Abstract
Objective
Treatment for COVID-19 is still urgent need for the critically ill and severe cases. UC-MSC administration has a therapeutic benefit for severe COVID-19 patients even in the recovery period. In this paper, we aimed to present our clinical experience with UC-MSC treatment in severe and critical severe COVID-19 patients.
Methods
In this study we evaluated the clinical outcome of severe/critically severe 210 COVID-19 patients treated with UC-MSCs, 1–2 × 106 per kilogram to 210 patients from 15/10/2020 until 25/04/2021.
Results
Out of 99 critically severe intubated patients we have observed good clinical progress/discharged from ICU in 52 (52.5%) patients. Where as 86 (77.5%) of 111 severe unintubated patients discharged from ICU. Intubated 47 (47.5%) patients and unintubated 25 (22.5%) patients pass away. Significantly higher survival was observed in patients who underwent UC-MSCs before intubation (OR = 1.475, 95% CI = 1.193–1.824 p < 0.001). It was observed that the SaO2 parameter tended to improve after UC-MSC therapy compared to all groups. But SaO2 parameter between intubated and unintubated groups was not statistically significant (p > 0.05), while in discharged cases SaO2 parameter was statistically significant (p = 0.01). Besides, there was a statistically significant relation with intubation status, age (OR = 3.868, 95% CI = 0.574–7.152 p = 0.02) and weigh (OR = 6.768, 95% CI = 3.423–10.112 p < 0.001) thus presented an elevated risk for COVID-19. The linear regression analysis confirmed that the high weight was associated with the risk of intubation in COVID-19 (p = 0.001).
Conclusions
According to our results and from recent studies, UC-MSC treatment is safe with high potential to be used as an added therapeutic treatment for severe COVID-19 patients. Our experience showed that UC-MSC therapy may restore oxygenation and downregulate cytokine storm in patients hospitalized with severe COVID-19. We advice wider randomised studies to discover the detailed therapeutic pathophysiology of the MSCs on COVID-19 patients.
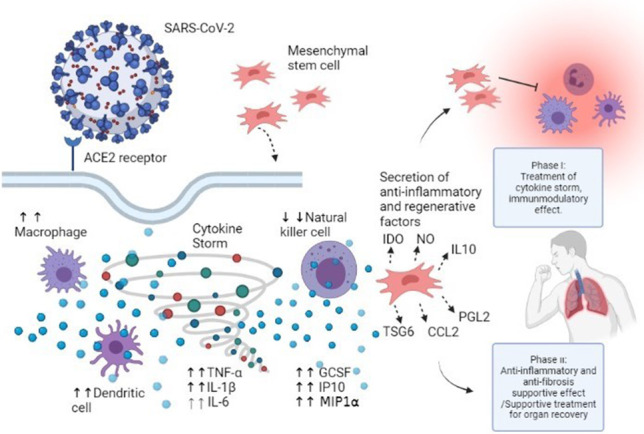
Graphical abstract
MSCs transplantation improves the damaging effects of the cytokine storm through immunomodulation and improving tissue and organ repair. Severe patients who were unintubated were in the Phase I, while critical patients who were intubated were in the Phase II. The figure is created via biorender application, (BioRender.com).
Keywords: MSCs, COVID-19, Stem cell therapy, SARS-CoV-2, Cytokine storm, Immunomodulatory effects
Introduction
COVID-19 causes fever, cough, respiratory tract inflammation, acute respiratory distress syndrome (ARDS), viral sepsis and multisystemic disease in which antigens related to the virus have been detected in most organs [1, 2].
As the virus grew and spread expeditiously in China and around the globe, the World Health Organization (WHO) officially declared COVID-19 a pandemic [3]. Currently, no effective drug alone has been proven to cure patients with COVID-19 infection. Thus, the virus is still spreading through population, as of 6 May 2021, there have been 155,506,494 confirmed cases and 3,247,228 deaths. [4]. New therapeutic strategies are needed to reduce the death rate and to improve the recovery of patients without side effects. COVID-19 triggers exaggerated and constant cytokine production suggesting the virus can stimulate a cytokine storm in the lung, which can also lead to severe organ injury and death [5–7].
The nasal epithelium was discovered to be the principal site of disease and transmission among people with suggestive and asymptomatic SARS-CoV-2 contaminations [8]. Similar to SARS-CoV, the S protein of SARS-CoV-2 ties to angiotensin-converting enzyme receptor 2 (ACE2) and enters cells in a way catalyzed by transmembrane protease serine 2 (TMPRSS2) in type II alveolar epithelial cells [8, 9]. The S protein of SARS-CoV-2 for ACE2 is the fundamental determinant of the replication rate of SARS-CoV-2 [10]. Regardless of critical advances in supportive treatment strategies, the incidence and mortality of ARDS stay high [11].
