Abstract
Background
Medication harm can lead to hospital admission, prolonged hospital stay and poor patient outcomes. Reducing medication harm is a priority for healthcare organisations worldwide. Recent Australian studies demonstrate cardiovascular (CV) medications are a leading cause of harm. However, they appear to receive less recognition as ‘high risk’ medications compared with those classified by the medication safety acronym, ‘APINCH’ (antimicrobials, potassium, insulin, narcotics, chemotherapeutics, heparin). Our aim was to determine the scale and type of medication harm caused by CV medications in healthcare.
Methods
A narrative review of adult (>16 years) medication harm literature identified from PubMed and CINAHL databases was undertaken. Studies with the primary outcome of measuring the incidence of medication harm were included. Harm caused by CV medications was described and ranked against other medication classes at four key stages of a patient’s healthcare journey. Where specified, the implicated medications and type of harm were investigated.
Results
A total of 75 studies were identified, including seven systematic reviews and three meta-analyses, with most focussing on harm causing hospital admission. CV medications were responsible for approximately 20% of medication harm; however, this proportion increased to 50% in older populations. CV medications were consistently ranked in the top five medication categories causing harm and were often listed as the leading cause.
Conclusion
CV medications are a leading cause of medication harm, particularly in older adults, and should be the focus of harm mitigation strategies. A practical approach to generate awareness among health professionals is to incorporate ‘C’ (for CV medications) into the ‘APINCH’ acronym.
Plain language summary
Patient harm from cardiovascular medications
Background
• Harm from medications can cause poor patient outcomes.
• Certain medications have been identified as ‘high risk’ and are known to cause high rates of harm.
• ‘High risk’ medications are included in medication guidelines used by health professionals.
• Cardiovascular medications (e.g. blood pressure and cholesterol medications) are important and have many benefits.
• Recent studies have found cardiovascular medications to cause high rates of harm.
• Cardiovascular medication harm is often under-recognised in clinical practice.
• Some guidelines do not consider cardiovascular medications to be ‘high risk’.
Method
• This review investigated the extent of harm caused by cardiovascular medications in adults across four healthcare settings:
(1) at the time of hospital admission;
(2) during hospital admission;
(3) after hospital; and
(4) readmission to hospital.
• Harm caused by cardiovascular medications was ranked against other medication classes.
• We investigated the type of cardiovascular medications to cause harm and the type of harm caused.
Results
• Seventy-five studies were reviewed across 41 countries.
• Cardiovascular medications were ranked within the top five medications to cause harm.
• Cardiovascular medications were a leading cause of harm in each healthcare setting investigated.
• Harm caused by cardiovascular medications was common in older adults (>65 years).
• Cardiovascular medications often caused preventable harm.
• Medications to treat high blood pressure and abnormal heart rhythms were the most common causes of harm.
• We reported kidney injury, electrolyte changes and low blood pressure as common types of harm.
Conclusion
• Increased focus on cardiovascular medications in clinical practice is needed.
• Health professionals need to carefully prescribe and frequently review cardiovascular medications, especially in older adults.
• Patient and health professional discussions should be based on both the benefits and harms of cardiovascular medications.
• Cardiovascular medications should be included in all ‘high risk’ medication guidelines.
Keywords: adverse drug events, adverse drug reactions, cardiovascular medications, high-risk medications, medication errors, medication harm
Key points
• Medication harm is a major priority area for healthcare organisations worldwide.
• Cardiovascular medications contribute to significant medication harm (~20–50%) across both ambulatory and inpatient clinical settings.
• Adapting a medication safety acronym is recommended to generate awareness about the optimisation and rationalisation of cardiovascular medications, particularly in older adults.
Introduction
Adverse events in healthcare are defined as ‘incidents in which harm resulted to a person receiving care’. 1 These events are associated with poor patient outcomes and are often preventable. 1 Medication harm is a major subset of adverse events affecting healthcare systems worldwide.2 –5 It can cause hospital admissions, longer hospital stays, increased patient morbidity and mortality and greater resource utilisation.2 –7 In Australia, the annual fiscal burden of medication harm has been estimated to be AUD$1.4 billion.7,8 The World Health Organization has identified medication harm as a global priority and the Australian government lists medication safety as the country’s tenth National Health Priority.9 –11
To help clinicians recognise and mitigate medication harm, ‘high alert’ or ‘high risk’ medication lists have been promoted in clinical settings.12 –14 The most commonly acknowledged list is published by the Institute of Safe Medication Practices (ISMP). 14 ‘High alert’ medications are those with a heightened risk of causing devastating harm if not used correctly. 14 In Australia, there is no standard list; however, the ‘APINCH’ acronym (Figure 1) is widely used to advertise medication safety, encourage harm prevention strategies and raise awareness to the potential for catastrophic harm caused by certain medication classes. 12 It is not intended to be an exhaustive list and does not incorporate every medication linked to harm.12,15
Figure 1.
The APINCHa ‘high risk’ medication acronym.
aAdapted from the Australian Commission on Safety and Quality in Health Care. 12
Cardiovascular (CV) medications are among the most frequently prescribed, particularly in the older population.16,17 Currently, 90% of Australians aged >75 years take a CV medication. 16 A strong evidence base for treating CV disorders (e.g. acute coronary syndrome and heart failure) promotes the concurrent use of multiple CV medications.18,19 However, polypharmacy is an independent risk factor for medication harm and the older population are more susceptible to adverse drug events (ADEs).20,21
Local studies have identified CV medications as prominent causes of patient harm during hospital admission, and it is likely that this extends into ambulatory care.22,23 The purpose of this narrative review was to investigate the international literature to determine the scale and type of medication harm caused by CV medications. A contemporary review is pertinent due to the increased use of CV medications over the last three decades. 16 We sought to identify common themes, and if necessary, propose an approach to promote awareness about the safe use of these medications in clinical practice.
Methods
Data sources
Given the breadth and heterogeneity of medication harm research, a systematic review of one clinical intervention was deemed too restrictive. Instead, a narrative review exploring and evaluating the major medication harm studies was considered more appropriate to capture the extent of the issue across multiple healthcare settings. A structured literature search (see Appendix 1) based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines was undertaken using PubMed and Cumulated Index to Nursing and Allied Health Literature (CINAHL) databases. Search terms such as ‘adverse drug reaction’, ‘adverse drug event’, ‘adverse reaction’, ‘adverse event’, ‘medication error’ and ‘medication harm’ were used. Citations and bibliography lists of identified articles were scanned for additional studies.
Inclusion criteria
Systematic reviews and meta-analyses, narrative reviews, case-control studies and observational cohort studies of adverse events and medication harm within both hospital and ambulatory settings were included. Included studies quantified the incidence of medication harm as a primary outcome measure and included information about the medication classes causing harm. If systematic reviews did not specify rank of CV medications, relevant observational studies within the systematic reviews were also analysed.
Exclusion criteria
We excluded studies only investigating paediatric medication harm (<16 years of age) and those using definitions such as ‘potential drug-related problems’ or ‘potential adverse drug events’ which indicated the potential for harm, not actual patient harm. Studies investigating specific patient groups (e.g. mental health or diabetic populations) or specific medication classes (e.g. antimicrobials) were excluded. Randomised clinical trials containing adverse drug event (ADE) data about specific CV medications and conference proceedings, editorials and magazines were also excluded. To link medication harm to contemporary prescribing patterns, studies published before 1990 were excluded.
Definitions and terminology
Multiple terms are often used synonymously to describe medication harm, including ADEs and adverse drug reactions (ADRs; Box 1, see Appendix 2). 24 For the purposes of this review, medication harm was defined inclusively as ‘any negative patient outcome or injury, related to medication use’. 24 Classification of medications as ‘high alert’ versus ‘high risk’ appears to be based on the preference of international patient safety bodies. Due to the similarities between definitions, ‘high alert’ and ‘high risk’ medications were considered interchangeable for the purposes of this study.
Studies investigating medication harm have used multiple categorisations for CV medications. In this review, CV medications were classified as those that directly act on the CV system. 25 This included antihypertensives [e.g. angiotensin-converting enzyme inhibitors (ACE-Is) and calcium-channel blockers (CCBs)], diuretics (e.g. loop and thiazide diuretics), antiarrhythmics (e.g. digoxin, amiodarone), hypolipidaemics (e.g. statins, fenofibrate and ezetimibe) and antianginals (e.g. nitrates). 25 Anticoagulant and antiplatelet medications were excluded, as these medications are already widely acknowledged as high-risk medications and are a focus of pre-existing harm mitigation strategies within existing literature.12,14,26 –28 Including anticoagulants and antiplatelets in this review would detract from the focus on the harm caused by CV medications.
Analysis of studies
Studies were separated according to healthcare setting to investigate both inpatient and ambulatory populations. These included medication harm causing hospital admission, occurring during hospital stay, after discharge or in ambulatory care and readmission. If studies investigated medication harm causing hospital presentation or admission, these were separated based on whether index admissions or readmissions were investigated.
All retrieved studies were reviewed to determine the type of medication harm investigated and the incidence rate of both ‘all cause’ medication harm and harm caused specifically by CV medications. CV medications were then ranked comparative with other medication classes to determine if CV medications were a leading cause of harm. If specified, the types of CV medications implicated were ascertained.
