Abstract
Background.
The frequency of "exhausted" or check-point-positive (PD-1+CTLA-4+) cytotoxic lymphocytes (Tex) in the tumor microenvironment is associated with response to anti-PD-1 therapy in metastatic melanoma. The current study determined whether pretreatment Tex cells in locally advanced melanoma predicted response to neoadjuvant anti-PD-1 blockade.
Methods.
Pretreatment tumor samples from 17 patients with locally advanced melanoma underwent flow cytometric analysis of pretreatment Tex and regulatory T cell frequency. Patients who met the criteria for neoadjuvant checkpoint blockade were treated with either PD-1 monotherapy or PD-1/CTLA-4 combination therapy. Best overall response was evaluated by response evaluation criteria in solid tumors version 1.1, with recurrence-free survival (RFS) calculated by the Kaplan–Meier test. The incidence and severity of adverse events were tabulated by clinicians using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.
Results.
Of the neoadjuvant treated patients, 10 received anti-PD-1 monotherapy and 7 received anti-CTLA-4/PD-1 combination therapy. Of these 17 patients, 12 achieved a complete response, 4 achieved partial responses, and 1 exhibited stable disease. Surgery was subsequently performed for 11 of the 17 patients, and 8 attained a complete pathologic response. Median RFS and overall survival (OS) were not reached. Immune-related adverse events comprised four grade 3 or 4 events, including pneumonitis, transaminitis, and anaphylaxis.
Conclusion.
The results showed high rates of objective response, RFS, and OS for patients undergoing immune profile-directed neoadjuvant immunotherapy for locally advanced melanoma. Furthermore, the study showed that treatment stratification based upon Tex frequency can potentially limit the adverse events associated with combination immunotherapy. These data merit further investigation with a larger validation study.
Immunotherapy by both cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) blockade induces durable antitumor immune responses and recently has revolutionized the treatment of melanoma and other malignancies. This has translated to dramatically improved survival in the metastatic setting,1–6 prompting clinical trials of adjuvant immunotherapy for regionally metastatic melanoma.
The benefit of adjuvant checkpoint blockade has been confirmed in two prospective clinical trials with significant prolongation of recurrence-free survival.7,8 Neoadjuvant immune and targeted therapies for melanoma, explored recently in clinical trials,9 offer several advantages over surgery followed by adjuvant therapy. These advantages include reduced surgical morbidity, better evaluation of outcome, reduced duration of treatment, and consequently reduced side effects from systemic therapy.
A randomized neoadjuvant phase 2 study examining the use of anti-PD-1 alone or anti-PD-1/CTLA-4 combination therapy for high-risk resectable stage 3 melanoma further indicated the feasibility of this approach. The anti-PD-1 monotherapy elicited modest responses, with an overall response rate (ORR) of 25% and a complete pathologic response (pCR) rate of 25%. Toxicity was low, with 8% of patients having immune-related adverse events (irAEs).10 Although combined ipilimumab and nivolumab augmented clinical responses [ORR, 73%; pCR, 45% by response evaluation criteria in solid tumors (RECIST)], this was at the expense of substantial toxicity, with 73% of the patients experiencing grade 3 irAEs.10. Another prospective study comparing combined CTLA-4 and PD-1 blockade in the neoadjuvant and adjuvant setting reported similar findings, with pCR observed in 78% of the patients in the neoadjuvant arm of the study.11 However, 90% of the patients in both cohorts experienced one or more grade 3 or 4 irAEs.
Given the modest response rates with PD-1 monotherapy and the substantial toxicity associated with combination checkpoint blockade, a genuine concern lies in delaying surgery to pursue systemic treatment in the setting of potentially curable regional disease. Several biomarkers have been evaluated as predictors of immune response.12–15 Recent research has shown that the presence of exhausted T lymphocytes (Tex), which are T cells characterized by a dysfunctional state together with high expression of inhibitory receptors such as PD-1, correlates with response to anti-PD-1 monotherapy or combination therapy.16–18 Prior functional analyses indicated that Tex cells can be reinvigorated by immune checkpoint blockade.16,19–21 The current study examined the efficacy of neoadjuvant immunotherapy guided by immune profiling and T cell analysis.
