Abstract
Objective: To determine whether severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) antibody levels after the first dose of vaccine can predict the final antibody response, and whether this is dependent on the vaccine type.
Methods: Sixty-nine recipients of BNT162b2 (Pfizer/BioNTech) and 55 recipients of AZD1222 (AstraZeneca), without previous infection or immunosuppressive medication, were included in this study. Antibody levels were quantified 3 weeks after the first dose [directly before boostering in the case of AZD1222 (11 weeks after the first dose)] and 3 weeks after the second dose using the Roche Elecsys SARS-CoV-2 S total antibody assay.
Results: Median pre-booster {BNT162b2: 80.6 [interquartile range (IQR) 25.5–167.0] binding antibody units (BAU)/mL; AZD1222: 56.4 (IQR 36.4–104.8) BAU/mL; not significant} and post-booster [BNT162b2: 2092.0 (IQR 1216.3–4431.8) BAU/mL; AZD1222: 957.0 (IQR 684.5–1684.8) BAU/mL; P<0.0001] levels correlated well in the recipients of BNT162b2 (ρ=0.53) but not in the recipients of AZD1222. Moreover, antibody levels after the first dose of BNT162b2 correlated inversely with age (ρ=-0.33, P=0.013), whereas a positive correlation with age was observed after the second dose in recipients of AZD1222 (ρ=0.26, P=0.030).
Conclusions: The results of this study suggest that antibody levels quantified by the Roche Elecsys SARS-CoV-2 S assay before the booster shot could infer post-booster responses to BNT162b2, but not to AZ1222. In addition, this study found a vaccine-dependent effect on antibody responses, where age seems to play an ambivalent role.
Keywords: SARS-CoV-2, Serology, BNT162b2, AZD1222, Antibody, Age
Background
Vaccines against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) have been available for several months (Zaqout et al., 2021). Determination of spike-protein-specific antibodies after SARS-CoV-2 vaccination, although not recommended unrestrictedly (Centers for Disease Control and Prevention, 2021), is commonly performed. The post-vaccination antibody levels, even when measured with standardized commercially available CE-certified quantitative test systems, differ significantly (Kristiansen et al., 2021; Perkmann et al., 2021a). Furthermore, in addition to these analytically related differences, there are significant differences in expected levels depending on the age and serostatus of the vaccine recipients (Krammer et al., 2021; Perkmann et al., 2021b; Subbarao et al., 2021), the vaccine used (Eyre et al., 2021), and the timing of blood collection (elapsed time interval since first or second dose).
To date, the extent to which antibody levels after the first dose are suitable to infer the booster response are not clear. Similarly, it is unclear whether this response depends on the type of vaccine used. Indeed, this would be likely because vector and mRNA vaccines elicit different immune responses, with vector vaccines also including a non-spike-specific response directed against the vector (Federico, 2021).
This article reports differences in the predictability of SARS-CoV-2 vaccine post-booster levels measured with a quantitative antibody assay (Roche Elecsys SARS-CoV-2 S) dependent on the vaccine used. A differential impact of age on the antibody response to AZD1222 (AZD1222, Astra Zeneca) or BNT162b2 (BNT162b2, Pfizer/BioNTech) is also demonstrated.