Results
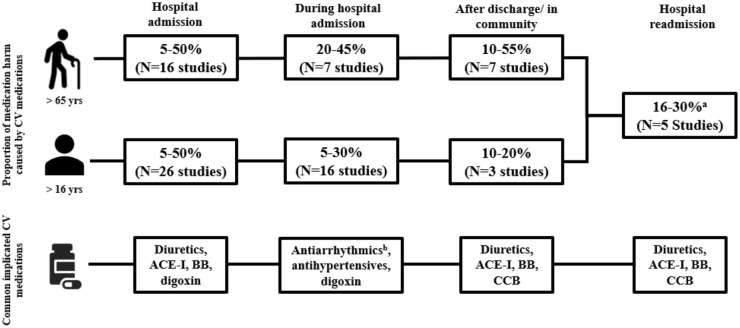
Study inclusion, exclusion and rationale are shown in a flow diagram included in Online Resource 1. Overall, 75 studies were included, of which 10 were systematic reviews/meta-analyses. Most studies investigated medication harm as a cause of hospital admission (n = 42) and five investigated both admission and inpatient medication harm. The majority of studies (not including systematic and literature reviews) were conducted in the United States (US; n = 19), Australia (n = 14) and the United Kingdom (n = 6; see Online Resource 2). A total of 13 studies had been included in the 10 identified systematic reviews; therefore, to prevent duplication of results, these studies were not included in tables but are referred to in the text. A broad overview of the proportion of medication harm caused by CV medications in each healthcare setting is shown in Figure 2. This figure also provides the differences between the adult (>16 years) and older person (>65 years) populations.
Figure 2.
An overview of cardiovascular medication harm across four healthcare settings.
aRate reported for all adults, as limited literature exists for older persons.
bIncludes intravenous and oral antiarrhythmics.
ACE-I, angiotensin-converting enzyme inhibitors; BB, beta-blockers; CCBs, calcium-channel blockers; CV, cardiovascular.
CV medication harm resulting in hospital presentation or admission
Forty-two studies were identified, including six systematic reviews and one meta-analysis that examined medication harm as a cause of admission to hospital. Thirty-five studies were analysed and tabulated (Table 1). The results of the remaining seven studies are included within the systematic reviews.29 –35 The majority of studies were undertaken in general medical or aged care populations. The rate of medication harm varied, with higher rates reported in older populations (range 0.16–41.3%).36,37
Table 1.
CV medication harm causing presentation or admission to hospital.
| Author(s) | Type of study | Population/(n) | Country | Type of medication harm | Incidence | % CV medication harm | CV medications rank against other drug classes | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Systematic reviews and meta-analysis | ||||||||
| Runciman et al. 38 | Review of multiple sources: SR, national data bases | Multiple data sources: refer to study | AU | ADR/ADE a | 2–4% of admissions; 30% in ⩾75 years | CV medications leading cause (% NR) | NR | Cost: >AUD$400 million per year Mortality: 27% of AE deaths in 1997–1998 |
| Howard et al. 39 | SR of 17 POS | ⩾16 years/(n = 17 studies) | – | Preventable DRAsa,b | 1.4–15.4% of admissions (3.7% median) | 33% (CV) 16% (diuretics) of preventable DRAs |
2nd (diuretics) 6th (BB) 7th (ACE-I) 9th (positive inotropes) 12th (CCB) 14th (nitrates) |
NR |
| Kongkaew et al. 36 | SR of 25 POS | All patients/(n = 25 studies, 106,586 patients) | – | ADR | 0.16–15.7% of admissions (5.3% median) | 45.7%
c
(median in all adults) 42.5% c (median in older adults) |
1st | Severity (reported by two studies): 3.3% and 38.1% of events were severe |
| Al Hamid et al. 40 | SR of ROS/POS | ⩾18 years/(n = 21 ADR studies, 6 ADE studies) | – | ADR and ADE a | ADR: 1.47% median (ROS), 12% median (POS) ADE: 12.4% median (POS) |
33.9% (median) of ADRs, 42.3% (median) of ADEs | 1st | Refer to study |
| Alhawassi et al. 41 | SR of ROS/POS | ⩾18 years/(n = 14 studies) | – | ADR | 10.0% of admissions (median) | CV medications leading cause (% NR) | NR | NR |
| Oscanoa et al. 42 | SR and MA | ⩾60 years/(n = 42 studies) | – | ADR a | 8.7% | BB, digoxin, ACE-I and CCB were frequently identified (% NR) | NR | NR |
| Literature reviews | ||||||||
| Roughead et al. 43 | Review of 14 studies | All patients/(n = 14 studies, 12,676 patients combined) | AU | DRA a | 2.4–3.6% of admissions | CV medications leading cause (% NR) | NR | NR |
| Wiffen et al. 44 | LR of 69 studies (54 POS, 15 ROS) | All patients/(n = 412,000) | – | ADR | 3.1% of admitted patients | Digoxin (22/69), Diuretics (15/69) identified as leading cause | NR | Cost: £380 million per year |
| Angamo et al. 45 | LR of studies in developed and developing countries | ⩾15 years (n = 43 studies) | – | ADR | Developed: 6.3% (median) Developing: 5.5% (median) | CV medications identified as leading cause (% NR) | NR | Severity: Developed: 20.0% severe (median) Developing: 10.0% (median) |
| Observational, cohort, and cross-sectional studies | ||||||||
| Larmour et al. 46 | PCS | All patients/(n = 5623 admissions) | AU | ADR | 1.6% of admissions | 11.1% of ADRs | 1st | Mortality: 5.6% |
| Stanton et al. 47 | PCS | Adult patients/(n = 691) | AU | ADR | 3.04% of admissions | 9.5% of ADRs | 4th | NR |
| Nelson et al. 48 | PCS | Patients admitted to ICU or internal medicine/(n = 452) | USA | DRA a (includes ADR and DTF) | DRA: 16.2% ADR: 5.3% of patients |
13.2% (diuretics) 10.5% (CV) of patients with DRA |
2nd 5th |
NR |
| Jha et al. 49 | PCS | All patients/(n = 3238 admissions) | USA | ADE a | 2.3% of admissions | 12% of ADEs | 2nd | Cost: $6.3 million per year for all ADEs Severity: 78% severe |
| Burgess et al. 50 | Retrospective secondary data analysis of case series | >60 years/(n = 43,380) | AU | ADR | 0.8% of all admissions for all age-groups | 17.5% of patients >60 years with ADRs | 1st | NR |
| Passarelli et al. 51 | PCS | ⩾60 years admitted to internal medicine/(n = 186) | Brazil | ADR | 11.3% of patients | 22.7% (digoxin) of ADRs causing admission | 1st | LOS: increased (p < 0.001) |
| Budnitz et al. 52 | PCS | All patients/(n = estimated 701,547 cases) | USA | ADE a | NR | 7.6% of ADE hospitalisations | 5th | NR |
| Ducharme et al. 53 | Retrospective analysis of preventability data | ADR data collected over three years at a teaching hospital/(n = 475 ADRs) | USA | pADR | 126 pADRs (% NR) | 11.9% | 4th | NR |
| Edwards et al. 54 | PCS | Patients ⩾17 years/(n = 62,064 admissions) | USA | ADE a | 2.4% of admissions | 28% of ADEs | 2nd | Mortality: 3.2% |
| Ocampo et al. 55 | CSS | Patients ⩾60 years/(n = 400) | Columbia | ADE and ADR | 6.8% | % NR | 3rd | NR |
| Ventura et al. 56 | PCS | All patients/(n = 56,031) | Italy | ADR | 21.2% | % NR | 3rd | NR |
| Budnitz et al. 6 | RCS | ⩾65 years/(n = estimated 99,628 cases) | USA | ADE | NR | 9.8% of ADE hospitalisations | 3rd | NR |
| Conforti et al. 57 | PCS | Patients ⩾65 years/(n = 1023) | Italy | ADR | 11.1% of patients | 46.2% of ADRs | 1st | LOS: increased (no p value reported) |
| Marcum et al. 58 | RCS | ⩾65 years veterans/(n = 6778) | USA | ADR a | 10% of patients | CV medications identified as leading cause (% NR) | NR | NR |
| McLachlan et al. 59 | PCS | All general medical patients/(n = 336) | New Zealand | ADE | 28.6% of admissions | 23% (vasodilators), 16% (diuretics), 11% (chronotropes) | 1st (vasodilators), 3rd (diuretics), 4th (chronotropes) | NR |
| Phillips et al. 60 | PCS | All patients/(n = 370) | AU | ADEa,d | 16% of patients (34.7% were ADRs) | 31.5% of ADEs | 3rd (ACE-I) 5th (BB) 6th (diuretics) 8th (CCB) |
Severity: 34.7% of ADEs were severe |
| Gustafsson et al. 37 | RCS | ⩾65 years with cognitive impairment/(n = 458) | Sweden | DRPa,e | DRP: 41.3% ADR: 18.8% of admissions |
29.5% of DRPs (% of ADRs NR) | 1st | NR |
| de Almeida et al. 61 | RCS | Adult patients/(n = 866) | Brazil | ADR | 2.3% | 14.3% of ADRs | 2nd | Cost: $5698.84 per ADR hospitalisation |
| Paradissis et al. 22 | PCS | ⩾65 years admitted to internal medicine/ (n = 164) | AU | ADE a | 15.2% of patients | 50% of ADEs | 1st | LOS: increased (p = 0.043) |
| Parameswaran et al. 62 | Prospective, CSS | ⩾65 years admitted to hospital/(n = 1008) | AU | ADR | 18.9% of admissions | 23.9% of ADRs (diuretics) 16.4% (ACE-I/ARB) 7.1% (BB) |
1st 2nd 3rd |
Preventable: 87.2% of ADRs Mortality: 2.1% Severity: 2.1% |
| Poudel et al. 63 | Descriptive RCS | All patients/(n = weighted estimate 150 259 899 hospitalisations) | USA | ADE a | 5.97–6.28% of hospitalisations | 13.24% of ADEs (combined) | 8th (diuretics) 11th (anti-HTN) 15th (cardiac glycosides) 17th (anti-arrhythmics) 18th (BB) 21st (antihyperlipidaemic) |
LOS: increased for ADE hospitalisations (p < 0.001) Cost: increased (p < 0.001) Mortality: increased (p < 0.001) |
| Ognibene et al. 64 | RCS | ⩾65 years admitted to internal medicine/(n = 1750) | Italy | ADR f | 6.1% of admissions | 17.6% (Diuretics) | 1st | Mortality: 10.4% of ADRs Severity: 27.4% with residual disability |
| Schurig et al. 65 | PCS | All patients presenting to emergency/(n = 10,174) | Germany | ADR a | 6.5% of admissions | ~19% (BB) ~17% (ACE-I) |
2nd 3rd |
NR |
| Mullan et al. 66 | RCS | ⩾65 years with and without dementia/(n = 228,165 admissions) | AU | MMa,g | 4.6% of admissions | With dementia: 3.9% of MM (anti-HTN) 3.4% (BB) 2.5% (ACE-I) Without dementia: 4.7% (anti-HTN) 4.1% (BB) 2.6% (cardiac glycosides) 2.1% (diuretics) 2.0% (ACE-I) |
With dementia: 4th 5th 8th Without dementia: 5th 6th 7th 8th 10th |
NR |
| Zhang et al. 67 | Population based RCS | All patients/(n = 315,274 ADR admissions) | AU | ADR | 432.3 per 100,000 residents | 4.8% of ADRs (anti-HTN) 3.9% (BB) 2.9% (cardiac stimulant) |
4th 7th 9th |
NR |
| Smeaton et al. 68 | PCS | Patients aged between 45–64 years/(n = 100) | Ireland | ADR | 21% | 52.2% of ADRs | 1st | Preventable: 52.2% of ADRs |
Includes medication errors.
