Abstract
Diene self-exchange reactions of the 17-electron, formally cobalt(0) cyclooctadienyl precatalyst, (R,R)-(iPrDuPhos)Co(COD) (P2CoCOD, (R,R)-iPrDuPhos = 1,2-bis((2R,5R)-2,5-diisopropylphospholano)benzene, COD = 1,5-cyclooctadiene) were studied using natural abundance and deuterated 1,5-cyclooctadiene. Exchange of free and coordinated diene was observed at ambient temperature in benzene-d6 solution and kinetic studies support a dissociative process. Both neutral P2CoCOD and the 16-electron, cationic cobalt(I) complex, [(R,R)-(iPrDuPhos)Co(COD)][BArF4] (BArF4 = B[(3,5-(CF3)2)C6H3]4) underwent instantaneous displacement of the 1,5-cyclooctadiene ligand by carbon monoxide and generated the corresponding carbonyl derivatives. The solid-state parameters, DFT-computed Mulliken spin density and analysis of molecular orbitals suggest an alternative description of P2CoCOD as low-spin cobalt(II) with the 1,5-cyclooctadiene acting as a LX2-type ligand. This view of the electronic structure provides insight into the nature of the ligand substitution processes and the remarkable stability of the neutral cobalt complexes toward protic solvents observed during catalytic alkene hydrogenation.
Keywords: Cobalt, ligand substitution, metallacyclopropane, hydrogenation
Graphical Abstract

Introduction
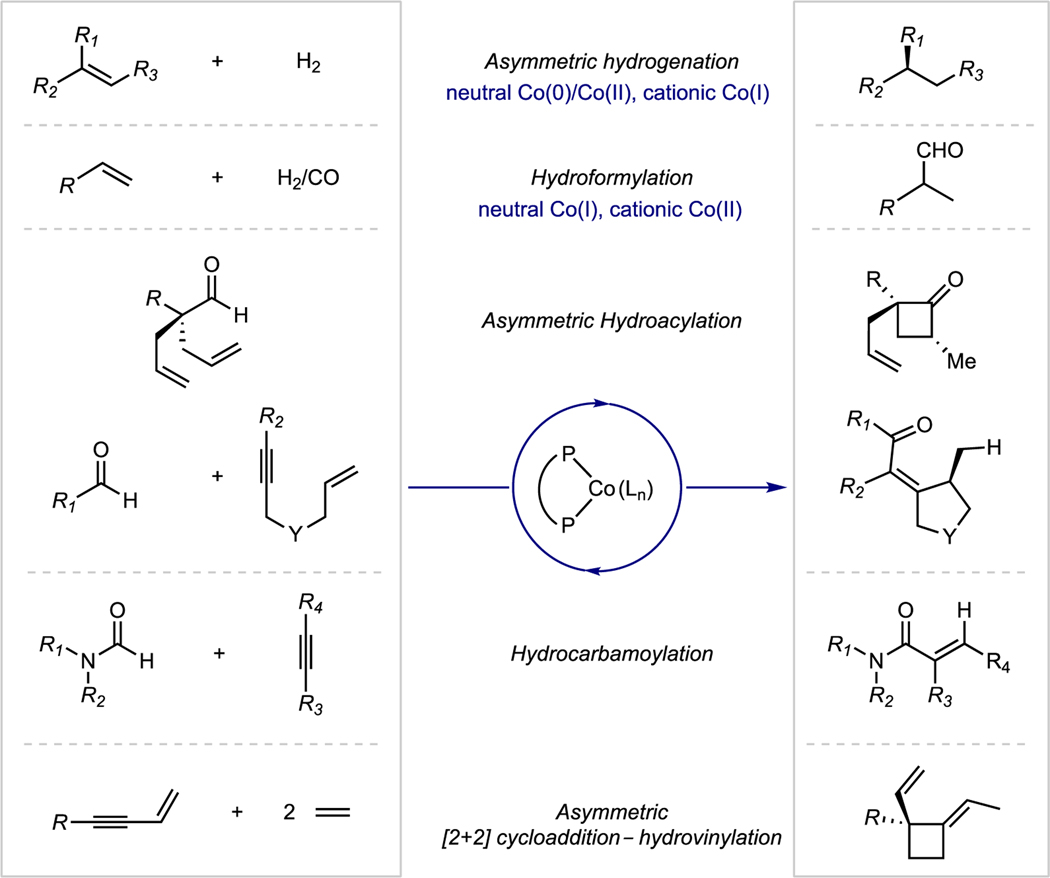
Catalysis with Earth-abundant, first-row transition metals has witnessed a renaissance of interest recently due to potential economic, environmental advantages as well as reactivity distinct from more widely used and studied precious metal catalysts.1 The asymmetric hydrogenation of carbon–carbon, carbon–oxygen and carbon–nitrogen multiple bonds has received considerable attention. Examples of manganese, iron, cobalt and nickel catalysts with high activity and enantioselectivities have been reported and in some cases offer performance or operating conditions superior to precious metals.2,3,4 Bis(phosphine)cobalt complexes have emerged as a powerful and versatile class of catalysts for asymmetric hydrogenation and offer remarkable properties such as stability in alcohol solvents and broad functional group tolerance.5 Compounds of this type have also been reported to be versatile catalysts for other catalytic transformations such as alkene hydroformylation6 and hydroacylation,7 alkene and alkyne hydrovinylation,8 [4+2] and [2+2] cycloadditions9 and other C–C bond-forming reactions (Scheme 1).10
Scheme 1.
Examples of catalytic chemistry promoted by bis(phosphine) cobalt complexes.
