Abstract
Although once daily anti-glaucoma drug therapy is a current clinical reality, most therapies require multiple dosing and there is an unmet need to develop convenient, safe, and effective sustained release drug delivery systems for long-term treatment to improve patient adherence and outcomes. One of the first sustained release drug delivery systems was approved for the reduction of intraocular pressure in glaucoma patients. It is a polymeric reservoir-type insert delivery system, Ocusert™, placed under the eyelid and on the ocular surface for zero-order drug release over one week. The insert, marketed in two strengths, released Pilocarpine on the eye surface. While many clinicians appreciated this drug product, it was eventually discontinued. No similar sustained release non-invasive drug delivery system has made it to the market to date for treating glaucoma. Drug delivery systems under development include punctal plugs, ring-type systems, contact lenses, implants, microspheres, nanospheres, gels, and other depot systems placed in the extraocular, periocular, or intraocular regions including intracameral, supraciliary, and intravitreal spaces. This article discusses the advantages and disadvantages of the various routes of administration and delivery systems for sustained glaucoma therapy. It also provides the reader with some examples and discussion of drug delivery systems that could potentially be applied for glaucoma treatment. Interestingly, one intracamerally injected implant, Durysta™, was approved recently for sustained intraocular pressure reduction. However, long-term acceptance of such devices has yet to be established. The ultimate success of the delivery system will depend on efficacy relative to eye drop dosing, safety, reimbursement options, and patient acceptance. Cautious development efforts are warranted considering prior failed approaches for sustained glaucoma drug delivery. Neuroprotective approaches for glaucoma therapy including cell, gene, protein, and drug-combination therapies, mostly administered intravitreally, are also rapidly progressing towards assessment in humans.
Keywords: drug delivery, topical, intraocular, intraocular pressure, sustained release
1.0. Introduction
1.1. The need for sustained release glaucoma therapy
Currently a variety of eye drops are available for reducing elevated intraocular pressure (IOP), which is the most important and only treated risk factor that if left untreated, will lead to glaucoma disease progression to irreversible blindness. The therapeutic molecules include various classes of pharmacological agents such as prostaglandins, beta-blockers, rho kinase inhibitor (i.e., netarsudil), nitric oxide donor (i.e., latanoprostene bunod), alpha-2 adrenergic agonist, carbonic anhydrase inhibitors, and muscarinic agonists. These therapeutic agents, either alone or in combination, are effective in reducing intraocular pressure. While some agents such as prostaglandins require once-a-day dosing, several others require multiple daily doses. Studies estimate patient adherence for the regimented use of glaucoma eye drops to be less than 50% (Robin and Grover, 2011, Hwang et al., 2014, Newman-Casey et al., 2015, Feehan et al., 2016, Nordstrom et al., 2005, Ribeiro et al., 2016, Rajurkar et al., 2018) with approximately 60% of the patients having difficulties administering them (Hennessy et al., 2011). Additionally, eye drops deliver drug to the eye in a high frequency, pulsatile fashion with a peak drug concentration followed by a valley before the next dose is administered the same day or the following day. With such a pulsatile time-course of drug concentrations or pharmacokinetics, drug effects can wax and wane, which could result in elevated intraocular pressure at different points during a day depending on the nature of the drug being administered. Perhaps a better alternative would be continuous drug delivery and the associated sustained suppression of IOP. Besides sustained IOP suppression, another potential advantage of the controlled release delivery systems is the reduced total dose and lower or slower systemic exposure, which reduces the risk of systemic side effects. As is usually the case, this type of drug administration also has negative consequences. For instance, continuous drug delivery may, in some cases, lead to the development of drug tolerance and any burst release from these systems could negatively affect the tissues. These concerns should be evaluated on a case by case basis.
Sustained drug delivery can be achieved using a variety of delivery systems including implants, microparticles, nanoparticles, and gels or their combination. These systems are prepared typically with carrier materials or by using pure drug (composite vs. pure drug delivery systems, Figure 1).
Figure 1.
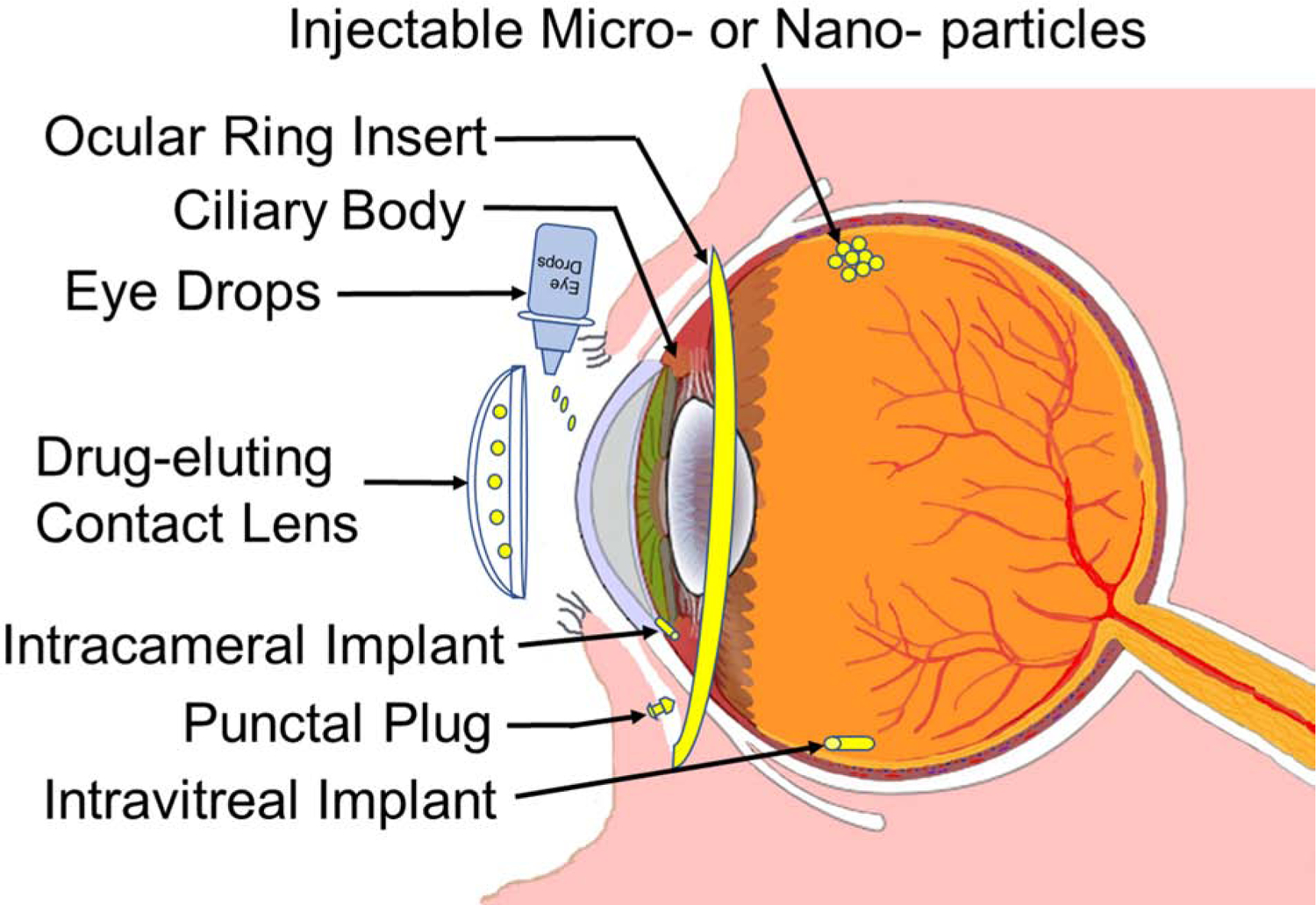
Diagram of the human eye with ocular drug delivery systems. Redrawn based on the eye image from Wikimedia common at; https://commons.wikimedia.org/wiki/File:Schematic_diagram_of_the_human_eye_en.svg
Composite drug delivery systems can be broadly classified as reservoir or matrix types of drug delivery systems. In a reservoir type delivery system, the drug is present in a core, surrounded by impermeable or rate limiting membranes that control rate of drug release. While in the case of matrix type of delivery systems, the drug is dispersed throughout the delivery system in a carrier material. Typically, reservoir type systems can be engineered to deliver the drug in a zero-order fashion (constant rate of drug release) for most of the life of the delivery system, while the matrix type of systems release the drug in a non-zero order fashion (typically a declining rate of drug release, Figure 2).
Figure 2.

Typical reservoir and matrix delivery systems for sustained drug delivery that can potentially release the drug at a zero-order or non-zero order rates (for instance only a few contact lens delivery systems can achieve zero-order release).
However, depending on the delivery system design and manufacturing, the release rates can be different from the previously mentioned general trends. Ideally, an anti-glaucoma drug delivery system will be a zero-order sustained release system. Such a system will maintain constant drug levels for about 4 months or longer near the targeted tissue site to continuously suppress the IOP following a single dose administration. Another characteristic of the best delivery system would be that it does not require invasive eye surgery or injection to position itself for drug delivery to the targeted ocular tissues, while being sufficiently effective with a low safety risk. While primarily discussing sustained drug delivery systems for reducing IOP, this article also discusses sustained neuroprotection in glaucoma, based on cell, gene, and polymeric microsphere therapies.
2. Ocular surface drug delivery systems
For the purpose of this review, extraocular or ocular surface drug delivery systems are those that allow noninvasive placement on or near the eye surface, including eye drops, in order to deliver the drug directly to nearby ocular tissues. Thus, extraocular drug delivery systems can be placed in multiple locations including but not limited to the corneal surface, conjunctival fornix or cul-de-sac, punctum or nasolacrimal duct, and on or underneath the eyelid. Extraocular routes allow the use of implants/inserts, particles, and gels as delivery systems for sustaining drug delivery. Of these systems, implants (i.e., reservoir type ring inserts) are most likely to offer the longest durations of drug release for therapeutic purposes, while using the least amount of carrier material. Materials should be judiciously selected depending on the treatment location. Accordingly, punctal plugs may be made of either hard or soft materials, while those delivery systems intended for use on the corneal surface and in the cul-de-sac should be composed entirely of soft materials. Gels and particles of various drugs are already in clinical use for daily dosing. However, gels and particles capable of releasing drug for several days to months following extraocular dosing are not in clinical use. Each of the potential sites of extraocular administration and representative delivery systems (Figure 1) are discussed below.
2.1. Contact lens delivery systems
Contact lenses are being evaluated as a potential alternative to eye drops for the delivery of ophthalmic drugs. At the present time more than 90% of ocular drug products are eye drops, which have proven to be a very ineffective drug delivery system due to rapid drainage and nonproductive absorption. Mainly due to the short residence time on the ocular surface, only less than 5% of drug is typically bioavailable to the ocular tissues, requiring the frequent administration of drops with high drug concentrations to maintain target drug concentration within the therapeutic window (Peng et al., 2012a, Peng et al., 2012b). Contact lenses can be very effective for treating corneal disease because the lenses can be placed directly on the cornea surface separated only by a thin fluid layer known as the post-lens tear film, which is not as well-mixed with the tear film unlike an instilled eye drop. The drug release from the contact lenses into this tear film is retained in front of the cornea for an estimated 30 min, compared to an estimated 2 min for the commercial eye drop (Peng et al., 2010, Peng et al., 2012a, Friedman et al., 2005, Creech et al., 2001). This increase in residence time can lead to an increase in drug bioavailability to an estimated 50% compared to about 5% or less typically provided by drops (Peng et al., 2012b, Creech et al., 2001, Li and Chauhan, 2006). This is one of the main reasons why contact lenses area being developed as drug delivery systems for several eye diseases (Creech et al., 2001, Li and Chauhan, 2006).
Although contact lens ocular drug delivery systems were first investigated by Wichterle in 1960 and the interest and research has continued over the years, with most activity evident at the present time, there have been no FDA-approved products using this drug delivery system. This could be due to the challenges and risk associated with this particular drug delivery platform (Witcherle and Lim, 1960, Lim et al., 2002), which are gradually being surmounted. Some of the challenges encountered for this type of platform have included; drug loading (i.e., some of the approaches have limited drug loading capabilities), drug delivery (i.e., sustained delivery for a specific time at a controlled rate), optical clarity, patient comfort, and biocompatibility. The risks encountered have included microbial keratitis and dry eye syndrome (Lim et al., 2002).
Contact lenses are made using hydrogels such as poly (2-hydroxyethyl methacrylate) (pHEMA) or silicone that allow water and nutrient movement to the corneal surface. Soft contact lenses are defined by the FDA as soft, flexible plastic materials that permit oxygen to penetrate through to the eye surface. Additionally, the FDA categorizes the lenses into four groups with subcategories based on production generations. For silicone hydrogels, there are three production generations: 1) Lotrafilcon A and Balafilcon A, 2) Senofilcon A and Galyfilcon A, and 3) Samfilcon A, Comfilcon A, and Enfilcon A (Szozotka-Flynn, 2008, Harvitt and Bonanno, 1999, Holden and Mertz, 1984, Tighe, 2013, Rex et al., 2018, Musgrave and Fang, 2019). Most contact lenses are intended for daily use, while several others are intended for extended wear up to 7 days; Biofinity by CooperVision made out of Comfilcon A or SiH48, Acuvue Oasys by Johnson and Johnson made out of Senofilcon A, and Bausch + Lomb Ultra made out of Samfilicon A fall into this category. At least two brands of silicone hydrogels, Air Optix Night & Day AQUA by Alcon made of Lotrafilcon A and PureVision2 by Bausch + Lomb made of Balafilcon A are contact lenses FDA-approved for continuous wear up to 30 days.
While some investigational new drug applications are approved for contact lens-based drug delivery, published data to date is in preclinical animal models. These studies assessed the possibility of using contact lens delivery systems for extended periods. One study assessed delivery up to 100 days (Xu et al., 2018, Ciolino et al., 2009, Ciolino et al., 2014). This lens was fabricated by coating PLGA films containing fluorescein or the antibiotic ciprofloxacin with pHEMA and then polymerizing with UV light exposure (Ciolino et al., 2009).
Contact lens delivery systems received significant attention for sustained drug delivery to the eye for a variety of purposes including anti-glaucoma drug therapy. The advantages of the contact lens delivery system include ease of wear, direct contact with corneal surface, the main port of entry for topically dosed anti-glaucoma drugs, and the amenability of hydrogels used in contact lens for drug incorporation. Drug in a contact lens delivery system is typically loaded in the periphery of the contact lens, away from the pupil and the visual axis, to not obstruct vision. Contact lens based delivery systems can include the drug in the lens in a variety of configurations including but not limited to coating or embedding of drug-polymer film in the contact lens, molecular imprinting of drug in polymeric hydrogel, surface adsorption of nano-carriers or drugs in contact lens with hydrogel, dispersion of nanoparticles, liposomes, or emulsions within contact lens hydrogel, dispersion of surfactant-drug complexes in the lens, and adsorption of drug to preformed contact lens material through soaking (Kompella et al., 2010) (Figure 3).
Figure 3.
Contact lenses as ocular drug delivery systems. Various types of drug molecules or delivery systems can be placed into the periphery of lenses using several approaches.
The simplest approach for loading contact lens involves the soaking of the polymeric hydrogel lenses (i.e., pHEMA or silicone hydrogel) in a concentrated solution of the active pharmaceutical ingredient (API) molecules. Although this approach is easy to perform, it has several limitations such as a limited drug loading capability and fast drug release from the lenses usually within 24 hours (Hehl et al., 1999, Sedlacek, 1965, Karlgard et al., 2003a, Karlgard et al., 2003b). However, some of the modified silicone hydrogel systems have provided extended drug delivery for 15–20 days or up to 200 days depending on the composition of the lens (Kim et al., 2008a). To better control drug release, Nakada and Sugiyama and some others developed compound lens wherein two lenses were bonded together with a hollow cavity between them providing a reservoir to increase the lens drug loading capacity (Nakada and Sugiyama, 1998). Nakada and Sugiyama used polymerizable methoxy silane compounds (Nakada and Sugiyama, 1998). Although higher drug levels were achieved with these lenses, the process also increased the lens thickness, which seemed to impede O2 and CO2 permeability. Changing the contact lens composition to a silicone hydrogel polymer that produces a thinner lens could make the fabrication more suitable for drug delivery and improve O2 and CO2 permeability (Rootman et al., 1992, Sano et al., 1996, Le Bourlais et al., 1998, Xinming et al., 2008). Some researchers developed and used a molecularly imprinted polymeric hydrogel approach (Hiratani and Alvarez-Lorenzo, 2002, Hiratani et al., 2005, Alvarez-Lorenzo et al., 2006). In this case, the imprinted hydrogels prepared from complexes of methacrylic acid polymerized with N,N-diethylacrylamide and ethylene glycol dimethacrylate were molded to recognize the drug structure and bonding features to improve drug molecule adsorption (Hiratani and Alvarez-Lorenzo, 2002). Lenses made using this method provided increased drug loading depending on the incorporated drug and prolonged delivery over the traditional lens soaking approach. A drawback with this approach was the variation in the drug release depending upon the active ingredient incorporated into the lens. Yet another approach required the conjugation of nanoparticles or drug molecules, which is accomplished by the surface functionalization of the contact lens to attach the drug or drug-loaded nano-carriers (i.e., liposomes) (Danion et al., 2007b, Danion et al., 2007a). But this approach also had several drawbacks like the rapid detachment of drug or disintegration of liposomes and the potential to impede O2 and CO2 permeability. Therefore, another group of researchers tried integrating a drug-polymer film onto the contact lens surface (Ciolino et al., 2009). This approach entailed the coating of a pHEMA hydrogel contact lens with a drug-65:35 poly (D, L-lactide-co-glycolide, or PLGA) polymer film. Drug release properties could be manipulated by changing the type or molecular weight of the polymers or the drug to polymer ratios. Finally, the last approach mentioned here requires the entrapment of drugs in liposomes, microparticles, nanoparticles, or surfactants that are added to the hydrogel preparation followed by polymerization to form the contact lens (Gulsen and Chauhan, 2005, Gulsen et al., 2005, Gulsen and Chauhan, 2004, Kapoor et al., 2009).
In addition to the above approaches, the contact lens hydrogel may be used to disperse two agents within the lens, one to hinder the diffusion or release of a second agent (Peng et al., 2010). Using such an approach, Peng et al., 2012 (Peng et al., 2012a) assessed a contact lens delivery system based on vitamin E (25% w/w loading) diffusion barriers to sustain the delivery of the anti-glaucoma drug timolol with a 67 or 200 μg drug loading. The lenses were compared with timolol eye drops for their efficacy in reducing IOP in spontaneously glaucomatous beagle dogs. This study indicated a similar IOP reduction at the end of 4 days with contact lens delivery system (5.02 mm Hg), which were replaced daily, and 0.5% timolol once-a-day eye drops (4.64 mm Hg). In subsequent studies using the same dog model, this team showed that both dorzolamide and timolol maleate can be co-delivered from a single contact lens preparation and that this system (containing 680 μg of dorzolamide and 200 μg timolol maleate), when placed continuously on the eye surface for 4 days, was 2-fold superior (5.22 mm Hg IOP reduction) compared to twice-a-day eye drop therapy (2.6 mm Hg reduction) with Cosopt topical eye drops (containing 400 μg of dorzolamide and 120 μg timolol maleate).
Ciolino’s group developed contact lenses to sustain the release of latanoprost, a prostaglandin analog and anti-glaucoma drug. These lenses containing either a high dose (149 μg) or a low dose (97 μg) of the drug were compared with a commercial 0.005% latanoprost daily eye drop in a glaucomatous monkey model (Ciolino et al., 2016). In their study, 1-week wear of high and low dose contact lenses resulted in a diurnal IOP drop range of 6.0–10.2 mm Hg and 4.0–7.8 mm Hg, respectively. Eye drops, on the other hand, resulted in an IOP reduction of 2.9–6.6 mm Hg on day 5 (Table 1). Thus, the contact lens delivery system exerts more prolonged effects, while using a lower drug amount compared to eye drops (Peng et al., 2012a).
Table 1.
Mean diurnal IOP reduction by high and low dose latanoprost eluting contact lens delivery system vs. topical eye drops. Based on a glaucomatous monkey model described by Ciolino et. al., 2016.
| Contact Lens High Dose (149 μg) | Contact Lens Low Dose (97 μg) | Topical Eye Drop (50 μl, 2.5 μg) | |
|---|---|---|---|
| Mean Diurnal IOP Reduction after the Treatment Period (mm Hg) | 6.0–10.2 | 4.0–7.8 | 2.9–6.6 |
| Dosing | Single sustained release lens | Single sustained release lens | Once daily |
| Treatment Period | 8 days | 8 days | 5 days |
Some other innovations in the contact lens area include a diamond nano-gel-embedded contact lenses that mediate lysozyme-dependent drug release (Kim et al., 2014a), self-implantable double-layered micro-drug-reservoirs for efficient and controlled ocular drug delivery (Than et al., 2018), co-delivery of latanoprost and timolol from micelles-loaded contact lenses for the treatment of glaucoma (Xu et al., 2019), and engineering and development of chitosan-based nano-coatings for ocular contact lenses (Mehta et al., 2019). While fancy material innovations are readily feasible, a practical manufacturing method and a device that is biologically superior to standard of care for improving patient outcomes are hard to come by.
Another approach to improve drug delivery employed polymeric films similar to contact lenses, to increase the drug residence time and achieve controlled drug release (Tighsazzadeh et al., 2019). These composite thin and erodible polymeric films were developed using two different polymers, hyaluronic acid (HA) and hydroxypropyl methylcellulose (HPMC), which are currently used as thickening agents in some eye drop formulations. Formulation compositions included single polymer preparations from 1% w/v HA and 1.5% w/v HPMC, and 1% composite gels with a 1:1 ratio HA: HPMC and the last, a 2% composite gel with a ratio of 3:1 HA: HPMC. All formulations included the addition of glycerol as a plasticizer at a 2:1 polymer to glycerol weight ratio and were loaded with 0.5% w/v of the anti-glaucoma drug timolol maleate (Tighsazzadeh et al., 2019). The study results revealed that ocular films of HA and HPMC could be produced either alone or in combination. Composite formulations were to some degree better performers because they combined the strong film forming properties of HPMC polymer with the remarkable swelling capacity of the HA polymer (Tighsazzadeh et al., 2019). Also, these films were generally biocompatible as was evidenced by the cell viability results. These composite films may be useful as a topical ocular drug delivery platform to enhance drug residence time and improve bioavailability (Tighsazzadeh et al., 2019). At the time of this publication the researchers were planning further evaluations using in vivo animal model studies. Like this study, several literature reports, while advancing materials, fall short on in vivo proof of an advantage relative to the standard of care.
While there are several advantages to using contact lens delivery systems including ease of placement, enhanced bioavailability, and sustained release and efficacy, for patients already wearing contact lenses for vision correction, the delivery systems need to be tailored to fit their visual correction needs. Accordingly, not all patients may prefer to use contact lens delivery systems. While the extended wear of contact lenses is feasible, the safety of such a drug delivery system has yet to be established and any roughness in the surface of the contact lens should be addressed to prevent complications (Choi and Kim, 2018). Additionally, the newly developed lenses should be characterized/improved to prevent bacterial transfer and drug loss during storage/distribution. However, with the growing use of contact lenses for vision correction in the current generation, contact lens drug delivery systems may receive wider acceptance in future.
2.2. Dendrimer nanofiber mats
Dendrimers are a class of polymers which are comprised of radially symmetrical multivalent molecules with well-defined branched structures that are in the nanometer size (Abbasi et al., 2014). They are also referred to as starburst polymers, cascade molecules, or arborols and synthesized initially around 1980 by Tomalia (Tomalia et al., 1985), Newkome (Newkome et al., 1985), and Vogle (Buhleier et al., 1978). Since that time numerous researchers have contributed to their development and biomedical applications. During the last two decades and more particularly the past five years, there has been an increasing number of biological and chemical related publications (around 1000) on dendrimers which have greatly advanced the field (Janaszewska et al., 2019). Their molecular construction consists of three different sections; a core, the branches, and terminal functional end groups that can be functionalized using a number of materials including therapeutic compounds (Nimesh, 2013, Klajnert and Bryszewska, 2001, Abbasi et al., 2014, Kumar et al., 2017). This versatility makes dendrimers attractive vehicles for drug delivery. Among various chemistries, polyamidoamine (PAMAM) dendrimers are most investigated for drug delivery. PAMAM dendrimer cytotoxicity is dependent on the generation (Gn), number of surface groups, and nature of terminal moieties (i.e., anionic, neutral, or cationic). Higher toxicity is associated with higher generation ≥ G4 dendrimers and a positive charge on the surface (Janaszewska et al., 2019, Madaan et al., 2014). To combat this problem, research focused on adding different chemical modifications to the periphery of the molecule, which led to the discovery that the cytotoxicity could be decreased with certain modifications. For instance, PAMAM dendrimers synthesized with polyethylene glycol (PEG), acetyl groups, carbohydrates, pyrrolidone, maltose, maltotriose, poly (propylene imine) (PPI), lauroyl chloride, poly (ethylene oxide) (PEO) and other biocompatible groups can significantly reduce cytotoxicity while maintaining other advantages (Jevprasesphant et al., 2003a, Jevprasesphant et al., 2003b, Malik et al., 2012, Ciolkowski et al., 2012, Stasko et al., 2007, Gillies et al., 2005, Gupta et al., 2010). Additionally, they can potentially be used in conjunction with drug-loaded nanoparticles or as degradable solid inserts (Lancina et al., 2017, Yang et al., 2012). A solid dendrimer-based material would have significant advantages over aqueous solutions with respect to storage stability and potentially retention at the site of administration.
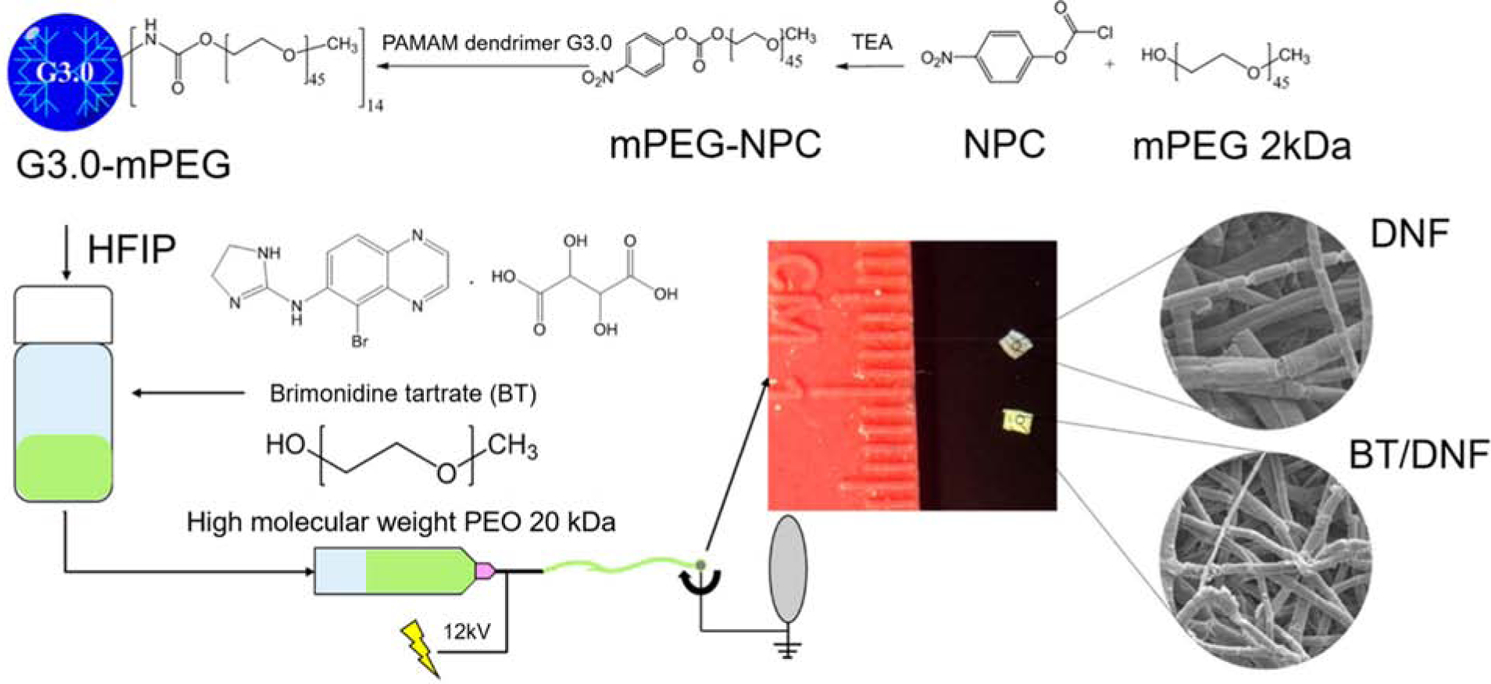
One unique example for sustained anti-glaucoma therapy following drug application to the ocular surface is the use of dendrimer nanofiber (DNF) mats (Lancina et al., 2017). In this study, fast dissolving, dendrimer nanofiber mats were prepared from modified polyamidoamine (PAMAM) G3.0 dendrimers co-spun with polyethylene oxide (PEO) and the anti-glaucoma drug brimonidine tartrate in a multistage process (Figure 4).
