Abstract
Context
CYP24A1 encodes 24-hydroxylase, which converts 25(OH)D3 and 1,25(OH)2D3 to inactive metabolites. Loss-of-function variants in CYP24A1 are associated with 24-hydroxylase deficiency (24HD), characterized by hypercalcemia, nephrolithiasis, and nephrocalcinosis. We retrospectively reviewed laboratory, imaging, and clinical characteristics of patients with suspected or confirmed 24HD and patients with other vitamin D−mediated hypercalcemia disorders: sarcoidosis, lymphoma, and exogenous vitamin D toxicity (EVT).
Objective
To identify features that differentiate 24HD from other vitamin D-mediated hypercalcemia disorders.
Methods
Patients seen at the Mayo Clinic (Rochester, MN) from January 1, 2008, to 31 December, 2016, with the following criteria were retrospectively identified: serum calcium ≥9.6 mg/dL, parathyroid hormone <30 pg/mL, and 1,25(OH)2D3 >40 pg/mL. Patients were considered to have 24HD if they had (1) confirmed CYP24A1 gene variant or (2) 25(OH)D3:24,25(OH)2D ratio ≥50. Patients with sarcoidosis, lymphoma, and EVT were also identified. Groups were compared using the Fisher exact test (categorical variables) or the Wilcoxon rank sum test (continuous variables).
Results
We identified 9 patients with 24HD and 28 with other vitamin D−mediated disorders. Patients with 24HD were younger at symptom onset (median 14 vs 63 years; P = .001) and had positive family history (88.9% vs 20.8%; P < .001), nephrocalcinosis (88.9% vs 6.3%; P < .001), lower lumbar spine Z-scores (median −0.50 vs 1.20; P = .01), higher peak serum phosphorus (% of peak reference range, median 107 vs 84; P = .01), and higher urinary calcium:creatinine ratios (median 0.24 vs 0.17; P = .047).
Conclusion
Patients with 24HD had clinical and laboratory findings that differed from other vitamin D−mediated hypercalcemia disorders. 24HD should be suspected in patients with hypercalcemia who present at younger age, have positive family history, and have nephrocalcinosis.
Keywords: Idiopathic infantile hypercalcemia, genetic, hypercalcemia, vitamin D, 24-hydroxylase, CYP24A1
Allelic variants in the human cytochrome P450 Family 24 Superfamily A member 1, or CYP24A1, were first shown to be associated with idiopathic infantile hypercalcemia (IIH) in 2011, when 10 patients with the disorder were described [1]. Most patients with IIH received medical attention for symptoms attributable to severe hypercalcemia, including lethargy, failure to grow and gain weight, dehydration, and hypotonia. Numerous reports established that loss-of-function mutations in CYP24A1 were associated with a familial hypercalcemia syndrome attributable to 24-hydroxylase deficiency (24HD); this syndrome is characterized by intermittent or persistent hypercalcemia, nephrolithiasis, and nephrocalcinosis (NC) [2-22]. Although primary hyperparathyroidism and malignancy account for 80% to 90% of hypercalcemia cases, 24HD is nevertheless an important addition to the differential diagnosis of non-parathyroid hormone (PTH)–mediated hypercalcemia and high (or inappropriately normal) 1,25(OH)2D3 [23]. Currently, however, the clinical and biochemical differences between 24HD and other disorders of vitamin D−mediated hypercalcemia are not well understood.
Physiologically, CYP24A1-encoded 24-hydroxylase (24H) works in conjunction with CYP27B1-encoded 25(OH)D3 1-α hydroxylase (1αH) to maintain appropriate vitamin D concentrations and calcium homeostasis [24, 25]. In response to low serum calcium, elevated PTH, or low serum phosphorus, 1αH converts 25(OH)D3 to its active metabolite, 1,25(OH)2D3 [26]. 1,25(OH)2D3 increases bone resorption, renal calcium and phosphorus reabsorption, and intestinal calcium and phosphorus absorption [27, 28]. When serum calcium is elevated, 24H converts 25(OH)D3 and 1,25(OH)2D3 to inactive metabolites (24,25[OH]2D3 and 1,24,25[OH]3D3, respectively) [24, 26, 29-31]. 24H is expressed in tissues containing the vitamin D receptor, including bone, kidney, and intestine, and its activity is increased by 1,25(OH)2D3 and FGF23, but reduced by hypophosphatemia [24, 26, 32-35].
Other disorders associated with vitamin D−mediated hypercalcemia include sarcoidosis, lymphoma, exogenous vitamin D toxicity (EVT), and fungal infections; however, distinguishing among these disorders can be difficult because of their similar biochemical profiles [26]. We thus aimed to assess the clinical presentation, laboratory parameters, and imaging studies of patients with vitamin D−mediated hypercalcemic disorders to identify potential differentiating factors. Such data may expedite evaluation of patients with 24HD, facilitating faster diagnosis and avoiding unnecessary testing.
Materials and Methods
This study was approved by the Mayo Clinic Institutional Review Board. The reporting of this study is in compliance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement [36].
Patient Identification
A search was performed of an institutional patient database to identify those seen between January 1, 2008, and December 31, 2016, at the Mayo Clinic (Rochester, Minnesota) who had a single blood sample showing a serum calcium level of 9.6 mg/dL or higher, PTH less than 30 pg/mL, and 1,25(OH)2D3 greater than 40 pg/mL. Though these laboratory parameters include values that are within the normal reference interval, they were chosen in order to include all patients with possible vitamin D–mediated hypercalcemia based on our review of our known patients with 24HD and recognition that, in these patients, these values sometimes fall in the normal range. We reviewed electronic health records to identify patients with confirmed or suspected 24HD, which was defined as at least 1 of the following: (1) genetic testing confirming a disease-associated CYP24A1 sequence variant based on ACMG guidelines [37], (2) elevated ratio of 25(OH)D3:24,25(OH)2D3 (values >50), or (3) high serum calcium, low PTH, and a family history of 24HD. Four patients in our 24HD cohort were members of the family reported in Tebben et al [38]: proband II 3, III 1, III 2, and III 3. Patients with a diagnosis of sarcoidosis, lymphoma, EVT, or fungal infection were also identified through manual record review.
