Abstract
Although the conjunctival fornix appears to contain the greatest proportion of stem cells, it is likely that pockets of conjunctival epithelial stem cells may also exist throughout the conjunctival epithelium. This study was to investigate the potential localization of putative stem/progenitor cells in the human bulbar conjunctival epithelium by evaluating 6 keratins and 13 molecules that have been previously proposed stem cell associated or differentiation markers. We found that cornea specific cytokeratin (CK) 3 was not expressed by the bulbar conjunctival epithelial cells. In contrast, CK4 and CK7 were expressed by the superficial cells of bulbar conjunctival epithelium. CK14 and CK15 were confined to the basal cell layer. CK19 was strongly expressed by all layers of the bulbar conjunctival epithelium. The expression patterns of molecular markers in the basal cells of human bulbar conjunctival epithelium were found to be similar to the corneal epithelium. Basal conjunctival epithelial cells strongly expressed stem cell associated markers, including ABCG2, p63, nerve growth factor (NGF) with its receptors tyrosine kinase receptor A (TrkA) and neurotrophin low-affinity receptor p75NTR, glial cell-derived neurotrophic factor (GDNF) with its receptor GDNF family receptor alpha 1 (GFRα−1), integrin β1, α-enolase, and epidermal growth factor receptor (EGFR). The differentiation associated markers nestin, E-cadherin and involucrin were not expressed by these cells. These findings indicate that the basal cells of bulbar conjunctival epithelium shares a similar expression pattern of stem cell associated markers to the corneal epithelium, but has a unique pattern of differentiation associated cytokeratin expression.
The nonkeratinized conjunctival epithelium extends from the corneal limbus to the lid margin, where there is a gradual transition to keratinized, stratified squamous epithelium. The conjunctival epithelium is important in providing a mechanical and immunological barrier to injury and infection, as well as in contributing to the production and stability of the tear film, with its numerous mucin-secreting goblet cells. Despite its importance in maintaining the homeostasis of the ocular surface, conjunctival stem cell biology has been less investigated than that of corneal stem cells (Ang et al., 2004).
An important advance is that corneal and conjunctival epithelia are now believed to belong to two separate distinct cell lineages that arise from different stem cell populations (Wei et al., 1996). The fornical conjunctiva has been shown to be the site that is enriched in conjunctival epithelial stem cells (SC) in rabbit and mice models. The fornical epithelium in mice eyes possessed a significantly higher percentage of slow cycling label-retaining cells, a well-established feature of stem cells, as compared to the bulbar and palpebral conjunctiva (Wei et al., 1995). Cells from the fornical conjunctiva were shown to have greater growth potential in culture, as demonstrated by a higher colony forming efficiency and greater number of serial subcultures, than cells from the bulbar and palpebral regions (Wei et al., 1993).
Although the conjunctival fornix appears to contain the greatest proportion of stem cells, it is likely that pockets of conjunctival epithelial stem cells may also exist throughout the conjunctival epithelium. This could explain the observation by other investigators who analyzed the in vitro proliferative capacities of the different conjunctival regions, and concluded that stem cells may be uniformly distributed over the bulbar and fornical conjunctiva (Pellegrini et al., 1999). The higher density of goblet cells in the fornix, as compared to the other regions of the conjunctiva, may be due to the concentrated bipotent conjunctival stem cells there (Huang et al., 1988; Wei et al., 1995). The stem cells scattered in the bulbar and palpebral conjunctiva may give rise to the dispersed pockets of goblet cells seen within these regions.
Although no definitive markers for ocular surface epithelial stem cells have been identified to date, our group has characterized three patterns of molecular markers expressed by human limbal basal epithelial cells (Chen et al., 2004; Qi et al., 2007). This study was to attempt to investigate the potential localization of putative stem/progenitor cells in the human bulbar conjunctival epithelium by evaluating 19 molecules that have been previously proposed stem cell associated or differentiation markers. These tentative findings may promote future functional studies to understand conjunctival SC phenotype.
Materials and Methods
Materials and reagents
Optimal cutting temperature (OCT) compound and cryomolds were purchased from Sakura Finetek (Torrance, CA; http://www.sakura-americas.com). Mouse monoclonal antibodies (mAbs) against CK4 (6B10), CK7 (K72.7), CK15 (LHK15), integrin β1(4B7R), p63 (4A4), epidermal growth factor receptor (EGFR, 528), involucrin (SY5), rabbit polyclonal antibody against glial cell-derived neurotrophic factor (GDNF) were purchased from Lab Vision (Fremont, CA; http://www.labvision.com). Anti-CK3 (AE5), anti-nestin (10C2), and anti-p75NTR (neurotrophin common low-affinity receptor, ME20.4) mAbs were from Chemicon International (Temecula, CA; http://www.chemicon.com). CK19 mAb (RCK108) was from DAKO (Carpinteria, CA; http://www.dakocytomation.com). CK14 mAb (RCK107) was from ICN Biomedicals, Inc. (Aurora, OH; http://www.icnbiomed.com). Human ABCG2 mAb (BXP-21) was from Calbiochem (San Diego, CA; http://www.calbiochem.com). Rabbit polyclonal antibodies against nerve growth factor (NGF, M-20), tyrosine kinase receptors A (TrkA, 763), GDNF family receptor alpha 1 (GFRα−1, H-70), goat polyclonal antibody against α-enolase (C-19) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA; http://www.scbt.com). Human E-cadherin mAb (4A2C7) was from Zymed Laboratories (Carlsbad, CA; http://www.invitrogen.com). Fluorescein Alexa-Fluor 488 conjugated secondary antibodies (goat anti-mouse or anti-rabbit immunoglobulin [IgG], donkey anti-goat IgG) were from Molecular Probes (Eugene, OR; http://www.probes.com). Propidium iodide (PI) was purchased from Sigma-Aldrich (St. Louis, MO; http://www.sigmaaldrich.com).