Methods
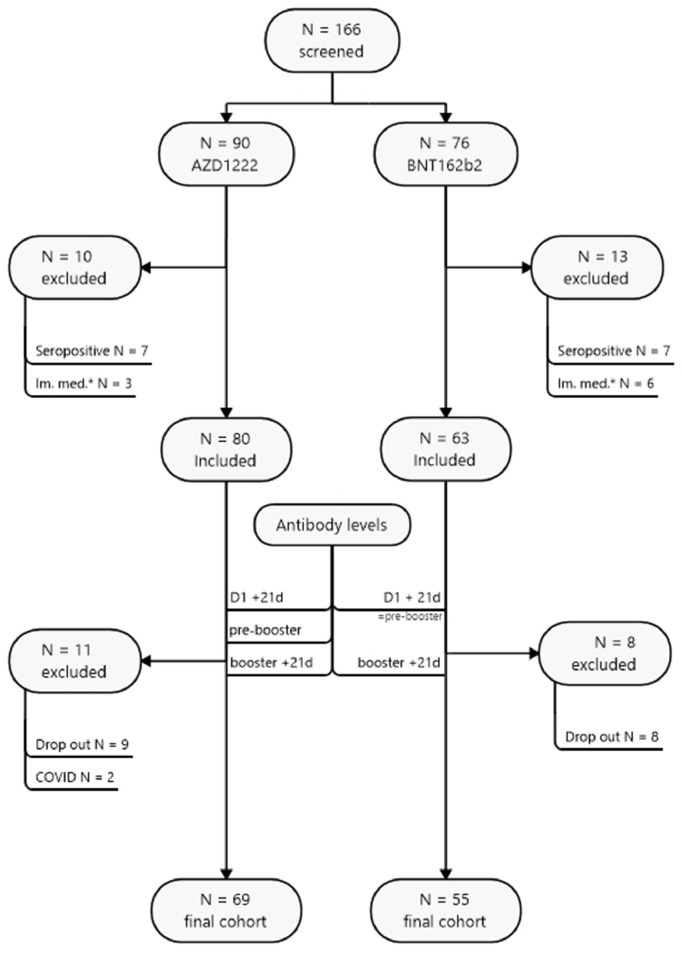
Of 166 participants recruited within the MedUni Wien Biobank's healthy donors' collection until 5 March 2021, 124 were eligible for inclusion. All subjects were aged >18 years and provided written informed consent to participate in the study. Reasons for exclusion were previous infection with SARS-CoV-2 and ongoing immunosuppressive medication, as these conditions are known to bias the average vaccination response. In addition, there were dropouts due to missed blood sampling and the onset of coronavirus disease 2019 between the first and second doses (see Figure 1 ). The prime-boost regimen specified an 11-week dosing interval for AZD1222 and a 3-week dosing interval for BNT162b2. The protocol of this performance evaluation study was reviewed and approved by the Ethics Committee of the Medical University of Vienna (EK 1066/2021).
Figure 1.
Study flow chart. COVID-19, coronavirus disease 2019.
Samples were processed and, if applicable, stored before analysis at <-70°C according to standard operating procedures by the MedUni Wien Biobank in an ISO 9001:2015-certified environment (Haslacher et al., 2018). Previous SARS-CoV-2 infection was ruled out or confirmed by the Roche Elecsys SARS-CoV-2 anti-nucleocapsid total antibody electrochemiluminescence assay (ECLIA), and assumed in all participants with SARS-CoV-2 infection proved using a polymerase chain reaction assay. Vaccine-induced anti-spike antibodies were quantified using the Roche Elecsys SARS-CoV-2 S total antibody ECLIA on Roche cobas e801 modular analysers. All analyses were performed at the Department of Laboratory Medicine, Medical University of Vienna, which operates a certified (ISO 9001:2015) and accredited (ISO 15189:2012) quality management system. Performance data of both tests have been published previously (Perkmann et al., 2020, 2021a).
Continuous data, given as median [interquartile range (IQR)], were compared by rank sign tests (Mann–Whitney U-test, Wilcoxon test). Categorical data, presented as counts and percentages, were compared by χ²-tests. Rank correlations were computed according to Pearson and presented by Pearson's ρ. P<0.05 was considered to indicate statistical significance. All calculations were performed using MedCalc 19.7 (MedCalc, Ostend, Belgium), and figures were drawn using Mindjet Manager 19 (Corel, Ottawa, Canada) and Prism 9 (GraphPad, La Jolla, CA, USA).