Data Collection
From each patient record, the following data were abstracted, if available: peak serum calcium (reference intervals 9.3-10.6 mg/dL for ages 1-17 and 8.6-10.0 mg/dL for ages 18-59); lowest serum PTH; serum calcium and phosphorus at the time of lowest PTH; peak serum phosphorus; alkaline phosphatase closest to peak calcium; peak serum 25(OH)D3; peak serum 1,25(OH)2D3; 24,25(OH)2D; urinary calcium-to-creatinine ratio (uCa:Cr) from a spot urine test; 24-hour urine calcium, creatinine, and phosphorus; computed tomographic or ultrasonographic imaging evidence of urinary stones, NC, or cysts; bone density Z-score of the hip and spine; age at onset of symptoms attributable to stones, hypercalcemia, or hypercalciuria; family history of symptoms attributable to stones, hypercalcemia, or hypercalciuria; and other presenting features.
Hypercalciuria was defined by a uCa:Cr value exceeding the 95th percentile for the patient’s age [39] or by a 24-hour urinary calcium excretion level exceeding 4 mg/kg per day [40]. Phosphorous at the time of lowest PTH, peak serum phosphorous, and alkaline phosphatase values were converted to percent of peak reference range before analysis because reference values differ significantly by age.
Statistical Analysis
Available data were summarized and reported as the median (interquartile range [IQR]) for continuous variables and number (percentage) for categorical variables. Comparisons between disease groups were evaluated with the Fisher exact test for categorical variables and the Kruskal–Wallis or Wilcoxon rank sum test for continuous variables, due to the skewed nature of the measurements. All calculated P values were 2-sided, and P< .05 was considered statistically significant. Data were analyzed with SAS version 9.4 software (SAS Institute Inc) and R 3.4.2 (The R Foundation).
Results
Demographic Characteristics
We identified 222 patients that fit the aforementioned criteria (serum calcium level ≥9.6 mg/dL, PTH <30 pg/mL, and 1,25(OH)2D3 >40 pg/mL); of these, 6 had genetic testing confirming 24HD and 3 had an elevated 25(OH)D3:24,25(OH)2D ratio without genetic verification available (ie, 9 patients had confirmed or suspected 24HD). CYP24A1-specific genetic and laboratory parameters in our 24HD cohort are presented in Table 1. We identified 37 patients with other disorders associated with vitamin D−mediated hypercalcemia: 6 with EVT, 7 with lymphoma, 15 with sarcoidosis, and 9 with fungal infection. We excluded patients with fungal infection from the subsequent analyses because nearly all had superficial infections without systemic involvement upon further review.
Table 1.
Genetic characteristics and laboratory parameters of patients with 24-hydroxylase deficiency (n = 9)
| Genetic variant or laboratory parameter | |
|---|---|
| CYP24A1 (24-hydroxylase) variant, n(%) a | |
| c.1226T>C (p.Leu409Ser) (homozygous) | 1 (11.1) |
| c.999_106del (p.Ser334Valfs*9) and c.1186C > T (p.Arg396Trp) | 1 (11.1) |
| IVS5 + 1G>A and IVS6-2A > G (N/A) | 1 (11.1) |
| IVS6-2A>G (N/A) (single mutation) | 2 (22.2) |
| IVS5 + 1G>A (N/A) (single mutation) | 1 (11.1) |
| Unknown | 3 (33.3) |
| 24,25(OH) 2 D 3 , ng/mL, median (IQR) | 0.20 (0.15, 0.23) |
| 25(OH)D 3 :24,25(OH) 2 D ratio, median (IQR) | 353 (336, 460) |
| Family history of 24-hydroxylase variant | |
| Yes | 4 (44.4) |
| Unknown | 5 (55.6) |
Summary statistics are presented as median (IQR) for continuous variables and n (%) for categorical variables.
Abbreviations: IQR, interquartile range; N/A, not applicable.
a Protein alteration, if applicable, is shown parenthetically after the variant.
Characteristics at Presentation for Patients with Vitamin D−mediated Hypercalcemia
Presenting characteristics of patients with 24HD, sarcoidosis, lymphoma, and EVT are shown in Table 2. Among patients with 24HD, 2 (22%) had IIH and 5 (56%) had urinary stones. For the 2 patients with IIH, symptoms included poor sleep, difficulty feeding, and weight loss.
Table 2.
Characteristics at presentation for patients with vitamin d−mediated hypercalcemia, stratified by disease group
| Feature, n(%) | 24HD (n = 9) | Sarcoidosis (n = 15) | Lymphoma (n = 7) | EVT (n = 6) |
|---|---|---|---|---|
| Urinary stones | 5 (55%) | 4 (27%) | 0 (0%) | 0 (0%) |
| Severe symptoms of hypercalcemiaa | 3 (33%) | 6 (40%) | 6 (86%) | 3 (50%) |
| Palpitation | 1 (11%) | 0 (0%) | 0 (0%) | 1 (17%) |
| Weight loss | 2 (22%) | 3 (20%) | 0 (0%) | 2 (33%) |
| Pulmonary symptomsb | 0 (0%) | 3 (20%) | 2 (29%) | 0 (0%) |
| Asymptomatic | 2 (22%) | 3 (20%) | 1 (14%) | 3 (50%) |
Abbreviations: 24HD, 24-hydroxylase deficiency; EVT, exogenous vitamin D toxicity.
a Symptoms included loss of appetite, nausea, vomiting, abdominal pain, constipation, polyuria, polydipsia, dehydration, constitutional symptoms (fatigue, weakness, muscle pain), altered mental status, or poor feeding or failure to thrive (in neonates).
b Symptoms included cough or shortness of breath.
