Abstract
Mitochondrial dysfunction is a well-established pathological event in Parkinson’s disease (PD). Proteins misfolding and its impaired cellular clearance due to altered autophagy/mitophagy/pexophagy contribute to PD progression. It has been shown that mitochondria have contact sites with endoplasmic reticulum (ER), peroxisomes and lysosomes that are involved in regulating various physiological processes. In pathological conditions, the crosstalk at the contact sites initiates alterations in intracellular vesicular transport, calcium homeostasis and causes activation of proteases, protein misfolding and impairment of autophagy. Apart from the well-reported molecular changes like mitochondrial dysfunction, impaired autophagy/mitophagy and oxidative stress in PD, here we have summarized the recent scientific reports to provide the mechanistic insights on the altered communications between ER, peroxisomes, and lysosomes at mitochondrial contact sites. Furthermore, the manuscript elaborates on the contributions of mitochondrial contact sites and organelles dysfunction to the pathogenesis of PD and suggests potential therapeutic targets.
Keywords: Parkinson’s disease, mitochondrial contact sites, mitochondria, endoplasmic reticulum, lysosome, peroxisome
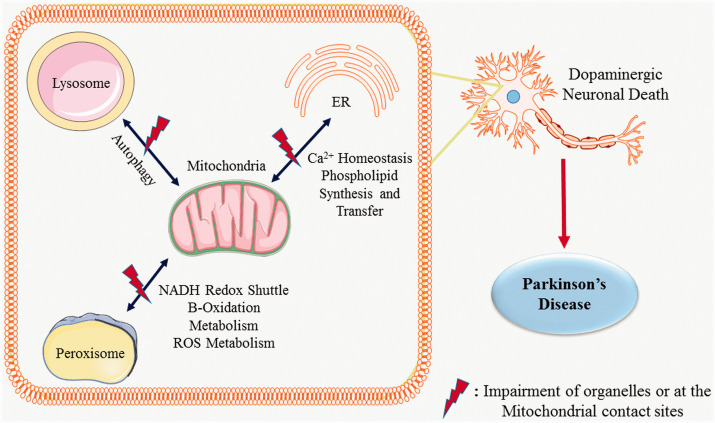
Loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and striatum (STr) and intra-neuronal accumulation of alpha-synuclein (α-syn) are the major pathological hallmarks of PD (Elfarrash et al., 2019; Wegrzynowicz et al., 2019). Dopamine plays a significant role in the regulation of voluntary movements and its decrease not only affects motor functions but also a spectrum of non-motor functions. Thus, it serves as a biomarker in the premotor phase of PD (Mulcahy et al., 2020; Schneider et al., 2021). Several recent reports suggest that dopaminergic dysfunction is associated with the accumulation of α-syn in the mitochondrial structure (Danyu et al., 2019; Ganjam et al., 2019). Mitochondrial dysfunction plays a critical role in the progression of PD by impairing fusion-fission, transport and selective autophagic degradation of misfolded proteins (Yamaguchi et al., 2020; Ahmed et al., 2021; Mani et al., 2021). Mitochondria communicate bi-directionally with endoplasmic reticulum, peroxisomes and lysosomes at the mitochondrial contact sites and maintain cellular homeostasis (Xia et al., 2019; Picca et al., 2020). ER is the largest store of Ca2+ in a cell. The ER-mitochondria contact site is called Mitochondria-associated membrane (MAMs) (Cherubini et al., 2020). Transfer of Ca2+ and phospholipid precursors to mitochondria from endoplasmic reticulum (ER) is the basic function at this site (Gomez-Suaga et al., 2017; Kostic et al., 2018; Che et al., 2021). Neurotoxins like rotenone (used to induce PD in rodent models) damage MAMs by altering mitochondrial Ca2+ homeostasis (Lee et al., 2018; Wu et al., 2018). Mitochondria and lysosomes interact at a contact site which is distinct from the mitophagy process, and that help to regulate cellular homeostasis (Wong et al., 2019). Additionally, lysosome and mitochondria form contact sites with ER for the transfer of calcium, phospholipids and cholesterol to the respective organelles (Atakpa et al., 2018; Deus et al., 2020). Lysosome-Mitochondria-ER contact sites are further regulated by lipid-transfer proteins such as the vacuolar protein sorting-associated protein 13 (VPS13) family, wherein VPS13A has been linked to ER-mitochondria contacts and VPS13C is linked to ER-lysosome contacts (Kumar et al., 2018). Mutations of VPS13 family genes are linked to different neurodegenerative diseases (Vonk et al., 2017) and interestingly, mutations in VPS13C are linked to PD pathology (Lesage et al., 2016). Lloyd-Evans and Waller-Evans (2019) reviewed lysosomal Ca2+ homeostasis and its role in various human diseases (Lloyd-Evans and Waller-Evans, 2019). Apart from Ca2+ signalling, lysosomal dysfunction was reported to affect the chaperone-mediated autophagic clearance of α-syn, which contributes to PD (Arias et al., 2015; Sun et al., 2019).
Mitochondria and peroxisomes work closely to maintain the lipid balance via fatty acid β-oxidation pathway (Cohen et al., 2014; Hosoi et al., 2017; Valm et al., 2017). Peroxisomes regulate mitochondrial dynamics and mitochondria-mediated apoptosis (Tanaka et al., 2019). Plasmalogen, a peroxisome-derived glycerophospholipid is an important component of mitochondrial membrane (Kimura et al., 2019) and its decrease has been reported in PD (Fabelo et al., 2011; Miville-Godbout et al., 2016). Altered metabolism and oxidative stress have been shown to play a crucial role in various neurodegenerative diseases including PD (Kurian et al., 2017; Kumar et al., 2019; L. Zhang et al., 2020; Xie et al., 2020).
Growing evidence suggests that the dysfunction in the mitochondrial contact sites with ER, lysosome and peroxisome are associated with PD. The present review summarises data on the individual organelle dysfunction and alterations in the inter-organellar crosstalk involving mitochondrial contact sites in PD. It also provides information on the role of redox signalling, autophagy, and potential therapeutic targets in pathogenesis of PD.
Mitochondrial Dysfunction in PD
Mitochondria specific genes such as PINK1, PARK2 (PARKIN), DJ-1 (PARK7) and leucine-rich repeat kinase 2 (LRRK2) play crucial roles in PD by regulating ROS homeostasis (Valente et al., 2004; Nichols et al., 2005; Guzman et al., 2010). PINK1 accumulation in the mitochondria activates PARKIN's E3 ubiquitin ligase activity and recruits PARKIN into the impaired mitochondria. This triggers the selective autophagic clearance of damaged mitochondria (Lazarou et al., 2012; Xi et al., 2021). Mutations in PINK1, PARKIN, DJ-1 and LRRK2 are linked to PD (Puspita et al., 2017). The involvement of mitochondrial dysfunction in PD was more clearly understood from an incident where several drug abusers self-injected 1-methyl-4-phenyl-1,2,3,6-tetrabydropyridine (MPTP). The bioactive form of MPTP is 1-methyl-4-phenylpyridinium (MPP+) which inhibits Complex I of the Electron Transport Chain (ETC) in mitochondria, resulting in increased ROS generation (Javitch et al., 1985; Mizuno et al., 1987). This in turn caused selective destruction of dopaminergic neurons in the SNPc and STr, resembling clinical symptoms of PD (Langston et al., 1983). Unlike nuclear DNA, mitochondrial DNA (mtDNA) is more vulnerable to oxidative damage due to lack of histones (Gureev et al., 2017). mtDNA encodes 13 essential proteins of ETC (Gustafsson et al., 2016) and their mutation/deletions triggers oxidative stress which results in neuronal damage (Iannielli et al., 2018). Polymerase γ1 (POLG1) enzyme is a nuclear-encoded gene product that plays an important role in polymerase synthesis and mtDNA maintenance (Ropp and Copeland, 1996). Mutations in POLG1 are shown to be associated with PD, and this corroborates with mtDNA mutation (Gui et al., 2015; Hsieh et al., 2019).
Impaired Mitophagy
The selective removal of damaged mitochondria by autophagosomes and the subsequent catabolism by lysosomes is termed mitophagy. Alterations in fission and fusion process (a crucial part of mitophagy) play an important role in neurodegenerative diseases like AD, PD, stroke etc. (Chen et al., 2021; Trombetta-Lima et al., 2021). The regulation of fission and fusion is governed by several key proteins including the dynamin-related protein 1 (DRP1), the mitochondrial fusion proteins 1, 2 (MFN1, MFN2) and the optic atrophy 1 (OPA1) protein (Yu-Wai-Man et al., 2014; Xi et al., 2018). Stressful conditions such as nutritional deficiency or exposure to toxins impairs mitochondria driven -MFN1, MFN2 and OPA1 mediate fusion process (Archer, 2013). Severe mitochondrial damage promotes DRP1 and FIS1 facilitates fission (X. Zhang et al., 2020) and produces smaller fragments of mitochondria for clearance by mitophagy (Chen et al., 2021). Despite this data, it still remains unclear as to how the defective mitochondria decide between fusion and fission. Deletion of the MFN2 gene in mouse induces ETC impairment and causes dopaminergic degeneration (Lee et al., 2012; Pham et al., 2012). Further, Berthet et al. (2014) showing that DRP1 gene knock-out in mouse resultss in depletion of mitochondria and dopaminergic degeneration leading to PD (Berthet et al., 2014).
Mutations in PINK1 and PARKIN, the major regulatory proteins in mitophagy are associated with PD pathogenesis (Vincow et al., 2013; Park and Koh, 2020). Furthermore, the involvement of other PD related genes like DJ-1, LRRK2 in mitophagy in PD pathogenesis is also well documented in various reports (Deas et al., 2011; J Liu et al., 2019; Wang et al., 2019).
Endoplasmic Reticulum Dysfunction in PD
In addition to its role in calcium storage and protein synthesis and regulation of mitochondrial energy metabolism, ER performs protein folding and trafficking (Field et al., 2007; Choi et al., 2017; Sowers et al., 2018; Lima et al., 2019). ER dysfunction or an unhealthy cellular environment produces misfolded proteins (Chen et al., 2019). The aggregation of misfolded proteins activates unfolded protein response (UPR) via ER stress sensors such as ATF6 (activating transcription factor 6), PERK (protein kinase RNA-like endoplasmic reticulum kinase) and IRE1 (inositol-requiring enzyme 1) (IRE1). This helps in protein-folding homeostasis. Activated sensors induce expression of genes that code for the proteins involved in the cellular clearance of misfolded proteins (Adams et al., 2019). ER stress-induced by glucose starvation, hypoxia, disruption of calcium homeostasis, or oxidative stress causes the accumulation of misfolded proteins. Prolonged ER stress and consistent accumulation of misfolded proteins stimulate the proapoptotic pathways in cells through activation of the transcription factor C/EBP homologous protein (CHOP) and cysteine proteases caspase-4/12 (Nakagawa et al., 2000; Ferri and Kroemer, 2001; Hitomi et al., 2004; Marciniak et al., 2004) leading to cell death. This was clearly demonstrated in 6-OHDA mouse model of PD, where the upregulated CHOP causes neuronal death through apoptosis (Sun et al., 2013; Aimé et al., 2020). Similarly, knockdown of ATF6, a UPR protein, is shown to aggravate MPTP induced neurotoxicity in a mouse model (Egawa et al., 2011). Consistent with the experimental reports on PD models (Bellucci et al., 2011), the post mortem examination of PD patients’ brains revealed the activation of UPR protein with α-syn co-localization (Hoozemans et al., 2012). Further, accumulation of misfolded α-syn is reported to activate IRE1-induced autophagy-dependent neuronal death in a drosophila model of PD. Thus inhibition of IRE1 is found to be neuroprotective (Yan et al., 2019). Knockdown of Herp (ER stress protein) and mutation in LRRK2 exacerbated the ER-stress mediated neuronal death and α-syn aggregation in PD (Belal et al., 2012; Lee et al., 2019). These data indicate that ER dysfunction has a crucial impact on PD pathogenesis.
ER–Mitochondria Contact Sites
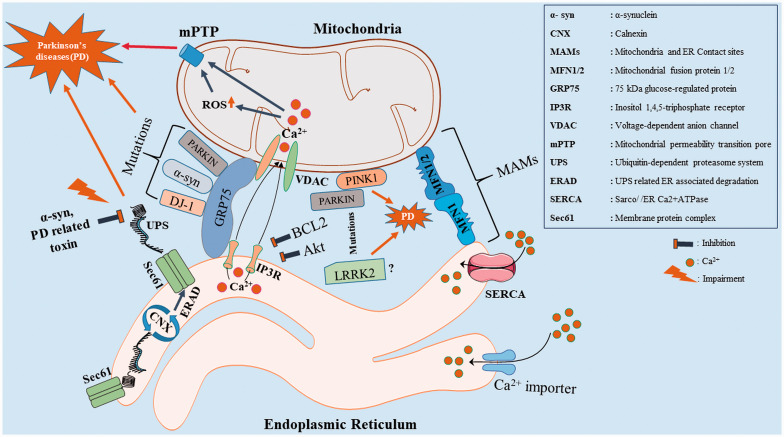
Bernhard and Rouiller (1956), initially explained the inter-organellar contact sites between mitochondria and ER (Bernhard and Rouiller, 1956). ER contact sites with mitochondria are termed as “Mitochondria-Associated Membranes (MAMs)”. About 5 to 20% of the mitochondrial surface is physically in contact with ER and varies depending on the cell type (Rizzuto et al., 1998). A symbiotic relationship between ER and mitochondria is crucial for the maintenance of cellular Ca2+ homeostasis (Calvo-Rodríguez et al., 2016; Wu and Kao, 2016; Butler et al., 2017). Calcium concentration in ER regulates several enzymes involved in the synthesis of secretory proteins such as N-glycosylation and oxidative protein folding like calnexin or calreticulin (Guo et al., 2020; Kweon et al., 2020). Cytosolic chaperone GRP75 is the first complex identified in mammalian cells that bridges the mitochondrial voltage-dependent anion channel (VDAC) of the outer mitochondrial membrane (OMM) with inositol 1,4,5-triphosphate receptor (IP3R) of ER and forms MAMs (Szabadkai et al., 2006; Yeo et al., 2021). MAMs allow the exchange of lipids (Shiao et al., 1995), calcium (Ca2+) (Rizzuto et al., 1998; Csordás et al., 1999) and reactive oxygen species (Verfaillie et al., 2012; Eisner et al., 2013) between both the organelles. This allows for = their adaptations in cellular stress. Knockdown of GRP75 is reported to decrease the transfer of Ca2+ from the ER to mitochondria (Csordás et al., 2006). MFN1 and MFN2, the two key mitochondrial fusion proteins are reported to strengthen the MAMs and facilitate mitochondrial Ca2+ uptake (Filadi et al., 2016; Che et al., 2021). MFN2 is localized at both ER and mitochondrial membranes. At the MAMs MFN1 and MFN2 form homo-and heterotypic interactions, the latter with MFN1 (Szabadkai et al., 2006; de Brito and Scorrano, 2008). In addition, knockout of MFN2 is reported to decrease ER-mitochondria contact and crosstalk (Krols et al., 2016). Calcium homeostasis is also assisted by cytosolic Ca2+ reuptake into ER through the sarco/endoplasmic reticulum (SR/ER) Ca2+ATPase pump (SERCA) (Chemaly et al., 2018). Alterations in SERCA due the mutations, toxins or DNA damage increases the Ca2+ transfer from ER to mitochondria. This causes mitochondrial Ca2+ overload leading to apoptosis through the opening of mPTP (mitochondrial permeabilization transition pore), cytochrome c release and other pro-apoptotic factors (Bittremieux et al., 2016).