Stem cells derived from different tissues, such as human bone marrow, adipose tissue, and umbilical cord tissue (UC-MSC) have been widely used for regulating immunomodulation by their paracrine effects with different types of cytokines in autoimmune diseases [12]. According to the clinical trials in COVID-19 patients, with UC-MSC and placebo group; an improvement in pulmonary involvement and a better recovery have been demostrated in patients with UC-MSC injection [13]. This finding indicates that UC-MSC administration has a therapeutic benefit for severe COVID-19 patients even in the recovery period [13–15]. In this paper, we aimed to present our clinical experience with UC-MSC treatment in severe and critical severe 210 COVID-19 patients.
Materials and Methods
Experimental Design
Intravenous UC-MSCs transplantation was performed on 210 patients followed in ICU and COVID wards. These 210 patients were clinically heterogeneous and unresponsive to unique COVID-19 medical treatment algorithms confirmed with Turkish Ministry of Health (advised clinical treatment). The patients were enrolled into the supportive treatment with each family’s signed written consent form, in accordance with the Declaration of Helsinki. The UC-MSC transplantations were performed in several hospitals in Turkey and approved by ministry of health stem cell commission (COVID-19) online registration system in 24 h.
Cell Preparation and Transplantation
The clinical grade UC-MSCs were supplied by the Atigen-Cell Technology Center, Trabzon, Turkey licensed by Turkish Health Ministry. The total number of cell count used was 1–2 × 106 cells per kilogram. The cells were prepared for injection in 100 ml of normal saline. The UC-MSCs were administered to the patients at a critical stage, when they did not show any improvement by the recommended clinical treatment for COVID-19, as to whether the effectiveness of the cellular treatment on this severe stage of infection and inflammation could be observed. The cells were infused in 50–60 min with a rate of 2 ml/minute, as described in the literature [5, 15]. In this study we evaluated the outcome of the MSC treatment after a single dose of clinical-grade UC-MSCs (1–2 × 106 cells/kg), intravenously.
Patients
The patients (ages 23–81) were tested by the real-time Reverse Transcription Polymerase Chain Reaction (RT-PCR) assay of nasal and pharyngeal swabs and evaluated when they were first admitted. In case of one of them confirmed with COVID-19 infection, the patient was started with the recommended treatment protocol by the ministry of health, and a second PCR test was scheduled to hospital. Patients were excluded from the study if they had a history of cancer or bacterial and yeast secondary infection. The patients were enrolled for the supportive UC-MSC transplantation due to their lack of response to advised treatments. Patients were followed in intensive care unit (ICU) and COVID wards for primary safety and efficacy outcomes as introduced in previous COVID-19 studies in the literature, until they were discharged from the hospital or passed away. All the patients were underwent MSCs transplantation after the approval by Stem Cell Treatment Comission of Turkish Health Ministry. This procedure is an active online protocol between the hospital clinic and Ministry Commission; followed by clinical doctor’s signed requisation form, patient representative signed consent and Licenced Stem Cell Center cell product signed form.
The cells are carried to the hospitals under the confirmed “transport protocol” of the Cell Center quality system. Intravenous MSC transplantation was performed on 210 PCR positive severe and critically severe COVID-19 patients followed in inpatient/ICU, unresponsive to treatment algorithms advised by the Turkish COVID-19 Scientific Committee. As the 17 patients excluded from the study, they had a history of cancer or bacterial and yeast secondary infection.
Statistical Analysis
The findings of this study were evaluated by using the software IBM SPSS Statistics (IBM Corporation version 20.0 SPSS Inc., Chicago, IL, USA). Quantitative data were shown as mean (± standard deviation), whereas categorical data were shown as numbers and percentiles. When comparing normally distributed continuous variables between the study groups, t-test was used. Qualitative data such as sex, intubated condition, outcome condition, safety and efficiency outcome were tested by using the Chi-square statistic. We evaluated the comparision of arterial oxygen saturation (SaO2) before and after UC-MSC therapy by Paired Sample t-test. A multivariate analysis was performed by the linear regression model. The model included age and weight as independent variables and intubation condition was used as a dependent variable. To determine the risk factor between groups, odds ratio (OR) and 95% confidence interval (95% CI) were used. A p-value of < 0.05 was regarded statistically significant.
Results
Participant Flow
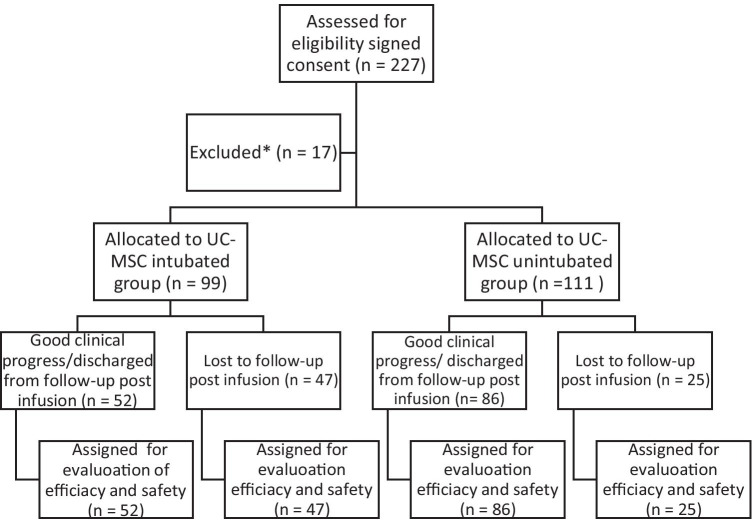
COVID-19 infected critically severe and severe patients were evaluated for this study. Participant flow chart with enrollment and randomization is shown in Fig. 1. They were followed with unique COVID-19 medical treatment algorithms confirmed by Turkish Ministry of Health (advised clinical treatment). Every patient received UC-MSCs (1–2 × 106 per kilogram) on average 6.4 days after being tested positive. Demographics and baseline characteristics for enrolled patients are presented in Table 1. 99 critically severe patients were intubated before the MSC transplantation. All the patients were observed until they were discharged from ICU or passed away.
