Abstract
A series of novel compounds with two halogen substituents have been designed and synthesized to further optimize the 2-phenylcyclopropylmethylamine scaffold in the quest for drug-like 5-HT2C agonists. Compound (+)-22a was identified as a potent 5-HT2C receptor agonist, with good selectivity against the 5-HT2B and the 5-HT2A receptors. ADMET assays showed that compound (+)-22a possessed desirable properties in terms of its microsomal stability, and CYP and hERG inhibition, along with an excellent brain penetration profile. Evaluation of (+)-22a in animal models of schizophrenia-related behaviors revealed that it had a desirable activity profile, as it reduced locomotor activity in d-amphetamine-stimulated hyperlocomotion in the open field test, it restored d-amphetamine-disrupted prepulse inhibition, it induced cognitive improvements in NR1-KD animals in the novel object recognition memory test, and it produced very little catalepsy relative to haloperidol. These data support the further development of (+)-22a as drug candidate for the treatment of schizophrenia.
Graphical Abstract
Introduction
Schizophrenia is a severe mental disease that affects 1% of the population in the US and more than 21 million people worldwide.1 It is a disease of complex etiology with likely more than 100 genetic loci and unspecified non-genetic contributing factors.2, 3 At least three types of symptoms exemplify schizophrenia: positive, negative and cognitive. The available antipsychotic drugs are effective against positive symptoms while having minimal effects at cognitive and negative symptoms.4, 5
The 5-HT2C serotonin receptor has recently been identified as a promising drug target for the treatment of a variety of central nervous system (CNS) disorders, including obesity, schizophrenia, and substance abuse.6, 7 Lorcaserin (1, Fig. 1) and vabicaserin (2) are two representative 5-HT2C agonists that have been developed. Lorcaserin has been approved as an anti-obesity drug, vabicaserin was studied in clinical trials for the treatment of acute schizophrenia.8, 9 However, development of vabicaserin was discontinued for unknown reasons. For many years it has been appreciated that 5-HT2C receptor function might be altered in schizophrenia and that the 5-HT2C antagonist activity of certain atypical antipsychotic drugs might be associated with weight gain and adverse metabolic consequences.10–13 The 5-HT2C receptor is a promising target for treating schizophrenia and related disorders because: (1) activation of 5-HT2C receptors specifically decreases mesolimbic dopamine release without affecting nigrostriatal dopamine,14 thus it is predicted to have antipsychotic efficacy while causing few extrapyramidal side effects (EPS); and (2) 5-HT2C agonists are known to induce weight loss, and thus such drugs should lack the undesired side effects of weight gain and associated metabolic disorders, which have been associated with most current antipsychotic drugs.13, 15 Additionally, activation of the 5-HT2C receptor has been demonstrated to counter cognitive deficits induced by NMDA antagonism,16 as well as to overcome cognitive deficits in animals bearing the human tryptophan hydroxylase 2 loss of function mutation.17 Therefore, developing safe 5-HT2C agonists for the treatment of schizophrenia could potentially address the cognitive deficits associated with this disease with fewer EPS and a lower risk of causing weight gain.
Figure 1.
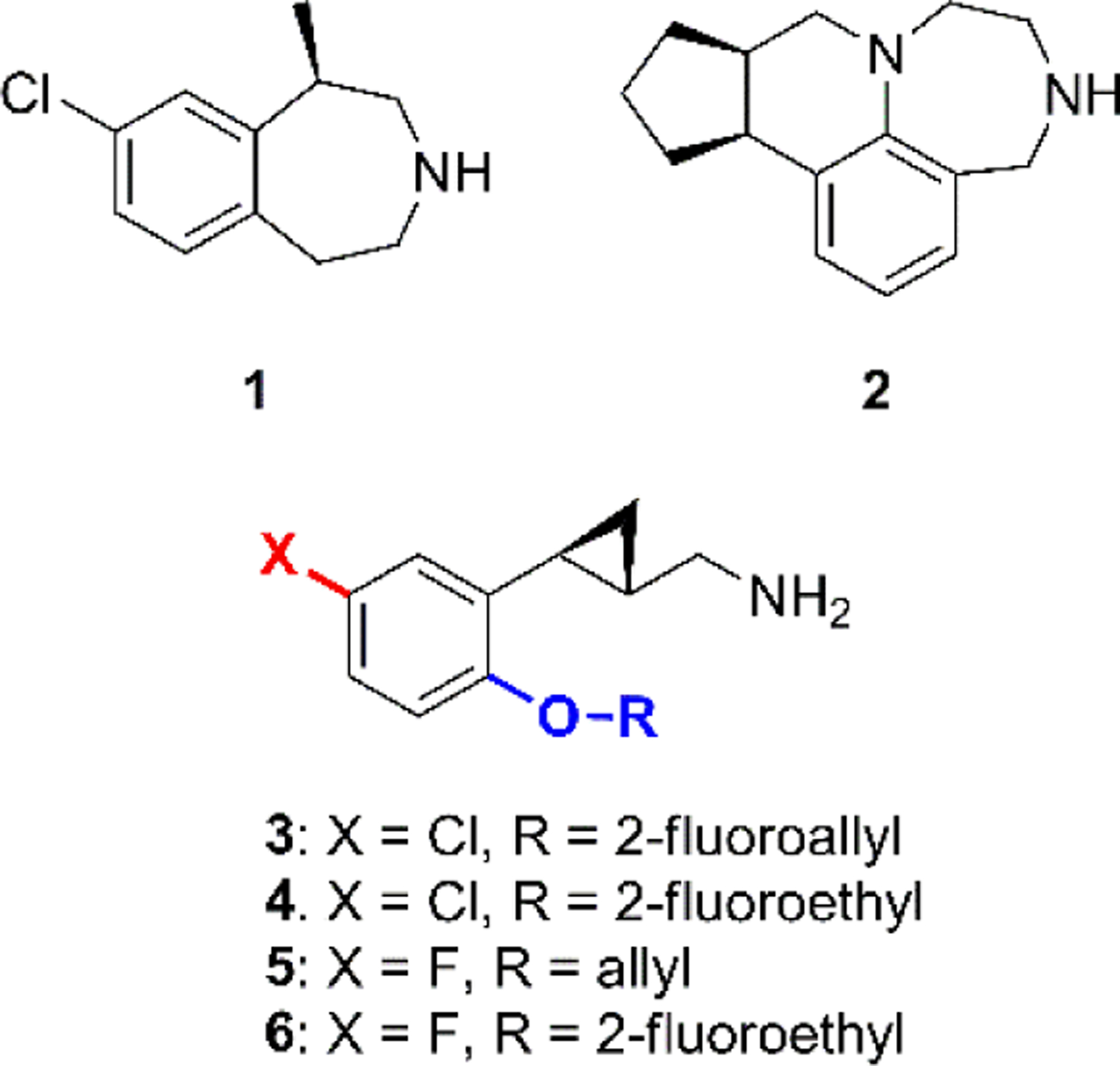
Structures of lorcaserin (1), vabicaserin (2) and compounds 3–6.
The greatest challenge for developing 5-HT2C receptor agonists is their selectivity against the other two related receptors of the 5-HT2 subfamily, namely 5-HT2A and 5-HT2B. Activation of 5-HT2A receptors has been reported to be associated with hallucinogenic effects,18 while activation of 5-HT2B receptors, which are mainly expressed in the periphery, produces valvulopathy and pulmonary hypertension as has been observed with the use of dexfenfluramine.7, 19 The FDA-approved drug lorcaserin was reported to have 100-fold selectivity for 5-HT2C relative to the 5-HT2B subtype, however it possesses full agonist activity at 5-HT2B with moderate potency (EC50 = 943 ± 90 nM, Emax = 100%),20 and lorcaserin was reported to cause a higher incidence of cardiac valve disorders in clinical trials compared to the placebo group.21 We previously reported on a series of novel compounds that have excellent selectivity for 5-HT2C receptors which are comprised of a 2-phenylcyclopropylmethylamine scaffold.22–25 Among the reported compounds, analogs 3–6 (Fig. 1) were identified to possess moderate to excellent pharmacological profiles, and they are now undergoing further evaluation in preclinical models.22
Due to the relatively small structural scaffold of the 2-phenylcyclopropylmethylamines and the very strict steric limitations applying to these compounds as revealed by previous structure-activity relationships (SARs) studies, adding a second halogen atom to the benzene ring appeared to offer a practical approach to further enhancing ligand potency and possibly metabolic stability. Decoration of the aromatic ring of a drug candidate with halogen atoms, especially fluorine and chlorine, has been used extensively in medicinal chemistry.26 The role of halogen substituents in enhancing ligand potency as well as in optimizing pharmacokinetic properties is well known. The incorporation of multi-halogen atoms is exemplified by drugs not only for peripheral diseases such as sitagliptin (7)27 and crizotinib (8)28, but also for CNS drugs such as aripiprazole (9)29 (Fig. 2). Moreover, the role of halogen binding in protein–ligand interactions has been studied recently,30 and halogen bonds have been proposed to provide orthogonal molecular interactions to hydrogen bonds.31 The lipophilicity of halogen atoms, which enhances the LogP values of small molecules, might also help to improve the blood-brain barrier (BBB) permeability of compounds designed for CNS indications.
Figure 2.
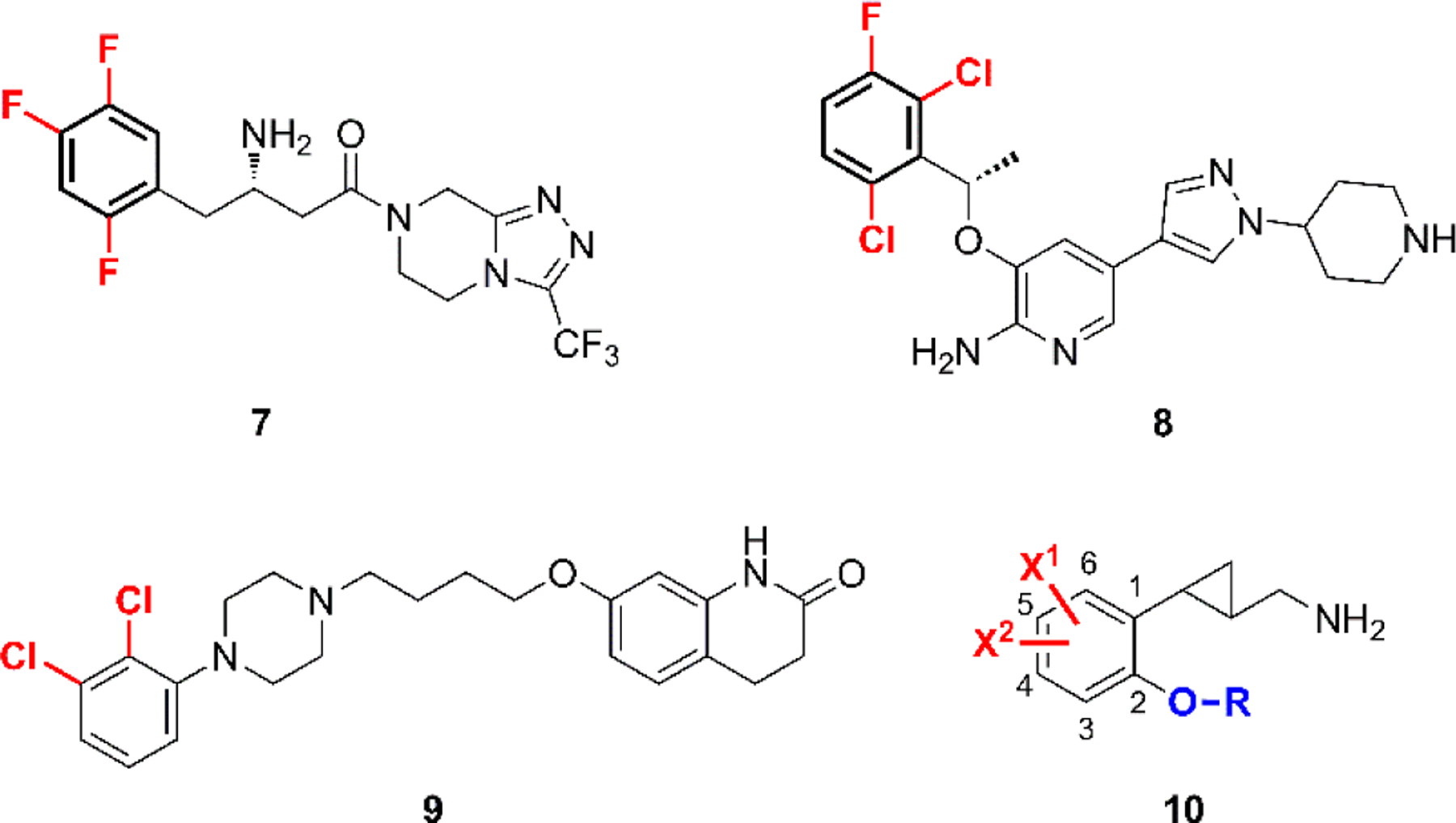
Representative drugs having a multi-halogen substitution pattern and the general structure of new 2-phenylcyclopropylmethylamines.
Encouraged by the promising results that had been achieved for the mono halogenated compounds,22 and the possible beneficial effect of a second halogen, we synthesized a new series of compounds containing two halogen substituents on the benzene ring, as depicted by the general structure 10 (Fig. 2). The synthesis and SAR results are described herein, along with the ADMET evaluation and efficacy study of a key compound in four different schizophrenia-related animal behavioral models.
Chemical Synthesis and Pharmacological Profiling.
The synthesis of the di-halogenated compounds was achieved employing methods similar to those reported by us previously, starting from the appropriate benzaldehydes. Thus, various di-halogenated benzaldehydes 11a–g were purchased or prepared according to literature methods (for details, see Supporting Information). As shown in Scheme 1, the benzaldehydes 11a–g were converted to the corresponding acrylamides 12a–g, with the double bond in E configuration, using the commercially available Wittig reagent N-methoxy-N-methyl(triphenylphosphoranylidene)acetamide. This reagent was preferred over ethyl 2-(triphenylphosphoranylidene)acetate due to the facts that better or complete E selectivity was observed for the newly formed double bond, and that the subsequent cyclopropanation step proceeds more efficiently with α,β-unsaturated Weinreb amides than with esters.32, 33 The acrylamides 12a–g were then subjected to the Corey-Chaykovsky cyclopropanation reaction to generate the cyclopropanes 13a–g as their trans isomers, using the sulfur ylide generated from trimethylsulfoxonium iodide by treatment with sodium hydride. Next sequential reduction using DIBAL-H followed by sodium borohydride provided alcohols 14a–g in good yields. Mitsunobu reaction of 14a–g with phthalimide produced the phthal imides 15a–g in excellent yields. De-protection of these imides with hydrazine hydrate afforded the primary amines, which were protected as their Boc intermediates 16a–g. De-methylation of intermediates 16a–g with BBr3 in dichloromethane afforded the phenol intermediates 17a–g. In our previous SAR studies,22 the analogs containing 2-fluoroethyl and allyl ether moieties were found to provide compounds with the best pharmacological profiles. Thus, the phenols 17a–g were converted to the 2-fluoroethyl ethers 18a–g using Mitsunobu conditions while alkylation of 17a–g with allyl bromide afforded allyl ethers 19a–g. The racemic compounds 16a–g, 18a–g and 19a–g were then resolved by chiral preparative-HPLC to provide both the (–)- and (+)-enantiomers, which were then de-protected with HCl in ether to afford both enantiomers of compounds 20a–g, 21a–g and 22a–g as their HCl salts.
Scheme 1.