METHODS
Study Design and Tumor Sample Acquisition
A retrospective study analyzed patients with locally advanced stage 3 or 4 oligometastatic melanoma who received neoadjuvant immunotherapy before intended surgical resection of their disease. To be included in the study, patients were required to have a pretreatment biopsy of their primary or locoregional disease within 30 days before the initiation of treatment. Samples were obtained after patients provided written consent under the University of California, San Francisco Committee on Human Research Protocol 138510.
Specimens were procured via core biopsy using a 16- or 18-gauge needle or punch biopsy using a 4-mm punch tool under sterile precautions. Fresh tumor samples were immediately placed on ice and transported to the laboratory for dissociation and analysis.
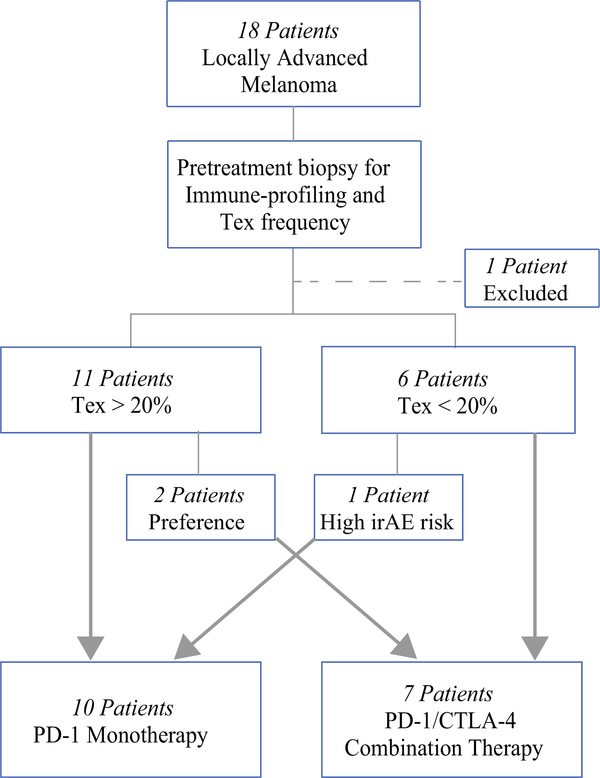
Between April 2013 and August 2018, 18 patients with locally advanced melanoma who received immunotherapy with neoadjuvant intent underwent pretreatment biopsy for immune profiling and Tex frequency determination as discussed in the “Flow Cytometric Analysis” section later. One patient was excluded due to an inadequate number of cells for analysis, defined as fewer than 200 CD8+ T cells within the live CD3+CD45+ gate. Of the patients with interpretable tumor-infiltrating lymphocyte profiling, nine received neoadjuvant treatment with anti-PD-1 antibody monotherapy (nivolumab or pembrolizumab), one underwent treatment with anti-PD-L1 antibody, and seven were treated with nivolumab in combination with ipilimumab. Treatment disposition was primarily determined by Tex frequency, with exceptions relating to patient preference or anticipated intolerance of combination immunotherapy.
Treatment Outcome Groups, Efficacy, and Adverse Events Analysis
Efficacy and immunologic data available as of August 2018 were included in all the analyses. The efficacy analysis was limited to best overall response (BOR), defined as the best tumor response according to RECIST version 1.1 criteria from the start of treatment to the time of disease progression or death. Recurrence free survival (RFS) was defined as the interval between the date of definitive surgery or complete response and the date of progression, death, or last clinic visit for which the patient was known not to have had radiographic or clinical progression. Overall survival (OS) was calculated as the time from the date of enrollment to the time of death or the last known date that the patient was known to be alive. Immune-related adverse events (irAEs) of any grade that occurred after initiation of neoadjuvant immunotherapy were extracted by retrospective chart review. The investigators determined the relatedness of an adverse event to treatment. The severity of adverse events was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
Flow Cytometric Analysis
Multiparameter flow cytometry was performed and analyzed on pretreatment samples obtained from metastatic tumors as previously described.17,18 Freshly isolated samples were minced and digested overnight with buffer consisting of collagenase type 4 (4188 Worthington Biochemical Corp, Lakewood, NJ), DNAse (SDN25–1G; Sigma-Aldrich, St. Louis, MO), 10% fetal bovine serum (FBS), 1% HEPES, and 1% penicillin–streptavidin in RPMI medium. Single-cell suspensions were double-filtered, centrifuged, and counted. Approximately 2 × 106 cells were stained with multiple fluorochrome-conjugated monoclonal antibodies (mAbs). The following antibodies were used, all from eBioscience unless otherwise stated: anti-human CD3 (anti-hCD3) (UCHT1), anti-hCD8 (RPA-T8), anti-hCD45 (HI30), anti-CD4 (SK3), anti-FOXP3 (PCH101), anti-hCTLA4 (14D3), anti-PD-1 (EH12.2H7; BioLegend, San Diego, CA), and LIVE/DEAD Fixable Aqua Dead Cell Stain Life Technologies (Thermo Fisher Scientific, Waltham, MA).
