Abstract
Objective
We assessed herpesvirus reactivation in severe SARS-CoV-2 infection.
Methods
Retrospective study including consecutive patients admitted to an onco-hematology intensive care unit (ICU) for severe COVID-19. Replication of EBV, CMV, and HSV was evaluated. Competing risk analyses were used to assess the cumulative risk of viral reactivation, and time-dependent Cox and Fine and Gray models to assess risk factors for viral reactivation.
Results
Among 100 patients, 38 were immunocompromised. Sixty-three patients presented viral reactivation (12% for HSV, 58% EBV and 19% CMV). Symptomatic patients received treatment. Overall cumulative incidence of viral reactivation was 56.1% [55.9–56.4] at 10 days. After adjustment, a preexisting hematological malignancy (sHR [95%CI] = 0.31 [0.11–0.85]) and solid organ transplantation (sHR [95% CI] = 2.09 [1.13–3.87]) remained independently associated with viral reactivation. Viral reactivation (P = 0.34) was not associated with mortality.
Conclusions
Incidence of herpesvirus reactivation in patients admitted to the ICU for severe COVID-19 was high, but rarely required antiviral treatment.
Keywords: COVID-19, SARS-CoV-2, Viral reactivation, CMV
1. Introduction
Patients with severe COVID-19 require intensive care unit (ICU) management. High rates of acquired infections have been reported in these patients, mainly related to bacterial and fungal nosocomial infections [1], [2], [3], [4]. Although secondary viral reactivation, mainly due to Herpesviridae, is common in critically ill patients, little is known about such events in patients with severe SARS-CoV-2 infection [5], [6], [7]. A recent study suggested a high incidence of herpesvirus reactivation in a small number of COVID-19 patients in the ICU [7]. The objective of our study was to assess cumulative incidence, risk factors, and prognosis of herpesvirus reactivation in severe COVID-19 patients with high rate of immune defect.
2. Methods
This study is a post-hoc analysis of a retrospective single-center study in the ICU of an onco-hematology academic hospital. This study was approved by an institutional review board (French Society of Anesthesia and Intensive Care Medicine–CE SRLF n°20–32).
Consecutive patients with confirmed SARS-CoV-2 infection admitted to the ICU between February 30 and May 10, 2020 were included.
Biological sampling was performed as previously described [8]. Quantification of EBV and CMV in whole blood was performed systematically for all patients at ICU admission, as part of the routine management of COVID-19 patients [9], monitored twice weekly and repeated in case of sepsis, using Abbott RealTime PCR EBV and CMV (Abbott) on the m2000 RealTime System platform (Abbott). Quantification of HSV was performed with Rotor-Gene® (Qiagen) using RealStar® alpha Herpesvirus PCR Kit (Altona) in case of sepsis or clinical signs after extraction with QIAsymphony (Qiagen).
Viral reactivation was defined when quantification reached the limit of detection. Significant reactivation was considered when quantitative PCR levels were above 3.5Log for CMV and 3.2Log for EBV twice consecutively or in case of clinical manifestations. For HSV, reactivation was considered in case of positive PCR in blood, bronchoalveolar lavage, or on the skin swab, with or without clinical signs.
Data are reported as absolute values with percentages for categorical variables or median with interquartile interval for quantitative variables. A competing risk analysis was performed to assess the cumulative risk of viral reactivation and depict it. Concomitant competing risks taken into account were “discharged alive from the ICU” and “ICU mortality”.
Fine and Gray model was used to assess risk factors for viral reactivation. Models were built using conditional backward stepwise variable selection process based upon variable influence in univariate analysis. Critical entry and exit P values were 0.2 and 0.1, respectively. It was preplanned to force patient's severity into the final model if this item was not previously selected. Data are reported as sub-Hazard ratios (sHR; 95%CI).
Statistical significance was considered on two-sided tests with a critical alpha risk of 0.05.
Statistical analyses were performed using R version 3.4.4 (R Foundation for Statistical Computing), “survival”, “cmprisk” packages.
