Abstract
Cancer associated fibroblasts (CAFs) and tumor associated macrophages (TAMs) are among the most important and abundant players of the tumor microenvironment. CAFs as well as TAMs are known to play pivotal supportive roles in tumor growth and progression. The number of CAF or TAM cells is mostly correlated with poor prognosis. Both CAFs and TAMs are in a reciprocal communication with the tumor cells in the tumor milieu. In addition to such interactions, CAFs and TAMs are also involved in a dynamic and reciprocal interrelationship with each other. Both CAFs and TAMs are capable of altering each other’s functions. Here, the current understanding of the distinct mechanisms about the complex interplay between CAFs and TAMs are summarized. In addition, the consequences of such a mutual relationship especially for tumor progression and tumor immune evasion are highlighted, focusing on the synergistic pleiotropic effects. CAFs and TAMs are crucial components of the tumor microenvironment; thus, they may prove to be potential therapeutic targets. A better understanding of the tri-directional interactions of CAFs, TAMs and cancer cells in terms of tumor progression will pave the way for the identification of novel theranostic cues in order to better target the crucial mechanisms of carcinogenesis.
Keywords: cancer associated fibroblasts, monocytes, tumor associated macrophages, tumor biology, tumor immunology, tumor microenvironment, macrophage polarization, M1/M2 cells
Introduction
It is known that the tumor microenvironment consists of several different types of cells in addition to cancer cells such as immune cells, fibroblasts as well as capillaries, basement membrane and extracellular matrix (ECM) (1–4). The dynamic and complex stroma interactions provide the conditions for tumor cell survival, growth and invasiveness. In addition to inflammatory cells, pro-inflammatory cytokines secreted from those cells are among the basic components of the tumor microenvironment (5). It is widely accepted that most neoplastic cells can only proliferate in a suitable microenvironment. Cancer cells recruit numerous cells to the tumor microenvironment and most of those cells become the cat’s-paw for the cancer cells; culminating in tumor survival, growth, invasion and metastasis. Cancer associated fibroblasts (CAFs) are one of the most crucial cells in the tumor milieu. CAFs in fact represent a heterogeneous population. The heterogeneity of CAFs might stem from their multiple origins. Similarly, tumor associated macrophages (TAMs) can also support tumor progression and increased number of TAMs is usually associated with poor outcome.
In this review; the origins, heterogeneity, activation and tumor-promoting effects of CAFs as well as tumor-supporting effects of TAMs are first outlined. Then, the interplay between CAFs and TAMs as well as their reciprocal interactions are discussed with accentuating their concerted effects on tumor progression and immune escape.
Cancer Associated Fibroblasts
Fibroblast cells, which are one the most common cells found in connective tissues, display a branched cytoplasm that surrounds an elliptical nucleus and they express vimentin (an intermediate filament protein). They are able to produce various constituents of the ECM. Fibroblasts become activated in the tumor milieu and activated fibroblasts found specifically in the tumor microenvironment are defined as CAFs (6). It can be proposed that fibroblasts are among the most abundant cell types found in tumor stroma (7, 8). In fact, desmoplasia (growth of rich stroma) has long been known to be associated with tumors (9–11). Since tumors were previously depicted as “wounds that never heal” (12), CAFs resemble myofibroblasts, which are spindle shaped activated fibroblasts (13). Although CAFs can be defined as the “cells that surround cancer epithelia”, they can also be regarded as those fibroblasts which are capable of promoting tumorigenesis (8). In line with this perspective, CAF derived factors can induce a tumor supportive microenvironment as well as facilitating cancer cell metastasis (14). CAFs also play key roles in sculpturing the tumor microenvironment (15, 16). Indeed, such a role of CAFs in promoting tumor progression might also be considered to be in agreement with the original “seed and soil” hypothesis that was proposed in 1889 by Stephen Paget, who suggested that the interactions between tumor cells (seed) and their microenvironment (soil) are crucial (17, 18).
Origin of CAFs
Fibroblasts were initially described in the mid-1800s by Virchow and Duval as the most common cell type embedded in connective tissue in animals, demonstrating a fusiform shape (19–22). In adult tissues, Virchow described cells that produced collagen and were resistant to apoptosis (19, 23). Fibroblasts have been identified in various tissue types; however, quiescent fibroblasts do not exist in embryonic tissue (24). Thus, it remains uncertain whether the activated fibroblasts originate from mesenchymal stem cells (MSCs) or fibrocytes in adults (24). In spite of the vast literature about the features and functions of CAFs, the debate about the multiple origins for CAFs is still ongoing ( Figure 1 ). The origin of CAFs can even vary depending on their location even in a single tumor and it is likely mixed (25). Most of the CAFs probably originate from mesoderm-derived precursor cells. During the tumorigenesis process, residing fibroblasts in the tissue also expand in response to injury caused by the tumor (24). In addition, CAFs can also be recruited from the bone marrow (24). Bone marrow derived MSCs can differentiate into CAFs and express α-smooth muscle actin (α-SMA) as well as fibroblast activation protein (FAP) (26). Furthermore, trans-differentiation of other cells of the tumor microenvironment may also give rise to CAF-like cells (25, 27–29). Conversion of adipocytes into CAFs has been reported in several studies (30–34). In addition, endothelial cells can also give rise to CAFs through endothelial to mesenchymal transition (EndMT) (27, 35–37). Zeisberg et al. demonstrated that transforming growth factor-β (TGF-β) 1 might promote proliferating endothelial cells to undergo phenotypic conversion into fibroblast-like cells (35). TGF-β is able to induce expressions of mesenchymal markers such as fibroblast-specific protein 1 (FSP1) and SMA; cause loss of endothelial markers such as CD31; resulting in EndMT (35). Another possible source of CAFs is pericytes (38–40). Furthermore, Wikström et al. suggested that smooth muscle cells could also give rise to CAFs (41). An interesting study by Kurashige et al. investigated the origin of CAFs in humans after sex-mismatched bone marrow transplantation (42). They reported that majority of α-SMA+ CAFs in liver, mammary gland and oral mucosa obtained 3–19 years after bone marrow transplantation were recipient derived cells; whereas, the peritumoral α-SMA- fibroblast-like cells were mostly bone marrow derived human leukocyte antigen (HLA)-DR+ myeloid cells (42).
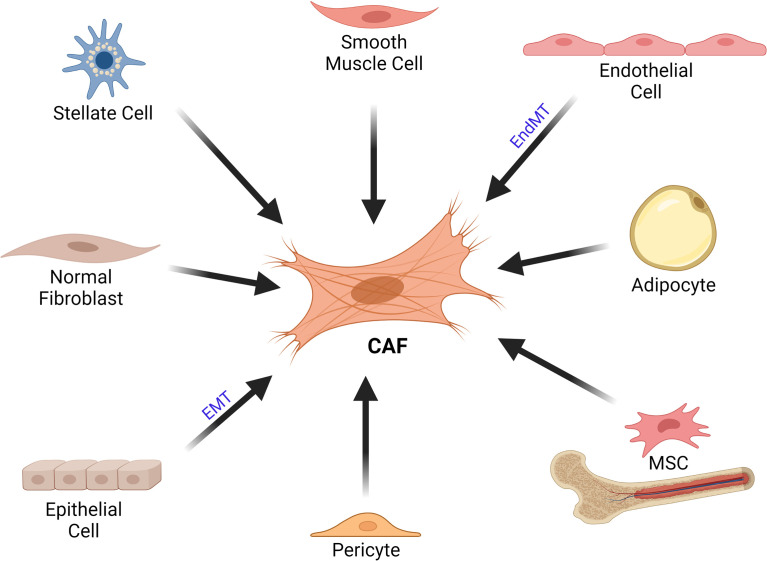
Figure 1.
Cellular Origins of CAFs. CAFs can originate from various cell types such as stellate cells, smooth muscle cells, endothelial cells, adipocytes, MSCs, pericytes, epithelial cells as well as normal fibroblasts. CAF, cancer associated fibroblast; EMT, epithelial-mesenchymal transition; EndMT, endothelial to mesenchymal transition; MSC, mesenchymal stem cell.
CAFs can originate via induction of tissue fibroblasts by tumor derived factors; in a way similar to the mechanism seen in myofibroblasts at wound healing (29). Tumor cell derived TGF-β, platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) can activate tissue fibroblasts. The mesenchymal–mesenchymal transition (MMT) results in the expression of CAF specific genes in fibroblasts such as α-SMA (43–46). Toullec et al. reported that stromal derived factor 1 (SDF-1) is an effective factor for the activation of resident fibroblasts in tumors (47). In a very recent study, Helms et al. investigated the differentiation of pancreatic stellate cells during tumor progression in vivo and reported that pancreatic stellate cells could give rise to a minor subset of pancreatic ductal adenocarcinoma CAFs (48). It was also suggested that miRNAs have regulatory roles in the transformation of normal fibroblasts to CAFs (49). Moreover, cells of epithelial origin are among the possible sources of CAFs and carcinomas may also contribute to the CAF population. Epithelial cells can obtain mesenchymal features and give rise to fibroblasts via epithelial-mesenchymal transition (EMT) (8, 50, 51), which can be induced by various factors such as PDGF, TGF-β, epidermal growth factor (EGF); resulting in the loss of E-cadherin expression (28, 52).
Finally, studies that utilize lineage tracing have also shed some light on the origin of CAFs (53, 54). Raz et al. investigated the origin of stromal subtypes. They reported that bone marrow derived mesenchymal stromal cells were recruited to primary breast tumors and lung metastases, and differentiated into CAF-like cells (55). Moreover, the expression profile of such bone marrow derived CAF-like cells was different from that of resident fibroblasts (55). In another study, Null et al. utilized in vivo genetic labelling of periostin+ subpopulations and their progeny in order to investigate their expansion/function during mammary tumor growth and metastasis (56). They proposed that highly metastatic cancer cells mobilized periostin expressing CAFs in the tumor microenvironment. Eventually, the dearth of specific markers for fibroblasts limits to some extent the broader application of the lineage tracing technique. Although we currently have a substantial understanding about the origins of CAF, the controversy concerning the importance of these mechanisms in terms of constituting the CAF population in a given tumor has not been resolved yet.
Heterogeneity of CAFs
CAFs as well as activated fibroblasts are known to be very heterogeneous, displaying different expression patterns ( Figure 2 ) (24, 57). Kalluri suggested that fibroblasts can be regarded as resting mesenchymal cells with the potential to be activated to give rise to MSCs (24). In line with this notion, it should be kept in mind that fibroblasts represent the most frequently used cell type as a source of induced pluripotent stem cells (iPSCs). In addition, fibroblast cells demonstrate a high level of plasticity and multipotency (58–61). Thus, various origins of precursor fibroblasts may explain the reason for the heterogeneity of fibroblasts. As discussed vide supra, activated fibroblasts can originate from various cell types (2, 8, 14, 62–66). Understanding the relative importance of each group of fibroblasts originating from different cell types will be crucial in deciphering the effects of activated fibroblasts in the disease setting. Furthermore, even the normal fibroblasts in various anatomic locations of the body can be classified as distinct cell types in light of differential gene expressions (57). As one can expect, such a heterogeneity persists also in terms of CAFs. Indeed, novel approaches such as single-cell RNA sequencing revealed significant transcriptional heterogeneity in CAF populations in murine and human studies (67–69).
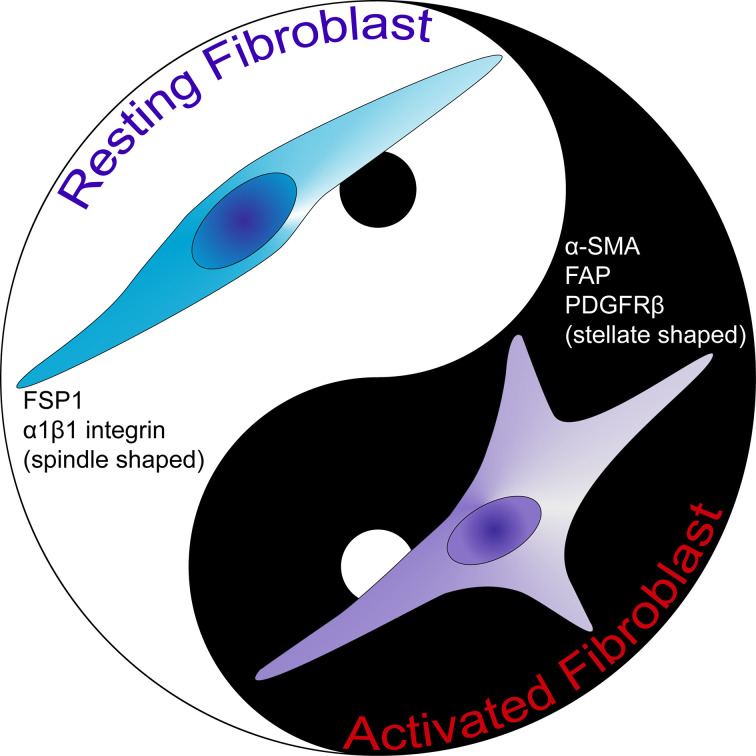
Figure 2.
The comparison of activated fibroblast and resting fibroblast cells. Resting fibroblast cells are morphologically spindle shaped in contrast to activated fibroblast cells which are stellate shaped. Activated fibroblast cells express α-SMA, FAP and PDGFRβ. FAP, fibroblast activation protein; FSP1, fibroblast-specific protein 1; PDGFRβ, platelet-derived growth factor receptor-β; α-SMA, α-smooth muscle actin.
In fact, CAFs are at present widely accepted to be a heterogeneous population. A recent seminal Consensus Statement on the basis of a meeting of experts in the field, underlined the degree of specialization among these cells (70). Such a specialization may explain the distinct functional sub-groups of CAFs identified by various markers (71). Our current understanding of these cells include the fact that most markers do not represent the whole CAF population. Several markers may be utilized to define CAFs such as α-SMA, FSP1, vimentin, desmin, FAP and platelet-derived growth factor receptor (PDGFR) (72). It should be kept in mind that these markers are not specific either for CAFs or for fibroblasts. Markers like FSP1 and α-SMA define diverse populations of CAFs (73). Due to the variation in terms of expressions of these markers, Sugimoto et al. described CAFs rather as a heterogeneous group of cells (7). Hence, it seems clear that more thorough classification of these cells as well as extensive analyses of functions of CAF subclasses are required in order to develop effective treatment options that target CAFs (74).
Currently, there are no markers that specifically define CAFs. The high heterogeneity and the lack of specific markers for these cells result in poorly understanding of this population of cells in respect to their biology and subtypes (75). Nevertheless, α-SMA is generally utilized as a CAF marker (73); since the signature expression profile of these cells is still vague. The critical problem is the fact that many of the proposed markers are either not expressed by all CAF cells or not specific solely to fibroblast cells. Even though FSP1 was suggested as a specific marker for fibroblasts (76), several activated fibroblasts were reported not to express FSP1 (73). In fact, there exists a unique FSP1 expressing CAF subpopulation in the tumor microenvironment, which is distinct form α-SMA+ myofibroblasts (7). The role of such FSP1+ CAFs should be further investigated. Several other markers such as vimentin and PDGFRβ can detect both fibroblasts and myofibroblasts in the tumor microenvironment. In fact, Orimo and Weinberg proposed that CAFs include populations of both myofibroblasts and fibroblasts present within tumor (73). Although tumor stromal fibroblasts in general may express α-SMA, FAP, vimentin, neuron-glial antigen-2 (NG2) chondroitin sulfate proteoglycan, PDGFRβ, prolyl 4-hydroxylase and FSP1; α-SMA and FAP can be used to discriminate myofibroblasts form fibroblasts in the tumor tissue ( Figure 3 ) (73).
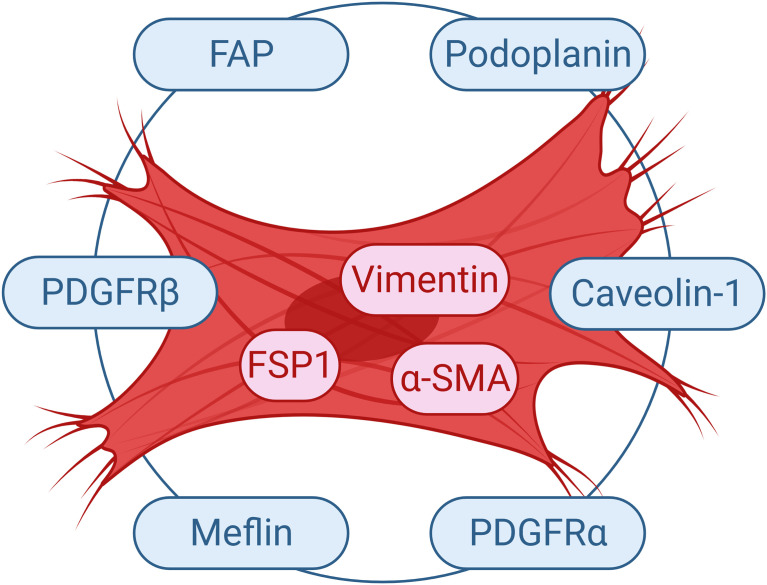
Figure 3.
Commonly Used Markers for CAFs. Selected markers used to study CAF cells are demonstrated. FAP, fibroblast activation protein; FSP1, fibroblast-specific protein 1; PDGFR, platelet-derived growth factor receptor; α-SMA, α-smooth muscle actin.
Activation of Fibroblasts
Resting fibroblast cells in tissues are spindle shaped single cells. In case of tissue injury, they become reversibly activated in order to take part in tissue repair (77). Activated fibroblasts turn into myofibroblasts; since they acquire contractile stress fibers, express α-SMA (78). When the wound healing process is completed, the activation of fibroblasts is reversed by either reprogramming or removal from the granulation tissue by a specific type of programmed cell death called nemosis (24, 79, 80). Activated fibroblasts are known to gain secretory functions such as production of higher amounts of ECM and also demonstrate increased proliferation (81). Such an increased bioactivity is called as fibroblast activation (81). In situations of prolonged injurious stimuli, such as fibrosis or cancer, they gain further proliferative and secretory functions ( Figure 4 ). Kalluri suggested that such fibrosis and cancer associated fibroblasts, respectively, bear epigenetic regulations which may result in irreversible activation (24, 82). For instance, resident fibroblasts become activated and myofibroblasts are generated from bone marrow derived cells, pericytes and endothelial cells in obstructive nephropathy (83). CAFs are similar to normal activated fibroblasts in terms of expressing α-SMA, but demonstrate higher proliferative capacity and secretory phenotype. On the other hand, the perspective proposing that CAFs include populations of both myofibroblasts and fibroblasts should also be kept in mind (73).
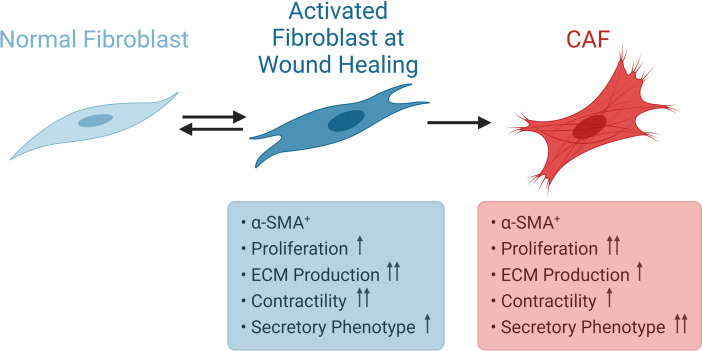
Figure 4.
Activation of Fibroblasts. Normal fibroblasts are spindle shaped cells. During wound healing, fibroblasts become reversibly activated and express α-SMA. CAFs also express α-SMA, but display higher proliferative capacity and secretory phenotype. CAF, cancer associated fibroblast; α-SMA, α-smooth muscle actin.
The Role of CAFs on Tumor Progression
Several aspects of multi-step carcinogenesis have been thoroughly investigated over the last decades. Identification of key oncogenes and tumor suppressor genes have led to the establishment of carcinogenesis models that incorporate gain or loss of various gene functions. Although such information is truly priceless, this seed-centric model does not sufficiently underline the importance of the microenvironment, “soil”, which the tumor cells reside in. Cancer cells cannot be regarded as isolated entities. They actively interact with their milieu, which contains several different types of cells including fibroblasts (84). The highly complex tumor microenvironment greatly affects tumor progression (85). Given the fact that Paget proposed the “Seed and Soil” hypothesis more than a century ago (17), this concept is in fact cannot be regarded as new.
The normal histological organization of most epithelial cells provides a way of separation from the stroma by a basement membrane, which contains stromal cells such as normal fibroblasts. In fact, tumor stroma seems to exert a bimodal influence on cancer development. It inhibits tumor growth in normal tissue, whereas it may facilitate tumor growth and migration during tumor progression (86). During the transition from normal to neoplastic epithelial cells, there exists a reciprocal and paracrine crosstalk between the epithelium and the stroma, which results in activation of members of the stroma such as fibroblasts and maturation of blood vessels. This, in turn, potentiates the development of cancer (86). In line with this reciprocal interaction, tumor progression is characterized with further epithelial proliferation and activation of stromal cells. In fact, CAFs may also be regarded as components of the host’s response to injury caused by the growing tumor (24, 87). Thus, CAFs may demonstrate anti-tumor responses during the early stages of neoplasia (24, 88). It is likely that fibroblasts become activated at early stages of carcinogenesis and give rise to CAFs, which take active part in tissue remodeling (89). The anti-tumor effects of CAFs at initial stages of neoplasia may be replaced by pro-tumoral effects as the tumor grows. CAF derived molecules can be implicated in cancer cell survival and proliferation under such circumstances. LeBleu and Kalluri suggested that pro-tumoral effects of CAFs might evolve gradually (89). In addition to several inherent changes in tumor cells, they also alter their microenvironment via secretory molecules such as growth factors, interleukins, colony stimulating factors. As a result, several mechanisms including the induction of angiogenesis (90, 91), inflammatory cell recruitment (92), immune modulation (93, 94) and ECM remodeling (95) facilitate tumor progression. In addition, CAF derived factors can promote therapy resistance and immune exclusion (96, 97). In an autochthonous model, Feig et al. demonstrated that immune control of pancreatic ductal adenocarcinoma growth could be achieved via depleting FAP+ CAFs (98). This depletion uncovered the anti-tumor effects of immunological checkpoint antagonists [α-cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and α-programmed cell death 1 ligand 1 (PD-L1)]. The researchers also suggested that CXCL12 (SDF-1) might account for the overriding immunosuppression by the FAP+ cells (98).
