Abstract
Context
Thyrotoxicosis is a common immune-related adverse event in patients treated with programmed cell death protein-1 (PD1) or programmed cell death protein ligand-1 (PD-L1) blockade. A detailed endocrinological assessment, including thyroid ultrasound and scintigraphy, is lacking, as are data on response to treatment and follow-up.
Objective
The aim of this study was to better characterize the thyrotoxicosis secondary to immune checkpoint inhibitors, gaining insights into pathogenesis and treatment.
Methods
We conducted a retrospective study of 20 consecutive patients who had normal thyroid function before starting immunotherapy and then experienced thyrotoxicosis on PD1 or PD-L1 blockade. Clinical assessment was combined with thyroid ultrasound, 99mtechnecium scintiscan, and longitudinal thyroid function tests.
Results
Five patients had normal or increased scintigraphic uptake (Sci+), no serum antibodies against the thyrotropin receptor, and remained hyperthyroid throughout follow-up. The other 15 patients had no scintigraphic uptake (Sci–) and experienced destructive thyrotoxicosis followed by hypothyroidism (N = 9) or euthyroidism (N = 6). Hypothyroidism was more readily seen in those with normal thyroid volume than in those with goiter (P = .04). Among Sci– individuals, a larger thyroid volume was associated with a longer time to remission (P < .05). Methimazole (MMI) was effective only in Sci+ individuals (P < .05).
Conclusion
Administration of PD1- or PD-L1–blocking antibodies may induce 2 different forms of thyrotoxicosis that appear similar in clinical severity at onset: a type 1 characterized by persistent hyperthyroidism that requires treatment with MMI, and a type 2, characterized by destructive and transient thyrotoxicosis that evolves to hypothyroidism or euthyroidism. Thyroid scintigraphy and ultrasound help in differentiating and managing these 2 forms of iatrogenic thyrotoxicosis.
Keywords: immunotherapy, immune check point inhibitors, immune related adverse event, thyroid, thyroid dysfunction
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies directed toward receptors expressed on T cells and other cell types of peripheral tissue, namely cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death protein-1 (PD1), and programmed cell death protein ligand-1 (PD-L1). By blocking these molecules, ICIs promote the activation of the immune system against cancer and as such are effective in the treatment of many solid tumors [1, 2]. The hyperactivation of the immune system induced by ICIs can also generate a broad spectrum of inflammatory tissue reaction acknowledged as immune-related adverse events (irAEs) [2]. Thyroid dysfunction and hypophysitis are the most common endocrine irAEs, followed by type 1 diabetes and adrenalitis, whereas autoimmune hypoparathyroidism and central diabetes insipidus have been less frequently reported [3-5]. Thyroid dysfunction can be a consequence of PD1 and PD-L1 blockade; however, it has not been fully characterized because trials have focused for the most part on the epidemiology of irAEs rather than on their clinical presentation and course.
An exhaustive endocrinological investigation, including a systematic analysis of thyroid ultrasound and scintigraphy, as well as data on the response to treatment and follow-up, are lacking.
The aim of the present study was to characterize ICI-induced thyrotoxicosis by hormonal and antibodies assessment and thyroid ultrasound and scintigraphic imaging in order to gain insights into its pathogenesis and to guide its management.
Material and Methods
Study Design and Population
This was a retrospective study. From January to December 2019, 41 patients were referred from Oncology and Pneumology to Endocrinology of The University Hospital of Pisa because of thyrotoxicosis due to anti-PD1 or anti–PD-L1 treatment. Thyrotoxicosis was defined as the finding of high levels of free thyroxine (FT4) and/or free 3,5,3′-triiodothyronine (FT3) associated with low to undetectable levels of thyrotropin (TSH). Inclusion criteria were: 1) patients 18 years or older with a cytologically/histologically confirmed type of solid cancer treated with an anti-PD1 or anti–PD-L1 drug as the first line of immunotherapy; 2) medical history unremarkable for thyroid disease; 3) FT4, FT3, and TSH in the normal range at the screening performed during the month preceding the start of immunotherapy; 4) neck ultrasound and thyroid scintigraphy performed at the onset of thyrotoxicosis; and 5) at least 6 months of follow-up. We decided to focus our study on patients treated with anti-PD1 or anti–PD-L1 drugs, which, compared to anti–CTLA-4 drugs, are currently employed more frequently and more commonly cause thyroid dysfunction [3]. Additionally, given the different role played by the CTLA-4 and PD1/PD-L1 pathways in immune regulation, we excluded patients previously treated with anti–CTLA-4 to avoid overlapping effects of the 2 drugs. Of 41 patients with thyrotoxicosis, 17 did not match the inclusion criteria because they lacked baseline data (N = 6), neck ultrasound or thyroid scintigraphy (N = 8), or because they were being treated with levothyroxine (N = 3). Of the remaining 24 patients, 4 were excluded because they had been previously treated with an anti–CTLA-4 drug, resulting in the 20 patients featured in this particular study (Table 1). Mean age was 62 years and 65% were male. Non–small cell lung cancer was the most common type of tumor, followed by melanoma, hepatocellular carcinoma, and renal carcinoma. Seventeen patients were treated with an anti-PD1 (10 nivolumab and 7 pembrolizumab) and 3 with an anti–PD-L1 drug (2 atezolizumab and 1 durvalumab). All patients had a computed tomography (CT) scan 7 to 21 days before the screening sample, and 4 patients had an additional CT scan between the screening and the onset of thyrotoxicosis (ie, time 0). The mean time from the start of immunotherapy to the onset of thyrotoxicosis was 2.5 months, while the mean time from the administration of last iodine contrast medium was 3.4 months. The mean time of follow-up was 9.6 months and no patient stopped ICIs during follow-up. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki. Data publication was approved by the local institutional review committee (Comitato Etico di Area Vasta Nord Ovest–CEAVNO). Patients were informed and gave their consent to participate in the study.