With respect to ER-Mitochondria contacts, pathogenic mutations of α-syn are reported to be localized in MAMs (Guardia-Laguarta et al., 2014; Paillusson et al., 2017). Dysfunction in MAMs alters the IP3R-GRP75-VDAC complex, mitochondrial Ca2+ homeostasis and leads to mitochondrial dysfunction and causes dopaminergic death in PD (Calì et al., 2012; Guardia-Laguarta et al., 2015). Furthermore, knockout of DJ-1 disrupts the IP3R-GRP75-VDAC complex and forms a causative factor for PD (Y. Liu et al., 2019). Another PD associated protein PINK1 is involved in mitophagy process.LRRK2 is also reported to be localized in MAMs and its dysfunction causes PD (Park and Koh, 2020; Toyofuku et al., 2020; Zhao et al., 2021). PARKIN, an E3 ubiquitin ligase is associated with MFN2 at MAMs and supports Ca2+ homeostasis. Hence, mutations in PARKIN are shown to decrease the tethering between ER and mitochondria, which is strongly linked to PD (Palacino et al., 2004; Calì et al., 2013; Basso et al., 2018). Hailey et al. (2010) demonstrated the recruitment of autophagy-related proteins (ATG14, ATG5) at MAMs during starvation in cells (Hailey et al., 2010; Hamasaki et al., 2013) and also reported that the knockdown of MFN2 gene inhibits the formation of autophagosomes. Here, it is important to mention that dysregulation in autophagy is one of the major pathological hallmark in PD (Filadi et al., 2018; Janikiewicz et al., 2018). PINK1 and Beclin1 (autophagy marker) localize in the MAMs. Silencing of PINK1 impairs Beclin1 enrichment and triggers cell death in SH-SY5Y cells (Gelmetti et al., 2017). These leads to disruption in the crosstalk between mitochondria and ER (MAMs) and plays a crucial role in PD (Figure 1 and Table 1).
Figure 1.
Diagram Shows the Physical Interactions Between the ER and Mitochondria (MAMs) From the Perspective of PD Associated Proteins (α-syn, PINK1, PARKIN, DJ-1, LRRK2). MAMs comprise of molecules like IP3R-GRP75-VDAC, MFN1-MFN1/2. IP3R-Grp75-VDAC complexes which are essential for Ca2+ flux between the ER and mitochondria. MFN 1&2 are involved in mitochondrial fission and fusion and mitophagy. Alterations in DJ-1, α-syn and PARKIN impair IP3R-GRP75-VDAC complex (weakening the MAMs and trigger the Ca2+ induced mitochondrial permeability transition pore (mPTP) dependent apoptosis) and lead to dopaminergic cell death. PINK1 recruits PARKIN in mitophagy process and their mutations alter the mitochondrial fission - fusion. LRRK2 (? – denotes that the exact function at MAMs is unclear), a mitochondrial specific protein associated with PD is also reported to be localized in MAMs. Furthermore, the ubiquitin-dependent proteasome system (UPS) is involved in the clearance of unfolded protein (proper folding of protein is confirmed through calnexin (CNX) folding cycle) degradation. Alterations in the UPS related ER associated degradation (ERAD) pathway cause α-syn accumulation which in implicated in PD.
Table 1.
List of PD Related Genes Associated With MAMs.
| Genes | Functions at ER-Mito contact site | Link with PD Pathophysiology | References |
|---|---|---|---|
| α-syn | α-syn associates with GRP75 protein. | The exact pathophysiology of α-syn mutations associated with MAMs in PD is yet to be studied | (Calì et al., 2013; Guardia-Laguarta et al., 2014, 2015) |
| DJ-1 | DJ-1 interacts with Grp 75 protein, promotes Ca2+ transfer | Mutations in DJ-1 alters Calcium homeostasis in MAMs | (Schon & Przedborski, 2011; Ottolini et al., 2013) |
| PARKIN | PARKIN associates with Grp 75 and MFN2 proteins | Mutations in PARKIN at MAMs are involved in altered autophagy | (Jin et al., 2007; Tanaka et al., 2010; Schon & Przedborski, 2011; Calì et al., 2012) |
| PINK1 | PINK1 recruits PARKIN to the OMM during mitophagy, where PARKIN ubiquitinates substrates including MFN2 | Mutations in PINK1 at MAMs are involved in altered autophagy | (Tanaka et al., 2010; Schon & Przedborski, 2011) |
Lysosomal Dysfunction in PD
The mitochondria-lysosomal contact sites through autophagosomes play a crucial role in the autophagy, biogenesis of both the organelles and exchanges of lipid, amino acids etc. Autophagy is essential for neuronal survival and serves as an adaptive response to nutrient deprivation (Clarke and Mearow, 2016; Fan et al., 2020). Lysosomes play an important role in clearing the damaged cellular components through autophagy (Li et al., 2019; Bi et al., 2021). Lysosomes contain more than 60 hydrolases which facilitate the removal of damaged cellular components and aggregated proteins (Eriksson et al., 2020). Interestingly, lysosomal proteolytic enzymes decline with ageing (Mazzulli et al., 2016). Mutations in lysosomal proteins - lysosomal type 5 P-type ATPase (ATP13A2) are reported to cause PD (Dehay et al., 2012; Mangone et al., 2020) while glucocerebrosidase (GBA), a lysosomal glycoside hydrolase is shown to be associated with pathology PD. Decrease in another chaperone-mediated autophagy protein and lysosomal-associated membrane protein 2 A (LAMP2) is linked to α-syn accumulations (Alvarez-Erviti et al., 2013). Thus, lysosomal dysfunction and associated impaired autophagy contribute to PD (He et al., 2018). The process of autophagy is regulated by mammalian target of the Rapamycin (mTOR) pathway in eukaryotes (Nicklin et al., 2009; Xi et al., 2019). The importance of attenuation of PI3K-Akt-mTOR activation has been well established in experimental models of PD (Inoki et al., 2005; Elstner et al., 2011; Heras-Sandoval et al., 2014; Xu et al., 2014). Rodríguez-Blanco et al. (2012) reported that MPP+ induces inhibition of the Akt/mTOR pathway due to increase in accumulation of ROS (Rodríguez-Blanco et al., 2012). Ablation of PTEN, a negative regulator of Akt (upstream) activates mTOR signalling and is neuroprotective in mouse models of PD (Domanskyi et al., 2011). Although the exact mechanisms involved in the pathogenesis and etiology of PD remain unknown, accumulating evidence suggests a major role of mTOR signalling (Bockaert and Marin, 2015; Dijkstra et al., 2015).
Lysosome–Mitochondria Contact Sites and PD
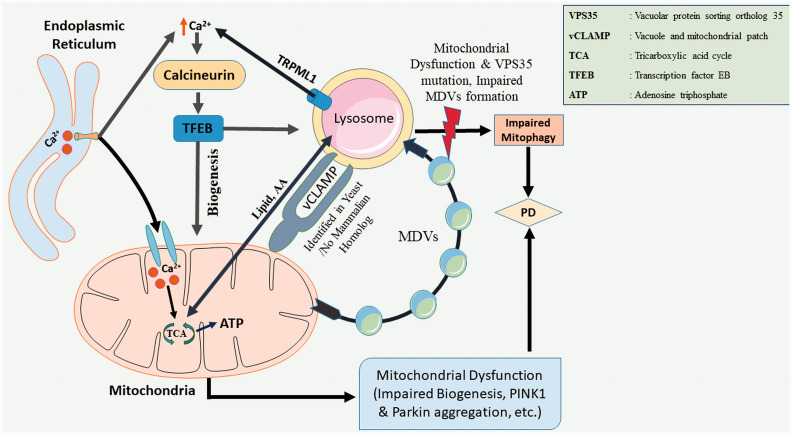
Mitochondria-lysosome contact sites can regulate mitochondrial dynamics by promoting mitochondrial fission and inter-mitochondrial contact site untethering (Wong et al., 2018, 2019). In yeast, mitochondria and vacuole (yeast version of lysosomes) develop a physical communication called “vacuole and mitochondrial patch” (vCLAMP) consisting of YPT7, LAM6, VPS39 proteins and an unknown mitochondrial component (Elbaz-Alon et al., 2014; Hönscher et al., 2014; Soto-Heredero et al., 2017). YPT7-VPS39 binds with a subunit of the translocase of outer membrane (TOM) of mitochondria (González Montoro et al., 2018). In mammalian cells, mitochondria-lysosome contact sites form with ∼ 15% of lysosomes in contact with mitochondria at any given time point (Wong et al., 2018). In addition, release of Ca2+ from lysosomes to mitochondria at these contact sites also play an important role in regulating mitochondrial functions (W. Peng et al., 2020). Efflux of Ca2+ from the lysosomes is triggered through TRPML1, transient receptor potential mucolipin (TRPML) subfamily, which is activated by an increase in ROS levels (Zhang et al., 2016). A decline in TRPML1 causes accumulation of ROS, which results in fragmented mitochondria and loss of mitochondrial membrane potential (Coblentz et al., 2014). Ca2+ release from the lysosome activates calcineurin, a Ca2+ dependent phosphatase which stimulates the transcription factor EB (TFEB) and initiates the transcription of PGC1α (Peroxisome proliferator-activated receptor gamma coactivator 1-alpha) and PPARα (peroxisome proliferator-activated receptor α) genes. Thus, Ca2+ release from lysosomes controls mitochondrial biogenesis (Mansueto et al., 2017; Todkar et al., 2017). However, excessive ROS levels may cause lysosomal dysfunction and autophagic failure and cell death (Li et al., 2015; Zhang et al., 2016).
MCOLN1calcineurin-dependent TFEB activation clears α-syn through initiation of autophagy (Ivankovic et al., 2016; Zhuang et al., 2020). Dephosphorylated form of TFEB translocates to the nucleus and regulates gene expressions for various genes (Napolitano and Ballabio, 2016). Under starvation, mTORC1 dephosphorylates and translocate TFEB to nucleus and facilitates the autophagy process (Martina et al., 2012).
In stress conditions, PARKIN and PINK1 facilitate the formation of mitochondrial-derived vesicles (MDVs) and these vesicles eliminate defective proteins and lipids from mitochondria via autophagy (McLelland et al., 2014; Matheoud et al., 2016; Jian et al., 2018). However, there is no evidence for these occurring at mitochondria-lysosome contact sites. Interestingly, a small fraction of MDVs is transported to peroxisomes (Sugiura et al., 2014), while the majority is transferred to lysosomes for degradation. Mitochondrial dysfunction causes a decrease in the formation of MDVs resulting in the accumulation of misfolded proteins. Mutations in PINK1 and PARKIN are directly linked to decrease in the formation of MDVs (Vincow et al., 2013; McLelland et al., 2014). In addition, vacuolar sorting protein 35 (VPS35) has a role in MDVs formation.
Acute mitochondrial stress induces lysosomal biogenesis whilst chronic mito-stress inhibits lysosomal biogenesis (Fernández-Mosquera et al., 2017). Defects in lysosomes disrupt the autophagy-lysosomal pathway (Plotegher and Duchen, 2017; Todkar et al., 2017) and impairs the autophagic clearance of misfolded proteins. This contributes to lysosomal dysfunction(Koike et al., 2008; Xie et al., 2015). Dysfunction of the lysosomal and mitochondrial contact sites impairs autophagy and triggers the accumulation of α-syn leading to PD (Decressac et al., 2013; Moors et al., 2017). Silencing of a mitochondrial localized (Junn et al., 2009) PD related gene, DJ-1 is found to be associated with the accumulation of autophagy markers in M17 neuroblastoma cell lines (Thomas et al., 2011). In support of the above-mentioned data, mutations in PARKIN gene (PARK2) are also reported to cause mitochondrial (Pacelli et al., 2011) and lysosomal (Guerra et al., 2019) dysfunction. To add, alterations in lysosomal (TFEB) and mitochondrial (PGC1α) transcription factors i.e impairment of inter-organelle communication influences PD progression advertently (Smolders and Van Broeckhoven, 2020). Mutations in VPS35 is shown to impair vacuolar transport. This is observed in PD (Vilariño-Güell et al., 2011; Zhou et al., 2017). Another protein, VPS13C (a human homolog of VPS13) encodes a member of the vacuolar protein sorting-associated 13 gene family. Mutations in VPS13 in yeast are associated with vCLAMP tethering between lysosome-mitochondria (Lang et al., 2015; Park et al., 2016; Peter et al., 2017) and triggers the mitochondrial dysfunction associated with PD (Lesage et al., 2016). RAB7 is a GTPase and is the key modulator of lysosomal maturation, positioning and network dynamics (Zhen and Stenmark, 2015). RAB7 also regulates the mitochondrial–lysosome tethering and untethering (Stroupe, 2018; Wong et al., 2018). An important PD related gene, LRRK2 knockout has been reported to deplete RAB7 (Dodson et al., 2012; Esteves et al., 2015), and cause aggregation of α‐syn (Dinter et al., 2016). Interestingly, increased levels of LRRK2 are also observed in larger granular structures along with RAB7 in the post-mortem brains of patients with Lewy body disease (Higashi et al., 2009). Deficiency of the mitochondrial specific protein PINK1 causes extension of vacuolar chamber and lysosomal dysfunction which impairs mitophagy and is a risk factor in PD (Demers-Lamarche et al., 2016). Altered organelle proteins at mitochondria-lysosomal contacts sites lead to impairment of autophagy/mitophagy and redox signalling, which are potential targets in PD drug discovery (Figure 2 and Table 2).
Figure 2.
Lysosome-Mitochondria Connection in PD. In yeast, mitochondria and lysosome establish a physical connection through a vacuole and mitochondria patch (vCLAMP), no mammalian homolog has been identified yet. This connection facilitates the exchange of lipids and nutrients between the organelles. TRPML1, a lysosomal calcium channel, acts as a ROS sensor releases Ca2+ in cytosol and activates Calcineurin/TFEB signalling cascade, and initiates autophagy. Mitochondrial-derived vesicles (MDV) remove damaged proteins and lipids from the intact mitochondria. Vacuolar sorting protein 35 (VPS35) plays a key role in MDVs formation. Impaired autophagy and mutations in VPS35 are associated with PD pathogenesis.
Table 2.
Mitochondria and Endo-Lysosomal Genes Associated PD.
| Genes | Functions | Pathophysiology | References |
|---|---|---|---|
| α-syn | Synaptic Vesicles recycling | Accumulation of α-syn | (Lin et al., 2019) |
| PINK1/ PARKIN | Mitophagy | Occasional α-syn accumulation | (Demers-Lamarche et al., 2016) |
| VPS35 | Protein trafficking, mediating MDVs to lysosome and in MQC | Mutations in VPS35 is associated with mitochondrial dysfunction; Dopaminergic cell death | (Tanner et al., 2011; Soubannier et al., 2012; Follett et al., 2014; Tang et al., 2015) |
| LRRK2 | Endo-lysosomal trafficking | α-syn accumulation, LRRK2 mutation is associated with DRP1/DLP1 in PD. Further DRP1 mediated mitochondrial dysfunction is associated with VPS35 mutations | (Niu et al., 2012; Yang et al., 2014; Roosen & Cookson, 2016; Wang et al., 2016) |
| ATP13A2 | Cation homeostasis | Mutations of ATP13A2 is linked with lysosome and mitochondrial dysfunctions and impairs autophagy. It is a late endosomal/lysosomal polyamine exporter. | (Gusdon et al., 2012; Ramonet et al., 2012; Korvatska et al., 2013; van Veen et al., 2020) |
| DJ-1 | Redox homeostasis | Mitochondrial dysfunction and impaired autophagy | (Hao et al., 2010; McCoy & Cookson, 2011; Thomas et al., 2011) |
Peroxisome Dysfunction in PD
Peroxisomes play important role in several biochemical pathways like fatty acid (FA) oxidation, glyoxylate metabolism, bile acid and docosahexaenoic acid synthesis, catabolism of amino acids, polyamines and purines, and synthesis of plasmalogens and cholesterol (Fransen et al., 2012; Waterham et al., 2016; Cipolla and Lodhi, 2017). Peroxisomes consume about 20% of the total cellular oxygen (Legakis et al., 2002) and account for 35% of total H2O2 generation in mammalian tissues (Boveris et al., 1972). Thus they were originally named based on their role in hydrogen peroxide production and catabolism (Fransen et al., 2012).
Peroxisomal biogenesis factor- 5 (PEX5), a redox-sensitive protein imports the ataxia-telangiectasia mutated (ATM), a phosphatidylinositol-3 kinase-like protein kinase which inhibits mTORC1, thereby peroxisomes participates in autophagy process (Zhang et al., 2013, 2015). Null mutations in either Peroxisomal Biogenesis Factor 2, 5 or 13 (PEX2, PEX5 or PEX13) genes are shown to increase the accumulation of α-syn, which in turn impairs pexophagy (Yakunin et al., 2010). Plasmalogens, a class of glycerophospholipids are an integral part of the cellular membrane (Lessig and Fuchs, 2009). Peroxisomes are involved in critical steps of plasmalogen biosynthesis. Decreased plasmalogens levels are recorded in the blood and brains of PD patients (Miville-Godbout et al., 2016, 2017). However, the pathogenic mechanism behind the decrease in plasmalogens levels in PD is still not clear. Furthermore, dysfunction of peroxisomes is a risk factor in PD (Yakunin et al., 2010). None-the-less, it is evident that peroxisomes play a crucial role in redox homeostasis and pexophagy.