Figure 4.

Synthesis and fabrication of dendrimer nanofiber (DNF) and brimonidine tartrate (BT)/DNF mats using electrospinning. Reprinted with permission from Lancina et al., 2017. Copyright 2017 American Chemical Society.
After fabrication, the dendrimer-based nanofiber mats were assessed in vitro using cultured cells and in vivo using a normotensive Brown Norway rat model for safety and efficacy. The DNF mats dissolved immediately after placement onto the eye. In vivo experiments compared daily brimonidine tartrate drops and DNF mat daily dosing up to 21 days (Lancina et al., 2017). As the test period progressed the repeated administration of the DNF mats produced an accumulative lowering of IOP which continued for the remainder of the test period (Figure 5). Overall, the in vivo, ex vivo, and in vitro evaluations suggested that the DNF mats provided good ocular compatibility and drug delivery efficacy.
Figure 5.
Brimonindine tartrate-dendrimer nanofiber (BT/DNF) mats reduce intraocular pressure. In vivo 3-week daily dose response is shown. Brown Norway rats (n=4) received a daily dose of brimonidine via saline eye drops or DNF mat for three weeks. IOP was recorded immediately prior to drug application. Values expressed are the difference between the experimental and contralateral eyes after normalizing individual eyes to baseline levels. The dash lines represent the mean IOP reduction values. DNF mat delivery system was able to sustain IOP reduction over the test period compared to saline eye drops (# indicates significant difference, P < 0.001). Error bars represent standard deviation. Reprinted with permission from Lancina et al., 2017. Copyright 2017 American Chemical Society.
2.3. Conjunctival fornix or cul-de-sac delivery systems
Implants, gels, or particles can be used for sustained drug delivery in the conjunctival fornix. Of these, implant like systems, commonly referred to as inserts, were previously approved for clinical use. Ocusert, a reservoir type of insert, provided a sustained 1-week zero-order release system for pilocarpine that is noninvasively placed in the conjunctival fornix was approved by the FDA on July 29, 1974, after a priority review (Figure 6).
Figure 6.
Descriptive diagram of the discontinued pilocarpine ocular therapeutic system (Ocusert® device) placed in the inferior fornix for sustained IOP reduction for one week.
The Ocusert system was available in two strengths to release 20 or 40 μg/hour (480 or 960 μg/day) of the drug in a 7 day period (Macoul and Pavanlangston, 1975). The delivery system was in use at least until 1993, beyond which it was discontinued by the manufacturer. Advantages of Ocusert system included continuous effectiveness, reliable dosing in children and the elderly, less effect on accommodation and less miosis relative to drops, and patient convenience (Pollack et al., 1976). Some disadvantages included requiring patient wear instruction, device movement and/or loss without patient knowledge, occasional cutting sensation, transient blurring of vision, miosis, and high cost.
Recently, another insert, referred to as a fornix ring, was in late stage development for clinical studies (Brandt et al., 2016, Brandt et al., 2017). The prototype fornix ring-type insert evaluated in the clinic by Allergan had a diameter of 24 to 29 mm, a cross-sectional thickness of 1 mm, with a central polypropylene support structure surrounded by silicone-bimatoprost matrix (Brandt et al., 2017, Brandt et al., 2016) (Figure 7). This insert is a matrix type system with drug distributed throughout the polymer, with a declining bimatoprost drug release rate, 35 μg/day on day 1 and 0–6 μg/day by day 180. The insert achieved a mean IOP reduction of 3.2 to 6.4 mm Hg at 6-months, compared to 4.2 to 6.4 mm Hg with the placebo insert and 0.5% timolol BID drops.
Figure 7.
A representative diagram of the Allergan’s Bimatoprost Ocular Insert under evaluation.
In addition to the above non-degradable inserts, degradable/erodible inserts may also be used. Drug release is controlled by diffusion for the non-degradable inserts and by dissolution/erosion as well as diffusion for other inserts (Khan et al., 2019, Morrison and Khutoryanskiy, 2014). Ocular inserts, especially the non-degradable systems, have several advantages and disadvantages (Khan et al., 2019, Morrison and Khutoryanskiy, 2014). The advantages include reproducible release kinetics, precise drug dosing, increased residence time, continuous slow release of drug, reduced daily fluctuations in tissue drug levels, prolonged drug activity, slower and/or lower systemic absorption, the possibility of combination therapies, increased shelf-life, avoidance of preservatives, reduced dosing frequency, and better patient compliance. Disadvantages of ocular inserts include the foreign body sensation experienced most often by oversensitive patients, burst release prior to controlled release, unwanted migration of the insert around the eye (e.g. vision interference or movement to the upper fornix), accidental loss while sleeping or after rubbing the eye, and in some cases difficulty placing or removing inserts (Khan et al., 2019, Morrison and Khutoryanskiy, 2014).
Besides some of the more classical type of inserts, there are some gels, particles or their combinations that can provide sustained release anti-glaucoma drug delivery. For instance, a topical dendrimer hydrogel containing plain drug or drug-loaded polymeric particles was used for sustained anti-glaucoma drug delivery or effects for a few days (Holden et al., 2012, Yang et al., 2012). The dendrimer hydrogel was tethered with three polyethylene glycol acrylate chains and it was designed to deliver two anti-glaucoma drugs, brimonidine and timolol. Because of the components of the hydrogel, the PBS solubility of bromonidine was improved. Compared to eye drops the dendrimer hydrogels brought about higher human corneal epithelial cell uptake. The in vitro drug release sustained over a period of 28–35 days (Holden et al., 2012, Yang et al., 2012). Similarly, another approach focused on a thermo-responsive hydrogel carrier with drug loaded polymer microspheres that is transformed to a solid non-degradable gel on the eye surface and provides a depot in the fornix, which is used for sustained anti-glaucoma drug delivery (Smolinsky, 2016, Fedorchak et al., 2017). This gel remains beneath the lower eyelid, releasing the drug for up to a month after which it can be removed. In preclinical rabbit studies, the IOP reduction provided by this delivery system was indicated to be comparable to twice-daily brimonidine drops for up to 28 days (Fedorchak et al., 2017).
2.4. Punctal delivery systems
To treat dry eye patients, punctal plugs (also known as lacrimal plugs) made of polymeric materials are routinely used (Kompella et al., 2010) (Figure 8). These plugs can be placed in one or both puncta present in each eye. Once in place, the punctal plug prevents drainage of tears via the nasolacrimal duct, thereby maintaining greater tear volume on the eye surface. This alleviates some of the dry eye symptoms. These plugs can be readily removed from the punctum. The plugs have structural elements that help insert and retain them in place. It can be envisioned that this plug material can be loaded with a drug and released in a unidirectional manner towards the eye surface, thereby allowing drug mixing with tear fluid and subsequent delivery to the eye surface and intraocular tissues. Such concepts were evaluated in clinical studies for latanoprost (Kompella et al., 2010).
Figure 8.

Punctal plug innovations for drug delivery. Punctal plugs can be either permanent (for dry eye) or temporary (for drug delivery). Common structural features, which can vary between different plugs, are the core (polymer matrix or drug matrix permeable to tear fluid), cap (semi- or im- permeable membrane with one or more pores), body (impermeable to drug and tear fluid) and nose (assists the insertion process). Plugs come in a variety of shapes and sizes. Some even change shape after insertion due to polymer activation at body temperature.
In a 2013 press release, it was indicated that QLT’s L-PPDS (Latanoprost Punctal Plug Delivery System), acquired by Mati Therapeutics Inc., was loaded with 70.5 μg of latanoprost per plug. When two such plugs (141 μg) were placed in an eye, after a month, the mean IOP reduction achieved was statistically significant at 5.7 mm Hg (Goldberg and Williams, 2012). Recently other punctal plug delivery systems have generated much interest and Dextenza from Ocular Therapeutix received FDA approval at the end of 2018. Dextenza is a punctal plug which elutes dexamethasone for the treatment of post-operative inflammation and pain. Ocular Therapeutix also has several variations including travoprost insert and travoprost implant in their pipeline, which could soon follow Dextenza with applications to the FDA.
Punctal plug delivery systems can be designed in a variety of ways with innovations incorporated for ease of plug insertion and prolonged retention with little or no failure rate (Kompella et al., 2010). Also, drug placement within the plug geometry can utilize innovative approaches comparable to the contact lens delivery systems. Drug solution, suspension, emulsion, nanoparticle or microparticle or liposome suspensions can potentially be loaded into the core of the plug. Yet another approach could potentially use various drug forms embedded throughout the plug matrix. Punctal plug delivery systems may have a selectively permeable homogenous membrane or an impermeable membrane with one or more pores that control drug release (Kompella et al., 2010).
Like other delivery systems, punctal plugs have their limitations or side effects which are usually quite rare and can be dependent on the type of plug insert (Jehangir et al., 2016). One of the biggest limitations of punctal plugs as drug carriers is that they can only handle the low drug doses typically required for potent drugs such as corticosteroids and prostaglandins. This is evidenced in the fact that the only approved plug system is Dextenza™, which delivers the corticosteroid dexamethasone, while success with other drugs is awaited (Sheppard et al., 2018, Talamo et al., 2017, Ocular Therapeutix, 2019). As for side effects, the most common one is a slight irritation or scratchiness in the tear duct area following the initial insertion which usually disappears after a period of acclimatization. Other potential side effects are inflammation, watery eyes, and allergic reaction to the plug material (Jehangir et al., 2016, Johnson, 2018, Boyd, 2020, Haddrill, 2017). Further, insertion of the wrong size of plug can cause it to stick out, all of which if experienced should be treated by a medical doctor.
2.5. Diagnostic IOP Monitoring
IOP monitoring in a nonclinical setting and continuous IOP monitoring would help understand the benefits of sustained anti-glaucoma drug delivery in a more comprehensive manner. At present, only a snapshot of the drug effects is captured in the clinical setting. Below, the need for IOP monitoring and the emerging approaches for continuous IOP monitoring are discussed.
Glaucoma ranks second as the leading cause of irreversible blindness worldwide (Sanchez and Martin, 2019, Dick et al., 2019). The most important and only treated risk factor is IOP and therefore, drug therapy is focused at reducing or maintaining IOP to prevent the optic nerve head damage and disease progression (Ha et al., 2012, Durairaj, 2015, Downs et al., 2011, Sanchez and Martin, 2019). External IOP measurement has been a reality since 1967 when Collins obtained the first wireless measurements (Collins, 1967). However, IOP measurements are typically recorded only during a clinical visit. This does not capture all the fluctuations in IOP, which has natural circadian rhythm, with maximum values at daybreak and minimum values by late afternoon (Konstas et al., 2004, Liu et al., 2003). Glaucoma is a 24 h disease and a continuous monitoring system for IOP could be very useful to improve diagnosis and treatment of this disease (Dick et al., 2019). However, this has remained a major challenge for at least the past 60 years (Sanchez and Martin, 2019, Maurice, 1958). Recently technological advances have been made for the continuous monitoring of IOP. For instance, biosensors with semiconductor components and several other devices using various measurement principles have been developed to provide a solution to this unmet need. Two devices have been introduced commercially, a contact lens known as the Sensimed Triggerfish contact lens sensor and a novel implantable sensor known as Eyemate (or Argos). Both monitors have received CE-Marking (a certification mark that indicates conformity with health, safety, and environmental protection standards for products manufactured within or outside the European Economic Area) even though they still have some obstacles to overcome before receiving wide acceptance.
The Sensimed Company introduced the Sensimed Triggerfish, a new contact lens sensor comprised of two platinum-titanium resistive strain-sensing gauges. (Chen et al., 2014, Sanchez and Martin, 2019). Embedded in the lens is a microprocessor that records circumferential area changes of the limbus and wirelessly sends a millivolt or ohm output signal that is proportional to them. The Sensimed Triggerfish contact lens sensor is being evaluated in 36 registered trials, but it has two major areas of concerns. The first concern is that no constant conversion factor between IOP (mm Hg) values and electronic current is in existence, which makes the results obtained non-intuitive for daily clinical use (Mansouri et al., 2012a). The second concern is that the contact lens sensor must be worn for extended periods of time which can cause corneal swelling and affect sensor measurements, leading to erroneous IOP measurements (Beltran-Agullo et al., 2017, Mertz, 1980, du Toit et al., 2003, Martin et al., 2007, Hubanova et al., 2014, Mansouri et al., 2012b).
The novel wireless IOP transducer (WIT) implantable sensor also known as the Eyemate (or Argos) is designed to be positioned in the sulcus space either concurrent to or after cataract extraction in patients with a history of primary open-angle glaucoma (Melki et al., 2014). It is designed to stay permanently in the patient’s eye. In the first safety study, the sensor demonstrated that it could reliably monitor IOP in a volunteer for 18 months following implantation. WIT sensors are comprised of three major parts, an ASIC chip, a circular micro-coil antenna, and eight pressure sensitive capacitors, all of which are encapsulated in silicone (Melki et al., 2014, Sanchez and Martin, 2019). The IOP measurements are generated by the mechanical deflection of a membrane located between arrays of capacitive pressure sensors in two parallel plates. This deflection causes a change in the distance between plates (Todani et al., 2011). The ARGOS study data obtained from clinical trials number NCT02945176 and NCT02434692, was presented by Koutsonas et al. (Koutsonas et al., 2015). It represented 1-year follow-up data from six patients implanted with the sensor and showed the potential of the sensor to monitor IOP continuously for the entire period. However, telemetry systems need to be simplified to prevent adverse conditions for patients and to facilitate the safe transfer of stored data (Sanchez and Martin, 2019). Even so the Eyemate product was launched in early 2019.
Despite their technical problems, the Sensimed Triggerfish contact lens sensor and Eyemate, have considerably advanced research in this area. Although the perfect IOP sensor device is not a current reality, it has been defined as a sensory device that is self-powered, noninvasive, biocompatible, provides direct and/or stable IOP measurements, and able to eliminate sudden fluctuations and/or signal drift while transferring the data at a safe frequency to some storage device (Sanchez and Martin, 2019).
3. Periocular drug delivery systems
Periocular routes of administration include subconjunctival, sub-Tenon, and posterior-juxtascleral route among others (Raghava et al., 2004). Of these, subconjuctival route can be used to place the drug close to limbus, cornea, and the ciliary body, allowing significant drug delivery to the anterior segment tissues including those contributing to ocular hypertension. The subconjunctival route allows dosing up to 0.5 mL volume, with a typical injection volume only around 100 μl. The subconjunctival route is amenable for dosing a variety of delivery systems including implants, microspheres, nanospheres, liposomes, and gels. Either in situ forming implants or preformed implants can be potentially dosed in this space. Due to crowding, removal of vehicle, and aggregation, even particulate delivery systems can form implant like structures in the subconjunctival space, as reported previously (Amrite and Kompella, 2005). The dosage forms administered in this space may be visible to the onlookers and dosing in this region may cause visible hemorrhage on the eye surface. However, the dosage form is away from the visual axis and will not interfere directly with vision. Placement at this site also allows retention of particles for prolonged periods, potentially allowing sustained drug delivery for a few months (Amrite et al., 2006, Amrite and Kompella, 2005).
Amrite and Kompella determined the ocular retention and distribution of microparticles and nanoparticles administered in the posterior subconjunctival region (Amrite and Kompella, 2005). In order to focus solely on the influence of particle size on disposition, non-biodegradable fluorescent polystyrene particles of various sizes (20, 200, and 2000 nm; carboxylate modified, negatively charged) obtained from commercial sources were used. Particles were administered to anaesthetized Sprague Dawley rats using a 27G needle. The disposition of the particles in the periocular and other ocular eye tissues was studied for 60 days with the quantification of particle amounts using liquid extraction followed by spectrofluorometric analysis. The effect on disposition of the particles was investigated with the dose set at 400 μg of particles. The penetration of the particles into the ocular tissues was negligible for microparticles and the nanoparticles. Some of the particles (200 and 2000 nm) were just about completely maintained at the site of administration after the 60 day study period, while the smaller 20 nm particles disappeared quickly (only 15 and 8% remained after 1 and 7 days, respectively and at 60 days they could not be detected) (Figure 9). Therefore, larger particles appear more suitable for sustained retention in the periocular space. Also, the effect of surface hydrophobicity of (20 nm; aldehyde sulfate modified; neutral charge) particles was investigated after 1-day post administration. Increasing the surface hydrophobicity increased the retention of at the end of the first day, relative to the negatively charged nanoparticles. Using budesonide as a model drug, Kompella’s group demonstrated that microparticles better sustain drug delivery from periocular space relative to nanoparticles (Kompella et al., 2003).
Figure 9.

Size-dependent prolonged retention of microparticles and nanoparticles at the site of administration following subconjunctival injection in rats. Following administration of a 400-μg dose of 2 μm, 200 nm and 20 nm particles, the percentage dose remaining at the site of administration was determined up to 60 days post administration. The data is expressed as mean ± SD, n=4. †P < 0.05, compared with 20 nm nanoparticles; * P < 0.05, compared with the particle fraction remaining at time 0. Reprinted with permission from Amrite and Kompella, 2005. Copyright 2005 Wiley.
In fact, the subconjunctival route has been investigated for sustained anti-glaucoma drug delivery (Fahmy et al., 2018, Lavik et al., 2016, Pek et al., 2016, Fu et al., 2016, Voss et al., 2015, Ng et al., 2015). Implants, microspheres, gels, and liposomes are some of the delivery systems assessed by this route for sustained glaucoma therapy. More efforts have been placed on non-implant delivery systems possibly because of the ease of injection. One example for sustained glaucoma therapy via this route is the use of liposomes. Using a normotensive rabbit model, Wong’s group in Singapore demonstrated that a subconjunctival injection of liposomal latanoprost is superior to daily eye drops in reducing intraocular pressure for up to about 80 days, with the liposomes injected on day 1 and then repeated on day 50 (Natarajan et al., 2011). However, these results should be interpreted with great caution since rabbits lack the receptor for prostaglandin analogs and are therefore poor responders to prostaglandin mediated IOP reduction.
Another example is a 100 nm liposome containing the drug latanoprost. In 2014, it was the focus of an open-label safety and efficacy study in six patients with either ocular hypertension or primary open-angle glaucoma. Each patient was subjected to a single subconjunctival injection of the liposome drug delivery system. The injection was well tolerated by all six patients. From a baseline IOP of 27.55 ± 3.25 mm Hg (range 24–31 mm Hg), there was a dramatic decrease after only one hour to 14.52 ± 3.31 mm Hg (range 10–18 mm Hg) or an IOP reduction percent range from 37–63%. A clinically and statistically significant IOP reduction was observed 3 months following the injection (≥ 20% IOP reduction, p = 0.001 to 0.049). These results were of great significance because they were likely the first reported nanomedicine that had an extended duration of action in humans (Wong et al., 2014, Shouchane-Blum et al., 2019). However, the subconjunctival liposome delivery system is not approved to date, suggesting inherent delivery and efficiency limitations associated with the delivery system.
4. Intraocular drug delivery systems
4.1. Intracameral delivery systems
Although eye drops are the gold standard of treatment for glaucoma, the barriers of the eye and the associated poor bioavailability and medical compliance problems have led to investigation of other delivery routes for improving patient outcomes. The intracameral approach is yet another delivery route with new therapeutic possibilities. The approach usually involves the administration by direct injection of a substance (typically an antibiotic) into the anterior chamber of the eye to prevent eye infection or endophthalmitis after cataract surgery and in some cases eye surgeons have administrated anesthesia in this manner (Karp et al., 2001). Some researchers have been investigating intracameral drug delivery systems for the treatment of glaucoma.
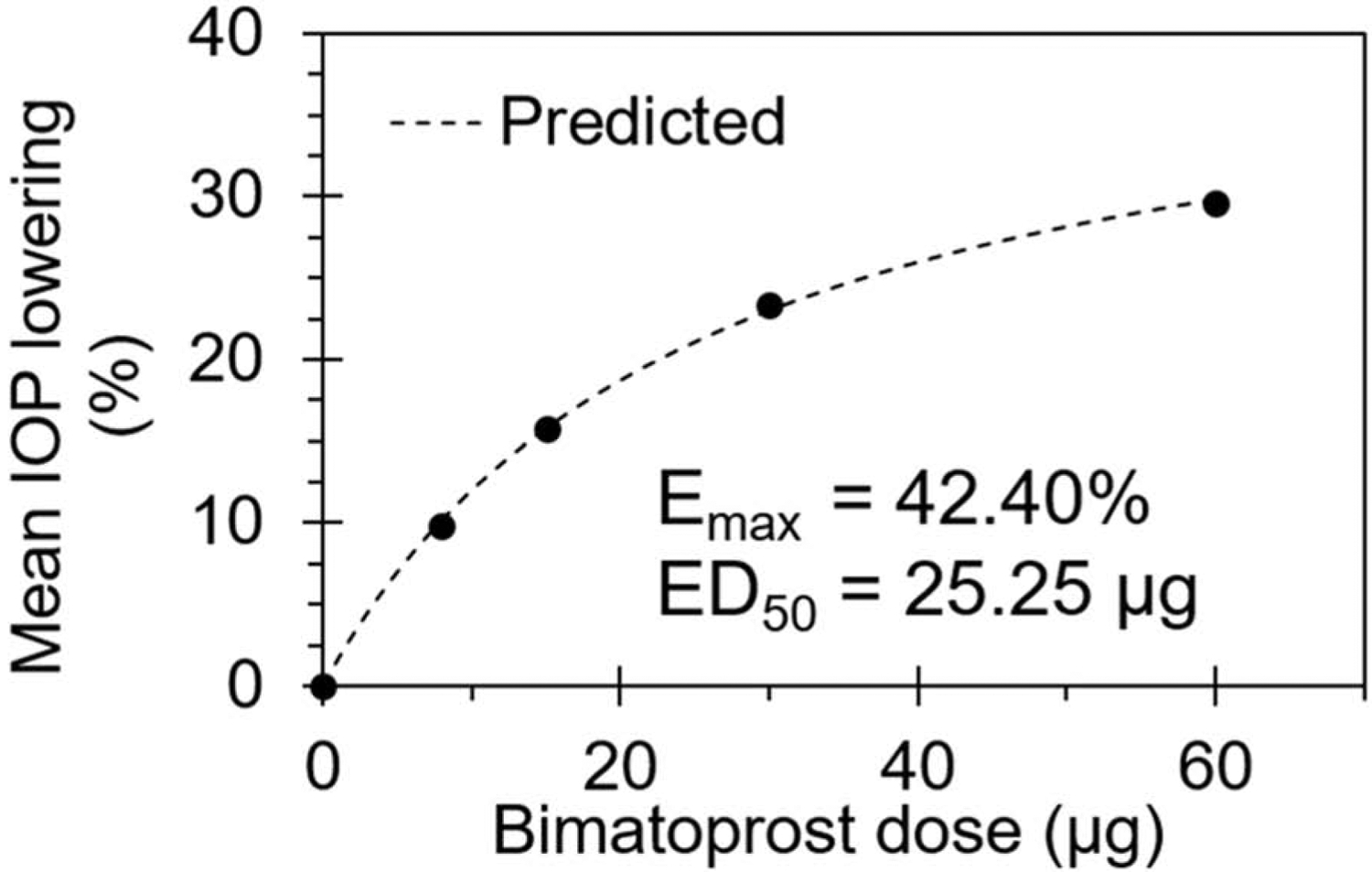
Allergan developed and assessed a sustained release implant delivery system for bimatoprost (Bimatoprost SR) in a Phase I/II study in patients with open angle glaucoma (Lewis et al., 2017). The delivery system is a biodegradable implant based on a poly (lactic-co-glycolic) acid matrix Novadur® platform, used in Ozurdex. The implant composition was apparently optimized to provide non-pulsatile steady drug release with zero-order kinetics. Unlike Ozurdex which requires a 22G needle, the Bimatoprost SR implant uses an applicator containing a 28G needle. Implants of various lengths allowed dosing at 6, 10, or 15 μg of drug. Also, dosing of 20 μg was achieved by injecting 2 implants containing 10 μg of drug each. All these doses were assessed for safety and efficacy. A dose-response relationship was evident for the mean overall IOP reduction from baseline at week 16 (Figure 10).
Figure 10.

Bimatoprost dose-response relationship for the mean overall IOP reduction from baseline at week 16, expressed in mm Hg. Based on data from Lewis et al., 2017.
IOP reduction increased with the dose, with the maximum mean overall IOP reduction being 9.5 mm Hg at 20 μg dose, which was higher than the 8.4 mm Hg reduction achieved by topical bimatoprost 0.03% QD. All doses of implant and eye drops resulted in statistically significant IOP reductions (p < 0.001). Beyond week 12, the mean change in IOP appeared to be superior for the implant group compared to the eye drop group. The implants were effective during the entire study, up to week 26 or 6.5 months, when the last measurement was reported. Over a 9-month period of observation, the implant visibly reduced in size, while retaining its shape.
