Summary:
Multi-resonance NMR experiments are powerful analytical and structural tools. Their conceptualization assumes that RF fields may be combined independently to manipulate spin interactions. However, practical implementation can compromise performance. One limitation is the generation of combination bands when two or more RF fields are applied simultaneously within the NMR probe. The combination bands can lead to significant interference with the detection circuitry. A facile approach to combined multi-band decoupling can resolve these problems and increase sensitivity two-fold (or more), by time sharing the application of the individual frequencies rather than time sharing decoupling and data acquisition.
Keywords: decoupling, multi-band, multinuclear decoupling, 13C-detection
Spin decoupling is an important and essential part of modern magnetic resonance spectroscopy. From the early discovery of scalar couplings, the ability to remove the presence of spin coupling in the observed NMR spectra has had powerful ramifications. Assignment of signals was made by selective decoupling one resonance and removing the couplings observed in the connected resonance 1,2, and one of the most powerful applications was the extensive development of broadband decoupling of one spin-type from another3–5. These tools were largely aimed at providing broad bandwidth coverage over the chemical shift range and simultaneously removing the relatively large one-bond scalar couplings, predominantly focusing on 1H-13C couplings. With the development of indirect 1H detection of heteronuclear correlations, related decoupling patterns were applied to the heteronucleus, typically 13C or 15N, during acquisition periods. The latter application has fueled the dramatic developments in multi-dimensional spectroscopy in the study of biomolecules6,7. Recent examples of tailored spin decoupling have also been developed to address band-selective homonuclear decoupling 8–14. Many of the examples referred to involve decoupling of single spin types within an experiment; however, the advent of perdeuteration in triple resonance biomolecular NMR15,16 also led to combinations of 2H and 15N decoupling within sequences, although normally at different segments of the sequence and usually not simultaneously. Similarly, in other areas of application of NMR, such as inorganic chemistry, numerous atoms within the system under study may possess NMR-active nuclei and it is desirable to decouple the spin-couplings both individually and collectively. The ability to have flexible decoupling of any or all spins within a molecule and at any desired time within the pulse sequence for a given experiment is a powerful and significant component of modern NMR spectroscopy.
Modern NMR spectrometers are multi-channel, highly programmable electronic systems that can create arbitrarily complex RF outputs to manipulate spin-spin interactions. Indeed, modern NMR spectroscopy depends on these capabilities; however, unfortunately, the nature of NMR detection hardware (the NMR probe) has a limited number of resonators (coils) to apply these RF fields to the sample (Figure 1). The probe generally relies on inductors, or inductive resonator structures17, to create magnetic fields responding to the applied RF from the console. The physical geometry of the probe and magnets limit the system to two orthogonal coils, which are then also orthogonal to the magnetic field. Orthogonality is used to minimize inductive coupling between the two coils. To apply more than two RF frequencies, the coils are double- or multiply-tuned to provide irradiation at multiple frequencies17. The designs have become quite sophisticated and efficient; nevertheless, it cannot be avoided that the experiment is an induction process and the two coils (inductors), although designed to be orthogonal, have finite coupling between them. Hence, as is known from basic electronics, there will be some transformer-like effects that lead to combination frequencies created in the probe when two frequencies are applied simultaneously. The probe circuitry can act as a mixer wherein combination sum and difference frequency RF will result from simultaneous application of any two (or more) RF frequencies. The relative intensity of the applied RF voltage compared to the detected NMR signal voltage (either observe or lock channel) is at least on the order of 106:1; hence, the very minor coupling between coils will lead to signals much larger than the desired signal. If these sum or difference frequencies happen to fall within the bandwidth of the associated electronics for the detected nucleus, then considerable interference can be observed during simultaneous decoupling and detection. The interference can occur for either the observe channel or the lock channel.
Figure 1.
Common probe coil arrangement. Two orthogonal coils are used to apply RF fields generated by RF patterns created in the console. A cartoon of the double tuned circuit (right) presents how the coils can act like transformer/mixers to create sums and differences of the frequencies applied to the coil inputs. This design applies for 1H frequencies up to approximately 600 MHz. Above 600 MHz, the circuits often have 1H alone on the inner coil (to optimize sensitivity) and 2H, 13C and 15N are multi-tuned on the outer coil.
