Abstract
Context
Glucagon is produced and released from the pancreatic alpha-cell to regulate glucose levels during periods of fasting. The main target for glucagon action is the liver, where it activates gluconeogenesis and glycogen breakdown; however, glucagon is postulated to have other roles within the body.
Objective
We sought to identify the circulating metabolites that would serve as markers of glucagon action in humans.
Methods
In this study (NCT03139305), we performed a continuous 72-hour glucagon infusion in healthy individuals with overweight/obesity. Participants were randomized to receive glucagon 12.5 ng/kg/min (GCG 12.5), glucagon 25 ng/kg/min (GCG 25), or a placebo control. A comprehensive metabolomics analysis was then performed from plasma isolated at several time points during the infusion to identify markers of glucagon activity.
Results
Glucagon (GCG 12.5 and GCG 25) resulted in significant changes in the plasma metabolome as soon as 4 hours following infusion. Pathways involved in amino acid metabolism were among the most affected. Rapid and sustained reduction of a broad panel of amino acids was observed. Additionally, time-dependent changes in free fatty acids and diacylglycerol and triglyceride species were observed.
Conclusion
These results define a distinct signature of glucagon action that is broader than the known changes in glucose levels. In particular, the robust changes in amino acid levels may prove useful to monitor changes induced by glucagon in the context of additional glucagon-like peptide-1 or gastric inhibitory polypeptide treatment, as these agents also elicit changes in glucose levels.
Keywords: glucagon, metabolomics, amino acids
Glucagon is a peptide hormone produced in the pancreatic α-cells with a well-established role in hepatic glucose production and glucose homeostasis. Glucagon is released from the pancreas during the early stage of fasting in response to falling plasma glucose levels. The predominant target tissue for glucagon action is the liver [1]. Activation of the glucagon receptor (GCGR), a Gαs-coupled G-protein coupled receptor, leads to a cellular rise in cyclic adenosine monophosphate (cAMP). Acutely, this stimulates glycogenolysis through inhibition of glycogen synthase and simultaneous activation of glycogen phosphorylase, leading to glycogen breakdown and glucose release. Early studies demonstrated that exogenous glucagon infusion in pancreatic clamped dogs resulted in a rapid increase in glycogenolysis and hepatic glucose production within 15 minutes [2]. Glucagon infusion in healthy human subjects also results in a rapid increase in hepatic glucose production as a result of glycogenolysis [3]. Activation of gluconeogenesis through increased gluconeogenic enzyme expression is a well-described consequence of glucagon in the liver. This effect is mediated through multiple transcriptional regulators including the cAMP-response element-binding protein (CREB) and the cAMP-regulated transcriptional co-activators (CRTCs), among others [4].
Dysregulation of glucagon signaling has also been implicated in the pathogenesis of metabolic disease and diabetes. For instance, patients with type 2 diabetes display higher glucagon levels throughout the day [5]. Insulin resistance is also associated with poor glucagon suppression following an oral glucose tolerance test [6]. In addition, diabetics have shown a diminished suppression of glucagon in response to insulin [7]. Given this data, inhibition of glucagon signaling has been pursued as a therapeutic target in diabetes and metabolic disease. Indeed, several inhibitors of the GCGR have been identified and result in lower plasma glucose levels [8]. However, in spite of effective blood glucose lowering, GCGR inhibition has resulted in untoward side effects, such as elevation of liver enzymes, increases in cholesterol, and concerns of pancreatic α -cell hyperplasia.
A role for glucagon outside of direct glucose homeostasis has also been suggested. For instance, glucagon infusion at high doses acutely increases energy expenditure [9, 10]. Glucagon also acts to reduce food intake [11, 12]. The effect of glucagon on energy intake and expenditure has made it an attractive target to treat obesity and other metabolic disturbances. Many studies are currently underway to investigate whether glucagon alone or in combination with glucagon-like peptide-1 (GLP-1) agonism can achieve body weight reduction superior to pure GLP-1 treatment alone. However, in particular, the combination with GLP-1 makes it quite difficult to measure isolated glucagon effects, as GLP-1 suppresses any glucagon-mediated increase in blood glucose. As such, there is a need for other markers of glucagon activity in humans.
We recently performed the longest to date (72-hour) glucagon infusion at tolerable doses (12.5 ng/kg/min and 25 ng/kg/min) on healthy individuals with overweight/obesity to determine the effects on energy expenditure, energy balance, and nausea [13]. Using blood samples collected during this infusion, we aimed to use nontargeted metabolomics and lipidomic analyses to ascertain markers of glucagon action in humans.
Methods
Participants
Healthy participants with overweight/obesity (body mass index [BMI] ≥27 to ≤45) were recruited to participate in the study. Subjects were nondiabetic (fasting blood glucose <126 mg/dL and HbA1c <6.5%) and in good health on the basis of medical history, physical examination, electrocardiogram, and normal laboratory values at screening. Full inclusion/exclusion criteria can be found at ClinicalTrials.gov (NCT03139305). Thirty-three participants completed the study for metabolomics analysis (Placebo, N = 10; GCG 12.5 ng/kg/min, N = 12; GCG 25 ng/kg/min, N = 11). The study protocol was approved by the AdventHealth Institutional Review Board and carried out in accordance with the Declaration of Helsinki. All participants provided their written consent.