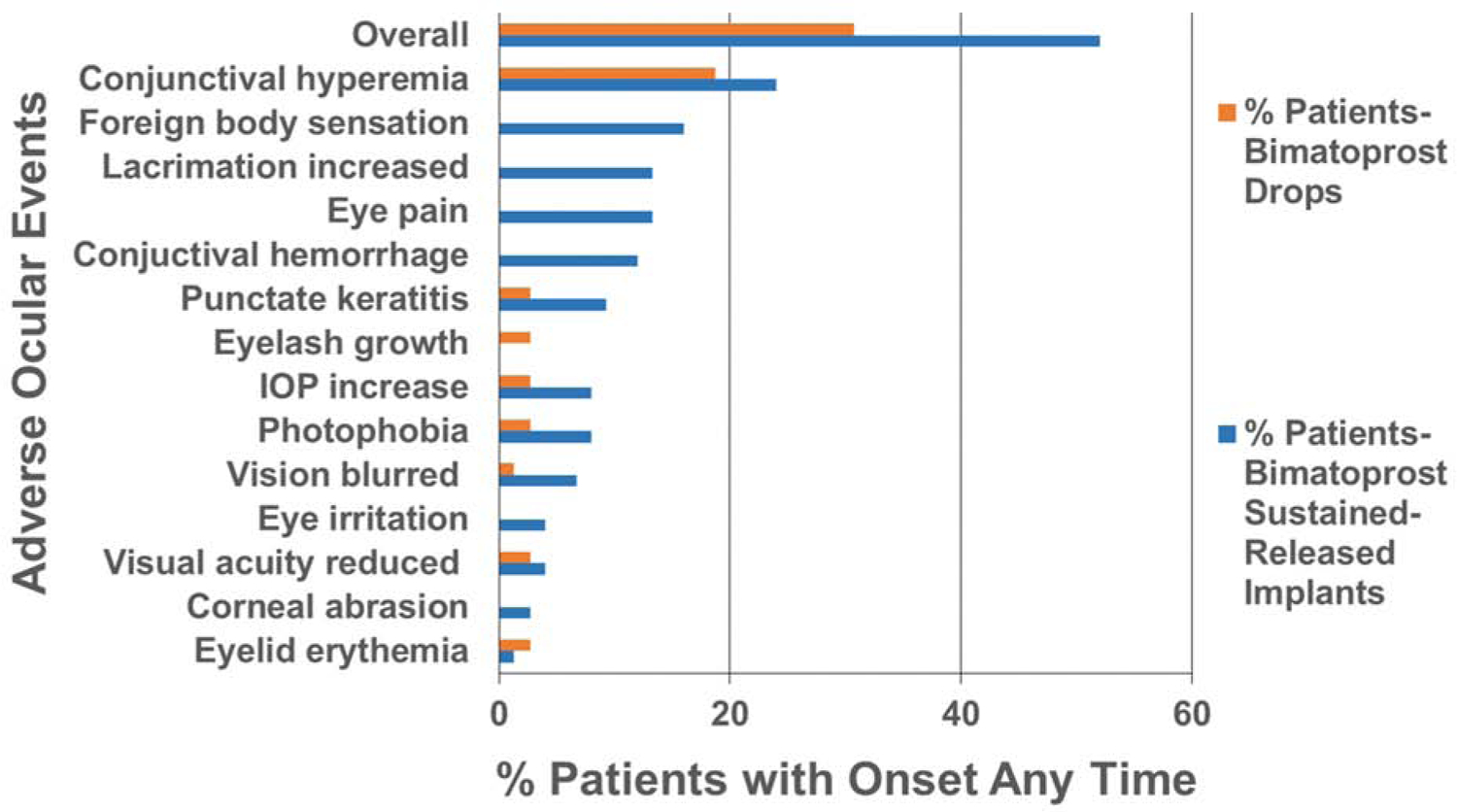
In addition to efficacy, Lewis et al., (Lewis et al., 2017) compared the safety of implants (study eyes) to eye drops (fellow eyes) of bimatoprost. Figure 11 compares the incidence of various side effects with onset at any time after dosing. Overall, adverse events were reported in 52% of patients for implants, when compared to 31% of patients with eye drops. These adverse events were in 32 and 29% of patients respectively, for implants and eye drops, when compared after 2 days post-dosing. Thus, the critical differences in the incidence of adverse events occur soon after implant dosing. The incidence of conjunctival hyperemia, foreign body sensation, eye pain, lacrimation increase, conjunctival hemorrhage, punctate keratitis, IOP increase, photophobia, blurring of vision, reduction of visual acuity, eye irritation, corneal abrasion, and eyelid erythema was higher overall in the implant group relative to eye drops in this report. Eye lash growth, however, was higher in the eye drop group.
Figure 11.
Comparison of side effects of bimatoprost sustained-release implants injected in the anterior chamber and eye drops in a Phase I/II study. Based on data from Lewis et al 2017.
The intracameral route requires a very low total dose of drugs such as prostaglandin analogs to achieve superior sustained IOP lowering compared to drops for at least 4 months (Seal et al., 2016). The adverse events are higher compared to eye drops, with key complications including elevated intraocular pressure and foreign body sensation, which are less frequent or absent for drops. Long term retention of the implant beyond the optimal drug effect period is another concern. If the implant is truly exhibiting zero-order kinetics throughout its life, it is unclear why the maximum IOP reduction is declining beyond 4 months, relative to eye drops.
A recent study report by Lee et al., 2019, in normotensive beagle dogs the dose-response efficacy of the sustained-release system Bimatoprost SR was compared to the topically administrated prostaglandin analogs (PGAs). The investigators observed that the topical bimatoprost dose-response curve demonstrated a U-Shape as the bimatoprost concentration was increased to 0.1% the result was a reduction in IOP-lowering efficacy. The opposite was the case for the Bimatoprost SR which demonstrated greater IOP lowering as the dose strength increased.
In July 2019 Allergan announced that the FDA accepted the companies New Drug Application (NDA) for their Bimatoprost Sustained-Release (SR) implant. The NDA was based on positive results from two Phase III clinical studies (ARTEMIS)(Allergan, Press Release 2019). These studies showed that Bimatoprost SR reduced IOP by 30% over a 12-week primary efficacy period, meeting the predefined criteria for non-inferiority to the comparator. The ARTEMIS studies evaluated 1,122 subjects for the safety and efficacy of Bimatoprost SR versus the standard comparator, which is timolol maleate the nonselective beta-adrenergic antagonist, which was first approved by the FDA in 1978. After only three treatments with Bimatoprost SR, greater than 80% of patients did not require other treatments to maintain IOP control for at least 12 months. Bimatoprost SR was well tolerated by most patients. Additionally, in the phase 1/2 trials, even though the implants were formulated to reduce IOP for 4- to 6- months, numerous patients experience sustained IOP suppression for longer than six months. The results also indicated that IOP was controlled in 40% of study patients for 12- months and for 28% of study patients for 24-months (Singh, 2020). In March 2020 Allergan received approval for the Bimatoprost SR 10 μg implant which will be marketed as Durysta and is the first biodegradable sustained released implant for the reduction of IOP in patients with primary open-angle glaucoma (POAG) or ocular hypertension. The product literature indicates that the delivery system is for one time use and that it should not be dosed in eyes that previously received Durysta. Thus, the success of repeated dosing with this intracameral sustained-release delivery system is awaited.
Another intracameral implant that was or might still be in a Phase II 12-month safety and efficacy evaluation (NCT02371746) is the ENV515 Travoprost Extended Release (XR) (Envisia Therapeutics, Research Triangle Park, NC, USA). It is a biodegradable, implant manufactured by the PRINT® technology that consists of an extended-release formulation of travoprost. Initial results were promising. The patients that received the low dose of the drug from the ENV515 exhibited a decrease in the mean IOP by 6.7 ± 3.7 mm Hg over 11-months. This ENV515 study demonstrated IOP lowering comparable to the Xalatin and Lumigan (latanoprost and bimatoprost topical prostaglandin analogs) and the in-study 0.5% timolol maleate topical daily drops. The most common adverse event experienced early on in the study was reported as transient hyperemia (Envisia, Press Release 2017).
Intracameral injections at the present time are not routine but with the approval Durysta that could change. Ophthalmic intracameral drug administration is like the other drug delivery routes with respect to providing advantages and disadvantages. One advantage is that medical adherence with the Durysta implant is not an issue because it provides IOP regulation that lasts for months post injection. Also, more of the dose has the chance to access the targeted tissues unlike eye drops that can be simply washed away. But with the intracameral drug delivery systems there are other issues of concern. One of which is the patient iridocorneal angle (Allergan, 2020a). Post administration, implants like the Durysta implant are intended to rest within the confines of the inferior angle. Accordingly, patients with small angles (Shaffer grade < 3) or any anatomical obstruction (e.g. scarring) that could restrict the implant from resting in the inferior angle should be considered before administering an implant to these patients (Allergan, 2020a). Another concern is that implants are injected near the corneal endothelium, which does not naturally regenerate, if damaged. Therefore, any adverse effects on the corneal endothelium causing loss need to be considered, especially if there is repeated dosing in this area. Other adverse reactions that have been experienced by some patients can include hypersensitivity to bimatoprost or other product components, cystoid macular edema, intraocular inflammation, pigmentation and endophthalmitis (Allergan, Press Release 2020, Allergan, 2020b).
4.2. Intravitreal delivery systems
The extraocular, periocular, and intracameral delivery approaches discussed above primarily enable drug delivery to the tissues of the anterior segment involved in glaucoma pathology. The tissues targeted by the above routes include ciliary body, trabecular meshwork, Schlemm’s canal, and uveoscleral outflow pathways. These routes, however, are inefficient in achieving significant drug delivery to the retinal ganglion cell layer and optic nerve head. Those tissues are best targeted by enhancing posterior segment drug delivery, as is the case with intravitreal route of drug administration. Additionally, drug present in the vitreous humor can access the ciliary body readily, provided the drug is cleared via the anterior pathway or present in adequate concentrations near the ciliary body. Moreover, sustained drug release systems are now routinely dosed to the vitreous humor in patients. It is currently feasible to sustain delivery of small molecule drugs such as corticosteroids for up to 3 years using non-degradable implants and for about 6 months using degradable implants in the vitreous humor. Unlike intracameral injections, intravitreal injections are now more routine and well accepted, despite the complication of endophthalmitis in some patients (Figure 12). The intravitreal route is a natural choice for neuroprotection of retinal ganglion cells (RGC), optic nerve head, and photoreceptors to prevent vision loss in glaucomatous eyes. This route may also be suitable for sustained IOP reduction.
Figure 12.

Description of four ocular drug delivery systems currently being tested or in use for the treatment of eye diseases, sized in comparison to the average human eye.
Despite the above merits, the intravitreal route is not widely being explored for sustained anti-glaucoma drug delivery, particularly to reduce intraocular pressure. This may mainly be due to inadequate efficacy achieved for some drugs, relative to eye drops or the difficulty in removing the drug product once dosed in the vitreous humor. The latter issue is pertinent to any invasively dosed drug product in the eye. Drug efficacy can potentially be improved by careful selection of the drug or drug form. For example, pure drug suspensions of drugs with low solubility can provide sustained drug exposure in the vitreous and hence, surrounding eye tissues including those responsible for controlling intraocular pressure. One such case is diclofenac acid vs. diclofenac sodium, with the former being less soluble (Durairaj et al., 2009)(Durairaj et al., 2009a). This is a good example of how easy it is to sustain a drug in the targeted tissue by forming a suspension of its base form. The duration of release for diclofenac acid is comparable to that of Ozurdex, a successful clinical implant. Briefly, in this study the influence of dosage form on intravitreal pharmacokinetics was investigated for diclofenac acid or diclofenac sodium after intravitreal injections. A diclofenac acid suspension (5 μm) resulted in persistent vitreal drug delivery for up to 21 days, as opposed to a diclofenac sodium salt solution, whose levels declined below the detection limits only after 24 hours in the vitreous humor and 4 hours in the choroid-retina. The apparent elimination half-life of the diclofenac acid suspension in the vitreous and choroid-retina was 24 and 18 hours, respectively, when compared to 2.9 and 0.9 hours, respectively, for diclofenac sodium salt solution dose (Durairaj et al., 2009a). From the pharmacokinetic modeling in this study it can be concluded that particle size, solubility, and dosage form resulted in an increased residence time and apparent elimination half-life, which meant a higher sustained drug release to the targeted tissues surrounding vitreous humor could be obtained. Drugs from the vitreous humor can be eliminated either via the posterior pathway through tissues in the back of the eye (e.g., retina and choroid) or via the anterior pathway via the aqueous humor entry. The anterior pathway of elimination is expected to contribute significant drug levels to the target tissues involved in intraocular pressure management, while the posterior pathway contributes towards neuroprotection. Based on the example of diclofenac, selection of drug form can affect the dosage form (solution vs. suspension), resulting in sustained drug delivery. Similar principles discussed here can potentially be applied to glaucoma drugs.
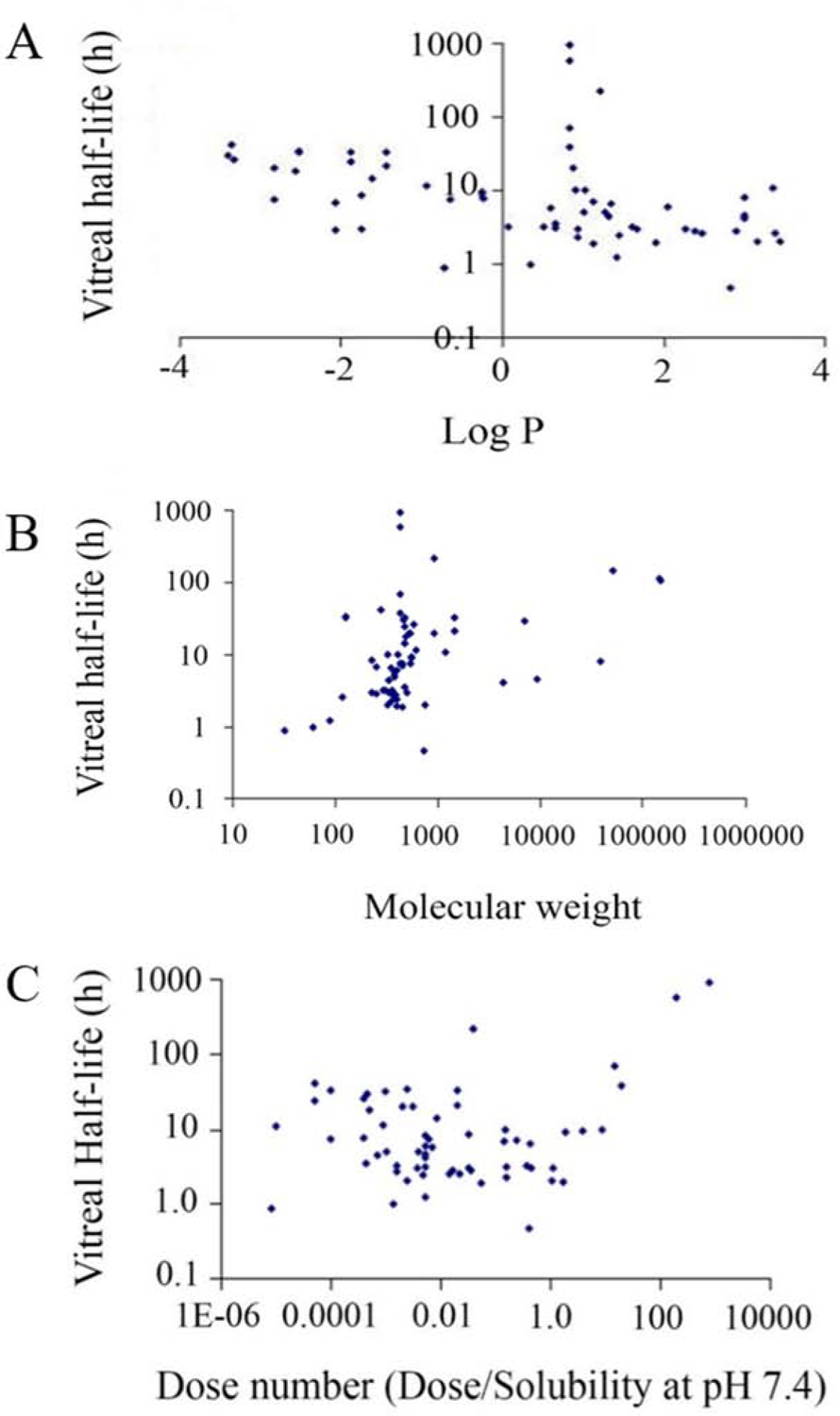
Drug delivery research has several important tools one of which is Quantitative structure-pharmacokinetic relationship (QSPKR) modeling. It can be used for the early prediction of pharmacokinetic behaviors for new drug candidates, which is of paramount importance in order to save research hours, development costs, and other developmental resources. Such is the case with the study by Kompella and team that developed best-fit validated models to predict the intravitreal half-life of structurally different drug compounds in order to understand the influences of the physicochemical properties, which included drug solubility, lipophilicity, and molecular weight (Durairaj et al., 2009b). A literature search provided the information necessary to build a database. This study identified 68 compounds administered as intravitreal injections in rabbit models. The statistical assessments focused on using the entire database or subsets thereof which isolated acids, bases, macromolecules, neutral compounds, pigmented/non-pigmented rabbit data, suspensions, and zwitterions. The best-fit models were cross validated against other subsets and the model for the entire database was tested for its ability to predict the results obtained in the smaller subsets. Analysis was carried out using multiple linear regression with non-collinear independent variables and models derived were based on correlation coefficients and goodness of fit statistics. Two sets of variables were used for these assessments. The first set was considered to be independent variables which included LogMW (MW- molecular weight), LogD (D- distribution coefficient), DN (dose number), PF (pigmentation factor) and SF (salt factor). In the second set LogP (P- partition coefficient) replaced LogD for the model development. The results indicated that the most influential factors on intravitreal half-life for the compounds were the group of variables, MW (LogMW), lipophilicity (LogP or LogD), and dose number (dose/solubility in PBS at pH 7.4) instead of a single factor. Additionally, it would be possible to prolong the intravitreal half-life of the drugs by increasing Log MW while decreasing LogP or LogD (Figure 13).
Figure 13.

Plot of vitreal half-life as a function of (A) Log P, (B) Molecular weight, and (C), Dose number (Dose/Solubility at pH 7.4) for about 68 drugs. Vitreal half-life, MW, and Dose Number are shown in logarithmic scale. Reprinted with permission from Durairaj et al., 2008. Copyright 2008, Springer Science Business Media, LLC, part of Springer Nature.
Durairaj et al., (Durairaj et al., 2009b), indicated that hydrophilic molecules and large molecules in particular have prolonged half-life in the vitreous. These molecules are preferentially eliminated via the anterior pathway from the vitreous humor since they do not partition well into retinal tissues to be removed via the posterior pathway. Such molecules may be ideal candidates to be dosed in the vitreous to achieve effects in anterior segment tissues for IOP reduction. Additionally, based on the work of Durairaj et al., drug exposure to the aqueous humor relative to vitreous humor is higher for drug molecules exhibiting longer vitreous half-life (t1/2) (Durairaj, 2017).
Other drug delivery research used intravitreally injected bimatoprost nanospheres, Lambert et al., (Lambert et al., 2015) showed that intraocular pressure can be reduced for at least a month in a mouse model. The dose requirements for the intravitreal route are anticipated to be higher, but only by a few folds, relative to intracameral dosing. Given the clinical success of 20 μg of bimatoprost via the intracameral route for sustained release (Lewis et al., 2017), and the feasibility of injecting a 700 μg dexamethasone-containing implant in the vitreous humor, there is a lot of room for dose optimization of prostaglandins in the intravitreal space. This, intravitreal dosing in conjunction with proper implant placement (Edelhauser et al., 2010), can potentially allow adequate sustained drug delivery to the target anterior segment eye tissues to achieve sustained IOP reduction following intravitreal dosing. For intravitreally dosed brimonidine, the vitreous humor-to-aqueous humor AUC ratio is about 4–5 fold in the monkey eye (Shen et al., 2014). Cantor and team (Cantor et al., 2008) determined the drug absorption from brimonidine purite (BP, Alphagan-P, Allergan, Irvine, CA) (0.15%) in the aqueous humor of cataract patients. The mean aqueous humor (AH) concentrations for brimonidine sampled around 52- and 54-minutes post administration of the 0.15% solutions were 95.5 and 87.5 ng/mL, respectively. Assuming a 1:1 scaling from monkey to human eye for simplicity, to achieve the above drug levels in the aqueous humor, a concentration of about 400–500 ng/mL brimonidine may have to be maintained in the vitreous humor.
So far in this review the systems discussed could best be described as passive diffusion/degradation controlled systems which may suffer from decreased biomedical activity with time in case of matrix type systems as opposed to reservoir systems and increased risk of adverse effects due to burst effect in case of reservoir systems and dose-dumping in the case of degradable systems (Witkin and Brown, 2011, Janoria et al., 2007). The last intravitreally administer drug delivery system mentioned in this review will focus on the novel potential pathway for targeted drug delivery in the human body using actively propelled micro and/or nanoparticles. Research in this area has consisted of microparticle propulsion in liquid or fluid-filled cavities of the body such as the stomach or blood using chemical or magnetically propelled structures (de Avila et al., 2017, Gao et al., 2015, Li et al., 2017, Venugopalan et al., 2014, Cheng et al., 2014, Ghosh and Fischer, 2009). While propulsion in the vitreous body of the eye over long distances (centimeters) was not realized because of the dense biopolymer network of the eye until recently. In 2018, Wu et al described their work using magnetic wirelessly activated surface treated micropropellers (i.e., slippery micropropellers) to penetrate the vitreous body of the eye to reach the retina (Wu et al., 2018). The propeller fabrication consisted of two main steps: preparation of the helical microstructures and the application of a coating. Fabrication was achieved using a technique known as physical vapor shadow growth via glancing angle deposition described elsewhere (Walker et al., 2015, Hawkeye and Brett, 2007, Robbie et al., 1998, Mark et al., 2013). Once inside the vitreous the micropropellers would be driven through the biological media using a rotating magnetic field. At first the researcher injected uncoated micropropellers and passive silica microparticles into porcine vitreous to confirm that they were propelled. But their data soon revealed that coating the micropropellers with a slippery fluorocarbon liquid layer was critical for the propulsion in the vitreous over long centimeter distances. Such nontoxic silicone oil and fluorocarbon coating are typically applied to various devices for medical applications (Chen et al., 2017, Chan et al., 2015). They reasoned that two major criteria had to be addressed for successful propulsion through a biological media: a.) match the propellers particle size to the macromolecular network, and b.) minimize the interaction between the propellers and biopolymer network. In a previous study that showed particles with a diameter of ~500 nm could pass through the biopolymeric network of the porcine vitreous, the micropropellers were fabricated with the above criteria and with this size consideration (Xu et al., 2013, Ullrich et al., 2013). Then they demonstrated that slippery helical micropropellers (0.5 μm in diameter by 2 μm in length) were propelled in the vitreous body of a porcine eye at a speed of ~10 μm/s. These slippery micropropellers could potentially be coated with drug incorporated into the coating layer for the treatment of various diseases of the eye and propelled to a location of close proximity to the targeted tissue site/s of the disease. The applicability of such complex, advanced materials for glaucoma drug therapy has yet to be evaluated.
It was estimated that ~6 million intravitreal injections were performed in the United States during 2016, with the worldwide figure considerably larger (Campbell et al., 2010, Kim, 2015, Williams, 2014, Hartman and Kompella, 2017). Intravitreal injectables are mainly approved for the treatment of branched or central retinal vein occlusion, diabetic macular edema, uveitis, and wet age-related macular degeneration. Most ophthalmic professionals would infer that the reason intravitreal injections have impacted the field of ophthalmology is because of: 1) established procedure guidelines, 2) persistent adherence to them, 3) injection method, and 4) the innovative design of needles, with smaller diameters, lengths, and controlled bevel angles (Aiello et al., 2004, Fagan and Al-Qureshi, 2013, Avery et al., 2014, Myers et al., January 6, 2015, Hartman and Kompella, 2017, Ozkaya et al., 2013). All of these have played a role to improve overall safety and patient acceptance of intravitreally injected ophthalmic drug products. However, due to the complex human eye anatomy, there can be complications for this type of drug administration including intraocular infection, subconjunctival or vitreous hemorrhage, vitreous incarceration, fluid reflux, scleral damage, endophthalmitis (EO), and pain (Hubschman et al., 2010).
4.3. Supraciliary delivery systems
The supraciliary route is analogous to the suprachoroidal route of drug delivery, wherein a microneedle or a regular needle is used to penetrate just beyond sclera. In the case of this route, the needle entry is near the ciliary body region, with the drug product deposition taking place above the ciliary body. Hence, the name, supraciliary route. For suprachoroidal delivery, the needle entry is further away from the cornea and limbus, towards the choroidal region. Since the ciliary body is the source of aqueous humor production, it is anticipated that drugs capable of suppressing aqueous humor production might benefit from supraciliary drug delivery. Additionally, the drug may have access to other sites of action including the trabecular meshwork and other aqueous humor outflow pathways. Using microneedles originally designed for suprachoroidal delivery, Prausnitz and his team injected brimonidine solution or microspheres in the supraciliary space and monitored IOP reduction in a rabbit model. After a single dose of brimonidine solution, the eye drop (75 μg dose) was as effective as a 100-fold lower supraciliary dose (0.75 μg), suggesting superior bioavailability of brimonidine via the supraciliary route (Kim et al., 2014b). Subsequent studies using brimonidine-loaded microspheres (i.e., SC-low dose and SC-high dose) formulation groups indicated that the formulations could reduce IOP for at least 14 days with the SC-low dose microspheres or 33 days with the SC-high dose after the administration of a single dose (Chiang et al., 2016, Fedorchak et al., 2017). Pek et al., (2016) also reported the subconjunctival delivery of brimonidine using a microsphere/carrier system providing a reduced IOP for 40 to 55 days. They demonstrated that the release rate and total release was dependent on PLGA molecular weight, initial drug/polymer weight ratio, buffer composition, and the microemulsion mixing speed (Pek et al., 2016).
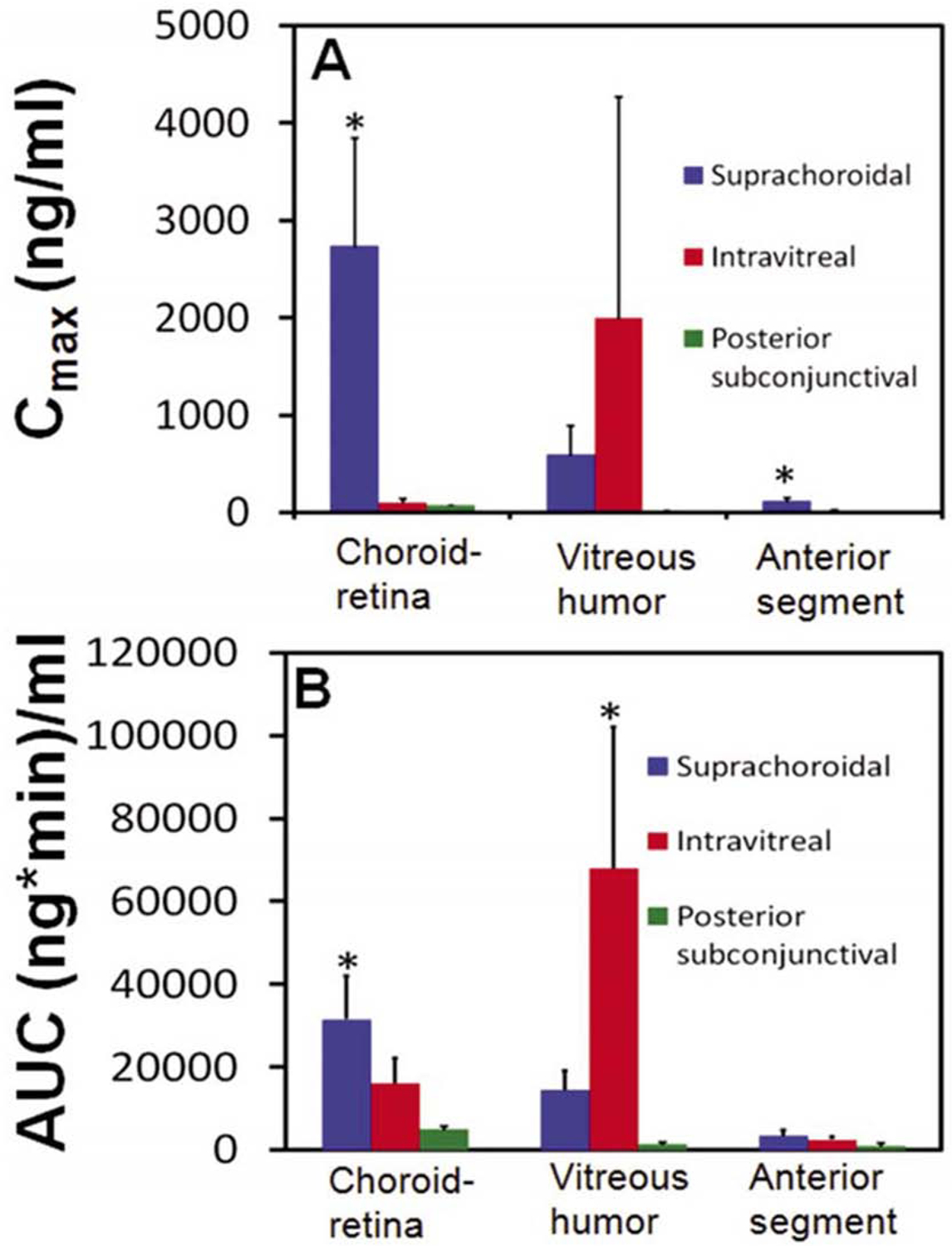
In this review we have thus far focused on different routes of drug administration, and the major issue of medical adherence to topical drops, but because of the limitations caused by the anatomy of the eye it is imperative to develop drug delivery systems that circumvent these structures and target the relevant eye tissues. In efforts to optimize drug delivery, researchers are continuously investigating these different routes of drug delivery to improve target tissue delivery for different eye diseases. Suprachoroidal injections are probably one of the newest approaches to this end. Prior to advancing this route, it is critical to understand pharmacokinetic advantages of this route relative to others. Following sodium fluorescein dosing in the suprachoroidal, posterior-subconjunctival, and intravitreal locations in a rat model (Tyagi et al., 2012), the drug exposure was higher for the suprachoroidal route relative to the other two routes in the anterior segment. The authors compared suprachoroidal space (SCS) drug delivery with subconjunctival and intravitreal (IVT) delivery routes by means of noninvasive fluorophotometry in Sprague Dawley rats. The sodium fluorescein delivery to the choroid-retina region was ranked as follows: suprachoroidal > IVT > posterior subconjunctival injection. The peak (Cmax) concentration of sodium fluorescein in the choroid-retina region was 36-fold and 25-fold higher after SCS injection compared to a posterior subconjunctival, and IVT injection, respectively. In addition, sodium fluorescein exposure (AUC 0–360 min) to this region after SCS injection was 6-fold and 2-fold higher than the posterior subconjunctival and IVT injections, respectively. Tmax was observed immediately after dosing for SCS injections. In comparison, for IVT injections the Tmax was 27.5 minutes and 10 minutes for subconjunctival injections (Figure 14).