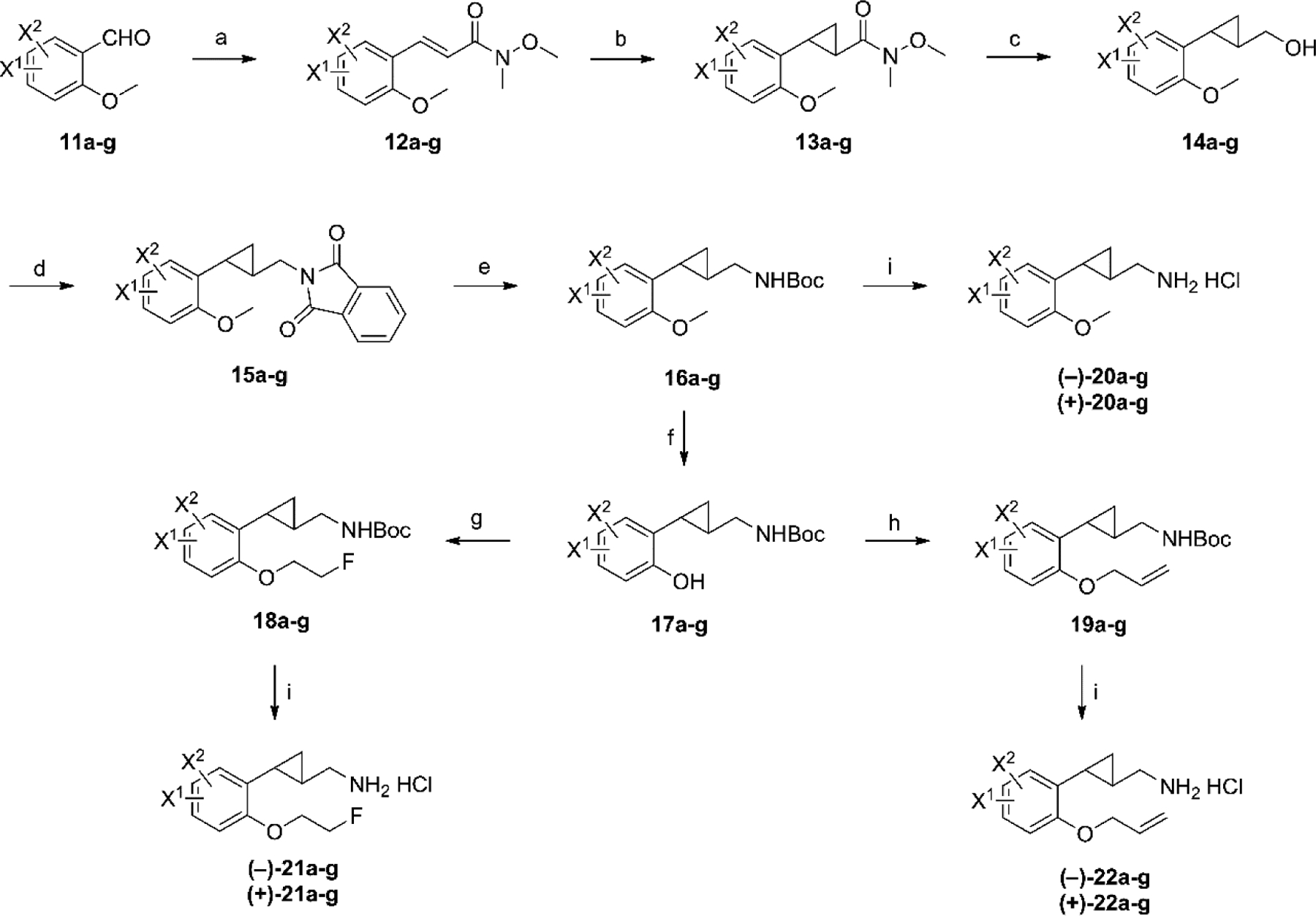
Synthesis of target compounds 20a–g, 21a–g, and 22a–g.a a Reagents and conditions: (a) Ph3P=CHC(O)N(OMe)Me, CH2Cl2, rt, overnight; (b) Me3S+(O)I−, NaH, DMSO, rt, overnight; (c) DIBAL-H, THF, −78 °C, 2 h; then NaBH4, MeOH, 0 °C to rt, 0.5 h; (d) phthalimide, PPh3, DEAD, THF, rt, overnight; (e) N2H4−H2O, EtOH, reflux, 3h; then Boc2O, Et3N, CH2Cl2, rt, 0.5 h; (f) BBr3, CH2Cl2, −78 °C to rt; then Boc2O, Et3N, CH2Cl2; (g) 2-fluoroethanol, Ph3P, DEAD, THF, 0 °C to 60 °C, 1h; (h) allyl bromide, Cs2CO3, DMF; (i) (1) chiral preparative-HPLC; (2) 2M HCl in Et2O, rt, 24 h. 11–22a: 4, 5-diF; 11–22b: 5, 6-diF; 11–22c: 4-F, 5-Cl; 11–22d: 5-Cl, 6-F; 11–22e: 4, 5-diCl; 11–22f: 5, 6-diCl; 11–22g: 5-F, 6-Cl.
All compounds were screened in functional assays at the 5-HT2C, 5-HT2B and 5-HT2A receptors. Data were acquired using recombinant, stably expressed human serotonin receptors in the HEK-293 cell line, using a fluorescence imaging plate reader (FLIPR) assay as described previously.22
As shown in Table 1, a 2-methoxy substituent provides analogs with good potency but low selectivity for 5-HT2C, while the 2-fluoroethoxy and allyloxy substituents afford compounds with sometimes reduced but in other cases still good 5-HT2C potency and improved selectivity.
Table 1.
Pharmacological Profiles of Di-halogenated Compounds at 5-HT2 Receptors.a
| Structure (HCl salt) | Compound | EC50, nM (Emax) | ||
|---|---|---|---|---|
| 5-HT2C | 5-HT2B | 5-HT2A | ||
| - | 5-HT | 0.18 (100%) | 1.0 (100%) | 1.9 (100%) |
| - | lorcaserin | 2.7 (98%) | 328 (80%) | 258 (67%) |
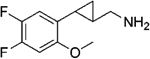 |
(–)-20a | 30 (104%) | 400 (59%) | 511 (76%) |
| (+)-20a | 3.4 (108%) | 27 (69%) | 81 (81%) | |
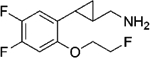 |
(–)-21a | 772 (73%) | NA | NA |
| (+)-21a | 7.3 (95%) | 453 (32%) | 361 (51%) | |
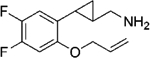 |
(–)-22a | 116 (94%) | NA | 831 (17%) |
| (+)-22a | 8.7 (97%) | 1745 (35%) | 491 (21%) | |
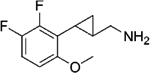 |
(–)-20b | 16 (108%) | 462 (62%) | 461 (72%) |
| (+)-20b | 9.5 (108%) | 209 (64%) | 437 (79%) | |
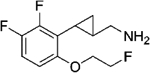 |
(–)-21b | 509 (78%) | 9871 (29%) | NA |
| (+)-21b | 25 (95%) | 2182 (36%) | 2332 (30%) | |
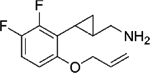 |
(–)-22b | 151 (89%) | 4464 (26%) | NA |
| (+)-22b | 36 (100%) | 3339 (35%) | NA | |
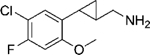 |
(–)-20c | 16 (99%) | 329 (44%) | 235 (88%) |
| (+)-20c | 7.6 (97%) | 25 (59%) | 81 (93%) | |
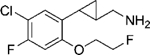 |
(–)-21c | 776 (70%) | 2521 (26%) | NA |
| (+)-21c | 24 (87%) | 506 (39%) | 654 (41%) | |
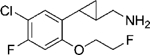 |
(–)-22c | 308 (77%) | 1957 (22%) | NA |
| (+)-22c | 42 (87%) | 1001 (44%) | NA | |
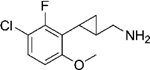 |
(–)-20d | 3.8 (93%) | 165 (64%) | 68 (86%) |
| (+)-20d | 13 (92%) | 354 (52%) | 212 (85%) | |
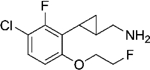 |
(–)-21d | 386 (80%) | NA | NA |
| (+)-21d | 71 (86%) | NA | 934 (40%) | |
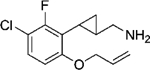 |
(–)-22d | 165 (87%) | NA | 1081 (16%) |
| (+)-22d | 116 (90%) | 6983 (69%) | NA | |
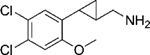
|
(–)-20e | 14 (89%) | 696 (16%) | 84 (75%) |
| (+)-20e | 9.7 (95%) | 31 (45%) | 21 (85%) | |
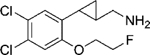 |
(–)-21e | 435 (75%) | NA | NA |
| (+)-21e | 18 (87%) | >10000 (26%) | 92 (58%) | |
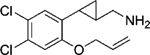 |
(–)-22e | 278 (84%) | NA | NA |
| (+)-22e | 61 (88%) | 714 (26%) | 167 (37%) | |
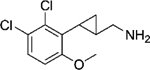 |
(–)-20f | 0.95 (104%) | 51 (62%) | 38 (88%) |
| (+)-20f | 21 (98%) | 534 (46%) | 203 (76%) | |
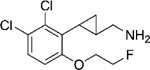 |
(–)-21f | 224 (85%) | >10,000 (62%) | NA |
| (+)-21f | 232 (90%) | 5523 (99%) | 949 (22%) | |
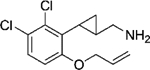 |
(–)-22f | 108 (93%) | 3962 (99%) | 426 (32%) |
| (+)-22f | 165 (70%) | NA | NA | |
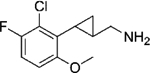 |
(–)-20g | 3.2 (96%) | 101 (91%) | 119 (72%) |
| (+)-20g | 24 (89%) | 2176 (60%) | 769 (77%) | |
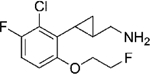 |
(–)-21g | 135 (80%) | 3486 (29%) | NA |
| (+)-21g | 49 (86%) | 961 (55%) | 798 (39%) | |
 |
(–)-22g | 104 (89%) | 5176 (57%) | NA |
| (+)-22g | 96 (92%) | 927 (55%) | NA | |
Data were acquired with recombinant, stably expressed human serotonin receptors in the HEK-293 cell line, using a fluorescence imaging plate reader (FLIPR) assay. “NA”, no activity (Emax < 15%) at 10 μM.
Compared to compounds bearing a single fluorine or chlorine group at position 5,22 those with an additional fluorine atom at position 4 or 6 exhibited slightly modified pharmacological profiles. In comparison to the 5, 6-difluorinated compounds, the 4, 5-difluoro substitution pattern led to better potency, as shown by the pairs (+)-20a vs (+)-20b, (+)-21a vs (+)-21b, and (+)-22a vs (+)-22b, wherein an ~ 3-fold difference can be observed within each pair. The 2-fluoroethyl and allyl ethers provided improved subtype selectivity. Thus, compounds (+)-21a, (+)-22a, (+)-21b, and (+)-22b showed good to excellent profiles as selective 5-HT2C agonists. Notably, compound (+)-22a represents the best of this series, exhibiting an EC50 value of 8.7 nM at the 5-HT2C receptors, with > 200-fold selectivity over 5-HT2B and > 50-fold selectivity over 5-HT2A. Given the fact that this compound displayed very weak activation of both the 5-HT2B receptors (Emax = 35%) and 5-HT2A receptors (Emax = 21%), (+)-22a is a good candidate for further studies. The superiority of compound (+)-22a over its 5-monofluoro analog relies on its very weak 5-HT2A activity, as the latter showed greater activation of the same receptor (EC50 = 374 nM, Emax = 56%).22
Similarly, the 4-fluoro-5-chloro substituted analogs provided an advantage in respect to potency compared to the 5-chloro-6-fluoro scaffold, which is illustrated by comparisons of (+)-20c vs (+)-20d, (+)-21c vs (+)-21d, and (+)-22c vs (+)-22d. Compound (+)-22c displayed an EC50 of 42 nM at 5-HT2C, no activity at 5-HT2A, and good selectivity over 5-HT2B (EC50 = 1001 nM, Emax = 44%).
The introduction of a second chlorine atom which is significantly larger than fluorine, into the 5-monochloro compound, led to a notable change in potency and selectivity. Compound (–)-20e, bearing a 4, 5-dichloro substitution pattern, showed good potency at the 5-HT2C receptor (EC50 = 14 nM), and good selectivity over 5-HT2B (EC50 = 696 nM, Emax = 16%). However, only moderate selectivity over 5-HT2A (EC50 = 84 nM) was found. Compound (+)-21e, with 2-fluroethoxy group at position 2, displayed an EC50 of 18 nM and excellent selectivity against 5-HT2B, but only moderate selectivity against 5-HT2A.
Most of the compounds above displayed the same enantiomer preference as we had described previously,22 with (+)-enantiomer showing the better potency. However, the introduction of a chlorine atom at position 6, as in the compound 20f, led to the reversal of this preference. (–)-20f showed excellent 5-HT2C potency (EC50 = 0.95 nM), 50-fold selectivity over 5-HT2B and 40-fold selectivity over 5-HT2A, while its enantiomer (+)-20f is 20-fold weaker at the 5-HT2C receptors. The introduction of slightly larger 2-alkoxy group led to a decrease in potency, as shown by compounds 21f and 22f. With the combination of a 5-fluoro and 6-chloro group, similar enantiomer preference was observed for compound 20g, with the (–)-enantiomer showing higher potency (EC50 = 3.2 nM). Although (+)-20g is 7.5-fold weaker (EC50 = 24 nM) compared to (–)-20g, it showed good selectivity against 5-HT2B (EC50 = 2176 nM, 91-fold) and 5-HT2A (EC50 = 769 nM, 32-fold). Compounds (–)-21g and (–)-22g showed only mid-nanomolar potency at 5-HT2C but good selectivity against both 5-HT2A and 5-HT2B receptors. In earlier work, based on the support of good internal consistency of our potency data, we tacitly assumed that enantiomers with the same sign of optical rotation as their parents devoid of substituents on the benzene ring had the same absolute configuration as those parents.25 An independent determination of the any of the new compounds’ absolute configuration has not yet been carried out, but the observed switch of the eutomer from the (+)- to the (–)-isomer in the two compounds mentioned above may result from the sign of optical rotation for a given absolute configuration having changed in response to the change in the substitution pattern, as from the eutomer possessing the opposite absolute configuration in those cases.
The SAR results of the above di-halogenated ligands further demonstrate the strict steric limitations applying to these cyclopropylmethylamine-based 5-HT2C ligands. (+)-22a is the best compound in this series with respect to its 5-HT2C potency and selectivity against the 5-HT2B (> 200-fold) and 5-HT2A receptors (> 50-fold). The low Emax values of this compound at the other two receptors make it unlikely to cause side-effects associated with their activation of those receptors. We thus selected (+)-22a for further studies of its ADMET properties and in vivo efficacy in animal behavioral models.
ADMET Study of Compound (+)-22a
The liver microsomal stability of (+)-22a using both human and mouse microsomes was assayed, and the data are shown in Table 2. Better stability was observed in the mouse liver microsomes in the absence of NADPH, indicating that oxidative mechanisms are likely involved in the metabolism of this compound. In the presence of NADPH, only 32.4% of (+)-22a remained after 2 h. In contrast, the compound’s stability in the presence of human liver microsomes is much better, with 70.5% of the parent compound remaining under the same conditions. Thus, good metabolic stability is anticipated in humans.
Table 2.
Liver Microsomal Stability of Compound (+)-22a.a
| Assay Format | Remaining Percentage (%) | |||||
|---|---|---|---|---|---|---|
| HLM | MLM | |||||
| 0 min | 30 min | 120 min | 0 min | 30 min | 120 min | |
| + NADPH | 100.0 | 91.9 | 70.5 | 100.0 | 68.1 | 32.4 |
| − NADPH | 100.0 | 94.7 | 81.9 | 100.0 | 97.8 | 79.6 |
Concentration of liver microsomes was 0.5 mg/mL and (+)-22a was tested at a concentration of 2 μM; HLM = human liver microsomes; MLM = mouse liver microsomes.