Study Design
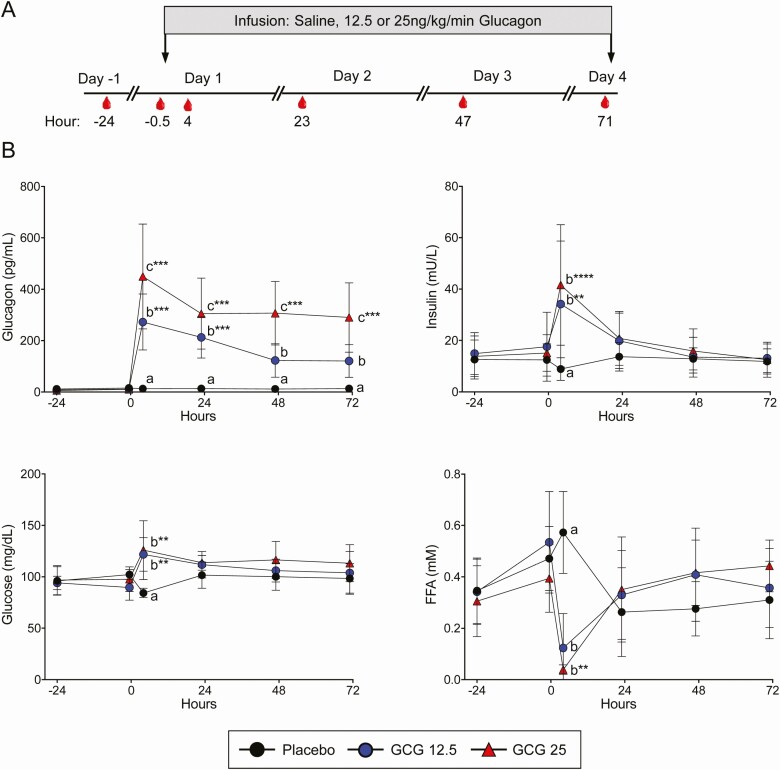
This was a prospective, randomized, placebo-controlled, 3-treatment parallel arm study to determine the effects of prolonged (72 hours) glucagon (GCG) administration at low and high doses. Study subjects were admitted to the Clinical Research Unit at the Translational Research Institute at AdventHealth for 5 overnight stays. Details of the full clinical protocol can be reviewed at [13]. Briefly, the first 2 overnight stays were used as baseline. On the second day (Day −1), a baseline blood draw (−24 hours) was performed. On Day 1, a blood draw was again taken approximately 30 minutes prior to the start of the glucagon infusion. The study subjects began a ramped infusion (first 4 hours) of either saline (placebo), low dose glucagon (12.5 ng/kg/min), or high dose glucagon (25 ng/kg/min) for the next 72 hours (Inpatient). The glucagon (GlucaGen, Novo Nordisk, Bagsværd Denmark) was administered under an FDA-approved Investigational New Drug Application (IND #136634). Intravenous (IV) bags of the study medication were hung together on an IV pole and changed out every 24 hours to provide a continuous infusion. Blood collections were taken as outlined in Fig. 1. With the exception of the 4-hour time point, all draws were performed in the overnight fasted state. Participants received 3 meals a day (50% carbohydrate, 35% fat, and 15% protein). Throughout the inpatient visit, vital sign measurements, metabolic weight assessments, continuous glucose monitoring, adverse event assessments, and urine collections were performed. Study subjects returned for a follow-up visit 2 to 5 days after discharge from the Clinical Research Unit for measurements of weight, vital and basic laboratory assessments, and determination of adverse events.
Figure 1.
Glucagon infusion results in a compensatory increase in insulin and suppression of lipolysis. A, Schematic of the protocol overview is shown. Blood draws were conducted at −24, −0.5, 4, 23, 47, and 71 hours relative to the beginning of the glucagon or placebo (saline) infusion. B, Plasma glucagon, insulin, glucose, and free fatty acids (FFA) were measured at each time point shown. Data points represent mean ± SD. Letters denote group differences at the indicated time point. **P < 0.01, ***P < 0.001, and ****P < 0.0001 vs the −24-hour time point for each treatment group.
Metabolomics
The global metabolomics analysis was performed as previously described [14]. Briefly, samples were homogenized and subjected to methanol extraction then split into aliquots for analysis by ultrahigh performance liquid chromatography/mass spectrometry (UHPLC/MS) in the positive (2 methods) and negative (2 methods) mode. Metabolites were then identified by automated comparison of ion features to a reference library of chemical standards, followed by visual inspection for quality control, as previously described [15]. For statistical analyses and data display, any missing values are assumed to be below the limits of detection; these values were imputed with the compound minimum (minimum value imputation). Statistical tests were performed in ArrayStudio (Omicsoft) or “R” to compare data between experimental groups; P < 0.05 is considered significant. An estimate of the false discovery rate (Q-value) is also calculated to account for the multiple comparisons that normally occur in metabolomic-based studies, with Q < 0.05 used as an indication of high confidence in a result.
Quantitative Measurement of Amino Acids
The targeted and quantitative metabolomics of amino acids was performed as previously described [16].
Extraction and derivatization of amino acids
A 100 µL aliquot of plasma was spiked with a 10 µL mixture of heavy isotope-labeled, amino acid internal standards followed by the addition of 800 µL of ice-cold methanol. Samples were vortexed and centrifuged at 18 000g for 5 minutes at 10 °C. A 100 µL aliquot of the methanol extract was dried under nitrogen and amino acids were reconstituted and derivatized using a MassTrak AAA Derivatization Kit (Waters Corp.). The samples were incubated at 55 °C for 10 minutes to complete the derivatization reaction.
Liquid chromatography
Derivatized amino acids were separated on a 2.1 × 100 mm, 1.7 µm Waters AccQTag column (T = 55 °C). The mobile phase gradient was 100% A (Solvent A, Cat# 186003838-Propriety Waters Corp.) and 0% B (Solvent B, Cat# 186003839-Propriety Waters Corp.) to 50% A and 50% B over 9.54 minutes. The gradient began at 0% B (0.7 mL min-1 flow rate) from 0 to 0.54 minutes, was increased from 0% to 9% B (0.7 mL min-1 flow rate) from 0.54 to 5.74 minutes, was increased from 9% to 21.2% B (0.7 mL min-1 flow rate) from 5.74 to 8.24 minutes, was increased from 21.2% B to 50% B (0.7 mL min-1 flow rate) from 8.24 minutes to 9.54 minutes, was increased from 50% B to 90% B (0.7 mL min-1 flow rate) from 9.54 minutes to 9.55 minutes and was held until 10.14 minutes. Re-equilibration was performed at 0% B from 10.14 to 10.15 minutes (0.7 mL min-1 flow rate) and was held until 10.15 minutes. The samples were injected (0.5 µL) by the autosampler maintained at 10 °C for the entire run.
