Abstract
Background and Aims
Post Covid-19 syndrome (PCS) is a major cause of morbidity. In this article we intend to review the association and consequences of PCS and diabetes.
Methods
We reviewed all studies on “Long Covid”, “Post COVID-19 Syndrome” and diabetes in PubMed and Google Scholar.
Results
The symptoms of PCS can be due to organ dysfunction, effects of hospitalisation and drugs, or unrelated to these. Type 2 diabetes mellitus has a bidirectional relationship with COVID-19. Presence of diabetes also influences PCS via various pathophysiological mechanisms. COVID-19 can add to or exacerbate tachycardia, sarcopenia (and muscle fatigue), and microvascular dysfunction (and organ damage) in patients with diabetes.
Conclusion
PCS in patients with diabetes could be detrimental in multiple ways. Strict control of diabetes and other comorbidities, supervised rehabilitation and physical exercise, and optimal nutrition could help in reducing and managing PCS.
Keywords: Diabetes, Long COVID, Post COVID syndrome, Sarcopenia, Fatigue, Tachycardia
Patients may suffer from several debilitating symptoms/signs that develop during or after an infection consistent with COVID-19. These symptoms continue for more than 12 weeks after COVID-19 infection, and are not explained by an alternative diagnosis [1]. Common symptoms include fatigue, breathlessness, myalgia, weakness, headache, cognitive blunting etc. This conglomeration of symptoms is called “Long COVID” or more appropriately as “Post COVID-19 Syndrome (PCS)” [2]. The aim of the brief review is to summarise current knowledge of health events occurring after COVID-19 in brief and relate them for patients with diabetes.
These symptoms often occur in clusters, overlap with each other, and a have tendency to wax and wane. These can be either persisting symptoms which occurred during acute COVID-19 infection, or new symptoms, and these can involve all organ systems [3]. In patients with health issues before SARS-CoV-2 infection, aggravation of pre-existing symptoms has also been noticed [4]. The most common symptoms is fatigue which is often profound and prolonged and shares characteristics with chronic fatigue syndrome (CFS) seen with other viral infections. Risk of PCS is more likely in those with more than 5 symptoms during acute COVID-19 and more in women, elderly, in obese individuals and in patients with diabetes [5,6]. Further, persistent symptoms were more common in people with severe disease at presentation and in those with increased convalescent antibody titres [7].
Based on the initial symptoms, duration of symptoms, period of quiescence and time of onset of symptoms, long COVID (PCS) can be divided into five categories (Table 1 ) [8]. As can be noted from the classifications, symptoms could last 3–12 months, and vary in severity from mild to severe, or even lead to death. Based on pathophysiological mechanisms, we propose classification of PCS, which may help clinicians to plan its management in a rational manner (Table 2 ).
Table 1.
Types of long COVID-19 syndrome [6].
| Type of long COVID-19 syndrome | Features |
|---|---|
| Type 1 | Symptoms with varying lengths of recovery and rehabilitation that directly correlate with the severity of infection, target organ damage and pre-existing medical conditions at the time of initial infection |
| Type 2 | Symptoms persisting for 6 weeks from the time of initial infection |
| Type 3 | Period of quiescence or near-complete recovery following initial infection, followed by a return of symptoms that persist for ≥3 months (Type 3A) or ≥ 6 months (Type 3B) |
| Type 4 | Initially asymptomatic at the time of a positive SARS-CoV-2 test but develop symptoms beginning 1–3 months (Type 4A) or ≥ 3 months (Type 4B) later that persist for varying lengths of time |
| Type 5 | Initially asymptomatic or minimally symptomatic at the time of a positive SARS-CoV-2 test and experience sudden death within the next 12 months. |
Table-2.
