Abstract
Background
Despite the rapid global growth of biobanking over the last few decades, and their potential for the advancement of health research, considerations specific to the sharing of benefits that accrue from biobanks have received little attention. Questions such as the types and range of benefits that can arise in biobanking, who should be entitled to those benefits, when they should be provided, by whom and in what form remain mostly unanswered. We conducted a scoping review to describe benefit sharing considerations and practices in biobanking in order to inform current and future policy and practice.
Methods
Drawing on the Arksey and O’Malley framework, we conducted a scoping review of the literature in three online databases (PubMed, Cochrane library, and Google Scholar). We extracted and charted data to capture general characteristics, definitions and examples of benefits and benefit sharing, justification for benefit sharing, challenges in benefit sharing, governance mechanisms as well as proposed benefit sharing mechanisms.
Results
29 articles published between 1999 and 2020 met the inclusion criteria for the study. The articles included 5 empirical and 24 non-empirical studies. Only 12 articles discussed benefit sharing as a stand-alone subject, while the remaining 17 integrated a discussion of benefits as one issue amongst others. Major benefit sharing challenges in biobanking were found to be those associated with uncertainties around the future use of samples and in resultant benefits.
Conclusion
Most of the benefit sharing definitions and approaches currently in use for biobanking are similar to those used in health research. These approaches may not recognise the distinct features of biobanking, specifically relating to uncertainties associated with the sharing and re-use of samples. We therefore support approaches that allow decisions about benefit sharing to be made progressively once it is apparent who samples are to be shared with, the intended purpose and expected benefits. We also highlight gaps in key areas informing benefit sharing in biobanking and draw attention to the need for further empirical research.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12910-021-00671-x.
Keywords: Benefits, Benefit sharing, Biobanking, Biobanks, Ethics, Sample sharing
Introduction
Biobanking refers to the storage, active sharing and re-use of biological specimens and associated data for research purposes [1, 2]. These specimens, collected specifically for biobanking or leftover from primary research or healthcare, support a wide range of research activities including basic, experimental and clinical research, as well as research applied in the development of tools for prevention, diagnosis and treatment of diseases; including personalized medicine [2, 3]. With increasing research activity and demand for biospecimens for research, the number of biobanks worldwide has increased significantly between 1980 and 1999 [4] with close to 70% of the world’s biobanks located in Europe [4]. Widely discussed ethical issues in literature include: nature of consent (broad, restricted, tiered); who can give informed consent; information to be contained in consent forms [3, 5–7]; privacy and confidentiality issues [3, 8]; ownership of samples [7]; the role of regulation in biobanking [9, 10] among others. An existing specific gap is in the understanding of the types of benefits and benefit sharing frameworks that should guide biobanking.
Schroeder (2007) notes that the concept of benefit sharing has not been well defined and provides a general definition for use with genetic resources; “the action of giving a portion of advantages/profits derived from the use of human genetic resources to the resource providers to achieve justice in exchange, with a particular emphasis on the clear provision of benefits to those who may lack reasonable access to resulting healthcare products and services without providing unethical inducements” [11]. In this definition, the terms ‘advantages/profits’’ are used deliberately to capture the notion that benefit sharing relates to both monetary and non-monetary benefits. Although several studies have explored benefit sharing in health research [11–17] and described benefit sharing frameworks [12, 18, 19], there is little consideration for benefit sharing in biobanking. There are significant debates in health research literature on benefit-sharing including in relation to the different forms of benefits (monetary, health care, infrastructure development, gifts etc.); who should receive the benefits (participants, communities, researchers, the ‘public’); who is responsible for their provision (researchers, sponsors, relevant government bodies, industry) and when the benefits should be provided (framed on a continuum of time of during and after the research) [17]. There are also important questions about how decisions about benefit sharing are made at institutional, national and supra-national levels. Yet these discourses, on nature of benefits and benefit-sharing mechanisms, have rarely been applied to the context of biobanking, leaving important questions about how benefit sharing should – and could – be considered in the context of biobanking. Thus, we undertook a scoping review of existing literature in order to explore further some of these issues, particularly the types and forms of benefits and benefit sharing considerations for biobanking.
Methods
The scoping review team comprised 3 authors who have varied experience in bioethics, biobanking and genomic research as proposed by Levac and colleagues [20]. The team discussed and agreed on the design of the scoping review including the research question, the search criteria, the databases to use and analytical approach. This scoping review was carried out according to the Arksey and O’Malley framework [21] improved upon by Levac et al. [20]. The review included the following five key phases: (1) identifying the research question, (2) identifying relevant literature, (3) literature selection, (4) charting the data, and (5) collating, summarizing, and reporting the results. The optional ‘consultation exercise’ recommended in the framework was not carried out.
The research question guiding this review was: what are the benefit sharing considerations and practices in biobanking described in literature? More specific questions were: (1) How are benefits and benefit sharing defined with relation to biobanking?; (2) Who are the stakeholders involved in biobanking and how are decisions on benefit sharing made?; (3) what are the motivators, barriers and enablers to benefit sharing in biobanking; and (4) what benefit sharing mechanisms have been proposed?
Data sources and search strategy
We conducted the initial literature search in September 2020 in three electronic databases, PubMed, Cochrane Library and Google Scholar. The databases were chosen due to their free access, comprehensiveness and were known to cover health-related matters. Google Scholar was included to cater for forms of literature that could not be obtained in the other databases. The search query consisted of terms that covered the two key areas of “benefit sharing” and “biobanking” and was expanded by use of Medical Subject Headings (MeSH) and relevant synonyms. The search was limited to the titles and abstracts of the articles within the databases. In keeping with the suggestion by Bramer et al. to include opposites of key search terms to avoid bias, we also included ‘risk sharing’ within the search criteria [22]. Table 1 describes the search terms used for the PubMed database, with the search query tailored to the specific requirements of each database. For Google Scholar, slightly different search strings were used because of the lack of MeSH terms within the database (see Additional file 2: Appendix 2). In addition to the search strategy described above, we also used the “cited by” function in Google Scholar and examined the reference lists of all the selected articles to identify additional articles that met our inclusion criteria. No date limits were placed on the database search and only articles in English were considered.
Table 1.
PubMed search criteria
| Search terms | Search details |
|---|---|
| Biobanks/Biobanking | biological specimen banks [MeSH] OR biobank*[tiab] OR biorepositor*[tiab] |
| Benefits/Benefit sharing | "beneficence"[Mesh] OR "benefit"[tiab] OR "benefit sharing"[tiab] OR "social value" OR "benefit distribution"[tiab] |
| Risk/Burden sharing | "risk assessment"[Mesh] OR "risk distribution"[tiab] OR "risk sharing"[tiab] OR "burden distribution"[tiab] OR "burden sharing"[tiab] |
| Final Query | ((biological specimen banks[MeSH] OR biobank*[tiab] OR biorepositor*[tiab]) AND ("beneficence"[Mesh] OR "benefit"[tiab] OR "benefit sharing"[tiab] OR "social value" OR "benefit distribution" [tiab])) OR ((biological specimen banks[MeSH] OR biobank*[tiab] OR biorepositor*[tiab]) AND ("risk assessment"[Mesh] OR "risk distribution"[tiab] OR "risk sharing"[tiab] OR "burden distribution"[tiab] OR "burden sharing"[tiab])) |
Citation management
All the citations from the different databases were exported to EndNote X9 and duplicates removed. This was followed by screening for relevant papers guided by a title and abstract screening tool (Additional file 1: Appendix 1).
