-Arylation of Oxindoles. J. Am. Chem. Soc. 2008, 130, 9613–9620. 10.1021/ja803179s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. M.; Altman R. A.; Buchwald S. L. Palladium-Catalyzed Enantioselective α-Arylation and α-Vinylation of Oxindoles Facilitated by an Axially Chiral P-Stereogenic Ligand. J. Am. Chem. Soc. 2009, 131, 9900–9901. 10.1021/ja903880q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P.-F.; Buchwald S. L. Continuous-Flow Synthesis of 3,3-Disubstituted Oxindoles by a Palladium-Catalyzed α-Arylation/Alkylation Sequence. Angew. Chem., Int. Ed. 2011, 50, 6396–6400. 10.1002/anie.201102401. [DOI] [PubMed] [Google Scholar]
- Beringer F. M.; Forgione P. S.; Yudis M. D. Diaryliodonium salts—XII: The Phenylation of Dimedone, Dibenzoylmethane and Tribenzoylmethane. Tetrahedron 1960, 8, 49–63. 10.1016/S0040-4020(01)93330-7. [DOI] [Google Scholar]
- Ochiai M.; Kitagawa Y.; Takayama N.; Takaoka Y.; Shiro M. Synthesis of Chiral Diaryliodonium Salts, 1,1′-Binaphthyl-2-yl(phenyl)iodonium Tetrafluoroborates: Asymmetric α-Phenylation of β-Keto Ester Enolates. J. Am. Chem. Soc. 1999, 121, 9233–9234. 10.1021/ja992236c. [DOI] [Google Scholar]
- Oh C. H.; Kim J. S.; Jung H. H. Highly Efficient Arylation of Malonates with Diaryliodonium Salts. J. Org. Chem. 1999, 64, 1338–1340. 10.1021/jo981065b. [DOI] [Google Scholar]
- Xie X.; Chen Y.; Ma D. Enantioselective Arylation of 2-Methylacetoacetates Catalyzed by CuI/trans-4-Hydroxy-L-proline at Low Reaction Temperatures. J. Am. Chem. Soc. 2006, 128, 16050–16051. 10.1021/ja066991j. [DOI] [PubMed] [Google Scholar]
- Beare M. A.; Hartwig J. F. Palladium-Catalyzed Arylation of Malonates and Cyanoesters Using Sterically Hindered Trialkyl- and Ferrocenyldialkylphosphine Ligands. J. Org. Chem. 2002, 67, 541–555. 10.1021/jo016226h. [DOI] [PubMed] [Google Scholar]
- For pioneering studies of racemic enamine arylations, see:; Kuehne M. E. The Arylation of Enamines. J. Am. Chem. Soc. 1962, 84, 837–847. 10.1021/ja00864a032. [DOI] [Google Scholar]
- For the α-arylation of enamines with arynes to give functionalized achiral enamines, see ref (119) and the following:; Ramtohul Y. K.; Chartrand A. Direct C-Arylation of β-Enamino Esters and Ketones with Arynes. Org. Lett. 2007, 9, 1029–1032. 10.1021/ol063057k. [DOI] [PubMed] [Google Scholar]
- Li R.; Wang X.; Wei Z.; Wu C.; Shi F. Reaction of Arynes with Vinylogous Amides: Nucleophilic Addition to the ortho-Quinodimethide Intermediate. Org. Lett. 2013, 15, 4366–4369. 10.1021/ol4018968. [DOI] [PubMed] [Google Scholar]
- Li L.; Li Y.; Fu N.; Zhang L.; Luo S. Catalytic Asymmetric Electrochemical α-Arylation of Cyclic β-Ketocarbonyls with Anodic Benzyne Intermediates. Angew. Chem., Int. Ed. 2020, 59, 14347–14351. 10.1002/anie.202006016. [DOI] [PubMed] [Google Scholar]