Results
Pre-booster antibody levels predict post-booster levels of BNT162b2 but not AZD1222
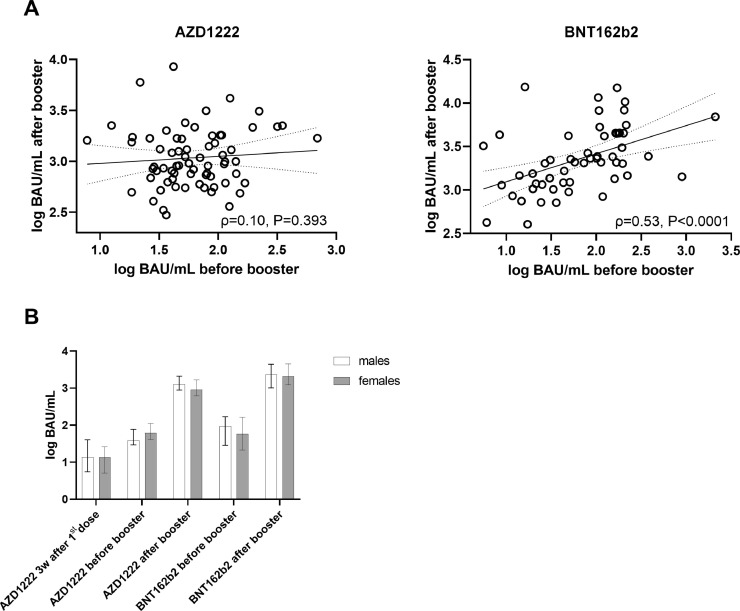
The 69 recipients of AZD1222 did not differ significantly from the 55 recipients of BNT162b2 in terms of age [median 42 (IQR 29–50) years vs. 42 (IQR 30–53.5) years, respectively; P=0.387]. However, the proportion of females was higher among the recipients of AZD1222 [57/69 (83%) vs. 31/55 (56%); χ²=10.1, P=0.001]. Median pre-booster levels (11 weeks and 3 weeks after the first dose, respectively) were 56.4 (IQR 36.4–104.8) binding antibody units (BAU)/mL for AZD1222 and 80.6 (IQR 25.5–167.0) BAU/mL for BNT162b2 (P=0.513). Twenty-one (IQR 21–22) days after the booster shot, median antibody levels increased to 957.0 (IQR 684.5–1684.8) BAU/mL in recipients of AZD1222 and 2092.0 (IQR 1216.3–4431.8) BAU/mL in recipients of BNT162b2 (P<0.0001). Antibody levels did not differ significantly between males and females at any of the assessed time points (all P>0.05, see Figure 2B ).
Figure 2.
(A) Correlation (according to Spearman) between pre- and post-booster antibody levels (Roche Elecsys S total antibody electrochemiluminescence assay) in recipients of AZD1222 (left) and BNT162b2 (right). (B) Medians and interquartile ranges of antibody levels according to sex. BAU, binding antibody units.
Correlation of SARS-CoV-2 antibody levels before the booster shot and the antibody response assessed 21 (IQR 21–22) days after the booster was calculated. The correlation was significant for recipients of BNT162b2 (ρ=0.53, P<0.0001), but not for recipients of AZD1222 (ρ=0.10, P=0.393) (Figure 2A). These findings were not altered after controlling for sex in non-parametric partial correlations (ρpartial=0.53, ρpartial=0.15), as this variable was differently distributed between recipients of BNT162b2 and AZD1222.
Antibody levels increase steadily between 3 and 12 weeks after AZD1222
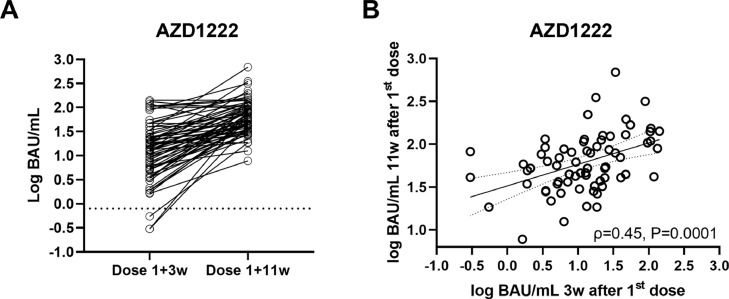
In the next step, changes in SARS-CoV-2 antibody levels between 3 and 11 weeks after the first dose of AZD1222 were assessed. Levels were significantly higher before the booster shot (11 weeks) compared with 3 weeks after the first dose [median 56.4 (IQR 36.4–104.8) BAU/mL vs. 13.4 (IQR 5.2–27.8) BAU/mL; P<0.0001; Figure 3A ]. Levels at both time points correlated at ρ=0.45 (Figure 3B). Regarding the pre-booster levels, the levels assessed 3 weeks after the first dose of AZD1222 did not correlate significantly with antibody responses to the booster shot (ρ=0.20, P=0.101). Again, controlling for sex did not significantly affect correlation coefficients (ρpartial=0.46, ρpartial=0.20)
Figure 3.