Table 1.
Thyroid features of the 20 patients at the onset of thyrotoxicosis induced by immunotherapy
| Patient | Sex, M/F | Age, y | Cancer | Drug | Time from ICI start, mo | FT4, ng/L | FT3, ng/L | TgAbs, IU/mL | TPOAbs, IU/mL | TRAbs, IU/mL | Tg, ng/mL | Urinary iodine, µg/L | Thyroid volume, mL | 99mTc-uptake, Sci+/Sci– |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 69 | NSCLC | Pembro | 1 | 30.5 | 10.7 | 69 | 125 | < 0.1 | 0.91 | 219 | 4.6 | Sci– |
| 2 | F | 46 | NSCLC | Nivo | 3 | 19.7 | 4.3 | 13 | < 1.0 | < 0.1 | 4.96 | 190 | 6.1 | Sci– |
| 3 | M | 73 | HCC | Pembro | 2 | 28.5 | 6.7 | < 1.0 | 24 | < 0.1 | 3.7 | 180 | 6.5 | Sci– |
| 4 | M | 53 | NSCLC | Atezo | 2 | 29.8 | 9.1 | 180 | < 1.0 | < 0.1 | 4.9 | 286 | 7.0 | Sci– |
| 5 | F | 41 | MEL | Nivo | 4 | 21.1 | 5.9 | < 1.0 | < 1.0 | < 0.1 | 2.2 | 290 | 9.0 | Sci– |
| 6 | F | 49 | MEL | Nivo | 5 | 29.9 | 11.0 | 20 | < 1.0 | < 0.1 | 11.0 | 190 | 11.4 | Sci– |
| 7 | M | 71 | NSCLC | Pembro | 6 | 33.0 | 8.8 | < 1.0 | < 1.0 | < 0.1 | 2.7 | 190 | 12.0 | Sci– |
| 8 | M | 77 | MEL | Nivo | 1 | 17.6 | 3.4 | 12 | < 1.0 | < 0.1 | 0.72 | 200 | 12.1 | Sci– |
| 9 | M | 62 | RCC | Nivo | 2 | 39.5 | 12.2 | 262 | 967 | < 0.1 | 6.97 | 252 | 22.0 | Sci– |
| 10 | M | 71 | NSCLC | Nivo | 2 | 19.1 | 3.9 | < 1.0 | < 1.0 | < 0.1 | 2.98 | 221 | 22.8 | Sci– |
| 11 | F | 71 | NSCLC | Pembro | 2 | 37.1 | 5.2 | 178 | < 1.0 | 0.12 | 79.7 | 276 | 23.7 | Sci– |
| 12 | M | 70 | MEL | Nivo | 9 | 30.9 | 8.8 | < 1.0 | < 1.0 | < 0.1 | 9.2 | 280 | 25.3 | Sci– |
| 13 | M | 65 | MEL | Nivo | 1 | 31.1 | 8.7 | 400 | 199 | < 0.1 | 1.3 | 190 | 36.2 | Sci– |
| 14 | M | 67 | NSCLC | Pembro | 2 | 35.5 | 5.7 | < 1.0 | < 1.0 | < 0.1 | 81.0 | 200 | 37.0 | Sci– |
| 15 | M | 74 | HCC | Durva | 1 | 43.8 | 7.3 | 2000 | < 1.0 | 0.1 | 1.8 | 283 | 38.7 | Sci– |
| 16 | M | 51 | NSCLC | Nivo | 2 | 29.3 | 11.2 | < 1.0 | < 1.0 | < 0.1a | 21.0 | 190 | 16.4 | Sci+ |
| 17 | F | 55 | NSCLC | Nivo | 1 | 26.6 | 5.6 | < 1.0 | < 1.0 | < 0.1a | 18.8 | 220 | 25.3 | Sci+ |
| 18 | F | 55 | NSCLC | Pembro | 2 | 30.7 | 7.2 | 1000 | 70 | < 0.1a | 2.7 | 290 | 27.9 | Sci+ |
| 19 | M | 51 | MEL | Pembro | 1 | 20.2 | 4.4 | < 1.0 | < 1.0 | < 0.1a | 3.8 | 298 | 42.0 | Sci+ |
| 20 | M | 71 | NSCLC | Atezo | 2 | 29.7 | 7.7 | < 1.0 | < 1.0 | < 0.1a | 79.1 | 216 | 48.0 | Sci+ |
Normal ranges: FT4 8 to 18 ng/L; FT3 2.5 to 5.0 pmol/L; TgAbs less than 30 IU/mL; TPOAbs less than 10 IU/mL; TRAbs less than 1.5 IU/mL; Tg less than 30 µg/L; urinary iodine 100 to 300 µg/L; normal thyroid volume: 12.1 mL (female); 16.5 mL (male).
