Abstract
Background
Late relapse (LR) of nonseminomatous germ cell tumour (NSGCT) is uncommon, with limited data published. LR is defined as relapse occurring after a disease-free interval of 2 yr.
Objective
To review features of NSGCT LR in a UK tertiary centre.
Design, setting, and participants
A total of 3064 patients were referred from January 2005 to December 2017. We identified patients who experienced LR after initial pathology demonstrated NSGCT and reviewed data for their original and LR presentation and management.
Outcome measurements and statistical analysis
Outcomes included time to LR measured from the date of diagnosis, and overall survival. This was assessed using Cox proportional Hazards modelling, with stratification or adjustment for potential confounders.
Results and limitations
We identified 101 patients with LR; the median time to LR was 96 mo. Forty-three patients (42.6%) experienced relapse after 10 yr. Univariable log-rank testing revealed that the median time to LR was significantly shorter for patients who had not received induction chemotherapy (iCTx; 54 mo, 95% confidence interval [CI] 48–108) than for those who did (112 mo, 95% CI 84–186; p = 0.04). Patients who had received iCTx were less likely to have elevated tumour markers (36% vs 46%) and more likely to undergo initial surgical resection at LR compared to CTx-naïve patients. Postpubertal teratoma (PPT), yolk sac, and dedifferentiated elements predominated for patients with iCTx exposure, whereas active GCT or fibrosis predominated in postchemotherapy resections for CTx-naïve patients at LR. Forty-one men underwent postchemotherapy retroperitoneal lymph node dissection (PC-RPLND) as part of their initial treatment for metastatic disease. Of these, 20 experienced LR in the retroperitoneum, with 18 undergoing repeat RPLND as part of their LR management. Fifteen of the repeat RPLND histopathology specimens had a PPT component. There have been 23 deaths overall; survival was worse for patients presenting with symptoms (13/36, 33%) and those receiving CTx and no surgery (10/17, 59%) at LR.
Conclusions
When LR of NSGCT occurs, it is frequently after an extended interval and is later among patients with prior iCTx, with PPT predominating. The high frequency of LR within the retroperitoneum following PC-RPLND reinforces the need for good-quality PC-RPLND.
Patient summary
We reviewed data for patients who had a late relapse of testicular cancer. We found that patients who did not receive chemotherapy as the first treatment for their initial diagnosis had a shorter time to relapse. Our results highlight the importance of long-term follow-up for testicular cancer.
Keywords: Cancer, Germ cell, Nonseminoma, Retroperitoneal lymph node dissection, Relapse
Take Home Message
Late recurrence of nonseminomatous germ cell tumours most frequently occurs more than 10 yr after the initial diagnosis.
1. Introduction
Late relapse (LR) of nonseminomatous germ cell tumour (NSGCT) is a relapse occurring after a disease-free interval of 2 yr. This is uncommonly reported but an increasingly recognised entity. Accurate determination of NSGCT LR is problematic owing to its rarity, the time interval since initial treatment, and its occurrence in a young, geographically mobile population.
For cases of clinical stage 1 (CS1) NSGCT at presentation, most relapses occur within 2 yr of diagnosis [1]. These early relapse (ER) cases exhibit aggressive behaviour, with features consistent with metastatic disease at presentation. LR in men with clinical stage 1 (CS1) disease without chemotherapy exposure is typically associated with slower-growing elements that may be less aggressive. These include seminomatous components of mixed GCTs and postpubertal teratoma (PPT) in the retroperitoneum that eventually manifest as LR [2], [3].
By contrast, LR in NSGCT with metastatic disease at original presentation appears to be a different entity. Chemotherapy alters the biology of LR, and it has been suggested that chemotherapy exposure should be included as a prerequisite for the definition of LR [4], [5], [6]. PPT, dedifferentiation of teratoma, and chemoresistant yolk sac tumours predominate in this scenario due to selection induced by previous induction chemotherapy.
