Abstract
Aim
- Patients with diabetes have increased morbidity and mortality from COVID-19. Case reports describe patients with simultaneous COVID-19 and diabetic acidosis (DKA), however there is limited data on the prevalence, predictors and outcomes of DKA in these patients.
Methods
- Patients with COVID-19 were identified from the electronic medical record. DKA was defined by standardized criteria. Proportional hazard regression models were used to determine risk factors for, and mortality from DKA in COVID-19.
Results
- Of 2366 patients admitted for COVID-19, 157 (6.6%) patients developed DKA, 94% of whom had antecedent type 2 diabetes, 0.6% had antecedent type 1 diabetes, and 5.7% patients had no prior diagnosis of diabetes. Patients with DKA had increased hospital length of stay and in-patient mortality. Higher HbA1c predicted increased risk of incident DKA (HR 1.47 per 1% increase, 95% CI 1.40–1.54). Risk factors for mortality included older age (HR 1.07 per 5 years, 95% CI 1.06–1.08) and need for pressors (HR 2.33, 95% CI 1.82–2.98). Glucocorticoid use was protective in patients with and without DKA.
Conclusion
- The combination of DKA and COVID-19 is associated with greater mortality, driven by older age and COVID-19 severity.
Keywords: Coronavirus; COVID-19; Diabetic ketoacidosis, DKA; SARS-CoV-2
Abbreviations
- T1D
type 1 diabetes
- T2D
type 2 diabetes
- DKA
diabetic ketoacidosis
- RRT
renal replacement therapy
Introduction
Type 2 diabetes (T2D) is a frequent comorbidity in patients with COVID-19, with a reported prevalence of 5.3–19.5% in China [1,2], 35.5% in Italy [3] and 10.9% in the United States [4]. T2D is associated with more severe COVID-19 disease [5,6], with twice the disease-related mortality as in patients without diabetes [7], [8], [9]. Mechanistic explanations for increased mortality in patients with T2D and how hyperglycemia contributes to this risk are unclear.
One possible mechanism for increased mortality due to COVID-19 in patients with T2D may be from the complication of diabetic ketoacidosis (DKA), a life-threatening hyperglycemic emergency. Although a more typical complication of type 1 diabetes (T1D), approximately 25% of DKA admissions were reported in patients with T2D in several cohort studies of patients without COVID-19 [10,11]. In patients with COVID-19, DKA prevalence and its risk factors and contribution to mortality are not known. A recent meta-analysis describing DKA in patients with COVID-19 included 110 cases from 19 studies [12]. These data were unable to describe DKA epidemiology or its clinical consequences in hospitalized patients with COVID-19. Furthermore, these data failed to address confounding that T2D may have on mortality in COVID-19.
In a large multi-ethnic cohort of patients with COVID-19 hospitalized at a single tertiary medical center, we report the prevalence of and risk factors for DKA. We also report mortality risk, both related to DKA and specific to patients with DKA. We hypothesized that increased severity of COVID-19 disease and a history of pre-existing diabetes would be associated with greater risk of DKA and that DKA would be a risk factor for mortality.
Methods
Cohort identification
We retrospectively identified all patients with a positive test result for SARS-CoV-2 from either a nasopharyngeal or oropharyngeal swab PCR who were admitted to Columbia University Irving Medical Center (New York Presbyterian-Columbia University Irving Medical Center and The Allen Pavilion Hospital) between March 1st and May 31st, 2020 who had bloodwork during their hospitalization. The study end date was June 10th, 2020, and any patient still alive and admitted without at least 30 days of follow-up by the end of the study end date was excluded (i.e. if a patient was admitted after May 10th and was still hospitalized). This study protocol was approved by the Institutional Review Board of Columbia University Irving Medical Center and the requirement for informed consent was waived.