Comparison of 24HD With Other Hypercalcemia Disorders
We compared clinical and biochemical parameters of patients with 24HD (n = 9) and the combined group of patients with sarcoidosis, lymphoma, or EVT (n = 28) (Table 3). Patients with 24HD were significantly younger at symptom onset (median [IQR], 14 [1-35] vs 63 [56-79] years; P = .001) and more commonly had a family history of symptoms (88.9% vs 20.8%; P < .001) and NC on imaging (88.9% vs 6.3%, respectively; P < .001) than other hypercalcemic disorders. Patients with 24HD also had lower Z-scores for total lumbar spine (median –0.50 [–0.80, 0.70] vs 1.20 [0.80, 2.10], respectively; P = .01), higher peak serum phosphorus levels (% of peak reference range, median 107 [98, 108] vs 84 [81, 91]; P = .01), and higher uCa:Cr ratios (median 0.24 [0.21, 1.70] vs 0.17 [0.14, 0.18]; P = .047).
Table 3.
Comparison of clinical and biochemical parameters for patients with 24HD or other hypercalcemia disorders
| Parameter | 24HD (n = 9) | All non-24HD disorders (n = 28) | P valuea | EVT (n = 6) | P valuea | Lymphoma (n = 7) | P valuea | Sarcoidosis (n = 15) | P valuea | Global P valueb |
|---|---|---|---|---|---|---|---|---|---|---|
| Age at onset of symptoms, years | 14 (1, 35) | 63 (56, 79) | .001 | 52 (30, 56) | .09 | 80 (78, 88) | .005 | 59 (56, 67) | .004 | <.001 |
| Family history of symptoms, n(%) | 8 (88.9) | 5/24 (20.8) | <.001 | 4 (66.7) | .53 | 0/5 (0) | .003 | 1/13 (7.7) | <.001 | <.001 |
| Imaging findings, n(%) | ||||||||||
| Stones, n(%) | 5 (55.6) | 6/15 (40.0) | .68 | 0/2 (0) | … | 2/5 (40.0) | … | 4/8 (50.0) | … | .73 |
| Nephrocalcinosis, n(%) | 8 (88.9) | 1/16 (6.3) | <.001 | 1/2 (50.0) | .35 | 0/6 (0) | .001 | 0/8 (0) | <.001 | <.001 |
| Cysts, n(%) | 7 (77.8) | 7/15 (46.7) | .21 | 0/2 (0) | … | 4/5 (80.0) | … | 3/8 (37.5) | … | .11 |
| Z-score | ||||||||||
| Left hip | −0.60 (−1.70, 0.60) | −0.25 (−0.70, 1.30) | .47 | 0.45 (−0.90, 1.80) | … | −0.70 () | … | 0.20 (−0.70, 1.30) | … | .81 |
| Right hip | −0.70 (−1.70, 0.40) | 0.80 (−0.30, 1.85) | .34 | 0.75 (−0.80, 2.30) | … | … | … | 0.80 (0.20, 1.40) | … | .56 |
| Spine (total lumbar) | −0.50 (−0.80, 0.70) | 1.20 (0.80, 2.10) | .01 | 1.10 (0.70, 1.50) | … | 0.80 () | … | 2.10 (0.90, 2.30) | … | .06 |
| Peak serum calcium, mg/dLd | 10.9 (10.6, 11.4) | 11.5 (10.9, 13.0) | .11 | 10.9 (10.6, 11.2) | .95 | 13.6 (11.3, 15.4) | .01 | 11.6 (10.8, 12.7) | .19 | .03 |
| Lowest PTH, pg/mL | 6.6 (6.0, 14.0) | 13.0 (8.6, 18.0) | .08 | 15.0 (12.0, 19.0) | … | 12.0 (7.9, 15.0) | … | 14.0 (7.9, 20.0) | … | .25 |
| Calcium at lowest PTH, mg/dL | 10.6 (10.4, 10.8) | 11.0 (10.4, 11.8) | .13 | 10.8 (10.6, 11.0) | … | 11.3 (11.0, 14.9) | … | 10.9 (10.1, 12.0) | … | .15 |
| Phosphorous at lowest PTH, %PRR | 96 (71, 96) | 80 (71, 89) | .43 | 90 (80, 94) | … | 71 (67, 91) | … | 80 (73, 84) | … | .25 |
| Peak serum phosphorous, %PRR | 107 (98, 108) | 84 (81, 91) | .01 | 90 (89, 94) | .11 | 82 (78, 91) | .04 | 84 (76, 89) | .02 | .03 |
| Alkaline phosphatasec, %PRR | 55 (30, 70) | 62 (51, 72) | .48 | 64 (48, 126) | … | 52 (48, 76) | … | 63 (56, 69) | … | .66 |
| Peak 25(OH)D3, ng/mL | 56 (54, 61) | 37 (29, 47) | .09 | 223 (109, 371) | .002 | 39 (35, 44) | .01 | 31 (28, 37) | .002 | <.001 |