Examination of the resonance frequencies of nuclei that are commonly investigated in biomolecular NMR, e.g. 1H, 2H, 13C, 15N, and 31P, reveals a number of complicating interference bands that arise due to these sum and difference frequencies (Figure 2). The majority of triple resonance 1H-detected biomolecular NMR experiments avoid these combination bands and use only 15N decoupling during acquisition. For the case of simultaneous 13C- and 15N-edited sequences18, the combination band is well out of the 1H frequency range, and the 1H detection channel of most spectrometers is bandwidth limited to avoid interference. Hence, other than power handling complications, little interference would be observed in the presence of simultaneous 15N- and 13C-decoupling. However, there is an opportunity for interference on the 2H lock channel, as the 15N and 13C difference frequency is near the deuterium frequency. For example, interference on the 2H lock channel can be observed in an experiment where simultaneous 13C- and 15N-decoupling is applied (Figure S1). The bandwidth of most 2H lock channels is approximately +/− 2 MHz; hence, it is not possible to filter out this interference. Additionally, in cases of perdeuterated proteins, many of the conventional triple-resonance experiments may utilize 2H decoupling, but it previously has been applied during evolution times within the pulse sequence that are separate from the detection period and the amide 1H is detected in the presence of 15N decoupling. Consequently, pulse sequences that employ simultaneous 13C- and 15N-decoupling (or 2H decoupling) typically employ a blanking procedure that puts the lock circuitry in a ‘LOCK_HOLD’ mode during the duration of such simultaneous sequences. The lock is switched back to normal ‘LOCK_ON’ operation during the recovery time.
Figure 2.
Combination frequencies occurring between some typical NMR frequencies in biomolecular corresponding to a 1H resonance frequency of 600 MHz.
A critical problem arises for the cases where the application of two decoupling frequencies create combination frequencies near or within the bandwidth of detection for the observed nucleus. Referring to figure 2, it is apparent that this may occur for 13C {15N, 2H}, 15N {13C,2H}, 2H {13C,15N}, 2H {31P,13C}, 13C {31P,2H}, or 31P {13C,2H}, where the nuclei within brackets are decoupled during the acquisition of the first nucleus. One of the most common experiments that is impacted by this interference is 13C {15N,2H}, corresponding to 13C-detected spectra in the context of 15N and 2H-labeled proteins19 These applications arise for large and/or paramagnetic proteins,20,21 particularly when Cα-detection is employed. Using a sample of uniformly 13C-labeled, 15N-labeled, 2H-labeled, and methyl-protonated valine (Cambridge Isotopte Laboratories, Inc.), the interference created from simultaneous 15N- and 2H-decoupling is illustrated in Figure 3. In the absence of decoupling, the FID and the transformed spectrum are of high quality and the full multiplicities of the resonances can be observed (Figure 3A,C and Figure 5). However, in the presence of combined composite pulse decoupling, using WALTZ for 2H and GARP for 15N, there is significant interference present in the FID, and the transformed spectrum is full of spikes and exhibits low quality (Figure 3B,D).
Figure 3.
13C-detection of the one-pulse FID for (*CH3)2*CD*CD(*NH2)*COOH valine in D2O, where *C represents 13C labeling. A, C) Direct detection of the 13C-FID and FT-spectrum acquired with no decoupling. B, D) Direct detection of 13C-FID and FT-spectrum acquired with simultaneous 2H and 15N decoupling.
Figure 5.
13C-detected one-dimensional spectra of (*CH3)2*CD*CD(*NH2)*COOH valine for different combinations of decoupling. Decoupling is applied using broadband WALTZ decoupling for 1H and the simultaneous COMB decoupling according to Figure 6 for 15N and 2H. The decoupling applied is indicated from top to bottom as {1H,2H,15N}, {2H,15N}, {1H,2H}, {1H,15N}, {2H}, {15N}, {1H}, and none.
One approach to the interference in the 13C {15N,2H} experiment would consider gating the acquisition sequence, wherein the decoupling frequencies are gated off for the sampling of each data point, as has been utilized in band-selective homonuclear decoupling8,9,13,14. While modern spectrometers have the flexibility to accomplish this programming during the acquisition of the FID, the additional programming complexity may place some restrictions on the spectral width, dwell time, and ease of operation. Time sharing each dwell point also inherently leads to sampling artifacts and a loss of sensitivity which is proportional to the off time of the receiver8,9.