Eligibility criteria
Any articles that described any aspect of biobanking and benefit sharing, allocation or distribution was included. Articles were not limited to geographical location and included peer reviewed journal publications, commentaries, editorials and reports. Any articles about benefit sharing not directly related to human health research were removed. Articles about financial banking, banking of animal tissue, banking of microorganisms (e.g. virus or microbiome archives), temporary banking of amputated parts, banking of tissue/organs for care and milk banks for dietary supplementation were excluded because they were out of the scope of the current review.
Title and abstract relevance screening
Only the titles and abstracts of citations identified in the database search were reviewed during the first stage of screening. Articles of which titles seemed to meet the inclusion criteria but where abstracts were missing were included for full article review in the data characterization phase. AS screened all the articles and the final list of selected articles was then reviewed by JDV and DK. Throughout the screening process AS, JDV and DK met to discuss and resolve any uncertainties related to study selection [20].
Data characterization
The full articles for all the citations deemed relevant via title and abstract screening and those missing abstracts were obtained for subsequent full text review. For articles that were not openly available, or those that could not be obtained through institutional library access, attempts were made to contact the author for assistance in obtaining them. Any articles that could not be obtained through these processes were excluded. Two separate templates were developed for data abstraction and characterization. The first was a Microsoft Excel sheet that captured study characteristics such as author name(s), publication year, publication type, geographic setting, and area of focus (see Table 2 below). The second was a coding framework developed in NVivo to capture the actual study content that related to the study questions. Both data charting forms (Excel and NVivo) were discussed extensively during analysis by the study team. The characteristics of each full-text article were extracted by AS and any additional studies that did not to meet the inclusion criteria were excluded at this phase. Frequencies were utilized to describe nominal data while narrative analysis was carried out on the qualitative content to draw out the themes.
Table 2.
General characteristics of included articles
| References | Type | Geographic setting | Relevance to benefit sharing in biobanking |
|---|---|---|---|
| Árnason [24] | Report | Iceland | Highlights the ethical issues around the Icelandic biobank project (deCODE) |
| Berg [32] | Opinion Article | Ireland | Practical difficulties in implementing benefit sharing |
| Boggio et al. [45] | Book Chapter | Geneva | Comparative analysis of 27 biobanking policies on various ethical legal and social issues (ELSI) |
| Capron et al. [29] | Peer Reviewed Article—Empirical | 27 countries | Ethical norms and the international governance of genetic databases and biobanks |
| Chalmers and Nicol [31] | E-book | Australia | Examines international best practice for the establishment, maintenance and use of human genetic research databases (HGRDs) and considers the measures that should be taken in Australia to comply with this best practice |
| Chen and Pang [48] | Peer Reviewed Article | Global | Discusses a fair, equitable and feasible biobank governance framework to ensure a fair balance of risks and benefits among all stakeholders |
| Emerson et al. [49] | Article-Debate | Not specified | Make a case for a tissue trust to respond to claims of exploitation through ‘scientific-imperialism’ and ‘bio-colonialism |
| Hobbs et al. [28] | Journal Article - Empirical | Germany and UK | Discusses appropriate methods of reciprocity in biobanking |
| Hugo ethics [38] | Opinion Article | N/A | Statement on benefit sharing by the Human Genome Organizations (HUGO) ethics committee |
| Joly et al. [47] | Peer Reviewed Article | N/A | Argues that open access can be considered benefit sharing in genomics research |
| Shickle (2014) | Book Section | N/A | Discusses various ethical legal and social issues (ELSI) in biobanking |
| Knoppers [39] | Peer Reviewed Article | Global | Discusses benefit sharing from the perspective of the Human Genome Organization’s (HUGO) ethics committee’s ‘Statement on Benefit-Sharing’ |
| Laurie et al. [46] | Peer Reviewed Article | N/A | Attempts to reconcile individual privacy and public interests in genetic research using biobanks as a case |
| Mahomed et al. [35] | Peer Reviewed Article | South Africa | Examines issues surrounding transfer of human tissues across national boundaries and describes what a South African Institution considered for its material transfer agreements |
| Moodley et al. [26] | Peer Reviewed Article - Empirical | South Africa | Unearths research participants (of biobanking) concerns with storage of their tissue and use for research |
| National Bioethics Advisory Commission [23] | Report and Recommendations | USA | Report on research involving human biological materials in the USA. Discusses ethical issues and gives policy guidance |
| Ndebele and Musesengwa [43] | Peer Reviewed Article | Developing countries | Addresses the issue of fairness in benefits sharing and argues for justice in the sharing of both burdens and benefits of genetics research |
| Nicol and Critchley [27] | Peer Reviewed Article -Empirical | Australia | Discusses benefit sharing and biobanking in Australia |
| Pullman and Latus [44] | Peer Reviewed Article | N/A | Suggests some ways in which benefit-sharing might be implemented for genetic add-on studies |
| Ravinetto and Dierickx [33] | Peer Reviewed Article | India | Reviews relevance and applicability of benefit sharing in the revised “Indian National Ethical Guidelines for Biomedical and Health Research Involving Human Participants” |
| Schroeder and Gefenas [50] | Peer Reviewed Article | N/A | Examines post study obligations as a mechanism for benefit sharing |
| Schroeder and Lasen-Diaz [41] | Peer Reviewed Article | Global | Considers whether Convention on Biological Diversity (CBD) should be expanded to include human biological resources |
| Schroeder and Lucas [37] | Book Chapter | Global | Encourages further empirical research in order to move from theoretical understandings of fair benefit sharing to better practice which benefits real people |
| Schuklenk and Kleinsmidt [42] | Peer Reviewed Article | Global | Critically analyses benefit sharing looking at some of the practical challenges in sharing benefits such as Intellectual Property (IP) with communities |
| Sheremeta and Knoppers [40] | Peer Reviewed Article | Global | Discusses population genetics and benefit sharing |
| Simm [36] | Peer Reviewed Article | Global | Examines and clarifies the notion of benefit-sharing by focusing on its justifications |
| Vaz et al. [25] | Peer Reviewed Article-Empirical | India | Elicits views of ethics committee members and researchers involved in biobanking |
| Xiaoyong [30] | Book Chapter | China | Examines benefits sharing under different health research models |
| Yakubu et al. [34] | Peer Reviewed Article | South Africa | Highlights governance issues in biobanking |
Results
Articles included in the scoping reviews
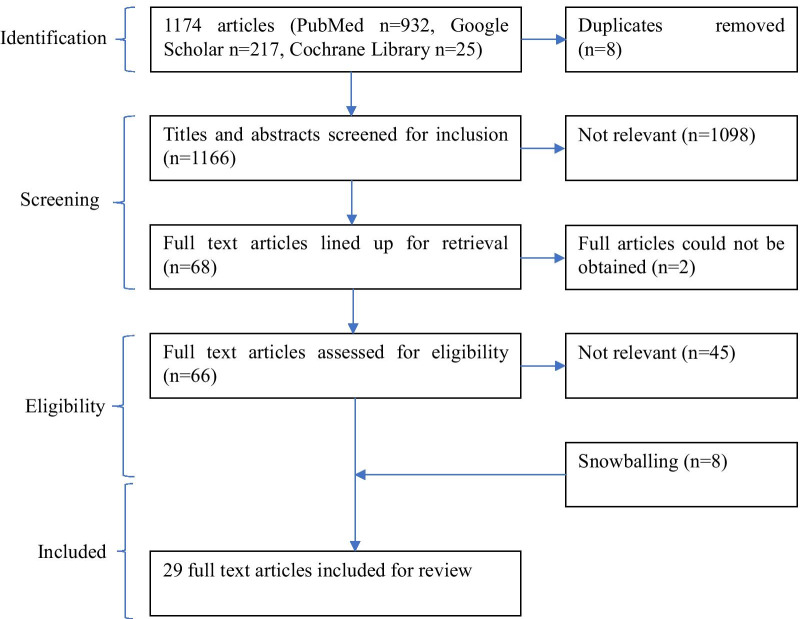
The search yielded 1174 potentially relevant citations. Eight duplicates were discarded after which the remaining citations were subjected to title and abstract relevance screening. At this stage a further 1098 citation were excluded. A total of 68 articles were lined up for retrieval of which 66 were eventually retrieved. Upon reading the full text, 21 articles met the inclusion criteria. An additional 8 articles were identified by going through the reference lists of the selected articles making the total number of articles included in the analysis 29 (see Fig. 1 below).