Clinical characteristics and outcomes
Details of the patients’ hospital course, demographics, clinical data, laboratory data and clinical outcomes were obtained using a combination of manual chart review of the electronic medical record (Epic®) and laboratory data extraction from the clinical data warehouse (charts accessed up until June 10, 2020) (Table S1; see supplementary materials associated with this article on line). Diabetes mellitus was defined by having either: 1) pre-hospitalization or admission ICD-10 billing codes for DM (Table S2; see supplementary materials associated with this article on line), 2) pre-hospitalization outpatient medications that treat DM, 3) pre-hospitalization (within 365 days) or inpatient A1c > 6.5, or 4) DKA as their DM defining event. Patients were classified by whether they experienced a DKA event during the hospitalization based on the DKA diagnostic guidelines by the American Diabetes Association [13] and available clinical data, with DKA defined when the following 3 criteria were met:
-
1)
a low serum bicarbonate (<19 mmol/L) OR a high anion gap (>18, which is the upper limit of normal at our laboratory), AND:
-
2)
elevated beta-OH-butyrate (>0.54 mmol/L which is x2 the upper limit of normal at our laboratory) OR urinary ketones (>trace) within a 48h window (+/- 24h) of criteria #1, AND:
-
3)
at least one elevated serum or fingerstick glucose >250 mg/dL within a 48 h window (+/- 24h) of criteria #1.
Time to DKA was defined as the duration in hours between admission and the first DKA event (i.e. earliest time when all 3 above criteria for DKA were met). Patients were then classified by vital status as dead or alive for the primary mortality outcome. For mortality outcome assessments, patients were censored on hospital discharge.
Statistical approach
Data from the electronic medical record and curated COVID-Care sources were merged, coded, cleaned and summarized for all patients. Collection date and time stamps of key laboratory tests were used to identify DKA-defining results occurring within a 48-hour window. Chi-square or Fisher's Exact test were used to compare DKA status groups on categorical variables and T-tests or Wilcoxon Sign Rank test for continuous variables. Cox proportional hazards models were used to estimate the associations of the following risks for DKA or mortality: age, sex, BMI, self-identified race (Black, Non-Black) and ethnicity (Hispanic, Non-Hispanic), hypertension (HTN), chronic kidney disease (CKD), pulmonary disease, liver disease, DM, inpatient glucocorticoid exposure, pressor or inotrope requirement, renal replacement therapy (RRT), acute kidney injury (AKI), hemoglobin A1c (HbA1c) and lactic acid (maximum value if multiple were checked). We first ran univariate models and all statistically significant risks (P < 0.05) and their first-order interactions were entered into a stepwise selection model requiring a P-value < 0.20 to enter and < 0.05 to stay in the model. The univariate and adjusted models for time to DKA diagnosis were repeated with death as a competing risk using the Fine-Gray method. The adjusted model for mortality was repeated forcing DKA status into the model to assess the influence of DKA on risk estimates.
Results are presented as percent of the cohort, means and standard deviations or median and interquartile ranges, and hazards ratios with 95% confidence limits for proportional hazards model estimates. Kaplan-Meier survival analysis was used to estimate and display survival stratified by DKA status. Cumulative incidence of time from admission to DKA diagnosis was graphed. BMI, maximum HbA1c and maximum lactate levels were not included in adjusted models of the full cohort due to missing data (Table S3; see supplementary materials associated with this article on line). No missing data were imputed in this study. All data management and statistical analyses used SAS (SAS Institute, Cary, NC).
Results
Baseline characteristics
During the study period, a total of 2366 patients were admitted who had bloodwork results available for analysis. One-hundred fifty-seven (6.6%) of the cohort met all 3 pre-defined criteria for DKA, with the majority of the cohort not experiencing DKA during their hospitalizations (2209 patients, 93.4%; Table 1). The majority of patients met both low bicarbonate and high AG criteria for DKA, with positive serum ketones. Full details of the frequency of combinations of diagnostic criteria that were met are presented in Table S4 (see supplementary materials associated with this article on line). The majority of patients were male (57%) with a mean age of 64.2 +/- 17.7 years. Forty percent of patients had known DM and 16% of patients with DM developed DKA. Only 1.1% and 0.6% of patients with DM and DM with DKA, respectively, were on an SGLT-2 inhibitor as an outpatient. Patients with compared to without DKA were in general male, younger and had lower prevalence of hypertension or pulmonary disease (Table 1). There were no differences in other baseline demographic or outpatient characteristics based on DKA status (Table 1).
Table 1.