Activated fibroblasts may even directly facilitate metastasis (86, 99, 100). Peña et al. reported that stanniocalcin-1 expression by CAFs could drive metastasis of colorectal cancer (101). Furthermore, tumors formed in the presence of stanniocalcin-1 deficient fibroblasts demonstrated fewer and smaller distant metastases in an orthotopic mouse model of colorectal cancer (101). In an interesting study, Pelon et al. described four CAF subpopulations in metastatic lymph nodes from breast cancer samples. They reported that CAF heterogeneity in axillary lymph nodes promotes metastases in breast cancer via complementary mechanisms (102). Studies utilizing single-cell RNA sequencing can provide important data about CAF functions in early, advanced and metastatic tumors (103–105). Our current knowledge concerning the CAFs at metastatic sites is very limited. Shani et al. recently investigated the transcriptional co-evolution of lung fibroblasts isolated from transgenic mice at defined stage-specific time points of metastases formation. They suggested that fibroblasts in lung metastases were transcriptionally plastic, showing stage-specific gene signatures (106). Indeed, it has long been known that cancer cells can migrate from the primary site with CAFs (107). In a mouse model, Duda et al. demonstrated that the viability of circulating metastatic cancer cells was higher if they were incorporated in tumor-stroma cell fragments (107). A seminal study by Kalluri et al. demonstrated the important role of local fibroblasts in providing the suitable microenvironment for metastatic colonization (108). VEGF-A and Tenascin-C produced by specific stromal cells were found to be important for metastatic colonization (108). In another study, Benedicto et al. reported that disruption of CXCR4/CXCL12 axis by CXCR4 antagonist AMD3100 inhibited the contribution of cancer and stromal cells to the metastatic cascade in the liver (109).
Our current understanding of the effects of tumor stroma on tumor progression and the course of metastatic events is rapidly expanding (110, 111). Cancer cells recruit different cell types which in turn help support tumor growth and progression (110). CAFs exert a myriad of effects on tumor progression. They were shown to directly facilitate tumor growth in several studies. In an important study, Weinberg et al. demonstrated that CAFs facilitated breast cancer progression more potently in a co-implantation tumor xenograft model, when compared with their normal counterparts (112). CAFs are also able to affect cancer cell invasion as well as metastasis (113). In an interesting study, Shapcott et al. utilized a deep learning cell identification algorithm on colon cancer diagnostic images in TCGA. They reported that increased fibroblast numbers were associated with invasion, metastasis and residual tumor (114). In a study by Gaggioli et al., fibroblast cells were shown to act as leading cells triggering proteolytic and structural modifications of the ECM to create tracks which carcinoma cells move within behind the fibroblasts, in order to achieve cell invasion into ECM (115). In a recent study, Wei et al. reported that periostin+ CAFs promoted lymph node metastasis (116). Periostin is an ECM protein produced by fibroblasts (117). It was previously shown to increase the proliferation and invasiveness of tumor cells (118). Wei et al. demonstrated that periostin+ CAFs affected lymphatic endothelial barriers in cervical squamous cell carcinoma via activating the integrin-FAK/Src-VE-cadherin signaling pathway in lymphatic endothelial cells, resulting in increased metastatic dissemination (116).
Tumor cells are well known to utilize exosomes to change the functions of other cells in the tumor microenvironment (119). In recent years, CAFs were also shown to take part in exosome mediated communications (120). CAF derived vesicles are able to promote the migration and invasion of cancer cells (121, 122). Qin et al. demonstrated that CAF derived exosomal miR-196a contributes to cisplatin resistance in head and neck cancer by targeting CDKN1B and ING5 (123). In another study, Wang et al. showed that CAF derived exosomes contain decreased miR-3188 levels compared to NFs. They also reported that loss of miR-3188 in exosomes contributed to malignant phenotypes of head and neck cancer cells (124). Such findings are not limited to head and neck cancer.
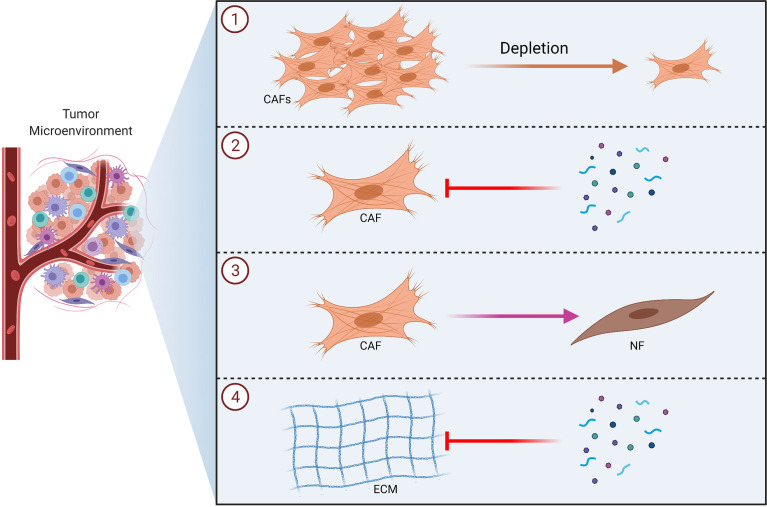
Various tumor derived factors can be responsible from the activation of CAFs, which may in turn support tumor growth. Cirri and Chiarugi proposed a model for the interaction of CAFs with tumor cells, which suggests that there exists a positive and reciprocal feedback between CAFs and cancer cells (82). Cancer cells induce fibroblast activation via factors like TGF-β; which in turn causes tumor survival, ECM remodeling, angiogenesis and EMT by secreting cytokines and growth factors (125–127). Tumor microenvironment also plays a crucial role in inducing drug resistance (128, 129). CAFs may support cancer cells in surviving from cancer drugs (130). Wang et al. reported that fibroblasts play an important role in lung cancer resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors via hepatocyte growth factor (HGF) (131). In another study, it was found that fibroblasts might cause resistance of cancer cells to gemcitabine (132). Nakasone et al. demonstrated that the microenvironment could be implicated in drug resistance by regulating vascular permeability and immune cell infiltration (133). Such studies showing the role of CAFs in terms of drug resistance underline the importance of therapeutic approaches that will also incorporate strategies to target the tumor microenvironment in order to overcome drug resistance. Therefore, therapeutic approaches that incorporate anti-CAF strategies have been extensively studied over the recent years (134–136). Such strategies may involve depletion of CAFs, blocking CAF functions, altering CAF activation status and targeting ECM ( Figure 5 ) (134, 137). In fact, several anti-CAF strategies have been investigated in the clinical setting (70).
Figure 5.
Therapeutic Strategies to Target CAFs in Tumors. Schematic illustrations of the CAF-targeting approaches including depletion of CAFs ①, blocking CAF functions ②, altering CAF activation status ③ and targeting ECM ④ are shown. CAF, cancer associated fibroblast; ECM, extracellular matrix; NF, normal fibroblast.
It should also be taken into consideration that organ specificity might play a major role in terms of the effects of CAFs on tumor progression. The stromal content of solid tumors can considerably differ and it may constitute 60–90% of the total tumor mass in several tumor types (e.g. breast, colon, stomach, pancreatic cancer) (1, 12, 138, 139). Moreover, CAFs are extremely influential in desmoplastic reactions and they may help to create a desmoplastic tumor microenvironment (140, 141). Desmoplasia is a prominent feature of the pancreatic ductal adenocarcinoma (142). Indeed, pancreatic ductal adenocarcinoma is characterized by an extensive deposition of ECM, which is probably more than any other type of malignancy (96). Both primary tumors and metastases of pancreatic ductal adenocarcinoma are characterized by increased levels of desmoplasia (143). Desmoplasia can be regarded to promote tumor invasion in pancreas, breast, lung, esophagus, stomach and prostate (144–150). On the other hand, the effect of desmoplasia in colorectal carcinoma progression is not very clear (151). Nonetheless, desmoplastic reaction seems to be an independent prognostic factor also for colorectal cancer (152–154). Maturity of CAFs and desmoplastic reactions were reported to be associated with cancer invasion in patients with colorectal cancer (155). Given the differences in various tissues concerning the presence and/or extent of desmoplasia, CAFs may also demonstrate tissue/organ and tumor type specific alterations. Indeed, it is very difficult to determine markers in order to label CAF subpopulations that take part in tumor progression in different organs (88), and to cap it off, it is currently unclear whether specific CAF populations are preserved across tissues (70). CAF subpopulations may also demonstrate differences in early or late stage primary tumors (156). Friedman et al. reported that CAF repertoire changes over time in breast cancer progression (157). As one might expect, it is not readily possible to investigate the alterations in CAF populations in human tissue samples during disease progression, since longitudinal analyses of the same lesion is very difficult. Future studies in murine models with temporal resolution may provide priceless information concerning the alterations in CAFs during tumor progression. Furthermore, there is very little information concerning the CAF composition in metastasis sites. Novel studies suggest the presence of resembling CAF populations in different malignancy types (96). Ikenaga et al. had previously reported that CD10+ pancreatic stellate cells enhanced the progression of pancreatic cancer cells (158). In a more recent study, CD10 and GPR77 were also reported to define a CAF population correlated with poor survival and chemoresistance in multiple cohorts of breast and lung cancer patients (159). Numerous studies showed that different types of malignancies contain both myofibroblasts and non-myofibroblasts (38, 160–162). In contrast, several other studies have demonstrated the existence of distinct CAF populations in various cancer types (96). The kinetics of CAF functions can vary in different malignancy types, since resident fibroblasts display different organ specific transcriptomic profiles (163). Rinn et al. investigated genome wide gene expression profiles of primary fibroblasts from distinct anatomical sites and demonstrated that differences in gene expression programs were related with anatomic divisions (i.e. anterior-posterior, proximal-distal, dermal-nondermal) (163). In fact, even different tumor subtypes may contain diverse fibroblast populations. Tchou et al. reported that gene expression in human breast CAFs significantly varied between breast cancer subtypes (ER+, Her2+ and triple-negative breast cancer) (164). Different subpopulations of CAFs may demonstrate distinct functions even within a tumor type (165). In light of these findings, investigation of differences and similarities between CAF subpopulations in different malignancy types may pave the way for innovative strategies to precisely target CAFs.
Tumor Associated Macrophages
Macrophages were first discovered by Ilya Mechnikov late in the 19th century (166). Mechnikov, who was awarded the 1908 Nobel Prize in Physiology or Medicine jointly with Paul Ehrlich in recognition of their work on immunity (167), championed the theory of phagocytes and described mobile cells battling invading pathogens (168). Indeed, macrophages are among the most important and abundant players of the tumor microenvironment. They are capable of affecting tumor progression, angiogenesis and therapy resistance (169–171). TAMs display a wide diversity and plasticity. TAMs were originally considered to originate from circulating monocyte precursors released from the bone marrow (172). It has also been demonstrated that many tissues contain embryonic derived populations of resident macrophages (173, 174). The exact causes of the diversity of TAMs are not clear; however, plasticity of TAMs and/or independent specific lineages generating multiple populations might account for such a heterogeneity (175). Indeed, deciphering the origin of TAMs might improve cancer immunotherapy strategies (176).
TAMs can demonstrate supportive and inhibitory effects on cancer, depending on several factors such as stage and microbiota (177). TAMs may also function as a barrier for anti-tumor immunity (178). More than 70% of human breast cancers express colony stimulating factor 1 (CSF-1), which is a key regulator of mononuclear phagocytic lineage; and expression of CSF-1 is correlated with poor prognosis (179). Macrophages are known to be highly plastic cells. They can be classically or alternatively activated. Classically activated anti-tumor macrophages (M1) and alternatively activated tumor promoting macrophages (M2) reflect the nomenclature of polarized immune responses (180, 181). In a seminal study, Hill et al. suggested that M1 or M2 dominant macrophage responses might influence the types of immune responses (182). In fact, Mantovani et al. suggested that M1 and M2 macrophages represent the extremes of a continuum of activation states. Therefore, the adaptability of macrophages in response to various conditions goes beyond such a dichotomy (181).
Polarization of Macrophages
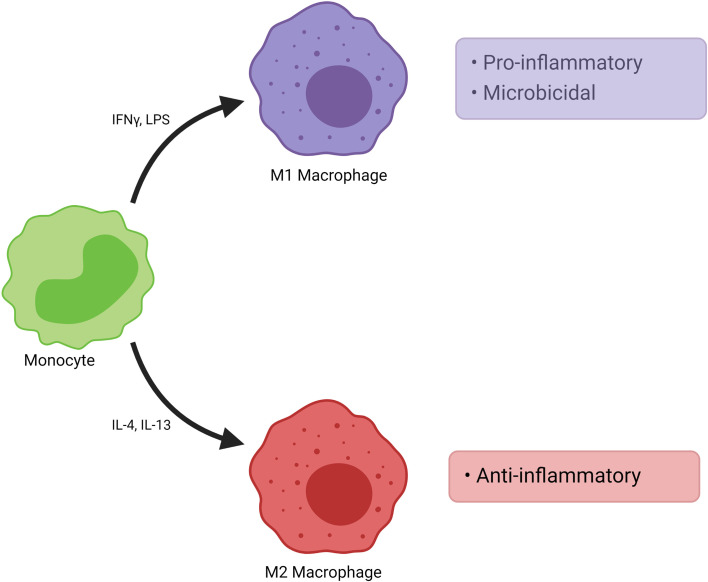
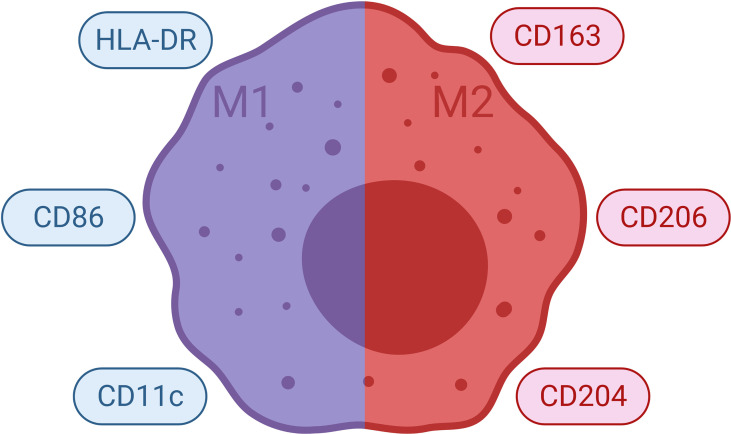
Monocytes from blood differentiate to M1 or M2 subtype macrophages, depending on their interactions with other cells present in tissues ( Figures 6 and 7 ). Such differentiation is dependent on the environmental stimuli these cells are subject to (183–190). The first type of antimicrobial macrophage activation became known as M1 macrophages (191). In an important study in 1992, Stein et al. reported that expression of macrophage mannose receptor was inhibited by interferon gamma (IFNγ); whereas, recombinant murine IL-4 significantly enhanced macrophage mannose receptor surface expression and activity (192). IL-4 caused inflammatory macrophages to show an alternative activation phenotype, unlike that induced by IFNγ (192). Such findings paved the way for the concept of alternatively activated macrophages (M2). High IFNγ levels result in M1 differentiation, whereas high levels of interleukin (IL)-4 and IL-13 polarize macrophages into M2 subtype (180). M1 macrophages produce several pro-inflammatory mediators such as tumor necrosis factor (TNF)-α, IL-1, IL-6; as well as microbicidal and tumoricidal molecules such as reactive nitrogen/oxygen intermediates (191). On the contrary, M2 macrophages express molecules which can be involved in parasite infestation, tissue remodeling and tumor progression such as resistin-like-α, Arginase 1, chitinase-like molecules, IL-10 and mannose receptor C-type 1 (Mrc1) (193). Human M1 macrophages show high expressions of CD14, CD16, CD64, CD86, and HLA-DRα. On the other hand, M2 macrophages express CD163 and CD206 (also known as Mrc1) (183, 194–196). Nevertheless, recent studies suggest that classification of macrophages is not dichotomous and different macrophage subtypes might demonstrate shared features (197). Indeed, macrophages can constitute nearly half of the tumor mass in breast cancer. In addition, it has been shown that there is a correlation between the number of TAMs and poor prognosis (198, 199).
Figure 6.
Polarization of Macrophages. Monocytes from blood differentiate into functionally distinct populations. M1 macrophages are induced by various stimuli such as IFNγ. They demonstrate pro-inflammatory and microbicidal effects. M2 macrophages are induced by stimuli such as IL-4, IL-13 and they show anti-inflammatory effects. M2 macrophages may be further divided into subpopulations such as M2a, M2b and M2c. IFNγ, interferon gamma; IL, interleukin; LPS, lipopolysaccharide.
Figure 7.
Commonly Used Markers for Macrophages. Selected markers used to distinguish macrophage phenotypes are demonstrated. HLA, human leukocyte antigen.
The Role of TAMs on Tumor Progression
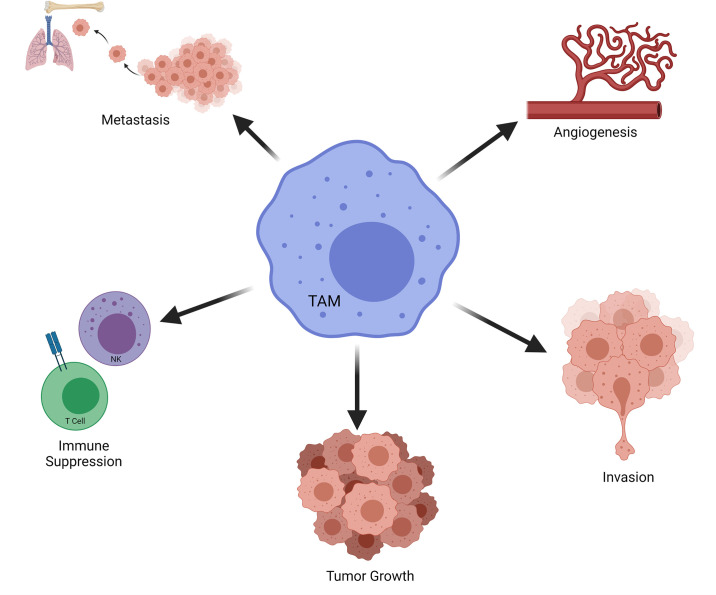
Macrophages may assume roles in all stages of tumor progression ( Figure 8 ) (200, 201). For instance, macrophages are able to induce angiogenesis and help tumor invasion in primary tumor. Subsequently, they may alter the pre-metastatic site, facilitate the dissemination and growth of tumor cells (202). Macrophages mostly favor tumor growth and are associated with poor outcome in various cancers (203). TAMs with an M2 phenotype can promote tumor metastasis and get involved in immune suppression as well as causing failure of radiation or checkpoint inhibitor therapy (204). M2-like TAMs that infiltrate metastatic sites can limit immune responses against high-grade serous carcinomas (205). Metastasis associated macrophages (MAMs) include bone marrow derived macrophages as well as tissue resident macrophages. MAMs can promote metastasis in secondary site (206). Thus, strategies that aim to target macrophages for cancer treatment has been explored over the last decades ( Figure 9 ) (203, 207–210). Current strategies to target TAMs for anti-cancer therapy usually involve inhibition/depletion of TAMs by various approaches or reprogramming them to assume an anti-tumor role instead of showing pro-tumoral effects (211–213). M2 macrophages secrete TGF-β, vascular endothelial growth factor (VEGF), IL-6, IL-10 as well as chemokines which facilitate angiogenesis and ECM remodeling. As a result, they can promote tumor invasion and metastasis (214). In addition, macrophages are very effective in creating an immunosuppressive microenvironment. For instance, TAMs may limit the activity of tumor infiltrating lymphocytes (203). Macrophages can stimulate regulatory T cell (Treg) development and expansion as well as disrupting natural killer (NK) and T cell functions (200, 210). TAMs may also overexpress inhibitory ligands for T cells such as PD-L1 and programmed cell death 1 ligand 2 (PD-L2) (210). High expression of PD‐L1 by TAMs was reported in hepatocellular carcinoma, glioblastoma and pancreatic cancer (215–218). PD-L1 expressed by antigen-presenting cells (e.g. macrophages) is widely regarded as an important regulatory molecule for T cells (219).
Figure 8.
Role of TAMs on Tumor Progression. Selected effects of TAMs in tumorigenesis are demonstrated. NK, natural killer; TAM, tumor associated macrophage.
Figure 9.
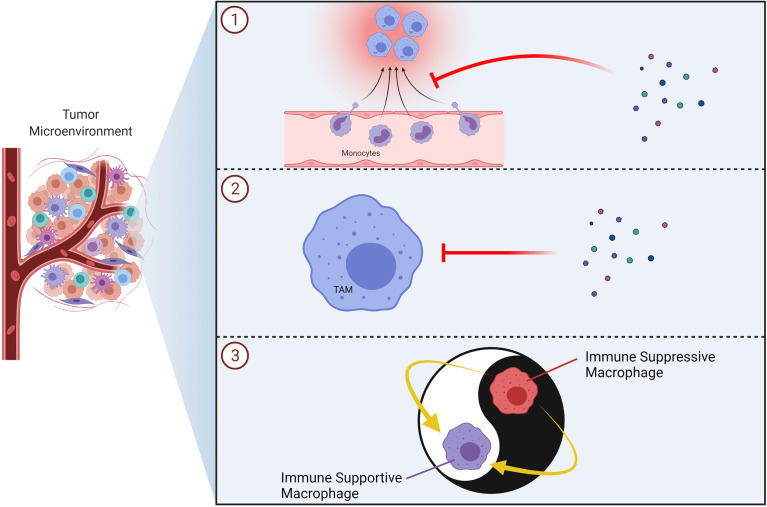
Therapeutic Strategies to Target TAMs in Tumors. Schematic illustrations of the TAM-targeting approaches including inhibition of monocyte/macrophage recruitment (e.g. CXCR4, CCR2 inhibitors) ①, inhibition/elimination of macrophages in the tumor microenvironment (e.g. bisphosphonates) ② and reprogramming of TAMs into immune supportive macrophages (e.g. TLR7/8 agonist) ③ are demonstrated. TAM, tumor associated macrophage; TLR, toll-like receptor.