Includes ADRs, overtreatment, under-treatment and adherence problems.
Definition for CV medications incorporated antithrombotics and anticoagulants.
Includes non-compliance, untreated indications, improper drug selection, sub/supratherapeutic dose, ADRs, drug interactions and drug use without indication.
Includes ADR, dosage too high, dosage too low, ineffective drug, needs additional drug therapy, unnecessary drug therapy and noncompliance.
Includes drug–drug interactions.
Includes ADR, ADEs and medication errors.
ACE-I, angiotensin-converting enzyme inhibitor; ADE, adverse drug event; ADR, adverse drug reaction; AE, adverse events; anti-HTN, antihypertensives; ARB, angiotensin receptor blocker; AU, Australia; AUD, Australian dollars; BB, beta-blockers; CCB, calcium channel blocker; CCS, case-control study; CSS, cross-sectional study/survey; CV, cardiovascular; DRA, drug-related admission; DRP, drug-related problem; DTF, drug therapeutic failure; ICU, intensive care unit; LOS, length of stay; LR, literature review; MA, meta-analysis; MM, medication misadventure; NR, not reported; OS, observational study; pADE, preventable adverse drug event; PCS, prospective cohort study; POS, prospective observational study (includes CCS/PCS); RCS, retrospective cohort study; ROS, retrospective observational study (includes CCS/RCS); SR, systematic review; USA, United States of America.
As per Table 1, 22 studies (63%) ranked CV medications in the top three causes of medication harm. Of the remaining 13 studies, a further five categorised CV medications within the top five causes of harm and another seven were unable to be ranked but described CV medications as a leading cause of harm. The majority of studies investigating older adults [73% (n = 8/11)] found CV medications to be the leading cause of harm.
In Australia, Runciman et al. 38 found CV medications, together with anticoagulants and anti-inflammatories, as responsible for over 50% of all ADEs on admission to hospitals, and identified CV medications as prominent causes of preventable and high-impact harm. A 2019 study investigating hospitalisation due to ADRs over 13 years concluded that drugs used to treat CV diseases were the leading therapeutic category contributing to medication harm, including deaths and disabilities. 67 Similarly, international studies with high rates of CV medication harm report fatal and preventable events, longer hospital stays and substantial costs linked to this harm.31,32,34,49,51,54
Types of CV medications causing harm
Diuretics, antihypertensives and digoxin were most frequently identified as causes of harm.32,34,51 Diuretics have been implicated in up to 30% of admissions due to medication harm, including renal failure and serious electrolyte imbalances.34,39 Included within the systematic reviews of Howard et al. 39 and Al Hamid et al. is a large, prospective observational study that showed ACE-Is and beta-blockers caused 7.7% and 6.8% of ADR-related admissions, respectively.34,40 ACE-I induced renal impairment, hypotension and angioedema, and beta-blocker induced bradycardia and heart block were common. 34 Digoxin and other antiarrhythmics were a leading cause of hospitalisation with a major US study finding 81% of emergency visits due to digoxin toxicity resulted in hospitalisation.6,31,51
CV medication harm occurring during hospital stay
A total of 23 inpatient studies were found, including one systematic review, two meta-analyses, 16 observational studies and three literature reviews. Twenty-one studies were analysed and tabulated and the results of the remaining two studies are included within the systematic reviews (Table 2).2,69 There were wide variations in the reported rates of inpatient medication harm (2–46% of patients).51,70,71 This may be attributed to the different methodologies used to identify harm and to the different patient populations studied. Studies investigating older adults reported higher rates of harm compared with those including all age groups.22,51,71,72
Table 2.
CV medication harm within hospitals.
| Author | Type of study | Patient population/(n) | Country | Type of medication harm | Incidence | % medication harm caused by CV medications | CV medications ranked against other drug classes | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Systematic reviews and meta-analyses | ||||||||
| Beijer et al. 73 | MA | All patients/(n = 68 studies, 123,794 admissions) | – | ADR a | 4.9% of admissions (mean) | CV identified in 38 studies | NR | Preventable: 28.9% (mean) from 12 studies |
| Alhawassi et al. 41 | SR of POS/ROS | ⩾18 years/(n = 14 studies) | – | ADR | 11.5% (median) | CV medications identified as leading cause (% NR) | NR | NR |
| Laatikainen et al. 74 | MA and SR of nine studies | All patients/(n = 9 studies, 46,626 patients) | – | ADE
a
ADR FADR |
ADEs: 21.6% (mean) ADRs: 23.4% (mean) FADRs: 9.6% (mean) | 16% of ADEs 20% of ADRs 9% of FADR (from three studies) |
2nd 3rd 4th |
Preventable: 12.0–75% Severity: 9.6% fatal (mean from 3 studies) |
| Literature reviews | ||||||||
| Wiffen et al. 44 | LR of 69 studies (54 POS and 15 ROS) | All patients/(n = 412,000) | – | ADR | 3.7% of inpatients | Digoxin (22 studies) Diuretics (15 studies) identified as leading cause of harm (% NR) | NR | Cost: £380 million |
| Kanjanarat et al. 70 | LR of 10 studies | All patients/(n = 10 studies, 117,259 patients) | – | pADE a | 1.8% (median) | 17.9% of pADEs | 1st | NR |
| Levkovich et al. 75 | Scoping LR | Hospitalised patients/(n = 12 studies) | – | Emergency call/respiratory arrest | 5–37% of deteriorations | CV medications identified as leading cause (% NR) | NR | NR |
| Observational/cohort and case-control studies | ||||||||
| Leape et al. 4 | RCS | All patients/(n = 30,195) | USA | AEs (including drug complications a ) | 19% of adverse events | 8.5% of drug complications | 4th | Severity: 14.1% caused serious disability |
| Wilson et al. 5 | RCS | All patients/(n = 14,000 admissions) | AU | AEs including drug related a | 10.8% of AEs | 11.6% (CV) 8.2% (antihypertensive) of drug-related AEs |
3rd (CV) 6th (anti-HTN) b |
Mortality: 8%, Severity: 17% caused permanent disability |
| Classen et al. 76 | Matched CCS | All patients/(n = 1580 cases and 20,197 controls) | USA | ADE a | 2.43% of patients | NR | 2nd (digoxin) | Cost: increase of $2262 linked with ADE (p < 0.001) Severity: 5.8% severe, 3.5% fatal LOS: increased (p < 0.01) |
| Doucet et al. 77 | PCS | ⩾70 years admitted to geriatric unit/(n = 2814) | France | ADE | 15.2% | 43.7% of ADEs | 1st | NR |
| Al-Tajir et al. 78 | PCS | All patients/(n = 736 ADE reports) | UAE | ADE | NR | 16.5% | 3rd | Preventable: 13.8% of ADEs |
| Passarelli et al. 51 | PCS | ⩾60 years admitted to internal medicine/(n = 186) | Brazil | ADR | 46.2% of patients | 11.8% (diuretics) 7.6% (captopril) of ADRs |
1st (diuretics) 2nd (captopril) |
LOS: increased (p < 0.001) |
| Cecile et al. 72 | RCS | Patients ⩾65 years/(n = 823) | France | ADEa,c | 13.6% of patients | 23.2% (CV) 15.2% (diuretics) of ADEs c |
1st (CV) 4th (diuretics) |
NR |
| Trivalle et al. 79 | Randomised prospective trial | Patients ⩾65 years/(n = 576) | France | ADE a | 223 ADEs identified | 19.8% of ADEs | 1st | NR |
| Morimoto et al. 80 | PCS | Patients ⩾15 years/(n = 3459 admissions) | Japan | ADE | 21% of patients | 5.1% of ADEs | 5th | Mortality: 1.6% of ADEs Severity: 4.9% life threatening |
| Conforti et al. 57 | PCS | Patients ⩾65 years/(n = 1023) | Italy | ADR | 25% of patients | 32.4% of ADRs | 1st (diuretics) | LOS: increased (no p value reported) |
| O’Connor et al. 71 | PCS | Patients ⩾65 years/(n = 513) | Ireland | ADR | 26% of patients | 25% (diuretics) 17% (anti-HTN) of ADRs |
1st (diuretics) 4th (anti-HTN) |
Severity: 24% were severe |
| Parikh et al. 81 | RCS | All patients/(n = 57,205) | AU | ADE a | 0.7% of admissions | 9% of ADEs | 4th | NR |
| Paradissis et al. 22 | RCS | Patients ⩾65 years/(n = 164) | AU | ADE a | 7.3% of patients | 44% of ADEs | 1st | LOS: increased (p = 0.043) |
| Rojas-Velandia et al. 82 | RCS | Patients admitted to ICU/(n = 697 patients) | Colombia | ADR | 11.0% of patients | 33.3% | 2nd | Preventable: 44% of ADRs |
| Robb et al. 83 | RCS | All patients/(n = 2659) | New Zealand | ADE/MRHa,d | 28% of patients e | 5.4% of MRH | 5th (furosemide) 8th (metoprolol) |
Severity: 1.6% permanent disability or death, 2.4% required an intervention to sustain life |
Includes medication errors.