Preparation of human corneoscleral tissues with bulbar conjunctival tissue
Four fresh normal human corneoscleral tissues with bulbar conjunctiva from donors (two men and two women) aged 9, 22, 47, and 67 years, preserved less than 8 h after death, were obtained from the National Disease Research Interchange (NDRI, Philadelphia, PA) for this study. The corneoscleral specimens were prepared using a previously described method (Chen et al., 2004) by cutting the tissues in the horizontal direction across the superior peripheral cornea and limbus. The tissue specimens were embedded in a mixture of 75% (v) OCT compound and 25% (v) Immu-Mount (Thermo-Shandon, Pittsburgh, PA), frozen in liquid nitrogen, and cut for frozen sections (10 μm thick) for hematoxylin–eosin staining and immunostaining.
Immunofluorescent staining and laser scanning confocal microscopy (LSCM)
Immunofluorescent staining was performed by a previously reported method (Chen et al., 2004; Qi et al., 2007) to evaluate the expression and location of different molecular markers that have been proposed to identify stem/progenitor cell or differentiated cells in human bulbar conjunctiva of corneoscleral frozen sections. Nineteen antigens were stained at the same time for each of the four specimens. In brief, human corneoscleral frozen sections were thawed, dehydrated, and fixed in cold methanol/acetone (1:1) at −30°C for 3 min (for cytoplasmic and nuclear protein staining) or 2% paraformaldehyde (for all membrane protein staining) at 4°C for 10 min. Sections were blocked with 20% normal goat or donkey serum in phosphate-buffered saline (PBS) for 1 h to reduce nonspecific antibody interaction. Primary antibodies against human CK3 (1:400, 2.5 μg/ml), CK4 (1:20), CK7 (1:100, 2 μg/ml), CK19 (1:200, 0.2 μg/ml), CK14 (1:50), CK15 (1:20, 10 μg/ml), ABCG2 (1:50, 5 μg/ml), p63 (1:1,000, 1 μg/ml), NGF (1:200, 1 μg/ml), TrkA (1:200, 1 μg/ml), p75NTR (1:100, 5 μg/ml), GDNF (1:100, 2 μg/ml), GFRα−1 (1:50, 4 μg/ml), integrin β1(1:200, 1 μg/ml), α-enolase (1:50, 4 μg/ml), EGFR (1:20, 10 μg/ml), nestin (1:100, 10 μg/ml), E-cadherin (1:200, 2.5 μg/ml), or involucrin (1:40, 5 μg/ml) were applied and incubated for 2 h at room temperature. Secondary antibodies, Alexa-Fluor 488 conjugated goat anti-rabbit or anti-mouse IgG, or donkey anti-goat IgG (1:300) were then applied and incubated in a dark chamber for 1 h, followed by counterstaining with PI (1:200) for 5 min. After washing with PBS, antifade Gel/Mount and a coverslip were applied. Sections without primary antibodies applied were used as negative controls. The staining was evaluated under an epifluorescent microscope (Eclipse E400; Nikon, Garden City, NY) and photographed with a digital camera (model DMX 1200; Nikon) or with a laser scanning confocal microscope (LSM 510; Carl Zeiss, Thornwood, NY) with excitation at 488 and 543 nm, and using emission filters LP505 and LP560, respectively. Photographs were taken of representative areas of the limbus and bulbar conjunctiva in the limbus–bulbar conjunctival transition zone. The staining intensity was assessed by a masked independent author. All staining was verified on all four independent tissues.
Results
The differential pattern of cytokeratins expressed by the bulbar conjunctival epithelium and the limbal epithelium
Figure 1 indicates the demarcation of the limbus and bulbar conjunctiva in the limbus–bulbar conjunctival transition zone utilized in this study. The horizontal cross-section cut through the superior corneoscleral tissue showed the papilla-like limbal epithelial columns with about 8–10 epithelial layers (Limbus) and the bulbar conjunctival epithelium consisting of six to nine epithelial layers (Bulbar Conjunctiva).
Fig. 1.
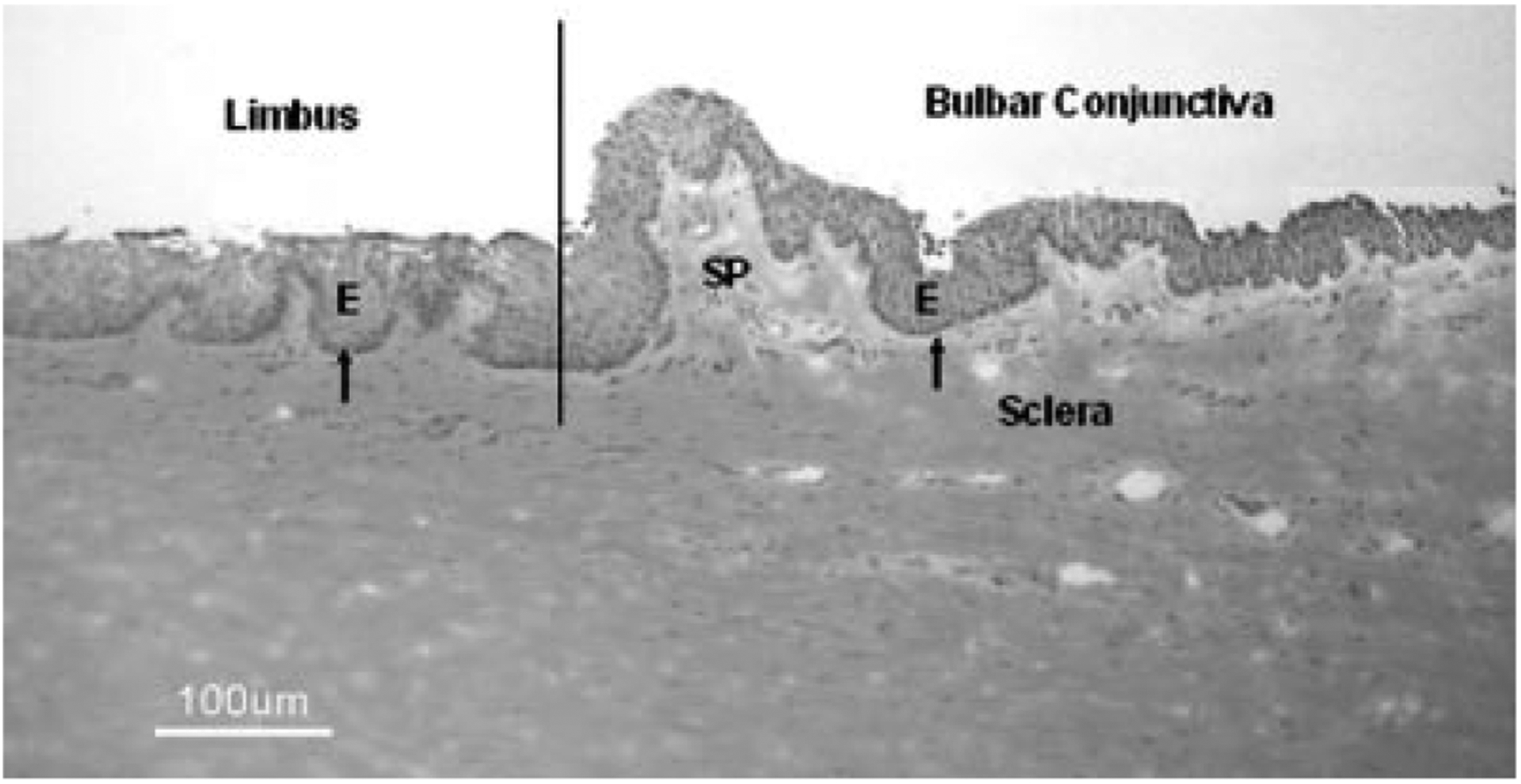
Histology of a representative human corneoscleral tissue with bulbar conjunctiva in hematoxylin–eosin staining showing the demarcation of the limbus and bulbar conjunctiva in the limbus–bulbar conjunctival transition zone utilized in this study. E, epithelium; arrow showing the basal layer of epithelial cells; SP, the substantia propria of the bulbar conjunctiva. Scale bar = 100 μm.