Data were acquired by an LSRFortessa (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (Tree Star Inc, Ashland, OR). All the samples were fresh and acquired by the LSRFortessa at different time points. Sphero Ultra Rainbow Beads (Spherotech, Lake Forest, IL) were used to calibrate and normalize to baseline-intensity voltages as samples were obtained at different time points. Gating strategy was determined using both isotype control antibody staining and an internal negative control cell population (i.e., PD-1 and CTLA-4 expression on CD3-cells). The frequency of exhausted T lymphocytes (Tex) within pretreatment samples was determined by calculating the percentage of CD8+ T cells that expressed both inhibitory receptors PD-1 and CTLA-4 within the total intratumoral CD8+ T cell population that was present. The study defined T regulatory cells as CD4+CD25+Foxp3+ T cells, and their proportion was calculated by comparing their frequency with the total population of intratumoral CD4+ T cells.
Statistical Analysis
Waterfall and spider plots were constructed with PRISM version 8 (Graphpad Inc, San Diego, CA). The patients had radiographic measurement of their target lesions evaluated at baseline and throughout their treatment. Change in tumor burden was defined as the percentage decrease in the summed reference diameters of the target lesion from baseline to nadir, observed up until the date of progression, as assessed by the investigator per RECIST version 1.1, the date of subsequent anticancer therapy (including tumor-directed surgery), or death, whichever occurred first. Both RFS and OS curves were constructed with the Kaplan–Meier method using PRISM (Graphpad Inc, San Diego, CA) version 8. Progression was recorded as the date of scans showing progression or the date that clinical progression or death was noted. All tests were two-sided, with P values lower than 0.05 considered statistically significant.
RESULTS
Patient Characteristics and Disposition
For this study, 17 patients with locally advanced melanoma met the inclusion criteria for analysis. All the patients received neoadjuvant immunotherapy. The baseline characteristics and demographics are presented in Table 1. In the neoadjuvant cohort, 88.2% of the patients had stage 3 disease, and 82.4% had disease that originated from a cutaneous primary. The two patients with stage 4 disease who underwent neoadjuvant therapy had limited metastatic disease and were considered potentially operable. Of the 17 patients, 71% were immunotherapy naïve, and 17.6% had previously received anti-CTLA-4 therapy with ipilimumab.
TABLE 1.
Baseline clinical characteristics of patients receiving neoadjuvant immunotherapy
| Variable | Neoadjuvant cohort (n = 17) n (%) |
|---|---|
| Age (years) | |
| Average | 53 |
| Range | 23–80 |
| Sex | |
| Male | 9 (52.9) |
| Female | 8 (47.1) |
| Primary site | |
| Cutaneous | 14 (82.4) |
| Mucosal | 1 (5.8) |
| Unknown | 2 (11.8) |
| Pretreatment stage | |
| 3 | 15 (88.2) |
| 4 | 2 (11.8) |
| Regional/distant disease | |
| Timing | |
| Synchronous | 5 (29.4) |
| Metachronous | 12 (70.6) |
| LDH (U/l)a | |
| Average | 239 |
| Range | 120–1452 |
| BRAF status | |
| Wild type | 13 (76.5) |
| Mutantb | 4 (23.5) |
| Neoadjuvant therapy | |
| PD-1 or PD-L1 | 10 (58.8) |
| PD-1/CTLA-4 | 7 (41.2) |
| Prior therapy | |
| Targeted therapy | |
| BRAF/MEK | 3 (17.6) |
| Immunotherapy | |
| PD-1 | 1 (5.8) |
| CTLA-4 | 3 (17.6) |
| Intralesional IL-12 | 1 (5.8) |
| None | 9 (52.9) |
Serum lactate dehydrogenase, normal range 105–333 U/L
Refers to V600E or V600K mutation
The disposition of the patients was primarily determined by the intratumoral frequency of exhausted T cells (Tex). A value greater than 20% was predictive of a response to anti-PD-1 monotherapy, and a value greater than 3% was predictive of combination anti-CTLA-4 and PD-1 therapy as previously reported.17,18
In keeping with these previously defined parameters, 10 patients were treated with either anti-PD-1 or anti-PD-L1 monotherapy, whereas the remaining 7 patients received combination immunotherapy with anit-CTLA-4 and anti-PD-1 (Fig. 1). Abundance of T regulatory cells (Treg) also was measured in pretreatment samples, and we sought to determine whether Tex and Treg frequencies were predictive of treatment response.