n.b. leading drug type was ‘other’.
Includes ADEs causing admission; inpatient rate not separated in analysis.
terms used interchangeably.
Includes ADEs causing admission, readmission and inpatient ADEs.
ADE, adverse drug event; ADR, adverse drug reaction; AE, adverse events; antiHTN, antihypertensives, AU, Australia; CCS, case-control study; CV, cardiovascular; FADR, fatal ADR; ICU, intensive care unit; LOS, length of stay; LR, literature review; MA, meta-analysis; MRH, medication-related harm; NR, not reported; OS, observational study; pADE, preventable ADE; pADR, preventable ADR; PCS, prospective cohort study; POS, prospective observational study (includes PCS/CCS/cross-sectional study); RCS, retrospective cohort study; ROS, retrospective observational study (includes RCS/CCS/cross sectional study); SR, systematic review; USA, United States of America.
CV medications were found to be one of the top five medications to cause harm during admission, and the prevalence of CV medication harm increased in older populations (Table 2).2,4,5,22,44,51,57,69 –72,74,76,83 A meta-analysis and systematic review found CV medications were the second and third most frequently implicated medications in inpatient ADEs and ADRs, respectively. 74 In addition, CV medications were the fourth most frequently involved drug class in fatal ADRs after antithrombotics, sedatives and antineoplastics. 74 Another literature review found CV medications to be implicated in causing 17.9% of preventable ADEs and recommended that they be a high-priority focus for harm prevention strategies. 70
In the Harvard Medical Practice Study, 3.7% of 30,195 patients experienced harm, with medications responsible for 19% of harm. 4 CV medications were the fourth highest cause (8.5%) of ADEs. 4 Antihypertensives, classified separately, were the seventh highest cause (5.0%). 4 In the Quality in Australian Health Care Study, CV medications were implicated in causing 20% (n = 46) of 230 ADEs of which, 13% resulted in permanent disability. 5 Events caused by CV medications were the most highly preventable. 5
Two studies investigated medication harm in critical care.75,82 A scoping review of 30 studies investigated medications as contributors to clinical deterioration or the need for critical care. 75 Sedatives, analgesics and CV medications were most commonly implicated, although the quality of evidence was low due to small sample sizes and few primary medication-related outcomes. 75 A Columbian study investigating ADRs as a cause of admission to the intensive care unit (ICU) found antiplatelet drugs (bleeding) and renin–angiotensin-receptor-blocking drugs (renal impairment) were most frequently responsible for harm. 82
Type of CV medication causing harm
Of the studies that delineated CV medications, antiarrhythmics, antihypertensives (e.g. beta-blockers, ACE-I) and diuretics were common causes of medication harm.22,44,74,76 Notably, digoxin was frequently implicated, likely a result of its narrow therapeutic range.44,76,84 Diuretics and antihypertensives have been reported to cause up to 33% and 17% of medication harm events, respectively.57,71 Renal impairment and electrolyte imbalances caused by these medications were frequent, and in severe cases, led to ICU admission and a prolonged length of hospital stay.22,44,51,71,82
CV medication harm after discharge from hospital
The transition of patients from hospital raises safety challenges due to the risk of medication harm.85 –88 Ten studies, one systematic review and nine observational studies, investigated medication harm after discharge or in ambulatory care. This included a systematic review and observational studies with a post discharge follow-up period of up to 365 days, and observational studies conducted in outpatient departments, multi-specialty clinics or a community setting (e.g. residential/continuing care facilities and general practitioners). Six studies were analysed and included in Table 3 as the remaining four observational studies were encompassed within the systematic review.87 –90 An examination of all adverse events in 400 general medical patients after discharge, found that ADEs accounted for the majority (66%) of events. 91 However, similar to medication harm on/during hospital admission, there was a wide variation in rates, with the highest rates reported in older populations (0.4–51.2%).88,92
Table 3.
CV medication harm after hospital discharge and in ambulatory care.
| Author | Type of study | Patient population/(n =) | Country | Type of medication harm | Incidence | % of medication harm caused by CV medications | CV medication ranked against other drug classes | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Systematic review | ||||||||
| Parekh et al. 92 | SR of 8 POS/ROS | ⩾65 years/(n = 8 studies, 10,945 patients combined) | – | ADRs and ADEs a | 0.4–51.2% of patients | 18.8–55.7% of events | 1st | Preventable: 35–59% |
| Observational studies | ||||||||
| Gandhi et al. 93 | PCS | Outpatients/(n = 1202) | USA | ADE a | 25% | 9% (BB) 8% (ACE-I) of ADEs |
2nd 3rd |
Severity: 3.6% Preventable: 3.0% |
| Gurwitz et al. 90 | RCS | Outpatients ⩾65 years/(n = 27,617) | USA | ADE | 50.1 events per 1000 person-years | 24.5% | 1st | Severity: 38% serious, life threatening or fatal |
| Carnovale et al. 94 | PCS | ⩾65 years/(n = 1073 cases) | Italy | ADR a | NR | 7.8% of ADRs | 6th | Severity: 18% of ADRs were serious Preventable: 7.3% |
| Mann et al. 95 | RCS | ⩾18 years admitted to Hospital at Home service/(n = 50) | USA | ADE a | 22% of patients | 21.4% of ADEs | 2nd (diuretics) | Preventable: 7.1% |
| Parekh et al. 96 | PCS | ⩾65 years/(n = 1280) | England | MRHa,b | 37% | 22.4% (anti-HTN), 12.2% (diuretics) of MRH | 1st (anti-HTN) 3rd (diuretics) |
Severity: 1.0% fatal, 2.2% life threatening Preventable: 14% |
Includes medication errors.
Includes ADR, medication errors or harm caused by non-adherence.
ACE-I, angiotensin converting enzyme inhibitors; ADE, adverse drug event; ADR, adverse drug reaction; AE, adverse events; anti-HTN, antihypertensives; BB, beta-blockers; CV, cardiovascular; ME, medication error; MRH, medication-related harm; NR, not reported; OS, observational study; pADE, preventable adverse drug event; PCS, prospective cohort study; POS, prospective observational study; RCS, retrospective cohort study; ROS, retrospective observational study; SR, systematic review; USA, United States of America.
CV medications were a leading cause of harm post discharge. Most studies found approximately one in five medication harm events were caused by CV medications, increasing in some studies to over half of events among older patients.88 –90,92,95 –97 The aforementioned systematic review of patients greater than 65 years of age found that CV medications were the leading cause of ADRs and ADEs, implicated in 18.8–55.7% of events. 92 Within the review, a 1999 study found that the number of newly prescribed medications at the time of discharge was a significant risk factor for medication harm. 97 CV medications were the most commonly prescribed medications at the time of discharge, a potential reason for the high rates of harm in this group (18.8%). 97
Types of CV medications causing harm
Medication harm was most frequently attributed to antihypertensives which included diuretics, ACE-Is, beta-blockers and CCBs.87,88,93 Harm caused by these medications was deemed highly preventable, causing 40.5% of preventable ADEs. 88 They were also implicated in causing 42% of serious ADEs. 93 While the high prevalence of harm caused by CV medications may simply reflect their high prescribing rates, one study found that, after correcting for this factor, CV medications still contributed to excess harm. 93
Readmission caused by CV medication harm
While a number of studies investigated medication harm that caused readmission, most of these studies were summarised in one systematic review. 86 This review of 19 studies showed a wide variation in the reported incidence of medication harm causing readmission (3–64%). 86 The mean readmission rate caused by medication harm at 30 days and 12 months was found to be approximately 20% (range 7–61%). 86 CV medications were frequent causes of preventable readmissions in six studies within the systematic review, causing as many as 30% of ADR readmissions.20,86,98,99 Diuretics causing renal impairment were common and, in severe cases, were linked with death.86,98 Postural hypotension, arrhythmias and peripheral oedema caused by ACE-Is/diuretics, beta-blockers and CCBs, respectively, were also reported.98,100
Discussion
This review identified CV medications as a leading cause of medication harm, consistent with results reported from recent, local studies.22,23 Irrespective of clinical setting, approximately one in five medication harm events were found to be caused by CV medications and they were also ranked within the top five classes of medications to cause harm. Antihypertensives, diuretics and antiarrhythmics (e.g. digoxin) were most frequently implicated. The latter is consistent with a 2014 study that found that despite decreasing rates of digoxin prescribing, emergency presentations due to toxicity remained high, with >5000 visits estimated annually in the US. 101
CV medications and the ageing population
An emergent theme was that older persons are particularly susceptible to CV medication harm, as shown in Figure 2. 17 This class accounted for greater than 50% of all events reported in some studies, and studies specifically involving older patients are summarised in Online Resource 3.22,62,96 Similar to the findings for all adults, antihypertensives, such as diuretics and ACE-Is, and antiarrhythmics, were frequently implicated (Figure 2).
