Abstract
The prevalence of pulmonary nontuberculous mycobacteria (NTM) disease is increasing in the United States. Associations were evaluated among residents of central North Carolina between pulmonary isolation of NTM and environmental risk factors including: surface water, drinking water source, urbanicity, and exposures to soils favorable to NTM growth. Reports of pulmonary NTM isolation from patients residing in three counties in central North Carolina during 2006 – 2010 were collected from clinical laboratories and from the State Laboratory of Public Health. This analysis was restricted to patients residing in single family homes with a valid residential street address and conducted at the census block level (n=13,495 blocks). Negative binomial regression models with thin-plate spline smoothing function of geographic coordinates were applied to assess effects of census block-level environmental characteristics on pulmonary NTM isolation count. Patients (n = 507) resided in 473 (3.4%) blocks within the study area. Blocks with >20% hydric soils had 26.8% (95% Confidence Interval (CI): 1.8%, 58.0%), p=0.03, higher adjusted mean patient counts compared to blocks with ≤20% hydric soil, while blocks with >50% acidic soil had 24.8% (−2.4%, 59.6%), p=0.08 greater mean patient count compared to blocks with ≤50% acidic soil. Isolation rates varied by county after adjusting for covariates. The effects of using disinfected public water supplies vs. private wells, and of various measures of urbanicity were not significantly associated with NTM. Our results suggest that proximity to certain soil types (hydric and acidic) could be a risk factor for pulmonary NTM isolation in central North Carolina.
Keywords: pulmonary nontuberculous mycobacteria, Southestern United States, hydric soil, acidic soil
1. Introduction
Nontuberculous mycobacteria (NTM) are a broad group of acid-fast bacilli (Johnson and Odell 2014) that comprise most of the more than 190 species in the genus Mycobacterium excluding Mycobacterium leprae and those classified in the Mycobacterium tuberculosis complex (Bergey et al. 2012). Nontuberculous mycobacteria can colonize any area of the body, but most commonly are isolated from the lungs (Good and Snider 1982; Griffith et al. 2007; O’Brien et al. 1987; Wassilew et al. 2016). They are considered an emerging pathogen of concern and when found in the lungs can lead to a variety of nonspecific and sometimes serious pulmonary symptoms, which may be exacerbated by other preexisting conditions (Chan and Iseman 2013; Griffith et al. 2007). Several patient characteristics, such as immune suppression, and chronic lung disease may increase the susceptibility to NTM colonization and infection (Adjemian et al. 2012a; Adjemian et al. 2012b; Dirac et al. 2012). The prevalence of pulmonary NTM infection increases with age (Donohue and Wymer 2016; O’Brien et al. 1987) and recent data suggests that many patients with pulmonary NTM may be genetically predisposed to this infection through combinations of variants at several gene loci (Szymanski et al. 2015). Since the year 2000, the prevalence of pulmonary NTM disease has been increasing steadily in the U.S. (Prevots and Marras 2015).
NTM are not generally transmitted person-to person (Meissner and Anz 1977; Tanaka et al. 2000); transmission of NTM is primarily associated with environmental exposure to soil colonized with NTM, and contaminated water and aerosols (Falkinham III 2013). Many physical characteristics of NTM aid in their ubiquity in the environment and their ability to aerosolize and be deposited into the respiratory tract (Falkinham III 2003; Parker et al. 1983; Poulain and Bourouiba 2018). First, NTM possess a lipid-dense outer membrane, making them extremely hydrophobic and therefore resistant to antibiotics, heavy metals, and disinfectants (Brennan and Nikaido 1995; Jarlier and Nikaido 1994). Their extreme hydrophobicity aids in their attachment to surfaces and the formation of biofilms (Steed and Falkinham 2006). Biofilm formation in water distribution pipes and the human respiratory tract further aids resistance to disinfection and antimicrobial therapy (Falkinham 2018). Second, NTM are oligotrophic, meaning they can survive in low nutrient conditions (Norton et al. 2004). Additionally, NTM are also known to grow well in the presence of humic and fulvic acids (Kirschner et al. 1992; Kirschner et al. 1999), and in relatively low pH environments (Portaels and Pattyn 1982).
Water is one of the most common reservoirs of NTM (Falkinham 2015; Parker et al. 1983), and is suspected to be a primary mode of exposure. Because of their hydrophobic membrane, NTM can readily aerosolize from water into the air by adhering to bubbles in the water column, which burst when reaching the water’s surface (Parker et al. 1983; Poulain and Bourouiba 2018). Since NTM are oligotrophs (Hall-Stoodley and Lappin-Scott 1998; Norton et al. 2004) and capable of forming biofilms (Williams et al. 2009), they are commonly isolated from surface water (Falkinham 2015; Gruft et al. 1979; Parker et al. 1983; Von Reyn et al. 1994). Additionally, NTM present in surface water can create biofilms present in complex drinking water distribution systems (Hilborn et al. 2006). Since NTM are also resistant to disinfection (Brennan and Nikaido 1995; Steed and Falkinham 2006), disinfectants introduced at the water treatment plant are unable to penetrate the thick cell membrane and can further select for growth of NTM by eliminating other competing organisms (Hall-Stoodley and Lappin-Scott 1998). Therefore, compared to private wells, NTM are more likely to be isolated from complex water systems relying on surface water sources (Falkinham III 2011; Gebert et al. 2018). It is hypothesized that individuals who receive drinking water from municipal water supplies using surface water sources are at increased risk of NTM exposure compared to those who receive drinking water from private wells. This is further supported by the fact that NTM are rarely isolated from groundwater sources (Martin et al. 1987). Consistent with this hypothesis, some studies reported higher prevalence of pulmonary NTM disease in urban environments where residents are more likely to receive municipal drinking water (Adjemian et al. 2012a; du Moulin et al. 1985; O’Brien et al. 1987; Winthrop et al. 2011).
Soil is another source of exposure to NTM (Brooks et al. 1984; Walsh et al. 2019). NTM are oligotrophic and their hydrophobic membranes cause them to readily attach to particles found in soil (Falkinham III 2013), making them easily aerosolized. Several soil types provide an adequate environment for NTM to flourish (Iivanainen et al. 1997). In particular, NTM can also survive in relatively low pH, making acidic soils suitable for their growth and proliferation (Brooks et al. 1984; Kirschner et al. 1992). Additionally, humic and fulvic acids present in swamps, pine forests, and peat bog soils have been found to stimulate growth of NTM (Kirschner et al. 1992; Kirschner et al. 1999). Peats, and acidic, boreal forest soils have been found to harbor high concentrations of NTM (Iivanainen et al. 1997).
The southeastern United States (U.S.) has been considered a rich area for environmental isolation of NTM, since peat (soil that is acidic and hydric) and coastal wetlands, both of which are abundant there, are common sources of NTM (Kirschner et al. 1992). The objective of this study was to assess if specific environmental characteristics around a patient’s home residence in central North Carolina were associated with increased risks of pulmonary NTM isolation. Specific environmental exposures considered included: surface water, drinking water supply, soil, and urbanicity.