FIG. 1.
Patient neoadjuvant immunotherapy disposition. For 18 patients with locally advanced melanoma, pretreatment biopsy was performed for immune-profiling and Tex frequency determination. One patient was excluded because of a pretreatment biopsy with an inadequate number of cells for analysis. The patients were stratified to a neoadjuvant immunotherapy group determined by their Tex frequency. The patients with Tex higher than 20% received PD-1 monotherapy, and those with Tex lower than 20% received anti-PD-1/CTLA-4 combination therapy. Three patients had neoadjuvant regimens not aligned with their Tex frequency due to patient preference or anticipated poor tolerance of the risk for immune-related adverse events (irAEs) with combination immunotherapy. Nine patients received neoadjuvant treatment with anti-PD-1 monotherapy. One patient received anti-PD-L1 monotherapy (grouped with anti-PD-1 for simplification), and seven patients received anti-PD-1/CTLA-4 combination therapy
Other factors that influenced treatment disposition included patient preference and expected tolerance of immunotherapy. In one case, a 55-year-old man who had stage 3 V600E BRAF WT melanoma and human immunodeficiency virus (HIV) with a Tex frequency of 18.6% (Table S1; patient 11) was thought to be at high risk for irAEs due to his HIV history. Because his Tex frequency was marginal and his risk of adverse events was high, he received PD-1 monotherapy and achieved a complete response. Relatedly, two patients in our cohort received treatment with combination PD-1/CTLA-4 therapy due to patient and provider preference. The first patient was a 44-year-old man (Table S1; patient 13) with stage 3C V600E BRAF WT melanoma who presented with a painful, rapidly enlarging axillary mass. Given the rapid progression of his disease and the severity of his symptoms, combination PD-1/CTLA-4 therapy was initiated at the discretion of his treating physician per his preference despite a Tex frequency of 42.3%. Similarly, a 52-year-old woman who had stage 4 V600E BRAF mutant melanoma with a Tex frequency of 34.1% (Table S1; patient 12) presented with a solitary pulmonary metastasis and elected to pursue combination immunotherapy due to her circumstances. She elected to pursue the Food and Drug Administration (FDA)-approved treatment with the highest reported efficacy, fully aware of the increased risk for irAEs.
Patient Outcomes from Immune Profile-Directed Neoadjuvant Immunotherapy
We have previously reported that the relative abundance of tumor-infiltrating Tex cells correlates with response to anti-PD-1 antibodies, both as monotherapy and in combination with ipilimumab in the metastatic setting. However, whether this metric is capable of predicting response in the context of advanced locoregional disease remains unknown. We therefore quantified Tex frequency and BOR by RECIST version 1.1 criteria to neoadjuvant immunotherapy either as anti-PD-1 monotherapy (n = 10) or in combination anti-CTLA-4 (n = 7) (Table 2).
TABLE 2.
