Abstract
Aim:
Prior research has primarily focused on static pain assessment, largely ignoring the dynamic nature of pain over time. We used a novel assessment tool for characterizing pain duration, frequency, and amplitude in women with dysmenorrhea and evaluated how these metrics were affected by naproxen treatment.
Methods:
Dysmenorrheic women (n=25) rated their menstrual pain by squeezing a pressure bulb proportional to the magnitude of their pain. To evaluate whether bulb squeezing was affected by naproxen, we compared parameters before and after naproxen. We also analyzed the correlation between pain relief on a numerical rating scale to changes in bulb squeezing parameters. Random bulb-squeezing activity in pain-free participants (n=14) was used as a control for nonspecific effects or bias.
Results:
In dysmenorrheic women, naproxen reduced the duration of the squeezing during a painful bout, the number of painful bouts and bout intensity. Before naproxen, the correlation between these bulb squeeze parameters and self-reported pain on numeric rating scale was not significant (R2 = 0.12, p = 0.304); however, there was a significant correlation between changes in bulb squeeze activity and self-reported pain relief after naproxen (R2 = 0.55, p < 0.001).
Conclusions:
Our study demonstrates a convenient technique for continuous pain assessment, capturing three different dimensions: duration, frequency, and magnitude. Naproxen may act by reducing the duration and frequency of episodic pain in addition to reducing the severity. After further validation, these methods could be used for other pain conditions for deeper phenotyping and assessing novel treatments.
Keywords: Dysmenorrhea, menstrual, muscle cramp, naproxen, pain measurement
Introduction:
Spontaneous pain consisting of continuous varying levels of pain even in the absence of an external stimulus, is often the largest component of severity in acute and chronic pain; however, it has rarely been captured continuously in human research over time.1,2 Survey data suggest that spontaneous pain represents a component of pain that particularly contributes to pain interference.3 However, prior research has focused generally on static pain assessment, failing to adequately evaluate changes in pain over time, especially visceral pain. A model for continuously measuring pain could shed light on pain phenotypes and underlying physiological mechanisms that may inform personalized medical interventions.4
The few studies that have measured spontaneous pain have had promising results. For example, one paradigm was developed to examine fluctuations in spontaneous pain in patients suffering from chronic back pain and chronic postherpetic neuropathy.5 Participants continuously stretched a finger span gauge over a 6-to-12-minute period to indicate their level of pain. This strategy demonstrated that measuring fractal complexity of spontaneous pain can be used to differentiate chronic back pain and postherpetic neuropathy. However, a method for determining the fundamental temporal characteristics (frequency, duration, and amplitude) of spontaneous pain has not been validated. A convenient technique for characterizing spontaneous pain may be particularly useful in a visceral pain condition with spontaneous cramping pain.
To validate a new metric for evaluating spontaneous pain and its temporal characteristics, we investigated women with menstrual period pain, clinically known as dysmenorrhea. Previously, a hand-held squeeze bulb was used to characterize the temporal relationship between cramping pain and uterine activity with MRI6 or abdominal muscle activity with EMG,7 however this method to measure pain intensity was not validated. We tested the hypothesis that worse global self-reported rating of menstrual cramping pain is associated with more prolonged, intense, and frequent bulb squeezing pain bouts in women with dysmenorrhea. We also hypothesized that the effects of an NSAID, naproxen, on overall pain severity would be mirrored by changes in reported pain bout frequency, duration, and magnitude. To test these hypotheses, we evaluated the time course of painful experiences in women with dysmenorrhea using a squeeze bulb device before and after an analgesic dosage of naproxen. Control participants without pain were included to verify that the analysis of bulb-squeeze parameters were conducted without bias. Since the presence of endometriosis may lessen medication responsiveness and pain behavior, we have also accounted for this as a covariate.8
Materials and Methods
Our Institutional Review Board approved this study. We recruited dysmenorrheic participants and healthy controls (ages 18-45) into a two-arm study that involved ultrasonography or MRI. Participants included in this study were enrolled between March 2015 and November 2017. Some participant data from the MRI and ultrasound study has already been published.6,7 The method in this paper was used in those prior studies6,7 to investigate the role of uterine contractions and abdominal muscle activity in menstrual pain. However, the findings in this manuscript uniquely focus on the effect of naproxen on spontaneous pain report using this new technique.