- Ross S. P.; Hoye T. R. Reactions of Hexadehydro-Diels–Alder Benzynes with Structurally Complex Multifunctional Natural Products. Nat. Chem. 2017, 9, 523–530. 10.1038/nchem.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony S. M.; Tona V.; Zou Y.; Morrill L. A.; Billingsley J. M.; Lim M.; Tang Y.; Houk K. N.; Garg N. K. Total Synthesis of (−)-Strictosidine and Interception of Aryne Natural Product Derivatives “Strictosidyne” and “Strictosamidyne”. J. Am. Chem. Soc. 2021, 143, 7471–7479. 10.1021/jacs.1c02004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huters A. D.; Styduhar E. D.; Garg N. K. Total Syntheses of the Elusive Welwitindolinones with Bicyclo[4.3.1] Cores. Angew. Chem., Int. Ed. 2012, 51, 3758–3765. 10.1002/anie.201107567. [DOI] [PubMed] [Google Scholar]
- Huters A. D.; Quasdorf K. W.; Styduhar E. D.; Garg N. K. Total Synthesis of (−)-N-Methylwelwitindolinone C Isothiocyanate. J. Am. Chem. Soc. 2011, 133, 15797–15799. 10.1021/ja206538k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quasdorf K. W.; Huters A. D.; Lodewyk M. K.; Tantillo D. J.; Garg N. K. Total Synthesis of Oxidized Welwitindolinones and (−)-N-Methylwelwitindolinone C Isonitrile. J. Am. Chem. Soc. 2012, 134, 1396–1399. 10.1021/ja210837b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styduhar E. D.; Huters A. D.; Weires N. A.; Garg N. K. Enantiospecific Total Synthesis of N-Methylwelwitindolinone D Isonitrile. Angew. Chem., Int. Ed. 2013, 52, 12422–12425. 10.1002/anie.201307464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weires N. A.; Styduhar E. D.; Baker E. L.; Garg N. K. Total Synthesis of (−)-N-Methylwelwitindolinone B Isothyiocyanate via a Chlorinative Oxabicycle Ring-Opening Strategy. J. Am. Chem. Soc. 2014, 136, 14710–14713. 10.1021/ja5087672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TePaske M. R.; Gloer J. B.; Wicklow D. T.; Dowd P. F. Tubingensin A: An Antiviral Carbazole Alkaloid from the Sclerotia of Aspergillus tubingensis. J. Org. Chem. 1989, 54, 4743–4746. 10.1021/jo00281a010. [DOI] [Google Scholar]
- TePaske M. R.; Gloer J. B.; Wicklow D. T.; Dowd P. F. The Structure of Tubingensin B: A Cytotoxic Carbazole Alkaloid from the Sclerotia of Aspergillus tubingensis. Tetrahedron Lett. 1989, 30, 5965–5968. 10.1016/S0040-4039(01)93829-8. [DOI] [Google Scholar]
- Bian M.; Wang Z.; Xiong X.; Sun Y.; Matera C.; Nicolaou K. C.; Li A. Total Synthesis of Anominine and Tubingensin A. J. Am. Chem. Soc. 2012, 134, 8078–8081. 10.1021/ja302765m. [DOI] [PubMed] [Google Scholar]
- Buszek K. R.; Brown N.; Luo D. Concise Total Synthesis of (±)-cis-Trikentrin A and (±)-Herbindole A via Intermolecular Indole Aryne Cycloaddition. Org. Lett. 2009, 11, 201–204. 10.1021/ol802425m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T.; Yamaguchi H.; Tanabe M.; Yasui Y.; Suzuki K. Synthetic Study of Aquayamycin. Part 3: First Total Synthesis. Tetrahedron Lett. 2000, 41, 8393–8396. 10.1016/S0040-4039(00)01480-5. [DOI] [Google Scholar]
- Sato S.; Sakata K.; Hashimoto Y.; Takikawa H.; Suzuki K. First Total Syntheses of Tetracenomycins C and X. Angew. Chem., Int. Ed. 2017, 56, 12608–12613. 10.1002/anie.201707099. [DOI] [PubMed] [Google Scholar]