Pretreatment immune profiles and treatment response of patients receiving neoadjuvant immunotherapy
| Tex percentage (average ± SD) | Treg percentage (average ± SD) | ORR patients (%) | |
|---|---|---|---|
| Neoadjuvant cohort | 25.7 ± 14.5 | 18.4 ± 11.2 | 16 (94.1) |
Tex, exhausted T lymphocytes, CD8+PD-1+CTLA-4+; Treg, regulatory T lymphocytes, CD4+CD25+Foxp3+; ORR, overall response rate to treatment as evaluated by RECIST v1.1
The patients who received neoadjuvant immunotherapy were enriched for high Tex with a mean frequency of 25.7%. The overall response rate determined by BOR was 94.1% compared with the previously reported overall response rate of 42% for pembrolizumab, 40% for nivolumab, and 58% for combination therapy.8,9 Of the 16 patients who responded, 12 achieved a complete response, and 4 were partial responders. Six complete responders underwent subsequent resection of the initially involved regional lymph node basin with no evidence of malignancy, confirming pCR. An additional patient with a stable oligometastatic adrenal mass that was no longer 18-Fluoro-2-deoxy-D-glucose positron emission tomography-avid also underwent resection of the residual mass, which demonstrated no evidence of viable tumor consistent with pCR. The remaining five patients opted against surgery given a negative on-treatment biopsy pathologic result and a complete radiographic response. Three of the four partial responders underwent consolidative resection, with the remaining patient opting to pursue brachytherapy.
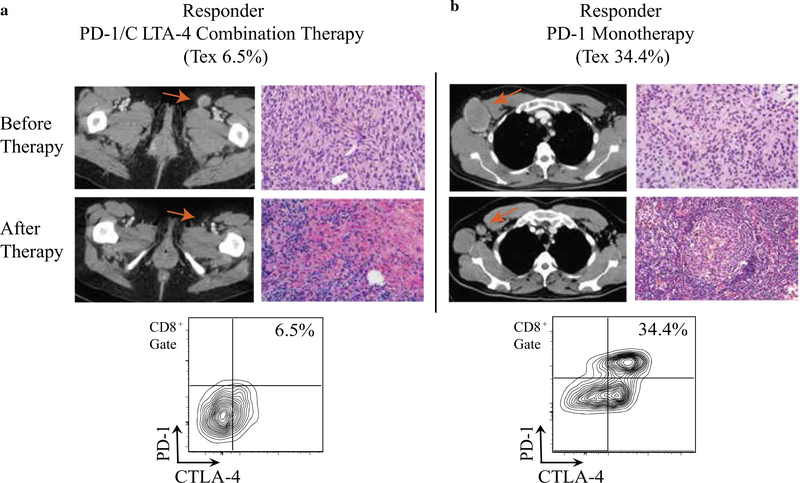
Figure 2 shows a patient with a Tex fraction of 6.54% who responded to combination immunotherapy (Fig. 2a) versus a patient with a Tex fraction of 34.4% who responded to PD-1 monotherapy (Fig. 2b), confirming our previously reported observation that a lower Tex threshold is required to mount an effective antitumor immune response to combination therapy. The solitary patient who did not respond to neoadjuvant immunotherapy initially presented with locally advanced V600E BRAF mutant stage 3C disease and involvement of the right inguinal lymph node basin (Fig. S1). Neoadjuvant PD-1 monotherapy was initiated for a pretreatment Tex of 33.1%, and the patient had a BOR of stable disease by RECIST version 1.1 criteria. He therefore underwent surgical salvage of the involved lymph node basin, and his surgical pathology was consistent with viable tumor. He relapsed locally 1 year later with the development of subcutaneous lesions in the right thigh. Subsequently, he was salvaged with the targeted MEK/BRAF inhibitors trametinib and dabrafenib and at this writing remains disease-free. Although his suboptimal response to PD-1 immunotherapy remains unclear, his pretreatment tumor biopsy notably had a markedly elevated Treg fraction at 41.1%.
FIG. 2.