Fig. 1.
Participant flow. UC-MSCs, umbilical cord mesenchymal stem cells. *Patients with cancer, bacterial and yeast secondary infection were not included in the study
Table 1.
Baseline characterictics of treatment group
| Characteristics | UC-MSC | Total (n) | P value | |
|---|---|---|---|---|
| Intubated (n = 99) | Unintubated (n = 111) | |||
| Sex, n (%) | 210 | 0,64a | ||
| Male | 74 (74.7%) | 79 (71.2%) | 153 | |
| Female | 25 (25.3%) | 32(28.8%) | 57 | |
| Age, mean ± SD, years | 61.22 ± 11.77 | 57.36 ± 12.00 | 0,02b | |
| Age range, years | 27–83 | 23–81 | ||
| Weight, mean ± SD, kg | 90.63 ± 12.86 | 83.86 ± 11.66 | < 0,001b | |
In this study, patients who were intubated were in the critical disease category, while patients who were not intubated were in the severe disease category. COVID-19 disease severity; Mild, moderate, severe and critically disease. Severe Illness: Individuals who have SpO2 < 94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) < 300 mm Hg, respiratory frequency > 30 breaths/min, or lung infiltrates > 50%. Critical Illness: Individuals who have respiratory failure, septic shock, and/or multiple organ dysfunction (3)
Abbreviations: UC-MSC umbilicial cord mesenchymal stem cell, n number of samples
a Pearson Chi-square test
b t-test
Recruitment
Patients were excluded from the study if they had a history of cancer or bacterial and yeast secondary infection, so excluded 17 out of 227 patients. 210 severe and critically severe patients recieved UC-MSCs. UC-MSCs derived from Wharton's jelly tissue were used in Turkey on 210 severe/critically ill COVID-19 patients, 47.1% of the patients were intubated in the ICU and 52,9% of the patients were unintubated.
Primary Safety Outcome
No adverse effects were observed related to infusion or allergic reactions, secondary infection, or life-threatening adverse events in patients, who received UC-MSC transplantation. The treatments were recorded within the predicted safety levels of UC-MSC transplantation treatments mentioned in the previous studies.
Efficacy Outcome
We evaluated patients in two main groups, the first group involved the patients who were unintubated. The second group involved the intubated patients. Patients in both groups were followed-up for at least 2–3 weeks after UC-MSC injection. We evaluated the patient exit with the clinical condition and comparision of SaO2 contributing to the efficacy outcome after UC-MSC injection.
A total of 210 cases diagnosed with COVID-19 were studied. 57 patients were female, and 153 patients were male with mean ages of 56.84 ± 14.12 and 59.99 ± 11.06, respectively. There was not statistically significant difference between men and women in the terms of age (p = 0.09). While the mean age of intubated patients is 61.22 ± 11.77, the mean age of unintubated patients is 57.36 ± 12.00. There was a statistically significant relation between intubation status in terms of age (OR = 3.868, 95% CI = 0.574–7.152 p = 0.02). High age is associated with the risk of intubation in COVID-19. The mean weight of intubated patients is 90.63 ± 12.86, while the mean weigh of unintubated patients is 83.86 ± 11.66. There was a statistically significant relation between intubation status in terms of weigh (OR = 6.768, 95% CI = 3.423–10.112 p < 0.001) (Table 1). High weight is associated with the risk of intubation in COVID-19.
It was observed that the SaO2 parameter tended to improve after UC-MSC therapy compared to all groups. But SaO2 parameter between intubated and unintubated groups was not statistically significant (p > 0.05), while in discharged cases SaO2 parameter was statistically significant (p = 0.01) (Table 2).
Table 2.