Proposed classification of post COVID-19 syndrome.
| A. Symptoms related to organ dysfunctions |
| 1. Symptoms related to various sequelae of COVID-19 |
| a. New onset diabetes |
| b. Kidney dysfunction |
| c. Pulmonary symptoms related to fibrosis |
| d. Cardiac dysfunction |
| e. Hepatic dysfunction |
| f. Neurological dysfunction |
| g. Sarcopenia |
| 2. Symptoms related to co-morbidity present before COVID-19 (renal, cardiac, diabetes, hepatic, pulmonary, neurological etc) |
| a. Status of the co-morbidity same as before COVID-19 |
| b. Status of the co-morbidity worsened after COVID-19 |
| 3. Symptoms related to acute complications of the co-morbidity and or COVID-19 |
| a. Diabetic ketoacidosis |
| b. Myocardial infarction |
| c. Stroke |
| d. Pulmonary embolism/thrombosis in other body areas |
| B. Symptoms related to hospitalisation and its consequences: drug therapy, concomitant and subsequent infections, nutritional deficiencies and psychological stress |
| a. Due to long hospitalisation, prolonged artificial ventilation, sequelae of critical illness, post intensive care syndrome |
| b. Infections: secondary infections, mucormycosis |
| c. Psychological issues -Anxiety, depression, post-traumatic stress disorder |
| d. Nutritional deficiencies |
| e. Drug side effects |
| C. Symptoms not readily explained by any known cause (list only includes common symptoms)a |
| a. Fatigue |
| b. ‘Brain fog’, memory lapses |
| c. Myalgias and arthralgias |
| d. Headache |
| e. Diarrhoea etc. |
Some of these may also be partly influenced by other conditions listed above.
Pathophysiologically, post COVID-19 syndrome is not a single clinical entity but a conglomeration of symptoms and signs due to multiple biological factors, which need more research. Commonly understood factors include organ damage, persistently dysregulated inflammatory and immune responses, and unrecognised microvascular thrombosis and endotheliitis [9]. Many other factors have been hypothesised; persistent tissue reservoirs of SARS-CoV-2, re-activation of other viruses, brainstem and/or vagus nerve dysfunctions and activation of autoimmunity due to molecular mimicry between pathogen and host proteins [10]. In addition, secondary infections (bacterial or fungal), effects of prolonged hospitalisation, sequelae of critical illness, post intensive care syndrome, drug side effects (e.g., corticosteroids), socioeconomic and psychological impact of the illness, may contribute [2,11]. Further, protein and micronutrient deficiencies due to prolonged hospitalisation and poor oral intake, cause nutrient deficient state in patients with severe COVID-19 [12,13]. These may be more common in Indian patients who have inadequate intake of protein and are often Vitamin B12 and Vitamin D deficient [14].
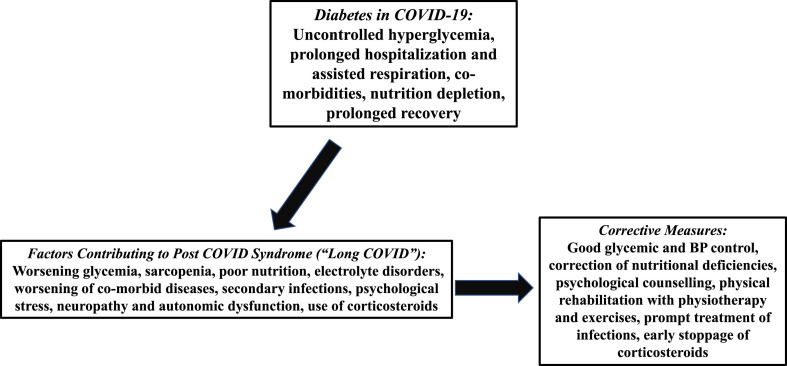
Type 2 diabetes mellitus (T2DM) has a bidirectional relationship with COVID-19 [15,16]. Poorly controlled diabetes increases the severity of COVID-19 and is associated with increased morbidity and mortality. COVID-19 pandemic has also resulted in poor control of diabetes, progression of prediabetes to diabetes, increase in number of new onset diabetes and rise of corticosteroid induced diabetes [15,17]. Theoretically, it is possible that patients with long standing diabetes, which may by itself cause debilitation, could predispose to PCS. Long duration of uncontrolled diabetes causes organ damage, and in particular microvascular injury, and these can be exacerbated in patients with SARS-CoV-2 infection. Further, diabetes increases the risk of development of severe and critical COVID-19 illness, increases the risk of hospitalisation and the need for mechanical ventilatory support, factors which can contribute to PCS. (Fig. 1 ). One of the sequelae of lung damage in patients with SARS-CoV-2 infection, i.e., pulmonary fibrosis, which can present with persistent breathlessness, requiring oxygen supplementation in post COVID-19 period, is more common in people with poorly controlled diabetes [18]. It is also reasonable to state that pre-existing low grade inflammatory state, as seen in T2DM, may get exacerbated and remains at a heightened state post COVID-19 that may cause several symptoms. Post COVID-19, infections (e.g., Rhinocerebral Mucormycosis) may also contribute [19]. Corticosteroid therapy for COVID-19, widely practiced in India, poses further problems [20]. High dose corticosteroid therapy causes severe hyperglycaemia, electrolyte imbalance and myopathy.