In this study we reviewed the features of LR in NSGCT in a tertiary referral centre.
2. Patients and methods
2.1. Patients
A total of 3064 patients were referred to our centre for NSGCT from January 2005 to December 2017. Patients with LR were identified from our institutional database. LR was defined as a recurrence occurring after a disease-free interval of >24 mo in the absence of a second primary tumour. Patients with pure seminomas and extragonadal GCT were excluded. All patients referred to our institution are discussed at a weekly multidisciplinary team meeting.
2.2. Data collection
Our electronic patient record for each case was interrogated to provide additional information not documented in meeting reports. Information regarding LR included presentation, sites of disease, management, histopathology, and mortality data. Retrospective data for the initial presentation and subsequent treatment were collected from historical records from our and referring institutions. The data included primary histology, clinical stage, and management, including early relapses and postchemotherapy retroperitoneal lymph node dissection (PC-RPLND) or other resections for residual masses. Staging was based on the 2016 International Union Against Cancer TNM staging scheme for testicular cancer. Induction chemotherapy was defined as three or four cycles of cisplatin-based chemotherapy as treatment for metastatic disease. Adjuvant chemotherapy was defined as a single cycle of chemotherapy for CS1 disease judged at risk of relapse.
2.3. Statistical analysis
Time to LR was measured from the date of diagnosis to the date of LR. The Kaplan-Meier method was used to plot time to LR and overall survival. For univariable analyses, a log-rank test was used to compare time to LR between groups. A Cox proportional-hazards model was used in multivariable analyses to assess time to LR. The proportional hazards assumption was tested for each variable separately and for all variables in a model by correlating Schoenfeld residuals with time. Analysis was conducted in R (R Foundation for Statistical Computing, Vienna, Austria), with p < 0.05 considered significant.
3. Results
Of the 3064 patients referred to our service for NSGCT during the study period, 101 experienced LR. Of these, 31 (30.7%) had CS1, 29 (28.7%) had CS2, and 41 (40.6%) had CS3 disease at presentation. The time to LR ranged from 25 to 504 mo following achievement of disease-free status (median 96 mo, interquartile range [IQR] 48–204). The patient characteristics at original presentation and at LR are summarised in Table 1.
Table 1.
Patient and tumour characteristics at original and late relapse presentation
| Parametexr | Result |
|---|---|
| All patients | |
| Men with late relapse (n) | 101 |
| Median age at initial presentation, yr (interquartile range) | 28 (23–25) |
| Clinical stage at original presentation, n (%) | |
| Clinical stage 1 | 31 (30.7) |
| Clinical stage 2 | 29 (28.7) |
| Clinical stage 3 | 41 (40.6) |
| Postchemotherapy RPLND as part of initial treatment, n (%) | 41 (40.6) |
| Symptomatic at late relapse, n (%) | 36 (35.6) |
| Detectable tumour markers at late relapse, n (%) | 39 (38.6) |
| Median time to late relapse, mo (interquartile range) | |
| All patients | 96 (48–204) |
| Patients with clinical stage 1 disease | 55 (42–108) |
| Patients with clinical stage 2 disease | 112 (60–204) |
| Patients with clinical stage 3 disease | 120 (72–252) |
| Deaths, n (%) | 23 (22.8) |
| No induction chemotherapy | |
| Number of men (n) | 24 |
| Clinical stage 1, surveillance | 18 (75) |
| Clinical stage 1, adjuvant therapy | 5 (20.8) |
| Clinical stage 2, radiotherapy | 1 (4.2) |
| Median time to late relapse - no induction chemotherapy exposure [IQR] | 54, [38-108] |
| Management of late relapse, n (%) | |
| Surgical resection | 7 (29.2) |
| Chemotherapy | 17 (70.8) |
| Postchemotherapy surgical resection | 9 |
| Deaths, n (%) | 6 (25) |
| Induction chemotherapy | |
| Number of men (n) | 77 |
| Clinical stage 1 with early relapse or elevated tumour markers after orchidectomy | 8 (10.4) |
| Clinical stage 2 | 28 (36.4) |
| Clinical stage 3 | 41 (53.2) |
| Median time to late relapse - induction chemotherapy exposure [IQR] | 112 [58-228] |
| Management of late relapse, n (%) | |
| Surgical resection | 63 (81.9) |
| Chemotherapy | 11 (14.3) |
| Postchemotherapy surgical resection | 3 |
| Chemoradiation | 1 (1.2) |
| Best supportive care | 2 (2.6) |
| Deaths, n (%) | 17 (22.1) |
RPLND = retroperitoneal lymph node dissection.