Comparision of SaO2 before and after UC-MSC therapy
| SaO2 | Before UC-MSC therapy | After UC-MSC therapy | P valuea |
|---|---|---|---|
| Intubated | 90.05 ± 6.49 | 90.34 ± 5.95 | 0.35 |
| Unintubated | 92.47 ± 4.52 | 92.76 ± 4.52 | 0.16 |
| Discharged | 92.09 ± 5.47 | 92.63 ± 4.81 | 0.01* |
| Total | 91.34 ± 5.64 | 91.63 ± 5.40 | 0.11 |
Paired Sample t Test. Values are included as mean ± standard deviation
Abbreviations: SaO2 Arterial oxygen saturation, UC-MSC umbilicial cord mesenchymal stem cell
Out of 99 critically severe intubated patients we have observed good clinical progress/discharged from ICU in 52 (52.5%) patients. Where as 86 (77.5%) of 111 severe unintubated patients discharged from ICU. Intubated 47 (47.5%) patients and unintubated 25 (22.5%) patients pass away. Significantly higher survival was observed in patients who underwent UC-MSCs before intubation (OR = 1.475, 95% CI = 1.193–1.824 p < 0.001) (Table 3). Besides, exit status in COVID-19 patients treated with MSCs according to intubation status was shown that Fig. 2.
Table 3.
Outcomes of patients who underwent UC-MSC according to the intubation condition
| Intubated condition | Discharged / Treatment Continues n (%) | Death n (%) | Total n (%) | P valuea | |
|---|---|---|---|---|---|
| < 0.001* | |||||
| Intubated | 52 (52.5%) | 47 (47.5%) | 99 (100%) | ||
| Unintubated | 86 (77.5%) | 25 (22.5%) | 111 (100%) | ||
| Total | 138 (61%) | 72 (39.0%) | 210 (100%) | ||
Abbreviations: n number of samples, UC-MSC umbilicial cord mesenchymal stem cell
a Pearson Chi-square test. Values are given as the number of subjects (n) and percentages (%)
*(OR = 1.475, 95% CI = 1.193–1.824 p < 0.001)
Fig. 2.
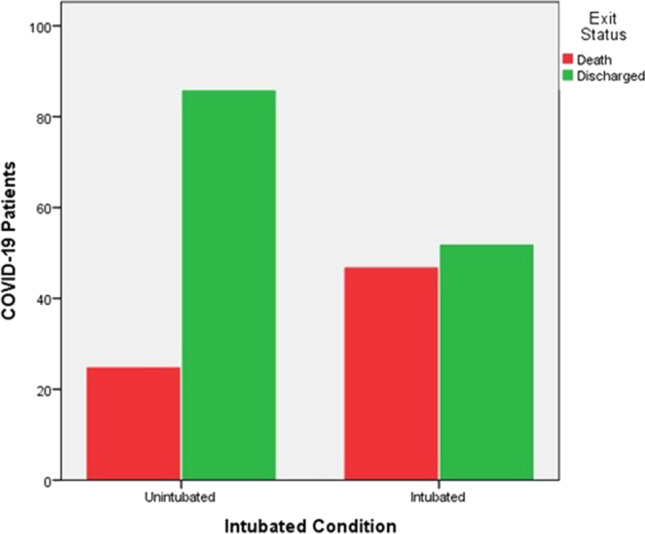
Exit status in COVID-19 patients treated with MSCs according to intubation status
In the linear regression model, the dependent variable, intubation condition was significantly associated with weight (p = 0.001). The linear regression analysis confirmed that the high weight was associated with the risk of intubation in COVID-19 (Table 4).
Table 4.
Linear regression analysis of risk factors for the COVID-19
| Independent variables | B | Std. Error | Standardized Beta | P value | 95% CI for B |
|---|---|---|---|---|---|
| Years | 0.004 | 0.003 | 0.089 | 0.208 | (-)0.002-(-)0.009 |
| Weight | 0.010 | 0.003 | 0.247 | 0.001 | 0.004–0.015 |
Dependent variable: Intubated condition
Discussion
COVID-19 causes fever, cough, respiratory tract inflammation, acute respiratory distress syndrome (ARDS), viral sepsis and multisystemic disease [1, 2]. Clinical experience thus far shows that COVID-19 is highly heterogeneous, ranging from being asymptomatic and mild to severe and causing death. Host factors including age, sex, and comorbid conditions are key determinants of disease severity and progression. Aging itself is a prominent risk factor for severe disease and death from COVID-19 [16]. In our study, A total of 210 cases diagnosed with COVID-19 were studied. 57 patients were female, and 153 patients were male with mean ages of 56.84 ± 14.12 and 59.99 ± 11.06, respectively. There was not statistically significant difference between men and women in the terms of age (p = 0.09). While the mean age of intubated patients was 61.22 ± 11.77, the mean age of unintubated patients was 57.36 ± 12.00. There was a statistically significant relation between intubation status in terms of age (OR = 3.868, 95% CI = 0.574–7.152 p = 0.02).