Baseline outpatient characteristics, DKA diagnosis, and clinical outcomes.
| Full Cohort | No DKA Full Cohort | No DKA Only DM Cohort | DKA | |
|---|---|---|---|---|
| n = 2366 | n = 2209 | n = 798 | n = 157 | |
| Demographics | ||||
| Age (years) | 64.2 ± 17.7 | 64.3 ± 17.9 | 69.3 + 13.8* | 63.6 ± 14.2 |
| Sex (female) | 43.2% | 44.1%* | 44.5%* | 31.2% |
| Race (Black) | 20.10% | 20.40% | 22.6% | 22.30% |
| Ethnicity (Hispanic/Latino) | 52.5% | 52.5% | 54.6% | 53.5% |
| Past Medical History | ||||
| Body mass index (kg/m2) | ||||
| <18.5 | 3.0% | 3.2% | 3.4% | 4.5% |
| 18.5 < 25.0 | 23.1% | 22.7% | 21.7% | 29.3% |
| 25.0 < 30.0 | 29.6% | 29.6% | 30.5% | 30.6% |
| >30.0 | 33.0% | 33.3% | 37.3% | 29.3% |
| missing | 11.0% | 11.3% | 7.1% | 6.4% |
| Diabetes mellitus† | 40.4% | 36.2%* | 100% | 100% |
| Chronic kidney disease | 13.2% | 13.2% | 22.8%* | 14.0% |
| Hypertension | 60.6% | 60.4% | 84.0%* | 63.7% |
| Pulmonary disease | 17.8% | 18.2%* | 19.8%* | 10.8% |
| Liver disease | 4.9% | 4.9% | 5.3% | 4.5% |
| COVID Treatment Medications | ||||
| Steroids‡ | 26.0% | 25.1%* | 26.6%* | 37.6% |
| Tocilizumab | 5.4% | 5.3% | 4.4% | 7.0% |
| Remdesivir | 2.2% | 2.2% | 1.6% | 1.9% |
| None | 72.4% | 73.3%* | 72.4%* | 59.9% |
| Illness Severity | ||||
| Vasopressors and/or inotropes§ | 17.2% | 16.3%* | 17.0%* | 28.7% |
| Invasive mechanical ventilation | 17.3% | 16.3%* | 17.4%* | 31.2% |
| Kidney replacement therapy|| | 5.2% | 4.7%* | 5.3%* | 12.1% |
| maximum Lactate (n=1981) | 2.8 ± 2.5 | 2.7 + 2.4* | 2.8 + 2.3* | 4.0 ± 3.0 |
| DKA Details | ||||
| Prior diabetes mellitus diagnosis | ||||
| Type 1 diabetes mellitus | 0.2% | 0.2% | 0.5% | 0.6% |
| Type 2 diabetes mellitus | 40.2% | 36.0%* | 99.5% | 99.4% |
| No prior diabetes mellitus | 59.6% | 63.8% | 0% | 0% |
| Initial glucose >250mg/dL | 15.80% | 11.3%* | 25.2%* | 79.0% |
| Inpatient HbA1c, % (n=780) | 8.0 ± 2.4 | 7.4 + 2.0* | 8.2 + 2.0* | 10.7 ± 2.8 |
| Time to DKA diagnosis (hours) | – | – | – | 6.0 ± 9.7 |
| Lactate at DKA diagnosis (n = 140) | – | – | – | 3.4 ± 2.4 |
| Outcomes | ||||
| Median hospital LOS (days) | 6.0 (8.0) | 6.0 (8.0)* | 6.0 (8.0)* | 7.0 (10.0) |
| Mortality | 26.3% | 25.6%* | 28.8%* | 36.9% |
represents P < 0.05 compared to the DKA group.
prevalance of DM was 100% in the DKA group as DKA was a DM defining event.
including prednisone, hydrocortisone, methylprednisone, and dexamethasone
including vasopressin, neosynephrine, norepinephrine, epinephrine, angiotensin II, dopamine, dobutamine, and milrinone
patients on kidney replacement therapy for ESRD excluded (i.e. only AKI indication)
DKA, diabetic ketoacidosis; SGLT-2, sodium glucose transporter-2; HbA1c, hemoglobin A1c; LOS, length of stay.
COVID-19 illness severity and clinical outcomes
The majority of patients in our cohort did not receive specific COVID-19 directed therapies (72%; Table 1) other than supportive care, consistent with recommendations during the early Spring 2020 pandemic surge. Use of glucocorticoids, however, was more common in patients who developed DKA (38% vs. 25%). Nearly 17% of patients required vasopressors or inotropes, 17% required invasive mechanical ventilation, and 5% developed acute kidney injury requiring renal replacement therapy (Table 1). Patients with DKA had higher rates of vasopressor or inotrope use, invasive mechanical ventilation, and renal replacement therapy (RRT): 29 vs. 16%; 31 vs. 16%; and 12 vs. 5%, respectively (Table 1).