| Peak 1,25(OH)2D3, pg/mL | 145 (114, 149) | 87 (69, 133) | .05 | 67 (46, 80) | … | 90 (82, 143) | … | 95 (70, 150) | … | .09 |
| Urinary spot test, mg/L | ||||||||||
| Calcium, mg/L | 207 (93, 325) | 214 (144, 288) | .78 | 160 () | … | … | … | 267 (127, 308) | … | .81 |
| Creatinine, mg/L | 1111 (370, 1525) | 870 (848, 1,679) | .52 | 625 (380, 870) | … | … | … | 1679 (848, 2048) | … | .26 |
| Calcium:creatinine ratio | 0.24 (0.21, 1.70) | 0.17 (0.14, 0.18) | .047 | 0.18 () | … | … | … | 0.15 (0.13, 0.18) | … | .10 |
| 24-Hour urinary test | ||||||||||
| Calcium, mg per 24 h | 248 (72, 407) | 385 (226, 521) | .23 | 237 (226, 399) | … | 385 (375, 536) | … | 391 (221, 521) | … | .47 |
| Creatinine, mg per 24 h | 1285 (351, 2219) | 1122 (832, 1536) | >.99 | 984 (811, 1157) | … | 1184 (832, 1536) | … | 1122 (1102, 1541) | … | .97 |
| Phosphorus, mg per 24 h | 1118 (311, 1332) | 956 (757, 1154) | >.99 | 757 () | … | … | … | 1154 () | … | .67 |
| Calcium excretion, mg/kg per h | 4.47 (3.52, 5.17) | 4.53 (2.67, 5.73) | >.99 | 4.53 (2.99, 7.33) | … | 5.27 (4.47, 7.20) | … | 4.24 (2.50, 5.25) | … | .53 |
| Hypercalciuria, n(%) | 8 (88.9) | 10/18 (55.6) | .19 | 2/4 (50.0) | … | 3/3 (100) | … | 5/11 (45.5) | … | .12 |
Reported statistics are median (IQR) for continuous variables and n (%) for categorical variables. Results followed by empty brackets indicate that only 1 sample was available for analysis. Laboratory measures taken closest to diagnosis. P values in bold indicate statistical significance at the 0.05 alpha level.
… Indicates that the global P value was not statistically significant using the Kruskal–Wallis test, so no further between-group tests were performed.
Abbreviations: 24HD, 24-hydroxylase deficiency; EVT, exogenous vitamin D toxicity; IQR, interquartile range; PTH, parathyroid hormone. %PRR = % of peak reference range.
a Comparison with 24HD values.
b Global P value (comparison across 4 disease groups).
c Lab taken closest to peak calcium.
d Reference intervals for serum calcium are 9.3-10.6 mg/dL for ages 1-17 and 8.6-10.0 mg/dL for ages 18-59.
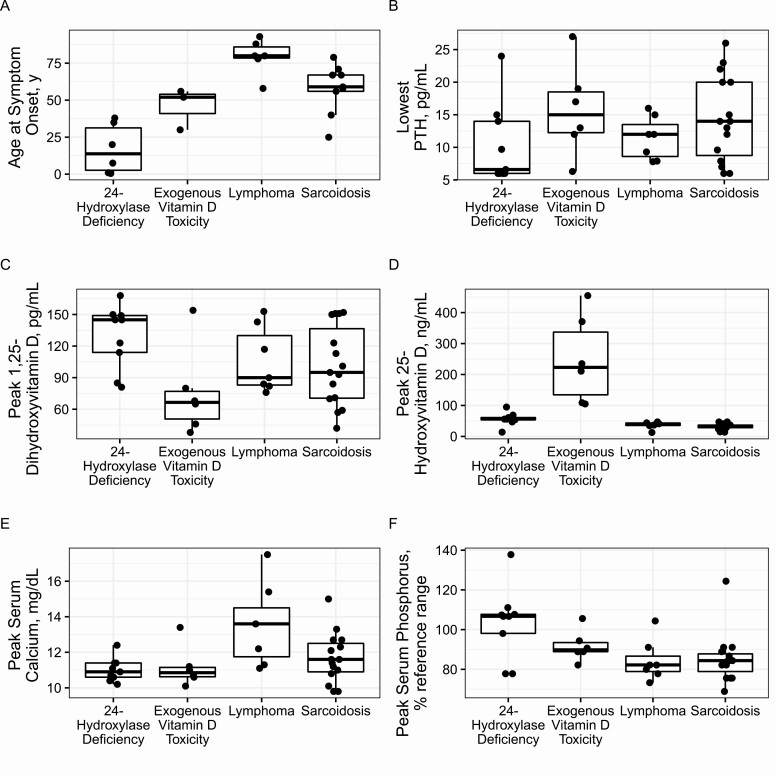
We also compared characteristics across each individual disease subgroup (24HD, lymphoma, sarcoidosis, EVT) (Table 3 and Fig. 1). Compared with the lymphoma and sarcoidosis groups, patients with 24HD were significantly younger at symptom onset (median 14 [1, 35] vs 80 [78, 88] and 59 [56, 67] years, respectively; P < .05 for both) and more commonly had a family history of symptoms (88.9% vs 0% and 7.7%, respectively; P < .05 for both), NC on imaging (88.9% vs 0% and 0%, respectively; P < .05 for both), and higher peak serum phosphorus levels (% of peak reference range, 107 [98, 108] vs 82 [78, 91] and 84 [76, 89], respectively; P < .05 for both). Compared with the lymphoma group, patients with 24HD had lower peak serum calcium levels (10.9 [10.6, 11.4] vs 13.6 [11.3, 15.4]; P = .01). Additionally, patients with 24HD had significantly higher peak 25(OH)D3 levels than the lymphoma and sarcoidosis groups (56 [54, 61] vs 39 [35, 44] and 31 [28, 37], respectively; P < .05 for both) but lower levels than the EVT group (223 [109, 371]; P = .002). Aside from having the highest peak 25(OH)D3 levels of any group, the EVT group did not reach statistical difference in any other observed category.