Examples of treatment response for two responders treated with neoadjuvant immunotherapy. a A patient with inguinal lymph node disease and a Tex frequency of 6.5% (as shown in the flow cytometry plot) who responded to anti-PD-1/CTLA-4 combination immunotherapy. b A patient with axillary lymph node disease and a Tex of 34.4% who responded to anti-PD-1 monotherapy. In both panels, the pre- and posttreatment imaging (computed tomography) and histology are shown, demonstrating marked radiographic and histologic responses. The red arrows mark the radiographic response
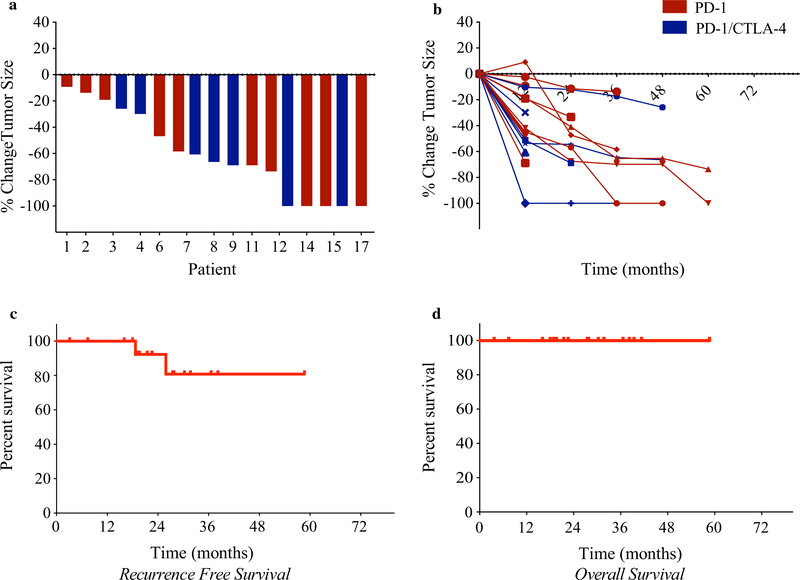
Overall, the vast majority of the patients experienced deep responses to treatment, with reduction in tumor burden exceeding 50% from baseline (Fig. 3a, b). The median RFS (defined as time of systemic treatment initiation to date of relapse or last known follow-up visit) was not reached (Fig. 3c). All the patients receiving neoadjuvant immunotherapy were alive at the time of the last follow-up visit (Fig. 3d). Notably, in agreement with our previous data for metastatic patients, Treg frequency was not independently predictive of response to immunotherapy (data not shown).
FIG. 3.
Reduction in tumor burden and survival benefit for patients receiving neoadjuvant immunotherapy. a A waterfall plot depicting the maximum percentage change in tumor size with neoadjuvant immunotherapy versus the pretreatment baseline for each patient. The x-axis represents the individual patients in the study. b Spider plots depicting percentage change in tumor burden per RECIST version 1.1 during each determined assessment period. Each line represents an individual patient. c Kaplan–Meier estimates of relapse-free survival (RFS) among patients receiving neoadjuvant immunotherapy. The median RFS was not reached. d Kaplan–Meier estimates of overall survival among patients receiving neoadjuvant immunotherapy compared with the adjuvant cohort. The median overall survival was not reached
Safety Profile
As with any medical intervention, analysis of both the risks and the benefits of the proposed approach must be carefully considered. Indeed, these calculations become even more critical when the clinician is faced with the option of proceeding directly to definitive resection and potential cure in the absence of any further systemic therapy. We therefore assessed the toxicity profile of neoadjuvant immunotherapy for patients with advanced locoregional disease. Of the 17 patients, 12 (70.6%) experienced adverse effects while receiving systemic therapy (Table 3). However, clinically severe (grade 3 or 4) events were uncommon and observed in only three (17.6%) of the patients undergoing treatment, predominantly among those receiving combination immunotherapy. These clinically severe events included an infusion reaction to anti-PD-L1 monotherapy as well as autoimmune mediated transaminitis and pneumonitis from the PD-1/CTLA-4 combination therapy. The most common grade 1 or 2 adverse effects reported were diarrhea (23.5%), arthralgias (17.6%) and pruritus (17.6%). Neoadjuvant treatment, especially anti-PD-1 antibody monotherapy, was generally well tolerated, showing an adverse event profile comparable with that of previously reported large phase 3 clinical studies.1–7
TABLE 3.