Figure 14.

Pharmacokinetic parameters (Cmax and AUC 0–360 min) estimated for sodium fluorescein after injection by suprachoroidal, intravitreal, and posterior subconjunctival routes in Sprague Dawley rats. Parameters for the three routes of administration were estimated using non-compartmental analysis using WinNonlin (version 1.5, Pharsight Inc., Sunnyvale, CA). Cmax is the maximum observed drug concentration and AUC 0–360 min is the area under the curve in a given tissue. Data are expressed as mean 6 SD for n = 4. * indicates p < 0.05 compared to other two groups. Reprinted from Tyagi et al., 2012, from PLOS ONE base on the open access license “CC-BY”.
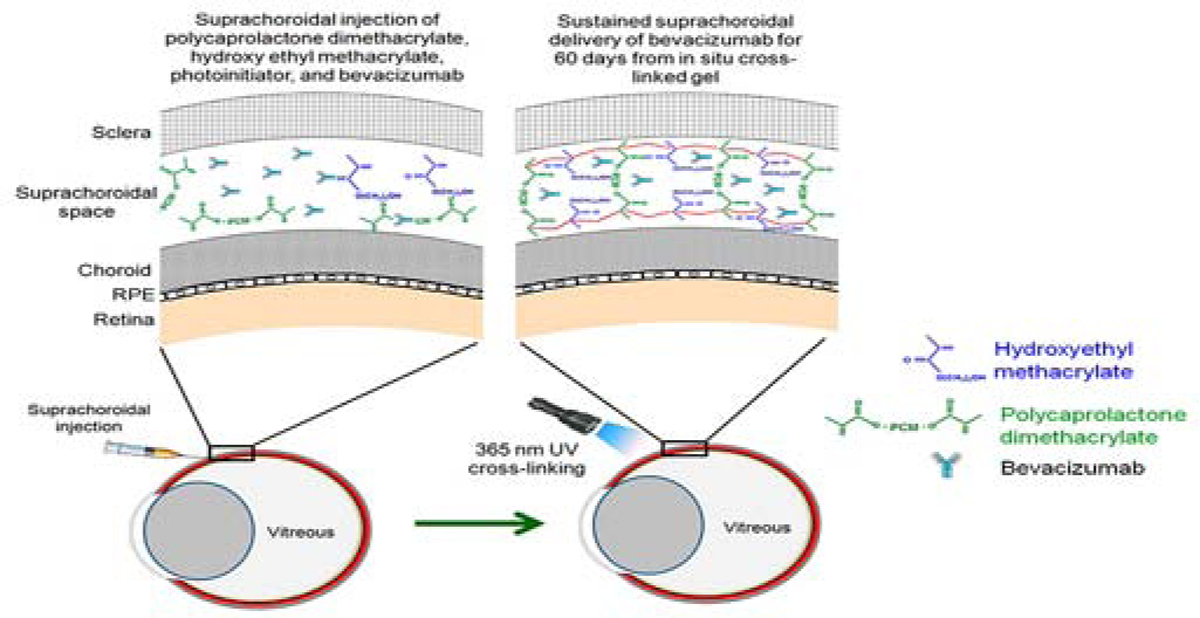
Suprachoroidal route and potentially the supraciliary route is amenable to dosing in situ forming implants following external activation of injected materials. A novel approach for sustained drug delivery to the eye is the injection of a drug containing mixture of polymeric solutions along with a UV or other photo-initiator followed by in situ exposure to UV light or another appropriate light source to initiate photopolymerization. Once cross-linked, a gel-like structure is formed that delivers sustained drug release to the targeted tissues (Tyagi et al., 2013). In this approach, two commonly used biomedical polymeric materials were selected for incorporation in the gel formulation, 2-hydroxyethyl methacrylate (HEMA) and polycaprolactone dimethacrylate at a ratio of 90:10. This gel forming polymer mixture supplemented with the photo-initiator 2, 2–dimethoxy-2-phenylacetophenone (DMPA) and a model drug was injected into the SCS of Sprague Dawley rat eyes and exposed to 365 nm wavelength UV light with an exposure intensity of 3.18 mW/cm2 (Tyagi et al., 2013). Actually, this exposure intensity is similar to that used during some human clinical trials where near-UV light at 365 nm with an exposure time of 30 minutes (exposure intensity of 3.0 ± 0.3 mW/cm2) was used to treat keratoconus and bacterial keratitis. Sustained drug release was assessed ex vivo in rabbit eyes and in vivo rat eyes following in situ gel formation. In vivo drug release was noninvasively monitored using Fluorotron Master and fundus photography and was sustained for at least 2-months in the SCS of the rats (Tyagi et al., 2013). The burst drug release from the gel crosslinked for 10 minutes was 21% while gels crosslinked with the 3- and 7-minute cure times had ≥ 62% burst drug release. This study was the first to demonstrate sustained drug delivery to the eye from a photo-responsive biodegradable gel formed in situ. A similar system could possibly be used to provide sustained anti-glaucoma drug delivery after injection into the suprachoroidal or more preferably, supraciliary space (Figure 15).
Figure 15.
Light activated, in situ forming gel for sustained drug delivery to the suprachoroidal space. Reprinted with permission from Tyagi et al., 2013. Copyright 2013 American Chemical Society.
The suprachoroidal route extended to the supraciliary location, will potentially result in greater drug exposure to the anterior segment eye tissues relative to intravitreal and periocular dosing. The supraciliary route is a new route of drug administration and its safety upon repeated dosing has yet to be established. However, because of the success of intravitreal injections and the development of microneedles or even nanoneedles, investigations are underway for suprachoroidal drug delivery applicability. Also, superior efficacy with sustained release dosage forms relative to daily eye drops has yet to be established. It is assumed that accurate placement of drug in the suprachoroidal space is expected to reduce injury to the underlying retinal layers. Some adverse effects similar to those exhibited following the intravitreal procedure may be observed after this procedure.
5. Neuroprotection
Glaucoma has been recognized as a progressive multifactorial neurodegenerative disease and researchers over the last few years have investigated a number of possible treatments in the area of neuroprotection to treat this unmet need (Lauzi et al., 2019, Weinreb et al., 2014, Arranz-Romera et al., 2019, Khatib and Martin, 2019). The multifactorial neurodegenerative processes that have been identified to contribute to the glaucomatous RGC loss include, aggregation of misfolded proteins, axonal transport dysregulation, glutamate excitotoxicity, inflammation, ischemia, oxidative stress, mitochondrial dysfunction, and neurotrophic deprivation (Baltmr et al., 2010, Russo et al., 2016, McMonnies, 2018, Arranz-Romera et al., 2019). It has also been discovered that these processes can interact, compounding their effect. This may indicate that effective treatment possibly could require a combination therapy (Tezel, 2006, Cuenca et al., 2014). Neuroprotection with regard to glaucoma refers to any therapeutic treatment independent of IOP reduction to prevent or delay retinal ganglion cell (RGC) and axonal death, which disrupts functional connectivity of neural circuits in the optic nerve and is critical feature of many degenerative disorders (Akopian et al., 2017, Calkins, 2012, Levkovitch-Verbin et al., 2001). Neuroprotection for some glaucoma patients is the key to controlling the progression of their disease. For these patients just reducing and maintaining IOP is not enough to prevent the disease progression to blindness (Almasieh and Levin, 2017, Garway-Heath et al., 2015, Heijl et al., 2002). It is estimated that at least one out of eight glaucoma patients will eventually go blind over a 20 year period (Khatib and Martin, 2020, Malihi et al., 2014). At the present time the neuroprotection strategies aimed at either making use of signaling pathway transmissions to stimulate cell survival or those that focus on protecting the target cell’s ability to withstand pathological assault, have shown great promise in animal models. Unfortunately, as is the case with some data obtained between species, the same efficacy has not been demonstrated in the corresponding human clinical trials (Quigley, 2012). Recently some of the neuroprotection strategies that have made it to the clinical trial stage are summarized below.
5.1. Systemic administration of the drug brimonidine
Several research studies have identified that the systemic administration of the drug brimonidine provides neuroprotection of RGC in animal models independent of IOP correction (Hernandez et al., 2008, WoldeMussie et al., 2001). It has been theorized that this neuroprotective effect could be based on a number of mechanisms (Gao et al., 2002, Feke et al., 2014, Wheeler et al., 1999, Dong et al., 2008). The administration of twice daily topical brimonidine has achieved what is considered adequate levels for neuroprotection in preclinical studies (Burke and Schwartz, 1996). Additionally, in the Low Pressure Glaucoma Study Group clinical trial, NCT00317577, which compared brimonidine with timolol during a 30 month period, indicated that the patients who were able to tolerate the treatment had lower incidences of visual field progression then those treated with timolol (Krupin et al., 2011). But this was not conclusive because timolol has shown tendencies to lower visual field progression in some other studies (Khatib and Martin, 2020).
5.2. Cell therapy
Since 2004 intravitreal injections as a treatment for some non-glaucoma eye diseases have increased significantly. Because of this a local administration to target RGC cells is not out of the question and has also been investigated to improve targeted cell delivery, eliminating some of the unwanted side effects from topical administration. Mesenchymal stromal cells (MSC) have been administered using this route and demonstrated neuroprotective capabilities in experimental glaucoma (Johnson et al., 2010, Emre et al., 2015, Yu et al., 2006). MSC cells when compared to other types of cell lines have certain advantages. For instance, they are easy to obtain, present no ethical problems, and can be used without any immune suppression. Initial results with these cells appeared to be very promising, but problems such as proinflammatory vitreous clumping were limiting. It was determined that these adverse effects could have been caused at least in part by inconsistencies in the MSC isolation and preparation. To control these factors, the International Society for Cellular therapy was developed to standardize the cell line (Tassoni et al., 2015, Tzameret et al., 2014, Dominici et al., 2006, Kim et al., 2017). Recently at least four clinical trials are in place evaluating stem cells for glaucoma, NCT02330978, NCT01920867, NCT03011541, and NCT02144103. It will be interesting to see the results from these trials.
5.3. Neurotrophin studies
Neurotrophin studies focusing on 1) ciliary neurotropic factor (CNTF) and 2) recombinant human nerve growth factor (rhNGF) – 1) It has been demonstrated that the ciliary neurotropic factor (CNTF) has neuroprotective properties in preclinical studies for glaucoma treatment (Pease et al., 2009). In 2019, Neurotech Pharmaceuticals initiated a randomized, sham controlled, masked Phase II study to determine the effects of encapsulated CNTF cell-based delivery in 54 glaucoma patients using their NT-501 device. Also, partnering with Neurotech, the Lowy Medical Research Institute, based on promising results from the Phase 1 and Phase 2 trials, began a Phase 3 clinical trial. The Phase 3 trial is to assess the safety and efficacy of CNTF in patients with type 2 macular telangiectasia. It is a multi-centered trial, with clinic locations in the United States, Europe, and Australia. CNTF is a therapeutic macromolecule that has been tested in numerous preclinical applications for its ability to decrease photoreceptor degeneration for various eye diseases. Among the many growth factors, cytokines, and neurotrophic factors that have been tested to determine their ability to minimize photoreceptor loss, CNTF was found to be one of the most effective. However, the problem with CNTF is that it degrades rapidly when injected into the eye. Because of this problem CNTF requires a special delivery device. The Neurotech NT-501 is an encapsulated cell technology implant (NT-501) that provides a solution to this problem. The retinal pigment epithelial (RPE) cell construct for the release of CNTF are first encapsulated in a semi-permeable membrane allowing the selective and sustained release of CNTF for the RGCs. This release then slows photoreceptor degradation, thereby stabilizing the visual field (Chew et al., 2019). The NT-501 device is surgically implanted into the vitreous and provides continuous release of CNTF into the vitreous cavity. Therefore, it provides controlled release and long-term delivery of CNTF. From the vitreous cavity, the CNTF diffuses to the retinal cells. 2) The FDA has approved eye drops to treat neurotrophic keratitis (Lambiase et al., 1998) that contain the recombinant human nerve growth factor (rhNGF) because of the desirable results from several clinical trials, NCT02101281, NCT03019627, and NCT03035864. Another clinical trial (NCT02855450) is designed to primarily assess the safety and tolerability of eye drops in progressive glaucoma patients. Several secondary objectives will also be assessed during the course of this trial.
5.4. Drug repurposing for glaucoma (i.e., memantine)
The repurposing of existing orally administered drugs is an approach that can speed up the FDA approval process. An example of this is the drug memantine, which is a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist (Vorwerk et al., 1996) used to treat Alzheimer patients in the moderate to severe stage of the disease and has demonstrated encouraging results for glaucoma in a monkey model (Hare et al., 2004). These receptors are commonly found throughout the central nervous system (CNS) and are required for healthy, functioning neuronal cells. Nevertheless, if it is excessively stimulated in the presence of the neurotransmitter glutamate and this can lead to moderate Ca2+ neurotoxicity. This response has been implicated for chronic glaucoma along with other neurodegenerative disorders. Unfortunately, based on the data from two Phase 3 clinical trials (NCT00141882 and NCT00168350), there was no statistical evidence that memantine was any different than the placebo.
5.5. Short term effect of Vitamin B3 (nicotinamide) administration
In 2019 a research team from the Centre for Eye Research in Australia started a pilot study to investigate the short term effect of taking Vitamin B3 (nicotinamide) the precursor for nicotinamide adenine dinucleotide (NAD), an essential cofactor for metabolism, which is found in all living cells. Retinal ganglion cell axon is unmyelinated and has a high energy requirement. Because if this the nicotinamide and NAD deficiency can potentially disrupt mitochondrial metabolism, energy, and RGC function. These observations were made by Nzoughet et al. in glaucoma patients that had lower plasma nicotinamide concentrations (Nzoughet et al., 2019). And Williams et al. determined that a nicotinamide supplement could significantly more neuroprotection in a glaucomatous mouse model (Williams et al., 2017). Also, primary outcomes for nicotinamide clinical trials measuring visual fields, electroretinography, OCT, and hyperspectral imaging have shown great promise.
5.6. Gene therapy
Some gene therapies have been very encouraging to treat non-glaucomatous, progressive retinal and optic nerve pathologies. But currently there are no gene therapies for glaucoma neuroprotection. Glaucoma patients could benefit from gene therapies that deliver neuroprotective therapeutics to patients. One gene therapy that could be beneficial was acquired by Astellas Pharmaceuticals and is a construct of brain-derived neurotrophic factor (BDNF) and its receptor TrkB (Osborne et al., 2018b, Osborne et al., 2018a), which the company is planning to assess in glaucoma clinical trials. This construct targets to maintain the natural neuroprotective levels of the BDNF, which typically can decline after the onset of glaucoma, for RGC survival (Quigley et al., 2000).
Other research reported by Tanigawa et al., 2020 described rare protein-altering variants in ANGPTL7 and their functional consequence and therapeutic effects. The study group consisted of more than 514,000 individuals in two population cohorts with European ancestries in the UK and Finland (Tanigawa et al., 2020). In the UK Biobank, the research group discovered a series of multiple rare ANGPTL7 variants that lower intraocular pressure and reduce the risk of glaucoma. Additionally, they identified a unique ANGPTL7 variant in the FinnGen cohort containing more than 50-fold enrichment in the population of Finland that provides them with protection against glaucoma. This research also places great emphasis on the benefits of multi-cohort analysis for discovering rare protein-altering variants for common diseases. Also, the results suggested that ANGPTL7 could be a good therapeutic target for treating (i.e., lowering IOP) and preventing glaucoma progression (i.e., providing neuroprotection).
5.7. New artificial intelligence algorithm to detect glaucoma progression
A new test method technology has been developed which can detect glaucoma progression essentially 18 months earlier than the current standard OCT retinal imaging technology (Normando et al., 2020). It is supported using an artificial intelligence (Al) algorithm, which provides the ability to accelerate clinical trials and could be eventually used for detection and diagnosing glaucoma (Normando et al., 2020). The technology, known as Detection of Apoptosing Retinal Cells (DARC), involves an injection in the arm of a fluorescent dye that attaches to retinal cells and illuminates those cells in the process of apoptosis, a form of programmed cell death (Normando et al., 2020). During the process, damaged cells appear bright white when viewed during an eye examination and the cells with the most severe damaged result in higher DARC counts. In the Phase II clinical trial of DARC (ISRCTN10751859), the AI was used to assess 60 of the study subjects, (from heathy control n = 40 and glaucoma n = 20 subjects with glaucoma). The first step in the process involved training the AI using retinal scans (post-dye injection) of the healthy control subjects (Normando et al., 2020). Following training the AI was then used to analyze the glaucoma subjects. Participants were followed up 18 months after the main trial period to determine whether their eye health had deteriorated. Every patient with a DARC count over a certain threshold was found to have progressive glaucoma at follow-up examination. Basically, the results were described as follows; “the algorithm had 97.0% accuracy, 91.1% sensitivity and 97.1% specificity to spot detection when compared to manual grading of 50% controls. It was next tested on glaucoma patient eyes defined as progressing or stable based on a significant (p < 0.05) rate of progression using OCT-retinal nerve fibre layer measurements at 18 months. It demonstrated 85.7% sensitivity, 91.7% specificity with area under the receiver operating characteristic curve of 0.89, and a significantly (p = 0.0044) greater DARC count in those patients who later progressed (Normando et al., 2020)”. As evidenced by these trial results the CNN-enabled algorithm provided automated and objective measurements of DARC, which validates its use as an AI-aided biomarker for detecting glaucoma progression and enables better testing of new drug therapies (Normando et al., 2020). At present the DARC Technology is being commercialized by a newly formed company called Novai with a member of the research group, Professor Francesca Cordeiro, serving as its Chief Scientific Officer.
5.8. Other strategies – combination therapies (non-clinical)
Although several combination therapies are currently in clinical practice to reduce IOP there is no equivalent combination therapy for neuroprotection (Hollo et al., 2014b, Hollo et al., 2014a, Yilmaz et al., 2018). All the aforementioned neuroprotection strategies have focused on a single material or approach to curb the neurodegenerative progression of glaucoma. However, there are others that are investigating novel therapeutic approaches to manage or protect the RGCs and other factors from neurodegeneration with combination therapy. Researchers described the development of simultaneous co-delivery of neuroprotective drugs (dexamethasone, melatonin, and coenzyme Q10 from multi-loaded PLGA microspheres for the treatment of glaucoma (Arranz-Romera et al., 2019) (Morrison et al., 1997). The microspheres had a mean particle size of 29.04 ± 1.89 μm, which made them suitable for an intravitreal injection using conventional 25G to 32G syringe needles. The group’s previous work indicated that there is an upper limit of multi-loaded microspheres that should not be exceeded when injecting intravitreally. At the upper limit of 0.5 mg, the multi-loaded microsphere particles induced retinal stress and photoreceptor dysfunction in rodents whereas with the lower dose of 0.1 mg, this phenomenon was not observed (Zhao et al., 2017). Additionally, multi-loaded microspheres had a co-delivery profile for sustained release over 30-days. In a chronic rodent model of ocular hypertension, 21-days after dosing, the multi-loaded microspheres showed a significant neuroprotective effect, while no protective effect was observed with single-drug microspheres or empty microspheres.
New strategies are being considered continuously and in the future glaucoma neuroprotection trials could result in the development of new glaucoma drug delivery systems to fill this unmet need. Also, it could become more common to treat the neuroprotection aspects of glaucoma similar to some cancers therapies where the treatment is tailored according to the patient’s unique physiological profile (Khatib and Martin, 2020).
6. Case Study - Pharmacokinetics and pharmacodynamics of bimatoprost, a sustained-release intracameral delivery system
Bimatoprost, a prostaglandin analog, approved for once daily eye drop dosing to reduce intraocular pressure, has several pharmacokinetic studies performed in multiple species including rabbit, monkey, beagle dog, and human models. Key drug delivery and pharmacokinetic data is summarized in Table 4 following topical as well as intracameral dosing of bimatoprost. Intracamerally dosed slow release implant of bimatoprost is the most advanced drug product under development for sustained lowering of IOP. Some preclinical pharmacokinetic data related to this implant is also summarized in Table 4 (Faulkner et al., 2010, Cantor et al., 2007, Shen et al., 2018, Shafiee et al., 2013, Seal et al., 2019, Shen et al., 2020, Woodward et al., 2003). Below, pharmacokinetics and pharmacodynamics of bimatoprost are further elaborated.
Table 4.
Pharmacokinetic parameters of bimatoprost (Bim) and bimatoprost acid (BimA) in aqueous humor after bimatoprost administration through topical or intracameral routes in pre-clinical and clinical studies. Key: NR-Not reported; Cmax- Maximum concentration; Tmax- Time for maximum concentration; AUC- Area under the concentration–time curve; BLQ- below limit of quantitation;
| Route (frequency and duration) | Species | Drug product | Cmax | Tmax | AUC | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| Bim | BimA | Bim | BimA | Bim | BimA | ||||
| Plain drug solution | |||||||||
| Topical (once daily for 21 days) | Human (cataract surgery patients) | Lumigan® (0.03 %) | 2.83 ± 0.57 ng/mL | 12.84 ± 6.82 ng/ml | 1 hr | 2 hr | 6.82 ng.hr/mL (0–5 hr) | 30.51 ng.hr/mL (0–5 hr) | Faulkner R et al., 2010 |
| Topical (single dose) | Human (cataract surgery patients) | Lumigan® (0.03 %) | 2.74 ± 1.50 ng/mL | 2.78 ±1.37 ng/mL | l hr | 3 hr | NR | NR | Cantor et al. 2007 |
| Topical (once daily for 4 days) | New Zealand white rabbit | Lumigan® (0.01 %) | NR (BLQ by 1 hr) | 13.9 ± 1.8 ng/mL | NR | 1 hr | NR | 42.8±3.1 ng.hr/mL (0–8 hr) | Shen et al. 2017 |
| Topical (twice daily for 3 days + single dose on day 4) | New Zealand white rabbit | Lumigan® (0.01 %) | NR (BLQ by 1 hr) | 18.3 ± 2.7 ng/mL | NR | 1 hr | NR | 62.3±4.8 ng.hr/mL (0–8 hr) | Shen et al. 2017 |
| Topical (single dose) | Pigmented rabbit (HY79b) | Bimatoprost (0.03 %) | 12.11 ± 21.72 ng/mL | 29.58 ± 26.37 ng/mL | l hr | l hr | 12.31 ng.hr/mL (0.25–24 hr) | 98.79 ng.hr/mL (0.25–24 hr) | Shafiee et al. 2013 |
| Topical (single dose) | Pigmented rabbit (HY79b) | Bimatoprost (0.03%) formulated in DuraSite | 26.57 ± 19.16 ng/mL | 103.4 ± 42.36 ng/mL | 0.5 hr | 2 hr | 24.29 ng.hr/mL (0.25–24 hr) | 302.6 ng.hr/mL (0.25–24 hr) | Shafiee et al. 2013 |
| Topical (single dose) | Cynomolgus monkey | Bimatoprost (0.1 %) | 41.8 ng/g=mL | NR | 0.5 hr | NR | 110 ng.hr/g (0–24 hr) | NR | Woodward et al. 2003 |
| Topical (twice daily for 9.5 days) | Cynomolgus monkey | Bimatoprost (0.1 %) | 14.2 ng/g=mL | NR | 6 hr | NR | 102 ng.hr/g (0–24 hr) | NR | Woodward et al. 2003 |
| Topical (once daily for 7 days) | Beagle dog | Lumigan® (0.03 %) | 0.285 ± 0.166 ng/g | 3.29 ± 0.35 ng/g | l hr | 9 hr | 1.45 ± 0.41 ng.hr/mL (0–9 hr) | 18.6 ± 2.6 ng.hr/mL (0–9 hr) | Seal et al. 2019 |
| Sustained-release implant | |||||||||
| Intracameral (single dose) | Beagle dog | Bimatoprost sustained release implant (15 μg) | 22.9 ± 19.6 ng/g | 1.27 ± 0.30 ng/g | 27 day | 52 day | 727 ± 511 ng.day/mL (0–6 month) | 44.0 ± 6.6 ng.day/mL (0–6 month) | Seal et al. 2019 |
| Intracameral (single dose) | Beagle dog | Bimatoprost sustained release implant (8 μg) | 3.92 ng/mL* | 1.83 ng/mL* | 11 week* | 11 week* | 25.19 ng.day/mL (2–14 week)* | 11.28 ng.day/mL (2–14 week)* | Shen J et al. 2020 |
| Intracameral (single dose) | Beagle dog | Bimatoprost sustained release implant (15 μg) | 4.61 ng/mL* | 1.26 ng/mL* | 11 week* | 9 week* | 34.75 ng.day/mL (2–14 week)* | 9.70 ng.day/mL (2–14 week)* | Shen J et al. 2020 |
| Intracameral (single dose) | Beagle dog | Bimatoprost sustained release implant (30 μg) | 8.61 ng/mL* | 1.95 ng/mL* | 11 week* | 11 week* | 65.83 ng.day/mL (2–14 week)* | 15.51 ng.day/mL (2–14 week)* | Shen J et al. 2020 |
| Intracameral (single dose) | Beagle dog | Bimatoprost sustained release implant (60 μg) | 10.80 ng/mL* | 3.61 ng/mL* | 7 week* | 11 week* | 85.06 ng.day/mL (2–14 week)* | 29.35 ng.day/mL (2–14 week)* | Shen J et al. 2020 |
indicates that parameters are estimated/extracted.
6.1. Pharmacokinetics of topical bimatoprost eye drop and intracameral bimatoprost sustained-release implant
The pharmacokinetics of topical bimatoprost eye drop and intracameral bimatoprost sustained-release implant was studied by Seal et al. (Seal et al., 2019). Beagle dogs with normal ophthalmic examination were used in the study. One drop each (~35 μl) of topical bimatoprost 0.03% ophthalmic solution (Lumigan; Allergan plc, Dublin, Ireland) was applied once daily for 7 days to both the eyes of animals (n=10). Following eye drop administration, eyes were closed for ~5 sec to allow dose distribution around the eye. Another set of animals (n=14) was subjected to intracameral injection of bimatoprost sustained-release implant (dose 15 μg) using sterile, preloaded ready-to-use applicator. Animals from topical dosing were sacrificed at 0.5, 1, 2, 4, and 9 h post-dose at day 7 and animals from intracameral dosing were sacrificed at 1, 2, 3, 4.5, and 6 months following the injection. Aqueous humor (AH), eyelid margins (upper and lower collected separately), periorbital fat, bulbar conjunctiva, iris-ciliary body (ICB), retina (area centralis region), and cornea were collected and frozen. The concentrations of bimatoprost and bimatoprost acid were estimated in these tissues using liquid chromatography–tandem mass spectrometer and normalized to tissue weight or volume. The data was analyzed using non-compartmental analysis.