Sci+ indicates patients with formation of a thyroid image due to a normal/increased uptake of technetium; Sci– indicates patients without formation of a thyroid image due to an absent uptake.
Abbreviations: Atezo, atezolizumab (anti-PD-L1); Durva, durvalumab (anti-PD-L1); F, female; FT3, free 3,5,3′-triiodothyronine; FT4, free thyroxine; HCC, hepatocellular carcinoma; ICI, immune checkpoint inhibitor; M, male; MEL, melanoma; Nivo, nivolumab; NSCLC, non–small cell lung cancer; Pembro, pembrolizumab (anti-PD1); PD1, programmed cell death protein-1; PD-L1, programmed cell death protein ligand-1; RCC, renal cell carcinoma; Tc, technecium; Tg, thyroglobulin; TgAbs, thyroglobulin antibodies; TPOAbs, thyroperoxidase antibodies; TRAbs, thyrotropin receptor antibodies.
a TRAb were negative at 3 different times during the follow-up.
Follow-up
Thyroid function (FT4, FT3, and TSH) was tested at screening performed before the start of immunotherapy (baseline) and then every 14 to 21 days at each drug infusion.
Data are therefore reported at day 0 (the time of onset of thyrotoxicosis), 14 (or 21), 28, 42, 56 (or 63), 70, 84, 98 (or 105), 112, 126, 140 (or 147), 154, 168, and 182. Thyroglobulin antibodies (TgAbs) and thyroperoxidase antibodies (TPOAbs) were tested at screening and at day 0 in all patients, while thyroglobulin (Tg) and urinary iodine were tested at day 0. Thyrotropin receptor antibodies (TRAbs) were measured at time 0 in all patients and retested during follow-up in selected patients when Graves disease was suspected. Neck ultrasound and thyroid scintigraphy were performed at the onset of thyrotoxicosis. Given the absence of a conventional treatment, an antithyroid drug was started in some patients during follow-up, based on the course of thyrotoxicosis. Hypothyroidism was diagnosed on the basis of 2 consecutive findings of low FT4 associated with slightly increased TSH (4-10 mIU/L), or of a single detection of low levels of FT4 associated with high TSH (> 10 mIU/L).
Laboratory Testing
Thyroid hormones and TSH were tested using immunoenzymatic assays (Ortho Clinical Diagnostics Inc). Reference ranges were 8 to 18 ng/L for FT4, 2.5 to 5.0 ng/L for FT3, and 0.4 to 4 mIU/L for TSH, respectively. Tg was measured by an immunometric assay (Access Thyroglobulin assay; Beckman Coulter Inc) (functional sensitivity 0.1 ng/mL). TgAbs were measured by an AIA-Pack 2000 TgAb-IgGs (Tosoh Corp); analytic, functional, and positive cutoffs were 6 IU/mL, 8 IU/mL, and 30 IU/mL, respectively. In this assay TgAbs interfere with Tg measurement when greater than or equal to 9.3 IU/mL [6]. TPOAbs were checked by an AIA-Pack 2000 TPOAb (Tosoh Corp) (positive cutoff > 10 IU/mL). TRAbs were tested by enzyme-linked immunosorbent assay (ElisaRSR TRAb 3rd Generation) (positivity cutoff > 1.5 IU/mL). Urinary iodine was measured by mass spectroscopy (reference range, 100-300 µg/L).
Thyroid Imaging
Neck ultrasound was performed by Technos (Esaote Biomedica) with a 7.5-MHz linear transducer. Thyroid volume was calculated using the ellipsoid volume formula. According to the upper limit of normal thyroid volume estimated in the reference Italian adult population (12.1 mL in women and 16.5 mL in men, respectively), patients were divided into 2 subgroups: 1) with normal thyroid volume; and 2) with goiter [7].
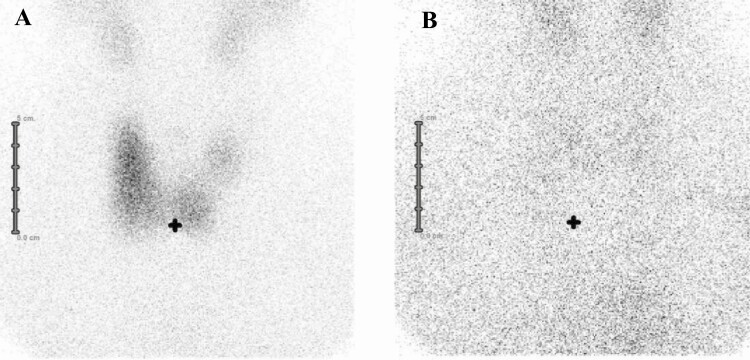
99mTechnecium (Tc) scintiscan was performed using a dedicated γ camera with a parallel-hole collimator, 20 minutes after the intravenous administration of 3 to 5 mCi (111-185 MBq) of 99mTc-pertechnetate. The field of view extended from the salivary glands level to the upper sternum. Anterior images were obtained for 100 000 counts (or 5 minutes) with the patient sitting and neck extended, and a radioactive marker was placed on the sternal notch. According to the image obtained, patients were classified into 2 groups: 1) Sci+: with a distinct thyroid image due to uptake of technetium (Fig. 1A); and 2) Sci–: with no thyroid image because of absent uptake (Fig. 1B).