As the population ages and the prevalence of CV disease increases, harm caused by CV medications will likely increase. As a result of the ageing process, body systems undergo a progressive decline in physiological functioning, including the CV, pulmonary and renal systems. 102 This results in altered pharmacokinetics/pharmacodynamics of medications which clinicians must consider when managing CV medications.17,102
The importance of individualising and rationalising therapy in older adults is essential for patient care. A number of deprescribing tools have been developed and incorporate CV medications.103 –105 The application of decision support is particularly prudent in frail older persons in whom risks of medications often outweigh their benefits.106,107 Of particular interest is optimising the use of antihypertensives by using agreed treatment goals, absolute CV disease risk and appropriate blood-pressure target levels. 107 Knowing when to review or deprescribe CV medications in older patients is challenging in light of the strong evidence base for these medications in reducing CV disease risk. 108 However, it is imperative to note that most clinical trials have not included the frail and multimorbid older patients who are frequently encountered in routine clinical practice.109,110 It is important to recognise that deprescribing is indeed concordant with ethical principles when serving patient-centred interests (i.e. beneficence, non-maleficence, autonomy, and justice). 111
The under-representation of multimorbid, older adults, the focus of drug approval processes on efficacy and the lack of long-term efficacy and data on harm in clinical trials of CV medications has been described in the literature.110,112,113 These factors result in a discrepancy between the incidence, type and severity of harm reported in clinical trials and that reported in clinical practice (post-marketing). 112 While the benefits of CV medications are widely acknowledged, a recent push for active pharmacovigilance programmes and deprescribing in patients with CV diseases has emphasised the need to recognise the harms linked with CV medications.110,112,113 Patient–clinician interactions should allow for informed treatment decisions about the benefits and harms of CV medications.110,114 In addition, opportunities for rationalisation through withdrawal or dose reduction should be considered regularly with a focus on realistic treatment goals.110,114 The studies included in this review are representative of real-world treatment populations, and the findings support the need for emphasis to be placed on the rationalisation of antihypertensives and other CV medications, particularly within older populations.
The implications of CV medication harm
While it is acknowledged that CV medications have a fundamental role in the reduction of major CV endpoints, such as morbidity and mortality, the harm caused by these medications should not be overlooked. Although medication harm, such as electrolyte imbalance and renal impairment caused by antihypertensives, can be potentially reversible and may not be considered severe, the findings of this review highlighted that these events are linked with poor outcomes. The included studies reported life-threatening events, admissions to the ICU, prolonged hospital stays and in some cases, fatal events, caused by antihypertensives and diuretics.22,34,39,44,51,71,82,88,98 Although underexplored, this medication harm has the potential to cause both unwanted physical and psychological ramifications for patients due to the distress associated with hospitalisation and intensive care stays. 115 Many studies also found a high proportion of preventable medication harm events to be caused by CV medications.5,62,70,77,88,98 The resultant financial impact and consumption of healthcare resources due to potentially avoidable hospital presentations and prolonged length of hospital stays should also be considered when measuring the magnitude of the harm. For patients where withdrawal of CV medications is not indicated (see above), prudent dose selection and stringent monitoring following guideline driven dose adjustments is warranted to reduce the risk of medication harm.
In this review, we wanted to investigate CV medication harm from an international perspective. The studies included in this review (including those within the systematic and literature reviews) were conducted across six continents including: North America, Europe, Australia/Oceania, South America, Asia and Africa, from 41 countries. This highlights that CV medication harm is a global challenge and does not discriminate across healthcare settings or healthcare systems worldwide. The World Health Organization’s third global patient safety challenge ‘Medication without harm’ has paved the way to improve medication harm from a global perspective. 9 The findings of this review suggest that improving CV medication harm should be prioritised along with other ‘high risk’ medications.
High risk medications
In responding to the high prevalence of medication harm, lists of ‘high risk/alert’ medications have been formulated. These include medications associated with an increased risk of patient harm, particularly if prescribed, dispensed or administered erroneously. 14 The lists are commonly promoted in hospitals to raise awareness about medication safety.
The ISMP list of ‘high alert’ medications is the most comprehensive and frequently used worldwide. 14 It is updated regularly based on medication error reports, medication harm literature and consensus from practitioners and safety experts. 14 The latest list consists of 21 different medication classes, with CV medications accounting for 19.0% of the listed medications. 14
A standardised list is important to remind clinicians of the risk of medication harm. In Australia, the current ‘high risk’ medications are within the acronym, ‘APINCH’ (Figure 1), which has been widely adopted nationwide. 12 It differs from the ISMP list in that it does not incorporate CV medications, such as antiarrhythmics (e.g. amiodarone), inotropes (e.g. digoxin) and adrenergic antagonists (e.g. beta-blockers).12 –14
The omitted ‘C’ in APINCH
In addition to the evidence for contributing to harm, there are other clinical reasons CV medications should be considered ‘high risk’. First, many CV medications require well-defined protocols to guide administration and often can only be prescribed by skilled staff. Second, ‘high risk’ medicines are defined as ‘medicines that have an increased risk of causing significant patient harm or death if they are misused or used in error’. 13 This criteria would apply to intravenously administered antiarrhythmics, such as digoxin, metoprolol and amiodarone. 116 Third, CV medication harm largely affects older populations who account for most hospital admissions and are particularly vulnerable to medication harm.36,50,117 Additionally, harm is prevalent across all healthcare settings and our findings suggest patients newly prescribed CV medications during hospital admission are at risk of medication harm in ambulatory care.92,97 This emphasises the need for clinicians to consider harm mitigation strategies at the time of discharge such as home medication reviews, additional follow-up general practitioner visits and ‘high-risk’ discharge clinics.
Given the results of our review, we propose that CV medications are brought to the forefront by incorporating ‘C’ into the ‘APINCH’ acronym. ‘CAPINCH’ or ‘APINCH-C’ would serve as a prompt to optimise the use of CV medications throughout hospitalisation, including at the time of discharge. We acknowledge that this may be a different application of the ‘APINCH’ acronym to what was originally intended. However, the addition of ‘C’ would provide a practical and timely initiative to generate awareness about the harm caused by CV medications.
Limitations
There are some limitations to this review. Medication harm literature is vast and heterogeneous, and studies differ in terminology and methodology which makes it difficult to compare studies. 24 Additionally, the breadth and exploratory nature of the research across four healthcare settings did not meet the explicit criterion for a systematic approach, such as PRISMA. Therefore, a narrative review was undertaken and consequently, some relevant studies may not have been included. To account for this, we employed a structured search strategy using elements of PRISMA (e.g. medical subject headings, abstract/title screening) with a focus on the major studies incorporating definitions that matched our medication harm definition. To aid in transparency, we distinguished between study design and definitions used throughout this review. Similarly, the classification of drug classes differed between studies. For example, some studies included antithrombotics (e.g. aspirin) as a CV medication. 36 As our focus was on medications directly acting on the CV system, antithrombotics were excluded; however, some studies did not specify what was included within the CV medication class. It should be noted that antiplatelets are not incorporated within the ‘APINCH’ acronym. 12 Due to the important role these medications play in practice, antiplatelets could be a potential focus for future reviews of medication harm. Finally, it is acknowledged that medication harm can be precipitated by drug–drug and drug–disease interactions and from medication omission (e.g. non-compliance). Unless it was specified by the authors of the study, we were unable to discern whether these underlying factors were major contributors to medication harm.
Conclusion
CV medications are frequently implicated in causing harm across all healthcare settings. A common theme was the high prevalence of harm in older adults, which leads to morbidity and hospital utilisation. A practical method for socialising the risk would be to adapt a well-accepted safety acronym.
Supplemental Material
Supplemental material, sj-pdf-1-taw-10.1177_20420986211027451 for Patient harm from cardiovascular medications by Chariclia Paradissis, Neil Cottrell, Ian Coombes, Ian Scott, William Wang and Michael Barras in Therapeutic Advances in Drug Safety
Supplemental material, sj-pdf-2-taw-10.1177_20420986211027451 for Patient harm from cardiovascular medications by Chariclia Paradissis, Neil Cottrell, Ian Coombes, Ian Scott, William Wang and Michael Barras in Therapeutic Advances in Drug Safety
Supplemental material, sj-pdf-3-taw-10.1177_20420986211027451 for Patient harm from cardiovascular medications by Chariclia Paradissis, Neil Cottrell, Ian Coombes, Ian Scott, William Wang and Michael Barras in Therapeutic Advances in Drug Safety
Acknowledgments
The authors thank Ms Christine Dalais, Liaison Librarian at The University of Queensland, who helped to develop, test and optimise the search strategy for this research.
Appendix 1
Example search strategy for manuscript ‘Patient harm from cardiovascular medications’
Manuscript title Patient harm from cardiovascular medications
Authors Paradissis, C; Coombes I; Cottrell, W.N.; Scott, I; Wang, W and Barras, M
Acknowledgements The authors thank Ms Christine Dalais, liaison librarian at the University of Queensland, who helped to develop, test and optimise the search strategy for this research.