Participant Recruitment
Reproductive-age women (18-45 years old) were recruited from physician referrals and flyers in the community and around local college campuses. Participants were considered to have moderate to severe dysmenorrhea if they reported an average pain of greater than or equal to 6 on a 0-10 numerical rating scale (NRS, 0 being no pain and 10 being the worst imaginable pain) during their menses or during withdrawal uterine bleeding from cyclical oral contraceptives while off analgesic drugs. Eligible dysmenorrheic participants were required to have pain in the region between the umbilicus and the perineum, above the inguinal ligament. Participants who reported an average pain less than 3 on a 0-10 NRS and had no chronic pelvic pain diagnoses were placed in the pain-free control group. These NRS thresholds were chosen based on our prior experience for identifying divergent phenotypes of uterine contractility or abdominal muscle activity in menstrual pain.6,7 After completing a phone screen, both pain-free and dysmenorrheic women participated in two scheduled visits, one during menses and one mid-cycle.
Exclusion criteria for all groups included: presence of active pelvic or abdominal malignancies (primary or metastatic), absence of regular menses (more than 45 days between periods), inability to read or comprehend the informed consent written in English, and unwillingness to take naproxen as part of the study. Participants were excluded if their BMI>40, they were claustrophobic, or had metal implants that would interfere with MRI.
Menses Study Visit
Participants were scheduled for a menses visit during the first 72 hours following the onset of menstrual bleeding. Participants were instructed to abstain from taking short-acting analgesic medications for at least 8 hours before the visit or 12 hours for long-acting analgesics.
After giving written informed consent, participants were asked to rate their current menstrual pain (pre-treatment) using a 0-10 NRS. All participants completed an initial MRI or ultrasound scan of their uterus. During these scans, subjects with dysmenorrhea (n=25) were instructed to indicate their baseline perceived menstrual pain on a 0-10 NRS and then squeeze a hand-held 5.8 cm diameter rubber bulb (RXPUMPBULB-MRI, Biopac Systems, Goleta, CA) whenever they experienced an increase in their cramping pain they typically experience with periods. Participants were further instructed to use their dominant hand while squeezing the bulb and to proportionally squeeze the bulb relative to how much pain they experienced above their baseline for the duration of a bout of pain.
Squeeze bulb measurements were recorded using a pressure transducer connected to a data acquisition system (BIOPAC, Goleta, CA). A diagram of the system is shown in Figure 1. Pain-free controls during menses (n=14) reported no pain and were instructed to squeeze the bulb randomly every 2-5 minutes throughout the recording session. This frequency was chosen because pilot observations suggested that this was the frequency of menstrual pain bouts in women with dysmenorrhea.
Figure 1: Diagram of apparatus used to monitor spontaneous pain.
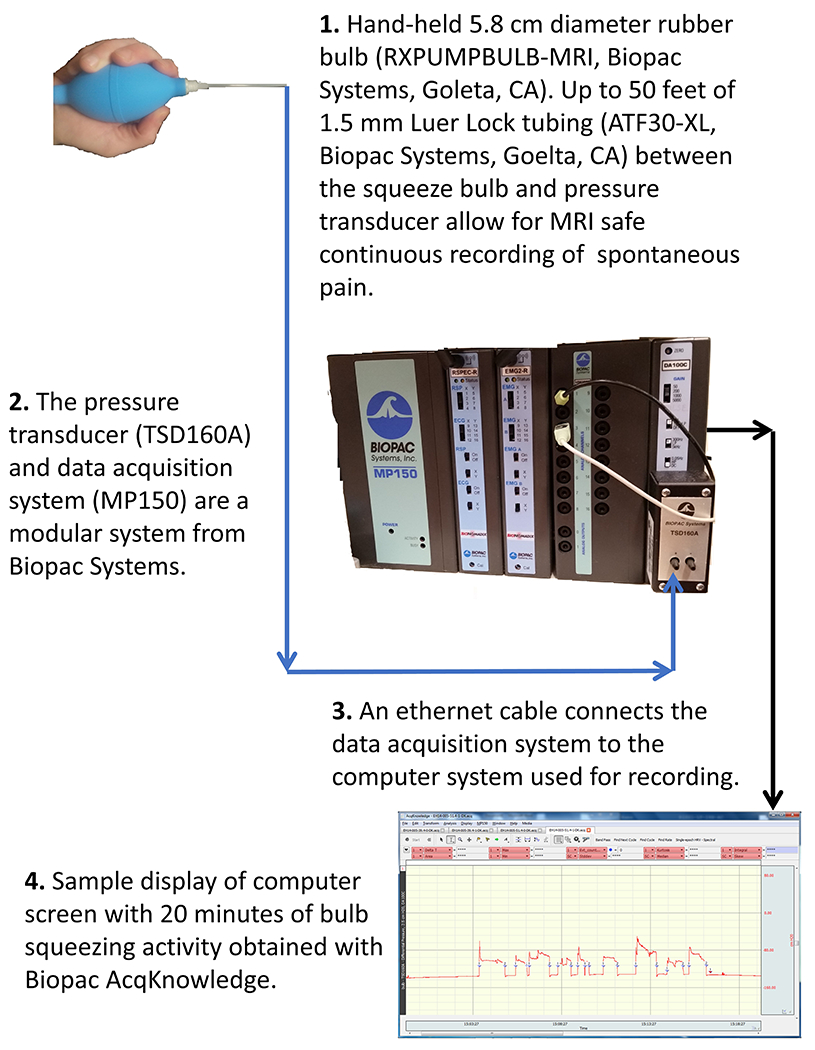
An MRI-safe squeeze bulb was connected to a pressure transducer via polyethylene tubing. The pressure transducer activity record was saved with a digital acquisition system.
