Abstract
Background
To investigate the global expression profile of miRNAs, their impact on cellular signaling pathways, and their association with poor prognostic parameters in African-American (AA) patients with triple negative breast cancer (TNBC).
Methods
Twenty-five samples of AA TNBC patients were profiled for global miRNA expression and stratified considering three clinical-pathological parameters: tumor size, lymph node (LN), and recurrence (REC) status. Differential miRNA expression analysis was performed for each parameter, and their discriminatory power was determined by Receiver Operating Characteristic (ROC) curve analysis. KMplotter was assessed to determine the association of the miRNAs with survival, and functional enrichment analysis to determine the main affected pathways and miRNA/mRNA target interactions.
Results
A panel of eight, 23 and 27 miRNAs were associated with tumor size, LN, and REC status, respectively. Combined ROC analysis of two (miR-2117, and miR-378c), seven (let-7f-5p, miR-1255b-5p, miR-1268b, miR-200c-3p, miR-520d, miR-527, and miR-518a-5p), and three (miR-1200, miR-1249-3p, and miR-1271-3p) miRNAs showed a robust discriminatory power based on tumor size (AUC = 0.917), LN (AUC = 0.945) and REC (AUC = 0.981) status, respectively. Enrichment pathway analysis revealed their involvement in proteoglycans and glycan and cancer-associated pathways. Eight miRNAs with deregulated expressions in patients with large tumor size, positive LN metastasis, and recurrence were significantly associated with lower survival rates. Finally, the construction of miRNA/mRNA networks based in experimentally validated mRNA targets, revealed nodes of critical cancer genes, such as AKT1, BCL2, CDKN1A, EZR and PTEN.
Conclusions
Altogether, our data indicate that miRNA deregulated expression is a relevant biological factor that can be associated with the poor prognosis in TNBC of AA patients, by conferring to their TNBC cells aggressive phenotypes that are reflected in the clinical characteristics evaluated in this study.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-021-08573-2.
Keywords: microRNA, Triple-negative breast cancer, African-American, Prognosis, Tumor size, Lymph node
Background
Breast cancer is one of the most frequent types of female cancer worldwide and one of the main causes of death in women [1]. Several new target therapies are currently in development with significant potential to reduce mortality in patients with varied breast cancer subtypes by significantly increasing their overall survival [2]. However, for triple negative breast cancer (TNBC), there are still few approved targeted therapies. Inhibitors of the programmed death-ligand 1 (PDL1) have been recently approved for the treatment of unresectable locally advanced or metastatic TNBC patients. This targeted therapy is, however, only beneficial for patients with PDL1 positive tumor expression [3, 4].
TNBC is considered one of the most aggressive subtypes of breast cancer, with high rates of progression and poor prognosis [5]. Comprehensive molecular-based studies have been extensively performed to identify new biomarkers that can be used for diagnosis, prognosis, and more efficient treatment regimens for this tumor subtype. One class of biomarkers that has emerged with promising therapeutic potential is the non-coding microRNAs (miRNAs) [6, 7]. With the technological advances in nanoparticle delivery systems, this class of biomarkers has been shown to be effective in inhibiting tumor progression and metastasis in several tumor models, including TNBC [8–10].
MiRNAs are conserved endogenous small RNA molecules that can regulate a range of developmental and physiological processes in the cells, in a tissue-specific manner [11]. MiRNAs expression alterations are associated with the development of pathological processes and chronic diseases [12]. In cancer, miRNAs play a critical role in tumor initiation and progression, through their regulatory role in the expression of gene targets involved in multiple signaling pathways, such as cell proliferation, differentiation, and cell death [13–17]. In TNBC, many miRNAs with deregulated expression have been identified and associated with aggressive cancer phenotypes, including larger tumor sizes, early tumor recurrence, lymph node, and metastatic invasion, and lower survival rates [18].
Several studies have shown that miRNAs present variable expression patterns according to race and/or ethnic groups [19–23]. However, few of them were conducted in patients’ tumor samples. We have previously shown in genomically characterized African-American (AA) and Latina patients with TNBC, differential patterns of tumor miRNA expression when compared to TNBC patients of European descent [24, 25]. These profiles were shown to be associated with distinct signaling pathways, commonly linked to poor prognosis and shorter survival. Although there are a limited number of studies evaluating the somatic miRNA expression in tumors of genomically ancestral characterized populations, this data supports the relevance of miRNAs as biological contributors to cancer disparities.
In this study, the main objective was to characterize the global patterns of miRNA expression, and their impact on cellular signaling pathways in the TNBC tissue specimens of AA patients. The miRNA patterns were also evaluated according to three selected clinical-pathological parameters: tumor size, lymph node (LN), and recurrence (REC) status.
Material and methods
Samples collection, clinical and demographic information
Formalin-fixed paraffin-embedded tissue blocks (FFPE) of 25 TNBC patients were collected from the surgical pathology archives of Howard University Hospital, Washington DC. Clinical and histopathological data were obtained for the patients in a codified manner according to the approved institutional IRB protocols and included age at diagnosis, histologic type, tumor size, Nottingham Histologic Score and Grade [26], lymph node (LN), recurrence (REC), and distant metastasis status. This study was approved by the Office of Regulatory Research Compliance – Institutional Review Board of Howard University (IRB-16-MED-39).
Clinical and histopathological data of the variables above were retrieved for all the cases analyzed. Most of the patients (81.8%) were diagnosed with infiltrating ductal carcinoma, with a mean age at diagnosis of 55.27 ± 9.96 years old (range 32 to 78 years old). All tumors, but one (grade 2), were classified as histological high grade (grade 3). Wide variability in tumor size was observed, with tumors presenting their largest dimension between 1.1 and 22 cm in size (average 5.86 ± 5.17 cm). Ten patients (62.5%) presented tumors smaller than 5 cm, while six patients (37.5%) presented tumors larger than 5 cm. LN involvement (LN+) was present in ten patients (47.6%) affecting one or more LNs while eleven patients (53.4%) were LN negative (LN-). Sixty-eight percent of the patients became disease-free after treatment and had no recurrence, while 32% relapsed (with local and/or distant metastasis) after the selected treatment regimen or never reached a disease-free condition since diagnosis (Table S1).
The classification of the TNBC phenotype was confirmed by immunohistochemistry analysis using estrogen (ER), progesterone (PR), and human epidermal growth factor receptor 2 (HER2) receptor markers, following current guidelines [27, 28].
The AA race information of the patients was initially obtained from self-reported medical records and later confirmed by genotyping analysis for a subset of the patients. The genotyping was performed using the SNP chip Illumina Infinium QC Array (Illumina Inc., CA), which contains 15,949 markers, including, 3000 ancestral informative markers (AIMs). The genotype calling was performed as we previously described [24, 25], using GenomeStudio Software v. 2011.1. Genotypes from the mitochondrial genome and on sex chromosomes were excluded, as well as genotypes with a call rate < 98%. The remaining autosomal genotypes (8687 in total) were integrated with the variant calls from ≥1900 individuals originating from 21 diverse populations in the 1000 Genomes Project. To explore population structure among individuals, Principal Component Analysis (PCA) was conducted on the genome-wide autosomal loci. First, a genetic relationship matrix between pairs of individuals (GRM files) was generated with the GCTA software [29]. Then, using the GRM files as input, the PCA method implemented in GCTA was applied. In these analyses, the default setting (n = 20) was accepted, which outputted the first 20 eigenvectors and all of the eigenvalues. Lastly, the top two Principal Components, PC1, and PC2 were plotted using RStudio [30]. PC1 (accounting for 69.1% of the extracted variation) broadly distinguished individuals of African descent from non-Africans, suggesting some genetic distance between African and non-African populations consistent with previous studies of autosomal loci [31, 32] (Figure S1).
RNA isolation and global miRNA expression analysis
The FFPE tumor specimens were evaluated by a pathologist for the presence of at least 80% of tumor cells. The selected tumor areas were microdissected from unstained 10 μm FFPE tissue sections and total RNA was isolated using TRIzol (Invitrogen Carlsbad, CA, USA) after deparaffinization with Xylene solution. RNA concentration and quality were tested by measuring 260/280 and 260/230 ratios using Nanodrop 2000 spectrophotometer (Willington, DE, USA).
MiRNA expression analysis was performed using Nanostring nCounter Human v3a miRNA Expression Assay (Seattle, WA, USA) that contains human probes derived from miRBase version 22 (http://www.mirbase.org) targeting 827 human miRNAs, six positive controls, eight negative controls, three ligation positive controls, three ligation negative controls, five internal reference genes (ACTB, B2M, GAPDH, RPL19, and RPL0), and five spike-in controls (ath-miR-159a, cel-miR-248, cel-miR-254, osa-miR-414, and osa-miR-442). Raw data were pre-processed using Nanostring’s Counter RCC collector and normalized using Nanostring nSolver 4.0 software (Background subtraction: the geometric mean of Negative Controls; Technical normalization: the geometric mean of Positive Controls; Codeset content normalization: all genes geometric mean). Normalized data was Log2 transformed and analyzed using the MultiExperiment Viewer software (MeV 4.9.0), GraphPad Prism 8.3.0, and IBM SPSS Statistics 25.
Differential miRNA expression analysis among the TNBC clinical groups
The tumor samples profiled for miRNA were stratified in different clinical groups according to three selected clinical-pathological parameters: tumor size (≥5 cm or < 5 cm), LN status [positive (LN+) or negative (LN-) and REC status (positive (REC+) or negative (REC-)]. Differential miRNA expression analysis was performed for each of these parameters, resulting in three distinct miRNA panels (Welch-based t-Test, p ≤ 0.05). Log2 Fold Change (Log2FC) was calculated for each differentially expressed miRNA. Unsupervised and Supervised Hierarchical Clustering analysis was performed using Pearson’s correlation coefficient and average linkage and visualized as heatmaps with a 3-color scheme blue-black-yellow representing values below the median-median-above median, respectively.