As shown in Figure 2, CK3, a corneal epithelial differentiation marker (Schermer et al., 1986), was strongly expressed by the superficial limbal epithelium, but the bulbar conjunctival epithelium was totally negative. CK4, a nonkeratinized stratified epithelial marker (Kasper, 1991; Krenzer and Freddo, 1997), was strongly expressed in the cytoplasm of the superficial layers of bulbar conjunctival epithelium, but no staining was detected in the limbal epithelium. CK7, a glandular epithelial marker (Krenzer and Freddo, 1997), was only weakly expressed by some apical bulbar conjunctival epithelial cells and was totally negative in the limbal epithelium. CK14, which is a marker of the basal cell layer of stratified epithelia (Fuchs et al., 1994; Lane and McLean, 2004), localized to the basal layer of the bulbar conjunctival epithelia. In contrast, CK14 was expressed by all limbal epithelial layers. CK15, which is also expressed by the basal epidermis (Porter et al., 2000), was detected in the basal layer of the bulbar conjunctival epithelia, and by all layers of the limbal epithelia. CK19, a simple epithelial marker (Krenzer and Freddo, 1997), was strongly expressed by all layers of the conjunctival epithelia but confined to the basal limbal cells. The expression pattern of CK19 in the limbus was consistent to our previous reports (Chen et al., 2004). The immuno-localizations of these CKs in bulbar conjunctival and limbal epithelia were summarized in Table 1.
Fig. 2.
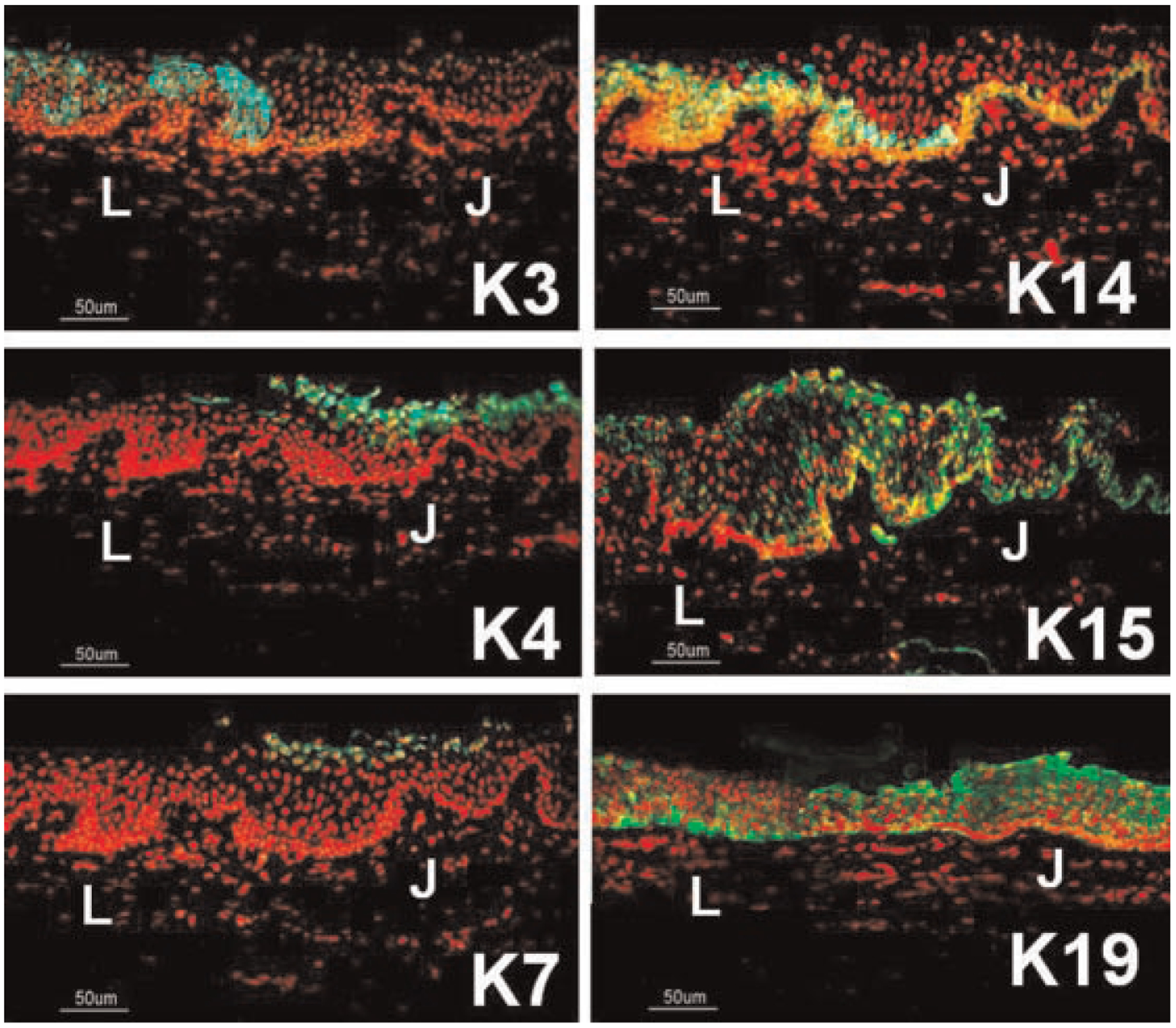
Representative images showing the immunofluorescent staining of cytokeratins (CKs) (green) in the epithelia at the limbus–bulbar conjunctiva transition zone. PI was used as nuclear counterstaining (red). CK3 was localized to the superficial limbal epithelial cells (L). CK4 and CK7 were only expressed by the superficial layers of bulbar conjunctival epithelium (J). CK14 and CK15 was confined to the basal layer of bulbar conjunctival epithelium (J), but was expressed by all layers of limbal epithelia (L). CK19 was strongly expressed by all layers of bulbar conjunctival epithelia (J), but confined to the basal limbal cells (L). Scale bar = 50 μm.