Mass spectrometry
Quantitation of derivatized amino acids was achieved using multiple reaction monitoring of calibration solutions and study samples with an Agilent 1290 HPLC/6490 triple quadrupole mass spectrometer (Waters Corp.). The mass spectrometer was operated in positive ion mode using electrospray ionization (ESI) with an ESI capillary voltage of 3500V. The electron multiplier voltage was set to 100V. The ion transfer tube temperature was 325 °C and vaporizer temperature was 325 °C. The ESI source sheath gas flow was set at 10 L/min. The mass spectrometer was operated with a mass resolution of 0.7 Da, a cycle time of 1.9 cycles/s, and nitrogen collision gas pressure was 30 psi for the generation and detection of product ions of each amino acid.
Data processing
The raw data was processed using Mass hunter quantitative analysis software (Agilent). Calibration curves (R2 = 0.99 or greater) were either fitted with a linear or a quadratic curve with a 1/X or 1/X2 weighting.
Blood and Urine Analyses
Blood glucose measurements were obtained bedside with a StatStrip glucose meter (Nova Biomedical). Plasma measurements of glucagon (Mercodia, Inc.), free fatty acids (FUJIFILM Wako Diagnostics USA), and insulin (Meso Scale Discovery) were performed per manufacturers’ instructions. Total urinary nitrogen was measured by pyrochemiluminescence as previously described [17].
Bioinformatic Analyses
A multistep analysis procedure was applied on the metabolic profile. First, the metabolite profile was log2-transformed and z-scaled to standardize every metabolite so that they were all on the same scale for statistical analysis. The differentially expressed metabolite analysis was then performed using Limma, an R/Bioconductor software package [18]. The objective was to identify metabolite levels that were significantly affected by glucagon infusion when compared to placebo control. The analyses were performed at the timepoints of 4, 23, and 71 hours with the low (12.5 ng/kg/min) and high (25 ng/kg/min) glucagon infusion dose. Once differentially expressed metabolite sets were identified, overrepresentation analysis was performed on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database [19] to identify significantly enriched biological pathways. Lastly, random forest prediction [20] was applied with split-variable randomization to find metabolites associated with the glucagon infusion and furthermore rank them according to their classification significances [21]. Principal component analysis was performed on the log2-transformed and z-scaled data with all metabolites, as well as the top 100 ranked metabolites, to assess the impact of glucagon infusion. Heat maps were prepared by Morpheus (Broad Institute, https://clue.io/morpheus).
Results
Study Design and Validation
Participants with overweight/obesity were recruited and randomized to receive placebo or 12.5 or 25 ng/kg/min glucagon (GCG 12.5 and GCG 25, respectively) infusion. The subject characteristics are shown in Table 1. The groups did not differ with regards to age, BMI, height, or weight. The participants received continually infused glucagon for 72 hours. Multiple blood draws were performed prior to and during the infusion period (Fig. 1). As shown in Fig. 1, plasma glucagon levels were rapidly increased and sustained throughout the infusion period in a dose-dependent fashion. Consistent with glucagon’s acute stimulatory effect on glycogenolysis, there is a sharp rise in plasma glucose levels at 4 hours (Fig. 1). As expected, there is concomitant rise in plasma insulin levels and suppression of lipolysis evident by a large decrease in circulating free fatty acids (FFA) 4 hours after the start of the glucagon infusion. At later time points in the infusion, however, glucose and insulin levels normalize and are similar to placebo. These results demonstrated that the glucagon infusion achieved the expected effects on hepatic glucose and glycogen metabolism. In addition, we see a rise of the FFA levels at the fasted time points that shows that glucagon infusion is leading to an activation of lipogenesis from adipose tissue under low insulin conditions.
Table 1.
Participant characteristics
| Variable | Placebo | GCG 12.5 | GCG 25 |
|---|---|---|---|
| Age, years | 45.5 ± 5.8 | 43.0 ± 11.0 | 41.7 ± 9.3 |
| BMI, kg/m2 | 34.89 ± 5.74 | 35.09 ± 2.70 | 34.86 ± 5.13 |
| Height, cm | 170.3 ± 9.7 | 164.0 ± 10.2 | 168.0 ± 9.5 |
| Weight, kg | 101.80 ± 24.26 | 94.11 ± 14.03 | 98.23 ± 21.02 |
| Sex, F/M | 6/4 | 10/2 | 7/4 |
Abbreviations: BMI, body mass index; GCG, glucagon.