- Torres-Ochoa R. O.; Buyck T.; Wang Q.; Zhu J. Heteroannulation of Aryne with α-Amino Imides: Synthesis of 2,2-Disubstituted Indolin-3-ones and Application to the Enantioselective Total Synthesis of (+)-Hinkdentine A. Angew. Chem., Int. Ed. 2018, 57, 5679–5683. 10.1002/anie.201800746. [DOI] [PubMed] [Google Scholar]
- Candito D. A.; Dobrovolsky D.; Lautens M. Development of an Intramolecular Aryne Ene Reaction and Application to the Formal Synthesis of (±)-Crinine. J. Am. Chem. Soc. 2012, 134, 15572–15580. 10.1021/ja306881u. [DOI] [PubMed] [Google Scholar]
- Xu H.; He J.; Shi J.; Tan L.; Qiu D.; Luo X.; Li Y. Domino Aryne Annulation via a Nucelophilic-Ene Process. J. Am. Chem. Soc. 2018, 140, 3555–3559. 10.1021/jacs.8b01005. [DOI] [PubMed] [Google Scholar]
- Goetz A. E.; Silberstein A. L.; Corsello M. A.; Garg N. K. Concise Enantiospecific Total Synthesis of Tubingensin A. J. Am. Chem. Soc. 2014, 136, 3036–3039. 10.1021/ja501142e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caubere P. Applications of Sodamide-Containing Complex Bases in Organic Synthesis. Acc. Chem. Res. 1974, 7, 301–308. 10.1021/ar50081a004. [DOI] [Google Scholar]
- Gregoire B.; Carre M.-C.; Caubere P. Arynic Condensation of Ketone Enolates. 17. New General Access to Benzocyclobutene Derivatives. J. Org. Chem. 1986, 51, 1419–1427. 10.1021/jo00359a008. [DOI] [Google Scholar]
- Ishida N.; Sawano S.; Masuda Y.; Murakami M. Rhodium-Catalyzed Ring Opening of Benzycyclobutenols with Site-Selectivity Complementary to Thermal Ring Opening. J. Am. Chem. Soc. 2012, 134, 17502–17504. 10.1021/ja309013a. [DOI] [PubMed] [Google Scholar]
- Corsello M. A.; Kim J.; Garg N. K. Total Synthesis of (−)-Tubingensin B Enabled by the Strategic Use of an Aryne Cyclization. Nat. Chem. 2017, 9, 944–949. 10.1038/nchem.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahatthananchai J.; Dumas A. M.; Bode J. W. Catalytic Selective Synthesis. Angew. Chem., Int. Ed. 2012, 51, 10954–10990. 10.1002/anie.201201787. [DOI] [PubMed] [Google Scholar]
- Trost B. M. Asymmetric Catalysis: An Enabling Science. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 5348–5355. 10.1073/pnas.0306715101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. J.; Overman L. E. Catalytic Asymmetric Synthesis of All-Carbon Quaternary Stereocenters. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 5363–5367. 10.1073/pnas.0307113101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caeiro J.; Peña D.; Cobas A.; Pérez D.; Guitián E. Asymmetric Catalysis in the [2 + 2 + 2] Cycloaddition of Arynes and Alkynes: Enantioselective Synthesis of a Pentahelicene. Adv. Synth. Catal. 2006, 348, 2466–2474. 10.1002/adsc.200600319. [DOI] [Google Scholar]
- Yubuta A.; Hosokawa T.; Gon M.; Tanaka K.; Chujo Y.; Tsurusaki A.; Kamikawa K. Enantioselective Synthesis of Triple Helicenes by Cross-Cyclotrimerization of a Helicenyl Aryne and Alkynes via Dynamic Kinetic Resolution. J. Am. Chem. Soc. 2020, 142, 10025–10033. 10.1021/jacs.0c01723. [DOI] [PubMed] [Google Scholar]