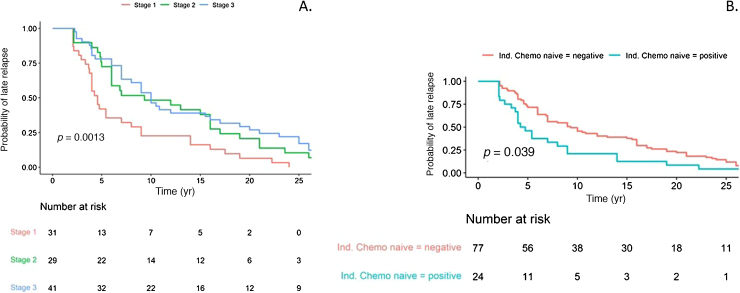
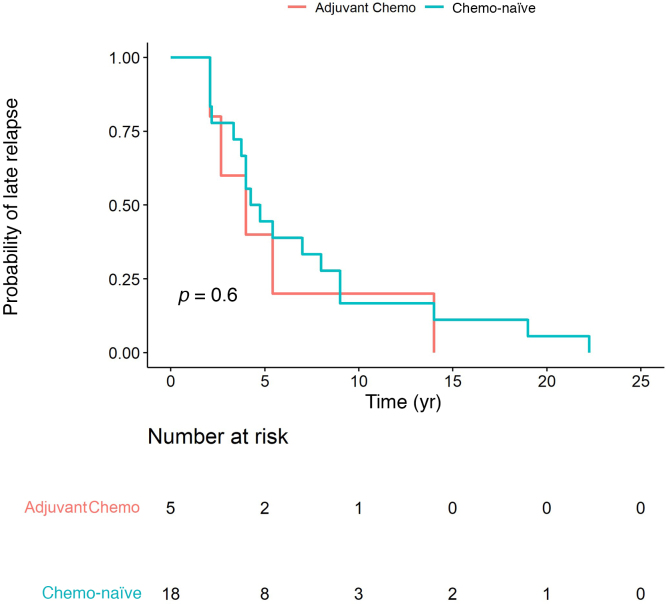
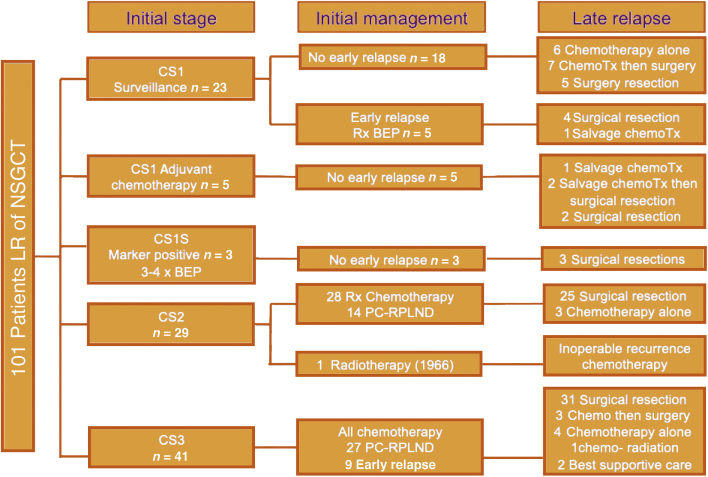
Of the 43 men (42.6%) with relapse beyond 10 yr, 16% had CS1, 33% had CS2, and 51% had CS3 disease at original presentation. A univariable log-rank test for the entire LR cohort revealed a significant difference in the time to LR in CS1 compared to CS2 and CS3 disease (Fig. 1A). The median time to relapse for CS1 disease was 55 mo (IQR 42-108), compared to 112 mo (IQR 60-204) for CS2 and 120 mo (IQR 72-252) for CS3 disease (p = 0.0013). There was a significant difference in time to LR between patients who had induction chemotherapy for metastatic disease at presentation (including CS1S patients) or for early relapse (ie, initially CS1) and those who were chemotherapy-naïve at LR (p = 0.039; median 112mo 95% [CI] 84-186 vs 54mo 95% [CI] 48-108), as shown in Figure 1B. There was no difference in time to LR in the CS1 group between chemotherapy-naïve patients and patients who received adjuvant chemotherapy as initial treatment (univariable log-rank test; Fig. 2). Figure 3 demonstrates initial and LR management by stage at presentation. Table 2 summarise the pattern and site of LR and Table 3 gives an overview of the histopathology.
Fig. 1.
Time to late relapse by (A) clinical stage and (B) induction chemotherapy exposure.
Fig. 2.
Time to late relapse in clinical stage 1 disease: chemotherapy-naïve versus adjuvant chemotherapy (Chemo).
Fig. 3.
Treatment flow chart.
BEP = bleomycin, etoposide, and platinum; ChemoTx = chemotherapy; CS = clinical stage; LR = late relapse; NSGCT = nonseminoma germ cell tumour; PC-RPLND = postchemotherapy retroperitoneal lymph node dissection; Rx = receipt.