Popkin et al. shown in meta-analysis that individuals with obesity were more at risk for COVID-19 positive, > 46.0% higher (OR = 1.46; 95% CI, 1.30–1.65; p < 0.0001); for hospitalization, 113% higher (OR = 2.13; 95% CI, 1.74–2.60; p < 0.0001); for ICU admission, 74% higher (OR = 1.74; 95% CI, 1.46–2.08); and for mortality, 48% increase in deaths (OR = 1.48; 95% CI, 1.22–1.80; p < 0.001) [17]. In our study, while the mean weight of intubated patients was 90.63 ± 12.86, the mean weigh of unintubated patients was 83.86 ± 11.66. There was a statistically significant relation between intubation status in terms of weigh (OR = 6.768, 95% CI = 3.423–10.112 p < 0.001). In the linear regression model, the dependent variable, intubation condition was significantly associated with weight (p = 0.001). The linear regression analysis confirmed that the high weight was associated with the risk of intubation in COVID-19.
Mesenchymal stem cells are considered to have extensive clinical application possibilities, including ARDS [18]. MSCs have broad bioactivities, including repair, immunomodulation, increased alveolar fluid clearance, and regulation of pulmonary vascular endothelial permeability [19]. MSCs have a wide range of sources, such as the bone marrow, umbilical cord, adipose tissue, amniotic membrane, and several other tissues [20]. MSCs from different sources have significant similarities, such as being adherent and teardrop- or spindle-shaped [21]. MSCs have the upsides of self-renewal, multidirectional differentiation, and immunomodulation towards suppressing proinflammatory factors. In Accord with these findings, it has been shown that stem cells from the placenta and human umbilical cord showed decreased lung tissue damage in mouse bleomycin models [22]. In clinical setting, immunomodulatory properties of MSCs have been shown in numerous pathological conditions, e.g., bronchopulmonary dysplasia, asthma, acute lung injury, chronic obstructive pneumonic illness, idiopathic aspiratory fibrosis [23, 24]. Studies suggest that MSCs from different sources have different levels of immunoregulatory efficiency. Najar and his colleques revealed in their study that Warton Jelly derived MSCs were found to be the most effective in immunomodulation [25].
Toll-like receptors (TLRs) family has a crucial activity in the innate immune system for the recognition of pathogen-associated molecular patterns (PAMPs), starting primary reaction to pathogens and helping to recognise virus, bacteria, protozoa, and fungi; and are commonly associated with chronic inflammatory and autoimmune diseases [26]. TLRs play an important role in immunomodulation by cell-to-cell contact and MSC-secreted soluble factors [27, 28].
It has been reported that some patients who survived acute phase of ARDS due to COVID-19 die afterwards due to progressive pulmonary fibrosis [29]. Disproportionate activation and proliferation of myofibroblasts results in deposition of the extracellular matrix (ECM) components which deteriorates functions of related tissues and fibrotic states are estimated to contribute to almost 50% of mortalities in the developed world [30]. During inflammatory phase of ARDS, dysregulation and overproduction of matrix metalloproteinases could occur and lead to a complex combination of epithelial and endothelial damage, thus uncontrolled fibrosis [31].
Overactive secretion of pro‐fibrotic growth factors, chemokines and procoagulant mediators are collectively referred as senescence-associated secretory phenotype (SASP) factors. Secretion of these mediators trigger progressive and excessive activation of epithelial cells characterized by dysregulated crosstalk between epithelial cells and mesenchymal cells. Consequent accumulation and activation of myofibroblasts affect the crosstalk between fibroblasts and epithelial cells to exhibit markers of stress and senescence which in turn lead to resistance to apoptosis and excessive production of extracellular matrix components [32, 33].
Inflammation in lung tissue results in alveolar pneumocyte damage and resultant fibroblast activation triggers pulmonary fibrosis through myofibroblast differentiation which might proceed for a long time [34]. Darwish et al. have shown that umbilical cord derived MSCs (UC-MSCs) have the potential to alleviate H5N1 infection induced acute lung injury. Inflammatory cytokine profiles seen in H5N1 infection is similar to COVID-19 such as high levels of IL-6, GCSF, IP10, MCP-1, MIP1 α, and TNF-α [6, 35, 36]. These inflammatory cytokines can activate MSCs to secrete immunosuppressive factors consisting of IDO, TSG6, NO, IL-10, CCL2, galectins, PGE2, and TGF-β and then modulating tissue homeostasis [37].
In recent studies clinical improvement was observed in severe COVID-19 patients, shortening the ICU stay [13, 14]. In a proof-of-concept study significant benefit of laboratory prognostic markers was evaluated consistent with other study findings in COVID-19 patients who had received MSCs transplantation [15, 38]. In another clinical trial with randomised, double-blind, placebo-controlled of phase 2 were done on 100 severe COVID-19 patients with lung damage. They were randomly assigned to receive either UC-MSCs 4 × 107 cells per infusion to 65 patients or placebo (35 patients) on day 0, 3, and 6. It was shown that UC-MSCs treatment is safe and have promissing reatment potential for COVID-19 patients. Improvement in whole lung lesion volume were reported with a better recovery rate compared to the placebo group [13].
Important pathological hallmark of COVID-19 in pulmonary vasculature is pulmonary hypertension as a part of systemic endothelial dysfunction [39, 40]. Endothelial dysfunction and damage explain most of the pathological processes triggered and involved in COVID-19 and mesenchymal cells seem to be promising agents to alleviate such widespread cellular damage [41, 42].
