Abstract
Background:
Among patients without an indication for a pacemaker, current evidence is inconclusive whether a dual-chamber implantable cardioverter-defibrillator (ICD) is superior to a single-chamber ICD. The current use of dual-chamber ICDs is not well characterized.
Methods:
We conducted a cross-sectional study exploring hospital-level variation in the use of dual-chamber ICDs across the United States. Patients receiving a primary prevention ICD from 2006 through 2009 without a documented indication for a pacemaker were included. Multivariate hierarchical logistic regression was used to explore patient, health care provider, and physician factors related to the use of a dual-chamber device.
Results:
Dual-chamber devices were implanted in 58% of the 87 115 patients without a pacing indication among 1293 hospitals, with hospital rates ranging from 0% in 33 centers to 100% in 109 centers. In multivariate analysis, geographic region was a strong independent predictor of dual-chamber device use, ranging from 36.4% in New England (reference region) to 66.4% in the Pacific region (odds ratio [OR], 5.25; 95% CI, 3.35-8.21). Hospital clustering was assessed using a median OR which was 3.96, meaning that 2 identical patients at different hospitals would have nearly a 4-fold difference in their chance of receiving a dual-chamber ICD.
Conclusions:
Use of dual-chamber ICDs for the primary prevention of sudden cardiac death among patients without an indication for permanent pacing varies markedly at the hospital level in the United States. This is a clear example of how practice can vary independent of patient factors.
Implantable cardioverter-defibrillators (ICDs) reduce mortality among patients with heart failure and left ventricular systolic dysfunction.1–3 As a result, the Center for Medicare and Medicaid Services (CMS) provides coverage for ICDs for the primary prevention of sudden cardiac death in selected patients.4 However, because most of the major clinical trials evaluated predominantly single-lead devices,1–3 the CMS National Coverage Decision for ICDs states that “providers must be able to justify the medical necessity of devices other than single-lead devices.”5 Dual-chamber ICDs, devices with a lead in the right atrium and right ventricle, are clearly indicated in patients receiving an ICD who also require pacing for bradyarrhythmias, such as heart block or symptomatic sinus node dysfunction.6 However, a recent study7 found that among patients undergoing ICD implantation for primary prevention in the United States and not receiving biventricular ICDs, nearly two-thirds received dual-chamber devices.
Dual-chamber devices may provide benefits for patients who do not have an indication for pacing.8 However, these postulated benefits have not been established in clinical trials. Enhanced rhythm detection is a benefit of dual-chamber ICDs, yet the extent to which this enhancement decreases inappropriate shocks remains controversial.9–15 Furthermore, although absolute differences are small, dual-chamber ICDs are associated with more frequent periprocedural complications (3.17% vs 2.11%; P < .001) and in-hospital mortality (0.40% vs 0.23%; P < .001).7 Thus, it remains unclear whether dual-chamber devices provide net benefit for patients without an indication for permanent pacing.
Geographic variability in the use of dual-lead devices among patients without a documented indication for permanent pacing is not well characterized; furthermore, the influences of patient, health care provider, hospital, and regional factors on the use of dual-chamber devices in community settings across the United States have not been described. Accordingly, this study explored hospital and regional level variation in the use of dual-chamber ICDs among patients without documented indications for pacing and defined the patient and physician factors associated with dual-chamber ICD placement in the United States.
METHODS
DATA SOURCES
Data from the National Cardiovascular Data Registry (NCDR) ICD Registry were used for this analysis.16 Participation in this registry is mandated by the CMS for reimbursement for all Medicare primary prevention ICDs, and implanting centers are required to enter complete data to receive Medicare reimbursement. However, more than 75% of hospitals report data on all ICD implantations (irrespective of indication and payer).17 The registry contains information on patient, health care provider, and hospital characteristics.
STUDY POPULATION
All patients receiving a first-time ICD for primary prevention from 2006 and 2009 were included. Patients receiving an ICD for secondary prevention, including those with a history of syncope, cardiac arrest, or sustained ventricular tachycardia, were excluded. We also excluded patients with an indication for permanent or biventricular pacing, specifically those with (1) a QRS interval duration of at least 120 milliseconds because these patients would be eligible for a biventricular pacer-ICD; (2) documented abnormal sinus node function; (3) second- or third-degree atrioventricular block; or (4) prior pacemaker implantation. To avoid the influence of low-volume outliers, patients treated at hospitals where fewer than 20 total ICDs received implants during the study period were also excluded.
GEOGRAPHIC REGIONS
Two types of regions were used for the analysis. For the multivariate model, the country was divided into 9 regions as defined by the US Census18 (New England, Mid Atlantic, South Atlantic, Northeast Central, Southeast Central, Northwest Central, Southwest Central, Mountain, and Pacific).
Hospital referral regions (HRRs) were used to explore smaller regional patterns of use of discretionary dual-chamber ICDs. A total of 306 different HRRs have been defined in the United States by the Dartmouth Atlas19 project and are based on referral patterns for tertiary care using major cardiovascular and neurosurgical procedures from the Medicare claims data. By using referral patterns to define regions, HRRs approximate a local culture of practice and are the standard in geographic variation research.20–22
OUTCOME MEASURE AND PREDICTOR VARIABLES
The primary outcome measure was implantation of a dual-chamber ICD. Candidate predictor variables included patient (demographic and clinical), health care provider, and hospital characteristics, including the geographic region described in the previous subsection. The registry contains information on patient characteristics, including demographics and cardiac history; procedural characteristics, including indications, device details, diagnostic studies, and complications; and characteristics of the index hospitalization, including other cardiac procedures and discharge medications using standardized data elements and definitions. Hospital characteristics were obtained from the hospital profile managed by the NCDR and include address, financing, community area, bed number, teaching status, ICD volume, and presence of an EP laboratory. The registry has also been supplemented with information on health care provider volume, and certification.23,24 Health care provider certification was determined with the American Board of Internal Medicine, Society for Thoracic Surgeons, and American College of Surgeons databases to determine physician certification, categorized as electrophysiologist (EP), non-EP, cardiologist, thoracic surgeon, and other.
