INTRODUCTION
Hepatitis C virus (HCV) infection affects approximately 170 million people worldwide, including 5 million in the U.S., and contributes significantly to morbidity and mortality, including end-stage liver disease, and hepatocellular carcinoma.[1–3] Several U.S. concerns about HCV are emerging. A recent CDC review of death certificates found that HCV mortality increased substantially from 1999-2007, with the median age of death being 57 years, or approximately 20 years below the national average life expectancy.[4] For the first time, HCV mortality surpassed HIV mortality in 2007,[5] and the number of deaths annually is expected to reach 34,900 by 2030, unless effective HCV treatment is provided.[6] HCV infection is the leading cause of liver transplantation,[7] and new HCV infections are on the rise with more than 16,500 new reported cases in 2011.[8] The total estimated cost of HCV is currently $6.5 billion, but it will exceed $9.1 billion annually by 2024.[9]
With the increasing availability of more tolerable and efficacious direct-acting antiviral (DAA) medications, eradicating HCV will require a strategic combination of prevention (e.g., harm reduction) and treatment strategies, including treatment as prevention, which requires improvements in diagnosis and linkage to care. Though improvements are needed, evidence of effective HCV treatment has been documented in primary care, specialty clinics, drug treatment programs,[10] and correctional facilities.[11]
Recent evidence suggests that receiving positive testing results for HCV significantly reduces risk behaviors among people who inject drugs (PWIDs).[12] PWIDs, however, are one of the least likely populations to be tested for HCV and even less likely to be treated due to fear of stigma or discrimination, co-existing mental illness, ongoing drug use or structural impediments,[13] including limited access or financial barriers to traditional health care services. Yet, active PWIDs with HCV are the most likely to transmit HCV infection to others.[14] A recent systematic review and meta-analysis suggests that sustained virological response (SVR), or “cure”, among PWIDs are similar to those who don’t inject drugs, providing empiric support that PWIDs should be screened and treated for HCV.[15] Furthermore, young PWIDs are a particularly high-risk group for new HCV infections and HCV progression, especially in the Northeast U.S., based on recent data from Massachusetts and Connecticut.[16,17] Young injectors are less likely to be screened for HCV where drug injection remains secretive, and these individuals are often unaware of HCV testing and treatment options. As a highly sensitive and specific (98%) rapid, point-of-care (POC) HCV antibody test (HCV test) that is comparable to traditional serological blood tests[18] became FDA-approved in 2012, new opportunities emerged to reach high risk marginalized populations outside traditional healthcare settings.
MMCs are non-traditional healthcare units that increase healthcare access removing geographic and social barriers associated with traditional, “brick and mortar” healthcare settings. According to the Mobile Health Map Project, there are approximately 1,500-2,000 MMCs across the U.S. providing a variety of prevention and treatment services to 6.5 million people (see http://www.mobilehealthmap.org). MMCs play a vital role in healthcare delivery, by accessing care for minorities, the poor, migrant workers, the homeless, adolescents, and uninsured/underinsured adults and children. For these people, MMCs create a convenient and unintimidating healthcare access point, while reducing disparities and healthcare costs. As such, they are frequently regarded as venues of convenience, delivering healthcare at the doorstep of communities that are otherwise limited by location,[19,20] cost,[21] insurance status,[21,22] literacy,[23] stigma,[24,25] or other structural barriers such as proximity and access to transportation.[26–28]
METHODS
New Haven is one of the poorest U.S. cities, with high rates of poverty, unemployment, crime, substance abuse and HIV/AIDS.[29,30] Recent data from New Haven also documented a particularly high prevalence of HCV and HCV/HIV coinfection.[31] When the new HCV POC test became commercially available, a “HepHome” program was initiated in the greater New Haven area, utilizing the Community Health Care Van (CHCV), a free MMC that has operated in marginalized communities since 1993.[32] The MMC is a 40-foot mobile clinic that travels weekdays to four of New Haven’s most vulnerable neighborhoods most profoundly impacted by HIV/HCV, substance abuse and poverty. It provides a variety of services including acute and primary care, prevention services, health fair screenings, tuberculosis screening and treatment,[33,34] directly administered antiretroviral therapy (DAART),[35–40] buprenorphine and extended-release naltrexone treatment of substance use disorders,[33,41,42] screening and treatment for HIV, mental illness, sexually transmitted infections (STIs),[43] HCV, and a variety of case management[40,44] and post-prison release services.[39,45–51] POC HCV testing has been largely understudied among PWIDs within MMCs and for difficult-to-reach populations.
With the availability of free, POC HCV testing kits, we initiated a pilot study to improve HCV testing among all patients presenting for any care on a MMC in New Haven, CT over the 12-month period from March 2012 to March 2013. All POC HCV testing was provided free of charge (patient insurance was leveraged as applicable), and confirmatory HCV testing was performed through the Connecticut Department of Public Health (CTDPH).
Using New Haven’s innovative MMC, our aims were to: 1) examine correlates of accepting HCV screening; 2) measure prevalence and correlates of reactive HCV status; 3) identify significant correlates of selecting POC HCV testing (OraQuick® HCV Rapid Antibody Test, OraSure Technologies, Inc., Bethlehem, Pennsylvania)[18] versus standard phlebotomy; and 4) compare the proportion linked to specialized HCV care between patients preferring POC HCV versus standard testing among those identified as being HCV-infected.
All MMC clients undergo a standardized medical intake assessment that includes demographic and social characteristics, drug and sexual behaviors, and past medical history. Variables for the comprehensive and follow-up questionnaires have been described previously.[52] Clients were classified as being a “baby boomer” if born from 1945 to 1965. Stably housed was defined as living in one’s own apartment, one’s own house, or with one’s family, while unstably housed was defined as living with a friend, in a hotel or shelter, or on a street or public place. Being in a relationship was defined as being married or cohabitating with a significant other. Health insurance status was self-reported. Standardized measures of type, route, and frequency of drug use were assessed using the drug component of the Addiction Severity Index.[53] Foreign-born were those self-reporting being born outside the United States or Puerto Rico. History of interpersonal violence was self-reported experience as a victim of domestic violence or sexual assault. Men-who-have-sex with men (MSM) were defined as males reporting having had sexual relations with another man. Additional variables required for mandatory state HCV surveillance reporting included tattoo history, occupational exposure to blood, and cohabitation with an individual known to be HCV-infected.
During the study period, all MMC clients were offered HCV testing, either using 1) standardized phlebotomy, in which other tests were “bundled”, including hepatitis A, hepatitis B, HIV, or syphilis where results returned within a week, or 2) fingerstick POC testing with immediate results provided in approximately 20 minutes. Clients self-selected either HCV testing strategy based on priorities and personal preferences. The trade-offs between the two screening strategies included waiting time for results, ability to bundle testing with other screenings, and fingerstick versus phlebotomy (Figure 1). All individuals with a reactive test result using either method underwent confirmatory HCV RNA testing and were referred to an HCV specialty clinic for additional treatment options (Figure 2). This study was approved by Yale University’s Institutional Review Board (#1306012179).