Following the initial MRI or ultrasound scan, participants ingested two tablets of naproxen sodium (220 mg each) with water. Next, they completed a set of questionnaires, including information about their prior medical, surgical, psychological, gynecological, and obstetrical history. Additionally, women were asked to rate their menstrual pain over the past three months with and without analgesic medications on a 0-100 mm visual analogue scale (VAS). When available, a lack of contributing anatomical factors (e.g., adenomyosis, leiomyoma) was corroborated with the MRI to confirm the diagnosis of primary dysmenorrhea. Since MRI cannot always be used for adequate diagnosis of endometriosis, surgical history was to clarify secondary dysmenorrhea due to endometriosis.
Ninety minutes after the administration of naproxen, participants reassessed their menstrual pain on the 0-10 NRS and repeated the bulb squeezing task during an MRI or ultrasound scan. Among the 14 pain-free controls on menses, only 1 participant did not complete the study, and 4 participants did not ingest naproxen due to time limitations or refusal.
Mid-Cycle Study Visit
After completing a menses visit, participants were scheduled for a mid-cycle visit. Participants could not be experiencing any menstrual bleeding and were not administered naproxen during this visit; therefore, they only completed one MRI or ultrasound scan. Of the original cohort, 12 pain-free controls and 19 dysmenorrheic participants completed the mid-cycle visit. During this visit, all participants received the same bulb squeeze instructions as the pain-free controls during their menses visit. They were instructed to squeeze the bulb randomly every 2-5 minutes throughout the recording session, even if participants experienced abdominal or pelvic pain symptoms.
Analyses
For each participant, a single fifteen-minute segment with visually identified squeezing bouts was analyzed by a reviewer blinded to participant group identity. We have labeled episodes of menstrual pain as “bouts” to maintain consistency throughout this study. The beginning of a self-reported pain bout was defined as the first time at which squeeze pressure increased, and the end of the pain bout was when squeeze pressure returned to baseline. Pain bouts were identified with a script by setting a threshold pressure for detection and confirmed with visual inspection with a BIOPAC AcqKnowledge script. The average and standard error of the mean of painful bout parameters (duration, frequency, and intensity measured by the amplitude of the applied pressure) were calculated for each group per study session and compared between groups (Stata V 13.1). Bout durations were capped at 60 seconds to avoid skewing, but only one participant with dysmenorrhea exceeded this threshold.
All complete available data was analyzed. Since this was a secondary analysis, instead of a power analysis, we have included effect sizes with bootstrapped 95% confidence intervals for the key analyses described below. The inclusion of effect size allows for better evaluation of null effects and promotes the replicability of significant effects by estimating the magnitude.9 We used Cohen’s terminology for describing the magnitude of effect size: small (d = 0.2, r = 0.1), medium (d = 0.5, r = 0.3 ), or large (d = 0.8, r= 0.5). 10 Linear regression modeling was used to evaluate the association of duration, frequency, and intensity of squeeze bulb-pressure to self-reported pain, while accounting for endometriosis as a covariate. A linear regression model was also used to evaluate the association of changes in these same parameters before and after naproxen ingestion. Paired t-tests were used to analyze nonspecific effects of naproxen or cycle day (menses vs. non-menses) in controls or specific effects in women with dysmenorrhea. We also explored whether there was a relationship between bulb squeezing activity and pain relief following acute naproxen ingestion. Groups were further subanalyzed based on no/mild relief and moderate/substantial relief medication response, based on IMMPACT guidelines.11 All results are expressed as mean ± standard error.