Despite ever increasing number of studies, randomized controlled multicenter clinical trials are certainly needed to achieve standardization of MSC therapy protocols for cell-based treatments in COVID-19 [5, 15, 43–45].
This study was investigated the impact of UC-MSCs therapy in 210 patients with COVID-19 (99 patient with critically severe, 111 patients with severe clinical symptoms). Patients were followed after by pulmonary function and clinical symptoms after the injection. Patients were observed for 2–3 weeks after MSCs transplantation. In our study we have revealed a clinical success rate of 61% in UC-MSC transplanted severe/critically severe COVID-19 patients. We have also observed that the response to MSC treatment is more accurate in severe patients during early stages of cytokine storm than the patients who were entubated and had lung incury due to the hyperimmunity tissue destruction. The results showed that significantly higher survival was observed in patients who underwent UC-MSCs before intubation (p < 0.001).
Here we could discuss about the two phases of UC-MSC treatment in COVID-19 disease due to the clinical aspects we have observed in these patients. In this study, severe patients who were unintubated were in the Phase I, while critical patients who were intubated were in the Phase II. In Phase I, we assume to alleviate the cytokine storm in severe COVID-19 patients with MSC transplantation. The immunmodulatory efficacy of MSCs has been revealed in many studies [7, 12, 46]. In this period of the disease, we could conclude to achieve a beneficial result of the UC-MSC treatment by a proven pathway of immun regulation. We could recommend one iv MSCs dose in the literature as 1–2 × 106/kg as effective for phase I COVID-19 patients [46]. Phase II (supportive treatment for organ recovery): Here we could comment on the treatment mechanism of the MSCs as a supportive treatment for original healing of the alveolar epithelial cells in critically severe COVID-19 patients. This may be achieved by anti inflammatory/antifibrosis supportive effect of MSCs which was determined in various studies [5, 6, 35, 36]. Anti inflammatory supportive effect provides to increase oxygenation in destroyed tissue, which gaines time for patient organ recovery. In our study, it was observed that the SaO2 parameter tended to improve after UC-MSC therapy compared to all groups. Especially, in discharged cases SaO2 parameter was statistically significant (p = 0.01).
UC-MSCs supportive treatment as prevention of fibrosis, which is seen in the COVID-19 patients as a disease complication is vital. We have scientific evidence that the accelerated regenerative original tissue healing may be achieved with MSCs treatment, but this may take some time not less than a couple of weeks, which was explored in many studies [47].
Conclusion
In the first stage of COVID-19 disease, alleviate UC-MSC transplantation is concluded as effective to alleviate the cytokine storm by immune regulation which leads improvement in patient clinical outcome. In the second stage of the disease, MSCs transplantation is evaluated as to increase oxygenation in mostly undamaged lung tissue by an anti-inflammatory effect. However, this effect does not occur constantly. In this condition we may advice repeated multiple doses supporting patient treatment.
In intubated COVID-19 patients with lung or organ injuries we may possibly support the original tissue healing by MSCs transplantation in long term follow up, but the dose and clinical application protocol is much different from the treatment protocol than we use in phase I. We could recommend single iv infussion with 1–2 × 106 cells/kg in phase I. Thus, we have observed that repeated doses may be recomended in phase II patients with tisuue injury for an ongoing healing support by mostly anti-inflammatory and immunregulatory effect. The dose and the application intervals could be experienced by each patient’s clinical response to the treatments.
Immunomodulatory/anti-infamatory and regenerative properties of UC-MSCs have demonstrated promising outcomes on numerous pulmonary disease states. Administration of UC-MSCs to COVID-19 patients has been shown to be well tolerated and safe without any detectable side effect. In the literature so far even patients with severe COVID-19 and pulmonary inflammation have demonstrated better rates of clinical outcome. UC-MSCs have the potential to alleviate inflammation and cytokine storm in COVID-19 patients, together with reducing pulmonary fibrosis processes. Still randomized clinical trials are needed to assess the exact mechanisms of action to determine the best route of application/dosage to enhance short- and long-term UC-MSCs therapeutic outcome.
We advise urgent randomised multicenter studies to evaluate the clinical experience achieved in our clinics and to discuss the mechanisms for the MSCs teatment strategies which we could have revealed some basic facts of the literature related by MSCs treatments.
Abbreviations
- COVID-19
Coronavirus Disease 2019
- MSCs
Mesechymalstem cells
- UC-MSCs
Umbilical Cord-derived mesenchymal stem cells
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- ARDS
Acute respiratory distress syndrome
- WHO
World Health Organization
- TMPRSS2
Transmembrane Serine Protease 2
- RT-PCR
Real Time PCR
- ICU
Intensive care unit
- TLRs
Toll-like receptors
- PAMPs
Pathogen-associated molecular patterns
- ECM
The extracellular matrix
- SASP
Senescence-associated secretory phenotype
- IL-6
Interleukin 6
- GCSF
Granulocyte colony-stimulating factor
- IP10
Interferon gamma-induced protein 10
- MCP-1
The monocyte chemoattractant protein-1
- MIP1α
Macrophage inflammatory protein 1α
- TNF- α
Tumor necrosis factor-alpha
Author Contributions
NOE: Project administration, Methodology, Writing original draft, Conceptualization, Data curation, Writing-review&editing.
