Abstract
Case series
Patients: Male, 29-year-old • Male, 54-year-old • Male, 84-year-old • Female, 79-year-old
Final Diagnosis: Atrial fibrillation • COVID-19 • heart failure
Symptoms: Dyspnea • palpitation
Medication: —
Clinical Procedure: —
Specialty: Cardiology
Objective:
Unusual clinical course
Background:
We report 4 family members, a 29-year-old son, 54-year-old father, 79-year-old grandmother, and 84-year-old grandfather, with COVID-19 pneumonia. Only the son had heart failure, with reduced ejection fraction and atrial fibrillation. This report aims to show that age and baseline comorbidities are not always predictors of severe COVID-19 disease.
Case Reports:
Case 1: The son, a 29-year-old man, presented with dyspnea and palpitation. His nasopharyngeal swab was positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). He required high-flow nasal cannula oxygen therapy and had new-onset atrial fibrillation and reduced ejection fraction. Case 2: The father, a 54-year-old man, presented with dyspnea. Nasopharyngeal swab was positive for SARS-CoV-2. He required regular nasal cannula oxygen therapy. Electrocardiogram showed sinus rhythm. Echocardiogram showed normal ejection fraction. Case 3: The grandfather, an 84-year-old man with a history of atrial flutter, chronic kidney disease, and hypertension, presented with dyspnea and fever. Nasopharyngeal swab was positive for SARS-CoV-2. He required regular nasal cannula oxygen therapy. Electrocardiogram showed sinus rhythm. Echocardiogram showed normal ejection fraction. Case 4: The grandmother, a 79-year-old woman with a history of hypertension, presented with dyspnea. Nasopharyngeal swab was positive for SARS-CoV-2. She required regular nasal cannula oxygen therapy. Electrocardiogram showed sinus rhythm.
Conclusions:
COVID-19 caused by SARS-CoV-2 is recognized to affect family members and can involve the heart, causing heart failure and cardiac arrhythmia like atrial fibrillation. This report highlights the importance of cardiac monitoring and consideration of cardiac damage, even without previous risk factors, in all hospitalized patients with COVID-19.
Keywords: Atrial Fibrillation; COVID-19; Heart Failure, Systolic; Severe Acute Respiratory Syndrome Coronavirus 2
Background
COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. It is a global pandemic disease that affected more than 65 million people worldwide and more than 14 million in the United States as of December 3, 2020, according to the Johns Hopkins coronavirus resource center [2]. There have been more than 1.5 million global deaths related to COVID-19 [2]. COVID-19 started in Wuhan, a city in the Hubei province of China, in December 2019 and then spread worldwide to affect 191 countries and regions [3]. The World Health Organization declared COVID-19 as a pandemic on March 11, 2020 [4].
COVID-19 disease can affect many organs, including the heart, kidneys, liver, and lungs, and the hematological, nervous, and gastrointestinal systems, but it usually starts within the respiratory tract [5]. Cardiac damage caused by COVID-19 can take multiple presentations, such as cardiac arrest, myocarditis, stress-induced cardiomyopathy, acute coronary syndrome, venous thromboembolism, arrhythmias, and subsequently heart failure [6–8]. Baseline cardiovascular diseases put patients with COVID-19 at a higher risk of increased complications and death [9]. Also, cardiac injury caused by COVID-19 increases the risk of morbidities and mortalities [10]. One of the most common conduction system diseases in patients with COVID-19 is atrial arrhythmia, such as atrial fibrillation and atrial flutter [11]. There is a known association between atrial fibrillation and SARS-CoV-2 infection [12]. Atrial fibrillation can be the presenting symptom of COVID-19 in some cases or can be a complication during the course of the disease [13]. Heart failure secondary to COVID-19 is yet to be studied at multiple levels, including epidemiology, mechanisms of injury, risk factors, and management. Heart failure with preserved ejection fraction was reported in patients with COVID-19 [14]. Some studies have shown that preexisting comorbidities in elderly patients are the main risk factors for cardiac injury from COVID-19 [15].
Herein, we present 4 cases of members from the same family, including a 29-year-old son, 54-year-old father, 84-year-old grandfather, and 79-year-old grandmother, who were hospitalized with COVID-19. Only the son had atrial fibrillation and heart failure, with reduced ejection fraction and without a previous history of cardiovascular disease.
COVID-19 testing in these patients was performed by viral RNA detection using a nasopharyngeal swab. Samples were tested by reverse transcription-polymerase chain reaction (RT-PCR) methodology using the Bio-Fire COVID-19 test device. This test was developed, and its performance characteristics were determined by Bio-Fire Defense, LLC (Salt Lake City, UT, USA). This test is authorized by the U.S. Food and Drug Administration under emergency use authorization (EUA) of in vitro diagnostic tests for the detection of SARS-CoV-2 (EUA Number: EUA200044) [16,17]. The specificity of most of the RT-PCR tests is 100%, but false-positive results can happen secondary to reagent contamination or technical errors [18]. In our cases, the pretest probability, and the clinical/radiology findings with a positive RT-PCR SARS-CoV-2 test in a high COVID-19 prevalence area confirmed the COVID-19 diagnosis.