Results:
We first compared patterns of pain bouts on menses in women with dysmenorrhea (n=25) vs. pain-free controls (n=14). The dysmenorrhea cohort contained a mix of 17 participants with primary dysmenorrhea and 8 participants with a prior diagnosis of endometriosis. Overall, the dysmenorrhea cohort represented women with moderate to severe menstrual pain. These patients reported over the previous 3 months that their average pain without medication as 71.3 ± 4.0 on a 0 - 100 VAS and that they missed 3.7 ± 1.9 days of school or work. In contrast, pain-free controls reported minimal menstrual pain (7.8 ± 2.9 on a 0 - 100 VAS) and never missed school or work due to menstrual pain. Women with dysmenorrhea had demographic characteristics similar to pain-free controls (Table 1).
Table 1. Participant demographics and self-reported pain scores for dysmenorrhea and bulb squeeze event study.
Absenteeism - days missed in last 3 months due to pain
| Demographics | Dysmenorrheic (n = 25) | Pain-Free (n = 14) | p value |
|---|---|---|---|
| Age | 27.0 ± 1.6 | 27.8 ± 3.6 | 0.732 |
| Race | 0.04 | ||
| African American | 3 (12%) | 1 (7%) | |
| White | 20 (80%) | 7 (50%) | |
| Other | 2 (8%) | 6 (43%) | |
| Menstrual pain without NSAIDs (0-100) | 71.3 ± 4.0 | 7.8 ± 2.9 | < 0.001 |
| Menstrual pain with NSAIDs (0-100) | 37.9 ± 5.8 | 5.8 ± 2.3 | < 0.001 |
| Absenteeism (days) | 3.7 ± 1.9 | 0 | |
| Primary Dysmenorrhea | 16 | ||
| Secondary Dysmenorrhea | 9 |
Control data from pain-free participants
We also examined bulb squeezing in pain-free controls during menses and mid-cycle days to look for possible effects of naproxen or menstrual cycle day on bulb squeezing activity (Table 2). The pain-free group reported no pain (VAS = 0 ± 0) on their menses visit and had no significant changes in bulb squeezing duration, frequency, or intensity after taking naproxen. Additionally, there were no significant changes in squeezing activity between pain-free participants’ mid-cycle and menses visits. Bootstrap analysis of effect sizes (95% CI of Cohen’s −0.19 to 0.27) suggested minimal and nonsignificant differences across all parameters and conditions in pain-free controls (average). Thus, it is unlikely that there are nonspecific effects of cycle day or naproxen on bulb squeezing activity.
Table 2. The relationship between bout duration, number of bouts, and bout intensity across conditions.
Asterisks indicate significant differences before vs after naproxen.
| Participant Groups | Time point | Bout duration (s/15 min) | Number of bouts | Bout intensity (cm H2O) |
|---|---|---|---|---|
| Pain-free Controls menses (n = 14) | Before | 12.7 ± 2.4 | 8.7 ± 1.1 | 133.4 ± 7.9 |
| After | 13.9 ± 2.7 | 7.3 ± 1.2 | 146.8 ± 13.5 | |
| Effect size | 0.128 | 0.382 | 0.401 | |
| Dysmenorrhea menses (n= 25) | Before | 10.8 ± 1.3 | 18.0 ± 2.5 | 52.5 ± 6.5 |
| After | 5.6 ± 1.2** | 9.4 ± 2.1* | 30.6 ± 5.7* | |
| Effect size | 0.847 | 0.754 | 0.734 |
= p<0.05,
= p < 0.01,
= p < 0.001
Pre-Naproxen bulb squeezing was not related to pain intensity
To evaluate the potential importance of duration, frequency, or intensity of bulb squeezing before naproxen, we analyzed the relationship between squeeze patterns and pain intensity among women with dysmenorrhea on menses. An example of painful menstrual bout activity is shown in Figure 2. Before naproxen administration at the menses visit, dysmenorrheic participants squeezed the bulb more frequently (18.0 ± 2.5 bouts / 15 min) than pain-free controls (8.7 ± 1.1 / 15 min; p = 0.002). However, within participants dysmenorrhea there was no significant correlation between either squeeze duration (r = 0.27, p = 0.192), squeeze frequency (r = 0.23, p = 0.269), or squeeze intensity (r = 0.16, p = 0.445) with self-reported pain on an NRS. A regression model for the association or duration, frequency, and intensity of bulb-squeezing with self-reported pain on an NRS had a small effect size and was not significant (R2 = 0.12 [95% CI −0.11 to 0.34], p = 0.304). Also, there were no significant differences in squeeze duration, frequency, or intensity between participants with primary dysmenorrhea and endometriosis before naproxen (p’s >0.40).