Figure 1:
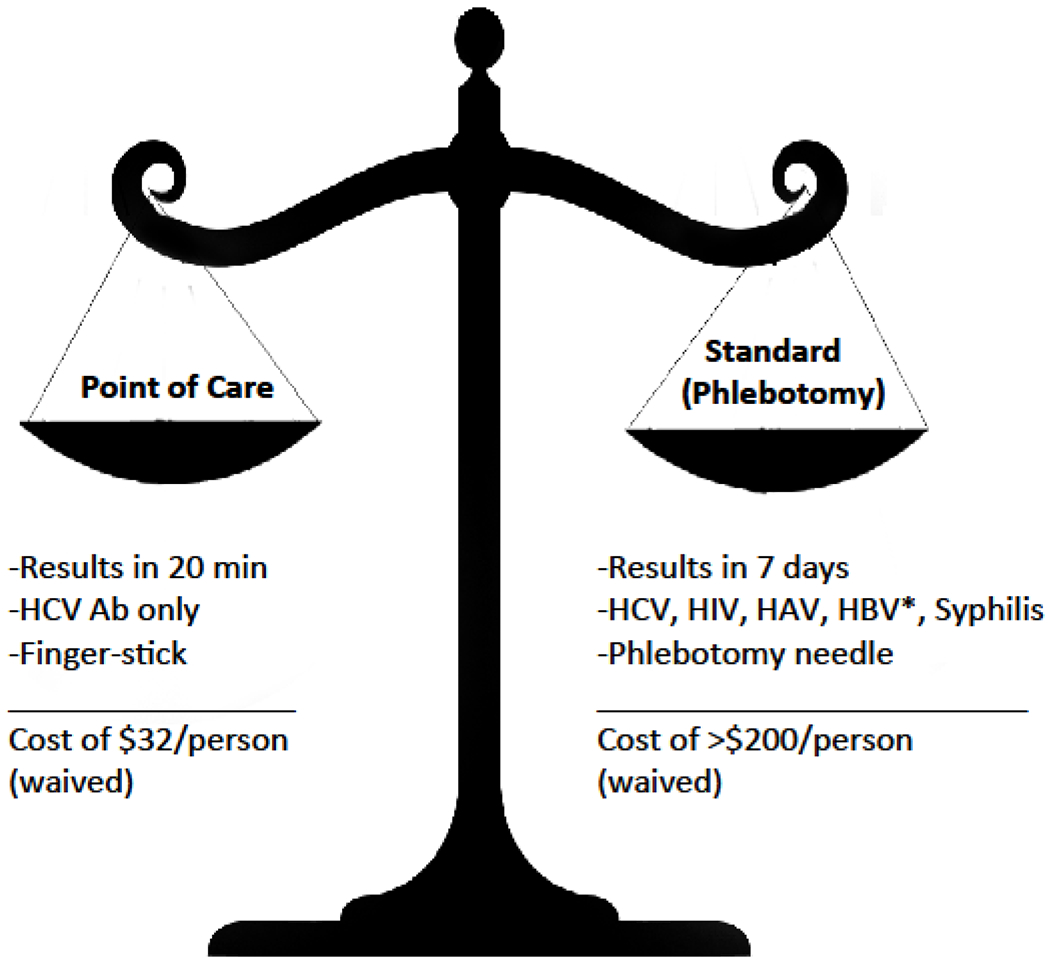
Decision-based trade-offs between HCV screening strategies, New Haven, Connecticut, 2012-2013.
HCV: Hepatitis C virus antibody; HAV: Hepatitis A virus IgM; HBV: Hepatitis B virus surface antigen
Figure 2:
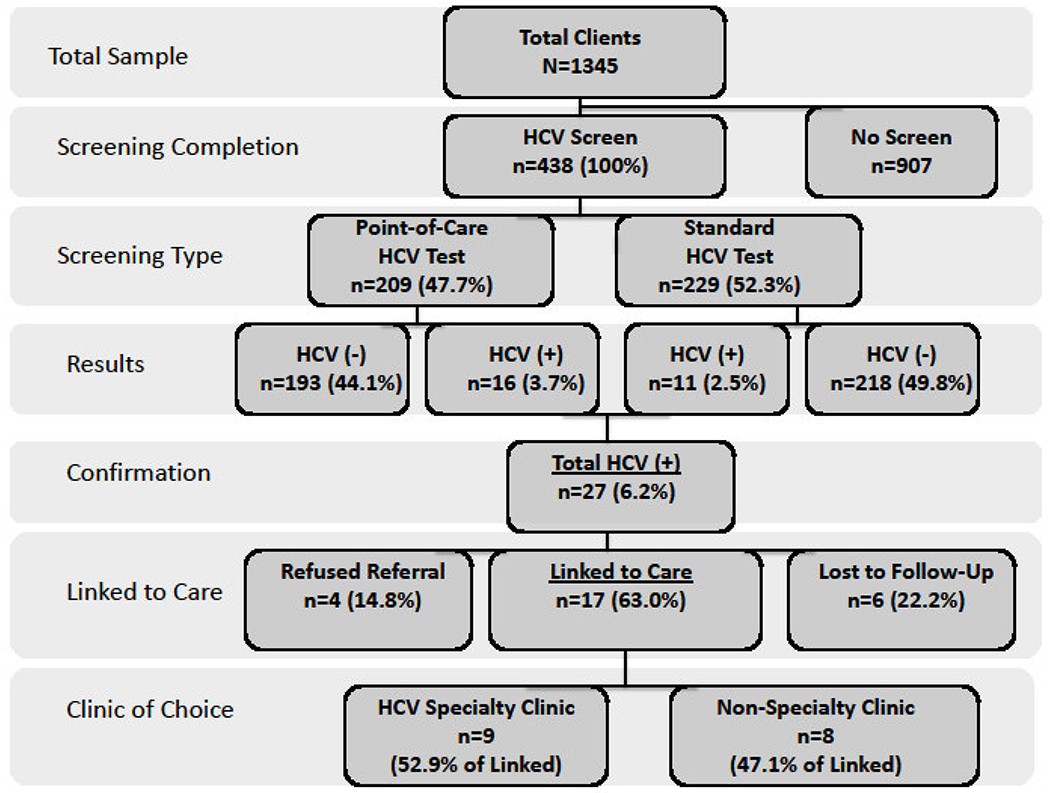
Screening and referral to care algorithm for point-of-care (POC) Hepatitis C virus (HCV) antibody testing versus standard phlebotomy testing in a mobile medical clinic, New Haven, Connecticut, 2012-2013.
DATA ANALYSIS
Because <10% of data had missing observations, with the frequency of missing data ranging from 0% to 17.7% across all variables, we did not find evidence that the difference between the clinical intake surveys was related to systematic heterogeneity among patients. Therefore, multiple imputations were implemented to address any missing data concerns.[54] Analytical results for the variables that were missing less than 10% of observations were not sensitive to the departures from missing at random (MAR) assumptions. For the small set of variables which were missing >10% of observations, we performed additional sensitivity analyses and found that the pattern in the imputed data were consistent with patterns of observed, non-imputed data. Rubin Rules were applied to combine the results from different imputations for each of our analyses.[54]
First, we used a Pearson Chi-squared test to (1) examine the differences between clients who accepted HCV testing and those who did not; (2) assess differences between clients testing positive versus negative for HCV antibody; (3) examine differences between clients who preferred POC versus phlebotomy HCV testing, using all available covariates. Second, we estimated two separate multivariate logistic models for the two outcomes: acceptance of HCV testing and preferring POC HCV testing over phlebotomy. The Akaike Information Criterion (AIC) was used as a measure of relative-goodness-of-fit to discriminate among various models that we estimated. Our final set of models is based on a combination of AIC goodness-of-fit as well as inclusion of clinically relevant variables. We report both the adjusted odds ratios (AOR), as well as marginal effects, which represent a change in probability of the outcome based on a unit of change in the covariate, while other variables are fixed at their means.[55]
In our final analysis, we examined the differences in the percentage of HCV-infected clients linked to specialty HCV care among those testing positive using the two HCV testing strategies. In addition to using Chi-squared testing, we constructed a 95% Bayesian credible intervals analysis using the Metropolis-Hastings algorithm that sampled from a posterior distribution specified by a uniform prior and a binomial likelihood.[56] All empirical analyses were conducted using STATA 12.[57]
RESULTS
Over the 12-month testing period, 438 (32.6%) of the 1345 unique clients seen on the MMC accepted HCV screening. Those agreeing to HCV testing (Table 1) were on average in their mid-thirties (35.6 years), and were more likely than those not being tested to be born after 1965 (83.2%), US-born (66.4%), black (44.5%), had more than 15 lifetime sexual partners (35.3%), and self-identified as PWID (22.0%). Of the 438 screened, 209 (47.7%) selected POC testing (Table 2) and were more likely to be white, US-born, and have a higher number of lifetime sexual partners, compared to those selecting standard phlebotomy testing.