Case Reports
Case 1. The 29-Year-Old Son
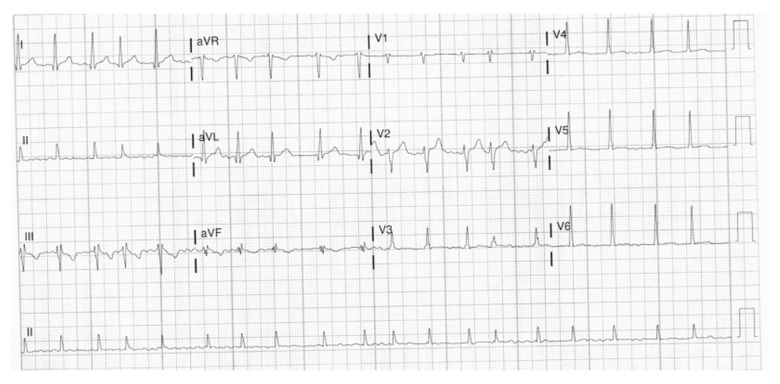
A 29-year-old man with no past medical history presented to the hospital in October 2020 with fever, chills, sweats, palpitation, and shortness of breath for 1 week before admission. In the Emergency Department (ED), his vital signs showed a temperature of 38.05°C, blood pressure of 124/72 mmHg, heart rate of 131 beats per min, respiratory rate of 28 breaths per min, and oxygen saturation (SaO2) of 91% on room air. His body mass index was 34.7. The physical examination showed a young man with mild respiratory distress. The lung examination showed diffuse rhonchi in both lungs, with decreased breath sounds at the bases bilaterally. The heart examination showed an irregularly irregular heart rate and rhythm. The physical examination was otherwise unremarkable. The laboratory examination showed a white blood cell (WBC) count of 7.3 K (reference range, 4.8–10.8 K), ferritin level of 807 ng/mL (range, 3–244 ng/mL), B-type natriuretic peptide (BNP) of 143 pg/mL (range, 0–100 pg/mL), C-reactive protein (CRP) level of 13.3 mg/dL (range, 0–0.9 mg/dL), erythrocyte sedimentation rate (ESR) of 20 mm/h (range, 0–20 mm/h), and troponin of 0.062 ng/mL (range, 0–0.056 ng/mL), which then normalized (Table 1). The arterial blood gas had a pH of 7.46, PCO2 of 26.5 mmHg, and PaO2 of 64.1 mmHg. The electrocardiogram (EKG) showed an irregularly irregular RR interval and no distinct P waves, consistent with atrial fibrillation (Figure 1). His heart rate, which was 155 beats per min initially, improved with intravenous (i.v.) cardizem to 109 beats per min. The patient had a chest computed tomography (CT) scan with a pulmonary embolism protocol, which showed extensive bilateral ground-glass opacities and no evidence of a pulmonary embolism (Figure 2).
Table 1.
The laboratory findings at hospital admission in 2020 of the 29-year-old son, 54-year-old father, 84-year-old grandfather, and 79-year-old grandmother.
| WBC | Ferritin | BNP | CRP | ESR | Troponin | |
|---|---|---|---|---|---|---|
| Normal values | (4.8–10.8 K) | (3–244 ng/ml) | (0–100 pg/ml) | (0–0.9 mg/dl) | (0–20 mm/hr) | (0–0.056 ng/ml) |
| Case 1 | 8 | 807 | 143 | 13.3 | 20 | 0.062 |
| Case 2 | 7.3 | 774 | 92 | 24.5 | 28 | 0 |
| Case 3 | 12.1 | 2940 | 322 | 16.6 | 24 | 0 |
| Case 4 | 4.1 | 302 | 57 | 6.5 | 109 | – |
Figure 1.
Case 1: EKG of the 29-year-old son on hospital admission in 2020. It shows atrial fibrillation with a heart rate of 109 beats per min.
Figure 2.
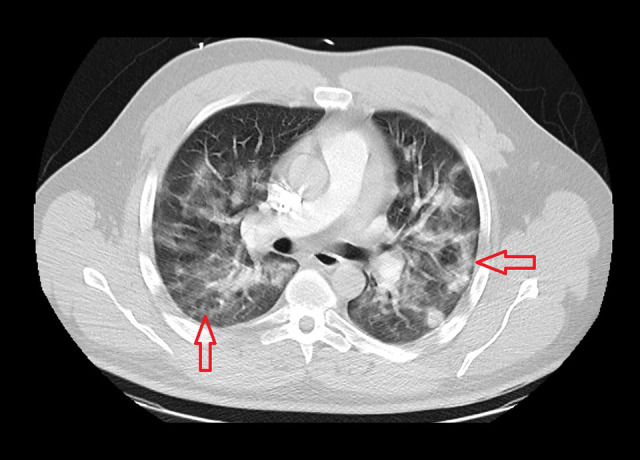
Case 1: Chest computed tomography scan of the 29-year-old son on hospital admission in 2020. It shows extensive bilateral ground-glass opacities.
His respiratory Bio-Fire panel using a nasopharyngeal swab was positive for SARS-CoV-2. He initially required only 2 L of O2 using a nasal cannula to keep his SaO2 above 94%. However, his SaO2 continued to drop to the high 80s even with a nasal cannula, venti-mask, and non-rebreather mask. He was started on high-flow nasal cannula O2 therapy and required 30 L of O2 and 70% of FiO2 to keep his SaO2 at more than 94%.