2. Materials and methods
2.1. Patient records
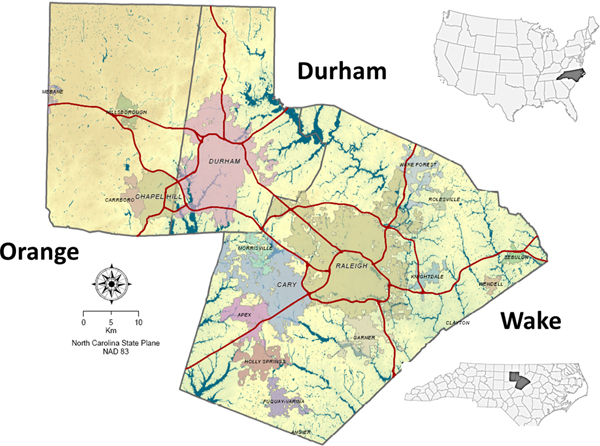
Reports of pulmonary NTM isolations were evaluated in this analysis. While pulmonary isolation alone is not enough to determine infection (Griffith et al. 2007), the analysis of pulmonary isolation patterns can aid in the understanding of environmental sources of exposures (Catanzaro 2002). However, the positive predictive value of a positive culture is relatively high (Prevots and Marras 2015), indicating that the presence of a positive culture is a strong indicator of environmental exposure. The North Carolina State Laboratory of Public Health and local clinical laboratories provided reports of patients living in Wake, Orange, and Durham counties with pulmonary isolation of NTM during 2006–2010 (Figure 1). Approval to access and analyze NTM isolation reports was granted by the Institutional Review Board (IRB) of the University of North Carolina School of Medicine, the IRB of the State of North Carolina, and the IRBs of participating clinical institutions. Further details about the study are available in Smith et al. (2016). Each patient’s demographic information, anatomical site of NTM isolation, and home address were extracted from the reports. The analysis was restricted to only include the first report of pulmonary isolation among each patient report; each report represented a unique individual.
Figure 1:
Three-county study area
2.2. Geographic information
Pulmonary NTM isolation data from patients were included in the analysis if their reported residence could be assigned to a street address within the three-county study area. Address assignment, or geocoding, was performed in ArcMap 10.0 (ESRI 2011) using a custom-built composite address locator that utilized Navteq 2010 street data (NAVTEQ 2010) as well as referenced parcel data provided by Durham, Orange, and Wake Counties. Patients not residing in ‘single-family homes’ were excluded. The single-family home category consisted of modular homes, mobile homes, duplex and triplex homes. We specifically excluded those who resided in apartment buildings, group homes, or prisons to increase the probability that patients had resided at the same address for a sufficiently long time for local exposure factors to affect the risk of isolation (McCarthy 1976).
The analysis was conducted at the census block level within the three-county study area. Census blocks are the smallest unit of aggregation that define a geographical area in the U.S. They can vary in size in urban compared to rural areas, and are not delimited based on population size (can have a population size of zero) (Rossiter 2011). We calculated the number of pulmonary NTM isolation patient reports per census block over a five-year period from 2006 – 2010. Blocks with zero population or without any single-family homes were excluded from further analysis.
2.3. Environmental data sources
Surface water exposure was assessed as the percent of the census block area estimated to be composed of ‘open water’ from the 2011 National Land Cover Database (NLCD) (U.S. Geological Survey 2011). Wetland exposure was considered as the percent of the census block composed of wetlands using a sum of the percent freshwater emergent wetlands, freshwater forested wetland, and shrub wetland categories from the National Wetlands Inventory (U.S. Fish and Wildlife Service).
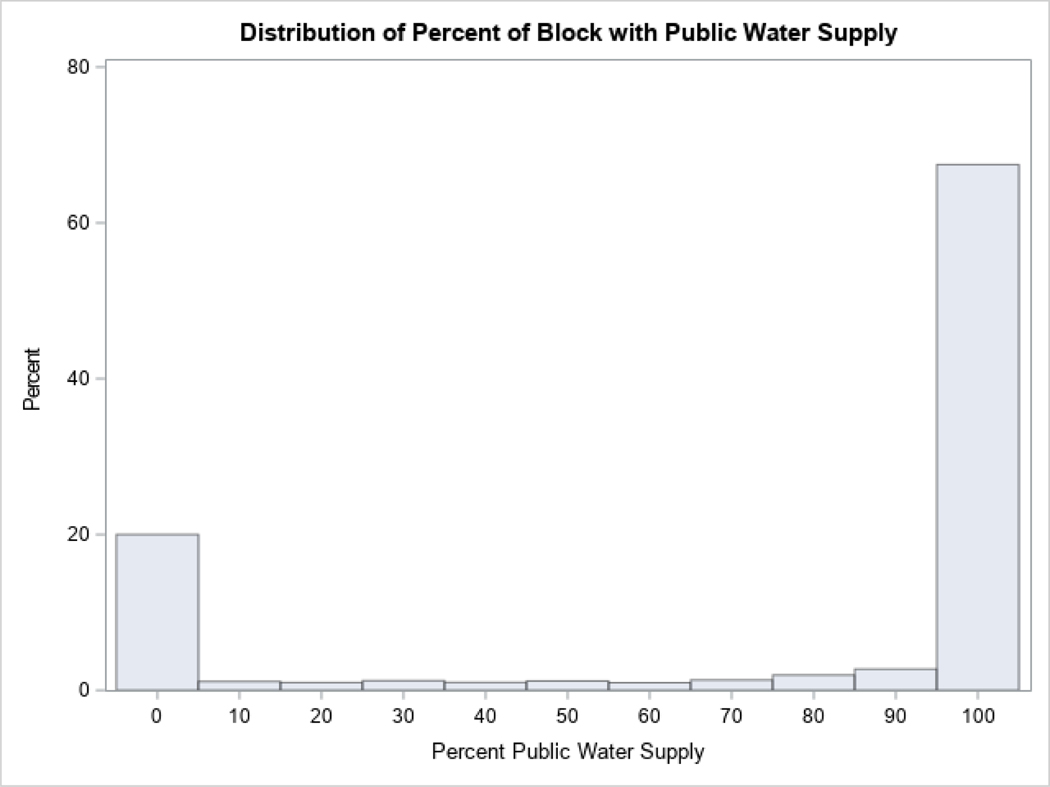
Drinking water sources were classified as municipal water supplies or as private wells for this analysis. Drinking water source was assigned to each residence in the study area by spatially joining municipal drinking water service areas to each patient’s geocoded address in GIS. Any household address that was not contained in a service area was assumed to be using a private well. We then calculated the percentage of residences in each census block utilizing each type of drinking water source. Based on the distribution (Appendix, Figure A.1), blocks were categorized either as having mainly municipal water supply if >50% of the block was composed of addresses receiving municipal water (67.0% of blocks), or as having mainly private wells.