Area under the concentration-time curve (AUC) and maximum concentration (Cmax) for bimatoprost and bimatoprost acid from Seal et al. (Seal et al., 2019) is presented in Figure 16. Higher Cmax and AUC for bimatoprost is evident in cornea, AH, and ICB after the administration of intracameral sustained-release implant compared to topical eye drop. Similarly, increased Cmax and AUC was observed for bimatoprost acid in ICB after the administration of intracameral sustained-release implant compared to topical eye drop. There was considerable amount of bimatoprost present in off-target tissues (upper and lower eyelid margin, bulbar conjunctiva, and periorbital fat) after the administration of topical bimatoprost eye drop. On the other hand, only trace amount (< 0.1 ng/ml) of bimatoprost was detected in these tissues following intracameral sustained-release implant. The amount of intact drug is more in cornea, AH, and ICB compared to its metabolized form following intracameral administration. On the other hand, the metabolized product is more in these tissues following topical administration. This indicates the larger contribution of corneal epithelium, and stroma in the degradation of bimatoprost to bimatoprost acid (Figure 16).
Figure 16.

Pharmacokinetic parameters for bimatoprost dosed as topical eye drops or slow release intracameral implant in beagle dogs. (A) Maximum concentration (Cmax) and (B) area under the concentration–time curve from time zero to the last measurable time point (AUC0-tlast) for bimatoprost and bimatoprost acid in various ocular tissues after the administration of 0.03% bimatoprost eye drops daily once for 7 days (daily dose of about 35 μg of 0.03% drug solution; ~ 10.5 μg) or single bimatoprost sustained-release implant (dose 15 μg) in beagle dogs. Bimatoprost to bimatoprost acid ratio of (C) AUC and (D) Cmax. Data is re-plotted from Seal JR et al., 2019. Missing bars indicate that the corresponding concentrations for bimatoprost and bimatoprost acid are below the limit of quantitation (≤ 0.1 ng/ml for each). Key: Bim- Bimatoprost; BimA- Bimatoprost acid; ED- Eye drop; and SR- Sustained-release.
6.2. Prediction of IOP response in humans
The pharmacokinetics and pharmacodynamics of different doses of intracameral bimatoprost sustained-release implant in beagle dogs was reported by Shen et al. (Shen et al., 2020). Normotensive beagle dogs were injected in one eye with different doses (8, 15, 30, and 60 μg) of bimatoprost sustained-release implant in the anterior eye, i.e., intracameral space, using applicator device. Animals were sacrificed at 2, 3, 5, 7, 9, 11, and 14 weeks post-dosing and aqueous humor samples were collected. The concentrations of bimatoprost and bimatoprost acid were measured using liquid chromatography-tandem mass spectrometry. Another set of animals receiving the same dosing were monitored three times every week noting IOP measurements until day 179 post-dosing.
Shen et al., (Shen et al., 2020) based on beagle dog data, predicted the average aqueous humor concentration in humans (2x the concentration in dog based on aqueous humor outflow differences) and using this estimated human concentration, predicted IOP response in humans based on the Emax model for dose-response in the dog model. Using the same Emax model for pharmacodynamic response in the dog, we estimated the concentration time course at the time points where dog IOP was reported. These concentrations were used to estimate human drug concentrations and IOP response using an approach similar to that used by Shen et al. (Shen et al., 2020). The predicted IOP response and concentration time course in humans over 179 days at different implant doses are shown in Figure 17. While the time course predictions are useful in understanding potential response to the slow release implant in humans, actual study outcomes in humans are not known. The assumptions include: 1) IOP lowering (%) correlates with the combined concentrations of bimatoprost and bimatoprost acid in aqueous humor as per the Emax model reported by Shen et al. (Shen et al., 2020). 2) The ratio of aqueous humor clearance of drug in humans to dog is 2:1, as per the assumption by Shen et al.(Shen et al., 2020). 3) The in vivo drug release rate for the implants is same in human and dog. 4) Humans have same EC50 and Emax for bimatoprost (Bim) and bimatoprost acid (BimA) as that of dog, similar to the assumption by Shen et al. (2020) (Figure 17).
Figure 17.

Predicted drug and response time-course for bimatoprost slow release implants in humans. (A) Predicted time-course of concentrations of bimatoprost plus bimatoprost acid in human aqueous humor and (B) corresponding %IOP lowering after anterior chamber administration of bimatoprost sustained-release implants containing 8, 15, 30, or 60 μg drug. The predictions are based on the IOP lowering (%) after the intracameral administration of bimatoprost sustained-release implants in Beagle dogs (Shen J, 2020). Assumptions: 1) IOP lowering (%) directly correlated with the combined concentrations of bimatoprost and bimatoprost acid in aqueous humor. 2) The ratio of clearance of drug for human to dog be 2:1. 3) The in vivo drug release rate for the implants is same in human and dog. 4) Humans have the same EC50 and Emax for bimatoprost and bimatoprost acid as that of dog.
6.3. Correlation of bimatoprost to bimatoprost acid levels in beagle dog
Using the time course concentrations extracted from Shen et al. (Shen et al., 2020), we correlated aqueous humor bimatoprost concentrations to bimatoprost acid formed in vivo following bimatoprost implant dosing in the intracameral space in beagle dogs. The concentrations of bimatoprost and bimatoprost acid in aqueous humor of beagle dogs for all the reported time points (2–14 weeks) and doses (8, 15, 30, and 60 μg) were used. The intact drug (Bim) and its metabolite product (BimA) were in good linear correlation with R2 = 0.8. Thus, it is difficult to dissect whether free acid or the parent drug contributed directly to drug activity based on this study alone (Figure 18).
Figure 18.
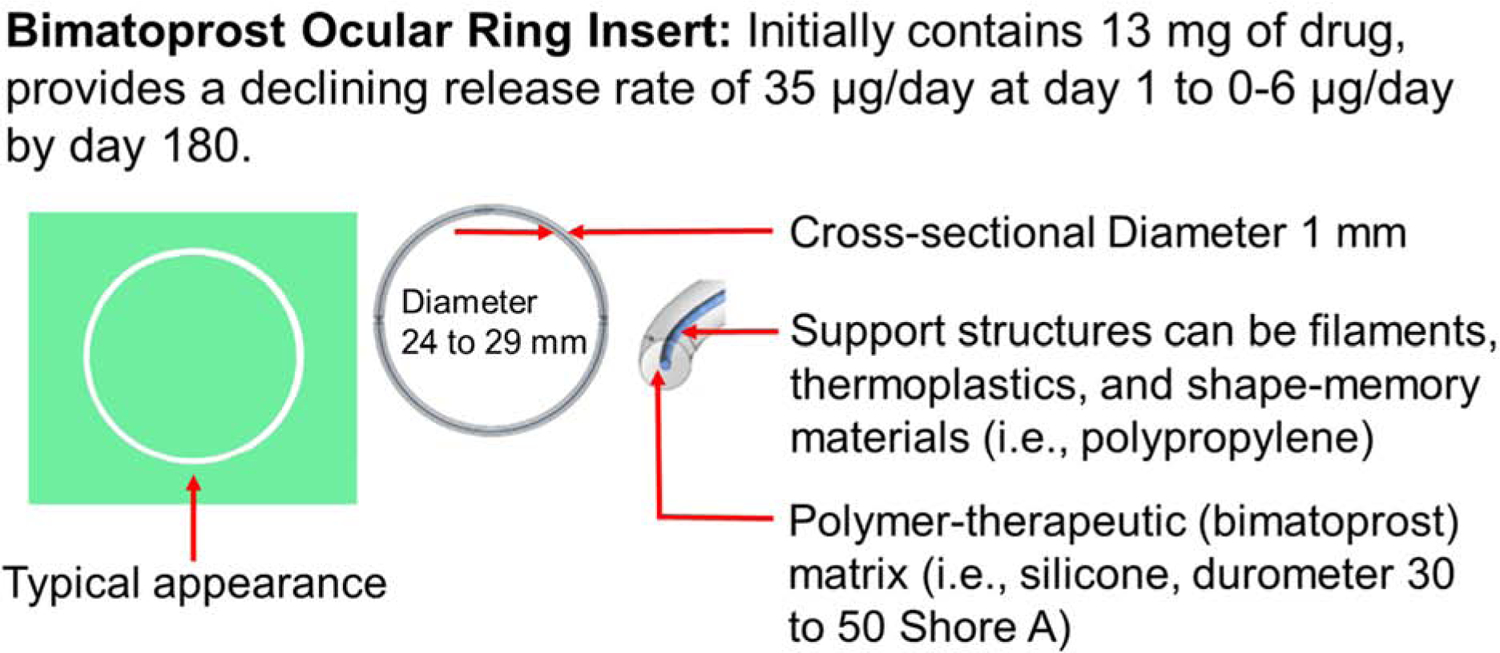
Correlation of bimatoprost to bimatoprost acid in aqueous humor of beagle dogs following intracameral dosing of slow release bimatoprost implants. Sustained-release implants containing 8, 15, 30, or 60 μg bimatoprost were used in beagle dogs. Drug concentrations from 2 to 14 weeks were extracted and correlated. Data is based on Shen et al., 2020.
6.4. Dose-response curve for intracameral bimatoprost sustained-release implant in beagle dog.
The average of IOP lowering (%) in beagle dog for the duration of 3–179 days after the intracameral administration of bimatoprost sustained-release implant reported by Shen et al. (Shen et al., 2020) for each dose was plotted against the dose administered to obtain a dose-response curve. A hyperbolic Emax model was fit to dose amount vs % IOP reduction for the implant (Figure 19). The model explained the data well. The same model was used by Shen et al. (Shen et al., 2020) to relate drug concentration to % IOP reduction (Figure 19).
Figure 19: Prediction of ED50 for bimatoprost sustained-release implant in beagle dog.
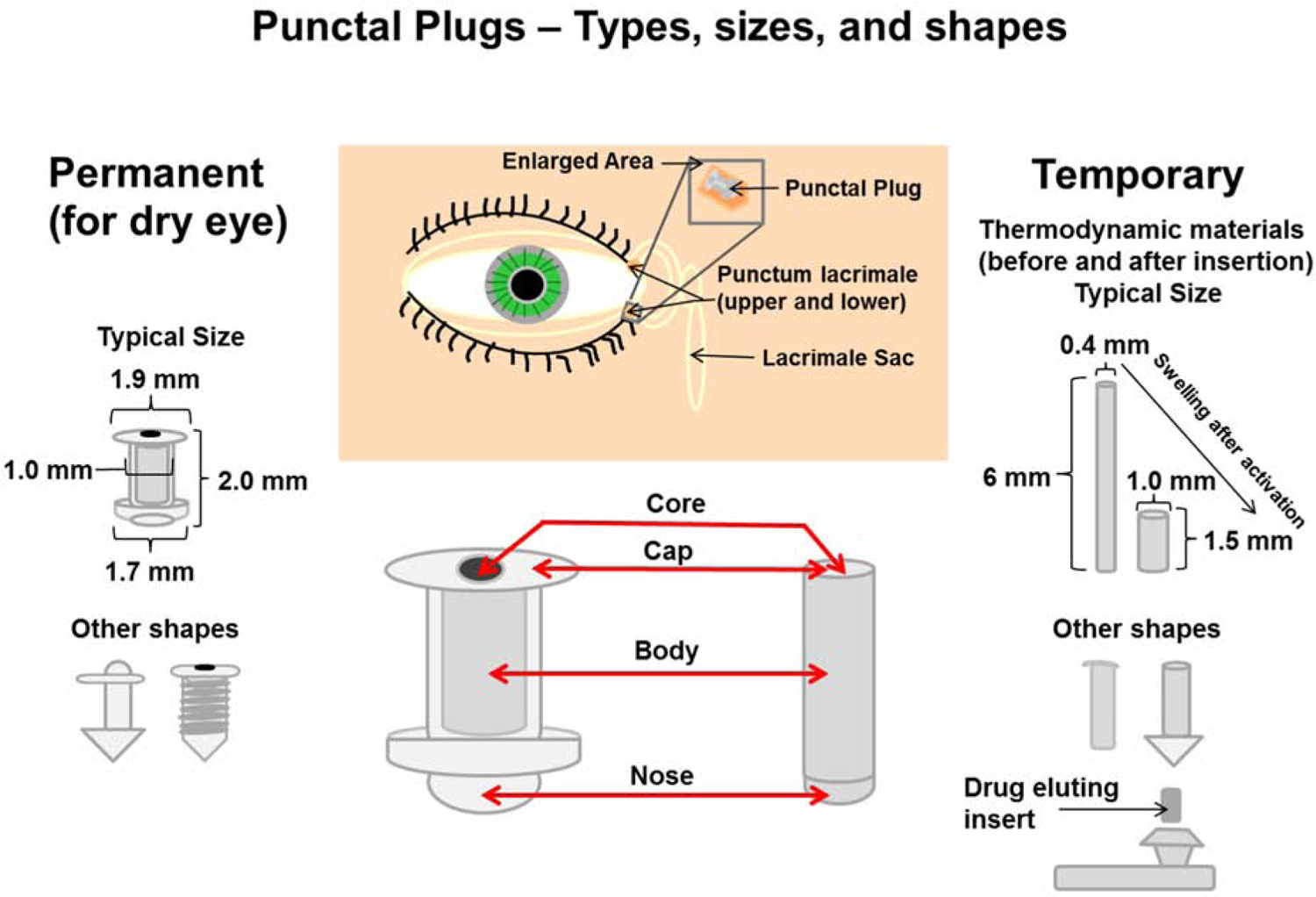
Bimatoprost sustained-release implants containing 8, 15, 30, or 60 μg drug were dosed intracamerally in beagle dogs and the IOP was monitored over 179 days. The average %IOP lowering (3–179 days) was extracted and data was fitted using simple Emax model to obtain the Emax and ED50. The data is based on Shen et al., 2020.
7. Comparative pharmacokinetics of extraocular, periocular, and intraocular routes
Anti-glaucoma drugs are commonly administered as eye drops. Key routes of administration for sustained-release anti-glaucoma drug delivery systems are topical, intracameral, intravitreal, subconjunctival (periocular), and supraciliary routes. Among these routes, only intracameral route has been used successfully with a recent clinically approved single-use drug product for anti-glaucoma drug delivery. While the head-to-head comparison of multiple routes for anti-glaucoma drug delivery are almost non-existent in humans and very sparse in rabbit models, some reasonable conclusions can be made about the relative effectiveness and dose requirements for the various routes. Below, the topical and intracameral routes are compared primarily, followed by some analysis for intravitreal and subconjunctival routes. Since the supraciliary route is new with limited exploration, no additional details are included. The general pharmacokinetics of various routes are compared using aqueous humor as the surrogate tissue in equilibrium with various targets relevant for intraocular pressure reduction. The discussion below is pertinent to small drug molecules with about 500 Da molecular weight or less. After comparing pharmacokinetics based on solution dosage form, the discussion is extended to sustained release systems. The following discussion is for aqueous humor drug concentration time-course.
7.1. Bioavailability
Bioavailability refers to the rate and extent of drug absorption and it is more commonly used to refer to the percent of dose absorbed. Time to reach peak concentration in aqueous humor, maximum concentration reached, and the area under the concentration vs. time plot relate to drug bioavailability. By the extraocular (e.g., topical eye drop) and periocular (e.g., subconjunctival) routes discussed above, peak drug concentrations in the aqueous humor are anticipated in about an hour or sooner for a low molecular weight drug (Schoenwald, 1993), with the delivery being instantaneous with intracameral injections. Since the peak drug concentrations are achieved relatively quickly, and the therapeutic duration desired for sustained drug delivery is at least a few months for glaucoma treatment, a critical factor in choosing a route of administration is the fraction of drug absorbed. Eye drops deliver a small fraction of the drug to the tissues of the aqueous humor, typically less than 5%, and at most about 10% (Schoenwald, 1993). If the drug is placed intracamerally, it is immediately available in the aqueous humor since the corneal absorption barrier is bypassed. Further, the fraction of dose delivered, or bioavailability is 100% by the intracameral route. Thus, the intracameral route allows the most rapid and complete delivery of the drug in solution form, with the rate and extent of delivery being lower with eye drops.
Subconjunctival route, while being 52-fold or more efficient than the systemic route for intraocular drug delivery to tissues of the anterior as well as posterior segment (Ayalasomayajula and Kompella, 2004), and less rapid (half-life of 22 min) (Kim et al., 2008b) for clearing the dose relative to eye drops (Snibson et al., 1992) (half-life in seconds for a solution drop and a few minutes for drug released from a viscous preparation) (Snibson et al., 1992, Zaki et al., 1986), is still inefficient in delivering drug to the intraocular tissues, like eye drops. Access to aqueous humor following subconjunctival dosing requires escaping conjunctival and episcleral vasculature, entry into the tear film, and subsequent delivery via the corneal pathway or delivery via sclera or noncorneal pathway (Raghava et al., 2004). The percent dose delivered by this route is also expected to be low, like eye drops.
Intravitreally administered drugs are cleared predominantly posteriorly (lipophilic drugs) via the retina-choroid circulation or anteriorly (hydrophilic drugs) via the aqueous humor outflow pathways (Maurice, 2001, Araie and Maurice, 1991). Thus, the extent of drug entry into the anterior chamber is expected to reduce with an increase in drug lipophilicity. For a lipophilic drug, only a small percentage of the dose may be cleared by the anterior pathway, where the drug can be exposed to the tissues influencing intraocular pressure control. Since the majority of the anti-glaucoma drugs, especially the most successful prostaglandin analogs, are lipophilic, intravitreal dosing is expected to result in the delivery of only a small fraction of the dose to the aqueous humor. Intavitreal route may result in peak times that are longer than those observed with topical and subconjunctival routes, since the drug at the site of administration is not lost rapidly.
7.2. Drug exposure
Depending on the route of entry, the drug exposure duration is expected to differ for the various routes for an immediate release dosage form such as a drug solution. Drug exposure can be evaluated based on area under the concentration vs. time curve or mean residence time (ratio of the area under the concentration x time vs. time curve and the area under the concentration vs. time curve) of the drug by different routes. Once the drug is dosed intracamerally, the only process it undergoes is distribution and elimination. On the other hand, all the other routes including topical, intravitreal, and subconjunctival routes must deliver drug across barriers into the aqueous humor. This absorption process may self-sustain drug levels, extending the mean residence time, relative to intracameral dose. However, the sustainment due to slow absorption is minimal following topical and subconjunctival dosing due to rapid drug loss from the site of administration, which halts the absorption prematurely. The intravitreal route, on the other hand, may sustain drug absorption for longer periods since the loss of drug to retina-choroid circulation is much slower relative to precorneal clearance of a topical eye drop. This is particularly true for intravitreally dosed hydrophilic drugs and macromolecules (Lamminsalo et al., 2018, Missel, 2012). Despite these innate differences in drug exposure durations by various routes, for a sustained release system providing drug delivery for a few months, the durations of drug exposure are controlled by the slow-release and expected to differ marginally for various routes.
7.3. Terminal slope of aqueous humor drug concentration decline
For a drug dosed intracamerally, the decline in concentrations reflects the drug removal process, primarily elimination from the aqueous humor. Thus, data obtained after intracameral dosing of a drug solution is useful in determining the aqueous humor elimination rate constant. If a drug resides mostly in the aqueous humor with little distribution to surrounding tissues, then the elimination half-life is expected to be close to that of aqueous humor drainage. With significant tissue distribution followed by redistribution when the aqueous humor drug levels decline, the drug may persist longer in the aqueous humor. However, when a drug is dosed topically, the terminal slope may or may not reflect the elimination rate constant, since drug absorption process across cornea is typically slower than the elimination process from the aqueous humor. The ocular surface loss rate also influences what is represented by the terminal slope in aqueous humor after topical dosing. High loss factors on the ocular surface and rapid absorption once in cornea, will result in the terminal slope being reflective of the elimination rate constant from aqueous humor. Following intravitreal injection, drug entry into the aqueous humor is typically rate limiting relative to aqueous humor elimination. Thus, for many drugs, the decline in the vitreous humor concentrations are expected to parallel the drug decline in the aqueous humor (Shen et al., 2014). In this case, the terminal slope is expected to represent a slower process than the true aqueous humor elimination rate constant. Despite these differences in the behavior of drug time-course in the aqueous humor, for slow release systems dosed by any of these routes, the drug release rate is expected to be rate-limiting. Thus, the terminal slope in aqueous humor would reflect drug release rates in each case.
7.4. Influence of absorption rate constant on drug exposure
Since the drug loss process on ocular surface exceeds by several fold relative to drug absorption process for eye drop dosing, with the bioavailability being very low, an increase in the absorption rate constant is expected to deliver more drug to the aqueous humor, thereby increasing bioavailability (Lee and Robinson, 2004, Makoid and Robinson, 1979). The same is the case for subconjunctival dosing and for intravitreal dosing. Further, the drug loss on the ocular surface is expected to be lower relative to eye drops for slow release systems, which may be considered as high viscosity preparations. Thus, slow release systems on ocular surface, by reducing precorneal loss, may achieve greater drug exposure.
7.5. Modeling sustained anti-glaucoma drug delivery after dosing by various routes of administration
Conventional pharmacokinetic modeling requires rate constants for drug loss at the site of administration that is remote from the aqueous humor, rate constant for drug elimination from the aqueous humor, fraction of drug absorbed from the site of administration into the aqueous humor, volume of aqueous humor compartment, and rate constant or rate of zero-order release of drug from the sustained release system. Of these parameters, rate constant for drug loss at the site of administration, fraction of dose absorbed, and the rate constant for drug absorption are rarely available. Some sites of administration are virtual spaces that do not allow a thorough quantification of the dose remaining (e.g., subconjunctival injection of a solution, which disappears soon after tissue dissection) due to sampling difficulties, making it complex to estimate the rate constant for drug loss from the site of administration. Similarly, the drug loss rate constant in the tear film are rarely estimated, although tear sampling is more routine. Precorneal drug loss rate constant is expected to differ widely for different formulations, especially based on viscosity. The rate constant for absorption differs based on the model used for estimation (e.g., a model with a loss factor at the site of administration vs. another without a loss factor). Estimation of fraction absorbed requires the aqueous humor AUC comparison of a route that requires drug absorption into aqueous humor with intracameral dosing. This comparison is rarely available for routes other than the topical route. Thus, a fair comparison of the various routes of administration is difficult in the absence of actual experimental data for relative dosing requirements in developing sustained release systems for anti-glaucoma drugs.
Considering the above limitations, simulations presented below should be interpreted with caution. Figure 20 shows pharmacokinetic models and assumptions for various pharmacokinetic parameters of a hypothetical anti-glaucoma drug administered by topical, intracameral, intravitreal, or subconjunctival routes. All routes included elimination from the aqueous humor. All extra-cameral routes included drug loss rate constant at the site of administration. The elimination half-life is fixed from the aqueous humor for all routes of administration. The bioavailability in aqueous humor was assumed to be 100, 10, 5, and 2%, respectively, for intracameral, topical, subconjunctival, and intravitreal routes, respectively. After fixing the first order drug release rate constant for the various routes of administration, drug concentrations in the aqueous humor were simulated for a fixed dose of 15 μg. It is evident that drug concentrations in aqueous humor decline similarly by the various routes of administration, consistent with the rate limiting nature of sustained drug release. The rank order for the drug exposure was: intracameral >> intravitreal > subconjunctival > topical, consistent with the critical influence of loss rate constants at the various sites of administration. Accordingly, the doses required for the same drug exposure or AUC are in the order: intracameral << intravitreal < subconjunctival < topical. Further, for intracameral route, the first-order drug release rate was compared with a zero-order release rate (Figure 20). Thus, a small drug dose is adequate for prolonged delivery to the aqueous humor for a few months. These models with a loss factor at the site of absorption may also be developed without assuming the fraction absorbed. Some of the rate constants (e.g., absorption rate constant) will differ in that case; thus, parameter values should be interpreted cautiously from one model to another.
Figure 20:
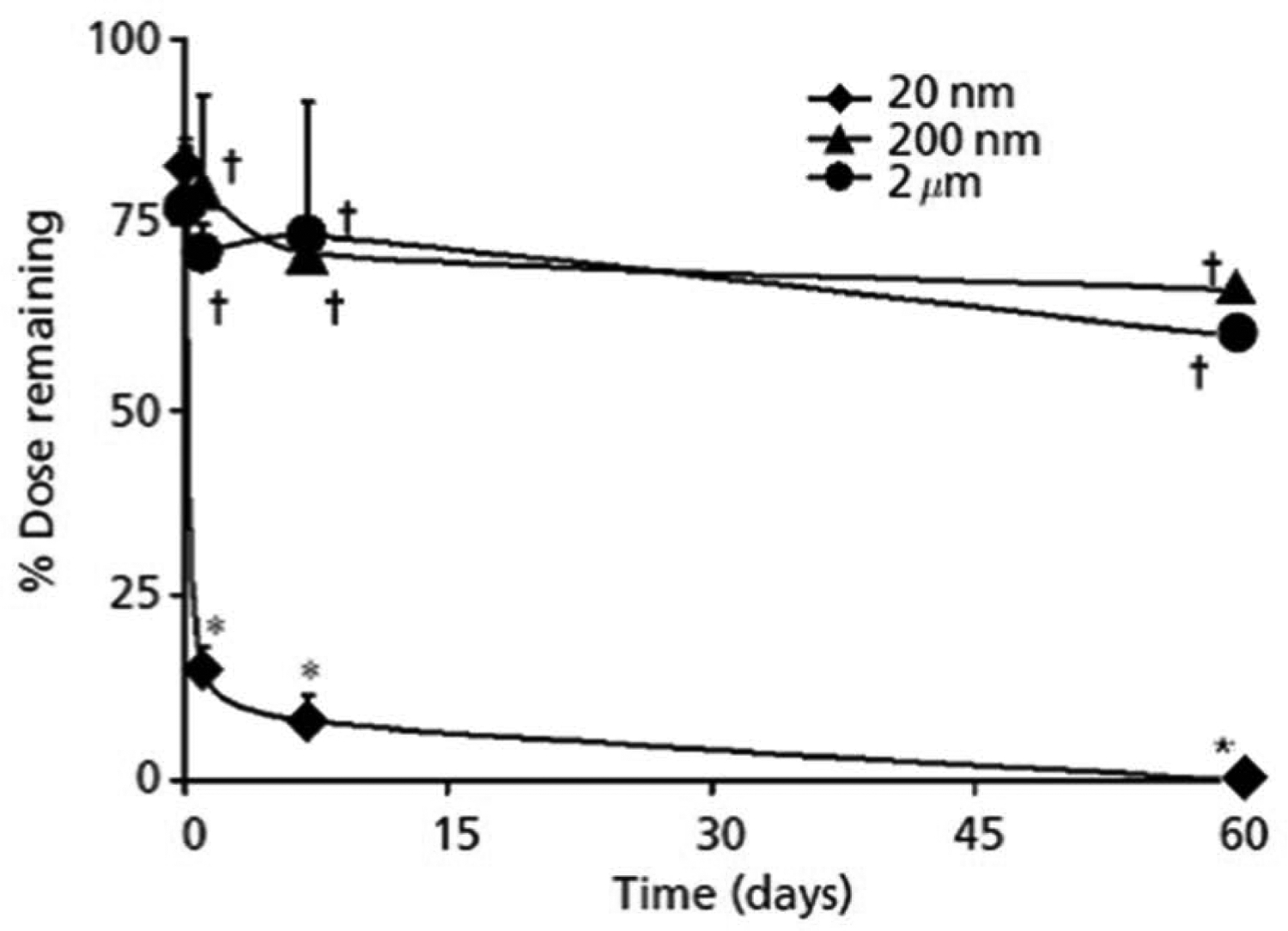
Simulation of sustained-release drug delivery to aqueous humor following topical, intracameral, intravitreal, and subconjunctival dosing in humans using Berkeley Madonna version 9. A) Pharmacokinetic models used for the simulation. B) Dose and pharmacokinetic parameters used for simulations. C) Aqueous humor concentration vs. time course data for the models in panel A using data in panel B. The sustained release system was assumed to release the drug as per a first-order process. D) Aqueous humor concentration vs. time course data for first-order vs. zero-order release rate following intracameral dosing.