High-throughput experimentation (HTE) has been an enabling technology for the rapid identification of in situ catalyst generation conditions and optimal ligands for asymmetric alkene hydrogenation. Two carbon-bridged, C2-symmetric chiral bis(phosphine)s in combination with cobalt(II) halides and alkyl lithium activating reagent emerged as effective for the hydrogenation of functionalized alkenes such as methyl 2-acetamidoacrylate.5a Greatly improved compatibility with essentially all of the 192 chiral bidentate ligands within the library was discovered when active cobalt catalysts were generated from zinc reduction in MeOH.5c A pilot-scale, 200 gram asymmetric hydrogenation affording the epilepsy medication levetiracetam in 98.2% ee was performed with only 0.08 mol% loading of the cobalt catalyst. Isolation of well-defined Co(I) and Co(0) precatalysts generated from sequential one-electron reduction provided insights into catalyst activation pathways. The asymmetric hydrogenation of α,β-unsaturated carboxylic acids with diverse substitution patterns has also been achieved with the Co(0) precatalysts,5d wherein good functional group tolerance was observed and deuterium labeling studies supported an unusual homolytic H2 cleavage pathway with cobalt carboxylates.
To gain additional mechanistic insights, our laboratory has also been exploring the chemistry of well-defined organometallic cobalt precatalysts. Bis(phosphine)cobalt(II) dialkyls have been prepared and structurally characterized as low-spin, planar compounds and applied to the diastereoselective hydrogenation of hydroxyl alkenes.5b In the absence of substrate, stirring (R,R)-(iPrDuPhos)Co(CH2SiMe3)2 in methanol resulted in dehydrogenation of the alcohol and isolation of [(R,R)-(iPrDuPhos)Co]2(μ-CO)2.11 Related, formally cobalt(0) 1,5-cyclooctadiene complexes have been prepared either from the hydrogenation of the cobalt(II) dialkyl derivatives in the presence of COD or from reduction of the corresponding cobalt(II) dihalides in the presence of the diene.5b,c,d These compounds are one electron reduced variants of widely used bis(phosphine) rhodium(I) cations and are effective single component catalysts for the hydrogenation of a range of alkenes with high activity and enantioselectivity. Despite their anticipated reducing nature, the Co(0) precatalysts showed unusual stability to protic medium as well as broad functional group tolerance.5c,d
Recently, our laboratory reported the synthesis, structural characterization and hydrogenation performance of [(R,R)-(iPrDuPhos)Co(COD)][BArF4] ([P2CoCOD]+), a pioneering example of a long-sought-after cobalt analog of the rhodium cations.12 An idealized square-planar geometry at cobalt was identified in the solid-state structure of [P2CoCOD]+,12 while D2d-distorted tetrahedral geometry was observed for all neutral P2CoCOD compounds, a result of the formally Co(0), d9 configuration.5b,c,d The one-electron oxidized, cationic [P2CoCOD]+exhibited much higher substitutional lability of 1,5-cyclooctadiene as compared to the neutral P2CoCOD complex, where displacement of the diene by enamides and arenes for [P2CoCOD]+ was instantaneous and substitution by tetrahydrofuran-d8 also occurred over time.12 “While the neutral cobalt(0) precatalysts were highly active in protic solvents such as MeOH and i-PrOH for the asymmetric hydrogenation of enamides and unsaturated carboxylic acids, optimal performance for the cobalt(I) precatalysts was observed in aprotic solvents such as tetrahydrofuran for the asymmetric hydrogenation of methyl 2-acetamidoacrylate and (Z)-methyl-2-acetamidocinnamate, highlighting the distinct reactivity/tolerance with protons for the neutral and cationic cobalt intermediates during catalysis. Isolation of otherwise identical complexes separated by one-electron highlights the unique electronic properties offered by first row transition metals. Catalytic transformations including hydrogenation in potentially two different redox cycles, initiated from Co(0) and Co(I) precatalysts, are highly desired features unique to first-row metals and likely offer new reactivity and selectivity. Here we describe a study in olefin substitution chemistry and electronic structures for both the neutral and cationic bis(phosphine) cobalt 1,5-cyclooctadiene complexes, P2CoCOD and [P2CoCOD]+, to better understand the basis of their distinct stability and reactivity profiles.
Results and Discussion
Kinetic Studies of Cyclooctadiene Substitution Reactions.
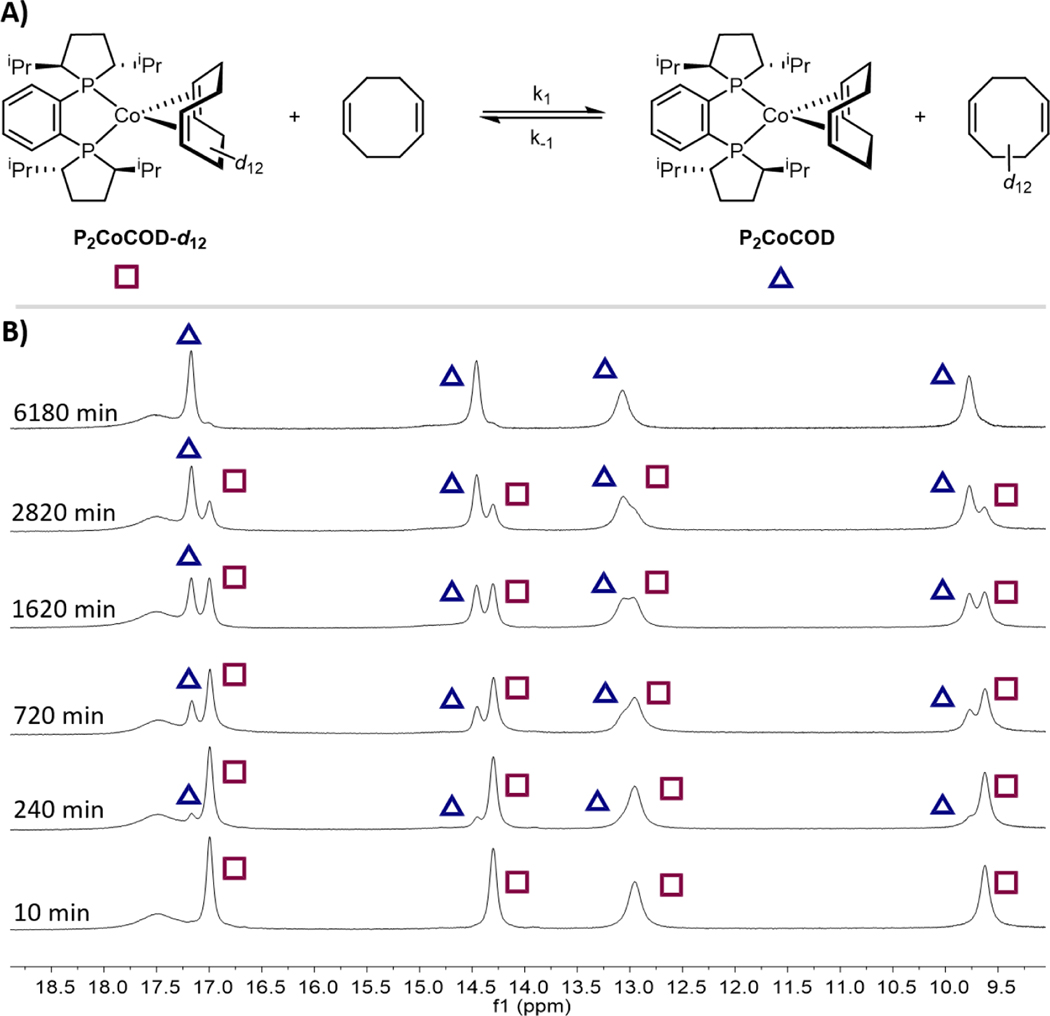