Table 1:
Baseline demographics and propensity to undergo Hepatitis C virus (HCV) antibody screening among clients within a mobile medical clinic (MMC) in New Haven, Connecticut, 2012-2013 (N=1345).
| Correlate | Total Patients Seen N=1345 |
HCV Screening Performed | Screened vs Not Screened | ||||
|---|---|---|---|---|---|---|---|
| Yes N=438 |
No N=907 |
||||||
| N observed (95% CI) | N imputed (%) | N observed (95% CI) | N imputed (%) | N observed (95% CI) | N imputed (%) | p-value | |
| Mean Age, years [S.D.] | 37.5 | 37.5 | 35.6 | 35.6 | 38.5 | 38.5 | <0.01 |
| (36.9,38.2) | [12.0] | (34.5,36.7) | [11.7] | (37.7,39.3) | [12.1] | ||
| Baby boomer generation | 278 | 294 | 57 | 74 | 221 | 221 | <0.01 |
| (263,324) | 21.8% | (58,90) | 16.8% | (196,246) | 24.4% | ||
| Gender | |||||||
| Male | 738 | 751 | 242 | 256 | 496 | 497 | 0.220 |
| (715,788) | 55.8% | (235,277) | 58.4% | (467,526) | 54.7% | ||
| Female | 607 | 594 | 196 | 182 | 411 | 410 | 0.220 |
| (557,630) | 44.2% | (161,203) | 41.6% | (381,440) | 45.3% | ||
| Race | |||||||
| White | 347 | 359 | 89 | 99 | 258 | 258 | 0.028 |
| (326,391) | 26.7% | (81,117) | 22.6% | (231,285) | 28.4% | ||
| Black | 528 | 546 | 175 | 195 | 353 | 353 | 0.050 |
| (510,582) | 40.6% | (173,216) | 44.5% | (324,382) | 38.9% | ||
| Hispanic | 411 | 425 | 126 | 142 | 285 | 285 | 0.644 |
| (391,459) | 31.6% | (121,163) | 32.4% | (258,312) | 31.4% | ||
| Foreign-born | 671 | 711 | 147 | 183 | 524 | 524 | <0.01 |
| (673,748) | 52.8% | (160,207) | 41.8% | (495,553) | 57.7% | ||
| Unstable housing | 409 | 496 | 63 | 144 | 346 | 350 | 0.177 |
| (453,539) | 36.8% | (111,178) | 32.9% | (321,379) | 38.6% | ||
| No health insurance | 584 | 708 | 112 | 235 | 472 | 472 | 0.232 |
| (661,755) | 52.6% | (195,275) | 53.6% | (442,502) | 52.0% | ||
| Not in a stable relationship | 933 | 1146 | 173 | 383 | 760 | 764 | 0.315 |
| (1114,1177) | 85.2% | (358,407) | 87.3% | (742,785) | 84.2% | ||
| Prior diagnosis of hepatitis C | 46 | 46 | 23 | 26 | 23 | 23 | <0.01 |
| (33,60) | 3.4% | (15,37) | 5.9% | (14,32) | 2.5% | ||
| Prior STI diagnosis | 253 | 363 | 105 | 228 | 148 | 148 | 0.004 |
| (257,468) | 27.0% | (110,345) | 52.0% | (126,170) | 16.3% | ||
| Injection drug history (PWID) | 188 | 204 | 76 | 96 | 112 | 113 | <0.01 |
| (177,231) | 15.2% | (78,115) | 22.0% | (93,133) | 12.5% | ||
| Heroin | 203 | 268 | 39 | 108 | 164 | 164 | 0.015 |
| (237,300) | 20.0% | (86,130) | 24.7% | (141,187) | 18.0% | ||
| Cocaine | 345 | 435 | 70 | 164 | 275 | 275 | 0.070 |
| (393,476) | 32.3% | (131,196) | 37.4% | (248,302) | 30.3% | ||
| Use of | 38 | 57 | 7 | 28 | 31 | 31 | 0.380 |
| NSEP | (26,88) | 4.2% | (0,59) | 6.3% | (20,42) | 3.4% | |
| Crack cocaine use | 268 | 334 | 50 | 118 | 218 | 218 | 0.166 |
| (296,371) | 24.8% | (87,148) | 26.8% | (193,243) | 24.0% | ||
| Non-injection drug use | 514 | 625 | 106 | 218 | 408 | 408 | 0.096 |
| (585,665) | 46.4% | (193,242) | 49.6% | (379,437) | 45.0% | ||
| History of incarceration | 531 | 676 | 106 | 246 | 425 | 433 | 0.091 |
| (628,725) | 50.3% | (205,287) | 56.1% | (404,463) | 47.8% | ||
| History of interpersonal violence | 251 | 315 | 44 | 108 | 207 | 207 | 0.313 |
| (280,351) | 23.4% | (82,134) | 24.7% | (182,232) | 22.8% | ||
| MSM | 47 | 51 | 20 | 24 | 27 | 27 | 0.037 |
| (36,65) | 3.7% | (13,35) | 5.6% | (17,37) | 3.0% | ||
| Lifetime sexual partners > 15 | 133 | 145 | 133 | 155 | 0 | 0 | na |
| (121,168) | 10.7% | (134,175) | 35.3% | na | 0% | ||
Note: Nimputed is sample size based on the frequency estimate from multiple imputation procedure. Cocaine and heroin numbers reflect total users of either intravenous, oral, or inhaled routes.
LEGEND: HCV: Hepatitis C Virus; NSEP: Needle Syringe Exchange Program; STI: Sexually Transmitted Infection; PWID: People Who Inject Drugs; MSM: Men Who Have Sex with Men
Table 2:
Decision to select point-of-care (POC) versus standard phlebotomy HCV antibody testing among clients in a mobile medical clinic (MMC) in New Haven, Connecticut, 2012-2013 (N=438).