Figure 2. Changes in bulb squeeze duration, frequency, and amplitude during a 15-minute segment during menses in a dysmenorrhea participant before and after naproxen administration.
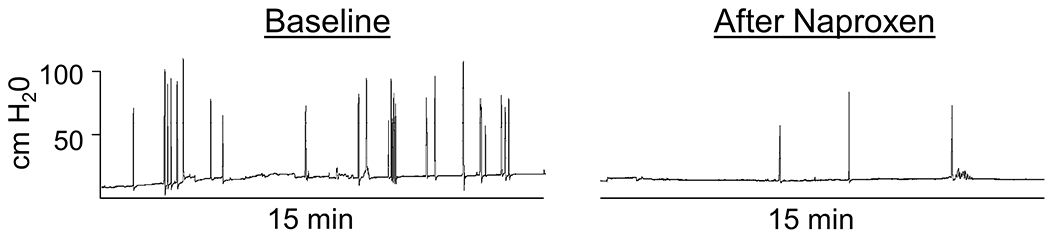
The Y axis (cm H20) is the same for both time points.
Changes in bulb squeezing after naproxen were related to changes in pain intensity
After naproxen, there was a reduction in menstrual pain among dysmenorrheic participants from 5.9 ± 0.4 to 3.5 ± 0.5 (p < 0.001) on a 0-10 NRS. As expected, there was a significantly greater analgesic effect of naproxen in participants with primary dysmenorrhea (reduction 53% ± 9) compared to participants with endometriosis (16% ± 8; p = 0.004). The differential effect of naproxen on primary vs. endometriosis was unlikely due to any differences in pre-treatment self-reported pain (p = 0.296).
Next, we compared individual duration, frequency, and intensity of bulb squeezes before and 90 minutes after naproxen administration in women with dysmenorrhea. Naproxen significantly reduced the duration of the painful bout (Table 2: duration before naproxen = 10.8 ± 1.3 sec, duration after naproxen = 5.6 ± 1.2; p = 0.004). Additionally, naproxen reduced the number of painful bouts (18.0 ± 2.5 to 9.4 ± 2.1 /15 min; p = 0.011) and bout intensity (52.5 ± 6.5 to 30.6 ± 5.7 cm H2O; p = 0.013).
Using a regression model, we explored how each of the bulb-derived dimensions of menstrual pain was related to reported pain intensity (NRS) after naproxen. The model analyzed the relationship between bout duration, frequency, and amplitude, with the change in pain intensity (NRS) after naproxen administration accounting for endometriosis status. Overall, there was a medium to strong correlation between changes in bulb squeeze activity and self-reported pain after naproxen (R2 = 0.55 [95% CI 0.28 to 0.82], p < 0.001). Specifically, changes in duration (p = 0.044) and frequency (p = 0.048) of bouts were negatively correlated with reported pain relief. In this regression model, changes in amplitude were not a significant predictor (p = 0.710). There was no endometriosis specific effect associated with changes in bulb squeezing activity (p = 0.19).
We also separately analyzed the groups according to IMMPACT guidelines: below 30% was indicative of a minimal improvement in pain, and 30% or higher was indicative of moderate pain relief.11 When we compared dysmenorrhea participants with mild/no relief vs. moderate/substantial pain relief (Figure 3), we observed significant differences between the groups in duration (p = 0.003), frequency (p = 0.003), and amplitude (p = 0.024). Significant reductions in bout duration (p < 0.001), frequency (p = 0.001), and intensity (p < 0.05) after naproxen were observed among participants that experienced moderate to substantial pain relief (Figure 3). However, in participants with mild to no relief, reductions in duration (p = 0.862), frequency (p = 0.549), and amplitude (p = 0.364) were not significant. A receiver operating characteristic curve of these parameters predicted group membership according to IMMPACT guidelines, with an area under the curve of 0.95 [95% CI 0.88 to 1.0] (p<0.001). The average effect size difference across all parameters between participants with mild/no relief vs. moderate/substantial pain relief (Cohen’s d= 1.3 [95% CI 0.84 to 1.76]) indicated that large differences in bulb-squeezing behavior are associated with self-reported pain relief after taking naproxen.
Figure 3. Responses in dysmenorrheic participants during menses are further broken out by IMMPACT pain relief categories.