Indeed, TAMs constitute a major portion of the tumor microenvironment. Clinical studies suggest that cytokines in the tumor milieu influence TAMs, which are inherently plastic cells, to assume an immunosuppressive role (220). It is known that TAMs are usually similar to M2 macrophages (221). Tumor-infiltrating M1 polarized macrophages are usually identified by an IL-12hi IL-10lo phenotype. During tumor development, these macrophages can enhance anti-tumor immune reactions. However, TAMs generally lean towards an M2-like phenotype, which is characterized by an IL-12lo IL-10hi phenotype, at later stages of tumor. Such macrophages demonstrate decreased tumoricidal activity (222). In an interesting study, Pinto et al. profiled macrophages in 150 colorectal cancer cases by immunohistochemistry; utilizing CD68 [as a marker of macrophage lineage (CD68 is one of good markers of TAMs (223))], CD80 (as a pro-inflammatory macrophage marker) and CD163 (as an anti-inflammatory macrophage marker). They found that CD163+ macrophages were predominantly found at the tumor invasive front, while CD80+ ones were almost exclusively located in the adjacent normal mucosa (224). Such findings suggested a chiefly anti-inflammatory polarization of TAMs. Given these findings, it is crucial to identify molecules that affect macrophage plasticity in the tumor milieu in order to design macrophage-oriented treatment and diagnosis strategies (225). Although FDA approved cancer immunotherapies primarily target the adaptive immunity, the importance of innate immunity in terms of anti-tumor strategies started to attract a great deal of attention (226). Various approaches have been explored over the recent years in order to target TAMs (227, 228). In line with such strategies, macrophages which express a chimeric antigen receptor (CAR) may prove to be useful in order to target solid tumors (229). In a recent inspiring study, Klichinsky et al. reported transducing human macrophages with an adenovirus vector carrying a CAR (230). They showed that a single infusion of human CAR macrophages decreased tumor burden and prolonged overall survival in two solid tumor xenograft mouse models (230). In another exciting study, Mitragotri et al. recently reported an engineered particle (called as “backpack”) which can adhere to the surfaces of macrophage and regulate cellular phenotypes in vivo. The “backpacks” released cytokines to guide the polarization of macrophages toward anti-tumor phenotypes, slowed tumor growth and improved overall survival in a murine breast cancer model (231). These findings will most likely open new avenues for next-generation CAR applications and adoptive cell transfer strategies in cancer therapy.
The Interplay Between CAFs and TAMs
In addition to playing important tumor-promoting roles in tumor initiation and progression, CAFs were also shown to sculpt the tumor microenvironment (93, 232). It has been known for some time that CAFs interact with tumor cells by cell-cell contact or via release of soluble factors. In addition to such interactions, CAFs also take active part in the crosstalk with other members of the tumor microenvironment. They secrete a plethora of soluble mediators (e.g. cytokines, chemokines); which affect various types of leukocytes including macrophages, thereby taking part in immune regulation (70, 197, 233). Moreover, various recent studies have demonstrated that CAFs function as regulators of immune cell recruitment and function (197).
In line with these findings, the effects of stromal fibroblasts on monocytes/macrophages have been investigated by several groups. CAFs may increase monocyte recruitment and differentiation into TAMs (234). CAFs can also promote skin tumor development by maintaining monocyte chemotactic protein-1 (MCP-1) mediated macrophage infiltration and chronic inflammation (235). Podoplanin+ CAFs were recently reported to be associated with CD204+ TAM infiltration in lung squamous cell carcinoma (236). Moreover, podoplanin+ CAFs were also found to be associated with monocyte recruitment and differentiation into CD204+ TAMs in lung adenocarcinoma (237). Yavuz et al. demonstrated that CAFs isolated from human invasive breast cancer could facilitate the differentiation of monocytes into M2-like pro-tumoral macrophages, in contrast to normal breast-derived fibroblasts. Such a differentiation was evident in terms of function as well as phenotypic features (15). Furthermore, these CAFs were demonstrated to be puissant in terms of recruiting monocytes. MCP-1 and SDF-1 were shown to be pivotal monocyte chemotactic cytokines which were secreted from stromal cells (15). Human CAFs and M2 macrophages were demonstrated to cooperate during prostate cancer progression (238). Similarly, CAFs and TAMs represent important partakers of tumorigenic processes also in head and neck cancer (239). The interplay between CAFs and TAMs seems to be very complex, as these two groups of cells are also able to alter each other’s functions (23).
Reciprocal Interactions
It has long been known that tumor cells interact with immune cells in the tumor milieu. On the other hand, relatively recent studies have discovered the key effects of the communication of immune cells and stromal cells (e.g. CAFs) on tumorigenesis. Stromal cells are able to recruit immune cells in addition to altering their functions. A specific example of such an influence exists in terms of CAFs, which are able to affect monocyte recruitment and polarization. Indeed, CAFs and TAMs are the main components of stromal cells (240–243). Tumor associated macrophages display high level of plasticity. Their activation status in the tumor microenvironment rather has an ephemeral nature (244). There exists a close relation between TAMs and CAFs, as TAMs constitute the most abundant innate immune cell type in the vicinity of CAF populated areas (134). Macrophage derived factors may promote the activation of resident hepatic stellate cells into myofibroblasts, resulting in a fibrotic environment in liver metastases of pancreatic ductal adenocarcinoma (245). Recently, Tokuda et al. showed the significance of osteopontin mediated cancer-TAM-CAF interactions in hepatocellular carcinoma (246).
M2 type TAMs can activate CAFs and thus help tumor progression (247). In addition, M2 macrophages can influence the MMT of fibroblasts, resulting in enhanced reactivity (238). Reciprocally, CAFs are also able to change M1 macrophages in the tumor microenvironment into M2-like macrophages (248). In neuroblastoma, bone marrow-derived MSCs (which become activated into CAFs at tumor site) were found to increase the invasiveness of macrophages; which in turn, induced the proliferation and invasion of CAFs (249). In addition to metabolically supporting the tumor growth, reciprocal interactions of CAFs with M2 macrophages result in a significant pro-tumoral effect (250). Stairs et al. reported that tumor cells recruited immature myeloid cells, which demonstrated immunosuppressive properties and facilitated desmoplasia by activating fibroblasts (251). It should be borne in mind that the reciprocal interactions of CAFs and TAMs in the tumor milieu have not been fully discovered, despite the fact that these two cell types are critical constituents of the tumor microenvironment. Thus, novel studies are required to decipher bilateral interactions between CAFs and TAMs as well as to understand the effects of such interactions on diverse functions of both CAFs and TAMs.
In addition to providing mechanistic insights, CAFs and CD163+ macrophages (M2) may also prove to be potential prognostic predictors; since expressions of CAF and M2 macrophage markers are correlated with poor prognosis in colorectal cancer and oral squamous cell carcinoma (252, 253). Given the effects of macrophage polarization in terms of anti-tumor immune responses, one can easily foresee that such a trans-differentiation of macrophages from anti-tumoral M1-like to pro-tumoral M2-like macrophages is a pivotal incident in the constitution of the tumor permissive microenvironment.
Recruitment and Polarization of TAMs by CAFs
It has been demonstrated that most of the macrophages located in tissues originated from yolk sac precursor cells (254, 255). Hence, the notion about adult tissue macrophages being only derived from bone marrow precursor cells has become obsolete. That being said, it should be kept in mind that macrophages found in pathogen associated inflammation chiefly originate from bone marrow monocytes (256). When TAM sub-populations were investigated, most of them were recently demonstrated to emerge from the Ly6C+ population of circulating mouse monocytes in grafted tumors, primary mouse mammary tumors and in lung metastases (176, 257, 258).
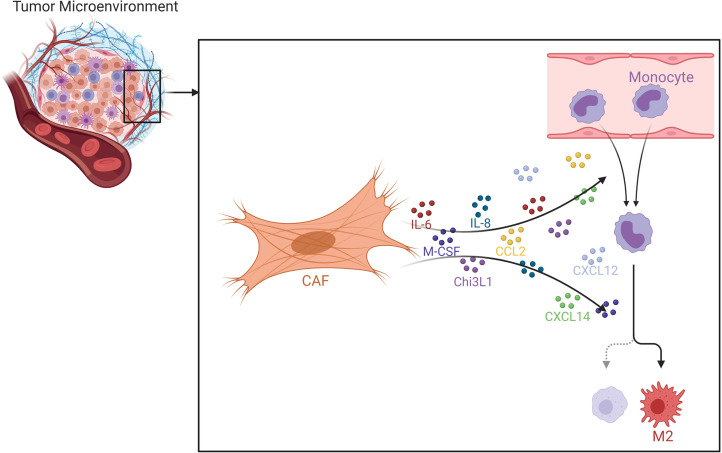
CAFs have been demonstrated to recruit macrophages to the tumor microenvironment in various studies with many mouse models, e.g. breast, prostate and squamous cell carcinomas ( Figure 10 ) (259–261). CAFs release high amounts of SDF-1, which was demonstrated to be implicated in monocyte recruitment (15). In addition to SDF-1, CAFs are also able to secrete CXCL14, which may promote monocyte recruitment and polarization into M2 macrophages in prostate cancer together with SDF-1 (238, 260). Such findings, in fact, accentuate the pivotal role of SDF-1/CXCR4 axis for the progression of various cancers (262). It is known that MCP-1 can facilitate the infiltration of blood monocytes into CAF spheroids and Ksiazkiewicz et al. demonstrated that MCP-1 is important for the recruitment of blood monocytes to tumor fibroblastic areas (263). Thus, MCP-1 as well as SDF-1 plays a key role in CAF-induced monocyte chemotaxis ( Figure 11 ) (15). CAF derived CXCL16 is also able to attract monocytes to promote stroma activation in triple negative breast cancer (264). Mace et al. demonstrated that pancreatic stellate cells, which represent a subset of pancreatic CAFs, produce macrophage colony-stimulating factor (M-CSF), IL-6, VEGF, SDF-1 and MCP-1; which may promote monocyte recruitment and macrophage differentiation as well as potentially facilitating M2 polarization (265, 266). Adenosine generated by CAFs in the tumor microenvironment can induce the expansion and/or differentiation of M2-like macrophages (267–270). Hegab et al. also reported that CAFs increased the conversion of TAMs to the M2 phenotype in a mouse model of lung adenocarcinoma (271). CAF derived IL-6, IL-8, TGF-β, and IL-10 also promote monocyte recruitment and differentiation into M2 macrophages (272, 273). In the human colorectal cancer microenvironment, CAF derived IL-6 and IL-8 were suggested to promote the differentiation of tumor infiltrating myeloid cells into M2 macrophages (274). In an interesting study, Nakamura et al. investigated the significance of expression of carbonic anhydrase IX, which is a marker of hypoxia, by CAFs. They reported that the numbers of CD204+ TAMs and podoplanin+ CAFs were significantly higher in the carbonic anhydrase IX+ CAFs group than in the carbonic anhydrase IX- CAFs group (275).
Figure 10.
Effects of CAFs on Macrophages. Various chemokines and cytokines secreted by CAFs may promote macrophage recruitment and M2 polarization. CAF, cancer associated fibroblast; Chi3L1, chitinase 3-like 1.
Figure 11.
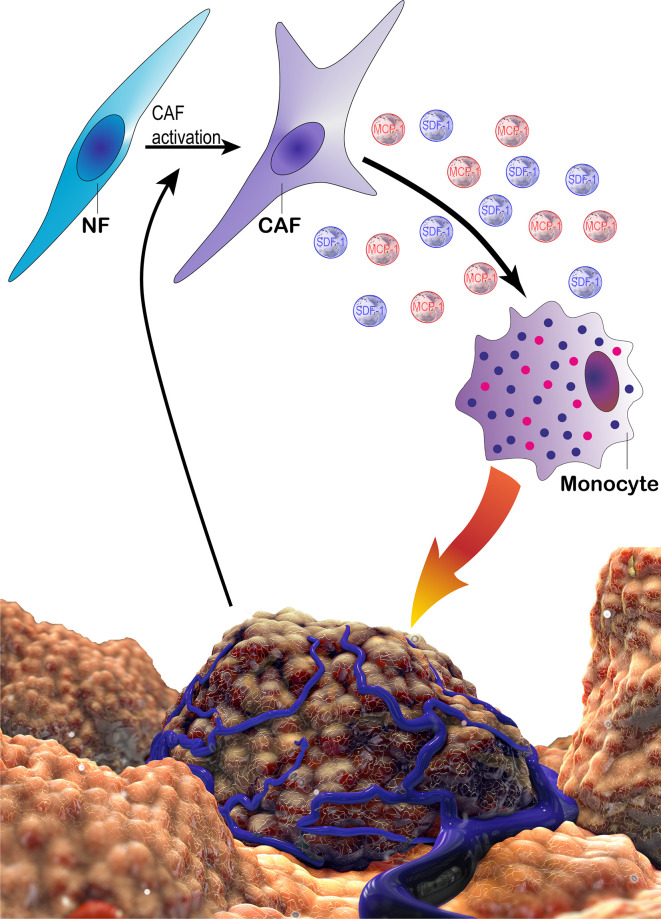
The schematic demonstration of potential tri-directional interactions of cancer cells, CAFs and monocytes. Cancer cells can take part in the activation of CAFs via several factors. CAFs may in turn recruit monocytes via MCP-1 and SDF-1. In addition, CAFs promote a pro-tumoral phenotype in monocytes/macrophages, which enhance cancer cell proliferation and motility as well as suppressing immune responses. CAF, cancer associated fibroblast; MCP-1, monocyte chemotactic protein-1; NF, normal fibroblast; SDF-1, stromal derived factor 1.
Higashino et al. reported that human FAP+ mesenchymal stem cells (CAF-like cells) helped the growth and migration of peripheral blood mononuclear cell derived macrophage-like cells (276). CAF-like cells promoted M2 polarization of macrophage-like cells. The CAF-like cells secreted CCL2, IL-6, CXCL8; which facilitated the migration of macrophage-like cells. Such effects were found to be associated with FAP expression, since FAP silencing decreased cytokine secretion in CAF-like cells (276). Yang et al. reported that FAP induced inflammatory CAFs by activating STAT3 via the uPAR–FAK–c-Src–JAK2 pathway. FAP+CAFs were the main source of CCL2, which facilitated the recruitment of myeloid-derived suppressor cells (MDSCs) to the tumor and promoted immunosuppression in a CCR2 dependent manner (277). Indeed, CAFs have a significant effect on polymorphonuclear MDSC infiltration of tumors (278). In another study, Borriello et al. reported isolating a population of αFAP- and FSP-1–expressing CAFs that share functional/phenotypic features with bone marrow derived mesenchymal stromal cells from primary human neuroblastoma tumors (279). In addition, they reported that the presence of αFAP- and FSP-1–positive cells in human neuroblastoma tumor stroma correlated with that of M2 TAMs (279). Similarly, CAFs were also found to be associated with infiltration of CD163+ macrophages in triple negative breast cancer and nasopharyngeal carcinoma patients (280, 281). Such studies might provide evidence for the association between CAFs and TAMs as well as their cooperation in generating a pro-tumorigenic milieu. Experiments with 3-D cultures of human breast cancer cells and fibroblasts revealed the CCL2-mediated recruitment of monocytes by CAFs (263). CAF derived CXCL12 and CXCL14 were also implicated in macrophage recruitment and M2 polarization (134). In hepatocellular carcinoma, human CAFs can attract monocytes by the SDF-1a/CXCR4 pathway and facilitate their differentiation into MDSCs via IL-6-mediated STAT3 activation (282). Zhang et al. reported that human CAFs could promote M2 polarization in pancreatic ductal adenocarcinoma partly via M-CSF (283).
In addition to their direct effects on macrophages, CAFs may also indirectly affect macrophage polarization. Pancreatic stellate cells (CAF-like cells) in pancreatic ductal adenocarcinoma were shown to stimulate mast cell activation (284). Mast cells, in turn, produce IL-13 which promotes M2 macrophage polarization (285). Furthermore, human CAF mediated ECM remodeling can increase the infiltration of TAMs, which may show pro-tumorigenic effects (286). Moreover, CAF derived ECM components may modulate macrophage polarization to M2-like (287). Kobayashi et al. demonstrated the impact of stroma derived hyaluronan mediated ECM remodeling on macrophage mobilization. Hyaluronan served as a signal for the recruitment of TAMs and its deficiency impaired macrophage recruitment (288). An interesting study investigated whether radiotherapy influences CAF mediated immunoregulatory effects on macrophages. Ionizing radiation did not alter the immunoregulatory effects of CAFs on macrophages (289). In line with this finding, Gorchs et al. reported that the strong immunosuppressive effect of CAFs on activated T cells remained unchanged after radiotherapy (290). On the other hand, radiotherapy was proposed to increase paracrine signaling between fibroblasts and tumor cells via insulin-like growth factor (IGF) and TGF-β pathways (291, 292). Chemotherapy can also modulate the tumor microenvironment (293). CAF derived growth arrest-specific protein 6, which is a ligand of TAM receptors, was reported to increase during cisplatin based chemotherapy (294). Peiris-Pagès et al. reported that tumor cells in contact with fibroblasts could elicit interferon mediated signaling in response to chemotherapy (295). Indeed, chemotherapy may promote the recruitment of monocytic cells and macrophages to tumors in vivo (133, 295, 296). In light of these findings, the trilateral crosstalk among TAMs, CAFs and tumor cells merits further investigation in patients treated with radiotherapy or chemotherapy.
Cheng et al. have shown that CAFs may recruit neutrophils and induce PD-L1+ neutrophils through the IL-6 - STAT3 pathway which facilitates immune suppression in hepatocellular carcinoma (297). STAT3 is known to affect tumor growth and invasion and the JAK/STAT3 pathway is activated in various cancer types (298–300). CAFs were recently shown to be able to induce the differentiation of recruited monocytes into programmed cell death protein 1 (PD-1) expressing M2-like macrophages, instead of M1 macrophages; in addition to recruiting those monocytes (15). Pathological evaluations of human mammary cancer tissues revealed that the number of CAFs is positively correlated with the numbers of both CD163+ and CD206+ macrophages. CD206 was demonstrated to be more correlated with M2a-like macrophages, whereas CD163 is noted on the surface of M2c-like macrophages (301, 302). Furthermore, M2a macrophages are known to be related with TH2 type immune responses and elimination of parasites. On the other hand, M2c macrophages rather take part in immune regulation and tissue remodeling (303). In light of the current literature, CAFs appear to be inducing the polarization of M1 macrophages more into the M2c phenotype than the M2a. Given the fact that M2c phenotype is associated with immune suppression, such a mechanism would best serve the interests of the tumor instead of anti-tumor immune responses. Moreover, the tissue remodeling roles of M2c macrophages also seem to be cut out for helping tumor progression since tumors are known as wounds which do not heal and; therefore, constitute a chronic wound healing or tissue remodeling process instead of an allergic response or a reaction for elimination of parasites.
In addition to tumor cell and T lymphocyte derived IL-4 (304), CSF-1 and granulocyte-macrophage colony-stimulating factor (GM-CSF), which are secreted from tumor cells (179, 305), were demonstrated to promote pro-tumoral differentiation of macrophages. In congruence with those studies, CAFs are also able to take active part in this re-polarization process. It was reported that the number of TAMs in breast carcinoma is significantly correlated with the presence of CAFs (15). A recent study showed that CAF derived Chitinase 3-like 1, which is implicated in inflammatory disorders, facilitated tumor growth in breast cancer. This effect was also associated with high infiltration of M2 polarized macrophages and TH2 type immune responses (261). Enhanced infiltration of breast tumors with TAMs may be caused by the presence of high number of CAFs in such tumor tissues; as CAFs are able to recruit monocytes and promote M2 macrophage polarization.
The Effects on Tumor Progression
Myriad of studies reported that CAFs and TAMs interact to promote cancer progression (306). In addition, various studies demonstrated that TAMs facilitate breast cancer invasion and metastasis via secreting numerous cytokines (307–309). 3-D cultures of human lung adenocarcinoma cells, human lung fibroblast cells and macrophages showed that macrophages and fibroblast cells can facilitate invasion and metastasis (310). CAFs were shown to play key roles in inducing the pro-tumoral phenotype of TAMs (248). Clinicopathological evaluations of oral squamous cell carcinoma tissue samples revealed that both high grade (0: negative, 1: scanty, 2: focal, 3: abundant) of CAFs and high number of CD68+ macrophages were correlated with the TNM stage. In addition, high numbers of CD68+ or CD163+ macrophages were correlated with Ki-67 labeling index. Furthermore, the number of CD68+ macrophages influenced progression free survival. Last but not least, the number of M2-polarized macrophages was associated with vascular invasion (248). Miyake et al. demonstrated that high expression of CXCL1 in urothelial cancer of the bladder was associated with increased recruitment of TAMs/CAFs and poor prognosis (311).
The interplay between CAFs and M2 macrophages, collectively promotes cancer cell motility, tumor invasion and metastasis. This collaboration may also activate endothelial cells and result in angiogenesis (238). Furthermore, collaboration of MAMs and CAFs increases the metastatic potential of arriving tumor cells in the secondary site (206). In addition to affecting the behavior of tumor cells and modifying the tumor microenvironment at primary/metastatic sites (125, 312, 313), CAFs can escort tumor cells in the bloodstream and help the tumor cells survive during the migration to metastatic sites (107). Podoplanin+ S100A4+ PDGF-Rα+ fibroblasts were shown to be present in bone metastasis sites of human breast cancer patients, while PDGFRβ+ fibroblasts were present in lung metastasis sites (314). Similarly, macrophages were shown to play an important role in tumor recurrence and metastatic outgrowth in murine models (206, 315–319). During the metastatic cascade, CAFs and M2 macrophages can collaborate in altering the functions of other stromal cells as well as the tumor cells (250, 320). Nielsen et al. reported that early recruitment of granulin secreting inflammatory monocytes to the liver is very important for pancreatic ductal adenocarcinoma liver metastasis. Granulin secretion by MAMs activated resident hepatic stellate cells into myofibroblasts that produced periostin, causing a fibrotic milieu that supports metastatic tumor growth (245).
The existence of a crosstalk among colon cancer derived exosomes, fibroblast-derived exosomes and macrophage phenotypes in colon cancer metastasis was also proposed (321). In addition, cancer derived exosomes were also shown to be taken up by liver resident Kupffer cells in a mouse model of pancreatic cancer metastasis. The exosomes bore macrophage inhibitory factor that promoted TGF-β generation from Kupffer cells, which activated resident hepatic stellate cells into myofibroblasts. In turn, myofibroblasts prepared the liver for metastatic disseminated tumor cells via production of fibronectin to recruit monocytes/macrophages (322–325). In addition, it was also reported that the primary tumor promoted fibroblast secretion of fibronectin, which took part in the recruitment of bone marrow derived macrophages to the secondary site in liver and lung metastases (322, 324, 326, 327). In a recent study, CAFs and TAMs were reported to interact in generating a perlecan (a heparan sulfate proteoglycan that stores and stabilizes growth factors implicated in regulation of prostate cancer cell growth) rich desmoplastic stroma at sites of prostate cancer bone metastasis (328). Indeed, the complex trilateral crosstalk among cancer cells, CAFs and M2 macrophages may activate endothelial cells and their bone marrow derived precursors to induce de novo angiogenesis and promote metastatic spread of tumor cells (238). CAFs can actively recruit endothelial progenitor cells to the tumor site. Endothelial progenitor cells synergize with CAFs to promote epigenetic modifications of tumor cells via mesenchymal-to-amoeboid transition, which provides an advantage to metastatic cells (329). In fact, Duda et al. demonstrated that metastatic cells can bring stromal components (e.g. activated fibroblasts) from the primary site to the lungs (107). The viability of circulating metastatic cancer cells was found to be higher if they were incorporated in heterotypic tumor stroma cell fragments. In addition, those stromal cells could provide a growth advantage to the metastatic tumor cells. Indeed, circulating tumor cells (CTCs) do not act independently and they are assisted by stromal and immune cells, which alter their metastatic potential. CAFs, which were reported to be associated with CTCs in heterotypic CTC clusters, seem to increase the metastatic efficiency (330). Interestingly, CAFs were found to be present in the brain metastases from lung carcinoma and other carcinomas, in contrast to primary brain tumors or normal brain tissue (107). Such findings may prove the direct involvement of primary tumor stroma in metastasis.