His atrial fibrillation was treated with metoprolol tartrate and flecainide, and he converted to a normal sinus rhythm. His CHA2DS2-VASc score was 1, and because of the possible high risk of thromboembolic complications in patients with COVID-19, he was started on full anticoagulation with a heparin drip initially and was then was switched to Eliquis. He was treated with dexamethasone 6 mg i.v. daily, i.v. remdesivir, and 1 unit of convalescent plasma transfusion (COVID-19).
His echocardiogram showed a mildly dilated left ventricle (LV) with severe global LV dysfunction (ejection fraction of 20–25%, LV internal diameter end-systole (LVIDs) of 5.2 cm (standard rage, 2–4 cm), moderate right ventricular (RV) hypokinesis, severe left atrial enlargement measuring 6.1 cm (reference range is <4.1 cm in males), trace tricuspid regurgitations, and trace mitral regurgitations (Figures 3–5). When comparing these echocardiogram findings to the echocardiogram he had in 2015, we found that, in 2015, his ejection fraction was within the normal range (62%), LVIDs was 4 cm, and his RV was not hypokinetic (Figure 6). Lisinopril was then administered. The patient did well during the hospitalization and was able to be weaned off the O2. His O2 saturation was good on room air, and he was discharged home.
Figure 3.
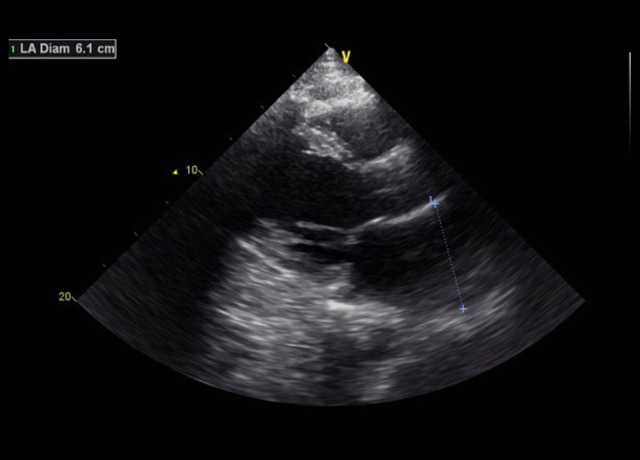
Case 1: Echocardiogram of the 29-year-old son on hospital admission in 2020. It shows elevated left atrial diameter to 6.1 cm (normal is 4.1 cm in males).
Figure 4.
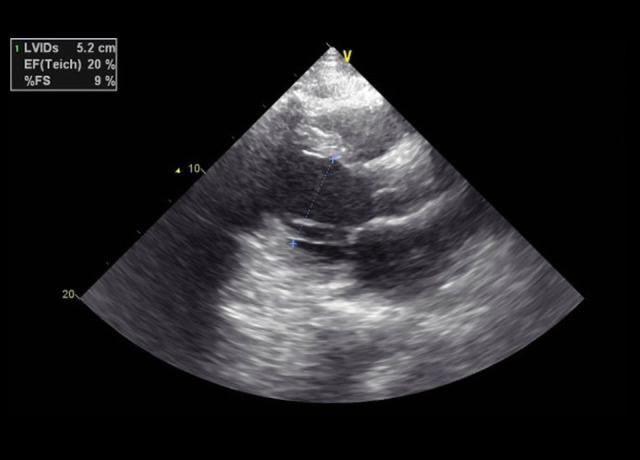
Case 1: Echocardiogram of the 29-year-old son on hospital admission in 2020. Elevated left ventricular internal diameter at end-systole (LVIDs) to 5.2 cm (normal range, 2–4 cm).
Figure 5.
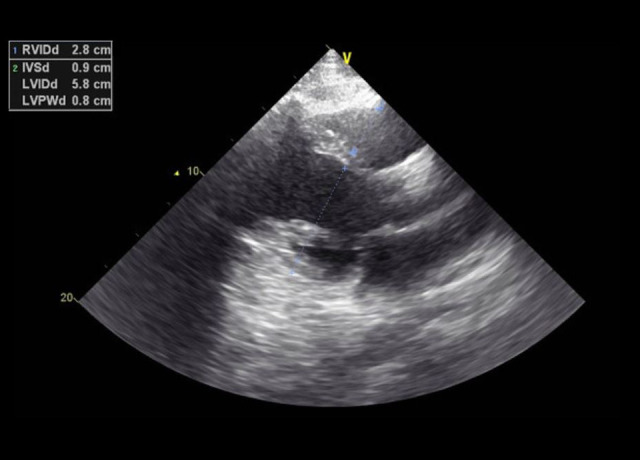
Case 1: Echocardiogram of the 29-year-old son on hospital admission in 2020. It shows elevated enddiastole right and left ventricular internal diameters.
Figure 6.