Peroxisome–Mitochondria Contact Sites and PD
Mitochondria and peroxisomes share similarities in common biochemical pathways such as fatty acid β-oxidation, ROS homeostasis and clearance of defective organelles(Pascual-Ahuir et al., 2017). The functional and physical interactions of peroxisomes with mitochondria was first reported in yeast (Ušaj et al., 2015). In yeast, peroxisomes localize near sites where mitochondria and ER contact each other (Costello et al., 2017; Ušaj et al., 2015). A recent report demonstrated the potential physical tether between peroxisomes and mitochondria. This tethering involves contact between PEX11 protein (which involve in peroxisomes biogenesis) and the mitochondrial MDM34 protein (Ušaj et al., 2015). Subsequently, MDM34 is a crucial structural constituent of the mitochondria-ER tether of ERMES (Kornmann et al., 2009). Fan et al. (2016) demonstrated the existence of peroxisomal-mitochondrial contact sites in mammalian cells (Fan et al., 2016). The contact sites facilitate the passage of ions and lipids (Cohen et al., 2014). Shai et al. (2018) reported that the mitochondria and peroxisomal contact sites are strengthened with two tethering proteins, FZO1 (the yeast mitofusin protein and a homolog of the human MFN1 and MFN2) (De Vecchis et al., 2017) and PEX34 (a peroxisomal membrane protein, whose molecular function is unclear) (Shai et al., 2018). Mitochondria and peroxisomes share the same fission proteins DRP1 and FIS1 (Schrader, 2006; Camões et al., 2009; Delille et al., 2009). Supporting the mitochondria and peroxisomal cross-talk, it was observed that ablation of peroxisomal biogenesis genes PEX3 or PEX5 triggers mitochondria-dependent apoptosis (Tanaka et al., 2019). Mao et al. (2014) reported that mitochondria and peroxisomes also share the same autophagy proteins Atg11 scaffold and the DRP1/DNM1-containing fission complex in selective autophagy (mitophagy and pexophagy). It is also reported that pexophagy process is associated with mitochondria-peroxisome contact sites (Mao et al., 2014; Zhong et al., 2016). Alterations in peroxisome biogenesis (Yakunin et al., 2010), pexophagy (Yakunin et al., 2010; Eun et al., 2018; Wang et al., 2020) and mitophagy(Vives-Bauza and Przedborski, 2011) are linked to the abnormal accumulations of α-syn in brain. Furthermore, recent investigations have demonstrated the importance of peroxisome in cancer, ageing and various other neurodegenerative diseases (Fransen et al., 2013; Islinger et al., 2018).
In yeast, the ERMES complex strengthens the contact site and facilitates the exchange of ions/proteins between mitochondria and ER in yeast. On the other hand, peroxin-11 on binding to the ERMES complex at MDM34 facilitates the transfer of information transfer between mitochondria and peroxisomes (Ušaj et al., 2015). The vCLAMP has also been reported to support phospholipid, iron and Ca2+ exchange at the ERMES (Elbaz-Alon et al., 2014). Dysfunctions in the communications or the contact sites of the involved organelles are reported in various neurodegenerative diseases including PD and involve several pathological pathways. Connecting the evidence, peroxisomal impairment in lipid and ROS metabolism impacts the mitochondrial redox homeostasis (Lismont et al., 2015). It is reported that excessive ROS production due to peroxisomal impairment leads to mitochondria-mediated cell death (López-Erauskin et al., 2012). Ganglioside-induced differentiation-associated protein 1 (GDAP1) regulates the mitochondrial network. While the mutation of GDAP1 impairs the mitochondrial fragmentations in neuroblastoma N1E-115 cells(Niemann et al., 2005), the absence of GDAP1 causes the elongation of peroxisomes(Huber et al., 2013). Mitochondrial and peroxisomal dysfunctions are linked to PD pathogenesis (Subramaniam and Chesselet, 2013). In addition to the MDVs transport to lysosome, vesicular transport is also reported in peroxisomes (Sugiura et al., 2014). However, the reason for the MDVs delivery to peroxisomes is unclear (Mohanty and McBride, 2013). At present, a membrane-anchored protein ligase called MUL1/MAPL (mitochondria-anchored protein ligase) is reported to be delivered to the peroxisomes (Neuspiel et al., 2008; Braschi et al., 2009). The retromer complex containing VPS35, VPS26 and VPS29 is identified as a MAPL binding partner using affinity chromatography approach (Braschi et al., 2010). It was reported that VPS35 participates in various cellular transport pathways and its defect have been linked to mitochondrial dysfunction and PD (Sugiura et al., 2014). Deletion of VPS35 gene in SH-SY5Y and NLT cell lines causes intracellular accumulation of α-syn (Tang et al., 2015). A separate report, in a Chinese cohort study, showed that the gene MUL1 is a risk factor for PD (Taximaimaiti and Li, 2019). Another protein, MIRO1, a Rho GTPase gets localized both in the peroxisomes and mitochondria in mammals (Castro et al., 2018; Okumoto et al., 2018; Farré et al., 2019). MIRO1 modulates the dynamics of both the organelles (Covill-Cooke et al., 2020). Here it is noteworthy that Gem1, a yeast orthologue of MIRO is an important regulator of mitochondrial and peroxisomal contact sites (Shai et al., 2018). In PD patients, α-syn accumulation in the brain is correlated with the upregulation of MIRO (Shaltouki et al., 2018). Very recently, Guillén-Samander et al. (2021) reported that VPS13D, an essential gene involved in autophagy/mitophagy process (Anding et al., 2018) establishes a bridge connecting ER, Mitochondria and peroxisome by MIRO, which is a PARKIN substrate directly associated with PD (Guillén-Samander et al., 2021). Although, indirectly there is substantial literature that provides evidence on the disturbances in redox homeostasis and autophagy processes and their link to PD, to the best of our knowledge, as of today, there is no data available that directly links the mitochondrial and peroxisomal contact sites dysfunctions to PD pathogenesis. Thus, mitochondria-peroxisome contact sites (Figures 3 and 4) provide novel research opportunities in relation to PD pathogenesis and therapeutics.
Figure 3.
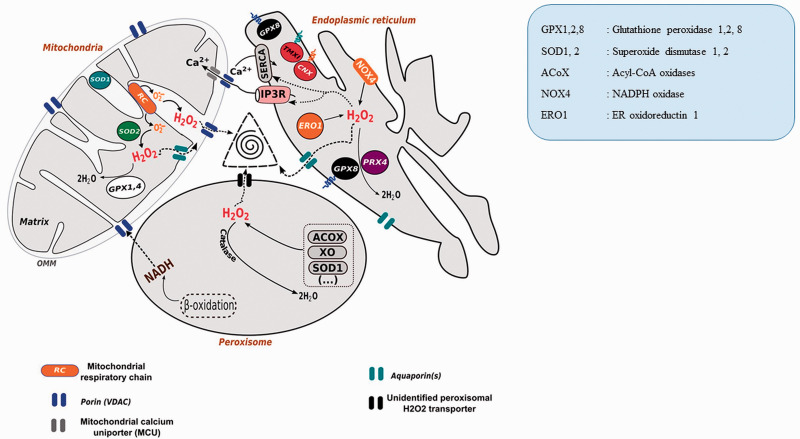
Redox Triangle (ER-Mitochondria-Peroxisome). ER, mitochondria, and peroxisome, generate ROS in their biochemical reactions. All the three organelles releases ROS through porin (ER), aquaporins (lysosome), unknown channel (Peroxisome) and form redox triangle causing oxidative damage of the associated organelles (Yoboue et al., 2018) (Open access article, reused the image as per terms of the Creative Commons CC BY license).
Figure 4.
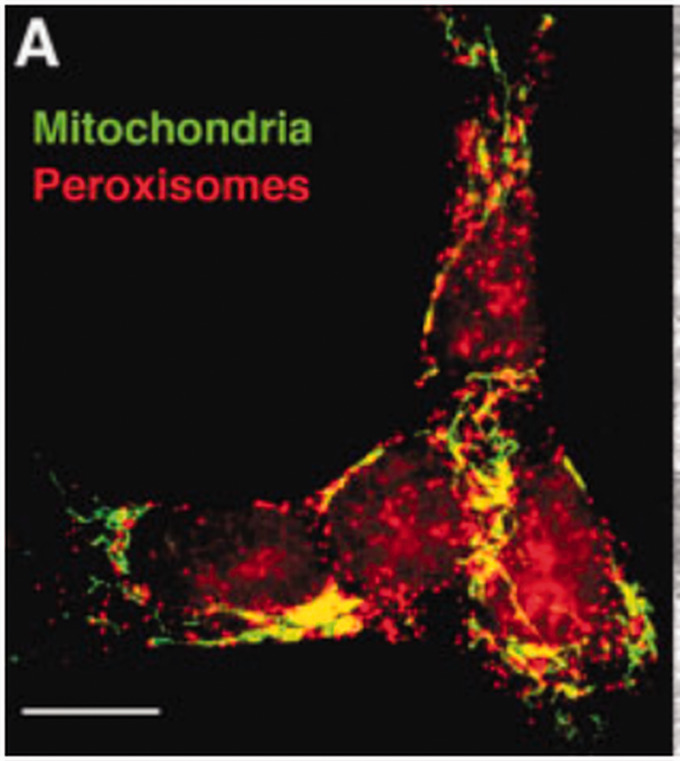
Mitochondria and Peroxisomal Contact Sites. A. Fluorescence microscope image showing close contact between peroxisome and mitochondrial in neuroblastoma cell line. The cells were stained with peroxisomal membrane protein (PMP70) that emitted red fluorescence and mitochondrial MnSOD, a mitochondrial antioxidant, emitted green fluorescence, that established the existence of contact sites in neuroblastoma cells captured by Fluorescence microscopy (10 μm). (Reused as per the BioEssays journal's copyright permission policy) (Schrader and Yoon, 2007).
PD and Organelle Specific Targeted Drug Delivery Strategies
Current treatments in PD are predominantly symptomatic and palliative care approaches. It is unfortunate that none of the therapeutic approaches have proven to be completely effective in preventing or slowing down the progression of PD. The prime obstacle that confronts the brain-targeted therapies is crossing the blood-brain barrier (Torres-Ortega et al., 2019). Towards this end, nano-drug delivery systems are being considered as potential options as they provide high bioavailability, have reduced adverse reactions and specifically target the desired tissues or organelles ( Chidambaram et al., 2020; Patra et al., 2018; Y. Peng et al., 2020 ). Functional groups can be attached to the surface of the nanoparticles (NPs) and this can help in reaching the desired target organelle. For example, mitochondria-specific delivery can be achieved by surface modification with triphenyl phosphonium (TPP) cations (Boddapati et al., 2005). Furthermore, targeted delivery approaches have been explored to target specific organelles like mitochondria, lysosome etc. Wittrup et al. (2010) studied antibody-conjugated magnetic nanoparticles to understand the role of GRP75, an important protein of MAMs, in endocytosis of macromolecules (Wittrup et al., 2010). Likewise, Au-functionalized siRNA particles were used to knock down GRP75 in PC-3 cells (Shah et al., 2020). Few other studies have been discussed in (Table 3). Future research in this area may lead to the development of specific organelle and mitochondrial membrane contact site delivery, especially for neurodegenerative diseases.
Table 3.
Organelle Specific Nano Drug Delivery in PD.
| Nanoparticles/organ-specific drug | Targeted organs | Molecules | References |
|---|---|---|---|
| Polymeric Nanoparticle-TPP | Mitochondria | Curcumin | (Marrache & Dhar, 2012; Tiwari et al., 2014) |
| TPP-modified synthetic molecule | Mitochondria | CoQ10 | (Kelso et al., 2002; Ghosh et al., 2010) |
| TPP-modified synthetic molecule | Mitochondria | Apocynin | (Jin et al., 2014) |
| Peptide-conjugated metallothionein 1 A | Mitochondria | — | (Kang et al., 2018) |
| Polyanhydride nanoparticle- folic acid (FA) | Mitochondria | Mito-Apocynin | (Brenza et al., 2017) |
| TPP-modified synthetic molecule | Mitochondria | Piperidine | (Gruber et al., 2013) |
| PLGA nanoparticle | Lysosome | Acidic nanoparticles | (Bourdenx et al., 2016) |
| Silica nanoparticles | Lysosome | (si) RNAs | (Schütz et al., 2016) |
| Ceria nanoparticles | Mitochondria | — | (Kwon et al., 2018) |
| Resveratrol nanoparticles (NRSV) | Mitochondria | Resveratrol | (Palle & Neerati, 2018) |
| TiO2-NP | Endoplasmic Reticulum | — | (Yu et al., 2015) |
| CeO2 NPs | Mitochondria | — | (Ruotolo et al., 2020) |
Conclusion
The communication between mitochondria and ER, lysosomes and peroxisomes is important for physiological homeostasis. However, not much research has been done in this area specifically with reference to PD pathology. Mitochondria are connected to ER, lysosomes, and peroxisomes bi-directionally at mitochondria contact site (MCS). In pathological conditions, alterations in Ca2+ homeostasis, impaired autophagy, redox imbalance, MDVs transport are observed at MCS. Interestingly, data suggests that the effects of structural and biochemical alterations at MAMs (mitochondria-ER contact site) are more prominent in PD pathology in comparison to alterations at mitochondria-lysosome and mitochondria-peroxisome sites. Derangements at MAMs impairs Ca2+ signalling which causes dopaminergic cell death. Also, mutations in PD associated genes such as α-syn, DJ-1, PARKIN are linked to IP3R-GRP75-VDAC complex dysfunction at MAMs. In addition, mutation in PINK and PARKIN impairs MFN1-MFN1/2 tethering at MAMs, consequently affecting autophagy and mitochondrial fission-fusion processes. Similarly, mitochondria-lysosomal contact sites regulate mitochondrial biogenesis and autophagy through Calcineurin/TFEB signalling and are crucial for clearing the damaged mitochondrial proteins by the MDVs transport mechanism. Dysfunction of this contact site and mutation in VPS35 are associated with PD pathogenesis. Although there is no direct evidence to show the involvement of derangement at mitochondria - peroxisome site in PD, alterations in proteins like VPS35, Mortalin/HSPA9, and sharing autophagy protein, Atg11 scaffold and DRP1/DNM1 impose deleterious effects and affect cellular homeostasis negatively. There are few investigations that have focused on the organ-specific nano delivery of molecule/genes to treat or understand the diseased condition. This review gives a glimpse of the role of mitochondrial contact sites in PD and establishes the role of mitochondria and organellar crosstalk in Parkinson’s disease. Further research may help to understand the role of mitochondrial contact sites in PD (Figure 5).
Figure 5.
Individual Organelle Dysfunction and Altered Communication at Mitochondrial Contact Sites Between Lysosome, ER, and Peroxisome in Terms of Autophagy and Redox Homeostasis Lead to Dopaminergic Cell Death in PD.
One key “chicken-and-egg problem” question is PD begins with the dysfunction of these organelles and/or MCS, or vice-versa? Detailed investigations on the mitochondrial crosstalk with these organelles and further studies on the regulation of these events will provide additional knowledge on PD pathogenesis and potential therapeutics for PD.
Acknowledgment
The authors also thank “Edit n Stat,” Chennai, for assistance in English proof reading.
Footnotes
Author Contributions: B. R.: Literature collection, compilation, analysis of data and manuscript drafting, images drawing; A. B.: Literature collection, images drawing; S. T.: Literature collection and compilation; A. M. M.: Literature collection and compilation; M. B.: Literature collection and compilation; S. K. M.: Manuscript draft reading; V. P. V.: Manuscript draft reading; R. C.: Manuscript draft reading; M. M. E.: Manuscript draft reading; S. B. C.: Designing, manuscript draft reading, copy right permission request for images and manuscript correction; M. K. S.: Manuscript draft reading and manuscript correction.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: B. R. acknowledges the Indian Council of Medical Research (ICMR), New Delhi, Govt. of India, for the Senior Research Fellowship. M.K.S acknowledges Discovery Grant (grant number: 417652) from the Natural Sciences and Engineering Research Council of Canada.