Receiver operating characteristic (ROC) curve analysis
The discriminatory power of the differentially expressed miRNAs between the different clinical groups was tested by constructing the ROC curve and calculating the Area Under the Curve (AUC). Sensitivity was plotted against specificity (%) for the binary classifiers (≥5 cm vs < 5 cm, LN+ vs LN-, and REC+ vs REC-). An AUC of 1.0 (100%) denotes perfect discrimination by the miRNA, whereas an AUC of 0.5 (50%) denotes a complete lack of discrimination by the miRNA. AUCs and 95% corresponding confidence intervals (CI) were calculated for each miRNA using GraphPad Prism v 8.3.0 and IBM SPSS Statistics 25.
Multiple binary logistic regression analysis was used to determine the effect of the panel of miRNAs in discriminating the samples according to their tumor size, LN, and REC status. The most robust miRNA panel combination was also determined for each clinical parameter studied.
Kaplan Meier (KM) plot analysis
The miRpower KM Plotter Tool [33] was used to calculate hazard ratios, confidence intervals, and log-rank P values for the miRNAs that were shown to present a high discriminatory power in each clinical group evaluated. This analysis was performed in relation to survival in the aggregated breast cancer clinical studies extracted from The Cancer Genome Atlas (TCGA) and Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) databases (extraction based on TNBC cases in general, and TNBC cases with known LN status).
Functional enrichment pathways analysis
The Diana miRPath v.3.0 [34] was used to identify the top signaling pathways that were affected by the significant differentially expressed miRNAs, which is based on the online tool Kyoto Encyclopedia of Genes and Genomes (KEGG) annotation. For these analyses, micro-T-CDS (v5.0) was used to identify the predicted gene targets regulated by the selected miRNAs, applying the p-value of 0.05 and a microT threshold of 0.8.
The miRpathDB v.2.0 [35] was used to determine the potential influence of the selected miRNAs in the Integrated Breast Cancer pathway (WP1984) from Wikipathways [36], considering experimentally validated and predicted target genes. This pathway presents relevant proteins and their interactions in breast cancer and has been widely used in miRNA prediction analyses [37, 38].
In silico functional analysis
The databases miRTarBase [39] and miRnet [40] were used to determine interactions between the selected miRNAs and target genes validated based on strong (reporter assays, Western Blot, and qPCR) and less strong experimental assays (microarray, NGS, pSilac). MirTargetLink Human online software was used to visualize miRNA-mRNA interaction networks [41].
The STRING v.11 database [42] was used to verify protein-protein interactions (PPI) between the validated target genes of each group, applying the minimum interaction score of 0.9 (highest confidence). Cytoscape v.3.8.0 [43] was used to construct molecular interaction networks of selected miRNAs and target genes.
Results
Differential miRNA expression analysis among the TNBC clinical groups
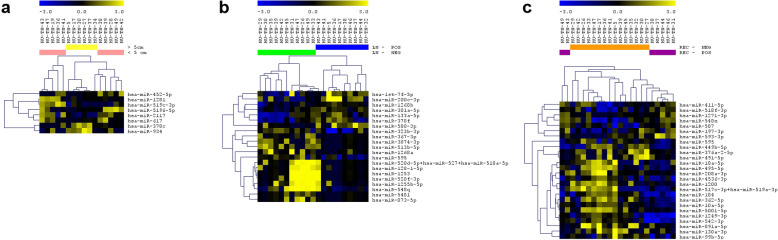
A total of 25 cases were successfully profiled for the expression of global miRNAs. The analysis of miRNAs expression was performed separately for each of the three selected clinical groups (tumor size, LN, and REC status) (Table 1, Fig. 1). In the analysis of the cases based on tumor size, eight miRNAs were observed differentially expressed between the large (≥5 cm) and small (< 5 cm) tumors, from which two miRNAs were upregulated and six downregulated in the large tumor size cases (t-Test p < 0.05, Fig. 1a). In the LN group analysis, 23 miRNAs were observed differentially expressed between the LN+ and LN- cases, seven upregulated and 14 downregulated in the LN+ cases (t-Test p < 0.05, Fig. 1b). Finally, 27 miRNAs were observed differentially expressed between REC+ and REC- cases, seven upregulated and 19 downregulated in the REC+ cases (t-Test, p < 0.05, Fig. 1c).
Table 1.
Log2FC, p-value, AUC and corresponding 95%CI for each differentially expressed miRNA observed in the tumor size, LN and REC status groups
| Tumor size (≥5 cm/< 5 cm) | Log2FC | p-value | AUC | 95% CI |
| miR-1281 | − 0.87487 | 0.0479 | 0.6833 | 0.4101 to 0.9565 |
| miR-2117 | −1.51124 | 0.0026 | 0.875 | 0.6826 to 1.0000 |
| miR-378c | 1.24370 | 0.0457 | 0.8083 | 0.5528 to 1.0000 |
| miR-452-5p | −0.81783 | 0.0329 | 0.7000 | 0.4376 to 0.9624 |
| miR-5196-5p | −1.27827 | 0.0132 | 0.7500 | 0.5050 to 0.9950 |
| miR-519c-3p | −1.27906 | 0.0187 | 0.8000 | 0.5804 to 1.0000 |
| miR-617 | −0.82711 | 0.0402 | 0.7167 | 0.4536 to 0.9797 |
| miR-934 | 1.610418 | 0.0193 | 0.8333 | 0.6295 to 1.0000 |
| LN status | Log2FC | p-value | AUC | 95% CI |
| let-7f-5p | 1.308153 | 0.0345 | 0.8091 | 0.6141 to 1.000 |
| miR-1253 | −2.23171 | 0.0436 | 0.7909 | 0.5984 to 0.9834 |
| miR-1255b-5p | −1.43984 | 0.0298 | 0.8091 | 0.6201 to 0.9981 |
| miR-1268a | −0.9362 | 0.0441 | 0.7682 | 0.5632 to 0.9732 |
| miR-1268b | 0.7279 | 0.0084 | 0.8636 | 0.6882 to 1.000 |
| miR-128-1-5p | −1.49312 | 0.0254 | 0.7818 | 0.5838 to 0.9799 |
| miR-133a-5p | 1.097722 | 0.0369 | 0.6727 | 0.4348 to 0.9106 |
| miR-200c-3p | 2.602164 | 0.0187 | 0.8182 | 0.6375 to 0.9988 |
| miR-301a-5p | 0.583352 | 0.0212 | 0.8091 | 0.6170 to 1.000 |
| miR-3074-3p | −0.85799 | 0.0301 | 0.7273 | 0.4976 to 0.9570 |
| miR-323b-3p | −1.30263 | 0.0324 | 0.7636 | 0.5366 to 0.9907 |
| miR-367-3p | −0.653 | 0.0438 | 0.7545 | 0.5444 to 0.9647 |
| miR-378f | 1.143995 | 0.0356 | 0.8000 | 0.6058 to 0.9942 |
| miR-513b-5p | −1.04378 | 0.0115 | 0.7864 | 0.5790 to 0.9938 |
| miR-520d-5p + miR-527 + miR-518a-5p | −1.53273 | 0.0161 | 0.8364 | 0.6645 to 1.000 |
| miR-520f-3p | −1.7368 | 0.0355 | 0.7909 | 0.5852 to 0.9966 |
| miR-548 l | −0.59929 | 0.0187 | 0.7909 | 0.5936 to 0.9882 |
| miR-548q | −1.98804 | 0.0105 | 0.7727 | 0.5600 to 0.9855 |
| miR-580-3p | 1.266989 | 0.0094 | 0.8045 | 0.5962 to 1.000 |
| miR-595 | −1.17619 | 0.0070 | 0.8182 | 0.6281 to 1.000 |
| miR-873-5p | −0.86919 | 0.0434 | 0.7045 | 0.4769 to 0.9321 |
| REC status | Log2FC | p-value | AUC | 95% CI |
| miR-10a-5p | −0.86382 | 0.022 | 0.7905 | 0.5801 to 1.000 |
| miR-1200 | −1.55119 | 0.0004 | 0.8571 | 0.7004 to 1.000 |
| miR-1249-3p | −1.54646 | 0.0211 | 0.8286 | 0.6493 to 1.000 |
| miR-1271-3p | 0.962012 | 0.0108 | 0.9143 | 0.7968 to 1.000 |
| miR-130a-3p | −1.45528 | 0.0144 | 0.7810 | 0.5850 to 0.9769 |
| miR-184 | −2.01337 | 0.0005 | 0.8857 | 0.7442 to 1.000 |
| miR-18a-5p | −1.2983 | 0.0226 | 0.7667 | 0.5637 to 0.9696 |
| miR-197-3p | 1.067639 | 0.0412 | 0.7714 | 0.5689 to 0.9740 |
| miR-208a-3p | −0.94776 | 0.0372 | 0.7333 | 0.5162 to 0.9505 |
| miR-362-5p | −1.31405 | 0.0486 | 0.7619 | 0.5401 to 0.9837 |
| miR-376a-2-5p | −1.07002 | 0.0055 | 0.7048 | 0.4876 to 0.9220 |
| miR-411-5p | 1.185286 | 0.0084 | 0.7810 | 0.5876 to 0.9743 |
| miR-449b-5p | −1.76057 | 0.0003 | 0.8571 | 0.6997 to 1.000 |
| miR-4536-3p | −1.25336 | 0.0292 | 0.8000 | 0.6039 to 0.9961 |
| miR-491-5p | −0.97535 | 0.0373 | 0.6952 | 0.4761 to 0.9144 |
| miR-495-5p | −1.18756 | 0.0291 | 0.7238 | 0.5092 to 0.9385 |
| miR-5001-5p | −1.12657 | 0.0165 | 0.7905 | 0.5968 to 0.9841 |
| miR-517c-3p + miR-519a-3p | −1.31722 | 0.0364 | 0.6952 | 0.4759 to 0.9146 |
| miR-518f-3p | 1.233145 | 0.003 | 0.8286 | 0.6538 to 1.000 |
| miR-542-3p | −1.47531 | 0.0253 | 0.8095 | 0.6106 to 1.000 |
| miR-548n | 1.427446 | 0.0044 | 0.8619 | 0.6799 to 1.000 |
| miR-587 | 1.032396 | 0.0112 | 0.7714 | 0.5633 to 0.9796 |
| miR-593-3p | 1.172516 | 0.0446 | 0.8190 | 0.6257 to 1.000 |
| miR-595 | −1.00655 | 0.0136 | 0.8381 | 0.6600 to 1.000 |
| miR-891a-5p | −1.23041 | 0.0142 | 0.7619 | 0.5570 to 0.9668 |
| miR-99b-5p | −1.3409 | 0.0267 | 0.8286 | 0.6574 to 0.9998 |
Fig. 1.