3. Results
One hundred patients were included in this analysis. Median age was 59 years [range 53–67] and most patients were of male gender (73%, n = 73). Thirty-eight patients had an immune defect, among which 24 patients (24%) had an underlying malignancy and 10% were solid organ transplant recipients. Among patients on valaciclovir (n = 10), nine had an hematological malignancy (n = 15) and one underwent solid organ transplantation (n = 10). Fifty-four (54%) patients required mechanical ventilation. Median ICU length of stay was 6 days [range 3–13] and ICU mortality reached 28% (n = 28).
A total of 63 patients (63%) presented viral reactivation during the ICU stay (HSV, n= 12; EBV, n = 58; CMV, n = 19). EBV and CMV reactivation reached significant thresholds (3.5Log and 3.2Log, respectively) in four and two patients, respectively. No patient received treatment for EBV reactivation. Both patients with significant CMV replication were solid organ transplant recipients and received valganciclovir. One patient without underlying immune defect had CMV disease (esophagitis) but died before diagnosis. All HSV reactivations (n = 10) were associated with cutaneous-mucous manifestation; seven were detected in the serum and one in the lung; none was receiving valaciclovir prophylaxis and all were treated with either acyclovir or valaciclovir. Viral reactivations were less frequent in patients with hematological malignancy (8% versus 27%) and more frequent in patients with higher leukocytosis (7.4 [5.8–9.7] versus 5.9 [4.4–8.4]), treated with dexamethasone in the ICU (44% versus 16%), and in patients with microbiologically documented secondary bacterial infection (44% versus 19%). Patients with viral reactivation had a longer ICU stay (7 [4–17] versus 5 [2–9] days) (Table 1 ).
Table 1.
Characteristics of patients admitted to the ICU for severe COVID-19 according to viral reactivation.
| Characteristics | No viral reactivation | Viral reactivation | P |
|---|---|---|---|
| n | 37 | 63 | |
| Age (years) | 58 [49–67] | 60 [53–67] | 0.39 |
| Male | 26 (70) | 47 (75) | 0.81 |
| Immunosuppression | 13 (36) | 25 (42) | 0.75 |
| Solid tumor | 4 (11) | 5 (8) | |
| Hematological malignancy | 10 (27) | 5 (8) | |
| Solid organ transplantation | 0 (0) | 10 (16) | |
| HIV infection | 0 (0) | 1 (2) | |
| Autoimmune or inflammatory disease | 4 (11) | 8 (13) | |
| Valaciclovir prophylaxis | 7 (19) | 3 (5) | 0.05 |
| Hematopoietic cell transplantation | 5 (14) | 1 (2) | 0.05 |
| Antibiotics prior to ICU admission | 14 (38) | 31 (49) | 0.37 |
| SAPSII at admission | 25 [19–39] | 29 [18–38] | 0.83 |
| ICU admission | |||
| Leukocytes (G/L) | 5.89 [4.43–8.40] | 7.37 [5.76–9.73] | 0.02 |
| Lymphocyte (G/L) | 0.82 [0.58–1.16] | 0.77 [0.50–1.05] | 0.42 |
| IL-6 (ng/mL) | 82 [54–157] | 85 [42–126] | 0.85 |
| Lactate | 1.1 [0.8–1.6] | 1.20 [1.0–1.4] | 0.60 |
| Ferritin | 1094 [499–1670] | 1311 [654–2305] | 0.16 |
| Therapeutics | |||
| dexamethasone | 6 (16) | 27 (44) | 0.01 |
| lopinavir/ritonavir | 5 (14) | 8 (13) | 1.00 |
| eculizumab | 2 (5) | 8 (13) | 0.41 |
| tocilizumab | 3 (8) | 2 (3) | 0.54 |
| Mechanical ventilation | 17 (46) | 37 (59) | 0.30 |
| Vasopressors | 15 (41) | 33 (53) | 0.31 |
| Renal replacement therapy | 1 (3) | 11 (18) | 0.06 |
| Infectious event | 8 (22) | 28 (44) | 0.04 |
| Bacterial event | 7 (19) | 28 (44) | 0.02 |
| Pneumoniaa | 6 (16) | 23 (37) | 0.05 |
| VAP | 6 (16) | 21 (33) | 0.10 |
| Fungal event | 1 (3) | 4 (6) | 0.74 |
| ICU stay (days) | 5 [2–9] | 7 [4–17] | 0.03 |
| ICU mortality | 7 (19) | 21 (33) | 0.19 |
Data are reported as absolute value with percentage for categorical variables or median with interquartile interval for quantitative variables. ICU: Intensive Care Unit; NSAIDs: non-steroid anti-inflammatory; SAPSII: Simplified Acute Physiology Score II; SOT: Solid Organ Transplantation; VAP: Ventilator-Associated Pneumonia.