STATISTICAL ANALYSIS
Patients receiving single-chamber ICDs were compared with those receiving dual-chamber ICDs, using χ2 tests for dichotomous outcomes and t tests for continuous outcomes.
Multivariate hierarchical logistic regression was used to determine the relationship between dual-chamber ICD and patient, health care provider, and hospital characteristics. Hierarchical models were used to account for the clustering of patients within physicians or hospitals. Physician and hospital clustering could not be accounted for in the same model because many physicians work in multiple hospitals. Therefore, 2 separate models were constructed, 1 accounting for clustering by physician and 1 for clustering by hospital. All variables in Table 1 were considered as candidates. Backward selection was used to determine which variables were retained in the final model (P < .05). Variables in the final model include age (<65, 65-75, or >75 years), sex, race/ethnicity (white, black, Hispanic, other), and select comorbidities (lung disease, atrial fibrillation, diabetes mellitus, and hemodialysis); health care provider variables included physician specialty (board-certified EP, fellowship-trained EP, surgeon, or credentialed other); hospital variables included American Heart Association region and profit type (government, university, or private).
Table 1.
Baseline Characteristics Stratified by Type of Implantable Cardioverter-Defibrillator (ICD)
| Characteristic | Total (n = 87 115) |
DC ICD (n = 50 626) |
SC ICD (n = 36 489) |
P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (SD), y | 62.81 (13.17) | 64.13 (12.72) | 60.97 (13.55) | <.001 |
| Female, No. (%) | 23 763 (27.28) | 13 410 (26.49) | 10 353 (28.37) | <.001 |
| Race/ethnicity, No. (%) | ||||
| White | 61 382 (70.46) | 36 543 (72.18) | 24 839 (68.07) | <.001 |
| Black | 17 115 (19.65) | 9041 (17.86) | 8074 (22.13) | |
| Hispanic | 5466 (6.27) | 3169 (6.26) | 2297 (6.30) | |
| Other | 3152 (3.62) | 1873 (3.70) | 1279 (3.51) | |
| Comorbidities, No. (%) | ||||
| Atrial fibrillation/atrial flutter | 14 804 (16.99) | 9942 (19.64) | 4862 (13.32) | <.001 |
| Cerebrovascular disease | 10 784 (12.38) | 6558 (12.95) | 4226 (11.58) | .06 |
| Chronic lung disease | 19 561 (22.45) | 11 835 (23.38) | 7726 (21.17) | <.001 |
| Diabetes mellitus | 32 939 (37.81) | 19 134 (37.79) | 13 805 (37.83) | .07 |
| Hypertension | 66 090 (75.87) | 39 226 (77.48) | 26 864 (73.62) | <.001 |
| Renal failure–hemodialysis | 3313 (3.80) | 1856 (3.67) | 1457 (3.99) | <.001 |
| Physician characteristics | ||||
| Physician volume, mean (SD), No. | 54.22 (42.73) | 52.89 (40.93) | 56.07 (45.03) | <.001 |
| EP operator ICD training, No. (%) | ||||
| Board-certified EP | 60 514 (74.05) | 34 654 (72.53) | 25 860 (76.18) | <.001 |
| EP fellowship only | 5157 (6.31) | 2943 (6.16) | 2214 (6.52) | |
| Surgery boards | 1644 (2.01) | 1130 (2.37) | 514 (1.51) | |
| Pediatrics cardiology boards | 66 (0.08) | 47 (0.10) | 19 (0.06) | |
| Credentialeda | 8917 (10.91) | 5683 (11.89) | 3234 (9.53) | |
| Other | 5425 (6.64) | 3321 (6.95) | 2104 (6.20) | |
| Hospital characteristics | ||||
| Hospital annual volume, mean (SD) | 213.2 (152.9) | 210.4 (151.8) | 217.1 (154.4) | <.001 |
| Geographic location, No. (%)b | ||||
| New England | 3449 (3.96) | 1254 (2.48) | 2195 (6.02) | <.001 |
| Mid Atlantic | 12 951 (14.88) | 5887 (11.64) | 7064 (19.38) | |
| South Atlantic | 20 819 (23.92) | 12 224 (24.17) | 8595 (23.58) | |
| Northeast Central | 15 734 (18.08) | 9546 (18.87) | 6188 (16.97) | |
| Southeast Central | 7867 (9.04) | 4636 (9.17) | 3231 (8.86) | |
| Northwest Central | 6479 (7.44) | 4463 (8.82) | 2016 (5.53) | |
| Southwest Central | 9571 (11.00) | 5819 (11.50) | 3752 (10.29) | |
| Mountain | 3518 (4.04) | 2340 (4.63) | 1178 (3.23) | |
| Pacific | 6649 (7.64) | 4412 (8.72) | 2237 (6.14) | |
| Profit type, No. (%) | ||||
| Government | 1574 (1.81) | 1091 (2.16) | 483 (1.32) | <.001 |
| Private/community | 72 559 (83.29) | 43 173 (85.28) | 29 386 (80.53) | |
| University | 12 982 (14.90) | 6362 (12.57) | 6620 (18.14) | |
| Community, No. (%) | ||||
| Rural | 8796 (10.10) | 5107 (10.09) | 3689 (10.11) | <.001 |
| Suburban | 23 561 (27.05) | 14 273 (28.19) | 9288 (25.45) | |
| Urban | 54 758 (62.86) | 31 246 (61.72) | 23 512 (64.44) | |
| Patient beds, mean (SD), No. | 481.2 (255.4) | 468.0 (249.4) | 499.5 (262.4) | <.001 |
| Teaching | 48 282 (55.42) | 26 413 (52.17) | 21 869 (59.93) | <.001 |
| With EP laboratory | 58 634 (67.31) | 34 503 (68.15) | 24 131 (66.13) | <.001 |
Abbreviations: DC, dual-chamber; EP, electrophysiologist; SC, single-chamber.
Credentialed refers to an alternative pathway for non-EP physicians to be “credentialed” to perform ICD implants. It was strictly voluntary and has subsequently expired, such that no additional physicians will be characterized as being credentialed through this alternative pathway.