Differentially expressed miRNAs in the analysis of the TNBC cases distributed by tumor size (a), LN (b) and REC (c) status
MiRNA differential expression analysis was also performed based on the four combined LN and REC status groups, considering the representative number of patients in each of these sub-groups (especially for the LN+/REC- which represented 23.8% of the patients): LN+/REC+, LN−/REC+, LN+/REC- and LN−/REC- (ANOVA, p < 0.01). Twenty-two miRNAs were found differentially expressed among these groups (Fig. 2). The comparison of the differentially expressed miRNAs among the LN, REC and combined LN/REC status groups resulted in eight miRNAs: miR-10a-5p, miR-1271-3p, miR-184, miR-18a-5p, miR-411-5p, and miR-542-3p present in the REC and LN/REC comparisons, and miR-1253 present in LN and LN/REC comparisons, and miR-595 present in all the three comparisons.
Fig. 2.
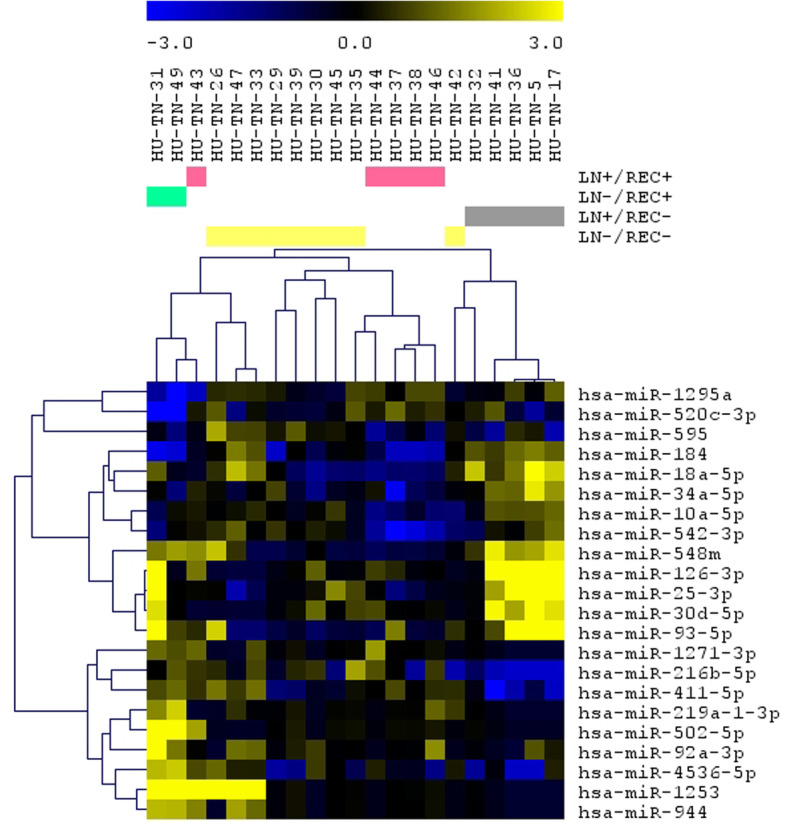
Differentially expressed miRNAs in the analysis of the TNBC cases distributed in the four combined LN and REC status groups
ROC analysis
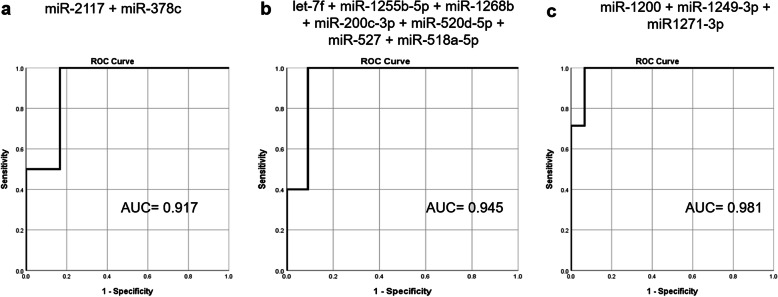
ROC analysis was performed for each miRNA in each of the clinical groups evaluated. The analysis of the eight miRNAs observed differentially expressed according to tumor size, resulted in AUC values from 0.683 (miR-1281) to 0.875 (miR-2117). Four miRNAs presented AUC higher than 0.800 and were used to determine the most robust miRNA panel for classifying the samples based on tumor size (≥5 cm or < 5 cm). As a result, the combination of miR-2117 and miR-378c showed the highest discriminatory power with an AUC value of 0.917(Fig. 3a).
Fig. 3.
Combined ROC analysis for the most robust discriminatory miRNA panels for tumor size (a), LN (b) and REC (c) status
Eleven out of the 23 differentially expressed miRNAs according to the LN status presented AUC ≥ 0.800, and the combination of let-7f-5p, miR-1255b-5p, miR-1268b, miR-200c-3p, miR-520d-5p + miR-527 + miR-518a-5p expression levels presented the highest discriminatory power (AUC = 0.945; Fig. 3b).
Finally, the ROC analysis of miRNAs differentially expressed according to REC status, showed an AUC ranging from 0.695 (miR-491-5p and miR-517c-3p + miR-519a-3p) to 0.914 (miR-1271-3p). Twelve miRNAs presented AUC values higher than 0.800, and a combined ROC analysis indicates that the most robust miRNA panel consisted of miR-1200, miR-1249-3p and miR-1271-3p (AUC = 0.981; Fig. 3c). The individual AUC values for all the miRNAs differentially expressed per clinical group and their corresponding 95% CI are presented in Table 1.
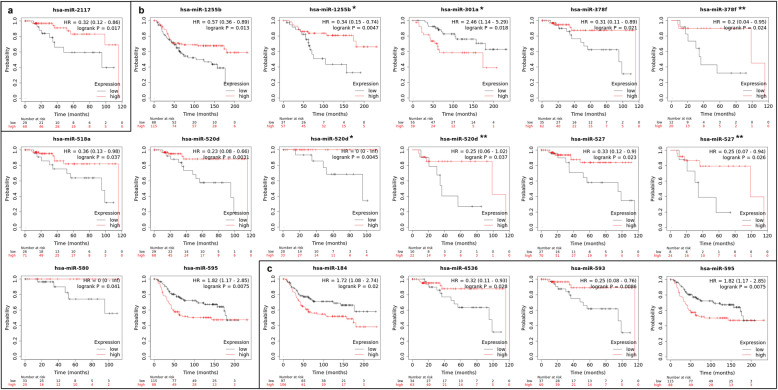
Association of the Target miRNA genes with survival using KM plot database
The miRNAs that were shown to present a high discriminatory power (AUC ≥0.8) in each clinical group analyzed were queried concerning survival in TNBC samples of the TCGA and METABRIC cohorts, using the KM Plot database. In the tumor size group, only one (miR-2117) out of four miRNAs, was observed in association with survival (Fig. 4a). Lower expression of this miRNA, as we observed in the group of patients with larger tumor sizes, was significantly associated with shorter survival. In the LN group, eight (miR-1225b, miR-301a, miR-378f, miR-518a, miR-520d, miR-527, miR-580; miR-595) out of 12 miRNAs were associated with survival, some of which specifically associated with LN- (miR-1225b, miR-301a, miR-520d, and miR-580) or LN+ (miR-378f, miR-527) patients (Fig. 4b). From these miRNAs, all, except miR-580a and miR-595, presented in the KM Plot, the same direction of the expression levels as we observed in our cases in the LN+ group. Finally, in the group of patients based on REC status, four out of 11 miRNAs, presented the association with survival in the TNBC samples of the TCGA and METABRIC cohorts. In this group, only miR-4536, which we observed with lower expression in the REC+ group of patients, was associated with shorter survival (Fig. 4c).
Fig. 4.
KM Plot graphics of the miRNAs that presented AUC > 0.8 in each clinical group analyzed (a tumor size, b LN, and c REC) and that were significantly associated with survival in the TNBC cases of TCGA and/or METABRIC. * indicates patients with LN- and ** with LN+
Functional enrichment pathways
The potentially affected KEGG pathways by the differentially expressed miRNAs were determined for each of the clinical groups evaluated. The top 10 KEGG pathways ranked by p-value for each analysis are shown in Table 2.
Table 2.