Figure 1.
Boxplots show comparisons across disease groups. The heavy line represents the median, boxes represent the first and third quartiles, and whiskers represent 1.5 × interquartile range values. (A) Age at symptom onset. (B) Lowest parathyroid hormone (PTH). (C) Peak 1,25 dihydroxyvitamin D3. (D) Peak 25-hydroxyvitamin D3. (E) Peak serum calcium. (F) Peak serum phosphorus.
Discussion
This study identifies features that may help distinguish patients with 24HD from those with other disorders of vitamin D−mediated hypercalcemia. In 2011, Schlingmann et al. [1] first associated CYP24A1 variants with IIH. With the subsequent recognition of late-onset disease in adults (characterized by hypercalcemia, NC, nephrolithiasis, or a combination) [3, 7, 9, 12, 14, 21, 38, 41] and studies that showed only select cases of IIH were associated with CYP24A1 sequence variants [4], it is important to characterize this disorder as 24HD, which in its most severe form may cause IIH. This distinction is important because not all patients with 24HD will have IIH and vice versa. Schlingmann et al. also reported variants in SLC34A1 encoding NaPi2a present in the renal proximal tubule and responsible for phosphate reabsorption to be another cause of IIH [42]. As in patients with CYP24A1 variants, the underlying cause of hypercalcemia and hypercalciuria is elevated 1,25(OH)2D3. However, in contrast to patients with CYP24A1 variants, those with SLC34A1 variants exhibit renal phosphate wasting with low FGF23 concentrations.
Hypercalcemia is common in the inpatient and outpatient settings and has a broad differential diagnosis [23]. Discovery of the abnormality often prompts an extensive and costly evaluation, particularly when non-PTH mediated. Although much about the prevalence and disease course of 24HD still remains unknown, a recent study from several European university centers reported 35% of patients with hypercalcemia (serum calcium >2.6 mmol/L) and low serum PTH (<20 pg/mL) harbored variants in CYP24A1 [15]. Additionally, Nesterova et al. [17] estimated that the prevalence of biallelic CYP24A1 variants in the general population is as high as 0.4% to 2%. Thus, despite limited data, 24HD is an important addition to the differential diagnosis of a patient with vitamin D–mediated hypercalcemia.
Recent studies have reported that the ratio of 25(OH)D3:24,25(OH)2D, as determined by liquid chromatography with tandem mass spectrometry, may sensitively identify patients with 24HD [15, 43]; ratios exceeding 50 (usually >80) are considered compatible with the disorder (reference range, 7-23) [44]. Thus, we used the ratio of 50 or higher in patients with clinical findings consistent with 24HD as an inclusion criterion for our study. More than 65% of our 24HD cohort had genetic testing that confirmed the condition. The average 24,25(OH)2D3 concentration for patients with 24HD in our cohort was 0.19 (range, 0.1-0.28), and all patient values were below the reference range for vitamin D−replete healthy individuals (>1.7 ng/mL) [44].
Our results indicate that presenting features overlap considerably for different forms of vitamin D−mediated hypercalcemia (Table 2). However, 55% of our cohort with 24HD initially presented with urinary stones and 22% with IIH, suggesting that stones may be a more common finding compared to other causes of vitamin D mediated hypercalcemia. Although the current cohort was small, our observation was consistent with findings of a recent literature review by Jobst-Schwan et al. [12]. Features of patients with sarcoidosis were less specific, although pulmonary symptoms may be a differentiating factor. Severe hypercalcemia symptoms were most common at presentation for patients with lymphoma (86% of cases).
Comparison of patients with 24HD with those having other forms of vitamin D−mediated hypercalcemia revealed several significant differences (Table 3). Given the genetic basis of the disease and the previously reported characteristics, we had suspected that patients with 24HD would be significantly younger at symptom onset and would be more likely to have a positive family history and NC than all other groups. However, the disease-specific subgroup analysis showed that patients with 24HD were younger and more likely to have a positive family history and NC than the lymphoma and sarcoidosis groups only (not EVT). The lack of significant difference between 24HD and EVT was likely driven by a few families that were included in an already small cohort: we had 1 family of 4 individuals in our 24HD group (n = 9) and 3 siblings in our EVT group (n = 6) who all received supplements with a very high vitamin D content. Furthermore, we had imaging studies (to assess for NC) available for only 2 individuals in the EVT group. Further studies of a larger cohort would be needed to adequately compare these 2 groups.
The effect of the CYP24A1 variant on bone density is also unclear, with previous studies reporting inconsistent results that ranged from low [17, 38] to normal [14, 41, 45] to increased [3] bone density. We observed significantly lower lumbar spine Z-scores in patients with 24HD, including some members of the family reported by Tebben et al. [38] plus 5 unrelated patients with suspected or confirmed 24HD. Of note, we did not observe significant differences in hip Z-scores when comparing 24HD with all other groups, nor did we observe differences in hip or lumbar spine Z-scores when comparing 24HD to individual groups, likely related to the relatively small groups sizes. Decreased bone density in 24HD would theoretically be caused by chronic high or high-normal 1,25(OH)2D3 levels resulting in increased osteoclastic resorption from bone. Our results from this small cohort suggest that 24HD may decrease bone density and affect trabecular bone to a greater degree than cortical bone. Nevertheless, with such varied results across the literature, the effects of 24HD on bone density remains incompletely understood and additional studies are needed for elucidation.