Fig. 1.
Relationship between post COVID-19 syndrome and diabetes mellitus and suggested corrective measures.
Three issues need further deliberation since both diabetes and COVID-19 may contribute to worsen them in an overlapping manner. First is postural tachycardia syndrome (POTS), which is diagnosed based on the following criteria: orthostatic tachycardia (heart rate increase of > 30 beats per minutes in adults [> 40 beats per minute in patients aged 12–19 years] within 10 minutes of assuming upright posture) in the absence of orthostatic hypotension, for at least 3 months [21]. Exact pathophysiology and long-term consequences of this syndrome are yet not known. Patients with long-standing diabetes may have autonomic neuropathy and dysfunction leading to tachycardia and postural hypotension, and these signs may get exacerbated in case POTS is co-existing. A combination of two will cause fatigue, tachycardia, and giddiness thus contributing to PCS.
Second issue is post-COVID-19 muscle weakness contributing to fatigue. In diabetes, neuropathy and myopathy contribute to muscle atrophy and sarcopenia [22]. COVID-19 infection, hospitalisation, protein deficiency and corticosteroid therapy often cause rapid onset sarcopenia in severe COVID-19 infections [23]. Further, many Indians, especially women, have low skeletal muscle mass and are sarcopenic [24]. Combined, skeletal muscles would become weaker than in pre-COVID-19 period in patients with long duration of diabetes and particularly in elderly and women. This would cause fatigue, and limitation of walking, further adding to delay in recovery.
A third situation is mostly hypothetical since not much research data are available. It is well known that diabetes can cause microvascular complications involving eyes, nerves and kidneys. What is not known is if these complications can be exacerbated by microvascular damage due to COVID-19. Interestingly, capillary dysfunction {capillary cell swelling and damage (endotheliitis), microthrombosis, pericyte damage, scarring etc.} has been hypothesised to cause multiple symptoms of PCS including fatigue, ‘brain fog’, and memory problems [9]. Theoretically, severe capillary damage can cause renal and other organ failures, a particularly worrying scenario in patients with diabetes [9].
Rehabilitation of such patients is often slow and requires a multidisciplinary effort. For patients with diabetes, basic management principles must be adhered to. Strict glycaemic control and control of co-morbidities during acute COVID-19 would reduce development of PCS and help manage it. Steroids should be used only when it is indicated, for the shortest duration while glycemia should be strictly controlled. Prompt treatment of infection is important. Proper nutrition should be ensured, in particular, increase in protein intake and correction of vitamin and micronutrient deficiencies. Depending on symptoms, psychological counselling should be included in management. Physical therapy and exercise should be started after an adequate period of rest but should not be delayed. Exercise may have multi-pronged beneficial effects; on sarcopenia, mental health, pulmonary efficiency, immunity, glycemia and blood pressure. It is preferable to start patient on individualised and supervised exercise training including aerobic and resistance exercises and chest physiotherapy [25]. On the positive side, PCS improves with time in a significant number of patients. One study showed that the persistence of symptoms was seen in 13% lasting >28 days, 4.5% lasting >8 weeks and 2.3% > 12 weeks [5].