M2 macrophages which were induced by CAFs were demonstrated to promote pancreatic tumor cell growth, migration and invasion (283). In another study, CCR2-dependent recruitment of monocytes/macrophages by tumor resident mesenchymal stromal cells was reported to facilitate tumor growth (331). A recent study showed that endosialin-positive CAFs recruited macrophages and promoted the M2 polarization; thus, promoting the progression of hepatocellular cancer (332). Hashimoto et al. suggested that TAMs and CAFs collectively facilitate the development of neuroblastoma (249). Myofibroblasts are known to secrete VEGF, which induces the accumulation of immune cells such as macrophages at sites of fibrosis (333). In turn, VEGF mediated activation of macrophages promotes skin cancer carcinogenesis, angiogenesis and invasion (334).
CAF-educated monocytes were demonstrated to enhance breast cancer cell invasion; to significantly increase the expressions of EMT-related genes and vimentin protein; while decreasing the expression of E-cadherin protein; in contrast to their normal counterparts (15). In addition, CAF-educated monocytes increased the motility of breast cancer cells. Linde et al. recently reported that CD206+ macrophages downregulated E-cadherin junctions in breast cancer cells (335), which in turn may promote EMT. In light of these findings, CAFs seem to facilitate EMT, invasion and metastasis (100); through promoting M2-polarized macrophages. It was also reported that CAF-educated monocytes increased breast cancer cell proliferation in vitro (15). Furthermore, the infiltration of CD163+ TAMs seemed to be associated with specific molecular subtypes of breast cancer; since their numbers were found to be the highest in triple negative, and the lowest in ER+PR+ breast cancer samples. Recent findings suggest that the tumor stroma also participates in the tumor resistance to therapy (23, 336). Ireland et al. demonstrated that TAMs and CAFs are major sources of IGF-1 and IGF-2 in breast and pancreatic tumors; and IGF signaling is implicated in the breast and pancreatic tumor resistance to paclitaxel and gemcitabine, respectively (337, 338).
The Effects on Immune Evasion
CAFs may be implicated in the establishment of an immunosuppressive milieu via inducing immunosuppressive macrophages. Takahashi et al. analyzed the effects of CAFs on functional polarization of TAMs in oral squamous cell carcinoma and found that infiltration of CAFs was associated with the numbers of CD68+ CD163+ macrophages. In addition, they demonstrated that CAFs promoted the accumulation of pro-tumoral macrophages, resulting in an immunosuppressive milieu (248). CAF-supernatant-treated CD14+ cells (CAF-educated cells) resembled pro-tumoral macrophages and these CAF-educated cells significantly suppressed both CD4+ and CD8+ T cell proliferations. Moreover, the suppression of T cell proliferation was mediated via the production of TGF-β, IL-10, and arginase I (248). CAF derived IL-33 was reported to cause TAMs to undergo the M1 to M2 transition (339). Genomic profiling of metastasis related genes revealed that these IL-33 stimulated TAMs demonstrated a significant increase in MMP9 expression. Moreover, genetic deletion of IL-33 or MMP9 blocked metastasis in mouse and human fibroblast rich pancreatic cancers (339). Indeed, these results may shed light on the TAM-CAF committed cancer metastasis as well as underlining the importance of targeting the TAM-CAF axis in terms of a potential cancer treatment. In a recent article, Shani et al. demonstrated that IL-33 was upregulated in metastasis associated fibroblasts in mouse models of spontaneous breast cancer metastasis and in patients with breast cancer with lung metastasis (340). IL-33 upregulation promoted type 2 inflammation in the metastatic microenvironment and took part in the recruitment of various cells including inflammatory monocytes to lung metastases. in vivo IL-33 targeting caused inhibition of lung metastasis and significant reduction of immune cell recruitment (340). In a recent study, Sun et al. reported that CXCL3 was highly upregulated in IL-33-stimulated macrophages and the receptor of CXCL3 (CXCR2) was mostly expressed by CAFs (341). In addition, CXCL3-CXCR2 signaling upregulated α-SMA and activation of CXCR2 by CXCL3 caused CAF-to-myoCAF transition (341). These findings underline the importance of the interaction between CAFs and immune cells in terms of generating a favorable inflammatory niche in breast cancer lung metastasis.
Adenosine, which is an immunosuppressive metabolite produced at high levels in the tumor microenvironment, is implicated in immune escape as well as in tumor growth and metastasis (342). Various cell types can generate adenosine in the tumor microenvironment such as CAFs as well as tumor cells (267, 268, 343–345). Adenosine can exert immunosuppressive effects on/via several immune cells including T cells, NK cells, dendritic cells (DCs) and MDSCs as well as macrophages (268, 346). In the extracellular space, adenosine exerts a plethora of immunomodulatory effects. It alters mononuclear phagocyte functions via 4 G-protein coupled cell membrane receptors (A1, A2A, A2B, and A3) (347). Adenosine downregulates classical macrophage activation majorly via A2A receptors, while upregulating alternative macrophage activation via A2B receptors (347). Hence, adenosine affects the process of tumorigenesis by regulating mononuclear phagocyte functions and extracellular adenosine generation is one of various immunosuppressive mechanisms that hinder anti-tumor immune responses in part due to the interactions between CAFs and TAMs (268).
Moreover, the interplay between these two cells types may also demonstrate critical effects on adaptive immune responses at various levels. One of such immunomodulatory effects on adaptive immunity is associated with the disruption of antigen presentation. CAF derived TGF-β, IL-6, tryptophan 2,3-dioxygenase (TDO2), indoleamine-2,3-dioxygenase (IDO) and VEGF can impede the function and maturation of DCs; resulting in the inhibition of T cell activation and causing T cell anergy (134, 234, 348–352). IL-6 signaling may also redirect monocyte differentiation into macrophages rather than DCs (24, 134, 353). In addition, Yavuz et al. demonstrated that CAF-educated monocytes significantly suppressed T cell proliferation in contrast to control monocytes (15). CAFs were previously reported to directly inhibit T lymphocyte proliferation (232, 354). In addition to their direct effects, CAFs also indirectly suppress immune responses via polarizing the monocytes to M2-like TAMs. Furthermore, CAF-educated M1 macrophages were also demonstrated to increase secretion of IL-10 and decrease secretion of IL-12 in addition to increasing the expression of M2 markers (15). Moreover, CAFs can induce a PD-1+ TAM phenotype by themselves, even without the presence of cancer cells (15). Nearly all PD-1+ TAMs express an M2-like surface profile (355). Thus, PD-1 axis may be pivotal in CAF mediated immune suppression in vivo in various types of cancer. Furthermore, CAF-educated monocytes demonstrated enhanced expressions of CD206 and CD163 (15). Given the fact that CD206 has anti-inflammatory effects, CAF mediated induction of higher expression of CD206 on monocytes might also represent another indirect mechanism of immune suppression by CAFs. Moreover, CAFs were shown to induce down regulation of major histocompatibility complex (MHC) class II expression on monocytes. This finding might demonstrate a mechanism of immune escape through decreased antigen presentation, in addition to confirming that CAFs are not implicated in any types of “good inflammatory response” (356).
CAFs and TAMs were also reported to localize in colorectal carcinomas where they promote the progression of tumor and suppress immune responses. Zhang et al. demonstrated that the recruitment of TAMs was associated with vascular cell adhesion molecule 1 (VCAM-1) expression (272). CAFs are able to induce monocyte adhesion by up-regulating VCAM-1 expression in cancer cells. In addition, CAFs are also able to promote the polarization to M2 macrophages, which take part in suppression of NK cell functions. Thus, CAF-TAM interplay in the tumor microenvironment seems to also have the potential to regulate NK cell functions (272).
Discussion and Concluding Remarks
Several issues still persist in terms of the effects of CAFs on tumor progression. One of the most important problems that needs to be solved is indeed the origins of CAFs in different cancer types. Given the heterogeneity of CAFs, subpopulations of these cells should also be thoroughly investigated in terms of their specific effects on tumor progression, immune suppression and tumor microenvironment organization. In fact, presence of different fibroblast subpopulations and the effect of the cancer type probably influence the net outcome (135). A solution for the issues concerning the origins and heterogeneity of CAFs could be profiling CAF populations at the single cell level in different cancer settings as well as utilizing multiplex immunofluorescence or brightfield immunohistochemistry (357–360). Indeed, Muhl et al. compared single-cell transcriptional profiles of fibroblasts among murine organs (i.e. heart, skeletal muscle, intestine and bladder) in a recent study in order to investigate the heterogeneity of fibroblasts within and between organs (21). As one would expect, they reported significant inter- and intra-organ heterogeneity amongst fibroblasts. In another study, Bartoschek et al. reported functionally and spatially distinct subclasses of CAFs by single cell RNA sequencing in a genetically engineered mouse model (38). They proposed that spatial separation of the CAF subclasses could be attributed to different origins. Such studies will help us understand the relevance of fibroblast heterogeneity in different organs and tissues. Novel studies which will focus on CAFs in addition to fibroblasts, especially in human tissues, will most likely shed new light on the importance of these quirky cells. Moreover, longitudinal studies are required in order to decipher the functions and heterogeneity of CAFs over time, yielding spatiotemporal resolution. Moreover, investigation of primary and metastatic tumors at single cell resolution could help in understanding the role of local and recruited stromal cell populations in carcinogenesis.
The current literature suggests that the collaboration of CAFs with TAMs plays a crucial role in tumor progression. It seems clear that CAFs and TAMs are pivotal components of the tumor microenvironment and they may prove to be potential valuable therapeutic targets. Given the reciprocal relationship of CAFs with TAMs in the tumor microenvironment in terms of promoting tumor growth, therapeutic strategies aimed at altering polarization of TAMs or reeducating M2 macrophages should also take into account the synergistic effects of CAFs in order to stimulate effective anti-tumor immune responses. Hence, combined targeting of CAFs and TAMs can be regarded as an option to consider. A better understanding of the mechanisms as well as signaling pathways implicated in the tripartite interplay of CAFs, TAMs and tumor cells will help decipher key concepts of tumor immunology. The fact that there are significant differences among monocytes/macrophages from distinct tumors and the existence of diverse TAM subsets should also be borne in mind (361). Utilization of novel techniques (e.g. multiplexed immunohistochemistry, mass cytometry by time-of-flight, single-cell RNA-seq, spatial transcriptomics and systems biology approaches) may indeed assist in better understanding the diversity of TAMs (228, 361).
Future in vivo studies will further provide us with novel mechanistic insights and distinct implications. Developing model systems which better simulate all the elements of the tumor microenvironment is crucial in order to reach such goals. These systems will potentially allow for the demonstration of the effects of cell-to-cell contact in addition to the cross-talk mediated though soluble factors. An approach utilizing a severe combined immunodeficiency (SCID) mice setting might help to better determine whether human monocytes co-injected s.c. with cancer cells display a potent pro-tumoral effect if preconditioned with CAFs in comparison to their normal counterparts. Given the recent exciting findings concerning the utilization of TAMs for cancer therapy (362–364), harnessing the power of innate immune cells (e.g. via CAR macrophages) holds great promise for the development of novel and attractive immunotherapeutic modalities. It is evident that CAFs are able to sculpt the tumor microenvironment via TAMs. Therefore, therapeutic strategies which antagonize the CAF-mediated immunosuppressive microenvironment may prove to be efficient in terms of increasing the effectiveness of conventional therapies and immunotherapies against cancer. In addition, a more thorough understanding of the tri-directional interactions of CAFs, TAMs and cancer cells in terms of tumor progression will pave the way for the identification of novel theranostic cues in order to better target the crucial mechanisms of carcinogenesis.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication. Figures 1 , 3 – 10 were created with BioRender.com.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AcknowLedgments
“There is remedy for all things except death”. Dedicated to the memory of my beloved Dad Nejat Sahin GUNAYDIN, MD (1953–2021).
Glossary
- CAF
Cancer associated fibroblast
- CAR
Chimeric antigen receptor
- CCL
C-C motif chemokine ligand
- CCR
C-C motif chemokine receptor
- CD
Cluster of differentiation
- CDKN1B
Cyclin dependent kinase inhibitor 1B
- Chi3L1
Chitinase 3-like 1
- CSF-1
Colony stimulating factor 1
- CTCs
Circulating tumor cells
- CTLA-4
Cytotoxic T-lymphocyte associated protein 4
- CXCL
C-X-C motif chemokine ligand
- CXCR
C-X-C motif chemokine receptor
- DC
Dendritic cell
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- EGFR
Epidermal growth factor receptor
- EMT
Epithelial-mesenchymal transition
- EndMT
Endothelial to mesenchymal transition
- ER
Estrogen receptor
- FAP
Fibroblast activation protein
- FGF
Fibroblast growth factor
- FSP1
Fibroblast-specific protein 1
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- GPR77
G protein-coupled receptor 77
- HGF
Hepatocyte growth factor
- HLA
Human leukocyte antigen
- IDO
Indoleamine-2,3-dioxygenase
- IFNγ
Interferon gamma
- IGF
Insulin-like growth factor
- IL
Interleukin
- ING5
Inhibitor of growth family member 5
- iPSCs
Induced pluripotent stem cells
- LPS
Lipopolysaccharide
- MAM
Metastasis associated macrophage
- MCP-1
Monocyte chemotactic protein-1
- M-CSF
Macrophage colony-stimulating factor
- MDSC
Myeloid-derived suppressor cell
- MHC
Major histocompatibility complex
- MMT
Mesenchymal–mesenchymal transition
- Mrc1
Mannose receptor C-type 1
- MSC
Mesenchymal stem cell
- NF
Normal fibroblast
- NG2
Neuron-glial antigen-2
- NK
Natural killer
- PD-1
Programmed cell death protein 1
- PDGF
Platelet-derived growth factor
- PDGFR
Platelet-derived growth factor receptor
- PD-L1
Programmed cell death 1 ligand 1
- PD-L2
Programmed cell death 1 ligand 2
- PR
Progesterone receptor
- SCID
Severe combined immunodeficiency
- SDF-1
Stromal derived factor 1
- SMA
Smooth muscle actin
- STAT3
Signal transducer and activator of transcription 3
- TAMs
Tumor associated macrophage
- TCGA
The Cancer Genome Atlas
- TDO2
Tryptophan 2,3-dioxygenase
- TGF-β
Transforming growth factor-β;
- TH
T helper cell
- TLR
Toll-like receptor
- TNF
Tumor necrosis factor
- Treg
Regulatory T cell
- VCAM-1
Vascular cell adhesion molecule 1
- VEGF
Vascular endothelial growth factor
References
- 1. Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular Changes Involved in Conversion of Normal to Malignant Breast: Importance of the Stromal Reaction. Physiol Rev (1996) 76(1):69–125. 10.1152/physrev.1996.76.1.69 [DOI] [PubMed] [Google Scholar]
- 2. Quail DF, Joyce JA. Microenvironmental Regulation of Tumor Progression and Metastasis. Nat Med (2013) 19(11):1423–37. 10.1038/nm.3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joyce JA, Fearon DT. T Cell Exclusion, Immune Privilege, and the Tumor Microenvironment. Science (2015) 348(6230):74–80. 10.1126/science.aaa6204 [DOI] [PubMed] [Google Scholar]
- 4. Xing S, Zheng X, Zeng T, Zeng MS, Zhong Q, Cao YS, et al. Chitinase 3-Like 1 Secreted by Peritumoral Macrophages in Esophageal Squamous Cell Carcinoma Is a Favorable Prognostic Factor for Survival. World J Gastroenterol (2017) 23(43):7693–704. 10.3748/wjg.v23.i43.7693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balkwill F, Mantovani A. Inflammation and Cancer: Back to Vircho? Lancet (2001) 357(9255):539–45. 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 6. Barsky SH, Green WR, Grotendorst GR, Liotta LA. Desmoplastic Breast Carcinoma as a Source of Human Myofibroblasts. Am J Pathol (1984) 115(3):329–33. [PMC free article] [PubMed] [Google Scholar]
- 7. Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of Fibroblast Heterogeneity in the Tumor Microenvironment. Cancer Biol Ther (2006) 5(12):1640–6. 10.4161/cbt.5.12.3354 [DOI] [PubMed] [Google Scholar]
- 8. Kalluri R, Zeisberg M. Fibroblasts in Cancer. Nat Rev Cancer (2006) 6(5):392–401. 10.1038/nrc1877 [DOI] [PubMed] [Google Scholar]
- 9. Ayala G, Tuxhorn JA, Wheeler TM, Frolov A, Scardino PT, Ohori M, et al. Reactive Stroma as a Predictor of Biochemical-Free Recurrence in Prostate Cancer. Clin Cancer Res (2003) 9(13):4792–801. [PubMed] [Google Scholar]
- 10. DeClerck YA. Desmoplasia: A Response or a Niche? Cancer Discov (2012) 2(9):772–4. 10.1158/2159-8290.CD-12-0348 [DOI] [PubMed] [Google Scholar]
- 11. Liu H, Ma Q, Xu Q, Lei J, Li X, Wang Z, et al. Therapeutic Potential of Perineural Invasion, Hypoxia and Desmoplasia in Pancreatic Cancer. Curr Pharm Des (2012) 18(17):2395–403. 10.2174/13816128112092395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dvorak HF. Tumors: Wounds That do Not Heal. Similarities Between Tumor Stroma Generation and Wound Healing. N Engl J Med (1986) 315(26):1650–9. 10.1056/NEJM198612253152606 [DOI] [PubMed] [Google Scholar]
- 13. De Wever O, Demetter P, Mareel M, Bracke M. Stromal Myofibroblasts are Drivers of Invasive Cancer Growth. Int J Cancer (2008) 123(10):2229–38. 10.1002/ijc.23925 [DOI] [PubMed] [Google Scholar]
- 14. Ostman A, Augsten M. Cancer-Associated Fibroblasts and Tumor Growth–Bystanders Turning Into Key Players. Curr Opin Genet Dev (2009) 19(1):67–73. 10.1016/j.gde.2009.01.003 [DOI] [PubMed] [Google Scholar]
- 15. Gok Yavuz B, Gunaydin G, Gedik ME, Kosemehmetoglu K, Karakoc D, Ozgur F, et al. Cancer Associated Fibroblasts Sculpt Tumour Microenvironment by Recruiting Monocytes and Inducing Immunosuppressive PD-1(+) TAMs. Sci Rep (2019) 9(1):3172. 10.1038/s41598-019-39553-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gok Yavuz B, Gunaydin G, Kosemehmetoglu K, Karakoc D, Ozgur F, Guc D. The Effects of Cancer-Associated Fibroblasts Obtained From Atypical Ductal Hyperplasia on Anti-Tumor Immune Responses. Breast J (2018) 24(6):1099–101. 10.1111/tbj.13139 [DOI] [PubMed] [Google Scholar]
- 17. Paget S. The Distribution of Secondary Growths in Cancer of the Breast. Lancet (1889) 133(3421):571–3. 10.1016/S0140-6736(00)49915-0 [DOI] [PubMed] [Google Scholar]
- 18. Langley RR, Fidler IJ. The Seed and Soil Hypothesis Revisited–The Role of Tumor-Stroma Interactions in Metastasis to Different Organs. Int J Cancer (2011) 128(11):2527–35. 10.1002/ijc.26031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Virchow R. Die Cellularpathologie in Ihrer Begruendung Auf Physiologische Und Pathologische Gewebelehr. Berlin: August Hirschwald; (1858). [Google Scholar]
- 20. Duval M. Atlas D’embryologie. France: Masson; (1889). 10.5962/bhl.title.51975 [DOI] [Google Scholar]
- 21. Muhl L, Genove G, Leptidis S, Liu J, He L, Mocci G, et al. Single-Cell Analysis Uncovers Fibroblast Heterogeneity and Criteria for Fibroblast and Mural Cell Identification and Discrimination. Nat Commun (2020) 11(1):3953. 10.1038/s41467-020-18511-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fernandes IR, Russo FB, Pignatari GC, Evangelinellis MM, Tavolari S, Muotri AR, et al. Fibroblast Sources: Where Can We Get Them? Cytotechnology (2016) 68(2):223–8. 10.1007/s10616-014-9771-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ireland LV, Mielgo A. Macrophages and Fibroblasts, Key Players in Cancer Chemoresistance. Front Cell Dev Biol (2018) 6:131. 10.3389/fcell.2018.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kalluri R. The Biology and Function of Fibroblasts in Cancer. Nat Rev Cancer (2016) 16(9):582–98. 10.1038/nrc.2016.73 [DOI] [PubMed] [Google Scholar]