TABLE 1.
The differential immuno-localization of 6 cytokeratins (CKs) and 13 proposed limbal stem/progenitor cell associated and differentiation markers in the human bulbar conjunctival epithelium and in the limbal epithelium
| Molecular markers | Conjunctival epithelium | Limbal epithelium | ||
|---|---|---|---|---|
| Basal | Suprabasal | Basal | Suprabasal | |
| CK3 | − | − | − | +++ |
| CK4 | − | +++ | − | − |
| CK7 | − | + | − | − |
| CK14 | +++ | − | + | +++ |
| CK15 | +++ | − | ++ | + |
| CK19 | +++ | +++ | ++ | ± |
| ABCG2 | +++ | − | +++a | −a |
| p63 | +++ | + | +++a | −a |
| NGF | +++ | − | +++a | −a |
| TrkA | +++ | + | +++a | −a |
| p75NTR | ++ | − | ++a | −a |
| GDNF | +++ | + | +++a | −a |
| GFRα−1 | ++ | − | +++a | −a |
| Integrin β1 | +++ | + | +++a | +a |
| α-Enolase | +++ | + | +++a | +a |
| EGFR | +++ | + | +++a | +a |
| Nestin | − | +++ | −a | +++a |
| E-cadherin | − | +++ | −a | +++a |
| Involucrin | − | +++ | −a | +++a |
The grading was based on the intensity of immunofluorescent staining: −, undetectable; +, weak positive; ++, moderate positive; +++, strong positive.
Data were published previously (Chen et al., 2004; Qi et al., 2007).
Stem cell associated markers positively expressed by the basal epithelial layer of the bulbar conjunctiva
Figure 3 shows the expression of proposed limbal stem cell associated markers (Moll et al., 1982; Chen et al., 2004) in the bulbar conjunctival epithelium near the limbus–bulbar conjunctival transition zone. ABCG2 transporter was expressed in the membrane and cytoplasm of certain basal cells, but not in the suprabasal layers of the bulbar conjunctival epithelium. p63 nuclear protein was strongly immunodetected in the nuclei of most basal cells, and weakly in some suprabasal epithelial cells, but not in the superficial cells of bulbar conjunctival epithelia.
Fig. 3.
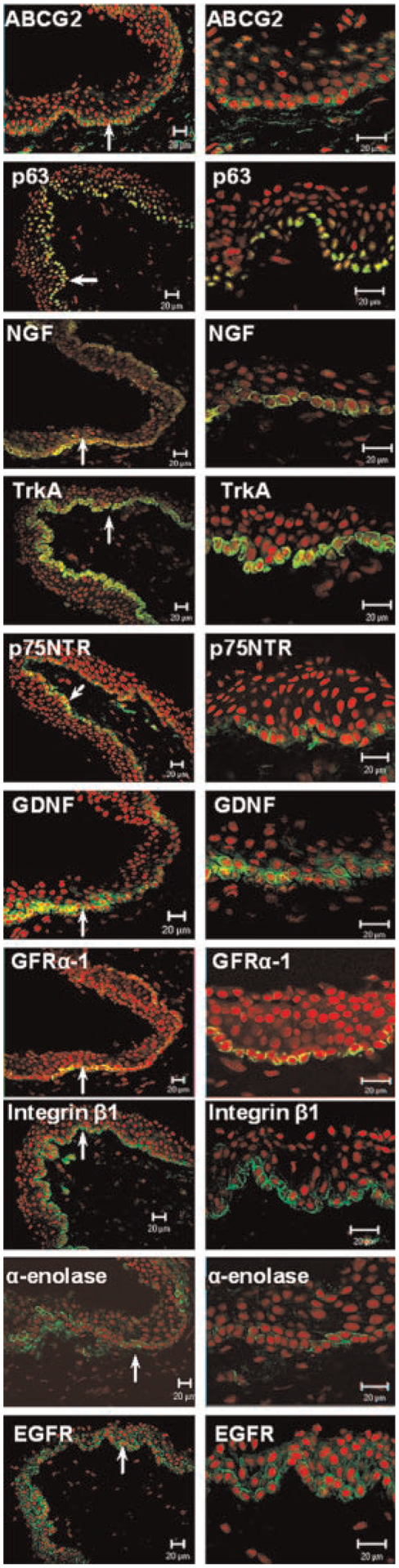
Representative low (left) and high (right) magnification images of immunofluorescent staining for stem/progenitor cell associated markers ABCG2, p63, NGF with its receptors TrkA and p75NTR, GDNF with its receptor GFRα−1, integrin β1, α-enolase, and EGFR (green) in bulbar conjunctival epithelium of human corneoscleral frozen sections. PI was used as a nuclear counterstaining (red). Basal conjunctival epithelial cells strongly express these stem cell associated markers (arrow indicates the area of basal epithelium which is magnified in the right column). Scale bar = 20 μm (magnification: left column 200×; right column 400×).