Date 5 November 2020
Database PubMed
Search
(((((((((“Inpatients”[Mesh])) OR “Ambulatory Care”[Mesh:NoExp]) OR “Patient Discharge”[Mesh]) OR “Patient Readmission”[Mesh]) OR “Outpatients”[Mesh]) OR “Patient Admission”[Mesh]) OR (hospital*[Title/Abstract] AND (discharge*[Title/Abstract] OR admission*[Title/Abstract] OR readmission*[Title/Abstract] OR re-admission*[Title/Abstract] OR inpatient*[Title/Abstract]) OR (outpatient*[Title/Abstract] OR ambulatory[Title/Abstract]))) AND ((((((((((“adverse drug reaction*”[Title/Abstract]) OR (“adverse drug event*” [Title/Abstract])) OR (“adverse reaction*” [Title/Abstract])) OR (“medication harm”[Title/Abstract])) OR (“medication related harm”[Title/Abstract])) OR (“medication-related harm”[Title/Abstract])) OR (“medication error*”[Title/Abstract])) OR (“adverse event*”[Title/Abstract]))) OR (“Drug-Related Side Effects and Adverse Reactions”[Mesh:NoExp]))) NOT (paediatric OR pediatric OR child OR children)
Filters Date restriction: 1990–present.
Number of results 14, 926.
Appendix 2
Box 1.
Medication harm definitions and terminology. a .
| Acronym | Definition |
|---|---|
| ADE | Any untoward medical occurrence that may present during treatment with a pharmaceutical product but that does not necessarily have a causal relation to the treatment |
| ADR | A response to a medicine which is noxious and unintended, and which occurs at doses normally used in man |
| ME | Any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the health care professional, patient, or consumer |
Based on definitions from the World Health Organization.
ADE, adverse drug event; ADR, adverse drug reaction; ME, medication error.
Footnotes
Author contributions: CP, MB, IC and NC were involved in the conception and planning of the manuscript. CP researched, analysed and wrote the manuscript under the guidance of MB, IC and NC. MB, IC, NC, IS and WW reviewed and edited the manuscript. IS and WW provided medical expertise in the analysis and presentation of results and discussion points.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Code availability: Not applicable
ORCID iDs: Chariclia Paradissis  https://orcid.org/0000-0001-9449-6761
https://orcid.org/0000-0001-9449-6761
Ian Scott  https://orcid.org/0000-0002-7596-0837
https://orcid.org/0000-0002-7596-0837
Availability of data and material: All data included and analysed for this research are incorporated within the manuscript and supplementary files provided. These published works were obtained from PubMed and CINAHL databases.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Chariclia Paradissis, School of Pharmacy, The University of Queensland, Pharmacy Australia Centre of Excellence, 20 Cornwall Street, Woolloongabba, Brisbane, QLD 4102, Australia; Pharmacy Department, Royal Brisbane and Women’s Hospital, Metro North Health, Brisbane, QLD, Australia.
Neil Cottrell, School of Pharmacy, The University of Queensland, Brisbane, QLD, Australia.
Ian Coombes, School of Pharmacy, The University of Queensland, Brisbane, QLD, Australia; Pharmacy Department, Royal Brisbane and Women’s Hospital, Metro North Health, Brisbane, QLD, Australia.
Ian Scott, Faculty of Medicine, The University of Queensland, Brisbane, QLD, Australia; Department of Internal Medicine and Clinical Epidemiology, Princess Alexandra Hospital, Metro South Health, Brisbane, QLD, Australia.
William Wang, Faculty of Medicine, The University of Queensland, Brisbane, QLD, Australia; Department of Cardiology, Princess Alexandra Hospital, Metro South Health, Brisbane, QLD, Australia.
Michael Barras, School of Pharmacy, The University of Queensland, Brisbane, QLD, Australia; Pharmacy Department, Princess Alexandra Hospital, Metro South Health, Brisbane, QLD, Australia.
References
- 1. Australian Institute of Health and Welfare. Australia’s health 2018. Australia’s health series no. 16. AUS 221. Canberra: AIHW, 2018. [Google Scholar]
- 2. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA 1995; 274: 29–34. [PubMed] [Google Scholar]
- 3. Davis P. Adverse events in New Zealand public hospitals I: occurrence and impact. N Z Med J 2002; 115: U271. [PubMed] [Google Scholar]
- 4. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med 1991; 324: 377–384. [DOI] [PubMed] [Google Scholar]
- 5. Wilson RM, Runciman WB, Gibberd RW, et al. The quality in Australian health care study. Med J Aust 1995; 163: 458–471. [DOI] [PubMed] [Google Scholar]
- 6. Budnitz DS, Lovegrove MC, Shehab N, et al. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med 2011; 365: 2002–2012. [DOI] [PubMed] [Google Scholar]
- 7. Roughead L, Semple S, Rosenfeld E. Literature review: medication safety in Australia. Sydney: Australian Commission on Safety and Quality in Health Care, 2013. [Google Scholar]
- 8. Roughead EE, Semple JS, Rosenfeld JE. The extent of medication errors and adverse drug reactions throughout the patient journey in acute care in Australia. Int J Evid Based Healthc 2016; 14: 113–122. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization. The third WHO global patient safety challenge: medication without harm, https://www.who.int/patientsafety/medication-safety/en/ (2019, accessed 29 August 2019).
- 10. Pharmaceutical Society of Australia. Medicine safety to be the 10th National Health Priority area, https://www.psa.org.au/ (2019, accessed 30 July 2020).
- 11. The Hon Greg Hunt MP. Doorstop interview about the COAG health ministers meeting, https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/doorstop-interview-about-the-coag-health-ministers-meeting (2019, accessed 30 July 2020).
- 12. Australian Commission on Safety and Quality in Health Care. APINCHS classification of high risk medicines, https://www.safetyandquality.gov.au/our-work/medication-safety/high-risk-medicines/apinchs-classification-high-risk-medicines (2019, accessed 8 November 2019).
- 13. Australian Commission on Safety and Quality in Health Care. High risk medicines, https://www.safetyandquality.gov.au/our-work/medication-safety/high-risk-medicines (2019, accessed 12 December 2019).
- 14. Institute for Safe Medication Practices. High-alert medications in acute care settings, https://www.ismp.org/recommendations/high-alert-medications-acute-list (2018, accessed 10 September 2019).
- 15. Clinical Excellence Commission. High-risk medicines, http://www.cec.health.nsw.gov.au/patient-safety-programs/medication-safety/high-risk-medicines/A-PINCH (2019, accessed 8 November 2019).
- 16. Australian Institute of Health and Welfare. Medicines for cardiovascular disease. Cat. no. CVD 80. Canberra: AIHW, 2017. [Google Scholar]
- 17. Holbeach E, Yates P. Prescribing in the elderly. Aust Fam Physician 2010; 39: 728–733. [PubMed] [Google Scholar]
- 18. Chew DP, Scott IA, Cullen L, et al. National Heart Foundation of Australia & Cardiac Society of Australia and New Zealand: Australian clinical guidelines for the management of acute coronary syndromes 2016. Heart Lung Circ 2016; 25: 895–951. [DOI] [PubMed] [Google Scholar]
- 19. Ali Raza J, Movahed A. Use of cardiovascular medications in the elderly. Int J Cardiol 2002; 85: 203–215. [DOI] [PubMed] [Google Scholar]
- 20. Ruiz B, Garcia M, Aguirre U, et al. Factors predicting hospital readmissions related to adverse drug reactions. Eur J Clin Pharmacol 2008; 64: 715–722. [DOI] [PubMed] [Google Scholar]
- 21. Wang Y, Singh S, Bajorek B. Old age, high risk medication, polypharmacy: a ‘trilogy’ of risks in older patients with atrial fibrillation. Pharm Pract (Granada) 2016; 14: 706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paradissis C, Coombes ID, Donovan P, et al. The type and incidence of adverse drug events in ageing medical inpatients and their effect on length of hospital stay. J Pharm Pract Res 2017; 47: 347–354. [Google Scholar]
- 23. Falconer N, Barras M, Abdel-Hafez A, et al. Prioritising patients at high-risk of medication harm: Development and validation of risk prediction models [dissertation]. University of Queensland, Brisbane, Australia, 2019. [Google Scholar]
- 24. Falconer N, Barras M, Martin J, et al. Defining and classifying terminology for medication harm: a call for consensus. Eur J Clin Pharmacol 2019; 75: 137–145. [DOI] [PubMed] [Google Scholar]
- 25. Australian medicines handbook online [Internet]. Adelaide (S. Australia): Australian Medicines Handbook Pty Ltd, 2020. Cardiovascular drugs, https://amhonline.amh.net.au/chapters/cardiovasculardrugs?menu=banner (2020, accessed 30 July 2020).