- Stará I. G.; Starý I. Helically Chiral Aromatics: The Synthesis of Helicenes by [2 + 2 + 2] Cycloisomerization of π-Electron Systems. Acc. Chem. Res. 2020, 53, 144–158. 10.1021/acs.accounts.9b00364. [DOI] [PubMed] [Google Scholar]
- Gingras M.; Félix G.; Peresutti R. One Hundred Years of Helicene Chemistry. Part 2: Stereoselective Syntheses and Chiral Separations of Carbohelicenes. Chem. Soc. Rev. 2013, 42, 1007–1050. 10.1039/C2CS35111K. [DOI] [PubMed] [Google Scholar]
- Peña D.; Pérez D.; Guitián E.; Castedo L. Synthesis of Hexabenzotriphenylene and Other Strained Polycyclic Aromatic Hydrocarbons by Palladium-Catalyzed Cyclotrimerization of Arynes. Org. Lett. 1999, 1, 1555–1557. 10.1021/ol990864t. [DOI] [Google Scholar]
- Peña D.; Cobas A.; Pérez D.; Guitián E.; Castedo L. Dibenzo[a,o]phenanthro[3,4-s]pycene, a Configurationally Stable Double Helicene: Synthesis and Determination of Its Conformation by NMR and GIAO Calculations. Org. Lett. 2003, 5, 1863–1866. 10.1021/ol034433t. [DOI] [PubMed] [Google Scholar]
- Hosokawa T.; Takahashi Y.; Matsushima T.; Watanabe S.; Kikkawa S.; Azumaya I.; Tsurusaki A.; Kamikawa K. Synthesis, Structures, and Properties of Hexapole Helicenes: Assembling Six [5]Helicene Substructures into Highly Twisted Aromatic Systems. J. Am. Chem. Soc. 2017, 139, 18512–18521. 10.1021/jacs.7b07113. [DOI] [PubMed] [Google Scholar]
- QUINAP has been used previously to access enantioenriched helicenes. For a recent example, see:; Karras M.; Holec J.; Bednárová L.; Pohl R.; Schmidt B.; Stará I. G.; Starý I. Asymmetric Synthesis of Nonracemic 2-Amino[6]helicenes and Their Self-Assembly into Langmuir Films. J. Org. Chem. 2018, 83, 5523–5538. 10.1021/acs.joc.8b00538. [DOI] [PubMed] [Google Scholar]
- Gandeepan P.; Cheng C.-H. Cobalt Catalysis Involving π Components in Organic Synthesis. Acc. Chem. Res. 2015, 48, 1194–1206. 10.1021/ar500463r. [DOI] [PubMed] [Google Scholar]
- Zheng T.; Sun H.; Chen Y.; Li X.; Dürr S.; Radius U.; Harms K. Synergistic Effect of a Low-Valent Cobalt Complex and a Trimethylphosphine Ligand on Selective C–F Bond Activation of Perfluorinated Toluene. Organometallics 2009, 28, 5771–5776. 10.1021/om900589z. [DOI] [Google Scholar]
- Gandon V.; Aubert C.; Malacria M. Recent Progress in Cobalt-Mediated [2 + 2 + 2] Cycloaddition Reactions. Chem. Commun. 2006, 2209–2217. 10.1039/b517696b. [DOI] [PubMed] [Google Scholar]
- Campbell C. D.; Rees C. W. Reactive Intermediates. Part I. Synthesis and Oxidation of 1- and 2-Aminobenzotriazole. J. Chem. Soc. C 1969, 5, 742–747. 10.1039/j39690000742. [DOI] [Google Scholar]
- Kato H.; Nakazawa S.; Kiyosawa T.; Hirakawa K. Heterocycles by Cycloaddition. Part II. Cycloaddition-Extrusion Reactions of Five-Membered Mesoionic Compounds with Benzyne: Preparation of Benz[c]azole and Benzo[c]thiophen Derivatives. J. Chem. Soc., Perkin Trans. 1 1976, 672–675. 10.1039/P19760000672. [DOI] [Google Scholar]