NGF, a member of the neurotrophin family, together with its two corresponding receptors: the high-affinity receptor TrkA and the low-affinity receptor p75NTR, were found primarily expressed in the cell membrane and cytoplasm of bulbar conjunctival basal epithelial cells. TrkA was also found weakly expressed in certain suprabasal epithelial cells. GDNF, a member of the GDNF family of neurotrophic factors, was primarily localized to the cytoplasm of basal and some suprabasal cells of the bulbar conjunctival epithelium. GDNF receptor GFRα−1 localized to the membrane of certain cells in bulbar conjunctival basal epithelial layer, but not in the suprabasal layers of the bulbar conjunctiva.
The integrin β1 was strongly expressed in the cell membrane of bulbar conjunctival basal epithelium, and weakly expressed in the suprabasal epithelial cells. α-Enolase was strongly expressed in cytoplasm of a subset of bulbar conjunctival basal epithelial cells, while a few suprabasal epithelial cells were also positive. The EGFR antibody stained the cell membranes of the bulbar conjunctival basal epithelia much stronger than superficial cells.
Differentiation associated markers negatively expressed by the basal epithelial layer of the bulbar conjunctiva
As demonstrated in Figure 4, nestin and E-cadherin were expressed in the cytoplasm of suprabasal and superficial cells of the bulbar conjunctival epithelium. The bulbar conjunctival basal cells were totally negative. The differentiation associated marker involucrin (Banks-Schlegel and Green, 1981; Watt and Green, 1981) strongly stained only the most superficial layers in the bulbar conjunctival epithelium.
Fig. 4.
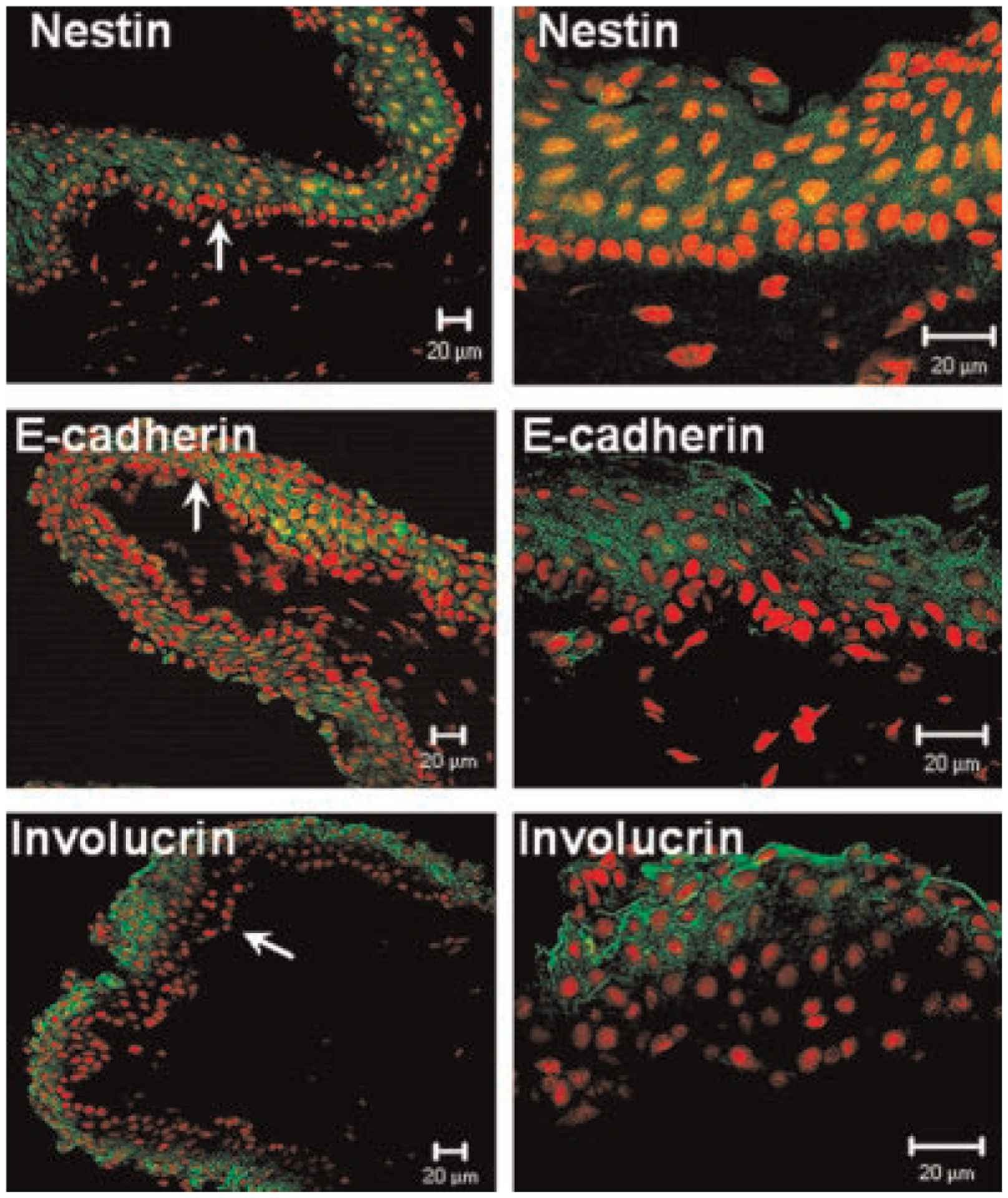
Representative low (left) and high (right) magnification images of immunofluorescent staining for differentiation markers nestin, E-cadherin and involucrin (green) in bulbar conjunctival epithelium of human corneoscleral frozen sections. PI was used for nuclear counterstaining (red). Basal conjunctival epithelial cells were negative for nestin, E-cadherin, and involucrin (arrow indicates the area of basal epithelium which is magnified in the right column). Scale bar = 20 μm (magnification: left column 200×; right column 400×).
Combining with the data we previously published (Chen et al., 2004; Qi et al., 2007), the immuno-localizations of stem/progenitor cell associated and differentiation markers in bulbar conjunctival and limbal epithelia were summarized in Table 1.
Discussion
This study evaluated the immuno-localization of 6 cytokeratins and 13 limbal stem/progenitor cell associated and differentiation markers in the human bulbar conjunctival epithelium. The findings provide a basic characterization of the bulbar conjunctival epithelial phenotype and suggest its basal cells with undifferentiated features may contain stem/progenitor cells for the conjunctival epithelium.