- 26. Guerrouij M, Uppal CS, Alklabi A, et al. The clinical impact of bleeding during oral anticoagulant therapy: assessment of morbidity, mortality and post-bleed anticoagulant management. (Report). J Thromb Thrombolysis 2011; 31: 419. [DOI] [PubMed] [Google Scholar]
- 27. Piazza G, Nguyen TN, Cios D, et al. Anticoagulation-associated adverse drug events. Am J Med 2011; 124: 1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Budnitz DS, Shehab N, Kegler SR, et al. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med 2007; 147: 755–765. [DOI] [PubMed] [Google Scholar]
- 29. Chan M, Nicklason F, Vial J. Adverse drug events as a cause of hospital admission in the elderly. Intern Med J 2001; 31: 199–205. [DOI] [PubMed] [Google Scholar]
- 30. Hallas J, Gram LF, Grodum E, et al. Drug related admissions to medical wards: a population based survey. Br J Clin Pharmacol 1992; 33: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McDonnell PJ, Jacobs MR. Hospital admissions resulting from preventable adverse drug reactions. Ann Pharmacother 2002; 36: 1331–1336. [DOI] [PubMed] [Google Scholar]
- 32. Mjorndal T, Boman MD, Hagg S, et al. Adverse drug reactions as a cause for admissions to a department of internal medicine. Pharmacoepidemiol Drug Saf 2002; 11: 65–72. [DOI] [PubMed] [Google Scholar]
- 33. Onder G, Pedone C, Landi F, et al. Adverse drug reactions as cause of hospital admissions: results from the Italian Group of Pharmacoepidemiology in the Elderly (GIFA). J Am Geriatr Soc 2002; 50: 1962–1968. [DOI] [PubMed] [Google Scholar]
- 34. Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 2004; 329: 15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wawruch M, Zikavska M, Wsolova L, et al. Adverse drug reactions related to hospital admission in Slovak elderly patients. Arch Gerontol Geriatr 2009; 48: 186–190. [DOI] [PubMed] [Google Scholar]
- 36. Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: a systematic review of prospective observational studies. Ann Pharmacother 2008; 42: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 37. Gustafsson M, Sjolander M, Pfister B, et al. Drug-related hospital admissions among old people with dementia. Eur J Clin Pharmacol 2016; 72: 1143–1153. [DOI] [PubMed] [Google Scholar]
- 38. Runciman WB, Roughead EE, Semple SJ, et al. Adverse drug events and medication errors in Australia. Int J Qual Health Care 2003; (15 Suppl. 1): i49–i59. [DOI] [PubMed] [Google Scholar]
- 39. Howard RL, Avery AJ, Slavenburg S, et al. Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol 2007; 63: 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Al Hamid A, Ghaleb M, Aljadhey H, et al. A systematic review of hospitalization resulting from medicine-related problems in adult patients. Br J Clin Pharmacol 2014; 78: 202–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alhawassi TM, Krass I, Bajorek BV, et al. A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin Interv Aging 2014; 9: 2079–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oscanoa TJ, Lizaraso F, Carvajal A. Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur J Clin Pharmacol 2017; 73: 759–770. [DOI] [PubMed] [Google Scholar]
- 43. Roughead EE, Gilbert AL, Primrose JG, et al. Drug-related hospital admissions: a review of Australian studies published 1988-1996. Med J Aust 1998; 168: 405–408. [DOI] [PubMed] [Google Scholar]
- 44. Wiffen P, Gill M, Edwards J, et al. Adverse drug reactions in hospital patients: a systematic review of the prospective and retrospective studies. In: Database of Abstracts of Reviews of Effects (DARE): quality-assessed reviews. York, UK: Centre for Reviews and Dissemination (UK), 2002, pp.1–14. [Google Scholar]
- 45. Angamo MT, Chalmers L, Curtain CM, et al. Adverse-drug-reaction-related hospitalisations in developed and developing countries: a review of prevalence and contributing factors. Drug Saf 2016; 39: 847–857. [DOI] [PubMed] [Google Scholar]
- 46. Larmour I, Dolphin RG, Baxter H, et al. A prospective study of hospital admissions due to drug reactions. Aust J Hosp Pharm 1991; 21: 90–95. [Google Scholar]
- 47. Stanton LA, Peterson GM, Rumble RH, et al. Drug-related admissions to an Australian hospital. J Clin Pharm Ther 1994; 19: 341–347. [DOI] [PubMed] [Google Scholar]
- 48. Nelson KM, Talbert RL. Drug-related hospital admissions. Pharmacotherapy 1996; 16: 701–707. [PubMed] [Google Scholar]
- 49. Jha AK, Kuperman GJ, Rittenberg E, et al. Identifying hospital admissions due to adverse drug events using a computer-based monitor. Pharmacoepidemiol Drug Saf 2001; 10: 113. [DOI] [PubMed] [Google Scholar]
- 50. Burgess CL, Holman CD, Satti AG. Adverse drug reactions in older Australians, 1981-2002. Med J Aust 2005; 182: 267–270. [DOI] [PubMed] [Google Scholar]
- 51. Passarelli MC, Jacob-Filho W, Figueras A. Adverse drug reactions in an elderly hospitalised population: inappropriate prescription is a leading cause. Drugs Aging 2005; 22: 767–777. 2005/09/15. DOI: 10.2165/00002512-200522090-00005 [DOI] [PubMed] [Google Scholar]
- 52. Budnitz DS, Pollock DA, Weidenbach KN, et al. National surveillance of emergency department visits for outpatient adverse drug events. JAMA 2006; 296: 1858–1866. [DOI] [PubMed] [Google Scholar]
- 53. Ducharme MM, Boothby LA. Analysis of adverse drug reactions for preventability. Int J Clin Pract 2007; 61: 157–161. [DOI] [PubMed] [Google Scholar]
- 54. Edwards DB, Heisler M, Guidry J, et al. Adverse drug events leading to admission at a community nonteaching hospital. J Clin Outcomes Manag 2007; 14: 389–394. Article. [Google Scholar]
- 55. Ocampo JM, Chacón JA, Gómez JF, et al. Adverse drug reactions and adverse drug events in elderly patients consulting a hospital emergency unit. Colomb Med 2008; 39: 135–146. [Google Scholar]
- 56. Ventura MT, Laddaga R, Cavallera ‘P, et al. Adverse drug reactions as the cause of emergency department admission: focus on the elderly. Immunopharmacol Immunotoxicol 2010; 32: 426–429. [DOI] [PubMed] [Google Scholar]
- 57. Conforti A, Costantini D, Zanetti F, et al. Adverse drug reactions in older patients: an Italian observational prospective hospital study. Drug, Healthcare and Patient Safety 2012; 4: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Marcum ZA, Amuan ME, Hanlon JT, et al. Prevalence of unplanned hospitalizations caused by adverse drug reactions in older veterans. J Am Geriatr Soc 2012; 60: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McLachlan CY, Yi M, Ling A, et al. Adverse drug events are a major cause of acute medical admission. Intern Med J 2014; 44: 633–638. [DOI] [PubMed] [Google Scholar]
- 60. Phillips AL, Nigro O, Macolino KA, et al. Hospital admissions caused by adverse drug events: an Australian prospective study. Aust Health Rev 2014; 38: 51–57. [DOI] [PubMed] [Google Scholar]
- 61. De Almeida SM, Romualdo A, De Abreu Ferraresi A, et al. Use of a trigger tool to detect adverse drug reactions in an emergency department. BMC Pharmacol Toxicol 2017; 18: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Parameswaran Nair N, Chalmers L, Bereznicki BJ, et al. Adverse drug reaction-related hospitalizations in elderly Australians: a prospective cross-sectional study in two Tasmanian hospitals. Drug Saf 2017; 40: 597–606. [DOI] [PubMed] [Google Scholar]
- 63. Poudel DR, Acharya P, Ghimire S, et al. Burden of hospitalizations related to adverse drug events in the USA: a retrospective analysis from large inpatient database. Pharmacoepidemiol Drug Saf 2017; 26: 635–641. [DOI] [PubMed] [Google Scholar]
- 64. Ognibene S, Vazzana N, Giumelli C, et al. Hospitalisation and morbidity due to adverse drug reactions in elderly patients: a single-centre study. Intern Med J 2018; 48: 1192–1197. [DOI] [PubMed] [Google Scholar]
- 65. Schurig AM, Bohme M, Just KS, et al. Adverse Drug Reactions (ADR) and emergencies. Dtsch Arztebl Int 2018; 115: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mullan J, Burns P, Mohanan L, et al. Hospitalisation for medication misadventures among older adults with and without dementia: a 5-year retrospective study. Australas J Ageing 2019; 38: e135–e141. [DOI] [PubMed] [Google Scholar]
- 67. Zhang H, Du W, Gnjidic D, et al. Trends in adverse drug reaction-related hospitalisations over 13 years in New South Wales, Australia. Intern Med J 2019; 49: 84–93. 2018/10/04. DOI: 10.1111/imj.14134. [DOI] [PubMed] [Google Scholar]
- 68. Smeaton T, McElwaine P, Cullen J, et al. A prospective observational pilot study of adverse drug reactions contributing to hospitalization in a cohort of middle-aged adults aged 45–64 years. Drugs Ther Perspect 2020; 36: 123–130. [Google Scholar]
- 69. Davies EC, Green CF, Taylor S, et al. Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient-episodes. PLoS One 2009; 4: e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kanjanarat P, Winterstein AG, Johns TE, et al. Nature of preventable adverse drug events in hospitals: a literature review. Am J Health Syst Pharm 2003; 60: 1750–1759. [DOI] [PubMed] [Google Scholar]
- 71. O’Connor MN, Gallagher P, Byrne S, et al. Adverse drug reactions in older patients during hospitalisation: are they predictable? Age Ageing 2012; 41: 771–776. [DOI] [PubMed] [Google Scholar]
- 72. Cecile M, Seux V, Pauly V, et al. Adverse drug events in hospitalized elderly patients in a geriatric medicine unit: study of prevalence and risk factors. Rev Med Interne 2009; 30: 393–400. [DOI] [PubMed] [Google Scholar]