We have explored a general solution to the problem that involves an interleaved, or time-shared, synchronous decoupling scheme applied to two hetero-nuclei, which avoids the creation of combination bands. The concept is illustrated in Figure 4. For the case of 13C {15N,2H,1H}, decoupling of 15N is initiated in a synchronous fashion at the start of the acquisition. Then, gaps are programmed into the decoupling pattern such that accurate, synchronously timed decoupling can be applied to 2H in the gap duration. The sequences then repeat for the full acquisition time, and there is no further programming required. This sequence is readily programmed as shown in Figure S2, and the synchronous timing is controlled by the command ‘cpds’ in the pulse program operating in TopSpin, which ensures that the sequence starts at the same point in the waveform at the beginning of each acquisition. We refer to this approach as Combined Multi-Band (COMB) decoupling. Since there is no simultaneous application of both 2H and 15N frequencies, there is no combination sum band at the frequency of 13C, and the acquisition of the FID can proceed normally without interference and without any explicit acquisition programming. The latter characteristic simplifies experimental setup and definition of data sizes in the acquisition domain. The small delay times, which are 6 us in Figure S2, may be adjusted depending on the generation and characteristics of the hardware; however, this value has worked on a range of Bruker AVIII and newer consoles operating TopSpin 3.x or 4.x software.
Figure 4.
Simultaneous decoupling of 1H, 2H, and 15N during direct 13C-detection. A) Conventional broadband sequences applied asynchronously simultaneously to the respective probe circuit. B) Combined Multi-Band (COMB) decoupling applied simultaneously and synchronously to 2H and 15N channels along with asynchronous broadband 1H decoupling.
Using the sample of uniformly 13C-labeled, 15N-labeled, 2H-labeled, and methyl-protonated valine, a series of one-dimensional 13C spectra were acquired for all possible single, dual, and triple combinations of 1H, 2H, and 15N decoupling during acquisition (Figure 5). The dramatic impact of combined 2H and 15N decoupling on the Cα resonance is evident. These spectra are acquired free of interference artifacts and retaining the full sensitivity of the spectrometer. One can readily envision that these high-quality spectra may then be further simplified or manipulated using various forms of homonuclear 13C decoupling22. Examples may include the IPAP or DIPAP tools developed by Bermel and coworkers19 for studying intrinsically disordered proteins or different forms of band selective decoupling8,9,13,14. These tools are fully compatible with multi-dimensional triple- and quadruple-resonance experiments
An important requirement in the application of COMB is the actual decoupling pattern used in the interleaved manner. Most optimized decoupling schemes, ranging from MLEV, WALTZ, and GARP to more sophisticated optimal-control-theory generated sequences23,24 are window-less sequences. The COMB approach is reminiscent of the CRAMPS methodology in ssNMR25,26, and it is important to consider the bandwidth complications of window-ed or gap-decoupling sequences. It is well established that decoupling sequences have been constructed using repetitive inversion of spins, and the common sequences, such as MLEV, WALTZ, and GARP3,5 (including rectangular and adiabatic pulses (e.g. WURST27)) were developed using phase cycling of spin inversion pulses. By inserting gaps to enable application of an inversion on the second spin, the behavior of the decoupling bandwidth can be impacted. We have explored several options and find that, for 15N decoupling, use of BIP720 pulses23, phase cycled according to the P5M4 supercyle28,29 provides an excellent compromise between RF power requirements and decoupling bandwidth. Using a 1.8 ms BIP720 pulse applied at an effective RF field of 800 Hz (equivalent to a rectangular π/2 pulse of 320 μs) provides very adequate bandwidth, while leaving a gap of up to 450 μs for a 2H π pulse. The effectiveness and bandwidth of decoupling for both 15N and 2H is demonstrated in Figure 6. Observation of the Cα resonance of the labeled Val sample, during on-resonance 2H decoupling and stepwise offsetting the 15N decoupling frequency, illustrates a bandwidth of approximately 90 ppm in offset at the 15N frequency. Similarly, observation of the offset dependence for 2H decoupling, while on-resonance 15N decoupling, illustrates a bandwidth of approximately 8.5 ppm. These bandwidths provide complete decoupling under conditions appropriate for protein NMR spectroscopy. Further comparisons of conventional continuous decoupling and the gapped decoupling in COMB are provided in the Supporting information Figures S3 to S25. These data illustrate bandwidth and residual linewidth comparisons for gap-less, gapped, and COMB sequences.