As defined by the US Census.18
To determine the proportion of total variance in outcomes attributable to clustering within physicians or hospitals, we calculated the intraclass correlation (ICC) coefficient before adjustment, after adjusting for patient factors, and after adjusting for patient, health care provider, and hospital factors in the 2 models. If variation could be explained by adjusting for any of these covariates, then the ICC should decrease as the covariates are added to the model. To further quantify the extent to which observed variation was due to clustering of patients within physicians or hospitals, the median odds ratio (MOR) was also calculated for the 2 models.25 The MORs are always greater than 1, do not have 95% CIs, and are more easily interpretable than ICCs for examining clustering effects from hierarchical models.26 For example, an MOR of 1.0 would signify no clustering effects, whereas an MOR of 2.0 would signify that a patient has 2-fold higher odds of receiving a dual-chamber ICD if he or she went to another randomly selected physician or hospital.
Many consider paroxysmal atrial fibrillation to be an indication for a dual-chamber ICD and permanent atrial fibrillation to be an indication for a single-chamber device.27 Because we could not distinguish permanent from paroxysmal atrial fibrillation in the registry, we repeated the analysis excluding all patients with atrial fibrillation. To further assess the robust-ness of the findings, we repeated the analysis but restricted it to the Medicare population because this is the population for which there is complete enrollment in the registry.
Statistical analyses were performed using the SAS statistical package (version 9.2; SAS Institute, Cary, North Carolina) and STATA 10.0 (StataCorp LP, College Station, Texas). Use of the NCDR database was approved by the ICD Registry Research and Publications Committee and analysis was approved by the Yale University School of Medicine Human Investigation Committee, New Haven.
RESULTS
From 2006 through 2009, 239 113 first-time primary prevention ICDs were entered in the ICD registry. After excluding 151 665 patients with an indication for a dual-chamber device (permanent or biventricular pacing indication) and 333 patients receiving their ICD at a low-volume hospital, a total of 87 115 patients were eligible for a single- or dual-chamber ICD (Figure 1).
Figure 1.
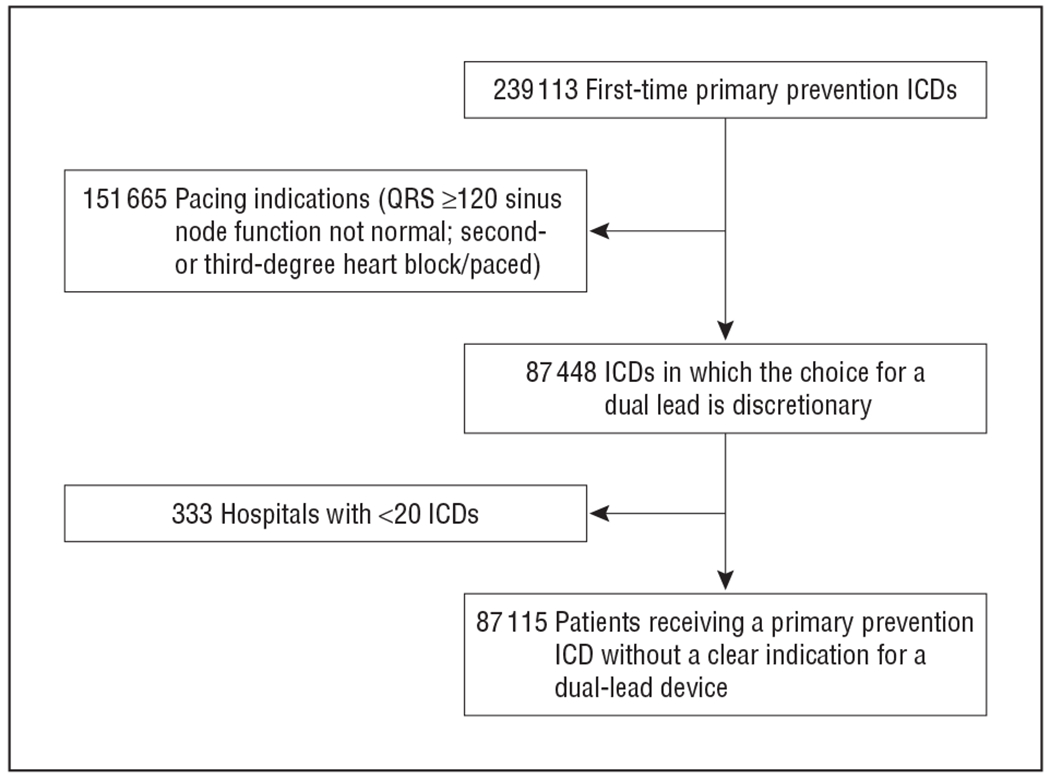
Study sample. ICD, implantable cardioverter-defibrillator; NCDR, National Cardiovascular Data Registry; QRS, QRS interval duration.
The use of dual-chamber devices occurred in 58% of the patients (50 626) without pacing indications across 1293 hospitals (Table 1). The proportion of dual-chamber devices increased steadily over the study period from 53% in the first quarter of 2006 to 62% in the fourth quarter of 2009 (P < .001). The hospital rate of dual-chamber ICD implantation ranged from 0% to 100%, with 33 hospitals (3%) exclusively implanting single-chamber ICDs, 109 hospitals (8%) exclusively implanting dual-chamber ICDs, and the remainder (89%) falling in between (Figure 2). The physician implantation rate ranged from 0% to 100% with 443 (11%) implanting single-chamber ICDs exclusively and 941 (23%) implanting dual-chamber ICDs exclusively, with the remainder falling in between (Figure 3). The lowest regional rate (by US Census region) of dual-chamber ICD implantation was 36.4% in New England compared with 66.4% in the Pacific region (odds ratio [OR], 5.25; 95% CI, 3.35-8.21). Patterns of variation by HRR are shown in Figure 4. While there seems to be lower use of dual-chamber ICDs in the Northeast region, there is otherwise no discernible pattern, with high-use regions appearing immediately adjacent to low-use regions.
Figure 2.