| Correlates | Total observed | HCV Testing Strategy Selected N=438 |
Pearson Chi-squared p-value | |||
|---|---|---|---|---|---|---|
| Point-of-Care N=209 |
Standard N=229 |
|||||
| N observed (95% CI) | N imputed (%) | N observed (95% CI) | N imputed (%) | |||
| HCV test | ||||||
| Positive | 27 | 16 | - | 11 | - | 0.219 |
| (8,24) | 7.7% | (5,17) | 4.8% | |||
| Negative | 411 | 193 | 193 | 218 | 218 | 0.219 |
| (185,201) | 92.3% | (212,224) | 95.2% | |||
| Mean age [S.D.] | 35.6 | 36.0 | 36.0 | 35.3 | 35.3 | 0.497 |
| (34.2,37.7) | [12.2] | (33.9,36.8) | [11.0] | |||
| Baby boomer generation | 57 | 32 | 36 | 25 | 38 | 0.857 |
| (25,47) | 17.2% | (26,49) | 16.6% | |||
| Gender | ||||||
| Male | 242 | 113 | 119 | 129 | 137 | 0.604 |
| (105,134) | 56.9% | (121,152) | 59.8% | |||
| Female | 173 | 86 | 90 | 87 | 92 | 0.604 |
| (75,104) | 43.0% | (77,108) | 40.2% | |||
| Race | ||||||
| Non-Hispanic White | 89 | 58 | 62 | 31 | 36 | <0.01 |
| (48,75) | 29.7% | (24,48) | 15.7% | |||
| Non-Hispanic Black | 175 | 87 | 92 | 88 | 103 | 0.853 |
| (78,106) | 44.0% | (87,119) | 45.0% | |||
| Hispanic | 126 | 50 | 53 | 76 | 89 | <0.01 |
| (40,65) | 25.4% | (73,106) | 38.9% | |||
| Foreign-born | 147 | 49 | 54 | 98 | 130 | <0.01 |
| (40,67) | 25.8% | (112,147) | 56.8% | |||
| Unstable housing | 63 | 18 | 68 | 45 | 76 | 0.244 |
| (39,98) | 32.5% | (59,93) | 33.2% | |||
| No health Insurance | 112 | 31 | 112 | 81 | 123 | 0.198 |
| (78,147) | 53.6% | (103,142) | 53.7% | |||
| Not in a stable relationship | 173 | 50 | 183 | 123 | 200 | 0.887 |
| (163,203) | 87.7% | (188,211) | 87.3% | |||
| Prior diagnosis of HCV | 23 | 12 | 12 | 11 | 14 | 0.351 |
| (5,19) | 5.7% | (5,22) | 6.1% | |||
| Prior diagnosis of STI | 105 | 23 | 95 | 82 | 133 | 0.660 |
| (0,205) | 45.5% | (103,162) | 58.1% | |||
| Injection drug history (PWID) | 76 | 47 | 53 | 29 | 44 | 0.187 |
| (40,66) | 25.4% | (30,57) | 19.2% | |||
| Heroin | 39 | 16 | 56 | 23 | 52 | 0.43 |
| (39,73) | 26.8% | (37,67) | 22.7% | |||
| Cocaine | 70 | 22 | 78 | 48 | 86 | 0.954 |
| (51,104) | 37.2% | (68,104) | 37.6% | |||
| Use of NSEP | 7 | 4 | 16 | 3 | 12 | 0.623 |
| (0,39) | 7.7% | (0,24) | 5.2% | |||
| Crack cocaine use | 50 | 21 | 63 | 29 | 55 | 0.453 |
| (34,91) | 30.1% | (38,72) | 24.0% | |||
| Non-injection drug use | 106 | 23 | 95 | 83 | 122 | 0.305 |
| (72,118) | 45.5% | (103,141) | 53.3% | |||
| History of incarceration | 106 | 30 | 119 | 76 | 127 | 0.779 |
| (90,149) | 57.0% | (107,146) | 55.5% | |||
| History of interpersonal violence | 44 | 20 | 66 | 24 | 42 | 0.085 |
| (39,94) | 31.6% | (28,56) | 18.3% | |||
| Occupational exposure to blood | 9 | 8 | 11 | 1 | 8 | 0.674 |
| (3,19) | 5.1% | (0,25) | 3.5% | |||
| Household known HCV+ individual | 20 | 17 | 39 | 3 | 50 | 0.972 |
| (0,82) | 18.6% | (0,146) | 21.8% | |||
| MSM | 20 | 12 | 13 | 8 | 11 | 0.460 |
| (6,20) | 6.3% | (3,19) | 4.8% | |||
| Tattoo history | 112 | 90 | 121 | 22 | 102 | 0.197 |
| (104,139) | 57.9% | (0,160) | 44.5% | |||
| Lifetime sexual partners > 15 | 133 | 106 | 115 | 27 | 40 | <0.01 |
| (100,129) | 55.0% | (26,54) | 17.5% | |||
Note: Nimputed is sample size based on the frequency estimate from multiple imputation procedure. Cocaine and heroin numbers reflect total users of either intravenous, oral, or inhaled routes.
LEGEND: HCV: Hepatitis C Virus; HCV Ab: Hepatitis C Antibody; STI: Sexually Transmitted Infection PWID: People Who Inject Drugs; NSEP: Needle Syringe Exchange Program; MSM: Men Who Have Sex with Men.
Among those tested, HCV prevalence was 6.2% (27/438), with no differences in HCV prevalence among the POC and standard testing groups (7.7% versus 4.8%; p=0.219) (Table 2). All those with positive HCV antibody tests had confirmatory HCV RNA levels drawn, consistent with HCV infection. Those with HCV infection (Table 3) were significantly older (43.2 vs 35.2 years old; p<0.01), baby boomers (33.3% vs 15.8%; p=0.027), white (55.5%; p<0.01), foreign-born (59.3%;p<0.047), and PWID (81.5% vs 18.2%; p<0.01); 81.5% (n=22) patients suspected that they were HCV-infected, but had not ever been confirmed or linked to care and were included in the testing assessment.
Table 3:
HCV antibody testing results (positive vs negative) stratified by test type and client characteristics in a mobile medical clinic (MMC) in New Haven, Connecticut, 2012-2013 (N=438).
| HCV Antibody Testing Result | |||||
|---|---|---|---|---|---|
| Correlates | HCV Positive N=27 |
HCV Negative N= 411 |
HCV+ vs HCV − | ||
| N observed (95% CI) | N imputed* (%) | N observed (95% CI) | N imputed* (%) | p-value | |
| HCV Ab Test | |||||
| Point-Of-Care | 16 | 16 | 193 | 193 | 0.215 |
| (11,21) | 59.3% | (173,213) | 46.9% | ||
| Standard Phlebotomy | 11 | 11 | 218 | 218 | 0.215 |
| (6,16) | 40.7% | (198,238) | 53.0% | ||
| Mean age, years [S.D.] | 43.2 | 43.2 | 35.2 | 35.1 | <0.01 |
| (38.8,47.6) | [10.6] | (34.0,36.2) | [11.6] | ||
| Baby boomer generation | 8 | 9 | 49 | 65 | 0.027 |
| (3,14) | 33.3% | (50,80) | 15.8% | ||
| Gender | |||||
| Male | 13 | 14 | 229 | 242 | 0.373 |
| (8,19) | 51.8% | (222,263) | 58.8% | ||
| Female | 13 | 13 | 160 | 169 | 0.373 |
| (8,19) | 48.1% | (148,189) | 41.1% | ||
| Race | |||||
| Non-Hispanic White | 14 | 15 | 75 | 84 | <0.01 |
| (9,21) | 55.5% | (67,100) | 20.4% | ||
| Non-Hispanic Black | 6 | 6 | 169 | 188 | 0.027 |
| (2,11) | 22.2% | (168,209) | 45.7% | ||
| Hispanic | 5 | 6 | 121 | 136 | 0.186 |
| (1,11) | 22.2% | (116,157) | 33.0% | ||
| Foreign-born | 15 | 16 | 132 | 167 | 0.047 |
| (10,22) | 59.3% | (145,189) | 40.6% | ||
| Unstable housing | 9 | 11 | 54 | 133 | 0.206 |
| (4,18) | 40.7% | (99,167) | 32.4% | ||
| No health insurance | 8 | 11 | 104 | 223 | 0.200 |
| (5,18) | 40.7% | (184,263) | 54.3% | ||
| Not in a stable relationship | 19 | 24 | 154 | 359 | 0.571 |
| (20,28) | 88.9% | (334,383) | 87.3% | ||
| Prior diagnosis HCV | 22 | 22 | 1 | 4 | <0.01 |
| (18,26) | 81.5% | (0,10) | 1.0% | ||
| Prior diagnosis STI | 10 | 13 | 95 | 215 | 0.414 |
| (7,20) | 48.1% | (99,330) | 52.3% | ||
| Injection drug history (PWID) | 19 | 22 | 57 | 75 | <0.01 |
| (17,26) | 81.5% | (58,91) | 18.2% | ||
| Heroin | 17 | 21 | 22 | 87 | <0.01 |
| (16,26) | 77.8% | (67,107) | 21.1% | ||
| Cocaine | 16 | 20 | 54 | 144 | <0.01 |
| (14,26) | 74.1% | (113,175) | 35.0% | ||
| Use of NESP | 5 | 6 | 2 | 21 | <0.01 |
| (1,12) | 22.2% | (0,53) | 5.1% | ||
| Crack cocaine use | 16 | 19 | 34 | 99 | <0.01 |
| (14,25) | 70.3% | (69,128) | 24.0% | ||
| Non-injection drug use | 2 | - | 104 | 215 | <0.01 |
| (0,6) | 7.4% | (190,240) | 52.3% | ||
| History of incarceration | 14 | 19 | 92 | 227 | 0.119 |
| (13,25) | 70.3% | (185,269) | 55.2% | ||
| History of interpersonal violence | 9 | 10 | 35 | 98 | 0.051 |
| (4,15) | 37.0% | (72,124) | 23.8% | ||
| Occupational exposure to blood | 2 | 3 | 7 | 19 | 0. 232 |
| (0,7) | 11.1% | (5,32) | 4.6% | ||
| Household known HCV+ individual | 7 | 11 | 13 | 78 | 0.274 |
| (5,16) | 40.1% | (0,227) | 19.0% | ||
| MSM | 1 | - | 19 | 23 | 0.658 |
| (0,3) | 3.7% | (12,34) | 5.6% | ||
| Tattoo history | 14 | 24 | 98 | 260 | 0.052 |
| (19,29) | 88.9% | (228,291) | 63.2% | ||
| Lifetime sexual partners > 15 | 4 | 5 | 129 | 150 | 0.035 |
| (0,9) | 18.5% | (130,170) | 36.5% | ||
Note: The number of individuals in each group (N-imputed) as well as 95% confidence interval have been estimated using Rubin Rules for combining multiple imputations (in this case 10 imputations).
