Abstract
Tree shrews (Tupaia spp.) have been used in neuroscience research since the 1960s due to their evolutionary proximity to primates. The use of and interest in this animal model have recently increased, in part due to the adaptation of modern neuroscience tools in this species. These tools include quantitative behavioral assays, calcium imaging, optogenetics and transgenics. To facilitate the exchange and development of these new technologies and associated research findings, we organized the inaugural “Tree Shrew Users Meeting” which was held online due to the COVID-19 pandemic. Here, we review this meeting and discuss the history of tree shrews as an animal model in neuroscience research and summarize the current themes being investigated using this animal, as well as future directions.
Keywords: Tree Shrew Users Meeting, Animal models, Tupaia, Neuroscience
INTRODUCTION
On March 29th, 2021, we organized the Tree Shrew Users Meeting, the inaugural international gathering of neuroscientists who use the tree shrew (Tupaia spp.) as a model organism in their research or are interested in doing so in the future. The goal of this meeting was to facilitate communication between these groups to foster a community and further establish this animal as a major model in neuroscience. Here, we briefly introduce the tree shrew animal model and its history in neuroscience research and follow with a review of the content of the inaugural meeting.
TREE SHREWS AS AN ANIMAL MODEL IN NEUROSCIENCE
Tree shrews are diurnal animals originating from South-East Asia. The earliest archived description of a tree shrew comes from William Ellis, a surgeon in Captain Cook’s third expedition in 1780 (Lyon, 1913). Given their similar appearance, tree shrews were initially thought to be squirrels and were later classified as insectivores and primates (Simpson, 1945). Today, they are classified within their own order, Scandentia (Van Valen, 1965) (Figure 1).
Figure 1.
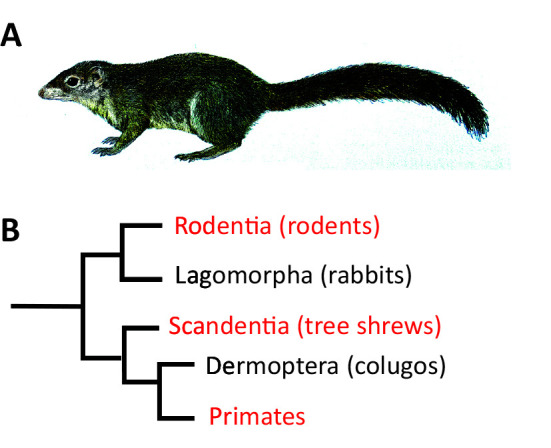
Tree shrew phylogeny
A: Illustration of a Northern Tree Shrew (Tupaia belangeri) adapted from Wilson & Mittermeier, (2009); B: Schematic representation of the phylogenetic position of tree shrews and relationship to primates.
Tree shrews present numerous advantages as a model organism in neuroscience; they are the closest phylogenetic relative of primates (Janečka et al., 2007; Ni & Qiu, 2012) that can be kept in a laboratory setting (Fuchs, 2015; Hubrecht & Kirkwood, 2010). Their breeding cycle is short and year-round and, as such, they can thrive in captivity. In addition, tree shrews are born in a relatively undeveloped state, allowing measurement and manipulation of neurodevelopmental processes (Drenhaus et al., 2006).
Tree shrews can be trained on different forms of detection and discrimination tasks, making them a suitable model to study the neuronal underpinning of sensory-motor and cognitive behaviors (Casagrande & Diamond, 1974; Khani & Rainer, 2012; Mustafar et al., 2018; Petry et al., 1984). Unlike rodents, they are diurnal (Emmons & Greene, 2000), possess a cone dominated retina (Müller & Peichl, 1989), and exhibit interesting visually guided behaviors (Mustafar et al., 2018). These properties have made tree shrews a suitable model particularly in the field of visual neuroscience.
Recent surge in the use of tree shrews in neuroscience research
Tree shrews have been a model organism for Neuroscience research in Europe and the United States since the 1960s. There has been a surge of interest in this animal model following recent publication of the tree shrew genome (Fan et al., 2013, 2019; Xu et al., 2012) and several novel findings using state-of-the-art methods (Dimidschstein et al., 2016; Lee et al., 2016; Smith & Fitzpatrick, 2016). However, since the use of tree shrews is not as widespread as mice, zebrafish or drosophila, there is a lack of standardized resources, methodology and genetic tools. To accelerate the development of such resources and further establish the tree shrew as a major model organism in neuroscience, we decided to gather the community of tree shrew researchers for an inaugural international meeting. The meeting was held virtually due to the COVID-19 pandemic and was attended by 91 participants. It contained four sessions: a “Community Introduction” session showcasing the work of 18 groups with brief presentations, two sessions dedicated to important questions for the community: creating transgenic animal models and tree shrew colonies and breeding in the US, as well as a keynote session on the history of tree shrews in visual neuroscience. To allow the continuation of information exchange beyond the meeting, an online channel was created for all interested participants.