A frequently confronted challenge in developing CNS targeted drugs is insufficient blood-brain barrier (BBB) penetration. Thus compound (+)-22a was tested in mice to verify its CNS exposure. The compound was administered intraperitoneally (ip) to CD1 mice at a dose of 10 mg/kg, and both brain and plasma concentrations were assayed at the 0.5 and 2 h time-points (Table 3). Excellent brain penetration was observed for compound (+)-22a, with brain/plasma concentration ratios of 29.9 and 45.0 at 0.5 and 2.0 h, respectively. The high brain penetrance of this compound should thus maximize its exposure in the CNS allowing for robust activation of central 5-HT2C receptors, while at the same time minimizing possible side effects due to compound interactions with peripheral systems.
Table 3.
Brain Penetration Study of Compound (+)-22a.
| Route | Time (h) | Animal ID | Brain (ng/g) | Plasma (ng/mL) | Ratio (Brain Plasma/)a | Mean |
|---|---|---|---|---|---|---|
|
ip 10 mg/kg |
0.5 | 1 | 10120 | 321 | 31.5 | 29.9 |
| 2 | 8480 | 300 | 28.3 | |||
| 2.0 | 1 | 3948 | 96.4 | 41.0 | 45.0 | |
| 2 | 2228 | 45.4 | 49.1 |
Density of brain tissue is calculated as 1.0 g/mL.
The inhibition of the cytochrome P450 enzymes by (+)-22a was tested at five different isoforms, and the data are shown in Table 4. Although a high inhibition of CYP 1A2 (86%) was observed with (+)-22a when it was tested at a concentration of 10 μM, low inhibition was observed for the other, more abundant isoforms, especially CYP 3A4 (3.4% inhibition at 10 μM). Furthermore, inhibition of the human Ether-à-go-go related gene (hERG) channel was also tested, and (+)-22a displayed a dose-dependent inhibition of hERG, with an IC50 value of 9.1 μM. This is not unexpected as the structure of (+)-22a falls into the common templates which are known to cause hERG inhibition.34 However, the high brain concentrations found for this compound should minimize the possible cardiac toxicity associated with hERG inhibition.
Table 4.
Cytochrome P450 Inhibition by Compound (+)-22a.a
| CYP Isoform (Substrate) | Inhibition (%) ± SEM |
|---|---|
| 1A2 (Phenacetin) | 86.0 ± 0.25 |
| 2C9 (Tolbutamide) | 29.2 ± 2.2 |
| 2C19 (Mephenytoin) | 32.0 ± 5.3 |
| 2D6 (Dextromethorphan) | 43.5 ± 3.0 |
| 3A4 (Midazolam) | 3.4 ± 1.6 |
Compound (+)-20a was tested at 10 μM; concentration of human liver microsomes was 0.2 mg/mL; concentration of phenacetin, tolbutamide, mephenytoin, dextromethorphan, and midazolam was 40, 200, 50, 10, and 5 μM respectively.
Efficacy Study of Compound (+)-22a in Animal Models of Schizophrenia-like Behaviors
To explore the possible use of (+)-22a in the treatment of schizophrenia, this compound was tested in four different animal behavioral paradigms. The open field test was used to evaluate the ability of the compound to decrease hyperlocomotion using d-amphetamine (AMPH) as the stimulant, whereas the AMPH-disrupted prepulse inhibition (PPI) test was used to analyze possible compound effects on abnormalities in sensorimotor gating.35, 36 The well-known novel object recognition memory (NORM) test using transgenic N-methyl-D-aspartate (NMDA) receptor NR1-knockdown (KD) mice was conducted to assess the ability of the compound to improve cognition.37 Lastly, the ability of (+)-22a to induce catalepsy was evaluated and compared to haloperidol. These data are detailed below.
Open Field Activity.
Baseline activity of the mice was tested for 15 min before the administration of AMPH. During this period, only those mice from the 30 mg/kg (+)-22a + AMPH group showed a small, albeit significant (p < 0.040) reduction in locomotion compared to the Veh + AMPH group; all other groups had similar levels of baseline activities (Fig. 3A–B). Administration of AMPH induced a significant increase in locomotor activity, as shown by the comparison of the Veh + AMPH group with the Veh + Veh group (Fig. 3A, C). Animals given (+)-22a showed a dose-dependent suppression of AMPH-stimulated hyperlocomotion; the 2 mg/kg dose showed no significant efficacy while 5, 10, 20, and 30 mg/kg significantly reduced the hyperactivity compared to the Veh + AMPH group. Notably, locomotor activities of mice treated with 20 or 30 mg/kg (+)-22a + AMPH did not significantly differ from animals in the 20 mg/kg (+)-22a + Veh or the Veh + Veh control groups which had similar activity levels. Thus a dose of 20 or 30 mg/kg of (+)-22a reduced the animal activity to normal levels.
Figure 3.
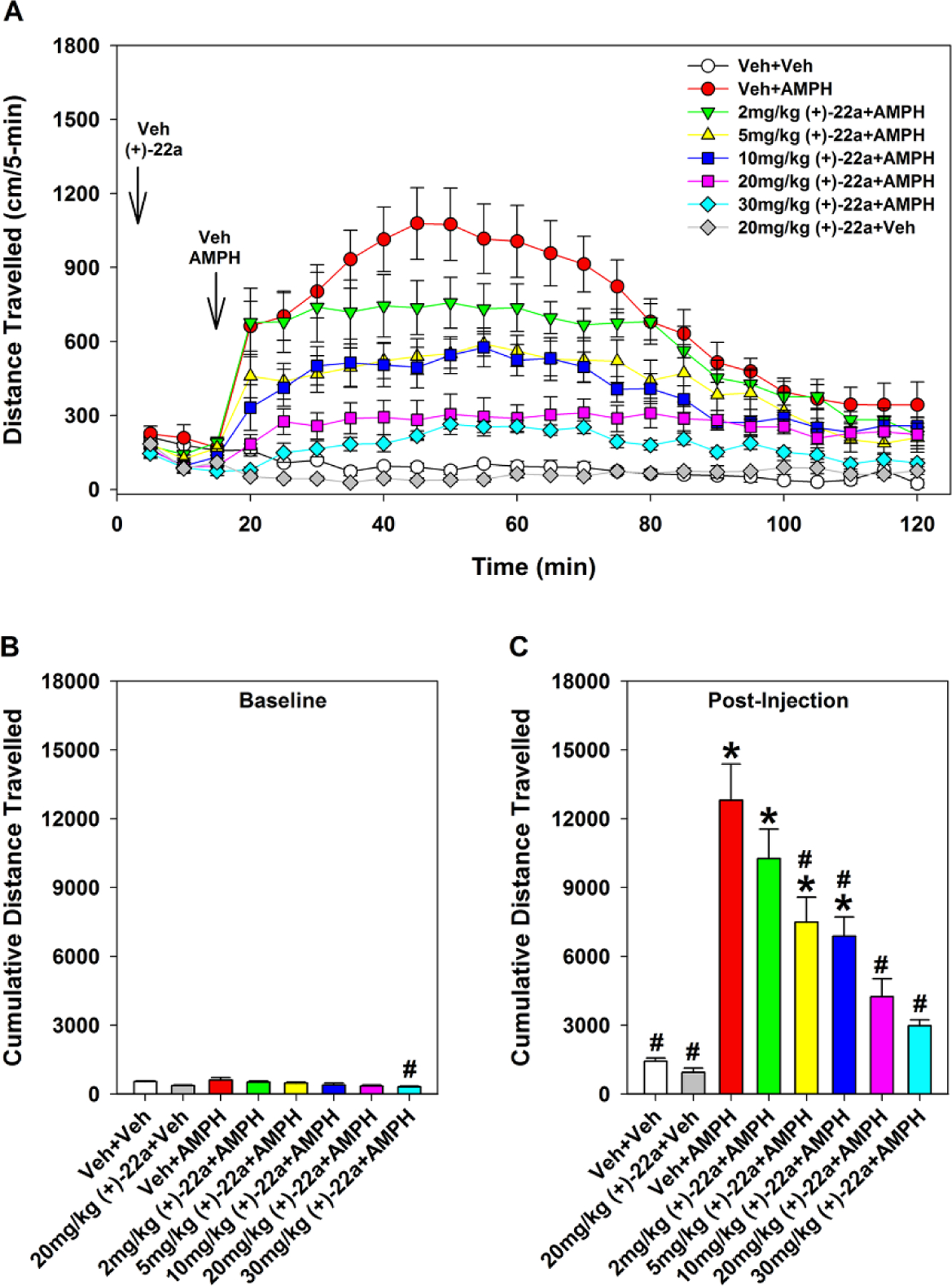
Effects of compound (+)-22a on d-amphetamine (AMPH)-stimulated hyperlocomotion in the open field. (A) Locomotor activity in the open field in 5-min blocks reflecting baseline responses (0–15 min) and AMPH stimulated activity with its reduction by (+)-22a (15–120 min). (B) Cumulative baseline locomotor activities (0–15 min). (C) Cumulative AMPH-stimulated activities and reductions in activity with (+)-22a (16–90 min). N = 7–10 mice/treatment; *, p < 0.05, compared to the Veh + Veh group; #, p < 0.05, compared to the Veh + AMPH group.
Prepulse Inhibition (PPI).
The Veh + AMPH group showed a marked suppression of PPI compared to Veh + Veh controls (p < 0.007) and a loss of prepulse dependency at the 8 and 12 dB prepulse intensities (Fig. 4A). The 0.5 (p < 0.01) and 1 mg/kg (p < 0.05) doses of (+)-22a restored this AMPH-disrupted PPI. Mice treated with the 1 mg/kg dose + AMPH showed full prepulse dependency of the PPI response, where the percentages of the PPI at 4, 8, and 12 dB were significantly distinguished from each other (p < 0.010). Although 2 mg/kg (+)-22a increased AMPH-disrupted PPI, this was not significantly different from the Veh + AMPH group. Mice in the 4 mg/kg (+)-22a + Veh group showed similarly high levels of PPI with the Veh + Veh group. However, the 4 mg/kg dose failed to increase AMPH-disrupted PPI.
Figure 4.
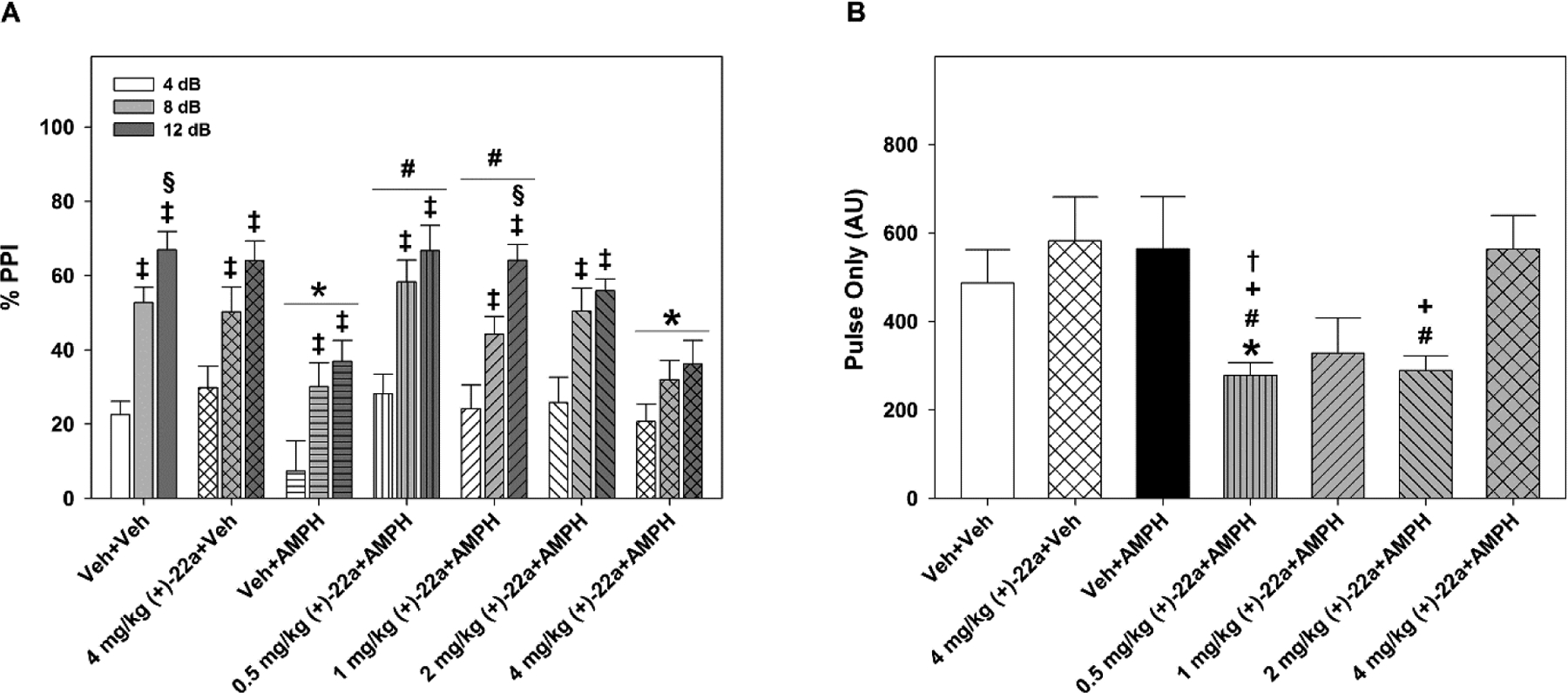
Compound (+)-22a restores d-amphetamine (AMPH)-disrupted PPI in C57BL/6 mice. (A) Mice treated with vehicle-vehicle (Veh + Veh) showed prepulse dependency in the PPI response. Four mg/kg (+)-22a alone did not affect PPI relative to the Veh + Veh controls. Mice administered Veh + AMPH had a marked reduction in PPI. Treatment with 0.5, 1, or 2 mg/kg (+)-22a restored overall AMPH-disrupted PPI to the levels of the Veh + Veh group; with 1 mg/kg fully rescuing prepulse dependency of the response. Mice given the 4 mg/kg dose + AMPH showed a reduction in PPI similar to that found in Veh + AMPH animals. N = 8–11 mice/treatment; *, p < 0.05, compared to the Veh + Veh control; #, p < 0.05, compared to the Veh + AMPH group; ‡, p < 0.05, within treatment 8 or 12 dB compared to the 4 dB response; §, p < 0.05, within treatment 12 dB compared to the 8 dB response. (B) Pulse-only responses during PPI testing were not affected by treatment in the (+)-22a+Veh or the Veh + AMPH groups but when (+)-22a was given before AMPH the startle-only response was attenuated in dose-dependent manner. N = 8–11 mice/treatment; *, p < 0.05, compared to the Veh + Veh control; #, p < 0.05, compared to the Veh + AMPH group; +, p < 0.05 compared to 4 mg/kg (+)-22a + Veh treatment; †, p < 0.05, compared to the 4 mg/kg (+)-22a + AMPH treatment.
The startle or “pulse-only” responses to the 120 dB acoustic stimulus (Fig. 4B) were similar among the Veh + Veh, 4 mg/kg (+)-22a + Veh, Veh + AMPH, and 4 mg/kg (+)-22a + AMPH groups. In contrast, the 0.5, 1, and 2 mg/kg doses of this compound decreased startle activities with the 0.5 mg/kg dose significantly reducing this response relative to the Veh + Veh, 4 mg/kg (+)-22a + Veh, Veh + AMPH, and 4 mg/kg (+)-22a + AMPH groups (ps < 0.035). Null activities were not distinguished across treatment conditions, with activities of 10.60 ± 0.53 AU across all animals, which accounted for less than 4% of the average pulse-only response. Together, these data show that (+)-22a can normalize AMPH-disrupted PPI and restore prepulse dependency of the response, with 1 mg/kg showed the best response.
Novel Object Recognition Memory (NORM).