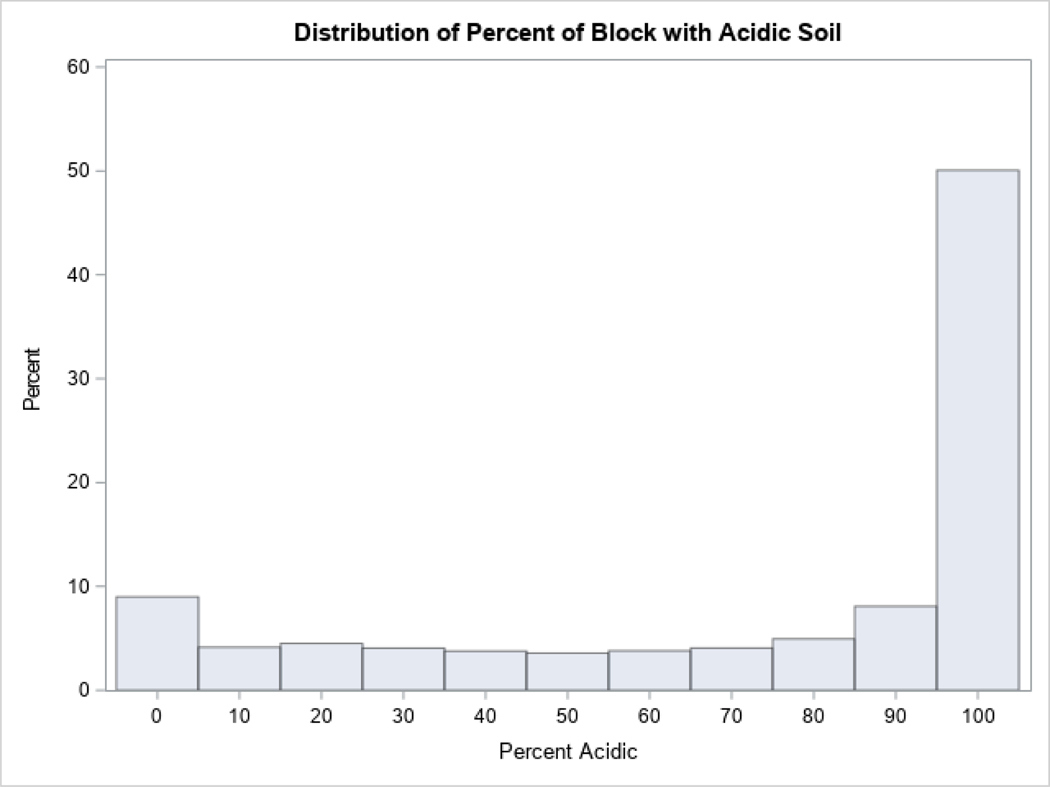
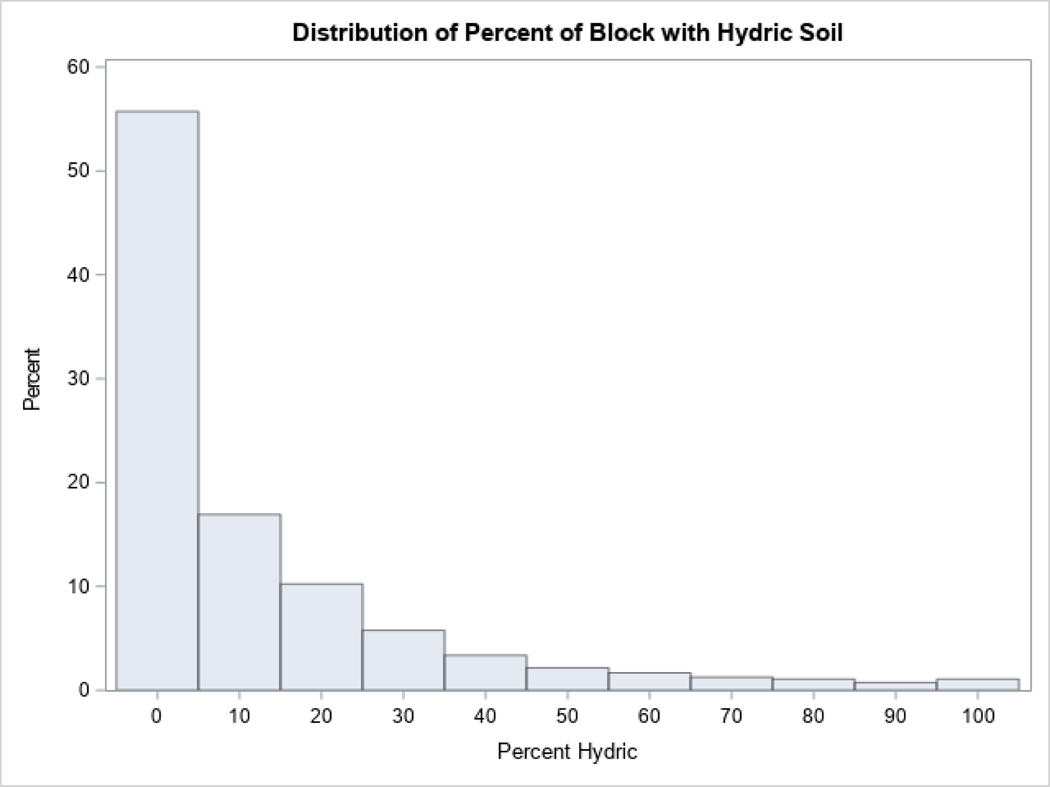
The Soil Survey Geographic Database (SSGD) was used to classified soils in the study area as acidic and/or hydric (Soil Survey Staff). Using pH data provided by the SSGD, soil with a pH<5.5 was considered acidic. Soil designated as being saturated with water and developing anaerobic conditions for at least some of the year was considered hydric (Natural Resources Conservation Service). We then geometrically intersected the soils data with Census blocks to produce an areal estimate of respective soil extents within each block. Based on the distribution analysis of bimodal variables (Appendix, Figure A.2, Figure A.3), blocks were classified either as acidic if >50% of the block was composed of acidic soil (72.5% of blocks) or as non-acidic; blocks were classified either as hydric if >20% of the block had hydric soil (20.3% of blocks) or as non-hydric.
Other soil and dust exposures were represented by the percent of the block composed of cultivated crops using data from the 2011 NLCD (U.S. Geological Survey 2011) and the density (km/km2) of dirt roads in the block based on 2010 Navteq street data (NAVTEQ 2010).
To assess if urbanicity was associated with pulmonary NTM isolation, we considered: 1) population density by block; 2) percentage of block area with impervious surfaces; and 3) paved road density (km/km2) in study blocks, based on the 2010 Navteq street data (NAVTEQ 2010). Impervious surface was defined according to the classification descriptions for developed land from the 2011 NLDC (U.S. Geological Survey 2011). Percent ‘impervious surface’ summed the percent of the census block’s area classified as “developed, low intensity (20–49% impervious surface)”, “developed, medium intensity (50–79% impervious surface)”, and “developed, high intensity” (80–100% impervious surface). The percentages of each block consisting of evergreen or deciduous forests were estimated using the 2011 NLCD (U.S. Geological Survey 2011). We also considered grasslands by combining the percent of the block consisting of herbaceous and pasture/hay cover from the 2011 NLCD (U.S. Geological Survey 2011).
2.4. Other data sources
As pulmonary isolation rates in our study area differed among counties, age categories, and races (Smith et al. 2016) we included these demographic covariates in the regression analysis. The data were abstracted from 2010 US census datasets. We included median age (U.S. Census Bureau 2010b) of persons in the block, the percent of the block that is female (U.S. Census Bureau 2010a), and percent of racial categories (White, Black, and other races ) (U.S. Census Bureau 2010c) for each census block. Since median household income was not available at the block level, we aggregated residential building values from county parcel data to blocks in GIS and calculated the median residence value per block as a proxy of socio-economic status.
2.5. Statistical analysis
Regression analyses were conducted using generalized additive models for a negative binomial-distributed outcome to estimate the relationship between the count of patients with pulmonary NTM isolations per census block and environmental characteristics of the census block. To control for spatial autocorrelation, regression models included a mean trend surface, a two-dimensional smoothing function (thin-plate spine) of geographic coordinates (Dormann et al. 2007; Webster et al. 2006). Models were fitted using the procedure gampl in SAS (SAS 2015). A set of sociodemographic and geographic covariates for the final regression model were selected using stepwise regression technique. If two variables were moderately correlated (absolute value of Spearman correlation coefficient ≥0.4) (Akoglu 2018), then each variable was analyzed in a separate model. The variable with the strongest association that best predicted NTM, was then chosen for further analysis, and could potentially be included in our final model. The natural log of the total population size of the block was used as an offset term in each model. The initial set of covariates included all available census block level demographic variables characterizing age, racial composition, and sex composition at the block level, as well as a set of land cover variables, population density, and median building value per block. The final model was selected based on a combination of statistical significance and a priori selection of suspected predictors of pulmonary NTM risk and considered: soil exposure (hydric soil, acidic soil, cultivated crops, dirt road density, deciduous forests, evergreen forests, and grasslands); water exposure (wetlands, open water, drinking water source); urbanicity (impervious surface, paved roads, and population density); and demographic characteristics (age, sex, and race). Multicollinearity was assessed using the variance inflation factor among the variables included in the final model. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) software.