The rate and mechanism of substitution of the cyclooctadiene ligands in both cobalt(0) and cobalt(I) was of fundamental interest and may provide insights into precatalyst activation and substrate coordination events. Our studies commenced with a self-exchange experiment between free and coordinated 1,5-cyclooctadiene with neutral P2CoCOD. Because of the paramagnetically shifted 1H NMR resonances of the formally cobalt(0) compound, 1,5-cyclooctadiene-d12 would provide additional spectroscopic handles for these experiments. The iron-catalyzed [4+4] cycloaddition of dienes, pioneered by tom Dieck13a and later optimized by Ritter13b and expanded by our laboratory13c was well suited for the preparation of cyclooctadiene-d12 from commercially available 1,3-butadiene-d6. The targeted cobalt(0) diene complex, (R,R)-(iPrDuPhos)Co(COD-d12) (P2CoCOD-d12), was successfully synthesized following the previously reported procedure5c in 92% isolated yield. The natural abundance compound, P2CoCOD exhibits paramagnetically shifted resonances in the benzene-d6 1H NMR spectrum at ambient temperature that range between −80.23 and 34.35 ppm. Comparing these data to the analogous spectrum of P2CoCOD-d12 identified the six resonances corresponding to the coordinated cyclooctadiene (see Supporting Information, Figure S2).
With the P2CoCOD-d12 in hand, exchange with natural abundance 1,5-cyclooctadiene was explored (Figure 2A). Addition of 20 equivalents of free 1,5-cyclooctadiene to a benzene-d6 solution of P2CoCOD-d12 and monitoring the progress of the reaction by 1H NMR spectroscopy established appearance of coordinated COD resonances over the course of 8 hours at ambient temperature (Figure S7). Notably, the isotopic composition of the cyclooctadiene ligand also impacted the 1H NMR chemical shifts of the iPrDuPhos protons (Figure 2B). The resolution of the 1H NMR resonances of the iPrDuPhos ligand in P2CoCOD and P2CoCOD-d12 provided a convenient measure for determining the degree of conversion for diene exchange. The reaction reached 10 and 50% conversions in approximately 4 and 27 hours, respectively and equilibrium was established after 96 hours.
Figure 2.
A) Diene self-exchange experiment between P2CoCOD-d12 and natural abundance 1,5-cyclooctadiene. B) Stack plot of 1H NMR spectra of (R,R)-iPrDuPhos ligand resonances (partial) showing disappearance of P2CoCOD-d12 signals (square) and appearance of P2CoCOD signals (triangle) as an indicator of conversion.
To determine the reaction order in both cobalt and 1,5-cyclooctadiene, initial rate (<10% conv.) measurements were performed. Three separate reactions: (A) P2CoCOD-d12 (32 mM) and 1,5-cyclooctadiene (640 mM); (B) P2CoCOD-d12 (32 mM) and 1,5-cyclooctadiene (1280 mM) and (C) P2CoCOD-d12 (64 mM) and 1,5-cyclooctadiene (640 mM) in benzene-d6 solutions were monitored by 1H NMR spectroscopy and the formation of [P2CoCOD] product as a function of time is shown in Figure 3. Doubling the concentration of 1,5-cycloctadiene did not result in a discernable acceleration in initial rate (A vs B) while an approximate two-fold enhancement in rate was observed when the initial P2CoCOD-d12 concentration was doubled (A vs C), indicating a 0th order in [COD-d12] and 1st order in [P2CoCOD-d12], respectively (Figure 3B). These findings support a dissociative substitution pathway where ligand coordination is fast compared to rate-determining cobalt-carbon bond breaking. Although 17-electron complexes have been reported to undergo associative substitutions through 19-electron intermediates,14 the steric properties of the ancillary ligands are known to dramatically influence the preferred substitution mechanism.15 The solid-state structure of P2CoCOD demonstrates that the iso-propyl groups on the rigid DuPhos ligand backbone impose a large steric hindrance above and below the P–Co–P plane, likely inhibiting formation a five-coordinate, 19-electron intermediate with two COD ligands coordinated in (η2, η2) and η2 arrangement. Substitution of P2CoCOD with 1,5-cyclooctadiene-d12 was also monitored and exhibited the same kinetic profile, suggesting there was no secondary kinetic isotope effect. An overall first order rate constant k = 6.6(±0.5) ×10−6 s−1 was derived from the linear fits of the initial rates, supporting slow diene substitution. The entire reaction time courses for trials A-C were followed over 103 hours until [P2CoCOD] plateaued and the equilibria were established (Figure 3C). The increase in [P2CoCOD] slowed significantly at higher conversions as the reverse reaction rate of the equilibrium became significant. Plotting conversions versus time for trials A-C showed similar traces but slightly different conversions (positions of final equilibrium) relevant to the initial [P2CoCOD-d12]:[COD] ratio. (Figure 3D).