Changes in the Plasma Metabolome With Glucagon Infusion
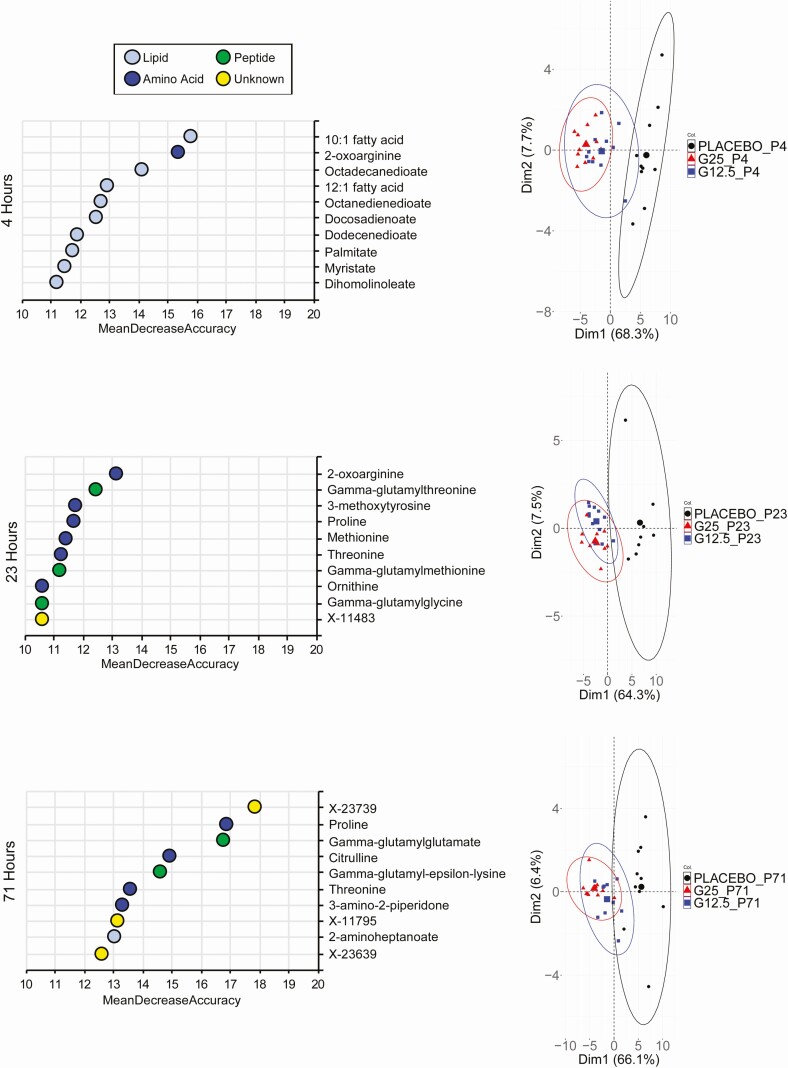
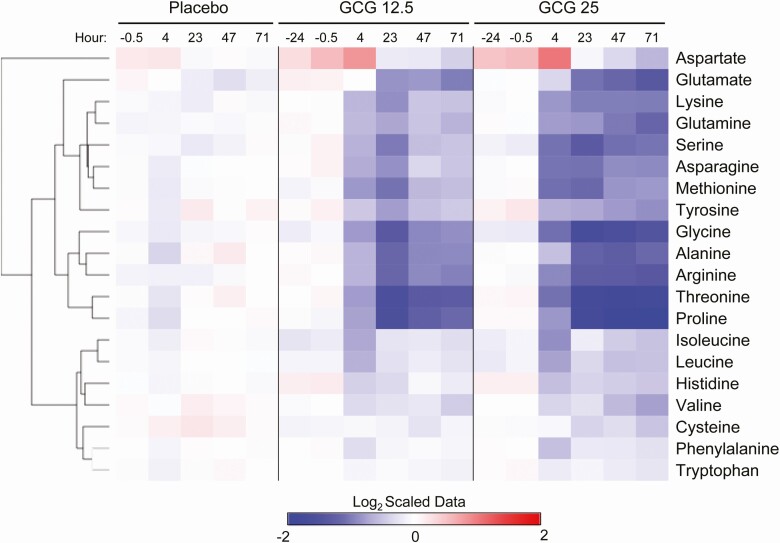
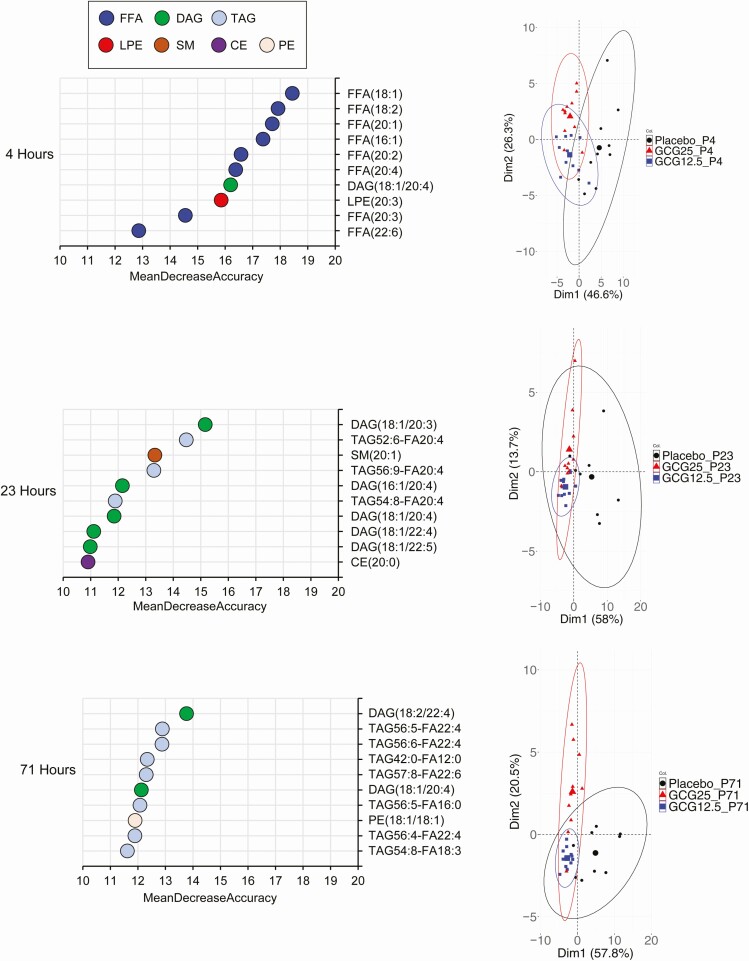
To gain a greater understanding of the effect of an extended glucagon exposure, we performed global metabolomic and lipidomic analysis. Metabolomic analysis of plasma samples obtained during the glucagon infusion confirmed the changes in glucose levels (Supplemental Figure 1 [22]). In addition, pyruvate and lactate levels were diminished at 23 hours and later, consistent with the activation of gluconeogenesis by glucagon. At the final time point of 71 hours, both GCG 12.5 and GCG 25 produced marked changes in the metabolomic profile as compared with the placebo group (Supplemental Figure 2 [22]). Strikingly, a large majority of metabolites were downregulated in the glucagon-treated samples, compared with placebo. Random forest algorithm was used at each time point to determine the metabolites with the greatest contribution to the distinction of the groups (Fig. 2). Principal component analysis (PCA) using the top 100 metabolites from the random forest analysis reveals that as soon as the 4-hour time point, separation of the glucagon groups from the placebo is evident (Fig. 2). Consistent with the FFA measurements, the top metabolites at 4 hours are fatty acids. At 23 and 71 hours, however, amino acids and peptides (namely gamma-glutamyl amino acids) predominate (Fig. 2). Examination of all essential amino acids revealed broad time-dependent decreases with both glucagon dose groups (Fig. 3). The magnitude of diminishment varied, with robust decreases of certain amino acids, such as proline and threonine, while others (eg, phenylalanine and tryptophan) displayed little change during the infusion period. We next used a quantitative and targeted metabolomic approach to confirm these findings in amino acid levels. As shown in Supplemental Figure 3 [22], the results confirmed the initial findings of a marked decrease in most amino acids at the 71-hour time point.