Fig. 1.
PRISMA Flow chart of study selection process
General characteristics of included articles
Of all the articles included in the review, only one was published in 1999 [23] while the rest were published between 2000 and 2020. Journal articles formed majority of documents included in the review (22/29; 75.8%), followed by book chapters/sections (5/29; 17.2%). There were also 2 reports specific to benefit sharing that were included in the review [23, 24]. Five of the 29 documents were findings from empirical studies [25–29] while the rest were either reports, commentaries, opinions or debates. In terms of geographic settings for the empirical studies, there was one study each from India [25], Australia [27], South Africa [26] and UK/Germany [28]. One study was done across 27 different countries classified as 18 High, 6 Medium, and 3 Low-income [29]. Some of the non-empirical articles were also country-specific, and included one each from China [30], Australia [31], Ireland [32], India [33] and two from South Africa [34, 35]. The two reports included in the review were from the United States [23] and Iceland [24]. Majority of the articles discussed benefit sharing from a global perspective, often comparing high-income and low-income countries. Less than half of the documents reviewed (12/29; 41.4%) addressed benefit sharing as a stand-alone subject [30, 32, 33, 36–44] while the rest discussed it as one of several ethical issues in biobanking. While most of the articles looked at biobanking in general, 11 documents (37.9%) discussed biobanking within genetics/genomics research.
Reported definition of benefits and benefit sharing
There were broad variation in definitions of the terms ‘benefit’ and ‘benefit sharing’ in the articles included in the review. Only three of the 29 articles specifically defined the term benefit [36, 40, 45]; all three made reference to the 2000 statement on benefit-sharing by the Human Genome Organization (HUGO) ethics committee that defined benefit as “a good that contributes to the wellbeing of an individual and/or a given community” [38]. A larger number of articles (n = 12) provided some form of definition or description of benefit sharing. Of those, 7 articles adopted, with slight variations, Schroeder et al.’s definition [11] earlier described [33, 37, 41, 43–46]. A further two articles listed the elements of the HUGO ethics committee statement on benefit sharing in providing their definitions [36, 40]. Three papers gave independent definitions of benefit sharing as “an equitable exchange in return for genetic resources” [47]; as a “process or action of sharing in the benefits that derive from the research in a manner that is fair and equitable” [35]; or as”mechanisms that might be put in place to ensure that benefits stemming from biobanking and use of biobank resources in biomedical research are not perceived to be the exclusive domain of the commercial sector” [27].
Reported benefits
The reviewed articles describe a wide range of benefits in biobanking spanning those that can be enjoyed at individual level, at community level and at a global scale. Table 3 below highlights the types of benefits mentioned within the articles and provides some specific examples.
Table 3.
Benefits and potential beneficiaries
| Benefit | Example(s) | Beneficiaries | Articles |
|---|---|---|---|
| Financial | Licensing fees, percentage of profits, annual fees, direct payments | State, pharmaceutical companies, general population, researchers | [24–27, 29, 32–35, 38, 40, 44–48, 50] |
| Capacity building | Improvement of healthcare infrastructure (databases, physical infrastructure, research infrastructure), research staff training and development, technology transfer, and joint ventures | Public health facilities, research staff, research facilities, communities that utilize improved capacity | [24, 29, 35, 38–50] |
| Treatment or Healthcare | Free medical service (medication or consultation), provision of vaccines, tests, drugs, and treatments, treatment for non-research-related conditions | Research participants, their families, communities | [24–26, 28, 30, 32, 33, 37, 38, 40, 45–48, 50] |
| Improved understanding, knowledge or therapies | Improved understanding/new insights of disease processes and a potential for new therapeutic modalities | Whole of mankind, researchers, pharmaceutical industry, individuals, communities, and populations from which they are derived | [25, 32, 35, 38–40, 46–48, 50] |
| Post study access | Provision of pharmaceutical and diagnostics products that emerge from the research, giving access to new genetic tests, access to interventions identified as beneficial or to other appropriate care or benefits at subsidized cost | Research participants/specimen donors and their communities | [24–26, 29, 33, 37, 40, 45, 47, 50] |
| Return of results/findings | Provision of individual results (information about own health, incidental findings) or aggregate findings (general research results, study outcome) from the use of their samples | Specimen donors, their families/communities, research community | [26, 28, 33, 40, 45, 46] |
| Intellectual property/royalties | Publication rights, royalties from intellectual property, recognition | Researchers, pharmaceutical industry | [39, 40, 42, 45, 48] |
| Humanitarian efforts | Donation of percentage of profits/royalties for humanitarian efforts such as general education or health campaigns, community development projects (schools, clean water, roads), support mechanisms for destitute community members, insurance | Communities participating in biobanking | [24, 38, 40, 42, 50] |
| Responsiveness to local needs | Diagnostic or therapeutic application of the research that are tailored to local health needs | Communities where samples are collected/research is conducted | [40, 48, 50] |
| Jobs | Jobs and related economic activities generated by the research industry such as employment within research facilities and/or biobanks. Scientists and other cadre of staff | Local community members, local researchers and professionals | [24, 44] |
| Compensation for costs | Reimbursement of individual’s time, inconvenience and expenses | Research participants/specimen donors | [38, 39] |
| Counselling, screening services and testing | Genetic counselling, screening tests, free tests, regular health checks | Specimen donors, their families | [28, 45] |
| Other benefits | Anything can be shared, as long as it is defined as a benefit by a substantial number of stakeholders. Even recognition of participants contribution and thanking them | Different stakeholders involved in biobanking | [24, 36, 38] |
Justification for benefit sharing
In the papers reviewed, a significant amount (18/29; 62.1%) justify the importance of benefit sharing in biobanking, with the majority (13/18; 72.2%) indicating that benefit sharing is important as a matter of justice and as a means of redressing existing inequalities, promoting fairness and equality and addressing potential for exploitation [25, 32, 33, 35–37, 39, 42, 43, 47–50]. Other reasons stated included sharing benefits as a moral duty/ethical obligation [25, 33, 39, 44, 45, 49]; in order to comply with existing regulation/ethical principles on benefit sharing [27, 39, 42, 43]; that we share a common heritage and therefore benefits should be shared in solidarity/or as a common good [27, 39, 40]; to respond to participant needs/requests [39, 48]; and that investments by private enterprises currently exceed contributions by governments and that the enterprises need to share the benefits [36, 38, 41].