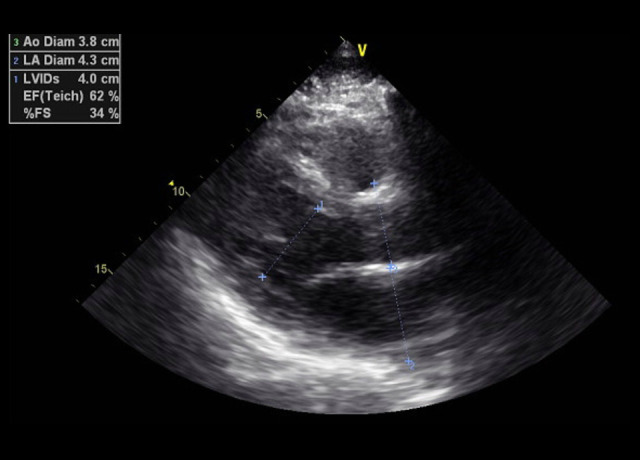
Case 1: Echocardiogram of the 29-year-old son in 2015. It shows normal left ventricular internal diamet
Case 2. The 54-Year-Old Father
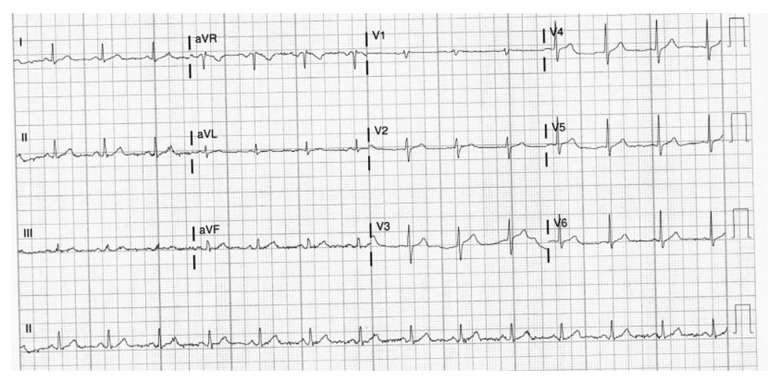
A 54-year-old man with no known past medical history presented to the hospital with exertional dyspnea for 1 week before admission. He did not have chest pain or cough. His vital signs in the ED showed a temperature of 38.3°C, blood pressure of 127/62 mmHg, heart rate of 76 beats per min, respiratory rate of 19 breaths per min, and SaO2 of 91% on room air. The physical examination showed decreased breath sounds at the lung bases. The heart examination showed a regular rate and rhythm. The physical examination was otherwise un-remarkable. The laboratory examination showed a WBC of 7.3 K, ferritin level of 774 ng/mL, BNP of 92 pg/mL, CRP level of 24.5 mg/dL, ESR of 28 mm/h, and twice-tested troponin levels of 0 ng/mL. His blood gas had a pH of 7.445, PCO2 of 30.7 mmHg, and PaO2 of 67.5 mmHg. The respiratory Bio-Fire panel using a nasopharyngeal swab was positive for SARS-CoV-2. The EKG showed a normal sinus rhythm (Figure 7). The chest X-ray showed patchy bibasilar opacities (Figure 8). The patient was given 2 L of nasal cannula O2 therapy, with an improvement of his PaO2 to 96 mmHg and SaO2 to 98%. The patient was treated with i.v. remdesivir and i.v. dexamethasone. He also had a unit of convalescent plasma transfusion (COVID-19).
Figure 7.
Case 2: EKG of the 54-year-old father on hospital admission in 2020. It shows sinus rhythm with a rate of 84 beats per min.
Figure 8.
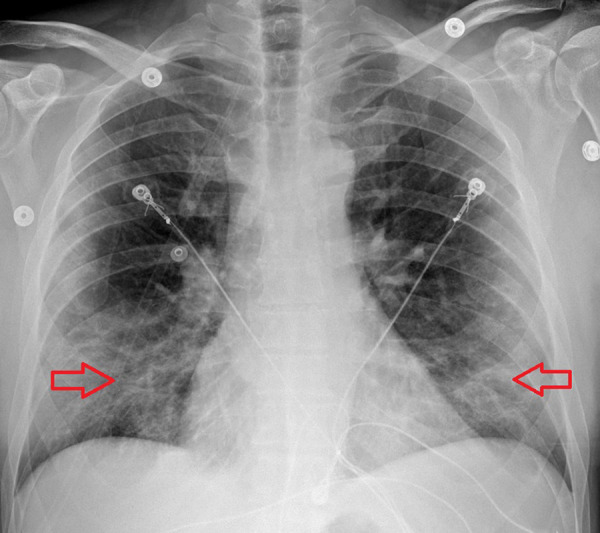
Case 2: Chest X-ray of the 54-year-old father. It shows patchy bibasilar opacities.
An echocardiogram showed a normal LV size and function (EF 65%), normal RV size and function, moderate left atrial enlargement, mild concentric LV hypertrophy, trace mitral regurgitations/tricuspid regurgitations, and a small pericardial effusion. The patient was weaned off O2, and his SaO2 was good on room air. He was discharged home.
Case 3. The 84-Year-Old Grandfather
An 84-year-old man with a history of atrial flutter, primary hypertension, and chronic kidney disease presented to the hospital with shortness of breath, fever, and generalized weakness.