Figure 1.
A, Formation of a scintigraphy image due to a normal/increased uptake of technetium (Sci+). B, Absent formation of a scintigraphy image due to an absent uptake of technetium (Sci–). In both patients neck ultrasound showed diffuse goiter.
Statistical Analysis
Statistical data analysis was performed using SPSS 21 (IBM Corp). Data are presented as mean ± SD or median with interquartile range, as indicated. The Shapiro-Wilk test was used to assess normality of data distribution of continuous variables. Statistical tests used to compare groups included the t test for normally distributed variables and Mann-Whitney U tests for variables with skewed distribution. The chi-square test or the Fisher exact test was used to compare counts and frequencies between groups for categorical variables as appropriate. Pearson (R) and Spearman (ρ) correlation coefficients were used to quantify association for Gaussian and skewed continuous variables, respectively. Multivariate regression analysis was conducted to identify the independent determinants of time to remission (dependent variable) among clinical parameters (independent variables). Repeated-measures mixed-model analysis was used to assess intergroup differences in thyroid hormone concentrations over time.
Results
Thyroid Features at Baseline and at Onset of Thyrotoxicosis
Thyroid features at baseline and at the onset of thyrotoxicosis of each patient are reported in Table 1 and summarized in Table 2. At baseline, all patients were euthyroid with negative TgAbs and TPOAbs. Thyroid volume at neck ultrasound was 22.4 mL (range, 10.8-29.9 mL) (median and interquartile range). Thyroid volume was normal in 9 patients, whereas 11 individuals had goiter. The echoic pattern was normal in 6 patients, slightly hypoechoic in 9, and frankly hypoechoic in 5. Thyroid nodules were detected in 5 patients; in 2 of them we performed fine-needle biopsy, which turned out benign. At 99mTc-scintiscan, 5 patients were classified as Sci+ and 15 as Sci–. Sci+ patients showed a larger thyroid volume compared to Sci– (27.9 vs 12.0 mL; P = .03). Evaluating only patients with TgAbs less than 9.3 IU/mL (the interfering cutoff of the assay), the Tg levels were similar in Sci+ (N = 4) and in Sci– (N = 7) patients (12.4 in Sci+ vs 6.7 in Sci–, P = .38). The FT3/FT4 ratio was similar in the 2 groups (0.26 in Sci+ vs 0.28 in Sci–, P = .79).
Table 2.
Thyroid features at baseline and at the onset of thyrotoxicosis
| Laboratory testing (normal range) | Baseline | Day 0 |
|---|---|---|
| FT4 (8-18 ng/L) | 12.7 (10.9-14.5) ng/L | 29.8 (25.2-30.5) ng/L |
| FT3 (2.5-5 ng/L) | 3.9 (3.6-4.3) ng/L | 7.3 (5.5-8.9) ng/L |
| FT3/FT4 ratio | 0.31 (0.28-0.34) | 0.25 (0.29-0.32) |
| TSH (0.4-4 mIU/L) | 1.5 (0.9-2.4) mIU/mL | 0 (0-0) mIU/L |
| TgAbs (< 30 IU/mL) | ||
| Positive | 0 | 7 |
| Level | 179 (32.2-365.5) IU/mL | |
| TPOAbs (< 10 IU/mL) | ||
| Positive | 0 | 5 |
| Level | 125 (70-199) IU/mL | |
| TRAbs (< 1.5 IU/mL) | ||
| Positive | 0 | |
| Urinary iodine (100-300 µg/L) | 219.5 (190-287) µg/L | |
| Thyroglobulin (< 30 ng/mL) | 4.4 (2.3-10.5) ng/mL | |
| Imaging | ||
| Ultrasound | ||
| Thyroid volume (6-16 mL) | 22.8 (10.8-29.9) mL | |
| Echoic pattern | ||
| Normoechoic | 6 | |
| Slightly hypoechoic | 9 | |
| Frankly hypoechoic | 5 | |
| Patients with nodules | 5 | |
| 99mTc-scintigraphy | ||
| Sci+ | 5 | |
| Sci– | 15 |
Laboratory tests with normal values, thyroid volume with normal value, different echoic pattern, nodules, and diverse scintigraphy pattern are reported.
Baseline: screening performed before the start of immunotherapy. FT4, FT3, FT3/FT4 ratio, TSH, and TgAbs are reported as median (25th-75th percentile). Number of patients with positive TgAbs and TPOAbs are also reported.
Day 0: time of onset of thyrotoxicosis.
FT4, FT3, FT3/FT4 ratio, TSH, TgAbs, TPOAbs, TRAbs, urinary iodine, thyroglobulin, and thyroid volume are reported as median (25th-75th percentile).