3. Results
During the study period there were a total of 577 unique patients within the study area identified through pulmonary NTM isolation reports. Of these, 507 (88%) patients resided in single family homes, and were included in further analysis. The mean age of patients in all three counties was 60 years (standard deviation (SD)=19 years) and 51.8% of cases were females (Table 1). The majority of patients were White (70.5%), followed by Black (19.9%), and other races (9.6%). The 70 patients excluded from the analysis, due to their not residing in single family homes, were similar to the 507 patients included with respect to age (p=0.50) but had a higher percentage of Black patients (p=0.04) (Table 1).
Table 1:
Characteristics of patients from reports of pulmonary NTM isolation
| Patients included in analysis | Patients excluded from analysis | ||||
|---|---|---|---|---|---|
| Descriptive Characteristics | All Counties | Durham County | Orange County | Wake County | All counties |
| Total number of patients | 507 | 164 | 95 | 248 | 70 |
| Incidence rate per 100,0001 | 8.8 | 14.3 | 15.3 | 6.2 | 9.5 |
| Average age in years (SD)2 | 60.0 (19.2) | 60.0 (18.2) | 63.0 (19.3) | 58.9 (19.7) | 61.7 (17.2) |
| Number of females (%)3 | 262 (51.8%) | 94 (57.3%) | 53 (55.8%) | 115 (46.6%) | 41 (59.4%) |
| Number of patients by race (%)4 | |||||
| White | 337 (70.5%) | 101 (65.6%) | 70 (76.9%) | 166 (71.2%) | 38* (57.6%) |
| Black | 95 (19.9%) | 36 (23.4%) | 11 (12.1%) | 48 (20.6%) | 22* (33.3%) |
| Other races | 46 (9.6%) | 17 (11.0%) | 10 (11.0%) | 19 (8.2%) | 6* (9.1%) |
Cumulative incidence rate over 5 years, total population only of included blocks in each county,
Age missing for 3,
Sex missing for 1,
Race missing for 29,
p<0.05 (compared to all included counties)
In the three-county area, there were 22,104 census blocks (2,653 in Orange County, 5,029 in Durham County, and 14,422 in Wake County). Blocks containing no single-family residences (n=8,120) and blocks with a total population of zero (n=489) were removed from analysis leaving 13,495 blocks (61.1%) with a total population of 1,155,620 residents including 1,748 blocks with 123,990 people in Orange County, 2,937 blocks with 229,968 residents in Durham County, and 8,810 blocks with 801,662 residents in Wake County. The 507 pulmonary NTM isolation patients resided in 473 blocks. There were 446 blocks with one patient, 22 blocks with two patients, 3 blocks with three patients, and 2 blocks with four patients. On average, within the included census blocks, the median age of was 40.6, and the majority of the population was white (65.0%), followed by black (20.9%) (Table 2).
Table 2:
Characteristics for census blocks included in analysis
| n | Mean | SD | 25th Percentile | Median | 75th percentile | |
|---|---|---|---|---|---|---|
| Hydric soil % | 13,495 | 12.2 | 20.3 | 0 | 1.0 | 16.0 |
| Acidic soil % | 13,495 | 71.9 | 36.0 | 43.0 | 95.0 | 100.0 |
| Wetland % | 13,495 | 1.5 | 6.2 | 0 | 0 | 0 |
| Cultivated crops % | 13,494 | 0.8 | 4.7 | 0 | 0 | 0 |
| Dirt road density (km/km2) | 13,495 | 0.5 | 3.7 | 0 | 0 | 0 |
| Deciduous forest % | 13,494 | 10.0 | 17.1 | 0 | 0 | 14.7 |
| Evergreen Forest % | 13,494 | 5.8 | 11.1 | 0 | 0 | 7.4 |
| Grassland % | 13,494 | 6.4 | 13.6 | 0 | 0 | 5.1 |
| Wetland % | 13,495 | 1.5 | 6.2 | 0 | 0 | 0 |
| Open water % | 13,494 | 0.2 | 1.4 | 0 | 0 | 0 |
| Public water supply, % of households | 12,953 | 74.6 | 40.9 | 50.0 | 100.0 | 100.0 |
| Impervious surface % | 13,494 | 34.0 | 31.4 | 4.9 | 25.8 | 57.1 |
| Paved road density (km/km2) | 13,495 | 29.4 | 24.0 | 12.2 | 24.1 | 40.3 |
| Population density (people/km2) | 13,495 | 1,245 | 1,267 | 292 | 919 | 1,796 |
| Median building value, $100,000 USD | 13,465 | 1.74 | 1.2 | 0.96 | 1.42 | 2.18 |
| Median age (years) | 13,495 | 40.6 | 11.2 | 33.0 | 39.0 | 48.0 |
| Female % | 13,495 | 51.4 | 10.3 | 47.3 | 51.0 | 56.0 |
| White race % | 13,495 | 65.0 | 32.3 | 43.0 | 76.0 | 91.0 |
| Black race % | 13,495 | 20.9 | 28.1 | 0 | 8.0 | 30.0 |
| Other races % | 13,495 | 12.3 | 16.6 | 0 | 7.0 | 17.0 |
We evaluated unadjusted models by comparing different block-level characteristics between patient and non-patient blocks and evaluating each candidate variable individually using models with a population offset term and thin-plate spline function of geographic coordinates (Table 3). These models indicated significant positive associations with the mean count of NTM patients for median age of the block (3.7% (95% Confidence Interval (CI): 2.9%, 4.5%), p <0.0001 per additional year of median age) and percent of females in the block (1.5% (0%, 3.1%), p=0.05). There were also notable differences between counties with Durham and Orange counties having significantly greater adjusted mean NTM counts than Wake county. In the unadjusted models, the effects of blocks with > 20% hydric soil, blocks with >50% acidic soil, and blocks with >50% municipal drinking water supply were short of being significant. The spline function of geographic coordinates was a significant predictor of pulmonary NTM isolation counts in all models (not shown).