- 25. Madar S, Goldstein I, Rotter V. ’Cancer Associated Fibroblasts’–More Than Meets the Eye. Trends Mol Med (2013) 19(8):447–53. 10.1016/j.molmed.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 26. Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, et al. Mesenchymal Stem Cell Transition to Tumor-Associated Fibroblasts Contributes to Fibrovascular Network Expansion and Tumor Progression. PloS One (2009) 4(4):e4992. 10.1371/journal.pone.0004992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Potenta S, Zeisberg E, Kalluri R. The Role of Endothelial-to-Mesenchymal Transition in Cancer Progression. Br J Cancer (2008) 99(9):1375–9. 10.1038/sj.bjc.6604662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kalluri R, Weinberg RA. The Basics of Epithelial-Mesenchymal Transition. J Clin Invest (2009) 119(6):1420–8. 10.1172/JCI39104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers (Basel) (2015) 7(4):2443–58. 10.3390/cancers7040902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bochet L, Lehuede C, Dauvillier S, Wang YY, Dirat B, Laurent V, et al. Adipocyte-Derived Fibroblasts Promote Tumor Progression and Contribute to the Desmoplastic Reaction in Breast Cancer. Cancer Res (2013) 73(18):5657–68. 10.1158/0008-5472.CAN-13-0530 [DOI] [PubMed] [Google Scholar]
- 31. Zhang Y, Daquinag AC, Amaya-Manzanares F, Sirin O, Tseng C, Kolonin MG. Stromal Progenitor Cells From Endogenous Adipose Tissue Contribute to Pericytes and Adipocytes That Populate the Tumor Microenvironment. Cancer Res (2012) 72(20):5198–208. 10.1158/0008-5472.CAN-12-0294 [DOI] [PubMed] [Google Scholar]
- 32. Jotzu C, Alt E, Welte G, Li J, Hennessy BT, Devarajan E, et al. Adipose Tissue Derived Stem Cells Differentiate Into Carcinoma-Associated Fibroblast-Like Cells Under the Influence of Tumor Derived Factors. Cell Oncol (Dordr) (2011) 34(1):55–67. 10.1007/s13402-011-0012-1 [DOI] [PubMed] [Google Scholar]
- 33. Motrescu ER, Rio MC. Cancer Cells, Adipocytes and Matrix Metalloproteinase 11: A Vicious Tumor Progression Cycle. Biol Chem (2008) 389(8):1037–41. 10.1515/BC.2008.110 [DOI] [PubMed] [Google Scholar]
- 34. Tan J, Buache E, Chenard MP, Dali-Youcef N, Rio MC. Adipocyte is a non-Trivial, Dynamic Partner of Breast Cancer Cells. Int J Dev Biol (2011) 55(7-9):851–9. 10.1387/ijdb.113365jt [DOI] [PubMed] [Google Scholar]
- 35. Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of Endothelial to Mesenchymal Transition as a Source for Carcinoma-Associated Fibroblasts. Cancer Res (2007) 67(21):10123–8. 10.1158/0008-5472.CAN-07-3127 [DOI] [PubMed] [Google Scholar]
- 36. Lin F, Wang N, Zhang TC. The Role of Endothelial-Mesenchymal Transition in Development and Pathological Process. IUBMB Life (2012) 64(9):717–23. 10.1002/iub.1059 [DOI] [PubMed] [Google Scholar]
- 37. Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, et al. Endothelial-To-Mesenchymal Transition Contributes to Cardiac Fibrosis. Nat Med (2007) 13(8):952–61. 10.1038/nm1613 [DOI] [PubMed] [Google Scholar]
- 38. Bartoschek M, Oskolkov N, Bocci M, Lovrot J, Larsson C, Sommarin M, et al. Spatially and Functionally Distinct Subclasses of Breast Cancer-Associated Fibroblasts Revealed by Single Cell RNA Sequencing. Nat Commun (2018) 9(1):5150. 10.1038/s41467-018-07582-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage Tracing and Genetic Ablation of ADAM12(+) Perivascular Cells Identify a Major Source of Profibrotic Cells During Acute Tissue Injury. Nat Med (2012) 18(8):1262–70. 10.1038/nm.2848 [DOI] [PubMed] [Google Scholar]
- 40. Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A Pericyte Origin of Spinal Cord Scar Tissue. Science (2011) 333(6039):238–42. 10.1126/science.1203165 [DOI] [PubMed] [Google Scholar]
- 41. Wikstrom P, Marusic J, Stattin P, Bergh A. Low Stroma Androgen Receptor Level in Normal and Tumor Prostate Tissue Is Related to Poor Outcome in Prostate Cancer Patients. Prostate (2009) 69(8):799–809. 10.1002/pros.20927 [DOI] [PubMed] [Google Scholar]
- 42. Kurashige M, Kohara M, Ohshima K, Tahara S, Hori Y, Nojima S, et al. Origin of Cancer-Associated Fibroblasts and Tumor-Associated Macrophages in Humans After Sex-Mismatched Bone Marrow Transplantation. Commun Biol (2018) 1:131. 10.1038/s42003-018-0137-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gallagher PG, Bao Y, Prorock A, Zigrino P, Nischt R, Politi V, et al. Gene Expression Profiling Reveals Cross-Talk Between Melanoma and Fibroblasts: Implications for Host-Tumor Interactions in Metastasis. Cancer Res (2005) 65(10):4134–46. 10.1158/0008-5472.CAN-04-0415 [DOI] [PubMed] [Google Scholar]
- 44. Buess M, Nuyten DS, Hastie T, Nielsen T, Pesich R, Brown PO. Characterization of Heterotypic Interaction Effects In Vitro to Deconvolute Global Gene Expression Profiles in Cancer. Genome Biol (2007) 8(9):R191. 10.1186/gb-2007-8-9-r191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Denk PO, Hoppe J, Hoppe V, Knorr M. Effect of Growth Factors on the Activation of Human Tenon’s Capsule Fibroblasts. Curr Eye Res (2003) 27(1):35–44. 10.1076/ceyr.27.2.35.15456 [DOI] [PubMed] [Google Scholar]
- 46. Ronnov-Jessen L, Petersen OW. Induction of Alpha-Smooth Muscle Actin by Transforming Growth Factor-Beta 1 in Quiescent Human Breast Gland Fibroblasts. Implications for Myofibroblast Generation in Breast Neoplasia. Lab Invest (1993) 68(6):696–707. [PubMed] [Google Scholar]
- 47. Toullec A, Gerald D, Despouy G, Bourachot B, Cardon M, Lefort S, et al. Oxidative Stress Promotes Myofibroblast Differentiation and Tumour Spreading. EMBO Mol Med (2010) 2(6):211–30. 10.1002/emmm.201000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Helms EJ, Berry MW, Chaw RC, DuFort CC, Sun D, Onate MK, et al. Mesenchymal Lineage Heterogeneity Underlies Non-Redundant Functions of Pancreatic Cancer-Associated Fibroblast. bioRxiv (2021). 2021.05.01.442252. 10.1101/2021.05.01.442252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tao L, Huang G, Song H, Chen Y, Chen L. Cancer Associated Fibroblasts: An Essential Role in the Tumor Microenvironment. Oncol Lett (2017) 14(3):2611–20. 10.3892/ol.2017.6497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Radisky DC, Kenny PA, Bissell MJ. Fibrosis and Cancer: Do Myofibroblasts Come Also From Epithelial Cells via EM? J Cell Biochem (2007) 101(4):830–9. 10.1002/jcb.21186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, et al. Rac1b and Reactive Oxygen Species Mediate MMP-3-Induced EMT and Genomic Instability. Nature (2005) 436(7047):123–7. 10.1038/nature03688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stockinger A, Eger A, Wolf J, Beug H, Foisner R. E-Cadherin Regulates Cell Growth by Modulating Proliferation-Dependent Beta-Catenin Transcriptional Activity. J Cell Biol (2001) 154(6):1185–96. 10.1083/jcb.200104036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Biffi G, Tuveson DA. Deciphering Cancer Fibroblasts. J Exp Med (2018) 215(12):2967–8. 10.1084/jem.20182069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mizutani Y, Kobayashi H, Iida T, Asai N, Masamune A, Hara A, et al. Meflin-Positive Cancer-Associated Fibroblasts Inhibit Pancreatic Carcinogenesis. Cancer Res (2019) 79(20):5367–81. 10.1158/0008-5472.CAN-19-0454 [DOI] [PubMed] [Google Scholar]
- 55. Raz Y, Cohen N, Shani O, Bell RE, Novitskiy SV, Abramovitz L, et al. Bone Marrow-Derived Fibroblasts Are a Functionally Distinct Stromal Cell Population in Breast Cancer. J Exp Med (2018) 215(12):3075–93. 10.1084/jem.20180818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Null JL, Kim DJ, Zhang Y, McCann JV, Barker TH, Dudley AC. Abstract PO051: Fibroblast Lineage Tracing in Metastatic and Pre-Metastatic Microenvironments. Cancer Res (2021) 81(5 Supplement):PO051–PO. 10.1158/1538-7445.TME21-PO051 [DOI] [Google Scholar]
- 57. Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, et al. Diversity, Topographic Differentiation, and Positional Memory in Human Fibroblasts. Proc Natl Acad Sci USA (2002) 99(20):12877–82. 10.1073/pnas.162488599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Raab S, Klingenstein M, Liebau S, Linta L. A Comparative View on Human Somatic Cell Sources for iPSC Generation. Stem Cells Int (2014) 2014:768391. 10.1155/2014/768391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ubil E, Duan J, Pillai IC, Rosa-Garrido M, Wu Y, Bargiacchi F, et al. Mesenchymal-Endothelial Transition Contributes to Cardiac Neovascularization. Nature (2014) 514(7524):585–90. 10.1038/nature13839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Miyake T, Kalluri R. Cardiac Biology: Cell Plasticity Helps Hearts to Repair. Nature (2014) 514(7524):575–6. 10.1038/nature13928 [DOI] [PubMed] [Google Scholar]
- 61. Lorenz K, Sicker M, Schmelzer E, Rupf T, Salvetter J, Schulz-Siegmund M, et al. Multilineage Differentiation Potential of Human Dermal Skin-Derived Fibroblasts. Exp Dermatol (2008) 17(11):925–32. 10.1111/j.1600-0625.2008.00724.x [DOI] [PubMed] [Google Scholar]
- 62. Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, et al. Bone Marrow-Derived Myofibroblasts Contribute to the Mesenchymal Stem Cell Niche and Promote Tumor Growth. Cancer Cell (2011) 19(2):257–72. 10.1016/j.ccr.2011.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. LeBleu VS, Taduri G, O’Connell J, Teng Y, Cooke VG, Woda C, et al. Origin and Function of Myofibroblasts in Kidney Fibrosis. Nat Med (2013) 19(8):1047–53. 10.1038/nm.3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The Myofibroblast: One Function, Multiple Origins. Am J Pathol (2007) 170(6):1807–16. 10.2353/ajpath.2007.070112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Anderberg C, Pietras K. On the Origin of Cancer-Associated Fibroblasts. Cell Cycle (2009) 8(10):1461–2. 10.4161/cc.8.10.8557 [DOI] [PubMed] [Google Scholar]
- 66. McAnulty RJ. Fibroblasts and Myofibroblasts: Their Source, Function and Role in Disease. Int J Biochem Cell Biol (2007) 39(4):666–71. 10.1016/j.biocel.2006.11.005 [DOI] [PubMed] [Google Scholar]
- 67. Neuzillet C, Tijeras-Raballand A, Ragulan C, Cros J, Patil Y, Martinet M, et al. Inter- and Intra-Tumoural Heterogeneity in Cancer-Associated Fibroblasts of Human Pancreatic Ductal Adenocarcinoma. J Pathol (2019) 248(1):51–65. 10.1002/path.5224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblast. Cancer Discov (2019) 9(8):1102–23. 10.1158/2159-8290.CD-19-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Croft AP, Campos J, Jansen K, Turner JD, Marshall J, Attar M, et al. Distinct Fibroblast Subsets Drive Inflammation and Damage in Arthritis. Nature (2019) 570(7760):246–51. 10.1038/s41586-019-1263-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, et al. A Framework for Advancing Our Understanding of Cancer-Associated Fibroblasts. Nat Rev Cancer (2020) 20(3):174–86. 10.1038/s41568-019-0238-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Irvine AF, Waise S, Green EW, Stuart B, Thomas GJ. Characterising Cancer-Associated Fibroblast Heterogeneity in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Sci Rep (2021) 11(1):3727. 10.1038/s41598-021-81796-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Han C, Liu T, Yin R. Biomarkers for Cancer-Associated Fibroblasts. Biomark Res (2020) 8(1):64. 10.1186/s40364-020-00245-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Orimo A, Weinberg RA. Heterogeneity of Stromal Fibroblasts in Tumors. Cancer Biol Ther (2007) 6(4):618–9. 10.4161/cbt.6.4.4255 [DOI] [PubMed] [Google Scholar]
- 74. Kanzaki R, Pietras K. Heterogeneity of Cancer-Associated Fibroblasts: Opportunities for Precision Medicine. Cancer Sci (2020) 111(8):2708–17. 10.1111/cas.14537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen X, Song E. Turning Foes to Friends: Targeting Cancer-Associated Fibroblasts. Nat Rev Drug Discov (2019) 18(2):99–115. 10.1038/s41573-018-0004-1 [DOI] [PubMed] [Google Scholar]
- 76. Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, et al. Identification and Characterization of a Fibroblast Marker: FSP1. J Cell Biol (1995) 130(2):393–405. 10.1083/jcb.130.2.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gabbiani G, Ryan GB, Majne G. Presence of Modified Fibroblasts in Granulation Tissue and Their Possible Role in Wound Contraction. Experientia (1971) 27(5):549–50. 10.1007/BF02147594 [DOI] [PubMed] [Google Scholar]
- 78. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and Mechano-Regulation of Connective Tissue Remodelling. Nat Rev Mol Cell Biol (2002) 3(5):349–63. 10.1038/nrm809 [DOI] [PubMed] [Google Scholar]
- 79. Rasanen K, Vaheri A. Activation of Fibroblasts in Cancer Stroma. Exp Cell Res (2010) 316(17):2713–22. 10.1016/j.yexcr.2010.04.032 [DOI] [PubMed] [Google Scholar]
- 80. Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis Mediates the Decrease in Cellularity During the Transition Between Granulation Tissue and Scar. Am J Pathol (1995) 146(1):56–66. [PMC free article] [PubMed] [Google Scholar]
- 81. Castor CW, Wilson SM, Heiss PR, Seidman JC. Activation of Lung Connective Tissue Cells In Vitro. Am Rev Respir Dis (1979) 120(1):101–6. 10.1164/arrd.1979.120.1.101 [DOI] [PubMed] [Google Scholar]
- 82. Cirri P, Chiarugi P. Cancer Associated Fibroblasts: The Dark Side of the Coin. Am J Cancer Res (2011) 1(4):482–97. [PMC free article] [PubMed] [Google Scholar]
- 83. Grande MT, Lopez-Novoa JM. Fibroblast Activation and Myofibroblast Generation in Obstructive Nephropathy. Nat Rev Nephrol (2009) 5(6):319–28. 10.1038/nrneph.2009.74 [DOI] [PubMed] [Google Scholar]
- 84. Pietras K, Ostman A. Hallmarks of Cancer: Interactions With the Tumor Stroma. Exp Cell Res (2010) 316(8):1324–31. 10.1016/j.yexcr.2010.02.045 [DOI] [PubMed] [Google Scholar]
- 85. Mueller MM, Fusenig NE. Friends or Foes - Bipolar Effects of the Tumour Stroma in Cancer. Nat Rev Cancer (2004) 4(11):839–49. 10.1038/nrc1477 [DOI] [PubMed] [Google Scholar]
- 86. Schauer IG, Sood AK, Mok S, Liu J. Cancer-Associated Fibroblasts and Their Putative Role in Potentiating the Initiation and Development of Epithelial Ovarian Cancer. Neoplasia (2011) 13(5):393–405. 10.1593/neo.101720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ishii G, Ochiai A, Neri S. Phenotypic and Functional Heterogeneity of Cancer-Associated Fibroblast Within the Tumor Microenvironment. Adv Drug Delivery Rev (2016) 99(Pt B):186–96. 10.1016/j.addr.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 88. Marsh T, Pietras K, McAllister SS. Fibroblasts as Architects of Cancer Pathogenesis. Biochim Biophys Acta (2013) 1832(7):1070–8. 10.1016/j.bbadis.2012.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. LeBleu VS, Kalluri R. A Peek Into Cancer-Associated Fibroblasts: Origins, Functions and Translational Impact. Dis Model Mech (2018) 11(4). 10.1242/dmm.029447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Folkman J. Angiogenesis in Cancer, Vascular, Rheumatoid and Other Disease. Nat Med (1995) 1(1):27–31. 10.1038/nm0195-27 [DOI] [PubMed] [Google Scholar]
- 91. Bergers G, Benjamin LE. Tumorigenesis and the Angiogenic Switch. Nat Rev Cancer (2003) 3(6):401–10. 10.1038/nrc1093 [DOI] [PubMed] [Google Scholar]
- 92. Coussens LM, Werb Z. Inflammation and Cancer. Nature (2002) 420(6917):860–7. 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gunaydin G, Dolen Y, Unver N, Kesikli SA, Guc D. The Effects of Cancer Associated Fibroblasts on Mechanisms of Immune Evasion in a Rat Chemical Mammary Carcinoma Model. Front Immunol (2013) 4. 10.3389/conf.fimmu.2013.02.01132 [DOI] [Google Scholar]
- 94. Mhaidly R, Mechta-Grigoriou F. Fibroblast Heterogeneity in Tumor Micro-Environment: Role in Immunosuppression and New Therapies. Semin Immunol (2020) 48:101417. 10.1016/j.smim.2020.101417 [DOI] [PubMed] [Google Scholar]
- 95. Theret N, Musso O, Turlin B, Lotrian D, Bioulac-Sage P, Campion JP, et al. Increased Extracellular Matrix Remodeling Is Associated With Tumor Progression in Human Hepatocellular Carcinomas. Hepatology (2001) 34(1):82–8. 10.1053/jhep.2001.25758 [DOI] [PubMed] [Google Scholar]
- 96. Biffi G, Tuveson DA. Diversity and Biology of Cancer-Associated Fibroblast. Physiol Rev (2021) 101(1):147–76. 10.1152/physrev.00048.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Fiori ME, Di Franco S, Villanova L, Bianca P, Stassi G, De Maria R. Cancer-Associated Fibroblasts as Abettors of Tumor Progression at the Crossroads of EMT and Therapy Resistance. Mol Cancer (2019) 18(1):70. 10.1186/s12943-019-0994-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, et al. Targeting CXCL12 From FAP-Expressing Carcinoma-Associated Fibroblasts Synergizes With Anti-PD-L1 Immunotherapy in Pancreatic Cancer. Proc Natl Acad Sci USA (2013) 110(50):20212–7. 10.1073/pnas.1320318110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wels J, Kaplan RN, Rafii S, Lyden D. Migratory Neighbors and Distant Invaders: Tumor-Associated Niche Cells. Genes Dev (2008) 22(5):559–74. 10.1101/gad.1636908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, Diamandis EP. Cancer-Associated Fibroblasts Drive the Progression of Metastasis Through Both Paracrine and Mechanical Pressure on Cancer Tissue. Mol Cancer Res (2012) 10(11):1403–18. 10.1158/1541-7786.MCR-12-0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pena C, Cespedes MV, Lindh MB, Kiflemariam S, Mezheyeuski A, Edqvist PH, et al. STC1 Expression by Cancer-Associated Fibroblasts Drives Metastasis of Colorectal Cancer. Cancer Res (2013) 73(4):1287–97. 10.1158/0008-5472.CAN-12-1875 [DOI] [PubMed] [Google Scholar]
- 102. Pelon F, Bourachot B, Kieffer Y, Magagna I, Mermet-Meillon F, Bonnet I, et al. Cancer-Associated Fibroblast Heterogeneity in Axillary Lymph Nodes Drives Metastases in Breast Cancer Through Complementary Mechanisms. Nat Commun (2020) 11(1):404. 10.1038/s41467-019-14134-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Bernard V, Semaan A, Huang J, San Lucas FA, Mulu FC, Stephens BM, et al. Single-Cell Transcriptomics of Pancreatic Cancer Precursors Demonstrates Epithelial and Microenvironmental Heterogeneity as an Early Event in Neoplastic Progression. Clin Cancer Res (2019) 25(7):2194–205. 10.1158/1078-0432.CCR-18-1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dominguez CX, Muller S, Keerthivasan S, Koeppen H, Hung J, Gierke S, et al. Single-Cell RNA Sequencing Reveals Stromal Evolution Into LRRC15(+) Myofibroblasts as a Determinant of Patient Response to Cancer Immunotherapy. Cancer Discov (2020) 10(2):232–53. 10.1158/2159-8290.CD-19-0644 [DOI] [PubMed] [Google Scholar]
- 105. Puram SV, Tirosh I, Parikh AS, Patel AP, Yizhak K, Gillespie S, et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell (2017) 171(7):1611–24 e24. 10.1016/j.cell.2017.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Shani O, Raz Y, Monteran L, Megides O, Shacham H, Cohen N, et al. Evolution of Metastases-Associated Fibroblasts in the Lung Microenvironment Is Driven by Stage-Specific Transcriptional Plasticity. bioRxiv (2020) 778936. 10.1101/778936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Duda DG, Duyverman AM, Kohno M, Snuderl M, Steller EJ, Fukumura D, et al. Malignant Cells Facilitate Lung Metastasis by Bringing Their Own Soil. Proc Natl Acad Sci USA (2010) 107(50):21677–82. 10.1073/pnas.1016234107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. O’Connell JT, Sugimoto H, Cooke VG, MacDonald BA, Mehta AI, LeBleu VS, et al. VEGF-A and Tenascin-C Produced by S100A4+ Stromal Cells Are Important for Metastatic Colonization. Proc Natl Acad Sci USA (2011) 108(38):16002–7. 10.1073/pnas.1109493108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Benedicto A, Romayor I, Arteta B. CXCR4 Receptor Blockage Reduces the Contribution of Tumor and Stromal Cells to the Metastatic Growth in the Liver. Oncol Rep (2018) 39(4):2022–30. 10.3892/or.2018.6254 [DOI] [PubMed] [Google Scholar]
- 110. Joyce JA, Pollard JW. Microenvironmental Regulation of Metastasis. Nat Rev Cancer (2009) 9(4):239–52. 10.1038/nrc2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Studebaker AW, Storci G, Werbeck JL, Sansone P, Sasser AK, Tavolari S, et al. Fibroblasts Isolated From Common Sites of Breast Cancer Metastasis Enhance Cancer Cell Growth Rates and Invasiveness in an Interleukin-6-Dependent Manner. Cancer Res (2008) 68(21):9087–95. 10.1158/0008-5472.CAN-08-0400 [DOI] [PubMed] [Google Scholar]