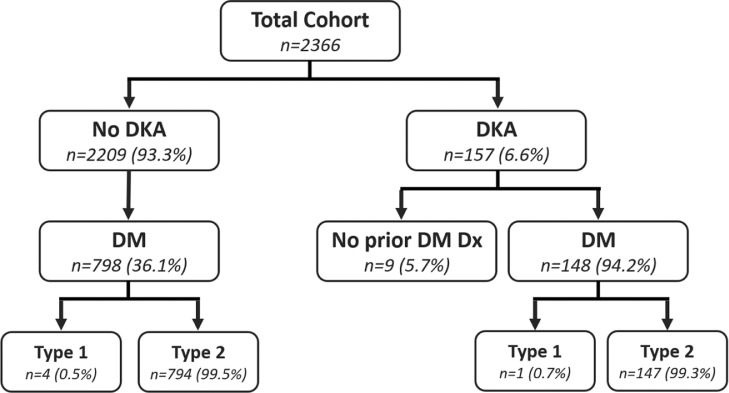
More patients who developed DKA (n = 157) had a prior diagnosis of DM (n = 148; 94.2%) and only 9 patients (5.7%) had no prior diagnosis of DM in their electronic medical records (Fig. 1 , Table 1). Levels of HbA1c were higher in patients with compared to without DKA (n = 718; 10.7% vs. 7.4%, respectively). DKA occurred early in the hospitalization, with a mean time from admission to DKA diagnosis of 6 h +/- 10 h (Table 1 and Fig. S1; see supplementary materials associated with this article on line). Seventy-nine percent of patients who experienced DKA had a glucose level > 250 mg/dL at the time of admission. The median hospital length of stay was longer in patients with DKA (7 vs. 6 days). Additionally, patients with DKA experienced an overall mortality rate of 37%, which was higher than the 25.6% and 28.8% mortality rates observed for both all patients without DKA and diabetes patients without DKA respectively (Table 1).
Fig. 1.
. Prevalence of diabetes among study patients who did and did not develop DKA. Of the 6.6% of patients in our study who developed DKA, 94% had a known prior diagnosis of DM (overwhelmingly T2D 99.3%). The overall majority of patients did not develop DKA (93.3%) and of these, only 36% had a prior known history of T2D (overwhelmingly T2D 99.5%). DM, diabetes mellitus; T2D, Type 2 diabetes mellitus; DKA, diabetic ketoacidosis.
DKA
We used proportional hazard regression to determine risk factors for DKA. Univariate models indicated that male sex, lower BMI, no history of pulmonary disease, and higher levels of HbA1c and lactate predicted greater risk of incident DKA. Adjusted models included all significant univariate predictors described previously and their first-order interactions except BMI and lactate due to missing values (Table S3; see supplementary materials associated with this article on line). Each unit increase in HbA1c predicted a 47% increased risk of DKA (HR 1.47, 95% CI 1.40–1.54; Table 2). When we restricted the cohort to the subset of patients with diabetes (n = 955), greater HbA1c remained a significant predictor of DKA (HR 1.39; 95% CI 1.31–1.47).
Table 2.
Multivariate cox proportional hazards models for outcomes of DKA and all-cause mortality.
| Adjusted Hazard Ratios |
||
|---|---|---|
| Developing DKA* | All-cause mortality* | |
| n = 2366 | n = 2366 | |
| DKA | 1.17 (0.89–1.53) | |
| Age (5 years) | 1.07 (1.06–1.08) | |
| Steroids | 1.20 (0.91–1.60) | |
| Pressors or Inotropes | 2.33 (1.82–2.98) | |
| Pressors or Inotropes x Steroids | ||
| Steroids = Yes | 1.53 (1.18–1.96) | |
| Steroids = No | 2.33 (1.82–2.98) | |
| Age (5 years) x Steroids | ||
| Steroids = Yes | 1.27 (1.21–1.34) | |
| Steroids = No | 1.42 (1.36–1.49) | |
| Max HbA1c (per unit) | 1.47 (1.40 - 1.54) | |
with DKA as a forced variable
Variables included in univariate analysis: DKA, Age, Sex, BMI, Race/Ethnicity, HTN, CKD, Pulmonary disease, Liver disease, DM, SGLT-2i, max HbA1c, Steroid exposure, Pressor or Inotrope requirement, RRT, AKI, Mechanical ventilation, and max Lactic acid.