ORCID iDs: Bipul Ray https://orcid.org/0000-0003-3553-4996
Saravana Babu Chidambaram https://orcid.org/0000-0003-2357-056X
References
- Adams C. J., Kopp M. C., Larburu N., Nowak P. R., Ali M. M. U. (2019). Structure and molecular mechanism of ER stress signaling by the unfolded protein response signal activator IRE1. Front Mol Biosci, 6, 11. 10.3389/fmolb.2019.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S., Kwatra M., Ranjan Panda S., Murty U. S. N., Naidu V. G. M. (2021). Andrographolide suppresses NLRP3 inflammasome activation in microglia through induction of Parkin-mediated mitophagy in in-vitro and in-vivo models of Parkinson disease. Brain Behav Immun, 91, 142–158. 10.1016/j.bbi.2020.09.017 [DOI] [PubMed] [Google Scholar]
- Aimé P., Karuppagounder S. S., Rao A., Chen Y., Burke R. E., Ratan R. R., Greene L. A. (2020). The drug adaptaquin blocks ATF4/CHOP-dependent pro-death Trib3 induction and protects in cellular and mouse models of Parkinson’s disease. Neurobiol Dis, 136, 104725. 10.1016/j.nbd.2019.104725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L., Seow Y., Schapira A. H. V., Rodriguez-Oroz M. C., Obeso J. A., Cooper J. M. (2013). Influence of microRNA deregulation on chaperone-mediated autophagy and α-synuclein pathology in Parkinson’s disease. Cell Death Dis, 4(3), e545–e545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anding A. L., Wang C., Chang T.-K., Sliter D. A., Powers C. M., Hofmann K., Youle R. J., Baehrecke E. H. (2018). Vps13D encodes a ubiquitin-binding protein that is required for the regulation of mitochondrial size and clearance. Curr Biol, 28(2), 287–295.e6. 10.1016/j.cub.2017.11.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer S. L. (2013). Mitochondrial dynamics—Mitochondrial fission and fusion in human diseases. N Engl J Med, 369(23), 2236–2251. 10.1056/NEJMra1215233 [DOI] [PubMed] [Google Scholar]
- Arias E., Koga H., Diaz A., Mocholi E., Patel B., Cuervo A. M. (2015). Lysosomal mTORC2/PHLPP1/akt regulate chaperone-mediated autophagy. Mol Cell, 59(2), 270–284. 10.1016/j.molcel.2015.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atakpa P., Thillaiappan N. B., Mataragka S., Prole D. L., Taylor C. W. (2018). IP3 receptors preferentially associate with ER-lysosome contact sites and selectively deliver Ca2+ to lysosomes. Cell Rep, 25(11), 3180–3193.e7. 10.1016/j.celrep.2018.11.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso V., Marchesan E., Peggion C., Chakraborty J., von Stockum S., Giacomello M., Ottolini D., Debattisti V., Caicci F., Tasca E., Pegoraro V., Angelini C., Antonini A., Bertoli A., Brini M., Ziviani E. (2018). Regulation of ER-mitochondria contacts by parkin via Mfn2. Pharmacol Res, 138, 43–56. 10.1016/j.phrs.2018.09.006 [DOI] [PubMed] [Google Scholar]
- Belal C., Ameli N. J., El Kommos A., Bezalel S., Al'Khafaji A. M., Mughal M. R., Mattson M. P., Kyriazis G. A., Tyrberg B., Chan S. L. (2012). The homocysteine-inducible endoplasmic reticulum (ER) stress protein herp counteracts mutant α-synuclein-induced ER stress via the homeostatic regulation of ER-resident calcium release channel proteins. Hum Mol Genet, 21(5), 963–977. 10.1093/hmg/ddr502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellucci A., Navarria L., Zaltieri M., Falarti E., Bodei S., Sigala S., Battistin L., Spillantini M., Missale C., Spano P. (2011). Induction of the unfolded protein response by α-synuclein in experimental models of Parkinson’s disease. J Neurochem, 116(4), 588–605. 10.1111/j.1471-4159.2010.07143.x [DOI] [PubMed] [Google Scholar]
- Bernhard W., Rouiller C. (1956). Close topographical relationship between mitochondria and ergastoplasm of liver cells in a definite phase of cellular activity. J Biophys Biochem Cytol, 2(4), 73–78. 10.1083/jcb.2.4.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet A., Margolis E. B., Zhang J., Hsieh I., Zhang J., Hnasko T. S., Ahmad J., Edwards R. H., Sesaki H., Huang E. J., Nakamura K. (2014). Loss of mitochondrial fission depletes axonal mitochondria in midbrain dopamine neurons. J Neurosci, 34(43), 14304–14317. 10.1523/JNEUROSCI.0930-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi D., Yao L., Lin Z., Chi L., Li H., Xu H., Du X., Liu Q., Hu Z., Lu J., Xu X. (2021). Unsaturated mannuronate oligosaccharide ameliorates β‐amyloid pathology through autophagy in Alzheimer’s disease cell models. Carbohydr Polym, 251, 117124. 10.1016/j.carbpol.2020.117124 [DOI] [PubMed] [Google Scholar]
- Bittremieux M., Parys J. B., Pinton P., Bultynck G. (2016). ER functions of oncogenes and tumor suppressors: Modulators of intracellular Ca2+ signaling. Biochim Biophys Acta Mol Cell Res, 1863(6), 1364–1378. 10.1016/j.bbamcr.2016.01.002 [DOI] [PubMed] [Google Scholar]
- Bockaert J., Marin P. (2015). mTOR in brain physiology and pathologies. Physiol Rev, 95(4), 1157–1187. 10.1152/physrev.00038.2014 [DOI] [PubMed] [Google Scholar]
- Boddapati S. V., Tongcharoensirikul P., Hanson R. N., D'Souza G. G. M., Torchilin V. P., Weissig V. (2005). Mitochondriotropic liposomes. J Liposome Res, 15(1–2), 49–58. 10.1081/LPR-64958 [DOI] [PubMed] [Google Scholar]
- Bourdenx M., Daniel J., Genin E., Soria F. N., Blanchard-Desce M., Bezard E., Dehay B. (2016). Nanoparticles restore lysosomal acidification defects: Implications for Parkinson and other lysosomal-related diseases. Autophagy, 12(3), 472–483. 10.1080/15548627.2015.1136769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Oshino N., Chance B. (1972). The cellular production of hydrogen peroxide. Biochem J, 128(3), 617–630. 10.1042/bj1280617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braschi E., Zunino R., McBride H. M. (2009). MAPL is a new mitochondrial SUMO E3 ligase that regulates mitochondrial fission. EMBO Rep, 10(7), 748–754. 10.1038/embor.2009.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braschi E., Goyon V., Zunino R., Mohanty A., Xu L., McBride H. M. (2010). Vps35 mediates vesicle transport between the mitochondria and peroxisomes. Curr Biol, 20(14), 1310–1315. 10.1016/j.cub.2010.05.066 [DOI] [PubMed] [Google Scholar]
- Brenza T. M., Ghaisas S., Ramirez J. E. V., Harischandra D., Anantharam V., Kalyanaraman B., Kanthasamy A. G., Narasimhan B. (2017). Neuronal protection against oxidative insult by polyanhydride nanoparticle-based mitochondria-targeted antioxidant therapy. Nanomed Nanotechnol Biol Med, 13(3), 809–820. 10.1016/j.nano.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M. R., Ma H., Yang F., Belcher J., Le Y.-Z., Mikoshiba K., Biel M., Michalakis S., Iuso A., Križaj D., Ding X.-Q. (2017). Endoplasmic reticulum (ER) Ca2+-channel activity contributes to ER stress and cone death in cyclic nucleotide-gated channel deficiency. J Biol Chem, 292(27), 11189–11205. 10.1074/jbc.M117.782326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calì T., Ottolini D., Negro A., Brini M. (2012). α-Synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions. J Biol Chem, 287(22), 17914–17929. 10.1074/jbc.M111.302794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calì T., Ottolini D., Negro A., Brini M. (2013). Enhanced Parkin levels favor ER-mitochondria crosstalk and guarantee Ca2+ transfer to sustain cell bioenergetics. Biochim Biophys Acta Mol Basis Dis, 1832(4), 495–508. [DOI] [PubMed] [Google Scholar]
- Calvo-Rodríguez M., García-Durillo M., Villalobos C., Núñez L. (2016). In vitro aging promotes endoplasmic reticulum (ER)-mitochondria Ca2+ cross talk and loss of store-operated Ca2+ entry (SOCE) in rat hippocampal neurons. Biochim Biophys Acta, 1863(11), 2637–2649. 10.1016/j.bbamcr.2016.08.001 [DOI] [PubMed] [Google Scholar]
- Camões F., Bonekamp N. A., Delille H. K., Schrader M. (2009). Organelle dynamics and dysfunction: A closer link between peroxisomes and mitochondria. J Inherit Metabol Dis, 32(2), 163–180. 10.1007/s10545-008-1018-3 [DOI] [PubMed] [Google Scholar]
- Castro I. G., Richards D. M., Metz J., Costello J. L., Passmore J. B., Schrader T. A., Gouveia A., Ribeiro D., Schrader M. (2018). A role for mitochondrial rho GTPase 1 (MIRO1) in motility and membrane dynamics of peroxisomes. Traffic, 19(3), 229–242. 10.1111/tra.12549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che L., Yang C.-L., Chen Y., Wu Z.-L., Du Z.-B., Wu J.-S., Gan C.-L., Yan S.-P., Huang J., Guo N.-J., Lin Y.-C., Lin Z.-N. (2021). Mitochondrial redox-driven mitofusin 2 S-glutathionylation promotes neuronal necroptosis via disrupting ER-mitochondria crosstalk in cadmium-induced neurotoxicity. Chemosphere, 262, 127878. 10.1016/j.chemosphere.2020.127878 [DOI] [PubMed] [Google Scholar]
- Chemaly E. R., Troncone L., Lebeche D. (2018). SERCA control of cell death and survival. Cell Calcium, 69, 46–61. 10.1016/j.ceca.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Guo Z., Luo Z., Zheng F., Shao W., Yu G., Cai P., Wu S., Li H. (2021). Drp1-mediated mitochondrial fission contributes to mitophagy in paraquat-induced neuronal cell damage. Environ Pollut, 272, 116413. 10.1016/j.envpol.2020.116413 [DOI] [PubMed] [Google Scholar]
- Chen T., Zhu J., Wang Y.-H., Hang C.-H. (2019). ROS-mediated mitochondrial dysfunction and ER stress contribute to compression-induced neuronal injury. Neuroscience, 416, 268–280. 10.1016/j.neuroscience.2019.08.007 [DOI] [PubMed] [Google Scholar]
- Cherubini M., Lopez-Molina L., Gines S. (2020). Mitochondrial fission in Huntington’s disease mouse striatum disrupts ER-mitochondria contacts leading to disturbances in Ca2+ efflux and reactive oxygen species (ROS) homeostasis. Neurobiol Dis, 136, 104741. 10.1016/j.nbd.2020.104741 [DOI] [PubMed] [Google Scholar]
- Chidambaram S. B., Ray B., Bhat A. (2020). Chapter 5—Mitochondria-targeted drug delivery in neurodegenerative diseases. In: Shegokar R. (Ed.), Delivery of drugs (pp. 97–117). Elsevier. 10.1016/B978-0-12-817776-1.00005-5. [DOI] [Google Scholar]
- Choi S., Quan X., Bang S., Yoo H., Kim J., Park J., Park K.-S., Chung J. (2017). Mitochondrial calcium uniporter in drosophila transfers calcium between the endoplasmic reticulum and mitochondria in oxidative stress-induced cell death. J Biol Chem, 292(35), 14473–14485. 10.1074/jbc.M116.765578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla C. M., Lodhi I. J. (2017). Peroxisomal dysfunction in age-related diseases. Trends Endocrinol Metab, 28(4), 297–308. 10.1016/j.tem.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J.-P., Mearow K. (2016). Autophagy inhibition in endogenous and nutrient-deprived conditions reduces dorsal root ganglia neuron survival and neurite growth in vitro. J Neurosci Res, 94(7), 653–670. 10.1002/jnr.23733 [DOI] [PubMed] [Google Scholar]
- Coblentz J., St Croix C., Kiselyov K. (2014). Loss of TRPML1 promotes production of reactive oxygen species: Is oxidative damage a factor in mucolipidosis type IV? Biochem J, 457(2), 361–368. 10.1042/BJ20130647 [DOI] [PubMed] [Google Scholar]
- Cohen Y., Klug Y. A., Dimitrov L., Erez Z., Chuartzman S. G., Elinger D., Yofe I., Soliman K., Gärtner J., Thoms S., Schekman R., Elbaz-Alon Y., Zalckvar E., Schuldiner M. (2014). Peroxisomes are juxtaposed to strategic sites on mitochondria. Mol BioSyst, 10(7), 1742–1748. 10.1039/c4mb00001c [DOI] [PubMed] [Google Scholar]
- Costello J. L., Castro I. G., Hacker C., Schrader T. A., Metz J., Zeuschner D., Azadi A. S., Godinho L. F., Costina V., Findeisen P., Manner A., Islinger M., Schrader M. (2017). ACBD5 and VAPB mediate membrane associations between peroxisomes and the ER. J Cell Biol, 216(2), 331–342. 10.1083/jcb.201607055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covill‐Cooke C., Toncheva V. S., Drew J., Birsa N., López‐Doménech G., Kittler J. T. (2020). Peroxisomal fission is modulated by the mitochondrial Rho-GTPases, Miro1 and Miro2. EMBO Rep, 21(2), e49865. 10.15252/embr.201949865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordás G., Thomas A. P., Hajnóczky G. (1999). Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J, 18(1), 96–108. 10.1093/emboj/18.1.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordás Grgy., Renken C., Várnai P., Walter L., Weaver D., Buttle K. F., Balla T., Mannella C. A., Hajnóczky G. (2006). Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol, 174(7), 915–921. 10.1083/jcb.200604016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danyu L., Yanran L., Xiuna J., Ying C., Sudan P., Tianen Z., Zhifen Z., Dezhi Z., Kaixun H., Yingyu X., Enxiang T. (2019). α-Synuclein induced mitochondrial dysfunction via cytochrome c oxidase subunit 2 in SH-SY5Y cells. Exp Cell Res, 378(1), 57–65. 10.1016/j.yexcr.2019.02.006 [DOI] [PubMed] [Google Scholar]
- de Brito O. M., Scorrano L. (2008). Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature, 456(7222), 605–610. 10.1038/nature07534 [DOI] [PubMed] [Google Scholar]
- De Vecchis D., Cavellini L., Baaden M., Hénin J., Cohen M. M., Taly A. (2017). A membrane-inserted structural model of the yeast mitofusin Fzo1. Sci Rep, 7(1), 10217. 10.1038/s41598-017-10687-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deas E., Wood N. W., Plun-Favreau H. (2011). Mitophagy and Parkinson’s disease: The PINK1–parkin link. Biochim Biophys Acta, 1813(4), 623–633. 10.1016/j.bbamcr.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decressac M., Mattsson B., Weikop P., Lundblad M., Jakobsson J., Bjorklund A. (2013). TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proc Natl Acad Sci, 110(19), E1817–E1826. 10.1073/pnas.1305623110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehay B., Ramirez A., Martinez-Vicente M., Perier C., Canron M.-H., Doudnikoff E., Vital A., Vila M., Klein C., Bezard E. (2012). Loss of P-type ATPase ATP13A2/PARK9 function induces general lysosomal deficiency and leads to Parkinson disease neurodegeneration. Proc Natl Acad Sci U S A, 109(24), 9611–9616. 10.1073/pnas.1112368109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delille H. K., Alves R., Schrader M. (2009). Biogenesis of peroxisomes and mitochondria: Linked by division. Histochem Cell Biol, 131(4), 441–446. 10.1007/s00418-009-0561-9 [DOI] [PubMed] [Google Scholar]
- Demers-Lamarche J., Guillebaud G., Tlili M., Todkar K., Bélanger N., Grondin M., Nguyen A. P., Michel J., Germain M. (2016). Loss of mitochondrial function impairs lysosomes. J Biol Chem, 291(19), 10263–10276. 10.1074/jbc.M115.695825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deus C. M., Yambire K. F., Oliveira P. J., Raimundo N. (2020). Mitochondria–lysosome crosstalk: From physiology to neurodegeneration. Trends Mol Med, 26(1), 71–88. 10.1016/j.molmed.2019.10.009 [DOI] [PubMed] [Google Scholar]
- Dijkstra A. A., Ingrassia A., de Menezes R. X., van Kesteren R. E., Rozemuller A. J. M., Heutink P., van de Berg W. D. J. (2015). Evidence for immune response, axonal dysfunction and reduced endocytosis in the substantia Nigra in early stage Parkinson’s disease. PLoS One, 10(6), e0128651. 10.1371/journal.pone.0128651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinter E., Saridaki T., Nippold M., Plum S., Diederichs L., Komnig D., Fensky L., May C., Marcus K., Voigt A., Schulz J. B., Falkenburger B. H. (2016). Rab7 induces clearance of α-synuclein aggregates. J Neurochem, 138(5), 758–774. 10.1111/jnc.13712 [DOI] [PubMed] [Google Scholar]