- 112. Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal Fibroblasts Present in Invasive Human Breast Carcinomas Promote Tumor Growth and Angiogenesis Through Elevated SDF-1/CXCL12 Secretion. Cell (2005) 121(3):335–48. 10.1016/j.cell.2005.02.034 [DOI] [PubMed] [Google Scholar]
- 113. Dimanche-Boitrel MT, Vakaet L, Jr., Pujuguet P, Chauffert B, Martin MS, Hammann A, et al. In Vivo and In Vitro Invasiveness of a Rat Colon-Cancer Cell Line Maintaining E-Cadherin Expression: An Enhancing Role of Tumor-Associated Myofibroblasts. Int J Cancer (1994) 56(4):512–21. 10.1002/ijc.2910560410 [DOI] [PubMed] [Google Scholar]
- 114. Shapcott M, Hewitt KJ, Rajpoot N. Deep Learning With Sampling in Colon Cancer Histology. Front Bioeng Biotechnol (2019) 7:52. 10.3389/fbioe.2019.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, et al. Fibroblast-Led Collective Invasion of Carcinoma Cells With Differing Roles for RhoGTPases in Leading and Following Cells. Nat Cell Biol (2007) 9(12):1392–400. 10.1038/ncb1658 [DOI] [PubMed] [Google Scholar]
- 116. Wei WF, Chen XJ, Liang LJ, Yu L, Wu XG, Zhou CF, et al. Periostin(+) Cancer-Associated Fibroblasts Promote Lymph Node Metastasis by Impairing the Lymphatic Endothelial Barriers in Cervical Squamous Cell Carcinoma. Mol Oncol (2021) 15(1):210–27. 10.1002/1878-0261.12837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Gonzalez-Gonzalez L, Alonso J. Periostin: A Matricellular Protein With Multiple Functions in Cancer Development and Progression. Front Oncol (2018) 8:225. 10.3389/fonc.2018.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hong L, Sun H, Lv X, Yang D, Zhang J, Shi Y. Expression of Periostin in the Serum of NSCLC and its Function on Proliferation and Migration of Human Lung Adenocarcinoma Cell Line (A549) In Vitro . Mol Biol Rep (2010) 37(5):2285–93. 10.1007/s11033-009-9721-1 [DOI] [PubMed] [Google Scholar]
- 119. Kalluri R. The Biology and Function of Exosomes in Cancer. J Clin Invest (2016) 126(4):1208–15. 10.1172/JCI81135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Su T, Zhang P, Zhao F, Zhang S. Exosomal MicroRNAs Mediating Crosstalk Between Cancer Cells With Cancer-Associated Fibroblasts and Tumor-Associated Macrophages in the Tumor Microenvironment. Front Oncol (2021) 11:631703. 10.3389/fonc.2021.631703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Dourado MR, Korvala J, Astrom P, De Oliveira CE, Cervigne NK, Mofatto LS, et al. Extracellular Vesicles Derived From Cancer-Associated Fibroblasts Induce the Migration and Invasion of Oral Squamous Cell Carcinoma. J Extracell Vesicles (2019) 8(1):1578525. 10.1080/20013078.2019.1578525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sun LP, Xu K, Cui J, Yuan DY, Zou B, Li J, et al. Cancerassociated Fibroblastderived Exosomal Mir3825p Promotes the Migration and Invasion of Oral Squamous Cell Carcinoma. Oncol Rep (2019) 42(4):1319–28. 10.3892/or.2019.7255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Qin X, Guo H, Wang X, Zhu X, Yan M, Wang X, et al. Exosomal miR-196a Derived From Cancer-Associated Fibroblasts Confers Cisplatin Resistance in Head and Neck Cancer Through Targeting CDKN1B and ING5. Genome Biol (2019) 20(1):12. 10.1186/s13059-018-1604-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wang X, Qin X, Yan M, Shi J, Xu Q, Li Z, et al. Loss of Exosomal miR-3188 in Cancer-Associated Fibroblasts Contributes to HNC Progression. J Exp Clin Cancer Res (2019) 38(1):151. 10.1186/s13046-019-1144-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, et al. Reciprocal Activation of Prostate Cancer Cells and Cancer-Associated Fibroblasts Stimulates Epithelial-Mesenchymal Transition and Cancer Stemness. Cancer Res (2010) 70(17):6945–56. 10.1158/0008-5472.CAN-10-0785 [DOI] [PubMed] [Google Scholar]
- 126. De Wever O, Mareel M. Role of Tissue Stroma in Cancer Cell Invasion. J Pathol (2003) 200(4):429–47. 10.1002/path.1398 [DOI] [PubMed] [Google Scholar]
- 127. Lohr M, Schmidt C, Ringel J, Kluth M, Muller P, Nizze H, et al. Transforming Growth Factor-Beta1 Induces Desmoplasia in an Experimental Model of Human Pancreatic Carcinoma. Cancer Res (2001) 61(2):550–5. [PubMed] [Google Scholar]
- 128. Kaushik S, Pickup MW, Weaver VM. From Transformation to Metastasis: Deconstructing the Extracellular Matrix in Breast Cancer. Cancer Metastasis Rev (2016) 35(4):655–67. 10.1007/s10555-016-9650-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Esim O, Gedik ME, Dogan AL, Gunaydin G, Hascicek C. Development of Carboplatin Loaded Bovine Serum Albumin Nanoparticles and Evaluation of its Effect on an Ovarian Cancer Cell Line. J Drug Deliv Sci Technol (2021) 102655. 10.1016/j.jddst.2021.102655 [DOI] [Google Scholar]
- 130. Kadel D, Zhang Y, Sun HR, Zhao Y, Dong QZ, Qin LX. Current Perspectives of Cancer-Associated Fibroblast in Therapeutic Resistance: Potential Mechanism and Future Strategy. Cell Biol Toxicol (2019) 35(5):407–21. 10.1007/s10565-019-09461-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wang W, Li Q, Yamada T, Matsumoto K, Matsumoto I, Oda M, et al. Crosstalk to Stromal Fibroblasts Induces Resistance of Lung Cancer to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. Clin Cancer Res (2009) 15(21):6630–8. 10.1158/1078-0432.CCR-09-1001 [DOI] [PubMed] [Google Scholar]
- 132. Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, et al. Tumour Micro-Environment Elicits Innate Resistance to RAF Inhibitors Through HGF Secretion. Nature (2012) 487(7408):500–4. 10.1038/nature11183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Nakasone ES, Askautrud HA, Kees T, Park JH, Plaks V, Ewald AJ, et al. Imaging Tumor-Stroma Interactions During Chemotherapy Reveals Contributions of the Microenvironment to Resistance. Cancer Cell (2012) 21(4):488–503. 10.1016/j.ccr.2012.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Liu T, Han C, Wang S, Fang P, Ma Z, Xu L, et al. Cancer-Associated Fibroblasts: An Emerging Target of Anti-Cancer Immunotherapy. J Hematol Oncol (2019) 12(1):86. 10.1186/s13045-019-0770-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Barrett RL, Pure E. Cancer-Associated Fibroblasts and Their Influence on Tumor Immunity and Immunotherapy. Elife (2020) 9. 10.7554/eLife.57243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sun W, Fu S. Role of Cancer-Associated Fibroblasts in Tumor Structure, Composition and the Microenvironment in Ovarian Cancer. Oncol Lett (2019) 18(3):2173–8. 10.3892/ol.2019.10587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Santana-Viera L, Ibba ML, Rotoli D, Catuogno S, Esposito CL. Emerging Therapeutic RNAs for the Targeting of Cancer Associated Fibroblast. Cancers (Basel) (2020) 12(6). 10.3390/cancers12061365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Powell DW, Adegboyega PA, Di Mari JF, Mifflin RC. Epithelial Cells and Their Neighbors I. Role of Intestinal Myofibroblasts in Development, Repair, and Cancer. Am J Physiol Gastrointest Liver Physiol (2005) 289(1):G2–7. 10.1152/ajpgi.00075.2005 [DOI] [PubMed] [Google Scholar]
- 139. D’Arcangelo E, Wu NC, Cadavid JL, McGuigan AP. The Life Cycle of Cancer-Associated Fibroblasts Within the Tumour Stroma and its Importance in Disease Outcome. Br J Cancer (2020) 122(7):931–42. 10.1038/s41416-019-0705-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Zeltz C, Primac I, Erusappan P, Alam J, Noel A, Gullberg D. Cancer-Associated Fibroblasts in Desmoplastic Tumors: Emerging Role of Integrins. Semin Cancer Biol (2020) 62:166–81. 10.1016/j.semcancer.2019.08.004 [DOI] [PubMed] [Google Scholar]
- 141. Liu T, Zhou L, Li D, Andl T, Zhang Y. Cancer-Associated Fibroblasts Build and Secure the Tumor Microenvironmen. Front Cell Dev Biol (2019) 7:60. 10.3389/fcell.2019.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Cannon A, Thompson C, Hall BR, Jain M, Kumar S, Batra SK. Desmoplasia in Pancreatic Ductal Adenocarcinoma: Insight Into Pathological Function and Therapeutic Potential. Genes Cancer (2018) 9(3-4):78–86. 10.18632/genesandcancer.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Whatcott CJ, Diep CH, Jiang P, Watanabe A, LoBello J, Sima C, et al. Desmoplasia in Primary Tumors and Metastatic Lesions of Pancreatic Cancer. Clin Cancer Res (2015) 21(15):3561–8. 10.1158/1078-0432.CCR-14-1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Iacobuzio-Donahue CA, Ryu B, Hruban RH, Kern SE. Exploring the Host Desmoplastic Response to Pancreatic Carcinoma: Gene Expression of Stromal and Neoplastic Cells at the Site of Primary Invasion. Am J Pathol (2002) 160(1):91–9. 10.1016/S0002-9440(10)64353-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Hasebe T, Sasaki S, Imoto S, Mukai K, Yokose T, Ochiai A. Prognostic Significance of Fibrotic Focus in Invasive Ductal Carcinoma of the Breast: A Prospective Observational Study. Mod Pathol (2002) 15(5):502–16. 10.1038/modpathol.3880555 [DOI] [PubMed] [Google Scholar]
- 146. Shimosato Y, Suzuki A, Hashimoto T, Nishiwaki Y, Kodama T, Yoneyama T, et al. Prognostic Implications of Fibrotic Focus (Scar) in Small Peripheral Lung Cancers. Am J Surg Pathol (1980) 4(4):365–73. 10.1097/00000478-198008000-00005 [DOI] [PubMed] [Google Scholar]
- 147. Koliopanos A, Friess H, di Mola FF, Tang WH, Kubulus D, Brigstock D, et al. Connective Tissue Growth Factor Gene Expression Alters Tumor Progression in Esophageal Cancer. World J Surg (2002) 26(4):420–7. 10.1007/s00268-001-0242-x [DOI] [PubMed] [Google Scholar]
- 148. Caporale A, Cosenza UM, Vestri AR, Giuliani A, Costi U, Galati G, et al. Has Desmoplastic Response Extent Protective Action Against Tumor Aggressiveness in Gastric Carcinoma? J Exp Clin Cancer Res (2001) 20(1):21–4. [PubMed] [Google Scholar]
- 149. Halvorsen TB, Seim E. Association Between Invasiveness, Inflammatory Reaction, Desmoplasia and Survival in Colorectal Cancer. J Clin Pathol (1989) 42(2):162–6. 10.1136/jcp.42.2.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Caporale A, Vestri AR, Benvenuto E, Mariotti M, Cosenza UM, Scarpini M, et al. Is Desmoplasia a Protective Factor for Survival in Patients With Colorectal Carcinoma? Clin Gastroenterol Hepatol (2005) 3(4):370–5. 10.1016/S1542-3565(04)00674-3 [DOI] [PubMed] [Google Scholar]
- 151. Hewitt RE, Powe DG, Carter GI, Turner DR. Desmoplasia and Its Relevance to Colorectal Tumour Invasion. Int J Cancer (1993) 53(1):62–9. 10.1002/ijc.2910530113 [DOI] [PubMed] [Google Scholar]
- 152. Ueno H, Kanemitsu Y, Sekine S, Ishiguro M, Ito E, Hashiguchi Y, et al. Desmoplastic Pattern at the Tumor Front Defines Poor-Prognosis Subtypes of Colorectal Cancer. Am J Surg Pathol (2017) 41(11):1506–12. 10.1097/PAS.0000000000000946 [DOI] [PubMed] [Google Scholar]
- 153. Ueno H, Shinto E, Shimazaki H, Kajiwara Y, Sueyama T, Yamamoto J, et al. Histologic Categorization of Desmoplastic Reaction: Its Relevance to the Colorectal Cancer Microenvironment and Prognosis. Ann Surg Oncol (2015) 22(5):1504–12. 10.1245/s10434-014-4149-9 [DOI] [PubMed] [Google Scholar]
- 154. Ueno H, Ishiguro M, Nakatani E, Ishikawa T, Uetake H, Murotani K, et al. Prognostic Value of Desmoplastic Reaction Characterisation in Stage II Colon Cancer: Prospective Validation in a Phase 3 Study (SACURA Trial). Br J Cancer (2021) 124(6):1088–97. 10.1038/s41416-020-01222-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Shin N, Son GM, Shin DH, Kwon MS, Park BS, Kim HS, et al. Cancer-Associated Fibroblasts and Desmoplastic Reactions Related to Cancer Invasiveness in Patients With Colorectal Cancer. Ann Coloproctol (2019) 35(1):36–46. 10.3393/ac.2018.09.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Hosein AN, Huang H, Wang Z, Parmar K, Du W, Huang J, et al. Cellular Heterogeneity During Mouse Pancreatic Ductal Adenocarcinoma Progression at Single-Cell Resolution. JCI Insight (2019) 5. 10.1172/jci.insight.129212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Friedman G, Levi-Galibov O, David E, Bornstein C, Giladi A, Dadiani M, et al. Cancer-Associated Fibroblast Compositions Change With Breast Cancer Progression Linking the Ratio of S100A4+ and PDPN+ CAFs to Clinical Outcome. Nat Cancer (2020) 1(7):692–708. 10.1038/s43018-020-0082-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Ikenaga N, Ohuchida K, Mizumoto K, Cui L, Kayashima T, Morimatsu K, et al. CD10+ Pancreatic Stellate Cells Enhance the Progression of Pancreatic Cancer. Gastroenterology (2010) 139(3):1041–51, 51 e1-8. 10.1053/j.gastro.2010.05.084 [DOI] [PubMed] [Google Scholar]
- 159. Su S, Chen J, Yao H, Liu J, Yu S, Lao L, et al. CD10(+)GPR77(+) Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell (2018) 172(4):841–56 e16. 10.1016/j.cell.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 160. Li H, Courtois ET, Sengupta D, Tan Y, Chen KH, Goh JJL, et al. Reference Component Analysis of Single-Cell Transcriptomes Elucidates Cellular Heterogeneity in Human Colorectal Tumors. Nat Genet (2017) 49(5):708–18. 10.1038/ng.3818 [DOI] [PubMed] [Google Scholar]
- 161. Peng J, Sun BF, Chen CY, Zhou JY, Chen YS, Chen H, et al. Single-Cell RNA-Seq Highlights Intra-Tumoral Heterogeneity and Malignant Progression in Pancreatic Ductal Adenocarcinoma. Cell Res (2019) 29(9):725–38. 10.1038/s41422-019-0195-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell (2018) 33(3):463–79 e10. 10.1016/j.ccell.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 163. Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY. Anatomic Demarcation by Positional Variation in Fibroblast Gene Expression Programs. PloS Genet (2006) 2(7):e119. 10.1371/journal.pgen.0020119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Tchou J, Kossenkov AV, Chang L, Satija C, Herlyn M, Showe LC, et al. Human Breast Cancer Associated Fibroblasts Exhibit Subtype Specific Gene Expression Profiles. BMC Med Genomics (2012) 5:39. 10.1186/1755-8794-5-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Ohlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, et al. Distinct Populations of Inflammatory Fibroblasts and Myofibroblasts in Pancreatic Cancer. J Exp Med (2017) 214(3):579–96. 10.1084/jem.20162024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Epelman S, Lavine KJ, Randolph GJ. Origin and Functions of Tissue Macrophages. Immunity (2014) 41(1):21–35. 10.1016/j.immuni.2014.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. NobelPrize.org . The Nobel Prize in Physiology or Medicine 1908. Nobel Media AB; (2021). Available at: https://www.nobelprize.org/prizes/medicine/1908/summary/. [Google Scholar]
- 168. Tauber AI. Metchnikoff and the Phagocytosis Theory. Nat Rev Mol Cell Biol (2003) 4(11):897–901. 10.1038/nrm1244 [DOI] [PubMed] [Google Scholar]
- 169. Cassetta L, Fragkogianni S, Sims AH, Swierczak A, Forrester LM, Zhang H, et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Target. Cancer Cell (2019) 35(4):588–602 e10. 10.1016/j.ccell.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Qian BZ, Pollard JW. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell (2010) 141(1):39–51. 10.1016/j.cell.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Chen P, Bonaldo P. Role of Macrophage Polarization in Tumor Angiogenesis and Vessel Normalization: Implications for New Anticancer Therapies. Int Rev Cell Mol Biol (2013) 301:1–35. 10.1016/B978-0-12-407704-1.00001-4 [DOI] [PubMed] [Google Scholar]
- 172. van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The Mononuclear Phagocyte System: A New Classification of Macrophages, Monocytes, and Their Precursor Cells. Bull World Health Organ (1972) 46(6):845–52. [PMC free article] [PubMed] [Google Scholar]
- 173. Hoeffel G, Ginhoux F. Ontogeny of Tissue-Resident Macrophages. Front Immunol (2015) 6:486. 10.3389/fimmu.2015.00486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling Mononuclear Phagocyte Heterogeneity. Nat Rev Immunol (2010) 10(6):453–60. 10.1038/nri2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Laviron M, Boissonnas A. Ontogeny of Tumor-Associated Macrophages. Front Immunol (2019) 10:1799. 10.3389/fimmu.2019.01799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, et al. The Cellular and Molecular Origin of Tumor-Associated Macrophages. Science (2014) 344(6186):921–5. 10.1126/science.1252510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-Associated Macrophages as Treatment Targets in Oncology. Nat Rev Clin Oncol (2017) 14(7):399–416. 10.1038/nrclinonc.2016.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Nakamura K, Smyth MJ. Myeloid Immunosuppression and Immune Checkpoints in the Tumor Microenvironment. Cell Mol Immunol (2020) 17(1):1–12. 10.1038/s41423-019-0306-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Lin EY, Gouon-Evans V, Nguyen AV, Pollard JW. The Macrophage Growth Factor CSF-1 in Mammary Gland Development and Tumor Progression. J Mammary Gland Biol Neoplasia (2002) 7(2):147–62. 10.1023/A:1020399802795 [DOI] [PubMed] [Google Scholar]
- 180. Biswas SK, Mantovani A. Macrophage Plasticity and Interaction With Lymphocyte Subsets: Cancer as a Paradigm. Nat Immunol (2010) 11(10):889–96. 10.1038/ni.1937 [DOI] [PubMed] [Google Scholar]
- 181. Locati M, Curtale G, Mantovani A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu Rev Pathol (2020) 15:123–47. 10.1146/annurev-pathmechdis-012418-012718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 Macrophages and the Th1/Th2 Paradigm. J Immunol (2000) 164(12):6166–73. 10.4049/jimmunol.164.12.6166 [DOI] [PubMed] [Google Scholar]
- 183. Mosser DM, Edwards JP. Exploring the Full Spectrum of Macrophage Activation. Nat Rev Immunol (2008) 8(12):958–69. 10.1038/nri2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Pollard JW. Tumour-Educated Macrophages Promote Tumour Progression and Metastasis. Nat Rev Cancer (2004) 4(1):71–8. 10.1038/nrc1256 [DOI] [PubMed] [Google Scholar]
- 185. Gordon S, Taylor PR. Monocyte and Macrophage Heterogeneity. Nat Rev Immunol (2005) 5(12):953–64. 10.1038/nri1733 [DOI] [PubMed] [Google Scholar]
- 186. Zhou J, Tang Z, Gao S, Li C, Feng Y, Zhou X. Tumor-Associated Macrophages: Recent Insights and Therapies. Front Oncol (2020) 10:188. 10.3389/fonc.2020.00188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated Regulation of Myeloid Cells by Tumours. Nat Rev Immunol (2012) 12(4):253–68. 10.1038/nri3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Zhong Q, Fang Y, Lai Q, Wang S, He C, Li A, et al. CPEB3 Inhibits Epithelial-Mesenchymal Transition by Disrupting the Crosstalk Between Colorectal Cancer Cells and Tumor-Associated Macrophages via IL-6r/STAT3 Signaling. J Exp Clin Cancer Res (2020) 39(1):132. 10.1186/s13046-020-01637-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Chen RH, Xiao ZW, Yan XQ, Han P, Liang FY, Wang JY, et al. Tumor Cell-Secreted ISG15 Promotes Tumor Cell Migration and Immune Suppression by Inducing the Macrophage M2-Like Phenotype. Front Immunol (2020) 11:594775. 10.3389/fimmu.2020.594775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Jayasingam SD, Citartan M, Thang TH, Mat Zin AA, Ang KC, Ch’ng ES. Evaluating the Polarization of Tumor-Associated Macrophages Into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front Oncol (2019) 9:1512. 10.3389/fonc.2019.01512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Liu YC, Zou XB, Chai YF, Yao YM. Macrophage Polarization in Inflammatory Diseases. Int J Biol Sci (2014) 10(5):520–9. 10.7150/ijbs.8879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 Potently Enhances Murine Macrophage Mannose Receptor Activity: A Marker of Alternative Immunologic Macrophage Activation. J Exp Med (1992) 176(1):287–92. 10.1084/jem.176.1.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Gordon S, Martinez FO. Alternative Activation of Macrophages: Mechanism and Functions. Immunity (2010) 32(5):593–604. 10.1016/j.immuni.2010.05.007 [DOI] [PubMed] [Google Scholar]
- 194. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity (2014) 41(1):14–20. 10.1016/j.immuni.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B, Dereuddre-Bosquet N, et al. Macrophage Activation Switching: An Asset for the Resolution of Inflammation. Clin Exp Immunol (2005) 142(3):481–9. 10.1111/j.1365-2249.2005.02934.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Ambarus CA, Krausz S, van Eijk M, Hamann J, Radstake TR, Reedquist KA, et al. Systematic Validation of Specific Phenotypic Markers for In Vitro Polarized Human Macrophages. J Immunol Methods (2012) 375(1-2):196–206. 10.1016/j.jim.2011.10.013 [DOI] [PubMed] [Google Scholar]
- 197. Monteran L, Erez N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front Immunol (2019) 10:1835. 10.3389/fimmu.2019.01835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Lewis CE, Leek R, Harris A, McGee JO. Cytokine Regulation of Angiogenesis in Breast Cancer: The Role of Tumor-Associated Macrophages. J Leukoc Biol (1995) 57(5):747–51. 10.1002/jlb.57.5.747 [DOI] [PubMed] [Google Scholar]