KÇPU: Methodology, Investigation, Writing- original draft, Conceptualization, Data curation, Writing-review&editing.
NA: Investigation, Validation.
GRG: Investigation, Validation.
MS: Investigation, Validation.
Data Availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics Approval
The study was in accordance with the Declaration of Helsinki for medical research involving human subjects. The protocol was approved by Stem Cell Treatment Comission of Turkish Health Ministry. This procedure is an active online protocol between the hospital clinic and Ministry Commission; followed by clinical doctor’s signed requisation form, patient representative signed consent and Licenced Stem Cell Center cell product signed form.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Consent for Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: Observations and hypotheses. Lancet. 2020;395(10235):1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odabasi Z, Cinel I. Consideration of Severe Coronavirus Disease 2019 As Viral Sepsis and Potential Use of Immune Checkpoint Inhibitors. Crit Care Explor. 2020;2(6):e0141. doi: 10.1097/CCE.0000000000000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO characterizes COVID-19 as a pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Accessed 5 May 2021.
- 4.WHO Coronavirus (COVID-19) (2021). Dashboard. https://covid19.who.int/. Accessed 5 May 2021.
- 5.Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging and Disease. 2020;11(2):216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30;:] Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta P, McAuley DF, Brown M, et al. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sungnak W, Huang N, Bécavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nature Medicine. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuyama S, Nagata N, Shirato K, Kawase M, Takeda M, Taguchi F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. Journal of Virology. 2010;84(24):12658–12664. doi: 10.1128/JVI.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothlin RP, Vetulli HM, Duarte M, Pelorosso FG. Telmisartan as tentative angiotensin receptor blocker therapeutic for COVID-19. Drug Dev Res. 2020;81(7):768–770. doi: 10.1002/ddr.21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou, C., Yang, B., Tian, Y., et al. (2011). Immunomodulatory effect of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells on lymphocytes. Celluar Immunology,272(1), 33–38. [DOI] [PMC free article] [PubMed]
- 13.Shi L, Huang H, Lu X, et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: A randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduction and Targeted Theraphy. 2021;6(1):58. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng F, Xu R, Wang S, et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: A phase 1 clinical trial. Signal Transduction and Targeted Theraphy. 2020;5(1):172. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ercelen NO, Bilgili B, Monteleone B, et al. MSC transplantation in eight severe COVID-19 patients: Can cytokine storm be reversed? Stem Cell Research & Therapy. 2020;10:460. [Google Scholar]
- 16.Chen Y, Klein SL, Garibaldi BT, et al. Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Research Reviews. 2021;65:101205. doi: 10.1016/j.arr.2020.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popkin BM, Du S, Green WD, et al. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obesity Reviews. 2020;21(11):e13128. doi: 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashemian SR, Aliannejad R, Zarrabi M, et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: A case series. Stem Cell Research and Theraphy. 2021;12(1):91. doi: 10.1186/s13287-021-02165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: Mechanisms of potential therapeutic benefit in ARDS and sepsis. The Lancet Respiratory Medicine. 2014;2(12):1016–1026. doi: 10.1016/S2213-2600(14)70217-6. [DOI] [PubMed] [Google Scholar]
- 20.Main, H., Munsie, M., & O’Connor, M. D. (2014). Managing the potential and pitfalls during clinical translation of emerging stem cell therapies. Clinical and Translation Medicine,3, 10. [DOI] [PMC free article] [PubMed]
- 21.Javazon EH, Colter DC, Schwarz EJ, Prockop DJ. Rat marrow stromal cells are more sensitive to plating density and expand more rapidly from single-cell-derived colonies than human marrow stromal cells. Stem Cells. 2001;19(3):219–225. doi: 10.1634/stemcells.19-3-219. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Jia Z. Cell-based therapy in lung regenerative medicine. Regenerative Medicine Research. 2014;2(1):7. doi: 10.1186/2050-490X-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behnke J, Kremer S, Shahzad T, et al. MSC based therapies-new perspectives for the injured lung. Journal of Clinical Medicine. 2020;9(3):682. doi: 10.3390/jcm9030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss DJ, Bertoncello I, Borok Z, et al. Stem cells and cell therapies in lung biology and lung diseases. Proceedings of the American Thoracic Society. 2011;8(3):223–272. doi: 10.1513/pats.201012-071DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Najar M, Raicevic G, Fayyad-Kazan H, et al. (2012). Immune-related antigens, surface molecules and regulatory factors in human-derived mesenchymal stromal cells: the expression and impact of inflammatory priming [published correction appears in Stem Cell Rev. 2012 Dec;8(4):1286. Kazan, Hussein Fayyad [corrected to Fayyad-Kazan, Hussein]]. Stem Cell Reviews and Reports, 8(4), 1188–1198. [DOI] [PubMed]