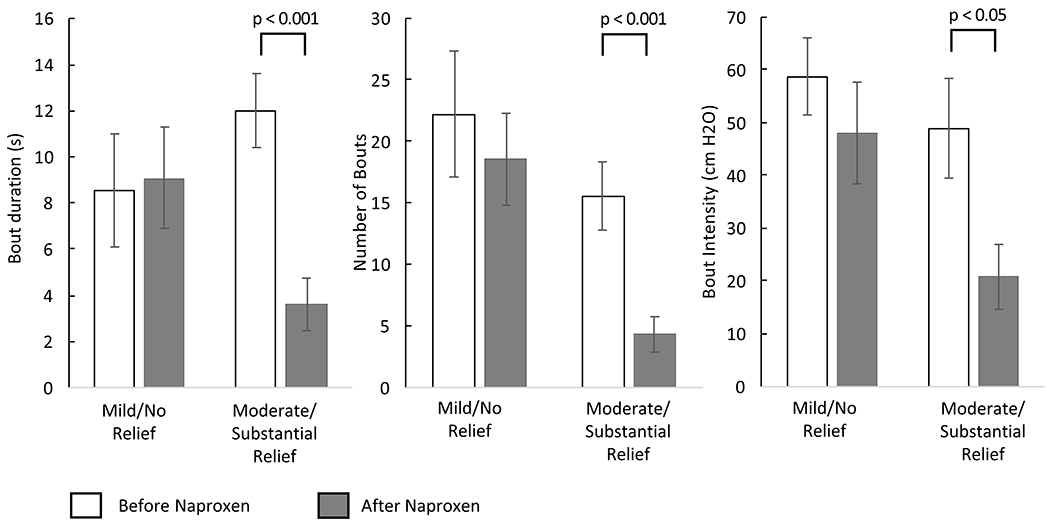
The results in Table 2 were recalculated based on reported relief to naproxen. Participants who demonstrated mild/no relief to naproxen had insignificant changes in bout duration, number of bouts, and bout intensity, while participants who demonstrated moderate/substantial response had significant changes in all measured parameters.
Discussion
Analysis of real-time bulb-squeezing in dysmenorrheic participants showed that naproxen reduces the duration, frequency, and intensity of painful bouts during menses. Our findings also further parse out the dimensions of analgesic effectiveness. Specifically, reductions in duration and frequency were correlated with analgesic effectiveness. These findings demonstrate the potential utility of using bulb-squeezing to characterize dynamic changes in self-reported pain in other pain states.
Although most pain conditions are associated with significant levels of spontaneous pain, spontaneous pain has rarely been objectively characterized.2 In one prior study of postherpetic neuropathy (PHN), subjects continuously rated their pain over 6-12 min segments using a finger-span device analogous to our bulb squeezing method. Using this technique, the authors concluded that PHN pain could be described with a fractal dimension, that paralleled the fractal dimension observed in brain activity. 5 As a control, the authors asked participants to rate an imagined pain which had a different dimension entirely. Although fractal analysis may provide some insight into the nature of power laws and pain, our metric instead provides a factor related to the severity of the pain and the effect of treatment. Electronic VAS capture has also been used to study evoked pain from cervical distension,12 intradermal injection of capsaicin,13 thermal stimulation,14 and ischemic cuff application.15 None of these evoked pain studies characterized pain experience metrics beyond a single instantaneous report, such as frequency, duration, or intensity. Although the idea of evaluating current levels of pain on a VAS or NRS is standard practice, repeated assessment involving dozens, or even hundreds of ticks with either a pencil/paper or event marks with a digital interface would be too tedious for most studies. Conversely, the use of a squeeze bulb allows for easy acquisition of continuous data akin to the smooth, continuous responsiveness of an automobile’s accelerator pedal. Thus, this technique’s key innovation is its convenience for continuous assessment, allowing the capture of three different dimensions of experienced pain.
Intriguingly, the actual amount of bulb squeezing did not reliably reflect absolute levels of pain on an NRS during the pre-naproxen period. Control participants were not instructed on how hard to squeeze the bulb, but pain participants were instructed to squeeze the bulb in proportion to their level of pain. This may have resulted in control participants squeezing the bulb harder. Control participants did not squeeze the bulb significantly less intensely due to passage of time, nonspecific effects of naproxen, or due to a bias in analysis; as such, this does not impact the significance of these findings. In any case, our results demonstrate the relative change in pressure squeezing in pain participants was more meaningful than the absolute pressure. Although establishing baseline pain scores may be informative, difference scores after treatment are more meaningful for measuring treatment effectiveness.11,16 Indeed, differences in pain report after naproxen were strongly correlated with the squeeze parameters with NRS (55% of the variance). Baseline self-report scores alone may have limited value because the relationship to baseline pain characteristics may be vulnerable to bias.17 A survey study suggests that spontaneous pain may also reflect a different component than self-report.3 Further work using our new technique, could help better clarify the relationship between spontaneous and self-reported pain.