- Dodson M. W., Zhang T., Jiang C., Chen S., Guo M. (2012). Roles of the drosophila LRRK2 homolog in Rab7-dependent lysosomal positioning. Hum Mol Genet, 21(6), 1350–1363. 10.1093/hmg/ddr573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanskyi A., Geiβler C., Vinnikov I. A., Alter H., Schober A., Vogt M. A., Gass P., Parlato R., Schütz G. (2011). Pten ablation in adult dopaminergic neurons is neuroprotective in Parkinson’s disease models. Faseb J, 25(9), 2898–2910. 10.1096/fj.11-181958 [DOI] [PubMed] [Google Scholar]
- Egawa N., Yamamoto K., Inoue H., Hikawa R., Nishi K., Mori K., Takahashi R. (2011). The endoplasmic reticulum stress sensor, ATF6α, protects against neurotoxin-induced dopaminergic neuronal death. J Biol Chem, 286(10), 7947–7957. 10.1074/jbc.M110.156430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner V., Csordás G., Hajnóczky G. (2013). Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle - pivotal roles in Ca2+ and reactive oxygen species signaling. J Cell Sci, 126(Pt 14), 2965–2978https://doi.org/10.1242/jcs.093609">">.">https://doi.org/10.1242/jcs.093609">. 10.1242/jcs.093609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz-Alon Y., Rosenfeld-Gur E., Shinder V., Futerman A. H., Geiger T., Schuldiner M. (2014). A dynamic interface between vacuoles and mitochondria in yeast. Dev Cell, 30(1), 95–102. 10.1016/j.devcel.2014.06.007 [DOI] [PubMed] [Google Scholar]
- Elfarrash S., Jensen N. M., Ferreira N., Betzer C., Thevathasan J. V., Diekmann R., Adel M., Omar N. M., Boraie M. Z., Gad S., Ries J., Kirik D., Nabavi S., Jensen P. H. (2019). Organotypic slice culture model demonstrates inter-neuronal spreading of alpha-synuclein aggregates. Acta Neuropathol Commun, 7(1), 213. 10.1186/s40478-019-0865-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstner M., Morris C. M., Heim K., Bender A., Mehta D., Jaros E., Klopstock T., Meitinger T., Turnbull D. M., Prokisch H. (2011). Expression analysis of dopaminergic neurons in Parkinson’s disease and aging links transcriptional dysregulation of energy metabolism to cell death. Acta Neuropathol, 122(1), 75–86. 10.1007/s00401-011-0828-9 [DOI] [PubMed] [Google Scholar]
- Eriksson I., Wäster P., Öllinger K. (2020). Restoration of lysosomal function after damage is accompanied by recycling of lysosomal membrane proteins. Cell Death Dis, 11(5), 1–16. 10.1038/s41419-020-2527-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves A. R., G-Fernandes M., Santos D., Januário C., Cardoso S. M. (2015). The upshot of LRRK2 inhibition to Parkinson’s disease paradigm. Mol Neurobiol, 52(3), 1804–1820. 10.1007/s12035-014-8980-6 [DOI] [PubMed] [Google Scholar]
- Eun S. Y., Lee J. N., Nam I.-K., Liu Z.-Q., So H.-S., Choe S.-K., Park R. K. (2018). PEX5 regulates autophagy via the mTORC1-TFEB axis during starvation. Exp Mol Med, 50(4), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabelo N., Martín V., Santpere G., Marín R., Torrent L., Ferrer I., Díaz M. (2011). Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson’s disease and incidental Parkinson’s disease. Mol Med, 17(9–10), 1107–1118. 10.2119/molmed.2011.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J., Li X., Issop L., Culty M., Papadopoulos V. (2016). ACBD2/ECI2-mediated peroxisome-mitochondria interactions in Leydig cell steroid biosynthesis. Mol Endocrinol (Baltimore, Md.), 30(7), 763–782. 10.1210/me.2016-1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X., Hou T., Zhang S., Guan Y., Jia J., Wang Z. (2020). The cellular responses of autophagy, apoptosis, and 5-methylcytosine level in zebrafish cells upon nutrient deprivation stress. Chemosphere, 241, 124989. 10.1016/j.chemosphere.2019.124989 [DOI] [PubMed] [Google Scholar]
- Farré J. ‐C., Mahalingam S. S., Proietto M., Subramani S. (2019). Peroxisome biogenesis, membrane contact sites, and quality control. EMBO Rep, 20(1), e46864. 10.15252/embr.201846864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Mosquera L., Diogo C. V., Yambire K. F. (2017) Acute and chronic mitochondrial respiratory chain deficiency differentially regulate lysosomal biogenesis. Sci Rep, 7(1), 1–11. 10.1038/srep45076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri K. F., Kroemer G. (2001). Organelle-specific initiation of cell death pathways. Nat Cell Biol, 3(11), E255–E263. 10.1038/ncb1101-e255 [DOI] [PubMed] [Google Scholar]
- Field F. J., Watt K., Mathur S. N. (2007). Ezetimibe interferes with cholesterol trafficking from the plasma membrane to the endoplasmic reticulum in CaCo-2 cells. J Lipid Res, 48(8), 1735–1745. 10.1194/jlr.M700029-JLR200 [DOI] [PubMed] [Google Scholar]
- Filadi R., Greotti E., Turacchio G., Luini A., Pozzan T., Pizzo P. (2016). Presenilin 2 modulates endoplasmic Reticulum-Mitochondria coupling by tuning the antagonistic effect of mitofusin 2. Cell Rep, 15(10), 2226–2238. 10.1016/j.celrep.2016.05.013 [DOI] [PubMed] [Google Scholar]
- Filadi R., Pendin D., Pizzo P. (2018). Mitofusin 2: From functions to disease. Cell Death Dis, 9(3), 1–13. 10.1038/s41419-017-0023-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett J., Norwood S. J., Hamilton N. A., Mohan M., Kovtun O., Tay S., Zhe Y., Wood S. A., Mellick G. D., Silburn P. A., Collins B. M., Bugarcic A., Teasdale R. D. (2014). The Vps35 D620N mutation linked to Parkinson’s disease disrupts the cargo sorting function of retromer. Traffic (Copenhagen, Denmark), 15(2), 230–244. 10.1111/tra.12136 [DOI] [PubMed] [Google Scholar]
- Fransen M., Nordgren M., Wang B., Apanasets O. (2012). Role of peroxisomes in ROS/RNS-metabolism: Implications for human disease. Biochim Biophys Acta Mol Basis Dis, 1822(9), 1363–1373. 10.1016/j.bbadis.2011.12.001 [DOI] [PubMed] [Google Scholar]
- Fransen M., Nordgren M., Wang B., Apanasets O., Van Veldhoven P. P. (2013). Aging, age-related diseases and peroxisomes. Sub-Cell Biochem, 69, 45–65. 10.1007/978-94-007-6889-5_3 [DOI] [PubMed] [Google Scholar]
- Ganjam G. K., Bolte K., Matschke L. A., Neitemeier S., Dolga A. M., Höllerhage M., Höglinger G. U., Adamczyk A., Decher N., Oertel W. H., Culmsee C. (2019). Mitochondrial damage by α-synuclein causes cell death in human dopaminergic neurons. Cell Death Dis, 10(11), 16. 10.1038/s41419-019-2091-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelmetti V., De Rosa P., Torosantucci L., Marini E. S., Romagnoli A., Di Rienzo M., Arena G., Vignone D., Fimia G. M., Valente E. M. (2017). PINK1 and BECN1 relocalize at mitochondria-associated membranes during mitophagy and promote ER-mitochondria tethering and autophagosome formation. Autophagy, 13(4), 654–669. 10.1080/15548627.2016.1277309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Chandran K., Kalivendi S. V., Joseph J., Antholine W. E., Hillard C. J., Kanthasamy A., Kanthasamy A., Kalyanaraman B. (2010). Neuroprotection by a mitochondria-targeted drug in a Parkinson’s disease model. Free Radic Biol Med, 49(11), 1674–1684. 10.1016/j.freeradbiomed.2010.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Suaga P., Paillusson S., Stoica R., Noble W., Hanger D. P., Miller C. C. J. (2017). The ER-mitochondria tethering complex VAPB-PTPIP51 regulates autophagy. Curr Biol, 27(3), 371–385. 10.1016/j.cub.2016.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González Montoro A., Auffarth K., Hönscher C., Bohnert M., Becker T., Warscheid B., Reggiori F., van der Laan M., Fröhlich F., Ungermann C. (2018). Vps39 interacts with Tom40 to establish one of two functionally distinct vacuole-mitochondria contact sites. Dev Cell, 45(5), 621–636.e7. 10.1016/j.devcel.2018.05.011 [DOI] [PubMed] [Google Scholar]
- Gruber J., Fong S., Chen C.-B., Yoong S., Pastorin G., Schaffer S., Cheah I., Halliwell B. (2013). Mitochondria-targeted antioxidants and metabolic modulators as pharmacological interventions to slow ageing. Biotechnol Adv, 31(5), 563–592. 10.1016/j.biotechadv.2012.09.005 [DOI] [PubMed] [Google Scholar]
- Guardia-Laguarta C., Area-Gomez E., Rub C., Liu Y., Magrane J., Becker D., Voos W., Schon E. A., Przedborski S. (2014). α-synuclein is localized to mitochondria-associated ER membranes. J Neurosci, 34(1), 249–259. 10.1523/JNEUROSCI.2507-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia-Laguarta C., Area-Gomez E., Schon E. A., Przedborski S. (2015). A new role for α-synuclein in Parkinson’s disease: Alteration of ER-mitochondrial communication. Movement Disord, 30(8), 1026–1033. 10.1002/mds.26239 [DOI] [PubMed] [Google Scholar]
- Guerra F., Girolimetti G., Beli R., Mitruccio M., Pacelli C., Ferretta A., Gasparre G., Cocco T., Bucci C. (2019). Synergistic effect of mitochondrial and lysosomal dysfunction in Parkinson’s disease. Cells, 8(5), 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui Y.-X., Xu Z.-P., Lv W., Zhao J.-J., Hu X.-Y. (2015). Evidence for polymerase gamma, POLG1 variation in reduced mitochondrial DNA copy number in Parkinson’s disease. Parkinson Relat Disord, 21(3), 282–286. 10.1016/j.parkreldis.2014.12.030 [DOI] [PubMed] [Google Scholar]
- Guillén-Samander A., Leonzino M., Hanna M. G., Tang N., Shen H., De Camilli P. (2021). VPS13D bridges the ER to mitochondria and peroxisomes via Miro. J Cell Biol, 220(5), e202010004. https://doi.org/10.1083/jcb.202010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X.-Y., Liu Y.-S., Gao X.-D., Kinoshita T., Fujita M. (2020). Calnexin mediates the maturation of GPI-anchors through ER retention. J Biol Chem, 295(48), 16393–16410. 10.1074/jbc.RA120.015577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureev A. P., Shaforostova E. A., Starkov A. A., Popov V. N. (2017). Simplified qPCR method for detecting excessive mtDNA damage induced by exogenous factors. Toxicology, 382, 67–74. 10.1016/j.tox.2017.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusdon A. M., Zhu J., Van Houten B., Chu C. T. (2012). ATP13A2 regulates mitochondrial bioenergetics through macroautophagy. Neurobiol Dis, 45(3), 962–972. 10.1016/j.nbd.2011.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson C. M., Falkenberg M., Larsson N.-G. (2016). Maintenance and expression of mammalian mitochondrial DNA. Annu Rev Biochem, 85(1), 133–160. 10.1146/annurev-biochem-060815-014402 [DOI] [PubMed] [Google Scholar]
- Guzman J. N., Sanchez-Padilla J., Wokosin D., Kondapalli J., Ilijic E., Schumacker P. T., Surmeier D. J. (2010). Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature, 468(7324), 696–700. 10.1038/nature09536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailey D. W., Rambold A. S., Satpute-Krishnan P., Mitra K., Sougrat R., Kim P. K., Lippincott-Schwartz J. (2010). Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell, 141(4), 656–667. 10.1016/j.cell.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki M., Furuta N., Matsuda A., Nezu A., Yamamoto A., Fujita N., Oomori H., Noda T., Haraguchi T., Hiraoka Y., Amano A., Yoshimori T. (2013). Autophagosomes form at ER-mitochondria contact sites. Nature, 495(7441), 389–393. 10.1038/nature11910 [DOI] [PubMed] [Google Scholar]
- Hao L.-Y., Giasson B. I., Bonini N. M. (2010). DJ-1 is critical for mitochondrial function and rescues PINK1 loss of function. Proc Natl Acad Sci U S A, 107(21), 9747–9752. 10.1073/pnas.0911175107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Yuan W., Li Z., Hou Y., Liu F., Feng J. (2018). 6-Hydroxydopamine induces autophagic flux dysfunction by impairing transcription factor EB activation and lysosomal function in dopaminergic neurons and SH-SY5Y cells. Toxicol Lett, 283, 58–68. 10.1016/j.toxlet.2017.11.017 [DOI] [PubMed] [Google Scholar]
- Heras-Sandoval D., Pérez-Rojas J. M., Hernández-Damián J., Pedraza-Chaverri J. (2014). The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signall, 26(12), 2694–2701. [DOI] [PubMed] [Google Scholar]
- Higashi S., Moore D. J., Yamamoto R. (2009). Abnormal localization of leucine-rich repeat kinase 2 to the endosomal-lysosomal compartment in lewy body disease. J Neuropathol Exp Neurol, 68(9), 994–1005. 10.1097/NEN.0b013e3181b44ed8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi J., Katayama T., Eguchi Y., Kudo T., Taniguchi M., Koyama Y., Manabe T., Yamagishi S., Bando Y., Imaizumi K., Tsujimoto Y., Tohyama M. (2004). Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Aβ-induced cell death. J Cell Biol, 165(3), 347–356. 10.1083/jcb.200310015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hönscher C., Mari M., Auffarth K., Bohnert M., Griffith J., Geerts W., van der Laan M., Cabrera M., Reggiori F., Ungermann C. (2014). Cellular metabolism regulates contact sites between vacuoles and mitochondria. Dev Cell, 30(1), 86–94. 10.1016/j.devcel.2014.06.006 [DOI] [PubMed] [Google Scholar]
- Hoozemans J. J. M., van Haastert E. S., Nijholt D. A. T., Rozemuller A. J. M., Scheper W. (2012). Activation of the unfolded protein response is an early event in Alzheimer’s and Parkinson’s disease. Neuro-Degenerat Dis, 10(1–4), 212–215. 10.1159/000334536 [DOI] [PubMed] [Google Scholar]
- Hosoi K.-I., Miyata N., Mukai S., Furuki S., Okumoto K., Cheng E. H., Fujiki Y. (2017). The VDAC2–BAK axis regulates peroxisomal membrane permeability. J Cell Biol, 216(3), 709–722. 10.1083/jcb.201605002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P. ‐C., Wang C. ‐C., Tsai C. ‐L., Yeh Y. ‐M., Lee Y. S., Wu Y. ‐R. (2019). POLG R964C and GBA L444P mutations in familial Parkinson’s disease: Case report and literature review. Brain Behav, 9(5), e01281. 10.1002/brb3.1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber N., Guimaraes S., Schrader M., Suter U., Niemann A. (2013). Charcot-Marie-Tooth disease-associated mutants of GDAP1 dissociate its roles in peroxisomal and mitochondrial fission. EMBO Rep, 14(6), 545–552. 10.1038/embor.2013.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannielli A., Bido S., Folladori L., Segnali A., Cancellieri C., Maresca A., Massimino L., Rubio A., Morabito G., Caporali L., Tagliavini F., Musumeci O., Gregato G., Bezard E., Carelli V., Tiranti V., Broccoli V. (2018). Pharmacological inhibition of necroptosis protects from dopaminergic neuronal cell death in Parkinson’s disease models. Cell Rep, 22(8), 2066–2079. 10.1016/j.celrep.2018.01.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K., Corradetti M. N., Guan K.-L. (2005). Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet, 37(1), 19–24. 10.1038/ng1494 [DOI] [PubMed] [Google Scholar]