Table 3:
Block-level characteristics for patient and non-patient blocks, and associations with pulmonary NTM counts by census block1
| Predictor variable | Level | Block Level Characteristics | Unadjusted Model2 | ||
|---|---|---|---|---|---|
| Patient Blocks (n=473) | Non-Patient Blocks (n=13,022) | Mean Count (95% Confidence Interval) | p-value | ||
| Hydric soil | |||||
| No. (% of blocks) | >20% | 119 (4.4%) | 2,619 (95.6%) | 17.3% (−5.0%, 44.9%) | 0.1 |
| ≤20% | 354 (3.3%) | 10,403 (96.7%) | Reference | ||
| Acidic soil | |||||
| No. (% of blocks) | >50% | 308 (3.1%) | 9,475 (96.2%) | 9.7% (−14.8%, 41.3%) | 0.5 |
| ≤50% | 165 (4.5) | 3,547 (95.5%) | Reference | ||
| Cultivated crops % | |||||
| mean (SD) | 0.7% (3.6%) | 0.8% (4.7%) | 0.8% (−1.5%, 3.2%) | 0.5 | |
| Dirt road density (km/km2) | |||||
| mean (SD) | 268 (1,950) | 483 (3,774) | 0% (0%, 0%) | 0.7 | |
| Deciduous forest % | |||||
| mean (SD) | 10.2% (15.2%) | 9.9% (17.2%) | 0.5% (−0.1%, 1.1%) | 0.1 | |
| Evergreen forest % | |||||
| mean (SD) | 6.0% (8.8%) | 5.8% (11.2%) | 0.2% (−0.7%, 1.2%) | 0.6 | |
| Grassland % | |||||
| mean (SD) | 5.2% (10.2%) | 6.4% (13.7%) | −0.1% (−1.0%, 0.7%) | 0.7 | |
| Wetlands % | |||||
| mean (SD) | 1.5% (5.2%) | 1.5% (6.2%) | −1.1% (−2.8%, 0.5%) | 0.2 | |
| Open water % | |||||
| mean (SD) | 0.4% (1.7%) | 0.2% (1.4%) | 5% (−0.1%, 11.2%) | 0.1 | |
| Public drinking water supply | |||||
| No. (% of blocks) | >50% households | 308 (3.4%) | 8,738 (96.6%) | 10.6% (−8.5%, 33.6%) | 0.3 |
| ≤50% households | 165 (4.5) | 4,284 (96.3%) | Reference | ||
| Impervious surface % | |||||
| mean (SD) | 32.7% (27.4%) | 34.0% (31.51%) | −0.2% (−0.6%, 0.1%) | 0.1 | |
| Paved road density (km/km2) | |||||
| mean (SD) | 21,083 (16,413) | 29,716 (24,126) | 0% (0%, 0%) | 0.4 | |
| Population density (people/km2) | |||||
| mean (SD) | 1,350 (1,205) | 1,241 (1,269) | 0% (0%, 0%) | 0.005 | |
| Median building value, per $100,000 USD | |||||
| mean (SD) | 1.8 (1.1) | 1.7 (1.20) | −1.4% (−9.6%, 7.6%) | 0.8 | |
| Median age in years | |||||
| mean (SD) | 41.0 (11.1) | 40.5 (11.2) | 3.7% (2.9%, 4.5%) | <.0001 | |
| Female % | |||||
| mean (SD) | 52.0% (7.2%) | 51.4% (10.4%) | 1.5% (0%, 3.1%) | 0.05 | |
| Race | |||||
| White | 62.9% (29.7%) | 65.0% (32.4%) | 0.2% (−0.1%, 0.5%) | 0.3 | |
| County | |||||
| No. (%) | |||||
| Durham | 148 (5.0%) | 2,789 (95.0%) | 183.7% (90.2%, 323.2%) | <.0001 | |
| Orange | 85 (4.9%) | 1,663 (95.1%) | 171.8% (58.5%, 366.1%) | 0.0003 | |
| Wake | 240 (2.7%) | 8,570 (97.3%) | Reference | ||
excludes blocks with zero total population and non-single-family residences
included an offset term for population size and a two-dimensional spline smoothing function of geographic coordinates as a single covariate
Many variables considered were highly correlated (Appendix, Table A.1). When two variables were correlated, we evaluated each variable separately and selected the one that had a stronger association with pulmonary NTM. Based on this analysis, we excluded variables for forest cover (deciduous and evergreen forests) and paved road density. Variance inflation factor (VIF) was less than 2 for all variables in the final model (data not shown).
The final multivariable regression model (Table 4) included hydric soil, acidic soil, percent of the block with wetlands, median age of the block municipal water source, and county of residence. This model indicated that blocks with >20% hydric soils had 26.8% (1.8%, 58.0%), p=0.03, higher adjusted mean patient counts compared to blocks with ≤ 20% hydric soil, while blocks with >50% acidic soil had 24.8% (−2.4%, 59.6%), p=0.08 higher mean count of patients compared to blocks with ≤ 50% acidic soil. One percent increase in proportion of the block area composed of wetlands was associated with an almost 2% decrease in mean count of patients: −1.9% (−3.7%, −0.01%), p = 0.03. Each additional year of median age of residents was associated with a 3.7% (95% CI: 2.9%, 4.5%), p<0.001 greater adjusted mean count of patients. The observed adjusted effect of municipal drinking water sources (>50%) was not significant with 14.9% (−4.9%, 39.0%), p = 0.15 greater mean count of cases in blocks with mainly municipal water supplies.
Table 4:
Association between environmental characteristics and pulmonary NTM isolations
| Variable | Level | Adjusted Mean Count1
(95% Confidence Interval) |
P-value |
|---|---|---|---|
| Hydric soils | >20% | 26.8% (1.8%, 58.0%) | 0.03 |
| ≤20% | Reference | ||
| Acidic soils | >50% | 24.8% (−2.4%, 59.6%) | 0.08 |
| ≤50% | Reference | ||
| Percent wetland | −1.9% (−3.7%, −0.01%) | 0.03 | |
| Public water supply | > 50% households | 14.9% (−4.9%, 39.0%) | 0.15 |
| ≤50% households | Reference | ||
| Median age (years) | 3.7% (2.9%, 4.5%) | <0.001 | |
| County | |||
| Durham | 260% (140%, 440%) | <0.001 | |
| Orange | 352% (104%, 507%) | <0.001 | |
| Wake | Reference |
model included an offset term for population size and a two-dimensional spline smoothing function of geographic coordinates
4. Discussion
This study examined the relationship between environmental risk factors (including water, soil, and urbanicity) and pulmonary NTM isolation among residents of three counties in central North Carolina. This study shows a significant statistical association between pulmonary NTM isolation in a population-based study and exposure to hydric soils and, and a suggestive (but non-significant) association between pulmonary NTM isolation and exposure to acidic soils. This supports previous evidence that certain soil characteristics may be important predictors of NTM isolation risk. Greater counts of NTM have been found to be highly correlated with peat, precipitation, the concentrations of certain metals (Al, Fe, Cu, Co, and Cr), and more acidic soils (Iivanainen et al. 1993). Recently, it has been reported that NTM are most commonly detected in more acidic, wetter, and cooler soils (Walsh et al. 2019).
Our a priori assumption was that patients are exposed to NTM from their household water and their local soil (Falkinham III 2013; Maekawa et al. 2011). Previous studies have produced inconsistent results on the effects of these exposure pathways. While NTM is commonly found in soil and water samples (Iivanainen 1995; Neumann et al. 1997), environmental samples are difficult to culture due bacterial and fungal overgrowth, and methods used to limit overgrown can inhibit some NTM from growing in culture (Hu et al. 2017; Thomson et al. 2008). Despite this limitation, several previous studies have demonstrated genetic similarity between pulmonary isolates from patients and isolates from soil (De Groote et al. 2006; Fujita et al. 2013) and tap water (du Moulin et al. 1985; Falkinham III 2011; Hilborn et al. 2008; Schulze-Röbbecke et al. 1995; Von Reyn et al. 1994) to which those individuals were exposed.
However, epidemiological case-control studies have yielded mixed results regarding soil and water exposure as risk factors for pulmonary NTM disease. Several studies evaluated patients with pulmonary Mycobacterium avium complex (MAC), a specific type of NTM. One study compared patients with MAC to controls with bronchiectasis and found that patients with MAC were more likely to have greater exposure to soil, compared to controls. However, this study also observed no difference in water use frequency between MAC patients and controls (Maekawa et al. 2011). Another study comparing patients with MAC lung disease to healthy controls found no association with exposure to either soil or water aerosols (Dirac et al. 2012). Yet, a population-based survey found that an occupational soil exposure was associated with greater risk of MAC infection, and that the risk increased with increasing years of participation in a soil-related occupation (Reed et al. 2006). Another analysis identified certain soil properties, including high copper and sodium, and low manganese, are associated with increased risk of pulmonary NTM disease (Adjemian et al. 2012a). In our study, metal and mineral soil characteristics were unavailable for two of three study counties (U.S. Geological Survey). These inconsistences in the epidemiologic literature shed light on the challenges in determining where patients are becoming infected in their environment, and that patients can have multiple exposures.
We evaluated a rather crude assessment of water supply exposure (municipal vs. well water), which, in our analysis, did not show a significant association with pulmonary NTM isolation. We did not have detailed information on distribution system and treatment characteristics of each municipal water system serving each block, but in the study area, most of the population is served by eight water sources, largely derived from surface water. We also lacked critical information on the presence of biofilms in different parts of the water supply system, organism concentration in relation to the distance from the water treatment system, and possible biofilm compositions in showerheads and sink taps, among others (Falkinham III et al. 2001; Iivanainen 1995; Johnson and Odell 2014; Kaevska et al. 2014) (Gebert et al. 2018).