Since menstrual pain is thought to arise from uterine contractions,18 our spontaneous pain report strategy may be useful for related conditions. A prior study measuring postpartum uterine contractions during breastfeeding19 suggested a relationship to pain. The frequency and duration of postpartum uterine contractions as suggested by the tocodynamometer were correlated with McGill Total Pain Index scores. Amplitude was not measured in Holdcroft et al. 2013 because of concerns of validity. Regardless, their results are supportive of the general idea that the frequency and duration of episodic pain are important factors in visceral pain experience. Our prior work using MRI in dysmenorrhea has suggested that sustained uterine contractions occur every 2-5 minutes.6 Intriguingly, bulb squeeze events occur more frequently (about once per minute) than uterine contraction frequency, suggesting that perceived menstrual pain could involve multiple events. For example, a contraction-related abdominal skeletal muscle component of pain has been identified by us previously, that precedes bouts of reported menstrual pain using this bulb squeezing method.7 This prior study demonstrated that pre-naproxen abdominal muscle activity was a predictor of NSAID effectiveness; thus, NSAIDs may act by inhibiting prostaglandin-mediated contractions and subsequent skeletal muscle activity. However, the mechanisms responsible for NSAID-resistance are not well understood.8 Further use of this bulb squeeze method, coupled with cine MRI, could be useful for identifying the mechanisms responsible for NSAID-resistant menstrual pain and even other visceral pain conditions such as angina and irritable bowel syndrome. Our ongoing study employs cine MRI to specifically study associations between uterine contractility and perfusion to cramping pain (https://clinicaltrials.gov/ct2/show/NCT04145518).
Our study was limited in terms of sample size (25 patients with dysmenorrhea and 14 pain-free controls). Thus, it would be prudent to validate this technique with a larger sample size. However, the large effect sizes and 95% confidence intervals suggest our results would likely be reproducible if performed in a larger study. The heterogeneity of racial distribution among women with dysmenorrhea has been reported in other studies and is possibly due to the role of leiomyoma in Black women.20 Although this could have a statistical impact, the use of within-subject analysis limits potential racial bias in establishing group effects. Although it was not possible to confirm pathology with laparoscopy in the dysmenorrhea cohort, the availability of MRI in some of our participants made it possible to identify potential causes of secondary dysmenorrhea. However, using comparable inclusion criteria from a similar but larger cohort (n=98), we established that only 3% of dysmenorrhea participants had evidence of occult uterine pathology by clinical exam with ultrasound follow up. 21 In any case, the primary hypothesis tested by this paper, the within-subject relationship between bulb-squeezing parameters and changes in pain, was not dependent on primary or secondary status. To mitigate any limitations in our sample size, we have included bootstrap analyses, which suggest that our sample size was adequate for demonstrating specific effects of pain relief on bulb-squeezing activity and that nonspecific effects in control observations are limited. This method is not meant to replace the evaluation of VAS, NRS, or clinical interview, but demonstrates how bulb-squeeze metrics of cramping can be useful for research purposes to objectively evaluate and study the mechanisms responsible for menstrual pain.
In sum, our results suggest that using a squeeze bulb for evaluation of spontaneous pain report provides reliable data that represents additional temporal and magnitude dimensions of the pain experience in dysmenorrhea. After additional validation with a larger sample size, further application of this method to other chronic pain conditions and treatments could expand our understanding of the spontaneous pain experience. Given that dynamic, spontaneous bouts of pain are a source of distress for most chronic pain conditions and recall of pain is known to be substantially biased,17,22 future studies should explore these dimensions of frequency, duration, and amplitude in other chronic pain conditions and treatment trials, especially for dysmenorrhea.
Acknowledgements:
The authors thank Dr. G.F. Gebhart for his sagacious advice and editorial assistance. This study was funded by the National Institute of Child and Human Development (HD081709, HD091502).
Funding:
This study was funded by the National Institute of Child and Human Development (HD081709, HD091502).
Footnotes
Disclosure
The authors report they have no financial conflicts of interest.