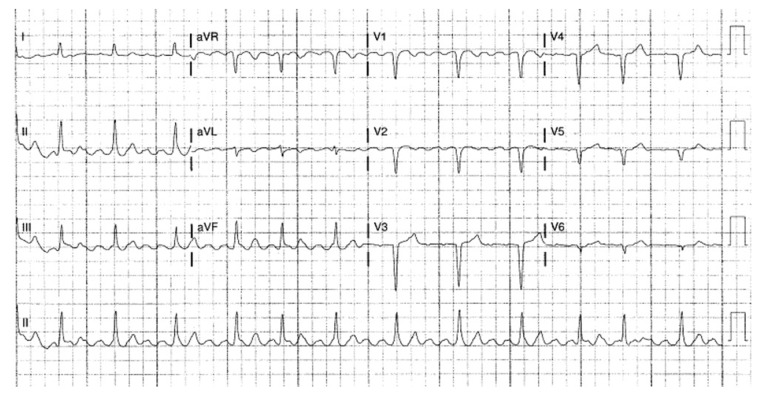
His medications included amiodarone and Xarelto for his atrial flutter. His vital signs in the ED were temperature of 36.8°C, blood pressure of 136/75 mmHg, heart rate of 68 beats per min, respiratory rate of 23 breaths per min, and SaO2 99% on room air. The physical examination showed an irregular heart rhythm. The lung examination showed fine crackles bilaterally. The laboratory examination showed a WBC of 12.1 K, ferritin level of 2940 ng/mL, BNP of 322 pg/mL, CRP level of 16.6 mg/dL, ESR of 24 mm/h, and troponin level of 0 ng/mL. His RT-PCR test was positive for SARS-CoV-2. The EKG showed atrial flutter, with a varied AV block and rate of 71 beats per min (Figure 9). The chest CT with a pulmonary embolism protocol showed no pulmonary embolism but bilateral multifocal pulmonary airspace disease. He had an echocardiogram which showed a normal LV size and function (ejection fraction of 60%), normal RV size and function, mild atrial enlargement, and mild tricuspid regurgitations. The patient was administered i.v. dexamethasone, and he continued his home anticoagulation medication. The patient did well and was discharged home.
Figure 9.
Case 3: EKG of the 84-year-old grandfather. It shows atrial flutter with a varied AV block and a rate of 71 beats per min.
Case 4: The 79-Year-Old Grandmother
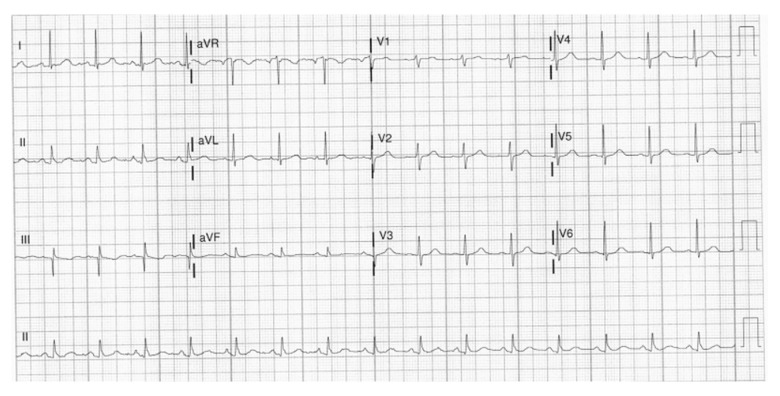
A 79-year-old woman with a past medical history of primary hypertension, morbid obesity, gastrointestinal bleed, and lumbar spinal disc herniation, who had recent surgery with laminectomy and discectomy complicated with postoperative epidural hematoma and abscess, presented to the hospital with shortness of breath and myalgia. Her vital signs in the ED were temperature of 37.1°C, blood pressure of 159/69 mmHg, heart rate of 74 beats per min, respiratory rate of 20 breaths per min, and SaO2 of 98% on room air. The physical examination showed a regular heart rhythm and coarse crackles bilaterally. The physical examination was otherwise unremarkable. The laboratory examination showed a WBC of 4.1, ferritin level of 302 ng/mL, BNP of 53 pg/mL, CRP level of 6.5 mg/dL, and ESR of 109 mm/h. The D-dimer was elevated to 1.49 mg/L (reference range, 0–0.52 mg/L). The EKG showed a sinus rhythm (Figure 10). She tested positive for SARS-CoV-2. The chest CT scan with a pulmonary embolism protocol was negative for pulmonary embolism but showed patchy ground-glass opacities in both lungs. Secondary to the elevated D-dimer, the patient had a 4-point venous Doppler ultrasound which showed evidence of right-sided basilic venous thrombosis, but the patient was asymptomatic and was treated with supportive care, especially because of her history of the gastrointestinal bleed and the recent epidural hematoma. Her SARS-CoV-2 infection was treated with i.v. Decadron. No echocardiogram was done, but she did not show any signs or symptoms of cardiac compromise. The patient did well and was discharged home.
Figure 10.
Case 4: EKG of the 79-year-old grandmother. It shows sinus rhythm with a heart rate of 94 beats per min.
Discussion
This report shows that age and baseline comorbidities are not always predictors of severe COVID-19 disease and cardiac injury, as shown in some studies [19–21]. This report highlights the importance of cardiac monitoring and consideration of cardiac damage, even without previous risk factors, in all hospitalized patients with COVID-19. In this report, we presented 4 cases of COVID-19 disease caused by SARS-CoV-2 infection within the same family but in different generations. Only the youngest patient, who did not have a baseline cardiovascular disease, had heart failure, with reduced ejection fraction and atrial fibrillation. He was the only family member that required high-flow nasal cannula O2 therapy.