We report a small but significant protective association with percent wetland in the final adjusted model. While coastal wetlands have been identified as a source of NTM in the southeast, inland wetlands have not (Kirschner et al. 1992). The protective effect of inland wetland exposure and NTM pulmonary isolation is a novel finding that can be considered for inclusion in future studies.
Several previous studies have also suggested that pulmonary NTM occurs at a higher rate in urban communities compared to rural areas (O’Brien et al. 1987; Adjemian et al. 2012a; Olivier et al. 2003; Winthrop et al. 2011). Our study, which took place at the census block level, opposed to the county level, also was conducted in a mixed urban/rural environment. In this study, indicators of urbanicity including population density, percent of impervious surface, and density of road network were not significant predictors of pulmonary NTM isolation in multivariable analysis.
Our analysis was restricted to a three-county study area in central North Carolina, with a rather small number of patients. We found that prevalence of pulmonary NTM isolation reports differed substantially by county. These differences could be due to variability in NTM detection rates in various health care institutions (Good and Snider 1982), unmeasured differences in the population composition among the counties, or biases associated with the greater accessibility of specialized care centers in Orange and Durham counties. Individuals living farther away from specialized centers could be less likely to be diagnosed and subsequently treated (Olivier et al. 2003).
Previous studies have also indicated that certain behaviors, such as gardening (De Groote et al. 2006) and occupational soil exposure (Reed et al. 2006) are risk factors for NTM isolation. Others have identified strong associations between MAC disease and certain host factors such as chronic obstructive pulmonary disease, previous pneumonia hospitalizations, or taking steroid medications (Dirac et al. 2012). It is likely that these behaviors and host factors play a large role in the risk of pulmonary NTM isolation, which can be a good indication of environmental exposure and subsequent progression to pulmonary NTM infection (Prevots and Marras 2015). Since we relied on laboratory records, we were unable to obtain any information on occupation, certain behaviors, and length of time at residence. The lack of data on these factors added to the challenge in assessing contributions of environmental exposures.
Since our cases were derived from review of reports of NTM isolation from clinical laboratories, we did not have a control group with which to compare environmental exposures. To overcome this, we analyzed our data at the census block-level. Therefore, we used area-level demographic and environmental data, and could not control for individual-level characteristics. This could result in residual confounding as demographic characteristics of case patients could systematically differ from aggregated block-level data. Percent of census block area covered with a specific soil type was used to characterize individual exposure, which could result in substantial exposure misclassification. This study also had notable strengths as it utilized detailed data on environmental characteristics as well as sociodemographic data in conjunction with a comprehensive, population-based dataset of NTM isolation reports.
5. Conclusions
We found that pulmonary NTM isolations were associated with patient residence near hydric soils, and potentially with acidic soils although the latter association was short of being significant. These findings are consistent with previous reports suggesting that NTM are commonly isolated from those soil types. This analysis did not produce evidence that using disinfected municipal drinking water was a risk factor for pulmonary NTM; although the association was in the direction of increased risk, it was far from being significant. Future studies should use individual level data and comprehensive environmental assessment to evaluate the relationship between environmental exposures and pulmonary NTM isolation.
Acknowledgments
We would like to thank Mr. Roy Vick, North Carolina State Soil Scientist, Natural Resources Conservation Service, United States Department of Agriculture for his assistance with securing and interpreting soil maps. This work was supported by U.S. Environmental Protection Agency [Contract #EP13D000041; IA#DW89922983]
Figure A.1:
Distribution of public water supply in each census block
Figure A.2:
Percent of block with acidic soil
Figure A.3:
Percent of block with hydric soil
Table A.1:
Spearman correlations between variables
| No. of pulmonary NTM isolation cases | Hydric soil >50% | Acidic soil >20% | Cultiv-ated crops % | Dirt road density (m/km2) | Decid-uous forest % | Ever-green forest % | Grass-land % | Wet-land % | Open water % | Public water supply >50% households | Impervious surface % | Paved road density (m/km2) | Population density (people/km2) | Med. Building value | Med. age | Female % | White race % | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of pulmonary NTM isolation cases | 1.00 | |||||||||||||||||
| Hydric soil >50% | 0.02* | 1.00 | ||||||||||||||||
| Acidic soil >20% | −0.03* | −0.03* | 1.00 | |||||||||||||||
| Cultivated crops % | 0.01 | 0.23* | 0.05* | 1.00 | ||||||||||||||
| Dirt road density (m/km2) | 0.02* | 0.02 | −0.25* | 0.10 | 1.00 | |||||||||||||
| Deciduous forest % | 0.03* | 0.20* | −0.25* | 0.24* | 0.25* | 1.00 | ||||||||||||
| Evergreen forest % | 0.04* | 0.14* | −0.11* | 0.24* | 0.19* | 0.61* | 1.00 | |||||||||||
| Grassland % | 0.02* | 0.19* | −0.16* | 0.39* | 0.24* | 0.59* | 0.53* | 1.00 | ||||||||||
| Wetland % | 0.03* | 0.30* | −0.04* | 0.26* | 0.11* | 0.37* | 0.32* | 0.35* | 1.00 | |||||||||
| Open water % | −0.01* | 0.13* | 0.03* | 0.37* | 0.17* | 0.35* | 0.34* | 0.39* | 0.37* | 1.00 | ||||||||
| Public water supply >50% of households | 0.05 | −0.12* | −0.06* | −0.28* | −0.17* | −0.46* | −0.44* | −0.48* | −0.26* | −0.29* | 1.00 | |||||||
| Impervious surface % | 0.01 | −0.14* | 0.16* | −0.22* | −0.18* | −0.61* | −0.54* | −0.47 | −0.27* | −0.28* | 0.38* | 1.00 | ||||||
| Paved road density (m/km2) | −0.07* | −0.22* | 0.21* | −0.31* | −0.28* | −0.72* | −0.63* | −0.63* | −0.43* | −0.40* | 0.46* | 0.58* | 1.00 | |||||
|
Population
density (people/ km2) |
0.03* | −0.18* | 0.20* | −0.31* | −0.23* | −0.64* | −0.57* | −0.60* | −0.32* | −0.35* | 0.42* | 0.67* | 0.67* | 1.00 | ||||
| Median building value | 0.01 | 0.00 | −0.01 | −0.05* | −0.06* | 0.08* | 0.07* | −0.01 | 0.03* | 0.03* | −0.05* | −0.06* | −0.11* | 0.01 | 1.00 | |||
| Median age | 0.00 | 0.00 | −0.08* | 0.06* | 0.06* | 0.21* | 0.21* | 0.15* | 0.05* | 0.10* | −0.12* | −0.37* | −0.19* | −0.44* | 0.09* | 1.00 | ||
| Female % | 0.03* | 0.01 | −0.03* | −0.04* | −0.02* | −0.07* | −0.06* | −0.05* | −0.04* | −0.04* | 0.05* | 0.08* | 0.07* | 0.08* | −0.03* | 0.05* | 1.00 | |
| White race % | −0.03* | 0.03* | −0.02 | 0.05* | −0.01 | 0.19* | 0.17* | 0.14* | 0.05* | 0.08* | −0.14* | −0.32* | −0.18* | −0.31* | 0.40* | 0.36* | −0.04* | 1.00 |
Correlations with an absolute value of Spearman correlation coefficient >0.4 have been bolded
denotes statistically significant correlations at p<0.05
Footnotes
DISCLAIMER
The views expressed in this manuscript are those of the individual authors and do not necessarily reflect the views and policies of the U.S. Environmental Protection Agency or the National Institutes of Health. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
References
- Adjemian J, Olivier KN, Seitz AE, Falkinham JO 3rd, Holland SM, Prevots DR. 2012a. Spatial clusters of nontuberculous mycobacterial lung disease in the United States. American Journal of Respiratory and Critical Care Medicine 186:553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots RD. 2012b. Prevalence of nontuberculous mycobacterial lung disease in US medicare beneficiaries. American Journal of Respiratory and Critical Care Medicine 185:881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akoglu H. 2018. User’s guide to correlation coefficients. Turk J Emerg Med 18:91–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergey DH, Goodfellow M, Kämpfer P, Busse H-Jr. 2012. Bergey’s manual of systematic bacteriology. Vol. 5, the actinobacteria. 2nd ed. New York:Springer. [Google Scholar]