Current and past status of research
The Community Introductions sessions provided brief updates from most of the research groups participating in the meeting. Broadly, the groups could be categorized in three research directions: retina (Sajdak et al., 2019), including retinal models of disease and injury (El Hamdaoui et al., 2021; Gawne et al., 2017; Norton et al., 2021) as well as retinal information processing (Johnson et al., 2019); development and function of cortical and subcortical sensory systems (Maher et al., 2021) including visual cortex and superior colliculus (Lee et al., 2016; Petry & Bickford, 2019; Sedigh-Sarvestani et al., 2021); and higher order cognitive functions including pattern and object discrimination and decision making (Mustafar et al., 2018).
The panel on transgenic tree shrews featured Dr. Yong-Gang Yao of the Kunming Institute of Zoology and Dr. Hirofumi Nishizono, of Kanazawa Medical University who are leading the effort in the creation of transgenic tree shrews. These groups are independently developing novel genetic engineering approaches to produce transgenic tree shrews (Darwish et al., 2019; Li et al., 2017). Dr. Yao is also working on the development of inbred lines of tree shrews to reduce genetic variability and facilitate the development of disease models.
In the panel about tree shrew colonies and breeding in the United States, Susan Freling from the Max Planck Florida Institute for Neuroscience, Dr. Alev Erisir from the University of Virginia, and Dr. Raphael Grytz from the University of Alabama at Birmingham summarized the current state of their colonies and highlighted challenges for the community.
The keynote was delivered by Dr. Jon Kaas of Vanderbilt University, who gave an extensive description of the contribution of tree shrews to our understanding of the visual system. LeGros Clark’s early studies of the visual pathway of tree shrews reported similarity to primates (Le Gros Clark, 1924), leading to adoption of the tree shrew as a model organism by visual neuroscientists including Irving Diamond (Snyder & Diamond, 1968). Indeed, recent studies suggest that ancestral primates had similar brain morphology compared to tree shrews (Mantilla et al., 2021). Dr. Kaas gave an overview of the early contributors of tree shrew research and the connections between the different laboratories in the United States that started using this animal model in the 1960s. He particularly highlighted the contributions of the late Dr. Viviana Casagrande, notably her work on the X and Y cells (Sherman et al., 1975) of the LGN as well as the contribution of the superior colliculus to vision (Casagrande & Diamond, 1974). He concluded with comparative work demonstrating the evolution of the tecto-pulvinar pathway, and the emergence of higher visual areas involved in the processing of motion in primates.
Future directions
The Tree Shrew Users Meeting gave an overview of the current efforts in the transfer of state-of-the-art neuroscience approaches from rodents to tree shrews. Viral targeting has become available through promoter-based approaches (Dimidschstein et al., 2016), allowing cell-type specific recordings and optogenetic manipulations. Additional tools, such as a 3D anatomical atlas are under development and will facilitate further establishment of the tree shrew as a major model organism in neuroscience. The upcoming transgenic lines will also open the door to the study of neuro-degenerative and neuro-developmental disorders. The recent surge of interest in tree shrews is related to a growing need for alternative animal models in neuroscience to complement investigations currently performed predominately in rodents (Yartsev, 2017). Given their phylogenetic proximity to primates, small size and short gestation cycle, tree shrews serve as an ideal animal model for neuroscience research.
Next meeting
We look forward to hosting an extended meeting in 2022 and further growing the community. This meeting will be hosted at the Max Planck Florida Institute of Neuroscience in the United States and will have a virtual component to allow participation of non-local attendees. We welcome all interested participants including prospective tree shrew users from broad subfields within neuroscience. Interested participants should contact treeshrewmeeting@gmail.com
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
E.S, M.S-S., R.W. and D.F. conceived the review and prepared the draft. All authors contributed to the discussions, read, and approved the final version of the manuscript.
Funding Statement
This work was supported by the National Institutes of Health Grant EY032327 (to D.F.)