Governance mechanisms for benefit sharing
Of the documents we reviewed majority (22/29; 75.9%) mention ways in which benefit sharing can be governed in biobanking. The governance mechanisms range from institutional level policies to international level guidelines and frameworks. A summary of the mechanisms, relevant clauses and articles that make reference to governance is presented in Table 4 below.
Table 4.
Governance mechanisms for benefit sharing
| Governance mechanism | Relevant provisions | Articles | ||
|---|---|---|---|---|
| 1 | Convention on Biological Diversity | (a) Bonn Guidelines on Access to Genetic Resources and Fair and Equitable Sharing of the Benefits Arising out of their Utilization[51] | Both monetary (e.g. access fees, upfront payment, joint ownership of relevant IP rights) and non-monetary (e.g. sharing of research and development results, collaboration, development programs to build local capacity) benefits ought to be shared | [41, 42, 46, 49] |
| (b) The Nagoya Protocol on Access and Benefit-sharing [52] | Various provisions on the conservation of biological diversity, the sustainable use of its components and the fair and equitable sharing of the benefits arising out of their utilization | [24, 30, 37, 40–42, 45, 47] | ||
| 2 | UNESCO | (a) Universal Declaration on the Human Genome and Human Rights[53] | Benefits from advances in biology, genetics and medicine, concerning the human genome, shall be made available to all, with due regard for the dignity and human rights of each individual | [24, 27, 31, 39, 40, 45–48] |
| (b) Universal Declaration on Bioethics and Human Rights 2005[54] | Benefits should be shared with society as a whole, within the international community and with developing countries. Such benefits include “special and sustainable assistance to, and acknowledgement of, the persons and groups that have taken part in the research” | [24, 27, 33, 37, 41, 43, 46, 50] | ||
| 3 | HUGO statement on benefit sharing [38] | Undue inducement for human genetic samples through compensation ought to be prohibited | [24, 27, 40–44, 47–49] | |
| Technology transfer, local training, joint ventures, health care provision, infrastructure provision, payment of expenses, and the use of royalties for humanitarian purposes ought to be encouraged | ||||
| 1–3% of net profits be donated to local, national and international humanitarian efforts | ||||
| 4 | The Nuffield Council report on Human Tissue Ethical and Legal Issues [55] | Discussions on the link between commercialization and benefit sharing: i.e. property rights over the actual tissue, claims of entitlement to share in any benefits arising from the exploitation of the tissue and, any consequent intellectual property rights | [45] | |
| 5 | WHO guidelines | (a) International Guidelines on Ethical Issues in Medical Genetics and Genetic Services [56] | Families or ethnic groups with a particular variant or disease, whose genetic information results in a patent, should receive some benefit in return | [39, 45, 50] |
| (b) European partnership on patients’ rights and citizens’ empowerment [57] | Some kind of benefit will ultimately be returned, either to the individual from who the materials were taken, or to the general class of person to which that individual belongs | |||
| 6 | Council for International Organisations of the Medical Sciences (CIOMS) guidelines[58] | Give priority to direct benefits over indirect benefits | [33, 43, 45, 50] | |
| All research in developing countries and sponsored by developed countries, should be of relevance to the developing countries | ||||
| 7 | OECD Guidelines for Human Biobanks and Genetic Research Databases[59] | Sharing of knowledge is an important benefit to be derived from human biobanks and genetic research databases (HBGRDs) | [27, 46, 48] | |
| 8 | UN Declaration on Human Rights[60] | Everyone has the right freely to “share in scientific advancement and its benefits” | [46] | |
| 9 | The Declaration of Helsinki[61] | At the end of any research study, every subject entered in the project should be assured of the best proven prophylactic, diagnostic and therapeutic methods identified by that study | [33, 37, 43, 50] | |
| 10 | Governmental laws, policies and regulations | Countries specific laws, policies and regulations including: | [24, 30, 31, 34, 35, 37, 39, 42, 43, 46, 48, 50] | |
| (a) Mandating return of benefits to participants and families | ||||
| (b) Regulations on proprietary claims in respect of human tissue | ||||
| (c) Laws on apportionment of profits or tools for benefit-sharing | ||||
| (d) Statutory categories of benefit | ||||
| 11 | Ethics committees and regulatory bodies | (a) Checking for presence of benefit sharing provisions in protocols | [25, 33, 35, 37, 39, 43, 44, 46, 50] | |
| (b) Ensuring communities and individuals are not exploited | ||||
| (c) Ensuring commercially exploitable projects make considerations for benefit-sharing | ||||
| (d) Approving benefit-sharing proposals presented to them | ||||
| (e) Providing guidance on who should provide benefits in order to ensure compliance | ||||
| 12 | Institutional policies and frameworks for benefit sharing | (a) Intellectual property policies | [25, 33, 40, 42, 44] | |
| (b) Information to be included in ICFs | ||||
| (c) Requirements for benefit sharing agreements | ||||
| (d) Return of results and compensation of participants | ||||
| 13 | Benefit sharing agreements | Specifies under which conditions benefits are to be shared, which benefits to be shared and proportion of sharing. Can exist at different levels: | [25, 29, 32, 41, 49] | |
| (a) International level e.g. conventions between governments and industry players | ||||
| (b) Between industry and researchers | ||||
| (c) Between biobanks and researchers | ||||
| (d) Between/among biobanks | ||||
| (e) Between all above players and research participants/specimen donors | ||||
| 14 | Material transfer agreements (MTAs) | Stipulates ethico‐legal requirements regarding the transfer of human biological materials | [35] | |
Challenges to benefit sharing
A wide variety of conceptual and practical challenges to benefit sharing in biobanking were highlighted in 24 of the articles reviewed (see Table 5 for a summary). These range from the long periods associated with the development of any meaningful interventions from biobanking, the difficulties in determining who is to benefit and the proportions of those benefits, and the often large numbers of stakeholders involved (from specimen donors to researchers in multiple countries) and the power dynamics between them [24, 25, 28, 32, 33, 35, 37, 39–41, 44–47, 50]. Apart from benefit sharing in biobanking often being described as impracticable, articles also described challenges relating to cost with concerns that implementing benefit sharing would inflate the cost of research thereby discouraging investment especially in research that may not have big or immediate returns [28, 29, 31, 32, 37, 39, 50]. Another barrier mentioned in 12 articles is the lack of regulatory support/infrastructure for benefit sharing, specifically the lack of details in international and national ethics and guidance documents to guide implementation of benefit-sharing agreements. Institutions involved in biobanking are therefore left to interpret and implement benefit sharing as they deem best [24, 32, 33, 35, 37, 39, 42, 43, 45, 46, 48, 50]. Other issues identified included tensions between benefit sharing and other ethical principles such as coercion, undermining altruism and concerns about the commodification of human tissues.