Table 2.
Pattern and sites of late relapse
| CTx-naïve (n = 24) | iCTx (n = 77) | |
|---|---|---|
| Elevated serum tumour markers at late relapse, n (%) | 11 (45.8) | 28 (36.4) |
| Number of late relapse sites, n (%) | ||
| Single site | 16 (66.7) | 55 (71.4) |
| Multiple sites | 8 (33.3) | 22 (28.6) |
| Location of single-site late relapse, n (%) | ||
| Retroperitoneal | 11 (68.7) | 34 (61.8) |
| Pelvic | 4 (25) | 4 (7.3) |
| Retrocrural | 0 | 4 (7.3) |
| Supradiaphragmatic | 1 (6.3) | 13 (23.6) |
| Men symptomatic at late relapse, n (%) | 9 (37.5) | 26 (33.7) |
iCTx = induction chemotherapy.
Table 3.
Histopathology of surgical resection specimens from late relapse cases
| Histopathology | Patients |
Deaths, | ||
|---|---|---|---|---|
| Total | iCTx-naïve | iCTx | n (%) | |
| PPT only | 45 | 6 | 39 | 3 (7) |
| DDFM | 7 | 0 | 7 | 2 (29) |
| Yolk sac ± DDFM | 15 | 1 | 14 (2 with DDFM) (including 2 Bx) | 5 (35) |
| Active germ cell tumour ± PPT | 11 | 4 (including 1 Bx) | 7 (1 with DDFM) (including 1 Bx) | 4 (37) |
| Benign/necrosis | 7 | 6 | 1 | 0 |
| Total | 85 | 17 (including 1 Bx) | 68 (nb. no pathology available in one resection) | |
Bx = diagnostic biopsy (no surgical resection); DDFM = dedifferentiated malignancy; iCTx = induction chemotherapy; PPT = postpubertal teratoma.
3.1. CS1 disease: all chemotherapy-naïve patients
In the group of 18 chemotherapy- naïve patients with CS1 disease, LR occurred at a median of 54 mo (95% CI 45–108) after diagnosis. The LR pattern in this group included seven cases with positive STMs. Four patients had multisite relapse; of the cases with single-site LR, ten were in the retroperitoneum, three were in the pelvis, and one was in the lung.