Pneumonia includes all lung infections occurring in non-ventilated patients and in patients with mechanical ventilation within 48 hours and after 48 hours of ventilation (VAP).
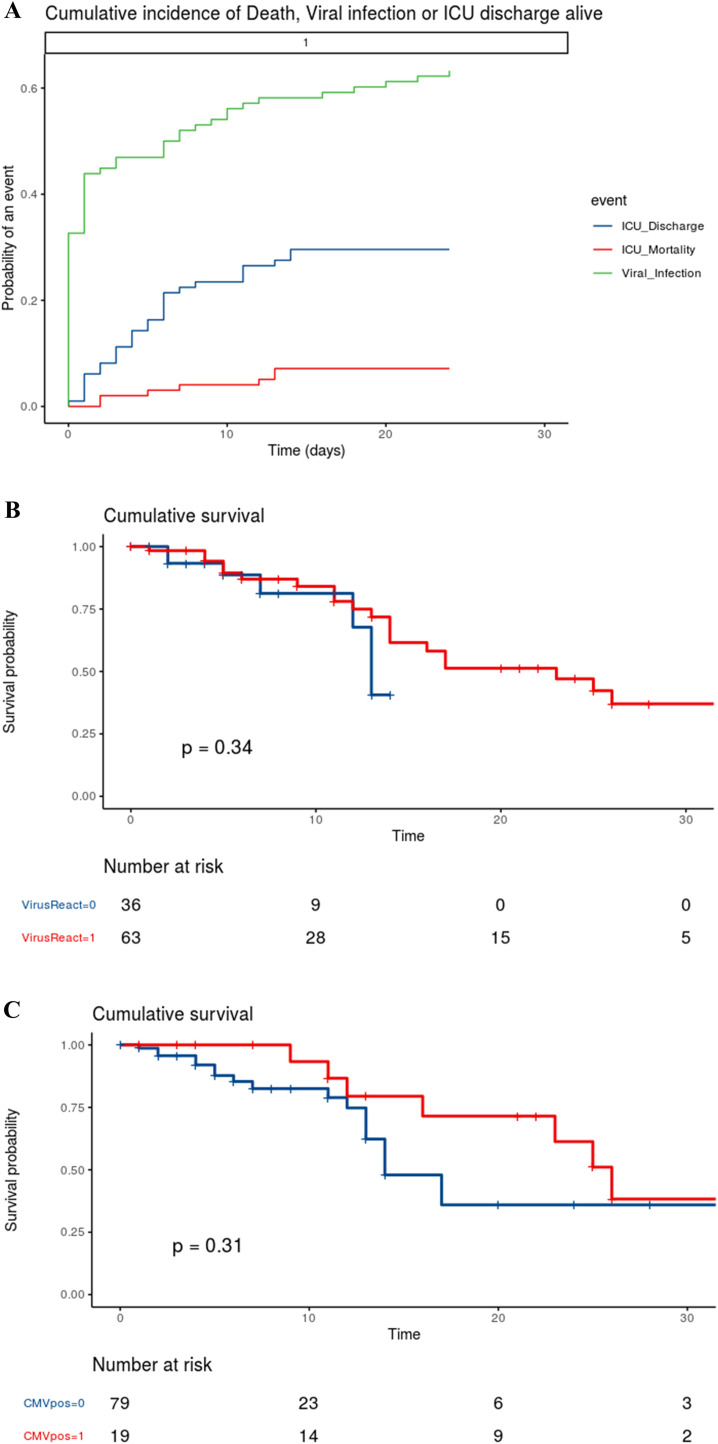
Using competing risk analysis with ICU discharge and mortality as competing events, the overall cumulative incidence of viral reactivation was 56.1% [55.9–56.4] at 10 days (Fig. 1A). Viral reactivation in patients with hematological malignancy occurred in 35.7% of them [33.9–37.5] at 10 days as compared to 59.5% [59.2–59.8] at 10 days in patients without any hematological malignancy.