References
- 1.Casagrande VA, Diamond IT Ablation study of the superior colliculus in the tree shrew (Tupaia glis) . The Journal of Comparative Neurology. 1974;156(2):207–237. doi: 10.1002/cne.901560206. [DOI] [PubMed] [Google Scholar]
- 2.Darwish M, Nishizono H, Uosaki H, Sawada H, Sadahiro T, Ieda M, et al Rapid and high-efficient generation of mutant mice using freeze-thawed embryos of the C57BL/6J strain. Journal of Neuroscience Methods. 2019;317:149–156. doi: 10.1016/j.jneumeth.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Dimidschstein J, Chen Q, Tremblay R, Rogers SL, Saldi GA, Guo LH, et al A viral strategy for targeting and manipulating interneurons across vertebrate species. Nature Neuroscience. 2016;19(12):1743–1749. doi: 10.1038/nn.4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drenhaus U, Rager G, Eggli P, Kretz R On the postnatal development of the striate cortex (V1) in the tree shrew (Tupaia belangeri) . European Journal of Neuroscience. 2006;24(2):479–490. doi: 10.1111/j.1460-9568.2006.04916.x. [DOI] [PubMed] [Google Scholar]
- 5.El Hamdaoui M, Levy AM, Gaonkar M, Gawne TJ, Girkin CA, Samuels BC, et al Effect of scleral crosslinking using multiple doses of genipin on experimental progressive myopia in tree shrews. Translational Vision Science & Technology. 2021;10(5):1. doi: 10.1167/tvst.10.5.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emmons LH, Greene HW. 2000. Tupai: A Field Study of Bornean Treeshrews. Berkeley: University of California Press.
- 7.Fan Y, Huang ZY, Cao CC, Chen CS, Chen YX, Fan DD, et al Genome of the Chinese tree shrew. Nature Communications. 2013;4:1426. doi: 10.1038/ncomms2416. [DOI] [PubMed] [Google Scholar]
- 8.Fan Y, Ye MS, Zhang JY, Xu L, Yu DD, Gu TL, et al Chromosomal level assembly and population sequencing of the Chinese tree shrew genome. Zoological Research. 2019;40(6):506–521. doi: 10.24272/j.issn.2095-8137.2019.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs E Tree shrews at the German Primate Center. Primate Biology. 2015;2(1):111–118. doi: 10.5194/pb-2-111-2015. [DOI] [Google Scholar]
- 10.Gawne TJ, Ward AH, Norton TT Long-wavelength (red) light produces hyperopia in juvenile and adolescent tree shrews. Vision Research. 2017;140:55–65. doi: 10.1016/j.visres.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubrecht R, Kirkwood J. 2010. The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals. 8th ed. Chichester: Wiley.
- 12.Janečka JE, Miller W, Pringle TH, Wiens F, Zitzmann A, Helgen KM, et al Molecular and genomic data identify the closest living relative of primates. Science. 2007;318(5851):792–794. doi: 10.1126/science.1147555. [DOI] [PubMed] [Google Scholar]
- 13.Johnson EN, Westbrook T, Shayesteh R, Chen EL, Schumacher JW, Fitzpatrick D, et al Distribution and diversity of intrinsically photosensitive retinal ganglion cells in tree shrew. Journal of Comparative Neurology. 2019;527(1):328–344. doi: 10.1002/cne.24377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khani A, Rainer G Recognition memory in tree shrew (Tupaia belangeri) after repeated familiarization sessions. Behavioural Processes. 2012;90(3):364–371. doi: 10.1016/j.beproc.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Le Gros Clark WE On the brain of the tree-shrew (Tupaia minor) . Proceedings of the Zoological Society of London. 1924;94(4):1053–1074. doi: 10.1111/j.1096-3642.1924.tb03328.x. [DOI] [Google Scholar]
- 16.Lee KS, Huang XY, Fitzpatrick D Topology of ON and OFF inputs in visual cortex enables an invariant columnar architecture. Nature. 2016;533(7601):90–94. doi: 10.1038/nature17941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li CH, Yan LZ, Ban WZ, Tu Q, Wu Y, Wang L, et al Long-term propagation of tree shrew spermatogonial stem cells in culture and successful generation of transgenic offspring. Cell Research. 2017;27(2):241–252. doi: 10.1038/cr.2016.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyon Jr MW Treeshrews: an account of the mammalian family Tupaiidae. Proceedings of the United States National Museum. 1913;45(1976):1–188. doi: 10.5479/si.00963801.45-1976.1. [DOI] [Google Scholar]