Overall, our understanding of PCS is improving but more research is needed. In particular, patients with diabetes would require care in glycemic and risk factor control to prevent the development and severity of PCS. Data on bidirectional adverse effects of diabetes and PCS are clearly needed.
References
- 1.COVID-19 rapid guideline: managing the long-term effects of COVID-19. National Institute for Health and Care Excellence (UK); London: 2020 Dec 18. https://www.ncbi.nlm.nih.gov/books/NBK567261/ [PubMed] [Google Scholar]
- 2.Raveendran A.V., Jayadevan R., Sashidharan S. Long COVID: An overview. Diabetes Metab Syndr. 2021;15(3):869–875. doi: 10.1016/j.dsx.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sisó-Almirall A., Brito-Zerón P., Conangla Ferrín L., Kostov B., Moragas Moreno A., Mestres J., Sellarès J., Galindo G., Morera R., Basora J., Trilla A., Ramos-Casals M. On behalf of the CAMFiC long covid-study group. Long covid-19: proposed primary care clinical guidelines for diagnosis and disease management. Int J Environ Res Publ Health. 2021 Apr 20;18(8):4350. doi: 10.3390/ijerph18084350.PMID:33923972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernández-de-Las-Peñas C., Florencio L.L., Gómez-Mayordomo V., Cuadrado M.L., Palacios-Ceña D., Raveendran A.V. Proposed integrative model for post-COVID symptoms [published online ahead of print, 2021 Jun 1] Diabetes Metab Syndr. 2021;15(4):102159. doi: 10.1016/j.dsx.2021.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudre C.H., Murray B., Varsavsky T. Attributes and predictors of long COVID. Nat Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman E.L., Savelieff M.G., Hayek S.S., Pennathur S., Kretzler M., Pop-Busui R. COVID-19 and diabetes: a collision and collusion of two diseases. Diabetes. 2020 Dec;69(12):2549–2565. doi: 10.2337/dbi20-0032. Epub 2020 Sep 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blomberg B., Mohn K.G.I., Brokstad K.A. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021 doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker R.C. COVID-19 and its sequelae: a platform for optimal patient care, discovery and training. J Thromb Thrombolysis. 2021:1–8. doi: 10.1007/s11239-021-02375-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Østergaard L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: consequences of capillary transit-time changes, tissue hypoxia and inflammation. Phys Rep. 2021 Feb;9(3) doi: 10.14814/phy2.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Proal A.D., VanElzakker M.B. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. 2021 Jun 23;12:698169. doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unnikrishnan R., Misra A. Infections and diabetes: risks and mitigation with reference to India. Diabetes Metab Syndr. 2020 Nov-Dec;14(6):1889–1894. doi: 10.1016/j.dsx.2020.09.022. Epub 2020 Sep 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Im J.H., Je Y.S., Baek J., Chung M.-H., Kwon H.Y., Lee J.-S. Nutritional status of patients with COVID-19. Int J Infect Dis. 2020;100:390–393. doi: 10.1016/j.ijid.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diabetes India National Diabetes Obesity and Cholesterol Foundation (NDOC), and Nutrition Expert Group, India. Balanced nutrition is needed in times of COVID19 epidemic in India: a call for action for all nutritionists and physicians. Diabetes Metab Syndr. 