Another simple approach to estimate the relative dose requirements for administration by various routes is based on the basis of the expression, dosing rate = average steady state concentration desired in aqueous humor x clearance from aqueous humor x dosing interval/fraction absorbed. Since only fraction absorbed is route dependent in this equation, the equation implies that dosing rate is inversely proportional to the fraction of drug absorbed. If the bioavailability of a drug in aqueous humor is assumed to be 100, 10, 5, and 2%, respectively, as above for intracameral, topical, subconjunctival, and intravitreal routes, 1-, 10-, 20- and 50-fold doses are required by these routes, respectively, relative to intracameral route.
7.6. Comparison of intracameral vs. topical route for bimatoprost dose reduction
Since only intracameral route has succeeded in clinically relevant sustained intraocular pressure reduction, limited insights can be derived by comparing the doses used in developing sustained release delivery systems for prostaglandins by various routes. A single 10 μg dose in a sustained release implant dosed intracamerally sustains IOP reduction that is non-inferior to twice daily timolol eye drops for 3 months (Medeiros et al., 2020). Eye drop dose of bimatoprost for 3 months is about 450 μg for a 0.01% drop of 50 μL volume. Thus, the intracameral route offers about 45- and 135-fold dose-reduction for bimatoprost relative to 0.01% eye drops and 0.03% eye drops, respectively, with the caveat that the peak IOP reduction may or may not be equivalent to the corresponding eye drops (Table 3). For the fornix ring insert of bimatoprost, assuming the drug amount in the implant is 13,000 μg, the dose reduction with intracameral dose is 650-fold (Brandt et al., 2016) (Table 3).
Table 3.
Prostaglandin eye drops and clinically advanced or late stage sustained release delivery systems. Dose ratio relative to intracameral route is included for bimatoprost, the most clinically successful prostaglandin for sustained delivery.
| Route | Product | Manufacturer | Strength/ Total dose/ Frequency | Daily dose (μg/Day) | Dose (μg/180 Days | Dose ratio relative to intracameral |
|---|---|---|---|---|---|---|
| Bimatoprost | ||||||
| Topical eye drop solution | Lumigan | Allergan | 0.1 mg/mL or 0.3 mg/mL, 1 drop q.d. (pm) | 5 μg/Day or 15 μg/Day | 900 or 2700 μg | 45 or 135 |
| Conjunctival fornix insert | Fornix ring insert | Allergan | 13 mg, 1 insert, delivers for 6 months | To - 35 μg/Day T180 - 6 μg/Day |
13,000 | 650 |
| Intracameral implant | Durysta | Allergan | 10 μg, 1 implant, delivers for 3 months | 0.1 μg/Day to 0.9 μg/Day | 20 | 1 |
| Latanoprost | ||||||
| Topical eye drop solution | Xalatan | Pfizer | 50 μg/mL | 2.5 μg/Day | ||
| Topical nondegradable insert | Eye-D VS101 | BioLight Life Sciences Ltd. | High, medium, and low doses, delivers for 3 months | N/A+ | ||
| Punctum or nasolacrimal duct plug | L-PPDS Punctal plug | Mati Therapeutics | Low dose: 70.5 μg (1 plug) High dose:141 μg (2 plugs), delivers for 4 weeks |
3.0 μg/Day* | ||
| EXP-LP Punctal plug | Eximore, Ltd. | Low dose: 250 μg High dose: 450 μg |
1.38 μg/Day 2.50 μg/Day |
|||
| Intracameral implant | PA5108 For latanoprost free acid | PolyActiva | Study 1 doses - 8.9, 14.7, 26.6, and 35.5 μg. Study 2 doses – 16.2, 30, 45, and 60 μg, delivers for 3 months |
N/A+ | ||
| Travoprost | ||||||
| Topical eye drop solution | Travatan Z | Novartis | 40 μg/mL | 2 μg/Day | ||
| Punctum or nasolacrimal duct plug | OTX-TP2 | Ocular Therapeutix | N/A, delivers for 2 or 3 months | N/A+ | ||
| Sclera-anchored implant | iDose | Glaukos Corp. | Two doses, delivers for 6–12 months | N/A+ | ||
| Intracameral implant | Travoprost XR-ENV515 OTX-TIC | Envisia Therapeutics Ocular Therapeutix |
42.5 μg, delivers for 6–12 months N/A, delivers for 3 months |
N/A+ N/A+ |
||
Punctal plugs were designed to release drug at this rate
Not available
7.7. Comparison of punctal plugs vs. eye drops for latanoprost dose reduction
Although punctal plug delivery system has not been approved for a prostaglandin, studies by QLT indicate that 141 μg of latanoprost for a 1-month release is not sufficiently effective. That is, compared to 2.5 μg daily dose of latanoprost over 30 days, punctal plugs may offer less than 2-fold dose-reduction advantage.
7.8. Comparison of contact lens vs. eye drops for latanoprost dose reduction
Contact lens with 149 μg of latanoprost was superior in reducing IOP compared to eye drops (2.5 μg daily dose with a 50 μL drop) in monkey eyes over 8 days (total eye drop dose = 20 μg) (Table 1). Compared to drops there is no dose reduction with the contact lens in this study, although not all the drug may have been utilized from the contact lens. There is room for improvement in dosing prostaglandins with contact lenses since the estimated bioavailability from contact lens dosing of drugs is as high as 50% (Dixon et al., 2018). Another study reported 6-fold dose reduction with contact lenses containing timolol and dorzolamide relative to eye drop for intraocular pressure reduction in Beagle dogs (Hsu et al., 2015).
7.9. Dosing considerations and selection of delivery system for various routes
While prescription of a delivery system for each route of administration is neither prudent nor the goal of this manuscript, the following are some key criteria that must be kept in mind in choosing a delivery system for each route of administration for reducing intraocular pressure. 1) The dose requirements are the highest for topical dosing and the lowest for intracameral dosing. Thus, the required size of a topical delivery system will be much larger than an intracameral delivery system. 2) For a topical delivery system, proximity of the system to cornea is beneficial for drug delivery to the aqueous humor. Thus, contact lenses provide the closest proximity to the cornea and most efficient delivery. Punctal plugs release drug away from the cornea and therefore, they are expected to be less efficient for aqueous humor drug delivery. 3) The drug loading capacity is approximately in the order: fornix delivery systems like bimatoprost ring > contact lens loaded with bimatoprost > punctal plug loaded with bimatoprost. Thus, some delivery systems like punctal plugs are inherently limited in their capacity for drug loading due to size restrictions of the associated anatomical spaces. Thus, only the most potent drugs are suitable for delivery by punctal plug delivery systems. 4) When limited by drug capacity, a switch from a matrix type of delivery system to a thin membrane-controlled reservoir system with or without a pore-mediated drug release can increase drug loading capacity. 5) Intracameral delivery systems have yet to evolve, to allow safe re-injection. The smallest drug load and size of the delivery system are desired in this space. Approval of Durysta at an ultra-low dose of 10 μg is a key milestone in this area, with more room for improvement in terms of device placement. 6) The smallest mass/size of delivery system adequate for retention, efficacy, and safety for each route of administration is what should be employed. 7) Each delivery system has its pros and cons, while some delivery systems may allow large drug loading capacity (e.g., fornix inserts), there may greater foreign body sensation. Thus, a tradeoff is anticipated between the goals of adequate delivery and adequate safety for each delivery system.
8. Current glaucoma drug delivery systems
In this review, the discussion has focused on a variety of sustained drug delivery systems that are under development or some that could be investigated to treat glaucoma and prevent disease progression. Additionally, some of the sustained drug delivery systems are in late stage clinical trials while others still have challenges to overcome for them to be tested for the treatment of this eye disease. For ophthalmologists, the primary tool in their toolbox remains a variety of topical eye drops and/or eye drop combinations, (Table 2). At least two of the therapeutic drug classes are administered once daily as a drop, which may enable more glaucoma patients to achieve medical adherence although the issue of drug level variability throughout that 24-hour period remains. As for the sustained release drug delivery systems, one question that will have to be addresses is how these delivery systems will be received by patients and clinicians. Any of the sustained drug delivery systems under development should provide ease of administration and superior patient comfort and therapeutic benefit to allow medical adherence. Also, they should provide a more consistent drug response and preferably consistent drug levels throughout the lifecycle of the sustained drug delivery system. The drug response with the sustained release systems should ideally be equal to or better than the peak effect achieved with eye drops. There should be mechanisms to obtain reimbursements for the cost of the sustained release systems.
Table 2.
FDA approved topical eye drops for the treatment of glaucoma. The information was obtained from product information and related websites*.
| Classification | Recommended Dose (1 Drop, gtt) | Brand Name | Manufacturer |
|---|---|---|---|
| Alpha-2 adrenergic agonists e.g. apraclondine (for short-term use) and brimonidine tartrate | t.i.d. t.i.d. b.i.d. t.i.d. |
Iopidine Alphagan, Alphagan-P Qoliana |
Alcon Allergan Allergan Alcon |
| Alpha- and beta- adrenergic agonists (sympathomimetic agents); e.g., epinephrine, dipivalyl epinephrine | b.i.d. (q.d. or b.i.d.varies between patients) b.i.d. |
Eppy/N, Glaucon, Epifrin, Epinal Propine |
----- Canadian brands Allergan |
| Beta-blockers; e.g., bextaxolol is a beta-1 blocker while carteolol, levobunolol, metipranolol, and timolol are mixed beta-1 and beta-2 blockers | 1 or 2 drops b.i.d. q.d. or b.i.d. 1 or 2 drops q.d. b.i.d. 1 or 2 drops b.i.d. q.d. or b.i.d. b.i.d. b.i.d. q.d. or b.i.d. q.d. or b.i.d. 1 or 2 drops q.d. |
Betoptic, Betoptic S Betimol Betagan Carteolol HCl Kerlone Istalol Ocupress OptiPranolol Timoptic Timoptic XE Vistagan |
Alcon Vistakon Pharm. Allergan Sandoz Inc. Merck ISTA pharm. Cadila Pharm. Bausch & Lomb Bausch & Lomb Merck Allergan |
| Carbonic anhydrase inhibitors**; e.g., acetazolamide, brinzolamide, and dorzolamide | t.i.d. t.i.d. |
Azopt Trusopt |
Alcon Merck |
| Cholinergic/parasympatho mimetic agents | Up to q.i.d. Up to q.i.d. Up to q.i.d. q.d. or b.i.d. 1 or 2 drops t.i.d. 1 or 2 drops b.i.d. or 1 or 2 drops b.i.w. |
Pilocarpine Isopto Carpine Isopto Atropine Phospholine Iodide Carbachol Echothiophate Demecarium (dc) |
Alcon/Sandoz Inc. Alcon Alcon Wyeth Pharm Alcon Wyeth Pharm. Merck |
| Prostaglandin analogs; e.g., bimatoprost, latanoprost, tafluprost, and travoprost | q.d. q.d. b.i.d. q.d. q.d. q.d. |
Travatan Z Lumigan Rescula (dc) Xalatan Vyzulta Zioptan |
Novartis Allergan Novartis Pfizer Bausch & Lomb Merck (Akorn Pharm.) |
| Rho kinase inhibitor; e.g., netarsudil | q.d. | Rhopressa | Aerie Pharm. |
| Prostaglandin and nitric oxide producer; e.g., latanoprstene bunod | q.d. | Vyzulta | Bausch + Lomb |
| Combination glaucoma drugs; e.g., brimonidine and timolol, dorzolamide and timolol, brinzolamide and brimonidine, netarsudil and latanoprost | b.i.d. b.i.d. t.i.d. q.d. |
Combigan Cosopt Simbrinza Rocklatan |
Allergan Akorn/Merck Alcon Aerie Pharm. |
Recommended dosage information obtained from; www.accessdata.fda.gov, www.webmd.com/eye-health/which-medicines-treat-glaucoma#1, http://www.drugs.com/dosage/, http://www.goodrx.com, http://www.news-medical.net, and http://www.rxlist.com/. Abbreviations; b.i.d - twice a day, b.i.w - twice a week, dc - discontinued, q.d.- once a day, q.i.d -four times a day, and t.i.d.- three times a day.
Acetazolamide (Diamox) and methazolamide (Neptazane) are two orally dosed carbonic anhydrase inhibitors for reducing intraocular pressure.
In Table 3, the drug dose required for eye drops are compared with those used in some of the slow release systems currently being assessed. Punctal plugs and contact lenses used up to about 100 times the latanoprost dose present in a single eye drop of 30 μL. Intracameral implants of bimatoprost reported in this paper employed up to 20 micrograms of drug, which corresponds to about 2.2-times the dose used in a single 30 μL eye drop of high strength bimatoprost. Fornix ring placed on the eye surface, on the other hand, used >1000-times the dose of bimatoprost in an eye drop. Travoprost intracameral implants employed about 35-times the amount of drug present in a 30 μL eye drop. It is evident that much smaller cumulative drug doses are required when the product is placed in the intracameral space as opposed to the eye surface. This is consistent with the high target bioavailability anticipated, once the drug is in intracameral space, relative to eye drops. It is anticipated that the doses required by supraciliary, intravitreal, and periocular routes will fall between intracameral and topical routes, depending on the drug type and the target involved. Given the high drug loads in a slow release system, the delivery systems should be foolproof for dose dumping of drug within the eye after administration, in order to avoid drug related severe adverse events. In this regard, the intracameral bimatoprost slow release system requiring a low multiple of the eye drop dose is attractive. Ocular surface delivery systems on the other hand are attractive for their relative ease of administration and removal when required. Overall, efforts toward a reduction of the total dose required, ease of product administration, and robust design that avoids dose dumping will drive the sustained drug delivery system development for glaucoma therapy.
9. Conclusions - future directions
In principle a variety of delivery systems including implants or inserts of various types such as fornix rings, punctal plugs, contact lenses, injectables, and cylindrical delivery systems can achieve sustained drug release for anti-glaucoma drugs in order to reduce intraocular pressure. Also, a variety of routes including extraocular, periocular, and intraocular can achieve sustained anti-glaucoma drug delivery with an appropriate delivery system. Any successful delivery system based on the above or related approaches should have the following three key attributes: 1) efficacy at least equal to or preferably superior to what is currently feasible with daily drops, 2) risks that are low and acceptable upon repeated administrations, and 3) reimbursement eligibility from third party payers for the drug product. Several delivery systems are currently in clinical trials or late stage development, with the clinical efficacy currently being the most optimal for intracamerally placed delivery systems. However, the safety of repeated dosing of intracameral implants has yet to be established. It is anticipated that repeat-use sustained anti-glaucoma drug products capable of exerting drug effects for a few months will be a reality within the next five years. While most of this article focused on approved intraocular pressure lowering drugs being repurposed for sustained therapy, there is ongoing research in sustaining the delivery of neuroprotective drugs to the back of the eye via intravitreal injections (Khatib and Martin, 2019, Checa-Casalengua et al., 2011). Once proof-of-concept is established in the market for the benefit of neuroprotective drugs, more activity in sustaining the delivery of such drugs is anticipated for improving outcomes in glaucoma patients.
Highlights for the manuscript “Extraocular, Periocular, and Intraocular Routes for Sustained Drug Delivery for Glaucoma”.
Intracamerally placed depot systems such as implants can provide sustained drug delivery to the anterior segment tissues including those contributing to elevated intraocular pressure.
Punctal plugs, contact lenses, fornix rings, and nanofiber mats are some drug delivery systems that can be dosed on or near the eye surface to deliver anti-glaucoma drugs to target tissues.
Subconjunctivally placed slow release systems can sustain drug delivery to the tear film or intraocular tissues to achieve therapeutic intraocular pressure reduction. Microparticles and larger delivery systems are more suitable for prolonged drug delivery by this route.
Intravitreal route, being capable of sustaining drug retention for several months from depot formulations such as drug suspensions or other delivery systems can potentially sustain drug exposure to target tissues for intraocular pressure reducing drugs as well as neuroprotective agents. This approach is particularly beneficial for hydrophilic drugs.
Supraciliary route, an extension of suprachoroidal route allows placement of anti-glaucoma drugs in the proximity of ciliary body, thereby allowing sustained drug delivery to target tissues.
Dose required for sustained anti-glaucoma drug delivery will be the lowest with intracameral dosing and the highest with ocular surface delivery systems.
Acknowledgements and Disclosures
The authors are thankful to Dr. Paul Kaufman of University of Wisconsin-Madison and Dr. Robert Ritch of New York Eye and Ear Infirmary of Mount Sinai for their valuable feedback during the initial preparation of this manuscript.
Funding
This work was supported in part by the National Institutes of Health (NIH) grants R01EY024072, R01EY022097, and R21EY029887.
Abbreviations
- AH
Aqueous humor
- AI
Artificial intelligence
- API
Active pharmaceutical ingredient
- AUC
Area under the curve
- AUC0-tlast
Area under the curve (to the last quantified time point)
- b.i.d.
Twice a day
- Bim
Bimatoprost
- BimA
Bimatoprost acid
- b.i.w.
Twice a week
- BLQ
Below limit of quantitation
- BT
Brimonidine tartrate
- Cmax
Maximum concentration
- CLS
Contact lens sensor
- CNS
Central nervous system
- CNTF
Ciliary neurotropic factor
- DARC
Detection of apoptosing retinal cells
- DMPA
2, 2–dimethoxy-2-phenylacetophenone
- DMSO
Dimethylsulfoxide
- DNFs
Dendrimer nanofibers
- DSC
Differential scanning calorimetry
- EC50
Concentration for half maximal response
- ED
Eye drop
- ED50
Effective dose for half maximal response
- Emax
Maximum response
- FDA
Food and Drug Administration
- Gn
Generation number
- HA
Hyaluronic acid
- HEMA
2-hydroxyethyl methacrylate
- HFIP
Hexafluoroisopropanol
- HPMC
Hydroxypropyl methylcellulose
- ICB
Iris-ciliary body
- IOP
Intraocular pressure
- IVT
Intravitreal
- L-PPDS
Latanoprost punctal plug delivery system
- MSC
Mesenchymal stem cells
- NAD
Nicotinamide adenine dinucleotide
- NDA
New drug application
- NMDA
N-methyl-D-aspartate
- NPC
4-nitrophenol chloroformate
- OHT
Ocular hypertension
- PAMAM
Poly (amidoamine) dendrimers
- PEG
Polyethylene glycol
- mPEG
methoxy polyethylene glycol
- PEO
Polyethylene oxide
- pHEMA
Poly (2-hydroxyethyl methacrylate)
- PLGA
Poly (lactic-co-glycolic acid)
- POAG
Primary open-angle glaucoma
- q.d.
Once a day
- Q10
Coenzyme Q10 or ubiquinone
- QSPKR
Quantitative structure-pharmacokinetic relationship
- RGC
Retinal ganglion cells
- rhNGF
Recombinant human nerve growth factor
- RPE
Retinal pigment epithelium
- SCS
Suprachoroidal space
- SD
Standard deviation
- SR
Sustained release
- TEA
Triethylamine
- t.i.d.
Three times a day
- Tmax
Time for maximum concentration
- WIT
Wireless IOP transducer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Statement
This manuscript has not been previously published in any language anywhere and it is not under consideration by another journal. The authors do not have any financial or other conflicts of interest.
References:
- Abbasi E, Aval SF, Akbarzadeh A, Milani M, Nasrabadi HT, Joo SW, Hanifehpour Y, Nejati-Koshki K & Pashaei-Asl R (2014) Dendrimers: synthesis, applications, and properties. Nanoscale Research Letters 9:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello LP, Brucker AJ, Chang S, Cunningham ET, D’amico DJ, Flynn HW, Grillone LR, Hutcherson S, Liebmann JM, O’brien TP, Scott IU, Spaide RF & Ta C (2004) Evolving guidelines for intravitreous injections. Retina-the Journal of Retinal and Vitreous Diseases 24(5):S3–S19. [DOI] [PubMed] [Google Scholar]
- Akopian A, Kumar S, Ramakrishnan H, Roy K, Viswanathan S & Bloomfield SA (2017) Targeting neuronal gap junctions in mouse retina offers neuroprotection in glaucoma. Journal of Clinical Investigation 127(7):2647–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allergan (2020a) Durysta Product Insert, See https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/211911s000lbl.pdf.