LEGEND: HCV: Hepatitis C Virus; HCV Ab: Hepatitis C Antibody; STI: Sexually Transmitted Infection PWID: People Who Inject Drugs; NSEP: Needle Syringe Exchange Program; MSM: Men Who Have Sex with Men. Cocaine and heroin numbers reflect total users of either intravenous, oral, or inhaled routes
Accepting HCV testing was positively and significantly correlated with having been diagnosed with a STI, being US-born, and being a PWID (Table 4). Covariates associated with choosing POC testing included having >15 lifetime sexual partners, being white, and being US-born (Figure 3 and Table 4). While there was no difference in HCV prevalence among the type of HCV testing strategy selected, those selecting POC testing were significantly more likely (93.8% vs 18.2%; p<0.0001) to be linked to HCV specialty care (Figure 4).
Table 4:
Multivariate Logistic Regression Analysis of HCV Screening and Selection of Point-of-Care HCV Testing among Clients on a Mobile Medical Clinic in New Haven, Connecticut, 2012-2013 (N=1345).
| Outcome: Decision to Accept HCV Ab Screening N=1345 |
Outcome: Choosing Point-of-Care HCV Ab Screen versus Standard Phlebotomy N=438 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Covariate | AOR | (95% CI) | Marginal Effects (dy/dx) | p-value | Covariate | AOR | (95% CI) | Marginal Effects (dy/dx) | p-value |
| Prior STI | 5.03 | (1.76,14.26) | 0.36 | <0.01 | > 15 sex partners | 5.84 | (3.14, 10.8) | 0.41 | <0.01 |
| Non-Hispanic White | 0.55 | (0.36,0.78) | −0.11 | <0.01 | Non-Hispanic White | 3.21 | (1.58, 5.96) | 0.27 | <0.01 |
| Non-Hispanic Black | 0.76 | (0.53, 1.09) | −0.05 | 0.13 | Non-Hispanic Black | 1.15 | (0.62, 1.85) | 0.04 | 0.65 |
| Hispanic | Referent | Hispanic | Referent | ||||||
| US-born | 1.76 | (1.25,2.46) | 0.12 | <0.01 | US-born | 2.31 | (1.26, 3.68) | 0.21 | <0.01 |
| Injection Drug Use (PWID) |
2.21 | (1.12,4.46) | 0.14 | 0.03 | Injection Drug Use (PWID) |
0.31 | (0.19, 1.01) | −0.20 | 0.01 |
| Baby Boomer | 0.67 | (0.46,0.97) | −0.07 | 0.04 | |||||
| Prior HCV Diagnosis | 1.94 | (0.94,4.31) | 0.16 | 0.09 | Prior HCV Diagnosis | 2.10 | (0.67, 6.58) | 0.18 | 0.2 |
| Non-injection Drug Use | 0.71 | (0.82,1.97) | 0.05 | 0.23 | |||||
| Unstable | 0.76 | (0.50,1.19) | −0.04 | 0.21 | |||||
| Housing | |||||||||
| Median AIC: 1392 | Median AIC: 67 | ||||||||
LEGEND: HCV: Hepatitis C Virus; HCV Ab: Hepatitis C Antibody; STI: Sexually Transmitted Infection; AOR: Adjusted Odds Ratio; CI: Confidence Interval; PWID: People Who Inject Drugs; AIC: Akaike Information Criterion; Covariates significant at the p<0.05 level are boldfaced.
Figure 3:
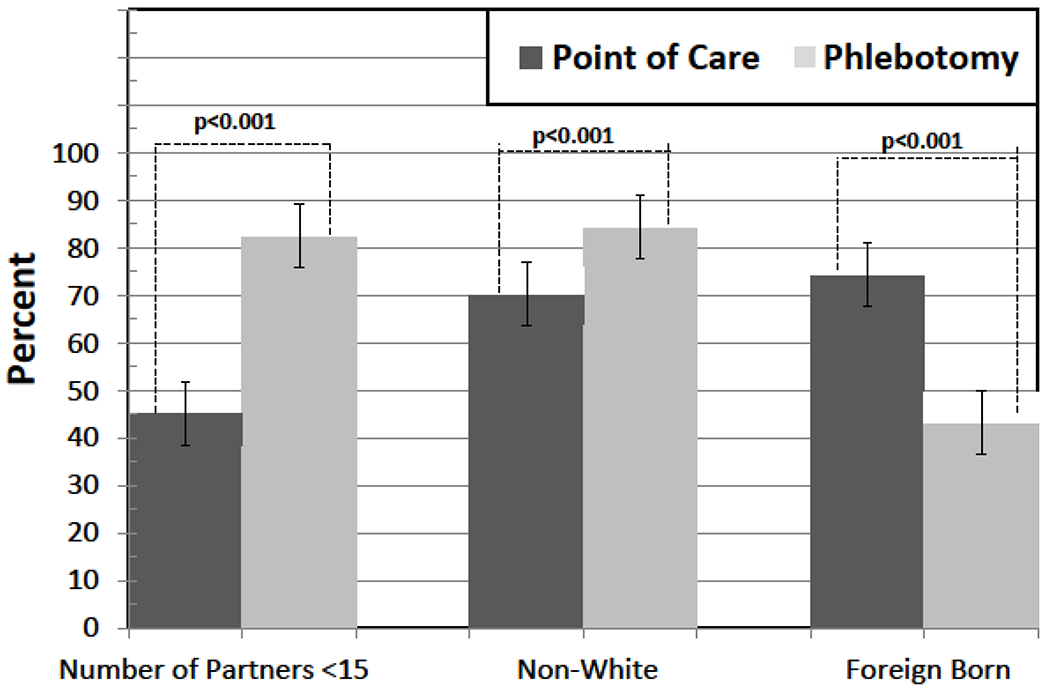
Key determinants of HCV testing strategy for clients on the mobile medical clinic model; point-of-care (POC) vs standard (phlebotomy) testing, New Haven, Connecticut, 2012-2013.