Figure 3.
A) Conditions and initial rates of ligand substitution. B) Initial rate experiments, P2CoCOD concentrations versus time followed until 10% conversion. C) Product concentrations versus time followed over entire reaction. D) Conversion to product versus time followed over entire reaction. Trials A, B and C reached equilibrium at 91% conv. (initial concentration [P2CoCOD-d12]:[COD] = 1:20), 94% conv. (initial concentration [P2CoCOD-d12]:[COD] = 1:40) and 88% conv. (initial concentration [P2CoCOD-d12]:[COD] = 1:10) respectively.
The observation of a dissociative substitution mechanism for the 17-electron, neutral cobalt(0) complex prompted related studies on cyclooctadiene exchange with the 16-electron, cationic cobalt(I) complex, [P2CoCOD]+. The high propensity of [P2CoCOD]+ to form the more stable 18-electron, η6-arene complexes [P2Co(arene)]+ precluded the use of benzene-d6 as solvent.12 While [P2CoCOD]+ was also unstable upon prolonged standing at ambient temperature in THF-d8 due to solvent substitution and formation of high-spin, paramagnetic cobalt species,12 adding 20 equivalents of 1,5-cyclooctadiene-d12 to a THF-d8 solution of [P2CoCOD]+ and monitoring the reaction by 1H NMR spectroscopy after 10 minutes demonstrated that the reaction had reached equilibrium (See Supporting Information, Figure S8). No additional spectroscopic changes were observed until competing THF-d8 substitution occurred. Although no quantitative kinetic data could be obtained due to the fast rate of substitution, these observations clearly establish the substantial increase in the substitutional lability of [P2CoCOD]+ as compared to its one-electron reduced analogue, P2CoCOD.
Cyclooctadiene Substitution Reactions with Carbon Monoxide.
Substitution of the diene with stronger field ligands such as CO was also explored. Substitution of the 1,5-cyclooctadiene ligand by carbon monoxide with P2CoCOD was previously studied and afforded the coordinatively saturated, dimeric cobalt(0) dicarbonyl complex with bridging, terminal CO ligands and a cobalt–cobalt bond, [(R,R)-iPrDuPhosCo(η1-CO)(μ2-CO)]2 (Scheme 2A).11 The carbonylation of [P2CoCOD]+ was studied for comparison. Treatment of a diethyl ether solution of [P2CoCOD]+ with 1 atm CO resulted in an instant color change from blue to orange. The product was isolated in 86% yield and X-ray diffraction established formation of the 18-electron cobalt carbonyl complex, [(R,R)-iPrDuPhosCo(η1-CO)3][BArF4]. (Scheme 2B, Figure 4) An idealized trigonal bipyramidal geometry at cobalt was observed. The 1H, 31P{1H} and 13C{1H} NMR spectra (THF-d8, 298K) of [(R,R)-(iPrDuPhos)Co(η1-CO)3][BArF4] suggest chemically equivalent phosphine atoms (singlet, 98.15 ppm) and carbon atoms of CO ligands (199.5 ppm) at ambient temperature (Supporting Information, Figures S3, S4, S5). The 1H and 13C NMR signals are consistent with an overall C2-symmetric environment for the ligand, despite the overall C1-symmetry of the molecule. This observation is consistent with previously reported for η6-Ph(BPh3) and η2, κ1-methyl 2-acetamidoacrylate coordination.12 Solution infrared spectra (Et2O, 298 K) of [(R,R)-iPrDuPhosCo(η1-CO)3][BArF4] showed terminal CO stretching frequencies at 2079, 2035 and 2011 cm−1 which are higher than those of the neutral [(R,R)-iPrDuPhosCo(η1-CO)(μ2-CO)]2 (Scheme 2), consistent with weaker back-bonding to CO from Co(I)+ than the neutral, formal Co(0) center. The observation that both the neutral P2CoCOD and cationic [P2CoCOD]+ underwent fast and complete displacement of COD ligand by CO to afford coordinatively saturated carbonyl complexes highlight their substitutional lability to the stronger field ligand.
Scheme 2.

A) Rapid substitution of P2CoCOD by CO and formation of bis(phosphine)cobalt dicarbonyl dimer (ref. 11). B) Fast substitution of [P2CoCOD]+ by CO and formation of cationic bis(phosphine)cobalt tricarbonyl.
Figure 4.
Solid-state structure of [(R,R)-iPrDuPhosCo(η1-CO)3][BArF4] at 30% probability ellipsoids with H atoms and BArF4− anion omitted for clarity.
Electronic Structure Studies of [P2CoCOD]+ and P2CoCOD.
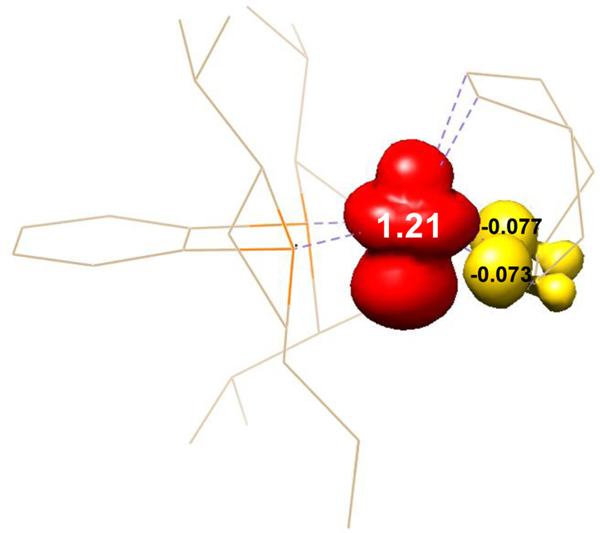
The electronic structures of the neutral and cationic cobalt complexes were investigated by full molecule density functional theory (DFT) using the B3LYP functional. The qualitative d-orbital splitting diagrams for [P2CoCOD]+ are shown in Figure 5A. The HOMO is principally dz2 in character as expected for a square-planar d8 metal complex with σ-donating phosphines and π-accepting alkene (diene) ligands.16 The qualitative d-orbital splitting diagram and the Mulliken spin density plot for P2CoCOD are likewise presented in Figures 5B and 6. The spin density distribution on cobalt approximates the shape of the dz2 orbital, suggesting dz2 as the singly occupied molecular orbital (SOMO) of the S = 1/2 complex. This contrasts expectations for a cobalt(0), d9 complex where Jahn-Teller distortion resulting in a D2d geometry would be expected and the dxy, dxz and dyz orbitals would have higher energies than dx2-y2, dz2 orbitals and dz2 would be filled.