Mortality
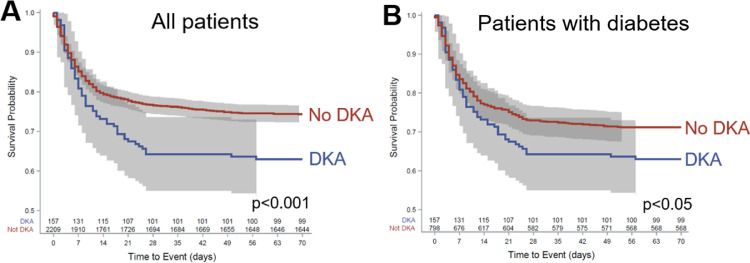
Death was more likely among patients who developed DKA (36.9% vs. 25.6%, P < 0.05) with a mean survival of 40.0 ± 1.9 days compared to patients who did not develop DKA (54.0 ± 1.9 days, P = 0.0008; Fig. 2 A). Among patients with diabetes (n = 955), death was also more common in those who developed DKA (36.9% vs. 28.8%, P < 0.05, OR = 1.45 (1.01–2.07); Table 1), with one-quarter of deaths occurring within 12 days (95% CI: 7–20) in comparison to 22 days (95% CI: 13–42, P = 0.03) for patients without DKA (Fig. 2B).
Fig. 2.
. Kaplan-Meier survival analysis of survival stratified by DKA and diabetes status. A) Death was more likely among patients who developed DKA (36.9% vs. 25.6%, P < 0.05) with a mean survival of 40.0 ± 1.9 days compared to patients who did not develop DKA (54.0 ± 1.9 days, P = 0.0008). B) Among patients with diabetes (n = 955), mean survival in patients with DKA was 40.0 ± 1.9 days compared to 40.6 ± 0.7 days in patients without DKA, with one-quarter of deaths occurring within 12 days (95% CI: 7–20) of admission in patients with DKA compared to 22 days (95% CI: 13–42) for patients without DKA (P = 0.03). DKA, diabetic ketoacidosis.
In a univariate regression analysis of survival, admission glucose predicted the likelihood of death (HR 1.066 (1.041–1.091) for every 50 mg/dL increase in glucose), but in a subgroup analysis of DKA patients, admission glucose was no longer predictive of inpatient mortality (HR 0.98 (0.92–1.04)). In light of these results and given that some admission glucoses were used in the scoring and determination of DKA status, admission glucose as a stand-alone variable was excluded from further multivariable models. Using proportional hazard regression, we determined predictors of mortality. Greater mortality risk included older age, higher BMI, non-Black race, a history of HTN and CKD, exposure to pressors/inotropes and higher levels of lactate. Exposure to glucocorticoids protected against mortality. Multivariate models included all univariate predictors, DKA forced into the model and all first-order interactions (Table 2). Risk factors for mortality included older age and pressor use. The interaction between glucocorticoid use and age and pressor use demonstrated that glucocorticoids conferred protection (Table 2). DKA did not predict mortality in either the univariate or multivariable models.
Discussion
We report for the first-time critical findings regarding the epidemiology of DKA in patients with COVID-19. We found that DKA prevalence was exceedingly high in patients with pre-existing diabetes, that poor diabetes control based on higher admission HbA1c predicted incident DKA and that DKA developed early after presentation to the hospital (i.e. within 6 hours). Although only 6.6% of the 2366 patients who had a positive test for SARS CoV2 developed DKA, 16% of the 955 patients with a history of diabetes developed DKA. Patients with COVID-19 and DKA had slightly longer lengths of stay, notable as hospital (and particularly ICU) bed availability was scarce. Among patients with diabetes, the DKA was associated with more rapid onset of death compared to those without DKA. Although mortality was higher in patients with compared to without DKA, it was driven by older age and severity of COVID-19 disease and glucocorticoid administration protected against death.
The prevalence of DKA in patients with diabetes in our cohort was substantially higher than the 0.9% prevalence of hyperglycemic emergency (encompassing DKA, and the related hyperosmolar hyperglycemic state) in patients hospitalized for all causes in National Inpatient Sample (NIS) datasets [14]. Patients with diabetes who developed DKA were younger (64 vs. 69 years old), had higher levels of HbA1c, and had fewer chronic medical conditions (14% with CKD, 63.7% with HTN and 10.8% with pulmonary disease, as compared to 22.8%, 84% and 19.8% respectively) as compared to patients with diabetes and without DKA. Despite being younger with fewer comorbidities, patients with DKA had higher rates of mechanical ventilation (31.2% vs. 17.4%), need for RRT (12.1% vs. 5.3%), treatment with glucocorticoids (37.6% vs. 26.6%) and vasopressors and/or inotropes (28.7% vs. 17.0%) and mortality (36.9% vs, 28.8%) than patients with diabetes and without DKA. In multivariable analysis, higher HbA1c during the hospitalization was associated with greater risk of DKA. These data suggest that diabetes control as assessed by HbA1c may be causally related to DKA development, and that DKA was associated with greater severity of COVID-19 disease, but further work to elucidate the underlying mechanisms responsible for this relationship is needed.