References
- 1.Bennett GJ. What is spontaneous pain and who has it? J Pain. 2012. October;13(10):921–9. [DOI] [PubMed] [Google Scholar]
- 2.Mogil JS, Crager SE. What should we be measuring in behavioral studies of chronic pain in animals? Pain. 2004. November;112(1–2):12–5. [DOI] [PubMed] [Google Scholar]
- 3.He D, Grant B, Holden RR, Gilron I. Methodology for self-report of rest pain (or spontaneous pain) vs evoked pain in chronic neuropathic conditions: a prospective observational pilot study. Pain Rep. 2017. March;2(2):e587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dworkin RH, Turk DC, Peirce-Sandner S, Burke LB, Farrar JT, Gilron I, et al. Considerations for improving assay sensitivity in chronic pain clinical trials: IMMPACT recommendations. Pain. 2012. June;153(6):1148–58. [DOI] [PubMed] [Google Scholar]
- 5.Foss JM, Apkarian AV, Chialvo DR. Dynamics of pain: fractal dimension of temporal variability of spontaneous pain differentiates between pain States. J Neurophysiol. 2006. February;95(2):730–6. [DOI] [PubMed] [Google Scholar]
- 6.Hellman KM, Kuhn CS, Tu FF, Dillane KE, Shlobin NA, Senapati S, et al. CINE MRI During Spontaneous Cramps in Women with Menstrual Pain. Am J Obstet Gynecol. 2018. January 31; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oladosu FA, Tu FF, Farhan S, Garrison EF, Steiner ND, Roth GE, et al. Abdominal skeletal muscle activity precedes spontaneous menstrual cramping pain in primary dysmenorrhea. Am J Obstet Gynecol. 2018. May 4; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oladosu FA, Tu FF, Hellman KM. Nonsteroidal antiinflammatory drug resistance in dysmenorrhea: epidemiology, causes, and treatment. Am J Obstet Gynecol. 2017. September 6; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007. November;82(4):591–605. [DOI] [PubMed] [Google Scholar]
- 10.Cohen J Statistical power analysis for the behavioral sciences. Routledge; 2013. [Google Scholar]
- 11.Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009. December;146(3):238–44. [DOI] [PubMed] [Google Scholar]
- 12.Bajaj P, Bajaj P, Madsen H, Arendt-Nielsen L. A comparison of modality-specific somatosensory changes during menstruation in dysmenorrheic and nondysmenorrheic women. Clin J Pain. 2002. June;18(3):180–90. [DOI] [PubMed] [Google Scholar]
- 13.Chang PF, Arendt-Nielsen L, Graven-Nielsen T, Svensson P, Chen ACN. Comparative EEG activation to skin pain and muscle pain induced by capsaicin injection. Int J Psychophysiol. 2004. January;51(2):117–26. [DOI] [PubMed] [Google Scholar]
- 14.Naert ALG, Kehlet H, Kupers R. Characterization of a novel model of tonic heat pain stimulation in healthy volunteers. Pain. 2008. August 15;138(1):163–71. [DOI] [PubMed] [Google Scholar]
- 15.Graven-Nielsen T, Vaegter HB, Finocchietti S, Handberg G, Arendt-Nielsen L. Assessment of musculoskeletal pain sensitivity and temporal summation by cuff pressure algometry: a reliability study. Pain. 2015. November;156(11):2193–202. [DOI] [PubMed] [Google Scholar]
- 16.Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001. November;94(2):149–58. [DOI] [PubMed] [Google Scholar]
- 17.Robinson ME, Myers CD, Sadler IJ, Riley JL, Kvaal SA, Geisser ME. Bias effects in three common self-report pain assessment measures. Clin J Pain. 1997. March;13(1):74–81. [DOI] [PubMed] [Google Scholar]
- 18.Woodbury RA, Torpin R. Myometrial physiology and its relation to pelvic pain. J Am Med Assoc. 1947. July 26;134(13):1081–5. [DOI] [PubMed] [Google Scholar]
- 19.Holdcroft A, Snidvongs S, Cason A, Doré CJ, Berkley KJ. Pain and uterine contractions during breast feeding in the immediate post-partum period increase with parity. Pain. 2003. August;104(3):589–96. [DOI] [PubMed] [Google Scholar]
- 20.Eltoukhi HM, Modi MN, Weston M, Armstrong AY, Stewart EA. The health disparities of uterine fibroid tumors for African American women: a public health issue. Am J Obstet Gynecol. 2014. March;210(3):194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hellman KM, Datta A, Steiner ND, Kane Morlock JN, Garrison EF, Clauw DJ, et al. Identification of experimental bladder sensitivity among dysmenorrhea sufferers. Am J Obstet Gynecol. 2018;219(1):84.e1–84.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coughlin SS. Recall bias in epidemiologic studies. J Clin Epidemiol. 1990;43(1):87–91. [DOI] [PubMed] [Google Scholar]