Figure 2.
Glucagon infusion produces dramatic changes in the plasma metabolite profile. Random forest analysis was performed to determine the metabolites with the greatest contribution to group separation. The top 10 metabolites for 4, 23, and 71 hours are shown (left panel). The color denotes metabolite classification. The top 100 metabolites from the random forest analysis were used for principal component analysis (PCA) at the same time points to visualize the separation of the 3 treatment groups (right panel).
Figure 3.
Glucagon infusion results in a marked reduction in amino acids. The heat map represents the change in plasma amino acid levels during the study period for all 3 treatment groups. The data displayed are log2-transformed of the z-scaled values normalized to the placebo at the −24-hour time point. The rows were Euclidian distance clustered.
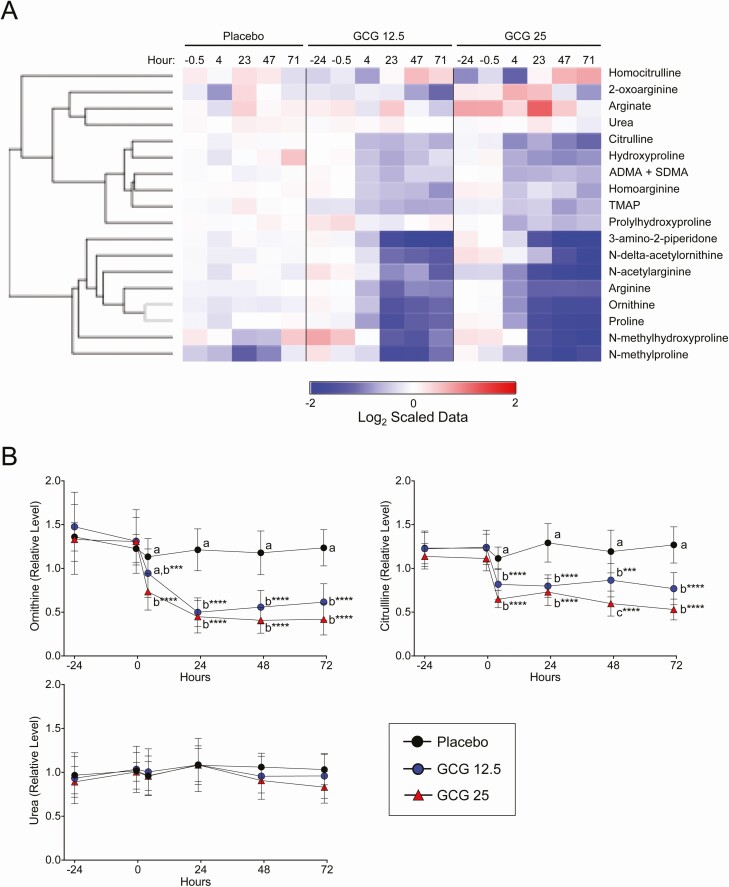
The decrease in amino acid levels during the glucagon infusion may be a consequence of increased gluconeogenesis from amino acids. The liver disposes of excess nitrogen from amino acid catabolism through the urea cycle. Therefore, we examined metabolites comprising and related to the urea cycle. Similar to the amino acid profile, there was a broad decrease in most of the metabolites examined, including ornithine and citrulline (Fig. 4A and 4B). Interestingly, plasma urea concentration changed very little with glucagon infusion. However, total urinary nitrogen did increase over time with glucagon infusion (Table 2).
Figure 4.
Regulation of urea cycle, arginine, and proline metabolites by glucagon. A, The heat map represents change in the indicated plasma metabolite during the study period for all 3 treatment groups. The data displayed are log2-transformed of the z-scaled values normalized to the placebo at the −24-hour time point. The rows were Euclidian distance clustered. B, Detailed changes in ornithine, citrulline, and urea are shown. Data points represent mean ± SD. Letters denote group differences at the indicated time point. ***P < 0.001, and ****P < 0.0001 vs the −24-hour time point for each treatment group.
Table 2.