Figure 5.
Qualitative molecular orbital diagrams representing the corresponding orbitals possessing significant d-orbital character of A) [P2CoCOD]+; B) P2CoCOD computed by DFT at B3LYP/def2-SVP/def2-TZVP level of theory. Calculations were initiated from optimized solid-state structures.
Figure 6.
Spin-density plot for P2CoCOD obtained from the Mulliken population analysis (red, positive spin density; yellow, negative spin density). DFT calculation was performed at the B3LYP/def2-SVP/def2-TZVP level of theory. Calculations were initiated from optimized solid-state structure.
A closer examination of the metrical parameters from the solid-state structure of P2CoCOD5c revealed that one alkene double bond of COD was longer than the other (1.417(3) Å vs. 1.396(3) Å), while the average Co–C bond length was shorter than the other (2.044(3) Å vs. 2.128(2) Å), indicating stronger back-bonding interaction and metallacyclopropane character17 of one “Co–C=C” bonds (Figure 7A). The elongated C=C bond length is comparable to that (1.423 Å) of a structurally characterized cobalt ethylene complex, (PMe)3Co(Ph)(η2-C2H4).18 By comparison, both alkene double bond distances of COD of the cationic [P2CoCOD]+ are 1.39(1) Å (Figure 7A), suggesting weaker back-bonding from Co(I)+ and a lack of metallacyclopropane character. The solid-state structure of P2CoCOD showed distortion from an idealized D2d geometry towards a more square-pyramidal configuration, where the phosphines, cobalt and one C=C bond of the cyclooctadiene define the basal plane while the other alkene is apical. A similar distortion towards square-pyramidal geometry and metallacyclopropane character have also been identified in the solid-state structures of (dppe)Co(COD)5b (dppe = 1,2-Bis(diphenylphosphino)ethane) and (R,R)-(BenzP*)Co(COD)5d ((R,R)-BenzP* = (R,R)-1,2-Bis(t-butylmethylphosphino)benzene) (Figure 7A). As such, these formally P2Co(0)COD complexes are best described as five-coordinate, d7, cobalt(II) compounds with the cyclooctadiene being viewed as an LX2-type ligand. The four strong-field donors consisting of phosphines and alkyls occupy the xy-plane and largely determine the relative d-orbital energies similar to those in a square-planar field with perturbation from the weaker field alkene donor coordinating through the z-axis and slightly raising the energies of the dz2, dxz and dyz parentage orbitals. Accordingly, the spin density analysis and qualitative d-orbital splitting diagram suggest that the SOMO of the low spin, d7 cobalt complex has primarily dz2 character with contributions from the cyclooctadiene carbon p orbitals, while the dxz, dyz and dxy orbitals are filled and dx2-y2 orbital is empty (Figures 6, 5B).
Figure 7.
A) Summary of bond distances of P2CoCOD complexes supporting the metallacyclopropane assignments. Bond distances of [P2CoCOD]+ are reported for comparison. B) Truncated solid-state structure of P2CoCOD at 30% probability ellipsoids with H atoms omitted for clarity.
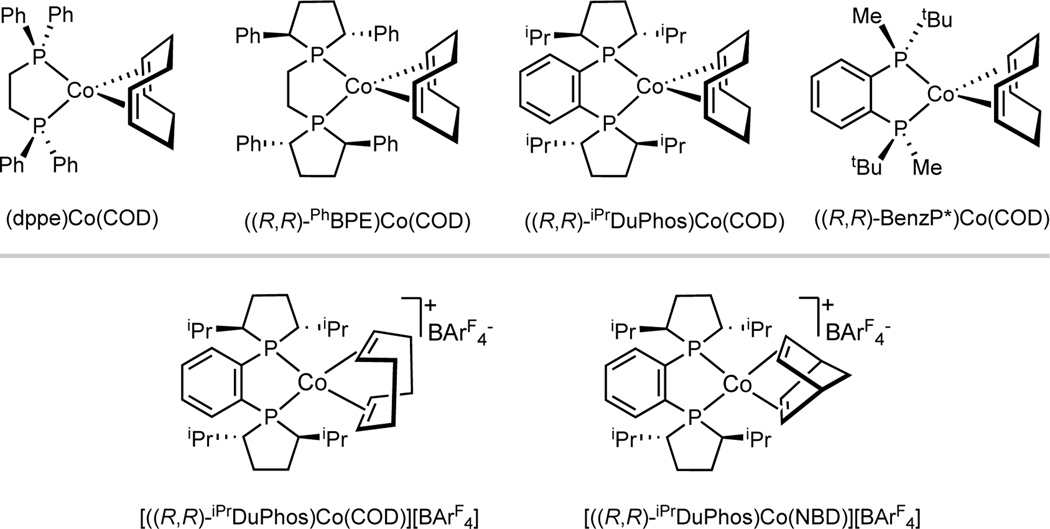
In addition, the X-band EPR spectra of P2CoCOD, (dppe)CoCOD, (R,R)-(BenzP*)CoCOD and (R,R)-(PhBPE)CoCOD ((R,R)-PhBPE = 1,2-bis[(2R,5R)-2,5-diphenylphospholano]-ethane) all exhibit characteristic hyperfine coupling of one g tensor to the 59Co nucleus (I = 7/2) but no coupling to the 31P nuclei,5b–d indicating a lack of dx2-y2 character(phosphines are on the xy plane) of the SOMO and further supporting a d7 instead of d9 electronic configuration. Nevertheless, the relatively small g anisotropy for all P2CoCOD complexes5b–d compared to the large g anisotropy characteristic of square-planar, low spin, d7 cobalt(II) complexes, including (dppe)Co(CH2SiMe3)2 5b and (iPrmPNP)Co(X) (X = CH3, Cl and H, iPrmPNP is an L2X-type ligand ),19 also suggest that the P2CoCOD compounds are electronically differentiated from low spin, planar L2CoX2 complexes. For a related structurally characterized P2Co(0)(η6-arene) precatalyst, (R,R)-(PhBPE)Co(η6-C6H6),5c DFT-computed Mulliken spin density distribution on cobalt approximate the shape of the dx2-y2 orbital (Supporting Information, Figure S10), supporting a Co(0), d9 assignment for the η6-arene complex.