Chemotherapy was the initial treatment for LR for 13 of these 18 patients, of whom six exhibited a complete response and seven/13 underwent postchemotherapy surgical resection. Surgical resection was the initial treatment in five/18 patients. Active GCT and PPT predominated the histopathology in this group, including three benign/necrosis resection specimens among those who had postchemotherapy surgeryTable 3.
3.2. CS1 disease: previous adjuvant chemotherapy
There were five CS1 patients who received adjuvant chemotherapy, of whom none experienced ER. LR in this group occurred at a median time of 48 months (95% CI 32–infinity). The LR pattern included three cases with positive STMs. Three patients had multisite relapse, while one had single-site LR in the retroperitoneum and one had single-site LR in the pelvis.
Histopathology for the four patients undergoing surgical resection showed two PPT and two benign/necrosis cases; two patients had chemotherapy as the initial treatment before surgery.
3.3. CS1 disease: previous induction chemotherapy
LR in the eight men with CS1 disease with previous exposure to three cycles of bleomycin, etoposide, and platinum (three CS1 cases with elevated STMs after orcidectomy [CS1S] and five CS1 cases with ER on surveillance) occurred at a median time of 55 mo (IQR 42–108). The LR pattern included four cases with positive STMs. LR sites were distributed predominantly below the diaphragm, with four exclusively in the retroperitoneum, one retrocrural, and one pelvic relapse. Two patients had multisite relapse, with only one experiencing a relapse above the diaphragm in a subclavicular node.
Histopathology for the seven patients managed with initial surgical resection revealed predominantly PPT (five patients); two case had yolk sac tumour, with one showing associated somatic dedifferentiation.
3.4. CS2 disease
LR in the group of 29 men with CS2 disease occurred at a median time of 112 mo (IQR 60–204). The LR pattern included nine cases with positive STMs and the distribution included 19 relapses exclusively in the retroperitoneum and one in the pelvis. Six relapses were supradiaphragmatic (3 retrocrural, 1 lung, and 2 mediastinal). Three patients experienced multisite relapse.
Histopathology data for the 21 patients undergoing initial surgical resection and the four postchemotherapy surgical resections with one biopsy are shown in Table 3. PPT predominated (n = 15), with yolk sac tumour in five cases. Three patients had predominantly dedifferentiated histopathology, including two of the yolk sac tumours.
3.5. CS3 disease
LR in the group of 41 patients with CS3 disease occurred at a median time of 120 mo (IQR 72–252). The LR pattern included 16 cases with positive STMs. The distribution of LR included 23 single-site relapses, of which 11 were in the retroperitoneum, two were in the pelvis, and the remaining ten were above the diaphragm (five lung, three mediastinal, and two retrocrural). In 18 patients, LR involved multiple sites.
Histopathology for the 31 patients who had initial surgery and three who had post salvage chemotherapy surgery including two biopsies revealed 19 PPT, seven yolk sac, five active GCT, and four dedifferentiated tumours. Of note one of the yolk sac cases and one active GCT exhibited dedifferentiation. Pathology was not available for one case (Table 3).
Among all the cases in the LR cohort who had had induction chemotherapy exposure at LR (CS1 with ER, CS1S, CS2, and CS3) and underwent surgical resection at LR (or biopsy) there was a similar pattern of histopathology: PPT predominated, with yolk sac and dedifferentiated tumours and less active GCT than in the CS1 chemotherapy-naïve and adjuvant subgroups.
3.6. Previous PC-RPLND
PC-RPLND for a residual mass was part of the initial treatment strategy for metastatic disease in 41 patients. The surgeon described a bilateral template for 12 of these cases; among the remaining 29, a modified template or resection of the residual mass without formal template dissection was reported, or the extent of the resection was not specified. Of the 41 patients with prior PC-RPLND, 20 had LR in the retroperitoneum (12 single-site LR and eight retroperitoneum plus other sites). Only six of the 20 patients with retroperitoneal recurrence had a formal bilateral template described at initial surgery. Eighteen patients with PC-RPLND for initial management had repeat retroperitoneal resection of their LR, with 15 having PPT as at least one component in the specimen.