This task in rodents is considered similar to testing declarative memory in humans, and individuals with schizophrenia are deficient on declarative memory tasks.38, 39 We used the transgenic NR1-KD mice since these mice represent a hypoglutamatergic model of schizophrenia and are deficient on the novel object recognition memory test.37
As expected, vehicle-treated wild type (WT) mice displayed a preference for the novel object at testing, whereas the NR1-KD animals showed no preference for either object (p < 0.001; Fig. 5A). Administration of (+)-22a produced an attenuation in NORM in WT animals with 1 mg/kg (p < 0.010). By comparison, these same doses increased novel object preference in NR1-KD mice, and 1 mg/kg (+)-22a significantly enhanced this preference relative to the vehicle-treated NR1-KD animals (p < 0.002) to a level that was indistinguishable from the WT vehicle control. Hence, the 1 mg/kg dose decreased NORM in WT mice, but augmented it in the hypoglutamatergic NR1-KD animals.
Figure 5.
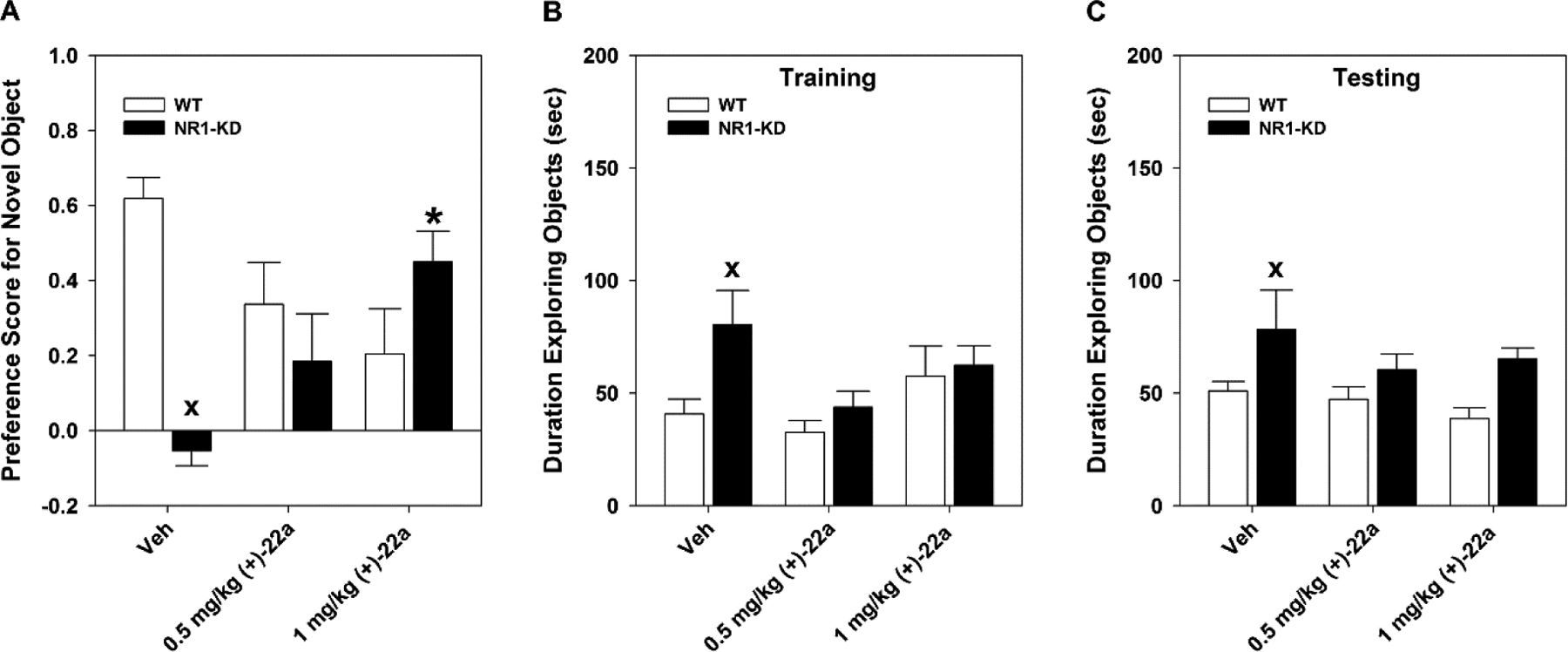
Compound (+)-22a rescues novel object recognition memory in NR1-KD mice. Mice were given vehicle (Veh) or 0.5 or 1 mg/kg (+)-22a 30 min before training or at testing for long-term memory (LTM). (A) At testing for LTM, Veh-treated wild-type (WT) controls displayed a preference for the novel object, whereas NR1-KD mice showed no preference for either object. In contrast, 1 mg/kg (+)-22a rescued this deficit in the mutants while producing some impairment in the WT animals. (B–C) As a control, the duration of object exploration was examined during (B) training and (C) LTM testing. At training and at testing Veh-treated NR1-KD mice spent more time exploring the objects than WT mice, while (+)-22a reduced this exploration time to levels that were not significantly different from the WT controls. N = 9–10 mice/genotype/treatment; *, p < 0.05, (+)-22a compared to the Veh within genotype; x, p < 0.05, NR1-KD compared to the WT vehicle.
To determine whether the differences in responses between the genotypes or among the doses of (+)-22a could be attributed to neophobia, total object exploration times were examined both at training and at testing for long-term memory (LTM). The vehicle-treated NR1-KD mice were observed to spend more time exploring objects than WT controls at both training (p < 0.006; Fig. 5B) and testing (p < 0.032; Fig. 5C). By contrast, following administration of 0.5 or 1 mg/kg (+)-22a, object exploration was similar between genotypes during training and testing. Hence, the rescue of NORM in NR1-KD mice cannot be attributed to neophobia in these mutants.
Catalepsy.
We next determined whether compound (+)-22a had any cataleptic activity as compared with the vehicle and haloperidol responses. Catalepsy at baseline prior to injection (time 0) was similar for all groups with time in catalepsy ≤ 1 second (data not shown). One hour after injection, catalepsy was very low in all groups given 0.01 to 30 mg/kg (+)-22a and this result was not different from that of the vehicle control (Fig. 6). In contrast, catalepsy was observed to increase in a dose-dependent fashion with haloperidol such that responses at the 1 and 10 mg/kg doses were significantly higher than those for all groups given (+)-22a as well as that for the vehicle control (ps < 0.001). These findings demonstrate that the cataleptic potential of (+)-22a is extremely low.
Figure 6.
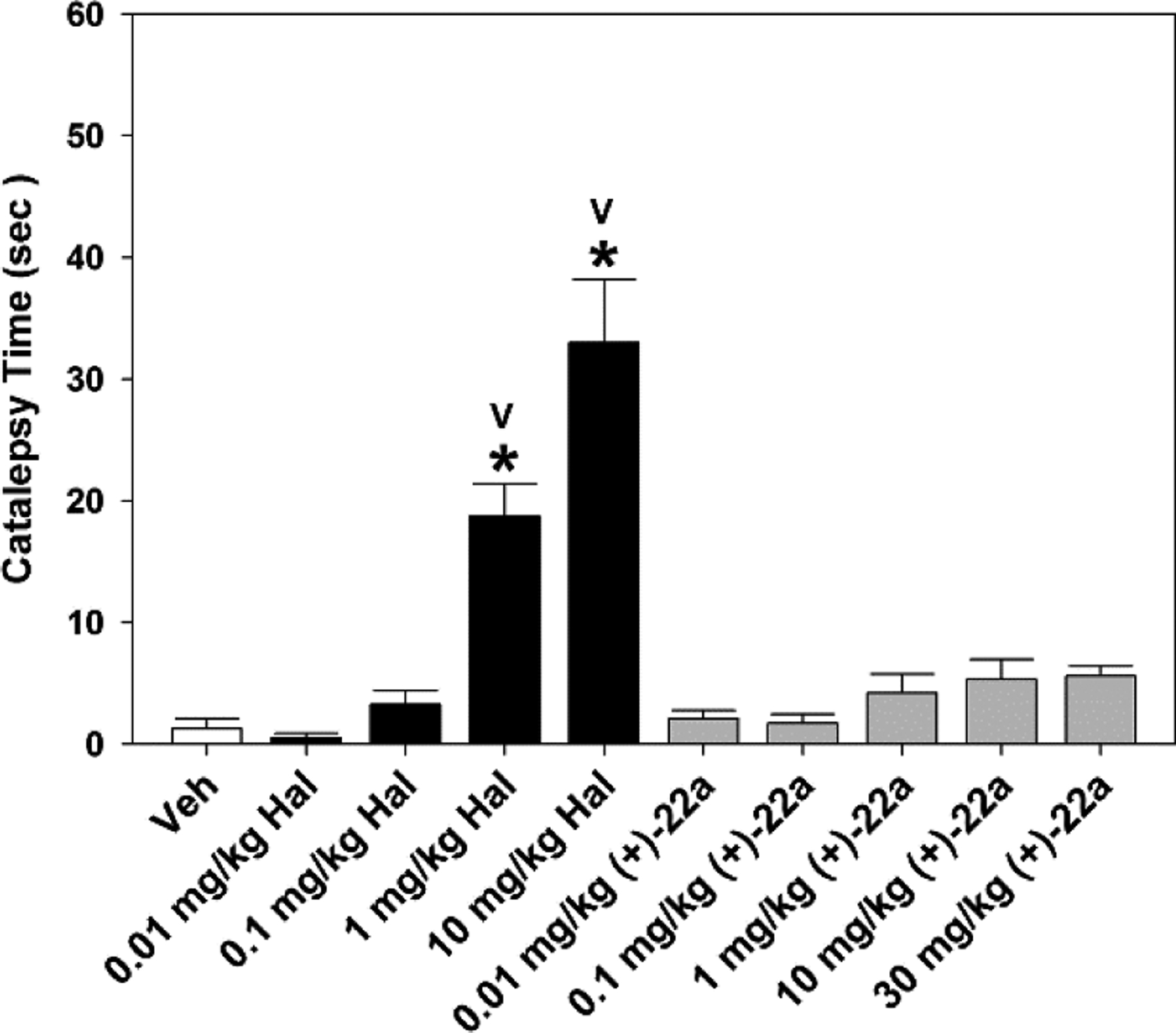
Comparison of catalepsy induced by haloperidol and (+)-22a in C57BL/6 mice with the horizontal bar test. Separate groups of mice were injected with vehicle (Veh), or 0.01, 0.1, 1, or 10 mg/kg haloperidol (Hal), or 0.01, 0.1, 1, 10, or 30 mg/kg compound (+)-22a and evaluated for catalepsy 60 min later. N = 8–10 mice/treatment; *, p < 0.05, compared to the Veh control; V, p < 0.05, compared to all other groups.
CONCLUSION
A new series of cyclopropylmethylamine-based 5-HT2C ligands bearing two halogen substituents on the phenyl ring have been designed and synthesized in order to further optimize this class of molecules. Compound (+)-22a was identified as a potent 5-HT2C receptor agonist (EC50 = 8.7 nM, Emax = 97%), with good selectivity against the 5-HT2B (EC50 = 1745 nM, Emax = 35%) and 5-HT2A receptors (EC50 = 491 nM, Emax = 21%). Improved selectivity over the 5-HT2A receptor has been achieved in comparison to the analog containing a single fluorine substituent, which showed moderate potency at the 5-HT2A receptor as well as higher efficacy (EC50 = 374 nM, Emax = 56%).22 In ADMET tests, compound (+)-22a displayed good stability in the liver microsome assays and low inhibition of the most abundant CYP isoforms. It inhibits the hERG channel with moderate potency (IC50 = 9.1 μM). However, a brain penetration study in mice indicated high brain concentration/plasma concentration ratios at 0.5 h (29.9) and 2.0 h (45.0), respectively.
Compound (+)-22a showed efficacy in two schizophrenia-related behavioral models. It suppressed AMPH-induced hyperlocomotion in the open field in a dose-dependent manner, and it normalized the AMPH-disrupted PPI. Furthermore, it rescued NORM of mice in the NR1-KD hypoglutamatergic animal model. Catalepsy was very low with compound (+)-22a as it was similar to that of the vehicle control and it was significantly lower than that for haloperidol. Taken together, these data support the further development of compound (+)-22a as a potential drug candidate for the treatment of schizophrenia. This agent may cause fewer side effects while improving cognition and avoiding weight gain in schizophrenia patients based upon its 5-HT2C-directed mechanism of action.
EXPERIMENTAL SECTION
General.
All chemicals and solvents were purchased from Sigma-Aldrich or Fisher Scientific, and were used as obtained without further purification. Microwave reactions were run in a Biotage Initiator microwave reactor. Synthetic intermediates were purified by CombiFlash flash chromatography on 230–400 mesh silica gel. 1H and 13C NMR spectra were recorded on Bruker DPX-400 or AVANCE-400 spectrometers; at 400 MHz and 100 MHz respectively. NMR chemical shifts were reported in δ (ppm) using residual solvent peaks as standard (CDCl3–7.26 (H), 77.23 (C); CD3OD–3.31 (H), 49.15 (C); DMSO-d6–2.50 (H), 39.52 (C)). Mass spectra were measured in the ESI mode at an ionization potential of 70 eV with an LC-MS MSD (Hewlett-Packard). Purity of all final compounds (greater than 95%) was determined by analytical HPLC (ACE 3AQ C18 column (150 × 4.6 mm, particle size 3 μM), 0.05% TFA in H2O/0.05% TFA in MeOH gradient eluting system). Optical rotation values were recorded on a Rudolph Research Autopol IV automatic polarimeter.
Compounds 16a–g, 18a–g and 19a–g were prepared according to the methods depicted in Scheme 1. The synthetic procedures and characterization data of all intermediates can be found in the Supporting Information.
General Methods.
(1) Chiral HPLC separation of racemic 16a–g, 18a–g, and 19a–g. Analytical conditions: RegisCell chiral column (25 cm × 4.6 mm, 10 μm particle size), 1.5–15% EtOH in n-hexane as the mobile phase; preparative conditions: RegisPack chiral column (25 cm × 21.1 mm, 10 μm particle size), 3.0–7.5% EtOH in n-hexane as the eluting system (isocratic eluent, stacked injections, flow rate = 18 mL/min, λ = 254 and 280 nm). Optical purity of both enantiomers were determined on analysis HPLC after the separation, and a second separation was done when necessary to guarantee an enantiomeric excess of > 90%. (2) Both enantiomers of compounds 16a–g, 19a–g, and 21a–g were dissolved in 2M HCl in diethyl ether and stirred at room temperature for 24–48 h. The white solids formed were collected by filtration, washed with diethyl ether, and dried under vacuum to give compounds (–)-20a–g, (+)-20a–g, (–)-21a–g, (+)-21a–g, (–)-22a–g, and (+)-22a–g as HCl salts.
(–)-[2-(4,5-Difluoro-2-methoxyphenyl)cyclopropyl]methanamine Hydrochloride ((–)-20a).
White solid. HPLC: 99.8%; 1H NMR (CD3OD) δ 6.94–6.88 (m, 2H), 3.87 (s, 3H), 3.08 (dd, J = 13.2, 7.2 Hz, 1H), 2.93 (dd, J = 13.2, 8.0 Hz, 1H), 2.09–2.03 (m, 1H), 1.27–1.23 (m, 1H), 1.13–1.08 (m, 1H), 1.04–1.00 (m, 1H); 13C NMR (CD3OD) δ 156.2 (d, JCF = 7.4 Hz), 150.2 (dd, JCF = 242.7, 13.5 Hz), 145.5 (dd, JCF = 236.5, 12.6 Hz), 127.1, 116.4 (d, JCF = 19.2 Hz), 101.6 (d, JCF = 21.4 Hz), 56.9, 45.1, 19.5, 17.8, 13.1; HRMS calcd for C11H14F2NO+ ([M+H]+): 214.1038, found: 214.1035; [α]D20 −7.9 (c 0.7, MeOH).
(+)-[2-(4,5-Difluoro-2-methoxyphenyl)cyclopropyl]methanamine Hydrochloride ((+)-20a).
White solid. HPLC: 99.8%; 1H NMR (CD3OD) δ 6.94–6.88 (m, 2H), 3.87 (s, 3H), 3.08 (dd, J = 13.2, 7.2 Hz, 1H), 2.93 (dd, J = 13.2, 8.0 Hz, 1H), 2.08–2.03 (m, 1H), 1.26–1.22 (m, 1H), 1.14–1.08 (m, 1H), 1.04–0.98 (m, 1H); HRMS calcd for C11H14F2NO+ ([M+H]+): 214.1038, found: 214.1030; [α]D20 +6.0 (c 0.2, MeOH).