- Brennan PJ, Nikaido H. 1995. The envelope of mycobacteria. Annual Review of Biochemistry 64:29–63. [DOI] [PubMed] [Google Scholar]
- Brooks RW, Parker BC, Gruft H, Falkinham JO III. 1984. Epidemiology of infection by nontuberculous mycobacteria: V. Numbers in eastern United States soils and correlation with soil characteristics. American Review of Respiratory Disease 130:630–633. [DOI] [PubMed] [Google Scholar]
- Catanzaro A. 2002. Diagnosis, differentiating colonization, infection, and disease. Clinics in Chest Medicine 23:599–601. [DOI] [PubMed] [Google Scholar]
- Chan ED, Iseman MD. Underlying host risk factors for nontuberculous mycobacterial lung disease. In: Proceedings of the Seminars in Respiratory and Critical Care Medicine 2013, Vol. 34 Thieme Medical Publishers, 110–123. [DOI] [PubMed] [Google Scholar]
- De Groote MA, Pace NR, Fulton K, Falkinham JO. 2006. Relationships between mycobacterium isolates from patients with pulmonary mycobacterial infection and potting soils. Applied and Environmental Microbiology 72:7602–7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirac MA, Horan KL, Doody DR, Meschke JS, Park DR, Jackson LA, et al. 2012. Environment or host? A case–control study of risk factors for mycobacterium avium complex lung disease. American Journal of Respiratory and Critical Care Medicine 186:684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue MJ, Wymer L. 2016. Increasing prevalence rate of nontuberculous mycobacteria infections in five states, 2008–2013. Ann Am Thorac Soc 13:2143–2150. [DOI] [PubMed] [Google Scholar]
- Dormann CF, McPherson JM, Araújo MB, Bivand R, Bolliger J, Carl G, et al. 2007. Methods to account for spatial autocorrelation in the analysis of species distributional data: A review. Ecography 30:609–628. [Google Scholar]
- du Moulin GC, Sherman IH, Hoaglin DC, Stottmeier KD. 1985. Mycobacterium avium complex, an emerging pathogen in massachusetts. Journal of Clinical Microbiology 22:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESRI. 2011. Arcgis desktop. Part Release 10.0 Redlands, CA:Environmental Systems Research Institute. [Google Scholar]
- Falkinham JO III, Norton CD, LeChevallier MW. 2001. Factors influencing numbers of mycobacterium avium, mycobacterium intracellulare, and other mycobacteria in drinking water distribution systems. Applied and Environmental Microbiology 67:1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkinham JO III. 2003. Mycobacterial aerosols and respiratory disease. Emerging infectious diseases 9:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkinham JO III. 2011. Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerg Infect Dis 17:419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkinham JO III. Ecology of nontuberculous mycobacteria—where do human infections come from? In: Proceedings of the Seminars in Respiratory and Critical Care Medicine, 2013, Vol. 34 Thieme Medical Publishers, 095–102. [DOI] [PubMed] [Google Scholar]
- Falkinham JO. 2015. Environmental sources of nontuberculous mycobacteria. Clinics in Chest Medicine 36:35–41. [DOI] [PubMed] [Google Scholar]
- Falkinham JO. 2018. Challenges of ntm drug development. Fronteirs in Microbiology 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K, Ito Y, Hirai T, Maekawa K, Imai S, Tatsumi S, et al. 2013. Genetic relatedness of mycobacterium avium‐intracellulare complex isolates from patients with pulmonary mac disease and their residential soils. Clinical Microbiology and Infection 19:537–541. [DOI] [PubMed] [Google Scholar]
- Gebert MJ, Delgado-Baquerizo M, Oliverio AM, Webster TM, Nichols LM, Honda JR, et al. 2018. Ecological analyses of mycobacteria in showerhead biofilms and their relevance to human health. mBio 9:e01614–01618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good RC, Snider DE. 1982. Isolation of nontuberculous mycobacteria in the United States, 1980. The Journal of Infectious Diseases 146:829–833. [DOI] [PubMed] [Google Scholar]
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. 2007. An official ats/idsa statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. American Journal of Respiratory and Critical Care Medicine 175:367–416. [DOI] [PubMed] [Google Scholar]
- Gruft H, Loder A, Osterhout M, Parker BC, Falkinham JO III. 1979. Postulated sources of mycobacterium intracellulaire and mycobacterium scrofulaceum infection: Isolation of mycobacteria from estuaries and ocean waters. American Review of Respiratory Disease 120:1385–1388. [DOI] [PubMed] [Google Scholar]
- Hall-Stoodley L, Lappin-Scott H. 1998. Biofilm formation by the rapidly growing mycobacterial species mycobacterium fortuitum. FEMS Microbiology Letters 168:77–84. [DOI] [PubMed] [Google Scholar]
- Hilborn ED, Covert TC, Yakrus MA, Harris SI, Donnelly SF, Rice EW, et al. 2006. Persistence of nontuberculous mycobacteria in a drinking water system after addition of filtration treatment. Applied and Environmental Microbiology 72:5864–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilborn ED, Yakrus MA, Covert TC, Harris SI, Donnelly SF, Schmitt MT, et al. 2008. Molecular comparison of mycobacterium avium isolates from clinical and environmental sources. Applied and Environmental Microbiology 74:4966–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Yu X, Zhao D, Li R, Liu Y, Ge M, et al. 2017. Isolation of nontuberculous mycobacteria from soil using middlebrook 7h10 agar with increased malachite green concentration. AMB Express 7:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iivanainen E, Martikainen P, Väänänen P, Katila M-L. 1993. Environmental factors affecting the occurrence of mycobacteria in brook waters. Applied and Environmental Microbiology 59:398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iivanainen E. 1995. Isolation of mycobacteria from acidic forest soil samples: Comparison of culture methods. Journal of Applied Bacteriology 78:663–668. [DOI] [PubMed] [Google Scholar]
- Iivanainen EK, Martikainen PJ, Räisänen ML, Katila M-L. 1997. Mycobacteria in boreal coniferous forest soils. FEMS Microbiology Ecology 23:325–332. [Google Scholar]
- Jarlier V, Nikaido H. 1994. Mycobacterial cell wall: Structure and role in natural resistance to antibiotics. FEMS Microbiology Letters 123:11–18. [DOI] [PubMed] [Google Scholar]
- Johnson MM, Odell JA. 2014. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis 6:210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaevska M, Lvoncik S, Slana I, Kulich P, Kralik P. 2014. Microscopy, culture, and quantitative real-time pcr examination confirm internalization of mycobacteria in plants. Applied and Environmental Microbiology 80:3888–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner RA Jr., Parker BC, Falkinham JO III. 1992. Epidemiology of infection by nontuberculous mycobacteria. Mycobacterium avium, mycobacterium intracellulare, and mycobacterium scrofulaceum in acid, brown-water swamps of the southeastern United States and their association with environmental variables. American Review of Respiratory Disease 145:271–275. [DOI] [PubMed] [Google Scholar]
- Kirschner RA Jr., Parker BC, Falkinham JO III. 1999. Humic and fulvic acids stimulate the growth of mycobacterium avium. FEMS Microbiology Ecology 30:327–332. [DOI] [PubMed] [Google Scholar]
- Maekawa K, Ito Y, Hirai T, Kubo T, Imai S, Tatsumi S, et al. 2011. Environmental risk factors for pulmonary mycobacterium avium-intracellulare complex disease. Chest 140:723–729. [DOI] [PubMed] [Google Scholar]
- Martin EC, Parker BC, Falkinham JO 3rd. 1987. Epidemiology of infection by nontuberculous mycobacteria. Vii. Absence of mycobacteria in southeastern groundwaters. American Review of Respiratory Disease 136:344–348. [DOI] [PubMed] [Google Scholar]
- McCarthy KF. 1976. The household life cycle and housing choices. Papers in Regional Science 37:55–80. [Google Scholar]
- Meissner G, Anz W. 1977. Sources of mycobacterium avium complex infection resulting in human diseases. 116:1057–1064. [DOI] [PubMed] [Google Scholar]
- Natural Resources Conservation Service. Hydric soils-introduction. Available: https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/use/hydric/?cid=nrcs142p2_053961 [accessed December 8, 2020.