Table 5.
Benefit sharing challenges in biobanking
| Challenge | Examples | Documents cited |
|---|---|---|
| Tensions with other ethical principles | (a) Payment or compensation of research participants/specimen donors: may be in conflict with conventions or guidelines that state that the human body should not be a source of income (commodification/commercialization of human body). Payments may also be considered coercing individuals to participate therefore a form of undue influence/coercion | [24–26, 29–33, 36–38, 41, 43–45, 50] |
| (b) Claims of ownership of samples: It becomes difficult to distribute benefits when it is unclear who owns the samples | ||
| (c) De-identification of samples (individuals and communities): makes it difficult to share benefits with donors or return results if they are unknown | ||
| (d) Providing healthcare as a benefit: May be seen as undue inducement if provided to vulnerable individuals who have no other means of accessing care. Also brings up question of whose responsibility it is to provide care; researcher or government? | ||
| (e) Conflict between protection against undue inducement on the one hand and exploitation on the other | ||
| (f) Conflict between business enterprise required to fund research and claims of commercialization of human body in the process | ||
| Practicality Issues | (a) Long periods between tissue collection and development of interventions: benefit not immediate or apparent | [24, 25, 28, 32, 33, 35, 37, 39–41, 44–47, 50] |
| (b) Low yield: numerous attempts before successful intervention | ||
| (c) Nature of research: Samples may be used for basic research where no intervention is developed. Benefits such as post study access therefore become impractical | ||
| (d) Absence of royalties, profits and patents: Difficult to distribute benefits | ||
| (e) Nature of sample collection: Small amounts of tissue may be collected over wide geographical regions. If and when an intervention is developed, it may be difficult to share benefits among all | ||
| (f) Oversight: Due to long periods between sample collection and development of intervention (sometimes decades) it becomes difficult for Ethics committees or governments to perform oversight of benefit sharing | ||
| (g) Uncertainty: Not possible to tell how samples will be used, what interventions will be developed and therefore what benefits may accrue | ||
| Weak governance | (a) No requirement for benefit sharing in legislation nor enforcement mechanisms | [24, 32, 33, 35, 37, 39, 42, 43, 45, 46, 48, 50] |
| (b) No protections for poor countries from exploitation by richer countries | ||
| (c) Guidelines do not describe which benefits shall be shared and how benefit sharing would work practically | ||
| (d) Lack of clarity in guidelines on whether direct or indirect benefits should be shared | ||
| (e) Poor or absent medical and patents laws and/or regulatory frameworks in most countries | ||
| (f) Organizations and policies provide inconsistent and incomplete frameworks, and none of them possess supra-national status, authority or enforceability | ||
| (g) Laws prohibiting sale of tissues may work against compensation of sample donors (misconstrued as payment) | ||
| (h) Non-committal language in legislation e.g. “may”, “could be considered” | ||
| (i) Non-reliance by states of international declarations e.g. Declaration of Helsinki by the United States of America | ||
| (j) Inability by ethics committees to enforce benefit sharing requirements | ||
| (k) Narrow focus on one kind of benefit e.g. post study obligations by international guidance documents precludes other benefits | ||
| (l) Explicit exclusion of human biological materials from CBD | ||
| (m) No means for redressing past injustices e.g. samples already shipped out | ||
| (n) Precedence provided by some court rulings in which specimen donors have been denied right to share in benefits from their genetic materials | ||
| Not current practice | (a) Superseded by other principles e.g. privacy, consent | [24, 27, 31–33, 35, 37, 39, 44, 50] |
| (b) No ethical precedence/ long-standing ethical tradition for paying/compensating donors of biological material | ||
| (c) Attitude: Seen as unworkable or idealist by detractors; even questioning the need for benefit sharing | ||
| (d) Scant attention to governance of benefit sharing | ||
| (e) Presumption that tissue donation is purely altruistic | ||
| (f) Benefit sharing is still poorly understood and implemented, including by many key research stakeholders, such as researchers, sponsors, regulators and, sometimes, ethics committees | ||
| (g) Guidelines, checklists and templates from most ECs and Institutional Review Boards (IRBs) do not include “benefit sharing” among the issues to be checked/reviewed | ||
| (h) Negotiations about benefit sharing not a part of informed consent processes | ||
| Undermining altruism | Focus on the sharing of financial benefits could attenuate people’s willingness to participate for idealistic reasons | [25, 26, 29, 31, 32, 36, 37, 45, 47] |
| Expensive | Implementing benefit sharing could increase cost of doing biobanking and research | [8, 28, 29, 31, 37, 39, 50] |
| Insufficient evidence to support/justify benefit sharing | (a) No empirical studies to demonstrate need for and how to execute benefit sharing in biobanking | [26–28, 32, 35, 37] |
| (b) Little empirical research on what types of benefit sharing arrangements members of the public may wish to see incorporated into biobank governance and regulatory frameworks | ||
| (c) Seems intuitive to compensate tissue donors when their samples lead to generation of revenue but there are no specific arguments for this | ||
| (d) Specific, strong arguments for financial compensation to individuals are hard to find | ||
| Procedural issues | (a) Who negotiates for benefit sharing? | [30, 37, 40, 42] |
| (b) How is community defined? | ||
| (c) How are representatives to negotiate benefit sharing selected given existing structures may be undemocratic or exclude certain members | ||
| (d) Need for inclusion of other interest groups e.g. religious leaders | ||
| Difficulty in quantifying contributions | (a) Donors vs donors: Should donors whose specimen can be directly attributed to intervention be the ones to be compensated? | [30, 32–37, 44, 47, 50] |
| (b) Virtually impossible to determine the relative importance of any one sample to the overall success of the study | ||
| (c) Researchers vs donors: Does the sample provided by the specimen donor have inherent value or is value created by what the researcher does? | ||
| (d) Researchers vs researchers: How should benefits shared between providing entity/biobank and recipient entity/biobank. Also, researchers from low-income countries compared to those from rich countries |
Mechanisms for benefit sharing
A total of 11 articles we reviewed propose mechanisms for benefit sharing. Benefit sharing agreements—described as formal engagements between two or more parties involved in biobanking in which decisions about how benefits that arise in biobanking would be shared—were described in 5 articles [25, 29, 32, 41, 49]. However, they did not provide information on what such agreements should contain, and how they could be negotiated including who would be involved. Some of the articles proposed consultations with stakeholders at different stages of the biobanking process [38, 41, 47, 49]. However, apart from participants/ specimen donors and their communities, the articles do not describe which other parties should be consulted, the specific issues to be discussed, at what stage of the biobanking process nor the context under which the consultations should be undertaken.