Top 10 KEGG pathways potentially affected by the differentially expressed miRNAs in each clinical group evaluated: tumor size, LN, and REC status (ranked by p-value)
| # | KEGG pathways | p-value | # genes | # miRNAs |
|---|---|---|---|---|
| Tumor size (≥5 cm, < 5 cm) | ||||
| 1 | Ubiquitin mediated proteolysis | 1.46E-05 | 40 | 7 |
| 2 | Circadian rhythm | 0.002970639 | 14 | 7 |
| 3 | Proteoglycans in cancer | 0.002970639 | 42 | 7 |
| 4 | Renal cell carcinoma | 0.004487894 | 18 | 7 |
| 5 | Signaling pathways regulating pluripotency of stem cells | 0.004646529 | 33 | 6 |
| 6 | Endocytosis | 0.004646529 | 44 | 8 |
| 7 | Glioma | 0.007370446 | 17 | 7 |
| 8 | Oocyte meiosis | 0.015388458 | 25 | 7 |
| 9 | RNA degradation | 0.022814164 | 21 | 6 |
| 10 | Prolactin signaling pathway | 0.022814164 | 17 | 6 |
| LN status | ||||
| 1 | Mucin type O-Glycan biosynthesis | 3.42E-10 | 14 | 8 |
| 2 | Proteoglycans in cancer | 6.78E-10 | 32 | 9 |
| 3 | Signaling pathways regulating pluripotency of stem cells | 3.68E-07 | 55 | 9 |
| 4 | TGF-beta signaling pathway | 3.17E-06 | 68 | 8 |
| 5 | Glycosaminoglycan biosynthesis – heparan sulfate/heparin | 5.72E-06 | 38 | 7 |
| 6 | Hippo signaling pathway | 6.54E-06 | 2 | 2 |
| 7 | FoxO signaling pathway | 6.94E-06 | 49 | 8 |
| 8 | Circadian rhythm | 2.90E-05 | 52 | 9 |
| 9 | ErB signaling pathway | 3.54E-05 | 44 | 9 |
| 10 | Phosphatidylinositol signaling system | 6.34E-05 | 25 | 7 |
| REC status | ||||
| 1 | Prion diseases | 1.07E-05 | 10 | 8 |
| 2 | Proteoglycans in cancer | 1.07E-05 | 91 | 20 |
| 3 | Mucin type O-Glycan biosynthesis | 1.34E-05 | 15 | 12 |
| 4 | Cell adhesion molecules (CAMs) | 1.84E-05 | 65 | 18 |
| 5 | TGF-beta signaling pathway | 3.45E-05 | 40 | 16 |
| 6 | Axon guidance | 3.45E-05 | 65 | 16 |
| 7 | Oxytocin signaling pathway | 0.001164 | 76 | 18 |
| 8 | N-Glycan biosynthesis | 0.003947 | 21 | 15 |
| 9 | Thyroid hormone signaling pathway | 0.003947 | 53 | 19 |
| 10 | Signaling pathways regulating pluripotency of stem cells | 0.004389 | 65 | 20 |
The analysis of differentially expressed miRNAs, in the tumor size group, resulted in 21 pathways, including ubiquitin-mediated proteolysis (hsa04120), proteoglycans in cancer (hsa05205), and signaling pathways regulating pluripotency of stem cells (hsa04550). In the LN group, 51 KEGG pathways potentially affected by these miRNAs were identified, including mucin type-O-glycan biosynthesis (hsa00512), TGF-beta signaling pathway (hsa04350), glycosaminoglycan biosynthesis – heparan sulfate/heparin (hsa00534), hippo signaling pathway (hsa04390), ErbB signaling pathway (hsa04012). Finally, considering the REC status of the patients, 41 KEGG pathways were identified potentially affected by these miRNAs, including proteoglycans in cancer (hsa05205), cell adhesion molecules (CAMs) (hsa04514), N-Glycan biosynthesis (hsa00510), and thyroid hormone signaling pathway (hsa04919). The thyroid hormone signaling pathway was predicted to be affected by eight miRNAs (miR-1200, miR-1271-3p, miR-449b-5p, miR-4536-3p, miR-542-3p, miR-548n, miR-593-3p and miR-595) indicating that this signaling pathway may be important in the development of breast cancer recurrence.
Considering all the miRNAs selected from the three comparisons among the three clinical groups, 15 miRNAs were found to be involved in the Integrated Breast Cancer Pathway, potentially targeting 133 genes, some of them targeted by more than one miRNA (Table S2).
In silico functional analysis and miRNA/mRNA target networks
To further elucidate the potential biological impact of each differentially expressed miRNA in the clinical groups evaluated, experimentally validated interactions between miRNA and their target genes were assessed (only strong evidence of interaction was considered for this analysis). Thirty-three of the 58 selected miRNAs (2/8 for the tumor size group, 13/23 for LN status and 18/27 for REC status) presented interactions experimentally validated, regulating a total of 295 target genes (Table S3). When considering only miRNAs with high power to discriminate among the cases in each clinical group (based on ROC analysis), a total of 13 miRNAs were observed (one for the tumor size group, seven for LN status and five for REC status) (Table 3).
Table 3.
MiRNAs and corresponding target genes identified in each clinical group evaluated (only miRNAs with high discriminatory power (AUC ≥0.8) were included)
| Clinical groups | miRNAs | Target Genes |
|---|---|---|
|
Tumor size (≥5 cm/< 5 cm) |
miR-519c-3p | HIF1A, ABCG2, ELAVL1, TIMP2, PTEN, CDKN1A |
| LN status | let-7f-5p | KLK10, KLK6, PRDM1, IL3, CYP19A1, COPS8, GPS1, CCND1, COPS6, MYH9, SOCS3, ELF4, CCL7, AGO1, IL6, POSTN |
| miR-200c-3p | TUBB3, BMI1, GEMIN2, BAP1, ZEB2, ZEB1, FN1, ZFPM2, PTPN13, RNF2, RCOR3, BRD7, ACVR2B, MSN, NTRK2, ERRFI1, CCNE2, XIAP, BCL2, TIMP2, FBLN5, VEGFA, NCAM1, IKBKB, FLT1, KLF9, TBK1, PMAIP1, NTF3, LPAR1, EDNRA, RHOA, KLHL20, PTPRD, ELMO2, ERBIN, WDR37, VAC14, TCF7L1, RASSF2, HOXB5, RIN2, KLF11, SEPT7, SHC1, MYB, ETS1, DUSP1, USP25, EFNA1, RND3, DNMT3A, DNMT3B, SP1, CFL2, CDH11, SEC23A, KDR, HFE, DLC1, ATRX, ZNF217, BTC, ZFPM1, PIN1, KRAS, NOTCH1, GATA4, SUZ12, ROCK2, UBQLN1, E2F3, MALAT1, CDK2, PRKCZ, NOS3, SIRT1, FOXO1, PDCD10, ADAM12, PTEN, LEPR, CRKL, MYLK, SH3PXD2A, DNAJC3, JAZF1, RPS6KB1, SLC1A2 | |
| miR-301a-5p | PTEN, BTG1, NDRG2 | |
| miR-520d-5p | PPIB | |
| miR-518a-5p | MCL1, PIK3C2A, CCL2 | |
| miR-580-3p | TWIST1 | |
| miR-595 | PARD6A | |
| REC status | miR-184 | AKT2, INPPL1, NFATC2, SOX7, AGO2, MYC, BCL2, EZR, SND1, GAS1, ZFPM2, PDGFB, PLPP3, AKT1, BIN3, PRKCB, PPP1R13L, TNFAIP2, PKM |
| miR-449b-5p | SIRT1, CCNE2, MET, GMNN, HDAC1, CDC25A, CDK6, MYCN, NEAT1 | |
| miR-542-3p | BIRC5, ILK, MTDH, PIM1, AKT1, BMP7, RPS23, ANGPT2, OTUB1, IGFBP1, CTTN, PIK3R1, FZD7 | |
| miR-593-3p | CDC274, PROP1 | |
| miR-595 | PARD6A |
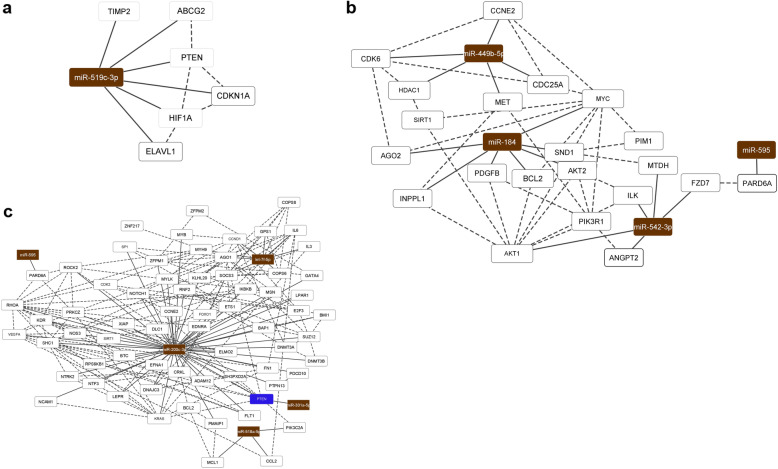
Protein-protein interactions (PPI) were evaluated considering the selected validated miRNA target genes for each clinical group and used to construct a miRNA/mRNA network (Fig. 5). For the tumor size, one network was generated based on the six target genes of miR-519c-3p (Table 3, Fig. 5a); considering their involvement in the biological process (GO), four of these targets were reported as related to negative regulation of growth (PTEN, CDKN1A, HIF1A), of the mitotic cell cycle (PTEN, CDKN1A, TIMP2), and vascular smooth muscle cell proliferation (PTEN, CDKN1A).
Fig. 5.
MiRNA/mRNA networks of the differentially expressed miRNAs observed in the tumor size (a), LN (b) and REC (c) clinical groups and their corresponding experimentally validated target genes (Cytoscape v. 3.8.0). Brown rectangle: miRNAs, white rectangles: genes targeted by one miRNA, blue rectangles: genes targeted by two miRNAs. True lines: protein-protein interaction (STRING database), doted lines: miRNA-target gene interaction
PPI analysis for the LN group was generated considering the 113 experimentally validated target genes of seven miRNAs (Table 3). Considering PPI with the highest confidence (interaction score ≥ 0.9000) and removing nodes without connections, a network was generated with 68 target genes of five miRNAs (Fig. 5b).
For the REC comparison, 41 experimentally validated target genes of five miRNAs (Table 3) were used for the PPI analysis. The miRNA/mRNA network was constructed considering the same settings as the LN network (interaction score ≥ 0.900, nodes without connection were hidden), resulting in a network with 21 genes and four miRNAs (Fig. 5c).
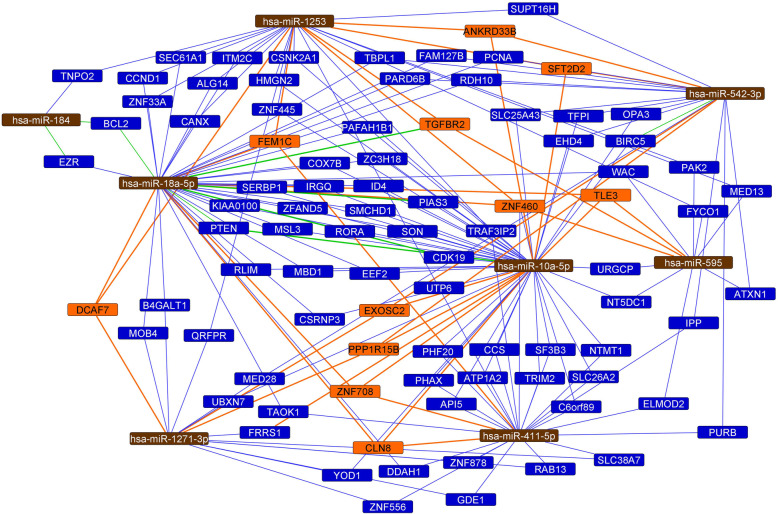
Finally, considering the eight miRNAs common to LN/REC, LN and REC comparisons and the miRTarbase data (Fig. 6), a network was generated with 89 genes experimentally validated as a target of the 8 selected miRNAs. Most of them were validated through weak evidence of interaction, however strong evidence of interaction was also reported for miR-10a and PTEN, miR-184 and BCL2 and EZR, miR-18a-5p and EZR, PTEN, TGFBR2, CDK19, PIAS3, and BCL2, and miR-542-3p and BIRC5.