Taken together, the slow substitution rate of the 17-electron P2CoCOD may be attributed to the strong binding of one cyclooctadiene C=C bond to the cobalt through strong π-back donation, resulting in a rate-determining alkene dissociation step. In addition, the shift of electron density from cobalt to the alkene fragment likely makes the metal less reducing and consequently less reactive towards protic solvents such as methanol. A stepwise substitution mechanism accounting for the overall dissociative process established from kinetic measurements is proposed in Scheme 3, where the transition states have primarily cobalt–carbon bond-breaking character. Dissociation of the apical alkene arm provided space for an incoming cyclooctadiene molecule affording a 17-electron bis(η2-cyclooctadiene) intermediate, while formation of a 15-electron mono(η2-cyclooctadiene) intermediate is less likely. Subsequent tautomerization between the apical and equatorial COD on the metallacyclopropane component and alkene dissociation generated the product. In comparison, oxidation by one electron to furnish the corresponding cation, [P2CoCOD]+, resulted in markedly higher substitutional lability and faster ligand self-exchange rate likely originating from the electrophilicity of the cobalt(I) center and a stabilization effect from coordination of another alkene. An associative substitution for [P2CoCOD]+ is likely operative and occurs through an 18-electron intermediate. The planar geometry observed with [P2CoCOD]+ may also provide greater access of an incoming ligand from the apical direction, resulting in an increased rate of substitution.
Scheme 3.
Proposed mechanism of ligand self-exchange reaction of P2CoCOD-d12 and COD.
Conclusions
A dissociative substitution mechanism for the 17-electron, neutral P2CoCOD complex was established by kinetic studies on the self-exchange reaction between 1,5-cyclooctadiene and 1,5-cyclooctadiene-d12. Rapid displacement of 1,5-cyclooctadiene by carbon monoxide and formation of coordinatively saturated bis(phosphine)cobalt carbonyl complexes was observed for both P2CoCOD and the 16-electron cobalt cation, [P2CoCOD]+. Electronic structure studies and metrical parameters from the solid-state structures of P2CoCOD complexes support a cobalt(II)–metallacyclopropane assignment. The strengthened interaction within the metallacyclopropane and a more electropositive cobalt(II) center provide a rationale for the unusual protic stability of these formally cobalt(0) precatalysts.
Supplementary Material
Figure 1.
Examples of previously reported well-defined bis(phosphine)cobalt diene compounds.
Acknowledgements
H.Z. and P.J.C. acknowledge financial support from a National Science Foundation (NSF) Grant Opportunities for Academic Liaison with Industry (GOALI) grant (CHE-1855719). M.M.B. acknowledges an NIH F32 fellowship (F32 GM134610).
Footnotes
Associated Content
Additional experimental and computational details, characterization data including NMR spectra of new complexes are available in the Supporting Information. CCDC 2022560 contain the supplementary crystallographic data for [(R,R)-iPrDuPhosCo(η1-CO)3][BArF4].
References
- 1.a) Chirik PJ; Morris RH Getting Down to Earth: The Renaissance of Catalysis with Abundant Metals. Acc. Chem. Res. 2015, 48, 2495–2495. [DOI] [PubMed] [Google Scholar]; b) Mukerjee A; Milstein D. Homogeneous Catalysis by Cobalt and Manganese Pincer Complexes. ACS Catal. 2018, 8, 11435–11469. [Google Scholar]; c) Chen J; Lu Z. Asymmetric hydrofunctionalization of minimally functionalized alkenes via earth abundant transition metal catalysis. Org. Chem. Front. 2018, 5, 260–272. [Google Scholar]; d) Obligacion JV; Chirik PJ Earth-abundant transition metal catalysts for alkene hydrosilylation and hydroboration. Nat. Rev. Chem. 2018, 2, 15–34.. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Fürstner A. Iron Catalysis in Organic Synthesis: A Critical Assessment of What It Takes To Make This Base Metal a Multitasking Champion. ACS Cent. Sci. 2016, 2, 778–789. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Hayler JD; Leahy DK; Simmons EM A Pharmaceutical Industry Perspective on Sustainable Metal Catalysis. Organometallics 2019, 38, 36–46. [Google Scholar]
- 2.a) Zuo W; Lough AJ; Li Y; Morris RH Amine(imine)diphosphine Iron Catalysts for Asymmetric Transfer Hydrogenation of Ketones and Imines. Science, 2013, 342, 1080–1083. [DOI] [PubMed] [Google Scholar]; b) Morris RH Exploiting Metal-Ligand Bifunctional Reactions in the Design of Iron Asymmetric Hydrogenation Catalysts. Acc. Chem. Res. 2015, 48, 1494–1502. [DOI] [PubMed] [Google Scholar]; c) Morris RH Iron Group Hydrides in Noyori Bifunctional Catalysis. Chem. Rec. 2016, 6, 2640–2654. [DOI] [PubMed] [Google Scholar]; d) Morris RH Mechanisms of the H2- and transfer hydrogenation of polar bonds catalyzed by iron group hydrides. Dalton Trans. 2018, 47, 10809–10826. [DOI] [PubMed] [Google Scholar]