(A) Intra-individual progression (A) and correlation (B) of antibody levels (Roche Elecsys S total antibody electrochemiluminescence assay) after AZD1222, measured 3 weeks after the first dose and before the booster dose (11 weeks). BAU, binding antibody units.
AZD1222 and BNT162b2 behave in opposite ways regarding the correlation of age and antibody response
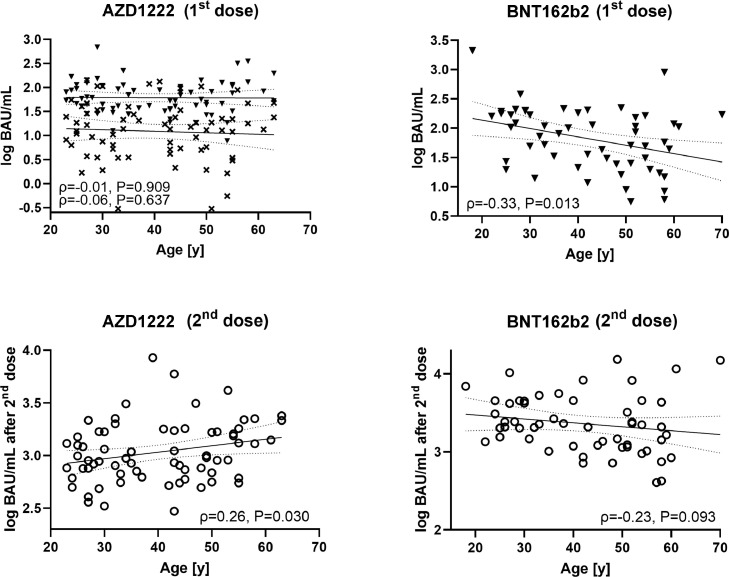
Finally, this study investigated if the age of the participants affected the antibody responses to the two vaccines. For AZD1222, age did not correlate with the antibody levels measured 3 weeks after the first dose (ρ=-0.06, P=0.637) or just before the second dose (ρ=-0.01, P=0.909). However, there was a weak positive association between age and the antibody response to the second dose of AZD1222 (ρ=0.26, P=0.030). In contrast, antibody levels assessed 3 weeks after the first dose of BNT162b2 correlated inversely with age (ρ=-0.33, P=0.013); however, this correlation was mitigated after the second dose (ρ=-0.23, P=0.093; Figure 4 ). As for the other reported associations, controlling for sex did not significantly affect correlation coefficients (ρpartial=-0.06, ρpartial=-0.02, ρpartial=0.27, ρpartial=-0.33, ρpartial=-0.23)
Figure 4.
Correlation (according to Spearman) between antibody levels (Roche Elecsys S total antibody electrochemiluminescence assay) and age for AZD1222 (left column) and BNT162b2 (right column) at different points in time. Crosses indicate 3 weeks after first dose of AZD1222, filled triangles indicate pre-booster, open circles indicate post-booster. BAU, binding antibody units.
Discussion
The relationship between the detection of SARS-CoV-2-specific antibodies and immunity is becoming increasingly well established based on evidence from numerous studies (Hall et al., 2021; Lumley et al., 2021). Thus, identifying neutralizing antibodies to SARS-CoV-2 as a surrogate marker for established immunity is under discussion (Feng et al., 2021). In this sense, the association with neutralization assays has been investigated previously for several SARS-CoV-2 antibody-binding assays (Bal et al. 2021; Perkmann et al., 2021c). It has also been shown for the Roche S assay that antibody levels >15 BAU/mL are strongly correlated with the presence of neutralizing antibodies (see Product Manual V2.0). However, the interpretation of antibody levels after vaccination is limited due to the many variables involved. For example, whether initially weak antibody responses also lead to lower levels after the booster, and whether this varies with different vaccines has been unclear to date. Additional data are needed to inform different health policies and clinical decisions (European Centre for Disease Prevention and Control, 2021).