The role of ethics/regulatory review in supporting benefit sharing was highlighted in 3 articles which discussed this role as limited to checking for specific benefit sharing provisions prior to approving primary research [33, 46, 50]. One of the articles proposed a tissue trust in which tissues are held in trust for the donors by a trustee who oversees uses in accordance with the wishes of the donors. The tissue trust is then capable of returning long term benefits to the source community that extend beyond the benefits of primary research, such as improvement of healthcare facilities and capacity building of local personnel [49].
Discussion
In this paper, we set out to explore the considerations and practices for benefit sharing in biobanking. To guide our discussions, we began by describing the definitions of ‘benefits’ and ‘benefit sharing’ in the context of biobanking. The articles we reviewed characterised ‘benefits’ in biobanking very broadly to include anything that contributes to the wellbeing of an individual or community at local, national or global level. This broad definition allows for the inclusion of a wide variety of benefits ranging from provision of basic, tangible household supplies such as soap, to more complex and less tangible items such as building research skills within the community [14, 17]. Although this non-specificity in the description of benefits expands the breadth of what could be considered as a benefit in biobanking, it also further complicates considerations for benefit-sharing in biobanking due to its broad nature.
Similarly, among the reviewed articles, benefit sharing in biobanking was broadly described to encompass the fair and equitable distribution of any benefits that accrue from biobanking. Although it was considered an ethical obligation and discussed alongside other ethical issues in research such as consent, privacy and confidentiality, it did not receive specific attention as a stand-alone ethical issue within the reviewed articles. Whilst biobanking is a supportive function of research rather than research, benefit sharing in biobanking has mostly been framed in a similar manner to benefit sharing in health research.
The challenges to benefit sharing outlined in the reviewed papers are not unique to biobanking but are magnified by the nature of biobanking. Biobanking typically tends to blend primary research, which is organised around specific research questions and methods, with more open-ended secondary research which is often unknown at the time of sample collection. It is not always clear at the time of sample collection exactly what kind of secondary research will be done in the future with the samples, when, by whom, and what product/intervention might be realised, if any. This lack of clarity coupled with the fact that there may be long intervening periods between sample collection and intervention development, means that biobanking benefits are often not immediately known or apparent and raises important unresolved questions such as whether benefit sharing ought to be contingent on intervention development. The pooling of samples from different studies, communities or even biobanks as well as requirements for de-identification mean that it might be difficult to attribute inventions to particular communities thereby further complicating return of benefits to sample donors or their communities.
Majority of the challenges plaguing benefit sharing in biobanking seem to be those stemming from the uncertainties about if, when, by whom and for what purposes stored samples will be used and the attendant difficulty in predicting benefits. Additionally, benefit sharing in biobanking is still poorly understood with some stakeholders perceiving it as idealistic or unworkable and further confounded by weak governance mechanisms.
Benefit sharing ideally involves various stakeholders who have different roles as providers, producers, and recipients of benefits. Various stakeholders are described as important in benefits sharing arrangements in biobanking. These stakeholders include sample donors, their families/communities, researchers, regulatory bodies, biobanks, research institutions, countries and the global community. However, a number of areas remain unclear including which stakeholders play what role- if any, extent to which they are involved in benefit sharing discourse/negotiations, when and how benefit sharing decisions would be made and the regulatory environment within which such stakeholder interactions should take place.
The reviewed articles refer to a wide variety of governance mechanisms that can be drawn upon to guide benefit sharing in biobanking. However, most of the stated mechanisms are from related fields such as genomics, health research, human rights and other benefit sharing conventions. The lack of specificity to biobanking underscores the need to develop governance mechanisms that are not only targeted at biobanking, but also context-specific and have the potential for international application especially when samples and data are shared across borders.
Limitations
Despite attempts to be as comprehensive as possible, this review only utilised free online databases to perform literature searches and may not have identified all relevant articles in the published and grey literature. Searching other bibliographic databases may have yielded additional literature. Similarly, our inclusion of articles published in English only constitutes a limitation to our analysis.
Conclusions
Biobanking is expanding rapidly globally whilst benefit sharing in biobanking is still at its infancy. This is reflected in the limited number of dedicated articles and empirical studies in the review. Based on the literature reviewed, we highlight the main gaps in key areas informing benefit sharing in biobanking and draw attention to the need for empirical research involving various categories of stakeholders in both LMICs and HICs in order to: support how stakeholders define what counts as appropriate benefits for them; demonstrate the need for benefit sharing; clarify contentious issues such as under what circumstances benefits should be shared, in what form, with which stakeholders; and how decisions about benefit sharing should made.
Traditional benefit sharing mechanisms such as material transfer agreements and ethics or regulatory review may be insufficient in addressing the complex nature of benefit sharing in biobanking; due to their focus on discrete research protocols. Alternative, novel mechanisms such as benefit sharing agreements, that can be developed and used concurrently, at micro-level (between specimen donors/communities and researchers), meso-level (between researchers and biobanks or between biobanks) and macro levels (between countries) have been proposed. Such approaches may provide the latitude to account for the different benefit sharing levels, the needs of the stakeholders within particular settings and can include clauses for amendments, allowing for progressive decision making as more information becomes available during the biobanking cycle. However, there is paucity of empiric information on these and other benefit sharing mechanisms. Further research could help inform the development, adoption, implementation and testing of benefit sharing mechanisms appropriate for biobanking within different contexts.
Supplementary Information
Additional file 1. Appendix 1: Title and Abstract screening tool.
Additional file 2. Appendix 2: Google Scholar and Cochrane Library search strings.
Acknowledgements
The authors appreciate colleagues at the KEMRI-Wellcome Trust Research Programme for their useful comments and suggestions during the development of this manuscript. The corresponding author would also like to acknowledge IDEAL (Initiative to Develop African Leaders) for the opportunity to conduct this research as part of his PhD studentship. We also acknowledge the Centre for Geographic Medicine (Coast), Kenya Medical Research Institute/Wellcome Trust Research Programme for providing an enabling environment to conduct this work.
Abbreviations
- CBD
Convention on Biological Diversity
- CIOMS
Council for International Organizations of Medical Sciences
- ECs
Ethics Committees
- ELSI
Ethical Legal and Social Issues
- HGRDs
Human genetic research databases
- HUGO
Human Genome Organizations
- IP
Intellectual property
- IRBs
Institutional Review Boards
- KEMRI
Kenya Medical Research Institute
- MeSH
Medical subject headings
- N/A
Not applicable
- OECD
Organisation for Economic Co-operation and Development
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- UK
United Kingdom
- UN
United Nations
- UNESCO
United Nations Educational, Scientific and Cultural Organization
- USA
United States of America
- WHO
World Health Organization
Authors' contributions
AS conceived and designed the study with the input of JDV and DK. AS carried out the study selection and data extraction. JDV & DK supported AS in analysis and writing of the draft manuscript. AS, JDV and DK reviewed the manuscript.