Distinguishable distribution of cytokeratins in the human bulbar conjunctival epithelium
Cytokeratins (CKs), a family of cytoskeletal component proteins of epithelial cells, are often used in the characterization of epithelial phenotype and differentiation. CKs are divided into two subfamilies: type I (acidic) and type II (basic to neutral). In vivo, a basic CK usually heterodimerizes or “pairs” with a particular acidic CK (Steinert, 1993). The expression of CK pairs is tissue specific, differentiation dependent, and developmentally regulated (Kao and Liu, 2003; Lane and McLean, 2004). While there is a large body of available data concerning CKs expression in human conjunctival or corneal epithelium, there are still unanswered questions regarding the differential distribution of CKs in these two types of epithelia. Our results indicate that the epithelia covering the limbal and the conjunctival surfaces are distinctly different. Although both epithelia are nonkeratinized and stratified, the two types of epithelia can be distinguished by their differential expression of CKs: (1) the limbal, but not conjunctival epithelium, expresses cytokeratin CK3; (2) CK4 and CK7 were only expressed by the superficial cells of bulbar conjunctival epithelium; (3) CK14 and CK15 were only expressed in the basal layers of the bulbar conjunctival epithelia, while detected in all layers of the limbal epithelia; (4) CK19 is abundantly found in all layers of the conjunctival epithelium, but confined to the basal limbal cells. The conjunctival and limbal epithelial phenotypes were shown to express unique patterns of CK3, CK4, CK7, CK14, CK15, and CK19 (summarized in Table 1).
Twenty different CKs are known to be expressed in human epithelia (Moll et al., 1982; Tseng et al., 1982; Mischke, 1998; Pitz and Moll, 2002). CK3 has been reported to be uniquely expressed by corneal epithelium (Moll et al., 1982; Schermer et al., 1986), as only minimal expression has been noted in other human tissues (Collin et al., 1992; Wei et al., 1993). As anticipated, CK3 was not detected in the conjunctival epithelium in our study.
In humans ocular surface, the expression patterns of CK19 and CK14 were controversial (Moll et al., 1982; Barnard et al., 2001; Chen et al., 2004; Yoshida et al., 2006) and the report on the expression of CK15 is scarce (Yoshida et al., 2006). CK14 was reported to be expressed in the basal layer of the corneal and conjunctival epithelia (Moll et al., 1982; Barnard et al., 2001), CK19 is expressed strongly in basal limbal cells (Barnard et al., 2001; Chen et al., 2004) and all layers of the conjunctival epithelium (Diebold et al., 2001; Pitz and Moll, 2002; Schlotzer-Schrehardt and Kruse, 2005; Yoshida et al., 2006), and CK15 is expressed in all layers of limbal cells and in the basal conjunctival epithelium (Yoshida et al., 2006). More or less, our finding was consistent to the reports above. However, we found CK14 in all layers of limbal epithelia, which was different from the previous reports (Moll et al., 1982; Barnard et al., 2001) but consistent to Yoshida’s finding (Yoshida et al., 2006). The difference in the immunohistochemical staining pattern is probably due to variations in technical procedure, which includes the method of tissue processing, the specificity and sensitivity of antibodies used, and conditions for visualization. Indeed, staining patterns of p63, another gene often used to stain the limbal epithelium, varies greatly in tissue-processing methods (Hsueh et al., 2004). In this study, the tissues we used were from donors in different ages and different sexes, the elapsed time between death and tissue embedded was within 8 h. We have confirmed the expression patterns of CK14 and CK15 by using the antibodies from other companies (data not shown here). We have prevented overfixation which often leads to loss or reduction of antigen reactivity. The variation of cytokeratin expression seemed to be associated with the sample quality, and thus we verified our results using four independent tissues. The CK expression pattern observed in the conjunctival epithelium in the present study is broadly equivalent to that seen in other nonkeratinizing stratified epithelia, that is, with a predominance of CK4, CK14, CK15, and CK19 (Kasper, 1992). We found that both CK14 and CK15 can serve as markers of the basal layer of the conjunctiva. It appears that CK19 is homogeneously distributed throughout all layers of bulbar conjunctival epithelium. It is possible that this extension of CK19 distribution into suprabasal layers in the conjunctiva is related in some way to the high proliferative activity of this epithelium (Pitz and Moll, 2002).
Basal conjunctival epithelial cells strongly express ABCG2, p63, NGF, TrkA, p75NTR, GDNF, GFRα−1, integrin β1, α-enolase, and EGFR
Our group has characterized three patterns of molecular markers expressed by human limbal basal epithelial cells (Chen et al., 2004; Qi et al., 2007): (i) exclusively positive for ABCG2, p63, NGF, TrkA, GDNF, and GFRα−1 by a subset of basal cells; (ii) relatively higher expression of integrin β1, EGFR, and α-enolase by most basal cells, and (iii) lack of expression of nestin, E-cadherin, connexin 43, involucrin, and CK3. Interestingly, the present study revealed the same expression patterns of molecular markers in the basal cells of human bulbar conjunctival epithelium as in the basal cells of corneal epithelium although they are formed by phenotypically different epithelial lineages (Schermer et al., 1986; Wei et al., 1996).
The conjunctiva is innervated by sensory, sympathetic, and parasympathetic nerves (Hsueh et al., 2004). NGF and GDNF are neurotrophic factors. NGF belongs to the neurotrophin (NT) family, which also comprises brain-derived neurotrophic factor, NT-3, NT-4/5, and NT-6 (Maness et al., 1994; Chao et al., 2006). NTs share the same low-affinity neurotrophin receptor p75NTR, but use different members of the Trk receptors (Huang and Reichardt, 2003) for high-affinity binding and signal transduction. NGF preferentially activates TrkA. Ligand specificity of GDNF is determined by GFRα−1 (Saarma and Sariola, 1999). Traditionally, neurotrophic factors have been considered to regulate survival and differentiation of neurons and neural SCs (Abe, 2000; Niles et al., 2004). However, in recent years, NGF receptor p75NTR expression has been noted as a characteristic of human esophageal and oral keratinocyte SCs (Okumura et al., 2003; Nakamura et al., 2006); TrkA has been shown to preferentially localize to limbal basal epithelial cells and was previously proposed as a potential marker for corneal limbal SCs (Lambiase et al., 1998; Touhami et al., 2002; Stepp and Zieske, 2005); GFRα−1 is strongly expressed by a subset of spermatogonia, including the SCs for spermatogenesis (Braydich-Stolle et al., 2005; Buageaw et al., 2005). Rios et al. (2007) verified by Western blot analysis that rat conjunctiva contains all NTs and NT receptors. Our immunostaining data revealed for the first time that the NGF and GDNF, together with their receptors, were primarily expressed by the basal cells of the bulbar conjunctival epithelium. They may serve as new molecular markers for the conjunctival basal epithelial phenotype and potentially as stem/progenitor cell associated markers for this tissue.