These data strongly emphasize that the antibody response to the first dose of the mRNA vaccine BNT162b2 can predict the final post-booster antibody levels. However, this relationship was not shown for the vector vaccine AZD1222. To the best of the authors’ knowledge, this finding has not been reported previously, and may be explained by the specific immune response to vector vaccines. In contrast to mRNA-based preparations, vector vaccines also induce an immune response directed against the viral vector, which in the case of AZD1222 is the chimpanzee adenovirus ChAdOx1 (Kaur and Gupta, 2020). De-novo anti-vector immunity could affect spike-protein induction and, therefore, strongly influence the association between pre- and post-booster values. Whether this demonstrated difference between the BNT162b and AZD1222 vaccines is transferable to other mRNA and vector vaccines remains to be investigated by further studies.
In addition, significant associations were found between antibody levels and age. With AZD1222, neither of the two pre-booster samples (3 weeks and 11 weeks after the first dose) correlated with age. However, after the booster shot, a significant positive association (ρ=0.26, P=0.013) was found, indicating that older age was associated with slightly higher antibody levels. A possible explanation might be reduced anti-vector immunity in older individuals (Dorrington and Bowdish, 2013; Connors et al., 2021); however, this correlation was, although significant, comparably weak and must therefore be interpreted with caution. Although suggested by the literature (Lustig et al., 2021), sex-dependent differences could not be identified, which may be the result of a potentially small effect size or may be due to the limited number of male recipients of AZD1222.
For BNT162b2, in contrast, an inverse correlation was found between antibody levels and age after the first dose (ρ=-0.33, P=0.013). However, this association nearly vanished after the second dose, and lost statistical significance (ρ=-0.23, P=0.093). These findings align with recent articles which found a gap in antibody levels of younger and older individuals after the first dose of BNT162b2 (Abu Jabal et al., 2021; Müller et al., 2021; Viana et al., 2021). A recently published prospective study by Lustig et al. (2021) confirmed that older adults yield lower antibody levels after the first dose of BNT162b2, with the age groups converging after the second dose.
In conclusion, these data suggest that antibody levels quantified by the Roche Elecsys SARS-CoV-2 S assay before the booster shot could infer post-booster responses to BNT162b2, but not to AZD1222. In addition, a vaccine-dependent effect on antibody responses was found, suggesting a possible link between vaccine response and vector immunity.
Conflict of interest statement
The Department of Laboratory Medicine received compensations for advertisement on scientific symposia from Roche, and holds a grant for evaluating an in-vitro diagnostic device from Roche.
Funding
None.
Ethical approval
The protocol of this performance evaluation study was reviewed and approved by the Ethics Committee of the Medical University of Vienna (EK 1066/2021).
Acknowledgments
The authors wish to thank all sample donors for their valuable contribution. We further thank Borka Radovanovic-Petrova, Monika Martiny, Jadwiga Konarski, Bernhard Haunold, Maedeh Iravany and Shohreh Lashgari for perfect technical and administrative assistance. The MedUni Wien Biobank is part of the Austrian biobanking consortium BBMRI.at.