- 3.a) Li Y-Y; Yu S-L; Shen W-Y; Gao J-X Iron-, Cobalt-, and Nickel-Catalyzed Asymmetric Transfer Hydrogenation and Asymmetric Hydrogenation of Ketones. Acc. Chem. Res. 2015, 48, 2587–2598. [DOI] [PubMed] [Google Scholar]; b) Liu W; Sahoo B; Junge K; Beller M. Cobalt Complexes as an Emerging Class of Catalysts for Homogeneous Hydrogenations. Acc. Chem. Res. 2018, 51, 1858–1869. [DOI] [PubMed] [Google Scholar]; c) Wei D; Darcel C. Iron Catalysis in Reduction and Hydrometalation Reactions. Chem. Rev. 2019, 119, 2550–2610. [DOI] [PubMed] [Google Scholar]; d) Alig L; Fritz M; Schneider S. First-Row Transition Metal (De)Hydrogenation Catalysis Based On Functional Pincer Ligands. Chem. Rev. 2019, 119, 2681–2751. [DOI] [PubMed] [Google Scholar]; e) Ai W; Zhong R; Liu X; Liu Q. Hydride Transfer Reactions Catalyzed by Cobalt Complexes. Chem. Rev. 2019, 119, 2876–2953. [DOI] [PubMed] [Google Scholar]; f) Mukherjee A; Milstein D. Homogeneous Catalysis by Cobalt and Manganese Pincer Complexes. ACS Catal. 2018, 8, 11435−11469. [Google Scholar]
- 4.a) Shevlin M; Friedfeld MR; Sheng H; Pierson NA; Hoyt JM; Campeau L-C; Chirik PJ Nickel-Catalyzed Asymmetric Alkene Hydrogenation of α, β-Unsaturated Esters: High-Throughput Experimentation-Enabled Reaction Discovery, Optimization, and Mechanistic Elucidation. J. Am. Chem. Soc. 2016, 138, 3562–3569. [DOI] [PubMed] [Google Scholar]; b) Liu Y; Yi Z; Tan X; Dong X; Zhang X. Nickel-Catalyzed Asymmetric Hydrogenation of Cyclic Sulfami-date Imines: Efficient Synthesis of Chiral Cyclic Sulfami-dates. iScience, 2019, 19, 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Du X; Xiao Y; Huang J; Zhang Y; Duan Y; Wang H; Shi C; Chen G; Zhang X. Cobalt-catalyzed highly enantioselective hydrogenation of α,β-unsaturated carboxylic acids. Nat. Commun. 2020, 11, 3239. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Li B; Chen J; Zhang Z; Gridnev ID; Zhang W. Nickel-Catalyzed Asymmetric Hydrogenation of N-Sulfonyl Imines. Angew. Chem. Int. Ed. 2019, 58, 7329–7334. [DOI] [PubMed] [Google Scholar]; e) Xu H; Yang P; Chuanprasit P; Hirao H; Zhou J. Nickel‐Catalyzed Asymmetric Transfer Hydrogenation of Hydrazones and Other Ketimines. Angew. Chem. Int. Ed. 2015, 54, 5112–5116. [DOI] [PubMed] [Google Scholar]
- 5.a) Friedfeld MR; Shevlin M; Hoyt JM; Krska SW; Tudge MT; Chirik PJ Cobalt precursors for high-throughput discovery of base metal asymmetric hydrogenation catalysts. Science 2013, 342, 1076–1080. [DOI] [PubMed] [Google Scholar]; b) Friedfeld MR; Margulieux GW; Schaefer BA; Chirik PJ Bis(phosphine) cobalt dialkyl complexes for directed alkene hydrogenation. J. Am. Chem. Soc. 2014, 136, 13178−13181. [DOI] [PubMed] [Google Scholar]; c) Friedfeld MR; Zhong H; Ruck RT; Shevlin M; Chirik PJ Cobalt-catalyzed asymmetric hydrogenation of enamides enabled by single-electron reduction. Science 2018, 360, 888–893. [DOI] [PubMed] [Google Scholar]; d) Zhong H; Shevlin M; Chirik PJ Cobalt-Catalyzed Asymmetric Hydrogenation of α,β-Unsaturated Carboxylic Acids by Homolytic H2 Cleavage. J. Am. Chem. Soc. 2020, 142, 5272–5281. [DOI] [PubMed] [Google Scholar]
- 6.a) Hood DM; Johnson RA; Carpenter AE; Younker JM; Vinyard DJ; Stanley GG Highly active cationic cobalt(II) hydroformylation catalysts. Science, 2020, 367, 542–548. [DOI] [PubMed] [Google Scholar]; b) MacNeil CS; Mendelsohn LN; Zhong H; Pabst TP; Chirik PJ Synthesis and Reactivity of Organometallic Intermediates Relevant to Cobalt-Catalyzed Hydroformylation. Angew. Chem. Int. Ed. 2020, 59, 8912–8916. [DOI] [PubMed] [Google Scholar]
- 7.a) Chen Q; Kim DK; Dong VM Regioselective Hydroacylation of 1,3-Dienes by Cobalt Catalysis. J. Am. Chem. Soc. 2014, 136, 3772–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kim DK; Riedel J; Kim RS; Dong VM Cobalt Catalysis for Enantioselective Cyclobutanone Construction. J. Am. Chem. Soc. 2017, 139, 10208−10211. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Santhoshkumar R; Mannathan S; Cheng C-H Ligand-Controlled Divergent C–H Functionalization of Aldehydes with Enynes by Cobalt Catalysts. J. Am. Chem. Soc. 2015, 137, 16116–16120. [DOI] [PubMed] [Google Scholar]; d) Yang J; Rerat A; Lim YJ; Gosmini C; Yoshikai N. Cobalt-Catalyzed Enantio- and Diastereoselective Intramolecular Hydroacylation of Trisubstituted Alkenes. Angew. Chem. Int. Ed. 2017, 56, 2449–2453. [DOI] [PubMed] [Google Scholar]; e) Whyte A; Bajohr J; Torelli A; Lautens M. Enantioselective Cobalt-Catalyzed Intermolecular Hydroacylation of 1,6-Enynes. Angew. Chem. Int. Ed. 2020, accepted article, 10.1002/anie.202006716. [DOI] [PubMed] [Google Scholar]