Funding
This work was supported through the DELTAS Africa Initiative [DEL-15-003]. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)'s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa's Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust [107769/Z/10/Z] and the UK government. The views expressed in this publication are those of the author(s) and not necessarily those of AAS, NEPAD Agency, Wellcome Trust or the UK government.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
As this study involved an analysis of publicly available documents only, this is not human subjects research and does not require ethics approval.
Consent for publication
This manuscript was written with the permission of Director KEMRI CGMRC. All authors read and approved the final manuscript for publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Watson PH, Wilson-McManus JE, Barnes RO, Giesz SC, Png A, Hegele RG, et al. Evolutionary concepts in biobanking—the BC BioLibrary. J Transl Med. 2009;7:95. doi: 10.1186/1479-5876-7-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinkorova J, Topolcan O. Biobanks in Horizon 2020: sustainability and attractive perspectives. EPMA J. 2018;9(4):345–353. doi: 10.1007/s13167-018-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olson JE, Bielinski SJ, Ryu E, Winkler EM, Takahashi PY, Pathak J, et al. Biobanks and personalized medicine. Clin Genet. 2014;86(1):50–55. doi: 10.1111/cge.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang B, Park J, Cho S, Lee M, Kim N, Min H, et al. Current status, challenges, policies, and bioethics of biobanks. Genomics Inform. 2013;11(4):211–217. doi: 10.5808/GI.2013.11.4.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joly Y, Dalpé G, So D, Birko S. Fair shares and sharing fairly: a survey of public views on open science, informed consent and participatory research in biobanking. PLoS ONE. 2015;10(7):e0129893. doi: 10.1371/journal.pone.0129893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tindana P, de Vries J. Broad consent for genomic research and biobanking: perspectives from low- and middle-income countries. Annu Rev Genomics Hum Genet. 2016;17:375–393. doi: 10.1146/annurev-genom-083115-022456. [DOI] [PubMed] [Google Scholar]
- 7.Tindana P, Molyneux S, Bull S, Parker M. 'It is an entrustment': broad consent for genomic research and biobanks in sub-Saharan Africa. Dev World Bioeth. 2019;19(1):9–17. doi: 10.1111/dewb.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budimir D, Polasek O, Marusic A, Kolcic I, Zemunik T, Boraska V, et al. Ethical aspects of human biobanks: a systematic review. Croat Med J. 2011;52(3):262–279. doi: 10.3325/cmj.2011.52.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cambon-Thomsen A, Rial-Sebbag E, Knoppers BM. Trends in ethical and legal frameworks for the use of human biobanks. Eur Respir J. 2007;30(2):373–382. doi: 10.1183/09031936.00165006. [DOI] [PubMed] [Google Scholar]
- 10.de Vries J, Tindana P, Littler K, Ramsay M, Rotimi C, Abayomi A, et al. The H3Africa policy framework: negotiating fairness in genomics. Trends Genet. 2015;31(3):117–119. doi: 10.1016/j.tig.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroeder D. Benefit sharing: it's time for a definition. J Med Ethics. 2007;33(4):205–209. doi: 10.1136/jme.2006.016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buck M, Hamilton C. The Nagoya protocol on access to genetic resources and the fair and equitable sharing of benefits arising from their utilization to the convention on biological diversity. Rev Eur Community Int Environ Law. 2011;20:47–61. doi: 10.1111/j.1467-9388.2011.00703.x. [DOI] [Google Scholar]
- 13.Pisani E, Aaby P, Breugelmans JG, Carr D, Groves T, Helinski M, et al. Beyond open data: realising the health benefits of sharing data. BMJ. 2016;355:i5295. doi: 10.1136/bmj.i5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molyneux S, Mulupi S, Mbaabu L, Marsh V. Benefits and payments for research participants: experiences and views from a research centre on the Kenyan coast. BMC Med Ethics. 2012;13:13. doi: 10.1186/1472-6939-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Njue M, Kombe F, Mwalukore S, Molyneux S, Marsh V. What are fair study benefits in international health research? Consulting community members in Kenya. PLoS ONE. 2014;9(12):e113112. doi: 10.1371/journal.pone.0113112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Njue M, Molyneux S, Kombe F, Mwalukore S, Kamuya D, Marsh V. Benefits in cash or in kind? A community consultation on types of benefits in health research on the Kenyan Coast. PLoS ONE. 2015;10(5):e0127842. doi: 10.1371/journal.pone.0127842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lairumbi GM, Parker M, Fitzpatrick R, English MC. Forms of benefit sharing in global health research undertaken in resource poor settings: a qualitative study of stakeholders' views in Kenya. Philos Ethics Humanit Med. 2012;7:7. doi: 10.1186/1747-5341-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millum J. Sharing the benefits of research fairly: two approaches. J Med Ethics. 2012;38(4):219–223. doi: 10.1136/medethics-2011-100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.London AJ, Zollman KJ. Research at the auction block: problems for the fair benefits approach to international research. Hast Cent Rep. 2010;40(4):34–45. doi: 10.1353/hcr.0.0281. [DOI] [PubMed] [Google Scholar]
- 20.Levac D, Colquhoun H, O'Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 22.Bramer WM, de Jonge GB, Rethlefsen ML, Mast F, Kleijnen J. A systematic approach to searching: an efficient and complete method to develop literature searches. J Med Libr Assoc. 2018;106(4):531–541. doi: 10.5195/jmla.2018.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Bioethics Advisory Commission. Research involving human biological materials: ethical issues and policy guidance. 1999.
- 24.Árnason G. Icelandic biobank. A report for GenBenefit Manchester: University of Central Lancashire. 2007.
- 25.Vaz M, Vaz M, Keith S. The views of ethics committee members and medical researchers on the return of individual research results and incidental findings, ownership issues and benefit sharing in biobanking research in a South Indian city. Dev World Bioeth. 2018;18(4):321–30. doi: 10.1111/dewb.12143. [DOI] [PubMed] [Google Scholar]
- 26.Moodley K, Sibanda N, February K, Rossouw T. "It's my blood": ethical complexities in the use, storage and export of biological samples: perspectives from South African research participants. BMC Med Ethics. 2014;15:4. doi: 10.1186/1472-6939-15-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicol D, Critchley C. Benefit sharing and biobanking in Australia. Public Underst Sci. 2012;21(5):534–555. doi: 10.1177/0963662511402425. [DOI] [PubMed] [Google Scholar]
- 28.Hobbs A, Starkbaum J, Gottweis U, Wichmann HE, Gottweis H. The privacy-reciprocity connection in biobanking: comparing German with UK strategies. Public Health Genomics. 2012;15(5):272–284. doi: 10.1159/000336671. [DOI] [PubMed] [Google Scholar]
- 29.Capron AM, Mauron A, Elger BS, Boggio A, Ganguli-Mitra A, Biller-Andorno N. Ethical norms and the international governance of genetic databases and biobanks: findings from an international study. Kennedy Inst Ethics J. 2009;19(2):101–124. doi: 10.1353/ken.0.0278. [DOI] [PubMed] [Google Scholar]
- 30.Xiaoyong Z. Benefit-sharing in human genetic research: international experiences and Chinese legal choices. Life Sciences in Translation—A Sino-European Dialogue on Ethical Governance of the Life Sciences. 2009:49.