- 73. Beijer HJ, de Blaey CJ. Hospitalisations caused by adverse drug reactions (ADR): a meta-analysis of observational studies. Pharm World Sci 2002; 24: 46–54. [DOI] [PubMed] [Google Scholar]
- 74. Laatikainen O, Miettunen J, Sneck S, et al. The prevalence of medication-related adverse events in inpatients-a systematic review and meta-analysis. Eur J Clin Pharmacol 2017; 73: 1539–1549. [DOI] [PubMed] [Google Scholar]
- 75. Levkovich BJ, Bingham G, Jones D, et al. Understanding how medications contribute to clinical deterioration and are used in rapid response systems: a comprehensive scoping review. Aust Crit Care 2019; 32: 256–272. [DOI] [PubMed] [Google Scholar]
- 76. Classen DC, Pestotnik SL, Evans RS, et al. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1997; 277: 301–306. [PubMed] [Google Scholar]
- 77. Doucet J, Jego A, Noel D, et al. Preventable and non-preventable risk factors for adverse drug events related to hospital admissions in the elderly. Clin Drug Investig 2002; 22: 385–392. [Google Scholar]
- 78. Al-Tajir GK, Kelly WN. Epidemiology, comparative methods of detection, and preventability of adverse drug events. Ann Pharmacother 2005; 39: 1169–1174. [DOI] [PubMed] [Google Scholar]
- 79. Trivalle C, Cartier T, Verny C, et al. Identifying and preventing adverse drug events in elderly hospitalised patients: a randomised trial of a program to reduce adverse drug effects. J Nutr Health Aging 2010; 14: 57–61. [DOI] [PubMed] [Google Scholar]
- 80. Morimoto T, Sakuma M, Matsui K, et al. Incidence of adverse drug events and medication errors in Japan: the JADE study. J Gen Intern Med 2011; 26: 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Parikh S, Christensen D, Stuchbery P, et al. Exploring in-hospital adverse drug events using ICD-10 codes. Aust Health Rev 2014; 38: 454–460. [DOI] [PubMed] [Google Scholar]
- 82. Rojas-Velandia C, Ruiz-Garzon J, Moscoso-Alcina JC, et al. Characterization of adverse drug reactions causing admission to an intensive care unit. Br J Clin Pharmacol 2017; 83: 1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Robb G, Loe E, Maharaj A, et al. Medication-related patient harm in New Zealand hospitals. N Z Med J 2017; 130: 21–32. [PubMed] [Google Scholar]
- 84. Cohen MR. Medication errors. 2nd ed. Washington, DC: American Pharmacists Association, 2007. [Google Scholar]
- 85. Australian Pharmaceutical Advisory Council. Guiding principles to achieve continuity in medication management. Canberra: Commonwealth of Australia, 2005. [Google Scholar]
- 86. El Morabet N, Uitvlugt EB, Van den Bemt BJF, et al. Prevalence and preventability of drug-related hospital readmissions: a systematic review. J Am Geriatr Soc 2018; 66: 602–608. [DOI] [PubMed] [Google Scholar]
- 87. Hanlon JT, Pieper CF, Hajjar ER, et al. Incidence and predictors of all and preventable adverse drug reactions in frail elderly persons after hospital stay. J Gerontol A Biol Sci Med Sci 2006; 61: 511–515. [DOI] [PubMed] [Google Scholar]
- 88. Kanaan AO, Donovan JL, Duchin NP, et al. Adverse drug events after hospital discharge in older adults: types, severity, and involvement of Beers Criteria Medications. J Am Geriatr Soc 2013; 61: 1894–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Forster AJ, Murff HJ, Peterson JF, et al. Adverse drug events occurring following hospital discharge. J Gen Intern Med 2005; 20: 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA 2003; 289: 1107–1116. [DOI] [PubMed] [Google Scholar]
- 91. Forster AJ, Murff HJ, Peterson JF, et al. The incidence and severity of adverse events affecting patients after discharge from hospital. Ann Intern Med 2003; 138: 161. [DOI] [PubMed] [Google Scholar]
- 92. Parekh N, Ali K, Page A, et al. Incidence of medication-related harm in older adults after hospital discharge: a systematic review. J Am Geriatr Soc 2018; 66: 1812–1822. [DOI] [PubMed] [Google Scholar]
- 93. Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care. N Engl J Med 2003; 348: 1556–1564. [DOI] [PubMed] [Google Scholar]
- 94. Carnovale C, Gentili M, Fortino I, et al. The importance of monitoring adverse drug reactions in elderly patients: the results of a long-term pharmacovigilance programme. Expert Opin Drug Saf 2016; 15: 131–139. [DOI] [PubMed] [Google Scholar]
- 95. Mann E, Zepeda O, Soones T, et al. Adverse drug events and medication problems in “Hospital at Home” patients. Home Health Care Serv Q 2018; 37: 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Parekh N, Ali K, Stevenson JM, et al. Incidence and cost of medication harm in older adults following hospital discharge: a multicentre prospective study in the UK. Br J Clin Pharmacol 2018; 84: 1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gray SL, Mahoney JE, Blough DK. Adverse drug events in elderly patients receiving home health services following hospital discharge. Ann Pharmacother 1999; 33: 1147–1153. [DOI] [PubMed] [Google Scholar]
- 98. Davies EC, Green CF, Mottram DR, et al. Emergency re-admissions to hospital due to adverse drug reactions within 1 year of the index admission. Br J Clin Pharmacol 2010; 70: 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zhang M, Holman CD, Preen DB, et al. Repeat adverse drug reactions causing hospitalization in older Australians: a population-based longitudinal study 1980-2003. Br J Clin Pharmacol 2007; 63: 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Crispo JAG, Thibault DP, Willis AW. Adverse drug events as a reason for adult hospitalization: a nationwide readmission study. Ann Pharmacother 2019; 53: 557–566. [DOI] [PubMed] [Google Scholar]
- 101. See RI, Shehab RN, Kegler SS, et al. Emergency department visits and hospitalizations for digoxin toxicity: United States, 2005 to 2010. Circ Heart Fail 2014; 7: 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mangoni AA, Jackson S. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol 2004; 57: 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. O’Mahony D, O’Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015; 44: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Fick DM, Semla TP, Beizer J, et al. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015; 63: 2227–2246. [DOI] [PubMed] [Google Scholar]
- 105. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med 2015; 175: 827. [DOI] [PubMed] [Google Scholar]
- 106. Nyborg G, Straand J, Brekke M. Inappropriate prescribing for the elderly–a modern epidemic? Eur J Clin Pharmacol 2012; 68: 1085–1094. [DOI] [PubMed] [Google Scholar]
- 107. Scott IA, Hilmer SN, Le Couteur DG. Going beyond the guidelines in individualising the use of antihypertensive drugs in older patients. Drugs Aging 2019; 36: 675–685. [DOI] [PubMed] [Google Scholar]
- 108. Goyal P, Anderson TS, Bernacki GM, et al. Physician perspectives on deprescribing cardiovascular medications for older adults. J Am Geriatr Soc. Epub ahead of print 11 September 2019. DOI: 10.1111/jgs.16157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Naganathan V. Cardiovascular drugs in older people. Aust Prescr 2013; 36: 190–194. [Google Scholar]
- 110. Krishnaswami A, Steinman MA, Goyal P, et al. Deprescribing in older adults with cardiovascular disease. J Am Coll Cardiol 2019; 73: 2584–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Reeve E, Denig P, Hilmer SN, et al. The ethics of deprescribing in older adults. J Bioeth Inq 2016; 13: 581–590. [DOI] [PubMed] [Google Scholar]
- 112. Garattini S, Garattini S, Bertele’ V, et al. Benefits, benefits, once more benefits… with no risk? Stop overlooking the harms of medicines. Eur J Clin Pharmacol 2018; 74: 373–375. [DOI] [PubMed] [Google Scholar]
- 113. Rossello X, Pocock SJ, Julian DG. Long-term use of cardiovascular drugs: challenges for research and for patient care. J Am Coll Cardiol 2015; 66: 1273–1285. [DOI] [PubMed] [Google Scholar]
- 114. Morgan DJ, Scherer LD, Korenstein D. Improving physician communication about treatment decisions: reconsideration of “Risks vs Benefits”. JAMA 2020; 324: 937–938. [DOI] [PubMed] [Google Scholar]
- 115. Rose L, Muttalib F, Adhikari NKJ. Psychological consequences of admission to the ICU: helping patients and families. JAMA 2019; 322: 213–215. [DOI] [PubMed] [Google Scholar]
- 116. Saedder E, Brock B, Nielsen L, et al. Identifying high-risk medication: a systematic literature review. Eur J Clin Pharmacol 2014; 70: 637–645. [DOI] [PubMed] [Google Scholar]
- 117. Australian Institute of Health and Welfare. Australian hospital statistics. Health Services Series no. 90. Cat. no. HSE 225. 90 ed. Canberra: AIHW, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-taw-10.1177_20420986211027451 for Patient harm from cardiovascular medications by Chariclia Paradissis, Neil Cottrell, Ian Coombes, Ian Scott, William Wang and Michael Barras in Therapeutic Advances in Drug Safety
Supplemental material, sj-pdf-2-taw-10.1177_20420986211027451 for Patient harm from cardiovascular medications by Chariclia Paradissis, Neil Cottrell, Ian Coombes, Ian Scott, William Wang and Michael Barras in Therapeutic Advances in Drug Safety
Supplemental material, sj-pdf-3-taw-10.1177_20420986211027451 for Patient harm from cardiovascular medications by Chariclia Paradissis, Neil Cottrell, Ian Coombes, Ian Scott, William Wang and Michael Barras in Therapeutic Advances in Drug Safety