COVID-19 disease is caused by SARS-CoV-2 infection [22]. It has been proposed that the disease originated in bats and then moved to Malayan pangolin as an intermediate host and then to humans [23]. SARS-CoV-2 binds to human cells at angiotensin-converting-enzyme (ACE) receptors and disrupts the kininkallikrein and renin-angiotensin systems [24]. The main portal of entry is the lungs [25]. SARS-CoV-2 infection can cause multiorgan failure, affecting the organs that have ACE receptors, such as the lungs, heart, kidneys, vascular endothelium, and intestinal epithelium [5]. Higher morbidities and mortalities have been observed in patients with preexisting conditions like diabetes mellitus, cardiovascular disease, hypertension, and obesity [19]. A study by Li et al showed that patients with COVID-19 with a history of cardiovascular disease, hypertension, and diabetes mellitus were prone to more severe disease and treatment in the Intensive Care Unit (ICU) [20].
Myocardial damage can be seen in at least 10% of hospitalized patients with COVID-19 and in up to 25% to 35%, or even more, of critically ill patients [26]. Myocardial injuries can be evidenced by elevated cardiac markers or new EKG or echo-cardiographic abnormalities. Up to 12% of patients without cardiovascular disease can have an acute cardiac injury (elevated cardiac markers or EKG abnormalities) during hospitalization, as reported by the National Health Commission of China in March 2020 [27]. There was a 17% risk of acute cardiac injury out of 191 patients with COVID-19 in a multicenter cohort study in China [28]. Another study with 416 patients with COVID-19 found that cardiac injury was present in 20% of cases, increasing the risk of mechanical ventilation by 5-fold and the risk of mortality by 11-fold [29]. Mortality increases in patients with COVID-19 if a cardiac injury is present [29]. The presence of preexisting cardiovascular disease is also associated with high mortality [30]. COVID-19 can induce a different spectrum of cardiovascular manifestations, including cardiac arrest, myocarditis, stress-induced cardiomyopathy, acute myocardial injury, acute coronary syndrome, venous thromboembolism, arrhythmias, and, subsequently, heart failure [6–8]. It is still unclear if the cardiac damage is secondary to a systemic, local, or inflammatory injury. Some studies have identified SARS-CoV-2 by RT-PCR in heart samples, and other studies showed mild myocardial inflammation but without cardiomyocyte apoptosis [31]. Causes of cardiac injury in patients with COVID-19 are not well established, but multiple hypotheses exist, including hypoxia, myocarditis, stress-induced cardiomyopathy, small vessel vasculitis, cardiac microvascular ischemia, endotheliitis, and cytokine storm [32–34]. Huayan et al studied the risk factors for cardiac involvement in 102 hospitalized patients with COVID-19 and found that older age with underlying comorbidities (hypertension, cardiovascular disease, diabetes mellitus, cardiovascular disease, chronic obstructive pulmonary disease, chronic kidney disease, and elevated CRP levels) were the main risk factors for cardiac involvement [21]. The 29-year-old man in this report was the youngest among the 4 family members with COVID-19. His CRP level was lower than most of his family members. He did not have underlying medical problems. His grandfather had hypertension and chronic kidney disease but did not develop the disease severity that his grandson had.
Heart failure is one of the most common cardiac complications of SARS-CoV-2 infection. It has been reported in 23% of all patients and in 49% of patients who died [35]. The etiologies of heart failure in COVID-19 are not yet well studied. Fever, tachycardia, kidney dysfunction, or dehydration can exacerbate a preexisting heart failure or unmask undiagnosed heart failure. New-onset heart failure can be seen in 25% of hospitalized patients with COVID-19 and in 33% of patients with COVID-19 admitted to the ICU [36,37]. Heart failure can be a result of the direct virus effect or secondary to the systemic inflammation impact on the heart. A cardiac MRI can show diffuse hypokinesis (ejection fraction, 33%), edema, and increased LV-wall thickness and volumes with no perfusion defects right after gadolinium injection but with early and late gadolinium enhancement [38]. Endomyocardial biopsy can show viral particles in the myocardium or diffuse inflammatory infiltrates, with a cluster of differentiation 3 [39,40]. Hypercoagulable status in COVID-19 can cause pulmonary embolism and thus acute right ventricular failure [41]. Stress-induced cardiomyopathy can be one of the reasons for acute decompensated heart failure. A study of 100 patients with COVID-19 with a mean age of 66 years found that 90% of the cases had normal LV function, and the most common findings were RV dilation in 39% of cases and LV diastolic dysfunction in 16% of the cases [42,43]. Respiratory distress in patients with COVID-19 can cause elevated pulmonary artery pressure, leading to RV failure and tricuspid regurgitations, which increase mortality. The presence of heart failure with reduced ejection fraction, RV failure, and tricuspid regurgitations >1 is associated with higher mortality [44].