We found that DKA was diagnosed within 6 ± 10 h of presentation to the hospital with COVID-19. This is notable as similar to the CORONADO study [15,16], admission glucose predicted inpatient death in a univariate regression analysis. However, because it was not significant in a subgroup analysis of only patients with DKA and because the operational definition of DKA may include admission glucose, this value was not included in subsequent multivariable regression analyses. Patients with DKA had higher rates of glucocorticoid and vasopressor use, both of which can cause hyperglycemia and precipitate DKA. However, since DKA was diagnosed within the first few hours of admission, it is likely that DKA developed prior to potential glucotoxic effects from exposure to these medications. It is noteworthy that besides vasopressors, we found that male sex was associated with DKA; multiple studies have reported higher mortality risk in men with COVID-19 [17], [18], [19], [20], but with unclear mechanism. Multivariate models found that increased HbA1c predicted increased risk of DKA in both the whole cohort and the subset of patients with diabetes, consistent with other studies that show higher outpatient HbA1c is associated with DKA [21], [22], [23].
We observed increased mortality in patients with concomitant DKA and COVID-19 despite comparable use of common COVID-19 treatments (i.e. remdesivir, toclizumab) and even higher use of therapies proven to affect mortality (glucocorticoids) [24]. However, in multivariate modeling, morality risk was driven by older age and severity of disease as defined by exposure to pressors/inotropes. Our findings also confirm that use of glucocorticoids protects against death [24].
In our cohort, 5.7% of patients who presented with DKA did not have a history of diabetes. These data are consistent with prior reports that describe new-onset diabetes with patients with COVID-19 [25], [26], [27]. However, in these studies a diagnosis of new-onset diabetes was assigned even in patients with an elevated HbA1c at the time of admission, which may be suggestive of pre-existing diabetes. In contrast, we considered patients who had an HbA1c in the diabetic range (> 6.4%) as having pre-existing diabetes and considered “new-onset” diabetes only in those patients who presented with DKA despite normal HbA1c and without any antecedent history of a diabetes diagnosis or use of anti-hyperglycemic medications. Despite application of stringent diagnostic criteria for new-onset diabetes, our reported rates of 5.7% are considered high. Moreover, it is likely that we are underestimating the incidence of new-onset diabetes in patients with COVID-19 since our analysis was restricted to patients with DKA and did not include patients with uncomplicated hyperglycemia. This is an area for further study, as new hyperglycemia in patients with COVID-19 may be associated with even greater mortality than in patients with a prior history of diabetes [28].
Our study has several limitations. This is a retrospective observational study and cannot assign causality to the underlying physiology. Further work is needed to clarify mechanisms of new-onset diabetes, and our data cannot contribute to the current debate [29], [30], [31] as to whether pancreatic beta cells show sufficient expression of the ACE2 receptor to facilitate direct SARS-CoV2 infection. We utilized the most common definition of DKA – glucose >250 mg/dl, metabolic acidosis and presence of ketones – [13] which may under-report euglycemic DKA [32]. We did not think euglycemic DKA was prevalent in our cohort, especially as the number of patients treated with SGLT-2 inhibitors, a known causal factor for euglycemic DKA [33,34] was trivial. Additionally, because patients were diagnosed with DKA based on multiple criteria (Table S4; see supplementary materials associated with this article on line), we could not determine if DKA severity variably affected outcomes. Further, we were not able to identify race or ethnicity in the majority of our patients; due to the emergent situations occurring during the Spring 2020 pandemic surge, race and ethnicity was not completely recorded for many admitted patients. This impaired our ability to discern whether specific races or ethnicities are more prone to COVID-19-related DKA, akin to the known predisposition for “Flatbush” or ketosis-prone diabetes in African-Americans [35]. Finally, due to the retrospective design and limitations imposed by the COVID pandemic, granular information of the timing of most recent diabetes medications pre-hospitalization was not available.