- Allergan (2020b) Prescribing Information.) Allergan, Inc., Irvine, CA. [Google Scholar]
- Allergan (Press Release 2019) U.S. FDA Accepts Allergan’s New Drug Application For Bimatoprost Sustained-Release in Patients with Open-Angle Glaucoma or Ocular Hypertension.) Allergan, Inc., Irvine, CA. [Google Scholar]
- Allergan (Press Release 2020) Allergan receives FDA approval for DURYSTA (bimatoprost implant) the first and only intracameral biodegradable sustained-release implant to lower intraocular pressure in open-angle glaucoma or ocular hypertension patients.), Dublin: PR Newswire. [Google Scholar]
- Almasieh M & Levin LA (2017) Neuroprotection in Glaucoma: Animal Models and Clinical Trials. Annual Review of Vision Science, Vol 3 3:91–120. [DOI] [PubMed] [Google Scholar]
- Alvarez-Lorenzo C, Hiratani H & Concheiro A (2006) Contact lenses for drug delivery. American Journal of Drug Delivery 4(3):131–151. [Google Scholar]
- Amrite AC, Ayalasomayajula SP, Cberuvu NPS & Kompella UB (2006) Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2,VEGF, and vascular leakage. Investigative Ophthalmology & Visual Science 47(3):1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrite AC & Kompella UB (2005) Size-dependent disposition of nanoparticles and microparticles following subconjunctival administration. Journal of Pharmacy and Pharmacology 57(12):1555–1563. [DOI] [PubMed] [Google Scholar]
- Araie M & Maurice DM (1991) THE LOSS OF FLUORESCEIN, FLUORESCEIN GLUCURONIDE AND FLUORESCEIN ISOTHIOCYANATE DEXTRAN FROM THE VITREOUS BY THE ANTERIOR AND RETINAL PATHWAYS. Experimental Eye Research 52(1):27–39. [DOI] [PubMed] [Google Scholar]
- Arranz-Romera A, Davis BM, Bravo-Osuna I, Esteban-Perez S, Molina-Martinez IT, Shamsher E, Ravindran N, Guo L, Cordeiro MF & Herrero-Vanrell R (2019) Simultaneous co-delivery of neuroprotective drugs from multi-loaded PLGA microspheres for the treatment of glaucoma. Journal of Controlled Release 297:26–38. [DOI] [PubMed] [Google Scholar]
- Avery RL, Bakri SJ, Blumenkranz MS, Brucker AJ, Cunningham ET, D’amico DJ, Dugel PU, Flynn HW, Freund KB, Haller JA, Jumper JM, Liebmann JM, Mccannel CA, Mieler WF & Ta CN (2014) Intravitreal injection technique and monitoring: Updated guidelines of an expert panel.) Retina, vol. 34, pp. S1–S18. [DOI] [PubMed] [Google Scholar]
- Ayalasomayajula SP & Kompella UB (2004) Retinal delivery of celecoxib is several-fold higher following subconjunctival administration compared to systemic administration. Pharmaceutical Research 21(10):1797–1804. [DOI] [PubMed] [Google Scholar]
- Baltmr A, Duggan J, Nizari S, Salt TE & Cordeiro MF (2010) Neuroprotection in glaucoma - Is there a future role? Experimental Eye Research 91(5):554–566. [DOI] [PubMed] [Google Scholar]
- Beltran-Agullo L, Buys YM, Jahan F, Shapiro CM, Flanagan JG, Cheng J & Trope GE (2017) Twenty-four hour intraocular pressure monitoring with the SENSIMED Triggerfish contact lens: effect of body posture during sleep. British Journal of Ophthalmology 101(10):1323–1328. [DOI] [PubMed] [Google Scholar]
- Boyd K (2020) Punctal Plugs. (Huffman James M., MD (reviewed)), American Academy of Ophthalmology, Online at: https://www.aao.org/eye-health/diseases/punctal-plugs. [Google Scholar]
- Brandt JD, Dubiner HB, Benza R, Sall KN, Walker GA & Semba CP (2017) Long-term Safety and Efficacy of a Sustained-Release Bimatoprost Ocular Ring. Ophthalmology 124(10):1565–1566. [DOI] [PubMed] [Google Scholar]
- Brandt JD, Sall K, Dubiner H, Benza R, Alster Y, Walker G & Semba CP (2016) Six-Month Intraocular Pressure Reduction with a Topical Bimatoprost Ocular Insert Results of a Phase II Randomized Controlled Study. Ophthalmology 123(8):1685–1694. [DOI] [PubMed] [Google Scholar]
- Buhleier E, Wehner W & Vogtle F (1978) CASCADE-CHAIN-LIKE AND NONSKID-CHAIN-LIKE SYNTHESES OF MOLECULAR CAVITY TOPOLOGIES. Synthesis-Stuttgart(2):155–158. [Google Scholar]
- Burke J & Schwartz M (1996) Preclinical evaluation of brimonidine. Survey of Ophthalmology 41:S9–S18. [DOI] [PubMed] [Google Scholar]
- Calkins DJ (2012) Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Progress in Retinal and Eye Research 31(6):702–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RJ, Bronskill SE, Bell CM, Paterson JM, Whitehead M & Gill SS (2010) Rapid Expansion of Intravitreal Drug Injection Procedures, 2000 to 2008: A Population-Based Analysis. Archives of Ophthalmology 128(3):359–362. [DOI] [PubMed] [Google Scholar]
- Cantor LB, Hoop J, Wudunn D, Yung C-W, Catoira Y, Valluri S, Cortes A, Acheampong A, Woodward DF & Wheeler LA (2007) Levels of bimatoprost acid in the aqueous humour after bimatoprost treatment of patients with cataract. The British journal of ophthalmology 91(5):629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor LB, Wudunn D, Catoira-Boyle Y & Yung CW (2008) Absorption of Brimonidine 0.1% and 0.15% Ophthalmic Solutions in the Aqueous Humor of Cataract Patients. Journal of Glaucoma 17(7):529–534. [DOI] [PubMed] [Google Scholar]
- Chan YK, Cheung N, Chan WSC & Wong D (2015) Quantifying silicone oil emulsification in patients: are we only seeing the tip of the iceberg? Graefe’s Archive for Clinical and Experimental Ophthalmology 253(10):1671–1675. [DOI] [PubMed] [Google Scholar]
- Checa-Casalengua P, Jiang CH, Bravo-Osuna I, Tucker BA, Molina-Martinez IT, Young MJ & Herrero-Vanrell R (2011) Retinal ganglion cells survival in a glaucoma model by GDNF/Vit E PLGA microspheres prepared according to a novel microencapsulation procedure. Journal of Controlled Release 156(1):92–100. [DOI] [PubMed] [Google Scholar]
- Chen GZ, Chan IS, Leung LKK & Lam DCC (2014) Soft wearable contact lens sensor for continuous intraocular pressure monitoring. Medical Engineering & Physics 36(9):1134–1139. [DOI] [PubMed] [Google Scholar]
- Chen JX, Howell C, Haller CA, Patel MS, Ayala P, Moravec KA, Dai EB, Liu LY, Sotiri I, Aizenberg M, Aizenberg J & Chaikof EL (2017) An immobilized liquid interface prevents device associated bacterial infection in vivo. Biomaterials 113:80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R, Huang WJ, Huang LJ, Yang B, Mao LD, Jin KL, Zhuge QC & Zhao YP (2014) Acceleration of Tissue Plasminogen Activator-Mediated Thrombolysis by Magnetically Powered Nanomotors. Acs Nano 8(8):7746–7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew EY, Clemons TE, Jaffe GJ, Johnson CA, Farsiu S, Lad EM, Guymer R, Rosenfeld P, Hubschman JP, Constable I, Wiley H, Singerman LJ, Gillies M, Comer G, Blodi B, Eliott D, Yan J, Bird A, Friedlander M & Macular Telangiectasia T. (2019) Effect of Ciliary Neurotrophic Factor on Retinal Neurodegeneration in Patients with Macular Telangiectasia Type 2 A Randomized Clinical Trial. Ophthalmology 126(4):540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang B, Kim YC, Doty AC, Grossniklaus HE, Schwendeman SP & Prausnitz MR (2016) Sustained reduction of intraocular pressure by supraciliary delivery of brimonidine-loaded poly(lactic acid) microspheres for the treatment of glaucoma. Journal of Controlled Release 228:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SW & Kim J (2018) Therapeutic Contact Lenses with Polymeric Vehicles for Ocular Drug Delivery: A Review. Materials 11(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciolino JB, Hoare TR, Iwata NG, Behlau I, Dohlman CH, Langer R & Kohane DS (2009) A Drug-Eluting Contact Lens. Investigative Ophthalmology & Visual Science 50(7):3346–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciolino JB, Ross AE, Tulsan R, Watts AC, Wang RF, Zurakowski D, Serle JB & Kohane DS (2016) Latanoprost-Eluting Contact Lenses in Glaucomatous Monkeys. Ophthalmology 123(10):2085–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciolino JB, Stefanescu CF, Ross AE, Salvador-Culla B, Cortez P, Ford EM, Wymbs KA, Sprague SL, Mascoop DR, Rudina SS, Trauger SA, Cade F & Kohane DS (2014) In vivo performance of a drug-eluting contact lens to treat glaucoma for a month. Biomaterials 35(1):432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciolkowski M, Petersen JF, Ficker M, Janaszewska A, Christensen JB, Klajnert B & Bryszewska M (2012) Surface modification of PAMAM dendrimer improves its biocompatibility. Nanomedicine-Nanotechnology Biology and Medicine 8(6):815–817. [DOI] [PubMed] [Google Scholar]
- Collins CC (1967) Miniature Passive Pressure Transensor for Implanting in the Eye. IEEE Transactions on Biomedical Engineering BME-14(2):74–83. [DOI] [PubMed] [Google Scholar]
- Creech JL, Chauhan A & Radke CJ (2001) Dispersive mixing in the posterior tear film under a soft contact lens. Industrial & Engineering Chemistry Research 40(14):3015–3026. [Google Scholar]
- Cuenca N, Fernandez-Sanchez L, Campello L, Maneu V, De La Villa P, Lax P & Pinilla I (2014) Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Progress in Retinal and Eye Research 43:17–75. [DOI] [PubMed] [Google Scholar]
- Danion A, Arsenault I & Vermette P (2007a) Antibacterial activity of contact lenses bearing surface-immobilized layers of intact Liposomes loaded with Levofloxacin. Journal of Pharmaceutical Sciences 96(9):2350–2363. [DOI] [PubMed] [Google Scholar]
- Danion A, Brochu H, Martin Y & Vermette P (2007b) Fabrication and characterization of contact lenses bearing surface-immobilized layers of intact liposomes. Journal of Biomedical Materials Research Part A 82A(1):41–51. [DOI] [PubMed] [Google Scholar]
- De Avila BEF, Angsantikul P, Li JX, Lopez-Ramirez MA, Ramirez-Herrera DE, Thamphiwatana S, Chen CR, Delezuk J, Samakapiruk R, Ramez V, Obonyo M, Zhang LF & Wang J (2017) Micromotor-enabled active drug delivery for in vivo treatment of stomach infection (vol 8, 272, 2017). Nature Communications 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick HB, Schultz T & Gerste RD (2019) Miniaturization in Glaucoma Monitoring and Treatment: A review of New Technologies That require a Minimal Surgical Approach. Ophthalmology and Therapy 8:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon P, Ghosh T, Mondal K, Konar A, Chauhan A & Hazra S (2018) Controlled delivery of pirfenidone through vitamin E-loaded contact lens ameliorates corneal inflammation. Drug Delivery and Translational Research 8(5):1114–1126. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ & Horwitz EM (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317. [DOI] [PubMed] [Google Scholar]
- Dong CJ, Guo YX, Agey P, Wheeler L & Hare WA (2008) alpha 2 adrenergic modulation of NMDA receptor function as a major mechanism of RGC protection in experimental glaucoma and retinal excitotoxicity. Investigative Ophthalmology & Visual Science 49(10):4515–4522. [DOI] [PubMed] [Google Scholar]
- Downs JC, Burgoyne CF, Seigfreid WP, Reynaud JF, Strouthidis NG & Sallee V (2011) 24-Hour IOP Telemetry in the Nonhuman Primate: Implant System Performance and Initial Characterization of IOP at Multiple Timescales. Investigative Ophthalmology & Visual Science 52(10):7365–7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Toit R, Vega JA, Fonn D & Simpson T (2003) Diurnal variation of corneal sensitivity and thickness. Cornea 22(3):205–209. [DOI] [PubMed] [Google Scholar]
- Durairaj C (2015) Optimal Sampling Scheme for Estimation of Intraocular Pressure Diurnal Curves in Glaucoma Trials. Clinical Pharmacokinetics 54(1):95–105. [DOI] [PubMed] [Google Scholar]
- Durairaj C (2017) Ocular Pharmacokinetics. In Pharmacologic Therapy of Ocular Disease. (Whitcup SM, and Azar DT (eds) 1 edn. Springer International Publishing, pp. 31–53. [Google Scholar]
- Durairaj C, Kim SJ, Edelhauser HF, Shah JC & Kompella UB (2009a) Influence of Dosage Form on the Intravitreal Pharmacokinetics of Diclofenac. Investigative Ophthalmology & Visual Science 50(10):4887–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durairaj C, Shah JC, Senapati S & Kompella UB (2009b) Prediction of Vitreal Half-Life Based on Drug Physicochemical Properties: Quantitative Structure-Pharmacokinetic Relationships (QSPKR). Pharmaceutical Research 26(5):1236–1260. [DOI] [PubMed] [Google Scholar]
- Edelhauser HF, Rowe-Rendleman CL, Robinson MR, Dawson DG, Chader GJ, Grossniklaus HE, Rittenhouse KD, Wilson CG, Weber DA, Kuppermann BD, Csaky KG, Olsen TW, Kompella UB, Holers VM, Hageman GS, Gilger BC, Campochiaro PA, Whitcup SM & Wong WT (2010) Ophthalmic Drug Delivery Systems for the Treatment of Retinal Diseases: Basic Research to Clinical Applications. Investigative Ophthalmology & Visual Science 51(11):5403–5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emre E, Yuksel N, Duruksu G, Pirhan D, Subasi C, Erman G & Karaoz E (2015) Neuroprotective effects of intravitreally transplanted adipose tissue and bone marrow-derived mesenchymal stem cells in an experimental ocular hypertension model. Cytotherapy 17(5):543–559. [DOI] [PubMed] [Google Scholar]
- Envisia (Press Release 2017) Envisia Therapeutics Press Releases; Interim ENV515 (Travoprost XR) Phase 2 Data Demonstrating 11-month Duration-of-Action After a Single Dose in Patients With Glaucoma.) PR Newswire, available at: https://www.prnewswire.com/news-releases/envWoSa-therapeutics-releases-interim-env515-travoprost-xr-phase-2-data-demonstrating-11-month-duration-of-action-after-a-single-dose-in-patients-with-glaucoma-300401812.html.
- Fagan XJ & Al-Qureshi S (2013) Intravitreal injections: a review of the evidence for best practice. Clinical and Experimental Ophthalmology 41(5):500–507. [DOI] [PubMed] [Google Scholar]
- Fahmy HM, Saad EAS, Sabra NM, El-Gohary AA, Mohamed FF & Gaber MH (2018) Treatment merits of Latanoprost/Thymoquinone - Encapsulated liposome for glaucomatus rabbits. International Journal of Pharmaceutics 548(1):597–608. [DOI] [PubMed] [Google Scholar]
- Faulkner R, Sharif NA, Orr S, Sall K, Dubiner H, Whitson JT, Moster M, Craven ER, Curtis M, Pailliotet C, Martens K & Dahlin D (2010) Aqueous Humor Concentrations of Bimatoprost Free Acid, Bimatoprost and Travoprost Free Acid in Cataract Surgical Patients Administered Multiple Topical Ocular Doses of LUMIGAN (R) or TRAVATAN (R). Journal of Ocular Pharmacology and Therapeutics 26(2):147–156. [DOI] [PubMed] [Google Scholar]
- Fedorchak MV, Conner IP, Schuman JS, Cugini A & Little SR (2017) Long Term Glaucoma Drug Delivery Using a Topically Retained Gel/Microsphere Eye Drop. Scientific Reports 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feehan M, Munger MA, Cooper DK, Hess KT, Durante R, Jones GJ, Montuoro J, Morrison MA, Clegg D, Crandall AS & Deangelis MM (2016) Adherence to Glaucoma Medications Over 12 Months in Two US Community Pharmacy Chains. Journal of Clinical Medicine 5(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feke GT, Bex PJ, Taylor CP, Rhee DJ, Turalba AV, Chen TC, Wand M & Pasquale LR (2014) Effect of Brimonidine on Retinal Vascular Autoregulation and Short-term Visual Function in Normal Tension Glaucoma. American Journal of Ophthalmology 158(1):105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DS, Nordstrom B, Mozaffari E & Quigley HA (2005) Glaucoma management among individuals enrolled in a single comprehensive insurance plan. Ophthalmology 112(9):1500–1504. [DOI] [PubMed] [Google Scholar]
- Fu J, Sun FY, Liu WH, Liu YF, Gedam M, Hu Q, Fridley C, Quigley HA, Hanes J & Pitha I (2016) Subconjunctival Delivery of Dorzolamide-Loaded Poly(ether-anhydride) Microparticles Produces Sustained Lowering of Intraocular Pressure in Rabbits. Molecular Pharmaceutics 13(9):2987–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Qiao X, Cantor LB & Wudunn D (2002) Up-regulation of Brain-Derived Neurotrophic Factor Expression by Brimonidine in Rat Retinal Ganglion Cells. Archives of Ophthalmology 120(6):797–803. [DOI] [PubMed] [Google Scholar]
- Gao W, Dong RF, Thamphiwatana S, Li JX, Gao WW, Zhang LF & Wang J (2015) Artificial Micromotors in the Mouse’s Stomach: A Step toward in Vivo Use of Synthetic Motors. Acs Nano 9(1):117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garway-Heath DF, Crabb DP, Bunce C, Lascaratos G, Amalfitano F, Anand N, Azuara-Blanco A, Bourne RR, Broadway DC, Cunliffe IA, Diamond JP, Fraser SG, Ho TA, Martin KR, Mcnaught AI, Negi A, Patel K, Russell RA, Shah A, Spry PG, Suzuki K, White ET, Wormald RP, Xing W & Zeyen TG (2015) Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet 385(9975):1295–1304. [DOI] [PubMed] [Google Scholar]
- Ghosh A & Fischer P (2009) Controlled Propulsion of Artificial Magnetic Nanostructured Propellers. Nano Letters 9(6):2243–2245. [DOI] [PubMed] [Google Scholar]
- Gillies ER, Dy E, Frechet JMJ & Szoka FC (2005) Biological evaluation of polyester dendrimer: Poly(ethylene oxide) “Bow-Tie” hybrids with tunable molecular weight and architecture. Molecular Pharmaceutics 2(2):129–138. [DOI] [PubMed] [Google Scholar]
- Goldberg DF & Williams R (2012) A Phase 2 Study Evaluating Safety and Efficacy of the Latanoprost Punctal Plug Delivery System (L-PPDS) in Subjects With Ocular Hypertension (OH) or Open-Angle Glaucoma (OAG). Investigative Ophthalmology & Visual Science 53(14):5095.22661464 [Google Scholar]
- Gulsen D & Chauhan A (2004) Ophthalmic drug delivery through contact lenses. Investigative Ophthalmology & Visual Science 45(7):2342–2347. [DOI] [PubMed] [Google Scholar]
- Gulsen D & Chauhan A (2005) Dispersion of microemulsion drops in HEMA hydrogel: a potential ophthalmic drug delivery vehicle. International Journal of Pharmaceutics 292(1–2):95–117. [DOI] [PubMed] [Google Scholar]
- Gulsen D, Li CC & Chauhan A (2005) Dispersion of DMPC liposomes in contact lenses for ophthalmic drug delivery. Current Eye Research 30(12):1071–1080. [DOI] [PubMed] [Google Scholar]
- Gupta U, Dwivedi SKD, Bid HK, Konwar R & Jain NK (2010) Ligand anchored dendrimers based nanoconstructs for effective targeting to cancer cells. International Journal of Pharmaceutics 393(1–2):185–196. [DOI] [PubMed] [Google Scholar]
- Ha D, De Vries WN, John SWM, Irazoqui PP & Chappell WJ (2012) Polymer-based miniature flexible capacitive pressure sensor for intraocular pressure (IOP) monitoring inside a mouse eye. Biomedical Microdevices 14(1):207–215. [DOI] [PubMed] [Google Scholar]
- Haddrill M (2017) Punctal plugs for dry eyes. (Epstein Robert L., MD (reviewed)), All about vision, Online at: https://www.allaboutvWoSon.com/conditions/punctal-plugs.htm. [Google Scholar]
- Hare WA, Woldemussie E, Lai RK, Ton H, Ruiz G, Chun T & Wheeler L (2004) Efficacy and safety of memantine treatment for reduction of changes associated with experimental glaucoma in monkey, I: Functional measures. Investigative Ophthalmology & Visual Science 45(8):2625–2639. [DOI] [PubMed] [Google Scholar]
- Hartman RR & Kompella UB (2017) Intravitreal, Subretinal, and Suprachoroidal Injections: Evolution of Microneedles for Drug Delivery. Journal of Ocular Pharmacology and Therapeutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvitt DM & Bonanno JA (1999) Re-evaluation of the oxygen diffusion model for predicting minimum contact lens Dk/t values needed to avoid corneal anoxia. Optometry and Vision Science 76(10):712–719. [DOI] [PubMed] [Google Scholar]
- Hawkeye MM & Brett MJ (2007) Glancing angle deposition: Fabrication, properties, and applications of micro- and nanostructured thin films. Journal of Vacuum Science & Technology A 25(5):1317–1335. [Google Scholar]
- Hehl EM, Beck R, Luthard K, Guthoff R & Drewelow B (1999) Improved penetration of aminoglycosides and fluoroquinolones into the aqueous humour of patients by means of Acuvue contact lenses. European Journal of Clinical Pharmacology 55(4):317–323. [DOI] [PubMed] [Google Scholar]
- Heijl A, Leske MC, Bengtsson B, Hyman L, Hussein M & Early Manifest Glaucoma Trial, G. (2002) Reduction of intraocular pressure and glaucoma progression - Results from the early manifest glaucoma trial. Archives of Ophthalmology 120(10):1268–1279. [DOI] [PubMed] [Google Scholar]
- Hennessy AL, Katz J, Covert D, Kelly CA, Suan EP, Speicher MA, Sund NJ & Robin AL (2011) A Video Study of Drop Instillation in Both Glaucoma and Retina Patients with Visual Impairment. American Journal of Ophthalmology 152(6):982–988. [DOI] [PubMed] [Google Scholar]
- Hernandez M, Urcola JH & Vecino E (2008) Retinal ganglion cell neuroprotection in a rat model of glaucoma following brimonidine, latanoprost or combined treatments. Experimental Eye Research 86(5):798–806. [DOI] [PubMed] [Google Scholar]
- Hiratani H & Alvarez-Lorenzo C (2002) Timolol uptake and release by imprinted soft contact lenses made of N,N-diethylacrylamide and methacrylic acid. Journal of Controlled Release 83(2):223–230. [DOI] [PubMed] [Google Scholar]
- Hiratani H, Fujiwara A, Tamiya Y, Mizutani Y & Alvarez-Lorenzo C (2005) Ocular release of timolol from molecularly imprinted soft contact lenses. Biomaterials 26(11):1293–1298. [DOI] [PubMed] [Google Scholar]
- Holden BA & Mertz GW (1984) CRITICAL OXYGEN LEVELS TO AVOID CORNEAL EDEMA FOR DAILY AND EXTENDED WEAR CONTACT-LENSES. Investigative Ophthalmology & Visual Science 25(10):1161–1167. [PubMed] [Google Scholar]
- Holden CA, Tyagi P, Thakur A, Kadam R, Jadhav G, Kompella UB & Yang H (2012) Polyamidoamine dendrimer hydrogel for enhanced delivery of antiglaucoma drugs. Nanomedicine-Nanotechnology Biology and Medicine 8(5):776–783. [DOI] [PubMed] [Google Scholar]
- Hollo G, Topouzis F & Fechtner RD (2014a) Fixed-combination intraocular pressure-lowering therapy for glaucoma and ocular hypertension: advantages in clinical practice. Expert Opinion on Pharmacotherapy 15(12):1737–1747. [DOI] [PubMed] [Google Scholar]
- Hollo G, Vuorinen J, Tuominen J, Huttunen T, Ropo A & Pfeiffer N (2014b) Fixed-Dose Combination of Tafluprost and Timolol in the Treatment of Open-Angle Glaucoma and Ocular Hypertension: Comparison with Other Fixed-Combination Products. Advances in Therapy 31(9):932–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu KH, Carbia BE, Plummer C & Chauhan A (2015) Dual drug delivery from vitamin E loaded contact lenses for glaucoma therapy. European Journal of Pharmaceutics and Biopharmaceutics 94:312–321. [DOI] [PubMed] [Google Scholar]
- Hubanova R, Aptel F, Chiquet C, Mottet B & Romanet JP (2014) Effect of overnight wear of the Triggerfish((R)) sensor on corneal thickness measured by Visante((R)) anterior segment optical coherence tomography. Acta Ophthalmologica 92(2):E119–E123. [DOI] [PubMed] [Google Scholar]
- Hubschman JP, Coffee RE, Bourges JL, Yu F & Schwartz SD (2010) Experimental model of intravitreal injection techniques. Retina-the Journal of Retinal and Vitreous Diseases 30(1):167–173. [DOI] [PubMed] [Google Scholar]
- Hwang DK, Liu CJL, Pu CY, Chou YJ & Chou P (2014) Persistence of Topical Glaucoma Medication A Nationwide Population-Based Cohort Study in Taiwan. Jama Ophthalmology 132(12):1446–1452. [DOI] [PubMed] [Google Scholar]
- Janaszewska A, Lazniewska J, Trzepinski P, Marcinkowska M & Klajnert-Maculewicz B (2019) Cytotoxicity of Dendrimers. Biomolecules 9(8):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoria KG, Gunda S, Boddu SH & Mitra AK (2007) Novel approaches to retinal drug delivery. Expert Opinion on Drug Delivery 4(4):371–388. [DOI] [PubMed] [Google Scholar]
- Jehangir N, Bever G, Jafarmahmood SM & Moshirfar M (2016) Comprehensive Review of the Literature on Existing Punctal Plugs for the Management of Dry Eye Disease. Journal of Ophthalmology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevprasesphant R, Penny J, Attwood D, Mckeown NB & D’emanuele A (2003a) Engineering of dendrimer surfaces to enhance transepithelial transport and reduce cytotoxicity. Pharmaceutical Research 20(10):1543–1550. [DOI] [PubMed] [Google Scholar]
- Jevprasesphant R, Penny J, Jalal R, Attwood D, Mckeown NB & D’emanuele A (2003b) The influence of surface modification on the cytotoxicity of PAMAM dendrimers. International Journal of Pharmaceutics 252(1–2):263–266. [DOI] [PubMed] [Google Scholar]
- Johnson J (2018) What to know about punctal plugs for dry eye. Medical News Today, Online, See https://www.medicalnewstoday.com/articles/322021.
- Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI & Martin KR (2010) Neuroprotective Effects of Intravitreal Mesenchymal Stem Cell Transplantation in Experimental Glaucoma. Investigative Ophthalmology & Visual Science 51(4):2051–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor Y, Thomas JC, Tan G, John VT & Chauhan A (2009) Surfactant-laden soft contact lenses for extended delivery of ophthalmic drugs. Biomaterials 30(5):867–878. [DOI] [PubMed] [Google Scholar]
- Karlgard CC, Jones LW & Moresoli C (2003a) Ciprofloxacin interaction with silicon-based and conventional hydrogel contact lenses. Eye Contact Lens 29(2):83–89. [DOI] [PubMed] [Google Scholar]
- Karlgard CCS, Wong NS, Jones LW & Moresoli C (2003b) In vitro uptake and release studies of ocular pharmaceutical agents by silicon-containing and p-HEMA hydrogel contact lens materials. International Journal of Pharmaceutics 257(1–2):141–151. [DOI] [PubMed] [Google Scholar]
- Karp CL, Cox TA, Wagoner MD, Ariyasu RG & Jacobs DS (2001) Intracameral anesthesia - A report by the American Academy of Ophthalmology. Ophthalmology 108(9):1704–1710. [DOI] [PubMed] [Google Scholar]
- Khan A, Raza SN, Itoo A, Bashir S, Wani TU & Khan NA (2019) Ocular Inserts - A Novel Approach In Ocular Drug Delivery. Journal of Drug Delivery & Therapeutics 9(4):693–703. [Google Scholar]
- Khatib T & Martin K (2019) Neuroprotection in Glaucoma: Towards Clinical Trials and Precision Medicine. Current Eye Research:1–12. [DOI] [PubMed] [Google Scholar]
- Khatib TZ & Martin KR (2020) Neuroprotection in Glaucoma: Towards Clinical Trials and Precision Medicine. Current Eye Research 45(3):327–338. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Zhang KY, Moore L & Ho D (2014a) Diamond Nanogel-Embedded Contact Lenses Mediate Lysozyme-Dependent Therapeutic Release. Acs Nano 8(3):2998–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Conway A & Chauhan A (2008a) Extended delivery of ophthalmic drugs by silicone hydrogel contact lenses. Biomaterials 29(14):2259–2269. [DOI] [PubMed] [Google Scholar]
- Kim J, You Y, Kim S & Kwon O (2017) Epiretinal Membrane Formation After Intravitreal Autologous Stem Cell Implantation In A Retinitis Pigmentosa Patient. Retinal Cases & Brief Reports: Summer 2017 11(3):227–231. [DOI] [PubMed] [Google Scholar]
- Kim SH, Csaky KG, Wang NS & Lutz RJ (2008b) Drug elimination kinetics following subconjunctival injection using dynamic contrast-enhanced magnetic resonance imaging. Pharmaceutical Research 25(3):512–520. [DOI] [PubMed] [Google Scholar]
- Kim SJ (2015) Intravitreal Injections and Endophthalmitis. Int. Ophthalmol. Clin 55(2):1–10. [DOI] [PubMed] [Google Scholar]
- Kim YC, Edelhauser HF & Prausnitz MR (2014b) Targeted Delivery of Antiglaucoma Drugs to the Supraciliary Space Using Microneedles. Investigative Ophthalmology & Visual Science 55(11):7387–7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klajnert B & Bryszewska M (2001) Dendrimers: properties and applications. Acta Biochimica Polonica 48(1):199–208. [PubMed] [Google Scholar]
- Kompella UB, Bandi N & Ayalasomayajula SP (2003) Subconjunctival nano- and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Investigative Ophthalmology & Visual Science 44(3):1192–1201. [DOI] [PubMed] [Google Scholar]
- Kompella UB, Kadam RS & Lee VH (2010) Recent advances in ophthalmic drug delivery.) Therapeutic Delivery, vol. 1, pp. 435–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstas AGP, Mylopoulos N, Karabatsas CH, Kozobolis VP, Diafas S, Papapanos P, Georgiadis N & Stewart WC (2004) Diurnal intraocular pressure reduction with latanoprost 0.005% compared to timolol maleate 0.5% as monotherapy in subjects with exfoliation glaucoma. Eye 18(9):893–899. [DOI] [PubMed] [Google Scholar]
- Koutsonas A, Walter P, Roessler G & Plange N (2015) Implantation of a Novel Telemetric Intraocular Pressure Sensor in Patients With Glaucoma (ARGOS Study): 1-Year Results. Investigative Ophthalmology & Visual Science 56(2):1063–1069. [DOI] [PubMed] [Google Scholar]
- Krupin T, Liebmann JM, Greenfield DS, Ritch R, Gardiner S & Low-Pressure Glaucoma Study, G. (2011) A Randomized Trial of Brimonidine Versus Timolol in Preserving Visual Function: Results from the Low-pressure Glaucoma Treatment Study (vol 151, pg 671, 2011). American Journal of Ophthalmology 151(6). [DOI] [PubMed] [Google Scholar]
- Kumar CG, Poornachandra Y & Pombala S (2017) Therapeutic Nanomaterials: From a Drug Delivery Perspective. In Nanostructures for Drug Delivery. (Andronescu E, and Grumezescu AM (eds)) Elsevier Inc. [Google Scholar]
- Lambert WS, Carlson BJ, Van Der Ende AE, Shih G, Dobish JN, Calkins DJ & Harth E (2015) Nanosponge-Mediated Drug Delivery Lowers Intraocular Pressure. Translational Vision Science & Technology 4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase A, Rama P, Bonini S, Caprioglio G & Aloe L (1998) Topical treatment with nerve growth factor for corneal neurotrophic ulcers. New England Journal of Medicine 338(17):1174–1180. [DOI] [PubMed] [Google Scholar]
- Lamminsalo M, Taskinen E, Karvinen T, Subrizi A, Murtomaki L, Urtti A & Ranta VP (2018) Extended Pharmacokinetic Model of the Rabbit Eye for Intravitreal and Intracameral Injections of Macromolecules: Quantitative Analysis of Anterior and Posterior Elimination Pathways. Pharmaceutical Research 35(8). [DOI] [PubMed] [Google Scholar]
- Lancina MG, Singh S, Kornpella UB, Husain S & Yang H (2017) Fast Dissolving Dendrimer Nanofiber Mats as Alternative to Eye Drops for More Efficient Antiglaucoma Drug Delivery. Acs Biomaterials Science & Engineering 3(8):1861–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzi J, Anders F, Liu HH, Pfeiffer N, Grus F, Thanos S, Arnhold S & Prokosch V (2019) Neuroprotective and neuroregenerative effects of CRMP-5 on retinal ganglion cells in an experimental in vivo and in vitro model of glaucoma. Plos One 14(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavik E, Kuehn MH, Shoffstall AJ, Atkins K, Dumitrescu AV & Kwon YH (2016) Sustained Delivery of Timolol Maleate for Over 90 Days by Subconjunctival Injection. Journal of Ocular Pharmacology and Therapeutics 32(10):642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourlais C, Acar L, Zia H, Sado PA, Needham T & Leverge R (1998) Ophthalmic drug delivery systems - Recent advances. Progress in Retinal and Eye Research 17(1):33–58. [DOI] [PubMed] [Google Scholar]
- Lee TWY & Robinson JR (2004) Drug delivery to the posterior segment of the eye III: The effect of parallel elimination pathway on the vitreous drug level after subconjunctival injection. Journal of Ocular Pharmacology and Therapeutics 20(1):55–64. [DOI] [PubMed] [Google Scholar]
- Levkovitch-Verbin H, Quigley HA, Kerrigan-Baumrind LA, D’anna SA, Kerrigan D & Pease ME (2001) Optic nerve transection in monkeys may result in secondary degeneration of retinal ganglion cells. Investigative Ophthalmology & Visual Science 42(5):975–982. [PubMed] [Google Scholar]
- Lewis RA, Christie WC, Day DG, Craven ER, Walters T, Bejanian M, Lee SS, Goodkin ML, Zhang J, Whitcup SM, Robinson MR & Bimatoprost Sr Study, G. (2017) Bimatoprost Sustained-Release Implants for Glaucoma Therapy: 6-Month Results From a Phase I/II Clinical Trial. American Journal of Ophthalmology 175:137–147. [DOI] [PubMed] [Google Scholar]
- Li CC & Chauhan A (2006) Modeling ophthalmic drug delivery by soaked contact lenses. Industrial & Engineering Chemistry Research 45(10):3718–3734. [Google Scholar]
- Li JX, De Avila BEF, Gao W, Zhang LF & Wang J (2017) Micro/nanorobots for biomedicine: Delivery, surgery, sensing, and detoxification. Science Robotics 2(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L, Loughnan MS & Sullivan LJ (2002) Microbial keratitis associated with extended wear of silicone hydrogel contact lenses. British Journal of Ophthalmology 86(3):355–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JHK, Zhang XY, Kripke DF & Weinreb RN (2003) Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Investigative Ophthalmology & Visual Science 44(4):1586–1590. [DOI] [PubMed] [Google Scholar]
- Macoul KL & Pavanlangston D (1975) PILOCARPINE OCUSERT SYSTEM FOR SUSTAINED CONTROL OF OCULAR HYPERTENSION. Archives of Ophthalmology 93(8):587–590. [DOI] [PubMed] [Google Scholar]
- Madaan K, Kumar S, Poonia N, Lather V & Pandita D (2014) Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. Journal of Pharmacy & Bioallied Sciences 6(3):139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makoid MC & Robinson JR (1979) PHARMACOKINETICS OF TOPICALLY APPLIED PILOCARPINE IN TH ALBINO RABBIT EYE. Journal of Pharmaceutical Sciences 68(4):435–443. [DOI] [PubMed] [Google Scholar]
- Malihi M, Moura ER, Hodge DO & Sit AJ (2014) Long-Term Trends in Glaucoma-Related Blindness in Olmsted County, Minnesota. Ophthalmology 121(1):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A, Chaudhary S, Garg G & Tomar A (2012) Dendrimers: A Tool for Drug Delivery. Advances in Biological Research 6(4):165–169. [Google Scholar]
- Mansouri K, Liu JHK, Weinreb RN, Tafreshi A & Medeiros FA (2012a) Analysis of Continuous 24-Hour Intraocular Pressure Patterns in Glaucoma. Investigative Ophthalmology & Visual Science 53(13):8050–8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri K, Medeiros FA, Tafreshi A & Weinreb RN (2012b) Continuous 24-Hour Monitoring of Intraocular Pressure Patterns With a Contact Lens Sensor. Archives of Ophthalmology 130(12):1534–1539. [DOI] [PubMed] [Google Scholar]
- Mark AG, Gibbs JG, Lee TC & Fischer P (2013) Hybrid nanocolloids with programmed three-dimensional shape and material composition. Nature Materials 12(9):802–807. [DOI] [PubMed] [Google Scholar]
- Martin R, De Juan V, Rodriguez G, Cuadrado R & Fernandez I (2007) Measurement of corneal swelling variations without removal of the contact lens during extended wear. Investigative Ophthalmology & Visual Science 48(7):3043–3050. [DOI] [PubMed] [Google Scholar]
- Maurice D (2001) Review: Practical issues in intravitreal drug delivery. Journal of Ocular Pharmacology and Therapeutics 17(4):393–401. [DOI] [PubMed] [Google Scholar]
- Maurice DM (1958) A recording tonometer. The British journal of ophthalmology 42(6):321–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcmonnies C (2018) Reactive oxygen species, oxidative stress, glaucoma and hyperbaric oxygen therapy. J. Opt 11:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros FA, Walters TR, Kolko M, Coote M, Bejanian M, Goodkin ML, Guo Q, Zhang J, Robinson MR & Weinreb RN (2020) Phase 3, Randomized, 20-Month Study of Bimatoprost Implant in Open-Angle Glaucoma and Ocular Hypertension (ARTEMIS 1). Ophthalmology. [DOI] [PubMed] [Google Scholar]
- Mehta P, Al-Kinani AA, Arshad MS, Singh N, Van Der Merwe SM, Chang MW, Alany RG & Ahmad Z (2019) Engineering and Development of Chitosan-Based Nanocoatings for Ocular Contact Lenses. Journal of Pharmaceutical Sciences 108(4):1540–1551. [DOI] [PubMed] [Google Scholar]
- Melki S, Todani A & Cherfan G (2014) An Implantable Intraocular Pressure Transducer Initial Safety Outcomes. Jama Ophthalmology 132(10):1221–1225. [DOI] [PubMed] [Google Scholar]
- Mertz GW (1980) OVERNIGHT SWELLING OF THE LIVING HUMAN CORNEA. Journal of the American Optometric Association 51(3):211–214. [PubMed] [Google Scholar]
- Missel PJ (2012) Simulating Intravitreal Injections in Anatomically Accurate Models for Rabbit, Monkey, and Human Eyes. Pharmaceutical Research 29(12):3251–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JC, Moore CG, Deppmeier LMH, Gold BG, Meshul CK & Johnson EC (1997) A rat model of chronic pressure-induced optic nerve damage. Experimental Eye Research 64(1):85–96. [DOI] [PubMed] [Google Scholar]
- Morrison PWJ & Khutoryanskiy VV (2014) Advances in ophthalmic drug delivery. Therapeutic Delivery 5(12):1297–1315. [DOI] [PubMed] [Google Scholar]
- Musgrave C & Fang F (2019) Contact Lens Materials: A Materials Science Perspective. Materials 12:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers L, Almeida D & Abramoff MD (January 6, 2015) Intravitreal injection technique: A primer for ophthalmology residents and fellows. EyeRounds.org, See http://www.Eyerounds.org/tutorials/intravitreal-injections/.