Immune-related adverse events (irAEs) associated with neoadjuvant immunotherapy
| irAE | Any (%) | Grade 3 or 4 (%) |
|---|---|---|
| Arthralgia | 3 (17.6) | 0 (0) |
| Abdominal pain | 1 (5.8) | 0 (0) |
| Rash | 2 (11.8) | 0 (0) |
| Pruritus | 3 (17.6) | 0 (0) |
| Fatigue | 2 (11.8) | 0 (0) |
| Diarrhea | 3 (17.6) | 0 (0) |
| Hypothyroidism | 2 (11.8) | 0 (0) |
| Pneumonitis | 1 (5.8) | 1 (5.8) |
| Transaminitis | 2 (11.8) | 1 (5.8) |
| Infusion reaction | 1 (5.8) | 1 (5.8) |
| Any event leading to delay in therapy | 1 (5.8) | 1 (5.8) |
irAE, immune-related adverse event as graded by CTACE v4.0
DISCUSSION
The increasing use of immune checkpoint inhibitors has drastically changed the treatment algorithm for advanced melanoma. These gains were initially established in the metastatic setting and currently demonstrate great benefit in the adjuvant setting.1–4 As with any intervention offered in a potentially curative setting, the benefit of improved survival must be carefully weighed against the risks of significant toxicity associated with these agents and disease progression with treatment that could preclude surgical resection. Therefore, patient selection using reliable predictive biomarkers is imperative for the success of this strategy because tumor PD-L1 expression level by immunohistochemical staining has not been validated in the neoadjuvant setting and has a low resolution3,4 in the context of metastatic disease.
Recent reports have shown that the frequency of Tex in freshly isolated melanoma tumors strongly correlate with response to both single-agent and combination checkpoint blockade in advanced melanoma, with a positive predictive value of 82% and a negative predictive value of 100%.17,18 Indeed, patients with high Tex frequency receiving neoadjuvant immunotherapy achieved response rates in excess of those generally reported. Notably, five patients who achieved complete radiographic responses by RECIST version 1.1 criteria declined surgery, and all remained disease-free during a median follow-up period of 27.6 months. Therefore, in our data set, pCR and radiographic response by RECIST criteria appeared to serve as equally predictive markers of recurrence-free response.
Neoadjuvant anti-PD-1 therapy was generally well tolerated, but irAEs of grade 3 severity or higher were noted for two of the seven patients receiving neoadjuvant combination immunotherapy. Although this toxicity profile may be acceptable in the metastatic setting, the risks of combined CTLA-4 and PD-1 blockade may not outweigh the survival benefits of this approach in the curative setting. Moreover, given the efficacy of combination immunotherapy in the context of metastatic disease,1–4 the risk of ipilimumab exposure with a lower disease burden, particularly with locoregional disease, may not be justified. Overall, our data suggest that if Tex frequency is greater than 20%, PD-1 inhibitor monotherapy may be sufficient. However, a Tex frequency of less than 3% requires careful consideration of patient-specific factors in the decision whether to pursue combination checkpoint blockade or to proceed directly to surgical resection. Further investigation with a larger prospective cohort validation study is required to address this question.
Recent study indicates that rapid, durable responses to even a single dose of nivolumab are associated with the early expansion of a Tex subset in the peripheral blood. At 3 weeks, these expanded cells have a immunophenotype similar to that of pretreatment baseline cells, which is highly suggestive of systemic reinvigoration of a preexisting Tex cell pool.16 This observation is also in agreement with the increased T cell diversity and higher clonality of responders to single-agent PD-1 blockade, suggestive of a more diverse T cell repertoire compared with the more heterogenous pattern of clonality displayed by responders to combination immunotherapy.12 However, the complex interplay of these adaptive subsets with one another and the tissue-resident antigen-presenting cells in the tumor microenvironment to coordinate effective tumor rejection remains incompletely understood, and further studies of other tumor types and animal models are necessary for further definition of these relationships. Moreover, a comprehensive understanding of the global immune dynamics and anatomic sites driving human anti-tumor immunity has yet to be established, and future preclinical and translational efforts are necessary to address these fundamental questions and further improve patient outcomes.
Neoadjuvant immunotherapy has gained increasing traction in the treatment of locally advanced melanoma, with several early-phase studies indicating the efficacy of this approach.9,10,21,22 However, when performed without stratification, these studies have uniformly demonstrated the toxicity of combination checkpoint blockade and poor response rates for PD-1 inhibitor monotherapy in this context. This is in contrast to certain patients for whom this approach has proved to be quite effective, with responses observed to even a single dose of treatment. Our approach permits an upfront pretreatment stratification option that may enhance response and limit toxicity if applied in future studies.