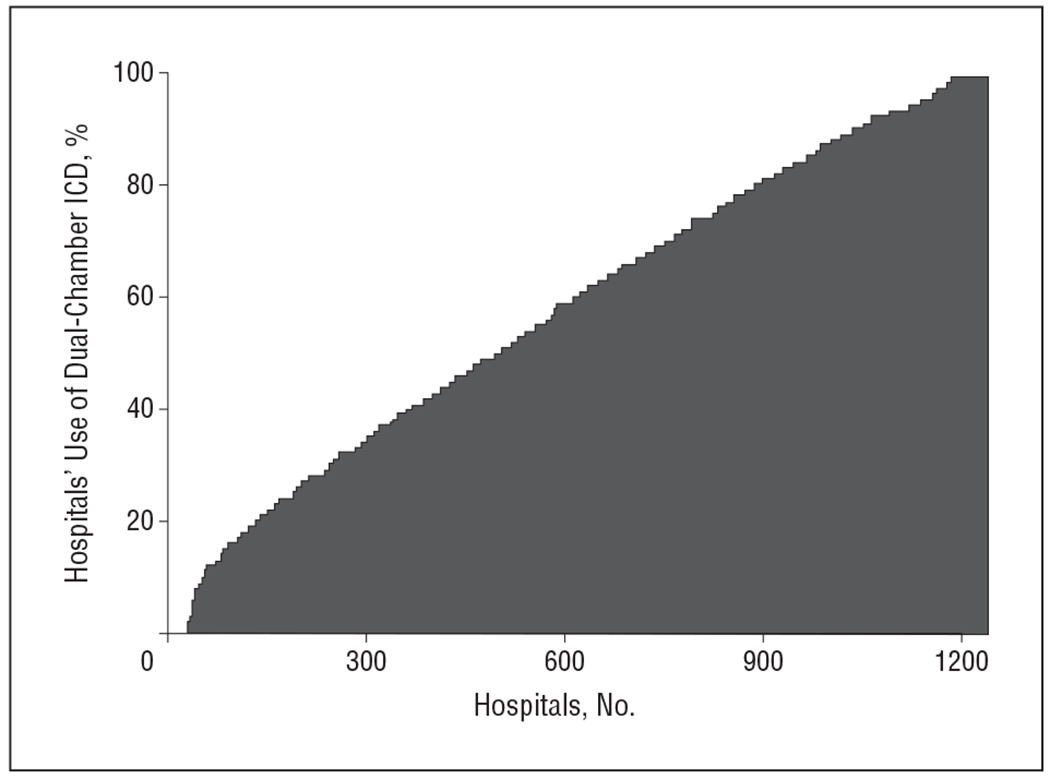
Percentage of primary prevention patients who received a discretionary dual-chamber implantable cardioverter-defibrillator (ICD) by hospital.
Figure 3.
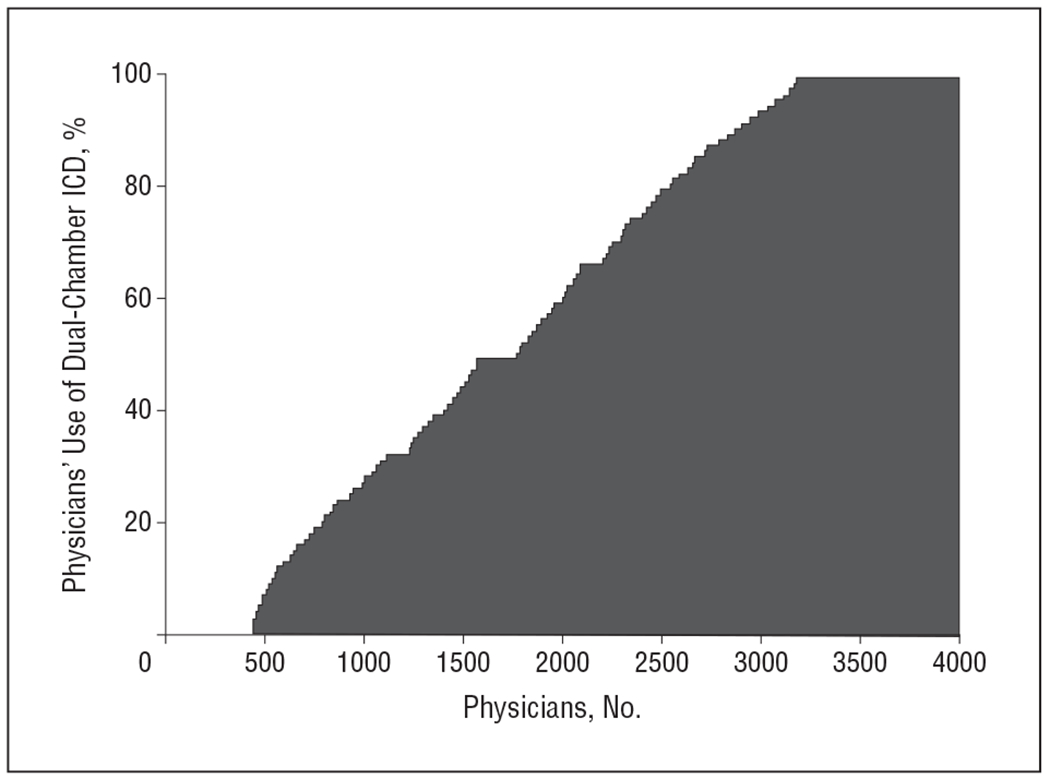
Percentage of primary prevention patients who received a discretionary dual-chamber implantable cardioverter-defibrillator (ICD) by physician.
Figure 4.