- Rigby J. H.; Holsworth D. D.; James K. Vinyl Isocyanates in Synthesis. [4 + 2] Cycloaddition Reactions with Benzyne Addends. J. Org. Chem. 1989, 54, 4019–4020. 10.1021/jo00278a005. [DOI] [Google Scholar]
- Sakurai H.; Sakaba H.; Nakadaira Y. Facile Preparation of 2,3-Benzo-1,4-diphenyl-7-silanorbornadiene Derivatives and the First Clear Evidence of Silylene to Disilene Thermal Rearrangement. J. Am. Chem. Soc. 1982, 104, 6156–6158. 10.1021/ja00386a072. [DOI] [Google Scholar]
- Cresp T. M.; Wege D. The Addition of Benzyne to Azulene. Tetrahedron 1986, 42, 6713–6718. 10.1016/S0040-4020(01)82112-8. [DOI] [Google Scholar]
- Engels B.; Schöneboom J. C.; Münster A. F.; Groetsch S.; Christl M. Computational Assessment of the Electronic Structures of Cyclohexa-1,2,4-triene, 1-Oxacyclohexa-2,3,5-triene (3δ2-Pyran), Their Benzo Derivatives, and Cyclohexa-1,2-diene. An Experimental Approach to 3δ2-Pyran. J. Am. Chem. Soc. 2002, 124, 287–297. 10.1021/ja011227c. [DOI] [PubMed] [Google Scholar]
- Hänninen M. M.; Peuronen A.; Tuononen H. M. Do Extremely Bent Allenes Exist?. Chem. - Eur. J. 2009, 15, 7287–7291. 10.1002/chem.200900928. [DOI] [PubMed] [Google Scholar]
- Schmidt M. W.; Angus R. O.; Johnson R. P. Small Ring Cyclic Allenes: An ab Initio Study of the Structure of 1,2-Cyclohexadiene. J. Am. Chem. Soc. 1982, 104, 6838–6839. 10.1021/ja00388a087. [DOI] [Google Scholar]
- Taskesenligil Y.; Kashyap R. P.; Watson W. H.; Balci M. Is the Intermediate in the Reaction of 3-Bromo-6,7-benzobicyclo[3.2.1]octa-2,6-diene with Potassium tert-Butoxide an Allene or an Alkyne?. J. Org. Chem. 1993, 58, 3216–3218. 10.1021/jo00063a058. [DOI] [Google Scholar]
- Daoust K. J.; Hernandez S. M.; Konrad K. M.; Mackie I. D.; Winstanley J.; Johnson R. P. Strain Estimates for Small-Ring Cyclic Allenes and Butatrienes. J. Org. Chem. 2006, 71, 5708–5714. 10.1021/jo060698k. [DOI] [PubMed] [Google Scholar]
- Dillon P. W.; Underwood G. R. Cyclic Allenes. I. The Electronic Structure and Probable Deformation of the Allene Linkage When Included in a Ring. An INDO–MO Study. J. Am. Chem. Soc. 1974, 96, 779–787. 10.1021/ja00810a023. [DOI] [Google Scholar]
- Angus R. O.; Schmidt M. W.; Johnson R. P. Small-Ring Cyclic Cumulenes: Theoretical Studies of the Structure and Barrier to Inversion in Cyclic Allenes. J. Am. Chem. Soc. 1985, 107, 532–537. 10.1021/ja00289a002. [DOI] [Google Scholar]
- Johnson R. P. Strained Cyclic Cumulenes. Chem. Rev. 1989, 89, 1111–1124. 10.1021/cr00095a009. [DOI] [Google Scholar]
- Nendel M.; Tolbert L. M.; Herring L. E.; Islam M. N.; Houk K. N. Strained Allenes as Dienophiles in the Diels–Alder Reaction: An Experimental and Computational Study. J. Org. Chem. 1999, 64, 976–983. 10.1021/jo982091c. [DOI] [PubMed] [Google Scholar]
- Christl M.Cyclic Allenes Up to Seven-Membered Rings. Modern Allene Chemistry; Krause N., Hashmi A. S. K.; Wiley-VCH Verlag GmbH & Co., 2004; Vol. 1, pp 243–357. [Google Scholar]