Fig. 6.
MiRNA/mRNA network of the 8 miRNAs commonly observed in the to LN/REC, LN and REC groups’ comparisons (miRTargetLink). Brown rectangle: miRNAs, blue rectangles: genes targeted by two miRNAs, orange rectangle: genes targeted by three or more miRNAs, blue and orange lines: weak evidence, green lines: strong evidence
To determine the interference of all differentially expressed miRNAs obtained in our analysis in the process related to breast tumorigenesis, a network was constructed considering only experimentally validated target genes presented in The Integrated Breast Cancer pathway (Table 4, Figure S2). In this network, several key oncogenes and tumor suppressor genes were found to be targeted by at least one of the differentially expressed miRNAs in our clinical group comparisons, indicating the importance of these miRNAs in breast tumor initiation and progression, affecting cell proliferation, migration and invasion capacity, and response to treatment.
Table 4.
MiRNAs and experimentally validated target genes involved in the Integrated Breast Cancer Pathway (Wikipathways) and their involvement in the clinical groups of this study
| miRNAs | Experimentally validated targets | Clinical groups |
|---|---|---|
| miR-1268a | CDC25B, MAPK1, RAP1A | LN status |
| miR-1268b | CDC25B, MAPK1, RAP1A | LN status |
| miR-130a-3p | ESR1, MYC, PTEN, SMAD4, TGFBR2 | REC status |
| miR-184 | AKT1, BCL2, MYC | REC status |
| miR-18a-5p | ATM, BCL2, CCND1, ESR1, PTEN, RAP1A, SMAD2, SMAD4, TFGBR2, TP53 | REC status |
| miR-18b-5p | ESR1, MDM2, SMAD2 | REC status |
| miR-200c-5p | CDH1, MDM2, PTEN | LN status |
| miR-449-5p | CDC25A, FOSL1, HDAC1, SIRT1, SMAD4, TGFBR2 | REC status |
| miR-452-5p | BMPR2, IRS1, KRAS, SMAD4 | Tumor size |
| miR-519a-3p | PTEN, RB1 | REC status |
| miR-99b-5p | CHEK1, MTOR, SP1 | REC status |
Discussion
In TNBC several miRNAs were observed with deregulated expression presenting with major roles in cancer progression [44, 45]. Most of the studies reporting on the miRNA expression in TNBC do not focus on minority groups or biological disparities based on the race of the patients. In fact, a search on PubMed database using the words “African American” OR “African women” OR “biological disparity” AND microRNA OR microRNAs OR miRNA OR miRNAs resulted in 83 studies related to AA women and microRNAs with only six of them with TNBC patients, which included the previous study of our group [24].
In this study, we report on the global miRNA profiling of genomically ancestral characterized AA patients with TNBC stratified in three clinical groups of patients based on tumor size, LN metastasis and breast cancer recurrence status.
Tumor size is one of the most important prognostic determinants for breast cancer [46]. Larger tumors can be a result of late diagnosis, high proliferation rate, lack of treatment, or poor response to neoadjuvant treatment. In this study, the average tumor size in the non-treated TNBC patients was 5.86 ± 5.17 cm; six of these patients were diagnosed with tumors larger than 5 cm, being categorized as at least Stage IIB (no regional LN metastasis and no clinical or radiological evidence of distant metastasis), which as expected, presents lower 5-year survival rates when compared to patients with smaller tumors [47]. Eight miRNAs were found differentially expressed between the groups of patients based on tumor size, among them, the miR-2117, miR-378c, miR519c-3p and miR-934 presented high power (AUC ≥ 0.8) to discriminate the patients in this group. Among these miRNAs, the expression of miR-2117 was observed in colorectal cancer inversely correlated with the expression levels of the target gene TGFBR1 [48]. This gene is found overexpressed in breast cancer [49] and is involved in the MAPK-signaling pathway [48]. Downregulation of miR-378c was observed in head and neck squamous cell carcinoma (HNSCC) and appeared as one of the most important prognostic variables for HNSCC [50]. However, in our study, we observed lower levels of miR-519c-3p from patients diagnosed with larger tumors (≥5 cm). Increased levels of miR-519c-3p were found to promote tumor growth and proliferation in hepatocellular carcinoma cells, by targeting the BTG3 gene [51]. Increased expression of miR-934 was previously reported on TNBC samples compared to ER+ tumors, however, its expression level was not associated with tumor size [52].
Lymph node (LN) status is still one of the strongest prognostic factors in breast cancer [53]. In this group, 23 miRNAs were found differentially expressed between the LN+ and LN- patients, among them, miR-1253, miR548l and mIR-873-5p, indicating that these miRNAs might be involved in conferring the invasion ability to the tumor cells. A combination of seven of these miRNAs (let-7f-5p, miR-1255b-5p, miR-1268b, miR-200c-3p and miR-520d-5p + miR-527 + miR-518a-5p), presented a more robust discriminatory power (AUC ≥ 0.9). It is important to mention, however, that miR-520d, irrespectively of the LN status, showed no differences in overall survival in the analysis of the Kmplot database. Interestingly, altered expression levels of miR-1253 and miR-548 l were reported to interfere in the migration and invasion capacity of non-small cell lung cancer (NSCLC). Altered expression of miR-548 l and miR-1253 was negatively associated with LN metastasis in NSCLC, by targeting the AKT1 and WNT5A genes, respectively [54, 55]. In our study, both of these miRNAs were observed with lower expression levels in the LN+ group, indicating as in NSCLC, a similar tumor suppressor activity in breast cancer. The long non-coding RNA DCST1-AS1 was reported to act as a “sponge” by binding miR-873-5p, resulting in the upregulation of other target genes such as IGF2BP1, MYC, LEF1, and CDD4 and conferring the increase of cell proliferation and metastasis capacity in TNBC cells [56]. The lower expression levels of this miRNA in the LN+ group of our study could be among other mechanisms, a consequence of high levels of this lncRNA.
In relation to recurrence (REC), 27 miRNAs were observed differentially expressed between REC+ and REC- groups, with 12 presenting an AUC value higher than 0.8; a combined analysis of three of these miRNAs (miR-1200, miR-1249-3p, and miR-1271-3p), presented a robust power (AUC ≥ 0.9) in discriminating the patients based on REC status. Deregulated expression levels of miR-1249-3p in breast cancer cells were associated with interference of lncRNA MIF-AS1 [57]. This lncRNA acts as a sponge resulting in lower expression levels of miR-1249-3p which impedes its interaction with the HOXB8 target gene. In vitro upregulation of miR-1249-3p resulted in suppression of proliferation and migration activity and reversion of the Epithelial-Mesenchymal Transition (EMT) progress, indicating a tumor suppressor role of this miRNA in breast tumors, and corroborating with our findings that showed lower expression levels of miR-1249-3p in the REC+ group. In gliomas and osteosarcomas, it was observed that the downregulation of miR-1200, led to the up-regulation of the HOXB2 gene, which increased proliferation and invasion capacity of the tumor cells [58, 59]. These data indicate a tumor suppressor activity of miR-1200, that as miR-1249-3p, presented lower expression levels in the REC+ group. Finally, for miR-1271, to our knowledge, there is no reported deregulation of its expression in tumor cells.
TNBC patients can show satisfactory response to chemotherapy, especially in the neoadjuvant setting [60–62]. However early recurrence is more frequent in this breast cancer subtype when compared to others, which usually occurs within the first 3 years after diagnosis [5, 63]. Chemotherapy adherence and uptake have also been shown to differ among patients’ racial/ethnic groups [64–67]. In TNBC, a previous study conducted by our group [68], showed however that although a substantial number of TNBC patients failed to receive and/or complete chemotherapy, AA patients presented a higher chemotherapy uptake than White patients. Interestingly, and aware of the limitation of the sample size of this present study, seven patients presented recurrence after treatment while fifteen patients were disease-free. Considering both LN and REC status, 22 miRNAs were found differentially expressed among the LN+/REC+, LN+/REC-, LN−/REC+, and LN−/REC- groups, eight of them also presented in the comparison of LN and REC clinical groups comparisons: miR-10a-5p, miR-1253, miR-1271-3p, miR-184, miR-18a-5p, miR-411-5p, miR-542-3p, and again miR-595. Finally, a query of the KM Plot database of the TCGA and METABRIC TNBC cases, showed that several of the miRNAs observed with deregulated expression in the patients with larger tumor size, positive LN and REC were significantly associated with lower survival rates. It is important to mention, however, that some miRNAs which expression levels were associated with REC of the patients, in the KM Plot showed higher survival rates. Considering that this database, which compiles cases from the TCGA and Metabric breast cancer patients, may not reflect the clinical and biological characteristics of our AA patients, as well as their socio-economic condition. The latter largely impacts health access and ultimately overall survival rates. In addition, the differences in specimen types, miRNA profiling, and other technical variables can largely affect miRNA expression levels.