(–)-[2-(2,3-Difluoro-6-methoxyphenyl)cyclopropyl]methanamine Hydrochloride ((–)-20b).
White solid. HPLC: 99.6%; 1H NMR (CD3OD) δ 7.08 (q, J = 9.6 Hz, 1H), 6.74–6.71 (m, 1H), 3.86 (s, 3H), 3.13 (dd, J = 13.2, 6.8 Hz, 1H), 2.92 (dd, J = 13.2, 8.4 Hz, 1H), 1.85–1.79 (m, 1H), 1.54–1.47 (m, 1H), 1.27–1.21 (m, 1H), 1.11–1.05 (m, 1H); 13C NMR (CD3OD) δ 156.7 (dd, JCF = 5.8, 2.0 Hz), 151.3 (dd, JCF = 243.3, 13.8 Hz), 146.7 (dd, JCF = 237.2, 13.9 Hz), 119.3 (d, JCF = 11.4 Hz), 115.6 (d, JCF = 18.3 Hz), 106.8 (dd, JCF = 6.4, 3.5 Hz), 56.8, 45.4, 18.3, 13.6, 12.9; HRMS calcd for C11H14F2NO+ ([M+H]+): 214.1038, found: 214.1044; [α]D20 −36.9 (c 0.7, MeOH).
(+)-[2-(2,3-Difluoro-6-methoxyphenyl)cyclopropyl]methanamine Hydrochloride ((+)-20b).
White solid. HPLC: 99.8%; 1H NMR (CD3OD) δ 7.08 (q, J = 9.6 Hz, 1H), 6.75–6.71 (m, 1H), 3.86 (s, 3H), 3.13 (dd, J = 12.8, 6.8 Hz, 1H), 2.91 (dd, J = 12.8, 8.4 Hz, 1H), 1.85–1.79 (m, 1H), 1.51–1.46 (m, 1H), 1.27–1.22 (m, 1H), 1.10–1.05 (m, 1H); HRMS calcd for C11H14F2NO+ ([M+H]+): 214.1038, found: 214.1041; [α]D20 +38.0 (c 0.2, MeOH).
(–)-[2-(5-Chloro-4-fluoro-2-methoxyphenyl)cyclopropyl]methanamine Hydrochloride ((–)-20c).
White solid. HPLC: 99.2%; 1H NMR (CD3OD) δ 7.06 (d, J = 8.4 Hz, 1H), 6.91 (d, J = 11.2 Hz, 1H), 3.89 (s, 3H), 3.08 (dd, J = 13.2, 7.2 Hz, 1H), 2.93 (dd, J = 13.2, 8.0 Hz, 1H), 2.06–2.00 (m, 1H), 1.29–1.21 (m, 1H), 1.13–1.08 (m, 1H), 1.04–0.98 (m, 1H); 13C NMR (CD3OD) δ 159.8, 158.6 (d, JCF = 235.0 Hz), 129.2, 128.0, 112.0 (d, JCF = 17.9 Hz), 101.2 (d, JCF = 25.4 Hz), 56.8, 45.1, 19.3, 17.7, 12.9; HRMS (ESI) m/z calcd for C11H14ClFNO+ ([M+H]+): 230.0742, found: 230.0719; [α]D20 −37.4 (c 0.5, MeOH).
(+)-[2-(5-Chloro-4-fluoro-2-methoxyphenyl)cyclopropyl]methanamine Hydrochloride ((+)-20c).
White solid. HPLC: 99.7%; 1H NMR (CD3OD) δ 7.06 (d, J = 8.4 Hz, 1H), 6.91 (d, J = 11.2 Hz, 1H), 3.89 (s, 3H), 3.08 (dd, J = 13.2, 7.2 Hz, 1H), 2.92 (dd, J = 13.2, 8.0 Hz, 1H), 2.06–2.00 (m, 1H), 1.28–1.21 (m, 1H), 1.14–1.08 (m, 1H), 1.04–0.98 (m, 1H); 13C NMR (CD3OD) δ 159.8, 158.6 (d, JCF = 234.8 Hz), 129.2, 128.0, 112.1 (d, JCF = 17.8 Hz), 101.2 (d, JCF = 25.7 Hz), 56.8, 45.1, 19.3, 17.7, 12.9; HRMS (ESI) m/z calcd for C11H14ClFNO+ ([M+H]+): 230.0742, found: 230.0724; [α]D20 +35.6 (c 0.5, MeOH).
(–)-[2-(3-Chloro-2-fluoro-6-methoxyphenyl)cyclopropyl]methanamine Hydrochloride ((–)-20d).
White solid. HPLC: 98.9%; 1H NMR (CD3OD) δ 7.28 (t, J = 8.8 Hz, 1H), 6.79 (dd, J = 9.2, 1.6 Hz, 1H), 3.87 (s, 3H), 3.11 (dd, J = 13.2, 6.8 Hz, 1H), 2.90 (dd, J = 13.2, 8.0 Hz, 1H), 1.82–1.76 (m, 1H), 1.48–1.43 (m, 1H), 1.23–1.18 (m, 1H), 1.09–1.03 (m, 1H); 13C NMR (CD3OD) δ 160.1 (d, JCF = 6.6 Hz), 158.7 (d, JCF = 243.8 Hz), 129.6, 118.9 (d, JCF = 20.1 Hz), 113.9 (d, JCF = 19.3 Hz), 108.5, 56.8, 45.4, 18.4, 13.7, 13.1; HRMS (ESI) m/z calcd for C11H14ClFNO+ ([M+H]+): 230.0742, found: 230.0740; [α]D20 −54.0 (c 0.15, MeOH).
(+)-[2-(3-Chloro-2-fluoro-6-methoxyphenyl)cyclopropyl]methanamine Hydrochloride ((+)-20d).
White solid. HPLC: 99.4%; 1H NMR (CD3OD) δ 7.29 (t, J = 8.8 Hz, 1H), 6.79 (dd, J = 8.8, 1.6 Hz, 1H), 3.88 (s, 3H), 3.11 (dd, J = 12.8, 6.4 Hz, 1H), 2.90 (dd, J = 12.8, 8.4 Hz, 1H), 1.82–1.77 (m, 1H), 1.48–1.44 (m, 1H), 1.24–1.18 (m, 1H), 1.09–1.04 (m, 1H); 13C NMR (CD3OD) δ 160.1 (d, JCF = 6.6 Hz), 158.7 (d, JCF = 243.8 Hz), 129.6, 118.9 (d, JCF = 20.1 Hz), 113.8 (d, JCF = 19.3 Hz), 108.4, 56.8, 45.4, 18.4, 13.7, 13.0; HRMS (ESI) m/z calcd for C11H14ClFNO+ ([M+H]+): 230.0742, found: 230.0744; [α]D20 +50.0 (c 0.1, MeOH).
(–)-[2-(4,5-Dichloro-2-methoxyphenyl)cyclopropyl]methanamine Hydrochloride ((–)-20e).
White solid. HPLC: 99.6%; 1H NMR (CD3OD) δ 7.11 (s, 2H), 3.88 (s, 3H), 3.08 (dd, J = 13.2, 7.2 Hz, 1H), 2.92 (dd, J = 13.2, 8.0 Hz, 1H), 2.09–2.04 (m, 1H), 1.28–1.26 (m, 1H), 1.16–1.10 (m, 1H), 1.06–1.00 (m, 1H); 13C NMR (CD3OD) δ 159.1, 131.6, 131.5 129.2, 124.5, 113.7, 56.8, 45.0, 19.5, 17.8, 13.2; HRMS (ESI) m/z calcd for C11H14Cl2NO+ ([M+H]+): 246.0447, found: 246.0438; [α]D20 −37.4 (c 0.3, MeOH).
(+)-[2-(4,5-Dichloro-2-methoxyphenyl)cyclopropyl]methanamine Hydrochloride ((+)-20e).
White solid. HPLC: 99.7%; 1H NMR (CD3OD) δ 7.11 (s, 2H), 3.89 (s, 3H), 3.08 (dd, J = 13.2, 7.6 Hz, 1H), 2.93 (dd, J = 12.8, 8.0 Hz, 1H), 2.09–2.04 (m, 1H), 1.29–1.26 (m, 1H), 1.15–1.10 (m, 1H), 1.06–1.02 (m, 1H); 13C NMR (CD3OD) δ 159.1, 131.6, 131.5, 129.2, 124.5, 113.7, 56.8, 45.0, 19.5, 17.8, 13.2; HRMS (ESI) m/z calcd for C11H14Cl2NO+ ([M+H]+): 246.0447, found: 246.0441; [α]D20 +37.7 (c 0.4, MeOH).
(–)-[2-(2,3-Dichloro-6-methoxyphenyl)cyclopropyl]methanamine Hydrochloride ((–)-20f).
White solid. HPLC: 99.9%; 1H NMR (CD3OD) δ 7.40 (d, J = 8.8 Hz, 1H), 6.96 (d, J = 8.8 Hz, 1H), 3.88 (s, 3H), 3.06 (d, J = 7.2 Hz, 2H), 1.78–1.73 (m, 1H), 1.44–1.40 (m, 1H), 1.21–1.15 (m, 1H), 1.11–1.06 (m, 1H); 13C NMR (CD3OD) δ 159.9, 135.9, 130.2, 129.6, 125.7, 111.7, 56.7, 45.4, 20.1, 18.3, 15.2; HRMS (ESI) m/z calcd for C11H14Cl2NO+ ([M+H]+): 246.0447, found: 246.0449; [α]D20 −62.0 (c 0.1, MeOH).
(+)-[2-(2,3-Dichloro-6-methoxyphenyl)cyclopropyl]methanamine Hydrochloride ((+)-20f).
White solid. HPLC: 99.8%; 1H NMR (CD3OD) δ 7.40 (d, J = 9.2 Hz, 1H), 6.96 (d, J = 9.2 Hz, 1H), 3.88 (s, 3H), 3.06 (d, J = 7.2 Hz, 2H), 1.77–1.74 (m, 1H), 1.44–1.42 (m, 1H), 1.21–1.15 (m, 1H), 1.09–1.05 (m, 1H); 13C NMR (CD3OD) δ 159.9, 135.9, 130.2, 129.6, 125.7, 111.7, 56.7, 45.4, 20.1, 18.3, 15.2; HRMS (ESI) m/z calcd for C11H14Cl2NO+ ([M+H]+): 246.0447, found: 246.0448; [α]D20 +69.0 (c 0.2, MeOH).
(–)-[2-(2-Chloro-3-fluoro-6-methoxyphenyl)cyclopropyl]methanamine Hydrochloride ((–)-20g).
White solid. HPLC: 99.7%; 1H NMR (CD3OD) δ 7.10 (t, J = 8.8 Hz, 1H), 6.92 (dd, J = 9.2, 4.4 Hz, 1H), 3.86 (s, 3H), 3.10–3.00 (m, 2H), 1.76–1.73 (m, 1H), 1.47–1.42 (m, 1H), 1.19–1.08 (m, 2H); 13C NMR (CD3OD) δ 157.1, 154.2 (d, JCF = 237.8 Hz), 129.1, 124.6 (d, JCF = 18.2 Hz), 115.5 (d, JCF = 22.9 Hz), 111.1 (d, JCF = 8.5 Hz), 56.8, 45.4, 19.6, 17.5, 14.7; HRMS (ESI) m/z calcd for C11H14ClFNO+ ([M+H]+): 230.0742, found: 230.0723; [α]D20 −66.4 (c 0.3, MeOH).
(+)-[2-(2-Chloro-3-fluoro-6-methoxyphenyl)cyclopropyl]methanamine Hydrochloride ((+)-20g).
White solid. HPLC: 99.6%; 1H NMR (CD3OD) δ 7.10 (t, J = 8.8 Hz, 1H), 6.92 (dd, J = 9.2, 4.4 Hz, 1H), 3.86 (s, 3H), 3.10–3.00 (m, 2H), 1.76–1.73 (m, 1H), 1.47–1.43 (m, 1H), 1.19–1.07 (m, 2H); 13C NMR (CD3OD) δ 157.1, 154.2 (d, JCF = 237.9 Hz), 129.1, 124.6 (d, JCF = 18.1 Hz), 115.5 (d, JCF = 22.9 Hz), 111.1 (d, JCF = 8.4 Hz), 56.8, 45.4, 19.6, 17.5, 14.7; HRMS (ESI) m/z calcd for C11H14ClFNO+ ([M+H]+): 230.0742, found: 230.0758; [α]D20 +69.4 (c 0.3, MeOH).
(–)-[2-[4,5-Difluoro-2-(2-fluoroethoxy)phenyl]cyclopropyl]methanamine Hydrochloride ((–)-21a).
White solid. HPLC: 99.7%; 1H NMR (CD3OD) δ 6.99–6.93 (m, 2H), 4.88–4.74 (m, 2H), 4.32–4.22 (m, 2H), 3.03–3.00 (m, 2H), 2.11–2.08 (m, 1H), 1.25–1.15 (m, 2H), 1.05–1.01 (m, 1H); 13C NMR (CD3OD) δ 155.0 (dd, JCF = 7.4, 2.0 Hz), 150.1 (dd, JCF = 243.0, 13.5 Hz), 145.8 (dd, JCF = 237.3, 12.6 Hz), 127.6, 116.6 (d, JCF = 19.5 Hz), 102.9 (d, JCF = 21.4 Hz), 83.4 (d, JCF = 166.9 Hz), 69.9 (d, JCF = 18.8 Hz), 44.9, 19.7, 17.8, 12.8; HRMS calcd for C12H15F3NO+ ([M+H]+): 246.1100, found: 246.1106; [α]D20 −1.5 (c 0.4, MeOH).
(+)-[2-[4,5-Difluoro-2-(2-fluoroethoxy)phenyl]cyclopropyl]methanamine Hydrochloride ((+)-21a).
White solid. HPLC: 99.5%; 1H NMR (CD3OD) δ 6.99–6.93 (m, 2H), 4.88–4.74 (m, 2H), 4.32–4.22 (m, 2H), 3.03–3.00 (m, 2H), 2.11–2.07 (m, 1H), 1.24–1.15 (m, 2H), 1.05–1.00 (m, 1H); HRMS calcd for C12H15F3NO+ ([M+H]+): 246.1100, found: 246.1086; [α]D20 +1.4 (c 0.1, MeOH).
(–)-[2-[2,3-Difluoro-6-(2-fluoroethoxy)phenyl]cyclopropyl]methanamine Hydrochloride ((–)-21b).
White solid. HPLC: 99.6%; 1H NMR (CD3OD) δ 7.09 (q, J = 9.6 Hz, 1H), 6.77–6.73 (m, 1H), 4.88–4.86 (m, 1H), 4.77–4.74 (m, 1H), 4.31–4.20 (m, 2H), 3.10 (dd, J = 13.2, 7.6 Hz, 1H), 2.99 (dd, J = 13.2, 7.6 Hz, 1H), 1.89–1.84 (m, 1H), 1.56–1.53 (m, 1H), 1.35–1.31 (m, 1H), 1.12–1.07 (m, 1H); 13C NMR (CD3OD) δ 155.6 (dd, JCF = 5.6, 2.1 Hz), 151.4 (dd, JCF = 243.7, 13.9 Hz), 147.0 (dd, JCF = 238.0, 13.8 Hz), 119.9 (d, JCF = 11.5 Hz), 115.6 (d, JCF = 18.3 Hz), 108.2 (dd, JCF = 3.6 Hz), 83.4 (d, JCF = 167.1 Hz), 69.7 (d, JCF = 18.9 Hz), 45.2, 18.4, 13.8, 12.9; HRMS calcd for C12H15F3NO+ ([M+H]+): 246.1100, found: 246.1099; [α]D20 −36.0 (c 0.5, MeOH).
(+)-[2-[2,3-Difluoro-6-(2-fluoroethoxy)phenyl]cyclopropyl]methanamine Hydrochloride ((+)-21b).