- Islinger M., Voelkl A., Fahimi H. D., Schrader M. (2018). The peroxisome: An update on mysteries 2.0. Histochem Cell Biol, 150(5), 443–471. 10.1007/s00418-018-1722-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivankovic D., Chau K.-Y., Schapira A. H. V., Gegg M. E. (2016). Mitochondrial and lysosomal biogenesis are activated following PINK1/parkin-mediated mitophagy. J Neurochem, 136(2), 388–402. 10.1111/jnc.13412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janikiewicz J., Szymański J., Malinska D., Patalas-Krawczyk P., Michalska B., Duszyński J., Giorgi C., Bonora M., Dobrzyn A., Wieckowski M. R. (2018). Mitochondria-associated membranes in aging and senescence: Structure, function, and dynamics. Cell Death Dis, 9(3), 1–12. 10.1038/s41419-017-0105-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitch JA, D’Amato RJ, Strittmatter SM,. (1985) Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine: Uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci, 82(7), 2173–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian F., Chen D., Chen L., Yan C., Lu B., Zhu Y., Chen S., Shi A., Chan D. C., Song Z. (2018). Sam50 regulates PINK1-Parkin-mediated mitophagy by controlling PINK1 stability and mitochondrial morphology. Cell Rep, 23(10), 2989–3005. 10.1016/j.celrep.2018.05.015 [DOI] [PubMed] [Google Scholar]
- Jin H., Kanthasamy A., Ghosh A., Anantharam V., Kalyanaraman B., Kanthasamy A. G. (2014). Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: Preclinical and clinical outcomes. Biochim Et Biophys Acta, 1842(8), 1282–1294. 10.1016/j.bbadis.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., Li G. J., Davis J., Zhu D., Wang Y., Pan C., Zhang J. (2007). Identification of novel proteins associated with both α-synuclein and DJ-1. Mol Cell Proteom, 6(5), 845–859. 10.1074/mcp.M600182-MCP200 [DOI] [PubMed] [Google Scholar]
- Junn E., Jang W. H., Zhao X., Jeong B. S., Mouradian M. M. (2009). Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J Neurosci Res, 87(1), 123–129. 10.1002/jnr.21831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y. C., Son M., Kang S., Im S., Piao Y., Lim K. S., Song M.-Y., Park K.-S., Kim Y.-H., Pak Y. K. (2018). Cell-penetrating artificial mitochondria-targeting peptide-conjugated metallothionein 1A alleviates mitochondrial damage in Parkinson’s disease models. Exp Mol Med, 50(8), 1–13. 10.1038/s12276-018-0124-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso G. F., Porteous C. M., Hughes G., Ledgerwood E. C., Gane A. M., Smith R. A. J., Murphy M. P. (2002). Prevention of mitochondrial oxidative damage using targeted antioxidants. Ann N Y Acad Sci, 959(1), 263–274. 10.1111/j.1749-6632.2002.tb02098.x [DOI] [PubMed] [Google Scholar]
- Kimura T., Kimura A. K., Ren M., Monteiro V., Xu Y., Berno B., Schlame M., Epand R. M. (2019). Plasmalogen loss caused by remodeling deficiency in mitochondria. Life Sci Alliance, 2(4), e201900348. 10.26508/lsa.201900348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike M., Shibata M., Tadakoshi M., Gotoh K., Komatsu M., Waguri S., Kawahara N., Kuida K., Nagata S., Kominami E., Tanaka K., Uchiyama Y. (2008). Inhibition of autophagy prevents hippocampal pyramidal neuron death after Hypoxic-Ischemic injury. Am J Pathol, 172(2), 454–469. 10.2353/ajpath.2008.070876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmann B., Currie E., Collins S. R., Schuldiner M., Nunnari J., Weissman J. S., Walter P. (2009). An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science, 325(5939), 477–481. 10.1126/science.1175088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korvatska O., Strand N. S., Berndt J. D., Strovas T., Chen D.-H., Leverenz J. B., Kiianitsa K., Mata I. F., Karakoc E., Greenup J. L., Bonkowski E., Chuang J., Moon R. T., Eichler E. E., Nickerson D. A., Zabetian C. P., Kraemer B. C., Bird T. D., Raskind W. H. (2013). Altered splicing of ATP6AP2 causes X-linked Parkinsonism with spasticity (XPDS). Hum Mol Genet, 22(16), 3259–3268. 10.1093/hmg/ddt180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic M., Katoshevski T., Sekler I. (2018). Allosteric regulation of NCLX by mitochondrial membrane potential links the metabolic state and Ca2+ signaling in mitochondria. Cell Rep, 25(12), 3465–3475.e4. 10.1016/j.celrep.2018.11.084 [DOI] [PubMed] [Google Scholar]
- Krols M., van Isterdael G., Asselbergh B., Kremer A., Lippens S., Timmerman V., Janssens S. (2016). Mitochondria-associated membranes as hubs for neurodegeneration. Acta Neuropathol, 131(4), 505–523. 10.1007/s00401-015-1528-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Gupta S., Sharma P., Prasad R., Pal A. (2019). In silico method for identification of novel copper and iron metabolism proteins in various neurodegenerative disorders. Neurotoxicology, 73, 50–57. 10.1016/j.neuro.2019.02.020 [DOI] [PubMed] [Google Scholar]
- Kumar N., Leonzino M., Hancock-Cerutti W., Horenkamp F. A., Li PQi., Lees J. A., Wheeler H., Reinisch K. M., De Camilli P. (2018). VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J Cell Biol, 217(10), 3625–3639. 10.1083/jcb.201807019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian P., Obisesan T. O., Craddock T. J. A. (2017). Oxidative species-induced excitonic transport in tubulin aromatic networks: Potential implications for neurodegenerative disease. J Photochem Photobiol B: Biol, 175, 109–124. 10.1016/j.jphotobiol.2017.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweon H.-J., Gu S., Witham E., Dhara M., Yu H., Mandon E. D., Jawhari A., Bredt D. S. (2020). NACHO engages N-Glycosylation ER chaperone pathways for α7 nicotinic receptor assembly. Cell Rep, 32(6), 108025. 10.1016/j.celrep.2020.108025 [DOI] [PubMed] [Google Scholar]
- Kwon H. J., Kim D., Seo K., Kim Y. G., Han S. I., Kang T., Soh M., Hyeon T. (2018). Ceria nanoparticle systems for selective scavenging of mitochondrial, intracellular, and extracellular reactive oxygen species in Parkinson’s disease. Angewandte Chemie Int Ed, 57(30), 9408–9412. [DOI] [PubMed] [Google Scholar]
- Lang A. B., Peter A. T. J., Walter P., Kornmann B. (2015). ER–mitochondrial junctions can be bypassed by dominant mutations in the endosomal protein Vps13. J Cell Biol, 210(6), 883–890. 10.1083/jcb.201502105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston J., Ballard P., Tetrud J., Irwin I. (1983). Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science, 219(4587), 979–980. [DOI] [PubMed] [Google Scholar]
- Lazarou M., Jin S. M., Kane L. A., Youle R. J. (2012). Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev Cell, 22(2), 320–333. 10.1016/j.devcel.2011.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H., Han J-h., Kim H., Park S. M., Joe E-h., Jou I. (2019). Parkinson’s disease-associated LRRK2-G2019S mutant acts through regulation of SERCA activity to control ER stress in astrocytes. Acta Neuropathol Commun, 7(1), 68. 10.1186/s40478-019-0716-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.-S., Huh S., Lee S., Wu Z., Kim A.-K., Kang H.-Y., Lu B. (2018). Altered ER-mitochondria contact impacts mitochondria calcium homeostasis and contributes to neurodegeneration in vivo in disease models. Proc Natl Acad Sci U S A, 115(38), E8844–E8853. 10.1073/pnas.1721136115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Sterky F. H., Mourier A., Terzioglu M., Cullheim S., Olson L., Larsson N.-G. (2012). Mitofusin 2 is necessary for striatal axonal projections of midbrain dopamine neurons. Hum Mol Genet, 21(22), 4827–4835. 10.1093/hmg/dds352 [DOI] [PubMed] [Google Scholar]
- Legakis J. E., Koepke J. I., Jedeszko C., Barlaskar F., Terlecky L. J., Edwards H. J., Walton P. A., Terlecky S. R. (2002). Peroxisome senescence in human fibroblasts. Mol Biol Cell, 13(12), 4243–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage S., et al. (2016). Loss of VPS13C function in autosomal-recessive parkinsonism causes mitochondrial dysfunction and increases PINK1/Parkin-dependent mitophagy. Am J Hum Genet, 98(3), 500–513. 10.1016/j.ajhg.2016.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessig J., Fuchs B. (2009). Plasmalogens in biological systems: Their role in oxidative processes in biological membranes, their contribution to pathological processes and aging and plasmalogen analysis. Curr Med Chem, 16(16), 2021–2041. 10.2174/092986709788682164 [DOI] [PubMed] [Google Scholar]
- Li L., Tan J., Miao Y., Lei P., Zhang Q. (2015). ROS and autophagy: Interactions and molecular regulatory mechanisms. Cell Mol Neurobiol, 35(5), 615–621. 10.1007/s10571-015-0166-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Chen M., Wang J., Guo X., Xiao L., Liu P., Liu L., Tang Y., Yao P. (2019). Quercetin ameliorates autophagy in alcohol liver disease associated with lysosome through mTOR-TFEB pathway. J Funct Foods, 52, 177–185. 10.1016/j.jff.2018.10.033 [DOI] [Google Scholar]
- Lima N. C. R., Melo T. Q., Sakugawa A. Y. S., Melo K. P., Ferrari M. F. R. (2019). Restoration of Rab1 levels prevents endoplasmic reticulum stress in hippocampal cells during protein aggregation triggered by rotenone. Neuroscience, 419, 5–13. 10.1016/j.neuroscience.2019.08.050 [DOI] [PubMed] [Google Scholar]
- Lin K.-J., Lin K.-L., Chen S.-D., Liou C.-W., Chuang Y.-C., Lin H.-Y., Lin T.-K. (2019). The overcrowded crossroads: Mitochondria, alpha-synuclein, and the endo-lysosomal system interaction in Parkinson’s disease. Int J Mol Sci, 20(21), 5312. 10.3390/ijms2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lismont C., Nordgren M., Van Veldhoven P. P., Fransen M. (2015). Redox interplay between mitochondria and peroxisomes. Front Cell Dev Biol, 3, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Liu W., Li R., Yang H. (2019). Mitophagy in Parkinson’s disease: From pathogenesis to treatment. Cells, 8(7), 712. 10.3390/cells8070712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Ma X., Fujioka H., Liu J., Chen S., Zhu X. (2019). DJ-1 regulates the integrity and function of ER-mitochondria association through interaction with IP3R3-Grp75-VDAC1. Proc Natl Acad Sci, 116(50), 25322–25328. 10.1073/pnas.1906565116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Evans E., Waller-Evans H. (2019). Lysosomal Ca2+ homeostasis and signaling in health and disease. Cold Spring Harb Perspect Biol, 12(6), a035311. 10.1101/cshperspect.a035311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Erauskin J., Galino J., Bianchi P., Fourcade S., Andreu A. L., Ferrer I., Muñoz-Pinedo C., Pujol A. (2012). Oxidative stress modulates mitochondrial failure and cyclophilin D function in X-linked adrenoleukodystrophy. Brain, 135(Pt 12), 3584–3598. 10.1093/brain/aws292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangone G., Bekadar S., Cormier-Dequaire F., Tahiri K., Welaratne A., Czernecki V., Pineau F., Karachi C., Castrioto A., Durif F., Tranchant C., Devos D., Thobois S., Meissner W. G., Navarro M. S., Cornu P., Lesage S., Brice A., Welter M. L., Corvol J.-C. (2020). Early cognitive decline after bilateral subthalamic deep brain stimulation in Parkinson’s disease patients with GBA mutations. Parkinson Relat Disord, 76, 56–62. 10.1016/j.parkreldis.2020.04.002 [DOI] [PubMed] [Google Scholar]
- Mani S., Swargiary G., Chadha R. (2021). Mitophagy impairment in neurodegenerative diseases: Pathogenesis and therapeutic interventions. Mitochondrion, 57, 270–293. 10.1016/j.mito.2021.01.001 [DOI] [PubMed] [Google Scholar]
- Mansueto G., Armani A., Viscomi C., D'Orsi L., De Cegli R., Polishchuk E. V., Lamperti C., Di Meo I., Romanello V., Marchet S., Saha P. K., Zong H., Blaauw B., Solagna F., Tezze C., Grumati P., Bonaldo P., Pessin J. E., Zeviani M., Sandri M., Ballabio A. (2017). Transcription factor EB controls metabolic flexibility during exercise. Cell Metab, 25(1), 182–196. 10.1016/j.cmet.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao K., Liu X., Feng Y., Klionsky D. J. (2014). The progression of peroxisomal degradation through autophagy requires peroxisomal division. Autophagy, 10(4), 652–661. 10.4161/auto.27852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak S. J., Yun C. Y., Oyadomari S., Novoa I., Zhang Y., Jungreis R., Nagata K., Harding H. P., Ron D. (2004). CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev, 18(24), 3066–3077. 10.1101/gad.1250704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrache S., Dhar S. (2012). Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. Proc Natl Acad Sci U S A, 109(40), 16288–16293. 10.1073/pnas.1210096109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina J. A., Chen Y., Gucek M., Puertollano R. (2012). MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy, 8(6), 903–914. 10.4161/auto.19653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheoud D., Sugiura A., Bellemare-Pelletier A., Laplante A., Rondeau C., Chemali M., Fazel A., Bergeron J. J., Trudeau L.-E., Burelle Y., Gagnon E., McBride H. M., Desjardins M. (2016). Parkinson’s disease-related proteins PINK1 and parkin repress mitochondrial antigen presentation. Cell, 166(2), 314–327. 10.1016/j.cell.2016.05.039 [DOI] [PubMed] [Google Scholar]
- Mazzulli J. R., Zunke F., Isacson O., Studer L., Krainc D. (2016). α synuclein–induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc Natl Acad Sci U S A, 113(7), 1931–1936. 10.1073/pnas.1520335113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy M. K., Cookson M. R., (2011). DJ-1 regulation of mitochondrial function and autophagy through oxidative stress. Autophagy, 77(5), 531–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLelland G.-L., Soubannier V., Chen C. X., McBride H. M., Fon E. A. (2014). Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. The EMBO Journal, 33(4), 282–295. 10.1002/embj.201385902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miville-Godbout E., Bourque M., Morissette M., Al-Sweidi S., Smith T., Mochizuki A., Senanayake V., Jayasinghe D., Wang L., Goodenowe D., Di Paolo T. (2016). Plasmalogen augmentation reverses striatal dopamine loss in MPTP mice. PLoS One, 11(3), e0151020. 10.1371/journal.pone.0151020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miville-Godbout E., Bourque M., Morissette M., Al-Sweidi S., Smith T., Jayasinghe D., Ritchie S., Di Paolo T. (2017). Plasmalogen precursor mitigates striatal dopamine loss in MPTP mice. Brain Res, 1674, 70–76. 10.1016/j.brainres.2017.08.020 [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Sone N., Saitoh T. (1987). Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on activities of the enzymes in the electron transport system in mouse brain. J Neurochem, 48(6), 1787–1793. 10.1111/j.1471-4159.1987.tb05737.x [DOI] [PubMed] [Google Scholar]
- Mohanty A., McBride H. M. (2013). Emerging roles of mitochondria in the evolution, biogenesis, and function of peroxisomes. Front Physiol, 4, 268. 10.3389/fphys.2013.00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moors T. E., Hoozemans J. J. M., Ingrassia A., Beccari T., Parnetti L., Chartier-Harlin M.-C., van de Berg W. D. J. (2017). Therapeutic potential of autophagy-enhancing agents in Parkinson’s disease. Mol Neurodegener, 12(1), 11. 10.1186/s13024-017-0154-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy G., Atwood B., Kuznetsov A. (2020). Basal ganglia role in learning rewarded actions and executing previously learned choices: Healthy and diseased states. PLoS One, 15(2), e0228081. 10.1371/journal.pone.0228081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Zhu H., Morishima N., Li E., Xu J., Yankner B. A., Yuan J. (2000). Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature, 403(6765), 98–103. 10.1038/47513 [DOI] [PubMed] [Google Scholar]