3.7. Survival
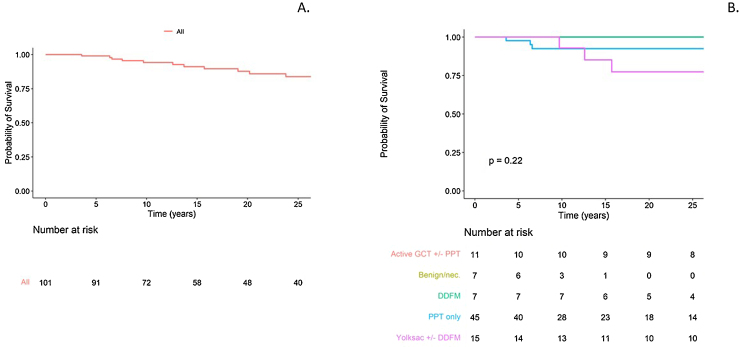
To date, 23 patients have died, of which 21 deaths are related to LR and two are unrelated. Five of these were in CS1, five in CS2, and 13 in CS3 cases. Figure 4 shows the overall survival for the LR cohort. Table 3 shows the mortality according to histopathology for those who underwent resection/biopsy. Patients presenting with symptoms (13/36, 33%) and those who received chemotherapy without surgery (10/17, 59%) at LR tended to have higher mortality.
Fig. 4.
Overall survival (A) for the whole cohort and (B) by histopathology. Note that the line for benign/necrosis (nec.) histopathology in B is not visible as there are no events and it sits at 1.0.
GCT = germ cell tumour; PPT = postpubertal teratoma; DDFM = dedifferentiated malignancy.
4. Discussion
We have presented a large series of patients experiencing NSGCT LR. There are significant limitations to our study that reflect the problems underpinning the paucity of data on NSGCT LR. These patients present many years after initial presentation and treatment, with more than 40% of recurrences occurring beyond 10 yr, with one case we have previously reported after 40 yr [7]. This, combined with the fact that the majority of cases in our series had their initial disease treated elsewhere, poses a challenge for retrospective data capture.
We could not determine the risk or incidence of NSGCT LR, as the size of the population and cohort of patients with testicular cancer from whom our cases have evolved is unclear. In a previous cohort study at our institution of 1263 patients treated between 1979 and 1993, after median follow-up of 10.3 yr we identified 53 (4%) with LR, including 38/732 (5.2%) of NSGCT patients [8].
Whilst this study cannot define the true incidence of LR, it demonstrates that initial clinical stage influences both the risk and time of LR. NSGCT LR incidence has been reported as approximately 2.4% among patients with CS1 disease undergoing surveillance in a population study [9]. As with our study, the majority of recurrences occurred within 5 yr, consistent with our median time to relapse of 55 mo. CS1 disease comprised only 30% of our LR cohort, whereas 50% of NSGCT cases are CS1 at presentation [10], [11]. The risk of LR in CS≥2, accounting for 70% of our cases, would therefore appear to be significantly greater and occurs much later, with a median interval of 10 yr in this group.
LR pathology differs between CS1 and higher stages, which is possibly related to previous exposure to chemotherapy. From our series, the two important pathological diagnoses at LR in the chemotherapy-naïve CS1 group are PPT and active GCT. Others have reported pathological findings suggesting less aggressive forms of active GCT in LR of CS1 disease. It has also been reported that characteristics of CS1 surveillance patients with LR (VLR) are similar to ER (9). Our results for the chemotherapy-naïve CS1 group support these observations; of 16/23 receiving first-line chemotherapy, seven experienced a complete response. Of the remaining nine patients who received initial chemotherapy subsequent surgical resection contained necrosis/fibrosis only in four indicating the presence of chemosensitive GCT within the recurrence.