White solid. HPLC: 99.3%; 1H NMR (CD3OD) δ 7.09 (q, J = 9.6 Hz, 1H), 6.77–6.73 (m, 1H), 4.88–4.85 (m, 1H), 4.76–4.74 (m, 1H), 4.31–4.20 (m, 2H), 3.09 (dd, J = 13.2, 7.6 Hz, 1H), 2.99 (dd, J = 12.8, 7.6 Hz, 1H), 1.89–1.84 (m, 1H), 1.56–1.53 (m, 1H), 1.35–1.31 (m, 1H), 1.12–1.07 (m, 1H); HRMS calcd for C12H15F3NO+ ([M+H]+): 246.1100, found: 246.1088; [α]D20 +30.2 (c 0.2, MeOH).
(–)-[2-[5-Chloro-4-fluoro-2-(2-fluoroethoxy)phenyl]cyclopropyl]methanamine Hydrochloride ((–)-21c).
White solid. HPLC: 99.2%; 1H NMR (CD3OD) δ 7.10 (d, J = 8.0 Hz, 1H), 6.96 (d, J = 11.2 Hz, 1H), 4.86–4.76 (m, 2H), 4.35–4.24 (m, 2H), 3.05–2.97 (m, 2H), 2.10–2.04 (m, 1H), 1.26–1.15 (m, 2H), 1.05–1.00 (m, 1H); 13C NMR (CD3OD) δ 158.6, 158.5 (d, JCF = 244.2 Hz), 129.5, 128.3, 112.7 (d, JCF = 17.8 Hz), 102.3 (d, JCF = 25.4 Hz), 83.3 (d, JCF = 167.0 Hz), 69.7 (d, JCF = 18.8 Hz), 45.0, 19.5, 17.8, 12.6; HRMS calcd for C12H15ClF2NO+ ([M+H]+): 262.0805, found: 262.0782; [α]D20 −26.4 (c 0.5, MeOH).
(+)-[2-[5-Chloro-4-fluoro-2-(2-fluoroethoxy)phenyl]cyclopropyl]methanamine Hydrochloride ((+)-21c).
White solid. HPLC: 99.3%; 1H NMR (CD3OD) δ 7.10 (d, J = 8.4 Hz, 1H), 6.96 (d, J = 10.8 Hz, 1H), 4.86–4.75 (m, 2H), 4.34–4.24 (m, 2H), 3.04–2.98 (m, 2H), 2.09–2.04 (m, 1H), 1.25–1.15 (m, 2H), 1.05–1.00 (m, 1H); 13C NMR (CD3OD) δ 158.6, 158.5 (d, JCF = 243.9 Hz), 129.5, 128.3, 112.7 (d, JCF = 17.5 Hz), 102.3 (d, JCF = 25.5 Hz), 83.3 (d, JCF = 167.1 Hz), 69.7 (d, JCF = 19.0 Hz), 45.0, 19.5, 17.8, 12.6; HRMS calcd for C12H15ClF2NO+ ([M+H]+): 262.0805, found: 262.0788; [α]D20 +28.4 (c 0.5, MeOH).
(–)-[2-[3-Chloro-2-fluoro-6-(2-fluoroethoxy)phenyl]cyclopropyl]methanamine Hydrochloride ((–)-21d).
White solid. HPLC: 98.8%; 1H NMR (CD3OD) δ 7.29 (t, J = 8.8 Hz, 1H), 6.81 (d, J = 9.2 Hz, 1H), 4.88–4.83 (m, 1H), 4.77–4.75 (m, 1H), 4.35–4.21 (m, 2H), 3.09 (dd, J = 13.2, 7.2 Hz, 1H), 2.98 (dd, J = 13.2, 7.6 Hz, 1H), 1.88–1.83 (m, 1H), 1.55–1.51 (m, 1H), 1.32–1.28 (m, 1H), 1.12–1.06 (m, 1H); 13C NMR (CD3OD) δ 159.0 (d, JCF = 6.4 Hz), 158.7 (d, JCF = 244.0 Hz), 129.5, 119.4 (d, JCF = 14.7 Hz), 114.4 (d, JCF = 19.2 Hz), 109.5, 83.3 (d, JCF = 167.4 Hz), 69.6 (d, JCF = 19.0 Hz), 45.3, 18.5, 13.9, 13.1; HRMS calcd for C12H15ClF2NO+ ([M+H]+): 262.0805, found: 262.0791; [α]D20 −53.0 (c 0.3, MeOH).
(+)-[2-[3-Chloro-2-fluoro-6-(2-fluoroethoxy)phenyl]cyclopropyl]methanamine Hydrochloride ((+)-21d).
White solid. HPLC: 99.3%; 1H NMR (CD3OD) δ 7.29 (t, J = 8.8 Hz, 1H), 6.81 (dd, J = 8.8, 1.4 Hz, 1H), 4.88–4.83 (m, 1H), 4.77–4.74 (m, 1H), 4.33–4.23 (m, 2H), 3.10 (dd, J = 12.8, 7.2 Hz, 1H), 2.98 (dd, J = 13.2, 7.6 Hz, 1H), 1.88–1.83 (m, 1H), 1.55–1.51 (m, 1H), 1.32–1.28 (m, 1H), 1.12–1.06 (m, 1H); 13C NMR (CD3OD) δ 159.0 (d, JCF = 6.5 Hz), 158.7 (d, JCF = 244.4 Hz), 129.5, 119.4 (d, JCF = 14.7 Hz), 114.4 (d, JCF = 19.3 Hz), 109.5, 83.3 (d, JCF = 167.3 Hz), 69.6 (d, JCF = 19.0 Hz), 45.3, 18.5, 13.9, 13.1; HRMS calcd for C12H15ClF2NO+ ([M+H]+): 262.0805, found: 246.0793; [α]D20 +53.5 (c 0.4, MeOH).
(–)-[2-[4,5-Dichloro-2-(2-fluoroethoxy)phenyl]cyclopropyl]methanamine Hydrochloride ((–)-21e).
White solid. HPLC: 99.6%; 1H NMR (CD3OD) δ 7.16 (s, 1H), 7.15 (s, 1H), 4.88–4.74 (m, 2H), 4.37–4.25 (m, 2H), 3.04–3.02 (m, 2H), 2.13–2.08 (m, 1H), 1.29–1.25 (m, 1H), 1.23–1.18 (m, 1H), 1.08–1.01 (m, 1H); HRMS calcd for C12H15Cl2FNO+ ([M+H]+): 278.0509, found: 278.0496; [α]D20 −32.4 (c 0.25, MeOH).
(+)-[2-[4,5-Dichloro-2-(2-fluoroethoxy)phenyl]cyclopropyl]methanamine Hydrochloride ((+)-21e).
White solid. HPLC: 99.4%; 1H NMR (CD3OD) δ 7.16 (s, 1H), 7.15 (s, 1H), 4.88–4.75 (m, 2H), 4.36–4.24 (m, 2H), 3.04–3.01 (m, 2H), 2.13–2.08 (m, 1H), 1.29–1.26 (m, 1H), 1.23–1.19 (m, 1H), 1.08–1.03 (m, 1H); 13C NMR (CD3OD) δ 158.1, 131.9, 131.6, 129.5, 125.1, 114.8, 83.3 (d, JCF = 167.1 Hz), 69.7 (d, JCF = 18.9 Hz), 44.9, 19.7, 17.9, 12.9; HRMS calcd for C12H15Cl2FNO+ ([M+H]+): 278.0509, found: 278.0508; [α]D20 +30.2 (c 0.5, MeOH).
(–)-[2-[2,3-Dichloro-6-(2-fluoroethoxy)phenyl]cyclopropyl]methanamine Hydrochloride ((–)-21f).
White solid. HPLC: 99.3%; 1H NMR (DMSO-d6) δ 8.09 (br, 3H), 7.48 (d, J = 8.8 Hz, 1H), 7.03 (d, J = 8.8 Hz, 1H), 4.90–4.73 (m, 2H), 4.32–4.22 (m, 2H), 3.26–3.22 (m, 1H), 2.64–2.59 (m, 1H), 1.78–1.73 (m, 1H), 1.45–1.41 (m, 1H), 1.18–1.12 (m, 1H), 1.04–1.00 (m, 1H); 13C NMR (DMSO-d6) δ 157.1, 133.8, 128.8, 128.6, 123.5, 112.4, 82.2 (d, JCF = 165.6 Hz), 68.1 (d, JCF = 18.6 Hz), 42.7, 18.7, 16.9, 14.0; HRMS calcd for C12H15Cl2FNO+ ([M+H]+): 278.0509, found: 278.0494; [α]D20 −96.0 (c 0.1, MeOH).
(+)-[2-[2,3-Dichloro-6-(2-fluoroethoxy)phenyl]cyclopropyl]methanamine Hydrochloride ((+)-21f).
White solid. HPLC: 99.0%; 1H NMR (DMSO-d6) δ 8.11 (br, 3H), 7.47 (d, J = 8.8 Hz, 1H), 7.03 (d, J = 8.8 Hz, 1H), 4.89–4.73 (m, 2H), 4.32–4.22 (m, 2H), 3.24 (dd, J = 12.4, 5.2 Hz, 1H), 2.64–2.59 (m, 1H), 1.78–1.73 (m, 1H), 1.45–1.41 (m, 1H), 1.18–1.13 (m, 1H), 1.04–1.00 (m, 1H); 13C NMR (DMSO-d6) δ 157.0, 133.7, 128.8, 128.6, 123.5, 112.4, 82.2 (d, JCF = 165.6 Hz), 68.1 (d, JCF = 18.6 Hz), 42.7, 18.6, 16.9, 13.9; HRMS calcd for C12H15Cl2FNO+ ([M+H]+): 278.0509, found: 278.0493; [α]D20 +70.0 (c 0.1, MeOH).
(–)-[2-[2-Chloro-3-fluoro-6-(2-fluoroethoxy)phenyl]cyclopropyl]methanamine Hydrochloride ((–)-21g).
White solid. HPLC: 99.1%; 1H NMR (CD3OD) δ 7.11 (t, J = 8.8 Hz, 1H), 6.94 (dd, J = 9.0, 4.2 Hz, 1H), 4.88–4.85 (m, 1H), 4.77–4.74 (m, 1H), 4.32–4.20 (m, 2H), 3.34 (dd, J = 13.2, 6.4 Hz, 1H), 2.85 (dd, J = 13.2, 8.8 Hz, 1H), 1.85–1.79 (m, 1H), 1.56–1.50 (m, 1H), 1.23–1.18 (m, 2H); 13C NMR (CD3OD) δ 156.0, 154.5 (d, JCF = 238.6 Hz), 129.7, 124.7 (d, JCF = 18.2 Hz), 115.5 (d, JCF = 22.9 Hz), 112.6 (d, JCF = 7.6 Hz), 83.4 (d, JCF = 167.3 Hz), 69.8 (d, JCF = 18.9 Hz), 45.3, 19.7, 17.7, 14.7; HRMS calcd for C12H15ClF2NO+ ([M+H]+): 262.0805, found: 262.0801; [α]D20 −54.5 (c 0.2, MeOH).
(+)-[2-[2-Chloro-3-fluoro-6-(2-fluoroethoxy)phenyl]cyclopropyl]methanamine Hydrochloride ((+)-21g).
White solid. HPLC: 99.2%; 1H NMR (CD3OD) δ 7.11 (t, J = 8.8 Hz, 1H), 6.94 (dd, J = 9.0, 4.2 Hz, 1H), 4.88–4.85 (m, 1H), 4.78–4.74 (m, 1H), 4.33–4.20 (m, 2H), 3.34 (dd, J = 13.2, 8.8 Hz, 1H), 2.85 (dd, J = 13.2, 8.8 Hz, 1H), 1.85–1.79 (m, 1H), 1.56–1.52 (m, 1H), 1.24–1.17 (m, 2H); 13C NMR (CD3OD) δ 156.0, 154.5 (d, JCF = 238.6 Hz), 129.7, 124.7 (d, JCF = 18.2 Hz), 115.5 (d, JCF = 22.8 Hz), 112.6 (d, JCF = 7.7 Hz), 83.4 (d, JCF = 167.3 Hz), 69.8 (d, JCF = 19.0 Hz), 45.3, 19.7, 17.7, 14.7; HRMS calcd for C12H15ClF2NO+ ([M+H]+): 262.0805, found: 262.0807; [α]D20 +61.4 (c 0.3, MeOH).
(–)-[2-[2-(Allyloxy)-4,5-difluorophenyl]cyclopropyl]methanamine Hydrochloride ((–)-22a).
White solid. HPLC: 99.3%; 1H NMR (CD3OD) δ 6.95–6.88 (m, 2H), 6.18–6.08 (m, 1H), 5.47 (dd, J = 17.2, 1.6 Hz, 1H), 5.33 (dd, J = 10.4, 1.6 Hz, 1H), 4.60 (d, J = 5.2 Hz, 2H), 3.07 (dd, J = 12.8, 7.2 Hz, 1H), 2.98 (dd, J = 13.2, 8.0 Hz, 1H), 2.17–2.09 (m, 1H), 1.33–1.29 (m, 1H), 1.13–1.02 (m, 2H); 13C NMR (CD3OD) δ 155.0 (dd, JCF = 7.6, 2.0 Hz), 150.0 (dd, JCF = 242.8, 13.5 Hz), 145.7 (dd, JCF = 236.9, 12.7 Hz), 134.5, 127.5, 118.6, 116.2 (d, JCF = 19.2 Hz), 103.1 (d, JCF = 21.3 Hz), 71.1, 45.1, 19.4, 18.0, 13.4; HRMS calcd for C13H16F2NO+ ([M+H]+): 240.1194, found: 240.1189; [α]D20 −14.0 (c 0.4, MeOH).
(+)-[2-[2-(Allyloxy)-4,5-difluorophenyl]cyclopropyl]methanamine Hydrochloride ((+)-22a).
White solid. HPLC: 99.4%; 1H NMR (CD3OD) δ 6.94–6.89 (m, 2H), 6.18–6.08 (m, 1H), 5.47 (dd, J = 17.2, 1.6 Hz, 1H), 5.33 (dd, J = 10.8, 1.6 Hz, 1H), 4.60 (d, J = 5.2 Hz, 2H), 3.06 (dd, J = 13.2, 7.2 Hz, 1H), 2.97 (dd, J = 13.2, 7.6 Hz, 1H), 2.17–2.08 (m, 1H), 1.33–1.28 (m, 1H), 1.13–1.02 (m, 2H); HRMS calcd for C13H16F2NO+ ([M+H]+): 240.1194, found: 246.1192; [α]D20 +12.0 (c 0.1, MeOH).
(–)-[2-[6-(Allyloxy)-2,3-difluorophenyl]cyclopropyl]methanamine hydrochloride ((–)-22b).
White solid. HPLC: 97.0%; 1H NMR (CD3OD) δ 7.06 (q, J = 9.6 Hz, 1H), 6.75–6.71 (m, 1H), 6.16–6.09 (m, 1H), 5.46 (dd, J = 17.2, 1.6 Hz, 1H), 5.32 (dd, J = 10.8, 1.6 Hz, 1H), 4.59 (d, J = 5.6 Hz, 2H), 3.08 (dd, J = 13.2, 7.6 Hz, 1H), 3.01 (dd, J = 13.2, 7.6 Hz, 1H), 1.91–1.86 (m, 1H), 1.61–1.56 (m, 1H), 1.31–1.27 (m, 1H), 1.12–1.08 (m, 1H); 13C NMR (CD3OD) δ 155.7 (d, JCF = 5.6 Hz), 151.3 (dd, JCF = 243.3, 14.0 Hz), 146.8 (dd, JCF = 237.6, 13.8 Hz), 134.6, 119.7 (d, JCF = 11.2 Hz), 118.6, 115.5 (d, JCF = 18.3 Hz), 108.5, 71.1, 45.3, 18.3, 13.9, 13.0; HRMS calcd for C13H16F2NO+ ([M+H]+): 240.1194, found: 240.1188; [α]D20 −46.8 (c 0.5, MeOH).