Figure 4:
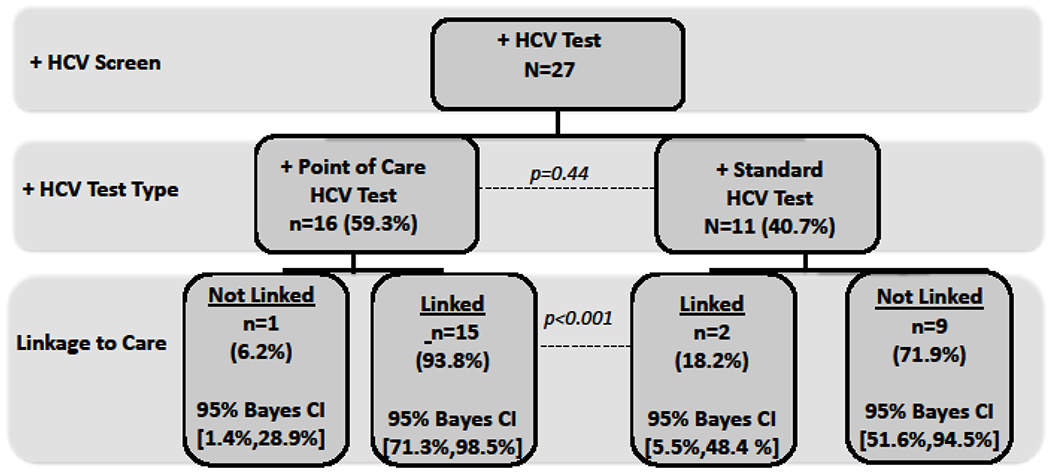
Linkage to care algorithm for point-of-care (POC) Hepatitis C virus (HCV) antibody testing versus traditional phlebotomy testing in a mobile medical clinic, New Haven, Connecticut, 2012-2013.
DISCUSSION
MMCs are highly acceptable to medically disenfranchised patients, including patients with HCV and underlying substance use disorders. They provide immediate, point-of-care treatment for patients across a range of medical conditions and are convenient for patients due to their user-friendly, street outreach, and non-judgmental nature. As such, they are emerging as important sources of non-specialty treatment and may provide an alternative patient-centered treatment strategy to traditional “brick and mortar” facilities.[58,59] MMCs, due to their mobility and user friendly services, innovatively increase healthcare accessibility and reduce health disparities for communities marginalized by geographic, social, and structural barriers through delivering essential services for preventative,[28,33,48,60,61] primary care,[19,25,26,62,63] and targeted disease-specific care.[20,43,64–67]
Overall, one third of all MMC clients opted for HCV testing. With expanded education and social marketing due to the more tolerable and efficacious HCV treatment strategies, HCV testing acceptability is likely to improve, especially among at-risk populations. Of concern here is the reduced likelihood of baby boomers to accept HCV testing in this population despite a high overall HCV prevalence among those tested. Strategies to improve HCV testing among this group is increased targeting of this high-risk group and our findings may reflect that current CDC messages about testing is not yet reaching marginalized populations.
POC HCV testing is an acceptable testing strategy in MMC settings with nearly half preferring POC testing. Importantly, a secondary and unanticipated benefit of our expanded HCV testing program was that it actively engaged HCV-infected patients and brought them into care, including acceptance of referrals to treatment. Thus, this modality of healthcare delivery is effective in both identifying new infections and also re-engaging those who have been previously diagnosed.
It is not surprising that patients have different preferences about HCV testing, specifically with different client profiles among those choosing one strategy over another. Importantly, those with the highest levels of perceived sexual risk (i.e., multiple sexual partners), preferred POC testing, yet those with injection-related risk selected standard phlebotomy testing. Though we did not specifically ask why clients chose one HCV testing strategy over another, one unsubstantiated speculation is that clients with many sexual partners may have been previously screened for other conditions (e.g., HIV, syphilis) but had not been tested for HCV since it is not traditionally associated with sexual risk, while PWIDs who are greatest risk for HCV plus numerous other conditions may have opted to “bundle” their screening for several conditions in favor of a more comprehensive assessment that could be obtained only through phlebotomy. The influence of patient preferences and expectations on patient reported outcomes remains an important issue in providing acceptable healthcare. Patients who actively engage in the decision-making process experience an empowerment effect, ultimately receive their preferred treatment, and may become more motivated to adhere to their selected treatment.[68]
There is increasing emphasis on patient preferences and choice in healthcare decision-making,[69] as recent research has shown evidence that expansion of choices can lead to improvements in patient health outcomes and quality of life. A 2008 systematic review and meta-analysis by the Preference Collaborative Review Group suggests that preferences positively affect patient outcomes in clinical trials, with those receiving their preferred treatment demonstrating better treatment outcomes.[70] For this reason, the patients in our setting were offered an option to be screened for HCV, as well as a choice between two HCV screening strategies.
In the context of this study, HCV-infected patients identified using POC testing were dramatically more likely to be linked to HCV specialty care. While it is unlikely that patient preference contributed to linkage to care, it is likely that immediate intervention once a diagnosis was made contributed to improved intervention outcomes. A recent systematic review of HIV rapid POC testing confirmed higher likelihood of linkage to care.[71] Taken together, these findings support that POC HCV testing using community outreach from a MMC is a complementary public health strategy to overcome health disparities for high risk, vulnerable populations. In particular with the increasing incidence of new HCV infections among young PWID, it is important for MMCs to promote early identification and linkage to care. Specific from this study was the ability to engage younger at-risk populations through the MMC, who might not otherwise be engaged in care, and effectively screen them for HCV. POC HCV testing at an MMC provides an important and complementary health delivery approach to access vulnerable populations who are otherwise estranged from healthcare
LIMITATIONS
The pilot nature of study lends itself to several important limitations. First, routine risk information was gleaned from routine clinical intake instrument on the MMC and not designed specifically to identify HCV-related risks. The addition of the HCV surveillance reporting information, however, supplemented the clinical information. Second, though there were few missing data, multiple imputations were deployed to enrich the data for completeness. Third, though we were able to determine how patient preferences influenced several outcomes, we collected insufficient data to determine “why” patients selected POC or standard HCV testing. Last, this study assessed POC HCV testing strategies only as the technology became available, and such findings may not persist as clinical care sites and patients have more experience with POC testing, including for HCV. Last, because HCV testing was provided for free, pragmatic studies may show lower acceptability of HCV testing, and if patients or providers had to consider costs, such constraints may influence HCV testing from a pragmatic perspective. Despite these limitations, however, important findings gleaned here provide insights into HCV testing acceptability by vulnerable populations, POC testing preferences and the ability of POC to improve linkage to care, which ultimately may improve individual and public health.
CONCLUSIONS
Acceptability of HCV testing, irrespective of strategy, was high and resulted in several newly identified HCV-infected patients. Differing demographic and motivations provided insights into preferences for POC and standard testing. POC testing, however, resulted in markedly higher linkage to HCV specialty care, but should be confirmed through an informed decision-making process, perhaps using an informed decision-making aid, to help guide future testing and linkage strategies.
Funding:
Funding for the study was provided by the National Institutes on Drug Abuse for Career Development (FLA: K24 DA017072) and Research (FLA: R01 DA13805, R01 DA017059) and The National Institutes of Allergy and Infectious Diseases (JPM: T32 A1007517) and Vertex Pharmaceuticals (FLA, JPM: Hepatitis C Circle of Care Grant). We thank the Connecticut Department of Public Health and Merck Pharmaceuticals for their generous donations of OraQuick® HCV Rapid Antibody Tests (OraSure Technologies, Inc., Bethlehem, Pennsylvania).
Competing Interests:
Vertex Pharmaceutical Circle of Care for Hepatitis C Grant for Educational Initiatives for Hepatitis C
Footnotes
IRB Approval: Yale University School of Medicine, Human Investigation Committee, Approval #1306012179
References
- 1.Wendt A, Adhoute X, Castellani P, Oules V, Ansaldi C, Benali S, et al. (2014). Chronic hepatitis C: future treatment. Clin Pharm, 6 (eCollection), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shepard C, Finelli L, Alter M (2005). Global epidemiology of hepatitis C virus infection. Lancet ID, 5 (9), 558–567. [DOI] [PubMed] [Google Scholar]
- 3.National Institute for Clinical Excellence (2004). Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of chronic hepatitis C. http://www.nice.org.uk/nicemedia/live/11524/32809/32809.pdf, accessed May 10, 2014.