Interestingly, the involvement of 12 of the miRNAs observed in the TNBC cases of our study and that were differentially expressed in the above clinical groups was among the ones observed with differential expression in our previous study in TNBC cases of AA and non-Hispanic White (NHW) patients [24]. Among these common miRNAs, 12 of them presented the same level of expression of this study, two of which associated with tumor size (miR-2117 and miR-617), eight with LN status (miR-1253, miR-1268a, miR-200c-3p, miR-520d-5p, miR-518a-5p, miR-528, miR-580 and miR-873-5p), and two with REC status (miR-1200 and miR-449b-5p). These associations could indicate that these 12 miRNAs are intrinsically regulated in the TNBC of AA patients, and could account for the observed more aggressive phenotype of their tumors when compared to the NHW patients. This suggestion can be supported by the analysis of the KEGG pathway of the differentially expressed miRNAs observed in each clinical group of this study, which revealed their involvement in signaling pathways often associated with tumor aggressiveness. In the LN group, for example, considering only the panel of seven miRNAs that presented the best capability to discriminate LN+ and LN- cases, among the top ten KEGG pathways observed, three were affected by six of these miRNAs: signaling pathways regulating pluripotency of stem cells, TGF-beta and Hippo signaling pathways. In the REC group, among the top ten KEGG pathways, the thyroid hormone signaling pathway was potentially affected by eight of the 12 miRNAs in the highest discriminatory panel (miR-1200, miR-1271-3p, miR-449b-5p, miR-4536-3p, miR-542-3p, miR-548n, miR-593-3p and miR-595). This signaling pathway can regulate tumor progression and apoptosis process through thyroid hormones (TR) genomic and non-genomic actions [69]. TR expression was associated with higher 5-year survival in metastasized BRCA1 mutated breast cancer [70]. BRCA1-mutation carriers frequently develop TNBC [71], and a disturbance of this signaling pathway may interfere with the survival of TNBC patients. Considering these miRNAs in the above clinical groups, miRNA/mRNA pairings that were experimentally validated showed a number of targets that play relevant roles in breast cancer progression, including genes that are part of the subclassification of the TNBC into the six molecular subtypes [72] among them, genes involved in the epithelial-to-mesenchymal transition (EMT) process, which is essential to confer cell migration and invasion and stem cell tumor capabilities [73, 74], and which miRNAs are important regulators [75, 76]. Of note, one of the most prevalent TNBC subtypes in AA patients, the mesenchymal-like (ML) subtype [77–79], is enriched in genes involved in the regulation of EMT and in the biology of cancer stem cells, both markers that confer clinical aggressiveness [80, 81]. In addition, some of the differentially expressed miRNAs observed in our study are highly involved in breast cancer progression as shown by their predicted interactions with relevant genes of the Integrated Breast Cancer Pathway. Some of these interactions were already experimentally validated, including miR-184 and AKT1K, BCL2 and MYC, and miR-200c, miR-130a-3p, miR-18a-5p, miR-519a-3p and PTEN. These interactions indicate an important role for these miRNAs in the tumorigenesis of TNBC in AA patients, which should be further explored in other independent well-characterized and large AA populations.
Conclusion
Altogether, our data indicate that alterations in miRNA expression are relevant biological factors that can be associated with the poor prognosis in TNBC of AA patients, by conferring to their TNBC cells, increase cell proliferation, elevate expression of angiogenesis markers and/or high migration and invasive cellular capabilities that are reflected in the clinical characteristics evaluated in this study. Therefore, apart from the socioeconomic and cultural factors that play a significant role in the observed disparities in incidence and mortality rates in TNBC of AA women, miRNAs deregulation can also be included as drivers of these disparities.
Supplementary Information
Additional file 1: Table S1. Summary of the clinical and histopathological characteristics of the TNBC-AA patients analyzed in this study.
Additional file 2: Figure S1. Principal Component Analysis (PCA) showing the clustering of the AA patients (black dots) with the Africans, African Americans and African Caribbean groups based on genotype analysis. The horizontal axis represents Principal Component 1 (PC1) and vertical axis Principal Component 2 (PC2).
Additional file 3: Table S2. MiRNAs that are significantly enriched and targets in Integrated Breast Cancer Pathway in each clinical group evaluated. (P < 0.05).
Additional file 4: Table S3. Experimentally validated target genes of 33 selected miRNAs differentially expressed in the three group comparisons. The Integrated Breast Cancer Pathway with miRNAs and experimentally validated target genes. Tumor size related miRs and target genes (pink), LN related miRs and target genes (orange), REC related miRs and target genes (blue), target genes associated with more than one comparison (green). Red lines represent inhibitory interaction, green lines represent stimulatory interaction, and black lines represent miRNA interaction with target.
Additional file 5: Figure S2. The Integrated Breast Cancer Pathway with miRNAs and experimentally validated target genes. Tumor size related miRs and target genes (pink), LN related miRs and target genes (orange), REC related miRs and target genes (blue), target genes associated with more than one comparison (green). Red lines represent inhibitory interaction, green lines represent stimulatory interaction, and black lines represent miRNA interaction with target.
Acknowledgments
We thank the support of the Genomics and Epigenomics Shared Resources (GESR) at Lombardi Comprehensive Cancer Center (LCCC) at Georgetown University, partially supported by the NIH/NCI grant P30-CA051008 for conducting the ancestral genomic analysis of the patients; and the Genomics Shared Resource at The Ohio State University Comprehensive Cancer Center (OSU CCC), supported in part by the OSU CCC and the NIH/NCI grant P30-CA16058, for conducting the miRNA profiling hybridizations.
Abbreviations
- AA
African-American
- TNBC
Triple negative breast cancer
- LN
Lymph node
- REC
Recurrence
- ROC
Receiver Operating Characteristic
- miRNAs
microRNAs
- ER
Estrogen receptor
- PR
Progesterone receptor
- HER2
Human epidermal growth factor receptor-2
- TCGA
The Cancer Genome Atlas
- METABRIC
Molecular Taxonomy of Breast Cancer International Consortium
- PPI
Protein-protein interactions
- GO
Biological process
- HNSCC
Head and neck squamous cell carcinoma
- NSCLC
Non-small cell lung cancer
- EMT
Epithelial-Mesenchymal Transition
- NHW
Non-Hispanic White
- ML
Mesenchymal-like
Authors’ contributions
All authors made substantial contributions to one or more of the following: conceptualization and study design: LRC, YK; Data curation: ST, BMS, RLCJ, MC; formal Analysis: ST, BMS; funding acquisition: YK; methodology: ST, BMS, PF, RM, RLCJ, MC; resources: AA, TN, VA; supervision: LRC, YK; writing-original draft: ST, BMS; writing – review and editing: LRC, YK. All authors read and approved the final manuscript.
Funding
This work was supported in part by the Charles & Mary Latham Fund (Ella O. Latham Trust). The Genomics and Epigenomics Shared Resources (GESR) at Lombardi Comprehensive Cancer Center (LCCC) at Georgetown University was partially supported by the NIH/NCI grant P30-CA051008. The Ohio State University Comprehensive Cancer Center (OSU CCC) was partially supported by the NIH/NCI grant P30-CA16058.
Availability of data and materials
The datasets generated and analyzed during the current study are available in the “Data repository for Manuscript: A Panel of miRNAs as Prognostic Markers for African-American Patients with Triple Negative Breast Cancer” (https://app.box.com/s/9h5wfr95ixqjrcjvjm26ul5lg73w8kbv).
Declarations
Ethics approval and consent to participate
All procedures and experimental protocols were approved by the Office of Regulatory Research Compliance – Institutional Review Board of Howard University (IRB-16-MED-39) and in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All the patients of this study signed informed consent for tissue procurement for research.
Consent for publication
Not applicable.