- 199. Bingle L, Brown NJ, Lewis CE. The Role of Tumour-Associated Macrophages in Tumour Progression: Implications for New Anticancer Therapies. J Pathol (2002) 196(3):254–65. 10.1002/path.1027 [DOI] [PubMed] [Google Scholar]
- 200. Noy R, Pollard JW. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity (2014) 41(1):49–61. 10.1016/j.immuni.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Nasrollahzadeh E, Razi S, Keshavarz-Fathi M, Mazzone M, Rezaei N. Pro-Tumorigenic Functions of Macrophages at the Primary, Invasive and Metastatic Tumor Site. Cancer Immunol Immunother (2020) 69(9):1673–97. 10.1007/s00262-020-02616-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Chen XW, Yu TJ, Zhang J, Li Y, Chen HL, Yang GF, et al. CYP4A in Tumor-Associated Macrophages Promotes Pre-Metastatic Niche Formation and Metastasis. Oncogene (2017) 36(35):5045–57. 10.1038/onc.2017.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Bercovici N, Guerin MV, Trautmann A, Donnadieu E. The Remarkable Plasticity of Macrophages: A Chance to Fight Cancer. Front Immunol (2019) 10:1563. 10.3389/fimmu.2019.01563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Liu KX, Joshi S. "Re-Educating" Tumor Associated Macrophages as a Novel Immunotherapy Strategy for Neuroblastom. Front Immunol (2020) 11:1947. 10.3389/fimmu.2020.01947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Hensler M, Kasikova L, Fiser K, Rakova J, Skapa P, Laco J, et al. M2-Like Macrophages Dictate Clinically Relevant Immunosuppression in Metastatic Ovarian Cancer. J Immunother Cancer (2020) 8(2). 10.1136/jitc-2020-000979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Doak GR, Schwertfeger KL, Wood DK. Distant Relations: Macrophage Functions in the Metastatic Niche. Trends Cancer (2018) 4(6):445–59. 10.1016/j.trecan.2018.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, et al. TLR7/8-Agonist-Loaded Nanoparticles Promote the Polarization of Tumour-Associated Macrophages to Enhance Cancer Immunotherapy. Nat BioMed Eng (2018) 2(8):578–88. 10.1038/s41551-018-0236-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Xiang X, Wang J, Lu D, Xu X. Targeting Tumor-Associated Macrophages to Synergize Tumor Immunotherapy. Signal Transduct Target Ther (2021) 6(1):75. 10.1038/s41392-021-00484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z. Tumor-Associated Macrophages: An Accomplice in Solid Tumor Progression. J BioMed Sci (2019) 26(1):78. 10.1186/s12929-019-0568-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Anfray C, Ummarino A, Andon FT, Allavena P. Current Strategies to Target Tumor-Associated-Macrophages to Improve Anti-Tumor Immune Response. Cells (2019) 9(1). 10.3390/cells9010046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Guerriero JL. Macrophages: The Road Less Traveled, Changing Anticancer Therapy. Trends Mol Med (2018) 24(5):472–89. 10.1016/j.molmed.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Hayashi N, Kataoka H, Yano S, Tanaka M, Moriwaki K, Akashi H, et al. A Novel Photodynamic Therapy Targeting Cancer Cells and Tumor-Associated Macrophages. Mol Cancer Ther (2015) 14(2):452–60. 10.1158/1535-7163.MCT-14-0348 [DOI] [PubMed] [Google Scholar]
- 213. Gunaydin G, Gedik ME, Ayan S. Photodynamic Therapy—Current Limitations and Novel Approaches. Front Chem (2021) 9. 10.3389/fchem.2021.691697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Welters MJP, Santegoets SJ, van der Burg SH. The Tumor Microenvironment and Immunotherapy of Oropharyngeal Squamous Cell Carcinoma. Front Oncol (2020) 10:545385. 10.3389/fonc.2020.545385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, et al. Activated Monocytes in Peritumoral Stroma of Hepatocellular Carcinoma Foster Immune Privilege and Disease Progression Through PD-L1. J Exp Med (2009) 206(6):1327–37. 10.1084/jem.20082173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT. Gliomas Promote Immunosuppression Through Induction of B7-H1 Expression in Tumor-Associated Macrophages. Clin Cancer Res (2013) 19(12):3165–75. 10.1158/1078-0432.CCR-12-3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, et al. Induction of T-Cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer Immunol Res (2015) 3(4):399–411. 10.1158/2326-6066.CIR-14-0215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Candido JB, Morton JP, Bailey P, Campbell AD, Karim SA, Jamieson T, et al. CSF1R(+) Macrophages Sustain Pancreatic Tumor Growth Through T Cell Suppression and Maintenance of Key Gene Programs That Define the Squamous Subtype. Cell Rep (2018) 23(5):1448–60. 10.1016/j.celrep.2018.03.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov (2018) 8(9):1069–86. 10.1158/2159-8290.CD-18-0367 [DOI] [PubMed] [Google Scholar]
- 220. Kumar S, Ramesh A, Kulkarni A. Targeting Macrophages: A Novel Avenue for Cancer Drug Discovery. Expert Opin Drug Discov (2020) 15(5):561–74. 10.1080/17460441.2020.1733525 [DOI] [PubMed] [Google Scholar]
- 221. Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-Associated Macrophages Are a Distinct M2 Polarised Population Promoting Tumour Progression: Potential Targets of Anti-Cancer Therapy. Eur J Cancer (2006) 42(6):717–27. 10.1016/j.ejca.2006.01.003 [DOI] [PubMed] [Google Scholar]
- 222. Sica A, Mantovani A. Macrophage Plasticity and Polarization: In Vivo Veritas. J Clin Invest (2012) 122(3):787–95. 10.1172/JCI59643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Fang J, Li X, Ma D, Liu X, Chen Y, Wang Y, et al. Prognostic Significance of Tumor Infiltrating Immune Cells in Oral Squamous Cell Carcinoma. BMC Cancer (2017) 17(1):375. 10.1186/s12885-017-3317-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Pinto ML, Rios E, Duraes C, Ribeiro R, Machado JC, Mantovani A, et al. The Two Faces of Tumor-Associated Macrophages and Their Clinical Significance in Colorectal Cancer. Front Immunol (2019) 10:1875. 10.3389/fimmu.2019.01875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Balkwill FR, Mantovani A. Cancer-Related Inflammation: Common Themes and Therapeutic Opportunities. Semin Cancer Biol (2012) 22(1):33–40. 10.1016/j.semcancer.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 226. Chen S, Lai SWT, Brown CE, Feng M. Harnessing and Enhancing Macrophage Phagocytosis for Cancer Therapy. Front Immunol (2021) 12:635173. 10.3389/fimmu.2021.635173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Liu Y, Wang R. Immunotherapy Targeting Tumor-Associated Macrophage. Front Med (Lausanne) (2020) 7:583708. 10.3389/fmed.2020.583708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Cassetta L, Pollard JW. Targeting Macrophages: Therapeutic Approaches in Cancer. Nat Rev Drug Discov (2018) 17(12):887–904. 10.1038/nrd.2018.169 [DOI] [PubMed] [Google Scholar]
- 229. Stower H. CARs for Macrophages. Nat Med (2020) 26(5):649. 10.1038/s41591-020-0905-5 [DOI] [PubMed] [Google Scholar]
- 230. Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, et al. Human Chimeric Antigen Receptor Macrophages for Cancer Immunotherapy. Nat Biotechnol (2020) 38(8):947–53. 10.1158/2326-6074.TUMIMM18-PR07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Shields CW4, Evans MA, Wang LL, Baugh N, Iyer S, Wu D, et al. Cellular Backpacks for Macrophage Immunotherapy. Sci Adv (2020) 6(18):eaaz6579. 10.1126/sciadv.aaz6579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Harper J, Sainson RC. Regulation of the Anti-Tumour Immune Response by Cancer-Associated Fibroblasts. Semin Cancer Biol (2014) 25:69–77. 10.1016/j.semcancer.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 233. Lazennec G, Richmond A. Chemokines and Chemokine Receptors: New Insights Into Cancer-Related Inflammation. Trends Mol Med (2010) 16(3):133–44. 10.1016/j.molmed.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Ziani L, Chouaib S, Thiery J. Alteration of the Antitumor Immune Response by Cancer-Associated Fibroblast. Front Immunol (2018) 9:414. 10.3389/fimmu.2018.00414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Zhang J, Chen L, Xiao M, Wang C, Qin Z. FSP1+ Fibroblasts Promote Skin Carcinogenesis by Maintaining MCP-1-Mediated Macrophage Infiltration and Chronic Inflammation. Am J Pathol (2011) 178(1):382–90. 10.1016/j.ajpath.2010.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Suzuki J, Aokage K, Neri S, Sakai T, Hashimoto H, Su Y, et al. Relationship Between Podoplanin-Expressing Cancer-Associated Fibroblasts and the Immune Microenvironment of Early Lung Squamous Cell Carcinoma. Lung Cancer (2021) 153:1–10. 10.1016/j.lungcan.2020.12.020 [DOI] [PubMed] [Google Scholar]
- 237. Sakai T, Aokage K, Neri S, Nakamura H, Nomura S, Tane K, et al. Link Between Tumor-Promoting Fibrous Microenvironment and an Immunosuppressive Microenvironment in Stage I Lung Adenocarcinoma. Lung Cancer (2018) 126:64–71. 10.1016/j.lungcan.2018.10.021 [DOI] [PubMed] [Google Scholar]
- 238. Comito G, Giannoni E, Segura CP, Barcellos-de-Souza P, Raspollini MR, Baroni G, et al. Cancer-Associated Fibroblasts and M2-Polarized Macrophages Synergize During Prostate Carcinoma Progression. Oncogene (2014) 33(19):2423–31. 10.1038/onc.2013.191 [DOI] [PubMed] [Google Scholar]
- 239. Utispan K, Koontongkaew S. Fibroblasts and Macrophages: Key Players in the Head and Neck Cancer Microenvironment. J Oral Biosci (2017) 59(1):23–30. 10.1016/j.job.2016.11.002 [DOI] [Google Scholar]
- 240. Komohara Y, Takeya M. CAFs and TAMs: Maestros of the Tumour Microenvironment. J Pathol (2017) 241(3):313–5. 10.1002/path.4824 [DOI] [PubMed] [Google Scholar]
- 241. Ge Z, Ding S. The Crosstalk Between Tumor-Associated Macrophages (TAMs) and Tumor Cells and the Corresponding Targeted Therapy. Front Oncol (2020) 10:590941. 10.3389/fonc.2020.590941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Oya Y, Hayakawa Y, Koike K. Tumor Microenvironment in Gastric Cancers. Cancer Sci (2020) 111(8):2696–707. 10.1111/cas.14521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Blavier L, Yang RM, DeClerck YA. The Tumor Microenvironment in Neuroblastoma: New Players, New Mechanisms of Interaction and New Perspective. Cancers (Basel) (2020) 12(10). 10.3390/cancers12102912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Zheng X, Turkowski K, Mora J, Brune B, Seeger W, Weigert A, et al. Redirecting Tumor-Associated Macrophages to Become Tumoricidal Effectors as a Novel Strategy for Cancer Therapy. Oncotarget (2017) 8(29):48436–52. 10.18632/oncotarget.17061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, et al. Macrophage-Secreted Granulin Supports Pancreatic Cancer Metastasis by Inducing Liver Fibrosis. Nat Cell Biol (2016) 18(5):549–60. 10.1038/ncb3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Tokuda K, Morine Y, Miyazaki K, Yamada S, Saito Y, Nishi M, et al. The Interaction Between Cancer Associated Fibroblasts and Tumor Associated Macrophages via the Osteopontin Pathway in the Tumor Microenvironment of Hepatocellular Carcinoma. Oncotarget (2021) 12(4):333–43. 10.18632/oncotarget.27881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Ueshima E, Fujimori M, Kodama H, Felsen D, Chen J, Durack JC, et al. Macrophage-Secreted TGF-Beta1 Contributes to Fibroblast Activation and Ureteral Stricture After Ablation Injury. Am J Physiol Renal Physiol (2019) 317(7):F52–64. 10.1152/ajprenal.00260.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Takahashi H, Sakakura K, Kudo T, Toyoda M, Kaira K, Oyama T, et al. Cancer-Associated Fibroblasts Promote an Immunosuppressive Microenvironment Through the Induction and Accumulation of Protumoral Macrophages. Oncotarget (2017) 8(5):8633–47. 10.18632/oncotarget.14374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Hashimoto O, Yoshida M, Koma Y, Yanai T, Hasegawa D, Kosaka Y, et al. Collaboration of Cancer-Associated Fibroblasts and Tumour-Associated Macrophages for Neuroblastoma Development. J Pathol (2016) 240(2):211–23. 10.1002/path.4769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Chiarugi P. Cancer-Associated Fibroblasts and Macrophages: Friendly Conspirators for Malignancy. Oncoimmunology (2013) 2(9):e25563. 10.4161/onci.25563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Stairs DB, Bayne LJ, Rhoades B, Vega ME, Waldron TJ, Kalabis J, et al. Deletion of P120-Catenin Results in a Tumor Microenvironment With Inflammation and Cancer That Establishes it as a Tumor Suppressor Gene. Cancer Cell (2011) 19(4):470–83. 10.1016/j.ccr.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Herrera M, Herrera A, Dominguez G, Silva J, Garcia V, Garcia JM, et al. Cancer-Associated Fibroblast and M2 Macrophage Markers Together Predict Outcome in Colorectal Cancer Patients. Cancer Sci (2013) 104(4):437–44. 10.1111/cas.12096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Fujii N, Shomori K, Shiomi T, Nakabayashi M, Takeda C, Ryoke K, et al. Cancer-Associated Fibroblasts and CD163-Positive Macrophages in Oral Squamous Cell Carcinoma: Their Clinicopathological and Prognostic Significance. J Oral Pathol Med (2012) 41(6):444–51. 10.1111/j.1600-0714.2012.01127.x [DOI] [PubMed] [Google Scholar]
- 254. Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al. A Lineage of Myeloid Cells Independent of Myb and Hematopoietic Stem Cells. Science (2012) 336(6077):86–90. 10.1126/science.1219179 [DOI] [PubMed] [Google Scholar]
- 255. Bertrand JY, Jalil A, Klaine M, Jung S, Cumano A, Godin I. Three Pathways to Mature Macrophages in the Early Mouse Yolk Sac. Blood (2005) 106(9):3004–11. 10.1182/blood-2005-02-0461 [DOI] [PubMed] [Google Scholar]
- 256. Wynn TA, Chawla A, Pollard JW. Macrophage Biology in Development, Homeostasis and Disease. Nature (2013) 496(7446):445–55. 10.1038/nature12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, et al. Different Tumor Microenvironments Contain Functionally Distinct Subsets of Macrophages Derived From Ly6C(high) Monocytes. Cancer Res (2010) 70(14):5728–39. 10.1158/0008-5472.CAN-09-4672 [DOI] [PubMed] [Google Scholar]
- 258. Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 Recruits Inflammatory Monocytes to Facilitate Breast-Tumour Metastasis. Nature (2011) 475(7355):222–5. 10.1038/nature10138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell (2010) 17(2):135–47. 10.1016/j.ccr.2009.12.041 [DOI] [PubMed] [Google Scholar]
- 260. Augsten M, Sjoberg E, Frings O, Vorrink SU, Frijhoff J, Olsson E, et al. Cancer-Associated Fibroblasts Expressing CXCL14 Rely Upon NOS1-Derived Nitric Oxide Signaling for Their Tumor-Supporting Properties. Cancer Res (2014) 74(11):2999–3010. 10.1158/0008-5472.CAN-13-2740 [DOI] [PubMed] [Google Scholar]
- 261. Cohen N, Shani O, Raz Y, Sharon Y, Hoffman D, Abramovitz L, et al. Fibroblasts Drive an Immunosuppressive and Growth-Promoting Microenvironment in Breast Cancer via Secretion of Chitinase 3-Like 1. Oncogene (2017) 36(31):4457–68. 10.1038/onc.2017.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 Pathway in Cancer. Clin Cancer Res (2010) 16(11):2927–31. 10.1158/1078-0432.CCR-09-2329 [DOI] [PubMed] [Google Scholar]
- 263. Ksiazkiewicz M, Gottfried E, Kreutz M, Mack M, Hofstaedter F, Kunz-Schughart LA. Importance of CCL2-CCR2A/2B Signaling for Monocyte Migration Into Spheroids of Breast Cancer-Derived Fibroblasts. Immunobiology (2010) 215(9-10):737–47. 10.1016/j.imbio.2010.05.019 [DOI] [PubMed] [Google Scholar]
- 264. Allaoui R, Bergenfelz C, Mohlin S, Hagerling C, Salari K, Werb Z, et al. Cancer-Associated Fibroblast-Secreted CXCL16 Attracts Monocytes to Promote Stroma Activation in Triple-Negative Breast Cancers. Nat Commun (2016) 7:13050. 10.1038/ncomms13050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Mace TA, Ameen Z, Collins A, Wojcik S, Mair M, Young GS, et al. Pancreatic Cancer-Associated Stellate Cells Promote Differentiation of Myeloid-Derived Suppressor Cells in a STAT3-Dependent Manner. Cancer Res (2013) 73(10):3007–18. 10.1158/0008-5472.CAN-12-4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Cho H, Seo Y, Loke KM, Kim SW, Oh SM, Kim JH, et al. Cancer-Stimulated CAFs Enhance Monocyte Differentiation and Protumoral TAM Activation via IL6 and GM-CSF Secretion. Clin Cancer Res (2018) 24(21):5407–21. 10.1158/1078-0432.CCR-18-0125 [DOI] [PubMed] [Google Scholar]
- 267. de Leve S, Wirsdorfer F, Jendrossek V. Targeting the Immunomodulatory CD73/Adenosine System to Improve the Therapeutic Gain of Radiotherapy. Front Immunol (2019) 10:698. 10.3389/fimmu.2019.00698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Allard B, Beavis PA, Darcy PK, Stagg J. Immunosuppressive Activities of Adenosine in Cancer. Curr Opin Pharmacol (2016) 29:7–16. 10.1016/j.coph.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 269. Della Latta V, Cabiati M, Rocchiccioli S, Del Ry S, Morales MA. The Role of the Adenosinergic System in Lung Fibrosis. Pharmacol Res (2013) 76:182–9. 10.1016/j.phrs.2013.08.004 [DOI] [PubMed] [Google Scholar]
- 270. Regateiro FS, Cobbold SP, Waldmann H. CD73 and Adenosine Generation in the Creation of Regulatory Microenvironments. Clin Exp Immunol (2013) 171(1):1–7. 10.1111/j.1365-2249.2012.04623.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Hegab AE, Ozaki M, Kameyama N, Gao J, Kagawa S, Yasuda H, et al. Effect of FGF/FGFR Pathway Blocking on Lung Adenocarcinoma and Its Cancer-Associated Fibroblasts. J Pathol (2019) 249(2):193–205. 10.1002/path.5290 [DOI] [PubMed] [Google Scholar]
- 272. Zhang R, Qi F, Zhao F, Li G, Shao S, Zhang X, et al. Cancer-Associated Fibroblasts Enhance Tumor-Associated Macrophages Enrichment and Suppress NK Cells Function in Colorectal Cancer. Cell Death Dis (2019) 10(4):273. 10.1038/s41419-019-1435-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Nagarsheth N, Wicha MS, Zou W. Chemokines in the Cancer Microenvironment and Their Relevance in Cancer Immunotherapy. Nat Rev Immunol (2017) 17(9):559–72. 10.1038/nri.2017.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Kim JH, Oh SH, Kim EJ, Park SJ, Hong SP, Cheon JH, et al. The Role of Myofibroblasts in Upregulation of S100A8 and S100A9 and the Differentiation of Myeloid Cells in the Colorectal Cancer Microenvironment. Biochem Biophys Res Commun (2012) 423(1):60–6. 10.1016/j.bbrc.2012.05.081 [DOI] [PubMed] [Google Scholar]
- 275. Nakamura H, Ichikawa T, Nakasone S, Miyoshi T, Sugano M, Kojima M, et al. Abundant Tumor Promoting Stromal Cells in Lung Adenocarcinoma With Hypoxic Regions. Lung Cancer (2018) 115:56–63. 10.1016/j.lungcan.2017.11.013 [DOI] [PubMed] [Google Scholar]
- 276. Higashino N, Koma YI, Hosono M, Takase N, Okamoto M, Kodaira H, et al. Fibroblast Activation Protein-Positive Fibroblasts Promote Tumor Progression Through Secretion of CCL2 and Interleukin-6 in Esophageal Squamous Cell Carcinoma. Lab Invest (2019) 99(6):777–92. 10.1038/s41374-018-0185-6 [DOI] [PubMed] [Google Scholar]
- 277. Yang X, Lin Y, Shi Y, Li B, Liu W, Yin W, et al. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3-CCL2 Signaling. Cancer Res (2016) 76(14):4124–35. 10.1158/0008-5472.CAN-15-2973 [DOI] [PubMed] [Google Scholar]
- 278. Kumar V, Donthireddy L, Marvel D, Condamine T, Wang F, Lavilla-Alonso S, et al. Cancer-Associated Fibroblasts Neutralize the Anti-Tumor Effect of CSF1 Receptor Blockade by Inducing PMN-MDSC Infiltration of Tumor. Cancer Cell (2017) 32(5):654–68 e5. 10.1016/j.ccell.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Borriello L, Nakata R, Sheard MA, Fernandez GE, Sposto R, Malvar J, et al. Cancer-Associated Fibroblasts Share Characteristics and Protumorigenic Activity With Mesenchymal Stromal Cell. Cancer Res (2017) 77(18):5142–57. 10.1158/0008-5472.CAN-16-2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Zhou J, Wang XH, Zhao YX, Chen C, Xu XY, Sun Q, et al. Cancer-Associated Fibroblasts Correlate With Tumor-Associated Macrophages Infiltration and Lymphatic Metastasis in Triple Negative Breast Cancer Patient. J Cancer (2018) 9(24):4635–41. 10.7150/jca.28583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Yu Y, Ke L, Lv X, Ling YH, Lu J, Liang H, et al. The Prognostic Significance of Carcinoma-Associated Fibroblasts and Tumor-Associated Macrophages in Nasopharyngeal Carcinoma. Cancer Manag Res (2018) 10:1935–46. 10.2147/CMAR.S167071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Deng Y, Cheng J, Fu B, Liu W, Chen G, Zhang Q, et al. Hepatic Carcinoma-Associated Fibroblasts Enhance Immune Suppression by Facilitating the Generation of Myeloid-Derived Suppressor Cells. Oncogene (2017) 36(8):1090–101. 10.1038/onc.2016.273 [DOI] [PubMed] [Google Scholar]