Total urinary nitrogen (TUN)
| Study day | Placebo | GCG 12.5 | GCG 25 | |
|---|---|---|---|---|
| TUN (g/day) | Day −2 | 14.28 ± 4.5a | 10.81 ± 2.9a | 12.35 ± 2.7a |
| Day −1 | 12.17 ± 4.1a | 11.14 ± 3.3a | 12.66 ± 1.8a | |
| Day 1 | 11.47 ± 4.9a | 15.58 ± 3.6b | 15.95 ± 4.1a,b | |
| Day 2 | 12.68 ± 4.8a | 15.48 ± 4.0b | 17.77 ± 4.4b | |
| Day 3 | 13.41 ± 6.0a | 14.62 ± 3.8a,b | 15.58 ± 3.8a,b |
Annotations show within-subject comparisons. Days with different letters differ significantly in TUN (g/day) within each group.
Abbreviation: GCG, glucagon.
As noted in Fig. 2, plasma levels of a large panel of the gamma-glutamyl amino acid derivatives were markedly diminished in both glucagon treatment groups (Supplemental Figure 4 [22]). Several of these specific dipeptides are also known to be present in the Meister cycle, important in glutathione metabolism. In addition to the gamma-glutamyl amino acids, levels of the Meister cycle intermediate 5-oxoproline were also reduced (Supplemental Figure 4 [22]). These results suggest that the effects on gamma-glutamyl amino acids and the Meister cycle may have impacts on glutathione metabolism.
Changes in the Plasma Lipidome With Glucagon Infusion
Lipidomic analysis was also performed at the identical time points during the infusion. This revealed dynamic regulation of plasma fatty acids and lipids. Clear separation of the glucagon treatment groups from placebo was observed as early as the 4-hour time point (Fig. 5). Random forest classification analysis identified a number of FFA as the largest contributors to the group classification. These data are consistent with the decrease in total FFA (Fig. 1) as a consequence of insulin-dependent suppression of lipolysis in the fed state and an increase in FFA levels under fasted conditions. Again, consistent with the total FFA data, the reduction of these species was restricted to the early time point and not evident at the later time points. A different pattern emerges in the analysis of the later time points. At 23 and 71 hours, suppression of almost all diacylglycerol and triacylglycerol species were observed (Fig. 5). This is consistent with glucagon’s inhibitory effect on lipogenesis and stimulation of fatty acid oxidation.
Figure 5.
Differentiation of glucagon effects on the plasma lipidomic profile. Random forest analysis was performed to determine the lipids with the greatest contribution to group separation. The top 10 lipids for 4, 23, and 71 hours are shown (left panel). The color denotes lipid classification. The top 100 lipids from the random forest analysis were used for principal component analysis (PCA) at the same time points to visualize separation of the 3 treatment groups (right panel).
Discussion
Glucagon, secreted from pancreatic alpha cells during periods of hypoglycemia and fasting, is a key regulator of glucose homeostasis, mainly through its action in the liver [23]. These actions include stimulation of glycogenolysis and gluconeogenesis to increase hepatic glucose production. The activation of glycogenolysis occurs acutely with glucagon stimulation while gluconeogenesis is activated through acute inhibition of glycolysis as well as increased expression of gluconeogenic enzymes. Amino acids are a predominant metabolic precursor for gluconeogenesis in the liver. Glucagon is also a well-known regulator of lipid metabolism in the liver. Glucagon stimulates fatty acid β -oxidation while inhibiting lipogenesis and secretion of triglycerides and very-low-density lipoproteins. The various actions of glucagon on hepatic metabolism results in overt changes in circulating metabolite levels. However, no broad and unbiased survey of circulating metabolites resultant from glucagon signaling has been performed in humans. Here we infuse 2 concentrations of glucagon and a placebo control for 72 hours to healthy individuals with overweight/obesity. Metabolomics and lipidomics analysis performed on plasma collected at various time points before and during the infusion period reveals substantial changes in human participants consistent with the known actions of glucagon in previous murine studies. First, we observe a broad and dramatic reduction in circulating amino acids. Second, there were significant reductions in urea cycle intermediates consistent with the effects on amino acid catabolism. Finally, dynamic changes in FFA and triglycerides were observed with glucagon infusion.
Previous studies have shown reductions in plasma amino acid levels with glucagon signaling [24-27]. In mice, either genetic deletion of the glucagon receptor or an inhibitory glucagon receptor antibody increased serum amino acids [28, 29]. However, not all amino acids were affected by the loss of glucagon signaling, as amino acids such as tryptophan and phenylalanine were minimally affected. Interestingly, excellent correlation is observed in our study where the levels of tryptophan and phenylalanine are not significantly altered by glucagon. Likewise, other amino acids, including alanine and threonine were among the most highly regulated in both the mouse and our study presented here. Flux measurements with isotopic tracers are necessary to determine the specific pathway of these diminished amino acids.
The increased amino acid catabolism produces a nitrogen excess that is eliminated largely through the urea cycle. In agreement, mice with glucagon receptor antagonist had reduced amino acid clearance concomitant with reduced urea production [30]. Consistent with the reduction of circulating amino acids, we also observed decreased levels of citrulline and ornithine. Although plasma urea concentrations are largely unchanged during the infusion period, total urinary nitrogen levels, however, are increased with glucagon. This is consistent with increased amino acid catabolism to support gluconeogenesis.
Finally, our lipidomic analysis demonstrated marked reduction of triacylglycerol and diacylglycerol species at the later time points (Fig. 5). Glucagon is known to stimulate fatty acid β-oxidation in the liver while suppressing lipogenesis. In mice, glucagon administration also diminishes hepatic triglyceride secretion while glucagon receptor knockout mice (Gcgr-/-) show an opposite pattern with increased triglyceride secretion following a fasting period [31]. This coincides with activation of PPARα signaling and increased β -oxidation. Chronic administration of glucagon in rats has similar effects with a 70% reduction of plasma triglycerides after 3 weeks [32]. Similar findings on triglyceride metabolism have also been observed in settings of hyperglucagonemia in human subjects [33].