The basal layer of bulbar conjunctival epithelium may be a source of progenitor cells for the conjunctival epithelium
To date, the location of the SCs for the human conjunctival epithelium and goblet cells has not been conclusively identified. However, it was reported that human bulbar and fornical conjunctival cells have equivalent in vitro proliferative capacity, suggesting that human conjunctival epithelial SCs might be evenly distributed within these two areas (Pellegrini et al., 1999). A recent report suggested that basal keratinocytes are undifferentiated and capable of proliferation, and this cell compartment includes SCs as well as TACs (Liu et al., 2007). Our findings demonstrate that the basal epithelial cells of the bulbar conjunctiva strongly expressed proposed stem/progenitor cell associated markers (ABCG2, p63, NGF, TrkA, p75NTR, GDNF, GFRα−1, integrin β1, α-enolase, EGFR, CK14, and CK15), but not differentiation markers (involucrin, nestin, E-cadherin, and CK4). This distribution pattern supports the hypothesis that the basal layer of bulbar conjunctival epithelium may be a source of progenitor cells for the conjunctival epithelium. While no single SC marker has been identified to date, the characterization of this putative SC phenotype in the present study may advance the understanding of conjunctival epithelial SC properties, and may have potential use in identification and isolation of conjunctival epithelial SCs.
Acknowledgments
This study was supported by Department of Defense CDMRP PRMRP grant FY06 PR064719 (DQL); NIH Grant EY11915 (SCP), National Eye Institutes, Bethesda, MD; by National Natural Science Foundation of China, Project 30872813 (HQ); by a grant from Lions Eye Bank Foundation (HQ); by an unrestricted grant from Research to Prevent Blindness, the Oshman Foundation and the William Stamps Farish Fund.
Contract grant sponsor: Department of Defense CDMRP PRMRP;
Contract grant number: FY06 PR064719.
Contract grant sponsor: NIH;
Contract grant number: EY11915.
Contract grant sponsor: National Natural Science Foundation of China;
Contract grant number: 30872813.
Literature Cited
- Abe K 2000. Therapeutic potential of neurotrophic factors and neural stem cells against ischemic brain injury. J Cereb Blood Flow Metab 20:1393–1408. [DOI] [PubMed] [Google Scholar]
- Ang LP, Tan DTH, Beuerman RW, Lavker RM. 2004. Ocular surface epithelial stem cells: Implications for ocular surface homeostasis. In: Pflugfelder SC, Beuerman RW, Stern ME, editors. Dry eye and ocular surface disorders. New York, NY: Marcel Dekker, Inc. pp 225–246. [Google Scholar]
- Banks-Schlegel S, Green H. 1981. Involucrin synthesis and tissue assembly by keratinocytes in natural and cultured human epithelia. J Cell Biol 90:732–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard Z, Apel AJG, Harkin DG. 2001. Phenotypic analyses of limbal epithelial cell cultures derived from donor corneoscleral rims. Clin Exp Ophthalmol 29:138–142. [DOI] [PubMed] [Google Scholar]
- Braydich-Stolle L, Nolan C, Dym M, Hofmann MC. 2005. Role of glial cell line-derived neurotrophic factor in germ-line stem cell fate. Ann NY Acad Sci 1061:94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buageaw A, Sukhwani M, Ben Yehudah A, Ehmcke J, Rawe VY, Pholpramool C, Orwig KE, Schlatt S. 2005. GDNF family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biol Reprod 73:1011–1016. [DOI] [PubMed] [Google Scholar]
- Chao MV, Rajagopal R, Lee FS. 2006. Neurotrophin signalling in health and disease. Clin Sci (Lond) 110:167–173. [DOI] [PubMed] [Google Scholar]
- Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li D-Q. 2004. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells 22:355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin C, Ouhayoun JP, Grund C, Franke WW. 1992. Suprabasal marker proteins distinguishing keratinizing squamous epithelia: Cytokeratin 2 polypeptides of oral masticatory epithelium and epidermis are different. Differentiation 51:137–148. [DOI] [PubMed] [Google Scholar]
- Diebold Y, Rios JD, Hodges RR, Rawe I, Dartt DA. 2001. Presence of nerves and their receptors in mouse and human conjunctival goblet cells. Invest Ophthalmol Vis Sci 42:2270–2282. [PubMed] [Google Scholar]
- Fuchs E, Chan YM, Paller AS, Yu QC. 1994. Cracks in the foundation: Keratin filaments and genetic disease. Trends Cell Biol 4:321–326. [DOI] [PubMed] [Google Scholar]
- Hsueh YJ, Wang DY, Cheng CC, Chen JK. 2004. Age-related expressions of p63 and other keratinocyte stem cell markers in rat cornea. J Biomed Sci 11:641–651. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. 2003. Trk receptors: Roles in neuronal signal transduction. Annu Rev Biochem 72:609–642. [DOI] [PubMed] [Google Scholar]
- Huang AJ, Tseng SC, Kenyon KR. 1988. Morphogenesis of rat conjunctival goblet cells. Invest Ophthalmol Vis Sci 29:969–975. [PubMed] [Google Scholar]
- Kao WW, Liu CY. 2003. The use of transgenic and knock-out mice in the investigation of ocular surface cell biology. Ocul Surf 1:5–19. [DOI] [PubMed] [Google Scholar]
- Kasper M 1991. Heterogeneity in the immunolocalization of cytokeratin specific monoclonal antibodies in the rat eye: Evaluation of unusual epithelial tissue entities. Histochemistry 95:613–620. [DOI] [PubMed] [Google Scholar]
- Kasper M 1992. Cytokeratins in intracranial and intraspinal tissues. Adv Anat Embryol Cell Biol 126:1–82. [DOI] [PubMed] [Google Scholar]