- 283. Zhang A, Qian Y, Ye Z, Chen H, Xie H, Zhou L, et al. Cancer-Associated Fibroblasts Promote M2 Polarization of Macrophages in Pancreatic Ductal Adenocarcinoma. Cancer Med (2017) 6(2):463–70. 10.1002/cam4.993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Ma Y, Hwang RF, Logsdon CD, Ullrich SE. Dynamic Mast Cell-Stromal Cell Interactions Promote Growth of Pancreatic Cancer. Cancer Res (2013) 73(13):3927–37. 10.1158/0008-5472.CAN-12-4479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Varricchi G, Galdiero MR, Loffredo S, Marone G, Iannone R, Marone G, et al. Are Mast Cells MASTers in Cancer? Front Immunol (2017) 8:424. 10.3389/fimmu.2017.00424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Acerbi I, Cassereau L, Dean I, Shi Q, Au A, Park C, et al. Human Breast Cancer Invasion and Aggression Correlates With ECM Stiffening and Immune Cell Infiltration. Integr Biol (Camb) (2015) 7(10):1120–34. 10.1039/c5ib00040h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Jiang H, Hegde S, DeNardo DG. Tumor-Associated Fibrosis as a Regulator of Tumor Immunity and Response to Immunotherapy. Cancer Immunol Immunother (2017) 66(8):1037–48. 10.1007/s00262-017-2003-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Kobayashi N, Miyoshi S, Mikami T, Koyama H, Kitazawa M, Takeoka M, et al. Hyaluronan Deficiency in Tumor Stroma Impairs Macrophage Trafficking and Tumor Neovascularization. Cancer Res (2010) 70(18):7073–83. 10.1158/0008-5472.CAN-09-4687 [DOI] [PubMed] [Google Scholar]
- 289. Berzaghi R, Ahktar MA, Islam A, Pedersen BD, Hellevik T, Martinez-Zubiaurre I. Fibroblast-Mediated Immunoregulation of Macrophage Function Is Maintained After Irradiation. Cancers (Basel) (2019) 11(5). 10.3390/cancers11050689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Gorchs L, Hellevik T, Bruun JA, Camilio KA, Al-Saad S, Stuge TB, et al. Cancer-Associated Fibroblasts From Lung Tumors Maintain Their Immunosuppressive Abilities After High-Dose Irradiation. Front Oncol (2015) 5:87. 10.3389/fonc.2015.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Tommelein J, De Vlieghere E, Verset L, Melsens E, Leenders J, Descamps B, et al. Radiotherapy-Activated Cancer-Associated Fibroblasts Promote Tumor Progression Through Paracrine IGF1R Activation. Cancer Res (2018) 78(3):659–70. 10.1158/0008-5472.CAN-17-0524 [DOI] [PubMed] [Google Scholar]
- 292. Barker HE, Paget JT, Khan AA, Harrington KJ. The Tumour Microenvironment After Radiotherapy: Mechanisms of Resistance and Recurrence. Nat Rev Cancer (2015) 15(7):409–25. 10.1038/nrc3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Linares J, Marin-Jimenez JA, Badia-Ramentol J, Calon A. Determinants and Functions of CAFs Secretome During Cancer Progression and Therapy. Front Cell Dev Biol (2020) 8:621070. 10.3389/fcell.2020.621070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Domen A, Quatannens D, Zanivan S, Deben C, Van Audenaerde J, Smits E, et al. Cancer-Associated Fibroblasts as a Common Orchestrator of Therapy Resistance in Lung and Pancreatic Cancer. Cancers (Basel) (2021) 13(5). 10.3390/cancers13050987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Peiris-Pages M, Sotgia F, Lisanti MP. Chemotherapy Induces the Cancer-Associated Fibroblast Phenotype, Activating Paracrine Hedgehog-GLI Signalling in Breast Cancer Cells. Oncotarget (2015) 6(13):10728–45. 10.18632/oncotarget.3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte Complexity Predicts Breast Cancer Survival and Functionally Regulates Response to Chemotherapy. Cancer Discov (2011) 1(1):54–67. 10.1158/2159-8274.CD-10-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Cheng Y, Li H, Deng Y, Tai Y, Zeng K, Zhang Y, et al. Cancer-Associated Fibroblasts Induce PDL1+ Neutrophils Through the IL6-STAT3 Pathway That Foster Immune Suppression in Hepatocellular Carcinoma. Cell Death Dis (2018) 9(4):422. 10.1038/s41419-018-0458-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Liu ZH, Chen ML, Zhang Q, Zhang Y, An X, Luo YL, et al. ZIC2 is Downregulated and Represses Tumor Growth via the Regulation of STAT3 in Breast Cancer. Int J Cancer (2020) 147(2):505–18. 10.1002/ijc.32922 [DOI] [PubMed] [Google Scholar]
- 299. Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 Signalling Axis in Cancer. Nat Rev Clin Oncol (2018) 15(4):234–48. 10.1038/nrclinonc.2018.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Wang Y, Shen Y, Wang S, Shen Q, Zhou X. The Role of STAT3 in Leading the Crosstalk Between Human Cancers and the Immune System. Cancer Lett (2018) 415:117–28. 10.1016/j.canlet.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Olmes G, Buttner-Herold M, Ferrazzi F, Distel L, Amann K, Daniel C. CD163+ M2c-Like Macrophages Predominate in Renal Biopsies From Patients With Lupus Nephritis. Arthritis Res Ther (2016) 18:90. 10.1186/s13075-016-0989-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Ohlsson SM, Linge CP, Gullstrand B, Lood C, Johansson A, Ohlsson S, et al. Serum From Patients With Systemic Vasculitis Induces Alternatively Activated Macrophage M2c Polarization. Clin Immunol (2014) 152(1-2):10–9. 10.1016/j.clim.2014.02.016 [DOI] [PubMed] [Google Scholar]
- 303. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The Chemokine System in Diverse Forms of Macrophage Activation and Polarization. Trends Immunol (2004) 25(12):677–86. 10.1016/j.it.2004.09.015 [DOI] [PubMed] [Google Scholar]
- 304. Coussens LM, Zitvogel L, Palucka AK. Neutralizing Tumor-Promoting Chronic Inflammation: A Magic Bullet? Science (2013) 339(6117):286–91. 10.1126/science.1232227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Su S, Liu Q, Chen J, Chen J, Chen F, He C, et al. A Positive Feedback Loop Between Mesenchymal-Like Cancer Cells and Macrophages is Essential to Breast Cancer Metastasis. Cancer Cell (2014) 25(5):605–20. 10.1016/j.ccr.2014.03.021 [DOI] [PubMed] [Google Scholar]
- 306. An Y, Liu F, Chen Y, Yang Q. Crosstalk Between Cancer-Associated Fibroblasts and Immune Cells in Cancer. J Cell Mol Med (2020) 24(1):13–24. 10.1111/jcmm.14745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Pittet MJ. Behavior of Immune Players in the Tumor Microenvironment. Curr Opin Oncol (2009) 21(1):53–9. 10.1097/CCO.0b013e32831bc38a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, et al. IL-4 Induces Cathepsin Protease Activity in Tumor-Associated Macrophages to Promote Cancer Growth and Invasion. Genes Dev (2010) 24(3):241–55. 10.1101/gad.1874010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Condeelis J, Pollard JW. Macrophages: Obligate Partners for Tumor Cell Migration, Invasion, and Metastasis. Cell (2006) 124(2):263–6. 10.1016/j.cell.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 310. Liu XQ, Kiefl R, Roskopf C, Tian F, Huber RM. Interactions Among Lung Cancer Cells, Fibroblasts, and Macrophages in 3D Co-Cultures and the Impact on MMP-1 and VEGF Expression. PloS One (2016) 11(5):e0156268. 10.1371/journal.pone.0156268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Miyake M, Hori S, Morizawa Y, Tatsumi Y, Nakai Y, Anai S, et al. CXCL1-Mediated Interaction of Cancer Cells With Tumor-Associated Macrophages and Cancer-Associated Fibroblasts Promotes Tumor Progression in Human Bladder Cancer. Neoplasia (2016) 18(10):636–46. 10.1016/j.neo.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Fiaschi T, Marini A, Giannoni E, Taddei ML, Gandellini P, De Donatis A, et al. Reciprocal Metabolic Reprogramming Through Lactate Shuttle Coordinately Influences Tumor-Stroma Interplay. Cancer Res (2012) 72(19):5130–40. 10.1158/0008-5472.CAN-12-1949 [DOI] [PubMed] [Google Scholar]
- 313. Fiaschi T, Giannoni E, Taddei ML, Cirri P, Marini A, Pintus G, et al. Carbonic Anhydrase IX From Cancer-Associated Fibroblasts Drives Epithelial-Mesenchymal Transition in Prostate Carcinoma Cells. Cell Cycle (2013) 12(11):1791–801. 10.4161/cc.24902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Kim HM, Jung WH, Koo JS. Expression of Cancer-Associated Fibroblast Related Proteins in Metastatic Breast Cancer: An Immunohistochemical Analysis. J Transl Med (2015) 13:222. 10.1186/s12967-015-0587-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Tham M, Khoo K, Yeo KP, Kato M, Prevost-Blondel A, Angeli V, et al. Macrophage Depletion Reduces Postsurgical Tumor Recurrence and Metastatic Growth in a Spontaneous Murine Model of Melanoma. Oncotarget (2015) 6(26):22857–68. 10.18632/oncotarget.3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Yin M, Li X, Tan S, Zhou HJ, Ji W, Bellone S, et al. Tumor-Associated Macrophages Drive Spheroid Formation During Early Transcoelomic Metastasis of Ovarian Cancer. J Clin Invest (2016) 126(11):4157–73. 10.1172/JCI87252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Valls AF, Shen Y, Schmidt T. A Core of Macrophages Facilitates Ovarian Cancer Metastases. Trans Cancer Res (2017) 6(1):S189–S96. 10.21037/tcr.2017.01.27 [DOI] [Google Scholar]
- 318. Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, et al. A Distinct Macrophage Population Mediates Metastatic Breast Cancer Cell Extravasation, Establishment and Growth. PloS One (2009) 4(8):e6562. 10.1371/journal.pone.0006562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Kruse J, von Bernstorff W, Evert K, Albers N, Hadlich S, Hagemann S, et al. Macrophages Promote Tumour Growth and Liver Metastasis in an Orthotopic Syngeneic Mouse Model of Colon Cancer. Int J Colorectal Dis (2013) 28(10):1337–49. 10.1007/s00384-013-1703-z [DOI] [PubMed] [Google Scholar]
- 320. Mukaida N, Zhang D, Sasaki SI. Emergence of Cancer-Associated Fibroblasts as an Indispensable Cellular Player in Bone Metastasis Proces. Cancers (Basel) (2020) 12(10). 10.3390/cancers12102896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Wang M, Su Z, Amoah Barnie P. Crosstalk Among Colon Cancer-Derived Exosomes, Fibroblast-Derived Exosomes, and Macrophage Phenotypes in Colon Cancer Metastasis. Int Immunopharmacol (2020) 81:106298. 10.1016/j.intimp.2020.106298 [DOI] [PubMed] [Google Scholar]
- 322. Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature (2015) 527(7578):329–35. 10.1038/nature15756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Nielsen SR, Schmid MC. Macrophages as Key Drivers of Cancer Progression and Metastasis. Mediators Inflammation (2017) 2017:9624760. 10.1155/2017/9624760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic Cancer Exosomes Initiate Pre-Metastatic Niche Formation in the Liver. Nat Cell Biol (2015) 17(6):816–26. 10.1038/ncb3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, et al. MMP9 Induction by Vascular Endothelial Growth Factor Receptor-1 Is Involved in Lung-Specific Metastasis. Cancer Cell (2002) 2(4):289–300. 10.1016/S1535-6108(02)00153-8 [DOI] [PubMed] [Google Scholar]
- 326. Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-Positive Haematopoietic Bone Marrow Progenitors Initiate the Pre-Metastatic Niche. Nature (2005) 438(7069):820–7. 10.1038/nature04186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, et al. Hypoxia-Induced Lysyl Oxidase is a Critical Mediator of Bone Marrow Cell Recruitment to Form the Premetastatic Niche. Cancer Cell (2009) 15(1):35–44. 10.1016/j.ccr.2008.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Brasil da Costa FH, Lewis MS, Truong A, Carson DD, Farach-Carson MC. SULF1 Suppresses Wnt3A-Driven Growth of Bone Metastatic Prostate Cancer in Perlecan-Modified 3D Cancer-Stroma-Macrophage Triculture Models. PloS One (2020) 15(5):e0230354. 10.1371/journal.pone.0230354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Giannoni E, Taddei ML, Parri M, Bianchini F, Santosuosso M, Grifantini R, et al. EphA2-Mediated Mesenchymal-Amoeboid Transition Induced by Endothelial Progenitor Cells Enhances Metastatic Spread Due to Cancer-Associated Fibroblasts. J Mol Med (Berl) (2013) 91(1):103–15. 10.1007/s00109-012-0941-9 [DOI] [PubMed] [Google Scholar]
- 330. Hurtado P, Martinez-Pena I, Pineiro R. Dangerous Liaisons: Circulating Tumor Cells (CTCs) and Cancer-Associated Fibroblasts (CAF). Cancers (Basel) (2020) 12(10). 10.3390/cancers12102861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Ren G, Zhao X, Wang Y, Zhang X, Chen X, Xu C, et al. CCR2-Dependent Recruitment of Macrophages by Tumor-Educated Mesenchymal Stromal Cells Promotes Tumor Development and is Mimicked by TNFalpha. Cell Stem Cell (2012) 11(6):812–24. 10.1016/j.stem.2012.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Yang F, Wei Y, Han D, Li Y, Shi S, Jiao D, et al. Interaction With CD68 and Regulation of GAS6 Expression by Endosialin in Fibroblasts Drives Recruitment and Polarization of Macrophages in Hepatocellular Carcinoma. Cancer Res (2020) 80(18):3892–905. 10.1158/0008-5472.CAN-19-2691 [DOI] [PubMed] [Google Scholar]
- 333. Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, et al. Tumor Induction of VEGF Promoter Activity in Stromal Cells. Cell (1998) 94(6):715–25. 10.1016/S0092-8674(00)81731-6 [DOI] [PubMed] [Google Scholar]
- 334. Linde N, Lederle W, Depner S, van Rooijen N, Gutschalk CM, Mueller MM. Vascular Endothelial Growth Factor-Induced Skin Carcinogenesis Depends on Recruitment and Alternative Activation of Macrophages. J Pathol (2012) 227(1):17–28. 10.1002/path.3989 [DOI] [PubMed] [Google Scholar]
- 335. Linde N, Casanova-Acebes M, Sosa MS, Mortha A, Rahman A, Farias E, et al. Macrophages Orchestrate Breast Cancer Early Dissemination and Metastasis. Nat Commun (2018) 9(1):21. 10.1038/s41467-017-02481-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Hanahan D, Coussens LM. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell (2012) 21(3):309–22. 10.1016/j.ccr.2012.02.022 [DOI] [PubMed] [Google Scholar]
- 337. Ireland L, Santos A, Campbell F, Figueiredo C, Hammond D, Ellies LG, et al. Blockade of Insulin-Like Growth Factors Increases Efficacy of Paclitaxel in Metastatic Breast Cancer. Oncogene (2018) 37(15):2022–36. 10.1038/s41388-017-0115-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Ireland L, Santos A, Ahmed MS, Rainer C, Nielsen SR, Quaranta V, et al. Chemoresistance in Pancreatic Cancer Is Driven by Stroma-Derived Insulin-Like Growth Factor. Cancer Res (2016) 76(23):6851–63. 10.1158/0008-5472.CAN-16-1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Andersson P, Yang Y, Hosaka K, Zhang Y, Fischer C, Braun H, et al. Molecular Mechanisms of IL-33-Mediated Stromal Interactions in Cancer Metastasis. JCI Insight (2018) 3(20). 10.1172/jci.insight.122375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Shani O, Vorobyov T, Monteran L, Lavie D, Cohen N, Raz Y, et al. Fibroblast-Derived IL33 Facilitates Breast Cancer Metastasis by Modifying the Immune Microenvironment and Driving Type 2 Immunity. Cancer Res (2020) 80(23):5317–29. 10.1158/0008-5472.CAN-20-2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Sun X, He X, Zhang Y, Hosaka K, Andersson P, Wu J, et al. Inflammatory Cell-Derived CXCL3 Promotes Pancreatic Cancer Metastasis Through a Novel Myofibroblast-Hijacked Cancer Escape Mechanism. Gut (2021). 10.1136/gutjnl-2020-322744 [DOI] [PubMed] [Google Scholar]
- 342. Leone RD, Emens LA. Targeting Adenosine for Cancer Immunotherapy. J Immunother Cancer (2018) 6(1):57. 10.1186/s40425-018-0360-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Hausler SF, Del Barrio IM, Diessner J, Stein RG, Strohschein J, Honig A, et al. Anti-CD39 and Anti-CD73 Antibodies A1 and 7G2 Improve Targeted Therapy in Ovarian Cancer by Blocking Adenosine-Dependent Immune Evasion. Am J Transl Res (2014) 6(2):129–39. [PMC free article] [PubMed] [Google Scholar]
- 344. Turcotte M, Spring K, Pommey S, Chouinard G, Cousineau I, George J, et al. CD73 is Associated With Poor Prognosis in High-Grade Serous Ovarian Cancer. Cancer Res (2015) 75(21):4494–503. 10.1158/0008-5472.CAN-14-3569 [DOI] [PubMed] [Google Scholar]
- 345. Mediavilla-Varela M, Luddy K, Noyes D, Khalil FK, Neuger AM, Soliman H, et al. Antagonism of Adenosine A2A Receptor Expressed by Lung Adenocarcinoma Tumor Cells and Cancer Associated Fibroblasts Inhibits Their Growth. Cancer Biol Ther (2013) 14(9):860–8. 10.4161/cbt.25643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-Derived Suppressor Cells in the Era of Increasing Myeloid Cell Diversity. Nat Rev Immunol (2021). 10.1038/s41577-020-00490-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Hasko G, Pacher P. Regulation of Macrophage Function by Adenosine. Arterioscler Thromb Vasc Biol (2012) 32(4):865–9. 10.1161/ATVBAHA.111.226852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The Polarization of Immune Cells in the Tumour Environment by TGFbeta. Nat Rev Immunol (2010) 10(8):554–67. 10.1038/nri2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Kitamura H, Kamon H, Sawa S, Park SJ, Katunuma N, Ishihara K, et al. IL-6-STAT3 Controls Intracellular MHC Class II Alphabeta Dimer Level Through Cathepsin S Activity in Dendritic Cells. Immunity (2005) 23(5):491–502. 10.1016/j.immuni.2005.09.010 [DOI] [PubMed] [Google Scholar]
- 350. Kitamura H, Ohno Y, Toyoshima Y, Ohtake J, Homma S, Kawamura H, et al. Interleukin-6/STAT3 Signaling as a Promising Target to Improve the Efficacy of Cancer Immunotherapy. Cancer Sci (2017) 108(10):1947–52. 10.1111/cas.13332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Dikov MM, Ohm JE, Ray N, Tchekneva EE, Burlison J, Moghanaki D, et al. Differential Roles of Vascular Endothelial Growth Factor Receptors 1 and 2 in Dendritic Cell Differentiation. J Immunol (2005) 174(1):215–22. 10.4049/jimmunol.174.1.215 [DOI] [PubMed] [Google Scholar]
- 352. Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, et al. Production of Vascular Endothelial Growth Factor by Human Tumors Inhibits the Functional Maturation of Dendritic Cells. Nat Med (1996) 2(10):1096–103. 10.1038/nm1096-1096 [DOI] [PubMed] [Google Scholar]
- 353. Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 Switches the Differentiation of Monocytes From Dendritic Cells to Macrophages. Nat Immunol (2000) 1(6):510–4. 10.1038/82763 [DOI] [PubMed] [Google Scholar]
- 354. Gunaydin G, Kesikli SA, Guc D. Cancer Associated Fibroblasts Have Phenotypic and Functional Characteristics Similar to the Fibrocytes That Represent a Novel MDSC Subset. Oncoimmunology (2015) 4(9):e1034918. 10.1080/2162402X.2015.1034918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 Expression by Tumour-Associated Macrophages Inhibits Phagocytosis and Tumour Immunity. Nature (2017) 545(7655):495–9. 10.1038/nature22396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Edin S, Wikberg ML, Rutegard J, Oldenborg PA, Palmqvist R. Phenotypic Skewing of Macrophages In Vitro by Secreted Factors From Colorectal Cancer Cells. PloS One (2013) 8(9):e74982. 10.1371/journal.pone.0074982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Wong PF, Wei W, Gupta S, Smithy JW, Zelterman D, Kluger HM, et al. Multiplex Quantitative Analysis of Cancer-Associated Fibroblasts and Immunotherapy Outcome in Metastatic Melanoma. J Immunother Cancer (2019) 7(1):194. 10.1186/s40425-019-0675-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Lorsakul A, Andersson E, Vega Harring S, Sade H, Grimm O, Bredno J. Automated Wholeslide Analysis of Multiplex-Brightfield IHC Images for Cancer Cells and Carcinoma-Associated Fibroblasts. SPIE; (2017). 10.1117/12.2254459 [DOI] [Google Scholar]
- 359. Huang H, Wang Z, Zhang Y, Brekken RA. Mesothelial Cell-Derived Antigen-Presenting Cancer-Associated Fibroblasts Induce Expansion of Regulatory T Cells in Pancreatic Cancer. bioRxiv (2021). 2021.02.04.429827. 10.1101/2021.02.04.429827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Yu M, Guo G, Huang L, Deng L, Chang CS, Achyut BR, et al. CD73 on Cancer-Associated Fibroblasts Enhanced by the A2B-Mediated Feedforward Circuit Enforces an Immune Checkpoint. Nat Commun (2020) 11(1):515. 10.1038/s41467-019-14060-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Wu K, Lin K, Li X, Yuan X, Xu P, Ni P, et al. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front Immunol (2020) 11:1731. 10.3389/fimmu.2020.01731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Stower H. New CARs in the Therapeutic Fleet. Nat Med (2020) 26(12):1804–5. 10.1038/s41591-020-01151-2 [DOI] [PubMed] [Google Scholar]
- 363. Mukhopadhyay M. Macrophages Enter CAR Immunotherapy. Nat Methods (2020) 17(6):561. 10.1038/s41592-020-0862-4 [DOI] [PubMed] [Google Scholar]
- 364. Laplagne C, Domagala M, Le Naour A, Quemerais C, Hamel D, Fournie JJ, et al. Latest Advances in Targeting the Tumor Microenvironment for Tumor Suppression. Int J Mol Sci (2019) 20(19). 10.3390/ijms20194719 [DOI] [PMC free article] [PubMed] [Google Scholar]