Adjuvant chemotherapy following orchidectomy was used in five of our 31 cases of CS1 LR, of whom none experienced ER. By contrast, five of 23 patients managed with surveillance alone experienced ER. Of the five CS1 cases who received adjuvant chemotherapy, two patients had only PPT in RPLND specimens. The development of PPT after adjuvant chemotherapy probably represents “growing teratoma”, as viable GCT has been eradicated by the adjuvant chemotherapy [12]. Thus, early consideration of surgical resection alone is warranted for LR in CS1 patients with prior adjuvant chemotherapy, unless there is evidence of active malignancy.
Patients with metastatic disease at presentation who have been exposed to induction chemotherapy represent a different entity. It is clear that LR can occur at extended intervals, with patients in our series developing LR at a median time of 10 yr. This is similar to the LR series in our previous cohort and those of Dieckmann et al [4] and Baniel et al [13].
Our series also highlights important issues regarding surgical management of the retroperitoneum. In 41 cases, LR occurred in patients who had undergone previous PC-RPLND for initial management of metastatic NSGCT. Of these, 20 had relapse in the retroperitoneum, which was the only LR site in 12 cases. Repeat salvage RPLND was performed in 18 of the 20 cases, among whom 15 had PPT on histopathology.
Half of the PC-RPLND procedures in our series were performed elsewhere. While some information was lacking, there was significant heterogeneity in the approach and templates used. Inadequate initial management of the retroperitoneum following chemotherapy is likely to contribute to LR. This reinforces the need for a complete and thorough PC-RPLND, particularly if teratoma is a component of the initial metastatic disease or early retroperitoneal recurrence of CS1 disease. These findings support a centralised approach with prospective data collection, as recommended after the British Association of Urological Surgeons RPLND audit [14].
While PPT was the most common element in LR histopathology, dedifferentiation with malignant transformation and active GCT, in particular yolk sac tumours, are also frequently present [8]. These can develop insidiously over a protracted time. It has been postulated that chemotherapy itself may foster these elements [4]. These elements are relatively chemoresistant and surgical resection is felt to be important for curative treatment. Thus, our practice, as reflected in this series, has been to restrict chemotherapy as first-line treatment for LR to patients who are chemotherapy-naïve and have elevated tumour markers or multiple sites of relapse.
5. Conclusions
NSGCT LR occurs after an extended and sometimes extremely protracted interval in many cases and thus may be under-reported in the literature. This reinforces the need for long-term follow-up. LR typically occurs earlier in patients with CS1 disease compared to patients with higher stages, who are typically treated with chemotherapy.
Patients with CS2 and CS3 disease appear to be at the highest risk of LR, with a median relapse interval of 10 yr after initial treatment. PPT including malignant transformations of teratoma is heavily represented and thus surgical intervention for LR should be used where feasible, as responses to chemotherapy are poor.
The high frequency of LR within the retroperitoneum following PC-RPLND reinforces the need for good-quality PC-RPLND restricted to high-volume specialist centres to ensure adequate resection. These institutions should maintain long-term prospective databases with collaborative analyses to further define NSGCT LR.
Author contributions: David L. Nicol had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Jay, Nicol.
Acquisition of data: Jay, Aldiwani, Pearce.
Analysis and interpretation of data: Jay, Aldiwani, Nicol, Reid, Mayer.
Drafting of the manuscript: Jay, Aldiwani, Mayer, Nicol.
Critical revision of the manuscript for important intellectual content: Huddart, Nicol, Reid.
Statistical analysis: Jay, O’Callaghan.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: None.
Other: None.
Financial disclosures: David L. Nicol certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: The authors thank Dr. Suranga Wijayarathna for assistance with data collection.