Fig. 1.
Panel A. Competing risk of cumulative incidence of viral reactivation in patients admitted to the ICU for severe COVID-19. Competing risk of cumulative incidence of viral reactivation (green), ICU mortality (red) and ICU discharge (blue) in all patients. Panel B. Survival curves for patients with (red) and without (blue) viral reactivation obtained by Kaplan-Meier analysis and compared using the Log Rank test. Panel C. Survival curves for patients with (red) and without (blue) CMV reactivation obtained by Kaplan-Meier analysis and compared using the Log Rank test.
After adjustment for the confounding factors using a Fine and Gray model, a preexisting hematological malignancy (sHR [95%CI] = 0.31 [0.11–0.85], P = 0.02) and solid organ transplantation (sHR [95%CI] = 2.09 [1.13–3.87], P = 0.02) remained independently associated with viral reactivation. Dexamethasone was no longer associated with viral reactivation (sHR [95%CI] = 1.09 [0.62–1.92], P = 0.77). Viral reactivation was not independently associated with patient's severity according to SAPSII score (sHR [95%CI] = 0.99 per point [0.98–1.01], P = 0.55).
Neither viral reactivation (P = 0.34; Fig. 1B) nor CMV reactivation (P = 0.31; Fig. 1C) were associated with mortality.
4. Discussion
We described a high incidence of herpesvirus reactivation (58% EBV, 19% CMV, and 12% HSV) in patients admitted in the ICU for severe COVID-19, but rarely to significant levels requiring antiviral treatment.
This work has limitations. First, the retrospective and single-centered design with a high rate of patients with immune defects (38%) may influence external validity of our findings. Second, patients with immune suppression were heterogenous and included patients with autoimmune and inflammatory diseases, different types of solid tumors and hematological malignancies. The latter may lead to an overestimation of virus reactivation, which seems unlikely as our results are in line with those of recent studies in this field [7], [12]. In addition, the incidence of virus reactivation reported in the ICU or in septic shock patients ranked from 48% to 71% in EBV-seropositive patients, 15–20% for CMV, and reached approximately 30% for HSV [5], [10], [11], which seems close to our observations within the limits of interpretation.
At last, CMV and EBV reactivations were assessed systematically for all patients and not guided by serologies to only select seropositive recipients. HSV was not systematically monitored, which could underestimate the rate of viral reactivation.
It should be noted that the majority of patients with hematological malignancies in our institution were receiving antiviral prophylaxis (n = 9/15), which may explain the low rate of HSV reactivation in these patients, but is not enough to explain the association between hematological malignancies and viral reactivation as it does not account for CMV et EBV reactivations. A post-hoc sensitivity analysis did not show any association between valaciclovir prophylaxis and viral reactivation but revealed an interaction between valaciclovir and hematological malignancies preventing adjustment on both variables. We did not find increased viral reactivation in patients specifically receiving steroids.
The pathogenic role of herpesvirus remains unclear, meaningly whether herpesviruses are colonizer activated in response to the severe underlying disease or the infectious agent with true attributable morbidity and/or mortality. The latter is unlikely as despite increased reactivation rate, more particularly in solid organ transplant recipients, no change in outcome was noted in patients with viral reactivation.