- 4.Ly K, Xing J, Klevens M, Jiles R, Ward J, Holmberg S (2012). The growing burden of mortality from viral hepatitis in the US, 1999-2007. Ann Int Med, 156, 271–278. [DOI] [PubMed] [Google Scholar]
- 5.Keeling M, Eames K (2005). Networks and epidemic models. J Royal Soc Int, 2, 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rein D, Wittenborn J, Weinbaum C, Sabin M, Smith B, Lesesne S (2011). Forecasting the morbidity and mortality associated with prevalent cases of pre-cirrhotic chronic hepatitis C in the United States. Dig Liver Dis, 43 (1), 66–72. [DOI] [PubMed] [Google Scholar]
- 7.Chou R, Cottrell E, Wasson N, Rahman B, Guise J (2013). Screening for hepatitis C virus infection in adults: A systematic review for the U.S. Preventive Services Task Force. Ann Int Med, 158, 101–108. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (2011). Viral hepatitis surveillance – United States, 2011. http://www.cdc.gov/HEPATITIS/Statistics/index.htm, accessed Apri 4, 2014.
- 9.Razavi H, ElKhoury A, Elbasha E, Estes C, Pasini K, Poynard T, et al. (2013). Chronic hepatitis C Virus (HCV) disease burden and cost in the United States. Hepatology, 57 (6), 2164–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruce RD, Eiserman J, Acosta A, Gote C, Lim JK, Altice FL (2012). Developing a modified directly observed therapy intervention for hepatitis C treatment in a methadone maintenance program: implications for program replication. Am J Drug Alcohol Abuse, 38 (3), 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maru DS, Bruce RD, Basu S, Altice FL (2008). Clinical outcomes of hepatitis C treatment in a prison setting: feasibility and effectiveness for challenging treatment populations. Clin Infect Dis, 47 (7), 952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruneau J, Zeng G, Abrahamowicz M, Jutras-Aswad D, Daniel M, Roy E (2014). Sustained drug use changes after Hepatitis C screening and counseling among recently infected persons who inject drugs: a longitudinal study. Clin Inf Dis, 58, 755–761. [DOI] [PubMed] [Google Scholar]
- 13.Sala A, Cao L, Wilson C, Zablit R, Zheng H, Zhao B Measurement-calibrated graph models for social network experiments 19th International Conference on World Wide Web. (2010). Raleigh, North Carolina. pp. 861–870. [Google Scholar]
- 14.Page K, Morris M, Hahn J, Maher L, Prins M (2013). Injection drug use and hepatitis C virus infection in young adult injectors: using evidence to inform comprehensive prevention. Clin Inf Dis, 57 (Suppl 2), S32–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrington P, Scott J, Wasserman S (2005). Models and methods in social network analysis. New York, NY: Cambridge University Press. [Google Scholar]
- 16.Hutchinson S, Bird S, Taylor A, Goldberg D (2006). Modelling the spread of hepatitis C virus infection among injecting drug users in Glasgow: Implications for prevention. Int J Drug Policy, 17, 211–221. [Google Scholar]
- 17.Centers for Disease Control and Prevention (2011). Hepatitis C virus infection among adolescents and young adults: Massachusetts, 2002-2009. MMWR, 60 (17), 537–541. [PubMed] [Google Scholar]
- 18.OraSure Technologies Inc. (2014). OraQuick® HCV Rapid Antibody Test. Bethlehem, Pennsylvania. http://www.orasure.com/company/company-overview-profile.asp, accessed April 4, 2014. [Google Scholar]
- 19.Leese GP, Ahmed S, Newton RW, Jung RT, Ellingford A, Baines P, et al. (1993). Use of mobile screening unit for diabetic-retinopathy in rural and urban areas. Brit Med J, 306 (6871), 187–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarnquist CC, Soni S, Hwang H, Topol BB, Mutima S, Maldonado YA (2011). Rural HIV-infected women’s access to medical care: ongoing needs in California. AIDS Care, 23 (7), 792–796. [DOI] [PubMed] [Google Scholar]
- 21.Edgerley LP, El-Sayed YY, Druzin ML, Kiernan M, Daniels KI (2007). Use of a community mobile health van to increase early access to prenatal care. Maternal and Child Health J, 11 (3), 235–239. [DOI] [PubMed] [Google Scholar]
- 22.Heller BR, Goldwater MR (2004). The Governor’s wellmobile: Maryland’s mobile primary care clinic. The Journal of Nursing Education, 43 (2), 92–94. [DOI] [PubMed] [Google Scholar]
- 23.Guruge S, Hunter J, Barker K, McNally MJ, Magalhaes L (2010). Immigrant women’s experiences of receiving care in a mobile health clinic. J Adv Nurs, 66 (2), 350–359. [DOI] [PubMed] [Google Scholar]
- 24.Whelan C, Chambers C, Chan M, Thomas S, Ramos G, Hwang S (2010). Why do homeless people use a mobile health unit in a country with universal health care? J Primary Care Comm Health, 1 (2), 78–82. [DOI] [PubMed] [Google Scholar]
- 25.Daiski I (2005). The health bus: healthcare for marginalized populations. Policy Polit Nurs Pract, 6 (1), 30–38. [DOI] [PubMed] [Google Scholar]
- 26.Hastings J, Zulman D, Wali S (2007). UCLA mobile clinic project. J of Health Care Poor Underserved, 18 (4), 744–748. [DOI] [PubMed] [Google Scholar]
- 27.Shannon K, Rusch M, Shoveller J, Alexson D, Gibson K, Tyndall MW (2008). Mapping violence and policing as an environmental-structural barrier to health service and syringe availability among substance-using women in street-level sex work. Int J Drug Policy, 19 (2), 140–147. [DOI] [PubMed] [Google Scholar]
- 28.Collinson S, Ward R (2010). A nurse-led response to unmet needs of homeless migrants in inner London. Br J Nurs, 19 (1), 36–41. [DOI] [PubMed] [Google Scholar]
- 29.(December 17, 2006). New Haven’s Problems and Promise. The New York Times. New York, NY. [Google Scholar]
- 30.Rosenfeld E New Haven fourth most dangerous city in U.S., according to preliminary FBI data. Yale Daily News. New Haven, CT. [Google Scholar]
- 31.Morano JP, Gibson BA, Altice FL (2013). The burgeoning HIV/HCV syndemic in the urban Northeast: HCV, HIV, and HIV/HCV coinfection in an urban setting. PloS One, 8 (5), e64321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heimer R, Kaplan EH, O’Keefe E, Khoshnood K, Altice F (1994). Three years of needle exchange in New Haven: what have we learned? AIDS Public Policy J, 9 (2), 59–74. [Google Scholar]
- 33.Schwarz RK, Bruce RD, Ball SA, Herme M, Altice FL (2009). Comparison of tuberculin skin testing reactivity in opioid-dependent patients seeking treatment with methadone versus buprenorphine: policy implications for tuberculosis screening. Am J Drug Alcohol Abuse, 35 (6), 439–444. [DOI] [PubMed] [Google Scholar]
- 34.Morano JP, Zelenev A, Walton MR, Bruce RD, Altice FL (2014). Latent tuberculosis infection screening in foreign-born populations: A successful mobile clinic outreach model. Am J Pub Health, 104 (8), 1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altice FL, Maru DS, Bruce RD, Springer SA, Friedland GH (2007). Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: a prospective, randomized, controlled trial. Clin Infect Dis, 45 (6), 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altice FL, Mezger JA, Hodges J, Bruce RD, Marinovich A, Walton M, et al. (2004). Developing a directly administered antiretroviral therapy intervention for HIV-infected drug users: implications for program replication. Clin Infect Dis, 38 (Suppl 5), S376–S387. [DOI] [PubMed] [Google Scholar]