- Christl M.; Braun M.; Fischer H.; Groetsch S.; Müller G.; Leusser D.; Deuerlein S.; Stalke D.; Arnone M.; Engels B. The Stereochemical Course of the Generation and Interception of a Six-Membered Cyclic Allene: 3δ2-1H-Napthalene (2,3-Didehydro-1,2-dihydronaphthalene). Eur. J. Org. Chem. 2006, 2006, 5045–5058. 10.1002/ejoc.200600443. [DOI] [Google Scholar]
- Christl M.; Fischer H.; Arnone M.; Engels B. 1-Phenyl-1,2-cyclohexadiene: Astoundingly High Enantioselectivities on Generation in a Doering–Moore–Skattebøl Reaction and Interception by Activated Olefins. Chem. - Eur. J. 2009, 15, 11266–11272. 10.1002/chem.200900718. [DOI] [PubMed] [Google Scholar]
- Christl M.; Schreck M.; Fischer T.; Rudolph M.; Moigno D.; Fischer H.; Deuerlein S.; Stalke D. 1-Phenyl-1,2-cyclohexadiene: Generation, Interception by Activated Olefins, Dimerisation and Trimerisation. Chem. - Eur. J. 2009, 15, 11256–11265. 10.1002/chem.200900717. [DOI] [PubMed] [Google Scholar]
- Christl M.; Schreck M. 7-Arylbicyclo[4.2.0]oct-1-ene – Synthese durch [2 + 2]-Cycloadditionen von 1,2-Cyclohezadien Sowie 1-Methyl-1,2-cyclohezadien und Thermische Äquilibrierung der Exo/Endo-Isomeren. Chem. Ber. 1987, 120, 915–920. 10.1002/cber.19871200609. [DOI] [Google Scholar]
- Hioki Y.; Mori A.; Okano K. Steric Effects on Deprotonative Generation of Cyclohexynes and 1,2-Cyclohexadienes from Cyclohexenyl Triflates by Magnesium Amides. Tetrahedron 2020, 76, 131103. 10.1016/j.tet.2020.131103. [DOI] [Google Scholar]
- Inoue K.; Nakura R.; Okano K.; Mori A. One-Pot Synthesis of Silylated Enol Triflates from Silyl Enol Ethers for Cyclohexynes and 1,2-Cyclohexadienes. Eur. J. Org. Chem. 2018, 2018, 3343–3347. 10.1002/ejoc.201800353. [DOI] [Google Scholar]
- Quintana I.; Peña D.; Pérez D.; Guitián E. Generation and Reactivity of 1,2-Cyclohexadiene under Mild Reaction Conditions. Eur. J. Org. Chem. 2009, 2009, 5519–5524. 10.1002/ejoc.200900631. [DOI] [Google Scholar]
- Peña D.; Iglesias B.; Quintana I.; Pérez D.; Guitián E.; Castedo L. Synthesis and Reactivity of New Strained Cyclic Allene and Alkyne Precursors. Pure Appl. Chem. 2006, 78, 451–455. 10.1351/pac200678020451. [DOI] [Google Scholar]
- Barber J. S.; Styduhar E. D.; Pham H. V.; McMahon T. C.; Houk K. N.; Garg N. K. Nitrone Cycloadditions of 1,2-Cyclohexadiene. J. Am. Chem. Soc. 2016, 138, 2512–2515. 10.1021/jacs.5b13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofstrand V. A.; West F. G. Efficient Trapping of 1,2-Cyclohexadienes with 1,3-Dipoles. Chem. - Eur. J. 2016, 22, 10763–10767. 10.1002/chem.201602201. [DOI] [PubMed] [Google Scholar]
- For recent studies of substituted allenes, see:; Wang B.; Constantin M.-G.; Singh S.; Zhou Y.; Davis R. L.; West F. G. West. Generation and Trapping of Electron-Deficient 1,2-Cyclohexadienes. Unexpected Hetero-Diels–Alder Reactivity. Org. Biomol. Chem. 2021, 19, 399–405. 10.1039/D0OB02285C. [DOI] [PubMed] [Google Scholar]
- Almehmadi Y. A.; West F. G. A Mild Method for the Generation and Interception of 1,2-Cycloheptadienes with 1,3-Dipoles. Org. Lett. 2020, 22, 6091–6095. 10.1021/acs.orglett.0c02172. [DOI] [PubMed] [Google Scholar]