Additional studies are required to further investigate the impact of viral reactivation in COVID-19 patients.
Human and animal rights
The authors declare that the work described has not involved experimentation on humans or animals.
Informed consent and patient details
The authors declare that this report does not contain any personal information that could lead to the identification of the patient(s) and/or volunteers.
Disclosure of interest
The authors declare that they have no competing interest.
Funding
This work did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
All authors attest that they meet the current International Committee of Medical Journal Editors (ICMJE) criteria for Authorship. Individual author contributions are as follows:
AS, GM, EA and MD contributed to the patients’ clinical management.
AS and GM collected patients’ data.
AS and MD analysed and interpreted patient's data.
AS and MD wrote the manuscript.
AS, GM, EA and MD reviewed the manuscript.
All authors read and approved the final manuscript.
Acknowledgments
We thank Docteur Linda Feghoul (Service de Virologie, hôpital Saint-Louis, Paris, France) for her expertise in virus monitoring.
References
- 1.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., et al. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71(9):2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luyt C.-E., Sahnoun T., Gautier M., Vidal P., Burrel S., Pineton de Chambrun M., et al. Ventilator-associated pneumonia in patients with SARS-CoV-2-associated acute respiratory distress syndrome requiring ECMO: a retrospective cohort study. Ann Intensive Care. 2020;10(1):158. doi: 10.1186/s13613-020-00775-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rouzé A., Martin-Loeches I., Povoa P., Makris D., Artigas A., Bouchereau M., et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: a European multicenter cohort study. Intensive Care Med. 2021;47(2):188–198. doi: 10.1007/s00134-020-06323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maes M., Higginson E., Pereira-Dias J., Curran M.D., Parmar S., Khokhar F., et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit Care Lond Engl. 2021;25(1):25. doi: 10.1186/s13054-021-03460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limaye A.P., Kirby K.A., Rubenfeld G.D., Leisenring W.M., Bulger E.M., Neff M.J., et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300(4):413–422. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luyt C.-E., Combes A., Deback C., Aubriot-Lorton M.-H., Nieszkowska A., Trouillet J.-L., et al. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am J Respir Crit Care Med. 2007;175(9):935–942. doi: 10.1164/rccm.200609-1322OC. [DOI] [PubMed] [Google Scholar]
- 7.Simonnet A., Engelmann I., Moreau A.-S., Garcia B., Six S., Kalioubie A.E., et al. High incidence of Epstein-Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically-ill patients with Covid-19. Infect Dis Now. 2021;51(3):296–299. doi: 10.1016/j.idnow.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saade A., Moratelli G., Dumas G., Mabrouki A., Tudesq J.-J., Zafrani L., et al. Infectious events in patients with severe COVID-19: results of a cohort of patients with high prevalence of underlying immune defect. Ann Intensive Care. 2021;11(1):83. doi: 10.1186/s13613-021-00873-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin Y.-H., Cai L., Cheng Z.-S., Cheng H., Deng T., Fan Y.-P., et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7(1):4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Libert N., Bigaillon C., Chargari C., Bensalah M., Muller V., Merat S., et al. Epstein-Barr virus reactivation in critically ill immunocompetent patients. Biomed J. 2015;38(1):70–76. doi: 10.4103/2319-4170.132905. [DOI] [PubMed] [Google Scholar]
- 11.Papazian L., Hraiech S., Lehingue S., Roch A., Chiche L., Wiramus S., et al. Cytomegalovirus reactivation in ICU patients. Intensive Care Med. 2016;42(1):28–37. doi: 10.1007/s00134-015-4066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hraiech S., Bonnardel E., Guervilly C., Fabre C., Loundou A., Forel J.-M., et al. Herpes simplex virus and Cytomegalovirus reactivation among severe ARDS patients under veno-venous ECMO. Ann Intensive Care. 2019;9(1):142. doi: 10.1186/s13613-019-0616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]