References
- Abu Jabal K, Ben-Amram H, Beiruti K, Batheesh Y, Sussan C, Zarka S, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Eurosurveillance. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.6.2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal A, Pozzetto B, Trabaud MA, Escuret V, Rabilloud M, Langlois-Jacques C, et al. Evaluation of high-throughput SARS-CoV-2 serological assays in a longitudinal cohort of patients with mild COVID-19: clinical sensitivity, specificity and association with virus neutralization test. Clin Chem. 2021;67:742–752. doi: 10.1093/clinchem/hvaa336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . CDC; Atlanta, GA: 2021. Interim guidelines for COVID-19 antibody testing in clinical and public health settings.https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html Available at. (accessed 18 June 2021) [Google Scholar]
- Connors J, Bell MR, Marcy J, Kutzler M, Haddad EK. The impact of immuno-aging on SARS-CoV-2 vaccine development. GeroScience. 2021;43:31–51. doi: 10.1007/s11357-021-00323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrington MG, Bowdish DME. Immunosenescence and novel vaccination strategies for the elderly. Front Immunol. 2013;4:171. doi: 10.3389/fimmu.2013.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control . ECDC; Stockholm: 2021. The use of antibody tests for SARS-COV-2 in the context of Digital Green Certificates –20 May 2021. [Google Scholar]
- Eyre DW, Lumley SF, Wei J, Cox S, James T, Justice A, et al. Quantitative SARS-CoV-2 anti-spike responses to Pfizer-BioNTech and Oxford-AstraZeneca vaccines by previous infection status. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federico M. The conundrum of current anti-SARS-CoV-2 vaccines. Cytokine Growth Factor Rev. 2021;60:46–51. doi: 10.1016/j.cytogfr.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. medRxiv 2021:2021. 06.21.21258528. [DOI] [PMC free article] [PubMed]
- Hall VJ, Foulkes S, Charlett A, Atti A, Monk EJM, Simmons R, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: a large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397:1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslacher H, Gerner M, Hofer P, Jurkowitsch A, Hainfellner J, Kain R, et al. Usage data and scientific impact of the prospectively established fluid bioresources at the hospital-based MedUni Wien Biobank. Biopreserv Biobank. 2018;16:477–482. doi: 10.1089/bio.2018.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur SP, Gupta V. COVID-19 vaccine: a comprehensive status report. Virus Res. 2020;288 doi: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Srivastava K, Alshammary H, Amoako AA, Awawda MH, Beach KF, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen PA, Page M, Bernasconi V, Mattiuzzo G, Dull P, Makar K, et al. WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Lancet. 2021;397:1347–1348. doi: 10.1016/S0140-6736(21)00527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley SF, O'Donnell D, Stoesser NE, Matthews PC, Howarth A, Hatch SB, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med. 2021;384:533–540. doi: 10.1056/NEJMoa2034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig Y, Sapir E, Regev-Yochay G, Cohen C, Fluss R, Olmer L, et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021 doi: 10.1016/S2213-2600(21)00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller L, Andrée M, Moskorz W, Drexler I, Walotka L, Grothmann R, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkmann T, Perkmann-Nagele N, Breyer MK, Breyer-Kohansal R, Burghuber OC, Hartl S, et al. Side-by-side comparison of three fully automated SARS-CoV-2 antibody assays with a focus on specificity. Clin Chem. 2020;66:1405–1413. doi: 10.1093/clinchem/hvaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkmann T, Perkmann-Nagele N, Koller T, Mucher P, Radakovics A, Marculescu R, et al. Anti-spike protein assays to determine SARS-CoV-2 antibody levels: a head-to-head comparison of five quantitative assays. Microbiol Spect. 2021 doi: 10.1128/spectrum.00247-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkmann T, Perkmann-Nagele N, Koller T, Mucher P, Radakovics A, Wolzt M, et al. Serum antibody response to BNT162b2 after natural SARS-CoV-2 infection. Eur J Clin Invest. 2021:e13632. doi: 10.1111/eci.13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkmann T, Koller T, Perkmann-Nagele N, Klausberger M, Duerkop M, Holzer B, et al. Spike protein antibodies mediate the apparent correlation between SARS-CoV-2 nucleocapsid antibodies and neutralization test results. Microbiol Spect. 2021:e00218–e00221. doi: 10.1128/spectrum.00218-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao S, Warrener LA, Hoschler K, Perry KR, Shute J, Whitaker H, et al. Robust antibody responses in 70–80-year-olds 3 weeks after the first or second doses of Pfizer/BioNTech COVID-19 vaccine, United Kingdom, January to February 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.es.2021.26.12.2100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana JF, Bergman M-L, Gonçalves LA, Duarte N, Coutinho TP, Borges PC, et al. Population homogeneity for the antibody response to COVID-19 BNT162b2/Comirnaty vaccine is only reached after the second dose, across all adult age ranges. medRxiv. 2021 doi: 10.1038/s41467-021-27761-z. 2021.03.19.21253680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaqout A, Daghfal J, Alaqad I, Hussein SAN, Aldushain A, Almaslamani MA, et al. The initial impact of a national BNT162b2 mRNA COVID-19 vaccine rollout. Int J Infect Dis. 2021;108:116–118. doi: 10.1016/j.ijid.2021.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]