Number of patients with positive TgAbs, TPOAbs, and TRAbs, echoic pattern, nodules, and distinct scintigraphy pattern are reported.
Abbreviations: FT3, 3,5,3′-free triiodothyronine; FT4, free thyroxine; Tc, technetium; Tg, thyroglobulin; TgAbs, thyroglobulin antibodies; TPOAbs, thyroperoxidase antibodies; TRAbs, thyrotrophin receptor antibodies; TSH, thyrotrophin.
Factors Influencing the Severity and Time Course of Thyrotoxicosis in Untreated Patients
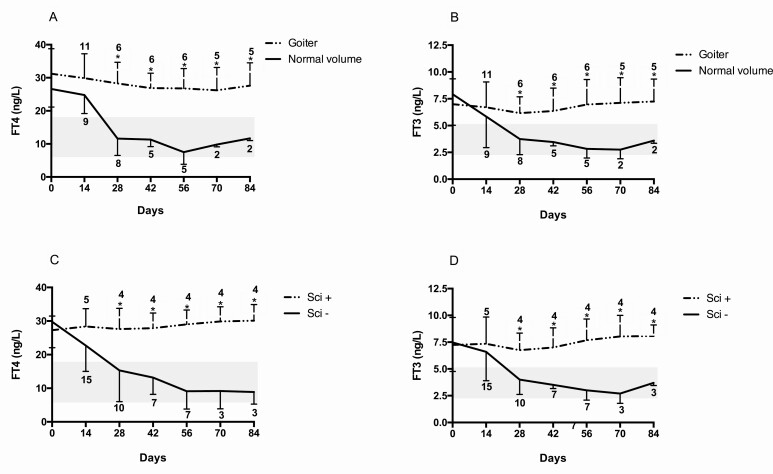
At baseline, FT4 and FT3 levels did not differ when comparing patients with goiter with those with normal thyroid volume. Additionally, FT4 and FT3 were similar in Sci+ and Sci– patients. The course of thyrotoxicosis was ascertained evaluating the levels of FT4 and FT3 during follow-up. Patients were excluded from the analysis once they started thyroid treatment (antithyroid drugs or levothyroxine). FT4 and FT3 were higher in patients with goiter compared to those with normal thyroid volume at days 28, 42, 56, 70, and 84 (P < .05 for all) (Fig. 2A and 2B). FT4 and FT3 were higher in Sci+ patients compared to Sci– patients at days 28, 42, 56 or 63, 70, and 84 (P < .05 for all) (Fig. 2C and 2D). Analysis was stopped at day 84 because only 4 patients were left untreated afterward.
Figure 2.
Changes in free thyroxine (FT4) and free 3,5,3′-triiodothyronine (FT3) concentrations according to A and B, thyroid volume, and C and D, uptake of technetium in patients with thyrotoxicosis induced by PD-1 or PD-L1 blockade. Time 0 indicates the onset of thyrotoxicosis. Patients were excluded when they started thyroid treatment (methimazole or levothyroxine). The number of patients at each time point in both groups is reported. Gray areas indicate normal values of FT4 and FT3. *P less than .05 between the 2 groups.
Efficacy of Treatment with Methimazole
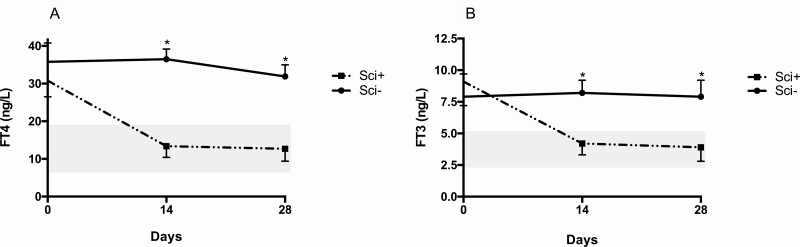
Five Sci+ and 6 Sci– patients started treatment with methimazole (MMI) at different times during follow-up. The 2 groups were treated with a comparable dosage of antithyroid drug (140 mg/week in Sci+ vs 145 mg/week in Sci–). The decrease in FT4 and FT3 levels was greater in Sci+ compared to Sci– patients at days 14 and 28 (P < .001 for both) (Fig. 3A and 3B). Whereas all 6 Sci– patients discontinued MMI after a median period of 40 days because it was ineffective on thyrotoxicosis, all 5 Sci+ patients were still treated with MMI at the end of follow-up at the median dosage of 35 mg/week. Their median levels of FT4 (13.8 ng/L), FT3 (4.3 ng/L), and TSH (2.9 mIU/mL) were all in the normal range.
Figure 3.
Changes in A, free thyroxine (FT4) and B, free 3,5,3′-triiodothyronine (FT3) concentrations after the start of methimazole therapy in 5 patients with normal/increased uptake of technetium (Sci+) and in 6 with absent uptake (Sci–). Gray areas indicate normal values of FT4 and FT3.*P less than .05 between the 2 groups.