- 26.Lotfinejad P, Asadzadeh Z, Najjary S, et al. (2020). COVID-19 infection: Concise review based on the immunological perspective [published online ahead of print, 2020 Sep 28]. Immunological Investigations, 1–20. [DOI] [PubMed]
- 27.Pevsner-Fischer M, Morad V, Cohen-Sfady M, et al. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007;109(4):1422–1432. doi: 10.1182/blood-2006-06-028704. [DOI] [PubMed] [Google Scholar]
- 28.Lombardo E, DelaRosa O, Mancheño-Corvo P, et al. Toll-like receptor-mediated signaling in human adipose-derived stem cells: Implications for immunogenicity and immunosuppressive potential. Tissue Engineering Part A. 2009;15(7):1579–1589. doi: 10.1089/ten.tea.2008.0340. [DOI] [PubMed] [Google Scholar]
- 29.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. The Lancet Respiratory Medicine. 2020;8(8):807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: Nearing the starting line. Science Translational Medicine. 2013;5(167):167sr1. doi: 10.1126/scitranslmed.3004700. [DOI] [PubMed] [Google Scholar]
- 31.Zemans RL, Colgan SP, Downey GP. Transepithelial migration of neutrophils: Mechanisms and implications for acute lung injury. American Journal of Respiratory Cell and Molecular Biology. 2009;40(5):519–535. doi: 10.1165/rcmb.2008-0348TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopes-Paciencia S, Saint-Germain E, Rowell MC, et al. The senescence-associated secretory phenotype and its regulation. Cytokine. 2019;117:15–22. doi: 10.1016/j.cyto.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 33.Sgalla G, Iovene B, Calvello M, et al. Idiopathic pulmonary fibrosis: Pathogenesis and management. Respiratory Research. 2018;19(1):32. doi: 10.1186/s12931-018-0730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuo W, Zhao X, Chen YG. SARS coronavirus and lung fibrosis. Molecular Biology of the SARS-Coronavirus. 2009;22:247–258. [Google Scholar]
- 35.Darwish I, Mubareka S, Liles WC. Immunomodulatory therapy for severe influenza. Expert Review of Anti-Infective Therapy. 2011;9(7):807–822. doi: 10.1586/eri.11.56. [DOI] [PubMed] [Google Scholar]
- 36.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China [published correction appears in JAMA. 2021 Mar 16;325(11):1113] JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang M, Yuan Q, Xie L. Mesenchymal stem cell-based immunomodulation: properties and clinical application. Stem Cells International. 2018;2018:3057624. doi: 10.1155/2018/3057624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang B, Chen J, Li T, et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine (Baltimore). 2020;99(31):e21429. doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamaoka-Tojo M. Vascular endothelial glycocalyx damage in COVID-19. International Journal of Molecuar Sciences. 2020;21(24):9712. doi: 10.3390/ijms21249712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varga Z. Endotheliitis bei COVID-19 [Endotheliitis in COVID-19] Pathologe. 2020;41(Suppl 2):99–102. doi: 10.1007/s00292-020-00875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang S, Liu X, Ge LL, et al. Mesenchymal stromal cell-derived exosomes improve pulmonary hypertension through inhibition of pulmonary vascular remodeling. Respiratory Research. 2020;21(1):71. doi: 10.1186/s12931-020-1331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukumitsu M, Suzuki K. Mesenchymal stem/stromal cell therapy for pulmonary arterial hypertension: Comprehensive review of preclinical studies. Journal of Cardiology. 2019;74(4):304–312. doi: 10.1016/j.jjcc.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 43.Sánchez-Guijo F, García-Arranz M, López-Parra M, et al. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine. 2020;25:100454. doi: 10.1016/j.eclinm.2020.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moll G, Drzeniek N, Kamhieh-Milz J, et al. MSC therapies for COVID-19: importance of patient coagulopathy, thromboprophylaxis, cell product quality and mode of delivery for treatment safety and efficacy. Frontires in Immunology. 2020;11:1091. doi: 10.3389/fimmu.2020.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qu W, Wang Z, Hare JM, et al. Cell-based therapy to reduce mortality from COVID-19: Systematic review and meta-analysis of human studies on acute respiratory distress syndrome. Stem Cells Translational Medicine. 2020;9(9):1007–1022. doi: 10.1002/sctm.20-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yadav P, Vats R, Bano A, Bhardwaj R. Mesenchymal stem cell immunomodulation and regeneration therapeutics as an ameliorative approach for COVID-19 pandemics. Life Sciences. 2020;263:118588. doi: 10.1016/j.lfs.2020.118588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basiri A, Pazhouhnia Z, Beheshtizadeh N. Regenerative medicine in COVID-19 treatment: real opportunities and range of promises. Stem Cell Reviews and Reports. 2021;17(1):163–175. doi: 10.1007/s12015-020-09994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.