- 19.Maher EE, Prillaman ME, Keskinoz EN, Petry HM, Erisir A Immunocytochemical and ultrastructural organization of the taste thalamus of the tree shrew (Tupaia belangeri) . Journal of Comparative Neurology. 2021;529(10):2558–2575. doi: 10.1002/cne.25109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mantilla GPW, Chester SGB, Clemens WA, Moore JR, Sprain CJ, Hovatter BT, et al Earliest Palaeocene purgatoriids and the initial radiation of stem primates. Royal Society Open Science. 2021;8(2):210050. doi: 10.1098/rsos.210050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller B, Peichl L Topography of cones and rods in the tree shrew retina. Journal of Comparative Neurology. 1989;282(4):581–594. doi: 10.1002/cne.902820409. [DOI] [PubMed] [Google Scholar]
- 22.Mustafar F, Harvey MA, Khani A, Arató J, Rainer G Divergent solutions to visual problem solving across mammalian species. eNeuro. 2018;5(4):e0167–18.2018. doi: 10.1523/ENEURO.0167-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ni XJ, Qiu ZD Tupaiine tree shrews (Scandentia, Mammalia) from the Yuanmou Lufengpithecus locality of Yunnan, China . Swiss Journal of Palaeontology. 2012;131(1):51–60. doi: 10.1007/s13358-011-0029-0. [DOI] [Google Scholar]
- 24.Norton TT, Khanal S, Gawne TJ Tree shrews do not maintain emmetropia in initially-focused narrow-band cyan light. Experimental Eye Research. 2021;206:108525. doi: 10.1016/j.exer.2021.108525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petry HM, Bickford ME The second visual system of the tree shrew. Journal of Comparative Neurology. 2019;527(3):679–693. doi: 10.1002/cne.24413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petry HM, Fox R, Casagrande VA Spatial contrast sensitivity of the tree shrew. Vision Research. 1984;24(9):1037–1042. doi: 10.1016/0042-6989(84)90080-4. [DOI] [PubMed] [Google Scholar]
- 27.Sajdak BS, Salmon AE, Cava JA, Allen KP, Freling S, Ramamirtham R, et al Noninvasive imaging of the tree shrew eye: wavefront analysis and retinal imaging with correlative histology. Experimental Eye Research. 2019;185:107683. doi: 10.1016/j.exer.2019.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sedigh-Sarvestani M, Lee KS, Satterfield R, Shultz N, Fitzpatrick D. 2021. A sinusoidal transform of the visual field in cortical area V2. <italic>bioRxiv</italic>, doi: <ext-link ext-link-type="uri" xlink:href="https://doi.org/10.1101/2020.12.08.416651">https://doi.org/10.1101/2020.12.08.416651</ext-link>.
- 29.Sherman SM, Norton TT, Casagrande VA X- and Y-cells in the dorsal lateral geniculate nucleus of the tree shrew (Tupaia glis) . Brain Research. 1975;93(1):152–157. doi: 10.1016/0006-8993(75)90294-2. [DOI] [PubMed] [Google Scholar]
- 30.Simpson GG The principles of classification and a classification of Mammals. Bulletin of the American Museum of Natural History. 1945;85(16):1–350. [Google Scholar]
- 31.Smith GB, Fitzpatrick D. 2016. Viral injection and cranial window implantation for in vivo two-photon imaging. In: Schwartzbach SD, Skalli O, Schikorski T. High-Resolution Imaging of Cellular Proteins. New York, NY: Springer New York, 171–185.
- 32.Snyder M, Diamond IT The organization and function of the visual cortex in the tree shrew. Brain, Behavior and Evolution. 1968;1(3):244–288. doi: 10.1159/000125507. [DOI] [Google Scholar]
- 33.Van Valen L Treeshrews, primates, and fossils. Evolution. 1965;19(2):137–151. doi: 10.1111/j.1558-5646.1965.tb01701.x. [DOI] [Google Scholar]
- 34.Wilson DE, Mittermeier RA. 2009. Handbook of the Mammals of the World. Barcelona: Lynx Edicions.
- 35.Xu L, Chen SY, Nie WH, Jiang XL, Yao YG Evaluating the phylogenetic position of chinese tree shrew (Tupaia belangeri chinensis) based on complete mitochondrial genome: implication for using tree shrew as an alternative experimental animal to primates in biomedical research . Journal of Genetics and Genomics. 2012;39(3):131–137. doi: 10.1016/j.jgg.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Yartsev MM The emperor’s new wardrobe: rebalancing diversity of animal models in neuroscience research. Science. 2017;358(6362):466–469. doi: 10.1126/science.aan8865. [DOI] [PubMed] [Google Scholar]