Thyrotoxicosis Remission
Remission of thyrotoxicosis was observed in all 15 Sci– patients: 9 never treated and 6 previously treated with MMI. Time to remission was 45 ± 15 days (mean ± SD) in the entire Sci– group and 70 ± 15 days in the cohort of the 6 Sci– patients previously treated with MMI. At univariate analysis, time to remission was associated with a larger thyroid volume and higher levels of FT4 and TgAbs at the onset of thyrotoxicosis. At multivariate analysis, only a larger thyroid volume was associated with a longer time to remission.
Onset of Hypothyroidism
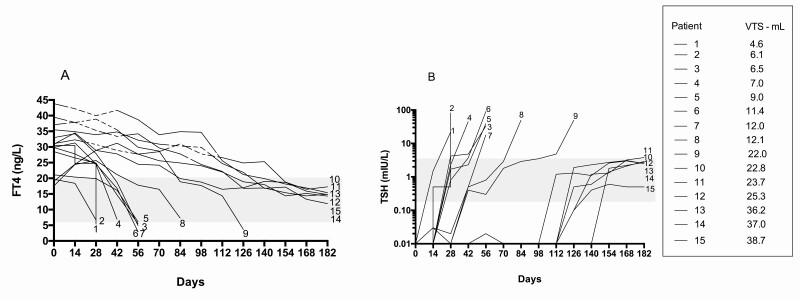
Of the 15 Sci– patients, 9 experienced hypothyroidism; none of them belonged to the cohort of patients treated with MMI. Hypothyroidism developed within days 14 to 84 (Fig. 4A and 4B) and was more common in patients with normal thyroid volume than in those with goiter (7/9 vs 2/11; P = .04). All patients started replacement treatment and showed normal thyroid tests at the end of follow-up while taking 75 µg/day of levothyroxine (median dosage).
Figure 4.
Trend of A, free thyroxine (FT4) and B, thyrotropin (TSH) in the 15 individuals with absent uptake of technetium (Sci–). Patients are listed according to increasing thyroid volume. Gray areas indicate normal values of FT4 and TSH. The dashed line in A indicates the time when the patients were on methimazole treatment.
Discussion
The incidence of thyrotoxicosis induced by ICIs is 10% to 20% in patients treated with anti–CTLA-4 (10%-20%) and, 30% to 40% in those treated with PD1 or PD-L1 blockade [3, 8, 9]. The different role of the CTLA-4 and PD1/PD-L1 pathways in immune regulation accounts for this difference; CTLA-4 plays a key role in central tolerance, whereas the PD1/PD-L1 pathway modulates the immune response in peripheral tissue [10, 11]. The term “thyrotoxicosis” reported in clinical trials includes several scenarios ranging from destructive thyroiditis to primary hyperthyroidism, which differ in their physiopathology, course, and treatment. The lack of a full endocrinological evaluation hampers the characterization of patients included in clinical trials of ICIs published to date [3]. For this reason, we have decided to investigate a cohort of patients who experienced thyrotoxicosis induced by anti-PD1 or anti–PD-L1 drugs.
In agreement with clinical trials reporting a peak of irAEs in the first 6 months of treatment with ICIs, we observed an early occurrence of thyrotoxicosis after the start of immunotherapy [3, 8].
Current guidelines and expert opinion on the management of irAEs recommend neck ultrasound and thyroid scintigraphy only in selected cases [3, 12]. The usefulness of thyroid scintigraphy is questioned because of the potential interference of the iodine contrast agent used for CT scans. The washout time of iodine contrast media is reported to range from 15 days to 6 to 12 months [13-15]. Instead, 3.5 months after the last CT scan, we found levels of urine iodine only slightly higher compared to the Italian population [16], but not high enough to influence the result of thyroid scintigraphy, as confirmed by our finding of similar levels in the 2 groups of patients (Sci– and Sci+). Scintigraphy with iodine isotopes that are organified by the thyroid (131I or 123I) may have provided a better characterization of our cohorts of patients. Specifically, 99mTc-pertechnetate is more available and less expensive than iodine isotopes, and 99mTc-scintiscan is carried out faster than iodine scintigraphy (20 minutes vs 24 hours), which is a favorable feature in oncological patients [15, 17]. Although measurement of technetium uptake might add useful data, the presence (Sci+) or absence (Sci–) of a thyroid image via 99mTc-scintiscan were sufficient to identify those patients who were responsive to methimazole treatment in our cohort.
A high rate of participants, mainly in the Sci– cohort, had the de novo appearance of TgAbs or, less frequently, of TPOAbs, likely as consequence of the humoral response to the release of antigens secondary to thyroid destruction [18, 19].
The rate of persistent hyperthyroidism was higher in our cohort compared to that previously reported [3]. This discrepancy might be due to the inclusion of patients with severe forms of thyrotoxicosis. All 5 Sci+ patients had no feature of thyroid autoimmunity before the start of ICIs, and only 1 showed positive TPOAbs and TgAbs at the onset of thyrotoxicosis. Moreover, in none of the 5 Sci+ patients did we observe the appearance of TRAbs (measured by a sensitive assay), a finding that would have enabled us to identify Graves disease [20, 21]. These findings are similar to those reported in most cases of persistent hyperthyroidism induced by anti-PD1 or anti–PD-L1 agents so far reported [22]. Interestingly enough, we did not observe a rise in Tg levels, which is typical of destructive thyrotoxicosis (131I treatment or subacute thyroiditis) as well as of autoimmune hyperthyroidism. The coexistence in some patients of serum TgAbs, which interfere with the detection of Tg, is the most likely reason for this finding [6, 23]. However, 6 patients in our cohort had low Tg levels in spite of undetectable TgAbs. This finding raises the possibility that these patients had serum M class TgAbs, which are not detected by commercial assays [24].