(+)-[2-[6-(Allyloxy)-2,3-difluorophenyl]cyclopropyl]methanamine hydrochloride ((+)-22b).
White solid. HPLC: 99.7%; 1H NMR (CD3OD) δ 7.06 (q, J = 9.6 Hz, 1H), 6.75–6.71 (m, 1H), 6.16–6.08 (m, 1H), 5.46 (dd, J = 17.2, 1.6 Hz, 1H), 5.32 (dd, J = 10.4, 1.6 Hz, 1H), 4.59 (d, J = 5.6 Hz, 2H), 3.07 (dd, J = 13.2, 7.6 Hz, 1H), 3.00 (dd, J = 13.2, 7.6 Hz, 1H), 1.91–1.85 (m, 1H), 1.60–1.56 (m, 1H), 1.31–1.26 (m, 1H), 1.11–1.06 (m, 1H); HRMS calcd for C13H16F2NO+ ([M+H]+): 240.1194, found: 240.1191; [α]D20 +41.6 (c 0.2, MeOH).
(–)-[2-[2-(Allyloxy)-5-chloro-4-fluorophenyl]cyclopropyl]methanamine Hydrochloride ((–)-22c).
White solid. HPLC: 99.8%; 1H NMR (CD3OD) δ 7.07 (d, J = 8.4 Hz, 1H), 6.90 (d, J = 11.2 Hz, 1H), 6.18–6.08 (m, 1H), 5.47 (dd, J = 13.6, 1.6 Hz, 1H), 5.34 (dd, J = 10.4, 1.6 Hz, 1H), 4.62 (d, J = 5.2 Hz, 2H), 3.06 (dd, J = 12.8, 7.2 Hz, 1H), 2.98 (dd, J = 12.8, 8.0 Hz, 1H), 2.11–2.05 (m, 1H), 1.32–1.28 (m, 1H), 1.13–1.01 (m, 2H); 13C NMR (CD3OD) δ 158.5, 158.4 (d, JCF = 243.8 Hz), 134.3, 129.2, 128.3, 118.8, 112.3 (d, JCF = 17.6 Hz), 102.4 (d, JCF = 25.4 Hz), 70.9, 45.0, 19.2, 17.9, 13.2; HRMS (ESI) m/z calcd for C13H16ClFNO+ ([M+H]+): 256.0899, found: 256.0875; [α]D20 −36.2 (c 0.5, MeOH).
(+)-[2-[2-(Allyloxy)-5-chloro-4-fluorophenyl]cyclopropyl]methanamine Hydrochloride ((+)-22c).
White solid. HPLC: 99.3%; 1H NMR (CD3OD) δ 7.07 (d, J = 8.4 Hz, 1H), 6.91 (d, J = 11.2 Hz, 1H), 6.17–6.08 (m, 1H), 5.47 (dd, J = 13.6, 1.6 Hz, 1H), 5.34 (dd, J = 11.2, 1.2 Hz, 1H), 4.62 (d, J = 5.2 Hz, 2H), 3.06 (dd, J = 11.2, 7.2 Hz, 1H), 2.99 (dd, J = 12.8, 5.6 Hz, 1H), 2.11–2.05 (m, 1H), 1.32–1.28 (m, 1H), 1.13–1.01 (m, 2H); 13C NMR (CD3OD) δ 158.5, 158.4 (d, JCF = 243.7 Hz), 134.3, 129.2, 128.3, 118.8, 112.3 (d, JCF = 17.6 Hz), 102.4 (d, JCF = 25.4 Hz), 70.9, 45.0, 19.2, 17.9, 13.2; HRMS (ESI) m/z calcd for C13H16ClFNO+ ([M+H]+): 256.0899, found: 256.0880; [α]D20 +34.6 (c 0.5, MeOH).
(–)-[2-[6-(Allyloxy)-3-chloro-2-fluorophenyl]cyclopropyl]methanamine Hydrochloride ((–)-22d).
White solid. HPLC: 98.5%; 1H NMR (CD3OD) δ 7.26 (t, J = 8.4 Hz, 1H), 6.79 (d, J = 9.2 Hz, 1H), 6.17–6.07 (m, 1H), 5.46 (d, J = 17.2 Hz, 1H), 5.33 (d, J = 10.4 Hz, 1H), 4.61 (d, J = 5.2 Hz, 2H), 3.08–2.97 (m, 2H), 1.88–1.83 (m, 1H), 1.59–1.52 (m, 1H), 1.28–1.22 (m, 1H), 1.11–1.06 (m, 1H); 13C NMR (CD3OD) δ 159.0 (d, JCF = 6.4 Hz), 158.7 (d, JCF = 243.9 Hz), 134.4, 129.4, 119.2 (d, JCF = 14.6 Hz), 118.7, 113.9 (d, JCF = 19.3 Hz), 109.7, 70.9, 45.3, 18.4, 13.9, 13.1; HRMS (ESI) m/z calcd for C13H16ClFNO+ ([M+H]+): 256.0899, found: 256.0900; [α]D20 −54.0 (c 0.3, MeOH).
(+)-[2-[6-(Allyloxy)-3-chloro-2-fluorophenyl]cyclopropyl]methanamine Hydrochloride ((+)-22d).
White solid. HPLC: 99.6%; 1H NMR (CD3OD) δ 7.26 (t, J = 8.6 Hz, 1H), 6.79 (dd, J = 9.2, 1.6 Hz, 1H), 6.17–6.07 (m, 1H), 5.46 (dd, J = 17.2, 1.4 Hz, 1H), 5.33 (dd, J = 10.4, 1.2 Hz, 1H), 4.61 (d, J = 5.6 Hz, 2H), 3.08–2.97 (m, 2H), 1.88–1.83 (m, 1H), 1.57–1.52 (m, 1H), 1.28–1.22 (m, 1H), 1.11–1.06 (m, 1H); 13C NMR (CD3OD) δ 159.0 (d, JCF = 6.6 Hz), 158.7 (d, JCF = 243.8 Hz), 134.4, 129.4, 119.2 (d, JCF = 14.5 Hz), 118.7, 113.9 (d, JCF = 19.2 Hz), 109.7, 70.9, 45.3, 18.4, 13.9, 13.1; HRMS (ESI) m/z calcd for C13H16ClFNO+ ([M+H]+): 256.0899, found: 256.0901; [α]D20 +55.0 (c 0.2, MeOH).
(–)-[2-[2-(Allyloxy)-4,5-dichlorophenyl]cyclopropyl]methanamine Hydrochloride ((–)-22e).
White solid. HPLC: 99.1%; 1H NMR (CD3OD) δ 7.12 (s, 1H), 7.10 (s, 1H), 6.17–6.07 (m, 1H), 5.47 (dd, J = 17.6, 1.6 Hz, 1H), 5.34 (dd, J = 10.4, 1.6 Hz, 1H), 4.63 (d, J = 5.2 Hz, 2H), 3.08–2.97 (m, 2H), 2.14–2.09 (m, 1H), 1.37–1.32 (m, 1H), 1.15–1.03 (m, 2H); 13C NMR (CD3OD) δ 157.9, 134.3, 131.9, 131.4, 129.2, 124.7, 118.7, 115.0, 70.9, 44.9, 19.4, 17.9, 13.5; HRMS (ESI) m/z calcd for C13H16Cl2NO+ ([M+H]+): 272.0603, found: 272.0596; [α]D20 −35.0 (c 0.4, MeOH).
(+)-[2-[2-(Allyloxy)-4,5-dichlorophenyl]cyclopropyl]methanamine Hydrochloride ((+)-22e).
White solid. HPLC: 99.2%; 1H NMR (CD3OD) δ 7.12 (s, 1H), 7.10 (s, 1H), 6.17–6.07 (m, 1H), 5.47 (dd, J = 17.2, 1.2 Hz, 1H), 5.34 (d, J = 10.4 Hz, 1H), 4.63 (d, J = 5.2 Hz, 2H), 3.08–2.96 (m, 2H), 2.14–2.09 (m, 1H), 1.39–1.32 (m, 1H), 1.15–1.03 (m, 2H); 13C NMR (CD3OD) δ 157.9, 134.3, 131.9, 131.4, 129.2, 124.7, 118.7, 115.0, 70.9, 44.9, 19.4, 17.9, 13.5; HRMS (ESI) m/z calcd for C13H16Cl2NO+ ([M+H]+): 272.0603, found: 272.0597; [α]D20 +37.6 (c 0.3, MeOH).
(–)-[2-[6-(Allyloxy)-2,3-dichlorophenyl]cyclopropyl]methanamine Hydrochloride ((–)-22f).
White solid. HPLC: 98.3%; 1H NMR (CD3OD) δ 7.37 (d, J = 9.2 Hz, 1H), 6.94 (d, J = 9.2 Hz, 1H), 6.18–6.08 (m, 1H), 5.46 (dd, J = 17.2, 1.6 Hz, 1H), 5.34 (dd, J = 10.8, 1.2 Hz, 1H), 4.62 (d, J = 5.2 Hz, 2H), 3.30 (dd, J = 13.2, 8.8 Hz, 1H), 2.86 (dd, J = 13.2, 8.8 Hz, 1H), 1.83–1.77 (m, 1H), 1.48–1.45 (m, 1H), 1.23–1.12 (m, 2H); 13C NMR (CD3OD) δ 158.8, 135.9, 134.4, 130.0, 129.9, 125.8, 118.9, 113.3, 70.9, 45.3, 20.2, 18.5, 15.3; HRMS (ESI) m/z calcd for C13H16Cl2NO+ ([M+H]+): 272.0603, found: 272.0611; [α]D20 −68.0 (c 0.1, MeOH).
(+)-[2-[6-(Allyloxy)-2,3-dichlorophenyl]cyclopropyl]methanamine Hydrochloride ((+)-22f).
White solid. HPLC: 99.1%; 1H NMR (CD3OD) δ 7.37 (d, J = 8.8 Hz, 1H), 6.94 (d, J = 8.8 Hz, 1H), 6.18–6.08 (m, 1H), 5.47 (dd, J = 17.2, 1.6 Hz, 1H), 5.34 (dd, J = 10.4, 1.2 Hz, 1H), 4.63 (d, J = 5.2 Hz, 2H), 3.30 (dd, J = 12.8, 8.8 Hz, 1H), 2.86 (dd, J = 12.8, 8.8 Hz, 1H), 1.83–1.79 (m, 1H), 1.48–1.46 (m, 1H), 1.23–1.15 (m, 2H); 13C NMR (CD3OD) δ 158.8, 135.9, 134.5, 130.0, 129.9, 125.8, 118.8, 113.3, 71.0, 45.4, 20.2, 18.6, 15.3; HRMS (ESI) m/z calcd for C13H16Cl2NO+ ([M+H]+): 272.0603, found: 272.0596; [α]D20 +64.0 (c 0.1, MeOH).
(–)-[2-[6-(Allyloxy)-2-chloro-3-fluorophenyl]cyclopropyl]methanamine Hydrochloride ((–)-22g).
White solid. HPLC: 99.7%; 1H NMR (CD3OD) δ 7.09 (t, J = 8.8 Hz, 1H), 6.92 (dd, J = 9.2, 4.4 Hz, 1H), 6.16–6.07 (m, 1H), 5.46 (dd, J = 17.6, 1.6 Hz, 1H), 5.33 (dd, J = 10.6, 1.4 Hz, 1H), 4.60 (d, J = 5.6 Hz, 2H), 3.29 (dd, J = 13.2, 6.4 Hz, 1H), 2.87 (dd, J = 12.8, 8.6 Hz, 1H), 1.84–1.77 (m, 1H), 1.53–1.47 (m, 1H), 1.22–1.17 (m, 2H); 13C NMR (CD3OD) δ 156.1, 154.3 (d, JCF = 238.3 Hz), 134.7, 129.5, 124.5 (d, JCF = 18.2 Hz), 118.7, 115.4 (d, JCF = 22.7 Hz), 112.8 (d, JCF = 7.5 Hz), 71.1, 45.4, 19.7, 17.7, 14.8; HRMS (ESI) m/z calcd for C13H16ClFNO+ ([M+H]+): 256.0899, found: 256.0895; [α]D20 −52.0 (c 0.2, MeOH).
(+)-[2-[6-(Allyloxy)-2-chloro-3-fluorophenyl]cyclopropyl]methanamine Hydrochloride ((+)-22g).
White solid. HPLC: 99.7%; 1H NMR (CD3OD) δ 7.09 (t, J = 8.8 Hz, 1H), 6.92 (dd, J = 9.2, 4.4 Hz, 1H), 6.17–6.08 (m, 1H), 5.46 (dd, J = 17.6, 1.6 Hz, 1H), 5.33 (dd, J = 10.4, 1.2 Hz, 1H), 4.60 (d, J = 4.8 Hz, 2H), 3.29 (dd, J = 13.6, 6.0 Hz, 1H), 2.87 (dd, J = 12.8, 8.8 Hz, 1H), 1.84–1.78 (m, 1H), 1.53–1.47 (m, 1H), 1.21–1.17 (m, 2H); 13C NMR (CD3OD) δ 156.1, 154.3 (d, JCF = 238.0 Hz), 134.7, 129.5, 124.5 (d, JCF = 18.1 Hz), 118.7, 115.4 (d, JCF = 22.7 Hz), 112.8 (d, JCF = 6.6 Hz), 71.1, 45.3, 19.7, 17.7, 14.8; HRMS (ESI) m/z calcd for C13H16ClFNO+ ([M+H]+): 256.0899, found: 256.0906; [α]D20 +61.8 (c 0.4, MeOH).
Calcium Flux Assay.
Calcium flux assay was recorded on a FLIPRTETRA fluorescence imaging plate reader (Molecular Dynamics) on Flp-In-293 cells stably expressing the human 5-HT2A, 5-HT2B, or 5-HT2C-INI as previously described.22
Animal Behavioral Studies Methods
Animals.
Adult male and female C57BL/6 and NR1 mice were used for behavioral testing. Mice were maintained on a 14 h light/10 h dark light-dark cycle with the onset of that dark cycle at 2000 h. Testing was conducted during the light cycle between 1000–1600 h. All animals were housed by genotype and sex. Forty-eight h before testing mice were weighed and moved into the appropriate test room. Mice remained in the test room for the duration of testing and had ad libitum access to food and water. All studies were conducted with an approved protocol from the Duke University Institutional Animal Care and Use Committee.
Drugs.
Haloperidol (Sigma-Aldrich, St. Louis MO) and compound (+)-22a were dissolved in 0.5% N, N-dimethylacetamide and 10% (2-hydroxypropyl)-β-cyclodextrin in sterile saline. d-Amphetamine (Tocris Bioscience, Bristol, UK) was dissolved in sterile saline. All treatments were delivered in a 4 mL/kg volume (ip).
Open Field Activity.
Activity in the open field was assessed as distance traveled in a novel environment (20 × 20 × 20 cm) using a Versamax Analyzer system (Omnitech Electronics, Columbus, OH). Locomotor activity was monitored by infrared diodes interfaced to a computer running Fusion software (Omnitech Electronics). Mice (n = 7–10 mice/treatment) were injected with vehicle (Veh) or 2, 5, 10, 20, or 30 mg/kg compound (+)-22a and placed into the open field. After 15 min each animal was removed and given Veh or 3 mg/kg d-amphetamine (AMPH) and returned immediately to the open field for an additional 105 min. Controls consisted of separate groups of mice administered Veh-Veh or 20 mg/kg compound (+)-22a + Veh. The results are depicted as total distance traveled in 5-min intervals over testing or cumulative distance traveled over the 15 min of baseline and 75 min following injection of AMPH or the vehicle.
Prepulse Inhibition.