Conclusion
In summary, we report that COVID-19 is frequently associated with DKA, mostly in patients with uncontrolled T2D, but may also present as new-onset diabetes. Patients with COVID-19 and DKA experience slightly longer hospitalizations and a higher risk of mortality, likely driven by increased COVID-19 illness severity. These data highlight the importance of outpatient glucose control to prevent DKA in these at-risk patients. If hospitalized, hyperglycemic patients who have COVID-19 and unexplained metabolic acidosis should be evaluated for DKA. A diagnosis of DKA should prompt aggressive therapy to manage the metabolic acidosis but should not preclude use of glucocorticoids to reduce risk of morbidity and mortality in patients with COVID-19 and DKA.
Disclosures
JSS, MBB, DJM, JZ, PK, SM, MTY and UBP have no disclosures
TLN: Amgen
Funding
None.
Data availability statement
The full dataset cannot be shared publicly because of the risk of identification of patients and their protected health information (PHI). Given the relatively specific patient sample, geographic constraints (single-site) with granular data on dates of death, the full dataset will be provided upon reasonable request following approval from The Columbia University IRB Human Research Protections Office (phone:212.305.5883, email: irboffice@cumc.columbia.edu).
Author contribution
JSS: study conceptualization and design; data acquisition, analysis and interpretation; writing and editing
MMB: study conceptualization and design; data acquisition, analysis and interpretation; writing and editing
DJM: data management, analysis and interpretation; writing and editing
JZ: data acquisition
PK: conceptualization, methodology, writing and editing
SM: data acquisition, writing and editing
MTY: study conceptualization and design; data analysis and interpretation; writing and editing
TLN: study conceptualization and design; data analysis and interpretation; writing and editing
UP: study conceptualization and design; data analysis and interpretation; writing and editing.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.diabet.2021.101267.
Appendix. Supplementary materials
References
- 1.Novel CPERE The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua liu xing bing xue za zhi= Zhonghua liuxingbingxue zazhi. 2020;41:145. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 4.Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 – United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh AK, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr. 2020;14:303–310. doi: 10.1016/j.dsx.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi Q, Zhang X, Jiang F, Zhang X, Hu N, Bimu C, et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care. 2020;43:1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020;14:535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bode B, Garrett V, Messler J, McFarland R, Crowe J, Booth R, et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14:813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan Y, Yang Y, Wang F, Ren H, Zhang S, Shi X, et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8 doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang ZH, Kihl-Selstam E, Eriksson JW. Ketoacidosis occurs in both Type 1 and Type 2 diabetes–a population-based study from Northern Sweden. Diabet Med. 2008;25:867–870. doi: 10.1111/j.1464-5491.2008.02461.x. [DOI] [PubMed] [Google Scholar]
- 11.Westphal SA. The occurrence of diabetic ketoacidosis in non-insulin-dependent diabetes and newly diagnosed diabetic adults. Am J Med. 1996;101:19–24. doi: 10.1016/s0002-9343(96)00076-9. [DOI] [PubMed] [Google Scholar]
- 12.Pal R, Banerjee M, Yadav U, Bhattacharjee S. Clinical profile and outcomes in COVID-19 patients with diabetic ketoacidosis: a systematic review of literature. Diabetes Metab Syndr. 2020;14:1563–1569. doi: 10.1016/j.dsx.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32:1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai R, Singh S, Syed MH, Dave H, Hasnain M, Zahid D, et al. Temporal trends in the prevalence of diabetes decompensation (diabetic ketoacidosis and hyperosmolar hyperglycemic state) among adult patients hospitalized with diabetes mellitus: a nationwide analysis stratified by age, gender, and race. Cureus. 2019;11:e4353. doi: 10.7759/cureus.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheen AJ, Marre M, Thivolet C. Prognostic factors in patients with diabetes hospitalized for COVID-19: findings from the CORONADO study and other recent reports. Diabetes Metab. 2020;46:265–271. doi: 10.1016/j.diabet.