- Napolitano G., Ballabio A. (2016). TFEB at a glance. J Cell Sci, 129(13), 2475–2481. 10.1242/jcs.146365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuspiel M., Schauss A. C., Braschi E., Zunino R., Rippstein P., Rachubinski R. A., Andrade-Navarro M. A., McBride H. M. (2008). Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Curr Biol, 18(2), 102–108. 10.1016/j.cub.2007.12.038 [DOI] [PubMed] [Google Scholar]
- Nichols W. C., Pankratz N., Hernandez D., Paisán-Ruíz C., Jain S., Halter C. A., Michaels V. E., Reed T., Rudolph A., Shults C. W., Singleton A., Foroud T. (2005). Genetic screening for a single common LRRK2 mutation in familial Parkinson’s disease. Lancet, 365(9457), 410–412. 10.1016/S0140-6736(05)17828-3 [DOI] [PubMed] [Google Scholar]
- Nicklin P., Bergman P., Zhang B., Triantafellow E., Wang H., Nyfeler B., Yang H., Hild M., Kung C., Wilson C., Myer V. E., MacKeigan J. P., Porter J. A., Wang Y. K., Cantley L. C., Finan P. M., Murphy L. O. (2009). Bidirectional transport of amino acids regulates mTOR and autophagy. Cell, 136(3), 521–534. 10.1016/j.cell.2008.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann A., Ruegg M., La Padula V., Schenone A., Suter U. (2005). Ganglioside-induced differentiation associated protein 1 is a regulator of the mitochondrial network . J Cell Biol, 170(7), 1067–1078. 10.1083/jcb.200507087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J., Yu M., Wang C., Xu Z. (2012). Leucine-rich repeat kinase 2 disturbs mitochondrial dynamics via dynamin-like protein. J Neurochem, 122(3), 650–658. 10.1111/j.1471-4159.2012.07809.x [DOI] [PubMed] [Google Scholar]
- Okumoto K., Ono T., Toyama R., Shimomura A., Nagata A., Fujiki Y. (2018). New splicing variants of mitochondrial rho GTPase-1 (Miro1) transport peroxisomes. J Cell Biol, 217(2), 619–633. 10.1083/jcb.201708122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolini D., Calì T., Negro A., Brini M. (2013). The Parkinson disease-related protein DJ-1 counteracts mitochondrial impairment induced by the tumour suppressor protein p53 by enhancing endoplasmic reticulum–mitochondria tethering. Hum Mol Genet, 22(11), 2152–2168. 10.1093/hmg/ddt068 [DOI] [PubMed] [Google Scholar]
- Pacelli C., De Rasmo D., Signorile A., Grattagliano I., di Tullio G., D'Orazio A., Nico B., Comi G. P., Ronchi D., Ferranini E., Pirolo D., Seibel P., Schubert S., Gaballo A., Villani G., Cocco T. (2011). Mitochondrial defect and PGC-1α dysfunction in parkin-associated familial Parkinson’s disease. Biochim Biophys Acta, 1812(8), 1041–1053. 10.1016/j.bbadis.2010.12.022 [DOI] [PubMed] [Google Scholar]
- Paillusson S., Gomez-Suaga P., Stoica R., Little D., Gissen P., Devine M. J., Noble W., Hanger D. P., Miller C. C. J. (2017). α-synuclein binds to the ER-mitochondria tethering protein VAPB to disrupt Ca2+ homeostasis and mitochondrial ATP production. Acta Neuropathol, 134(1), 129–149. 10.1007/s00401-017-1704-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacino J. J., Sagi D., Goldberg M. S., Krauss S., Motz C., Wacker M., Klose J., Shen J. (2004). Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem, 279(18), 18614–18622. [DOI] [PubMed] [Google Scholar]
- Palle S., Neerati P. (2018). Improved neuroprotective effect of resveratrol nanoparticles as evinced by abrogation of rotenone-induced behavioral deficits and oxidative and mitochondrial dysfunctions in rat model of Parkinson’s disease. Naunyn-Schmiedeberg’s Arch Pharmacol, 391(4), 445–453. 10.1007/s00210-018-1474-8 [DOI] [PubMed] [Google Scholar]
- Park J.-S., Thorsness M. K., Policastro R., McGoldrick L. L., Hollingsworth N. M., Thorsness P. E., Neiman A. M. (2016). Yeast Vps13 promotes mitochondrial function and is localized at membrane contact sites. Mol Biol Cell, 27(15), 2435–2449. 10.1091/mbc.E16-02-0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. Y., Koh H. C. (2020). FUNDC1 regulates receptor-mediated mitophagy independently of the PINK1/parkin-dependent pathway in rotenone-treated SH-SY5Y cells. Food Chem Toxicol, 137, 111163. 10.1016/j.fct.2020.111163 [DOI] [PubMed] [Google Scholar]
- Pascual-Ahuir A., Manzanares-Estreder S., Proft M. (2017). Pro- and antioxidant functions of the peroxisome-mitochondria connection and its impact on aging and disease. Oxidat Med Cell Longev, 2017, 1–17. 10.1155/2017/9860841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra J. K., Das G., Fraceto L. F., Campos E. V. R., Rodriguez-Torres M. d P., Acosta-Torres L. S., Diaz-Torres L. A., Grillo R., Swamy M. K., Sharma S., Habtemariam S., Shin H.-S. (2018). Nano based drug delivery systems: Recent developments and future prospects. J Nanobiotechnol, 16(1), 1–33. 10.1186/s12951-018-0392-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W., Wong Y. C., Krainc D. (2020). Mitochondria-lysosome contacts regulate mitochondrial Ca2+ dynamics via lysosomal TRPML1. Proc Natl Acad Sci, 117(32), 19266–19275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Chen L., Ye S., Kang Y., Liu J., Zeng S., Yu L. (2020). Research and development of drug delivery systems based on drug transporter and nano-formulation. Asian J Pharmaceut Sci, 15(2), 220–236. 10.1016/j.ajps.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John Peter A. T., Herrmann B., Antunes D., Rapaport D., Dimmer K. S., Kornmann B. (2017). Vps13-Mcp1 interact at vacuole-mitochondria interfaces and bypass ER-mitochondria contact sites. J Cell Biol, 216(10), 3219–3229. 10.1083/jcb.201610055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham A. H., Meng S., Chu Q. N., Chan D. C. (2012). Loss of Mfn2 results in progressive, retrograde degeneration of dopaminergic neurons in the nigrostriatal circuit. Hum Mol Genet, 21(22), 4817–4826. 10.1093/hmg/dds311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picca A., Calvani R., Coelho-Junior H. J., Landi F., Bernabei R., Marzetti E. (2020). Inter-organelle membrane contact sites and mitochondrial quality control during aging: A geroscience view. Cells, 9(3), 598. 10.3390/cells9030598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotegher N., Duchen M. R. (2017). Crosstalk between lysosomes and mitochondria in Parkinson’s disease. Frontiers in Cell and Developmental Biology, 5, 110. 10.3389/fcell.2017.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puspita L., Chung S. Y., Shim J. (2017). Oxidative stress and cellular pathologies in Parkinson’s disease. Molecular Brain, 10(1), 53. 10.1186/s13041-017-0340-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramonet D., Podhajska A., Stafa K., Sonnay S., Trancikova A., Tsika E., Pletnikova O., Troncoso J. C., Glauser L., Moore D. J. (2012). PARK9-associated ATP13A2 localizes to intracellular acidic vesicles and regulates cation homeostasis and neuronal integrity. Hum Mol Genet, 21(8), 1725–1743. 10.1093/hmg/ddr606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R., Pinton P., Carrington W. (1998). Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science (New York, N.Y.), 280(5370), 1763–1766. 10.1126/science.280.5370.1763 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Blanco J., Martín V., García-Santos G., Herrera F., Casado-Zapico S., Antolín I., Rodriguez C. (2012). Cooperative action of JNK and AKT/mTOR in 1-methyl-4-phenylpyridinium-induced autophagy of neuronal PC12 cells. J Neurosci Res, 90(9), 1850–1860. 10.1002/jnr.23066 [DOI] [PubMed] [Google Scholar]
- Roosen D. A., Cookson M. R. (2016). LRRK2 at the interface of autophagosomes, endosomes and lysosomes. Mol Neurodegener, 11(1), 73. 10.1186/s13024-016-0140-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropp P. A., Copeland W. C. (1996). Cloning and characterization of the human mitochondrial DNA polymerase, DNA polymerase gamma. Genomics, 36(3), 449–458. 10.1006/geno.1996.0490 [DOI] [PubMed] [Google Scholar]
- Ruotolo R., De Giorgio G., Minato I., Bianchi M., Bussolati O., Marmiroli N. (2020). Cerium oxide nanoparticles rescue α-synuclein-induced toxicity in a yeast model of Parkinson’s disease. Nanomaterials, 10(2), 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J. S., Marshall C. A., Keibel L., Snyder N. W., Hill M. P., Brotchie J. M., Johnston T. H., Waterhouse B. D., Kortagere S. (2021). A novel dopamine D3R agonist SK609 with norepinephrine transporter inhibition promotes improvement in cognitive task performance in rodent and non-human primate models of Parkinson’s disease. Exp Neurol, 335, 113514. 10.1016/j.expneurol.2020.113514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon E. A., Przedborski S. (2011). Mitochondria: The next (neurode)generation. Neuron, 70(6), 1033–1053. 10.1016/j.neuron.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader M. (2006). Shared components of mitochondrial and peroxisomal division. Biochim Biophys, 1763(5–6), 531–541. 10.1016/j.bbamcr.2006.01.004 [DOI] [PubMed] [Google Scholar]
- Schrader M and Yoon Y (2007) Mitochondria and peroxisomes: Are the ‘Big Brother’ and the ‘Little Sister’ closer than assumed? BioEssays, 29(11): 1105–1114. DOI: 10.1002/bies.20659. [DOI] [PubMed]
- Schütz I., Lopez-Hernandez T., Gao Q., Puchkov D., Jabs S., Nordmeyer D., Schmudde M., Rühl E., Graf C. M., Haucke V. (2016). Lysosomal dysfunction caused by cellular accumulation of silica nanoparticles. J Biol Chem, 291(27), 14170–14184. 10.1074/jbc.M115.710947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. S., Cultrara C. N., Ramos J. A., Samuni U., Zilberberg J., Sabatino D. (2020). Bifunctional Au-templated RNA nanoparticles enable direct cell uptake detection and GRP75 knockdown in prostate cancer. J Mater Chem B, 8(10), 2169–2176. 10.1039/C9TB02438G [DOI] [PubMed] [Google Scholar]
- Shai N., Yifrach E., van Roermund C. W. T., Cohen N., Bibi C., IJlst L., Cavellini L., Meurisse J., Schuster R., Zada L., Mari M. C., Reggiori F. M., Hughes A. L., Escobar-Henriques M., Cohen M. M., Waterham H. R., Wanders R. J. A., Schuldiner M., Zalckvar E. (2018). Systematic mapping of contact sites reveals tethers and a function for the peroxisome-mitochondria contact. Nat Commun, 9(1), 1–13. 10.1038/s41467-018-03957-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltouki A., Hsieh C.-H., Kim M. J., Wang X. (2018). Alpha-synuclein delays mitophagy and targeting miro rescues neuron loss in Parkinson’s models. Acta Neuropathol, 136(4), 607–620. 10.1007/s00401-018-1873-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiao Y. J., Lupo G., Vance J. E. (1995). Evidence that phosphatidylserine is imported into mitochondria via a mitochondria-associated membrane and that the majority of mitochondrial phosphatidylethanolamine is derived from decarboxylation of phosphatidylserine. J Biol Chem, 270(19), 11190–11198. 10.1074/jbc.270.19.11190 [DOI] [PubMed] [Google Scholar]
- Smolders S., Van Broeckhoven C. (2020). Genetic perspective on the synergistic connection between vesicular transport, lysosomal and mitochondrial pathways associated with Parkinson’s disease pathogenesis. Acta Neuropathol Commun, 8(1), 63. 10.1186/s40478-020-00935-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Heredero G., Baixauli F., Mittelbrunn M. (2017). Interorganelle communication between mitochondria and the endolysosomal system. Front Cell Dev Biol, 5, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubannier V., McLelland G.-L., Zunino R., Braschi E., Rippstein P., Fon E. A., McBride H. M. (2012). A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr Biol, 22(2), 135–141. 10.1016/j.cub.2011.11.057 [DOI] [PubMed] [Google Scholar]
- Sowers C. R., Wang R., Bourne R. A., McGrath B. C., Hu J., Bevilacqua S. C., Paton J. C., Paton A. W., Collardeau-Frachon S., Nicolino M., Cavener D. R. (2018). The protein kinase PERK/EIF2AK3 regulates proinsulin processing not via protein synthesis but by controlling endoplasmic reticulum chaperones. J Biol Chem, 293(14), 5134–5149. 10.1074/jbc.M117.813790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroupe C. (2018). This is the end: Regulation of Rab7 nucleotide binding in endolysosomal trafficking and autophagy. Front Cell Dev Biol, 6, 129. 10.3389/fcell.2018.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S. R., Chesselet M.-F. (2013). Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Progr Neurobiol, 106–107, 17–32. 10.1016/j.pneurobio.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura A., McLelland G.-L., Fon E. A., McBride H. M. (2014). A new pathway for mitochondrial quality control: Mitochondrial-derived vesicles. EMBO J, 33(19), 2142–2156. 10.15252/embj.201488104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Lian Y., Ding J., Meng Y., Li C., Chen L., Qiu P. (2019). The role of chaperone-mediated autophagy in neurotoxicity induced by alpha-synuclein after methamphetamine exposure. Brain Behav, 9(8), e01352. 10.1002/brb3.1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Liu J., Crary J. F., Malagelada C., Sulzer D., Greene L. A., Levy O. A. (2013). ATF4 protects against neuronal death in cellular Parkinson’s disease models by maintaining levels of parkin. J Neurosci, 33(6), 2398–2407. 10.1523/JNEUROSCI.2292-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G., Bianchi K., Várnai P., De Stefani D., Wieckowski M. R., Cavagna D., Nagy A. I., Balla T., Rizzuto R. (2006). Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol, 175(6), 901–911. 10.1083/jcb.200608073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Cleland M. M., Xu S., Narendra D. P., Suen D.-F., Karbowski M., Youle R. J. (2010). Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by parkin. J Cell Biol, 191(7), 1367–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Okazaki T., Aoyama S. (2019). Peroxisomes control mitochondrial dynamics and the mitochondrion-dependent apoptosis pathway. J Cell Sci, 132(11), jcs224766. [DOI] [PubMed] [Google Scholar]
- Tang F.-L., Liu W., Hu J.-X., Erion J. R., Ye J., Mei L., Xiong W.-C. (2015). VPS35 deficiency or mutation causes dopaminergic neuronal loss by impairing mitochondrial fusion and function. Cell Rep, 12(10), 1631–1643. 10.1016/j.celrep.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner C. M., Kamel F., Ross G. W., Hoppin J. A., Goldman S. M., Korell M., Marras C., Bhudhikanok G. S., Kasten M., Chade A. R., Comyns K., Richards M. B., Meng C., Priestley B., Fernandez H. H., Cambi F., Umbach D. M., Blair A., Sandler D. P., Langston J. W. (2011). Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect, 119(6), 866–872. 10.1289/ehp.1002839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taximaimaiti R., Li H. (2019). MUL1 gene polymorphisms and Parkinson’s disease risk. Acta Neurol Scand, 139(5), 483–487. 10.1111/ane.13081 [DOI] [PubMed] [Google Scholar]