Competing interests
The authors declare that all experiments comply with the current laws of the United States of America Conflict of Interest. The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Safaa Turkistani and Bruna M. Sugita contributed equally to this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Kim M, Suh DH, Lee KH, Eom KY, Lee JY, Lee YY, et al. Major clinical research advances in gynecologic cancer in 2019. J Gynecol Oncol. 2020;31(2):e48. doi: 10.3802/jgo.2019.30.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heimes AS, Schmidt M. Atezolizumab for the treatment of triple-negative breast cancer. Expert Opin Investig Drugs. 2019;28(1):1–5. doi: 10.1080/13543784.2019.1552255. [DOI] [PubMed] [Google Scholar]
- 4.Marra A, Viale G, Curigliano G. Recent advances in triple negative breast cancer: the immunotherapy era. BMC Med. 2019;17(1):90. doi: 10.1186/s12916-019-1326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anders CK, Abramson V, Tan T, Dent R. The evolution of triple-negative breast Cancer: from biology to novel therapeutics. Am Soc Clin Oncol Educ B. 2016;36(36):34–42. doi: 10.1200/EDBK_159135. [DOI] [PubMed] [Google Scholar]
- 6.Hashemi A, Gorji-bahri G. MicroRNA: promising roles in Cancer therapy. Curr Pharm Biotechnol. 2020;21(12):1186–1203. doi: 10.2174/1389201021666200420101613. [DOI] [PubMed] [Google Scholar]
- 7.To KKW. Fong W, Tong CWS, Wu M, Yan W, Cho WCS. Advances in the discovery of microRNA-based anticancer therapeutics: latest tools and developments. Expert Opin Drug Discov. 2020;15(1):63–83. doi: 10.1080/17460441.2020.1690449. [DOI] [PubMed] [Google Scholar]
- 8.Ramchandani D, Lee SK, Yomtoubian S, Han MS, Tung CH, Mittal V. Nanoparticle delivery of miR-708 mimetic impairs breast cancer metastasis. Mol Cancer Ther. 2019;18(3):579–591. doi: 10.1158/1535-7163.MCT-18-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin H, Xiong G, Guo S, Xu C, Xu R, Guo P, Shu D. Delivery of anti-miRNA for triple-negative breast Cancer therapy using RNA nanoparticles targeting stem cell marker CD133. Mol Ther. 2019;27(7):1252–1261. doi: 10.1016/j.ymthe.2019.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahir M, Upadhyay P, Ghosh A, Sarker S, Bhattacharya S, Gupta P, Ghosh S, Chattopadhyay S, Adhikary A. Delivery of dual miRNA through CD44-targeted mesoporous silica nanoparticles for enhanced and effective triple-negative breast cancer therapy. Biomater Sci. 2020;8(10):2939–2954. doi: 10.1039/d0bm00015a. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne) 2018;9:1–12. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svoronos AA, Engelman DM, Slack FJ. OncomiR or tumor suppressor? The duplicity of MicroRNAs in Cancer. Cancer Res. 2016;76(13):3666–3670. doi: 10.1158/0008-5472.CAN-16-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng Y, Croce CM. The role of microRNAs in human cancer. Signal Transduct Target Ther. 2016;1(1):15004. doi: 10.1038/sigtrans.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Roosbroeck K, Calin GA. Cancer hallmarks and MicroRNAs: the therapeutic connection. Adv Cancer Res. 2017;135:119–149. doi: 10.1016/bs.acr.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Teoh SL, Das S. The role of MicroRNAs in diagnosis, prognosis, metastasis and resistant cases in breast Cancer. Curr Pharm Des. 2017;23(12):1845–1859. doi: 10.2174/1381612822666161027120043. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Yao F, Xiao Z, Sun Y, Ma L. MicroRNAs and metastasis: small RNAs play big roles. Cancer Metastasis Rev. 2018;37(1):5–15. doi: 10.1007/s10555-017-9712-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vannini I, Fanini F, Fabbri M. Emerging roles of microRNAs in cancer. Curr Opin Genet Dev. 2018;48:128–133. doi: 10.1016/j.gde.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piasecka D, Braun M, Kordek R, Sadej R, Romanska H. MicroRNAs in regulation of triple-negative breast cancer progression. J Cancer Res Clin Oncol. 2018;144(8):1401–1411. doi: 10.1007/s00432-018-2689-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao S, Graham K, Shen J, Campbell LES, Singh P, Zirpoli G, Roberts M, Ciupak G, Davis W, Hwang H, Khoury T, Bovbjerg DH, Jandorf L, Pawlish KS, Bandera EV, Liu S, Ambrosone CB, Zhao H. Genetic variants in microRNAs and breast cancer risk in African American and European American women. Breast Cancer Res Treat. 2013;141(3):447–459. doi: 10.1007/s10549-013-2698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans-Knowell A, LaRue AC, Findlay VJ. MicroRNAs and their impact on breast Cancer, the tumor microenvironment, and disparities. Adv Cancer Res. 2017;133:51–76. doi: 10.1016/bs.acr.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bensen JT, Graff M, Young KL, Sethupathy P, Parker J, Pecot CV, Currin K, Haddad SA, Ruiz-Narváez EA, Haiman CA, Hong CC, Sucheston-Campbell LE, Zhu Q, Liu S, Yao S, Bandera EV, Rosenberg L, Lunetta KL, Ambrosone CB, Palmer JR, Troester MA, Olshan AF. A survey of microRNA single nucleotide polymorphisms identifies novel breast cancer susceptibility loci in a case-control, population-based study of African-American women. Breast Cancer Res. 2018;20(1):45. doi: 10.1186/s13058-018-0964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Telonis AG, Rigoutsos I. Race disparities in the contribution of miRNA isoforms and tRNA-derived fragments to triple-negative breast cancer. Cancer Res. 2018;78(5):1140–1154. doi: 10.1158/0008-5472.CAN-17-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong Z, Wang J, Wang D, Buas MF, Ren X, Freudenheim JL, Belinsky SA, Liu S, Ambrosone CB, Higgins MJ. Differences in microRNA expression in breast cancer between women of African and European ancestry. Carcinogenesis. 2019;40(1):61–69. doi: 10.1093/carcin/bgy134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugita B, Gill M, Mahajan A, Duttargi A, Kirolikar S, Almeida R, et al. Differentially expressed miRNAs in triple negative breast cancer between African-American and non-Hispanic white women. Oncotarget. 2016;7(48):79274–79291. doi: 10.18632/oncotarget.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugita BM, Pereira SR, de Almeida RC, Gill M, Mahajan A, Duttargi A, et al. Integrated copy number and miRNA expression analysis in triple negative breast cancer of Latin American patients. Oncotarget. 2019;10(58):6184–6203. doi: 10.18632/oncotarget.27250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, et al. American Joint Committee on Cancer (AJCC). AJCC Cancer Staging Manual. 8. New York: Springer; 2017. [Google Scholar]
- 27.Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FCG, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28(16):2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF, American Society of Clinical Oncology. College of American Pathologists Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;31(31):3997–4013. doi: 10.5858/arpa.2013-0953-SA. [DOI] [Google Scholar]
- 29.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rstudio Team . RStudio: integrated development for R. Boston: RStudio, Inc.; 2019. [Google Scholar]
- 31.Zakharia F, Basu A, Absher D, Assimes TL, Go AS, Hlatky MA, Iribarren C, Knowles JW, Li J, Narasimhan B, Sidney S, Southwick A, Myers RM, Quertermous T, Risch N, Tang H. Characterizing the admixed African ancestry of African Americans. Genome Biol. 2099;10(12):R141. doi: 10.1186/gb-2009-10-12-r141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen DC, Libiger O, Tindall EA, Hardie RA, Hannick LI, Glashoff RH, Mukerji M, Indian Genome Variation Consortium. Fernandez P, Haacke W, Schork NJ, Hayes VM. Complex patterns of genomic admixture within southern Africa. PLoS Genet. 2013;9(3):e1003309. doi: 10.1371/journal.pgen.1003309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagy A, Lánczky A, Menyhárt O, Győrffy B. Validation of miRNA prognostic power in hepatocellular carcinoma using expression data of independent datasets. Sci Rep. 2018;8(1):9227. doi: 10.1038/s41598-018-27521-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vlachos IS, Zagganas K, Paraskevopoulou MD, Georgakilas G, Karagkouni D, Vergoulis T, Dalamagas T, Hatzigeorgiou AG. DIANA-miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res. 2015;43(W1):W460–W466. doi: 10.1093/nar/gkv403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kehl T, Kern F, Backes C, Fehlmann T, Stöckel D, Meese E, et al. miRPathDB 2.0: a novel release of the miRNA Pathway Dictionary Database. Nucleic Acids Res. 2020;48(D1):D142–D147. doi: 10.1093/nar/gkz1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slenter DN, Kutmon M, Hanspers K, Riutta A, Windsor J, Nunes N, et al. WikiPathways: a multifaceted pathway database bridging metabolomics to other omics research. Nucleic Acids Res. 2018;46(D1):D661–D667. doi: 10.1093/nar/gkx1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang H, Wu X, Ibrahim S, McKenzie M, Chen JY. Predicting drug efficacy based on the integrated breast Cancer pathway model. IEEE. 2011:42–5. 10.1109/GENSiPS.2011.6169437.
- 38.Kahraman M, Röske A, Laufer T, Fehlmann T, Backes C, Kern F, Kohlhaas J, Schrörs H, Saiz A, Zabler C, Ludwig N, Fasching PA, Strick R, Rübner M, Beckmann MW, Meese E, Keller A, Schrauder MG. MicroRNA in diagnosis and therapy monitoring of early-stage triple-negative breast cancer. Sci Rep. 2018;8(1):11584. doi: 10.1038/s41598-018-29917-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chou CH, Shrestha S, Yang CD, Chang NW, Lin YL, Liao KW, Huang WC, Sun TH, Tu SJ, Lee WH, Chiew MY, Tai CS, Wei TY, Tsai TR, Huang HT, Wang CY, Wu HY, Ho SY, Chen PR, Chuang CH, Hsieh PJ, Wu YS, Chen WL, Li MJ, Wu YC, Huang XY, Ng FL, Buddhakosai W, Huang PC, Lan KC, Huang CY, Weng SL, Cheng YN, Liang C, Hsu WL, Huang HD. MiRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018;46(D1):D296–D302. doi: 10.1093/nar/gkx1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fan Y, Xia J. miRNet—functional analysis and visual exploration of miRNA–target interactions in a network context. Methods Mol Biol. 2018;1819:215–233. doi: 10.1007/978-1-4939-8618-7_10. [DOI] [PubMed] [Google Scholar]
- 41.Hamberg M, Backes C, Fehlmann T, Hart M, Meder B, Meese E, Keller A. miRTargetLink-miRNAs, Genes and Interaction Networks. Int J Mol Sci. 2016; 17(4). doi: 10.3390/ijms17040564. [DOI] [PMC free article] [PubMed]
- 42.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering C. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan C, Liu N. Identification of dysregulated microRNAs associated with diagnosis and prognosis in triple-negative breast cancer: an in silico study. Oncol Rep. 2019;41(6):3313–3324. doi: 10.3892/or.2019.7094. [DOI] [PubMed] [Google Scholar]
- 45.Tang Q, Ouyang H, He D, Yu C, Tang G. MicroRNA-based potential diagnostic, prognostic and therapeutic applications in triple-negative breast cancer. Artif Cells Nanomed Biotechnol. 2019;47(1):2800–2809. doi: 10.1080/21691401.2019.1638791. [DOI] [PubMed] [Google Scholar]