- 31.Chalmers D, Nicol D. Human genetic research databases and biobanks: towards uniform terminology and Australian best practice. J Law Med. 2008;15(4):538–555. [PubMed] [Google Scholar]
- 32.Berg K. The ethics of benefit sharing. Clin Genet. 2001;59(4):240–243. doi: 10.1034/j.1399-0004.2001.590404.x. [DOI] [PubMed] [Google Scholar]
- 33.Ravinetto R, Dierickx K. Benefit sharing in the revised Indian National Ethical Guidelines for Biomedical and Health Research Involving Human Participants. Indian J Med Ethics. 2018;3(3):204–209. doi: 10.20529/ijme.2018.066. [DOI] [PubMed] [Google Scholar]
- 34.Yakubu A, Munung NS, De Vries J. How should biobanking be governed in low-resource settings? AMA J Ethics. 2020;22(2):E156–E163. doi: 10.1001/amajethics.2020.156. [DOI] [PubMed] [Google Scholar]
- 35.Mahomed S, Behrens K, Slabbert M, Sanne I. Managing human tissue transfer across national boundaries—an approach from an institution in South Africa. Dev World Bioeth. 2016;16(1):29–35. doi: 10.1111/dewb.12080. [DOI] [PubMed] [Google Scholar]
- 36.Simm K. Benefit-sharing: an inquiry regarding the meaning and limits of the concept in human genetic research. Genomics Soc Policy. 2005;1(2):29. doi: 10.1186/1746-5354-1-2-29. [DOI] [Google Scholar]
- 37.Schroeder D, Lucas JC. Towards best practice for benefit sharing involving access to human biological resources: conclusions and recommendations. In: Benefit sharing. Springer; 2013. p. 217–29.
- 38.Hugo Ethics Committee Hugo ethics committee statement on benefit sharing: April 9, 2000. Clin Genet. 2000;58(5):364–366. doi: 10.1034/j.1399-0004.2000.580505.x. [DOI] [PubMed] [Google Scholar]
- 39.Knoppers BM. Population genetics and benefit sharing. Public Health Genomics. 2000;3(4):212–214. doi: 10.1159/000051141. [DOI] [PubMed] [Google Scholar]
- 40.Sheremeta L, Knoppers BM. Beyond the rhetoric: population genetics and benefit-sharing. Health Law J. 2003;11:89–117. [PubMed] [Google Scholar]
- 41.Schroeder D, Lasen-Diaz C. Sharing the benefits of genetic resources: from biodiversity to human genetics. Dev World Bioeth. 2006;6(3):135–143. doi: 10.1111/j.1471-8847.2006.00148.x. [DOI] [PubMed] [Google Scholar]
- 42.Schuklenk U, Kleinsmidt A. North-South benefit sharing arrangements in bioprospecting and genetic research: a critical ethical and legal analysis. Dev World Bioeth. 2006;6(3):122–134. doi: 10.1111/j.1471-8847.2006.00149.x. [DOI] [PubMed] [Google Scholar]
- 43.Ndebele P, Musesengwa R. Will developing countries benefit from their participation in genetics research? Malawi Med J. 2008;20(2):67–69. doi: 10.4314/mmj.v20i2.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pullman D, Latus A. Clinical trials, genetic add-ons, and the question of benefit-sharing. Lancet. 2003;362(9379):242–244. doi: 10.1016/s0140-6736(03)13916-5. [DOI] [PubMed] [Google Scholar]
- 45.Boggio A, Biller-Andorno N, Elger B, Mauron A, Capron AM. Comparing guidelines on biobanks: emerging consensus and unresolved controversies. Geneva: Réseau Universitaire International de Genève; 2005. [Google Scholar]
- 46.Laurie G, Mallia P, Frenkel DA, Krajewska A, Moniz H, Nordal S, et al. Managing access to biobanks: How can we reconcile individual privacy and public interests in genetic research? Med Law Int. 2010;10(4):315–337. doi: 10.1177/096853321001000404. [DOI] [Google Scholar]
- 47.Joly Y, Allen C, Knoppers BM. Open access as benefit sharing? The example of publicly funded large-scale genomic databases. J Law Med Ethics. 2012;40(1):143–147. doi: 10.1111/j.1748-720X.2012.00652.x. [DOI] [PubMed] [Google Scholar]
- 48.Chen H, Pang T. A call for global governance of biobanks. Bull World Health Organ. 2015;93(2):113–117. doi: 10.2471/blt.14.138420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Emerson CI, Singer PA, Upshur RE. Access and use of human tissues from the developing world: ethical challenges and a way forward using a tissue trust. BMC Med Ethics. 2011;12:2. doi: 10.1186/1472-6939-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schroeder D, Gefenas E. Realizing benefit sharing—the case of post-study obligations. Bioethics. 2012;26(6):305–314. doi: 10.1111/j.1467-8519.2010.01857.x. [DOI] [PubMed] [Google Scholar]
- 51.CBD S, editor. Bonn guidelines on access to genetic resources and fair and equitable sharing of the benefits arising out of their utilization. Montreal: Secreteriat of Convention on Biological Diversity; 2002.
- 52.Convention on Biological Diversity-United Nations. Nagoya protocol on access to genetic resources and the fair and equitable sharing of benefits arising from their utilization to the convention on biological diversity. Montreal; 2011.
- 53.Lenoir N. UNESCO’s Universal Declaration on the human genome and human rights. Revista de Derecho y Genoma Humano. 1998;9:141. [PubMed] [Google Scholar]
- 54.United Nations Educational Scientific and Cultural Organization (UNESCO). Universal Declaration on Bioethics and Human Rights. Paris. 2005. http://portal.unesco.org/en/ev.php-URL_ID=31058&URL_DO=DO_TOPIC&URL_SECTION=201.html. Accessed 8 June 2021.
- 55.Grubb A. The Nuffield Council report on human tissue. Med Law Rev. 1995;3(3):235–236. doi: 10.1093/medlaw/3.3.235. [DOI] [PubMed] [Google Scholar]
- 56.Organization WH. Proposed international guidelines on ethical issues in medical genetics and genetic services-report of a WHO Meeting on Ethical Issues and Medical Genetics; 2001.
- 57.Organization WH. European partnership on patients’ rights and citizens’ empowerment 2003. Genetic Databases-Assessing the Benefits and the Impact on Human Rights and Patient Rights; 2003.
- 58.Council for International Organizations of Medical Sciences (CIOMS). International Ethical Guidelines for Health-related Research Involving Humans. Fourth Edition ed. Geneva; 2016.
- 59.OECD. OECD guidelines on human Biobanks and genetic research databases. Eur J Health Law. 2010;17(2):191–204. 10.1163/157180910X12665776638821. [PubMed]
- 60.Assembly UG. Universal declaration of human rights. UN Gener Assembly. 1948;302(2):14–25. [Google Scholar]
- 61.World Medical A World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Appendix 1: Title and Abstract screening tool.
Additional file 2. Appendix 2: Google Scholar and Cochrane Library search strings.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.