As previous studies reported that most cases of thyrotoxicosis induced by anti-PD1 or anti–PD-L1 agents are self-limited and transient, treatment with MMI was inappropriately delayed in some Sci+ patients. On the other hand, given the absence of a standardized treatment of thyrotoxicosis induced by PD1 and PD-L1 blockade, some patients with more severe thyrotoxicosis were treated with MMI despite an absent uptake at scintigraphy. These misjudgments enabled us to identify scintigraphy as a useful tool to select those patients who would benefit from treatment with MMI.
Patients with destructive thyrotoxicosis developed hypothyroidism in agreement with several studies reporting a rate of hypothyroidism after destructive thyroiditis of 60% to 80% [3, 8, 18]. For the first time, we show that the risk of developing hypothyroidism is inversely related to thyroid volume, individuals with normal volume being at higher risk compared to those with goiter. Noteworthy, should overt hypothyroidism abruptly ensue after thyrotoxicosis, we advise a close monitoring of thyroid function after remission of thyrotoxicosis in order to promptly identify and treat hypothyroidism.
The relevance of ultrasound and scintigraphy performed at the onset of thyrotoxicosis in allowing the proper diagnosis, in the prediction of the course of the disease, as well as choosing the appropriate treatment are the main findings of this study. Based on these findings, we identified 2 different types of thyrotoxicosis induced by immunotherapy. Similarly to amiodarone-induced thyrotoxicosis (AIT), we identified a type 1 characterized by persistent hyperthyroidism that responds to antithyroid drugs, and a type 2, characterized by destructive thyroiditis that evolves into euthyroidism or hypothyroidism [25]. Measurement of Tg and calculation of the FT3/FT4 ratio might be useful to differentiate these 2 forms. However, our data showed that both Tg and FT3/FT4 were not reliable in differentiating the 2 types of thyrotoxicosis; neither were they helpful in distinguishing AIT type 1 from AIT type 2 [25]. Since treatment with anti-PD1 and anti–PD-L1 drugs has been reported to be associated with a change in the levels of cytokines, it would be interesting to evaluate in future studies whether the onset and the phenotype of thyrotoxicosis is associated with a specific change in cytokine pattern [26].
In conclusion, patients treated with anti-PD1 or anti–PD-L1 drugs may undergo 2 types of thyrotoxicosis that do not differ in severity at their onset. Thyroid scintigraphy and ultrasound are useful, low-invasive, and low-cost benefit tools in the management of thyrotoxicosis induced by PD1 and PD-L1 blockade.
Acknowledgments
The authors wish to thank Maria Di Blasi and the nursing staff for their help in the management of the patients included in the study. We would like to thank Mrs Stephanie Marino for editing this manuscript.
Financial Support: This work was supported by “Fondi di Ateneo,” the University of Pisa No. 539901/2020 to FL and “Contributo Shire International GmbH” to CM.
Author Contributions: A.B., I.L., and F.L. planned the study. A.B., I.L., L.M., F.S., P.C., and F.L. wrote the manuscript. P.P. performed the statistical analysis. All authors discussed the results of the study.
Glossary
Abbreviations
- AIT
amiodarone-induced thyrotoxicosis
- CT
computed tomography
- CTLA-4
cytotoxic T lymphocyte antigen 4
- FT3
free 3,5,3′-triiodothyronine
- FT4
free thyroxine
- ICI
immune checkpoint inhibitor
- irAEs
immune-related adverse events
- MMI
methimazole
- PD1
programmed cell death protein-1
- PD-L1
programmed cell death protein ligand-1
- Sci+
normal or increased scintigraphic uptake
- Sci–
no scintigraphic uptake
- Tg
thyroglobulin
- TgAbs
thyroglobulin antibodies
- TPOAbs
thyroperoxidase antibodies
- TRAbs
thyrotropin receptor antibodies
- TSH
thyrotropin
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”
References
- 1. Johnson DB, Reynolds KL, Sullivan RJ, et al. Immune checkpoint inhibitor toxicities: systems-based approaches to improve patient care and research. Lancet Oncol. 2020;21(8):e398-e404. [DOI] [PubMed] [Google Scholar]
- 2. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158-168. [DOI] [PubMed] [Google Scholar]
- 3. Chang LS, Barroso-Sousa R, Tolaney SM, Hodi FS, Kaiser UB, Min L. Endocrine toxicity of cancer immunotherapy targeting immune checkpoints. Endocr Rev. 2019;40(1):17-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lupi I, Brancatella A, Cosottini M, et al. Clinical heterogeneity of hypophysitis secondary to PD-1/PD-L1 blockade: insights from four cases. Endocrinol Diabetes Metab Case Rep. 2019;2019:19-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lupi I, Brancatella A, Cetani F, Latrofa F, Kemp EH, Marcocci C. Activating antibodies to the calcium-sensing receptor in immunotherapy-induced hypoparathyroidism. J Clin Endocrinol Metab. 2020;105(5):1581-1588. [DOI] [PubMed] [Google Scholar]