We also noted significantly higher age-adjusted peak serum phosphorus levels in patients with 24HD than in all other groups (Table 2 and Fig. 1F). Phosphorus levels are often not reported in previously published papers regarding 24HD and reviewing the literature reveals that, among those reporting serum phosphorus, there is variability, with some reporting normal levels, some elevated, and some low [3, 37, 38, 46]. The higher serum phosphorus concentration could be related to chronic elevation of 1,25(OH)2D3 increasing intestinal phosphate absorption. Additionally, suppressed PTH would decrease renal phosphate loss, though elevated FGF23 would be expected to induce phosphaturia [14]. Unfortunately, we do not have data regarding FGF23 for this cohort and it is difficult to predict with certainty whether FGF23 would be elevated due to higher serum phosphorus and 1,25(OH)2D3 or decreased due to low PTH, while acknowledging that the impact on FGF23 of 24H and 24,25(OH)2D, remain unknown. Further investigation into phosphate homeostasis including FGF23 analysis in this population is needed.
Hypercalciuria has been noted in patients with 24HD [3, 4, 7, 22]. In our cohort, more 24HD patients presented with hypercalciuria compared with all other groups (88.9% vs 55.6%). However, the difference was not statistically significant and hypercalciuria therefore does not appear to be a differentiating factor. We did observed significantly higher uCa:Cr in patients with 24HD. This result is challenging to interpret because patients in the 24HD group were younger at symptom onset, and normal uCa:Cr values are higher in infants and slowly decline during the first few years of life [39]. Thus, age alone may be the primary reason for this result. Weight-adjusted 24-hour urinary calcium excretion values, which are less influenced by age variation, were not significantly different, and uCa:Cr ratios were not significantly different when comparing individual groups. In summary, urine studies likely do not meaningfully contribute to differentiation of 24HD from other disorders of vitamin D−mediated hypercalcemia.
Comparison of individual groups showed that patients with 24HD, while hypercalcemic, had significantly lower serum calcium levels than patients with lymphoma (Table 3 and Fig. 1E). Markedly elevated calcium levels are a hallmark of hypercalcemia of malignancy, with multiple mechanisms implicated beyond elevated 1,25(OH)2D3, including PTH-related peptide and osteolytic metastases [47]. Thus, marked hypercalcemia with severe symptoms, in the absence of strong 24HD-differentiating factors (eg, family history, NC, younger age) should prompt investigation for malignancy.
Comparisons among individual disease groups also showed that patients with 24HD had significantly higher 25(OH)D3 concentrations than those with lymphoma or sarcoidosis but significantly lower concentrations than patients with EVT. 25(OH)D3 concentration exceeding at least 80 ng/mL (and usually much higher) is typically required to produce hypercalcemia from EVT [48]. Thus, high-normal 25(OH)D3 levels may prompt suspicion for 24HD, but values >80 ng/mL may be more consistent with an exogenous source. Furthermore, high 1,25(OH)2D3 levels are anticipated in 24HD and have been documented in multiple patients [2, 3, 38, 41]. Although patients in our cohort with 24HD did have relatively higher 1,25(OH)2D3 values, the difference was not statistically different compared with other vitamin D–mediated causes of hypercalcemia. Figure 1C shows the high-normal range of 1,25(OH)2D3 values for patients with 24HD. Thus, as previously reported, 1,25(OH)2D3 concentrations within the reference range should not exclude the diagnosis [1, 4, 9]. Dauber et al. [4] explained this phenomenon with the hypothesis that serum 1,25(OH)2D3 concentrations were not indicative of tissue levels.
Though generally perceived as an autosomal recessive disease, we described a kindred that included several affected members with a single allele variant, suggesting dominant inheritance in some is possible [38]. Although it may be that a second pathogenic mutation was not identified, the milder phenotype described in this cohort suggests that those with single allele variants have reduced penetrance. Nonetheless, given that the vast majority of publications regarding patients with 24HD report autosomal recessive inheritance, further evidence from nuanced genotype–phenotype studies will be required to support single allele disease causation in this condition.
Limitations of this study include the retrospective study design, which affected the availability of data for each disease group. Additionally, our cohort size was small and included a family in the 24HD subgroup (4 individuals) and another in the EVT subgroup (3 individuals), which may bias findings due to genetic overlap. Future prospective studies with larger nonrelated cohorts are needed to further clarify the defining characteristics of this disease relative to other vitamin D–mediated hypercalcemia disorders.
Conclusions
Our study identified disease characteristics that may help differentiate 24HD from other disorders of vitamin D–mediated hypercalcemia, and thus may assist in the clinical evaluation of these patients. 24HD should be considered early in the diagnostic process, especially in younger patients, those with a positive family history of hypercalcemia or hypercalciuria, and those with nephrocalcinosis.
Acknowledgments
Preliminary data from this study were presented on Saturday November 9, 2019, at the 2019 ASN Kidney Week conference in Washington, DC. The abstract was published as follows: Azer SM, Vaughan LE, Tebben P, Sas DJ. 24 Hydroxylase Deficiency: Comparison with Other Disorders of Vitamin D-Mediated Hypercalcemia [SA-PO258]. J Am Soc Nephrol. 2019;30:830.
Funding: None. On behalf of all my coauthors, I certify that we have no affiliation with, or financial involvement in, any organization or entity with a direct financial interest in the subject matter or materials discussed in the manuscript (eg, employment, consultant services, stock ownership, honoraria). Any financial project support of this research is identified in the manuscript.
Conflict of Interest: None.