- 37.Maru DS, Bruce RD, Walton M, Springer SA, Altice FL (2009). Persistence of virological benefits following directly administered antiretroviral therapy among drug users: results from a randomized controlled trial. J Acquir Immune Defic Syndr, 50 (2), 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maru DS, Kozal MJ, Bruce RD, Springer SA, Altice FL (2007). Directly administered antiretroviral therapy for HIV-infected drug users does not have an impact on antiretroviral resistance: results from a randomized controlled trial. J Acquir Immune Defic Syndr, 46 (5), 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saber-Tehrani AS, Springer SA, Qiu J, Herme M, Wickersham J, Altice FL (2012). Rationale, study design and sample characteristics of a randomized controlled trial of directly administered antiretroviral therapy for HIV-infected prisoners transitioning to the community - a potential conduit to improved HIV treatment outcomes. Contemp Clin Trials, 33 (2), 436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith-Rohrberg D, Mezger J, Walton M, Bruce RD, Altice FL (2006). Impact of enhanced services on virologic outcomes in a directly administered antiretroviral therapy trial for HIV-infected drug users. J Acquir Immune Defic Syndr, 43 Suppl 1, S48–53. [DOI] [PubMed] [Google Scholar]
- 41.Altice FL, Bruce RD, Lucas GM, Lum PJ, Korthuis PT, Flanigan TP, et al. (2011). HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr, 56 (Suppl 1), S22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCoy C, Metsch L, Chitwood D, Pereyra M (2000). Use of preventive health services by chronic drug users. Drug and Alcohol Dep, 60 (Suppl 1), S143. [Google Scholar]
- 43.Liebman J, Pat Lamberti M, Altice F (2002). Effectiveness of a mobile medical van in providing screening services for STDs and HIV. Public Health Nurs, 19 (5), 345–353. [DOI] [PubMed] [Google Scholar]
- 44.Thompson AS, Blankenship KM, Selwyn PA, Khoshnood K, Lopez M, Balacos K, et al. (1998). Evaluation of an innovative program to address the health and social service needs of drug-using women with or at risk for HIV infection. J Community Health, 23 (6), 419–440. [DOI] [PubMed] [Google Scholar]
- 45.Springer SA, Altice FL, Herme M, Di Paola A (2013). Design and methods of a double blind randomized placebo-controlled trial of extended-release naltrexone for alcohol dependent and hazardous drinking prisoners with HIV who are transitioning to the community. Contemp Clin Trials, 37 (2), 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Springer SA, Qiu J, Saber-Tehrani AS, Altice FL (2012). Retention on buprenorphine is associated with high levels of maximal viral suppression among HIV-infected opioid dependent released prisoners. PloS One, 7 (5), e38335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Springer SA, Chen S, Altice FL (2010). Improved HIV and substance abuse treatment outcomes for released HIV-infected risoners: the impact of buprenorphine treatment. J Urban Health, 87 (4), 592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Grady J, Hoelscher M, Atun R, Bates M, Mwaba P, Kapata N, et al. Tuberculosis in prisons in sub-Saharan Africa - the need for improved health services, surveillance and control. Tuberculosis, 91 (2), 173–178. [DOI] [PubMed] [Google Scholar]
- 49.Zelenev A, Marcus R, Kopelev A, Cruzado-Quinones J, Spaulding A, Desabrais M, et al. (2013). Patterns of homelessness and implications for HIV health after release from jail. AIDS Behav, 17 (Suppl 2), S181–194. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Althoff AL, Zelenev A, Meyer JP, Fu J, Brown SE, Vagenas P, et al. (2013). Correlates of retention in HIV care after release from jail: results from a multi-site study. AIDS Behav, 17 (Suppl 2), S156–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Draine J, Ahuja D, Altice FL, Arriola KJ, Avery AK, Beckwith CG, et al. (2011). Strategies to enhance linkages between care for HIV/AIDS in jail and community settings. AIDS Care, 23 (3), 366–377. [DOI] [PubMed] [Google Scholar]
- 52.Morano J, Gibson B, Altice F (2013). The burgeoning HIV/HCV syndemic in the urban northeast: HCV, HIV, and HIV/HCV coinfection in an urban setting. PloS One, 8 (3), e64321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. (1992). The fifth edition of the addiction severity index. J Subst Abuse Treat, 9 (3), 199–213. [DOI] [PubMed] [Google Scholar]
- 54.Rubin D (1987). Multiple imputation for non-response in surveys. New York, NY: Wiley. [Google Scholar]
- 55.Greene WH (2007). Econometric analysis, 4th edition. Upper Saddle River, NJ: Prentice-Hall. [Google Scholar]
- 56.Gelman A, Carlin JB, Stern HS, Rubin DB (1997). Bayesian data analysis. London, U.K.: Chapman and Hall. [Google Scholar]
- 57.Stata Corporation (2011). STATA: Release 12. Statistical Software College Station, TX: StataCorp LP. [Google Scholar]
- 58.Angora B, Assemien JD, Laurent A, Febro V, Coulibaly-Offia M, Masumbuko JM, et al. HIV in prison in low income countries. AIDS, 25 (9), 1244–1246. [DOI] [PubMed] [Google Scholar]
- 59.Gibson B, Ghosh D, Morano J, Altice F (2014). Accessibility and utilization patterns of a mobile medical clinic among vulnerable populations. Health & Place, 28, 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jit M, Stagg HR, Aldridge RW, White PJ, Abubakar I (2011). Dedicated outreach service for hard to reach patients with tuberculosis in London: observational study and economic evaluation. Brit Med J, 343, d5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vyas A, Madhavan S, Lemasters T, Atkins E, Gainor S, Kennedy S, et al. (2011). Factors influencing adherence to mammography screening guidelines in Appalachian women participating in a mobile mammography program. J Community Health, 37 (3), 632–646. [DOI] [PubMed] [Google Scholar]
- 62.Simsek Z, Koruk I, Doni NY (2012). An operational study on implementation of mobile primary healthcare services for seasonal migratory farmworkers, Turkey. Maternal Child Health J, 16 (9), 1906–1912. [DOI] [PubMed] [Google Scholar]
- 63.Pollack HA, Khoshnood K, Blankenship KM, Altice FL (2002). The impact of needle exchange-based health services on emergency department use. J Gen Intern Med, 17 (5), 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maheswaran H, Thulare H, Stanistreet D, Tanser F, Newell ML (2012). Starting a home and mobile HIV testing service in a rural area of South Africa. J Acquir Immune Defic Syndr, 59 (3), e43–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruiz M, Briones-Chavez CS (2010). How to improve the health of undocumented Latino immigrants with HIV in New Orleans: an agenda for action. Rev Panam Salud Publ, 28 (1), 66–70. [DOI] [PubMed] [Google Scholar]
- 66.Ruiz P, Vazquez W, Vazquez K (1973). The mobile unit: a new approach in mental health. Com Mental Health J, 9 (1), 18–24. [DOI] [PubMed] [Google Scholar]
- 67.Massie HN (1972). Neighborhood psychiatry in a mobile health unit: a report on psychiatric contact with adolescents and young adults in the Judson Mobile Health Unit in New York’s Lower East Side in 1969 and 1970. Comprehensive Psychiatry, 13 (5), 429–433. [DOI] [PubMed] [Google Scholar]
- 68.Torgerson D, Klaber-Moffett J, Russell IT (1996). Patient preferences in randomised trials: threat or opportunity? J of Health Serv Res Pol, 1, 194–197. [DOI] [PubMed] [Google Scholar]
- 69.Gallos L, Liljeros F, Argyrakis P, Bunde A, Havlin S (2007). Improving immunization strategies. Physical Review E, 7 (045104-R), 1–4. [DOI] [PubMed] [Google Scholar]
- 70.Preference Collaborative Review Group (2008). Patients’ preferences within randomised trials: systematic review and patient level meta-analysis. Brit Med J, 337, a1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wynberg E, Cooke G, Shroufi A, Reid SD, Ford N (2014). Impact of point-of-care CD4 testing on linkage to HIV care: a systematic review. J Int AIDS Soc, 17, 18809. [DOI] [PMC free article] [PubMed] [Google Scholar]