Figure 6.
Decoupling bandwidth for 15N and 2H under COMB conditions. A) Offset dependence of the Ca resonance of Valine under conditions of on-resonance 2H decoupling combined with stepped offset of 15N decoupling using a 1.8 ms BIP720 pulse incremented in steps of 5 ppm. B) Offset dependence of the Ca resonance of Valine under conditions of on-resonance 15N decoupling combined with stepped offset of 2H decoupling using a 428 ms square p pulse incremented in steps of 0.5 ppm.
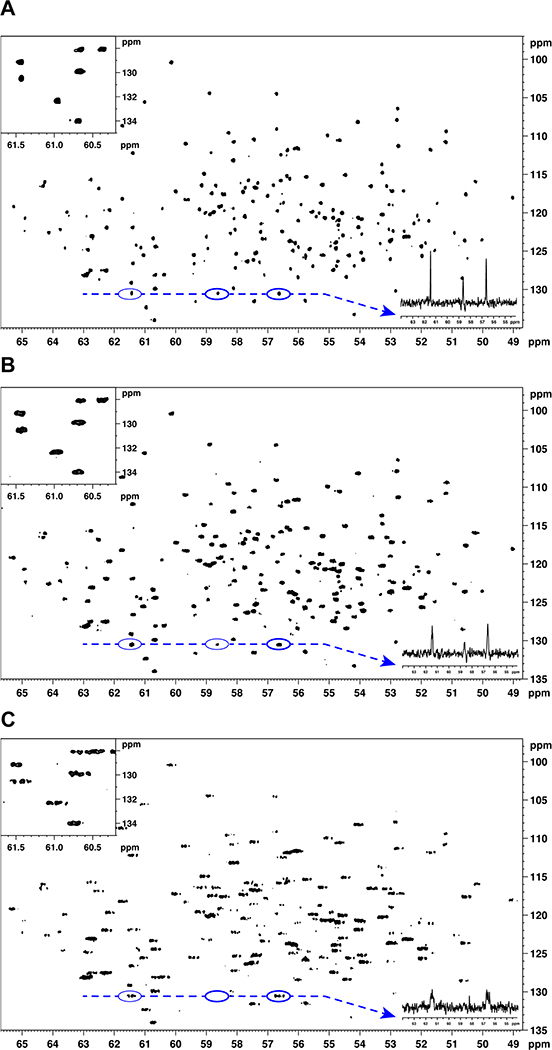
As a practical example, we chose the 13C direct-detected HNCOCA experiment19 acquired on a sample of uniformly 13C-, 15N- and 2H-labeled human carbonic anhydrase II (HCA-II) at 16.45 T (Figure 7). In this experiment, the acquisition dimension is set on the alpha carbons, and the 1JC-C couplings of approximately 53 Hz and 35 Hz to C’ and Cβ, respectively, were removed by a double IPAP scheme19,22. Previously, this experiment was acquired with only 15N-decoupling, for the reasons presented above, which leaves the 1JC-D couplings (~20 Hz) active. Without simultaneous decoupling of 15N and 2H, the 13Cα signals are split into complex multiplets. Each spectrum was acquired as the Cα-N plane: direct 13Cα acquisition dimension 2048 points (1024 complex), spectral width 28.4 ppm (5000 Hz) and acquisition time 0.2048 sec; indirect 15N dimension 138 points ×4 for double IPAP (69 complex – limited by the constant time), spectral width 40 ppm (2838.6 Hz) and acquisition time 0.02043 sec. The spectra differ only in the decoupling scheme applied during acquisition. Figure 7 demonstrates the gain in signal intensity and resolution by successive incorporation of the respective modes of decoupling. The 1J C-N, 2JC-N and the 1JC-D couplings were removed by COMB decoupling during the acquisition time (Figure 7A). The low average power of the COMB decoupling scheme also allows longer acquisition times and permits even higher resolution in these spectra. The sensitivity difference between 15N only and optimally COMB decoupled spectra corresponds to approximately a factor of ~2.5. Furthermore, the raw data may be processed without application of the linear combination of data (DIPAP), thus yielding the fully coupled spectrum and allowing very clear observation of the J(Cα,C’) and J(Cα,Cβ) couplings.
Figure 7.