- Lofstrand V. A.; McIntosh K. C.; Almehmadi Y. A.; West F. G. Strain-Activated Diels–Alder Trapping of 1,2-Cyclohexadienes: Intramolecular Capture by Pendent Furans. Org. Lett. 2019, 21, 6231–6234. 10.1021/acs.orglett.9b02085. [DOI] [PubMed] [Google Scholar]
- Uyegaki M.; Ito S.; Sugihara Y.; Murata I. 1-Benzoxepin and its Valence Isomers, 4,5-Benz-3-oxatricyclo[4.1.0.02,7]heptene and 3,4-Benz-2-oxabicyclo[3.2.0]hepta-3,6-diene. Tetrahedron Lett. 1976, 17, 4473–4476. 10.1016/0040-4039(76)80146-3. [DOI] [Google Scholar]
- Schreck M.; Christl M. Generation and Interception of 1-Oxo-3,4-cyclohexadiene. Angew. Chem., Int. Ed. Engl. 1987, 26, 690–692. 10.1002/anie.198706901. [DOI] [Google Scholar]
- Christl M.; Braun M. Friesetzung und Abfangreaktionen von 1-Oxa-2,3-cyclohexadien. Chem. Ber. 1989, 122, 1939–1946. 10.1002/cber.19891221019. [DOI] [Google Scholar]
- Ruzziconi R.; Naruse Y.; Schlosser M. 1-Oxa-2,3-cyclohexadiene (″2H-isopyran″): A Strained Heterocyclic Allene Undergoing Cycloaddition Reactions with Characteristic Typo-, Regio-, and Stereoselectivities. Tetrahedron 1991, 47, 4603–4610. 10.1016/S0040-4020(01)86466-8. [DOI] [Google Scholar]
- Jamart-Grégoire B.; Mercier-Girardot S.; Ianelli S.; Nardelli M.; Caubère P. Aggregative Activation and Heterocyclic Chemistry II Nucleophilic Condensation of Ketone Enolates on Dehydrodihydropyran Generated by Complex Bases. Tetrahedron 1995, 51, 1973–1984. 10.1016/0040-4020(94)01077-D. [DOI] [Google Scholar]
- Christl M.; Braun M.; Wolz E.; Wagner W. Cycloallene, 9. 1-Phenyl-1-aza-3,4-cyclohexadien, das Erste Isodihydropyridin: Ertzeugung und Abfangreaktionen. Chem. Ber. 1994, 127, 1137–1142. 10.1002/cber.19941270626. [DOI] [Google Scholar]
- Drinkuth S.; Groetsch S.; Peters E.-M.; Peters K.; Christl M. 1-Methyl-1-azacyclohexa-2,3-diene(N–B)borane - Generation and Interception of an Unsymmetrical Isodihydropyridine. Eur. J. Org. Chem. 2001, 2001, 2665–2670. . [DOI] [Google Scholar]
- Barber J. S.; Yamano M. M.; Ramirez M.; Darzi E. R.; Knapp R. R.; Liu F.; Houk K. N.; Garg N. K. Diels–Alder Cycloadditions of Strained Azacyclic Allenes. Nat. Chem. 2018, 10, 953–960. 10.1038/s41557-018-0080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano M. M.; Knapp R. R.; Ngamnithiporn A.; Ramirez M.; Houk K. N.; Stoltz B. M.; Garg N. K. Cycloadditions of Oxacyclic Allenes and a Catalytic Asymmetric Entryway to Enantioenriched Cyclic Allenes. Angew. Chem., Int. Ed. 2019, 58, 5653–5657. 10.1002/anie.201900503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez M.; Svatunek D.; Liu F.; Garg N. K.; Houk K. N. Origins of Endo Selectivity in Diels–Alder Reactions of Cyclic Allene Dienophiles. Angew. Chem., Int. Ed. 2021, 10.1002/anie.202101809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal M. V.; Hudson L.; Mason J. W.; Pradeilles J. A.; Zécri F. J.; Briner K.; Schreiber S. L. Water-Compatible Cycloadditions of Oligonucleotide-Conjugated Strained Allenes for DNA-Encoded Library Synthesis. J. Am. Chem. Soc. 2020, 142, 7776–7782. 