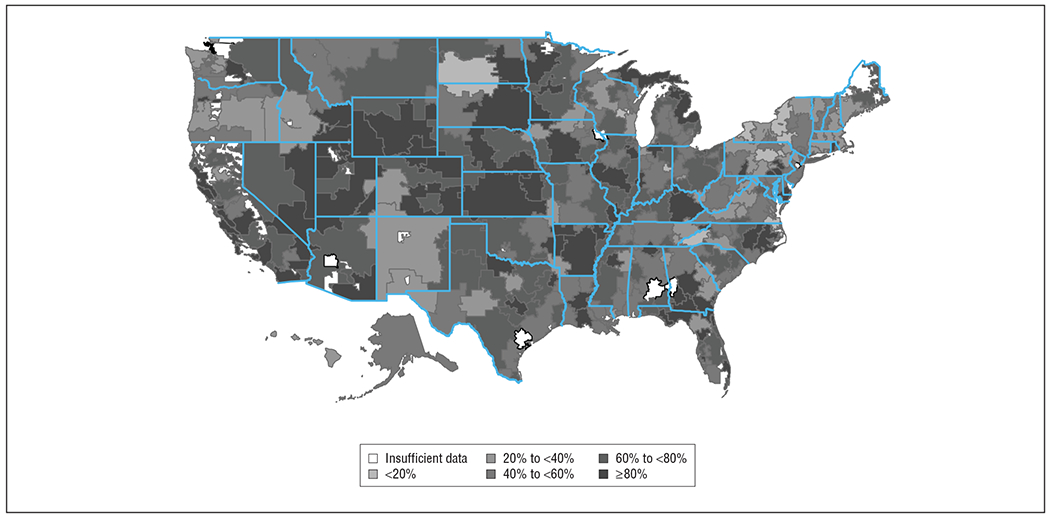
Proportion of patients receiving a dual-chamber implantable cardioverter-defibrillator by hospital referral region.
Using multivariate hierarchical logistic regression, patients were more likely to receive a dual-chamber ICD if they were older (65.4% for age >75 years vs 53.5% for age <65 years; OR, 1.52; 95% CI, 1.46-1.60), had atrial fibrillation or flutter (66.4% vs 56.3%; OR, 1.66; 95% CI, 1.59-1.73), had hypertension (59.4% vs 54.2%; OR, 1.11; 95% CI, 1.07-1.15) or if their ICD was implanted by a surgeon (68.7% vs 57.3% for an EP; OR, 1.95; 95% CI, 1.67-2.27). Those less likely to receive a dual-chamber ICD were black patients (52.8% vs 59.5% for white; OR, 0.81; 95% CI, 0.78-0.85), patients receiving hemodialysis (56.0% vs 58.2%; OR, 0.85; 95% CI, 0.78-0.92), and patients at academic medical centers (49.0% vs 59.7% for private or community hospitals; OR, 0.65; 95% CI, 0.49-0.86) (Table 2).
Table 2.
Patient, Health Care Provider, and Hospital Characteristics Associated With Receiving a Discretionary Dual-Chamber Implantable Cardioverter-Defibrillator (DC ICD)
| Description | % of Discretionary DC ICDs | Adjusted OR |
|---|---|---|
| Patient characteristics | ||
| Overall | 58.1 | |
| Age, y | ||
| <65 | 53.5 | 1 [Reference] |
| 65-75 | 61.7 | 1.35 (1.30-1.40) |
| >75 | 65.4 | 1.52 (1.46-1.60) |
| Female | 56.4 | NAa |
| Race/ethnicity | ||
| White | 59.5 | 1 [Reference] |
| Black | 52.8 | 0.81 (0.78-0.85) |
| Hispanic | 58.0 | 0.91 (0.84-0.98) |
| Other | 59.4 | 1.01 (0.92-1.11) |
| Comorbidities | ||
| Atrial fibrillation/atrial flutter | 67.2 | 1.66 (1.59-1.73) |
| Diabetes mellitus | 58.1 | 0.95 (0.92-0.98) |
| Hypertension | 59.4 | 1.11 (1.07-1.15) |
| Hemodialysis | 56.0 | 0.85 (0.78-0.92) |
| Physician characteristics | ||
| Specialty | ||
| EP | 57.3 | 1 [Reference] |
| EP fellowship only | 57.1 | 0.87 (0.80-0.95) |
| Surgeons | 68.7 | 1.95 (1.67-2.27) |
| Credentialedb | 0.30 (0.25-0.36) | |
| Hospital characteristics | ||
| Regionc | ||
| New England | 36.4 | 1 [Reference] |
| Mid Atlantic | 45.5 | 1.60 (1.02-2.52) |
| South Atlantic | 58.7 | 2.74 (1.80-4.18) |
| Northeast Central | 60.7 | 2.76 (1.81-4.20) |
| Southeast Central | 58.9 | 3.01 (1.87-4.86) |
| Northwest Central | 68.9 | 5.75 (3.59-9.21) |
| Southwest Central | 60.8 | 3.63 (2.33-5.64) |
| Mountain | 66.5 | 3.67 (2.24-5.99) |
| Pacific | 66.4 | 5.25 (3.35-8.21) |
| Hospital type | ||
| Private/community | 69.3 | 1 [Reference] |
| Government | 59.5 | 1.07 (0.61-1.89) |
| University | 49.0 | 0.65 (0.49-0.86) |
Abbreviations: EP, electrophysiologist; NA, not available; OR, odds ratio.
Some variables do not have associated ORs because they were not associated with the outcome on univariate analyses and were not included in the final model.
Credentialed refers to an alternative pathway for non-EP physicians to be “credentialed” to perform ICD implants. It was strictly voluntary and has subsequently expired such that no additional physicians will be characterized as credentialed through this alternative pathway.
As defined by the US Census.18
In unadjusted analysis, the ICC was 0.41. This remained largely unchanged after adjusting for patient-level factors (ICC = 0.42) and after adjusting for physician- and hospital-level factors (ICC = 0.39). This signifies that 39% of the variance between hospitals was attributable to clustering within hospitals. The MOR was 3.96, signifying that a randomly selected patient receiving an ICD at one hospital would have a nearly 4-fold higher odds of receiving a dual-chamber ICD than an identical patient at a different randomly selected hospital in the sample. Similarly, when the analysis was repeated among only the Medicare population, the hospital-level MOR was 3.85. When patients with atrial fibrillation were excluded, the hospital-level MOR was 4.51. When the analysis was performed accounting for clustering of use among physicians (rather than hospitals), the MOR was 4.89, signifying that a randomly selected patient receiving an ICD from one physician would have a nearly 5-fold higher odds of receiving a dual-chamber ICD than an identical patient at a different randomly selected physician in the sample.