Due to its central role in glucose homeostasis, glucagon signaling has also been an area of intense focus for therapeutic intervention. Multiple glucagon receptor antagonists have been identified and tested in humans for glucose lowering effects. These compounds have been shown to reduce fasting blood glucose and HbA1c levels in patients with type 2 diabetes [34, 35]. However, the other actions of glucagon, including its action on fatty acid oxidation and lipid metabolism have resulted in untoward side effects such as hepatosteatosis [36]. Activation of the glucagon receptor has also been pursued as a therapeutic intervention, based on observations that glucagon decreases food intake. Rationale for this approach is provided by oxyntomodulin, a naturally occurring peptide produced in the colon, that activates the GLP-1 and glucagon receptors and reduces both food intake and body weight in human subjects [37, 38]. Synthetic GLP-1/glucagon dual agonists have been developed and demonstrated robust weight loss and glucose lowering in mice and cynomolgus monkeys [39]. Dual agonists also demonstrated weight loss and glucose lowering in healthy subjects [40, 41]. Collectively, these studies show the promise and limitations of targeting the glucagon pathway in metabolic disease. Our results here provide additional information and data to help guide those efforts and monitor the pharmacodynamic actions of glucagon-targeting therapies in individuals with overweight/obesity.
Acknowledgments
We wish to thank Sanofi for their support of this study and insightful discussions. This study was made possible through the efforts and dedication of the Translational Research Institute. We also wish to extend our gratitude to the volunteers who participated in this study.
Financial Support: This research was funded through support from Sanofi.
Clinical Trial Information: ClinicalTrials.gov registration no. NCT03139305.
Glossary
Abbreviations
- BMI
body mass index
- cAMP
cyclic adenosine monophosphate
- ESI
electrospray ionization
- FFA
free fatty acid
- GCG
glucagon
- GCGR
glucagon receptor
- GLP-1
glucagon-like peptide-1
Additional Information
Disclosures: S.R.S. received research support from Sanofi. J.G., B.G., and J.T. are employees of Sanofi. R.B.V. is an employee at AstraZeneca.
Data Availability
All data generated or analyzed during this manuscript are included in this published article or in the data repositories listed in References. Data is available at the NIH Common Fund’s National Metabolomics Data Repository (NMDR) website, the Metabolomics Workbench, https://www.metabolomicsworkbench.org where it has been assigned Project ID (PR001022). The data can be accessed directly via the project doi:10.21228/M8KT26. This work is supported by NIH grant U2C-DK119886.
References
- 1. Ramnanan CJ, Edgerton DS, Kraft G, Cherrington AD. Physiologic action of glucagon on liver glucose metabolism. Diabetes Obes Metab. 2011;13(Suppl 1):118-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cherrington AD. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes. 1999;48(5):1198-1214. [DOI] [PubMed] [Google Scholar]
- 3. Magnusson I, Rothman DL, Gerard DP, Katz LD, Shulman GI. Contribution of hepatic glycogenolysis to glucose production in humans in response to a physiological increase in plasma glucagon concentration. Diabetes. 1995;44(2):185-189. [DOI] [PubMed] [Google Scholar]
- 4. Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12(3):141-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1987;64(1):106-110. [DOI] [PubMed] [Google Scholar]
- 6. Færch K, Vistisen D, Pacini G, et al. . Insulin resistance is accompanied by increased fasting glucagon and delayed glucagon suppression in individuals with normal and impaired glucose regulation. Diabetes. 2016;65(11):3473-3481. [DOI] [PubMed] [Google Scholar]
- 7. Raskin P, Fujita Y, Unger RH. Effect of insulin-glucose infusions on plasma glucagon levels in fasting diabetics and nondiabetics. J Clin Invest. 1975;56(5):1132-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sammons MF, Lee EC. Recent progress in the development of small-molecule glucagon receptor antagonists. Bioorg Med Chem Lett. 2015;25(19):4057-4064. [DOI] [PubMed] [Google Scholar]
- 9. Cegla J, Troke RC, Jones B, et al. . Coinfusion of low-dose GLP-1 and glucagon in man results in a reduction in food intake. Diabetes. 2014;63(11):3711-3720. [DOI] [PubMed] [Google Scholar]
- 10. Tan TM, Field BC, McCullough KA, et al. . Coadministration of glucagon-like peptide-1 during glucagon infusion in humans results in increased energy expenditure and amelioration of hyperglycemia. Diabetes. 2013;62(4):1131-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Geary N, Le Sauter J, Noh U. Glucagon acts in the liver to control spontaneous meal size in rats. Am J Physiol. 1993;264(1 Pt 2):R116-R122. [DOI] [PubMed] [Google Scholar]
- 12. Schulman JL, Carleton JL, Whitney G, Whitehorn JC. Effect of glucagon on food intake and body weight in man. J Appl Physiol. 1957;11(3):419-421. [DOI] [PubMed] [Google Scholar]
- 13. Whytock KL, Carnero EA, Vega RB, et al. . Prolonged glucagon infusion does not affect energy expenditure in individuals with overweight/obesity: a randomized trial. Obesity. 2021;29(6):1003-1013. [DOI] [PubMed] [Google Scholar]
- 14. Long T, Hicks M, Yu HC, et al. . Whole-genome sequencing identifies common-to-rare variants associated with human blood metabolites. Nat Genet. 2017;49(4):568-578. [DOI] [PubMed] [Google Scholar]