Supplementary Material
ACKNOWLEDGMENT
We gratefully acknowledge the patients who participated in this study. This work was supported by grants to Michael D. Rosenblum (NIH DP2-AR068130, K08-AR062064, AR066821 Burroughs Wellcome CAMS-1010934), Adil I. Daud (Amoroso and Cook fund, Parker Institute for Cancer Immunotherapy), and Lauren S. Levine (Conquer Cancer Foundation Young Investigator Award CA-0122026). Kelly M. Mahuron was supported by the NIH T32 training grant in Gastrointestinal Surgery T32DK007573.
DISCLOSURES Alain P. Algazi discloses institutional research funding from Novartis, Merck, BMS OncoSec, Acerta, AstraZeneca, MedImmune, Tessa, Celldex, and Celgene; consulting with stock options for Oncosec and Valitor; paid advisory board positions for Array and Regeneron; and an unpaid advisory role with Sensei Biotherapeutics. Adil I. Daud discloses research funding from the Amoroso and Cook fund, Parker Institute for Cancer Immunotherapy, Novartis, Merck, BMS, Incyte, AbbVie, OncoSec, Xencor, Pfizer, Roche/Genentech, and Exelixis; Advisory Board participation from Amgen, Array, and Roche/Genentech; and stock ownership in Trex Bio and Pionyr Immunotherapeutics. Matthew F. Krummel is the founder and board member of Pionyr Immunotherapeutics. Lauren S. Levine has received research funding from the Conquer Cancer Foundation CA-0122026. Kelly M. Mahuron received support by the NIH T32 training grant in Gastrointestinal Surgery T32DK007573. Michael D. Rosenblum is a consultant with equity ownership for Trex Bio, holds equity in Sitryx Bio, and has received funding on grants NIH DP2-AR068130, K08-AR062064, AR066821 Burroughs Wellcome CAMS-1010934. Matthew H. Spitzer discloses research funding from Genentech/Roche, Pfizer, Valitor Inc., and Bristol-Myers Squibb, and is a paid consultant for Five Prime Therapeutics, Ono Pharmaceutical, and January, Inc. Katy K. Tsai discloses institutional research funding from BMS, Parker Institute for Cancer Immunotherapy, Oncosec, and Regeneron. The remaining authors have no conflicts of interest.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1245/s10434-020-08648-7) contains supplementary material, which is available to authorized users.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
REFERENCES
- 1.Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;105:3005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–17. [DOI] [PubMed] [Google Scholar]
- 6.Ribas A, Hamid O, Daud A, et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600–9. [DOI] [PubMed] [Google Scholar]
- 7.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–35. [DOI] [PubMed] [Google Scholar]
- 8.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–801. [DOI] [PubMed] [Google Scholar]
- 9.Amaria RN, Menzies AM, Burton EM, et al. Neoadjuvant systemic therapy in melanoma: recommendations of the International Neoadjuvant Melanoma Consortium. Lancet Oncol. 2019;20:e378–89. [DOI] [PubMed] [Google Scholar]
- 10.Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med. 2018;24:1649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rozeman EA, Menzies AM, van Akkooi ACJ, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019;20:948–60. [DOI] [PubMed] [Google Scholar]
- 12.Carlino MS, Long GV, Schadendorf D, et al. Outcomes by line of therapy and programmed death ligand 1 expression in patients with advanced melanoma treated with pembrolizumab or ipilimumab in KEYNOTE-006: a randomised clinical trial. Eur J Cancer. 2018;101:236–43. [DOI] [PubMed] [Google Scholar]
- 13.Daud AI, Wolchok JD, Robert C, et al. Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 2016;34:4102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGranahan N, Furness AJS, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016;351:1463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daud AI, Loo K, Pauli ML, et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J Clin Investig. 2016;126:3447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loo K, Tsai KK, Mahuron K, et al. Partially exhausted tumor-infiltrating lymphocytes predict response to combination immunotherapy. JCI Insight. 2017;2(14):e93433. 10.1172/jci.insight.93433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pauken KE, Sammons MA, Odorizzi PM, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354:1160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang AC, Orlowski RJ, Xu X, et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat Med. 2019;25:454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24:1655–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.