COMMENT
To our knowledge, this is the first study to document physician, hospital, and regional variation in the use of dual-chamber ICDs among those without a pacing indication. The degree of variation is particularly striking, with some physicians and hospitals implanting a dual-chamber device in all of their patients and others implanting them in none. While some patient, health care provider, and hospital characteristics were associated with this “discretionary” dual-chamber device use, regional effects and clustering within hospitals accounted for substantially more variability than these factors. Even after adjustment for a wide range of patient, health care provider, and hospital characteristics, 39% of the variance was attributable to clustering within hospitals.
A particularly important contribution of this study is that it demonstrates that patient characteristics explain little of the marked regional variability in the use of dual-chamber ICDs observed in this study. Research on practice variation using administrative data is often criticized for the inability to account for all the pertinent patient-level factors.28–30 A unique strength of the ICD Registry is the availability of a broad range of clinical patient characteristics. Interestingly, we found that regional effects far outweighed the influence of the patient characteristics in influencing the use of dual-chamber ICDs, demonstrating that patient characteristics generally play a relatively small role in this decision. While patient-level variability may be important in explaining variation in some interventions,29,30 our data add to a larger body of literature demonstrating that practice varies markedly for reasons independent of patients’ clinical characteristics.19–21,31
While physicians presumably drive the decision to place a single- or dual-chamber ICD, it is unclear why variability by physician is as great as it is. Our prior work has demonstrated that physicians’ attitudes and recommendations around primary prevention ICDs do not vary in relation to ICD use when patients clearly meet the guideline criteria.32 However, when the evidence is less clear and the procedure becomes more discretionary, as among patients who are frail or with a shortened life expectancy, physicians were more likely to recommend a primary prevention ICD in the higher-use regions. This is consistent with research demonstrating that regional variations are more pronounced when decisions are discretionary.22
If clinical factors traditionally collected in a registry do not explain the variation in dual-chamber ICD use, other factors must be considered. The potential factors that could influence a physician’s behavior are many.33 In regard to financial incentives, while device manufacturers may benefit from the implantation of the more expensive dual-chamber devices, hospitals and physicians do not directly benefit because neither CPT nor DRG codes for ICD implantation distinguish between single- and dual-lead ICDs. For nearly half a century, social scientists have argued that opinion leaders and practice norms are the important contributors to the “diffusion of innovations” into a health care system.34,35 Perhaps the variation seen in the use of dual-chamber ICDs is a function of opinion leaders with strong opinions one way or another. In addition, implicit professional norms or the practice culture at the hospital level may be central drivers of practice variation.36,37 While the cause of this variation remains unknown, understanding why and how a procedure such as discretionary dual-chamber ICDs can vary from 0% to 100% at both the physician and hospital levels remains an important research endeavor.
The literature on the risks and benefits of single- vs dual-chamber devices is inconclusive. A theoretical benefit of dual-chamber devices is improved rhythm recognition, particularly among those with atrial arrhythmias, with the hope of reducing inappropriate device therapies. One study randomized patients with dual-chamber ICDs to either single- or dual-chamber rhythm detection and demonstrated that dual-chamber detection led to a reduction in inappropriate detection of atrial arrhythmias.12 However, while dual-chamber devices improve rhythm recognition, to our knowledge, no study has demonstrated that these devices reduce inappropriate shocks.10–12 An additional potential benefit of implanting a dual-chamber ICD is avoiding the need for revision if a patient develops an indication for permanent pacing. One analysis found that dual-chamber ICDs might be cost-effective at an upgrade rate as low as 5%.38 However, evidence suggests that dual-chamber devices could result in higher in-hospital complications and death.7 Finally, although trials demonstrated that dual-chamber ICDs can be safely programmed to pacing strategies that minimize ventricular pacing, dual-chamber devices offered no additional advantage with regard to incidence of hospitalization or mortality.14,15 The guidelines do not specify whether a single- or dual-chamber device should be implanted in patients without a pacing indication.6 In sum, there is little definitive evidence to guide clinicians as to whether they should implant a single- or a dual-chamber device.
We identified patient, clinician, and hospital factors associated with receipt of a dual-chamber ICD. The finding that older patients are more likely to receive a dual-chamber ICD may be directly related to a belief that older patients are more likely to develop conduction disease.39 The finding that black patients are less likely to receive a dual-chamber ICD may be due to the fact that they are often seen at hospitals that do fewer procedures in general.40 It is unclear why surgeons are more likely to implant dual-chamber ICDs and academic hospitals less likely. The marked variability found in the use of dual-chamber devices in this study demonstrates the need for research to define the population who will benefit from receiving an atrial lead. Furthermore, this analysis highlights how practice varies markedly when the evidence is inconclusive.22,41 What this report adds is a clear understating of how little patient characteristics contribute to practice variation, thus quieting prior criticisms of practice variation research.28,30,42 This work has implications beyond ICDs. According to a review of 3000 procedures,43 51% of the interventions performed today are without clear evidence.
Several issues should be considered when interpreting these findings. First, data in the ICD Registry are self-reported by each of the participating hospitals. Second, while robust patient and clinical data are collected in the registry, the decision to implant a dual-chamber device may at times be justified by factors that were not collected. Third, patient preferences were not included in this analysis. However, while patient preferences vary from individual to individual, in aggregate, patient preferences do not vary meaningfully by location.44,45 Finally, we were not able to assess the long-term effectiveness of single- vs dual-chamber ICDs.
Use of dual-chamber ICDs in patients without pacing indications varies widely and seems to be unrelated to patient factors. Research to understand this variation is needed to produce a deeper understanding of the influences on discretionary decision making, including an exploration of the hospital culture and norms and the importance of opinion leaders. Given the lack of clear data to support the use of one device type over the other, comparative effectiveness studies designed to inform the real-world clinical decision making about whether to use a single- or a dual-chamber ICD accounting for differences in device programming are also essential.
Funding/Support:
Dr Matlock was supported as a Hartford Geriatrics Health Outcomes Research Scholar and by K23 funding (AG040696-01) from the National Institute on Aging.
Funding for Less Is More:
Staff support for topics research funded by grants from the California Health Care Foundation and the Parsemus Foundation.