- 8.a) Röse P; Hilt G. Cobalt-Catalysed Bond Formation Reactions; Part 2. Synthesis 2016, 48, 463–492. [Google Scholar]; b) Jing SM; Balasanthiran V; Pagar VV; Gallucci JC; RajanBabu TV; Catalytic Enantioselective Hetero-dimerization of Acrylates and 1,3-Dienes. J. Am. Chem. Soc. 2017,139,18034; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Biswas S; Page JP; Dewese KR; RajanBabu TV Asymmetric Catalysis with Ethylene. Synthesis of Functionalized Chiral Enolates. J. Am. Chem. Soc. 2015, 137, 14268; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Page JP; RajanBabu TV Asymmetric Hydrovinylation of 1-Vinylcycloalkenes. Reagent Control of Regio- and Stereoselectivity. J. Am. Chem. Soc. 2012, 134, 6556; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Hilt G; Lüers S. Cobalt(I)-catalyzed 1,4-Hydrovinylation Reactions of 1,3-Dienes with Functionalized Terminal Alkenes under Mild Conditions. Synthesis 2002, 609. [Google Scholar]
- 9.a) Pagar VV; RajanBabu TV Tandem catalysis for asymmetric coupling of ethylene and enynes to functionalized cyclobutanes. Science 2018, 361, 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Fiebig L; Kuttner J; Hilt G; Schwarzer MC; Frenking G; Schmalz H; Schäfer M. Cobalt Catalysis in the Gas Phase: Experimental Characterization of Cobalt(I) Complexes as Intermediates in Regioselective Diels–Alder Reactions. J. Org. Chem. 2013, 78,10485. [DOI] [PubMed] [Google Scholar]
- 10.a) Wu C; Yoshikai N. Cobalt-Catalyzed Intramolecular Reactions between a Vinylcyclopropane and an Alkyne: Switchable [5+2] Cycloaddition and Homo-Ene Pathways. Angew. Chem. Int. Ed. 2018, 57, 6558–6562. [DOI] [PubMed] [Google Scholar]; b) Wang C; Monaco SD; Thai AN; Rahman MS; Pang BP; Wang C; Yoshikai N. Cobalt/Lewis Acid Catalysis for Hydrocarbofunctionalization of Alkynes via Cooperative C−H Activation. J. Am. Chem. Soc. 2020, 142, 12878–12889. [DOI] [PubMed] [Google Scholar]; c) Whyte A; Torelli A; Mirabi B; Prieto L; Rodríguez JF; Lautens M. Cobalt-Catalyzed Enantioselective Hydroarylation of 1,6-Enynes. J. Am. Chem. Soc. 2020, 142, 9510–9517. [DOI] [PubMed] [Google Scholar]
- 11.Zhong H; Friedfeld MR; Camacho-Bunquin J; Sohn H; Yang C; Delferro M; Chirik PJ Exploring the Alcohol Stability of Bis(phosphine) Cobalt Dialkyl Precatalysts in Asymmetric Alkene Hydrogenation. Organometallics 2019, 38, 149. [Google Scholar]
- 12.Zhong H; Friedfeld MR; Chirik PJ Syntheses and Catalytic Hydrogenation Performance of Cationic Bis(phosphine) Cobalt(I) Diene and Arene Compounds. Angew. Chem., Int. Ed. 2019, 58, 9194–9198. [DOI] [PubMed] [Google Scholar]
- 13.a) tom Dieck H; Dietrich J. Selectivity and Mechanism of Diene Cyclodimerization on Iron(0) Complexes. Angew. Chem., Int. Ed. Engl. 1985, 24, 781–783. [Google Scholar]; b) Lee H; Campbell MG; Hernández Sánchez R; rgel J; Raynaud J; Parker SE; Ritter T. Mechanistic Insight Into High-Spin Iron(I)-Catalyzed Butadiene Dimerization. Organometallics 2016, 35, 2923–2929. [Google Scholar]; c) Kennedy CR; Zhong H; Macaulay RL; Chirik PJ Regio- and Diastereoselective Iron-Catalyzed [4+4]-Cycloaddition of 1,3-Dienes. J. Am. Chem. Soc. 2019, 141, 8557–8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Q; Richmond TG; Trogler WC; Basolo F. Mechanism of carbon monoxide substitution in metal carbonyl radicals: vanadium hexacarbonyl and its phosphine-substituted derivatives. J. Am. Chem. Soc. 1984, 106, 71–76. [Google Scholar]
- 15.a) Zhao J; Goldman AS; Hartwig JF Oxidative Addition of Ammonia to Form a Stable Monomeric Amido Hydride Complex. Science, 2005, 307, 1080–1082. [DOI] [PubMed] [Google Scholar]; b) Göttker-Schnetmann I; Brookhart M. Mechanistic Studies of the Transfer Dehydrogenation of Cyclooctane Catalyzed by Iridium Bis(phosphinite) p-XPCP Pincer Complexes. J. Am. Chem. Soc. 2004, 126, 9330–9338. [DOI] [PubMed] [Google Scholar]
- 16.Börgel J; Campbell MG; Ritter T. Transition Metal d-Orbital Splitting Diagrams: An Updated Educational Resource for Square Planar Transition Metal Complexes. J. Chem. Educ. 2016, 93, 118–121. [Google Scholar]
- 17.(a) Dewar MJS A review of π Complex Theory. Bull. Soc. Chim. Fr. 1951, 18, 71–79. [Google Scholar]; (b) Chatt J; Duncanson LA Olefin coordination compounds. Part III. Infra-red spectra and structure: attempted preparation of acetylene complexes. J. Chem. Soc. 1953, 2939–2947. [Google Scholar]
- 18.Klein H-F; Groβ J; Hammer R; Schubert R. Methylcobaltverbindungen mit nicht chelatisierenden Liganden, IV. Chem. Ber. 1983, 116, 1441–1449. [Google Scholar]
- 19.Semproni SP; Milsmann C; Chirik PJ Four-Coordinate Cobalt Pincer Complexes: Electronic Structure Studies and Ligand Modification by Homolytic and Heterolytic Pathways. J. Am. Chem. Soc. 2014, 136, 25, 9211–9224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.