- 15. Dehaven CD, Evans AM, Dai H, Lawton KA. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J Cheminform. 2010;2(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gardell SJ, Zhang X, Kapoor N, Petucci C, Coen PM.. Metabolomics analyses of muscle atrophy induced by hind limb unloading. Methods Mol Biol. 2019;1996:297-309. [DOI] [PubMed] [Google Scholar]
- 17. Lovejoy JC, Smith SR, Bray GA, et al. . A paradigm of experimentally induced mild hyperthyroidism: effects on nitrogen balance, body composition, and energy expenditure in healthy young men. J Clin Endocrinol Metab. 1997;82(3):765-770. [DOI] [PubMed] [Google Scholar]
- 18. Ritchie ME, Phipson B, Wu D, et al. . limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Svetnik V, Liaw A, Tong C, Culberson JC, Sheridan RP, Feuston BP. Random forest: a classification and regression tool for compound classification and QSAR modeling. J Chem Inf Comput Sci. 2003;43(6):1947-1958. [DOI] [PubMed] [Google Scholar]
- 21. Liaw A, Weiner M. Classification and regression by randomForest. R News. 2002;2(3):18-22. [Google Scholar]
- 22. Vega RB, Whytock KL, Gassenhuber J, et al. . Data from: A Metabolomic Signature of Glucagon Action in Healthy Individuals with Overweight/Obesity figshare. Deposited May 27, 2021. https://figshare.com/s/ae752c9f925f006ddd78
- 23. Galsgaard KD, Pedersen J, Knop FK, Holst JJ, Wewer Albrechtsen NJ. Glucagon receptor signaling and lipid metabolism. Front Physiol. 2019;10:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Couet C, Fukagawa NK, Matthews DE, Bier DM, Young VR. Plasma amino acid kinetics during acute states of glucagon deficiency and excess in healthy adults. Am J Physiol. 1990;258(1 Pt 1):E78-E85. [DOI] [PubMed] [Google Scholar]
- 25. Boden G, Rezvani I, Owen OE. Effects of glucagon on plasma amino acids. J Clin Invest. 1984;73(3):785-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liljenquist JE, Lewis SB, Cherrington AD, Sinclair-Smith BC, Lacy WW. Effects of pharmacologic hyperglucagonemia on plasma amino acid concentrations in normal and diabetic man. Metabolism. 1981;30(12):1195-1199. [DOI] [PubMed] [Google Scholar]
- 27. Landau RL, Lugibihl K. Effect of glucagon on concentration of several free amino acids in plasma. Metabolism. 1969;18(4):265-276. [DOI] [PubMed] [Google Scholar]
- 28. Galsgaard KD, Winther-Sørensen M, Ørskov C, et al. . Disruption of glucagon receptor signaling causes hyperaminoacidemia exposing a possible liver-alpha-cell axis. Am J Physiol Endocrinol Metab. 2018;314(1):E93-E103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Solloway MJ, Madjidi A, Gu C, et al. . Glucagon couples hepatic amino acid catabolism to mtor-dependent regulation of α-cell mass. Cell Rep. 2015;12(3):495-510. [DOI] [PubMed] [Google Scholar]
- 30. Winther-Sørensen M, Galsgaard KD, Santos A, et al. . Glucagon acutely regulates hepatic amino acid catabolism and the effect may be disturbed by steatosis. Mol Metab. 2020;42:101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Longuet C, Sinclair EM, Maida A, et al. . The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab. 2008;8(5):359-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guettet C, Mathe D, Riottot M, Lutton C. Effects of chronic glucagon administration on cholesterol and bile acid metabolism. Biochim Biophys Acta. 1988;963(2):215-223. [DOI] [PubMed] [Google Scholar]
- 33. Xiao C, Pavlic M, Szeto L, Patterson BW, Lewis GF. Effects of acute hyperglucagonemia on hepatic and intestinal lipoprotein production and clearance in healthy humans. Diabetes. 2011;60(2):383-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kazierad DJ, Chidsey K, Somayaji VR, Bergman AJ, Calle RA. Efficacy and safety of the glucagon receptor antagonist PF-06291874: A 12-week, randomized, dose-response study in patients with type 2 diabetes mellitus on background metformin therapy. Diabetes Obes Metab. 2018;20(11):2608-2616. [DOI] [PubMed] [Google Scholar]
- 35. Kazda CM, Ding Y, Kelly RP, et al. . Evaluation of efficacy and safety of the glucagon receptor antagonist ly2409021 in patients with type 2 diabetes: 12- and 24-week phase 2 studies. Diabetes Care. 2016;39(7):1241-1249. [DOI] [PubMed] [Google Scholar]
- 36. Guzman CB, Zhang XM, Liu R, et al. . Treatment with LY2409021, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19(11):1521-1528. [DOI] [PubMed] [Google Scholar]
- 37. Wynne K, Park AJ, Small CJ, et al. . Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes. 2005;54(8):2390-2395. [DOI] [PubMed] [Google Scholar]
- 38. Cohen MA, Ellis SM, Le Roux CW, et al. . Oxyntomodulin suppresses appetite and reduces food intake in humans. J Clin Endocrinol Metab. 2003;88(10):4696-4701. [DOI] [PubMed] [Google Scholar]
- 39. Henderson SJ, Konkar A, Hornigold DC, et al. . Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes Obes Metab. 2016;18(12):1176-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ambery P, Parker VE, Stumvoll M, et al. . MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet. 2018;391(10140):2607-2618. [DOI] [PubMed] [Google Scholar]
- 41. Tillner J, Posch MG, Wagner F, et al. . A novel dual glucagon-like peptide and glucagon receptor agonist SAR425899: results of randomized, placebo-controlled first-in-human and first-in-patient trials. Diabetes Obes Metab. 2019;21(1):120-128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this manuscript are included in this published article or in the data repositories listed in References. Data is available at the NIH Common Fund’s National Metabolomics Data Repository (NMDR) website, the Metabolomics Workbench, https://www.metabolomicsworkbench.org where it has been assigned Project ID (PR001022). The data can be accessed directly via the project doi:10.21228/M8KT26. This work is supported by NIH grant U2C-DK119886.