Financial Disclosure:
Dr Curtis has received salary support under contract with the American College of Cardiology (ACC) to provide data analytic services for the ICD registry and has significant stock holdings (in Medtronic, a maker of ICDs. Dr Reynolds has received consulting fees from Medtronic. Dr Masoudi has had contracts with and has received grants from the ACC Foundation and the Oklahoma Foundation for Medical Quality; and has received grants from the Agency for Healthcare Research and Quality the National Heart, Lung, and Blood Institute, and the Heart Rhythm Society.
Footnotes
Publisher's Disclaimer: Disclaimer: The views expressed in this manuscript represent those of the authors and do not necessarily represent the official views of the NCDR or its associated professional societies identified at www.ncdr.com, or the Department of Veterans Affairs.
Additional Information: The ICD Registry is an initiative of the ACC Foundation and the Heart Rhythm Society.
Previous Presentation: This study was presented at the 2010 American Heart Association Quality of Care and Outcomes Research Conference; May 20, 2010; Washington, DC.
Contributor Information
Dan D. Matlock, Department of Medicine, University of Colorado Denver School of Medicine, Aurora; Colorado Cardiovascular Outcomes Research Group, Denver; Institute for Health Research, Kaiser Permanente Colorado, Denver.
Pamela N. Peterson, Department of Medicine, University of Colorado Denver School of Medicine, Aurora; Colorado Cardiovascular Outcomes Research Group, Denver; Institute for Health Research, Kaiser Permanente Colorado, Denver; Department of Medicine, Denver Health Medical Center, Denver.
Yongfei Wang, Department of Internal Medicine, Yale University, New Haven, Connecticut.
Jeptha P. Curtis, Department of Internal Medicine, Yale University, New Haven, Connecticut.
Matthew R. Reynolds, Department of Medicine, Division of Cardiology, Beth Israel Deaconess Medical Center and the Boston VA Healthcare System, Boston, Massachusetts.
Paul D. Varosy, Department of Medicine, University of Colorado Denver School of Medicine, Aurora; Colorado Cardiovascular Outcomes Research Group, Denver; The Medical Service, Section of Cardiology, Veterans Affairs Eastern Colorado Health Care System, Denver.
Frederick A. Masoudi, Department of Medicine, University of Colorado Denver School of Medicine, Aurora; Colorado Cardiovascular Outcomes Research Group, Denver; Institute for Health Research, Kaiser Permanente Colorado, Denver.
REFERENCES
- 1.Bardy GH, Lee KL, Mark DB, et al. ; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–237. [DOI] [PubMed] [Google Scholar]
- 2.Kadish A, Dyer A, Daubert JP, et al. ; Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350(21):2151–2158. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Zareba W, Hall WJ, et al. ; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–883. [DOI] [PubMed] [Google Scholar]
- 4.McClellan MB, Tunis SR. Medicare coverage of ICDs. N Engl J Med. 2005;352(3):222–224. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Medicare and Medicaid Services. National coverage decision for implantable automatic defibrillators. http://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=110&ncdver=3&CoverageSelection=National&KeyWord=defibrillators&KeyWordLookUp=Title&KeyWordSearchType=And&ncd_id=20.4&ncd_version=3&basket=ncd%25253A20%25252E4%25253A3%25253AImplantable+Automatic+Defibrillators&bc=gAAAABAAAAAA&. Accessed January 9, 2012.
- 6.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices); American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117(21):e350–e408. [DOI] [PubMed] [Google Scholar]
- 7.Dewland TA, Pellegrini CN, Wang Y, Marcus GM, Keung E, Varosy PD. Dual-chamber implantable cardioverter-defibrillator selection is associated with increased complication rates and mortality among patients enrolled in the NCDR implantable cardioverter-defibrillator registry. J Am Coll Cardiol. 2011;58(10):1007–1013. [DOI] [PubMed] [Google Scholar]
- 8.Kadish A, Mehra M. Heart failure devices: implantable cardioverter-defibrillators and biventricular pacing therapy. Circulation. 2005;111(24):3327–3335. [DOI] [PubMed] [Google Scholar]
- 9.Almendral J, Arribas F, Wolpert C, et al. ; DATAS Steering Committee; DATAS Writing Committee; DATAS Investigators. Dual-chamber defibrillators reduce clinically significant adverse events compared with single-chamber devices: results from the DATAS (Dual chamber and Atrial Tachyarrhythmias Adverse events Study) trial. Europace. 2008;10(5):528–535. [DOI] [PubMed] [Google Scholar]
- 10.Theuns DAMJ, Klootwijk AP, Goedhart DM, Jordaens LJLM. Prevention of inappropriate therapy in implantable cardioverter-defibrillators: results of a prospective, randomized study of tachyarrhythmia detection algorithms. J Am Coll Cardiol. 2004;44(12):2362–2367. [DOI] [PubMed] [Google Scholar]
- 11.Deisenhofer I, Kolb C, Ndrepepa G, et al. Do current dual chamber cardioverter defibrillators have advantages over conventional single chamber cardioverter defibrillators in reducing inappropriate therapies? a randomized, prospective study. J Cardiovasc Electrophysiol. 2001;12(2):134–142. [DOI] [PubMed] [Google Scholar]
- 12.Friedman PA, McClelland RL, Bamlet WR, et al. Dual-chamber vs single-chamber detection enhancements for implantable defibrillator rhythm diagnosis: the detect supraventricular tachycardia study. Circulation. 2006;113(25): 2871–2879. [DOI] [PubMed] [Google Scholar]
- 13.Wilkoff BL, Cook JR, Epstein AE, et al. ; Dual Chamber and VVI Implantable Defibrillator Trial Investigators. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288(24):3115–3123. [DOI] [PubMed] [Google Scholar]
- 14.Wilkoff BL, Kudenchuk PJ, Buxton AE, et al. ; DAVID II Investigators. The DAVID (Dual Chamber and VVI Implantable Defibrillator) II trial. J Am Coll Cardiol. 2009; 53(10):872–880. [DOI] [PubMed] [Google Scholar]
- 15.Olshansky B, Day JD, Moore S, et al. Is dual-chamber programming inferior to single-chamber programming in an implantable cardioverter-defibrillator? results of the INTRINSIC RV (Inhibition of Unnecessary RV Pacing With AVSH in ICDs) study. Circulation. 2007;115(1):9–16. [DOI] [PubMed] [Google Scholar]
- 16.The American College of Cardiology. National Cardiovascular Data Registry. http://www.ncdr.com/webncdr/ICD/Default.aspx. Accessed January 9, 2012.