10.1021/jacs.9b13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behenna D. C.; Mohr J. T.; Sherden N. H.; Marinescu S. C.; Harned A. M.; Tani K.; Seto M.; Ma S.; Novák Z.; Krout M. R.; McFadden R. M.; Roizen J. L.; Enquist J. A.; White D. E.; Levine S. R.; Petrova K. V.; Iwashita A.; Virgil S. C.; Stoltz B. M. Enantioselective Decarboxylative Alkylation Reactions: Catalyst Development, Substrate Scope, and Mechanistic Studies. Chem. - Eur. J. 2011, 17, 14199–14223. 10.1002/chem.201003383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong A. Y.; Stoltz B. M. The Construction of All-Carbon Quaternary Stereocenters by Use of Pd-Catalyzed Asymmetric Allylic Alkylation Reactions in Total Synthesis. Eur. J. Org. Chem. 2013, 2013, 2745–2759. 10.1002/ejoc.201201761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunge J. A.; Burger E. C. Transition Metal Catalyzed Decarboxylative Additions of Enolates. Eur. J. Org. Chem. 2005, 2005, 1715–1726. 10.1002/ejoc.200400865. [DOI] [Google Scholar]
- Weaver J. D.; Recio A.; Grenning A. J.; Tunge J. A. Transition Metal-Catalyzed Decarboxylative Allylation and Benzylation Reactions. Chem. Rev. 2011, 111, 1846–1913. 10.1021/cr1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost B. M. Metal Catalyzed Allylic Alkylation: Its Development in the Trost Laboratories. Tetrahedron 2015, 71, 5708–5733. 10.1016/j.tet.2015.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorat V. H.; Upadhyay N. S.; Murakami M.; Cheng C.-H. Nickel-Catalyzed Denitrogenative Annulation of 1,2,3-benzotriazin-4-(3H)-ones with Benzynes for Construction of Phenanthridinone Scaffolds. Adv. Synth. Catal. 2018, 360, 284–289. 10.1002/adsc.201701143. [DOI] [Google Scholar]
- Yamano M. M.; Kelleghan A. V.; Shao Q.; Giroud M.; Simmons B. J.; Li B.; Chen S.; Houk K. N.; Garg N. K. Intercepting Fleeting Cyclic Allenes with Asymmetric Nickel Catalysis. Nature 2020, 586, 242–247. 10.1038/s41586-020-2701-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleghan A. V.; Witkowski D. C.; McVeigh M. S.; Garg N. K. Palladium-Catalyzed Annulations of Strained Cyclic Allenes. J. Am. Chem. Soc. 2021, 10.1021/jacs.1c04896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Many of the silyl triflate precursors discussed herein (or close derivatives thereof) are currently commercially available. Benzyne precursor (53): CAS # 88284-48-4; 2-chlorobenzyne precursor: CAS # 1449472-59-6; 3,4-pyridyne precursor: CAS # 1211583-40-2; 2,3-pyridyne precursor: CAS # 134391-76-7; 2-bromobenzyne precursor: CAS # 1092542-31-8; 5-bromo-3,4-pyridyne precursor: CAS # 1413439-82-3; 4,5-indolyne precursor: CAS # 1259448-37-7; 4-methylbenzyne precursor: CAS # 262373-15-9; 4-methoxybenzyne precursor: CAS # 556812-41-0; 3-methoxybenzyne precursor: CAS # 217813-03-1; 2-methylbenzyne precursor: CAS # 556812-44-3; 4,5-dimethoxybenzyne precursor: CAS # 866252-52-0; 3-morpholinobenzyne precursor: CAS # 2047348-50-3; naphthalyne precursor: CAS # 780820-43-1; azacyclohexyne precursor (51): Sigma–Aldrich product number 803928.