Prepulse inhibition (PPI) was assessed using the SR Startle Lab Response system for mice (San Diego Instruments, San Diego, CA). Adult C57BL/6 males (n = 8–11 mice/treatment group) were administered the vehicle or 0.5, 1, 2, or 4 mg/kg compound (+)-22a followed 10 min later with vehicle or 3 mg/kg AMPH. Mice were placed into a Plexiglas holder which was positioned into the test chamber that was interfaced to a computer running SR Lab Startle Reflex software.40 After a 10 min acclimatization period, mice were exposed to 42 test trials composed of 18 pulse-only trials, 18 prepulse-pulse trials, and 6 null trials. A 62 dB white noise background was present during testing. Pulse-only trials consisted of a single 40 ms 120 dB burst of white noise. Prepulse-Pulse trials consisted of a 20 dB prepulse of white noise that was 4, 8, or 12 dB above the 62 dB background, followed 100 ms later with the 120 dB pulse. The white noise background was the only stimulus present during the null only trials. Testing began and ended with a block of 5 pulse-only trials; the remaining pulse-only trials were randomized with the null trials and the prepulse-pulse trials between these two blocks of pulse-only trials. The percent PPI for each prepulse intensity was calculated as the ratio of the average response during the prepulse-pulse trials to the average pulse-only responses, subtracted from 1, and expressed as a percentage. Null activity and pulse-only responses are reported in arbitrary units (AU) as measured by the SR Startle Lab Response system. The peak measure of the startle response (AU) was measured within a 65 ms window following onset of the pulse-only stimulus.41
Novel Object Recognition Memory.
Wild type (WT) and NR1-KD mice (n = 9–10 mice/genotype/treatment) were randomly assigned to three treatment groups that received Veh or 0.5 or 1 mg/kg compound (+)-22a. Testing was conducted over three days with a single trial (5 min) on each day.40 Mice were acclimated to the empty test chambers (38 × 22 × 20 cm) on day 1 and exposed to two identical objects on day 2. Twenty-four h later mice were presented with one of the same objects from day 2 which was paired with a novel object to evaluate long-term memory (LTM). Mice were administered Veh or compound (+)-22a 30 min prior to the trials on days 2 and 3. All trials were video-recorded using the Noldus Media Recorder (Noldus Technologies, Wageningen, Netherlands) and behavioral data were analyzed using Noldus Ethovision 10. Duration of time spent investigating the objects was scored. The results are presented as preference scores; Object Preference = (time spent with novel object – time spent with familiar object) / total time with both objects. Positive scores denote preference for the novel object, negative scores signify preference for the familiar object, and scores approaching zero indicate no preference for either object.
Catalepsy.
Male C57BL/6J mice (Jackson Laboratories; n = 8–10 mice/treatment/time-point) were assessed for cataleptic responses. Baseline cataleptic responses were assessed following removal from the home-cage by placing both front paws on a 60 cm horizontal bar (2.5 mm diameter) elevated above the bench with both rear paws in direct contact with the bench. Immediately following the baseline assessment, each animal was injected with vehicle, haloperidol, or compound (+)-22a and returned to its home cage. Catalepsy was evaluated in separate groups of mice at 60 min. The time spent in catalepsy was measured as the time following placement to removal of the front paws from the bar, with a maximum cut-off time of 60 seconds.
Statistics.
All data were analyzed using SPSS 22 (IBM SPSS, Chicago, IL) and are presented as means and standard errors of the mean (SEM). Distance traveled in the open field was assessed with RMANOVA with time as the within subject effect and treatment condition as the between subject effect. For PPI, null and startle activities were assessed using ANOVA with treatment as the between subject variable. Differences in PPI were examined with RMANOVA with prepulse intensity as the within subject variable and treatment condition as the between subject variable. Responses in NORM were evaluated using ANOVA for preference scores in the LTM test with genotype and treatment as independent variables. Duration of contact with the objects during the training session and the LTM test were analyzed with RMANOVA using test-session as the within subject variable and genotype and treatment as the between subject variables. For time spent in catalepsy, a one-way ANOVA was performed examining the effects of treatment. Post-hoc comparisons were performed with Bonferroni corrected pair-wise comparisons for all analyses. In all cases, a p < 0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
Financial support from the National Institute of Health (NIH) (No. R01MH99993) and NIMH Psychoactive Drug Screening Program is gratefully acknowledged. We thank Dr. Werner Tueckmantel for proofreading the manuscript and providing valuable suggestions.
ABBREVIATIONS USED
- 5-HT
serotonin
- ADMET
absorption, distribution, metabolism, excretion and toxicity
- AMPH
d-amphetamine
- BBB
blood–brain barrier
- CNS
central nervous system
- CYP
cytochrome
- FDA
US Food and Drug Administration
- GPCR
G-protein coupled receptor
- HEK-293
Human Embryonic Kidney 293 cells
- hERG
human ether-à-go-go-related gene
- HPLC
high-performance liquid chromatography
- KD
knockdown
- LTM
long-term memory
- NMDA
N-methyl-D-aspartate
- NORM
novel object recognition memory
- SAR
structure–activity relationship
- Veh
vehicle
- WT
wild type
Footnotes
Supporting Information
Synthetic procedures for compounds 16a–g, 18a–g, and 19a–g, and characterization data of all chemical intermediates can be found in this section. This material is available free of charge on the ACS Publications website at http://pubs.acs.org.
References
- 1. http://www.who.int/mental_health/management/schizophrenia/en/
- 2.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray JA; Roth BL The pipeline and future of drug development in schizophrenia. Molecular Psychiatry 2007, 12, 904–922. [DOI] [PubMed] [Google Scholar]
- 4.Harvey PD; Keefe RSE Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. Am J Psychiatry 2001, 158, 176–184. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg TE; Goldman RS; Burdick KE; Malhotra AK; Lencz T; Patel RC; Woerner MG; Schooler NR; Kane JM; Robinson DG Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia - Is it a practice effect? Arch Gen Psychiatry 2007, 64, 1115–1122. [DOI] [PubMed] [Google Scholar]
- 6.Meltzer HY; Roth BL Lorcaserin and pimavanserin: emerging selectivity of serotonin receptor subtype-targeted drugs. J Clin Invest 2013, 123, 4986–4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger M; Gray JA; Roth BL The Expanded Biology of Serotonin. Ann Rev Med 2009, 60, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunlop J; Watts SW; Barrett JE; Coupet J; Harrison B; Mazandarani H; Nawoschik S; Pangalos MN; Ramamoorthy S; Schechter L; Smith D; Stack G; Zhang J; Zhang G; Rosenzweig-Lipson S Characterization of vabicaserin (SCA-136), a selective 5-hydroxytryptamine 2C receptor agonist. J Pharmacol Exp Ther 2011, 337, 673–680. [DOI] [PubMed] [Google Scholar]
- 9.Shen JH; Zhao Y; Rosenzweig-Lipson S; Popp D; Williams JB; Giller E; Detke MJ; Kane JM A 6-week randomized, double-blind, placebo-controlled, comparator referenced trial of vabicaserin in acute schizophrenia. J Psychiatr Res 2014, 53, 14–22. [DOI] [PubMed] [Google Scholar]
- 10.Lee MA; Jayathilake K; Sim MY; Meltzer HY Decreased serotonin2C receptor responses in male patients with schizophrenia. Psychiatry Res 2015, 226, 308–315. [DOI] [PubMed] [Google Scholar]
- 11.Huang M; Dai J; Meltzer HY 5-HT2A and 5-HT2C receptor stimulation are differentially involved in the cortical dopamine efflux-Studied in 5-HT2A and 5-HT2C genetic mutant mice. Eur J Pharmacol 2011, 652, 40–45. [DOI] [PubMed] [Google Scholar]
- 12.Rauser L; Savage JE; Meltzer HY; Roth BL Inverse agonist actions of typical and atypical antipsychotic drugs at the human 5-hydroxytryptamine2C receptor. J Pharmacol Exp Ther 2001, 299, 83–89. [PubMed] [Google Scholar]
- 13.Kroeze WK; Hufeisen SJ; Popadak BA; Renock S; Steinberg SA; Ernsberger P; Jayathilake K; Meltzer HY; Roth BL HI-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology 2003, 28, 519–526. [DOI] [PubMed] [Google Scholar]
- 14.Marquis KL; Sabb AL; Logue SF; Brennan JA; Piesla MJ; Comery TA; Grauer SM; Ashby CR; Nguyen HQ; Dawson LA; Barrett JE; Stack G; Meltzer HY; Harrison BL; Rosenzweig-Lipson S WAY-163909 [(7bR,10aR)-1,2,3,4,8,9,10,10a-Octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1hi]indole]: A novel 5-hydroxytryptamine 2C receptor-selective agonist with preclinical antipsychotic-like activity. J Pharmacol Exp Ther 2007, 320, 486–496. [DOI] [PubMed] [Google Scholar]
- 15.Musil R; Obermeier M; Russ P; Hamerle M Weight gain and antipsychotics: a drug safety review. Expert Opin Drug Saf 2015, 14, 73–96. [DOI] [PubMed] [Google Scholar]
- 16.Calcagno E; Carli M; Baviera M; Invernizzi RW Endogenous serotonin and serotonin2C receptors are involved in the ability of M100907 to suppress cortical glutamate release induced by NMDA receptor blockade. J Neurochem 2009, 108, 521–532. [DOI] [PubMed] [Google Scholar]
- 17.Del’Guidice T; Lemay F; Lemasson M; Levasseur-Moreau J; Manta S; Etievant A; Escoffier G; Dore FY; Roman FS; Beaulieu JM Stimulation of 5-HT2C receptors improves cognitive deficits induced by human tryptophan hydroxylase 2 loss of function mutation. Neuropsychopharmacology 2014, 39, 1125–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nichols DE Hallucinogens. Pharmacol Ther 2004, 101, 131–181. [DOI] [PubMed] [Google Scholar]
- 19.Rothman RB; Baumann MH; Savage JE; Rauser L; McBride A; Hufeisen SJ; Roth BL Evidence for possible involvement of 5-HT2B receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation 2000, 102, 2836–2841. [DOI] [PubMed] [Google Scholar]
- 20.Thomsen WJ; Grottick AJ; Menzaghi F; Reyes-Saldana H; Espitia S; Yuskin D; Whelan K; Martin M; Morgan M; Chen W; Al-Shamma H; Smith B; Chalmers D; Behan D Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther 2008, 325, 577–587. [DOI] [PubMed] [Google Scholar]
- 21.DiNicolantonio JJC, S.; O’Keefe, J. H.; Meier, P. Lorcaserin for the treatment of obesity? A closer look at its side effects. Open Heart 2014, 1, e000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng J; Giguere PM; Onajole OK; Lv W; Gaisin A; Gunosewoyo H; Schmerberg CM; Pogorelov VM; Rodriguiz RM; Vistoli G; Wetsel WC; Roth BL; Kozikowski AP Optimization of 2-phenylcyclopropylmethylamines as selective serotonin 2C receptor agonists and their evaluation as potential antipsychotic agents. J Med Chem 2015, 58, 1992–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen G; Cho SJ; Huang XP; Jensen NH; Svennebring A; Sassano MF; Roth BL; Kozikowski AP Rational Drug Design Leading to the Identification of a Potent 5-HT(2C) Agonist Lacking 5-HT2 B Activity. ACS Med Chem Lett 2011, 2, 929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozikowski AP; Cho SJ; Jensen NH; Allen JA; Svennebring AM; Roth BL HTS and rational drug design to generate a class of 5-HT2C-selective ligands for possible use in schizophrenia. ChemMedChem 2010, 5, 1221–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho SJ; Jensen NH; Kurome T; Kadari S; Manzano ML; Malberg JE; Caldarone B; Roth BL; Kozikowski AP Selective 5-hydroxytryptamine 2C receptor agonists derived from the lead compound tranylcypromine: identification of drugs with antidepressant-like action. J Med Chem 2009, 52, 1885–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagmann WK The many roles for fluorine in medicinal chemistry. J Med Chem 2008, 51, 4359–4369. [DOI] [PubMed] [Google Scholar]
- 27.Kim D; Wang L; Beconi M; Eiermann GJ; Fisher MH; He H; Hickey GJ; Kowalchick JE; Leiting B; Lyons K; Marsilio F; McCann ME; Patel RA; Petrov A; Scapin G; Patel SB; Roy RS; Wu JK; Wyvratt MJ; Zhang BB; Zhu L; Thornberry NA; Weber AE (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)- yl]-1-(2,4,5-trifluorophenyl)butan-2-amine: a potent, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem 2005, 48, 141–151. [DOI] [PubMed] [Google Scholar]
- 28.Cui JJ; Tran-Dube M; Shen H; Nambu M; Kung PP; Pairish M; Jia L; Meng J; Funk L; Botrous I; McTigue M; Grodsky N; Ryan K; Padrique E; Alton G; Timofeevski S; Yamazaki S; Li Q; Zou H; Christensen J; Mroczkowski B; Bender S; Kania RS; Edwards MP Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J Med Chem 2011, 54, 6342–6363. [DOI] [PubMed] [Google Scholar]
- 29.Lawler CP; Prioleau C; Lewis MM; Mak C; Jiang D; Schetz JA; Gonzalez AM; Sibley DR; Mailman RB Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacology 1999, 20, 612–627. [DOI] [PubMed] [Google Scholar]
- 30.Wilcken R; Zimmermann MO; Lange A; Joerger AC; Boeckler FM Principles and applications of halogen bonding in medicinal chemistry and chemical biology. J Med Chem 2013, 56, 1363–1388. [DOI] [PubMed] [Google Scholar]
- 31.Voth AR; Khuu P; Oishi K; Ho PS Halogen bonds as orthogonal molecular interactions to hydrogen bonds. Nat Chem 2009, 1, 74–79. [DOI] [PubMed] [Google Scholar]
- 32.Toy PH; Dhanabalasingam B; Newcomb M; Hanna IH; Hollenberg PF A substituted hypersensitive radical probe for enzyme-catalyzed hydroxylations: Synthesis of racemic and enantiomerically enriched forms and application in a cytochrome P450-catalyzed oxidation. Journal of Organic Chemistry 1997, 62, 9114–9122. [Google Scholar]
- 33.Gooden DM; Schmidt DMZ; Pollock JA; Kabadi AM; McCafferty DG Facile synthesis of substituted trans-2-arylcyclopropylamine inhibitors of the human histone demethylase LSD1 and monoamine oxidases A and B. Bioorganic & Medicinal Chemistry Letters 2008, 18, 3047–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aronov AM Predictive in silico modeling for hERG channel blockers. Drug Discov Today 2005, 10, 149–55. [DOI] [PubMed] [Google Scholar]
- 35.Allen JA; Yost JM; Setola V; Chen X; Sassano MF; Chen M; Peterson S; Yadav PN; Huang XP; Feng B; Jensen NH; Che X; Bai X; Frye SV; Wetsel WC; Caron MG; Javitch JA; Roth BL; Jin J Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci U S A 2011, 108, 18488–18493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chitty K; Albrecht MA; Graham K; Kerr C; Lee JWY; Iyyalol R; Martin-Iverson MT Dexamphetamine effects on prepulse inhibition (PPI) and startle in healthy volunteers. Psychopharmacology 2014, 231, 2327–2337. [DOI] [PubMed] [Google Scholar]
- 37.Ramsey AJ NR1 knockdown mice as a representative model of the glutamate hypothesis of schizophrenia. Genetic Models of Schizophrenia 2009, 179, 51–58. [DOI] [PubMed] [Google Scholar]
- 38.Danion JM; Huron C; Vidailhet P; Berna F Functional mechanisms of episodic memory impairment in schizophrenia. Can J Psychiatry 2007, 52, 693–701. [DOI] [PubMed] [Google Scholar]
- 39.Horiguchi M; Meltzer HY The role of 5-HT1A receptors in phencyclidine (PCP)-induced novel object recognition (NOR) deficit in rats. Psychopharmacology (Berl) 2012, 221, 205–215. [DOI] [PubMed] [Google Scholar]
- 40.Park SM; Chen M; Schmerberg CM; Dulman RS; Rodriguiz RM; Caron MG; Jin J; Wetsel WC Effects of β-arrestin biased dopamine D2 receptor ligands on schizophrenia-like behavior in hypoglutmatergic mice. Neuropsychopharmacology 2015, in press, 10.1038/npp.2015.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geyer MA; Swerdlow NR Measurement of startle response, prepulse inhibition, and habituation. Curr Protoc Neurosci 2001, Chapter 8, 8.7.1–8.7.15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