Arrhythmias and conduction system disease are not uncommon in patients with COVID-19. Arrhythmia can present in 17% of hospitalized patients with COVID-19 and in up to 44% of patients with COVID-19 admitted to the ICU [45]. The exact mechanism of cardiac arrhythmia in patients with COVID-19 is not well established but it can be triggered by fevers, hypoxia, electrolytes abnormalities, and sepsis or be caused by antiviral medications or antibiotics used to treat patients with COVID-19 or its complications [12,46]. Cardiac myocyte injury caused by SARS-CoV-2 can also increase the risk of arrhythmia. It is not known if SARS-CoV-2 itself causes the conduction system disease or arrhythmia.
Sinus tachycardia is the most observed arrhythmia in these patients, which could be secondary to the physiologic response of the infection. Atrial fibrillation and atrial flutter are among the most common pathologic arrhythmias seen in patients with COVID-19. The development of new-onset atrial fibrillation in patients with COVID-19 can cause worse cardiovascular outcomes according to an observational study of 160 hospitalized patients with COVID-19 [47]. Preexisting atrial fibrillation can be seen in 11% of patients with COVID-19 and is linked to higher short-term mortality [48]. It is more common for elderly patients with a history of hypertension to have atrial fibrillation during their SARS-CoV-2 infection [13]. Our patient was young and without a significant medical history. Atrial arrhythmia is more commonly reported in mechanically ventilated patients (17.7%) [49]. Ventricular tachycardia and ventricular fibrillation are more common in patients with elevated troponin levels (12%) [50]. New-onset atrial tachyarrhythmia (atrial fibrillation, atrial flutter, or atrial tachycardia) were present in 16.5% of patients and mainly in patients admitted to the ICU, not those admitted to the regular medical ward [51]. Atrial fibrillation is the most common tachyarrhythmia in patients with COVID-19 according to a survey from 76 countries (50% were from outside the United States), but 31% were using chloroquine and azithromycin regularly [52]. Other arrhythmias that can be seen are sinus bradycardia, intermittent high-degree AV block, and sinus-node dysfunction [51,53]. From 4250 patients with COVID-19 in a multicenter New York study, 6.1% had QT prolongation to more than 500 milliseconds [54]. The risk factors and mechanisms of atrial fibrillation and heart failure in patients with COVID-19 need to be studied further.
Conclusions
COVID-19 disease caused by SARS-CoV-2 infection is recognized to affect family members and can involve the heart, causing heart failure and cardiac arrhythmia, such as atrial fibrillation. Atrial fibrillation and heart failure are not uncommon in patients with COVID-19, and more data are required in the future to determine the risk factors of cardiac involvement in COVID-19. This report highlights the importance of cardiac monitoring and consideration of cardiac damage, even in patients without previous risk factors, in all hospitalized patients with COVID-19.
Footnotes
Conflicts of Interest
None.
Declaration of Figures Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Morens DM, Breman JG, Calisher CH, et al. The origin of COVID-19 and why it matters. Am J Trop Med Hyg. 2020;103(3):955–59. doi: 10.4269/ajtmh.20-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins University Medical Center . Global and US Daily COVID-19 Data. 2021. Coronavirus Resource Center. https://coronavirus.jhu.edu. [Google Scholar]
- 3.Zhu H, Wei L, Niu P. The novel coronavirus outbreak in Wuhan, China. Glob Health Res Policy. 2020;5:6. doi: 10.1186/s41256-020-00135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–60. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–32. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thakkar S, Arora S, Kumar A, et al. A systematic review of the cardiovascular manifestations and outcomes in the setting of Coronavirus-19 disease. Clin Med Insights Cardiol. 2020;14:1179546820977196. doi: 10.1177/1179546820977196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhakal BP, Sweitzer NK, Indik JH, et al. SARS-CoV-2 infection and cardiovascular disease: COVID-19 heart. Heart Lung Circ. 2020;29(7):973–87. doi: 10.1016/j.hlc.2020.05.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giustino G, Pinney SP, Lala A, et al. Coronavirus and cardiovascular disease, myocardial injury, and arrhythmia: JACC Focus Seminar. J Am Coll Cardiol. 2020;76(17):2011–23. doi: 10.1016/j.jacc.2020.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng P, Zhu H, Witteles RM, et al. Cardiovascular risks in patients with COVID-19: Potential mechanisms and areas of uncertainty. Curr Cardiol Rep. 2020;22(5):34. doi: 10.1007/s11886-020-01293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Guo T, Dong D, et al. Defining heart disease risk for death in COVID-19 infection. QJM. 2020;113(12):876–82. doi: 10.1093/qjmed/hcaa246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dherange P, Lang J, Qian P, et al. Arrhythmias and COVID-19: A review. JACC Clin Electrophysiol. 2020;6(9):1193–204. doi: 10.1016/j.jacep.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lakkireddy DR, Chung MK, Gopinathannair R, et al. Guidance for cardiac electrophysiology during the COVID-19 pandemic from the Heart Rhythm Society COVID-19 Task Force; Electrophysiology Section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm. 2020;17(9):e233–41. doi: 10.1016/j.hrthm.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taha ME, Alsafi W, Taha M, et al. coronavirus disease and new-onset atrial fibrillation: Two cases. Cureus. 2020;12(5):e8066. doi: 10.7759/cureus.8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freaney PM, Shah SJ, Khan SS. COVID-19 and heart failure with preserved ejection fraction. JAMA. 2020;324(15):1499–500. doi: 10.1001/jama.2020.17445. [DOI] [PubMed] [Google Scholar]
- 15.Fu L, Liu X, Su Y, et al. Prevalence and impact of cardiac injury on COVID-19: A systematic review and meta-analysis. Clin Cardiol. 2021;44(2):276–83. doi: 10.1002/clc.23540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FDA U.S. Food and Drug Administration BioFire COVID-19 test – letter of authorization. Mar 12, 2021. https://www.fda.gov/media/136356/download.