- 17.Hammill SC, Kremers MS, Kadish AH, et al. Review of the ICD Registry’s third year, expansion to include lead data and pediatric ICD procedures, and role for measuring performance. Heart Rhythm. 2009;6(9):1397–1401. [DOI] [PubMed] [Google Scholar]
- 18.US Census, 2010. http://2010.census.gov/2010census/. Accessed February 15, 2012.
- 19.The Dartmouth Atlas of Health Care. http://www.dartmouthatlas.org. Accessed January 9, 2012.
- 20.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending: part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138(4):273–287. [DOI] [PubMed] [Google Scholar]
- 21.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending: part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138(4):288–298. [DOI] [PubMed] [Google Scholar]
- 22.Sirovich B, Gallagher PM, Wennberg DE, Fisher ES. Discretionary decision making by primary care physicians and the cost of U.S. Health care. Health Aff (Millwood). 2008;27(3):813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curtis JP, Luebbert JJ, Wang Y, et al. Association of physician certification and outcomes among patients receiving an implantable cardioverter-defibrillator. JAMA. 2009;301(16):1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Khatib SM, Hellkamp A, Curtis J, et al. Non-evidence-based ICD implantations in the United States. JAMA. 2011;305(1):43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen K, Petersen JH, Budtz-Jørgensen E, Endahl L. Interpreting parameters in the logistic regression model with random effects. Biometrics. 2000;56(3):909–914. [DOI] [PubMed] [Google Scholar]
- 26.Chan PS, Nichol G, Krumholz HM, Spertus JA, Nallamothu BK; American Heart Association National Registry of Cardiopulmonary Resuscitation (NRCPR) Investigators. Hospital variation in time to defibrillation after in-hospital cardiac arrest. Arch Intern Med. 2009;169(14):1265–1273. [DOI] [PubMed] [Google Scholar]
- 27.Ricci RP, Quesada A, Almendral J, et al. ; DATAS study Investigators. Dual-chamber implantable cardioverter defibrillators reduce clinical adverse events related to atrial fibrillation when compared with single-chamber defibrillators: a subanalysis of the DATAS trial. Europace. 2009;11(5):587–593. [DOI] [PubMed] [Google Scholar]
- 28.Bach PB. A map to bad policy: hospital efficiency measures in the Dartmouth Atlas. N Engl J Med. 2010;362(7):569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ong MK, Mangione CM, Romano PS, et al. Looking forward, looking back: assessing variations in hospital resource use and outcomes for elderly patients with heart failure. Circ Cardiovasc Qual Outcomes. 2009;2(6):548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson HV. Regional variation: only moderately interesting: a word of caution. Circ Cardiovasc Qual Outcomes. 2010;3(1):6–7. [DOI] [PubMed] [Google Scholar]
- 31.Sutherland JM, Fisher ES, Skinner JS. Getting past denial: the high cost of health care in the United States. N Engl J Med. 2009;361(13):1227–1230. [DOI] [PubMed] [Google Scholar]
- 32.Matlock DD, Kutner JS, Emsermann CB, et al. Regional variations in physicians’ attitudes and recommendations surrounding implantable cardioverter-defibrillators. J Card Fail. 2011;17(4):318–324. [DOI] [PubMed] [Google Scholar]
- 33.Godin G, Bélanger-Gravel A, Eccles M, Grimshaw J. Healthcare professionals’ intentions and behaviours: a systematic review of studies based on social cognitive theories. Implement Sci. 2008;3(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenhalgh T, Robert G, Macfarlane F, Bate P, Kyriakidou O. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q. 2004;82(4):581–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers E Diffusion of Innovations. New York, NY: Free Press; 1962. [Google Scholar]
- 36.Barnato AE, Bost JE, Farrell MH, et al. Relationship between staff perceptions of hospital norms and hospital-level end-of-life treatment intensity. J Palliat Med. 2007;10(5):1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin CY, Farrell MH, Lave JR, Angus DC, Barnato AE. Organizational determinants of hospital end-of-life treatment intensity. Med Care. 2009;47(5):524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldberger Z, Elbel B, McPherson CA, Paltiel AD, Lampert R. Cost advantage of dual-chamber versus single-chamber cardioverter-defibrillator implantation. J Am Coll Cardiol. 2005;46(5):850–857. [DOI] [PubMed] [Google Scholar]
- 39.Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: promoting understanding of sick sinus syndrome. Circulation. 2007;115(14): 1921–1932. [DOI] [PubMed] [Google Scholar]
- 40.Groeneveld PW, Laufer SB, Garber AM. Technology diffusion, hospital variation, and racial disparities among elderly Medicare beneficiaries: 1989-2000. Med Care. 2005;43(4):320–329. [DOI] [PubMed] [Google Scholar]
- 41.Lucas FL, Sirovich BE, Gallagher PM, Siewers AE, Wennberg DE. Variation in cardiologists’ propensity to test and treat: is it associated with regional variation in utilization? Circ Cardiovasc Qual Outcomes. 2010;3(3):253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Critics question study cited in health debate. Abelson R, Harris G. New York Times. June 2, 2011. http://www.nytimes.com/2010/06/03/business/03dartmouth.html?pagewanted=all. Accessed February 15, 2012. [Google Scholar]
- 43.Maynard A The powers and pitfalls of payment for performance. Health Econ. 2012;21(1):3–12. [DOI] [PubMed] [Google Scholar]
- 44.Anthony DL, Herndon MB, Gallagher PM, et al. How much do patients’ preferences contribute to resource use? Health Aff (Millwood). 2009;28(3):864–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fowler FJ Jr, Gallagher PM, Anthony DL, Larsen K, Skinner JS. Relationship between regional per capita Medicare expenditures and patient perceptions of quality of care. JAMA. 2008;299(20):2406–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]