Associate Editor: Guillaume Ploussard
References
- 1.Kollmannsberger C., Tandstad T., Bedard P.L. Patterns of relapse in patients with clinical stage I testicular cancer managed with active surveillance. J Clin Oncol. 2015;33:51–57. doi: 10.1200/JCO.2014.56.2116. [DOI] [PubMed] [Google Scholar]
- 2.Rice K.R., Beck S.D., Pedrosa J.A., Masterson T.A., Einhorn L.H., Foster R.S. Surgical management of late relapse on surveillance in patients presenting with clinical stage I testicular cancer. Urology. 2014;84:886–890. doi: 10.1016/j.urology.2014.05.054. [DOI] [PubMed] [Google Scholar]
- 3.Stephenson Aj, Bosl Gj, Motzer Rj. Retroperitoneal lymph node dissection for nonseminomatous germ cell testicular cancer: impact of patient selection factors on outcome. J Clin Oncol. 2005;23:2781–2788. doi: 10.1200/JCO.2005.07.132. [DOI] [PubMed] [Google Scholar]
- 4.Dieckmann K.P., Albers P., Classen J. Late relapse of testicular germ cell neoplasms: a descriptive analysis of 122 cases. J Urol. 2005;173:824–829. doi: 10.1097/01.ju.0000154013.96349.36. [DOI] [PubMed] [Google Scholar]
- 5.Sharp D.S., Carver B.S., Eggener S.E. Clinical outcome and predictors of survival in late relapse of germ cell tumor. J Clin Oncol. 2004;26:552–559. doi: 10.1200/JCO.2007.15.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronnen E.A., Kondagunta G.V., Bacik J. Incidence of late-relapse germ cell tumor and outcome to salvage chemotherapy. J Clin Oncol. 2005;23:6999–7004. doi: 10.1200/JCO.2005.21.956. [DOI] [PubMed] [Google Scholar]
- 7.Mukhtar S., Beatty J., Agrawal S., Christmas T.J., Jameson C., Huddart R.A. Germ cell tumour: late recurrence after 43 years. Ann R Coll Surg Engl. 2011;93:e24–6. doi: 10.1308/147870811X580442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shahidi M., Norman A.R., Dearnaley D.P., Nicholls J., Horwich A., Huddart R.A. Late recurrence in 1263 men with testicular germ cell tumors. Multivariate analysis of risk factors and implications for management. Cancer. 2002;95:520–530. doi: 10.1002/cncr.10691. [DOI] [PubMed] [Google Scholar]
- 9.Mortensen M.S., Lauritsen J., Kier M.G. Late relapses in stage I testicular cancer patients on surveillance. Eur Urol. 2016;70:365–371. doi: 10.1016/j.eururo.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Leveridge M.J., Siemens D.R., Brennan K. Temporal trends in management and outcomes of testicular cancer: a population-based study. Cancer. 2018;124:2724–2732. doi: 10.1002/cncr.31390. [DOI] [PubMed] [Google Scholar]
- 11.Osswald M., Harlan L.C., Penson D., Stevens J.L., Clegg L.X. Treatment of a population based sample of men diagnosed with testicular cancer in the United States. Urol Oncol. 2009;27:604–610. doi: 10.1016/j.urolonc.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huddart R.A., Reid A.M. Adjuvant therapy for stage IB germ cell tumours: one versus two cycles of BEP. Adv Urol. 2018;2018 doi: 10.1155/2018/8781698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baniel J., Foster R.S., Gonin R., Messemer J.E., Donohue J.P., Einhorn L.H. Late relapse of testicular cancer. J Clin Oncol. 1995;13:1170–1176. doi: 10.1200/JCO.1995.13.5.1170. [DOI] [PubMed] [Google Scholar]
- 14.Wells H., Hayes M.C., O’Brien T., Fowler S. Contemporary retroperitoneal lymph node dissection (RPLND) for testis cancer in the UK – a national study. BJU Int. 2017;119:91–99. doi: 10.1111/bju.13569. [DOI] [PubMed] [Google Scholar]