Comparison of decoupling modes in 13C-detected HNCOCA. 15N, 13Cα planes acquired with (A) COMB decoupling of both 15N and 2H, (B) 2H decoupling only, and (C) 15N decoupling only. The data were acquired as 2D experiments, and the 13C-13C couplings between C’-Cα and Cα-Cβ were removed with a double IPAP scheme. The insets provide a clearer visualization of peak shapes. The dashed lines correspond to traces shown to the right of each panel.
We have explicitly dealt with the combination bands relative to common labeling schemes in biomolecular NMR; however, there are numerous examples of combinations of frequencies that will lead to interference in the general application of NMR spectroscopy. These frequencies arise due to the ratios of the gyromagnetic ratio of nuclei, and these will occur at all magnetic field strengths. The approach of COMB can be extended and adapted to most of these cases. A bandwidth complication may be encountered for decoupling 13C in the cases of 15N {13C,2H}30 and 31P {13C,2H}. Achieving a sufficient bandwidth with windowed decoupling to cover the full 13C chemical shift range might prove difficult; however, when the chemical shift range of coupled 13C sites is moderate, COMB will be quite effective. The 31P {13C,2H} case may be easily optimized by selection of different windowing, since J31P-2H tend to be rather small and may require either no or minimal decoupling. The application of simultaneous 13C and 2H decoupling during acquisition for proteins would be envisioned only for samples where amide nitrogens were deuterated and direct 15N-detection was utilized. This case would represent a very low sensitivity case and is not likely of practical concern. Furthermore, for cases within a pulse sequence where simultaneous 13C and 2H decoupling is desired, COMB decoupling would still be an effective approach to avoid power handling problems depending on the specific probe configuration.
In conclusion, the Combined Multi-Band decoupling method provides a simple, efficient scheme to achieve simultaneous decoupling of two hetero-nuclei, and COMB avoids significant RF interference that can result in large artifacts and compromise the sensitivity of the experiment. COMB allows the greatest flexibility, elimination of sampling artifacts, and up to a 2.5-fold gain in sensitivity for demanding 13C-direct detection {15N,2H} experiments.
Supplementary Material
Acknowledgements:
The sample of HCA-II was generously provided by Drs. Ron Venters and Len Spicer, Duke University. This research was supported in part by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health, under Project ZIA BC 011132.
References
- 1.Ernst RR Nuclear Magnetic Double Resonance with an Incoherent Radio-Frequency Field. Journal of Chemical Physics 45, 3845–& (1966). [Google Scholar]
- 2.Weigert FJ, Jautelat M & Roberts JD Natural-abundance C nuclear magnetic resonance spectra of medium-molecular-weight organic compounds. Proc Natl Acad Sci U S A 60, 1152–5 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levitt MH, Freeman R & Frenkiel T Broad-Band Decoupling in High-Resolution Nuclear Magnetic-Resonance Spectroscopy. Advances in Magnetic Resonance 11, 47–110 (1983). [Google Scholar]
- 4.Levitt MH, Bodenhausen G & Ernst RR The Illusions of Spin Decoupling. Journal of Magnetic Resonance 53, 443–461 (1983). [Google Scholar]
- 5.Freeman R & Kupce E Decoupling: theory and practice. I. Current methods and recent concepts. NMR Biomed 10, 372–80 (1997). [DOI] [PubMed] [Google Scholar]
- 6.Arthanari H, Takeuchi K, Dubey A & Wagner G Emerging solution NMR methods to illuminate the structural and dynamic properties of proteins. Curr Opin Struct Biol 58, 294–304 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alderson TR & Kay LE Unveiling invisible protein states with NMR spectroscopy. Curr Opin Struct Biol 60, 39–49 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Vogeli B, Kovacs H & Pervushin K Simultaneous (1)H- or (2)H-, (15)N- and multiple-band-selective (13)C-decoupling during acquisition in (13)C-detected experiments with proteins and oligonucleotides. J Biomol NMR 31, 1–9 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Ying J, Li F, Lee JH & Bax A (1)(3)Calpha decoupling during direct observation of carbonyl resonances in solution NMR of isotopically enriched proteins. J Biomol NMR 60, 15–21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuo H, Kupce E, Li H & Wagner G Increased sensitivity in HNCA and HN(CO)CA experiments by selective C beta decoupling. J Magn Reson B 113, 91–6 (1996). [DOI] [PubMed] [Google Scholar]