- Krenzer KL, Freddo TF. 1997. Cytokeratin expression in normal human bulbar conjunctiva obtained by impression cytology. Invest Ophthalmol Vis Sci 38:142–152. [PubMed] [Google Scholar]
- Lambiase A, Bonini S, Micera A, Rama P, Bonini S, Aloe L. 1998. Expression of nerve growth factor receptors on the ocular surface in healthy subjects and during manifestation of inflammatory diseases. Invest Ophthalmol Vis Sci 39:1272–1275. [PubMed] [Google Scholar]
- Lane EB, McLean WH. 2004. Keratins and skin disorders. J Pathol 204:355–366. [DOI] [PubMed] [Google Scholar]
- Liu S, Li J, Tan DT, Beuerman RW. 2007. The eyelid margin: A transitional zone for 2 epithelial phenotypes. Arch Ophthalmol 125:523–532. [DOI] [PubMed] [Google Scholar]
- Maness LM, Kastin AJ, Weber JT, Banks WA, Beckman BS, Zadina JE. 1994. The neurotrophins and their receptors: Structure, function, and neuropathology. Neurosci Biobehav Rev 18:143–159. [DOI] [PubMed] [Google Scholar]
- Mischke D 1998. The complexity of gene families involved in epithelial differentiation. Keratin genes and the epidermal differentiation complex. Subcell Biochem 31:71–104. [PubMed] [Google Scholar]
- Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. 1982. The catalog of human cytokeratins. Patterns of expression in normal epithelia, tumors and cultured cells. Cell 31:11–24. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Endo KI, Kinoshita S. 2006. Identification of human oral keratinocyte stem/progenitor cells by neurotrophin receptor p75 and the role of neurotrophin/p75 signaling. Stem Cells 25:628–638. [DOI] [PubMed] [Google Scholar]
- Niles LP, Armstrong KJ, Rincon Castro LM, Dao CV, Sharma R, McMillan CR, Doering LC, Kirkham DL. 2004. Neural stem cells express melatonin receptors and neurotrophic factors: Colocalization of the MT1 receptor with neuronal and glial markers. BMC Neurosci 5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura T, Shimada Y, Imamura M, Yasumoto S. 2003. Neurotrophin receptor p75(NTR) characterizes human esophageal keratinocyte stem cells in vitro. Oncogene 22:4017–4026. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Golisano O, Paterna P, Lambiase A, Bonini S, Rama P, De Luca M. 1999. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol 145:769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitz S, Moll R. 2002. Intermediate-filament expression in ocular tissue. Prog Retin Eye Res 21:241–262. [DOI] [PubMed] [Google Scholar]
- Porter RM, Lunny DP, Ogden PH, Morley SM, McLean WH, Evans A, Harrison DL, Rugg EL, Lane EB. 2000. K15 expression implies lateral differentiation within stratified epithelial basal cells. Lab Invest 80:1701–1710. [DOI] [PubMed] [Google Scholar]
- Qi H, Chuang EY, Yoon KC, de Paiva CS, Shine HD, Jones DB, Pflugfelder SC, Li DQ. 2007. Patterned expression of neurotrophic factors and receptors in human limbal and corneal regions. Mol Vis 13:1934–1941. [PMC free article] [PubMed] [Google Scholar]
- Rios JD, Ghinelli E, Gu J, Hodges RR, Dartt DA. 2007. Role of neurotrophins and neurotrophin receptors in rat conjunctival goblet cell secretion and proliferation. Invest Ophthalmol Vis Sci 48:1543–1551. [DOI] [PubMed] [Google Scholar]
- Saarma M, Sariola H. 1999. Other neurotrophic factors: Glial cell line-derived neurotrophic factor (GDNF). Microsc Res Tech 45:292–302. [DOI] [PubMed] [Google Scholar]
- Schermer A, Galvin S, Sun T-T. 1986. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol 103:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Kruse FE. 2005. Identification and characterization of limbal stem cells. Exp Eye Res 81:247–264. [DOI] [PubMed] [Google Scholar]
- Steinert PM. 1993. Structure, function, and dynamics of keratin intermediate filaments. J Invest Dermatol 100:729–734. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Zieske JD. 2005. The corneal epithelial stem cell niche. Ocul Surf 3:15–26. [DOI] [PubMed] [Google Scholar]
- Touhami A, Grueterich M, Tseng SC. 2002. The role of NGF signaling in human limbal epithelium expanded by amniotic membrane culture. Invest Ophthalmol Vis Sci 43:987–994. [PubMed] [Google Scholar]
- Tseng SC, Jarvinen MJ, Nelson WG, Huang JW, Woodcock-Mitchell J, Sun TT. 1982. Correlation of specific keratins with different types of epithelial differentiation: Monoclonal antibody studies. Cell 30:361–372. [DOI] [PubMed] [Google Scholar]
- Watt FM, Green H. 1981. Involucrin synthesis is correlated with cell size in human epidermal cultures. J Cell Biol 90:738–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z-G, Wu R-L, Lavker RM, Sun T-T. 1993. In vitro growth and differentiation of rabbit bulbar, fornix, and palpebral conjunctival epithelia. Implication on conjunctival epithelial transdifferentiation and stem cells. Invest Ophthalmol Vis Sci 34:1814–1828. [PubMed] [Google Scholar]
- Wei ZG, Cotsarelis G, Sun TT, Lavker RM. 1995. Label-retaining cells are preferentially located in fornical epithelium: Implications on conjunctival epithelial homeostasis. Invest Ophthalmol Vis Sci 36:236–246. [PubMed] [Google Scholar]
- Wei Z-G, Sun T-T, Lavker RM. 1996. Rabbit conjunctival and corneal epithelial cells belong to two separate lineages. Invest Ophthalmol Vis Sci 37:523–533. [PubMed] [Google Scholar]
- Yoshida S, Shimmura S, Kawakita T, Miyashita H, Den S, Shimazaki J, Tsubota K. 2006. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Invest Ophthalmol Vis Sci 47:4780–4786. [DOI] [PubMed] [Google Scholar]