Glossary
Abbreviations
- 1αH
25(OH)D3 1-α hydroxylase
- 24H
24-hydroxylase
- 24HD
24-hydroxylase deficiency
- EVT
exogenous vitamin D toxicity
- IIH
idiopathic infantile hypercalcemia
- IQR
interquartile range
- NC
nephrocalcinosis
- PTH
parathyroid hormone
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- uCa:Cr
urinary calcium-to-creatinine ratio.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Schlingmann KP, Kaufmann M, Weber S, et al. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med. 2011;365(5):410-421. [DOI] [PubMed] [Google Scholar]
- 2. Castanet M, Mallet E, Kottler ML. Lightwood syndrome revisited with a novel mutation in CYP24 and vitamin D supplement recommendations. J Pediatr. 2013;163(4):1208-1210. [DOI] [PubMed] [Google Scholar]
- 3. Colussi G, Ganon L, Penco S, et al. Chronic hypercalcaemia from inactivating mutations of vitamin D 24-hydroxylase (CYP24A1): implications for mineral metabolism changes in chronic renal failure. Nephrol Dial Transplant. 2014;29(3):636-643. [DOI] [PubMed] [Google Scholar]
- 4. Dauber A, Nguyen TT, Sochett E, et al. Genetic defect in CYP24A1, the vitamin D 24-hydroxylase gene, in a patient with severe infantile hypercalcemia. J Clin Endocrinol Metab. 2012;97(2):E268-E274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di Maio F, Vittori M, Bassi P, Fulignati P, D’Alonzo S, Ferraro PM. [A young girl with recurrent calculosis and hypercalcemia]. G Ital Nefrol. 2018;35(3). [PubMed] [Google Scholar]
- 6. Dinour D, Beckerman P, Ganon L, Tordjman K, Eisenstein Z, Holtzman EJ. Loss-of-function mutations of CYP24A1, the vitamin D 24-hydroxylase gene, cause long-standing hypercalciuric nephrolithiasis and nephrocalcinosis. J Urol. 2013;190(2):552-557. [DOI] [PubMed] [Google Scholar]
- 7. Dowen FE, Sayers JA, Hynes AM, Sayer JA. CYP24A1 mutation leading to nephrocalcinosis. Kidney Int. 2014;85(6):1475. [DOI] [PubMed] [Google Scholar]
- 8. Fencl F, Bláhová K, Schlingmann KP, Konrad M, Seeman T. Severe hypercalcemic crisis in an infant with idiopathic infantile hypercalcemia caused by mutation in CYP24A1 gene. Eur J Pediatr. 2013;172(1):45-49. [DOI] [PubMed] [Google Scholar]
- 9. Figueres ML, Linglart A, Bienaime F, et al. Kidney function and influence of sunlight exposure in patients with impaired 24-hydroxylation of vitamin D due to CYP24A1 mutations. Am J Kidney Dis. 2015;65(1):122-126. [DOI] [PubMed] [Google Scholar]
- 10. Gigante M, Santangelo L, Diella S, et al. Mutational spectrum of CYP24A1 gene in a cohort of Italian patients with idiopathic infantile hypercalcemia. Nephron. 2016;133(3):193-204. [DOI] [PubMed] [Google Scholar]
- 11. Jacobs TP, Kaufman M, Jones G, et al. A lifetime of hypercalcemia and hypercalciuria, finally explained. J Clin Endocrinol Metab. 2014;99(3):708-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jobst-Schwan T, Pannes A, Schlingmann KP, Eckardt KU, Beck BB, Wiesener MS. Discordant clinical course of vitamin-d-hydroxylase (CYP24A1) associated hypercalcemia in two adult brothers with nephrocalcinosis. Kidney Blood Press Res. 2015;40(5):443-451. [DOI] [PubMed] [Google Scholar]
- 13. Madsen JOB, Sauer S, Beck B, Johannesen J. CYP24A1 mutation in a girl infant with idiopathic infantile hypercalcemia. J Clin Res Pediatr Endocrinol. 2018;10(1):83-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meusburger E, Mündlein A, Zitt E, Obermayer-Pietsch B, Kotzot D, Lhotta K. Medullary nephrocalcinosis in an adult patient with idiopathic infantile hypercalcaemia and a novel CYP24A1 mutation. Clin Kidney J. 2013;6(2):211-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Molin A, Baudoin R, Kaufmann M, et al. CYP24A1 mutations in a cohort of hypercalcemic patients: evidence for a recessive trait. J Clin Endocrinol Metab. 2015;100(10):E1343-E1352. [DOI] [PubMed] [Google Scholar]
- 16. Molin A, Nowoczyn M, Coudray N, et al. Molecular characterization of a recurrent 10.9 kb CYP24A1 deletion in idiopathic infantile hypercalcemia. Eur J Med Genet. 2019;62(11):103577. [DOI] [PubMed] [Google Scholar]
- 17. Nesterova G, Malicdan MC, Yasuda K, et al. 1,25-(OH)2D-24 hydroxylase (CYP24A1) deficiency as a cause of nephrolithiasis. Clin J Am Soc Nephrol. 2013;8(4):649-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pronicka E, Ciara E, Halat P, et al. Biallelic mutations in CYP24A1 or SLC34A1 as a cause of infantile idiopathic hypercalcemia (IIH) with vitamin D hypersensitivity: molecular study of 11 historical IIH cases. J Appl Genet. 2017;58(3):349-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schlingmann KP, Cassar W, Konrad M. Juvenile onset IIH and CYP24A1 mutations. Bone Rep. 2018;9:42-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shah AD, Hsiao EC, O’Donnell B, et al. Maternal hypercalcemia due to failure of 1,25-Dihydroxyvitamin-D3 catabolism in a patient with CYP24A1 mutations. J Clin Endocrinol Metab. 2015;100(8):2832-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wolf P, Müller-Sacherer T, Baumgartner-Parzer S, et al. A case of “late-onset” idiopathic infantile hypercalcemia secondary to mutations in the CYP24A1 gene. Endocr Pract. 2014;20(5):e91-e95. [DOI] [PubMed] [Google Scholar]