- 11.Matsuo H, Kupce E & Wagner G Resolution and sensitivity gain in HCCH-TOCSY experiments by homonuclear C beta decoupling. J Magn Reson B 113, 190–4 (1996). [DOI] [PubMed] [Google Scholar]
- 12.Kupce E & Wagner G Wideband homonuclear decoupling in protein spectra (vol 109, pg 329, 1995). Journal Of Magnetic Resonance Series B 111, 208–208 (1996).8661284 [Google Scholar]
- 13.Bermel W, Bertini I, Felli IC, Kummerle R & Pierattelli R 13C direct detection experiments on the paramagnetic oxidized monomeric copper, zinc superoxide dismutase. J Am Chem Soc 125, 16423–9 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Struppe JO et al. Long-observation-window band-selective homonuclear decoupling: increased sensitivity and resolution in solid-state NMR spectroscopy of proteins. J Magn Reson 236, 89–94 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sattler M & Fesik SW Use of deuterium labeling in NMR: overcoming a sizeable problem. Structure 4, 1245–9 (1996). [DOI] [PubMed] [Google Scholar]
- 16.Venters RA, Farmer BT 2nd, Fierke CA & Spicer LD Characterizing the use of perdeuteration in NMR studies of large proteins: 13C, 15N and 1H assignments of human carbonic anhydrase II. J Mol Biol 264, 1101–16 (1996). [DOI] [PubMed] [Google Scholar]
- 17.Zens AP Using magnetic coupling to improve multiple resonance NMR probe circuits. J Magn Reson 316, 106741 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Pascal SM, Muhandiram DR, Yamazaki T, Formankay JD & Kay LE Simultaneous Acquisition of N-15-Edited and C-13-Edited Noe Spectra of Proteins Dissolved in H2O. J Magn Resonance Ser B 103, 197–201 (1994). [Google Scholar]
- 19.Bermel W et al. Complete assignment of heteronuclear protein resonances by protonless NMR spectroscopy. Angew Chem Int Ed Engl 44, 3089–92 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Bertini I, Luchinat C, Parigi G & Pierattelli R Perspectives in paramagnetic NMR of metalloproteins. Dalton Transactions, 3782–3790 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Machonkin TE, Westler WM & Markley JL Paramagnetic NMR spectroscopy and density functional calculations in the analysis of the geometric and electronic structures of iron-sulfur proteins. Inorganic Chemistry 44, 779–797 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Felli IC & Pierattelli R Spin-state-selective methods in solution- and solid-state biomolecular 13C NMR. Prog Nucl Magn Reson Spectrosc 84–85, 1–13 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Smith MA, Hu H & Shaka AJ Improved broadband inversion performance for NMR in liquids. Journal of Magnetic Resonance 151, 269–283 (2001). [Google Scholar]
- 24.Schilling F et al. Next-generation heteronuclear decoupling for high-field biomolecular NMR spectroscopy. Angew Chem Int Ed Engl 53, 4475–9 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Waugh JS, Huber LM & Haeberlen U Approach to High-Resolution Nmr in Solids. Physical Review Letters 20, 180– (1968). [Google Scholar]
- 26.Gerstein BC, Pembleton RG, Wilson RC & Ryan LM High-Resolution Nmr in Randomly Oriented Solids with Homonuclear Dipolar Broadening - Combined Multiple Pulse Nmr and Magic Angle Spinning. Journal of Chemical Physics 66, 361–362 (1977). [Google Scholar]
- 27.Kupce E & Freeman R Adiabatic Pulses For Wide-Band Inversion And Broad-Band Decoupling. Journal Of Magnetic Resonance Series A 115, 273–276 (1995). [Google Scholar]
- 28.Tycko R, Pines A & Guckenheimer J Fixed-Point Theory of Iterative Excitation Schemes in Nmr. Journal of Chemical Physics 83, 2775–2802 (1985). [Google Scholar]
- 29.Tenailleau E & Akoka S Adiabatic 1H decoupling scheme for very accurate intensity measurements in 13C NMR. J Magn Reson 185, 50–8 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi K, Arthanari H, Shimada I & Wagner G Nitrogen detected TROSY at high field yields high resolution and sensitivity for protein NMR. J Biomol NMR 63, 323–31 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.