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cariou B, Hadjadj S, Wargny M, Pichelin M, Al-Salameh A, Allix I, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahrenfeldt LJ, Otavova M, Christensen K, Lindahl-Jacobsen R. Sex and age differences in COVID-19 mortality in Europe. Wien Klin Wochenschr. 2020:1–6. doi: 10.1007/s00508-020-01793-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108 doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bienvenu LA, Noonan J, Wang X, Peter K. Higher mortality of COVID-19 in males: sex differences in immune response and cardiovascular comorbidities. Cardiovasc Res. 2020;116:2197–2206. doi: 10.1093/cvr/cvaa284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, et al. Gender differences in patients with COVID-19: focus on severity and Mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Giovanni P, Meo F, Cedrone F, D'Addezio M, Di Martino G, Scampoli P, et al. Predictors and trend of ketoacidosis hospitalization rate in type 2 diabetes mellitus patients from 2006 to 2015 in abruzzo region, Italy. Clin Ter. 2020;171:e53–e58. doi: 10.7417/CT.2020.2189. [DOI] [PubMed] [Google Scholar]
- 22.Semenkovich K, Berlin KS, Ankney RL, Klages KL, Keenan ME, Rybak TM, et al. Predictors of diabetic ketoacidosis hospitalizations and hemoglobin A1c among youth with Type 1 diabetes. Health Psychol. 2019;38:577–585. doi: 10.1037/hea0000719. [DOI] [PubMed] [Google Scholar]
- 23.Ehrmann D, Kulzer B, Roos T, Haak T, Al-Khatib M, Hermanns N. Risk factors and prevention strategies for diabetic ketoacidosis in people with established type 1 diabetes. Lancet Diabetes Endocrinol. 2020;8:436–446. doi: 10.1016/S2213-8587(20)30042-5. [DOI] [PubMed] [Google Scholar]
- 24.Sterne J, Murthy S, Diaz J, Slutsky A, Villar J, Angus D, et al. WHO rapid evidence appraisal for COVID-19 therapies (REACT) working group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubino F, Amiel SA, Zimmet P, Alberti G, Bornstein S, Eckel RH, et al. New-onset diabetes in Covid-19. N Engl J Med. 2020;383:789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suwanwongse K, Shabarek N. Newly diagnosed diabetes mellitus, DKA, and COVID-19: causality or coincidence? A report of three cases. J Med Virol. 2021;93:1150–1153. doi: 10.1002/jmv.26339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sathish T, Tapp RJ, Cooper ME, Zimmet P. Potential metabolic and inflammatory pathways between COVID-19 and new-onset diabetes. Diabetes Metab. 2021;47 doi: 10.1016/j.diabet.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh AK, Singh R. Hyperglycemia without diabetes and new-onset diabetes are both associated with poorer outcomes in COVID-19. Diabetes Res Clin Pract. 2020;167 doi: 10.1016/j.diabres.2020.108382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fignani D, Licata G, Brusco N, Nigi L, Grieco GE, Marselli L, et al. SARS-CoV-2 receptor angiotensin i-converting enzyme type 2 (ACE2) is expressed in human pancreatic β-cells and in the human pancreas microvasculature. Front Endocrinol. 2020;11 doi: 10.3389/fendo.2020.596898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coate KC, Cha J, Shrestha S, Wang W, Gonçalves LM, Almaça J, et al. SARS-CoV-2 cell entry factors ACE2 and TMPRSS2 are expressed in the microvasculature and ducts of human pancreas but are not enriched in β cells. Cell Metab. 2020;32 doi: 10.1016/j.cmet.2020.11.006. 1028-40.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Modi A, Agrawal A, Morgan F. Euglycemic diabetic ketoacidosis: a review. Curr Diabetes Rev. 2017;13:315–321. doi: 10.2174/1573399812666160421121307. [DOI] [PubMed] [Google Scholar]
- 33.Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic Diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1687–1693. doi: 10.2337/dc15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma PV, Jobanputra YB, Lewin K, Bagatell S, Lichtstein DM. Diabetic ketoacidosis in patients with type 2 diabetes on sodium-glucose cotransporter-2 inhibitors - a case series. Rev Recent Clin Trials. 2018;13:156–160. doi: 10.2174/1574887113666180314101436. [DOI] [PubMed] [Google Scholar]
- 35.Lebovitz HE, Banerji MA. Ketosis-Prone diabetes (Flatbush Diabetes): an emerging worldwide clinically important entity. Curr Diab Rep. 2018;18:120. doi: 10.1007/s11892-018-1075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full dataset cannot be shared publicly because of the risk of identification of patients and their protected health information (PHI). Given the relatively specific patient sample, geographic constraints (single-site) with granular data on dates of death, the full dataset will be provided upon reasonable request following approval from The Columbia University IRB Human Research Protections Office (phone:212.305.5883, email: irboffice@cumc.columbia.edu).