- 22. Woods GN, Saitman A, Gao H, Clarke NJ, Fitzgerald RL, Chi NW. A young woman with recurrent gestational hypercalcemia and acute pancreatitis caused by CYP24A1 deficiency. J Bone Miner Res. 2016;31(10):1841-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lafferty FW. Differential diagnosis of hypercalcemia. J Bone Miner Res. 1991;6(Suppl 2):S51-59; discussion S61. [DOI] [PubMed] [Google Scholar]
- 24. Jones G, Kottler ML, Schlingmann KP. Genetic diseases of vitamin D metabolizing enzymes. Endocrinol Metab Clin North Am. 2017;46(4):1095-1117. [DOI] [PubMed] [Google Scholar]
- 25. Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96(1):365-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tebben PJ, Singh RJ, Kumar R. Vitamin D-mediated hypercalcemia: mechanisms, diagnosis, and treatment. Endocr Rev. 2016;37(5):521-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumar R. Vitamin D metabolism and mechanisms of calcium transport. J Am Soc Nephrol. 1990;1(1):30-42. [DOI] [PubMed] [Google Scholar]
- 28. Bell NH. Renal and nonrenal 25-hydroxyvitamin D-1alpha-hydroxylases and their clinical significance. J Bone Miner Res. 1998;13(3):350-353. [DOI] [PubMed] [Google Scholar]
- 29. Kumar R. Metabolism of 1,25-dihydroxyvitamin D3. Physiol Rev. 1984;64(2):478-504. [DOI] [PubMed] [Google Scholar]
- 30. Makin G, Lohnes D, Byford V, Ray R, Jones G. Target cell metabolism of 1,25-dihydroxyvitamin D3 to calcitroic acid. Evidence for a pathway in kidney and bone involving 24-oxidation. Biochem J. 1989;262(1):173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reddy GS, Tserng KY. Calcitroic acid, end product of renal metabolism of 1,25-dihydroxyvitamin D3 through C-24 oxidation pathway. Biochemistry. 1989;28(4):1763-1769. [DOI] [PubMed] [Google Scholar]
- 32. Kumar R, Schaefer J, Grande JP, Roche PC. Immunolocalization of calcitriol receptor, 24-hydroxylase cytochrome P-450, and calbindin D28k in human kidney. Am J Physiol. 1994;266(3 Pt 2):F477-F485. [DOI] [PubMed] [Google Scholar]
- 33. Tanaka Y, Deluca HF. The control of 25-hydroxyvitamin D metabolism by inorganic phosphorus. Arch Biochem Biophys. 1973;154(2):566-574. [DOI] [PubMed] [Google Scholar]
- 34. DeLuca HF, Schnoes HK. Vitamin D: recent advances. Annu Rev Biochem. 1983;52:411-439. [DOI] [PubMed] [Google Scholar]
- 35. Ribovich ML, DeLuca HF. 1,25-Dihydroxyvitamin D3 metabolism. The effect of dietary calcium and phosphorus. Arch Biochem Biophys. 1978;188(1):164-171. [DOI] [PubMed] [Google Scholar]
- 36. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344-349. [DOI] [PubMed] [Google Scholar]
- 37. Richards S, Aziz N, Bale S, et al. ; ACMG Laboratory Quality Assurance Committee . Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tebben PJ, Milliner DS, Horst RL, et al. Hypercalcemia, hypercalciuria, and elevated calcitriol concentrations with autosomal dominant transmission due to CYP24A1 mutations: effects of ketoconazole therapy. J Clin Endocrinol Metab. 2012;97(3):E423-E427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sargent JD, Stukel TA, Kresel J, Klein RZ. Normal values for random urinary calcium to creatinine ratios in infancy. J Pediatr. 1993;123(3):393-397. [DOI] [PubMed] [Google Scholar]
- 40. Leslie SW, Gossman WG.. Hypercalciuria. StatPearls; 2019. [Google Scholar]
- 41. Streeten EA, Zarbalian K, Damcott CM. CYP24A1 mutations in idiopathic infantile hypercalcemia. N Engl J Med. 2011;365(18):1741-2; author reply 1742. [DOI] [PubMed] [Google Scholar]
- 42. Schlingmann KP, Ruminska J, Kaufmann M, et al. Autosomal-recessive mutations in SLC34A1 encoding sodium-phosphate cotransporter 2A cause idiopathic infantile hypercalcemia. J Am Soc Nephrol. 2016;27(2):604-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaufmann M, Gallagher JC, Peacock M, et al. Clinical utility of simultaneous quantitation of 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D by LC-MS/MS involving derivatization with DMEQ-TAD. J Clin Endocrinol Metab. 2014;99(7):2567-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tang JCY, Nicholls H, Piec I, et al. Reference intervals for serum 24,25-dihydroxyvitamin D and the ratio with 25-hydroxyvitamin D established using a newly developed LC-MS/MS method. J Nutr Biochem. 2017;46:21-29. [DOI] [PubMed] [Google Scholar]
- 45. Cools M, Goemaere S, Baetens D, et al. Calcium and bone homeostasis in heterozygous carriers of CYP24A1 mutations: a cross-sectional study. Bone. 2015;81:89-96. [DOI] [PubMed] [Google Scholar]
- 46. Wilbanks J, Hillyer J, Hashim F, Sas D, Hanna C. A toddler with severe hypercalcemia and pyelonephritis: Questions. Pediatr Nephrol. 2021;36(4):857-858. [DOI] [PubMed] [Google Scholar]
- 47. Mirrakhimov AE. Hypercalcemia of malignancy: an update on pathogenesis and management. N Am J Med Sci. 2015;7(11):483-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Galior K, Grebe S, Singh R. Development of vitamin D toxicity from overcorrection of vitamin D deficiency: a review of case reports. Nutrients. 2018;10(8):953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.