- 46.Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63(1):181–187. doi: 10.1002/1097-0142(19890101)63:1<181::AID-NCR2820630129>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 47.Elkin EB, Hudis C, Begg CB, Schrag D. The effect of changes in tumor size on breast carcinoma survival in the U.S.: 1975-1999. Cancer. 2005;104(6):1149–1157. doi: 10.1002/cncr.21285. [DOI] [PubMed] [Google Scholar]
- 48.Slattery ML, Mullany LE, Sakoda LC, Wolff RK, Samowitz WS, Herrick JS. The MAPK-signaling pathway in colorectal Cancer: dysregulated genes and their association with MicroRNAs. Cancer Inform. 2018;26:17. doi: 10.1177/1176935118766522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pasche B, Pennison MJ, Jimenez H, Wang M. TGFBR1 and Cancer susceptibility. Trans Am Clin Climatol Assoc. 2014;125:300–312. [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez YON, Victoria B, Golusinski P, Golusinski W, Masternak MM. Characteristic miRNA expression signature and random forest survival analysis identify potential cancer-driving miRNAs in a broad range of head and neck squamous cell carcinoma subtypes. Rep Pract Oncol Radiother. 2018;23(1):6–20. doi: 10.1016/j.rpor.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L, Mo H, Jiang Y, Wang Y, Sun L, Yao B, Chen T, Liu R, Li Q, Liu Q, Yin G. MicroRNA-519c-3p promotes tumor growth and metastasis of hepatocellular carcinoma by targeting BTG3. Biomed Pharmacother. 2019;118:109267. doi: 10.1016/j.biopha.2019.109267. [DOI] [PubMed] [Google Scholar]
- 52.Castilla MA, López-García MA, Atienza MR, Rosa-Rosa JM, Díaz-Martín J, Pecero ML, Vieites B, Romero-Pérez L, Benítez J, Calcabrini A, Palacios J. VGLL1 expression is associated with a triple-negative basal-like phenotype in breast cancer. Endocr Relat Cancer. 2014;21(4):587–599. doi: 10.1530/ERC-13-0485. [DOI] [PubMed] [Google Scholar]
- 53.Zahoor S, Haji A, Battoo A, Qurieshi M, Mir W, Shah M, et al. Sentinel lymph node biopsy in breast cancer: a clinical review and update. J Breast Cancer. 2017;20(3):217–227. doi: 10.4048/jbc.2017.20.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu C, Yang H, Xu Z, Li D, Zhou M, Xiao K, Shi Z, Zhu L, Yang L, Zhou R. microRNA-548l is involved in the migration and invasion of non-small cell lung cancer by targeting the AKT1 signaling pathway. J Cancer Res Clin Oncol. 2015;141(3):431–441. doi: 10.1007/s00432-014-1836-7. [DOI] [PubMed] [Google Scholar]
- 55.Liu M, Zhang Y, Zhang J, Cai H, Zhang C, Yang Z, et al. MicroRNA-1253 suppresses cell proliferation and invasion of non-small-cell lung carcinoma by targeting WNT5A. Cell Death Dis. 2018;9(2):189. doi: 10.1038/s41419-017-0218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang L, Chen Y, Tang X, Wei D, Xu X, Yan F. Long noncoding RNA DCST1-AS1 promotes cell proliferation and metastasis in triple-negative breast Cancer by forming a positive regulatory loop with miR-873-5p and MYC. J Cancer. 2020;11(2):311–323. doi: 10.7150/jca.33982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ding J, Wu W, Yang J, Wu M. Long non-coding RNA MIF-AS1 promotes breast cancer cell proliferation, migration and EMT process through regulating miR-1249-3p/HOXB8 axis. Pathol Res Pract. 2019;215(7):152376. doi: 10.1016/j.prp.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 58.Pan B, Zhao M, Wang N, Xu L, Wu T, Li Z. LncRNA RGMB-AS1 promotes glioma growth and invasion through miR-1200/HOXB2 Axis. Onco Targets Ther. 2019;12:10107–10114. doi: 10.2147/OTT.S230098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li S, Pei Y, Wang W, Liu F, Zheng K, Zhang X. Circular RNA 0001785 regulates the pathogenesis of osteosarcoma as a ceRNA by sponging miR-1200 to upregulate HOXB2. Cell Cycle. 2019;18(11):1281–1291. doi: 10.1080/15384101.2019.1618127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loibl S. Neoadjuvant treatment of breast cancer: maximizing pathologic complete response rates to improve prognosis. Curr Opin Obstet Gynecol. 2015;27(1):85–91. doi: 10.1097/GCO.0000000000000147. [DOI] [PubMed] [Google Scholar]
- 61.Harbeck N, Gluz O. Neoadjuvant therapy for triple negative and HER2-positive early breast cancer. Breast. 2017;34:S99–S103. doi: 10.1016/j.breast.2017.06.038. [DOI] [PubMed] [Google Scholar]
- 62.Lebert JM, Lester R, Powell E, Seal M, McCarthy J. Advances in the systemic treatment of triple-negative breast cancer. Curr Oncol. 2018;25(11):S142–S150. doi: 10.3747/co.25.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13(8):2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 64.Sheppard VB, Isaacs C, Luta G, Willey SC, Boisvert M, Harper FWK, Smith K, Horton S, Liu MC, Jennings Y, Hirpa F, Snead F, Mandelblatt JS. Narrowing racial gaps in breast cancer chemotherapy initiation: the role of the patient-provider relationship. Breast Cancer Res Treat. 2013;139(1):207–216. doi: 10.1007/s10549-013-2520-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Killelea BK, Yang VQ, Wang SY, Hayse B, Mougalian S, Horowitz NR, Chagpar AB, Pusztai L, Lannin DR. Racial differences in the use and outcome of neoadjuvant chemotherapy for breast cancer: results from the National Cancer Data Base. J Clin Oncol. 2015;33(36):4267–4276. doi: 10.1200/JCO.2015.63.7801. [DOI] [PubMed] [Google Scholar]
- 66.Yee MK, Sereika SM, Bender CM, Brufsky AM, Connolly MC, Rosenzweig MQ. Symptom incidence, distress, cancer-related distress, and adherence to chemotherapy among African American women with breast cancer. Cancer. 2017;123(11):2061–2069. doi: 10.1002/cncr.30575. [DOI] [PubMed] [Google Scholar]
- 67.Gallups SF, Connolly MC, Bender CM, Rosenzweig MQ. Predictors of adherence and treatment delays among African American women recommended to receive breast Cancer chemotherapy. Womens Health Issues. 2018;28(6):553–558. doi: 10.1016/j.whi.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 68.Sheppard VB, Cavalli LR, Dash C, Kanaan YM, Dilawari AA, Horton S, Makambi KH. Correlates of triple negative breast Cancer and chemotherapy patterns in black and white women with breast Cancer. Clin Breast Cancer. 2017;17(3):232–238. doi: 10.1016/j.clbc.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu YC, Yeh CT, Lin KH. Molecular functions of thyroid hormone signaling in regulation of Cancer progression and anti-apoptosis. Int J Mol Sci. 2019;20(20):4986. doi: 10.3390/ijms20204986. [DOI] [Google Scholar]
- 70.Heublein S, Mayr D, Meindl A, Angele M, Gallwas J, Jeschke U, Ditsch N. Thyroid hormone receptors predict prognosis in BRCA1 associated breast Cancer in opposing ways. PLoS One. 2015;10(6):e0127072. doi: 10.1371/journal.pone.0127072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Talhouet S, Peron J, Vuilleumier A, Friedlaender A, Viassolo V, Ayme A, et al. Clinical outcome of breast cancer in carriers of BRCA1 and BRCA2 mutations according to molecular subtypes. Sci Rep. 2020;10(1):7073. doi: 10.1038/s41598-020-63759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lim J, Thiery JP. Epithelial-mesenchymal transitions: insights from development. Development. 2012;139(19):3471–3486. doi: 10.1242/dev.071209. [DOI] [PubMed] [Google Scholar]
- 74.Craene BD, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer. 2013;13(2):97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 75.Tang J, Li Y, Wang J, Wen Z, Lai M, Zhang H. Molecular mechanisms of microRNAs in regulating epithelial-mesenchymal transitions in human cancers. Cancer Lett. 2016;371(2):301–313. doi: 10.1016/j.canlet.2015.11.043. [DOI] [PubMed] [Google Scholar]
- 76.Zhao X, Lu Y, Nie Y, Fan D. MicroRNAs as critical regulators involved in regulating epithelial- mesenchymal transition. Curr Cancer Drug Targets. 2014;13(9):935–944. doi: 10.2174/15680096113136660099. [DOI] [PubMed] [Google Scholar]
- 77.Field LA, Love B, Deyarmin B, Hooke JA, Shriver CD, Ellsworth RE. Identification of differentially expressed genes in breast tumors from African American compared with Caucasian women. Cancer. 2012;118(5):1334–1344. doi: 10.1002/cncr.26405. [DOI] [PubMed] [Google Scholar]
- 78.Lindner R, Sullivan C, Offor O, Lezon-Geyda K, Halligan K, Fischbach N, Shah M, Bossuyt V, Schulz V, Tuck DP, Harris LN. Molecular phenotypes in triple negative breast cancer from African American patients suggest targets for therapy. PLoS One. 2013;8(11):e71915. doi: 10.1371/journal.pone.0071915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stewart PA, Luks J, Roycik MD, Sang QXA, Zhang J. Differentially expressed transcripts and dysregulated signaling pathways and networks in African American breast cancer. PLoS One. 2013;8(12):e82460. doi: 10.1371/journal.pone.0082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang SS, Jiang J, Liang XH, Tang YL. Links between cancer stem cells and epithelial– mesenchymal transition. Onco Targets Ther. 2015;8:2973–2980. doi: 10.2147/OTT.S91863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu X, Fan D. The Epithelial-Mesenchymal Transition and Cancer Stem Cells: Functional and Mechanistic Links. Curr Pharm Des. 2015;21(10):1279–1291. doi: 10.2174/1381612821666141211115611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Summary of the clinical and histopathological characteristics of the TNBC-AA patients analyzed in this study.
Additional file 2: Figure S1. Principal Component Analysis (PCA) showing the clustering of the AA patients (black dots) with the Africans, African Americans and African Caribbean groups based on genotype analysis. The horizontal axis represents Principal Component 1 (PC1) and vertical axis Principal Component 2 (PC2).
Additional file 3: Table S2. MiRNAs that are significantly enriched and targets in Integrated Breast Cancer Pathway in each clinical group evaluated. (P < 0.05).
Additional file 4: Table S3. Experimentally validated target genes of 33 selected miRNAs differentially expressed in the three group comparisons. The Integrated Breast Cancer Pathway with miRNAs and experimentally validated target genes. Tumor size related miRs and target genes (pink), LN related miRs and target genes (orange), REC related miRs and target genes (blue), target genes associated with more than one comparison (green). Red lines represent inhibitory interaction, green lines represent stimulatory interaction, and black lines represent miRNA interaction with target.
Additional file 5: Figure S2. The Integrated Breast Cancer Pathway with miRNAs and experimentally validated target genes. Tumor size related miRs and target genes (pink), LN related miRs and target genes (orange), REC related miRs and target genes (blue), target genes associated with more than one comparison (green). Red lines represent inhibitory interaction, green lines represent stimulatory interaction, and black lines represent miRNA interaction with target.
Data Availability Statement
The datasets generated and analyzed during the current study are available in the “Data repository for Manuscript: A Panel of miRNAs as Prognostic Markers for African-American Patients with Triple Negative Breast Cancer” (https://app.box.com/s/9h5wfr95ixqjrcjvjm26ul5lg73w8kbv).