- Nakada K & Sugiyama A (1998) Process for producing controlled drug-release contact lens, and controlled drug-release contact lens thereby produced. US Patent 6,027,745.
- Natarajan JV, Chattopadhyay S, Ang M, Darwitan A, Foo S, Zhen M, Koo M, Wong TT & Venkatraman SS (2011) Sustained Release of an Anti-Glaucoma Drug: Demonstration of Efficacy of a Liposomal Formulation in the Rabbit Eye. Plos One 6(9):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newkome GR, Yao ZQ, Baker GR & Gupta VK (1985) MICELLES .1. CASCADE MOLECULES - A NEW APPROACH TO MICELLES - A 27 -ARBOROL. Journal of Organic Chemistry 50(11):2003–2004. [Google Scholar]
- Newman-Casey PA, Robin AL, Blachley T, Farris K, Heisler M, Resnicow K & Lee PP (2015) The Most Common Barriers to Glaucoma Medication Adherence A Cross-Sectional Survey. Ophthalmology 122(7):1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng XW, Liu KL, Veluchamy AB, Lwin NC, Wong TT & Venkatraman SS (2015) A biodegradable ocular implant for long-term suppression of intraocular pressure. Drug Delivery and Translational Research 5(5):469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimesh S (2013) 13 - Dendrimers. In Gene Therapy. (Nimesh S (ed)) Woodhead Publishing, pp. 259–285. [Google Scholar]
- Nordstrom BL, Friedman DS, Mozaffari E, Quigley HA & Walker AM (2005) Persistence and adherence with topical glaucoma therapy. American Journal of Ophthalmology 140(4):598–606. [DOI] [PubMed] [Google Scholar]
- Normando EM, Yap TE, Maddison J, Miodragovic S, Bonetti P, Almonte M, Mohammad NG, Ameen S, Crawley L, Ahmed F, Bloom PA & Cordeiro MF (2020) A CNN-aided method to predict glaucoma progression using DARC (Detection of Apoptosing Retinal Cells). Expert Review of Molecular Diagnostics:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nzoughet JK, De La Barca JMC, Guehlouz K, Leruez S, Coulbault L, Allouche S, Bocca C, Muller J, Amati-Bonneau P, Gohier P, Bonneau D, Simard G, Milea D, Lenaers G, Procaccio V & Reynier P (2019) Nicotinamide Deficiency in Primary Open-Angle Glaucoma. Investigative Ophthalmology & Visual Science 60(7):2509–2514. [DOI] [PubMed] [Google Scholar]
- Oculartherapeutix (2019) Ocular Therapeutix: Dextenza Prescribing Information. Ocular Therapeutix, Online, See https://www.ocutx.com/products/dextenza/.
- Osborne A, Khatib TZ, Songra L, Barber AC, Hall K, Kong GYX, Widdowson PS & Martin KR (2018a) Neuroprotection of retinal ganglion cells by a novel gene therapy construct that achieves sustained enhancement of brain-derived neurotrophic factor/tropomyosin- related kinase receptor-B signaling. Cell Death & Disease 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne A, Wang AXZ, Tassoni A, Widdowson PS & Martin KR (2018b) Design of a Novel Gene Therapy Construct to Achieve Sustained Brain-Derived Neurotrophic Factor Signaling in Neurons. Human Gene Therapy 29(7):828–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaya A, Alkin Z, Celik U, Yuksel K, Ozgurhan EB, Agca A, Yazici AT & Demirok A (2013) Comparing the Effects of Three Different Intravitreal Injection Techniques on Vitreous Reflux and Intraocular Pressure. Journal of Ocular Pharmacology and Therapeutics 29(3):325–329. [DOI] [PubMed] [Google Scholar]
- Pease ME, Zack DJ, Berlinicke C, Bloom K, Cone F, Wang YX, Klein RL, Hauswirth WW & Quigley HA (2009) Effect of CNTF on Retinal Ganglion Cell Survival in Experimental Glaucoma. Investigative Ophthalmology & Visual Science 50(5):2194–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pek YS, Wu H, Mohamed ST & Ying JY (2016) Long-Term Subconjunctival Delivery of Brimonidine Tartrate for Glaucoma Treatment Using a Microspheres/Carrier System. Advanced Healthcare Materials 5(21):2823–2831. [DOI] [PubMed] [Google Scholar]
- Peng CC, Ben-Shlomo A, Mackay EO, Plummer CE & Chauhan A (2012a) Drug Delivery by Contact Lens in Spontaneously Glaucomatous Dogs. Current Eye Research 37(3):204–211. [DOI] [PubMed] [Google Scholar]
- Peng CC, Burke MT, Carbia BE, Plummer C & Chauhan A (2012b) Extended drug delivery by contact lenses for glaucoma therapy. Journal of Controlled Release 162(1):152–158. [DOI] [PubMed] [Google Scholar]
- Peng CC, Kim J & Chauhan A (2010) Extended delivery of hydrophilic drugs from silicone-hydrogel contact lenses containing Vitamin E diffusion barriers. Biomaterials 31(14):4032–4047. [DOI] [PubMed] [Google Scholar]
- Pollack IP, Quigley HA & Harbin TS (1976) OCUSERT PILOCARPINE SYSTEM - ADVANTAGES AND DISADVANTAGES. Southern Medical Journal 69(10):1296–1298. [DOI] [PubMed] [Google Scholar]
- Quigley HA (2012) Clinical trials for glaucoma neuroprotection are not impossible. Current Opinion in Ophthalmology 23(2):144–154. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Mckinnon SJ, Zack DJ, Pease ME, Kerrigan-Baumrind LA, Kerrigan DF & Mitchell RS (2000) Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Investigative Ophthalmology & Visual Science 41(11):3460–3466. [PubMed] [Google Scholar]
- Raghava S, Hammond M & Kompella U (2004) Periocular routes for retinal drug delivery. Expert Opinion on Drug Delivery Volume 1, Iss. 1:99–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajurkar K, Dubey S, Gupta PP, John D & Chauhan L (2018) Compliance to topical anti-glaucoma medications among patients at a tertiary hospital in North India. Journal of Current Ophthalmology 30(2):125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex J, Knowles T, Zhao X, Lemp J, Maissa C & Perry SS (2018) Elemental Composition at Silicone Hydrogel Contact Lens Surfaces. Eye Contact Lens 44 Suppl 2:S221–s226. [DOI] [PubMed] [Google Scholar]
- Ribeiro MVMR, Ribeiro LEF, Ribeiro ÊAN, Ferreira CV, Barbosa F & Timbó (2016) Adherence assessment of eye drops in patients with glaucoma using 8 item Morisky Score: A cross sectional study. In Revista Brasileira de Oftalmologia),vol. 75, pp. 432–437. [Google Scholar]
- Robbie K, Sit JC & Brett MJ (1998) Advanced techniques for glancing angle deposition. Journal of Vacuum Science & Technology B 16(3):1115–1122. [Google Scholar]
- Robin A & Grover DS (2011) Compliance and adherence in glaucoma management. Indian Journal of Ophthalmology 59:S93–S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rootman DS, Willoughby RPN, Bindlish R, Avaria M, Basu PK & Krajden M (1992) CONTINUOUS-FLOW CONTACT-LENS DELIVERY OF GENTAMICIN TO RABBIT CORNEA AND AQUEOUS-HUMOR. Journal of Ocular Pharmacology 8(4):317–323. [DOI] [PubMed] [Google Scholar]
- Russo R, Varano GP, Adornetto A, Nucci C, Corasaniti MT, Bagetta G & Morrone LA (2016) Retinal ganglion cell death in glaucoma: Exploring the role of neuroinflammation. European Journal of Pharmacology 787:134–142. [DOI] [PubMed] [Google Scholar]
- Sanchez I & Martin R (2019) Advances in diagnostic applications for monitoring intraocular pressure in Glaucoma: A review. Journal of Optometry 12:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano K, Tokoro T & Imai Y (1996) A new drug delivery system utilizing piggyback contact lenses. Acta Ophthalmologica Scandinavica 74(3):243–248. [DOI] [PubMed] [Google Scholar]
- Schoenwald RD (1993) Ocular pharmacokinectics/pharmacodyamics. In Ophthalmic Drug Delivery Systems. (Mitra AK (ed)) Marcel Dekker, Inc., New York, NY, pp. 83–110. [Google Scholar]
- Seal JR, Robinson MR, Burke J, Bejanian M, Coote M & Attar M (2019) Intracameral Sustained-Release Bimatoprost Implant Delivers Bimatoprost to Target Tissues with Reduced Drug Exposure to Off-Target Tissues. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics 35(1):50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedlacek J (1965) Possibility of the application of ophthalmic drugs with the use of gel contact lenses. Cesk Oftalmol 21(6):509–512. [PubMed] [Google Scholar]
- Shafiee A, Bowman LM, Hou E & Hosseini K (2013) Ocular pharmacokinetics of bimatoprost formulated in DuraSite compared to bimatoprost 0.03% ophthalmic solution in pigmented rabbit eyes. Clinical ophthalmology (Auckland, N.Z.) 7:1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Durairaj C, Lin T, Liu Y & Burke J (2014) Ocular Pharmacokinetics of Intravitreally Administered Brimonidine and Dexamethasone in Animal Models With and Without Blood-Retinal Barrier Breakdown. Investigative Ophthalmology & Visual Science 55(2):1056–1066. [DOI] [PubMed] [Google Scholar]
- Shen J, Lu GW & Hughes P (2018) Targeted Ocular Drug Delivery with Pharmacokinetic/pharmacodynamic Considerations. Pharm Res 35, Article number 217. [DOI] [PubMed] [Google Scholar]
- Shen J, Robinson MR, Struble C & Attar M (2020) Nonclinical Pharmacokinetic and Pharmacodynamic Assessment of Bimatoprost Following a Single Intracameral Injection of Sustained-Release Implants. Translational Vision Science & Technology 9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard JD, Torkildsen GL, Metzinger JL, Stein L, Ousler G & Goldstein MH (2018) Multicenter, Phase 2 exploratory study evaluating the safety and efficacy of DEXTENZA for the treatment of dry eye disease. Clinical and Experimental Rheumatology 36(3):S320–S320. [Google Scholar]
- Shouchane-Blum K, Geffen N & Zahavi A (2019) Sustained drug delivery platforms - A new era for gluacoma treatment. Clinical and Experimental Vision and Eye Research 2:22–29. [Google Scholar]
- Singh IP (2020) Perspective: I. Paul Singh, MD, on the FDA approval of Durysta.) Healio Press Release, Online at: https://www.healio.com/news/ophthalmology/20200325/perspective-i-paul-singh-md-onthe-fda-approval-of-durysta.
- Smolinsky M (2016) What’s New with Three Sustained-Release Drug Platforms.) Ophthalmology Innovation Summit (OIS), Online.
- Snibson GR, Greaves JL, Soper NDW, Tiffany JM, Wilson CG & Bron AJ (1992) OCULAR SURFACE RESIDENCE TIMES OF ARTIFICIAL TEAR SOLUTIONS. Cornea 11(4):288–293. [DOI] [PubMed] [Google Scholar]
- Stasko NA, Johnson CB, Schoenfisch MH, Johnson TA & Holmuhamedov EL (2007) Cytotoxicity of polypropylenimine dendrimer conjugates on cultured endothelial cells. Biomacromolecules 8(12):3853–3859. [DOI] [PubMed] [Google Scholar]
- Szozotka-Flynn LOD, M.S. FAAO (2008) Looking At Silicone Hydrogels Across Generations:Demystify the various polymers and lens treatments that characterize silicone hydrogel lenses.) Highlights From the Optometric Management Symposium On Contemporary Eye Care, Online at: www.optometricmanagement.com.
- Talamo JH, Tyson SL, Bafna S, Joseph GP, Goldberg DF, Jones JJ, Jones MP, Kim JK, Martel JB, Nordlund ML, Piovanetti IK, Singh IP, Metzinger JL & Mulani D (2017) Results of a Phase 3, Randomized, Double-Masked, Vehicle Controlled Study (Phase 3c) Evaluating the Safety and Efficacy of DEXTENZA (TM) (dexamethasone insert) 0.4 mg for the Treatment of Ocular Inflammation and Pain after Cataract Surgery. Investigative Ophthalmology & Visual Science 58(8). [Google Scholar]
- Tanigawa Y, Wainberg M, Karjalainen J, Kiiskinen T, Venkataraman G, Lemmelä S, Turunen JA, Graham RR, Havulinna AS, Perola M, Palotie A, Finngen, Daly MJ & Rivas MA (2020) Rare protein-altering variants in ANGPTL7 lower intraocular pressure and protect against glaucoma. PLOS Genetics 16(5):e1008682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassoni A, Gutteridge A, Barber AC, Osborne A & Martin KR (2015) Molecular Mechanisms Mediating Retinal Reactive Gliosis Following Bone Marrow Mesenchymal Stem Cell Transplantation. Stem Cells 33(10):3006–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G (2006) Oxidative stress in glaucomatous neurodegeneration: Mechanisms and consequences. Progress in Retinal and Eye Research 25(5):490–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than A, Liu CH, Chang H, Duong PK, Cheung CMG, Xu CJ, Wang XM & Chen P (2018) Self-implantable double-layered micro-drug-reservoirs for efficient and controlled ocular drug delivery. Nature Communications 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe BJ (2013) A decade of silicone hydrogel development: surface properties, mechanical properties, and ocular compatibility. Eye Contact Lens 39(1):4–12. [DOI] [PubMed] [Google Scholar]
- Tighsazzadeh M, Mitchell JC & Boateng JS (2019) Development and evaluation of performance characteristics of timolol-loaded composite ocular films as potential delivery platforms for treatment of glaucoma. International Journal of Pharmaceutics 566:111–125. [DOI] [PubMed] [Google Scholar]
- Todani A, Behlau I, Fava MA, Cade F, Cherfan DG, Zakka FR, Jakobiec FA, Gao YQ, Dohlman CH & Melki SA (2011) Intraocular Pressure Measurement by Radio Wave Telemetry. Investigative Ophthalmology & Visual Science 52(13):9573–9580. [DOI] [PubMed] [Google Scholar]
- Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J & Smith P (1985) A NEW CLASS OF POLYMERS - STARBURST-DENDRITIC MACROMOLECULES. Polymer Journal 17(1):117–132. [Google Scholar]
- Tyagi P, Barros M, Stansbury JW & Kompella UB (2013) Light-Activated, In Situ Forming Gel for Sustained Suprachoroidal Delivery of Bevacizumab. Molecular Pharmaceutics 10(8):2858–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi P, Kadam RS & Kompella UB (2012) Comparison of Suprachoroidal Drug Delivery with Subconjunctival and Intravitreal Routes Using Noninvasive Fluorophotometry. Plos One 7(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzameret A, Sher I, Belkin M, Treves AJ, Meir A, Nagler A, Levkovitch-Verbin H, Barshack I, Rosner M & Rotenstreich Y (2014) Transplantation of human bone marrow mesenchymal stem cells as a thin subretinal layer ameliorates retinal degeneration in a rat model of retinal dystrophy. Experimental Eye Research 118:135–144. [DOI] [PubMed] [Google Scholar]
- Ullrich F, Bergeles C, Pokki J, Ergeneman O, Erni S, Chatzipirpiridis G, Pane S, Framme C & Nelson BJ (2013) Mobility Experiments With Microrobots for Minimally Invasive Intraocular Surgery. Investigative Ophthalmology & Visual Science 54(4):2853–2863. [DOI] [PubMed] [Google Scholar]
- Venugopalan PL, Sai R, Chandorkar Y, Basu B, Shivashankar S & Ghosh A (2014) Conformal Cytocompatible Ferrite Coatings Facilitate the Realization of a Nanovoyager in Human Blood. Nano Letters 14(4):1968–1975. [DOI] [PubMed] [Google Scholar]
- Vorwerk CK, Lipton SA, Zurakowski D, Hyman BT, Sabel BA & Dreyer EB (1996) Chronic low-dose glutamate is toxic to retinal ganglion cells - Toxicity blocked by memantine. Investigative Ophthalmology & Visual Science 37(8):1618–1624. [PubMed] [Google Scholar]
- Voss K, Falke K, Bernsdorf A, Grabow N, Kastner C, Sternberg K, Minrath I, Eickner T, Wree A, Schmitz KP, Guthoff R, Witt M & Hovakimyan M (2015) Development of a novel injectable drug delivery system for subconjunctival glaucoma treatment. Journal of Controlled Release 214:1–11. [DOI] [PubMed] [Google Scholar]
- Walker D, Kasdorf BT, Jeong HH, Lieleg O & Fischer P (2015) Enzymatically active biomimetic micropropellers for the penetration of mucin gels. Science Advances 1(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb RN, Aung T & Medeiros FA (2014) The Pathophysiology and Treatment of Glaucoma A Review. Jama-Journal of the American Medical Association 311(18):1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler LA, Lai R & Woldemussie E (1999) From the lab to the clinic: Activation of an alpha-2 agonist pathway is neuroprotective in models of retinal and optic nerve injury. European Journal of Ophthalmology 9(1):S17–S21. [DOI] [PubMed] [Google Scholar]
- Williams GA (2014) IVT injections: health policy implications. Review of Ophthalmolgy 21:62–64. [Google Scholar]
- Williams PA, Harder JM, Foxworth NE, Cochran KE, Philip VM, Porciatti V, Smithies O & John SWM (2017) Vitamin B-3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 355(6326):756–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witcherle O & Lim D (1960) Hydrophilic gels for biological use. Nature 185:117–118. [Google Scholar]
- Witkin AJ & Brown GC (2011) Update on nonsurgical therapy for diabetic macular edema. Current Opinion in Ophthalmology 22(3):185–189. [DOI] [PubMed] [Google Scholar]
- Woldemussie E, Ruiz G, Wijono M & Wheeler LA (2001) Neuro-protection of retinal ganglion cells by brimonidine in rats with laser-induced chronic ocular hypertension. Investigative Ophthalmology & Visual Science 42(12):2849–2855. [PubMed] [Google Scholar]
- Wong TT, Novack GD, Natarajan JV, Ho CL, Htoon HM & Venkatraman SS (2014) Nanomedicine for glaucoma: sustained release latanoprost offers a new therapeutic option with substantial benefits over eyedrops. Drug Delivery and Translational Research 4(4):303–309. [DOI] [PubMed] [Google Scholar]
- Woodward DF, Krauss AHP, Chen J, Liang Y, Li C, Protzman CE, Bogardus A, Chen R, Kedzie KM, Krauss HA, Gil DW, Kharlamb A, Wheeler LA, Babusis D, Welty D, Tang-Liu DDS, Cherukury M, Andrews SW, Burk RM & Garst ME (2003) Pharmacological characterization of a novel antiglaucoma agent, Bimatoprost (AGN 192024). Journal of Pharmacology and Experimental Therapeutics 305(2):772–785. [DOI] [PubMed] [Google Scholar]
- Wu ZG, Troll J, Jeong HH, Wei Q, Stang M, Ziemssen F, Wang ZG, Dong MD, Schnichels S, Qiu T & Fischer P (2018) A swarm of slippery micropropellers penetrates the vitreous body of the eye. Science Advances 4(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xinming L, Yingde C, Lloyd AW, Mikhalovsky SV, Sandeman SR, Howel CA & Liewen L (2008) Polymeric hydrogels for novel contact lens-based ophthalmic drug delivery systems: A review. Contact Lens & Anterior Eye 31(2):57–64. [DOI] [PubMed] [Google Scholar]
- Xu JW, Ge Y, Bu RX, Zhang AN, Feng SS, Wang J, Gou JX, Yin T, He HB, Zhang Y & Tang X (2019) Co-delivery of latanoprost and timolol from micelles-laden contact lenses for the treatment of glaucoma. Journal of Controlled Release 305:18–28. [DOI] [PubMed] [Google Scholar]
- Xu JW, Xue YY, Hu GY, Lin TY, Gou JX, Yin T, He HB, Zhang Y & Tang X (2018) A comprehensive review on contact lens for ophthalmic drug delivery. Journal of Controlled Release 281:97–118. [DOI] [PubMed] [Google Scholar]
- Xu QG, Boylan NJ, Suk JS, Wang YY, Nance EA, Yang JC, Mcdonnell PJ, Cone RA, Duh EJ & Hanes J (2013) Nanoparticle diffusion in, and microrheology of, the bovine vitreous ex vivo. Journal of Controlled Release 167(1):76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Tyagi P, Kadam RS, Holden CA & Kompella UB (2012) Hybrid Dendrimer Hydrogel/PLGA Nanoparticle Platform Sustains Drug Delivery for One Week and Antiglaucoma Effects for Four Days Following One-Time Topical Administration. Acs Nano 6(9):7595–7606. [DOI] [PubMed] [Google Scholar]
- Yilmaz SG, Degirmenci C, Karakoyun YE, Yusifov E & Ates H (2018) The efficacy and safety of bimatoprost/timolol maleate, latanoprost/timolol maleate, and travoprost/timolol maleate fixed combinations on 24-h IOP. International Ophthalmology 38(4):1425–1431. [DOI] [PubMed] [Google Scholar]
- Yu S, Tanabe T, Dezawa M, Ishikawa H & Yoshimura N (2006) Effects of bone marrow stromal cell injection in an experimental glaucoma model. Biochemical and Biophysical Research Communications 344(4):1071–1079. [DOI] [PubMed] [Google Scholar]
- Zaki I, Fitzgerald P, Hardy JG & Wilson CG (1986) A COMPARISON OF THE EFFECT OF VISCOSITY ON THE PRECORNEAL RESIDENCE OF SOLUTIONS IN RABBIT AND MAN. Journal of Pharmacy and Pharmacology 38(6):463–466. [DOI] [PubMed] [Google Scholar]
- Zhao M, Rodriguez-Villagra E, Kowalczuk L, Le Normand M, Berdugo M, Levy-Boukris R, El Zaoui I, Kaufmann B, Gurny R, Bravo-Osuna I, Molina-Martinez IT, Herrero-Vanrell R & Behar-Cohen F (2017) Tolerance of high and low amounts of PLGA microspheres loaded with mineralocorticoid receptor antagonist in retinal target site. Journal of Controlled Release 266:187–197. [DOI] [PubMed] [Google Scholar]