- NAVTEQ. 2010. Navteq navstreets 2010 quarter2. Chicago, IL. [Google Scholar]
- Neumann M, Schulze-Robbecke R, Hagenau C, Behringer K. 1997. Comparison of methods for isolation of mycobacteria from water. Applied and Environmental Microbiology 63:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton CD, LeChevallier MW, Falkinham JO III. 2004. Survival of mycobacterium avium in a model distribution system. Water Research 38:1457–1466. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Geiter LJ, Snider DE Jr. 1987. The epidemiology of nontuberculous mycobacterial diseases in the United States: Results from a national survey. American Review of Respiratory Disease 135:1007–1014. [DOI] [PubMed] [Google Scholar]
- Olivier KN, Weber DJ, Wallace J, Richard J, Faiz AR, Lee J-H, Zhang Y, et al. 2003. Nontuberculous mycobacteria i: Multicenter prevalence study in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 167:828–834. [DOI] [PubMed] [Google Scholar]
- Parker BC, Ford MA, Gruft H, Falkinham JO III. 1983. Epidemiology of infection by nontuberculous mycobacteria: Iv. Preferential aerosolization of mycobacterium intracellulare from natural waters. American Review of Respiratory Disease 128:652–656. [DOI] [PubMed] [Google Scholar]
- Portaels F, Pattyn SR. 1982. Growth of mycobacteria in relation to the ph of the medium. Ann Microbiol (Paris) 133:213–221. [PubMed] [Google Scholar]
- Poulain S, Bourouiba L. 2018. Biosurfactants change the thinning of contaminated bubbles at bacteria-laden water interfaces. Physical review letters 121:204502. [DOI] [PubMed] [Google Scholar]
- Prevots DR, Marras TK. 2015. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: A review. Clinics in Chest Medicine 36:13–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed C, Von Reyn CF, Chamblee S, Ellerbrock TV, Johnson JW, Marsh BJ, et al. 2006. Environmental risk factors for infection with mycobacterium avium complex. American Journal of Epidemiology 164:32–40. [DOI] [PubMed] [Google Scholar]
- Rossiter K. 2011. What are census blocks? Available: https://www.census.gov/newsroom/blogs/random-samplings/2011/07/what-are-census-blocks.html July 2019].
- SAS. 2015. The gampl procedure. In: Sas/stat 141 user’s guide. Cary, NC, USA:SAS Institute Inc. [Google Scholar]
- Schulze-Röbbecke R, Feldmann C, Fischeder R, Janning B, Exner M, Wahl G. 1995. Dental units: An environmental study of sources of potentially pathogenic mycobacteria. Tubercle and Lung Disease 76:318–323. [DOI] [PubMed] [Google Scholar]
- Smith GS, Ghio AJ, Stout JE, Messier KP, Hudgens EE, Murphy MS, et al. 2016. Epidemiology of nontuberculous mycobacteria isolations among central north carolina residents, 2006–2010. Journal of Infection 72:678–686. [DOI] [PubMed] [Google Scholar]
- Soil Survey Staff. Soil survey geographic (ssurgo) database. Available: https://sdmdataaccess.sc.egov.usda.gov [accessed March 11 2011].
- Steed KA, Falkinham JO. 2006. Effect of growth in biofilms on chlorine susceptibility of mycobacterium avium and mycobacterium intracellulare. Applied and Environmental Microbiology 72:4007–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski EP, Leung JM, Fowler CJ, Haney C, Hsu AP, Chen F, et al. 2015. Pulmonary nontuberculous mycobacterial infection. A multisystem, multigenic disease. Am J Respir Crit Care Med 192:618–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E, Kimoto T, Matsumoto H, Tsuyuguchi K, Suzuki K, Nagai S, et al. 2000. Familial pulmonary mycobacterium avium complex disease. American Journal of Respiratory and Critical Care Medicine 161:1643–1647. [DOI] [PubMed] [Google Scholar]
- Thomson R, Carter R, Gilpin C, Coulter C, Hargreaves M. 2008. Comparison of methods for processing drinking water samples for the isolation of mycobacterium avium and mycobacterium intracellulare. Appl Environ Microbiol 74:3094–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. 2010a. Sex by age Available: https://data.census.gov/cedsci/table?q=p12&hidePreview=false&tid=DECENNIALSF12010.P12&vintage=2010 July 7, 2020]. [Google Scholar]
- U.S. Census Bureau. 2010b. Median age by sex.. Available: https://data.census.gov/cedsci/map?q=p13&hidePreview=false&tid=DECENNIALSF12010.P13&vintage=2010&layer=VT_2010_040_00_PP_D1&cid=P013001 July 7, 2020].
- U.S. Census Bureau. 2010c. Hispanic or latino, and not hispanic or latino by race. Available: https://data.census.gov/cedsci/table?q=p12&hidePreview=false&tid=DECENNIALSF12010.P9&vintage=2010 [accessed July 7, 2020.
- U.S. Fish and Wildlife Service. 2013. National wetlands inventory database. 20131224. Washington, D.C. [Google Scholar]
- U.S. Geological Survey. 2011. Nlcd 2011 land cover (2011 edition). 20140331. Sioux Falls, SD. [Google Scholar]
- U.S. Geological Survey. Application of the National-Scale Soil Geochemical and Mineralogical Data for the Conterminous U.S. Available: https://www.usgs.gov/energy-and-minerals/mineral-resources-program/science/application-national-scale-soil-geochemical?qt-science_center_objects=0#qt-science_center_objects [accessed December 10, 2020]
- Von Reyn CF, Marlow J, Arbeit R, Barber T, Falkinham J. 1994. Persistent colonisation of potable water as a source of mycobacterium avium infection in aids. The Lancet Respiratory Medicine 343:1137–1141. [DOI] [PubMed] [Google Scholar]
- Walsh CM, Gebert MJ, Delgado-Baquerizo M, Maestre FT, Fierer N. 2019. A global survey of mycobacterial diversity in soil. Applied and Environmental Microbiology 85:e01180–01119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassilew N, Hoffmann H, Andrejak C, Lange C. 2016. Pulmonary disease caused by non-tuberculous mycobacteria. Respiration 91:386–402. [DOI] [PubMed] [Google Scholar]
- Webster T, Vieira V, Weinberg J, Aschengrau A. 2006. Method for mapping population-based case-control studies: An application using generalized additive models. International Journal of Health Geographics 5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MM, Yakrus MA, Arduino MJ, Cooksey RC, Crane CB, Banerjee SN, et al. 2009. Structural analysis of biofilm formation by rapidly and slowly growing nontuberculous mycobacteria. Applied and Environmental Microbiology 75:2091–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winthrop KL, Varley CD, Ory J, Cassidy PM, Hedberg K. 2011. Pulmonary disease associated with nontuberculous mycobacteria, oregon, USA. Emerg Infect Dis 17:1760. [DOI] [PMC free article] [PubMed] [Google Scholar]