- 17.Creager HM, Cabrera B, Schnaubelt A, et al. Clinical evaluation of the BioFire® Respiratory Panel 2.1 and detection of SARS-CoV-2. J Clin Virol. 2020;129:104538. doi: 10.1016/j.jcv.2020.104538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARSCoV-2. JAMA. 2020;323(22):2249–51. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 19.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–38. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu H, Hou K, Xu R, et al. Clinical characteristics and risk factors of cardiac involvement in COVID-19. J Am Heart Assoc. 2020;9(18):e016807. doi: 10.1161/JAHA.120.016807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–44. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seyed Hosseini E, Riahi Kashani N, Nikzad H, et al. The novel coronavirus disease-2019 (COVID-19): Mechanism of action, detection and recent therapeutic strategies. Virology. 2020;551:1–9. doi: 10.1016/j.virol.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–73. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shereen MA, Khan S, Kazmi A, et al. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomasoni D, Italia L, Adamo M, et al. COVID-19 and heart failure: From infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur J Heart Fail. 2020;22(6):957–66. doi: 10.1002/ejhf.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–60. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–10. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inciardi RM, Adamo M, Lupi L, et al. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy [published correction appears in Eur Heart J. 2020;41(48):4591] Eur Heart J. 2020;41(19):1821–29. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fox SE, Lameira FS, Rinker EB, Vander Heide RS. cardiac endotheliitis and multisystem inflammatory syndrome after COVID-19. Ann Intern Med. 2020;173(12):1025–27. doi: 10.7326/L20-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tersalvi G, Vicenzi M, Calabretta D, et al. elevated troponin in patients with coronavirus disease 2019: Possible mechanisms. J Card Fail. 2020;26(6):470–75. doi: 10.1016/j.cardfail.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41(32):3038–44. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study [published correction appears in BMJ. 2020;368:m1295] BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612–14. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bader F, Manla Y, Atallah B, Starling RC. Heart failure and COVID-19. Heart Fail Rev. 2020;26(1):1–10. doi: 10.1007/s10741-020-10008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garot J, Amour J, Pezel T, et al. SARS-CoV-2 fulminant myocarditis. JACC Case Rep. 2020;2(9):1342–46. doi: 10.1016/j.jaccas.2020.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta P, McAuley DF, Brown M, et al. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–34. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tavazzi G, Pellegrini C, Maurelli M, et al. Myocardial localization of corona-virus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911–15. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abou-Ismail MY, Diamond A, Kapoor S, et al. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management [published correction appears in Thromb Res. 2020 Nov 26] Thromb Res. 2020;194:101–15. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szekely Y, Lichter Y, Taieb P, et al. Spectrum of cardiac manifestations in COVID-19: A systematic echocardiographic study. Circulation. 2020;142(4):342–53. doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Argulian E, Sud K, Vogel B, et al. Right ventricular dilation in hospitalized patients with COVID-19 infection. JACC Cardiovasc Imaging. 2020;13(11):2459–61. doi: 10.1016/j.jcmg.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rath D, Petersen-Uribe Á, Avdiu A, et al. Impaired cardiac function is associated with mortality in patients with acute COVID-19 infection. Clin Res Cardiol. 2020;109(12):1491–99. doi: 10.1007/s00392-020-01683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–69. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clerkin KJ, Fried JA, Raikhelkar J, et al. COVID-19 and cardiovascular disease. Circulation. 2020;141(20):1648–55. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 47.Pardo Sanz A, Salido Tahoces L, Ortega Pérez R, et al. New-onset atrial fibrillation during COVID-19 infection predicts poor prognosis. Cardiol J. 2021;28(1):34–40. doi: 10.5603/CJ.a2020.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zuin M, Rigatelli G, Bilato C, et al. Pre-existing atrial fibrillation is associated with increased mortality in COVID-19 patients. J Interv Card Electrophysiol. 2021 doi: 10.1007/s10840-021-00992-2. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N Engl J Med. 2020;382(24):2372–74. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo T, Fan Y, Chen M, et al. Cardiovascular Implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–18. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kir D, Mohan C, Sancassani R. Heart Brake: An unusual cardiac manifestation of COVID-19. JACC Case Rep. 2020;2(9):1252–55. doi: 10.1016/j.jaccas.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gopinathannair R, Merchant FM, Lakkireddy DR, et al. COVID-19 and cardiac arrhythmias: A global perspective on arrhythmia characteristics and management strategies. J Interv Card Electrophysiol. 2020;59(2):329–36. doi: 10.1007/s10840-020-00789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peigh G, Leya MV, Baman JR, et al. Novel coronavirus 19 (COVID-19) associated sinus node dysfunction: A case series. Eur Heart J Case Rep. 2020;4(FI1):1–6. doi: 10.1093/ehjcr/ytaa132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area [published correction appears in JAMA 2020;323(20): 2098] JAMA. 2020;323(20):2052–59. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]