- Thomas K. J., McCoy M. K., Blackinton J., Beilina A., van der Brug M., Sandebring A., Miller D., Maric D., Cedazo-Minguez A., Cookson M. R. (2011). DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy. Hum Mol Genet, 20(1), 40–50. 10.1093/hmg/ddq430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S. K., Agarwal S., Seth B., Yadav A., Nair S., Bhatnagar P., Karmakar M., Kumari M., Chauhan L. K. S., Patel D. K., Srivastava V., Singh D., Gupta S. K., Tripathi A., Chaturvedi R. K., Gupta K. C. (2014). Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical wnt/β-catenin pathway. ACS Nano, 8(1), 76–103. 10.1021/nn405077y [DOI] [PubMed] [Google Scholar]
- Todkar K., Ilamathi H. S., Germain M. (2017). Mitochondria and lysosomes: Discovering bonds. Front Cell Dev Biol, 5, 106. 10.3389/fcell.2017.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Ortega P. V., Saludas L., Hanafy A. S., Garbayo E., Blanco-Prieto M. J. (2019). Micro- and nanotechnology approaches to improve Parkinson’s disease therapy. J Controll Release, 295, 201–213. 10.1016/j.jconrel.2018.12.036 [DOI] [PubMed] [Google Scholar]
- Toyofuku T., Okamoto Y., Ishikawa T., Sasawatari S., Kumanogoh A. (2020). LRRK2 regulates endoplasmic reticulum–mitochondrial tethering through the PERK-mediated ubiquitination pathway. EMBO J, 39(2), e100875. 10.15252/embj.2018100875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombetta-Lima M., Sabogal-Guáqueta A. M., Dolga A. M. (2021). Mitochondrial dysfunction in neurodegenerative diseases: A focus on iPSC-derived neuronal models. Cell Calcium, 94, 102362. 10.1016/j.ceca.2021.102362 [DOI] [PubMed] [Google Scholar]
- Mattiazzi Ušaj M., Brložnik M., Kaferle P., Žitnik M., Wolinski H., Leitner F., Kohlwein S. D., Zupan B., Petrovič U. (2015). Genome-wide localization study of yeast Pex11 identifies peroxisome–mitochondria interactions through the ERMES complex. J Mol Biol, 427(11), 2072–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente E. M., Abou-Sleiman P. M., Caputo V. (2004). Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science, 304(5674), 1158–1160. 10.1126/science.1096284 [DOI] [PubMed] [Google Scholar]
- Valm A. M., Cohen S., Legant W. R., Melunis J., Hershberg U., Wait E., Cohen A. R., Davidson M. W., Betzig E., Lippincott-Schwartz J. (2017). Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature, 546(7656), 162–167. 10.1038/nature22369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen S., Martin S., Van den Haute C., Benoy V., Lyons J., Vanhoutte R., Kahler J. P., Decuypere J.-P., Gelders G., Lambie E., Zielich J., Swinnen J. V., Annaert W., Agostinis P., Ghesquière B., Verhelst S., Baekelandt V., Eggermont J., Vangheluwe P. (2020). ATP13A2 deficiency disrupts lysosomal polyamine export. Nature, 578(7795), 419–424. 10.1038/s41586-020-1968-7 [DOI] [PubMed] [Google Scholar]
- Verfaillie T., Rubio N., Garg A. D., Bultynck G., Rizzuto R., Decuypere J.-P., Piette J., Linehan C., Gupta S., Samali A., Agostinis P. (2012). PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ, 19(11), 1880–1891. 10.1038/cdd.2012.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilariño-Güell C., et al. (2011). VPS35 mutations in Parkinson disease. Am J Hum Genet, 89(1), 162–167. 10.1016/j.ajhg.2011.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincow E. S., Merrihew G., Thomas R. E., Shulman N. J., Beyer R. P., MacCoss M. J., Pallanck L. J. (2013). The PINK1-Parkin pathway promotes both mitophagy and selective respiratory chain turnover in vivo. Proc Natl Acad Sci U S A, 110(16), 6400–6405. 10.1073/pnas.1221132110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives-Bauza C., Przedborski S. (2011). Mitophagy: The latest problem for Parkinson’s disease. Trends Mol Med, 17(3), 158–165. 10.1016/j.molmed.2010.11.002 [DOI] [PubMed] [Google Scholar]
- Vonk J. J., Yeshaw W. M., Pinto F., Faber A. I. E., Lahaye L. L., Kanon B., van der Zwaag M., Velayos-Baeza A., Freire R., van IJzendoorn S. C., Grzeschik N. A., Sibon O. C. M. (2017). Drosophila Vps13 is required for protein homeostasis in the brain. PLoS One, 12(1), e0170106. 10.1371/journal.pone.0170106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Wang X., Fujioka H., Hoppel C., Whone A. L., Caldwell M. A., Cullen P. J., Liu J., Zhu X. (2016). Parkinson’s disease-associated mutant VPS35 causes mitochondrial dysfunction by recycling DLP1 complexes. Nat Med, 22(1), 54–63. 10.1038/nm.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wang P., Zhang Z., Farré J.-C., Li X., Wang R., Xia Z., Subramani S., Ma C. (2020). The autophagic degradation of cytosolic pools of peroxisomal proteins by a new selective pathway. Autophagy, 16(1), 154–166. 10.1080/15548627.2019.1603546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Liu N., Lu B. (2019). Mechanisms and roles of mitophagy in neurodegenerative diseases. CNS Neurosci Therapeut, 25(7), 859–875. 10.1111/cns.13140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham H. R., Ferdinandusse S., Wanders R. J. A. (2016). Human disorders of peroxisome metabolism and biogenesis. Biochim Biophys Acta, 1863(5), 922–933. 10.1016/j.bbamcr.2015.11.015 [DOI] [PubMed] [Google Scholar]
- Wegrzynowicz M., Bar-On D., Calo’ L., Anichtchik O., Iovino M., Xia J., Ryazanov S., Leonov A., Giese A., Dalley J. W., Griesinger C., Ashery U., Spillantini M. G. (2019). Depopulation of dense α-synuclein aggregates is associated with rescue of dopamine neuron dysfunction and death in a new Parkinson’s disease model. Acta Neuropathol, 138(4), 575–595. 10.1007/s00401-019-02023-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittrup A., Zhang S.-H., Svensson K. J., Kucharzewska P., Johansson M. C., Mörgelin M., Belting M. (2010). Magnetic nanoparticle-based isolation of endocytic vesicles reveals a role of the heat shock protein GRP75 in macromolecular delivery. Proc Natl Acad Sci U S A, 107(30), 13342–13347. 10.1073/pnas.1002622107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. C., Ysselstein D., Krainc D. (2018). Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature, 554(7692), 382–386. 10.1038/nature25486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. C., Peng W., Krainc D. (2019). Lysosomal regulation of inter-mitochondrial contact fate and motility in Charcot-Marie-tooth type 2. Dev Cell, 50(3), 339–354.e4. 10.1016/j.devcel.2019.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. C., Kim S., Peng W., Krainc D. (2019). Regulation and function of Mitochondria-Lysosome membrane contact sites in cellular homeostasis. Trends Cell Biol, 29(6), 500–513. 10.1016/j.tcb.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P.-C., Kao L.-S. (2016). Calcium regulation in mouse mesencephalic neurons—Differential roles of Na+/Ca2+ exchanger, mitochondria and endoplasmic reticulum. Cell Calcium, 59(6), 299–311. 10.1016/j.ceca.2016.03.008 [DOI] [PubMed] [Google Scholar]
- Wu S., Lei L., Song Y., Liu M., Lu S., Lou D., Shi Y., Wang Z., He D. (2018). Mutation of hop-1 and pink-1 attenuates vulnerability of neurotoxicity in C. elegans: The role of mitochondria-associated membrane proteins in parkinsonism. Exp Neurol, 309, 67–78. 10.1016/j.expneurol.2018.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi J., Rong Y., Zhao Z., Huang Y., Wang P., Luan H., Xing Y., Li S., Liao J., Dai Y., Liang J., Wu F. (2021). Scutellarin ameliorates high glucose-induced vascular endothelial cells injury by activating PINK1/parkin-mediated mitophagy. J Ethnopharmacol, 271, 113855. 10.1016/j.jep.2021.113855 [DOI] [PubMed] [Google Scholar]
- Xi X., Zou C., Ye Z., Huang Y., Chen T., Hu H. (2019). Pioglitazone protects tubular cells against hypoxia/reoxygenation injury through enhancing autophagy via AMPK-mTOR signaling pathway. Eur J Pharmacol, 863, 172695. 10.1016/j.ejphar.2019.172695 [DOI] [PubMed] [Google Scholar]
- Xi Y., Feng D., Tao K., Wang R., Shi Y., Qin H., Murphy M. P., Yang Q., Zhao G. (2018). MitoQ protects dopaminergic neurons in a 6-OHDA induced PD model by enhancing Mfn2-dependent mitochondrial fusion via activation of PGC-1α. Biochim Biophys Acta Mol Basis Dis, 1864(9), 2859–2870. 10.1016/j.bbadis.2018.05.018 [DOI] [PubMed] [Google Scholar]
- Xia M., Zhang Y., Jin K., Lu Z., Zeng Z., Xiong W. (2019). Communication between mitochondria and other organelles: A brand-new perspective on mitochondria in cancer. Cell Biosci, 9(1), 27. 10.1186/s13578-019-0289-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K., Kapetanou M., Sidiropoulou K., Bano D., Gonos E. S., Djordjevic A. M., Ehninger D. (2020). Signaling pathways of dietary energy restriction and metabolism on brain physiology and in age-related neurodegenerative diseases. Mech Ageing Dev, 192, 111364. 10.1016/j.mad.2020.111364 [DOI] [PubMed] [Google Scholar]
- Xie Y., Zhou B., Lin M.-Y., Wang S., Foust K. D., Sheng Z.-H. (2015). Endolysosomal deficits augment mitochondria pathology in spinal motor neurons of asymptomatic fALS mice. Neuron, 87(2), 355–370. 10.1016/j.neuron.2015.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Liu C., Chen S., Ye Y., Guo M., Ren Q., Liu L., Zhang H., Xu C., Zhou Q., Huang S., Chen L. (2014). Activation of AMPK and inactivation of akt result in suppression of mTOR-mediated S6K1 and 4E-BP1 pathways leading to neuronal cell death in in vitro models of Parkinson’s disease. Cell Signall, 26(8), 1680–1689. 10.1016/j.cellsig.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakunin E., Moser A., Loeb V., Saada A., Faust P., Crane D. I., Baes M., Sharon R. (2010). Alpha-synuclein abnormalities in mouse models of peroxisome biogenesis disorders. J Neurosci Res, 88(4), 866–876. 10.1002/jnr.22246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A., Ishikawa K.-I., Inoshita T., Shiba-Fukushima K., Saiki S., Hatano T., Mori A., Oji Y., Okuzumi A., Li Y., Funayama M., Imai Y., Hattori N., Akamatsu W. (2020). Identifying therapeutic agents for amelioration of mitochondrial clearance disorder in neurons of familial Parkinson disease. Stem Cell Rep, 14(6), 1060–1075. 10.1016/j.stemcr.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C., Liu J., Gao J., Sun Y., Zhang L., Song H., Xue L., Zhan L., Gao G., Ke Z., Liu Y., Liu J. (2019). IRE1 promotes neurodegeneration through autophagy-dependent neuron death in the drosophila model of Parkinson’s disease. Cell Death Dis, 10(11), 1–15. 10.1038/s41419-019-2039-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Xia C., Li S., Du L., Zhang L., Hu Y. (2014). Mitochondrial dysfunction driven by the LRRK2-mediated pathway is associated with loss of purkinje cells and motor coordination deficits in diabetic rat model. Cell Death Dis, 5, e1217. 10.1038/cddis.2014.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo A. J., Chong K. L., Gatei M., Zou D., Stewart R., Withey S., Wolvetang E., Parton R. G., Brown A. D., Kastan M. B., Coman D., Lavin M. F. (2021). Impaired endoplasmic reticulum-mitochondrial signaling in ataxia-telangiectasia. iScience, 24(1), 101972. 10.1016/j.isci.2020.101972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoboue E. D., Sitia R., Simmen T. (2018). Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages. Cell Death Dis, 9(3), 3. 10.1038/s41419-017-0033-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K.-N., Chang S.-H., Park S. J., Lim J., Lee J., Yoon T.-J., Kim J.-S., Cho M.-H. (2015). Titanium dioxide nanoparticles induce endoplasmic reticulum stress-mediated autophagic cell death via mitochondria-associated endoplasmic reticulum membrane disruption in normal lung cells. PLoS One, 10(6), e0131208. 10.1371/journal.pone.0131208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu-Wai-Man P., Carelli V., Chinnery P. F. (2014). 197th ENMC international workshop: Neuromuscular disorders of mitochondrial fusion and fission—OPA1 and MFN2 molecular mechanisms and therapeutic strategies: 26–28 april 2013, naarden, The Netherlands. Neuromusc Disord, 24(8), 736–742. 10.1016/j.nmd.2014.05.004 [DOI] [PubMed] [Google Scholar]
- Zhang J., Kim J., Alexander A., Cai S., Tripathi D. N., Dere R., Tee A. R., Tait-Mulder J., Di Nardo A., Han J. M., Kwiatkowski E., Dunlop E. A., Dodd K. M., Folkerth R. D., Faust P. L., Kastan M. B., Sahin M., Walker C. L. (2013). A TSC signaling node at the peroxisome regulates mTORC1 and autophagy in response to ROS. Nat Cell Biol, 15(10), 1186–1196. 10.1038/ncb2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Tripathi D. N., Jing J., Alexander A., Kim J., Powell R. T., Dere R., Tait-Mulder J., Lee J.-H., Paull T. T., Pandita R. K., Charaka V. K., Pandita T. K., Kastan M. B., Walker C. L. (2015). ATM functions at the peroxisome to induce pexophagy in response to ROS. Nat Cell Biol, 17(10), 1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yang S., Huang L., Ho P. C.-L. (2020). Poly (ethylene glycol)-block-poly (D, L-lactide) (PEG-PLA) micelles for brain delivery of baicalein through nasal route for potential treatment of neurodegenerative diseases due to oxidative stress and inflammation: An in vitro and in vivo study. Int J Pharmaceut, 591, 119981. 10.1016/j.ijpharm.2020.119981 [DOI] [PubMed] [Google Scholar]
- Zhang X., Cheng X., Yu L., Yang J., Calvo R., Patnaik S., Hu X., Gao Q., Yang M., Lawas M., Delling M., Marugan J., Ferrer M., Xu H. (2016). MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat Commun, 7(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Huang W., Shao Q., Yang Y., Xu Z., Chen J., Zhang X., Ge X. (2020). Drp1, a potential therapeutic target for Parkinson’s disease, is involved in olfactory bulb pathological alteration in the rotenone-induced rat model. Toxicol Lett, 325, 1–13. 10.1016/j.toxlet.2020.02.009 [DOI] [PubMed] [Google Scholar]
- Zhao N., Xia J., Xu B. (2021). Physical exercise may exert its therapeutic influence on Alzheimer’s disease through the reversal of mitochondrial dysfunction via SIRT1–FOXO1/3–PINK1–Parkin-mediated mitophagy. J Sport Health Sci, 10(1), 1–3. 10.1016/j.jshs.2020.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Y., Stenmark H. (2015). Cellular functions of rab GTPases at a glance. J Cell Sci, 128(17), 3171–3176. 10.1242/jcs.166074 [DOI] [PubMed] [Google Scholar]
- Zhong K., Li X., Le X., Kong X., Zhang H., Zheng X., Wang P., Zhang Z. (2016). MoDnm1 dynamin mediating peroxisomal and mitochondrial fission in complex with MoFis1 and MoMdv1 is important for development of functional appressorium in magnaporthe oryzae. PLoS Pathog, 12(8), e1005823. 10.1371/journal.ppat.1005823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Wang W., Hoppel C., Liu J., Zhu X. (2017). Parkinson’s disease-associated pathogenic VPS35 mutation causes complex I deficits. Biochim Biophys Acta Mol Basis Dis, 1863(11), 2791–2795. 10.1016/j.bbadis.2017.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X.-X., Wang S.-F., Tan Y., Song J.-X., Zhu Z., Wang Z.-Y., Wu M.-Y., Cai C.-Z., Huang Z.-J., Tan J.-Q., Su H.-X., Li M., Lu J.-H. (2020). Pharmacological enhancement of TFEB-mediated autophagy alleviated neuronal death in oxidative stress-induced Parkinson's disease models. Cell Death Dis, 11(2), 1–18. 10.1038/s41419-020-2322-6 [DOI] [PMC free article] [PubMed] [Google Scholar]