2020 Nov-Dec;14(6):1747–1750. doi: 10.1016/j.dsx.2020.08.030. Epub 2020 Aug 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayawardena R., Misra A. Balanced diet is a major casualty in COVID-19. Diabetes Metab Syndr. 2020 Sep-Oct;14(5):1085–1086. doi: 10.1016/j.dsx.2020.07.001. Epub 2020 Jul 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh A., Anjana R.M., Shanthi Rani C.S. Glycemic parameters in patients with new-onset diabetes during COVID-19 pandemic are more severe than in patients with new-onset diabetes before the pandemic: NOD COVID India Study. Diabetes Metab Syndr. 2021;15(1):215–220. doi: 10.1016/j.dsx.2020.12.033. Epub 2020 Dec 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unnikrishnan R., Misra A. Diabetes and COVID19: a bidirectional relationship. Nutr Diabetes. 2021 Jun 23;11(1):21. doi: 10.1038/s41387-021-00163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Misra A., Ghosh A., Gupta R. Heterogeneity in presentation of hyperglycaemia during COVID-19 pandemic: a proposed classification. Diabetes Metab Syndr. 2021;15(1):403–406. doi: 10.1016/j.dsx.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mrigpuri Parul, Sonal Sonal, Spalgais Sonam, Goel Nitin, Menon Balakrishnan, Kumar Raj. Uncontrolled diabetes mellitus: a risk factor for post covid fibrosis. Monaldi Arch Chest Dis. 2021;91(1) doi: 10.4081/monaldi.2021.1607. [DOI] [PubMed] [Google Scholar]
- 19.Singh A.K., Singh R., Joshi S.R., Misra A. Mucormycosis in COVID-19: a systematic review of cases reported worldwide and in India [published online ahead of print, 2021 May 21] Diabetes Metab Syndr. 2021;15(4):102146. doi: 10.1016/j.dsx.2021.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sosale A., Sosale B., Kesavadev J., Chawla M., Reddy S., Saboo B., Misra A. Steroid use during COVID-19 infection and hyperglycemia - what a physician should know. Diabetes Metab Syndr. 2021 Jun 10;15(4):102167. doi: 10.1016/j.dsx.2021.06.004. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raj S.R., Guzman J.C., Harvey P., Richer L., Schondorf R., Seifer C., Thibodeau-Jarry N., Sheldon R.S. Canadian cardiovascular society position statement on postural orthostatic tachycardia syndrome (POTS) and related disorders of chronic orthostatic intolerance. Can J Cardiol. 2020 Mar;36(3):357–372. doi: 10.1016/j.cjca.2019.12.024.PMID:32145864. [DOI] [PubMed] [Google Scholar]
- 22.Anagnostis P., Gkekas N.K., Achilla C., Pananastasiou G., Taouxidou P., Mitsiou M., Kenanidis E., Potoupnis M., Tsiridis E., Goulis D.G. Type 2 diabetes mellitus is associated with increased risk of sarcopenia: a systematic review and meta-analysis. Calcif Tissue Int. 2020 Nov;107(5):453–463. doi: 10.1007/s00223-020-00742-y. Epub 2020 Aug 9. [DOI] [PubMed] [Google Scholar]
- 23.Welch C., Greig C., Masud T., Wilson D., Jackson T.A. COVID-19 and acute sarcopenia. Aging Dis. 2020 Dec 1;11(6):1345–1351. doi: 10.14336/AD.2020.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gulati S., Misra A. Dietary proteins, metabolic syndrome, and sarcopenia: focus on Asian Indians. Diabetes Metab Syndr. 2019 Nov-Dec;13(6):3091–3092. doi: 10.1016/j.dsx.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Jimeno-Almazán A., Pallarés J.G., Buendía-Romero Á, Martínez-Cava A., Franco-López F., Sánchez-Alcaraz Martínez B.J., Bernal-Morel E., Courel-Ibáñez J. Post-COVID-19 syndrome and the potential benefits of exercise. Int J Environ Res Publ Health. 2021 May 17;18(10):5329. doi: 10.3390/ijerph18105329.PMID:34067776. [DOI] [PMC free article] [PubMed] [Google Scholar]