- 6. Latrofa F, Ricci D, Bottai S, et al. Effect of thyroglobulin autoantibodies on the metabolic clearance of serum thyroglobulin. Thyroid. 2018;28(3):288-294. [DOI] [PubMed] [Google Scholar]
- 7. Rago T, Chiovato L, Aghini-Lombardi F, Grasso L, Pinchera A, Vitti P. Non-palpable thyroid nodules in a borderline iodine-sufficient area: detection by ultrasonography and follow-up. J Endocrinol Invest. 2001;24(10):770-776. [DOI] [PubMed] [Google Scholar]
- 8. Morganstein DL, Lai Z, Spain L, et al. Thyroid abnormalities following the use of cytotoxic T-lymphocyte antigen-4 and programmed death receptor protein-1 inhibitors in the treatment of melanoma. Clin Endocrinol (Oxf). 2017;86(4):614-620. [DOI] [PubMed] [Google Scholar]
- 9. Lee H, Hodi FS, Giobbie-Hurder A, et al. Characterization of thyroid disorders in patients receiving immune checkpoint inhibition therapy. Cancer Immunol Res. 2017;5(12):1133-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182(2):459-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27(4):195-201. [DOI] [PubMed] [Google Scholar]
- 12. Castinetti F, Albarel F, Archambeaud F, et al. French Endocrine Society Guidance on endocrine side effects of immunotherapy. Endocr Relat Cancer. 2019;26(2):G1-G18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nimmons GL, Funk GF, Graham MM, Pagedar NA. Urinary iodine excretion after contrast computed tomography scan: implications for radioactive iodine use. JAMA Otolaryngol Head Neck Surg. 2013;139(5):479-482. [DOI] [PubMed] [Google Scholar]
- 14. Padovani RP, Kasamatsu TS, Nakabashi CC, et al. One month is sufficient for urinary iodine to return to its baseline value after the use of water-soluble iodinated contrast agents in post-thyroidectomy patients requiring radioiodine therapy. Thyroid. 2012;22(9):926-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343-1421. [DOI] [PubMed] [Google Scholar]
- 16. Iodine Global Network. Global Scorecard 2020. https://www.ign.org/cm_data/Global-Scorecard-2020–3-June-2020.pdf. Accessed February 2, 2021.
- 17. Huysmans DA, Hermus AR. Iodine and technetium scintigraphy of the thyroid. In: de Herder WW, ed. Functional and Morphological Imaging of the Endocrine System. Endocrine Updates. Vol 7. Springer; 2000. 10.1007/978-1-4615- [DOI] [Google Scholar]
- 18. Delivanis DA, Gustafson MP, Bornschlegl S, et al. Pembrolizumab-induced thyroiditis: comprehensive clinical review and insights into underlying involved mechanisms. J Clin Endocrinol Metab. 2017;102(8):2770-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamazaki H, Iwasaki H, Yamashita T, et al. Potential risk factors for nivolumab-induced thyroid dysfunction. In Vivo. 2017;31(6):1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beever K, Bradbury J, Phillips D, et al. Highly sensitive assays of autoantibodies to thyroglobulin and to thyroid peroxidase. Clin Chem. 1989;35(9):1949-1954. [PubMed] [Google Scholar]
- 21. Tozzoli R, Bagnasco M, Giavarina D, Bizzaro N. TSH receptor autoantibody immunoassay in patients with Graves’ disease: improvement of diagnostic accuracy over different generations of methods. Systematic review and meta-analysis. Autoimmun Rev. 2012;12(2):107-113. [DOI] [PubMed] [Google Scholar]
- 22. Brancatella A, Viola N, Brogioni S, et al. Graves’ disease induced by immune checkpoint inhibitors: a case report and review of the literature. Eur Thyroid J. 2019;8(4):192-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Latrofa F, Ricci D, Sisti E, et al. Significance of low levels of thyroglobulin autoantibodies associated with undetectable thyroglobulin after thyroidectomy for differentiated thyroid carcinoma. Thyroid. 2016;26(6):798-806. [DOI] [PubMed] [Google Scholar]
- 24. Ricci D, Brancatella A, Marinò M, et al. The detection of serum IgMs to thyroglobulin in subacute thyroiditis suggests a protective role of IgMs in thyroid autoimmunity. J Clin Endocrinol Metab. 2020;105(6):dgaa038. [DOI] [PubMed] [Google Scholar]
- 25. Martino E, Bartalena L, Bogazzi F, Braverman LE. The effects of amiodarone on the thyroid. Endocr Rev. 2001;22(2):240-254. [DOI] [PubMed] [Google Scholar]
- 26. Bridge JA, Lee JC, Daud A, Wells JW, Bluestone JA. Cytokines, chemokines, and other biomarkers of response for checkpoint inhibitor therapy in skin cancer. Front Med (Lausanne). 2018;5:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”